Translate this page into:
CXCR4 inhibition suppresses Cd-induced renal oxidative stress, apoptosis, and fibrosis by inhibiting the TGF-β1/Smad pathway
⁎Corresponding author. Address: No. 439, Shuangxi West Road, Wucheng District, Jinhua, Zhejiang, China. yangbiaohe@126.com (Yangbiao He)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study aimed to investigate the role and mechanism of CXC chemokine receptor 4 (CXCR4) in cadmium (Cd)-induced renal injury. CXCR4 and TGF-β1/Smad pathway protein levels were detected by western blotting. Indicators related to renal function and oxidative stress factors were assessed and reactive oxygen species (ROS) level was evaluated by staining. TUNEL was used to measure apoptosis rate. PAS and Masson's trichrome staining were used to detect the level of renal fibrosis. The expression of Bcl-2, Bax, Cleaved-caspase 3, fibronectin, and collagen I proteins were detected by western blotting, immunohistochemistry, or immunofluorescence. The expression of CXCR4 was increased in a Cd-induced chronic renal injury model in rats. Si-CXCR4 decreased levels of TGF-β1, TGF-βR1, p-Smad2/Smad2, p-Smad3/Smad3, the renal weight index, urine protein, blood urea nitrogen, blood creatinine, and levels of MDA but raised the levels of SOD and GSH-Px. In addition, si-CXCR4 inhibited apoptosis in Cd-treated rats. CXCR4 inhibition alleviated fibrosis levels in Cd-treated rats. In Cd-treated cells, TGF-β attenuated the suppressive effect of CXCR4 inhibition on the TGF-β1/Smad pathway. TGF-β intervention increased MDA and ROS, and downregulated SOD and GSH-Px. TGF-β attenuated the inhibitory effect of CXCR4 on apoptosis and fibrosis. CXCR4 inhibition decreased levels of Cd-induced renal oxidative stress, apoptosis, and fibrosis by inhibiting the TGF-β1/Smad pathway.
Keywords
CXCR4
Renal injury
Oxidative stress
Fibrosis
1 Introduction
As an organ of essential metabolism in the body, the kidney is involved in excreting metabolites and toxic substances through urine and regulating water-electrolyte and acid-base balance functions. In contrast, the dysfunction of renal excretion and regulation caused by various pathogenic factors will severely threaten the health of patients, leading to irreversible renal failure and uremia (Plata et al., 2019; Spence, 2021). Chronic kidney disease (CKD) affects 26 to 30 million adults in the United States and remains a major public health concern. The Centers for Disease Control and Prevention predict that 47% of people 30 will develop CKD in their lifetime. Eleven percent of patients with stage 3 CKD will eventually progress to end-stage renal disease (ESRD) requiring dialysis or kidney transplantation. CKD is also one of the strongest risk factors for cardiovascular disease {Zhao, 2020 #60}{Bai, 2019 #61}. Therefore, the clinical treatment of renal impairment is of great importance and value. It has long been established that abnormal exposure to heavy metal cadmium (Cd) ions in the environment is a factor of high tendency for kidney injury, and studies have found that abnormal intake of heavy metal Cd ions can lead to the synthesis and binding of metallothionein, which is widely distributed throughout the organ (Chen et al., 2021b; Kim et al., 2018). The kidney is the main route of excretion of metallic Cd ions, and studies have found that more than a third of Cd ions accumulate in the renal tubules, leading to severe damage to the tubular epithelium and kidney (Aparicio-Soto et al., 2016; Chen et al., 2021a). Therefore, Cd has a general pathological effect on renal function.
CXC chemokine receptor 4 (CXCR4) is widely present on the surface of different cell types and plays a crucial part in embryonic development, inflammation, immune response, angiogenesis, and hematopoietic regulation by binding to stromal cell-derived factor-1 and activating downstream signaling pathways (Mousavi, 2020; Pozzobon et al., 2016). However, Zheng et al. revealed that in a rat model of renal ischemia–reperfusion injury, CXCR4 downregulation promoted the growth of renal tubular epithelial cells (Zheng et al., 2018). In addition, Liu et al. showed that CXCR4 improved acute kidney injury recovery in bone marrow mesenchymal stem cells (Liu et al., 2013). Nevertheless, the function and mechanism of CXCR4 in Cd-induced kidney injury need to be further explored.
Transforming growth factor-β (TGF-β) can affect many biological processes, including extracellular matrix remodeling and apoptosis, and participates in development and pathological reactions such as embryogenesis, immune regulation, fibrosis, wound healing, and tumor progression (Haque and Morris, 2017; Lichtman et al., 2016). Furthermore, a study has shown that TGF-β can induce renal fibrosis (Jung et al., 2020). Similarly, a study by Chuang et al. confirmed that TGF-β can promote fibrosis after severe acute renal injury by stimulating renal macrophage infiltration (Chung et al., 2018). Astragalus polysaccharide extract (APS) can inhibit TGF-β1 and reduce the formation of extracellular matrix in diabetic rats, thus reducing inflammatory reaction and renal fibrosis, renal injury and dysfunction in rats {Zheng, 2021 #67}.In consideration of the above research, we constructed a chronic Cd-induced renal injury model in rats and a Cd-treated cell model as research objects to evaluate the mechanism of CXCR4 inhibition in Cd-induced renal oxidative stress, apoptosis, and fibrosis. The results showed that si-CXCR4 can improve rat renal function by inhibiting Cd-induced oxidative stress, fibrosis and apoptosis in rats with kidney injury. In addition, TGF-β was also studied in the regulation of CXCR4 inhibition. The results will provide a unique perspective and basis for the clinical setting of renal injury.
2 Materials and methods
2.1 Animals
This study used 30 adult male Wistar albino rats (CDC, Hubei, China) weighing 220 ± 15 g (10 weeks). The rats were housed in a control room at a temperature of 23 ± 2℃ and a humidity of 45 ± 15%. A 12 h light and 12 h dark cycle was maintained and the rats were fed a standard diet and water. After adapting to the surrounding environmental conditions, the rats were used for subsequent experiments.
2.2 Establishment of Cd-induced chronic renal injury model in rats and CXCR4 treatment
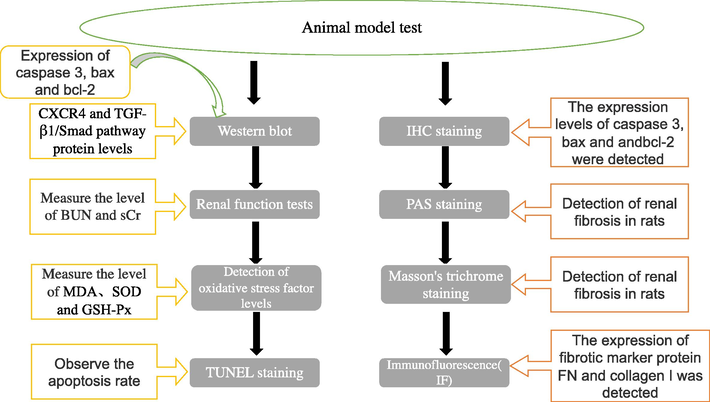
Thirty rats were randomly divided into five groups (six rats in each group), Con group the rats were first administered orally 3 ml isotonic NaCl solution kg−1 day−1 and after 2 h they intraperitoneally (i.p.) injected with 1 ml isotonic NaCl solution kg−1 day−1 over 30 successive days. Cd group the rats received CdCl2 (dissolved in an isotonic NaCl solution) at a dose of 3.5 mg kg−1 bw day−1 via i.p. injections for 30 successive days, at the same time 2 h before the CdCl2 injection, the rats were received orally 3 ml isotonic NaCl solution kg−1 day−1. Control animals received only the vehicle (saline). Intraperitoneal injection of 15 mg/kg CXCR4 inhibitor(AMD-3100; Sigma-aldridge) twice weekly in the Cd+si-CXCR4 group. In the Cd+enalapril group, enalapril was used as the positive control drug (intragastrically administered once daily at 10 mg/kg). TGF-β1 group was injected 6 ng/mL of TGF-β 1 through a caudal vein (Sigma Aldrich, USA). One-day after receiving the last treatment after 2 weeks of treatment rats were euthanized with pentobarbital (200 mg/kg bodyweight). Blood and tissue samples were collected from the inferior vena cava of each rat, clots were allowed to form, and then samples were centrifuged. Serum was harvested and used in renal functional tests. The kidney was immediately dissected and weighted. Renal ti ssue was placed in ice-cold 50 mM Tris-HCl buffer (pH 7.4) and homogenates were generated using a tissue homogenizer, producing a 10% (w/v) tissue homogenate. The homogenate was centrifuged 10 min at 4 °C and the supernatant was stored at −70 °C for biochemical tests.
The schematic diagram is as follows:
2.3 Western blot analysis
The kidney tissues were homogenized and centrifuged. The supernatant was discarded and 1000 μL of RIPA lysis solution containing l% phenylmethylsulfonyl fluoride was added (Beyotime Biotechnology, Shanghai, China). The total protein of the cells was collected. The protein concentration of each group was measured using a BCA (Rockford, IL, USA) assay. Samples of protein (20 μL of 30 mg) were used in SDS-PAGE(Sigma Aldrich, St. Louis, MO, USA) electrophoresis and electrotransferred to PVDF membranes (Millipore, Bedford, MA, USA). Connect CXCR41: (ab181020; 1:1000, Abcam, Shanghai, China), TGF-β1(ab142139; 1:1000, Abcam, Shanghai, China), TGF-βR1(ab14319; 1:1000, Abcam, Shanghai, China), P- Smad2(ab280888; 1:1000, Abcam, Shanghai, China), P- Smad 3(ab52903; 1:1000, Abcam, Shanghai, China), Smad2 (ab40855; 1:1000, Abcam, Shanghai, China), Smad3(ab40854; 1:1000, Abcam, Shanghai, China), Bcl-2(ab; 1:1000, Abcam, Shanghai, China), Bax (ab40855; 1:1000, Abcam, Shanghai, China), Cleaved caspase-3(ab40854; 1:1000, Abcam, Shanghai, China), Fibronectin (FN) (AB268200; 1:1000, Abcam, Shanghai, China) and collagen I(ab138492; 1:1000, Abcam, Shanghai, China) were incubated overnight with the membrane and then with the secondary antibody, GOAT anti-rabbit IgG H&L (AB150077, 1: 2000, Abcam Inc, Cambridge, UK). The protein is color imaged using an ECL chemiluminescent solution {Rhodes, 2011 #69}{Fellous, 2020 #70}.
2.4 Renal function tests
Before the rats were euthanized at week 12, 24-h urine was collected and the levels of protein (24 h-Pro) were measured. Blood was obtained from the abdominal aorta and the serum was centrifuged at 3000g for 10 min at 4 °C. The insoluble material was discarded and the serum was collected. Levels of blood urea nitrogen (BUN) and serum creatinine (sCr) were measured with BUN detection kit and serum creatinine detection kit (StressNarq Bioscience, British Columbia, Canada).
2.5 Detection of oxidative stress factor levels
Kidney samples were weighed to 0.1 g and used to prepare a 10% tissue homogenate. Kidney malondialdehyde (MDA) content was measured by using thiobarbituric acid, superoxide dismutase (SOD) activity was measured by biphenyl triol autoxidation and glutathione peroxidase (GSH-Px) by using a colorimetric method.
2.6 TUNEL staining
Paraffin-embedded tissue sections were dewaxed and hydrated routinely, rinsed twice with PBS, Proteinase K working solution was added, and left to react at 37 °C for 15 min. The samples were blotted with absorbent paper. TdT enzyme reaction solution (50 μL) was added dropwise to each sample and incubated with coverslips at 37 °C for 60 min. The samples were rinsed with PBS three times. Nuclei were stained with DAPI and then rinsed three times with PBS. A quenching agent was added, sealed, protected from light, and then examined on a Leica (DM2500) fluorescence microscope and photographed. Ten high magnification fields were randomly selected for each sample and the number of positive cells was counted.
2.7 Immunohistochemical (IHC) staining
The rabbit anti-rat caspase 3, Bax, and Bcl-2 immunohistochemistry kits (Xin'aosheng, Shanghai, China) were used to observe the expression of the above proteins. The manufacturer’s procedure was followed. After IHC staining, kidney tissue sections were observed under a microscope. The total number of positive cells was calculated using the HPIAS-1000 image analysis system (In Situ Cell Death Detection Kit; Roche Diagnostics, San Francisco, CA, USA).
2.8 PAS staining
Sections were routinely dewaxed, oxidized in periodate solution for 10 min, and rinsed in distilled water for 1 min. The samples were then stained in Schiff's reagent for 20 min and rinsed in running water for 1 min. Then hematoxylin was used to stain the nuclei for 5 min. The samples were then rinsed in running water for 1 min and routinely dehydrated. The transparent samples were sealed in neutral glue.
2.9 Masson's trichrome staining
Masson's trichrome staining was used to visualize the extent of glomerulosclerosis and interstitial fibrosis. The dewaxed and dehydrated sections were then stained with a mild alkaline magenta solution for 3 min, rinsed with deionized water, and treated with 1% phosphomolybdic acid solution for 1 min. Deionized water was used to remove the residual phosphomolybdic acid solution, the samples were stained with 2% blue aniline solution for 2 min, rinsed and dehydrated with 95% ethanol, dried, and embedded. After staining, the collagen fibers of the kidney tissue appeared blue-green under light microscopy and the kidney tissue appeared red.
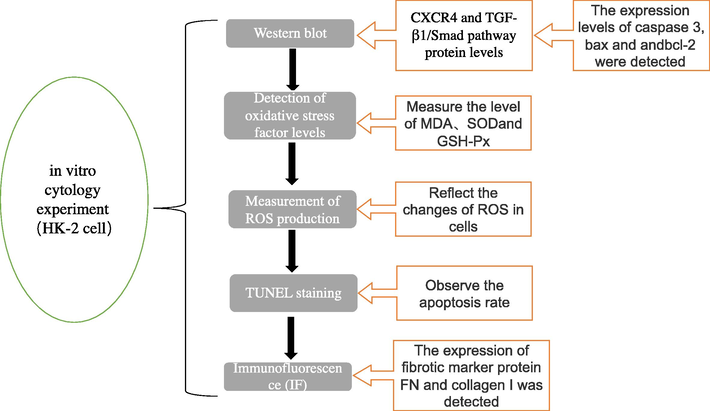
2.10 Cell culture and Cd treatment
Human renal proximal tubular epithelial (HK-2) cells from Sino Biological (Beijing, China) were stored in RPMI-1640 medium (Manassas, VA, USA) containing 10% FBS and 1% penicillin (Gibco, Carlsbad, CA, USA)/streptomycin (Gibco, Carlsbad, CA, USA) at 37 °C and 5% CO2. Cells were treated with CdCl2 for 24 h, and then Cd (2–10 µM) was added to serum-free medium for 12 h. A micromolar concentration (1–10 µM) of Cd was selected. The schematic diagram is as follows:
2.11 Cell transfection
After cells had grown to 50% confluence, 100 pmol of targeting CXCR4 (si-CXCR4) or TGF-β or negative control siRNA (NC) (Ribobio, Guangdong, China) was transfected with HK-2 cells using Lipo6000 transfection reagent ((Invitrogen, Carlsbad, CA, USA)) in conformity with the manufacturer's instructions. After 4 h of transfection, the original medium was replaced with fresh medium for 24 h until treatment with CdCl2.
2.12 Measurement of ROS production
To detect changes in intracellular ROS, DCFH-DA fluorescent dye was diluted to 10 μmol/L using serum-free culture medium. Each well was incubated with DCFH-DA 200 μL (Sigma, USA) for 60 min. The appropriate field of view was photographed under a microscope. The fluorescence in images was measured by optical density using Image J software, and the fluorescence intensity of each group was analyzed to indirectly reflect the changes of ROS in cells.
2.13 Immunofluorescence (IF)
HK-2 cells and cells derived from rat tissues were digested with trypsin and re-inoculated in 24-well plates. After 48 h of incubation at 37 °C and 5% CO2, they were then fixed in 4% paraformaldehyde for 20 min at room temperature. The fixed cells were blocked for 1 h in 5% goat serum and then incubated in primary antibody FN and collagen I (1:500 dilution) overnight at 4 °C. The secondary antibody was incubated for 1 h at room temperature. Diaminobenzidine solution was used for color development. Nuclei were stained with hematoxylin and then cells were observed under a light microscope and images were collected.
2.14 Statistical analysis
SPSS 22.0 software was used in the analysis and all data were expressed as the mean ± SD. One-way ANOVA was used to compare measured indicators between multiple groups and the LSD test was used for two-way comparison between groups. A P-value <0.05 was considered statistically different.
3 Results
3.1 CXCR4 inhibition suppressed the TGF-β1/Smad pathway in renal tissues of Cd-treated rats.
To assess the role of CXCR4 in renal tissue, CXCR4 inhibition was used in a chronic kidney injury model constructed by using Cd-treated rats. Western blotting results demonstrated that CXCR4 expression was upregulated in the Cd-induced chronic kidney injury model and downregulated after CXCR4 inhibition (Fig. 1A and B). Subsequently, the role of the TGF-β1/Smad signaling pathway in CXCR4 regulation of Cd-induced chronic kidney injury was assessed. The results showed that TGF-β1, TGF-βR1, p-Smad2/3, and Smad2/Smad3 expression was upregulated in the Cd-treated group compared with the control group. Compared with the Cd-treated si-control group, the Cd-treated si-CXCR4 group exhibited decreased expression, In addition, the same was true for the cadmium-treated enalapril group (Fig. 1A, C–F). This suggested that si-CXCR4 could inhibit Cd-induced chronic kidney injury by suppressing the TGF-β1/Smad signaling pathway.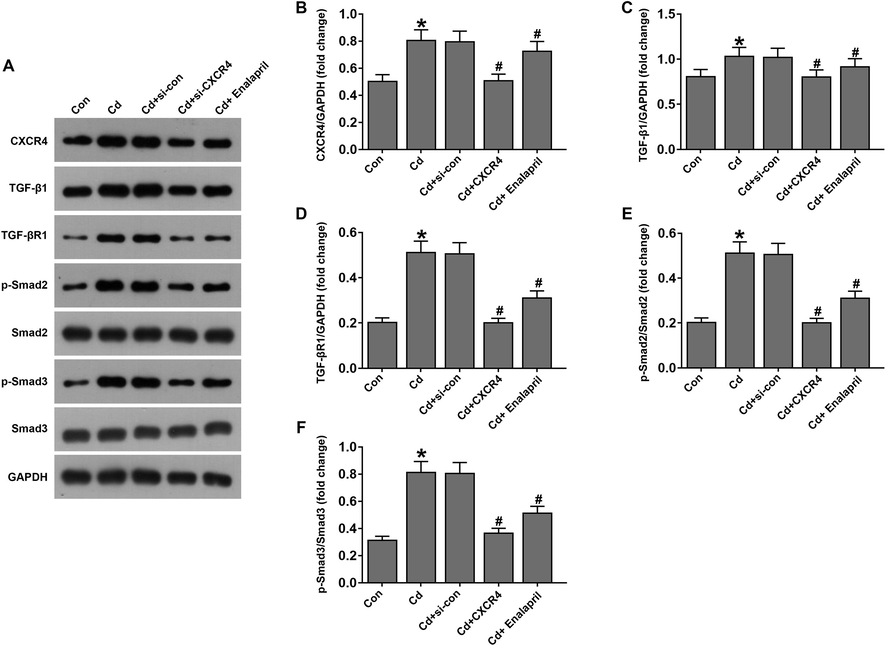
Inhibition of CXCR4 suppressed the TGF-β1/Smad pathway in renal tissues of cadmium-treated rats. (A) Western blotting was used to detect CXCR4, TGF-β1, TGF-βR1, p-Smad2/Smad2, and p-Smad3/Smad3 levels. (B–F) Western blotting-based statistical plots of CXCR4, TGF-β1, TGF-βR1, p-Smad2/Smad2, and p-Smad3/Smad3 protein expression. N = 6/per group. *P < 0.05 vs Control (Con); #P < 0.05 vs Cd + si-con.
3.2 Effect of CXCR4 inhibition on renal function and oxidative stress in Cd-treated rats
Subsequently, the regulation of renal function by si-CXCR4 was assessed in a Cd-induced chronic kidney injury model. The results showed that the renal function-related renal weight index (KW/DW), urinary protein, Scr, and BUN were prominently elevated in the Cd-treated group compared to the control group. Yet si-CXCR4 downregulated and enalapril KW/DW, urinary protein, Scr, and BUN levels. This suggested that CXCR4 downregulation could promote the recovery of renal function in Cd-induced chronic kidney injury rats, the same was true for the cadmium-treated enalapril group (Fig. 2A–D). Previous studies suggest that treatment against oxidative stress can effectively protect renal function and alleviate renal injury (Liu et al., 2019; Wu et al., 2018). Therefore, the role of CXCR4-regulated oxidative stress in Cd-induced chronic kidney injury was assessed. The results showed that oxidative stress factor MDA expression was increased and levels of SOD and GSH-Px expression were lowered in the Cd-treated group. However, si-CXCR4 and enalapril intervention could downregulate MDA and upregulate SOD and GSH-Px levels (Fig. 2E–G), suggesting that CXCR4 inhibition was able to inhibit oxidative stress to improve renal function in a Cd-induced kidney injury rat model.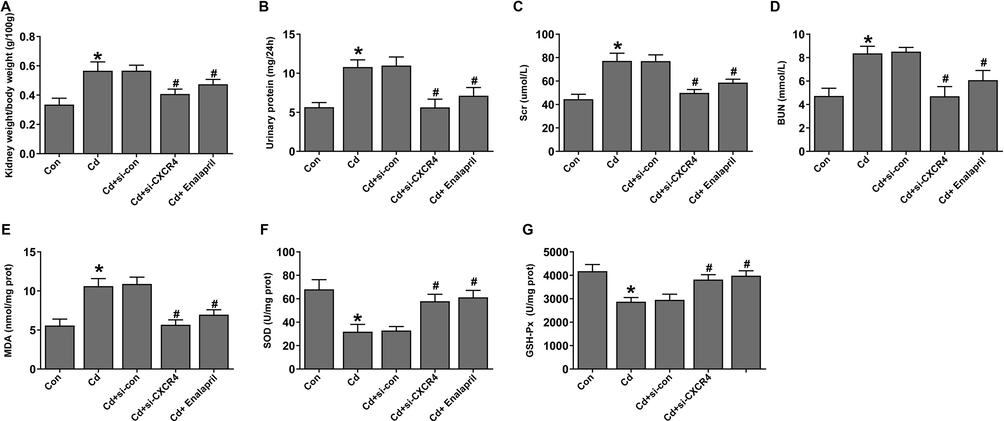
Effect of CXCR4 inhibition on renal function and oxidative stress in cadmium-treated rats. (A–D) Tests for renal function indicators KW/BW, urine protein, creatinine, and BUN. (E–G) Expression of stress-related markers MDA, SOD, and GSH-Px. *P < 0.05 vs Control (Con); #P < 0.05 vs Cd + si-con.
3.3 CXCR4 inhibition inhibited renal apoptosis in the Cd-treated rats
Kidney injury will produce a large amount of oxygen free radicals. At the same time, the ability of the body to scavenge oxygen free radicals is reduced, leading to a considerable accumulation of oxygen free radicals, which induces apoptosis and ultimately leads to tissue dysfunction (Chen et al., 2019; Shahzad et al., 2016). Subsequently, the effect of CXCR4 on renal apoptosis in Cd-treated rats was evaluated. In the rats with Cd-induced kidney injury, TUNEL staining showed increased apoptosis but apoptosis was reduced by the si-CXCR4 and enalapril intervention (Fig. 3A and B). Likewise, IHC staining (Fig. 3A, C–E) and western blot (Fig. 4A–D) results indicated that Bax and Cleaved-caspase-3 levels were increased, whereas, Bcl-2 levels decreased in the Cd-treated group. However, levels of Bax and Cleaved-caspase-3 decreased and Bcl-2 levels increased in the si-CXCR4 and enalapril treated group. This suggests that si-CXCR4 can inhibit apoptosis through inhibition in Cd-treated rats.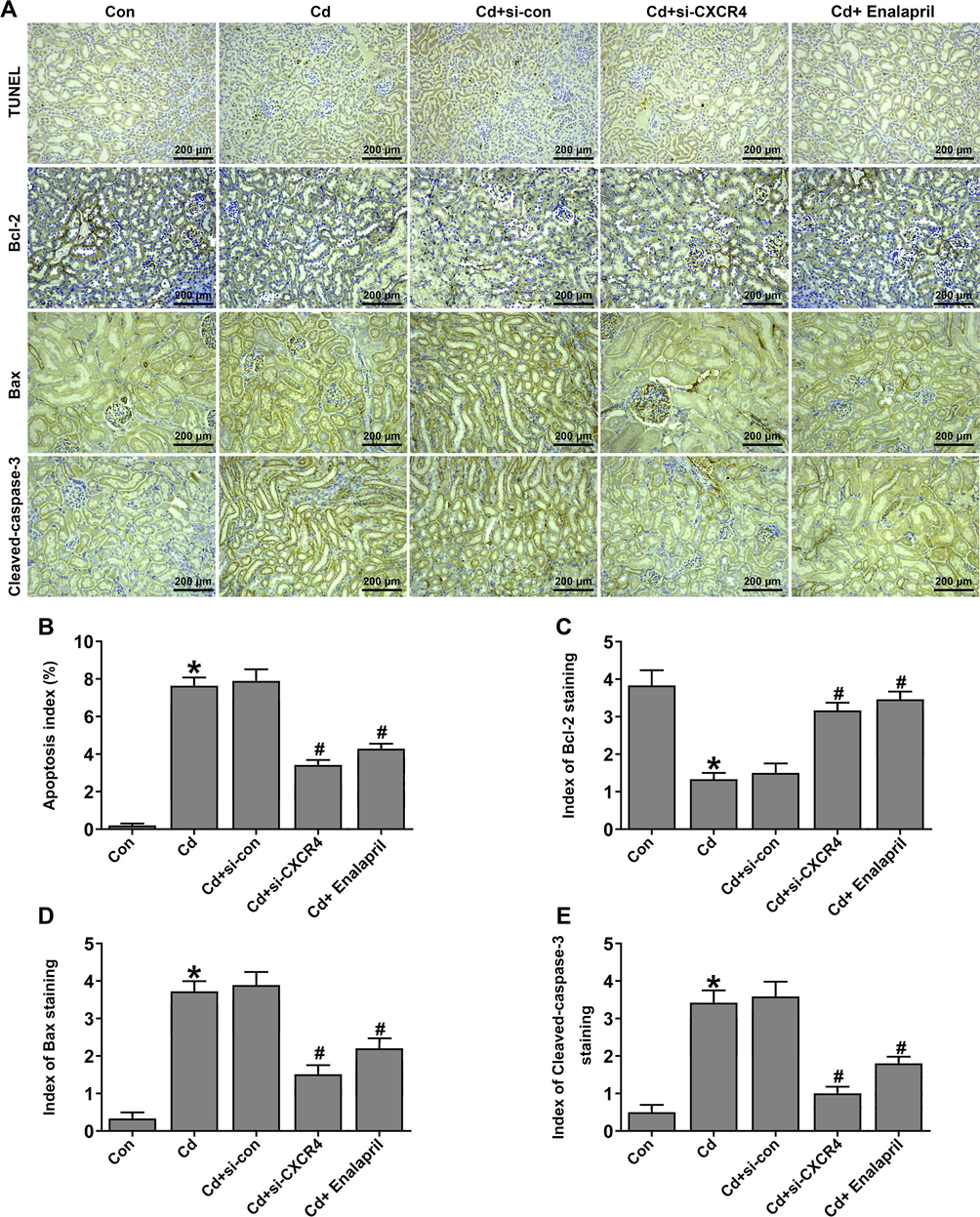
CXCR4 inhibition inhibited renal apoptosis in cadmium-treated rats. (A) TUNEL and immunohistochemical staining to detect apoptosis. (B) Statistical plots of TUNEL staining. (C–E) The statistical plots of immunohistochemical detection of apoptosis-associated protein expression. (F–I) Western blot analysis to detect the expression of apoptosis-related proteins Bcl-2, Bax, and Cleaved-caspase-3. *P < 0.05 vs Control (Con); *P < 0.05 vs Control (Con); #P < 0.05 vs Cd + si-con.
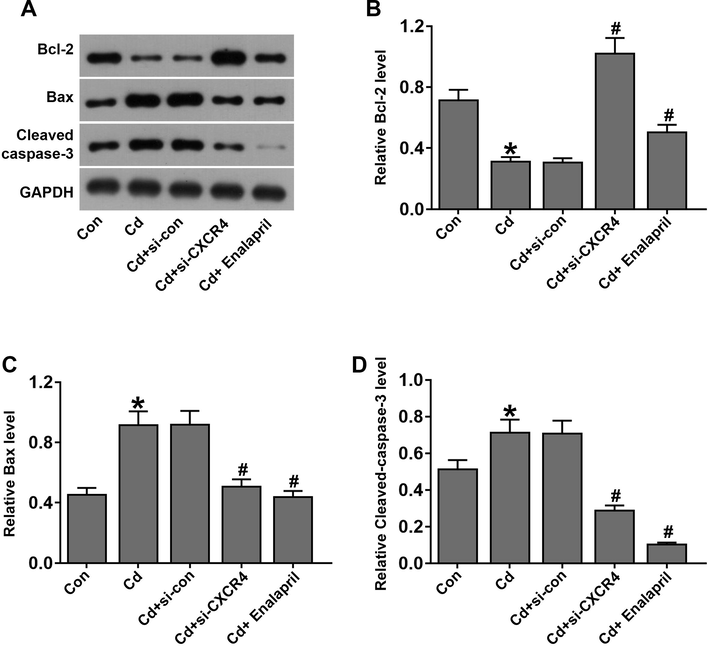
CXCR4 inhibition inhibited renal apoptosis in cadmium-treated rats. (A–D) Western blot analysis to detect the expression of apoptosis-related proteins Bcl-2, Bax, and Cleaved-caspase-3. *P < 0.05 vs Control (Con); #P < 0.05 vs Cd + si-con.
3.4 CXCR4 inhibition inhibited renal fibrosis in the Cd-treated rats
The regulation of CXCR4 inhibition on renal fibrosis was further assessed in Cd-treated rats. PAS staining indicated that si-CXCR4 intervention reduced glomerular basement membrane thickening in Cd-treated rats. Masson's trichrome staining showed that glomerular collagen fibers increased in the model group and si-CXCR4 and enalapril intervention reduced collagen fibrils in the model rats (Fig. 5A). In addition, si-CXCR4 and enalapril alleviated glomerulosclerosis and interstitial fibrosis in Cd-treated rats (Fig. 5B and C). Furthermore, IF (Fig. 6A–C) and western blotting (Fig. 6D–F) indicated that the expression of fibrosis marker proteins FN and collagen I were upregulated in Cd-treated rats. In addition, FN and collagen I expression was downregulated in the Cd-treated si-CXCR4 and enalapril group compared to the Cd-treated si-control. These results suggest that si-CXCR4 inhibits fibrosis in rats with Cd-induced kidney injury.
Inhibition of CXCR4 suppressed renal fibrosis in cadmium-treated rats. (A) PAS and Masson's trichrome staining. (B, C) Glomerulosclerosis and interstitial fibrosis. *P < 0.05 vs Control (Con); #P < 0.05 vs Cd + si-con.
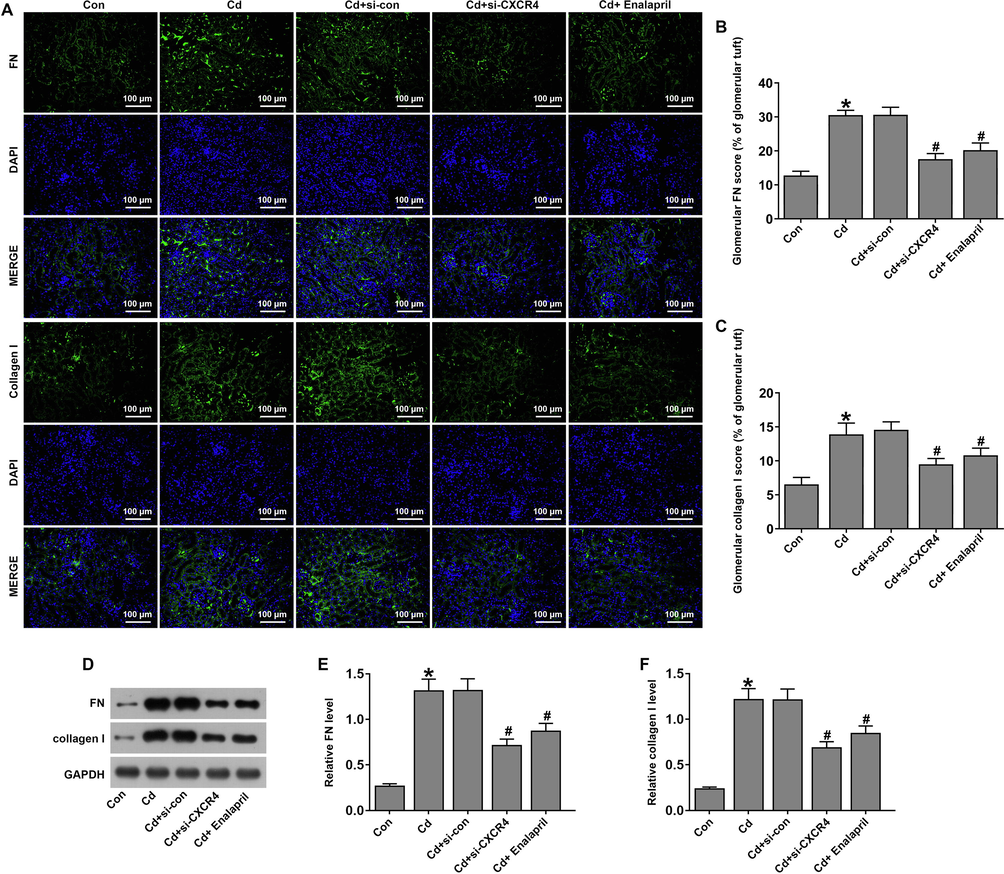
Inhibition of CXCR4 suppressed renal fibrosis in cadmium-treated rats. (A-B) Statistical plots of fibronectin (FN) and collagen I protein expression detected by immunofluorescence. (D–F) Statistical plots of FN and collagen I protein expression detected by western blotting. *P < 0.05 vs Control (Con); #P < 0.05 vs Cd + si-con.
3.5 TGF-β weakened the effects of CXCR4 inhibition of the TGF-β1/Smad pathway in Cd-treated cells
Previous studies suggest that TGF-β exacerbates renal injury (Chung et al., 2018). Subsequently, we assessed the role of TGF-β in the si-CXCR4 protection of Cd-induced cells. The results of western blotting showed that the TGF-β1/Smad pathway proteins were downregulated in the Cd-treated si-CXCR4 and enalapril group compared to the Cd-treated group. However, TGF-β interfered with TGF-β1, TGF-βR1, p-Smad2/Smad2, and p-Smad3/Smad3 expression that was upregulated in Cd-treated cells (Fig. 7A–E). This suggests that TGF-β attenuated the inhibitory effect of CXCR4 inhibition on the TGF-β1/Smad pathway in Cd-treated cells.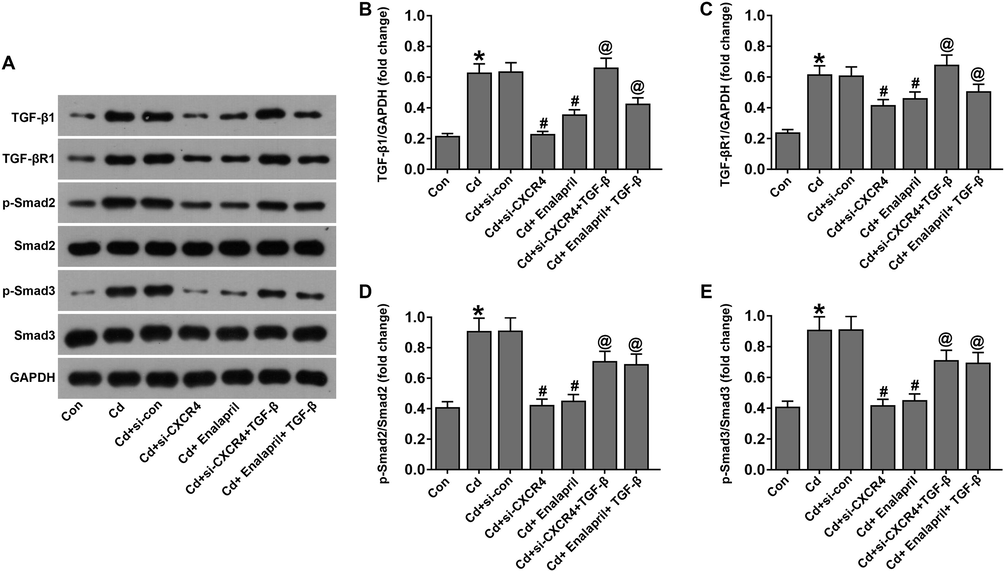
TGF-β weakened the effects of CXCR4 inhibition on the TGF-β1/Smad pathway in cadmium-treated cells. (A) Western blot detection of TGF-β1, TGF-βR1, p-Smad2/Smad2, and p-Smad3/Smad3 levels. (B–E) Western blot analysis gave statistical plots of TGF-β1, TGF-βR1, p-Smad2/Smad2, and p-Smad3/Smad3 protein expression. *P < 0.05 vs Control (Con); #P < 0.05 vs Cd + si-con; @P < 0.05 vs Cd + si-CXCR4.
3.6 TGF-β weakened the effects of CXCR4 inhibition on oxidative stress in Cd-treated cells
Subsequently, the role of TGF-β in the inhibition of oxidative stress by si-CXCR4 in Cd-treated cells was assessed. The results showed that levels of the oxidative stress factors MDA and ROS were increased and SOD and GSH-Px expression was downregulated in the Cd-treated group. However, si-CXCR4 and enalapril intervention in Cd-treated cells was able to downregulate MDA and ROS levels and upregulate SOD and GSH-Px levels. Compared with the Cd-treated si-CXCR4 group, the Cd-treated si-CXCR4 + TGF-β group increased MDA, ROS expression, and the downregulation of SOD and GSH-Px expression (Fig. 8A–E).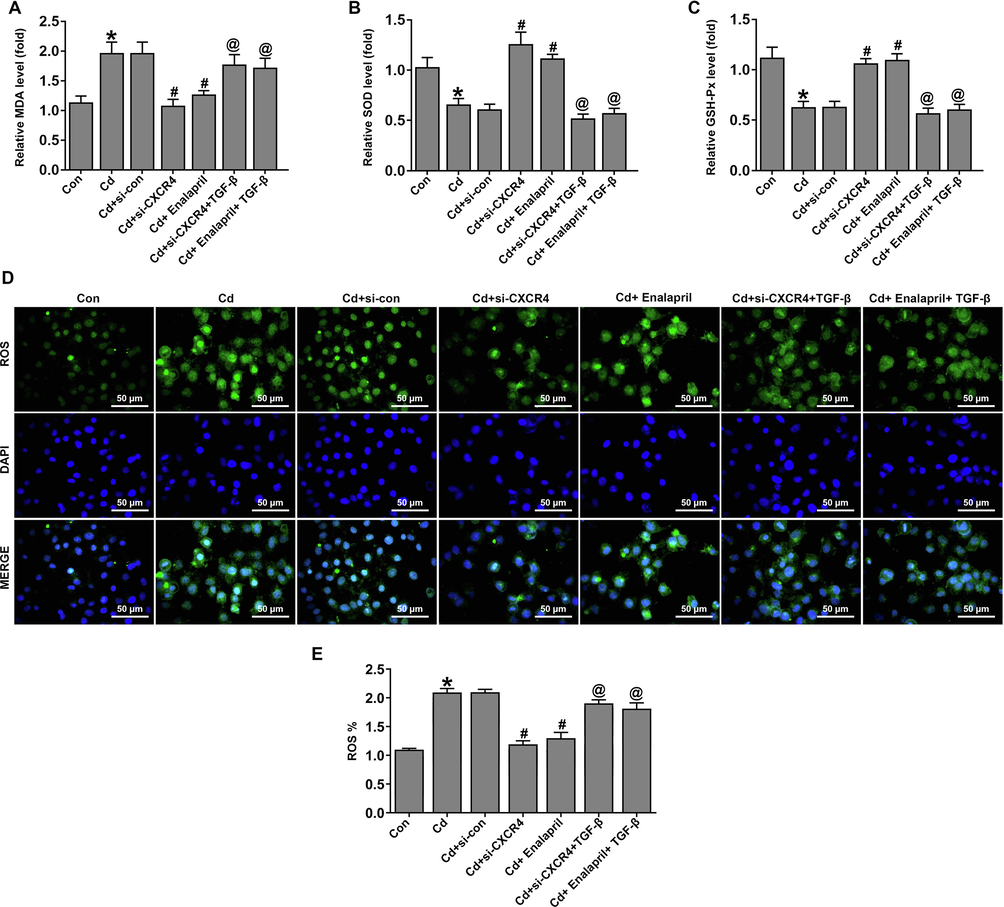
TGF-β weakened the effects of CXCR4 inhibition on apoptosis in cadmium-treated cells. (A–C) Expression of stress-related indicators MDA, SOD, and GSH-Px were detected. (D, E) ROS levels were detected by ROS staining. *P < 0.05 vs Control (Con); #P < 0.05 vs Cd + si-con; @P < 0.05 vs Cd + si-CXCR4.
3.7 TGF-β weakened the effects of CXCR4 inhibition on apoptosis in Cd-treated cells
Next, the role of TGF-β in si-CXCR4 inhibited apoptosis in Cd-treated cells was assessed. TUNEL staining (Fig. 9A–B) showed that apoptosis was remarkably increased in Cd-treated cells and si-CXCR4 and enalapril could inhibit the apoptosis rate. However, the apoptosis rate was remarkably increased in the TGF-β group of cells. Similarly, western blot results (Fig. 9C–F) showed that levels of Bax and Cleaved-caspase-3 levels were increased and Bcl-2 levels were downregulated in Cd-treated cells. However, the opposite trend occurred in the si-CXCR4 and enalapril-treated group. The Cd-treated si-CXCR4 + TGF-β group partially increased Bax and Cleaved-caspase-3 and downregulated Bcl-2 levels. This suggests that TGF-β partially reverses the ability of si-CXCR4 to inhibit apoptosis in Cd-treated cells.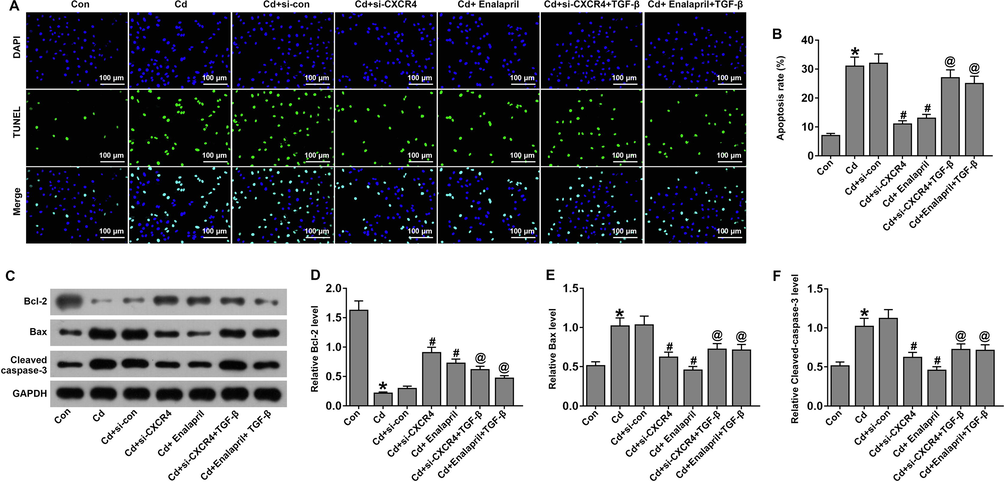
TGF-β weakened the effects of CXCR4 inhibition on apoptosis in cadmium-treated cells. (A, B) TUNEL staining to detect apoptosis. (C–F) Western blot analysis for expression of apoptosis-associated proteins Bcl-2, Bax, and Cleaved-caspase-3. *P < 0.05 vs Control (Con); #P < 0.05 vs Cd + si-con; @P < 0.05 vs Cd + si-CXCR4.
3.8 TGF-β weakened the effects of CXCR4 inhibition of fibrosis in Cd-treated cells
In addition, the role of TGF-β and si-CXCR4 was assessed in the inhibition of fibrosis in Cd-treated cells. Western blot (Fig. 10A–C) and IF (Fig. 10D–F) results showed that fibrosis marker proteins FN and collagen I expression were upregulated in Cd-treated rats, compared to the control group. Furthermore, FN and collagen I expression were downregulated in the Cd-treated si-CXCR4 and enalapril group compared to the Cd-treated si-control group. However, TGF-β intervention could upregulate FN and collagen I expression in the Cd-treated si-CXCR4 and enalapril group. These results suggest that TGF-β was able to attenuate the effect of CXCR4 inhibition on fibrosis in Cd-treated cells.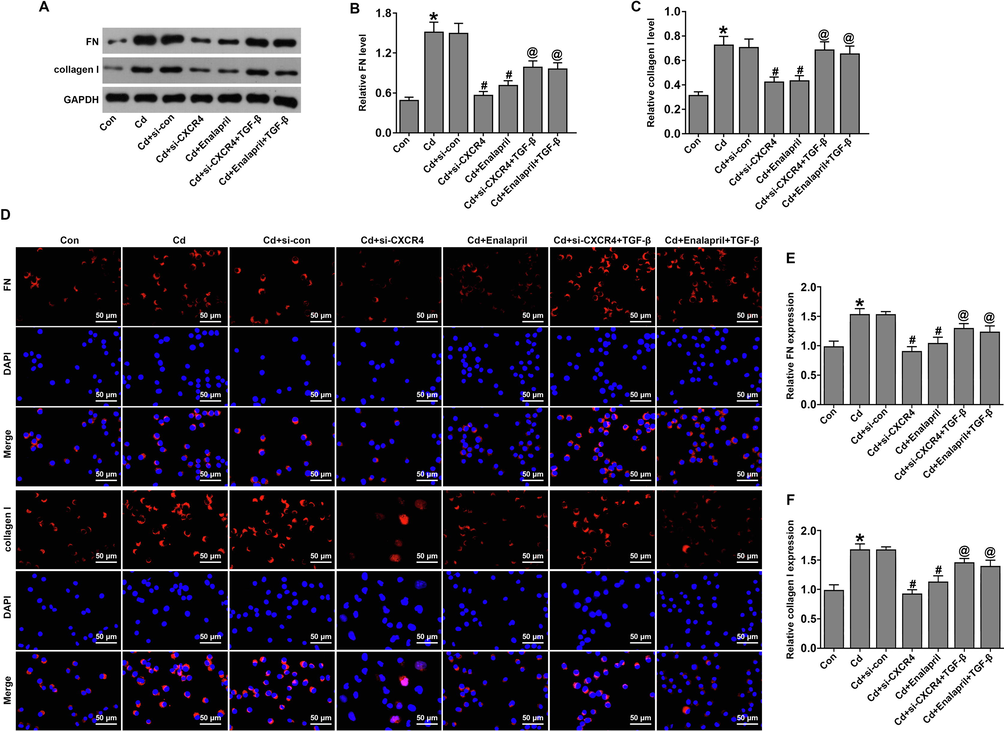
TGF-β weakened the effects of CXCR4 inhibition on fibrosis in cadmium-treated cells. (A–C) Western blot analysis of fibronectin (FN) and collagen I expression. (D-F) Immunofluorescence detection of FN and collagen I expression. *P < 0.05 vs Control (Con); #P < 0.05 vs Cd + si-con; @P < 0.05 vs Cd + si-CXCR4.
4 Discussion
Cd is now used widely in chemical and industrial production, and the resulting Cd residues are a serious environmental pollutant. Due to the pollution of soil and water resources, people have long been in contact with Cd from food and beverages. Cd is a metallic element that is toxic and non-essential to the organism (Kimura et al., 2019). In addition, Cd is very difficult to biodegrade. The consequent Cd accumulation in the body will lead to serious health problems. Cd is one of the most common nephrotoxic heavy metals in humans. Ingesting a large amount of Cd in a short period of time can lead to acute kidney damage. At the same time, long-term contact with Cd may lead to renal failure caused by fibrosis (Dastan et al., 2019; Elkhadragy et al., 2018). In recent years, molecularly targeted therapies have shown promising results in Cd-induced kidney injury. For example, microRNA-363-3p promotes apoptosis after Cd-induced kidney injury through PI3K expression (Chen et al., 2021b). The current study found that CXCR4 inhibition alleviated Cd-associated kidney injury.
CXCR4 is a critical G protein-coupled receptor. Studies have shown that CXCR4 can serve as an essential regulator in the pathogenic response of kidney and renal cells to external stimuli (Takabatake et al., 2009). For example, a study has recently identified that CXCR4 can maintain glomerular capillary integrity in chronic kidney disease (Chen et al., 2014). Moreover, CXCR4-mediated excessive proliferation of podocytes might in turn facilitate the development of glomerular disease (Sanchez-Nino et al., 2013). In renal tubules, CXCR4 inactivation damages nephrogenesis (Kamel et al., 2019). In the current study, we assessed the role and mechanism of CXCR4 in Cd-induced kidney injury. First, we found that CXCR4 expression was upregulated in the Cd-induced chronic kidney injury model and CXCR4 expression was downregulated after CXCR4 inhibition. In addition, CXCR4 inhibition downregulated the renal function indices KW/DW, urinary protein, and Scr. In addition, CXCR4 inhibition downregulated the levels of KW/DW, urinary protein, Scr, and BUN, suggesting that CXCR4 downregulation could promote the recovery of kidney function in rats with Cd-induced chronic kidney injury.
The kidney is one of the major organs damaged by Cd accumulation in the body. On the one hand, Cd brings about oxidative damage to tissues by inhibiting the activity of antioxidant enzymes such as SOD and GSH-Px in the organism and leads to increased levels of oxidative stress in the system and local environment (Pavon et al., 2019).
In addition, previous studies have proved that oxidative stress plays a vital role in CKD, and excessive ROS will cause irreversible damage to the renal structure, mainly manifested as strip-shaped interstitial fibrosis {Gao, 2020 #68}. In the current study, we found that oxidative stress factor MDA expression was increased and SOD and GSH-Px expression was downregulated in the Cd group, compared to the control group. However, si-CXCR4 intervention could downregulate MDA and upregulate SOD and GSH-Px levels, suggesting that CXCR4 inhibition can inhibit oxidative stress to improve renal function in the rat Cd-induced injury model. On the other hand, kidney injury produces large amounts of oxygen free radicals, leading to a considerable accumulation of oxygen free radicals, which induces apoptosis (Chen et al., 2019; Wang et al., 2020). In the current study, TUNEL staining showed increased apoptosis in Cd rats and si-CXCR4 intervention reduced apoptosis in Cd-treated rat kidney cells. Likewise, IHC staining and western blot analysis showed increased Bax and Cleaved-caspase-3 levels and downregulated Bcl-2 levels in the Cd-treated group. However, the opposite trend was found in the si-CXCR4-treated group. This suggests that si-CXCR4 can inhibit apoptosis by suppressing cell death in Cd-treated rats.
In addition, Recent studies have reported that renal fibrosis is characterized by excessive accumulation and deposition of extracellular matrix components {Jin, 2022 #63}{Cao, 2022 #65}. Previous studies have confirmed that Cd accumulation disrupts epithelial integrity and accelerates ECM deposition, thereby inducing the fibrotic process in the kidney {Thijssen, 2007 #66}. FN and collagen I, critical regulators of fibrosis, are upregulated during fibrosis (Lu et al., 2018; Sun et al., 2018).
In the current study, si-CXCR4 intervention reduced glomerular basement membrane thickening in Cd-treated rats. In the current study, si-CXCR4 intervention reduced glomerular basement membrane thickening in Cd-treated rats. Masson staining showed that glomerular collagen fibers increased in the model group and were reduced in the si-CXCR4 intervention group in Cd-treated rats. Likewise, the fibrosis marker proteins, FN and collagen I, were upregulated in Cd-treated rats. In addition, FN and collagen I expression were downregulated in the Cd-induced si-CXCR4 group compared to Cd-treated si-control. These results suggest that si-CXCR4 inhibits fibrosis in Cd-induced kidney injured rats.
TGF-β1, a fibrogenic cytokine, is closely associated with renal fibrosis injury. Smad family molecules are activated downstream of the TGF-β1 pathway through phosphorylation of Smad3 (Ma and Meng, 2019). Previous studies have suggested that TGF-β1/Smad signaling is reported to be activated in renal injury and fibrosis (Ma et al., 2018). Previous studies have suggested that TGF-β promotes fibrosis after severe acute kidney injury (Chung et al., 2018). The present study found that TGF-β1, TGF-βR1, p-Smad2/Smad2, and p-Smad3/Smad3 expression was upregulated in Cd-exposed cells and a Cd-induced kidney injury model, and si-CXCR4 interfered with TGF-β1, TGF-βR1, p-Smad2/3, and Smad2/3 to downregulate expression. However, TGF-β interfered with the upregulation of TGF-β1, TGF-βR1, p-Smad2/3, Smad2/3 expression in Cd-treated cells, suggesting that TGF-β attenuates the inhibitory effect of CXCR4 inhibition on the TGF-β1/Smad pathway in Cd-associated kidney injury.
However, there are limitations to this study. First, the function and mechanism of CXCR4 were investigated in Cd-exposed cells and in a Cd-induced kidney injury model, which needs to be further investigated in a multicenter clinical trial. Furthermore, previous studies confirmed that CXCR4 induced renal injury and proteinuria by activating the β-catenin signaling pathway (Mo et al., 2022). Whether CXCR4 can modulate other signaling pathways in Cd-related kidney injury must be further explored. In conclusion, CXCR4 suppresses Cd-induced renal oxidative stress, apoptosis, and fibrosis by inhibiting the TGF-β1/Smad pathway and provides insights into the clinical implications for molecularly targeted therapies in renal injury.
Acknowledgments
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Xujun Lang, and Dong Cheng. The first draft of the manuscript was written by Yangbiao He and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The Authors declare that there is no conflict of interest.
References
- Dietary extra virgin olive oil attenuates kidney injury in pristane-induced SLE model via activation of HO-1/Nrf-2 antioxidant pathway and suppression of JAK/STAT,NF-kappaB and MAPK activation. J. Nutr. Biochem.. 2016;27:278-288.
- [Google Scholar]
- SDF-1/CXCR4 signaling preserves microvascular integrity and renal function in chronic kidney disease. PLoS One. 2014;9:e92227.
- [Google Scholar]
- MicroRNA-363-3p promotes apoptosis in response to cadmium-induced renal injury by down-regulating phosphoinositide 3-kinase expression. Toxicol. Lett.. 2021;345:12-23.
- [Google Scholar]
- Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact.. 2019;308:269-278.
- [Google Scholar]
- Nrf2 deficiency aggravates the kidney injury induced by subacute cadmium exposure in mice. Arch. Toxicol.. 2021;95:883-893.
- [Google Scholar]
- Chung, S. et al., 2018. TGF-beta promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration JCI Insight 3.
- Protective effects of Allium hirtifolium Boiss extract on cadmium-induced renal failure in rats. Environ. Sci. Pollut. Res. Int.. 2019;26:18886-18892.
- [Google Scholar]
- Protective effects of Fragaria ananassa extract against cadmium chloride-induced acute renal toxicity in rats. Biol. Trace Elem. Res.. 2018;181:378-387.
- [Google Scholar]
- Transforming growth factor-beta: a therapeutic target for cancer. Hum. Vac. Immunother.. 2017;13:1741-1750.
- [Google Scholar]
- Novel repair mechanisms in a renal ischaemia/reperfusion model: subsequent saxagliptin treatment modulates the pro-angiogenic GLP-1/cAMP/VEGF, ANP/eNOS/NO, SDF-1alpha/CXCR4, and Kim-1/STAT3/HIF-1alpha/VEGF/eNOS pathways. Eur. J. Pharmacol.. 2019;861:172620
- [Google Scholar]
- Kim, E.N. et al., 2018. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury Aging (Albany NY) 10, 83–99.
- Protective effect of polaprezinc on cadmium-induced injury of lung epithelium. Metallomics. 2019;11:1310-1320.
- [Google Scholar]
- Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis. Wound Repair Regen.. 2016;24:215-222.
- [Google Scholar]
- Inhibition of Brd4 alleviates renal ischemia/reperfusion injury-induced apoptosis and endoplasmic reticulum stress by blocking FoxO4-mediated oxidative stress. Redox. Biol.. 2019;24:101195
- [Google Scholar]
- CXCR4-overexpressing bone marrow-derived mesenchymal stem cells improve repair of acute kidney injury. Am. J. Physiol. Renal. Physiol.. 2013;305:F1064-F1073.
- [Google Scholar]
- Live imaging of Type I collagen assembly dynamics in osteoblasts stably expressing GFP and mCherry-tagged collagen constructs. J. Bone Miner. Res.. 2018;33:1166-1182.
- [Google Scholar]
- Emodin ameliorates renal fibrosis in rats via TGF-beta1/Smad signaling pathway and function study of Smurf 2. Int. Urol. Nephrol.. 2018;50:373-382.
- [Google Scholar]
- CXCR4 induces podocyte injury and proteinuria by activating beta-catenin signaling. Theranostics. 2022;12:767-781.
- [Google Scholar]
- CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted-therapy. Immunol. Lett.. 2020;217:91-115.
- [Google Scholar]
- On the oxidative damage by cadmium to kidney mitochondrial functions. Biochem. Cell. Biol.. 2019;97:187-192.
- [Google Scholar]
- The gut microbiota and its relationship with chronic kidney disease. Int. Urol. Nephrol.. 2019;51:2209-2226.
- [Google Scholar]
- MIF, CD74 and other partners in kidney disease: tales of a promiscuous couple. Cytokine Growth Factor Rev.. 2013;24:23-40.
- [Google Scholar]
- Protection against oxidative stress-induced apoptosis in kidney epithelium by Angelica and Astragalus. J. Ethnopharmacol.. 2016;179:412-419.
- [Google Scholar]
- Reducing the risk of stroke in patients with impaired renal function: nutritional issues. J. Stroke Cerebrovasc. Dis.. 2021;30:105376
- [Google Scholar]
- miR-133b and miR-199b knockdown attenuate TGF-beta1-induced epithelial to mesenchymal transition and renal fibrosis by targeting SIRT1 in diabetic nephropathy. Eur. J. Pharmacol.. 2018;837:96-104.
- [Google Scholar]
- The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J. Am. Soc. Nephrol.. 2009;20:1714-1723.
- [Google Scholar]
- Raw and salt-processed Achyranthes bidentata attenuate LPS-induced acute kidney injury by inhibiting ROS and apoptosis via an estrogen-like pathway. Biomed. Pharmacother.. 2020;129:110403
- [Google Scholar]
- Mechanism of cyclosporine A nephrotoxicity: oxidative stress, autophagy, and signalings. Food Chem. Toxicol.. 2018;118:889-907.
- [Google Scholar]
- MicroRNA-381-induced down-regulation of CXCR4 promotes the proliferation of renal tubular epithelial cells in rat models of renal ischemia reperfusion injury. J. Cell. Biochem.. 2018;119:3149-3161.
- [Google Scholar]







