Translate this page into:
β-cyclodextrin/alginate nanoparticles encapsulated 5-fluorouracil as an effective and safe anticancer drug delivery system
⁎Corresponding authors at: Institute of Chemical Technology, Vietnam Academy of Science and Technology, 1A, TL 29 Thanh Loc Ward, District 12, Ho Chi Minh 729110, Viet Nam. dangchihien@gmail.com (Chi-Hien Dang), ntdanh@ict.vast.vn (Thanh-Danh Nguyen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Although 5-Fluorouracil (5-FU) is one of the most frequently used cytotoxic chemotherapy drugs in the treatment of cancer, it possesses a short biological half-life and toxic side effects against normal healthy cells. In this work, β-cyclodextrin/alginate (β-CD/Alg) nanoparticles loaded with 5-fluorouracil (5-FU) were prepared to evaluate release properties and bioactivity in different pH media. Stable nanocomposites with the best loading efficiency (36%) and encapsulation efficiency (90%) were successfully fabricated. The size of the nanocomposite solution was determined via dynamic light scattering (DLS) to be in the range 30–120 nm with a mean size of 70 nm, while TEM images showed the particle size of the nanocomposite to be in the range 30–80 nm with a mean size of 50 nm. The release profile of 5-FU in a simulated gastric fluid (pH 1.2) was much lower than in a simulated colorectal fluid (pH 7.4). The release behaviour of 5-FU from the nanocomposite was confirmed by the change in morphology in the pH media. A cytotoxicity assay indicated that the nanocomposite is an effective delivery system for 5-FU with strong antiproliferative activity against MCF-7 cells and negligible effects on normal healthy cells.
Keywords
Ionotropic gelation
Anticancer
β-cyclodextrin
Alginate
5-fluorouracil
Drug delivery
1 Introduction
5-Fluorouracil (5-FU) is one of the most frequently used cytotoxic chemotherapy drugs in the treatment of cancer, particularly gastric cancer (Ukai et al., 2020), pancreatic cancer (Wainberg et al., 2020), cervical cancer (Wainberg et al., 2020) and breast cancer (Luo et al., 2020). However, 5-FU has a short biological half-life (5–10 min) due to its rapid metabolism by dihydropyrimidine dehydrogenase (Li et al., 2008). 5-FU also has toxic side effects, which are evident in the bone marrow and gastrointestinal tract, and it exhibits non-selective bioactivity against normal healthy cells (Katona et al., 1998). To resolve these issues and obtain high clinical efficiency, numerous polymer-based nanocomposites encapsulating 5-FU have been developed to control its release. Such drug carriers should possess a high drug loading capacity, long circulation with good stability in the bloodstream, selective accumulation at the target site and effective release of the drug in suitable media. Wang and coworkers (Wang et al., 2015) developed a conjugate folic acid - poly(lactic-co-glycolic acid) based nanoparticles for 5-FU delivery. These nanoparticles were crosslinked using 1,3-diaminopropane as a cross-linker and tested against HT-29 cancer cells. Mattos and coworkers (de Mattos et al., 2016) successfully loaded 5-FU onto poly (lactic acid)–poly (ethylene glycol) composite against Hep-2 tumor cells.
Polymeric nanoparticles are of great interest for drug, peptide and protein delivery systems in applications that require controllable release. In particular, nanocomposites made from natural, biodegradable polysaccharides have attracted the interest of many researchers in the pharmaceutical and medical fields (Zheng et al., 2015, Muhamad et al., 2019, Pathak and Malviya 2020, Shariatinia 2020, Nazir et al., 2021). The natural polysaccharides and oligosaccharides that are widely used for controllable drug release include chitosan (Ali and Ahmed 2018, Quadrado and Fajardo 2020), alginate (Uyen et al., 2020), cellulose (Wsoo et al., 2020) and cyclodextrin (CD) (Tian et al., 2021). Of these, Alg and CD are particularly suitable as drug delivery carriers due to their low toxicity. Alginic acid, a polysaccharide containing guluronic acid and mannuronic acid units, has a pKa in the range 3.4–4.4 and its salt presents in the mixture (Sanchez-Ballester et al., 2021). Alg gelispheres (beads) can easily be fabricated via crosslinks with divalent and trivalent metal ions, such as Ca2+, Cu2+, Zn2+ and Al3+ (Urbanova et al., 2019). Kim and Jung (Kim and Jung 2020) have effectively loaded 5-FU into crosslinked Alg hydrogels via succinoglycan dialdehyde which showed complete release of drug in pH 2.0 and 7.4. Alginate based nanocomposites as effective 5-FU systems has been reported in the literature (Dodov et al., 2009, Hosseinifar et al., 2018). CD is a cyclic oligosaccharide that consists of a macrocyclic ring of glucose subunits with α-1,4-glycosidic bonds. CD forms inclusion complexes with organic molecules by taking up these molecules into the apolar central cavity without forming or breaking covalent bonds (Tian et al., 2020). Many researchers have fabricated composites based on Alg or CD for the controllable release of drugs, peptides and proteins (Mura 2020, Raus et al., 2021). However, the use of a composite combining Alg and CD for drug delivery has rarely been reported in the literature. Our recent studies have reported an easy method for synthesising CD/ALG nanoparticles via ionic gelation (Nguyen et al., 2015, Nguyen et al., 2018, Nguyen et al., 2019a, Nguyen et al., 2019b). However, this nanocomposite has not been used to load 5-FU, with evaluation of efficacy against cancer cells.
The aim of this study was to develop 5-FU loaded onto a nanocomposite made from β-CD and Alg through a pH-sensitive linker and to investigate stability and controlled release in vitro, as well as to provide evidence of anti-tumour efficacy in a cell culture.
2 Materials and methods
2.1 Materials
Materials (calcium chloride (CaCl2), β-cyclodextrin and sodium alginate) were purchased from Shanghai Chemical Co. (China). 5-FU and sulforhodamine B (SRB) were provided by Sigma Co. (USA). Trichloroacetic acid and acetic acid were purchased from Merck (Germany). All chemicals were used without further purification. Deionized water was used in all experiments.
2.2 Fabrication of 5-FU encapsulated β-CD/Alg nanoparticles
The synthesis of β-CD/Alg nanocomposites was performed via the ionotropic gelation mechanism previously reported, with slight modification (Nguyen et al., 2018, Nguyen et al., 2019a). Briefly, different concentrations of 5-FU solution were added dropwise to 5 mL of CaCl2 solution (6.5 mg/mL) and stirred at 1200 rpm for 90 min. Then, the mixture was added to 13.7 mL of Alg solution (10 mg/mL), stirred for 90 min, and irradiated with ultrasound for 60 min. The 5-FU@Ca/Alg mixture was centrifugated and washed with water (3 × 10 mL) before the addition of the β-CD solution (10.7 mL, 1.6 mg/mL). The mixture was stirred then left for 24 h, centrifugated at 3200 rpm for 15 min and washed with water (3 × 10 mL). The nanocomposites were obtained via a freeze-drying process after 14 h.
2.3 Association efficiency and loading efficiency
The association efficiency of the drug is defined as the ratio of 5-FU by weight in the nanocomposite to the weight of the nanocomposite obtained, as shown in Eq. (1). The weight of 5-FU in the nanocomposite was estimated from the difference between the total amount of 5-FU (Wtotal 5-FU) and the amount of 5-FU in the supernatant separated from the nanocomposite after centrifugation (Wfree 5-FU). The amount of the drug in the supernatant was determined via a calibration curve of absorption intensity at a peak of 266 nm in UV–Vis measurement (Yusefi et al., 2021).
The loading efficiency of the drug is the ratio of 5-FU by weight in the nanocomposite to the weight of the dry nanocomposite, obtained via Eq. (2).
2.4 Physicochemical characterisation of the nanocomposite
Zeta potential was used to determine the electrochemical equilibrium at the interfaces and molecular vibrations, while DLS was used to determine the particle size distribution in the colloidal solution. The nanocomposite was diluted in deionized water to attain a solution with a concentration of 0.5 mg/mL. A nanoPartica Horiba SZ-100 (Japan) was used for the measurements. Zeta potential was measured at 25 °C with an applied voltage of 3.3 V, and DLS was performed at an angle of 173°.
To determine the presence of possible functional groups in the synthesized nanocomposite, the samples including blank β-CD/Alg and 5-FU@β-CD/Alg were characterized by Fourier-transform infrared (FTIR) analysis. The measurement was carried out on a Tensor 27 FTIR spectrophotometer (Brucker, Germany) with a wavenumber range of 500–4000 cm−1 and resolution of 0.5 cm−1.
The X-ray diffraction (XRD) patterns of 5-FU, β-CD/Alg and 5-FU@β-CD/Alg crystals was measured on an X-ray diffractometer (Bruker, Model-D8 Advance).
The particle size distribution and morphology of the nanocomposite 5-FU@β-CD/Alg were evaluated using TEM (S-4800 JEOL JEM1400) set at an accelerating voltage of 120 kV and FESEM (S-4800 HI-9057–0006).
Thermogravimetry analysis (TGA) and differential scanning calorimetry (DSC) were performed using a LabSys Evo 1600 thermal analyser (SETARAM, France) at a temperature range of 30–800 °C and a heating rate of 10 °C min−1 in air.
2.5 In vitro release profile and kinetic evaluation of 5-fluorouracil
A drug release study was performed in simulated colorectal fluid (phosphate-buffered saline at pH 7.4) and gastric fluid (hydrochloride/potassium chloride buffer at pH 1.2). The drug release efficiency of 5-FU@β-CD/Alg was evaluated using a 5 mL-dialysis bag (cut-off molecular weight between 3000 and 5000 Da) as previously reported, with slight modification (Fu and Kao 2010, Yusefi et al., 2020). Before the experiment, the dialysis bags were soaked in a release medium for 12 h. The solution mixture comprising the nanocomposite 5-FU@β-CD/Alg (3 mg) and the release medium (3 mL) was added to the dialysis bag with the two ends tied. The dialysis bag was immersed in 12 mL of the release medium maintained at 37 °C and slowly stirred. To detect the released drug, an aliquot (0.5 mL) was withdrawn at different times in range of 0.25–96 h and the outer solution was immediately replaced by the same volume of fresh medium. The withdrawn samples were characterized by UV–Vis spectroscopy with a wavelength of 266 nm. Runs were performed in triplicate. The release profile was plotted as the relative release percentages of 5-FU over time. The same procedure was performed for blank β-CD/Alg and 5-FU as standard references. The drug release from the different media was calculated via Eq. (3).
For release kinetic study, the following models including zero order (Eq. (4)), first order (Eq. (5)), Hixson-Crowell (Eq. (6)), Higuchi (Eq. (7)) and Korsmeyer-Peppas (Eq. (8)) were investigated via an origin software. The mathematical equations representing these models is listed as follows:
2.6 Cytotoxicity assay
Fibroblast and MCF-7 (HTB-22) cells were provided and cultured by the University of Science, Vietnam National University, Ho Chi Minh City, Vietnam. Cells were cultured at 37 °C and 5% CO2 in Eagle’s minimal essential medium (EMEM) supplemented with 20 mM HEPES (Sigma), 0.025 μg/mL amphotericin B (Sigma), 2 mM L-glutamine (Sigma), 100 IU/mL penicillin G (Sigma), 100 μg/mL streptomycin (Sigma), and 10 % (v/v) FBS (Sigma). Samples with β-CD/Alg, 5-FU@β-CD/Alg and the solutions after release at pH 7.4 and pH 1.2 were used to test cytotoxicity.
The SRB assay was carried out as previously reported, with some modification (Nguyen and Ho-Huynh 2016, Thach et al., 2021). Cells were seeded in 96-well plates with 10,000 cells/well and cultured for 24 h before being incubated with the samples at various concentrations for 48 h. The cells were placed in cold trichloroacetic acid solution (50 %, w/v) for 1–3 h, washed, and stained with SRB (0.2%, w/v) for 20 min. Water and camptothecin (Calbiochem) were used as negative and positive controls, respectively. Absorption intensity was determined using an ELISA plate reader (Synergy HT, Biotek Instruments) at wavelengths of 492 nm and 620 nm. The percentage of growth inhibition (%) was determined at a concentration of 100 μg/mL via Eq. (9).
3 Results and discussion
3.1 Synthesis of nanocomposite 5-FU@β-CD/Alg
Synthesis of nanocomposite 5-FU@β-CD/Alg was carried out via an ionotropic gelation mechanism using Ca2+/Alg gelispheres and β-CD molecules as previously reported, with slight modification (Nguyen et al., 2015, Nguyen et al., 2019a). In the present work, the gelispheres were loaded with 5-FU before the nanoparticles were synthesised, as illustrated in Fig. 1. β-CD molecules have a negative zeta potential and can interact to cross-link a Ca2+ ion and alginate in the insoluble gelispheres to form a nanocomposite that is well dispersed in water. 5-FU can be encapsulated in the matrix of the polysaccharides via bonds with Ca2+ ions and functional groups in the CD molecules and alginate chain. The nanocomposite 5-FU@β-CD/Alg is easily purified and obtained using centrifugation. Because the quantity of the drug can significantly influence the efficiency of nanocomposite synthesis, the loading process for the drug was investigated using different ratios of drug weight to carrier weight. The loading efficiency and association efficiency of the drug were used to evaluate the performance of synthesis. The best nanocomposite was used to investigate the physicochemical characterisation and in vitro drug release behaviour and kinetics.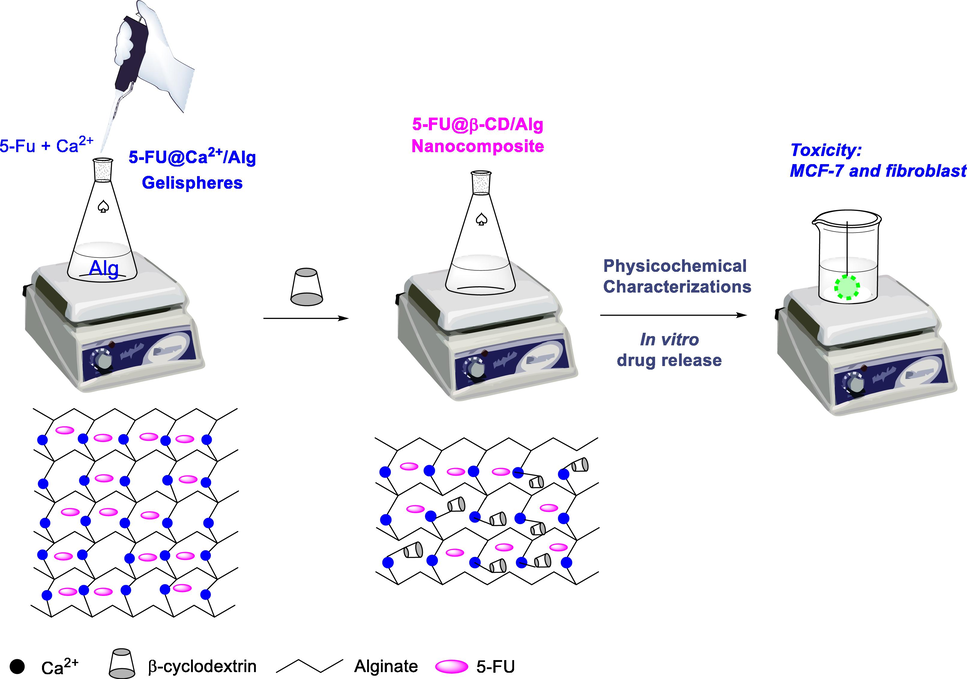
Study scheme and proposed formation mechanism for the 5-FU@β-CD/Alg nanocomposite.
To fabricate the nanocomposite, the same procedure as a previously reported was used, with a hydrophobic drug (ketoprofen) loaded onto β-CD before the 5-FU@β-CD complex was added to the Ca2+/Alg galispheres to form the nanocomposite (Nguyen et al., 2015). However, only a very low loading efficiency of the 5-FU was obtained and no nanoparticles were formed in the solution; this is likely because the solubility of 5-FU is greater than ketoprofen and its molecular size is smaller. Recent research (Jin et al., 2010, Di Donato et al., 2016) has confirmed that β-CD can easily encapsulate 5-FU in the solid state. However, it is not a good host for the complexation of 5-FU in an aqueous solution due to the low stability of the complex (Melnikova et al., 2020). Thus, 5-FU can interrupt the interaction between Ca2+/Alg galispheres and β-CD molecules during nanoparticle formation in solution, impeding nanocomposite synthesis.
In recent research (Nguyen, Vo, Huynh, et al. 2019), nanoparticles have been fabricated with changes in the synthesis procedure and a different order of addition of materials. With this change, 5-FU was entrapped into Ca2+/Alg galispheres before β-CD molecules were added to form the nanoparticles (Fig. 1); this procedure performed well in synthesising the 5-FU@β-CD/Alg nanocomposite. To study the effect of drug concentration, feeding ratios of 5-FU in the range 5–40% (w/w) were used to determine drug association efficiency and drug loading efficiency. The results show a non-linear relationship between the drug content added to the nanocomposite and encapsulation and loading efficiency (Fig. 2). Raising feeding ratios of 5-FU from 5 % to 40 % decreases the association efficiency of the drug from 96.8 ± 1.5% to 81.7 ± 2.0%, due to the saturation of 5-FU in the matrix of alginate chains. Meanwhile, increase in these ratios increases the loading efficiency, with a maximum of 36.4 ± 1.7 % at a feeding ratio of 20% (w/w). Although a non-linear relationship between feeding ratios and encapsulated efficiency can be observed for the β-CD-based nanocomposite, this result represents a different trend for 5-FU encapsulation than for other nanocomposites reported previously. Indeed, Prabha and Raj (Prabha and Raj 2016) showed that the association and loading efficiencies of 5-FU from the nanocomposite β-CD/Fe3O4/PEG increase with an increase in 5-FU concentration. Similarly, Hosseinifar et al. (Hosseinifar et al., 2018) also found that an increase in drug ratios induces better encapsulation performance in β-CD-Alginate nanogel.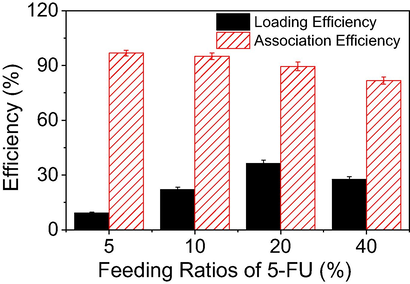
Influence of 5-FU concentration on loading efficiency and association efficiency of the drug from the 5-FU@β-CD/Alg nanocomposite.
3.2 Physicochemical characterisation of the nanocomposite 5-FU@β-CD/Alg
Zeta potential and particle size are the principal criteria for evaluating effective anticancer drug delivery targeting tumour tissue. Fig. 3 shows spectra of the zeta potential and particle size of blank nanocomposite β-CD/Alg and nanocomposite 5-FU@β-CD/Alg. Because of the negative zeta potential of the components β-CD and Alg (Lakkakula et al., 2017), the blank nanocomposite β-CD/Alg has a great surface charge of −48.7 mV while the presence of 5-FU (20%) slightly decreases the surface charge (−42.1 mV). The highly negative zeta potential induces repulsion between nanoparticles, greatly reducing the propensity to aggregate and making the nanocomposite highly stable in an aqueous solution. Thus, the nanocomposite based on β-CD and Alg protects the drug and is an ideal carrier for a drug delivery system.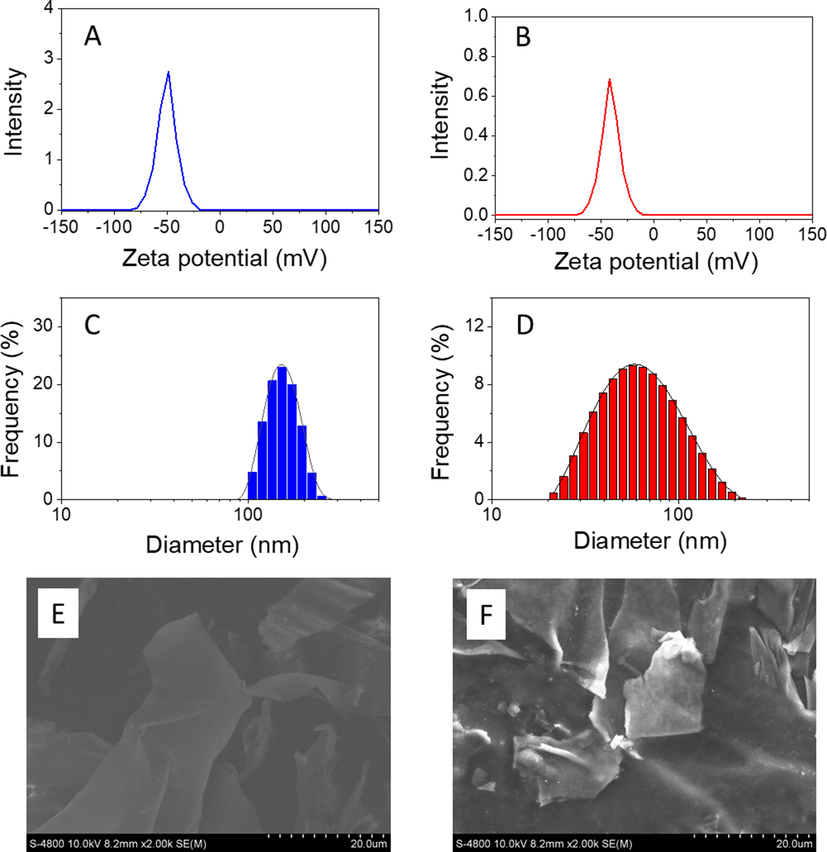
Zeta potential, dynamic light scattering and FESEM images of an aqueous solution of β-CD/Alg (A, C & E) and 5-FU@β-CD/Alg nanocomposite (B, D & F).
The particle size of the nanocomposites in an aqueous solution was determined by DLS measurement. The result shows that the particle size of 5-FU@β-CD/Alg is in the range 20–200 nm with a mean size of 57 nm, while the particle size of β-CD/Alg is larger, at 100–250 nm with a mean size of 150 nm. The smaller size of the nanocomposite 5-FU@β-CD/Alg indicates that the presence of 5-FU may significantly reduce the size of the nanoparticle, confirming the encapsulation of 5-FU into the matrix of crosslinks formed by calcium ions and alginate chains in β-CD/Alg. The FESEM images (Fig. 3E and 3F) show that the nanocomposites possess regular fibres with sheet-like shapes which were formed during the drying process.
The FTIR spectra of blank β-CD/Alg, 5-FU and 5-FU@β-CD/Alg nanocomposite are shown in Fig. 4. The FTIR spectrum of blank β-CD/Alg has the characteristic absorption peaks of glucose units at 3425, 2925, 1418 and 1030 cm−1. The bands at 3425 cm−1 and 1418 cm−1 can be attributed to the stretching and bending vibrations, respectively, of OH groups and the band at 1030 cm−1 to the stretching vibration of glucose C-O-C groups. The band at 2925 cm−1 is characteristic of C-H stretching vibration. In particular, the peak at 1627 cm−1 corresponds to the stretching vibration of the carboxyl groups (COO) in alginate chains. The FTIR spectrum of 5-FU has bands at 3142, 2936, 1776, 1349 and 1249 cm−1, where the bands at 3142 cm−1 and 2936 cm−1 are characteristic of N-H and C—H stretching vibrations, respectively, the band at 1776 cm−1 is characteristic of —C⚌O group of ketone, and the bands at 1349 and 1249 cm−1 are characteristic of stretching vibration of C—N and C—F, respectively. Analysis of the FTIR spectrum of the 5-FU@β-CD/Alg nanocomposite shows that the absorption bands shift to new positions compared to the blank nanocomposite, namely 3413, 2934, 1613, 1424, 1034 cm−1. The broad band at 3413 cm−1 can be assigned to the stretching vibration of N—H and O—H groups, the band at 2934 cm−1 to the stretching vibration of C—H, and the bands at 1613 cm−1 and 1424 cm−1 to the stretching vibration of C⚌O groups and bending vibration of the OH groups, respectively. The band at 1034 cm−1 is characteristic of the stretching vibration of C—O—C groups. Notably, the spectrum has bands from 1350 to 1250 cm−1 corresponding to the characteristic vibrations of C—N and C—F groups in the 5-FU molecule. The results indicate that the 5-FU drug is successfully encapsulated into the carrier β-CD/Alg.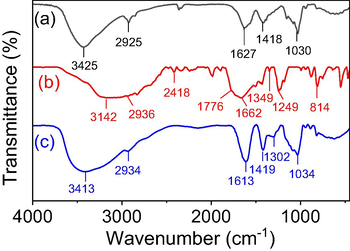
FTIR Spectra of (a) β-CD/Alg, (b) 5-FU and (c) 5-FU@β-CD/Alg nanocomposite.
XRD patterns of 5-FU, β-CD/Alg and 5-FU@β-CD/Alg nanocomposite are shown in Fig. 5. The XRD pattern of 5-FU showed the intense peaks at 2θ value of 16.5 and 28.6° that relates to its crystalline nature. The data of β-CD/Alg nanocomposite shows an abroad diffraction peak appeared at 2θ value of 43.50 which relates to crystalline structure glucuronate and mannuronate units in alginate chains with a crystalline diameter of 1.47 nm. For the diffractogram XRD of 5-FU@β-CD/Alg nanocomposite, the peaks observed at 16.2, 28.5 and 43.40 indicates the successful loading of 5-FU into the matrix of β-CD/Alg composite and maintaining crystalline nature of drug and the polysaccharides. The crystal diameter calculated at peak 43.40 was determined to be 1.36 nm.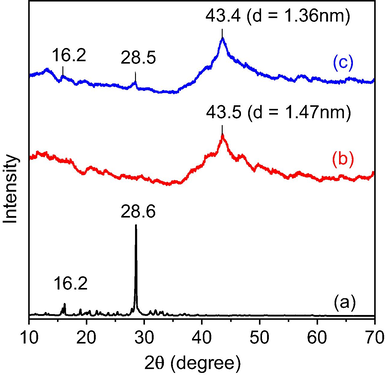
XRD patterns of (a) 5-FU, (b) β-CD/Alg and (c) 5-FU@β-CD/Alg nanocomposite.
The size and morphology of the 5-FU@β-CD/Alg nanocomposite were determined using TEM. Images and particle size distribution are shown in Fig. 6. The TEM images indicate that the nanocomposite has a uniform spherical morphology with low aggregation. The nanoparticles distribute in the range 30–80 nm with a mean diameter of 50 nm. The result confirms the successful synthesis of the 5-FU@β-CD/Alg nanocomposite.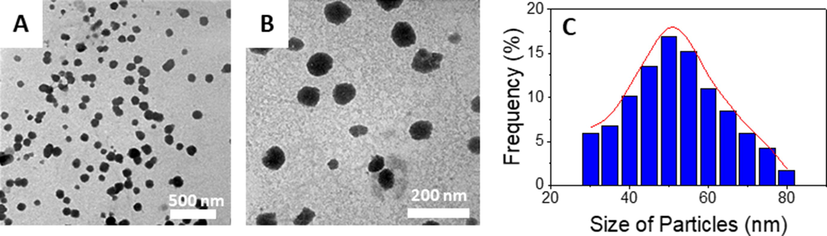
Transmission electron micrograph images at different amplification and particle size distribution of 5-FU@β-CD/Alg nanocomposite.
The thermal stability of the nanocomposites was analysed using TGA and DSC (Fig. 7). The TGA curves of both nanocomposites show three main weight-loss steps. The first weight loss step in both samples occurs between 30 and 220 °C and is estimated to be 22%. This step reflects the evaporation of adsorbed moisture from the surface of the samples, as confirmed by the presence of a corresponding endothermic peak in the DSC curves. The second weight-loss steps for β-CD/Alg and 5-FU@β-CD/Alg occur in the temperature range 220–545 °C with a weight loss of 43% and 220–440 °C with a weight loss of 40%, respectively. This weight loss can be attributed to the oxidation of sugar molecules in polysaccharide chains which relate to the small exothermic peaks in the DSC curves. In the final step, β-CD/Alg shows a weight loss of 20% in the range 545–800 °C and 5-FU@β-CD/Alg shows a weight loss of 25% in the much lower range 440–800 °C. The DSC curves have intense exothermic peaks in the corresponding temperature regions that can be attributed to oxidation of the drug and polysaccharide components. Moreover, enthalpy value of the drug loaded nanocomposite was found to be greater than the value of the blank nanocomposite that indicated the interaction between drug molecules and polysaccharides in the nanocomposite. The low-temperature stability of the 5-FU@β-CD/Alg nanocomposite indicates the presence of the drug in the nanocomposite. On the other hand, 5-FU is oxidised at a much low temperature, in the range 250–350 °C, as previously reported (Obireddy and Lai 2021). Thus, the thermal behaviour of the 5-FU@β-CD/Alg nanocomposite indicates the interaction of the drug with sugar molecules in polysaccharides that leads to a significant increase in the thermal stability of 5-FU.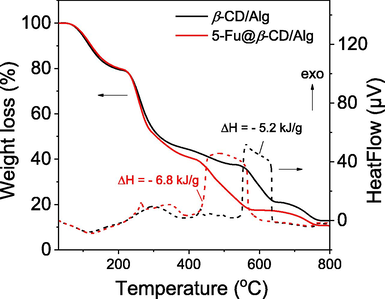
TGA and DSC curves of β-CD/Alg and 5-FU@β-CD/Alg nanocomposite in airflow of 20 mL/min at a heating rate of 10 °C/min.
3.3 In vitro drug release of nanocomposite 5-FU@β-CD/Alg
The drug release profile of 5-FU from the 5-FU@β-CD/Alg nanocomposite was investigated in different media at a constant temperature (37 °C). The 5-FU drug without a carrier in a phosphate buffer (pH 7.4) was also explored as a reference, with 5-FU release of 92.0% and 98.7% at 2 h and 4 h, respectively. Fig. 8A shows release profiles of the nanocomposite in phosphate buffer solutions at different pH values, including simulated colorectal fluid medium (pH 7.4) and simulated gastric fluid medium (pH 1.2) for 24 h. It was found that rapid release occurs during the first 60 min, followed by slower release. In the colorectal fluid medium (pH 7.4), drug release of 37.7% and 52.1% was obtained after 1 h and 4 h, respectively, with 69.8% after 24 h and 80.7% after 96 h. A much slower rate of release was observed in pH 1.2, with 20.0% released in the first 4 h and 34.1% in 96 h. Thus, the results indicate that the 5-FU drug in the nanocomposite is well protected from the physiological environment, and release behaviour from the nanocomposite is modulated by changes in environmental pH media, meaning that the nanocomposite can be used effectively in the targeted treatment of cancer cells.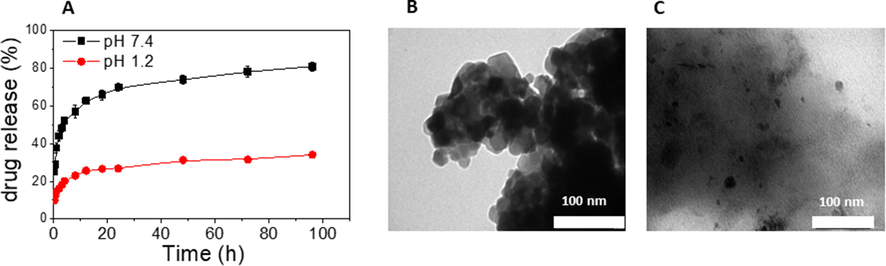
Drug release profile of the 5-FU@β-CD/Alg nanocomposite at pH 7.4 and pH 1.2 (A); TEM images of the 5-FU@β-CD/Alg nanocomposite recovered after 48 h from pH 1.2 (B) and pH 7.4 (C).
It is notable that the drug release behaviour from the 5-FU@β-CD/Alg nanocomposite is similar to previous reports on alginate-based nanocomposites (Azhar and Olad 2014, Anirudhan and Nair 2019, Wu et al., 2019), which have shown that at pH 7.4, 5-FU is released more rapidly than it is at pH 1.2. However, the release of the 5-FU drug is different from the release behaviour of ketoprofen from the nanocomposite ketoprofen@β-CD/Alg reported previously, where a lower pH leads to a faster drug release. This phenomenon demonstrates that 5-FU is loaded onto the matrix of Ca2+/Alg but is not forming an inclusion complex with β-CD.
In order to understand 5FU@β-CD/Alg release behaviour, the samples were recovered after release in both media and further characterised by TEM. The TEM images are shown in Fig. 8B and C. It is clear that the nanoparticles are clustered at pH 1.2 while the composite is sufficiently swelled at pH 7.4 to facilitate the diffusion of the drug into the solution. The formation of clusters at pH 1.2 contributes to the protonation of COO– groups in alginate, leading to the recombination of particles to form clusters of greater size and network holes to be minified, restricting pervasion of 5-FU from the interior of the nanocomposite. It can be concluded that the release behaviour of the drug from the 5-FU@CD/Alg nanocomposite is mainly by way of changes in the morphology of the nanocomposite.
The release kinetics of 5-FU drug in the various media were modelled into the five principal mathematical models. The kinetic parameters are listed in Table 1. The drug release behaviour fits both Hugichi and Korsmeyer-Peppas models but the later release model is best fitted. The results show that the release of 5-FU from the nanocomposite at pH 1.2 possesses slightly higher coefficient in comparison with release behaviour at pH 7.4. However, the difference is not significant, thus it can be concluded that the release kinetic is well fitted in both media. In addition, for all environmental pH values, the diffusion exponent is found to be lower than 0.45, indicating that the 5-FU release from the nanocomposite follows Fickian diffusion mechanism.
Kinetic models
pH 7.4
pH 1.2
Constant
n
R2
Constant
n
R2
Zero order
0.607
–
0.533
0.218
–
0.617
First order
3.0 × 10-3
–
0.400
2.8 × 10-3
–
0.516
Hixson-Croswell
0.010
–
0.496
8.1 × 10-3
–
0.531
Hugichi
6.764
–
0.817
3.640
–
0.946
Korsmeyer-Peppas
37.1
0.188
0.956
14.7
0.192
0.980
3.4 Cytotoxicity assay
The cytotoxic effects of β-CD/Alg, 5-FU@β-CD/Alg and release solutions at different pH values after 48 h against a normal cell line (fibroblast) and a breast cancer cell line (MCF-7) are shown in Table 2. As can be seen, the β-CD/Alg nanocomposite displays no inhibition of the normal cell line but a slight inhibition of 26.83 ± 3.58 against the cancer cell line at a concentration of 100 μg/mL. However, IC50 values of the blank nanocomposite are over 100 μg/mL for both cell lines, demonstrating the high biocompatibility of the carrier material. The results also show that although the IC50 value of 5-FU against fibroblasts is >100 µg/mL, inhibition against this normal cell of about 45.31 ± 5.52% at a concentration of 100 µg/mL is much higher than inhibition with 5-FU@β-CD/Alg. This result reveals the drawbacks of using the drug alone in chemotherapy treatment without a drug delivery system. As expected, the 5-FU@β-CD/Alg nanocomposite shows high toxicity against the cancer cell lines but almost no toxicity against normal cells. Indeed, this nanocomposite inhibits 71.75 ± 3.73% and 20.59 ± 3.07% for MCF-7 and fibroblasts cells, respectively, while the IC50 values are 21.68 ± 2.22 μg/mL and over 100 μg/mL, respectively. These results indicate that the nanocomposite is safe for normal cells but has a good ability against cancer cells. To evaluate the efficiency of drug release from the nanocomposite, the toxicity of the solution after release at pH 7.4 and pH 1.2 after 48 h was tested for both cell lines. Good toxicity of these release solutions was found against MCF-7, with inhibition of 78.48 ± 0.97% and 70.32 ± 0.98 %, respectively, and IC50 values of 2.02 ± 0.38 and 10.96 ± 0.69, respectively, while this solution shows very low toxicity against normal cells. Additionally, statistical analysis of the data showed that IC50 value of the 5-FU@β-CD/Alg nanocomposite released at pH 7.4 against MCF-7 is similar to the alone 5-FU drug but significantly different from the solution released at pH 1.2. Therefore, the results suggest the safety and high potential of using β-CD/Alg as an anticancer drug delivery system with high antiproliferation activity against MCF-7 cells and negligible effects on normal healthy cells.
No.
Samples
Inhibition %*
IC50 (μg/mL)
MCF-7
Fibroblast
MCF-7
Fibroblast
1
β-CD/Alg
26.83 ± 3.58a
−0.48 ± 3.99a
>100d
>100
2
5-FU
83.02 ± 2.61c
45.31 ± 5.52c
1.28 ± 0.18a
>100
3
5-FU@β-CD/Alg
71.75 ± 3.73b
20.59 ± 3.07b
21.68 ± 2.22c
>100
4
Release solution at pH 7.4
78.48 ± 0.97c
37.42 ± 2.53c
2.02 ± 0.38a
>100
5
Release solution at pH 1.2
70.32 ± 0.98b
39.68 ± 5.7c
10.96 ± 0.69b
>100
6
Camptothecin
61.45 ± 0.57**
50.71 ± 1.78***
0.007 ± 0.002
1.57 ± 0.84
4 Conclusion
This work successfully loaded 5-FU onto the β-CD/Alg nanocomposite to evaluate release behaviour and anticancer activity. The structure of the nanocomposite was characterised using analysis technologies. A stable colloidal solution was confirmed by highly negative zeta potential. The size of the nanocomposite was found by TEM to be in the range 30–80 nm with a mean size of 50 nm. The release profile of 5-FU in a strongly acidic medium (pH 1.2) was much lower than in a neutral medium (pH 7.4). The release of 5-FU from the nanocomposite is facilitated by the change in morphology of the nanocomposite, with the formation of clusters in an acidic medium and the destruction of nanoparticles at a neutral pH after 48 h. The cytotoxicity assay demonstrates that the nanocomposite is an effective delivery system for 5-FU, with a high level of anticancer activity against MCF-7 cells, while being safe for normal cells. Therefore, the nanocomposite has the potential for application in the chemotherapy treatment of cancer patients.
Acknowledgements.
The authors are thankful to Vietnam Academy of Science and Technology (VAST) for funding this project (VAST03.02/20-21).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol.. 2018;109:273-286.
- [Google Scholar]
- Polyelectrolyte complexes of carboxymethyl chitosan/alginate based drug carrier for targeted and controlled release of dual drug. J. Drug Delivery Sci. Technol.. 2019;51:569-582.
- [Google Scholar]
- A study on sustained release formulations for oral delivery of 5-fluorouracil based on alginate–chitosan/montmorillonite nanocomposite systems. Appl. Clay Sci.. 2014;101:288-296.
- [Google Scholar]
- Polymeric nanoparticles for oral delivery of 5-fluorouracil: formulation optimization, cytotoxicity assay and pre-clinical pharmacokinetics study. Eur. J. Pharm. Sci.. 2016;84:83-91.
- [Google Scholar]
- Alpha-and beta-cyclodextrin inclusion complexes with 5-fluorouracil: characterization and cytotoxic activity evaluation. Molecules. 2016;21:1644.
- [Google Scholar]
- Wheat germ agglutinin-conjugated chitosan–Ca–alginate microparticles for local colon delivery of 5-FU: development and in vitro characterization. Int. J. Pharm.. 2009;381:166-175.
- [Google Scholar]
- Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opinion Drug Deliv.. 2010;7:429-444.
- [Google Scholar]
- Pressure responsive nanogel base on alginate-cyclodextrin with enhanced apoptosis mechanism for colon cancer delivery. J. Biomed. Mater. Res. Part A. 2018;106:349-359.
- [Google Scholar]
- Preparation of 5-fluorouracil/β-cyclodextrin complex intercalated in layered double hydroxide and the controlled drug release properties. Ind. Eng. Chem. Res.. 2010;49:11176-11181.
- [Google Scholar]
- Putative role of dihydropyrimidine dehydrogenase in the toxic side effect of 5-fluorouracil in colorectal cancer patients. Oncology. 1998;55:468-474.
- [Google Scholar]
- Biocompatible and self-recoverable succinoglycan dialdehyde-crosslinked alginate hydrogels for pH-controlled drug delivery. Carbohydr. Polym.. 2020;250:116934.
- [Google Scholar]
- Cationic cyclodextrin/alginate chitosan nanoflowers as 5-fluorouracil drug delivery system. Mater. Sci. Eng., C. 2017;70:169-177.
- [Google Scholar]
- Pharmacokinetic characteristics and anticancer effects of 5-fluorouracil loaded nanoparticles. BMC Cancer. 2008;8:1-9.
- [Google Scholar]
- LncRNA SNORD3A specifically sensitizes breast cancer cells to 5-FU by sponging miR-185-5p to enhance UMPS expression. Cell Death Dis.. 2020;11:1-12.
- [Google Scholar]
- On Complex formation between 5-fluorouracil and β-cyclodextrin in solution and in the solid state: IR markers and detection of short-lived complexes by diffusion NMR. Molecules. 2020;25:5706.
- [Google Scholar]
- Muhamad, I.I., N.A.M. Lazim, Selvakumaran, S., 2019. Natural polysaccharide-based composites for drug delivery and biomedical applications. In: Natural Polysaccharides in Drug Delivery and Biomedical Applications, Elsevier, pp. 419–440.
- Advantages of the combined use of cyclodextrins and nanocarriers in drug delivery: a review. Int. J. Pharm.. 2020;579:119181.
- [Google Scholar]
- Nanocomposite hydrogels for melanoma skin cancer care and treatment: In-vitro drug delivery, drug release kinetics and anti-cancer activities. Arab. J. Chem.. 2021;14:103120.
- [Google Scholar]
- Selective cytotoxicity of a Vietnamese traditional formula, Nam Dia long, against MCF-7 cells by synergistic effects. BMC Complement. Alternat. Med.. 2016;16:1-10.
- [Google Scholar]
- Biosynthesized AgNP capped on novel nanocomposite 2-hydroxypropyl-β-cyclodextrin/alginate as a catalyst for degradation of pollutants. Carbohydr. Polym.. 2018;197:29-37.
- [Google Scholar]
- Synthesis and characterization of β-cyclodextrin/alginate nanoparticle as a novel drug delivery system. Chem. Biochem. Eng. Q.. 2015;29:429-435.
- [Google Scholar]
- Effect of capping methods on the morphology of silver nanoparticles: study on the media-induced release of silver from the nanocomposite β-cyclodextrin/alginate. New J. Chem.. 2019;43:16841-16852.
- [Google Scholar]
- Biogenic palladium nanoclusters supported on hybrid nanocomposite 2-hydroxypropyl-β-cyclodextrin/alginate as a recyclable catalyst in aqueous medium. J. Mol. Liq.. 2019;276:927-935.
- [Google Scholar]
- Multi-component hydrogel beads incorporated with reduced graphene oxide for pH-responsive and controlled co-delivery of multiple agents. Pharmaceutics. 2021;13:313.
- [Google Scholar]
- Formation and characterization of β-cyclodextrin (β-CD)–polyethyleneglycol (PEG)–polyethyleneimine (PEI) coated Fe3O4 nanoparticles for loading and releasing 5-Fluorouracil drug. Biomed. Pharmacother.. 2016;80:173-182.
- [Google Scholar]
- Microparticles based on carboxymethyl starch/chitosan polyelectrolyte complex as vehicles for drug delivery systems. Arab. J. Chem.. 2020;13:2183-2194.
- [Google Scholar]
- Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci.. 2021;16:280-306.
- [Google Scholar]
- Sodium alginate and alginic acid as pharmaceutical excipients for tablet formulation: Structure-function relationship. Carbohydr. Polym.. 2021;270:118399.
- [Google Scholar]
- Biopolymeric nanocomposites in drug delivery. In: Advanced Biopolymeric Systems for Drug Delivery. Springer; 2020. p. :233-290.
- [Google Scholar]
- Synthesis and antimicrobial, antiproliferative and anti-inflammatory activities of novel 1, 3, 5-substituted pyrazoline sulphonamides. Arab. J. Chem.. 2021;14:103408.
- [Google Scholar]
- Smart stimuli-responsive drug delivery systems based on cyclodextrin: a review. Carbohydr. Polym.. 2021;251:116871.
- [Google Scholar]
- The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: a review. Polym. Int.. 2020;69:597-603.
- [Google Scholar]
- Molecular biological analysis of 5-FU-resistant gastric cancer organoids; KHDRBS3 contributes to the attainment of features of cancer stem cell. Oncogene. 2020;39:7265-7278.
- [Google Scholar]
- Interaction pathways and structure–chemical transformations of alginate gels in physiological environments. Biomacromolecules. 2019;20:4158-4170.
- [Google Scholar]
- Fabrication of alginate microspheres for drug delivery: a review. Int. J. Biol. Macromol.. 2020;153:1035-1046.
- [Google Scholar]
- Meta-analysis examining overall survival in patients with pancreatic cancer treated with second-line 5-fluorouracil and oxaliplatin-based therapy after failing first-line gemcitabine-containing therapy: effect of performance status and comparison with other regimens. BMC Cancer. 2020;20:1-9.
- [Google Scholar]
- Targeted delivery of 5-fluorouracil to HT-29 cells using high efficient folic acid-conjugated nanoparticles. Drug Deliv.. 2015;22:191-198.
- [Google Scholar]
- A review on the properties of electrospun cellulose acetate and its application in drug delivery systems: a new perspective. Carbohydr. Res.. 2020;491:107978.
- [Google Scholar]
- An alginate-based hydrogel composite obtained by UV radiation and its release of 5-fluorouracil. Polym. Bull.. 2019;76:1167-1182.
- [Google Scholar]
- 5-Fluorouracil encapsulated chitosan-cellulose fiber bionanocomposites: synthesis, characterization and in vitro analysis towards colorectal cancer cells. Nanomaterials. 2021;11:1691.
- [Google Scholar]
- The potential anticancer activity of 5-fluorouracil loaded in cellulose fibers isolated from rice straw. Int. J. Nanomed.. 2020;15:5417.
- [Google Scholar]
- Polysaccharide-based nanocomposites and their applications. Carbohydr. Res.. 2015;405:23-32.
- [Google Scholar]







