Translate this page into:
Deep eutectic solvents as efficient extractants of caffeoylquinic acids from Blumea aromatica: A comparative analysis of content and antioxidant potential
⁎Corresponding authors. dai_gdpu_2018@gdpu.edu.cn (Wei Dai), h.f.chen@scbg.ac.cn (Hongfeng Chen), zhengxl2020@gdpu.edu.cn (Xilong Zheng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This study conducted a comparative analysis of the extraction efficiency and antioxidant potential of caffeoylquinic acids (CQAs) from Blumea aromatica using deep eutectic solvents (DESs) and traditional solvents. Utilizing UPLC-Q-Orbitrap HRMS, the quantification of seven CQAs revealed concentrations ranging from 0.46 to 7.60 mg/g, with 1,5-diCQA identified as the most abundant. DESs demonstrated significant advantages (P < 0.05) over traditional solvents. The optimal extraction occurred with DES-6 (choline chloride / 1,4-butanediol) for 3-CQA (4.71 ± 0.31 mg/g) and 4,5-diCQA (2.28 ± 0.19 mg/g), DES-7 (choline chloride / oxalic acid) for 4-CQA (2.05 ± 0.05 mg/g), and DES-5 (choline chloride / glycerol) for 5-CQA (1.70 ± 0.14 mg/g). Antioxidant activity assessment through DPPH, ABTS, and reducing power assays indicated that DES extracts outperformed conventional solvents. Notably, DES-3 (choline chloride / ethylene glycol) displayed remarkable activity, with IC50 values of 197.36 ± 1.05 and 14.86 ± 3.33 μg/mL for DPPH and ABTS radicals, respectively. DES-6 exhibited the highest reducing power. Correlation analysis established positive relationships between phenolic acid content and antioxidant activity, notably for 3-CQA and 5-CQA (p < 0.001 and p < 0.05). Additionally, 4-CQA, 1,3-diCQA, 3,4-diCQA, and 4,5-diCQA displayed specific and correlated antioxidant activities. Crucially, the environmentally friendly DESs extraction method proposed in this study offers a sustainable approach for obtaining CQAs from B. aromatica, concurrently ensuring their antioxidant potential is fully realized. This research not only advances our understanding of B. aromatica but also highlights a green and efficient method for extracting bioactive compounds with potential applications in the pharmaceutical and nutraceutical industries.
Keywords
Blumea aromatica
Caffeoylquinic acids
Antioxidant activity
Deep eutectic solvents
UPLC-Q-Orbitrap HRMS
1 Introduction
Blumea aromatica DC., a perennial herb within the Blumea genus, thrives in the regions of Southwest China and Southeast Asia (Editorial Committee of Flora of China, 1979). Referred to as “Shanfeng” in Traditional Chinese Medicine (TCM), it has been extensively employed in the treatment of conditions such as rheumatism, arthralgia, and eczema (Lan et al., 2012). Despite its recognized potential, there exists a significant gap in comprehending the chemical constituents and bioactive properties of B. aromatica. Notably, labdane diterpenoids and bisnorditerpenoids have been explicitly identified among the compounds isolated from B. aromatica in prior studies, revealing the diverse biological activities encompassing anti-inflammatory, immunosuppressive, and adenylate cyclase activation properties (Shen et al., 2019; Song et al., 2021). Our recent research on the chemical constituents and bioactivity of the Blumea genus has unveiled a variety of phenolic compounds in these plants (Dai et al., 2023). Earlier studies on B. aromatica primarily focused on the examination of specific terpenoids, with no available reports on the types and biological activities of phenolic compounds in B. aromatica. Therefore, a systematic investigation of the phenolic compounds in B. aromatica is imperative.
Phenolics are secondary metabolites that plants produce during their growth. They are abundant in vegetables, fruits, and medicinal plants. Phenolics have diverse structures and biological activities (Kim and Son, 2011). They can act as antioxidants, antimicrobials, anticancer agents, anti-aging agents, anti-inflammatory agents, and antihypertensive agents (Leri et al., 2020; Di Lorenzo et al., 2021; Shakoor et al., 2021; Rana et al., 2022). Thus, plant phenolics have wide applications in food and medicine. Caffeoylquinic acids (CQAs) represent a important component of Phenolics, originating from the phenylpropanoid biosynthesis pathway. These compounds consist of quinic acid acylated with one to four caffeic acid moieties, leading to 15 possible combinations, including four monoCQAs, six diCQAs, four triCQAs, and one tetraCQA (Alcazar Magana et al., 2021). CQAs possess various therapeutic attributes such as antioxidant, antibacterial, anticancer, antiviral, anti-Alzheimer, and neuroprotective activities (Taira et al., 2014; Murad et al., 2015; Bulgakov et al., 2018; Liu et al., 2020; Matthews et al., 2020; Metwally et al., 2020; Nzekoue et al., 2020; Trendafilova et al., 2020). CQAs are commonly consumed through plant-based foods like fruits, vegetables, and prominently, coffee beverages. This consumption pattern has established a significant inverse correlation between coffee intake and the incidence of several degenerative diseases, as well as a positive correlation with longevity (Farah and de Paula Lima, 2019). Owing to their potent antioxidant properties, CQAs are attracting increasing attention for their potential in mitigating cognitive decline and lifestyle-related diseases, and for their role in drug development (Alcazar Magana et al., 2021).
Phenolics are typically extracted from plant matrices through either organic solvent extraction or physical-assisted extraction. However, organic solvent extraction presents several drawbacks, including high volatility, toxicity, flammability, and low biodegradability (Chandrasekara et al., 2015). A promising alternative is the use of deep eutectic solvents (DESs), a novel class of green solvents known for their environmental friendliness. DESs typically consist of a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA), and their melting point is lower than that of any individual component, allowing them to remain in a stable liquid state at room temperature (Ammar et al., 2018). In comparison to organic solvents, DESs are easily synthesized, cost-effective, stable, non-volatile, biodegradable, and environmentally friendly. In recent years, DESs have garnered increased attention and have found wide application in the extraction of natural products (Zurob et al., 2020; Vazquez-Gonzalez et al., 2020; Khare et al., 2021; Zannou et al., 2022).
Plants serve as crucial sources of natural phenolics, and the Blumea genus within the Compositae family has been confirmed to exhibit rich phenolic content with specific and diverse biological activities (Kusumawati et al., 2018; Jirakitticharoen et al., 2022). While the use of DESs for extracting CQAs from plants has shown promising results, with a focus on compounds such as chlorogenic acid (3-CQA), isochlorogenic acid A (3,5-diCQA), isochlorogenic acid B (3,4-diCQA), and isochlorogenic acid C (4,5-diCQA), limited attention has been given to systematic studies. Duan et al. demonstrated the simultaneous extraction of four phenolic acids (3-CQA, 3,4-diCQA, 3,5-diCQA, and 4,5-diCQA) from Artemisia argyi leaves using binary and ternary DESs, highlighting the superior extraction efficiency of the ternary DES with a molar ratio of choline chloride, malic acid, and urea at 2:1:2 (Duan et al., 2019). Rashid et al. successfully employed DESs to extract chlorogenic acid from apple pomace, noting that formulations such as choline chloride: glycerol (1:2), choline chloride: lactic acid (1:3), and choline chloride: citric acid (1:1) exhibited superior extraction efficiency compared to traditional solvents (Rashid et al., 2023). Similarly, Maimulyanti et al. employed various DESs to extract polyphenols from coffee grounds, achieving a notable extraction rate of polyphenols (5.88 mg GAE g-1) and a concentration of polyphenols in DES of 294.02 mg/L when using a choline chloride to proline ratio of 1:1 (Maimulyanti et al., 2023).
Despite the diversity of reported qualitative and quantitative methods for studying CQAs, the reliable characterization and accurate quantitative analysis of CQA isomers remain challenging. This is primarily attributed to several factors, including the presence of multiple isomers with similar physicochemical properties, the lack of commercially available standards, and the degradation and transformation of CQAs during sample processing (Alcazar Magana et al., 2021). These challenges hinder the unambiguous characterization of CQA isomers and the development of comprehensive characterization methods for CQAs. To overcome these challenges and advance the study of CQA in diverse fields including food additives, healthcare products, and pharmaceuticals, it is imperative to conduct further investigations into the extraction methodologies as well as qualitative and quantitative analytical techniques employed for CQAs. Currently, despite the absence of an established official analysis method for CQAs, certain integrated approaches, notably LC-MS-based detection, are widely regarded as the most robust and dependable.
This study aims to investigate the potential of DESs as efficient extractants of CQAs from B. aromatica, while also evaluating their antioxidant activity. Nine CQAs were extracted from B. aromatica using seven DESs and 3 traditional extraction solvents. A UPLC-Q-Orbitrap HRMS method was developed to simultaneously determine the main components of the 7 CQAs in B. aromatica, and the DESs suitable for extracting these CQAs were identified. The antioxidant activities of the different solvent extracts were evaluated using DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis(3-ethylbenzothiazole-6-sulfonic acid)) free radical scavenging, and reducing power assays. Furthermore, the correlation between antioxidant activity and CQA content was analyzed to identify key components affecting antioxidant activity. This study holds significant value in exploring green extraction methods for CQAs and investigating the chemical constituents and biological activities of the medicinal plant B. aromatica. It provides valuable insights for further research and development of this medicinal plant.
2 Materials and methods
2.1 Chemicals, Reagents, and materials
The acetonitrile, methanol, and formic acid utilized in chromatography were obtained from Thermo Fisher Scientific (Waltham, Massachusetts, USA). The chromatographic water was procured from Guangzhou Watsons Food & Beverage Co., Ltd. (Guangzhou, China). The reference standards for 3-O-caffeoylquinic acid (chlorogenic acid, 3-CQA), 4-O-caffeoylquinic acid (cryptochlorogenic acid, 4-CQA), 5-O-caffeoylquinic acid (neochlorogenic acid, 5-CQA), 1,5-dicaffeoylquinic acid (1,5-diCQA), 1,3-dicaffeoylquinic acid (1,3-diCQA), 1,4-dicaffeoylquinic acid (1,4-diCQA), 3,5-dicaffeoylquinic acid (isochlorogenic acid A, 3,5-diCQA), 3,4-dicaffeoylquinic acid (isochlorogenic acid B, 3,4-diCQA), and 4,5-dicaffeoylquinic acid (isochlorogenic acid C, 4,5-diCQA) were acquired from Chengdu Alpha Biological Co., Ltd. (Chengdu, China) with batch numbers AZ22011851, AFBL221, AZ21102901, AFBG1302, AFBL1284, AFCC1605, AZ22042903, AZ21092203, and AFCB0705, respectively, all with a minimum purity of 98 %. Choline chloride was sourced from Alashan (Guangzhou) Biotechnology Co., Ltd. (Guangzhou, China). Lactic acid, acetic acid, ethylene glycol, urea, glycerol, 1,4-butanediol, and oxalic acid were purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China). 2,2-diphenyl-1-picrylhydrazyl, and 2,2′-azino-bis(3-ethylbenzothiazole-6-sulfonic acid) (purity > 98 %) from Alsan Biotechnology Co., Guangzhou, China; ascorbic acid from Guangzhou Chemical Reagent Factory, Guangzhou, China.
The plant material used in this experiment, B. aromatica DC., was collected from Zhaoqing, China, and identified as a species belonging to the Asteraceae family, specifically the Blumea genus, by Associate Professor Xilong Zheng from the College of Traditional Chinese Medicine Resources, Guangdong Pharmaceutical University.
2.2 Content determination of CQAs
2.2.1 Chromatographic conditions
The chromatographic separation system employed the Vanquish Flex ultra-high-performance liquid chromatograph from Thermo Fisher Scientific, USA. The chromatographic separation uses the Thermo Fisher Scientific Bremen Hypersill GOLD AQ column (100 mm × 2.1 mm, 1.9 μm) and Hypersil GOLD C18 column (100 mm × 2.1 mm, 1.9 μm). The mobile phase consisted of two components: A, a 0.1 % formic acid acetonitrile solution, and B, a 0.1 % formic acid (v/v).aqueous solution. Gradient elution was employed using the following conditions: 0–15 min, 97 % B; 15–20 min, 97 % to 96 % B; 20–25 min, 96 % to 90 % B; 25–50 min, 90 % to 87 % B; 50–54 min, 87 % to 5 % B. The flow rate was set at 0.3 mL/min, with an injection volume of 2 μL. The column temperature was maintained at 42 °C.
2.2.2 Mass spectrometric conditions
The Orbitrap Exploris 120 quadrupole–electrostatic orbitrap high–resolution mass spectrometer from Thermo Fisher Scientific, USA was utilized. The scanning mode employed in this study was negative ion mode. The detection conditions were as follows: HESI ion source, electrospray voltage set at 3.5 kV, capillary temperature maintained at 325 °C, and auxiliary gas temperature set at 300 °C. The mass spectrometry scanning range was set from m/z 200 to 600. Nitrogen was used for both the sheath gas, auxiliary gas, and purge gas. The sheath gas flow rate was set at 50 L/min, the auxiliary gas flow rate was set at 8 L/min, and the purge gas flow rate was set at 1 L/min. A resolution of 60,000 was employed.
2.2.3 Preparation of DESs
In the present study, choline chloride was employed as the HBA. Choline chloride stands out for its low toxicity, cost-effectiveness, and ready availability, making it a commonly chosen HBA in the formulation of DESs. For the HBD selection, 3 acids, 3 alcohols, and urea, known for their superior extraction efficacy on phenolic acids, were identified. Subsequently, seven DESs were synthesized using a specific molar ratio in combination with choline chloride (Ali Redha, 2021). The method described in Reference was followed with improvements (Ivanovic et al., 2022). The designated amount of reactants was placed in a 150 mL beaker and a magnetic stir bar was added. The mixture was then covered with a preservative film and a sealing film, heated, and stirred on a magnetic stirrer at 60 °C for 40 min to achieve a uniform transparent liquid. After cooling to room temperature, 30 % water was added and thoroughly mixed. The resulting mixture was transferred to a 50 mL centrifuge tube and stored in a refrigerator at 4 °C for future use. Table 1 presents the composition of the different types of DESs.
Type
HBA
HBD
molar ratio
DES-1
choline chloride
lactic acid
1: 3
DES-2
acetic acid
1: 3
DES-3
ethylene glycol
1: 3
DES-4
urea
1: 3
DES-5
glycerol
1: 3
DES-6
1,4-butanediol
1: 3
DES-7
oxalic acid
1: 2
2.2.4 Preparation of sample solutions and standard solutions
The dried whole plant of B. aromatica was crushed and sieved through a 50-mesh sieve. Precisely weighed B. aromatica powder (0.1 g, three parallel groups) was placed in a 15 mL centrifuge tube. Then, 5 mL of ethanol, methanol, water, and 7 different DESs were added individually. The tube was tightly sealed, mixed by oscillation, and weighed. Ultrasound treatment was performed at specific conditions, including a power of 400 W, frequency of 40 KHz, and a temperature of 50 °C for 20 min. After cooling to room temperature, the tube was reweighed, and the corresponding extraction solvent was added to compensate for weight loss. Centrifugation was conducted at a speed of 4000 r/min for 15 min, and 2 mL of the resulting supernatant was collected and stored in a separate centrifuge tube. This process was replicated 3 times in parallel. From the preservation solution, a total of 200 μL of the sample was transferred to a 2 mL centrifuge tube. Subsequently, 800 μL of 50 % methanol was added and thoroughly mixed by vortexing. The mixture was then filtered through a 0.22 μm microporous membrane to obtain the test solution. Reference substances of 7 CQAs were accurately weighed at 1.00 mg each and placed in 5 mL volumetric flasks, the name and structure of 7 CQAs are shown in Table 2 (Alcazar Magana et al., 2021). These substances were dissolved in 50 % methanol and diluted to the mark, resulting in a mother liquor solution with a concentration of 0.2 mg/mL. Next, 0.4 mL of the mother liquor of isochlorogenic acid A, isochlorogenic acid B, and isochlorogenic acid C reference solutions, and 0.625 mL of the mother liquor of the other six reference solutions were transferred into separate 5 mL volumetric flasks. The volume was adjusted to the calibration line using 50 % methanol and shaken to obtain a mixed reference solution with varying concentrations. Finally, all samples were stored in a refrigerator at a temperature of 4 °C.
Compound
type
R1
R2
R3
R4
structure
3-O-caffeoylquinic acid (chlorogenic acid, 3-CQA)
monoCQA
H
caffeic acid
H
H
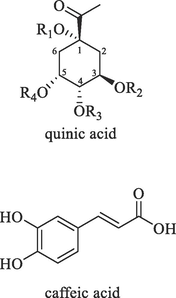
4-O-caffeoylquinic acid (cryptochlorogenic acid, 4-CQA)
H
H
caffeic acid
H
5-O-caffeoylquinic acid (neochlorogenic acid, 5-CQA)
H
H
H
caffeic acid
1,5-dicaffeoylquinic (1,5-diCQA)
diCQA
caffeic acid
H
H
caffeic acid
1,3-dicaffeoylquinic (1,3-diCQA)
caffeic acid
caffeic acid
H
H
1,4-dicaffeoylquinic (1,4-diCQA)
caffeic acid
H
caffeic acid
H
3,5-dicaffeoylquinic
(isochlorogenic acid A, 3,5-diCQA)H
caffeic acid
H
caffeic acid
3,4-dicaffeoylquinic
(isochlorogenic acid B, 3,4-diCQA)H
caffeic acid
caffeic acid
H
4,5-dicaffeoylquinic
(isochlorogenic acid C, 4,5-diCQA)H
H
caffeic acid
caffeic acid
2.2.5 Method validation
The study investigated calibration curves, limits of detection (LODs) and quantification (LOQs), precision (including intraday and interday precision), stability, and recovery. The calibration curves for 7 compounds were generated by diluting the stock solution with methanol–water to achieve suitable concentrations. The LOD represents the minimum analyte concentration at which the Signal-to-Noise ratio (S/N) equals 3:1, while the LOQ corresponds to the lowest analyte concentration at which the S/N ratio is equal to 10:1. Intraday precision was assessed by calculating the relative standard deviation (RSD%) using data from 6 injections conducted in a single day. Interday precision was evaluated by averaging the RSD% from 6 injections conducted daily for 3 consecutive days. Stability was evaluated by calculating the RSD% from 6 injections conducted at 0, 2, 4, 8, 12, 24, and 48 h. The method's accuracy was validated using 3 sample levels (80 %, 100 %, 120 %). The recovery rate was calculated using the equation (1).
where A represents the quantity of the tested component in the sample; B represents the amount of pure substance added; and C represents the actual measured value.
2.3 Antioxidative activity evaluation
2.3.1 DPPH free radical scavenging assay
The DPPH radical scavenging experiment is a simple, effective, economic and reliable method to determine the antioxidant activity of substances (Xu et al., 2021). Reference Method (Li and Li, 2022), 10 different solvents (3 traditional solvents and 7 DESs) were diluted to different concentrations (3.33, 166.67, 333.33, 500, 666.66, 1000, and 1666.67 μg/mL) respectively, 1 mL of different concentration of extract was absorbed into 5 mL tube, then 1 mL of DPPH solution (2 mM) was added into the tube. After shaking well, it was left in the dark for 30 min, and the absorbance was measured at 517 nm. The clearance rate is calculated according to the following equation (2).
where A0 is the absorbance of the blank control; A1 is the absorbance of the sample to be measured; A2 is the absorbance of the control sample to be measured.
2.3.2 ABTS free radical scavenging assay
The ABTS is green in solution and is lightened by antioxidants that remove free radicals. Similar to ABTS is often used in in vitro tests to characterize the antioxidant capacity of substances (Wang et al., 2020a). The method in reference is slightly modified (Jo et al., 2021). The mixture of ABTS solution (7 mM) and K2S2O8 solution (2.45 mM) was used as ABTS reaction solution at 734 nm. The mixture was kept in a dark room for 12 to 16 h and diluted with absolute ethanol until the absorbance of the diluted solution was (0.70 ± 0.20). 1 mL of different concentrations of extracts (3.33, 66.67, 100, 133.33, 250, 333.33, and 500 μg/mL) were aspirated, then 1 mL of ABTS reaction solution was added, and the absorbance was measured at 734 nm for 30 min, with ascorbic acid solution as a positive control, and each group of tests was performed three times. The clearance rate was calculated according to equation (3).
where A0 is the absorbance of the blank control; A1 is the absorbance of the sample to be measured; A2 is the absorbance of the control sample to be measured.
2.3.3 Reducing power assay
In this experiment, the antioxidant activity of the sample solution was determined by measuring the amount of Fe4[Fe(CN)6]3 produced, and the higher absorbance value indicated the stronger antioxidant power (Tsai et al., 2006). The method was slightly modified with reference to the literature (Oyaizu, 1986). The extracts of different concentrations (0.2, 2, 4, 10, 16, and 20 mg/mL) were aspirated 3 mL, 1 mL of phosphate buffer solution (PBS, PH 6.0), 2 mL of 1 % potassium ferricyanide solution were added in turn, mixed well, thermostatic water bath at 50 °C for 20 min, quickly removed and cooled, 1 mL of 10 % trichloroacetic acid solution was added, mixed well, centrifuged at 3000 r/min for 10 min, 2.5 mL of supernatant was taken, 1 mL of distilled water was added in turn The absorbance value was measured at 700 nm. Ascorbic acid was used as the positive control. The greater the absorbance value, the stronger the reducing ability of the sample.
2.4 Data processing and analysis
Excel 2019 and Origin 8.0 software (OriginLab Corporation, MA, USA) were used for graphic evaluation, one-way analysis of variance (ANOVA) and correlation analysis were performed using SPSS26.0, and IC50 values were calculated with GraphPad Prism 8. All experiments were repeated three times, and the results were expressed as mean ± SD.
3 Results and discussion
3.1 Quantitative analysis of 7 CQAs in b. Aromatica
3.1.1 Optimization of chromatographic conditions
To enhance the resolution and peak shape of the sample, we considered two columns: the Thermo Fisher Hypersill GOLD AQ column (100 mm × 2.1 mm, 1.9 μm) and the Fisher Hypersil GOLD C18 column (100 mm × 2.1 mm, 1.9 μm). Both columns underwent identical conditions of column temperature, mobile phase, and gradient elution. Comparative analysis demonstrated that the Thermo Fisher Hypersill GOLD AQ column (100 mm × 2.1 mm, 1.9 μm) exhibited superior separation efficiency, improved peak shape, and shorter retention time. Consequently, we selected the Thermo Fisher Hypersill GOLD AQ column (100 mm × 2.1 mm, 1.9 μm) for sample separation, the chromatogram is shown in Fig. 1.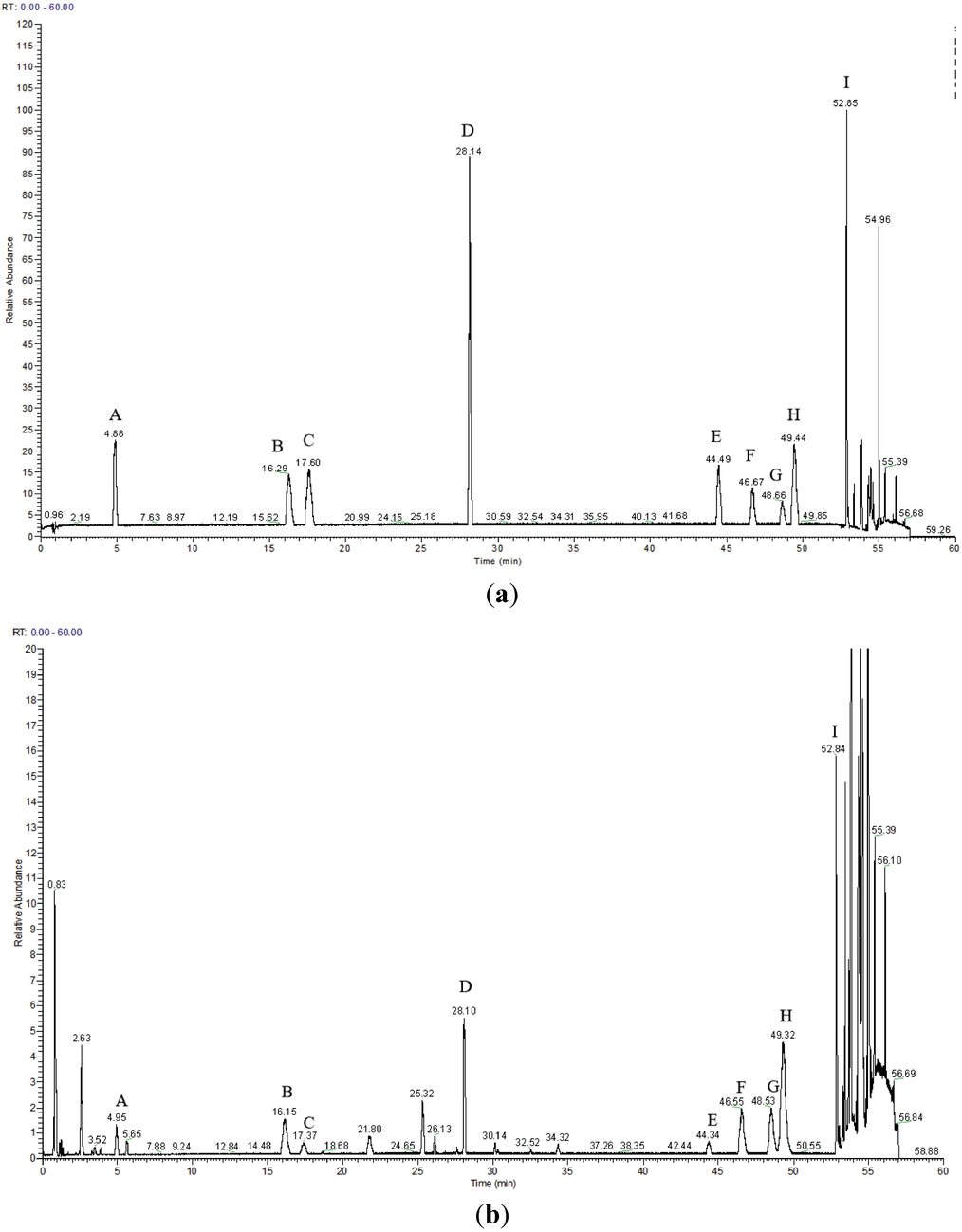
Total ion chromatograms of 9 kinds of CQAs mixed reference solution (a) and methanol extract of B. aromatica (b) in negative ion mode. (A: 4-CQA; B: 3-CQA; C: 5-CQA; D: 1,3-diCQA; E: 1,4-diCQA; F: 3,4-diCQA; G: 3,5-diCQA; H: 1,5-diCQA; I: 4,5-diCQA).
3.1.2 Method validation
The UPLC-Q-Orbitrap HRMS analysis technology is widely employed for the comprehensive analysis of chemical components in plants (Aydogan, 2020; Alvarez-Rivera et al., 2019). This technology presents numerous advantages over LC-UV and other detection and quantitative techniques, including rapid detection speed, high sensitivity, exceptional accuracy, and a lower limit of quantitation (Jia et al., 2021; Schram et al., 2004). In this study, we developed a UPLC-Q-Orbitrap HRMS method to simultaneously determine the content of 7 CQAs in B. aromatica. Methodological investigations demonstrated that the developed method exhibited outstanding precision, accuracy, repeatability, and stability. The calibration curves for 7 CQAs were generated within a specific concentration range using the aforementioned method in this experiment, as presented in Table 3. The obtained results demonstrated a strong linear relationship and a favorable correlation coefficient (R2 > 0.9990). The ranges for LOD and LOQ were found to be 0.0040–0.0149 µg/mL and 0.0130–0.0498 µg/mL, respectively. Excellent intraday and interday precisions, as well as stability within 48 h, were observed. The relative standard deviation (RSD%) values ranged from 0.69 % to 2.45 %, 0.52 % to 3.04 %, and 0.85 % to 3.21 %, respectively. Additionally, Table 4 presents the recovery rates, which ranged from 78.18 % to 113.90 %.
Compound
Calibration curves
R2
Linear range
(μg/mL)
LOD
(μg/mL)
LOQ
(μg/mL)
Precision
(RSD%)
Stability
(RSD%)
Intraday
(n = 6)
Interday
(n = 3)
3-CQA
y = 16139x − 5087
0.9992
0.0498 ∼ 25
0.0149
0.0498
1.97
1.69
0.48
4–CQA
y = 14391x − 2790.4
0.9994
0.0498 ∼ 25
0.0149
0.0498
1.86
1.75
1.52
5–CQA
y = 13083x − 2922.4
0.9995
0.0498 ∼ 25
0.0149
0.0498
1.34
1.6
0.93
1,5-diCQA
y = 27093x − 9632.1
0.9991
0.0498 ∼ 25
0.0149
0.0498
1.52
3.21
1.35
1,3-diCQA
y = 48121x − 14857
0.9993
0.0498 ∼ 25
0.0076
0.0253
0.78
1.37
0.96
1,4-diCQA
y = 37.04x − 13114
0.9991
0.0498 ∼ 25
0.0149
0.0498
1.27
1.48
2.10
3,5–diCQA
y = 17412x − 5348.5
0.9990
0.0316 ∼ 16
0.0081
0.0269
2.45
2.02
1.34
3,4–diCQA
y = 33968x − 5252.2
0.9995
0.0316 ∼ 16
0.0095
0.0316
0.69
1.19
0.34
4,5–diCQA
y = 74276x + 6735.2
0.9994
0.0316 ∼ 16
0.0040
0.0130
1.62
0.85
0.67
Compound
Unspiked (μg/mL)
Spiked (μg/mL)
Found (μg/mL)
Recovery (%)
RSD (%, n = 3)
3-CQA
3.4716
2.7772
6.1288
95.68
2.40
3.4720
7.1971
107.30
5.04
4.1670
7.4603
95.72
2.02
4–CQA
0.5945
0.4756
1.0992
106.12
1.23
0.5946
1.1963
101.21
2.94
0.7136
1.4058
113.69
0.90
5–CQA
0.4245
0.3396
0.7212
87.36
2.51
0.4244
0.8315
95.89
2.33
0.5095
0.9518
103.49
4.06
1,5-diCQA
2.5652
2.0522
4.4924
93.91
1.93
2.5655
4.5709
78.18
3.21
3.0784
5.9185
108.93
1.66
1,3-diCQA
0.6074
0.4855
1.0187
84.71
3.10
0.6076
1.2405
104.19
2.71
0.7290
1.2983
94.78
2.82
1,4-diCQA
0.7864
0.6291
1.4488
105.30
1.90
0.7862
1.4730
87.33
1.75
0.9438
1.7568
102.82
0.80
3,5–diCQA
0.6237
0.4990
1.1921
113.90
2.47
0.6230
1.2781
105.04
1.86
0.7486
1.3476
96.70
4.37
3,4–diCQA
1.6554
1.3243
2.9998
101.52
1.64
1.6553
3.2051
93.62
2.20
1.9868
3.6041
98.08
0.81
4,5–diCQA
0.5173
0.4138
0.9681
108.94
3.01
0.5173
1.0553
104.01
2.86
0.6207
1.1276
98.33
2.93
The analysis revealed two groups of isomers within the 7 CQAs identified in this study: three monoCQAs and six diCQAs. Despite minimal structural differences among components within each group, variations were observed in the positions of substituents. The content of these 7 CQAs in B. aromatica displayed notable fluctuations, including the presence of trace components. Consequently, the simultaneous determination of these 7 CQAs posed a considerable challenge. In this study, we successfully achieved the simultaneous quantitative analysis of the 7 CQAs through continuous optimization of chromatographic separation conditions and leveraging the analytical advantages offered by UPLC-Q-Orbitrap HRMS technology. Our findings serve as a valuable reference for the advancement and exploration of analytical techniques for similar compounds.
3.1.3 Quantitative analysis
DESs exhibit notable characteristics, including variable composition, ease of preparation, and biodegradability (Ruesgas-Ramon et al., 2020; Rashid et al., 2023). These solvents possess the capability to engage in intermolecular interactions, such as hydrogen bonding forces and van der Waals forces, with chemical components in plants (Ivanovic et al., 2020). This property enhances the dissolution efficiency of specific chemical components, facilitating a more thorough extraction of bioactive substances. Consequently, deep eutectic solvents find extensive application in the extraction of active ingredients from medicinal plants, encompassing alkaloids, flavonoids, polysaccharides, saponins, and polyphenols (Duan et al., 2016; Torres-Vega et al., 2020; Dheyab et al., 2021; Zuo et al., 2022; Liu et al., 2023). By employing a diverse composition of DESs, our aim was to explore their effectiveness in extracting CQAs from B. aromatica and compare it with that of traditional extraction solvents.
Each batch of samples was prepared in triplicate, and the compound content in each batch was determined using a calibration curve. The results are presented in Table 5 and Fig. 2, unveiling significant variations in the content of the 7 CQAs extracted from B. aromatica using diverse solvents (P < 0.05). The CQAs displayed a concentration range of 0.46–7.60 mg/g. The descending order of 7 CQAs was as follows: 1,5-diCQA > 3,5-diCQA > 3-CQA > 3,4-diCQA > 4,5-diCQA > 1,3-diCQA > 4-CQA > 1,4-diCQA. Furthermore, the total phenolic acid content in the samples ranged from 15.35 to 27.08 mg/g. Among the solvents examined, the extraction solvent comprised of choline chloride-1,4-butanediol (DES-6) demonstrated the highest efficiency in extracting phenolic acids. The monomeric CQAs present in DESs extract exhibited significantly higher levels compared to conventional extraction solvents (P < 0.05). Notably, choline chloride / 1,4-butanediol (DES-6) showed the highest concentrations of 3-CQA and 4,5-diCQA at 4.71 ± 0.31 and 2.28 ± 0.19 mg/g, respectively. The choline chloride / oxalic acid (DES-7) had the highest content of 4-CQA, measuring 2.05 ± 0.05 mg/g. The choline chloride / glycerol (DES-5) exhibited the highest levels of 5-CQA, 1,3-diCQA, 1,4-diCQA, and 3,4-diCQA, with concentrations of 1.70 ± 0.14, 2.17 ± 0.04, 1.39 ± 0.14, and 2.30 ± 0.06 mg/g, respectively. Furthermore, The choline chloride / acetic acid (DES-2) displayed the highest contents of 1,5-CQA and 3,5-diCQA, measuring 7.60 ± 0.38 and 5.70 ± 0.23 mg/g, respectively. The results indicate that the utilization of DESs can achieve higher extraction efficiency when extracting these specific caffeoylquinic acid derivatives. The use of seven DESs in this study demonstrated their superior extraction efficiency compared to 3 traditional solvents for the extraction of 7 CQAs from B. aromatica. a-g: Different lowercase letters indicate that the content of 7 CQAs in B. aromatica extracts with different extraction solvents is significantly different (P < 0.05); *: The total phenolic acid content was the sum of all 7 monomeric CQA contents.
solvent types
3-CQA
4–CQA
5–CQA
1,5-diCQA
1,3-diCQA
1,4-diCQA
3,5–diCQA
3,4–diCQA
4,5–diCQA
Total phenolic acid*
Methanol
1.34 ± 0.13a
0.46 ± 0.06a
0.51 ± 0.14a
5.41 ± 0.28a
0.93 ± 0.13a
0.81 ± 0.03a
3.97 ± 0.09c
0.96 ± 0.02a
0.96 ± 0.13a
15.35 ± 1.01a
Ethanol
2.94 ± 0.04b
0.70 ± 0.03b
0.77 ± 0.02b
7.27 ± 0.34df
1.27 ± 0.01b
1.12 ± 0.09c
4.53 ± 0.13d
1.50 ± 0.08c
1.08 ± 0.08a
21.18 ± 0.82bc
Water
3.00 ± 0.17b
1.41 ± 0.06e
1.21 ± 0.11c
5.16 ± 0.32a
2.02 ± 0.11ef
1.08 ± 0.04bc
3.37 ± 0.11b
2.30 ± 0.07f
1.39 ± 0.12b
20.94 ± 1.11b
DES-1
3.87 ± 0.09c
1.06 ± 0.04c
1.10 ± 0.13c
7.00 ± 0.19 cd
1.41 ± 0.12b
1.06 ± 0.01bc
5.32 ± 0.04f
1.53 ± 0.08c
1.66 ± 0.09c
24.01 ± 0.79de
DES-2
4.19 ± 0.15de
0.96 ± 0.09c
1.15 ± 0.08c
7.60 ± 0.38f
1.41 ± 0.05b
1.10 ± 0.10bc
5.70 ± 0.23 g
1.62 ± 0.05c
1.67 ± 0.14c
25.40 ± 1.27 g
DES-3
4.39 ± 0.13de
1.30 ± 0.02de
1.63 ± 0.11de
6.82 ± 0.14 cd
1.96 ± 0.23de
1.36 ± 0.07d
4.55 ± 0.09d
2.22 ± 0.17f
2.06 ± 0.08e
26.29 ± 1.04 g
DES-4
4.20 ± 0.08de
0.97 ± 0.12c
1.16 ± 0.05c
7.39 ± 0.45df
1.39 ± 0.16b
1.09 ± 0.05bc
5.67 ± 0.31 g
1.63 ± 0.21c
1.76 ± 0.01 cd
25.26 ± 1.32eg
DES-5
4.45 ± 0.20ef
1.55 ± 0.04f
1.70 ± 0.14e
6.52 ± 0.11bc
2.17 ± 0.04f
1.39 ± 0.14d
0.93 ± 0.01a
2.30 ± 0.06f
1.92 ± 0.03de
22.93 ± 0.77 cd
DES-6
4.71 ± 0.31f
1.24 ± 0.08d
1.51 ± 0.02d
7.33 ± 0.43df
1.63 ± 0.06c
1.32 ± 0.12d
5.00 ± 0.26e
2.06 ± 0.13d
2.28 ± 0.19f
27.08 ± 1.60 g
DES-7
4.13 ± 0.16c
2.05 ± 0.05 g
1.67 ± 0.12de
6.06 ± 0.26b
1.79 ± 0.01 cd
0.95 ± 0.12ab
4.33 ± 0.07d
1.29 ± 0.02b
1.10 ± 0.11a
23.37 ± 0.92de
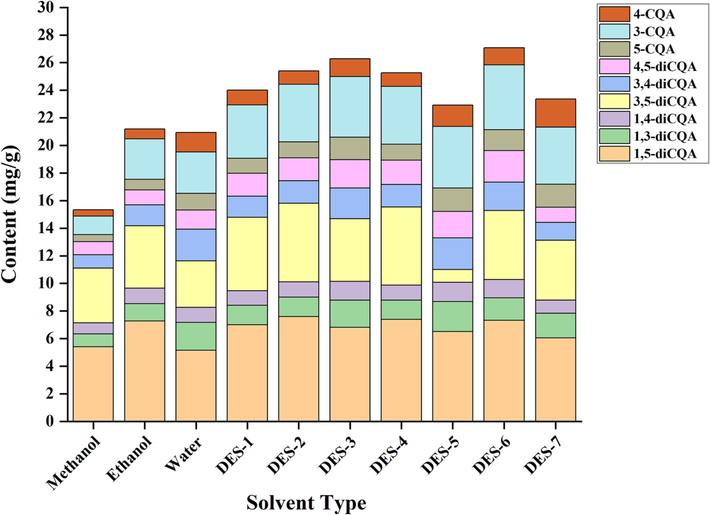
The content of 7 CQAs in different solvent extracts of B. aromatica.
In prior investigations, it has been substantiated that Deep Eutectic Solvents (DESs) composed of choline chloride-polyols can effectively extract neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, and other components (Ali Redha, 2021). Notably, when the molar ratio of choline chloride and glycerol is 1:2, the extraction efficiency of phenolics from mulberry leaves is reported to be optimal (Gao et al., 2020). The findings of this study align with the notion that the combination of choline chloride and various polyols exhibits commendable extraction efficiency for phenolics. Among them, DES-6 (choline chloride / 1,4-butanediol) demonstrated the highest extraction efficiency for phenolics. Chlorogenic acid, a pivotal component of CQA, has been the subject of relatively mature extraction techniques using DESs. For instance, Wu et al. utilized choline chloride / 1,4-butanediol (molar ratio of 1:2), with a water content of 30 %, achieving an extraction rate of 6.16 mg/g for chlorogenic acid (Wu et al., 2022). The results of this study corroborate these findings, showcasing that the extraction rate of chlorogenic acid by DES-6 (choline chloride / 1,4-butanediol) surpassed that of other solvents. Hence, DESs composed of choline chloride / 1,4-butanediol can be deemed effective extraction solvents for chlorogenic acid. Additionally, various other DESs have demonstrated utility in chlorogenic acid extraction. Luo et al. employed DES with a molar ratio of 2:1, comprising choline chloride and L-(+)-ascorbic acid, for extracting compounds in Eucommia ulmoides leaves, including chlorogenic acid (Luo et al., 2022). Fanali et al. conducted a screening of 15 different DESs based on choline chloride and betaine as hydrogen bond acceptors, ultimately identifying a DES composed of betaine and triethylene glycol (molar ratio 1:2) with favorable extraction effects on chlorogenic acid in coffee beans (Fanali et al., 2020).
In this study, we successfully extracted 7 CQAs from B. aromatica using DESs in a single process, significantly expanding our understanding of DESs in extracting CQA components. This contributes valuable insights to the efficient and environmentally friendly extraction of CQA components. The results underscore the potential of DESs as effective solvents for the extraction of bioactive compounds in an environmentally conscious manner. Continued research and exploration in this field hold the promise of developing novel and efficient extraction methods with applications across pharmaceuticals, nutraceuticals, and functional food industries.
3.2 Antioxidant activity of different solvent extracts
3.2.1 DPPH radical scavenging activity
The DPPH radical scavenging activity of B. aromatica extracts using different solvents is depicted in Fig. 3. The extracts obtained from various solvents exhibited certain scavenging capabilities towards DPPH radicals. However, both were comparatively lower than that of ascorbic acid. Within the concentration range of 0 to 500 μg/mL, the scavenging rate exhibited significant variations corresponding to the concentration, displaying a dose-dependent relationship. Upon surpassing a concentration of 500 μg/mL, the rate of clearance increment slowed down. Notably, ascorbic acid achieved a maximum clearance of 96.88 % ± 0.002 when the concentration exceeded 1666.7 μg/mL. Among the extracts obtained using different solvents, DES 1–6 demonstrated clearances exceeding 90 %, whereas DES-7 exhibited a clearance of 84.69 % ± 0.005. However, it is still higher compared to conventional solvents such as water, ethanol, and methanol.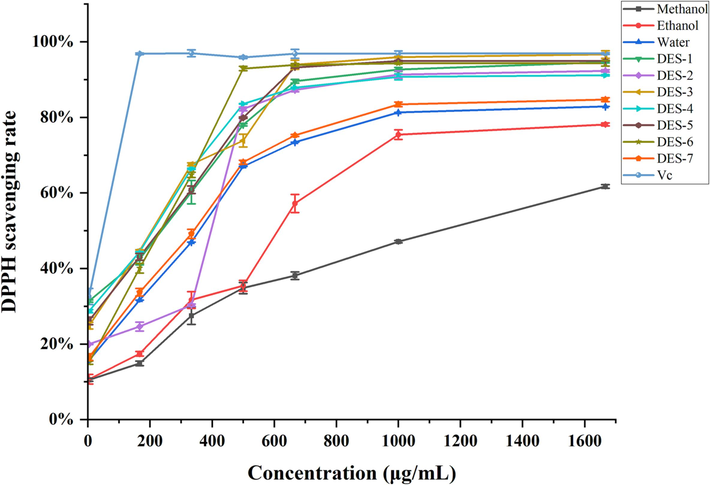
The DPPH radical scavenging activities of the ascorbic acid (VC) control and different solvent extracts of B. aromatica.
The antioxidant activity becomes stronger as the IC50 value decreases. The IC50 values for ascorbic acid and the various solvent extracts in terms of scavenging DPPH radicals, from lowest to highest, are as follows: ascorbic acid (7.42 ± 0.70 μg/mL) < DES-4 (185.63 ± 1.88 μg/mL) < DES-3 (197.36 ± 1.05 μg/mL) < DES-6 (210.90 ± 4.88 μg/mL) < DES-5 (211.75 ± 4.26 μg/mL) < DES-1 (214.24 ± 12.29 μg/mL) < DES-7 (294.44 ± 10.43 μg/mL) < water (314.67 ± 12.17 μg/mL) < DES-2 (355.60 ± 3.71 μg/mL) < ethanol (585.41 ± 28.85 μg/mL) < methanol (1065.32 ± 32.53 μg/mL), as illustrated in Table 6. a-g:Different lowercase letters indicate that the content of 7 CQAs in B. aromatica extracts with different extraction solvents is significantly different (P < 0.05); ※: represents the mass of dry material powder contained per 1 mL of solvent; Δrepresents the mass of the compound contained in each 1 mL of solvent.
Samples
DPPH
ABTS
Methanol※
1065.32 ± 32.53 g
762.24 ± 44.17c
Ethanol※
585.41 ± 28.85f
420.03 ± 19.57d
Water※
314.67 ± 12.17d
81.57 ± 1.91b
DES-1※
214.24 ± 12.29e
84.45 ± 20.80b
DES-2※
355.60 ± 3.71c
103.10 ± 3.42a
DES-3※
197.36 ± 1.05bc
14.86 ± 3.33a
DES-4※
185.63 ± 1.88b
73.91 ± 1.35b
DES-5※
211.75 ± 4.26bc
76.19 ± 3.94b
DES-6※
210.90 ± 4.88bc
21.29 ± 1.77a
DES-7※
293.44 ± 10.43d
103.33 ± 25.42b
Ascorbic acidΔ
7.42 ± 0.70a
3.07 ± 0.03a
3.2.2 ABTS radical scavenging activity
As depicted in Fig. 4, most of the various solvent extracts of B. aromatica exhibited scavenging ability towards ABTS radicals. The scavenging rate exhibited an initial faster and then slower increase with rising concentration within the range of 0 ∼ 500 μg/mL. At a concentration of 500 μg/mL, ascorbic acid achieved a maximum scavenging rate of 100 %. Among the extracts obtained using different solvents, the scavenging rate of the aqueous extract and the majority of the seven DES extracts exceeded 95 %. DES 3–6 exhibited a scavenging rate exceeding 99 %. In contrast, ethanol and methanol displayed lower scavenging rates, both below 60 %. The IC50 values for the scavenging of ABTS radicals, ranging from lowest to highest, are as follows: ascorbic acid (3.07 ± 0.03 μg/mL) < DES-3 (14.86 ± 3.33 μg/mL) < DES-6 (21.29 ± 1.77 μg/mL) < DES-4 (73.91 ± 1.35 μg/mL) < DES-5 (76.19 ± 3.94 μg/mL) < water (81.57 ± 1.91 μg/mL) < DES-1 (84.45 ± 20.80 μg/mL) < DES-2 (103.10 ± 3.42 μg/mL) < DES-7 (103.33 ± 25.42 μg/mL) < ethanol (420.03 ± 19.57 μg/mL) < methanol (762.24 ± 44.17 μg/mL), as presented in Table 6. DES-3 exhibited an IC50 value of 14.86 ± 3.33 μg/mL, which is in close proximity to ascorbic acid (3.07 ± 0.03 μg/mL), indicating that DES-3 had superior ABTS radical scavenging activity.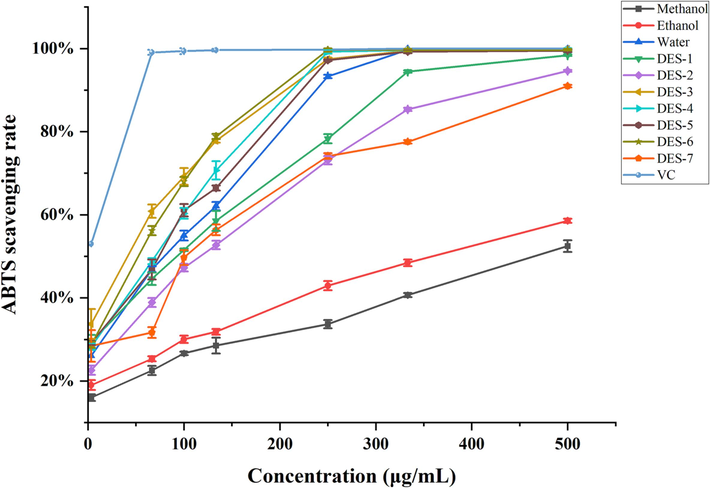
The ABTS radical scavenging activities of the ascorbic acid (VC) control and different solvent extracts of B. aromatica.
3.2.3 Reducing power determination
The reducing power of various solvent extracts of B. aromatica and ascorbic acid is presented in Fig. 5. Especially, both the ascorbic acid and methanol extracts exhibited a significant dose–response relationship in their linear reducing power. Ascorbic acid reached saturation at a concentration of 500 μg/mL, displaying a maximum absorbance of 1.412 ± 0.003. On the other hand, the different extracting solvents reached saturation at a concentration of 20 mg/mL, with DES-6 displaying the most potent reducing power, exhibiting a maximum absorbance of 1.593 ± 0.006. The remaining six DES and water extracts had maximum absorbance values ranging from 1.161 to 1.555. The descending order of the reducing power of different solvent extracts of B. aromatica is as follows: DES-6 > DES-3 > DES-4 > DES-5 > DES-1 > DES-2 > water > DES-7 > ethanol > methanol.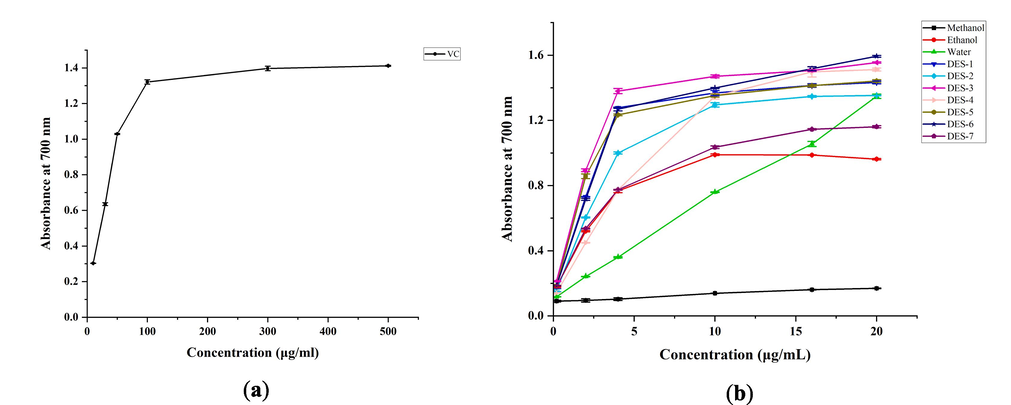
Reducing power of (a) the ascorbic acid (VC) control and (b) Different solvent extracts of B. aromatica.
3.3 Correlation analysis between CQAs and antioxidant activity
The Pearson correlation coefficient was used to analyze the relationship between the content of phenolic acids and the antioxidant activity of extracts from B. aromatica using different solvents. Table 7 presents the results. The total phenolic acid content in DESs extract showed a positive correlation with the reduction ability, DPPH free radical scavenging ability, and ABTS free radical scavenging ability (p < 0.01). This indicates that a higher content of total phenolic acids in the DESs extract corresponds to stronger antioxidant activity, suggesting that total phenolic acids contribute significantly to the in vitro antioxidant activity. Furthermore, a significant positive correlation (p < 0.001) was observed between 3-CQA and the total phenolic acid content, reduction ability, DPPH free radical scavenging ability, and ABTS free radical scavenging ability. These results suggest that 3-CQA is one of the main antioxidant components of total phenolic acids in the DESs extract of B. aromatica. Higher content of 3-CQA in the DESs extract corresponds to stronger antioxidant activity. Similarly, 5-CQA exhibited positive correlations (p < 0.05) with total phenolic acids, reductive capacity, DPPH free radical scavenging capacity, and ABTS free radical scavenging capacity, highlighting its importance in B. aromatica. The free radical scavenging ability of 4-CQA,
Correlation analysis
3-CQA
4–CQA
5–CQA
1,5-diCQA
1,3-diCQA
1,4-diCQA
3,5–diCQA
3,4–diCQA
4,5–diCQA
Total phenolic acid
Reducing power
DPPH
ABTS
3-CQA
1.000***
0.597
0.842**
0.617
0.590
0.725⁎
0.089
0.559
0.784**
0.948***
0.914***
0.921***
0.914***
4–CQA
1.000***
0.877***
−0.189
0.811**
0.305
−0.343
0.416
0.213
0.415
0.517
0.642⁎
0.670⁎
5–CQA
1.000***
0.132
0.861**
0.673⁎
−0.293
0.643⁎
0.622
0.695⁎
0.744⁎
0.804**
0.826**
1,5-diCQA
1.000***
−0.171
0.443
0.515
0.059
0.521
0.717⁎
0.515
0.437
0.385
1,3-diCQA
1.000***
0.671⁎
−0.572
0.839**
0.470
0.427
0.652⁎
0.693⁎
0.724⁎
1,4-diCQA
1.000***
−0.306
0.852**
0.840**
0.672⁎
0.740⁎
0.666⁎
0.650⁎
3,5–diCQA
1.000***
−0.414
0.003
0.355
0.103
0.052
0.069
3,4–diCQA
1.000***
0.712⁎
0.508
0.734⁎
0.669⁎
0.698⁎
4,5–diCQA
1.000***
0.801**
0.798**
0.715⁎
0.724⁎
Total phenolic acid
1.000***
0.914***
0.880***
0.886***
Reducing power
1.000***
0.979***
0.971***
DPPH
1.000***
0.979***
ABTS
1.000***
1,3-diCQA, 3,4-diCQA, 4,5-diCQA, and DPPH showed significant positive correlations with ABTS free radical scavenging ability (p < 0.05). Additionally, the reduction ability of 1,3-diCQA and 4,5-diCQA exhibited significant positive correlations with ABTS free radical scavenging ability (p < 0.05). These findings suggest that 4-CQA, 1,3-diCQA, 3,4-diCQA, and 4,5-diCQA possess certain antioxidant activity. The antioxidant activity of CQAs has been consistently confirmed in previous studies, and in this study, the correlation between the content of the 7 CQAs and antioxidant activity has been reaffirmed. It is important to note, however, that the components extracted from B. aromatica using the seven DESs and 3 traditional solvents in this study may not only include CQAs but also other components that could contribute to antioxidant activity. Thus, the observed antioxidant effects may be attributed to a combination of CQAs and other bioactive compounds present in the extracted components.
CQAs demonstrate a range of biological activities, with a significant focus on their antioxidant potential (Alcazar Magana et al., 2021). This study employed different solvents to extract CQA compounds from B. aromatica. The analysis of CQA content in the extracts revealed noteworthy concentrations of 3-CQA, 3,5-diCQA, and 1,5-diCQA, with values of 4.71 ± 0.31 mg/g, 5.70 ± 0.23 mg/g, and 7.60 ± 0.38 mg/g, respectively. Previous research has underscored the substantial biological activities associated with these CQAs, establishing them as essential molecular foundations for the pharmacological effects of many medicinal plants (Slanina et al., 2001; Singh and Bodiwala, 2010; Wang et al., 2015; Clifford et al., 2017; Wang et al., 2020b).
Particularly, 3-CQA (3-O-caffeoylquinic acid, chlorogenic acid) has garnered attention and been extensively studied in food and drug-related research contexts (Upadhyay and Rao, 2013; Naveed et al., 2018; Nwafor et al., 2022). To assess the antioxidant potential of B. aromatica extracts, this study employed various assays, including DPPH, ABTS free radical scavenging, and reducing power assays, evaluating the antioxidant activity of extracts obtained using different solvents. Numerous plant extracts have demonstrated robust antioxidant capacity in previous reports. For instance, the extract of Blumea laciniata exhibited strong scavenging effects on DPPH and ABTS free radicals, with IC50 values of 0.410 and 0.130 mg/mL, respectively (Zhou et al., 2020). The extract of the Piperis longi fructus-Rhei radix et rhizoma drug pair, extracted by deep eutectic solvent, also displayed potent DPPH free radical scavenging activity, reaching a scavenging rate of 90.29 % ± 0.22 at a mass concentration of 45.45 mg/mL (Wei et al., 2023). Therefore, extracts from Blumea aromatica obtained using DESs exhibit promising antioxidant activity, potentially even surpassing that of other extracts.
The results from the antioxidant activity assays provided valuable insights into the relationship between the antioxidant capacity of the extracts and the presence of CQAs. Positive correlations were observed between the concentration of phenolic acids and antioxidant activity, particularly for 3-CQA and 5-CQA, which exhibited significant associations (p < 0.001 and p < 0.05, respectively). These findings contribute to a deeper understanding of the antioxidant activity of B. aromatica and its correlation with the presence of CQAs, establishing CQAs as crucial contributors to the antioxidant potential of the extracts.
Overall, this study offers valuable insights into the antioxidant activity of B. aromatica extracts and establishes a clear connection between the observed antioxidant capacity and the presence of CQAs. These findings underscore the potential of B. aromatica as a valuable source of natural antioxidants, indicating its prospective application in the development of functional foods, nutraceuticals, or antioxidant-related drugs. Future research can further explore the mechanisms underlying the antioxidant effects of CQAs and investigate their potential therapeutic applications in conditions related to oxidative stress. The information generated from such studies can contribute to the development of novel strategies for harnessing the antioxidant properties of B. aromatica and its bioactive compounds for human health and well-being.
4 Conclusions
This study provides valuable insights into the chemical composition and bioactivity of B. aromatica, a plant renowned for its traditional medicinal applications. By utilizing seven DESs as extractants, a significant advantage was observed in the extraction of specific CQAs from B. aromatica compared to 3 traditional solvents. The UPLC-Q-Orbitrap HRMS analysis was effectively employed to identify and quantify 7 CQAs in the extracts, with 1,5-diCQA being the most abundant. Among the tested DESs, DES-6 demonstrated the optimal extraction efficiency for 3-CQA and 4,5-diCQA, while DES-7 showed the highest extraction efficiency for 4-CQA. DES-5 was most effective for extracting 5-CQA. The DES extracts exhibited remarkable antioxidant activity, surpassing that of conventional solvents. Indeed, while the significant antioxidant effects of B. aromatica extracts, particularly attributed to CQAs, have been established, the underlying mechanisms remain a subject for further investigation. Future studies should delve into a comprehensive exploration of the molecular and cellular mechanisms through which these compounds exert their potent antioxidant activities. Correlation analysis revealed positive relationships between phenolic acid content and antioxidant activity, particularly with 3-CQA and 5-CQA, indicating their potential as key contributors to the observed antioxidant effects. Importantly, the environmentally friendly DES extraction method presented in this study offers a sustainable approach for obtaining CQAs from B. aromatica, while maximizing its antioxidant potential.
Acknowledgements
This investigation was supported by grants from this research was funded by the following funds: the Guangzhou Basic Research Program Fund Project (No. 202102020630); the research Fund Project of Guangdong Provincial Administration of Traditional Chinese Medicine (No. 20201199); project support from Guangdong Rural Science and Technology Commissioner (No. KTP20200175); and the research Project of Online Open Course Steering Committee of Undergraduate Colleges and Universities in Guangdong Province (No. 20222ZXKC248); the Foundation of Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences (No. PCU201903).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Caffeoylquinic acids: chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J.. 2021;107(5):1299-1319.
- [Google Scholar]
- Review on extraction of phenolic compounds from natural sources using green deep eutectic solvents. J. Agric. Food Chem.. 2021;69(3):878-912.
- [Google Scholar]
- Recent applications of high resolution mass spectrometry for the characterization of plant natural products. TrAC-Trends Anal. Chem.. 2019;112:87-101.
- [Google Scholar]
- Solvent effects on antioxidant activities and phenolic contents of the alhydwan (Boerhavia elegana choisy) seed flour. J. Food Meas. Charact.. 2018;12(3):2121-2127.
- [Google Scholar]
- Recent advances and applications in LC-HRMS for food and plant natural products: a critical review. Anal. Bioanal. Chem.. 2020;412(9):1973-1991.
- [Google Scholar]
- Anticancer polyphenols from cultured plant cells: production and new bioengineering strategies. Curr. Med. Chem.. 2018;25(36):4671-4692.
- [Google Scholar]
- Solvent and extraction conditions control the assayable phenolic content and antioxidant activities of seeds of black beans, canola, and mille. J. Am. Oil Chem. Soc.. 2015;93:275-283.
- [Google Scholar]
- Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep.. 2017;34(12):1391-1421.
- [Google Scholar]
- Editorial Committee of Flora of China, 1979. Flora of China, 1st ed., Science Press, Beijing, China, Volume 75, pp. 20–21.
- Identification of Chemical Constituents in Blumea balsamifera Using UPLC-Q-Orbitrap HRMS and Evaluation of Their Antioxidant Activities. Molecules. 2023;28(11):4504.
- [Google Scholar]
- Deep eutectic solvents (DESs) as green extraction media of beneficial bioactive phytochemicals. Separations. 2021;8(10):176.
- [Google Scholar]
- Polyphenols and Human Health: The Role of Bioavailability. Nutrients. 2021;13(1):273.
- [Google Scholar]
- Comprehensive evaluation of deep eutectic solvents in extraction of bioactive natural products. ACS Sustain. Chem. Eng.. 2016;4(4):2405-2411.
- [Google Scholar]
- Green extraction of phenolic acids from Artemisia argyi leaves by tailor-made ternary deep eutectic solvents. Molecules. 2019;24(15):2842.
- [Google Scholar]
- Choline-chloride and betaine-based deep eutectic solvents for green extraction of nutraceutical compounds from spent coffee ground. J. Pharmaceut. Biomed.. 2020;189:113421
- [Google Scholar]
- Consumption of chlorogenic acids through coffee and health implications. Beverages. 2019;5(1):11.
- [Google Scholar]
- A green and integrated strategy for enhanced phenolic compounds extraction from mulberry (Morus alba L.) leaves by deep eutectic solvent. Microchem. J.. 2020;154:104598
- [Google Scholar]
- Innovative extraction techniques using deep eutectic solvents and analytical methods for the isolation and characterization of natural bioactive compounds from plant material. Plants. 2020;9(11):1428.
- [Google Scholar]
- Extraction of Bioactive Metabolites from Achillea millefolium L. with Choline Chloride Based Natural Deep Eutectic Solvents: A Study of the Antioxidant and Antimicrobial Activity. Antioxidants. 2022;11(4):724.
- [Google Scholar]
- UHPLC-Q-Orbitrap HRMS-based quantitative lipidomics reveals the chemical changes of phospholipids during thermal processing methods of Tan sheep meat. Food Chem.. 2021;360:130153
- [Google Scholar]
- Phenolics, Antioxidant and Antibacterial Activities of Immature and Mature Blumea balsamifera Leaf Extracts Eluted with Different Solvents. J. Trop. Med.. 2022;2022:7794227.
- [Google Scholar]
- Antioxidant activity of β-cyclodextrin inclusion complexes containing trans-cinnamaldehyde by DPPH. ABTS and FRAP. Food Sci. Biotechnol.. 2021;30(6):807-814.
- [Google Scholar]
- Menthol based hydrophobic deep eutectic solvent for extraction and purification of ergosterol using response surface methodology. Food Chem.. 2021;340:127979
- [Google Scholar]
- Antioxidant effects of solvent extracts from the dried jujube (Zizyphus jujube) sarcocarp, seed, and leaf via sonication. Food Sci. Biotechnol.. 2011;20(1):167-173.
- [Google Scholar]
- The effect of extraction methods on total phenolic, flavonoid and antioxidant capacity of Loloh Sembung (Blumea balsamifera) Int. Food Res. J.. 2018;25(5):2013-2017.
- [Google Scholar]
- Study on chemical constituents of the ethyl acetate extract from Blumea aromatica. J. Chin. Med. Mater.. 2012;35(2):229-231.
- [Google Scholar]
- Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci.. 2020;21(4):1250.
- [Google Scholar]
- Ultrasonic-Assisted Efficient Extraction of Coumarins from Peucedanum decursivum (Miq.) Maxim Using Deep Eutectic Solvents Combined with an Enzyme Pretreatment. Molecules. 2022;27(17):5715.
- [Google Scholar]
- Current advances in naturally occurring caffeoylquinic acids: structure, bioactivity, and synthesis. J. Agric. Food Chem.. 2020;68(39):10489-10516.
- [Google Scholar]
- Deep eutectic solvent-based ultrasound-assisted extraction of polyphenols from Cosmos sulphureus. J. Appl. Res. Med. Aroma.. 2023;32:100444
- [Google Scholar]
- Study on enhanced extraction and seasonal variation of secondary metabolites in Eucommia ulmoides leaves using deep eutectic solvents. J. Pharmaceut. Biomed.. 2022;209:114514
- [Google Scholar]
- Development of natural deep eutectic solvent (NADES) based on choline chloride as a green solvent to extract phenolic compound from coffee husk waste. Arab. J. Chem.. 2023;16(4):104634
- [Google Scholar]
- Caffeoylquinic acids in Centella asiatica reverse cognitive deficits in male 5XFAD Alzheimer's disease model mice. Nutrients. 2020;12(11):3488.
- [Google Scholar]
- Chlorogenic acid confers robust neuroprotection against arsenite toxicity in mice by reversing oxidative stress, inflammation, and apoptosis. J. Funct. Foods. 2020;75:104202
- [Google Scholar]
- Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr. Cancer. 2015;67(3):532-542.
- [Google Scholar]
- Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother.. 2018;97:67-74.
- [Google Scholar]
- Chlorogenic acid: Potential source of natural drugs for the therapeutics of fibrosis and cancer. Transl. Oncol.. 2022;15(1):101294
- [Google Scholar]
- Coffee silverskin extracts: quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int.. 2020;133:109128
- [Google Scholar]
- Studies on product of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. J. Nutr.. 1986;44(6):307-315.
- [Google Scholar]
- Health benefits of polyphenols: A concise review. J. Food Biochem.. 2022;46(10):e14264.
- [Google Scholar]
- Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: Optimization, comparison and bioactivity. Food Chem.. 2023;398:133871
- [Google Scholar]
- Biomolecules extraction from coffee and cocoa by-and co-products using deep eutectic solvents. J. Sci. Food Agric.. 2020;100(1):81-91.
- [Google Scholar]
- Mass spectrometry of 1,3-and 1,5-dicaffeoylquinic acids. J. Mass Spectrom.. 2004;39(4):384-395.
- [Google Scholar]
- Diterpenoids and Bisnorditerpenoids from Blumea aromatica. J. Nat. Prod.. 2019;82(11):3181-3185.
- [Google Scholar]
- Recent advances in anti-HIV natural products. Nat. Prod. Rep.. 2010;27(12):1781-1800.
- [Google Scholar]
- New and facile method of preparation of the anti-HIV-1 agent, 1,3-dicaffeoylquinic acid. Tetrahedron Lett.. 2001;42(19):3383-3385.
- [Google Scholar]
- Aromatin D-J: Seven previously undescribed labdane diterpenoids isolated from Blumea aromatica. Phytochemistry. 2021;184:112659
- [Google Scholar]
- Inhibition of the β-catenin/Tcf signaling by caffeoylquinic acids in sweet potato leaf through down-regulation of the Tcf-4 transcription. J. Agric. Food Chem.. 2014;62(1):167-172.
- [Google Scholar]
- Green extraction of alkaloids and polyphenols from Peumus boldus leaves with natural deep eutectic solvents and profiling by HPLC-PDA-IT-MS/MS and HPLC-QTOF-MS/MS. Plants. 2020;9(2):242.
- [Google Scholar]
- Caffeoylquinic acids, cytotoxic, antioxidant, acetylcholinesterase, and tyrosinase enzyme inhibitory activities of six inula species from Bulgaria. Chem. Biodivers.. 2020;17(4):e2000051.
- [Google Scholar]
- Antioxidant properties of hot water extracts from Agrocybe cylindracea. Food Chem.. 2006;98(4):670-677.
- [Google Scholar]
- An Outlook on Chlorogenic Acids-Occurrence, Chemistry, Technology, and Biological Activities. Crit. Rev. Food Sci. Nutr.. 2013;53(9):968-984.
- [Google Scholar]
- Utilization of strawberry and raspberry waste for the extraction of bioactive compounds by deep eutectic solvents. LWT-Food Sci. Technol.. 2020;130:109645
- [Google Scholar]
- Flavonoids and antioxidant activity of rare and endangered fern: Isoetes sinensis. PLoS One.. 2020;15(5):e0232185.
- [Google Scholar]
- Isochlorogenic acid (ICGA): natural medicine with potentials in pharmaceutical developments. Chin. J. Nat. Med.. 2020;18(11):860-871.
- [Google Scholar]
- Analysis of Brain Pharmacokinetics of Hyperoside and 1,5-Dicaffeoylquinic Acid and Treatment Effects of Acanthopanax Senticosus Leaves on Cerebral Ischemia by On-line Microdialysis-Tandem Mass Spectrometry. Chin. J. Anal. Chem.. 2015;43(11):1754-1760.
- [Google Scholar]
- Ultrasonic-assisted hydrophobic deep eutectic solvents for the extraction of seven compounds from Piperis longi fructus-Rhei radix et rhizoma drug pair and their vitro antioxidant evaluation. Sustain. Chem. Pharm.. 2023;32:100996
- [Google Scholar]
- Extraction and recovery of chlorogenic acid from sunflower disks using a high-efficiency system composed of deep eutectic solvents and macroporous resins. J. Food Process. Prese.. 2022;46(10):e16856.
- [Google Scholar]
- Optimization of extraction of bioactive compounds from Baphicacanthus cusia leaves by hydrophobic deep eutectic solvents. Molecules. 2021;26(6):1729.
- [Google Scholar]
- Green and highly extraction of phenolic compounds and antioxidant capacity from kinkeliba (Combretum micranthum G. Don) by natural deep eutectic solvents (NADESs) using maceration, ultrasound-assisted extraction and homogenate-assisted extraction. Arab. J. Chem.. 2022;15(5):103752
- [Google Scholar]
- Simultaneous optimization of extraction and antioxidant activity from Blumea laciniata and the protective effect on Hela cells against oxidative damage. J. Agric. Food Chem.. 2020;13(12):9231-9242.
- [Google Scholar]
- Optimization of the extraction process of flavonoids from Trollius ledebouri with natural deep eutectic solvents. J. Sep. Sci.. 2022;45(3):717-727.
- [Google Scholar]
- Design of natural deep eutectic solvents for the ultrasound-assisted extraction of hydroxytyrosol from olive leaves supported by COSMO-RS. Sep. Purif. Technol.. 2020;248:117054
- [Google Scholar]







