Translate this page into:
Dependence of solvents, pH, molar ratio and temperature in tuning metal organic framework architecture
⁎Corresponding author. Tel.: +91 9447287247; fax: +91 481 2732278. sureshmathewmgu@gmail.com (S. Mathew)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
One of the strategic goals in crystal engineering is to synthesize the crystals with predictable structures and valuable properties. Metal Organic Frameworks (MOFs), a new class of crystalline porous materials, is a recent upsurge of interest in materials research. The challenges in the area encompass the deliberate control of structure, and therefore the properties and function, as many synthetic factors play a subtle role in the crystallization of MOFs. Among many influencing factors, nature of solvents, pH, molar ratio of reactants and temperature are four crucial parameters that determine the overall architectures of MOFs. This review presents the effect of these major parameters on the synthesis of several MOFs to clearly understand their influence on the nature of binding and formation of different MOF structures.
Keywords
Metal organic frameworks
Influential factors
Solvents
pH
Molar ratio
Temperature
1 Introduction
Metal Organic Frameworks (MOFs), also called as Porous Coordination Polymers (PCPs) or coordination networks, are crystalline materials which can be readily self-assembled from metal ions or metal clusters with organic ligands (Guillerm et al., 2014; Zhou et al., 2012; Wang et al., 2013a). They display permanent porosity with the enormous internal surface area and large structural diversity, and thus lead to a wide spectrum of applications including gas capture and storage (Gándara et al., 2014; He et al., 2014b; Peng et al., 2013; Li et al., 2013b), molecule separations (Bloch et al., 2014; Li et al., 2012a), ion-exchange (Brozek et al., 2014; Zhao et al., 2013), drug delivery (Cunha et al., 2013; Zhuang et al., 2014; Horcajada et al., 2006; Vandana et al., 2015), sensing (Dou et al., 2014; Li et al., 2013b; Kreno et al., 2012), catalysis (Dhakshinamoorthy et al., 2012; Nguyen et al., 2012; Tan et al., 2012; Harding and Reynolds, 2012), luminescence (Tian et al., 2014; Guo et al., 2014; Kim et al., 2013) (Scheme 1). These features of MOFs have attracted tremendous interest in most prolific areas of materials chemistry research. The remarkable progress of MOFs could be explained by their efficient tailorable chemistry, i.e. the ability to fine tune the structure with different metal cations and organic ligands.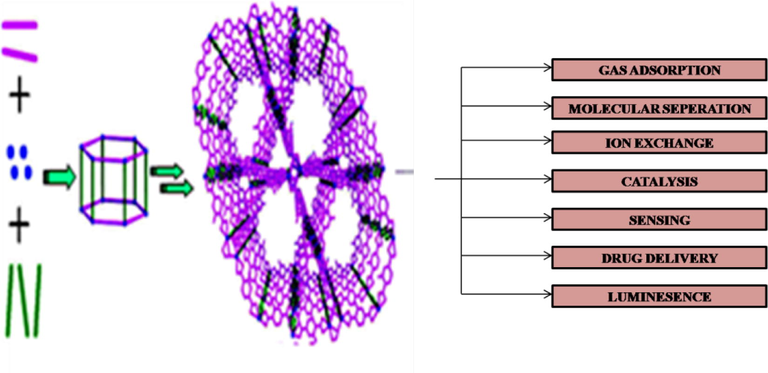
Illustration of MOF synthesis and its applications.
It is well known that the crystallization, structure and morphology of MOFs do not depend only on the modulus building blocks, but they are based on many synthetic conditions such as solvent type (Li et al., 2013a; Sun and Zhu, 2013; Liu et al., 2013), pH value of reaction mixture (Chen et al., 2013; Li et al., 2010; Gabriel et al., 2012; Kan et al., 2011), temperature (Sun and Sun, 2014; Liu et al., 2012b; Calderone et al., 2013), reagent concentration (Kim et al., 2012), time (Mahata et al., 2008; Burrows et al., 2011), molar ratio of starting materials (Lü et al., 2005; Cheng et al., 2011b; Liu et al., 2010; Gao et al., 2012), the presence of counter ions (Bourne and Moitsheki, 2005; Awaleh et al., 2006; Yi et al., 2005) and pressure (McKellar et al., 2014). These parameters have been categorized into compositional (e.g. solvent, pH, substituents of ligands, the molar ratio of starting materials, counter ions, concentration) and process parameters (e.g. pressure, time, temperature) (Scheme 2). These parameters play a profound effect on the structural chemistry of ligands and assembly process of ligands with metal centres leading to products with diverse structures.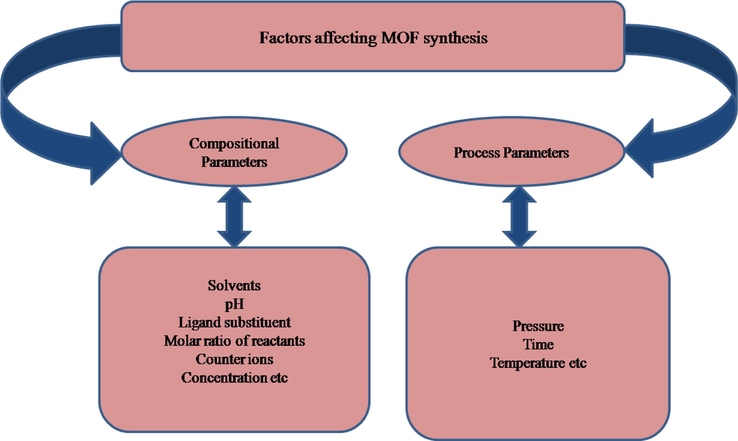
Various parameters influencing the formation of MOFs.
Although high throughput methods in the synthesis of MOF provide an idea of the effect of individual synthetic parameters on phase formation and morphology of crystals, it is quite difficult to follow a systematic investigation in new MOF structures that have been published frequently (Bauer et al., 2008; Ahnfeldt et al., 2009; Maniam and Stock, 2011). Antony. K. Cheetham and coworkers produced five phases of Co-succinate by using temperature as only the independent variable in 2004 (Forster et al., 2004). Awhile later they carried out 288 reactions via a high throughput method by selecting eight different Co(OH)2/C4H6O4 ratios, six different concentrations and six different temperatures ranging from 75 to 200 °C. They were able to produce two new phases in addition to five phases previously reported (Forster et al., 2005). Their effects show the role of parameters such as temperature, acid/base ratio, time and concentration in determining the structure of the compound. The amount of water included in the final structure has decreased for phases formed at progressively higher temperatures which have significant consequence in the overall structure i.e. the overall dimensionality of the structures in this series increases, with 1D chains up to 100 °C, two dimensional (2D) sheets at 150 °C, and three dimensional (3D) materials at 190 °C and above. The products from the reaction between Cadmium nitrate and Benzenedicarboxylic acid (H2BDC) in DMF medium are found to depend on the synthetic conditions, temperature, pressure and solvent quality (Burrows et al., 2008). Three dimensional network structures were obtained when the reagents were heated to 115 °C for 5 h while supramolecular isomers were obtained when the reaction was carried out at 95 °C and 115 °C respectively.
In this review our effort is to go through several syntheses of MOFs/PCPs to better understand the factors such as temperature, pH, solvents and molar ratio of reactants that influence the way of binding and formation of different MOF structures.
2 Various methods for MOF synthesis
Several types of methods were followed for the synthesis of MOFs. Each method can lead to compounds with different particle sizes, size distributions and morphologies which intern leads to different applications. Among them, some important synthetic routes are mentioned here.
2.1 The slow evaporation method
It is the commonly used conventional method for the MOF synthesis where slow evaporation of solvents of a reaction solution or slow diffusion of the solvent/solution occurs at room temperature without any external energy supply. Metal salts and organic linkers are mixed together in the liquid phase with or without the aid of additional auxiliary molecules. Usually, highly soluble precursors are used in this method which is concentrated by slow evaporation. The major disadvantage regarding this method is that it is a time-consuming process compared to other conventional synthesis methods. It can be overcome by using low-boiling solvents (Zou et al., 2005; Wang et al., 2009; Michaelides and Skoulika, 2005; Li et al., 2014a; Zhang et al., 2015; Fan et al., 2013).
2.2 Hydro/solvothermal method
In this liquid-phase synthesis method the reaction is carried out at higher temperatures (temperature higher that 100 °C) and pressure for several hours or days with the aid of closed vessels. Usually Teflon-lined autoclaves are used in which reactants in high boiling, polar solvents such as DMF, DEF, DMSO, H2O, acetone, acetonitrile, alcohols are taken. Sometimes a mixture of these solvents has also been used when solubility of the starting materials is different. Solution polarity would also be tuned and thus lead to intensify the crystal growth. The major advantage of this method is that it offers high solubility of the precursors and the formation of good quality MOF crystals suitable for structural characterization (Bo et al., 2015; Saha et al., 2014).
2.3 Microwave assisted synthesis
Unless other synthesis methods, microwave-assisted synthesis is a very energy efficient method of heating and a way to achieve fast crystallization due to higher nucleation rate. Recently MW assisted method is widely applied to obtain nano scale MOFs with high purity, identical morphology and very uniform distribution. This method makes use of energy in the form microwaves for a period of about an hour. Here conversion of this electromagnetic energy to thermal energy is occurring, i.e. when microwaves are applied dipole moment of the precursors in a Teflon vessel rotates itself to align with this field. As a consequence of frequent alignment collision between molecules occurs which leads to increase in kinetic energy or heating in the sample. One of the potential advantages of this technique is the adjustable power outputs, pressure and a wide range of temperatures (Klinowski et al., 2011; Liang and D’Alessandro, 2013; Bag et al., 2015; Palomino Cabello et al., 2015; Dong et al., 2015).
2.4 Sonochemical synthesis
Recently sonochemical synthesis has been efficiently employed for the rapid synthesis of MOFs. This is also an efficient method to shorten the crystallization time by the application of ultrasound. This ultrasound enhances the chemical or physical changes in a liquid medium through a process called cavitation. In this process growth and subsequent destruction of bubbles throughout the liquid occur after sonication. The collapse of bubbles results in rapid release of energy with temperatures of about 4000 K and pressure in excess of 1000 atmospheres. This is an energy efficient, environmental friendly method to generate homogeneous nucleation centres as there is no direct interaction between ultrasound and molecules (Bigdeli and Morsali, 2015; Karizi et al., 2015; Mehrani et al., 2014; Hu et al., 2012; Yang et al., 2012).
2.5 Mechanochemical synthesis
As a solvent free synthesis mechanochemical method is the simplest, economical and environmental friendly method compared to other liquid phase synthesis. Nowadays this method is employed for large scale synthesis of MOF materials. Here mechanical force has been applied for grinding the mixture of metal salts and organic linkers together in a mechanical ball mill. As a result, mechanical breakage of intramolecular bonds and construction of new bonds occurs. Sometimes liquid assisted grinding is also followed for the acceleration of mechanochemical reactions. The main advantages of this method are that (1) organic solvents can be avoided, (2) room temperature is sufficient, (3) side products formed are harmless and (4) MOF can be obtained in short reaction time (Klimakow et al., 2010; Pilloni et al., 2015; Friščić, 2011; Yan et al., 2013; Singh et al., 2013).
2.6 Electrochemical synthesis
The electrochemical synthesis has been recently employed for the fast and continuous production of large amounts of MOF crystals. Instead of metal salts, metal ions are used as a metal source in this method. The metal ions provided through anodic dissolution which react with organic linkers, and electrolytes in the reaction medium. Micro crystalline powders and films can be obtained by this continuous process (Martinez Joaristi et al., 2012; Carson et al., 2011; Campagnol et al., 2014).
Besides these methods a number of other synthesis routes are also followed such as ionothermal method (ionic liquids are used as solvents), precipitation reaction followed by recrystallization and layering of the solution.
3 Important influential factors effecting MOF synthesis and structure
3.1 Effect of solvent
Judicious choice of reaction solvents is a major concern in the synthesis of MOFs since they directly or indirectly influence the coordination behaviour of metal and ligand (Scheme 3). Although the cause behind the solvent choice for each synthesis still remains unclear, a number of examples of MOF synthesis show that each solvent system has a role in regulating the formation of different coordination environment. The solvents used in the assembly process may participate in coordination with metal ions or can also act as a guest molecule in the final lattice structure (Yakovenko et al., 2014; Akhbari and Morsali, 2011). Although most often the solvents may not be incorporated in the as-synthesized MOF, they act as a structure directing agent or a medium for the crystal growth process. i.e., the extent of deprotonation of organic carboxylate ligands can be controlled by selecting suitable solvents such as DMF, DEF and DMA or by adjusting the basicity of solvent medium. These solvents can be converted to its corresponding amines at higher temperatures and thus lead to deprotonation of carboxylates.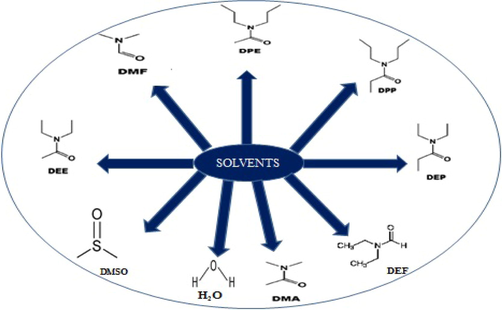
Different solvents used in MOF synthesis.
The structures of two fluorinated MOFs, Cu2(hfbba)2(3-mepy)2](DMF)2(3-mepy) (1) named as F-MOF-4 and [Cu2(hfbba)2(3-mepy)2], (2) named as Cu-F-MOF-4B {hfbba-4′4″-(hexaflouroisopropylidine)bis (benzoic acid), (3-mepy-3-methyl pyridine)} (Crane et al., 2015) have been found to depend on different solvents used in the reaction mixture (Pachfule et al., 2011). Cu-MOF-4B and F-MOF-4 were obtained with and without interdigitation by using solvents DEF and DMF respectively under the same reaction conditions. Gas adsorption is also different for these two MOFs depending on their structural variety. The changes in the structure may be due to the different degree of deprotonation of H2hfbba under different solvents. In order to investigate the influence of solvent on coordination connectivity Junhua Luo’s group followed a number of reactions of biphenyl tricarboxylic acid (H3BPT) and Cd(NO3)2·4H2O with three different solvents DMF, DMA and DEF (Li et al., 2012b). Then three entirely different coordination architectures, {[Cd3(BPT)2(DMF)2]·2H2O} (3), [Cd3(BPT)2(DMA)2] (4) and [(CH3CH2)2NH2]· {[Cd(BPT)]·2H2O} (5) had been produced in which (3) and (4) possess solvent as coordinating agents.
The complex (3) is a three-dimensional (3D) framework consists of infinite Cd−O−Cd chains with the solvent DMF molecule bridging the neighbouring Cd1 and Cd2 centres. The complex (4) is a 3D network consists of infinite metal-carboxylate chains, the solvent DMA molecule coordinated with one of the Cd(II) centres. The complex (5) possesses a two-dimensional (2D) honeycomb type net formed by the mononuclear metal ion with the BPT ligand, which are further stacked to form a 3D supramolecular architecture (Fig. 1). In the framework formed by DEF solvent, DEF decomposed and form protonated diethylamine cation. This makes the complex achieve charge neutrality without coordinating to a metal ion.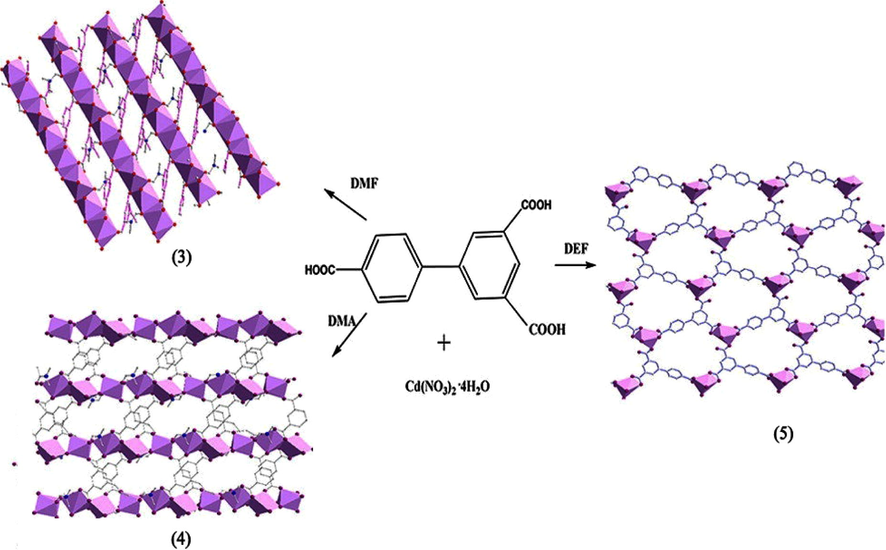
Coordination of Cd(NO3)2·4H2O and Biphenyl tricarboxylic acid by using different solvents in (3)-(5).
Reprinted with permission from reference, Li et al., 2012b. Copyright (2012) © American Chemical Society.
A number of reactions were carried out at mixed solvent system also. The combination of two or more solvents can contribute to the various coordination modes of ligands. For example, two zinc based coordination polymers namely [Zn2(tfbdc)2(DMF)2 (EtOH)]n (6) and [Zn(tfbdc)(MeOH)4]n (7) had been isolated by the reactions of Zn(NO3)2·6H2O, tetrafluoroterephthalic acid (H2tfbdc) and hexamethylene tetra- mine(hmt) in different solvent systems (Cheng et al., 2011a). First complex prepared by mixture of anhydrous ethanol and DMF showed μ2 and μ4 bridging modes of tfbdc ligand which led to a 3D pillar-layered framework with a rare topology, while second coordination polymer has a 1D zig-zag chain structure with the interchain hydrogen-bonding interactions when anhydrous methanol media were used instead of the DMF/EtOH mixture. Here the difference in the solvent polarity plays an important role in changing the dimensionalities from 1D to 3D structures by affecting the bridging modes of ligand.
Effect of mixed solvent system was also studied by Paul M. Forster’s group (Banerjee et al., 2011). They synthesized four Mg based coordination networks, three 3D {[Mg3(3,5-PDC)3(DMF)3].DMF}(8), {[Mg(3,5-PDC)(H2O)].(H2O)} (9), {[Mg4(3,5-PDC)4(DMF)2 (H2O)2]·2DMF·4.5H2O} (10) and a 2D [Mg(3,5-PDC)(H2O)2] (11) (3,5-PDC-pyridine dicarboxylate) using a combination of DMF, MeOH, EtOH and H2O. Compound (8) is a 3D network which consists of isolated and corner-sharing magnesium octahedra connected by the organic linkers and coordinated and free DMF molecules occupy in the channel. Compound (11) is a layered structure in which PDC connects isolated seven coordinated magnesium polyhedral. Compound (9) consists of isolated magnesium octahedra connected by the organic linker with each other forming a 3D network. Compound (10) also exhibits a 3D network based on isolated magnesium octahedra with disordered DMF and water molecules in cavities (Fig. 2). They found that the dimensionality of these four networks was determined by the relative coordination ability of the solvents with the metal. H2O is found to have high affinity to coordinate with the metal centres. Compared to H2O, DMF has less affinity towards Mg, while MeOH and EtOH do not show any affinity towards the metal centre when MeOH/H2O and EtOH/DMF mixtures were used.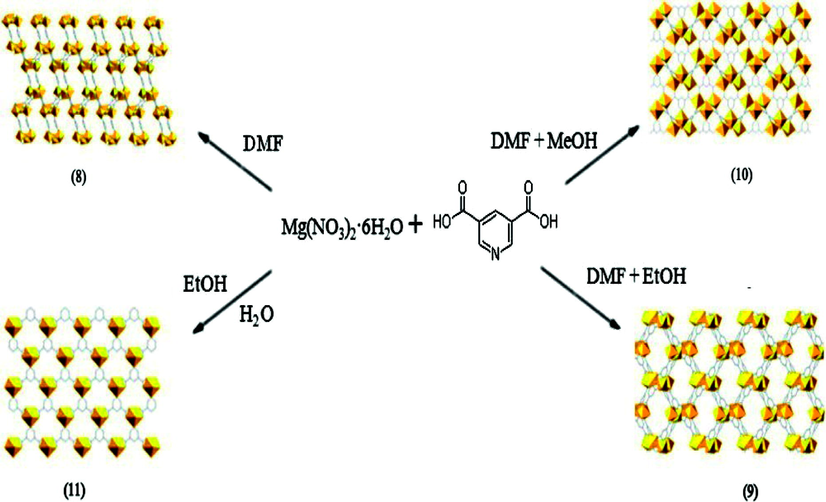
The self-assembly of Mg(NO3)2·6H2O and 3,5-PDC under different solvents in (8)-(11).
Reprinted with permission from reference, Banerjee et al., 2011. Copyright (2011) © American Chemical Society.
Different MOF structures may be obtained by the dissimilarities in coordination environments of central metal controlled by solvent system. In order to explore the role of solvent, P. Cui et al. synthesized two supramolecular isomeric metal organic frameworks under similar conditions, but with different DMF-EtOH-H2O ratio (Cui et al., 2011). They carried out a series of experiments changing solvent volumetric ratios from 5:2:10. Two isomeric Zn-tci (tci-tris(2-carboxyethyl) isocyanuric acid) with a rare 2D sheet structure and 3D framework with unusual Lonsdaleite (Ion) topology were obtained at volumetric ratios of 5:2:1 and 5:2:0 respectively. The experiments revealed that water in the mixed solvent system may influence the solvation process and regulate the formation of different environments for the self-assembly process. The self-assembly of benzene dicarboxylic acid (BDC) and Zinc (II) ion has also been found to depend on the ratio of solvents when binary solvent mixture is used (Wang et al., 2008). Local coordination environment of Zinc ion has varied with respect to the ratio of water/DMA leading to a variety of coordination polymers namely [Zn(BDC)(DMA)]n (12), [Zn3(BDC)3(DMA)3]n (13) and [Zn(BDC)(H2O)(DMA)]n (14) (Scheme 4). As the ratio of water/DMA increased DMA molecules are gradually substituted by water, which shows the definite relationship between the ratio of solvents and the composition of the products. A similar effect had been discussed for Mg based benzene tricarboxylate frameworks when the ratio of H2O/EtOH has changed (Mazaj et al., 2013). Four new frameworks were obtained with H2O/EtOH volume ratios 1:0, 1:1.6, 1:2.2, 0:1 respectively without any changes in Mg/BTC ratios. As the amount of EtOH increased from 0% to 100%, the structural dimensionality of the frameworks is also increased from zero dimensional to three dimensional. Therefore dimensionality of the final structures seems to be tuned by varying H2O/EtOH ratio. The effect of the choice of solvent has been clearly reflected in the above obtained structures. A unary, binary and ternary solvent system was used to prepare four 2D coordination polymers with Zn/Cd nitrates and an achiral angular ditopic ligand 2,6-bis(imidazole-1-yl)pyridine (pyim2) (Tripathi et al., 2014). Using MeOH/DMF system isostructural [trans-Zn(pyim2)2(NO3)2]n (15) and [trans-Cd(pyim2)2(NO3)2]n (16) were obtained respectively. When H2O was incorporated along with MeOH/DMF solvents a racemic mixture of Zn and Cd coordination polymers, [trans-Zn(pyim2)2(H2O)2](NO3)2]n (17) and [cis-Cd(pyim2)2(H2O)2](NO3)2]n (18) were obtained as products. The results confirm the effect of different solvent system on metal coordination environment and structures.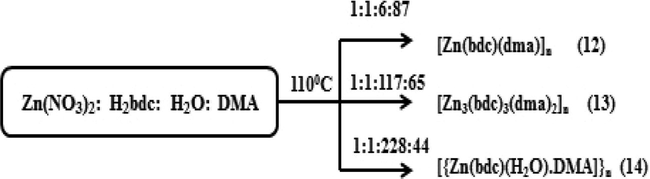
Different coordination polymers obtained by Zinc ion and Benzene dicarboxylic acid with respect to the ratio of water/DMA in (12)-(14).
Reprinted with permission from reference. Wang et al. (2008). Copyright (2008) © Royal Society of Chemistry.
Solvents with different molecular sizes may lead to the formation of MOFs with dissimilar structures. Two micro porous yttrium trimesates having different crystallographic symmetries and different spatial arrangements of linkers around Y3+ centres had been synthesized by mixed solvent of DMF/H2O and DEF/H2O (Dong et al., 2010). Due to the bigger steric hindrance of DEF compared to DMF there are no direct coordination between DEF and Y3+ in crystal structure [Y2(BTC)2(H2O)2] (19) using DEF/H2O as solvent. But in another compound with formula [Y2(BTC)2(DMF)(H2O)] (20) there has one coordinated DMF molecule which leads to different crystal structure than (19). The steric hindrance of the coordinating solvent is also found to be playing a role in the crystal-growth process. It is demonstrated in coordinated-solvent influenced crystal engineering of Cd(II)-4,4′-dipyridyl sulphide coordination network (Tzeng et al., 2009). With same starting materials, Cd(ClO4)2 and Py2S(4,4′-dipyridylsulphide), three various dimensional networks from 1D to 2D to 3D were able to develop when solvent mixtures of DMF/CH2Cl2, CH3CN/CH2Cl2 and CH3OH/CHCl3 were used as the reaction media. The difference in dimensionality in this case may be attributed to the steric hindrance of solvents used. DMF with more steric hindrance is responsible for the formation of a 1-D double zig-zag chain polymer, {[Cd(Py2S)2(DMF)2](ClO4)2}n (21) which is crystallized in the space group of P21/c yt while less steric hindered CH3CN and CH3OH favour to build 2D and 3D networks of {[Cd(Py2S)3 (CH3CN)2] (ClO4)2}n (22) and {[Cd2(Py2S)5 (CH3OH)2(ClO4)4}n (23) crystallized in P21/n and P4,2,2 space groups respectively.
In addition to structural differences the pore size variations have also been demonstrated. Reaction of Co(NO3)2·6H2O with 4,4′-((5-carboxy-1,3-phenylene)bis(Oxy))dibenzoic acid (H3CPBDA) in three solvents with different sizes (DMF, DMP and DMA) resulted in porous coordination polymers with different pore size (Huang et al., 2013). The sizes of the channels are decreased from 76.84 Å for {[Co1.5(CPBDA)(DMF)(H2O)3](DMP)}n (24) to 74.37 Å for {[Co1.5(CPBDA)(DMA)(H2O)3] (DMA)n (25) to 72.76 Å for {[Co1.5(CPBDA)(DMA)(H2O)3](DMF)}n (26) when the solvents used are DMP, DMA and DMF respectively. Hydroxyl solvents also have been investigated under the same reaction conditions but no crystals were produced which show the stronger coordination ability of carbonyl solvents compared to hydroxyl solvents. Solvent accessible pore volumes are found to differ by the solvent size in a series of isomorphic MOFs prepared from Cd(NO3)2·4H2O, isonicotinic acid (IN) and 4-tetrazole pyridine (TP) using three polar aprotic solvents DMI, NMP and DMA. Since the sizes of solvents are in the order of DMI > NMP > DMA, the pore volumes of the synthesized MOFs also reduced in the order [Cd3(IN)4(N3)2(DMI)2]·2DMI (29) > [Cd3(IN)4(N3)2(NMP)2]. 2NMP (28) > [Cd3(IN)4(N3)2 (DMA)2]·2DMA (27) and [Cd3(TP)4 (N3)2(DMI)2]·4DMI (32) > [Cd3(TP)4(N3)2(NMP)2]·4NMP.H2O (31) > [Cd3(TP)4(N3)2(DMA)2]. 4DMA. H2O (30) (He et al., 2014a) (Scheme 5).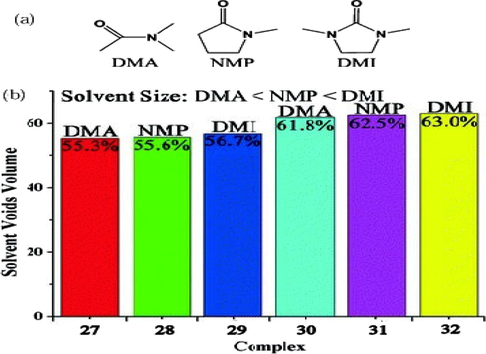
(a) The solvents used in the study of (27)-(32). (b) The solvent void volumes of (27)-(32) varying with the sizes of the solvent molecules.
Reprinted with permission from reference, He et al. (2014a). Copyright (2014) © Royal Society of Chemistry.
A similar size-controlled strategy is observed in three 3D MOFs obtained by the combination of MnCl2·4H2O and (1,1′,4′,1″- terphenyl)-2′,4,4″,5′-tetracarboxylic acid (H4TPTC) in three differently sized solvents NMP (N-methyl-2-pyrrolidine), DMA and DMF under similar reaction conditions. It is observed that the sizes of the channels of three MOFs 35.37 Å for [Mn2(TPTC)(H2O)(NMP)]NMP (33), 35.17 Å for [Mn2(TPTC) (H2O)(DMA)].DMA (34), and 33.11 Å for [Mn2(TPTC) (DMF)2] (35) have changed with respect to the decrease in sizes of solvent molecules (NMP > DMA > DMF) (Zhou et al., 2013).
Template effect of different solvents may generate new structures with different pore size, shape and even connectivity because these solvent guest molecules behave like structure directing agents during the self-assembly process (Biswas et al., 2009; Chaudhari et al., 2013; Li et al., 2013a). One anionic framework using DMF and two neutral frameworks using DEF and PrOH (Isopropanol) has been synthesized by Susumu Kitawaga and Sujit K.Ghosh under the same reaction conditions with same ligand and metal ion (Ghosh and Kitagawa, 2008). Although three frameworks were constructed by Cd-tris(2-carboxyethyl)isocyanurate (TCIH3) chains connected by the tripodal arms of the ligand, the products were different. It shows that the solvent molecules as guests sometime behave as structure directing agents. Chongyu Zuo’s group had systematically studied on change in inorganic building units of Mg-4,4′,4″-benzene-1,3,5-triyl-tribenzoate system to elucidate the role of different solvent system (Zuo et al., 2015). Using DMF/H2O (5:0.7) mixture (NH2Me2)2[Mg2(BTB)4/3 (H2O)2]·7DMF·4H2O (36) could be obtained in which a dinuclear unit of Mg2 was present. Incorporating DMSO in the solvent system (NH2Me2)2[Mg6O3(BTB)8/3(H2O)4]·15DMSO·5H2O (37) has emerged as product with hexanuclear block Mg6O3. Although the two complexes have been crystallized in a cubic space group with same framework structure and shape of pores, they are differentiated by Mg-containing nodes as Mg2 and Mg6 units. The difference in complexes relies on the template reagent roles of DMF and DMSO.
Sometimes crystal to crystal transformation can be possible by desolvation and resolvation process (Zhang et al., 2014; Wang et al., 2013c; Park et al., 2010). MOFs that cannot be obtained through conventional methods can be prepared through a crystal to crystal transformation. Reactions performed by Yao-Yu Wang and group yielded two interpenetrated 3D microporous MOFs based on 3-(2′,5′-dicarboxyphenyl)pyridine, 1,2-di(4-pyridyl)ethylene and Zn(NO3)36H2O by simply changing the reaction solvents. The two frameworks, {[Zn(dcpy) (bpe)0.5]. 3H2O} (38) formed by water as solvent and {[Zn(dcpy)(bpe)0.5 (CH3OH)0.5(H2O)0.5}(39) formed by mixed solvents DMF/CH3OH/H2O are isomeric and both show 2-nodal (3,4)-connected networks (Liu et al., 2012a). The network (38) can be reversibly transformed by dehydration and it shows a high uptake of H2 over N2 at 77 K and CO2 over N2 at 293 K, while the framework (39) irreversibly transformed into a new phase after dehydration which possess only surface adsorption. When this new phase is immersed in initial reaction solvents another phase is formed. Thus, they could prepare two MOFs having same topology, but with different properties. A solvent mediated anion-induced single crystal to single crystal is observed in Ag-4,4′-bis(1,2,4-triazole) system when monometallic cationic MOF Ag2(btr)2·2ClO4·3H2O (40) is immersed in aqueous KPF6–K2Cr2O7 and NaBF4–K2Cr2O7 solutions. Here interaction of ions with the anions in the solution due to the reversible nature of metal coordination in the precursor MOF has contributed in crystal to crystal structural transformations (Li et al., 2014b). An interesting example showing the role of solvents in crystal to crystal transformation is studied in Zn-Benzene tricarboxylate system. When a single crystal of [Zn2(μ2-OH2)(HBTRI)(BTRI) (H2O)2]. DMA·3H2O (41) is submerged in the solution of Gd(NO3)3 in 1:1 DMF/H2O solvent system a compound [Gd(BTRI)(H2O)6] (42) was obtained by transformation from (41) to (42) over time. The transformation, that is clear from the hot stage microscopy study, is probably related to the dehydration and rehydration occurred in the presence of solvent and vapour present in the system (Davies et al., 2012).
It has been demonstrated that quality of solvent is important in synthesizing coordination frameworks by carboxylic acids. Sometimes these solvents may undergo hydrolysis in the air and form counter cations that would play a templating role in the framework formation. For example diethyl ammonium cation (NH2Et2+) and dimethyl ammonium cation (NH2Me2+) were present in reactions carried out by Andrew D. Burrows and coworkers when they used DEF and DMF that had been kept in the laboratory for several weeks (Burrows et al., 2005; Cai et al., 2012). They observed the same result when they added (NH2Et2)Cl and (NH2Me2)Cl along with fresh DEF and DMF to reactions under the same conditions as in former reaction. From this we can understand the sensitivity of reactants to the purity of solvents used in MOF synthesis.
3.2 Effect of pH
It is well known that the crystallization and growth of inorganic–organic hybrid materials are highly influenced by the acidity/basicity of the reaction media. The deprotonation extent of an organic ligand and sometimes generation of an OH-ligand in aqueous solution with respect to the pH of the reaction media will favour the connectivity of polycarboxylate ligand to metal ion on the basis of acid-base concept. Some interesting works regarding the influence of pH value on MOF synthesis have been studied by many research groups (Luo et al., 2013; Chen et al., 2011; Gu et al., 2012; Fang et al., 2010).pH value of the reaction, as one of the external stimuli, has a remarkable influence on determining the coordination modes of carboxylic acid ligands involved in the synthesis of MOFs. For example recently H.M. Hu and his group synthesized two MOFs using CdCl2·2.5H2O, 4-carboxy-4,2′,6′,4″-terpyridine and oxalic acid (CTPY) by changing the pH values (Yuan et al., 2013). Frameworks of the formula [Cd2(CTPY)4]n·2nH2O] (43) and [Cd2(CTPY)2(ox)]n (44) were obtained at pH 7.5 and 5.5 respectively. It is clear that oxalate does not appear in (43). Here, depending on pH CTPY adopts different coordination modes. The participation of oxalic acid as template agent also depends on pH. Oxalic acid may act as a medium for the formation of (43) at higher pH value, 7.5 leading to 3D interpenetrating framework, while it is coordinated with Cd(II) atom in (44) at lower pH value, 5.5. Hence higher pH values have favoured the formation of interpenetrating networks and lower pH value tends to form a simple uninterpenetrated 3D framework.
Systematic investigation of the coordination ability of the flexible ligand, 1,3-adamantanediacetic acid (H2ADA) shows that the various coordination preferences are mainly governed by the pH value of the solution (Zhang et al., 2010b). Three neutral polymeric complexes, [Co(HADA)2(bpp)]n (45), {[Co(ADA)(bpp)(CH3OH)]H2O}n (46) and [Co2(ADA)2(bpp)]n (47) with different composition and dimensionality were formed at pH values 5,6 and 7 respectively. Similar effect had been reported by F.J. Meng et al. They synthesized two 3D cadmium frameworks by pyridine-2,4- dicarboxylic acid (2,4-PYDC) namely [Cd3(OH)2(2,4-PYDC)2] (48) (at pH 8.5) and {[Cd2(2,4-PYDC)2(bde)]·2H2O} (49) (at pH 4.5) (Meng et al., 2012). Out of nineteen distinct coordination modes, μ5-coordination mode of 2,4-PYDC ligand present in [Cd3(OH)2(2,4-PYDC)2] and μ3 and μ4 coordination mode in {[Cd2(2,4-PYDC)2(bde)]·2H2O} were observed for the first time. Four coordination polymers {[Cu(tza)2(Htza)2]·2H2O}n (50), [Cu(tza)2]n (51),{[Cu4(tza)4(CH3COO)2(μ3-OH)2(H2O)2]·2H2O}n (52), {[Cu4(tza)6(μ3-OH)2]·4H2O}n (53) formed by Cu(II) ions and multifunctional tetrazole-1-acetic acid showed the partial deprotonation of the ligand at pH value 1.5 in (50). A comparatively higher pH value of 2.5 in (51) leads to full deprotonation of the ligand and when the pH was increased to 4.0 and 5.0 in (52) and (53) there was full deprotonation as well as the coordination of some hydroxylgroup to the Cu(II) ions (Yu et al., 2008).
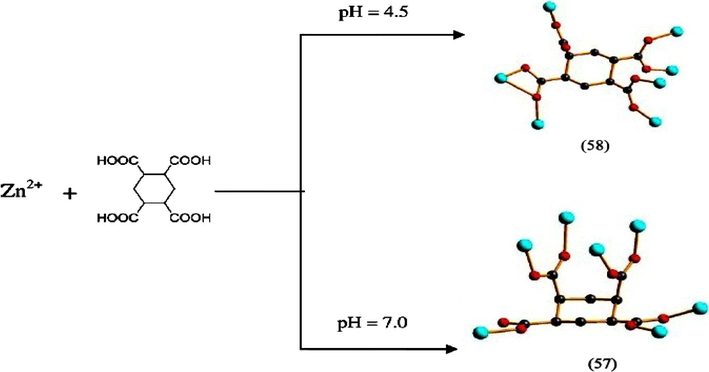
Colour of the compound was also found to depend on the pH value. Three differently coloured Co-BTC-3,3′,5,5′-tetra(1H-imidazole-1-yl)-1,1′-biphenyl(L) compounds were synthesized by tuning the pH value. Pink coloured crystals of {[Co(L)(HBTC)2(μ2-H2O)(H2O)2]·3H2O} (54) at pH = 5, purple coloured crystals of {[Co3(L)2(BTC)2]·4H2O} (55) at pH = 7 and brown coloured crystals of {[Co2(L)(BTC)(μ2-OH)(H2O)2]·2H2O}(56) by adjusting the pH to 9 were obtained. BTC was partially deprotonated at pH = 5 and fully deprotonated at both pH = 7 & 9 (Fig. 3). Here the coordination mode of BTC ligand along with the colour of the compounds is found to be tuned by the pH value of the reaction system (Luo et al., 2013). B. Liu et al., through their work illustrated the effect of pH on the different conformations of a flexible ligand, cyclohexane-1,2,4,5-tetracarboxylic acid (H4CTA) on reaction with Zn(II) metal ion. Coordination polymers formulated as {Zn4(μ7-CTA)(μ3-OH) (μ2-OH)3(H2O)2]n2nH2O} (57) and [Zn2(μ7-CTA)(H2O)3]n (58) were the products when analogous reactions were carried out at pH 7 and 4.5 respectively. Out of four possible conformations of H4CTA, in (57) the ligand adopts (a,e,e.a) conformation and in (58) the ligand conformation was transformed to a more stable (e,a,e,e) type when the pH is changed to 4.5. The final structure has also been varied from 2D network for (57) to 3D framework for (58) (Liu et al., 2011a) (Fig. 4).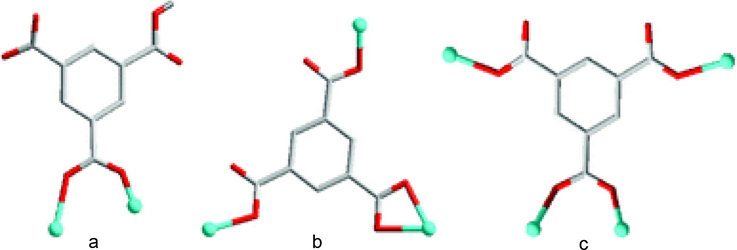
(a) The coordination mode of HBTC2− in (54). (b) The coordination mode of BTC3− in (55). (c) The coordination mode of BTC3− in (56).
Reprinted with permission from reference, Luo et al., 2013. Copyright (2013) © Royal Society of Chemistry.
Synthetic conditions for different conformations and coordination modes of the CTA ligand in (57)-(58).
Reprinted with permission from reference, Liu et al., 2011a. Copyright (2010) © Elsevier B.V.
W.Y. Sun and co-workers proposed that high pH value of the reaction would lead to higher dimensionality (Chu et al., 2008). They synthesized four new coordination polymers namely [Cd(HL)(H2O)] (59), [Co(HL)(H2O)] (60), [Cd(HL)(H2O)4] (61) and {[Cd3(L)2 (H2O)9]·7H2O} (62) under different reaction pH. The ligand N-(3-carboxyphenyl) iminodiacetic (HL) acid is only partially deprotonated in first three complexes prepared at low pH(<7) whereas in (62) all the carboxylate groups have deprotonated and exhibited a 2D network structure at pH 7 which leads to a more complicated network. The investigation on the influence of pH by Rong-Guang Lin et al. also revealed that the higher the pH value of the reaction, the higher would be the dimension of the complex (Lin et al., 2007). The reaction of multifunctional supramolecular synthon, 4-sulfocalixarene with manganese in which the pH of the solution increased by NH3 solution, one dimensional ({H[(C28H20O16S4)Mn(H2O)4 Mn0.5(H2O)2]}n·6nH2O (63) at pH 2 and {NH4[(C28H20 O16S4)Mn(H2O)4Mn0.5(H2O)2]}n·5nH2O (64) at pH 4 to two dimensional ([(C28H20O16S4)Mn2(H2O)8]n·6nH2O (65) at pH 5 structures were formed. It was a failure when they tried to obtain (64) through transformation of (63) although both having 1D structure. They found that failure in the transformation of one complex to another also is the proof for pH dependence. Increase in dimensionality from 0D to 2D with increase in pH is observed in Cu(II)/benzoate/4,4′-bipyridine complexes. pH value was found to have a pronounced effect on the ratio of 4,4′-bipyridine to benzoate. For example, 0D [Cu(H2O)(benzoate)2(4,4′-bipyridine)2)(benzoic acid)2(4,4′-bipy)] (66) at pH = 5.5, [Cu2(H2O)2(benzoate)4(4,4′-bipyridine)3](H2O)9] (67) and [Cu2(benzoate)4 (4,4′ bipyridine)3] (68) at pH = 6, 1D chain of {[Cu3(H2O)4(benzoate)6 (4,4′-bipyridine)4.5].(4,4′-bipy)(H2O)5} (69) at pH = 7.5 and 2D layer structure of [Cu3(OH)2(H2O)2(benzoate)4(4,4′-bipy)2] (70) at pH = 8 were obtained (Fig. 5). The results of crystal structure analysis show that there is a significant increase in 4,4′-bipyridine to benzoate ratio (from 1:1 to 1:2) when the pH is increased and the tendency of 4,4′-bipyridine to act as a bridge between metal and ligand increased at higher pH value (Wu et al., 2007). pH of hydrothermal reaction of Zn(NO3)2·6H2O and biphenyl-3,3′,4,4′-tetracarboxylic acid with bipyridyl ligand was adjusted by adding NaOH in order to get different bilayer architectures [Zn(bptc)0.5(H2O)]n (71), {[Zn(bptc)0.5(4,4′-bpy)0.5(H2O)]·1.5H2O}n (72), {[Zn2(H2bptc)2(4,4′-bpy)4]. H2O}n (73), [Zn(H2bptc) (bpe)]n (74) (Wang et al., 2007). The polymer (71) with the 2D bilayer network pillared by (bptc)4-anions and (72) with 2D supramolecular bilayer network by H-bonding interactions were formed at same pH value of 7 but with different concentration of 4,4′-bipyridine. At pH 5, (73) with the 2D supramolecular bilayer network by combination of two 1D structures and (74) with 2D bilayer framework connected by (H2bptc)2− anions were formed. Here coligand has also a profound effect along with pH value. A series of Cd coordination polymers had been obtained by the combination of a mixed ligand system, 3,3′,4,4′-diphenylsulfonetetracarboxylic acid (H4dpstc) and 1,10-phenanthroline (phen) (Zhang et al., 2013b). Firstly, at a neutral pH condition (pH = 7) of the mixture, the resultant MOF manifests a 0D binuclear molecule due to the partially deprotonated H2dpstc2− anions formed. The increase of pH value to 8, 10 and greater than 12 resulted in the structure variation from 1D chain to two types of 2D layers. When estimated, the pKa values of the each carboxylic group have shown to be different which affect the extend of deprotonation under different pH conditions. When pH is greater than 12, due to the precipitation of metal salt to M(OH)2, which is hard to participate in self assembly process, low dimensional structure was obtained.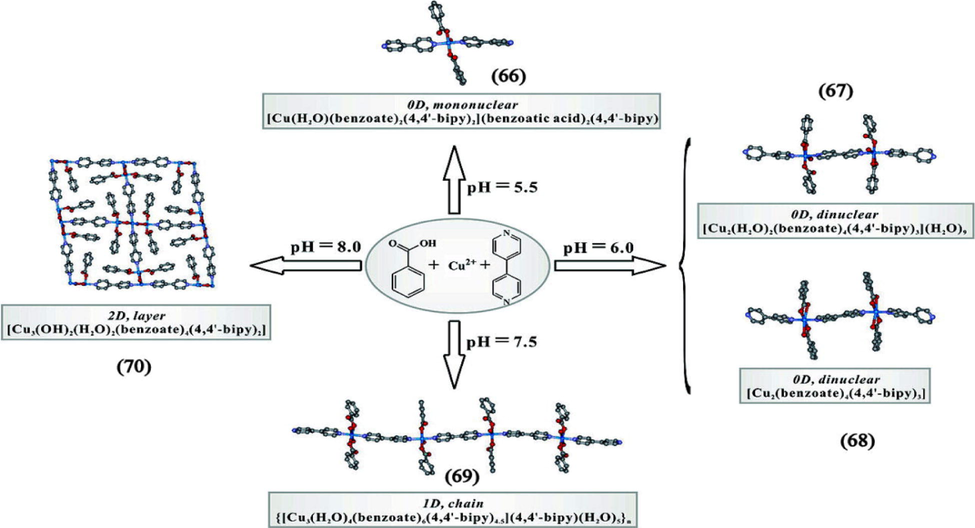
Systematic illustration of the assemble tendency from (66)-(70) under different pH Values.
Reprinted with permission from reference, Wu et al., 2007. Copyright (2007) © American Chemical Society.
Z.M. Su and coworkers, through their work demonstrated that different phases were formed during the crystallization process by base induced heteronucleation. Heating the solution of Zn(NO3)2·4H2O and H3BTC in the presence of DMA with different molar masses of NaOH led to three different crystalline phases (Wang et al., 2013b). Here NaOH acts as the medium for changing pH. It not only creates a basic environment which favours the deprotonation but takes part in the coordination also. In the two phases the presence of OH-ions can be observed. Among the three phases one has been used to explore drug release due to the inherent porosity of its cages. A 2D threefold interpenetrated network, {[Cu3(BTC)2(H2O)3(NH3)4]n·2nH2O} (75) at pH 8.3 and a 2D bilayer framework, [Cu4(BTC)2(OH)2 (H2O)2(NH3)4]n (76) at pH 9.2 had been synthesized by Chen et al., 2007. Unlike the difference in structures due to deprotonation of the organic ligand at various pH, here the structural diversity of the frameworks has been achieved by a hydroxyl group in (76) at a different pH conditions. The structural diversity is due to the changes caused in the Cu to BTC ratio in the structures when an hydroxyl group is introduced as a result of increase in pH.
Sometimes supramolecular isomers (same composition and building blocks, but with dissimilar architectures) could be obtained at different pH values of the reaction mixture. For example, the reaction of 5-methyl isophthalic acid (H2moip) and 1,3-bis(1,2,4-triazoi-1-yl)propane coligands with Zn(II) ion generated two supramolecular isomers {α[Zn2(moip)2(btp)(H2O)]·2H2O} (77) having 2D bilayer structure and a 3D polythreaded network, β-{[Zn2(moip)2(btp)(H2O)2]·2H2O} (78), at different pH values (Han et al., 2014). Z. Guo et al. reported that it would be more difficult to have coordination of water with metal ion at high pH values (Guo et al., 2007). The three coordination polymers obtained when Cd(NO3)2·4H2O is reacted with 1H-benzimidazole-5-carboxylic acid (Hbic) were 2D {[Cd(Hbic)2.(H2O)2]2H2O}n (79) at pH = 5.0, [Cd(Hbidc)2]n (80) with 44 rhombus network structure at pH = 6.5 and a 3D [Cd(Hbidc)2.(H2O)]n (81) at pH = 7.2. The effect of choice of pH on water coordination has been clearly reflected in the structures obtained.
3.3 Effect of temperature
In addition to the compositional parameters such as pH, solvents and molar ratio of starting materials, process parameters such as temperature, pressure and time are also key parameters that need to be analysed when developing synthetic procedures for the preparation of metal organic frameworks. Hydrothermal synthesis of MOFs offers a number of advantages over other conventional and non-conventional synthetic methods. As the reaction is carried out in a closed system at temperature above 100 °C and pressures above 1 atm, the probability of crystallization would be higher because of the high solubility of reactants and large crystals of high quality would be obtained in hydrothermal synthesis (Wang et al., 2015; Zhang et al., 2013a; Yang et al., 2013b). Hydrothermal synthesis is also necessary when the ligands used are not completely soluble in ordinary conditions.
A number of works have been reported where succinic acid is used as the ligand in order to find out the importance of hydrothermal method in MOF synthesis (Livage et al., 2001). Succinic acid is widely reported in comparing hydrothermal and non-hydrothermal synthesis of MOFs because of their flexibility and various modes of coordination under different temperature conditions. A comparative study was carried out using succinic acid ligand and holmium metal. G.E. Narda and his group prepared two HoIII succinate coordination polymers namely {[Ho2(C4H4O4)3 (H2O)4] 6H2O} (82) and {[Ho2(C4H4O4)3 (H2O)2]. H2O} (83) on mild and hydrothermal conditions respectively. The compounds differ in their dimensionality which in turn affected thermal stability and magnetic properties. The thermal stability of the framework formed under hydrothermal reaction is found to be high compared to the framework (82) formed under room temperature, which shows that the hydrothermal method is favourable for the formation of stable open frameworks with empty channels (Bernini et al., 2007). The compound formed by hydrothermal method has led to the formation of inorganic networks with open channels instead of polymeric layers in (82) formed under mild conditions. The reaction of Zn (II) ion and a new 5-substituted benzene-1,3-dicarboxylic ligand, 5-iodoisophthalic acid(H2IIP) in both hydrothermal and room temperature synthesis yielded a 2D (4,4′)- network and interpenetrated 3D structures. As the thermal energy is directly proportional to temperature, thermodynamically favoured products at high temperature and kinetically favoured products at low temperatures would be obtained as different conformers (Zhang et al., 2012). According to the works done, it is clear that hydrothermal conditions are more favourable for obtaining denser, less hydrated and higher dimensional solids with extended M-O-M networks and better thermal stability.
There are also examples of MOFs obtained from succinate ligand under different temperature conditions because of their flexibility and various modes of coordination. In 2013 S.A. Junior and coworkers synthesized Tm- Succinate in aqueous solution under two different temperatures (de Oliveira et al., 2013). The reaction of TmCl3·6H2O with succinic acid in aqueous solution at 100 °C gave a 3D MOF {[Tm2(L)3(H2O)].H2O} (84). Whether the same reaction carried out at 180 °C adopted again a 3D MOF with same formula. Even though they obtained two compounds with same empirical formula and dimensionality they are crystallized in two different space groups (Fig. 6). Here the coordination of succinate with the metal ion is in two distinct manner, i.e. the succinate anion adopts gauche and anti-conformations under two different temperature conditions. So controlling the experimental temperature enabled them to obtain monoclinic or triclinic structures by influencing the crystalline growth of both compounds. This was the first time report of two lanthanide succinate with same empirical formula but with different space groups.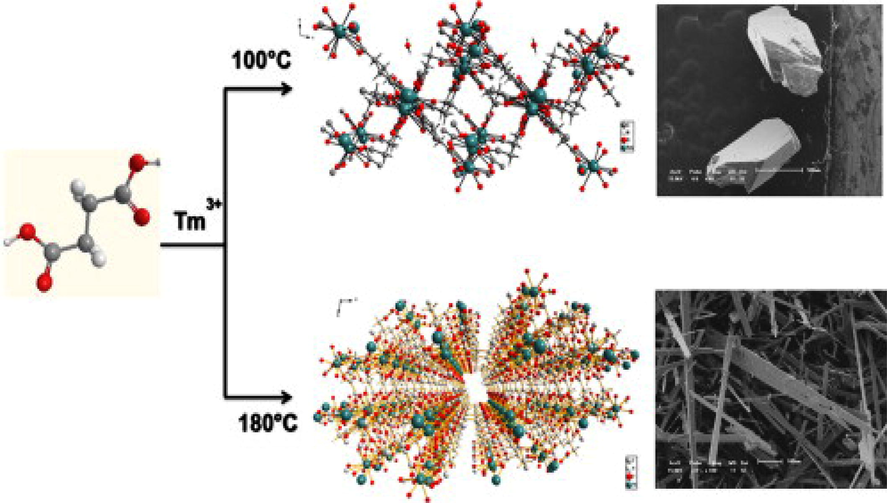
Two Tm-succinates obtained under different temperature conditions.
Reprinted with permission from reference, de Oliveira et al., 2013. Copyright (2013) © Elsevier Inc.
Another interesting example where the coordination polymers exhibiting supramolecular isomerism based on temperature is given by Kanoo et al. Four Ni coordination polymers under room temperature and hydrothermal conditions were isolated as supramolecular isomers namely {[Ni(bipy)(H2O)4](2,6-NDS)·4H2O} (85) at room temperature, {[Ni(bipy)(H2O)4](2,6-nds)·2H2O} (86) at 140 °C, {[Ni(bipy) (H2O)4](2,6-nds)} (87) at 120 °C and {[Ni(bipy)(H2O)4](2,6-NDS)·2H2O} (88) at 100 °C (2,6-NDS = naphthalene disulphonate, bipy = 4,4′-bipyridyl) (Kanoo et al., 2009). These four coordination polymers are stable porous structures with the same basic building unit, [Ni(bipy)(H2O)4]2+ coordinated with NDS ligands forming different orientations of Ni-bipyridine chains and the ligand through non-covalent interactions. (Fig. 7).![The arrangement of 1D [Ni(bipy)(H2O)4]2+chains (pink colour) and 2,6-nds counter anions (green colour) in supramolecular isomers (85)-(88) viewed along the crystallographic a-axis.](/content/184/2019/12/3/img/10.1016_j.arabjc.2016.01.003-fig12.png)
The arrangement of 1D [Ni(bipy)(H2O)4]2+chains (pink colour) and 2,6-nds counter anions (green colour) in supramolecular isomers (85)-(88) viewed along the crystallographic a-axis.
Reprinted with permission from reference, Kanoo et al., 2009. Copyright (2009)© American Chemical Society.
A detailed study was carried out by Vivek Polshettiwar and group to examine the influence of reaction temperature and time on size, shape and morphology of MOF microcrystals formed by microwave synthesis (Sarawade et al., 2013). The SEM images of MOFs formed indicate that layer-by-layer growth from small primary nanosheets has changed to the layered microrods as the temperature increased from 120 to 160 °C. Further increase in temperature leads to continuous growth of micro crystals.
Recently urothermal synthesis, that uses urea as the derivatives has been identified as an effective method for developing MOFs with promising applications (Yang et al., 2011; 2013a; Liu et al., 2011b; Zhang et al., 2010a). The influence of temperature in urothermal synthesis in MOF was reported by Hou et al. (2013). They obtained two differently structured MOFs, [La2(NDC)3(urea)3] (89) at 120 °C and [La(NDC)1.5(e-urea)] (90) at 140 °C (H2NDC = 2,6-naphthalene dicarboxylic acid; e-urea = ethylene urea). The difference in coordination numbers of the La atoms in two compounds is mainly attributed to the reaction temperature, although they are formed from same reactants. The compound synthesized under high temperature has high thermal stability compared to synthesized under low reaction temperature. There is H-bonding interaction between ethylene urea ligands and carboxylate groups of NDC shows different coordination modes of e-urea in the two compounds. Thermal and magnetic properties of two cobalt coordination polymers, [Co2(H2O)4(HBIDC)2]n (91) and [Co(HBIDC)] (92) (HBIDC = 1H-benzimidazole-5,6-dicarboxylate), are found to be different as they are prepared at different temperatures (160 °C and 220 °C) from same metal ion and organic ligand (Wei et al., 2009). The polymer (92) exhibits high thermal stability and anti ferro-magnetic property compared to ferromagnetic (91). The high thermal stability of (92) refers to the water free and compact 2D polymeric network and the ferromagnetic nature of (91) is due to the coupling between two CoII ions as a result of the short Co---Co distance (3.114 Å) and a small Co–O–Co angle.
Ligand adopting various coordination modes under different temperature condition was also illustrated by S.M. Zhang et al. The reaction between CuI and 2,3-bis(ter-butyl thiomethyl) quinoxaline resulted in two complexes [(Cu(L)I]2 (93) and {[Cu2(L)I2][CH3CN]3}∞ (94) having two different architectures prepared at room temperature and 0 °C respectively. The same procedure was followed by using the ligand 2,3-bis(n- propylthiomethyl)quinoxaline. Then also two different complexes [Cu2(L)I2]∞ (95) and {[Cu(L)I][CH3CN]}∞ (96) were obtained (Fig. 8). But the acetonitrile solvent molecules are incorporated only for complexes obtained at 0 °C. The ligand and acetonitrile solvent forms C-H---N and N-H---N hydrogen bonding in complexes (94) and (96) stabilize the orientation of terminal groups in ligands (Zhang et al., 2009).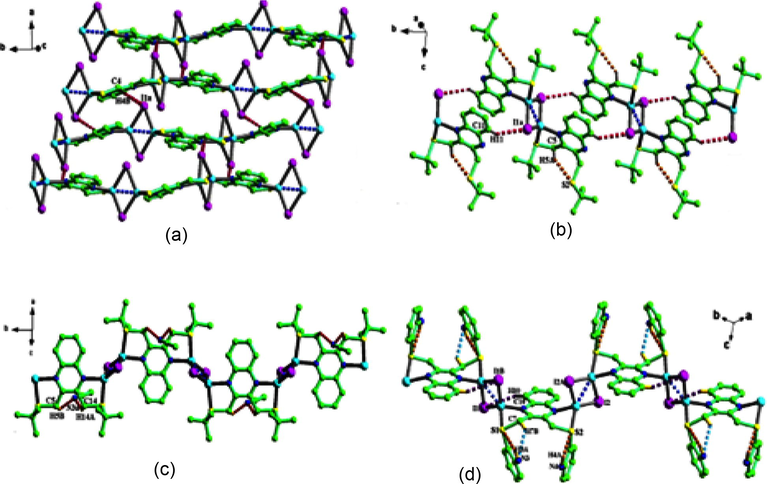
(a) 2D H-bonded supramolecular network of (93) connected by the C–H⋯I weak interaction, (b) the 1D H-bonded supramolecular chain of (94) connected by the C–H⋯I weak interaction, (c) the 1D chain structure of (95) including C–H⋯N hydrogen bond, (d) the 1D chain structure of (96) including intramolecular hydrogen bonds.
Reprinted with permission from reference, Zhang et al., 2009. Copyright (2010) © Elsevier B.V.
Hydrothermal synthesis is found to be an effective method for the construction of high dimensional MOFs. H.J. Mo et al. examined the reaction of Co(NO3)2.6H2O with a multidentate ligand 2,2′-bibenzimidazole (H2bbim) in the presence of 4-cyanopyridine under hydrothermal conditions using temperature as the only independent variable where the starting mixtures and molar ratio of the synthesis are the same (Mo et al., 2009). When the temperature is 130 °C the ligand had monoprotonated and a 0D complex {[Co(Hbbim)3].H2O} (97) is obtained. In order to get the higher dimensional MOFs they changed the reaction temperatures as 140 °C, 150 °C and 160 °C. At 150 °C four different types of crystals were obtained where the main product is [Co2(Hbbim)(bbim)3(ina)2(μ3-OH)]n (98) in which the ligand is found to be multi coordinated. The results encouraged them to observe the reaction at a range of temperatures from 160 °C to 200 °C. The higher dimensional MOFs were the products which indicated the easier deprotonation of the ligand at higher temperatures. 4-cyanopyridine is also found to be participating in coordination at higher temperatures.
A series of lanthanide coordination polymers using imidazole-4,5-dicarboxylic acid were synthesized by R. Q. Fan and group. Several of the obtained frameworks are temperature dependent. They found that high dimensional coordination polymers were obtained at high temperature compared to low temperature (Han et al., 2014). The self assembly of Zn(II) ion with 5-methyl isophthalic acid and 1,4-bis-(1,2,4-triazol-1-yl) butane as coligand under hydrothermal conditions at different temperature produced two dissimilar coordination polymers, {[(Zn(mip)(btb)]2H2O} (99) at 120 °C and [Zn2(mip)2(btb)1.5] (100) at 140 °C in which (100) displays an unusual (6.82)(6.82.10)(6.85) topology (Mahata et al., 2007). Three new manganese oxy-bis(benzoate) compounds {[Mn(H2O)3(C12H8O) (COO)2]H2O} (101), {[Mn(H2O) (C12H8O(COO)2]} (102) had been produced as pure phases by increasing the temperature (Fig. 9). Removal of some aqua molecules at higher temperature led to the formation of higher dimensional structure {Mn(OH)}2{C12H8O (COO)2}] (103). This is regarded as a thermodynamically entropy driven dehydration route which intern shortened the Mn-Mn distances in the framework. The dimensionality of the structures is found to be a pseudo 2D for (103) and two 2D layer structures for (102) and (103). The conversion of structures with highly hydrated one at low temperature to a metal hydroxide structure at higher temperature is the clear evidence of the influence of classical thermodynamic factors at higher temperature. Crystal growth under thermodynamic control is also evidenced in the reaction between Cu(II) ions and 1,5-bis(5-tetrazolo)-3-oxapentane (H3btz). Cu2+ and btz2− ligand adopt a μ3− bridging mode in {CuII(btz)]·0.5H2O}n (104) synthesized between 80 and 140 °C. When the heating temperature is between 160 and 180 °C, btz2− was connected to Cu2+ and Cu+ ions in μ4− bridging mode, leading to another product, CuIICuI2(btz)2]n (105). But at a temperature of 150 °C these two products are in coexistence and the transformation from (104) to (105) is found to be possible at 170 °C (Cui et al., 2012).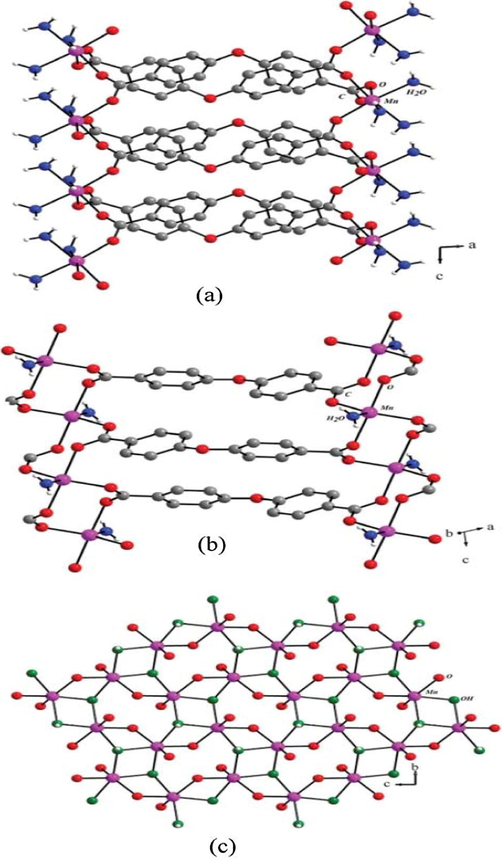
(a) Structure of (101) in the ac plane; (b) the two-dimensional structure of (102); (c) view of the two-dimensional layer of (103).
Reprinted with permission from reference, Mahata et al., 2007. Copyright (2007) © Royal Society of Chemistry.
Most of the synthesis suggests that high temperature favours higher dimensionality, but it is not always so. For example, the combination of Nickel acetate and 1,4-cyclohexanedicarboxylate (CDC) in the presence of cyclohexanol and water solvents led to 3D {Ni3(OH)2(CDC)2(H2O)4]·4H2O} (107) at 140 °C and 2D {[Ni6(OH)6(CDC)3(H2O)6]·2H2O} (106) at 170 °C (Scheme 6) (Chen et al., 2006). Another example, which shows the non-agreement of dimensionality with temperature is three 3D homochiral cadmium camphorates synthesized from Cd(NO3)2.4H2O and D-camphoric acid (D-H2Cam) at 25 °C, 140 °C and 180 °C (Zhang and Bu, 2008) (Scheme 7). The only changes in the structures are the degree of hydration controlled by temperature.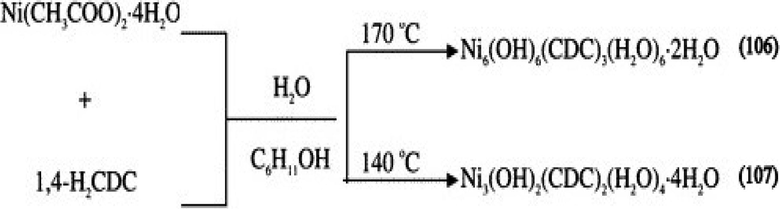
Assembly of Ni(CH3COO)2·4H2O and 1,4-H2CDC at different temperatures in (106)-(107).
Reprinted with permission from reference, Chen et al. (2006). Copyright (2006) © American Chemical Society.
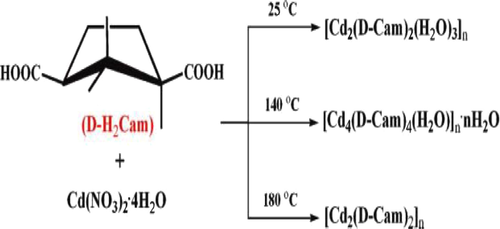
Synthesis conditions for the three Cd-Camphor compounds.
Reprinted with permission from reference, Zhang and Bu (2008). Copyright (2007) © Royal Society of Chemistry.
3.4 Effect of molar ratio of reactants
Molar ratio of the reactants is also important factor for the synthesis of MOFs since the topological pattern of MOFs depends on the stoichiometry of reactants. Luan et al. developed a facile route for the synthesis of Cu based coordination polymers with interlaced triple-stranded molecular braid architectures at controlled ligand to metal molar ratio. Three coordination polymers namely {[Cu4(bpp)4(maa)8(H2O)2]n·2nH2O} (108) {[Cu3(maa)6(bpp)2]n}(109), {[Cu2(maa)4(bpp)]n} (110) (where bpp = 1,3-bis(4-pyridyl)propane, Hmaa = 2-methylacrylic acid), were obtained by incorporating different molar ratios of bridging bpp ligand. Among these, (108) was synthesized with a bpp: [Cu (maa)4. 2H2O] stoichiometry of 2:1 in a mixed methanol acetonitrile solution, which contain a neutral, interlaced, triple-stranded molecular braid linked by three single-stranded meso-helical chains that contain only a mononuclear node. The compound (109) was isolated by changing the molar ratio of the reactants to 1.5:1, an interlaced 3D supramolecular structure containing 1D zigzag polymeric chain with both mono- and dinuclear nodes formed (Fig. 10). The compound (110) consists of 1D zigzag chains based on the dinuclear nodes with the parallel stacking fashion by decreasing the molar ratio to 1:1 (Luan et al., 2006).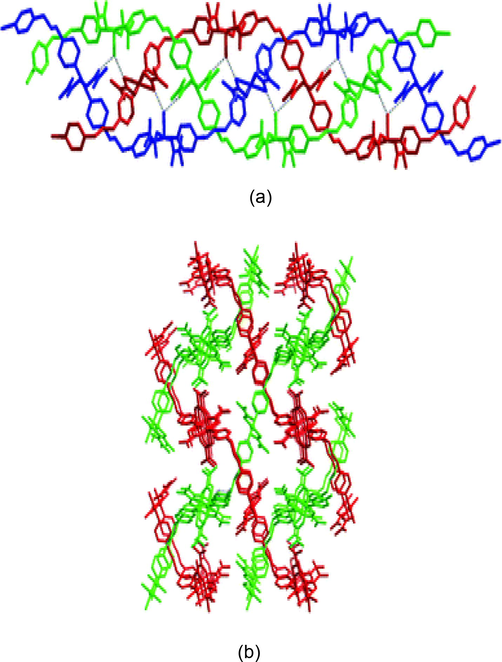
a) The perspective view of the triple-stranded molecular braid in (108), (b) interlaced 3D supramolecular structure containing 1D zigzag polymeric chain in (109).
Reprinted with permission from reference, Luan et al. (2006). Copyright (2006) @ Wiley-VCH Verlag GmbH &Co. KGaA.
The reactions of Mn (II) nitrate with the flexible ligand 1,4-bis(imidazol-1-ylmethyl)benzene (bix) in a molar ratio of 1: 3 in ethanol/chloroform giving rise to novel coordination framework, {[Mn2(1,4 bix)3(NO3)4]n2n.CHCl3} (111) which consists of 1D ladder like polymers and 2D brick wall networks resulted in unique 3D polycatenated motif. When the molar ratio was changed into 1: 1.5, the resulting compound {[Mn(1,4-bix)1.5(NO3)2]n} (112) consists of the 1D parallel molecular ladders (Carlucci et al., 2008).
Liu et al. have studied that how metal-to-ligand molar ratio influences the framework structure of final products assembled from Co (II) ions and flexible 1, 2-bis(tetrazol- 1-yl)ethane (btze) in the presence of thiocyanate ions. Two coordination polymers namely {[Co(btze)2(SCN)2]n} (113), {[Co(btze)3(SCN)2]n} (114) were obtained by incorporating the starting materials (Co(II)/btze) in a molar ratio of 1:1 and 1:2. (113) was obtained in a molar ratio of 1:1, that could give a 2D MOF because the short btze ligand gives singly bridged square grid layers with twofold parallel interpenetration (Fig. 11). The compound (114) consists of 1D linear chains with deep interdigitation to generate a 3D architecture by increasing the starting molar ratio of 1:2 or the excess btze ligand was used in the same reaction conditions (Liu et al., 2008).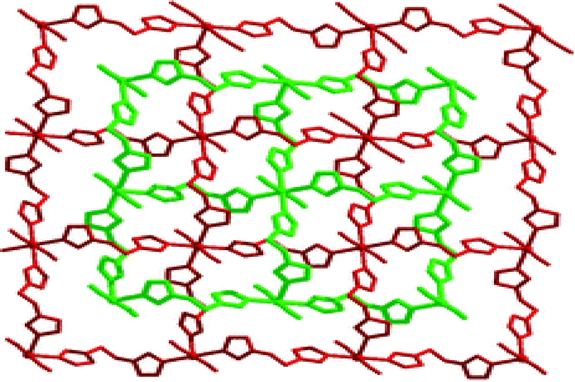
The twofold parallel interpenetration in 2D MOF (113).
Reprinted with permission from reference, Liu et al. (2008). Copyright (2008) © American Chemical Society.
To explore the molar ratio of metals and ligands affect the framework structure, Yin et al. synthesized two Cd compounds such as {[Cd3Cl3(TAZ)3(DMF)2]n} (115), {[CdCl2(TAZ)]n. n(H2O)} (116) based on 1,2,4 triazole ligand (TAZ). Among these, (115) displays 3D framework which was isolated in a molar ratio of metal/ligand 1:1.2, whereas in (116), one dimensional coordination polymer resulted where the molar ratio of metal/ligand was adjusted to 1:1.7. Comparing the structure of (116) with that of (115), the coordination number of TAZ decreased from 3 in (115) to 2 in (116). As a result, great structural change in framework from 1D to 3D was observed in (115), however they were synthesized from the direct reaction of Cd with TAZ by changing only the molar ratio of reactants (Scheme 8). The results indicated that they were molar ratio dependent compounds (Yin et al., 2011).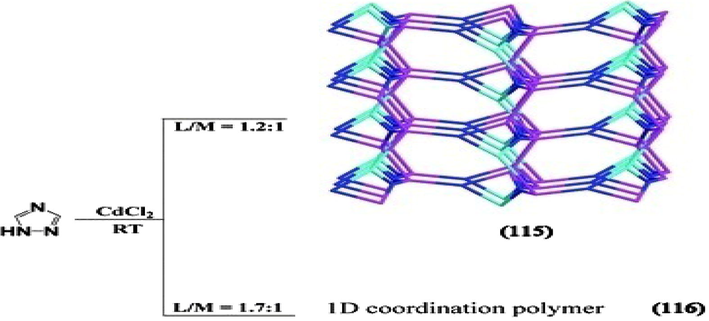
Schematic drawing of the synthesis and topologies in (115)-(116).
Wu et al. conducted a series of solvothermal reaction of zinc salts and 4,4′- (hexafluoroisopropylidene) bis(benzoic acid) (H2hfipbb) with different stoichiometry’s of 4,4′-dipyridylsulphide (dps) as co-ligands to explore the effect of ligand ratio on the final structure of MOFs. As a result, three different framework structures were produced, among these, [Zn(hfipbb)(H2hfipbb)0.5]n (117) which exhibited a 3D two fold parallel interpenetrating pillared network in a molar ratio of 1:1:0.25 (Zn/H2hfipbb/dps). Two isomeric networks [Zn2(hfipbb)2(dps)(H2O)]n (118 & 119) were also formed which exhibited non- interpenetrated 3D bimodal-connected network and 3D twofold interpenetrated bimodal-connected network respectively by adjusting the molar ratio of coligand (Wu et al., 2011) (Fig 12).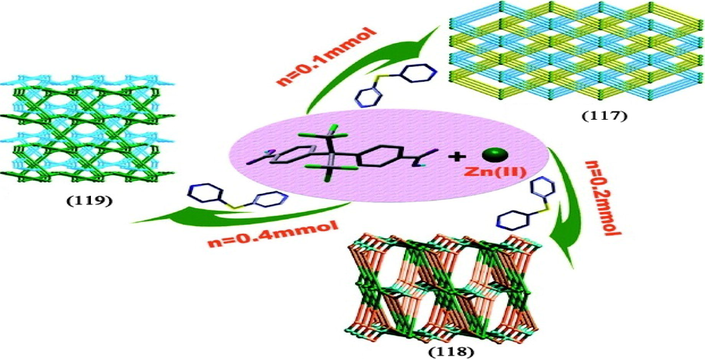
Different coordination polymers obtained by Zinc ion and H2hfipbb with different stoichiometry of 4,4′-dipyridyl sulphide as co-ligand in (117)-(119).
Reprinted with permission from reference, Wu et al. (2011). Copyright (2011) © American Chemical Society.
4 Conclusion and outlook
In this review, we have made an attempt to study the formation and structure of a series of MOFs/Coordination polymers. The structural differences when each parameter is varied imply that each reaction condition has great influence on the structure or dimensionality of metal organic frameworks. Literature study suggests that there is no universal law governing the choice of parameters for any MOF synthesis. The effect of parameters would vary depending on the reactants participating in the crystal formation. From the above mentioned examples for solvent effect, the impact of choice of solvents is clearly reflected in all the structures obtained. It is suggested that different solvent system may regulate the crystallization through dissimilar self-assembly process, i.e. specific reactants will be influenced by the solvents used in the reactions in an incompatible manner. A number of works suggest that the size of the carbonyl solvents has a significant role in the structural self-assembly process. The sizes of the channels are found to be increased with the increase in solvent sizes. Most of the work revealed that high pH value leads to coordination polymer with higher dimensionality and also different coordination modes would be obtained by ligands at different pH values. Like pH, temperature also acts as a structure directing factor by affecting the coordination mode of organic ligand and coordination number of the central metal ion. Thus multidimensional structures can be purposefully obtained by tuning the reaction temperature. In fact, hydrothermal synthesis is a straightforward and effective method for the synthesis of MOF crystals where the ligand is poorly soluble. Most of the structures show that hydrothermal conditions favour an increase in the overall dimensionality of these hybrids. Topological patterns of MOF could also be tuned by varying the molar ratio of reactants since it plays crucial role in directing the formation of various MOFs under the same reaction conditions.
Although both process and compositional parameters are known to have non-ignorable influences on final framework dimensionality, it is often hard to identify the proper role of solvents, temperature and pH all together in a synthesis. Compared to other conventional methods for the synthesis of porous metal organic frameworks, high throughput methods can be successfully applied to some extent for the systematic and efficient investigation and better understanding of the effect of both process and compositional parameters in a short period of time. A large amount of information can be obtained about the reaction trends in such rapid and systematic investigations. Anyways it is still a great challenge for the construction of the preferred structures in a truly deliberate manner as each factor has its own role in the synthesis of MOF structures.
Acknowledgements
We thank University Grants Commission (UGC) for funding. Our sincere thanks to all students in the research group of Prof. (Dr.) Suresh Mathew, School of Chemical Sciences (SCS) Mahatma Gandhi University, Kottayam, Kerala, India, for their kind support and encouragement during this work. We are indebted to those authors mentioned in the references who supported this work with the literature upon request.
References
- [Al4(OH)2(OCH3)4(H2N-bdc)3]⋅x H2O: A 12-connected porous metal-organic framework with an unprecedented aluminum-containing brick. Angew. Chem. Int. Ed.. 2009;48(28):5163-5166.
- [Google Scholar]
- Effect of the guest solvent molecules on preparation of different morphologies of ZnO nanomaterials from the [Zn2(1,4-bdc)2(dabco)] metal-organic framework. J. Coord. Chem.. 2011;64(20):3521-3530.
- [Google Scholar]
- Influence of the anion on the structure of bis(methylthio)methane supramolecular coordination complexes. Cryst. Growth Des.. 2006;6(12):2674-2685.
- [Google Scholar]
- Microwave-assisted large scale synthesis of lanthanide metal-organic frameworks (Ln-MOFs), having a preferred conformation and photoluminescence properties. Dalton Trans.. 2015;44(26):11954-11962.
- [Google Scholar]
- Synthesis and structural characterization of magnesium based coordination networks in different solvents. Cryst. Growth Des.. 2011;11(6):2572-2579.
- [Google Scholar]
- High-throughput assisted rationalization of the formation of metal organic frameworks in the iron(III) aminoterephthalate solvothermal system. Inorg. Chem.. 2008;47(17):7568-7576.
- [Google Scholar]
- The effect of hydrothermal and non-hydrothermal synthesis on the formation of holmium(III) succinate hydrate frameworks. Eur. J. Inorg. Chem.. 2007;2007(5):684-693.
- [Google Scholar]
- Sonochemical synthesis of a nano-structured zinc(II) amidic pillar metal-organic framework. Ultrason. Sonochem.. 2015;27:416-422.
- [Google Scholar]
- A cubic coordination framework constructed from benzobistriazolate ligands and zinc ions having selective gas sorption properties. Dalton Trans.. 2009;33:6487-6495.
- [Google Scholar]
- Reversible CO binding enables tunable CO/H2 and CO/N2 separations in metal-organic frameworks with exposed divalent metal cations. J. Am. Chem. Soc.. 2014;136(30):10752-10761.
- [Google Scholar]
- Hydrothermal synthesis, characterization and photoluminescent properties of the microporous metal organic frameworks with 1,3-propanediaminetetraacetate ligand and its auxiliary ligand. Inorg. Chim. Acta. 2015;428:170-175.
- [Google Scholar]
- Anion dependent structural diversity in cobalt(ii) complexes of 4,4[prime or minute]-bipyridine-N, N[prime or minute]-dioxide. CrystEngComm. 2005;7(112):674-681.
- [Google Scholar]
- Solvent-dependent cation exchange in metal-organic frameworks. Chem. – A Eur. J.. 2014;20(23):6871-6874.
- [Google Scholar]
- Syntheses, structures and properties of cadmium benzenedicarboxylate metal-organic frameworks. Dalton Trans.. 2008;18:2465-2474.
- [Google Scholar]
- Solvent hydrolysis and templating effects in the synthesis of metal-organic frameworks. CrystEngComm. 2005;7(89):548-550.
- [Google Scholar]
- The effect of reaction conditions on the nature of cadmium 1,3,5-benzenetricarboxylate metal-organic frameworks. Inorg. Chim. Acta. 2011;366(1):303-309.
- [Google Scholar]
- Construction of Ba(II) coordination polymers based on imidazole-based dicarboxylate ligands: structural diversity tuned by alcohol solvents. Cryst. Growth Des.. 2012;12(7):3575-3582.
- [Google Scholar]
- Temperature dependent structure formation and photoluminescence studies of a series of magnesium-based coordination networks. Inorg. Chim. Acta. 2013;394:452-458.
- [Google Scholar]
- A new polycatenated 3D array of interlaced 2D Brickwall layers and 1d molecular ladders in [Mn2(bix)3(NO3)4]n 2n.CHCl3 [bix=1,4-bis(imidazol-1-ylmethyl) benzene] that undergoes supramolecular isomerization upon guest removal. Cryst. Growth Des. 2008;8:162-165.
- [Google Scholar]
- Luminescent terbium-containing metal-organic framework films: new approaches for the electrochemical synthesis and application as detectors for explosives. Chem. Commun.. 2014;50(83):12545-12547.
- [Google Scholar]
- Sonochemical synthesis and characterization of submicrometer crystals of the metal-organic framework Cu[(hfipbb)(H2hfipbb)0.5] Cryst. Growth Des.. 2011;11(10):4505-4510.
- [Google Scholar]
- Bi-porous metal-organic framework with hydrophilic and hydrophobic channels: selective gas sorption and reversible iodine uptake studies. CrystEngComm. 2013;15(45):9465-9471.
- [Google Scholar]
- Polynuclear core-based nickel 1,4-cyclohexanedicarboxylate coordination polymers as temperature-dependent hydrothermal reaction products. Cryst. Growth Des.. 2006;6(3):664-668.
- [Google Scholar]
- Construction of one pH-independent 3-D pillar-layer lead-organic framework containing tetrazole-1-acetic acid. Inorg. Chem. Commun.. 2013;27:22-25.
- [Google Scholar]
- PH dependent structural diversity of metal complexes with 5-(4H–1,2,4-Triazol-4-yl)benzene-1,3-dicarboxylic acid. Cryst. Growth Des.. 2011;11(5):1901-1912.
- [Google Scholar]
- Construction of a three-fold parallel interpenetration network and bilayer structure based on copper(II) and trimesic acid. Cryst. Growth Des.. 2007;7(6):1171-1175.
- [Google Scholar]
- Two coordinated-solvent directed zinc(II) coordination polymers with rare gra topological 3D framework and 1D zigzag chain. Inorg. Chem. Commun.. 2011;14(1):300-303.
- [Google Scholar]
- Stoichiometry-dominated in situ formation of iodocuprate clusters and dimethyl-2,2[prime or minute]-biimidazoles as building units of coordination architectures. CrystEngComm. 2011;13(7):2644-2648.
- [Google Scholar]
- Structure modulation of metal–organic frameworks via reaction pH: Self-assembly of a new carboxylate containing ligand N-(3-carboxyphenyl)iminodiacetic acid with cadmium(II) and cobalt(II) salts. Polyhedron. 2008;27(2):812-820.
- [Google Scholar]
- Hydrothermal synthesis and characterization of a metal-organic framework by thermogravimetric analysis, powder X-ray diffraction, and infrared spectroscopy: an integrative inorganic chemistry experiment. J. Chem. Educ.. 2015;92(2):373-377.
- [Google Scholar]
- Temperature-controlled chiral and achiral copper tetrazolate metal-organic frameworks: syntheses, structures, and I2 adsorption. Inorg. Chem.. 2012;51(4):2303-2310.
- [Google Scholar]
- Two solvent-dependent zinc(II) supramolecular isomers: rare kgd and lonsdaleite network topologies based on a tripodal flexible ligand. Cryst. Growth Des.. 2011;11(12):5182-5187.
- [Google Scholar]
- Rationale of drug encapsulation and release from biocompatible porous metal-organic frameworks. Chem. Mater.. 2013;25(14):2767-2776.
- [Google Scholar]
- Solvent- and vapor-mediated solid-state transformations in 1,3,5-benzenetricarboxylate metal-organic frameworks. Cryst. Growth Des.. 2012;12(4):1999-2003.
- [Google Scholar]
- Effect of temperature on formation of two new lanthanide metal-organic frameworks: synthesis, characterization and theoretical studies of Tm(III)-succinate. J. Solid State Chem.. 2013;197(0):7-13.
- [Google Scholar]
- Commercial metal-organic frameworks as heterogeneous catalysts. Chem. Commun.. 2012;48(92):11275-11288.
- [Google Scholar]
- Solvent effect on the construction of two microporous yttrium-organic frameworks with high thermostability viain situ ligand hydrolysis. Dalton Trans.. 2010;39(24):5683-5687.
- [Google Scholar]
- Metal-organic framework MIL-53(Fe): facile microwave-assisted synthesis and use as a highly active peroxidase mimetic for glucose biosensing. RSC Adv.. 2015;5(23):17451-17457.
- [Google Scholar]
- Luminescent metal-organic framework films as highly sensitive and fast-response oxygen sensors. J. Am. Chem. Soc.. 2014;136(15):5527-5530.
- [Google Scholar]
- Hydrothermal synthesis and characterization of a novel 3D chiral inorganic-organic hybrid cadmium(II) framework with achiral 1,4-naphthalenedicarboxylic acid ligand. Synth. React. Inorg., Met.-Org., Nano-Met. Chem.. 2013;44(3):371-375.
- [Google Scholar]
- PH-dependent assembly and conversions of six cadmium(II)-based coordination complexes. Cryst. Growth Des.. 2010;10(7):3277-3284.
- [Google Scholar]
- The role of temperature in the synthesis of hybrid inorganic-organic materials: the example of cobalt succinates. Chem. Commun.. 2004;4:368-369.
- [Google Scholar]
- A high-throughput investigation of the role of pH, temperature, concentration, and time on the synthesis of hybrid inorganic–organic materials. Angew. Chem. Int. Ed.. 2005;44(46):7608-7611.
- [Google Scholar]
- Friščić, T., 2011. Metal-Organic Frameworks: Mechanochemical Synthesis Strategies. In: Encyclopedia of Inorganic and Bioinorganic Chemistry: John Wiley & Sons, Ltd.
- PH-specific hydrothermal assembly of binary and ternary Pb(II)-(O, N-carboxylic acid) metal organic framework compounds: correlation of aqueous solution speciation with variable dimensionality solid-state lattice architecture and spectroscopic signatures. Inorg. Chem.. 2012;51(17):9282-9296.
- [Google Scholar]
- High methane storage capacity in aluminum metal-organic frameworks. J. Am. Chem. Soc.. 2014;136(14):5271-5274.
- [Google Scholar]
- Tuning the formations of metal-organic frameworks by modification of ratio of reactant, acidity of reaction system, and use of a secondary ligand. Cryst. Growth Des.. 2012;12(1):281-288.
- [Google Scholar]
- Solvent as structure directing agent for the synthesis of novel coordination frameworks using a tripodal flexible ligand. CrystEngComm. 2008;10(12):1739-1742.
- [Google Scholar]
- PH and auxiliary ligand influence on the structural variations of 5(2′-Carboxylphenyl) nicotate coordination polymers. Cryst. Growth Des.. 2012;12(6):3312-3323.
- [Google Scholar]
- A supermolecular building approach for the design and construction of metal-organic frameworks. Chem. Soc. Rev.. 2014;43(16):6141-6172.
- [Google Scholar]
- Tuning the luminescence of metal-organic frameworks for detection of energetic heterocyclic compounds. J. Am. Chem. Soc.. 2014;136(44):15485-15488.
- [Google Scholar]
- A series of cadmium(II) coordination polymers synthesized at different ph values. Eur. J. Inorg. Chem.. 2007;2007(5):742-748.
- [Google Scholar]
- Temperature and pH driven self-assembly of Zn(ii) coordination polymers: crystal structures, supramolecular isomerism, and photoluminescence. CrystEngComm. 2014;16(9):1687-1695.
- [Google Scholar]
- Metal organic frameworks as nitric oxide catalysts. J. Am. Chem. Soc.. 2012;134(7):3330-3333.
- [Google Scholar]
- Tuning the void volume in a series of isomorphic porous metal-organic frameworks by varying the solvent size and length of organic ligands. CrystEngComm. 2014;16(24):5450-5457.
- [Google Scholar]
- Methane storage in metal-organic frameworks. Chem. Soc. Rev.. 2014;43(16):5657-5678.
- [Google Scholar]
- Metal-organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed.. 2006;45(36):5974-5978.
- [Google Scholar]
- Temperature-dependent urothermal synthesis of two distinct La(III)-naphthalenedicarboxylate frameworks. Inorg. Chem. Commun.. 2013;29:148-150.
- [Google Scholar]
- Facile synthesis of highly luminescent nanowires of a terbium-based metal–organic framework by an ultrasonic-assisted method and their application as a luminescent probe for selective sensing of organoamines. Inorg. Chem. Commun.. 2012;17:147-150.
- [Google Scholar]
- Solvent influence on sizes of channels in three new Co(II) complexes, exhibiting an active replaceable coordinated site. Cryst. Growth Des.. 2013;13(1):66-73.
- [Google Scholar]
- PH-Dependent assembly of two octamolybdate hybrid materials: A self-threading CdSO4-type framework and a 3D 4-connected framework. CrystEngComm. 2011;13(23):7037-7043.
- [Google Scholar]
- Temperature-controlled synthesis of metal-organic coordination polymers: crystal structure, supramolecular isomerism, and porous property. Cryst. Growth Des.. 2009;9(9):4147-4156.
- [Google Scholar]
- Ultrasound-assisted synthesis of nano-structured 3D zinc(II) metal–organic polymer: Precursor for the fabrication of ZnO nano-structure. Ultrason. Sonochem.. 2015;23:238-245.
- [Google Scholar]
- 3,6-Connected metal-organic frameworks based on triscarboxylate as a 3-connected organic node and a linear trinuclear Co3(COO)6 secondary building unit as a 6-connected node. Cryst. Growth Des.. 2012;12(8):4186-4193.
- [Google Scholar]
- Luminescent Li-based metal-organic framework tailored for the selective detection of explosive nitroaromatic compounds: direct observation of interaction sites. Inorg. Chem.. 2013;52(2):589-595.
- [Google Scholar]
- Mechanochemical synthesis of metal−organic frameworks: a fast and facile approach toward quantitative yields and high specific surface areas. Chem. Mater.. 2010;22(18):5216-5221.
- [Google Scholar]
- Microwave-assisted synthesis of metal-organic frameworks. Dalton Trans.. 2011;40(2):321-330.
- [Google Scholar]
- Metal-organic framework materials as chemical sensors. Chem. Rev.. 2012;112(2):1105-1125.
- [Google Scholar]
- Solvent-dependent formation of Cd(II) coordination polymers based on a C2-symmetric tricarboxylate linker. Cryst. Growth Des.. 2012;12(8):4109-4115.
- [Google Scholar]
- Co(II)-tricarboxylate metal–organic frameworks constructed from solvent-directed assembly for CO2 adsorption. Microporous Mesoporous Mater.. 2013;176:194-198.
- [Google Scholar]
- A homochiral microporous hydrogen-bonded organic framework for highly enantioselective separation of secondary alcohols. J. Am. Chem. Soc.. 2014;136(2):547-549.
- [Google Scholar]
- PH-dependent binary metal−organic compounds assembled from different helical units: structural variation and supramolecular isomers. Cryst. Growth Des.. 2010;10(4):1699-1705.
- [Google Scholar]
- Solvent-mediated crystal-to-crystal transformations from a cationic homometallic metal-organic framework to heterometallic frameworks. CrystEngComm. 2014;16(37):8818-8824.
- [Google Scholar]
- Microporous metal-organic frameworks with open metal sites as sorbents for selective gas adsorption and fluorescence sensors for metal ions. J. Mater. Chem. A. 2013;1(3):495-499.
- [Google Scholar]
- Microwave-assisted solvothermal synthesis of zirconium oxide based metal-organic frameworks. Chem. Commun.. 2013;49(35):3706-3708.
- [Google Scholar]
- PH-controlled the formation of 4-sulfocalix[4]arene-based 1D and 2D coordination polymers. Inorg. Chem. Commun.. 2007;10(11):1257-1261.
- [Google Scholar]
- Two solvent-dependent zinc(ii) supramolecular isomers: structure analysis, reversible and nonreversible crystal-to-crystal transformation, highly selective CO2 gas adsorption, and photoluminescence behaviors. CrystEngComm. 2012;14(19):6246-6251.
- [Google Scholar]
- Two new pH-controlled metal–organic frameworks based on polynuclear secondary building units with conformation-flexible cyclohexane-1,2,4,5-tetracarboxylate ligand. Inorg. Chim. Acta. 2011;367(1):127-134.
- [Google Scholar]
- Temperature-induced assembly of MOF polymorphs: Syntheses, structures and physical properties. CrystEngComm. 2012;14(5):1856-1864.
- [Google Scholar]
- Cobalt (II) coordination networks dependent upon the spacer length of flexible bis(tetrazole) ligands. Cryst. Growth Des.. 2008;8(1668)
- [Google Scholar]
- Solvent induced structural variation in magnesium carboxylate frameworks. Inorg. Chem. Commun.. 2013;29:110-113.
- [Google Scholar]
- Five novel cobalt coordination polymers: effect of metal-ligand ratio and structure characteristics of flexible bis(imidazole) ligands. CrystEngComm. 2010;12(10):3283-3290.
- [Google Scholar]
- Urothermal synthesis of a photoluminescent zinc coordination polymer. Inorg. Chem. Commun.. 2011;14(2):355-357.
- [Google Scholar]
- Hydrothermal versus nonhydrothermal synthesis for the preparation of organic–inorganic solids: the example of cobalt(II) succinate. Chem. Mater.. 2001;13(2):410-414.
- [Google Scholar]
- 3D coordination polymers with nitrilotriacetic and 4,4′-bipyridyl mixed ligands: structural variation based on dinuclear or tetranuclear subunits assisted by Na−O and/or O−H···O interactions. Inorg. Chem.. 2005;44(13):4515-4521.
- [Google Scholar]
- An investigation of the self-assembly of neutral, interlaced, triple-stranded molecular braids. Chem. Eur. J.. 2006;12:6281-6289.
- [Google Scholar]
- PH-Dependent cobalt(ii) frameworks with mixed 3,3[prime or minute],5,5[prime or minute]-tetra(1H-imidazol-1-yl)-1,1[prime or minute]-biphenyl and 1,3,5-benzenetricarboxylate ligands: synthesis, structure and sorption property. CrystEngComm. 2013;15(45):9537-9543.
- [Google Scholar]
- Role of temperature and time in the formation of infinite −M−O−M− linkages and isolated clusters in MOFs: a few illustrative examples. Inorg. Chem.. 2008;47(19):8451-8463.
- [Google Scholar]
- The role of temperature on the structure and dimensionality of MOFs: an illustrative study of the formation of manganese oxy-bis(benzoate) structures. Chem. Commun.. 2007;43:4471-4473.
- [Google Scholar]
- Investigation of porous Ni-based metal-organic frameworks containing paddle-wheel type inorganic building units via high-throughput methods. Inorg. Chem.. 2011;50(11):5085-5097.
- [Google Scholar]
- Electrochemical synthesis of some archetypical Zn2+, Cu2+, and Al3+ metal organic frameworks. Cryst. Growth Des.. 2012;12(7):3489-3498.
- [Google Scholar]
- Control of the crystallization process and structure dimensionality of Mg–Benzene–1,3,5-tricarboxylates by tuning solvent composition. Cryst. Growth Des.. 2013;13(8):3825-3834.
- [Google Scholar]
- The effect of pressure on the post-synthetic modification of a nanoporous metal-organic framework. Nanoscale. 2014;6(8):4163-4173.
- [Google Scholar]
- Sonochemical temperature controlled synthesis of pellet-, laminate- and rice grain-like morphologies of a Cu(II) porous metal–organic framework nano-structures. Ultrason. Sonochem.. 2014;21(4):1430-1434.
- [Google Scholar]
- PH-controlled synthesis of two new coordination polymers modeled by pyridine-2,4-dicarboxylic acid. Inorg. Chem. Commun.. 2012;21:186-190.
- [Google Scholar]
- Reactive microporous rare-earth coordination polymers that exhibit single-crystal-to-single-crystal dehydration and rehydration. Cryst. Growth Des.. 2005;5(2):529-533.
- [Google Scholar]
- Hydrothermal syntheses and structural diversity of cobalt complexes with 2,2′-bibenzimidazole ligand by temperature tuning strategy. Cryst. Growth Des.. 2009;9(1):488-496.
- [Google Scholar]
- Metal-organic frameworks for catalysis: the Knoevenagel reaction using zeolite imidazolate framework ZIF-9 as an efficient heterogeneous catalyst. Catal. Sci. Technol.. 2012;2(3):521-528.
- [Google Scholar]
- Solvothermal synthesis, structure, and properties of metal organic framework isomers derived from a partially fluorinated link. Cryst. Growth Des.. 2011;11(4):1215-1222.
- [Google Scholar]
- A rapid microwave-assisted synthesis of a sodium-cadmium metal-organic framework having improved performance as a CO2 adsorbent for CCS. Dalton Trans.. 2015;44(21):9955-9963.
- [Google Scholar]
- Post-synthetic reversible incorporation of organic linkers into porous metal-organic frameworks through single-crystal-to-single-crystal transformations and modification of gas-sorption properties. Chem. – A Eur. J.. 2010;16(38):11662-11669.
- [Google Scholar]
- Methane storage in metal-organic frameworks: current records, surprise findings, and challenges. J. Am. Chem. Soc.. 2013;135(32):11887-11894.
- [Google Scholar]
- Liquid-assisted mechanochemical synthesis of an iron carboxylate metal organic framework and its evaluation in diesel fuel desulfurization. Microporous Mesoporous Mater.. 2015;213:14-21.
- [Google Scholar]
- Alkaline earth metal-based metal-organic framework: hydrothermal synthesis, X-ray structure and heterogeneously catalyzed Claisen-Schmidt reaction. Dalton Trans.. 2014;43(34):13006-13017.
- [Google Scholar]
- Shape- and morphology-controlled sustainable synthesis of Cu Co, and in metal organic frameworks with high CO2 capture capacity. ACS Sust. Chem. Eng.. 2013;1(1):66-74.
- [Google Scholar]
- Mechanochemical synthesis of an yttrium based metal-organic framework. Chem. Commun.. 2013;49(10):972-974.
- [Google Scholar]
- Solvent-directed synthesis of chiral and non-centrosymmetric metal–organic frameworks based on pyridine-3,5-dicarboxylate. Inorg. Chem. Commun.. 2013;38:115-118.
- [Google Scholar]
- Influence of temperature on metal-organic frameworks. Chin. Chem. Lett.. 2014;25(6):823-828.
- [Google Scholar]
- Cluster-organic framework materials as heterogeneous catalysts for high efficient addition reaction of diethylzinc to aromatic aldehydes. Chem. Mater.. 2012;24(24):4711-4716.
- [Google Scholar]
- A luminescent metal-organic framework demonstrating ideal detection ability for nitroaromatic explosives. J. Mater. Chem. A. 2014;2(5):1465-1470.
- [Google Scholar]
- Chiral and achiral helical coordination polymers of zinc and cadmium from achiral 2, 6-bis (imidazol-1-yl) pyridine: solvent effect and spontaneous resolution. J. Chem. Sci.. 2014;126(5):1423-1431.
- [Google Scholar]
- Novel coordinated-solvent induced assembly of Cd(II) coordination polymers containing 4,4′-dipyridylsulfide. Cryst. Growth Des.. 2009;9(6):2552-2555.
- [Google Scholar]
- Current trends in nano metal – organic frameworks for biomedical applications. ScienceLetters. 2015;4:165.
- [Google Scholar]
- Series metal–organic frameworks constructed from 1,10-phenanthroline and 3,3′,4,4′-biphenyltetracarboxylic acid: Hydrothermal synthesis, luminescence and photocatalytic properties. J. Mol. Struct.. 2015;1080:44-51.
- [Google Scholar]
- Metal-organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc.. 2013;135(36):13222-13234.
- [Google Scholar]
- Control of the topologies and packing modes of three 2D coordination polymers through variation of the solvent ratio of a binary solvent mixture. CrystEngComm. 2008;10(9):1211-1215.
- [Google Scholar]
- Metal-organic frameworks based on the pyridine-2,3-dicarboxylate and a flexible bispyridyl ligand: syntheses, structures, and photoluminescence. CrystEngComm. 2009;11(2):292-297.
- [Google Scholar]
- PH-induced different crystalline behaviors in extended metal-organic frameworks based on the same reactants. Dalton Trans.. 2013;42(18):6294-6297.
- [Google Scholar]
- Ligand and pH-controlled znii bilayer coordination polymers based on biphenyl-3,3′,4,4′-tetracarboxylate. Cryst. Growth Des.. 2007;7(8):1514-1521.
- [Google Scholar]
- Two-/three-dimensional open lanthanide-organic frameworks containing rigid/flexible dicarboxylate ligands: synthesis, crystal structure and photoluminescent properties. CrystEngComm. 2013;15(10):1931-1949.
- [Google Scholar]
- Two cobalt(II) coordination polymers [Co2(H2O)4(Hbidc)2]n and [Co(Hbidc)]n (Hbidc = 1H-benzimidazole-5,6-dicarboxylate): syntheses, crystal structures, and magnetic properties. CrystEngComm. 2009;11(6):1054-1060.
- [Google Scholar]
- PH-dependent assembly of supramolecular architectures from 0D to 2D networks. Cryst. Growth Des.. 2007;7(9):1746-1752.
- [Google Scholar]
- Stoichiometry of N-donor ligand mediated assembly in the ZnII – Hfipbb system: from a 2-fold interpenetrating pillared-network to unique (3, 4) – connected isomeric nets. Cryst. Growth Des.. 2011;11:3850-3857.
- [Google Scholar]
- Study of guest molecules in metal-organic frameworks by powder X-ray diffraction: analysis of difference envelope density. Cryst. Growth Des.. 2014;14(11):5397-5407.
- [Google Scholar]
- Mechanochemical synthesis of a fluorenone-based metal organic framework with polarized fluorescence: an experimental and computational study. J. Mater. Chem. C. 2013;1(5):997-1004.
- [Google Scholar]
- Urothermal synthesis of two photoluminescent cadmium coordination polymers. Inorg. Chem. Commun.. 2013;30:152-155.
- [Google Scholar]
- Urothermal in situ ligand synthesis to fabricate a metal-organic framework with (3,4)-connected tfi topology. Inorg. Chem. Commun.. 2011;14(11):1695-1697.
- [Google Scholar]
- Rapid hydrothermal synthesis of MIL-101(Cr) metal–organic framework nanocrystals using expanded graphite as a structure-directing template. Inorg. Chem. Commun.. 2013;35:265-267.
- [Google Scholar]
- Facile synthesis of covalent organic frameworks COF-1 and COF-5 by sonochemical method. RSC Adv.. 2012;2(27):10179-10181.
- [Google Scholar]
- Self-assembly of right-handed helical infinite chain, one- and two-dimensional coordination polymers tuned via anions. Cryst. Growth Des.. 2005;5(3):1215-1219.
- [Google Scholar]
- Role of molar ratio, temperature and solvent on the Zn/Cd 1,2,4-triazolate system with novel topological architectures. CrystEngComm. 2011;13:3536-3544.
- [Google Scholar]
- PH-dependent Cu(II) coordination polymers with tetrazole-1-acetic acid: synthesis, crystal structures, EPR and magnetic properties. Cryst. Growth Des.. 2008;8(4):1140-1146.
- [Google Scholar]
- Effect of pH/metal ion on the structure of metal-organic frameworks based on novel bifunctionalized ligand 4[prime or minute]-carboxy-4,2[prime or minute]:6[prime or minute],4[prime or minute][prime or minute]-terpyridine. CrystEngComm. 2013;15(7):1460-1467.
- [Google Scholar]
- Hydrothermal synthesis, crystal structure, and luminescent properties of two zinc(II) and cadmium(II) 3D metal-organic frameworks. Zeitschrift für anorganische und allgemeine Chemie. 2013;639(5):826-831.
- [Google Scholar]
- Urothermal synthesis of crystalline porous materials. Angew. Chem. Int. Ed.. 2010;49(47):8876-8879.
- [Google Scholar]
- Temperature dependent charge distribution in three-dimensional homochiral cadmium camphorates. Chem. Commun.. 2008;4:444-446.
- [Google Scholar]
- Temperature and auxiliary ligand-controlled supramolecular assembly in a series of Zn(ii)-organic frameworks: syntheses, structures and properties. CrystEngComm. 2012;14(2):590-600.
- [Google Scholar]
- Tuning the formation of copper(I) coordination architectures with quinoxaline-based N, S-donor ligands by varying terminal groups of ligands and reaction temperature. Inorg. Chim. Acta. 2009;362(11):3915-3924.
- [Google Scholar]
- PH modulated assembly in the mixed-ligand system Cd(ii)-dpstc-phen: structural diversity and luminescent properties. CrystEngComm. 2013;15(19):3992-4002.
- [Google Scholar]
- Mixed-metal-organic frameworks (M[prime or minute]MOFs) from 1D to 3D based on the “organic” connectivity and the inorganic connectivity: syntheses, structures and magnetic properties. CrystEngComm. 2015;17(17):3312-3324.
- [Google Scholar]
- Single-crystal to single-crystal transformation from a hydrophilic–hydrophobic metal–organic framework to a layered coordination polymer. Inorg. Chim. Acta. 2014;411:128-133.
- [Google Scholar]
- Interaction of 1,3-adamantanediacetic acid (H2ADA) and ditopic pyridyl subunits with cobalt nitrate under hydrothermal conditions: pH influence, crystal structures, and their properties. Cryst. Growth Des.. 2010;10(1):76-84.
- [Google Scholar]
- Selective anion exchange with nanogated isoreticular positive metal-organic frameworks. Nat. Commun.. 2013;4
- [Google Scholar]
- Solvents influence on sizes of channels in three fry topological Mn(ii)-MOFs based on metal-carboxylate chains: syntheses, structures and magnetic properties. CrystEngComm. 2013;15(40):8125-8132.
- [Google Scholar]
- Optimized metal–organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8(3):2812-2819.
- [Google Scholar]
- Rational assembly of a 3D metal-organic framework for gas adsorption with predesigned cubic building blocks and 1D open channels. Chem. Commun.. 2005;28:3526-3528.
- [Google Scholar]
- Solvent-induced generation of two magnesium-based metal–organic frameworks with doubly inter-penetrated ReO3 nets constructed from the same linkers but distinct inorganic nodes. Inorg. Chem. Commun.. 2015;52:41-45.
- [Google Scholar]







