Translate this page into:
Degradation of parathion methyl by reactive sorption on the cerium oxide surface: The effect of solvent on the degradation efficiency
⁎Corresponding author. pavel.janos@ujep.cz (Pavel Janoš)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A simple and easily scalable “wet” procedure was used to prepare nanocrystalline cerium oxide capable of destroying the toxic organophosphate pesticide parathion methyl. The synthetic procedure consists of the direct precipitation of cerous salt with aqueous ammonia in the absence of CO2. The prepared cerium oxide was able to decompose the organophosphate compounds both in nonpolar (e.g., heptane) and polar aprotic (e.g., acetonitrile) solvents. However, in solvents with hydrogen-bond donating ability, the –OH groups on the cerium oxide surface were solvated and inactivated. The preferential solvation model was used to express the experimental dependencies of the cerium oxide degradation efficiency on the composition of the water-acetonitrile mixture. In certain solvent systems, some empirical polarity scales, such as the alpha-scale or the Dimrodth-Richardt parameter ET(30), may be correlated with the degradation efficiency of cerium oxide.
Keywords
Cerium oxide
Toxic organophosphate destruction
Solvent effect
Reaction mechanisms
Nucleophilic substitution SN2
1 Introduction
Nanostructured metal oxides with specific designs have been extensively used for the destruction of toxic chemicals since the pioneering works of Klabunde et al. (1996) at the turn of the century (Khaleel et al., 1999; Rajagopalan et al., 2002; Lucas et al., 2001). Magnesium oxide and titanium dioxide are representatives of the first generation of so-called “reactive sorbents” that are able not only to accumulate toxic substances on their surfaces but can also to destroy them (at least partially) and convert them into less toxic residuals. The family of reactive sorbents was extended substantially by Štengl et al. (2016; Houšková et al., 2007; Štengl et al., 2012); who also introduced new environmentally friendly wet synthetic procedures for their preparation.
Nanostructured metal oxides are especially effective in the decomposition of toxic organophosphates, including organophosphate pesticides (Janoš et al., 2014), nerve-paralytic chemical warfare agents, some kinds of flame retardants (Janoš et al., 2014; Ederer et al., 2019) and other derivatives (triesters) of phosphoric or thiophosphoric acids. Denet et al. (2020) recently compared the decontamination activity of several metal oxides (MgO, TiO2, CeO2, ZnO and ZrO2) against both chemical and biological agents. They also discussed the effects of various solvents on the chemical degradation efficiency of metal oxides and attempted to explain them using the concept of eluent strength, which is derived from liquid chromatography.
In our first study on the utilization of cerium oxide as a reactive sorbent (Janos et al., 2014); we showed that the degradation of toxic organophosphates may be performed effectively both in nonpolar solvents, such as heptane, and polar aprotic (and thus water-compatible) solvents such as acetonitrile. While the reactivity of reactive sorbents in aqueous media is crucial for typical environmental applications such as wastewater treatment, the utilization of organic solvents for the decontamination of military equipment or for the destruction of stockpiled toxic chemicals may be more advantageous. However, a detailed understanding of the solvent effects on heterogeneously catalysed liquid phase reactions is still lacking (Gould et al., 2020). Therefore, we examined the degradation of parathion methyl, a representative toxic organophosphate compound, in the presence of cerium oxide in various solvents and solvent mixtures with the goal of clarifying the reaction mechanism(s) more exactly. The preferential solvation model was used to evaluate the experimental dependencies and to identify the main forces driving parathion methyl degradation in acetonitrile–water mixed solvent.
2 Materials and methods
2.1 Materials and chemicals
Cerium nitrate, Ce(NO3)3·6H2O, was obtained from Sigma-Aldrich (Steinheim, Germany) as a reagent-grade chemical with 99.9% purity (trace metal basis). The organophosphate pesticide parathion methyl (PM) and its degradation product 4-nitrophenol (4-NP) were obtained from Sigma-Aldrich as chromatographic standards. Aqueous ammonia solution (25–29%), NH4OH, was obtained from Penta (Czech Republic) with p.a. purity. HPLC-grade methanol and acetonitrile were used as mobile phase constituents in liquid chromatography. The various organic solvents used as reaction media in this study were of reagent-grade or HPLC-grade purity and were obtained from Sigma-Aldrich.
2.2 Synthesis of cerium oxide
Cerium oxide was prepared by the direct precipitation of the cerium nitrate solution with aqueous ammonia under ambient conditions using the procedure described in our previous papers (Janoš et al., 2019; Tolasz et al., 2020) with slight modifications. Briefly, one litre of the cerium nitrate solution (0.05 mol/L) was agitated in a beaker with a thermostat at 22 ± 2 °C and purged with air that had been cleaned of carbon dioxide by passing it through KOH solution (40%). Concentrated aqueous ammonia was added in several portions until the pH reached ca. 10. Extensive agitation and purging with de-carbonated air continued until the colour of the precipitate changed to a pale yellow (typically for ca 2 h). The precipitate was washed with deionized water several times and air-dried at 60 °C. The air-dried cerium oxide was stored in tightly closed PE bottles and used without any additional thermal treatment unless otherwise specified. The sample was denoted as LT-CeO2.
For comparison, another kind of cerium oxide was prepared by the conventional “precipitation/calcination” method (Ederer et al., 2021) from cerous(III) carbonate by annealing in an open crucible at 500 °C for 2 h. The sample was denoted as HT-CeO2.
More detailed descriptions of the synthetic procedures can be found in the Supplementary data.
2.3 Methods of characterization
Diffraction patterns were collected with a Bruker D2 diffractometer equipped with a conventional X-ray tube (Cu Kα radiation, 30 kV, 10 mA) and a LYNXEYE 1-dimensional detector. A primary divergence slit module width 0.6 mm, Soler Module 2.5, Airscatter screen module 2 mm, Ni-Kβ filter 0.5 mm, in the 2θ range of 5°-90° with step 0.00808° and a time per step of 1.0 s were used. The crystallite sizes were calculated form the broadening of the diffraction line using the Scherrer formula (d = K λ/βcosθ where K is the shape factor, λ is the wavelength, θ is the diffraction angle and β is the diffraction line broadening (Johnson and Mooi, 1987; Janoš and Petrák, 1991), calculations were performed on the X'Pert HighScore Plus software. The crystalline structure, shape and size of nanoceria were inspected by high-resolution transmission electron microscopy (HRTEM) using a 200 kV TEM microscope (FEI Talos F200X). As a specimen support for TEM investigations, a microscopic copper grid covered by a thin, transparent, holey carbon film was used. Suspensions of all the samples were diluted, ultrasonicated for a few minutes and dropped onto the copper grids. The specific surface areas and porosities of the powder samples were measured using a Belsorp maxII instrument at liquid nitrogen temperature. The samples were degassed for 4 h at 120 °C. The Brunauer–Emmett–Teller (BET) method was used for surface area calculation. The acid-base properties of the cerium oxide samples were examined by potentiometric titration using an automatic titrator controlled by a PC (794 Basic Titrino, Metrohm, Switzerland) using the method described in our previous works (Ederer et al., 2021; Ederer et al., 2017).
2.4 Parathion-methyl degradation tests
The original procedure was derived from the military standard STANAG 4653 (Stanag, 2012), which is used to test chemical warfare agents. In principle, a toxic substance is mixed with a relatively high excess of the decontamination agent, and its conversion to the reaction product(s) is followed by a suitable analytical method. The modification of the testing procedure for organophosphate pesticides is described in detail in our previous paper (Janos et al., 2014); therefore; we summarize only briefly the main characteristics of the test: 50 mg of the sorbent (cerium oxide) was weighed into a series of glass vials, and 150 µL of the pesticide solution in heptane (6660 mg/L) was added (the ratio of 1 mg of pesticide to 50 mg of the sorbent was maintained). At predetermined time intervals ranging from 0.5 to 128 min, the reaction was terminated by the addition of 2 mL of 2-propanol, the sorbent was separated by centrifugation, and the supernatant was transferred into a volumetric flask. The sorbent was redispersed in methanol (4 mL) and centrifuged again; the extraction of the cerium oxide with methanol was repeated 3 times, and all the supernatants were combined in one volumetric flask, which was made up with mobile phase used for the subsequent determination of parathion methyl and 4-nitrophenol by HPLC.
2.4.1 Degradation tests in mixed solvents
In a typical arrangement, the stock solution of parathion methyl (5 g/L) in heptane was prepared. 50 mg of the sorbent was weighed into a glass vial and 0.5 mL of the pesticide solution in heptane was added, then 1 mL of other solvent was added (co-solvent). At a predetermined time, the reaction was terminated by the addition of 2-propanol, and the sorbent was extracted with methanol in a similar way as described above. The rate constant was calculated from the degree of conversion with the assumption that the reaction follows the pseudo-first order kinetics equation. In a similar way, the series of experiments was performed with the stock solution of parathion methyl in acetonitrile.
A slightly different procedure was used for measurements of kinetic dependencies: 100 mg of the sorbent was dispersed in 4.5 mL of the respective solvent, and the reaction was initiated by the addition of 0.5 mL of pesticide solution (5 g/L) in the same solvent. The rection mixture was agitated with a small magnetic mixer. At predetermined time intervals, small amounts of the reaction mixture were taken and analysed immediately by HPLC.
3 Results and discussion
3.1 Characterization of cerium oxide
As seen from the X-ray diffraction pattern in Fig. 1, nanoparticulate cerium oxide with the well-developed fluorite-type crystalline structure can be prepared by the direct precipitation method under ambient conditions. The crystallite sizes estimated from the broadening of the diffraction peaks were 5.2 nm, which is in good agreement with microscopic (HRTEM) observations (see Fig. 1b, where poorly aggregated nanoparticles with cubic morphology can be distinguished). The number of surface hydroxyl groups was determined by acid-base titration. The value of 0.254 mmol/g is lower (but not dramatically – about 14%) than that determined for the cerium oxide annealed at 500 °C, whereas the pH values corresponding to the point of zero charge (PZC) were almost identical – 4.64 and 4.52 for LT-CeO2 and HT-CeO2, respectively. A comparison of the respective titration and TOTH curves is given in Fig. 2. Some other characteristics of the cerium oxides are given in Supplementary data.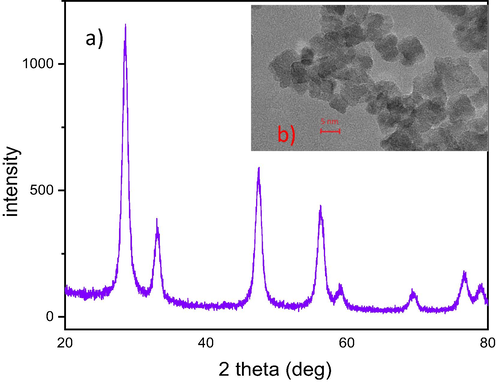
(a) XRD pattern of air-dried cerium oxide LT-CeO2; (b) HRTEM image of the same cerium oxide.
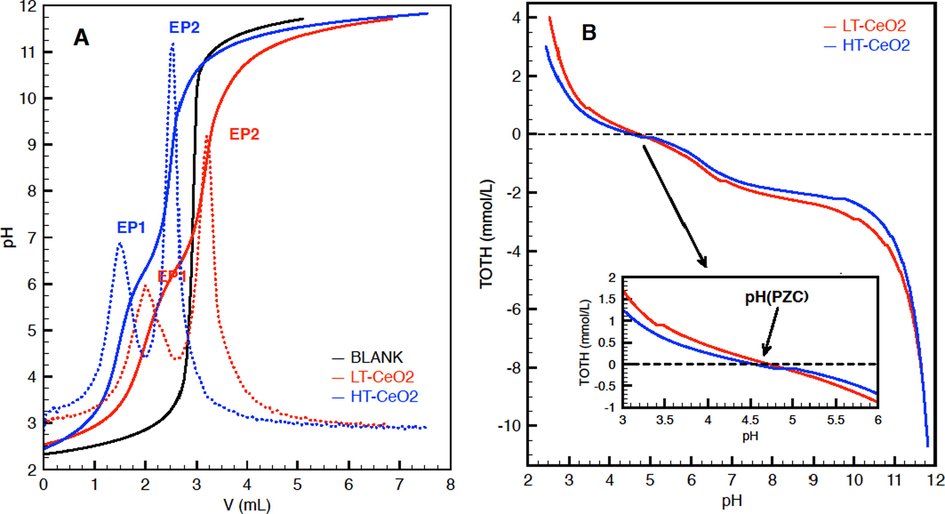
(a) Titration curves (full lines) and their 1st derivations (dotted lines) of cerium oxides. EP1 and EP2 are the equivalent points corresponding to the base consumption; the content of the –OH groups was calculated from the difference between EP2 and EP1. (b) TOTH (total concentration of protons consumed in the titration process) curves derived from the titration curves. The inset plot details show the pH that corresponds to the point of zero charge (PZC).
3.2 Degradation of parathion methyl
The standardized testing procedure showed that cerium oxide synthetized at a low temperature destroys parathion methyl efficiently both in heptane (with a half-life of less than 5 min) and in acetonitrile (with a half-life of approximately 25 min) (see Fig. 3).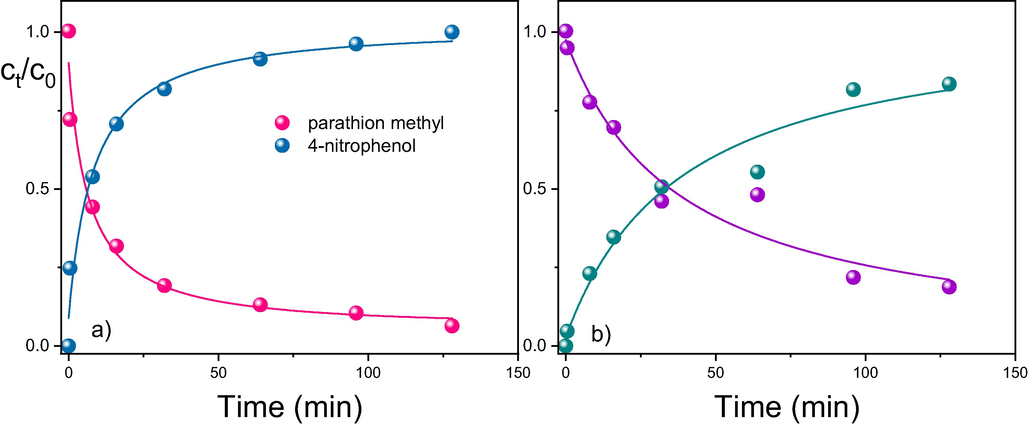
Degradation of parathion methyl in n-heptane (a) and acetonitrile (b) in the presence of cerium oxide LT-CeO2. Weight of sorbent = 0.05 g, 1 mg of parathion methyl dissolved in 0.15 mL of the respective solvent.
The degradation of parathion methyl in the presence of cerium oxide can be described as.
where A is parathion methyl, B is 4-nitrophenol and S are active sites on the cerium oxide surface. Assuming that
the conversion of parathion methyl to 4-nitrophenol may be described by the following kinetic equation:
with the integral form.
where [A]0 and [A]t are the concentrations of parathion methyl at the beginning of the reaction and at time t, respectively, w is the mass concentration of cerium oxide in the reaction mixture, and NS is the number of active sites in the cerium oxide. Eq. (3) was used to fit the experimental data in Fig. 3; some model parameters obtained from the nonlinear fitting are listed in Table 1.
Solvent
Parathion methyl destruction
4-nitrophenol creation
Rate constant (min-1mol-1L)
Da
R2
Rate constant (min-1mol-1L)
D
R2
n-heptane
0.185 (0.061)b
0.079 (0.068)
0.9717
0.137 (0.052)
0.054 (0.146)
0.9803
acetonitrile
0.029 (0.007)
−0.122 (0.159)
0.9926
0.026 (0.006)
−0.224 (0,154)
0.9959
For comparison, the degradation of parathion methyl was performed in the presence of cerium oxide prepared by calcination at 500 °C from the carbonate precursor. It was found that both cerium oxides (the air-dried LT-CeO2 and the high-temperature-treated HT-CeO2) exhibited almost identical degradation efficiencies. To elucidate in more details this somewhat counterintuitive phenomenon, we performed additional experiments, in which we examined the degradation efficiency of the cerium oxides prepared by the calcination of the air-dried cerium oxide or cerium carbonate at different temperatures (for more detail see Supplementary data).
As can be seen from Fig. 4, the post-synthetic thermal treatment does not significantly affect the degradation efficiency of cerium oxide in a relatively broad temperature range from 200 to ca 600 °C. At higher temperatures (above 600 °C), however, the degradation efficiency of cerium oxide decreases steeply with increasing temperature. This behaviour is almost independent on the precursor used for the cerium oxide preparation. In the high-temperature region, the degradation efficiency may be clearly related to the number of hydroxyl groups on the cerium oxide surface, as shown in our previous work (Ederer et al., 2021).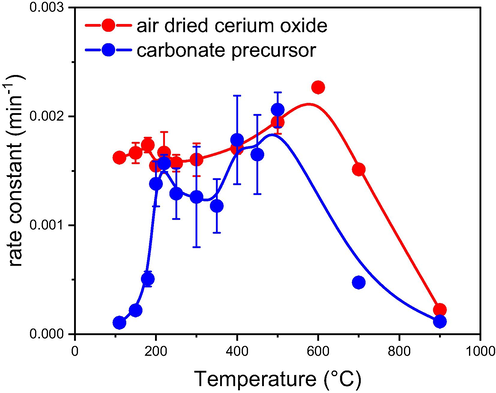
Effect of the (additional) thermal treatment on the degradation efficiency of cerium oxides prepared from various precursors: Red points – samples prepared by the additional thermal treatment of the air-dried cerium oxide; blue points – samples prepared by the calcination of cerium carbonate.
At temperatures below 200 °C, on the other hand, the carbonate precursor is not destroyed, and therefore cerium oxide is not created. On the other hand, there is no such limitation for the cerium oxides prepared by the purely wet process (e.g., via precipitation of cerium hydroxides accompanied by the aqueous-phase oxidation with a subsequent dehydration and creation of the crystalline cerium oxide). Therefore, effective cerium oxides can be prepared even at temperatures close to zero (Janoš et al., 2019; Tolasz et al., 2020).
3.2.1 Proposed mechanisms of degradation of parathion methyl
The complex dependence of the degradation efficiency on the temperature of treatment suggests that several properties of cerium oxide affect its degradation efficiency. In general, morphology (including the crystal shapes) and surface chemistry (surface non-stoichiometry) govern the activity of the ceria nanoparticles, together with the crystalline structure that facilitates the mobility of oxygen vacancies and other defects on the cerium oxide surface (Seal et al., 2020).
In the phosphoester cleavage process, both cerium cations as well as surface hydroxyls are involved (Janos et al., 2014): the cerium cation coordinates the sulfur atom in the thiophosphate group of the parathion methyl, making the central phosphorus atom more susceptible to nucleophilic attack, whereas the hydroxyl groups on the cerium oxide surface serve as nucleophilic agents that attack the P atom of the thiophosphate group. Recently, Plakhova et al. (2019) and Artiglia et al. (2021) emphasized the role of surface hydroxyls not only for the biological activity of cerium oxide, but also for some industrial applications.
3.3 Degradation of parathion methyl in mixed solvents
In our previous paper (Janos et al., 2014); we demonstrated that the addition of even small amounts of polar solvent (typically water) may deteriorate the degradation efficiency of cerium oxide (see also Fig. 5). In the following series of experiments, we tested the degradation efficiency in mixed solvents composed of heptane and another co-solvent (the ratio of heptane to co-solvent was 1:2) or acetonitrile with another co-solvent (the ratio was also 1:2). As shown in Fig. 5a, the systems with heptane as the main constituent are rather insensitive to changes in the composition of the reaction medium. Even the presence of polar solvents (acetonitrile) or higher alcohols does not disturb the degradation efficiency of cerium oxide. Mixed solvents with acetonitrile as the main constituent, however, are much more sensitive to the presence of co-solvents. The addition of polar and protic solvents significantly reduces the degradation ability of cerium oxide. This result is fully consistent with the presupposed reaction mechanism.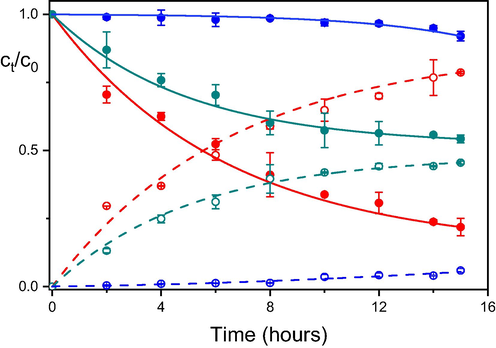
Degradation of parathion methyl in the presence of cerium oxide in acetonitrile (red points and curves); in an acetonitrile-methanol mixture 50/50% v/v (green points and curves); and in an acetonitrile–water mixture 50/50% v/v (blue points and curves).
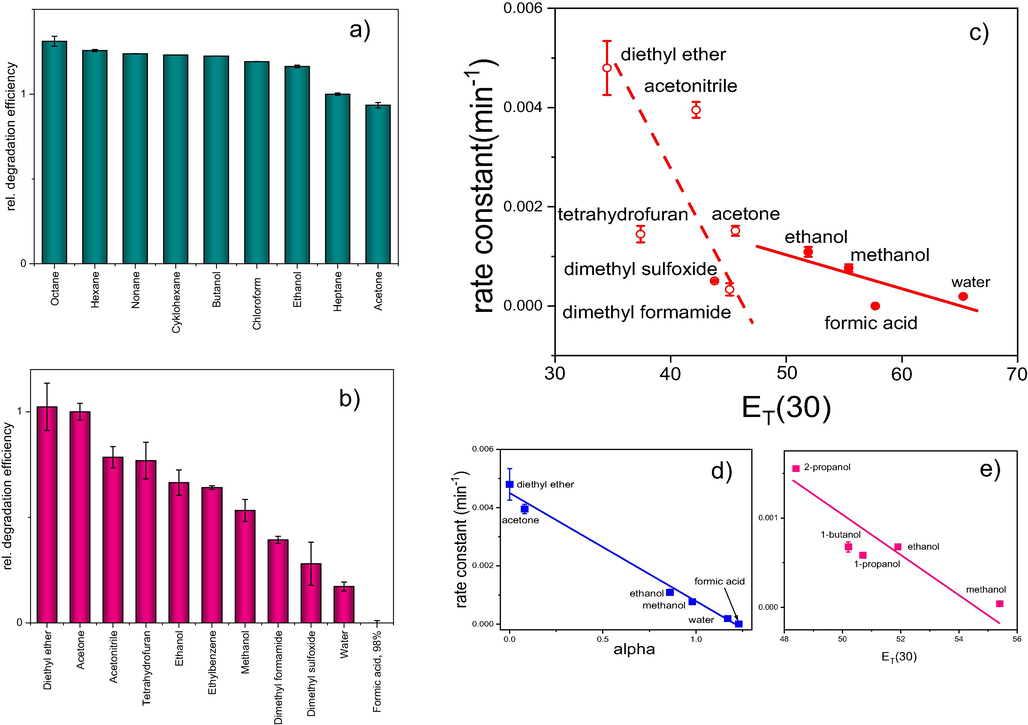
(a) Relative degradation efficiency of the air-dried cerium oxide in various mixed solvents consisting of heptane and other co-solvent. Heptane to co-solvent ratio 1: 2. (b) Relative degradation efficiency of the air-dried cerium oxide in various mixed solvents consisting of acetonitrile and other co-solvent. Acetonitrile to co-solvent ratio 1: 2.==== (c) Correlation between the degradation efficiency of cerium oxide (rate constant) and the hydrogen bond donation (HBD) ability of co-solvents expressed as the ET(30) parameter. (d) Similar correlation of selected co-solvents between the rate constant and the α parameter. Both α and ET(30) were taken from Taft and Kamlet, 1979. (e) Correlation between the rate constant of parathion methyl degradation and the polarity parameter ET(30) of selected alcohols.
According to the theory of nucleophilic reactions, binuclear nucleophilic substitution reactions (SN2) are suppressed in the presence of protic solvents (Hamlin et al., 2018), which are able to solvate the nucleophile via hydrogen bonding. The hydrogen bond donating (HBD) ability of solvents can be expressed, for example, with the aid of the α-scale introduced by Taft and Kamlet (1979). Marcus (Marcus, 1991) correlated the α-scale with other polarity scales, such as the ET(30), Z or AN scales. We attempted to correlate the degradation efficiency of cerium oxide with the polarity parameters of the co-solvents used as reaction media for parathion methyl degradation (Fig. 6).
3.4 Study of parathion methyl degradation in an acetonitrile–water mixture
The following figure (Fig. 7) demonstrates that the addition of water (and, to a lesser degree, methanol) to acetonitrile dramatically deteriorates the degradation efficiency of cerium oxide as a result of the solvation of active sites mainly via hydrogen bonding and slows down the kinetics of the reaction.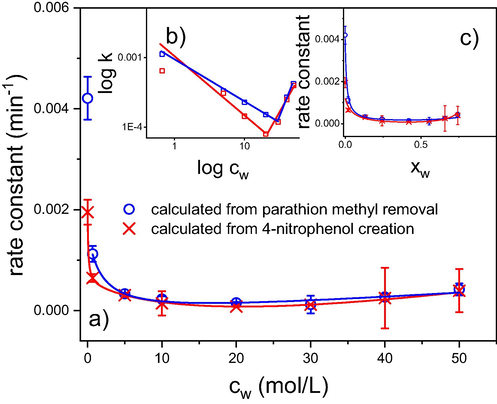
(a) Degradation of parathion methyl in the presence of cerium oxide in the acetonitrile – H2O mixture. Experimental conditions: The initial concentration of cerium oxide was 33 mg/mL, the initial concentration of parathion methyl was 1.33 mg/mL, and the equilibration time was 16 h. The degradation efficiency was expressed as a rate constant determined from the degree of conversion at a fixed time. The solid lines were fitted using an equation for the dependence of the hydrogen bond donating (HBD) ability of the acetonitrile – water binary solutions. (b) The same dependence was subdivided into the contributions of specific interactions (1) and electrostatic interactions (2); the red squares = experimental data for parathion methyl degradation.
To examine this effect in more detail, we measured the dependence of the degradation rate on the composition of the water-acetonitrile mixture over a wide concentration range. To express an effect of water addition on the reaction kinetics at least semi-quantitatively, a model that assumes the solvation of active sites in mixed solvents was proposed and used to fit the experimental data.
3.4.1 Solvation of active sites in the acetonitrile–water mixture
Water-acetonitrile mixtures are popular solvents in organic synthesis as well as in separation sciences because of the mutual miscibility of their components at any ratio and their solubilizing ability towards many organic and inorganic compounds. Despite the great number of studies devoted to this system, its properties are still not completely understood because of the inherently complex interactions of the components, which have quite different natures. Depending on the composition of the mixture (expressed in molar concentrations or mole fractions), various interactions (dipole–dipole interactions, hydrogen bonding) predominate in various concentration regions. It was deduced from X-ray diffraction and IR measurements (Takamuku et al., 1998) and from X-ray absorption spectrometry (Nagasaka et al., 2020) and computer simulations (Ahn and Lee, 2007) that acetonitrile, water and mixed clusters are present in the water-acetonitrile binary mixture. In a relatively large range of compositions (for xw = 0.25–0.8; xw – mole fraction of water), the presence of “microheterogeneities” is supposed.
The creation of these microconstituents and their contribution to the solvation of the site on the cerium oxide surface may be described as follows:
where Eqs. (1) and (2) describe the solvation of the active site S with the molecules of acetonitrile and water, respectively, Eq. (3) describes the creation of the acetonitrile–water clusters, and Eq. (4) describes the solvation of the active site with these clusters.
It holds for the active site:
= [S] + [SA] + [SW] + [SAW] + … + [SAWi] =.
where . As the concentrations of active sites are typically low in comparison with the concentrations of solvents, the concentrations may be approximated with cA and cW, respectively.
It is further assumed that various solvation species summarized in Eq. (8) contribute to the overall (observed) rate constant according to the following equation:
where
are the fractions of the respective species (e.g.,
=
) and kj are the constants specific for the respective species. When Eqs. (5) and (6) are combined, we obtain.
Eq. (10) relates the degradation efficiency of cerium oxide to the composition of the acetonitrile–water mixture; ki reflects the fact that various microheterogeneities or various arrangements of the acetonitrile–water clusters may be more or less effective in the solvation of the binding site on the cerium oxide surface. This idea is also quite compatible with the presumption that a specific spatial arrangement of the Ce3+/Ce4+ pairs and –OH groups is required to create the active site.
It was found that Eq. (10) gives a reasonable fit to the experimental data for n = 3. However, ki and other parameters in Eq. (10) are not accessible experimentally and are not found in the literature. Therefore, we tried to relate the degradation efficiency to other parameters commonly used to compare the behaviour of solvents or their mixtures. The most frequently used parameter is the Dimrodth-Richardt empirical parameter ET(30), which corresponds to the excitation energy of the solvatochromic indicator (2,6-diphenyl-4-(2,4,6-triphenylpyridin-1-ium)-1-phenolate, also known as ET(30) dye) in various solvents, which is usually considered a measure of polarity, and it was successfully related to various thermodynamic and kinetic properties not only in single-component solvents but also in solvent mixtures (Skwierczynski and Connors, 1994; Bosch and Rosés, 1992).
An extensive comparison was published by Roses et al. (1995); who used the solvent-exchange model to derive equations for the ET(30) parameter in binary mixtures. In their work, an alternative approach based on preferential solvation was also described. For the water-acetonitrile mixture, the equation was derived in the following form (ET is used instead of ET(30) for simplicity):
where.
and.
is the mole fraction of the more polar solvent (water) in the binary mixture. ET1 and ET2 are values of the ET(30) parameter for pure solvents (acetonitrile, water), whereas ET12 corresponds to the (hypothetical) mixed solvent, and f2/1 and f12/1 are parameters of preferential solvation. Numerical values of these parameters for several solvent mixtures are listed in the aforementioned paper (Rosés et al., 1995); including those of acetonitrile and water. Using these parameters, Eq. (8) gives a relatively simple monotonous curve that can be transformed to the curve expressing the experimental dependence of degradation efficiency on the composition of the solvent mixture. Nevertheless, this equation can be used to fit the experimental data on the condition that ET and f are treated as adjustable parameters (see Fig. 7c).
The experimental dependence in Fig. 7 reflects the inherently complex nature of mixtures consisting of organic solvents and water (Salmar et al., 2012; Saleh et al., 2006). The dependence of the reaction rate on the water content may be divided into two (or even more) regions, in which various interactions predominate. In the water-rich region, nonspecific (mainly electrostatic) interactions predominate, which was also confirmed by the linear dependence between the logarithm of the rate constant and the reciprocal value of the dielectric constant (Fig. 8). In the acetonitrile-rich region, however, solvent–solute interactions play an important role. The addition of even small amounts of water into the reaction mixture causes preferential solvation of the active sites accompanied by a dramatic decrease in the degradation efficiency. An almost identical dependence was found for the reaction of sodium 4-nitrophenoxide with iodomethane in the acetone–water mixture; this reaction presumably proceeded by the SN2 mechanism (Eduardo Humeres, 2001).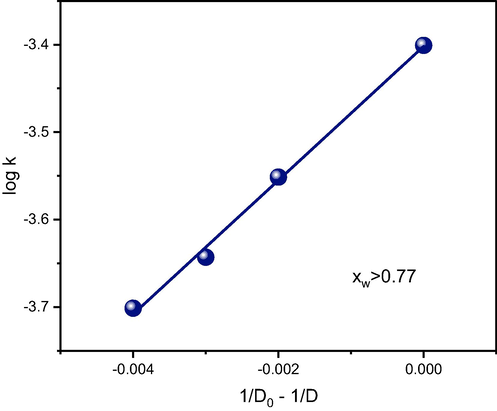
Dependence of the rate constant on the dielectric constant in the acetonitrile–water mixture for the water-rich region (compare with the similar dependence for the acetone–water mixture in Eduardo Humeres, 2001).
4 Conclusions
It was shown that surface hydroxyls play a crucial role in the destruction of the molecules of toxic organophosphates in the presence of cerium oxide. However, the –OH groups are consumed during phosphoester destruction, and thus, the respective materials should be considered stoichiometric sorbents (reagents) (Klabunde et al., 1996) rather than catalysts.
Parathion methyl degradation proceeds well in nonpolar solvents as well as in aprotic solvents. In solvents with hydrogen bond donating ability, however, the –OH groups on the cerium oxide surface are solvated and inactivated. As shown for the acetonitrile–water reaction mixture, the addition of even small amounts of protic solvent, such as water, dramatically deteriorates the cerium oxide degradation efficiency. The model of preferential solvation was used to express the experimental dependencies of the cerium oxide degradation efficiency on the mixture composition in the whole concentration range. In certain solvent systems, some empirical polarity scales, such as the alpha-scale or the Dimrodth-Richardt parameter ET(30), may be correlated with the degradation efficiency of cerium oxide.
Acknowledgements
The authors acknowledge the assistance provided by the Research Infrastructure NanoEnviCz, supported by the Ministry of Education, Youth and Sports of the Czech Republic under Project No. LM2018124 and Czech Scientific Foundation (grant No. 19-07460S).
The authors have no conflicts of interest to declare.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- K.J. Klabunde, J. Stark, O. Koper, C. Mohs, D.G. Park, S. Decker, Y. Jiang, I. Lagadic, D. Zhang, Nanocrystals as stoichiometric reagents with unique surface chemistry, J. Phys. Chem. 100 (1996) 12142–12153. http://www.scopus.com/inward/record.url?eid=2-s2.0-0030198228&partnerID=tZOtx3y1.
- Nanocrystals as stoichiometric reagents with unique surface chemistry, New adsorbents for air purification. Nanostructured Mater.. 1999;12:463-466.
- [CrossRef] [Google Scholar]
- Nanocrystalline Metal Oxides as Destructive Adsorbents for Organophosphorus Compounds at Ambient Temperatures. Chem. - A Eur. J.. 2002;8:2602.
- [CrossRef] [Google Scholar]
- Nanocrystalline metal oxides as unique chemical reagents/sorbents. Chemistry.. 2001;7:2505-2510.
- [CrossRef] [Google Scholar]
- Nanostructured Metal Oxides for Stoichiometric Degradation of Chemical Warfare Agents. Rev. Environ. Contam. Toxicol.. 2016;236:239-258.
- [CrossRef] [Google Scholar]
- Nanostructure materials for destruction of warfare agents and eco-toxins prepared by homogeneous hydrolysis with thioacetamide: Part 1—zinc oxide. J. Phys. Chem. Solids. 2007;68:716-720.
- [CrossRef] [Google Scholar]
- Mesoporous manganese oxide for warfare agents degradation. Microporous Mesoporous Mater.. 2012;156:224-232.
- [CrossRef] [Google Scholar]
- Some environmentally relevant reactions of cerium oxide. Nov. Biotechnol. Chim.. 2014;13
- [CrossRef] [Google Scholar]
- Mesoporous cerium oxide for fast degradation of aryl organophosphate flame retardant triphenyl phosphate. RSC Adv.. 2019;9:32058-32065.
- [CrossRef] [Google Scholar]
- Metal oxide nanoparticles for the decontamination of toxic chemical and biological compounds. Int. J. Pharm.. 2020;583:119373
- [CrossRef] [Google Scholar]
- Cerium dioxide as a new reactive sorbent for fast degradation of parathion methyl and some other organophosphates. J. Rare Earths. 2014;32:360-370.
- [CrossRef] [Google Scholar]
- N.S. Gould, S. Li, H.J. Cho, H. Landfield, S. Caratzoulas, D. Vlachos, P. Bai, B. Xu, Understanding solvent effects on adsorption and protonation in porous catalysts, Nat. Commun. 2020 111. 11 (2020) 1–13. https://doi.org/10.1038/s41467-020-14860-6.
- Can cerium oxide serve as a phosphodiesterase-mimetic nanozyme? Environ. Sci. NANO. 2019;6:3684-3698.
- [CrossRef] [Google Scholar]
- Room-temperature synthesis of nanoceria for degradation of organophosphate pesticides and its regeneration and reuse. RSC Adv.. 2020;10:14441-14450.
- [CrossRef] [Google Scholar]
- Nanocrystalline cerium oxide for catalytic degradation of paraoxon methyl: Influence of CeO2 surface properties. J. Environ. Chem. Eng.. 2021;9:106229
- [CrossRef] [Google Scholar]
- Cerium dioxide crystallite sizes by temperature-programmed reduction. J. Catal.. 1987;103:502-505.
- [CrossRef] [Google Scholar]
- P. Janoš, M. Petrák, Preparation of ceria-based polishing powders from carbonates, J. Mater. Sci. 1991 2615. 26 (1991) 4062–4066. https://doi.org/10.1007/BF02402947.
- Determination of amino groups on functionalized graphene oxide for polyurethane nanomaterials: XPS quantitation vs. functional speciation. RSC Adv.. 2017;7:12464-12473.
- [CrossRef] [Google Scholar]
- Combined Operational Characteristics, Technical specifications, Test Procedures and Evaluation Criteria for Chemical, Biological. Radiological Nuclear Decontamination Equipment 2012
- [Google Scholar]
- Engineered defects in cerium oxides: Tuning chemical reactivity for biomedical, environmental, & energy applications. Nanoscale. 2020;12:6879-6899.
- [CrossRef] [Google Scholar]
- Towards the surface hydroxyl species in CeO2 nanoparticles. Nanoscale.. 2019;11:18142-18149.
- [CrossRef] [Google Scholar]
- Size of Ceria Particles Influences Surface Hydroxylation and Hydroxyl Stability. J. Phys. Chem. C. 2021;125:9303-9309.
- [CrossRef] [Google Scholar]
- Nucleophilic Substitution (S N 2): Dependence on Nucleophile, Leaving Group, Central Atom, Substituents, and Solvent. ChemPhysChem. 2018;19:1315-1330.
- [CrossRef] [Google Scholar]
- Linear solvation energy relationships. Part 4. Correlations with and limitations of the α scale of solvent hydrogen bond donor acidities. J. Chem. Soc. Perkin Trans.. 1979;2:1723-1729.
- [CrossRef] [Google Scholar]
- The effectiveness of solvents as hydrogen bond donors. J. Solution Chem.. 1991;20:929-944.
- [CrossRef] [Google Scholar]
- Liquid structure of acetonitrile-water mixtures by X-ray diffraction and infrared spectroscopy. J. Phys. Chem. B. 1998;102:8880-8888.
- [CrossRef] [Google Scholar]
- Microheterogeneity in Aqueous Acetonitrile Solution Probed by Soft X-ray Absorption Spectroscopy. J. Phys. Chem. B. 2020;124:1259-1265.
- [CrossRef] [Google Scholar]
- Density Functional Theory Study of Acetonitrile -Water Clusters: Structures and Infrared Frequency Shifts. Bull. Korean Chem. Soc.. 2007;28:725-729.
- [CrossRef] [Google Scholar]
- Solvent effects on chemical processes. Part 7. Quantitative description of the composition dependence of the solvent polarity measure ET(30) in binary aqueous-organic solvent mixtures. J. Chem. Soc. Perkin Trans.. 1994;2:467-472.
- [CrossRef] [Google Scholar]
- Relationship between ET polarity and composition in binary solvent mixtures. J. Chem. Soc., Faraday Trans.. 1992;88:3541-3546.
- [CrossRef] [Google Scholar]
- Solute-solvent and solvent-solvent interactions in binary solvent mixtures. Part 1. A comparison of several preferential solvation models for describing ET(30) polarity of dipolar hydrogen bond acceptor-cosolvent mixtures. J. Chem. Soc. Perkin Trans.. 1995;2:1607-1615.
- [CrossRef] [Google Scholar]
- Role of water in determining organic reactivity in aqueous binary solvents. Cent. Eur. J. Chem.. 2012;10:1600-1608.
- [CrossRef] [Google Scholar]
- Density, viscosity and thermodynamic activation of viscous flow of water + acetonitrile. Phys. Chem. Liq.. 2006;44:551-562.
- [CrossRef] [Google Scholar]
- *,† Eduardo Humeres, † Ricardo J. Nunes, ‡ Vanderlei G. Machado, § and Marcelo D. G. Gasques, C. Machado§, Ion-Dipole SN2 Reaction in Acetone−Water Mixtures. Electrostatic and Specific Solute−Solvent Interactions, (2001). https://doi.org/10.1021/JO0012501.







