Translate this page into:
Design, synthesis and preclinical evaluations of (s)-2-((s)-1-benzyl-2,5-dioxopyrrolidin-3-yl)-3-(4-isopropylphenyl)-2-methylpropanal (succ-5) as cardioprotective, hepatoprotective and lipid lowering molecule. in-vivo and in-silico approaches
⁎Corresponding authors. samiullah@uop.edu.pk (Sami Ullah), matermaha@gmail.com (Mater H. Mahnashi), sadiquom@yahoo.com (Abdul Sadiq)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, the newly synthesized compound (Succ-5) was analyzed through spectral methods, seen for potential receptor targets via molecular docking, and pre-clinically evaluated for therapeutic effects and safety profile using biochemical and histopathological techniques. The biochemical analysis included assessment of cardiac biomarkers, hepatic enzymes, and lipid profiles, while histopathology included evaluation of cardiac and liver tissues. The toxic dose was determined pre-clinically, followed by dividing albino rats into five treatment groups (each having n = 6). The control group received oral saline for eight days. The 5-FU (5-Fluorouracil) group received oral saline for 8 days and 5-FU (150 mg/kg I.P.) on day 5. The atenolol group was administered with atenolol (20 mg/kg) for 8 days and 5-FU (150 mg/kg I.P.) on day 5. Two groups of rats were administered with the test compound (Succ-5) at doses of 5 mg/kg I.P and 10 mg/kg I.P (for 8-days), followed by 5-FU (150 mg/kg I.P.) on day 5. Elevated serum levels of CK-MB (creatinine kinase myocardial band), cTnI (troponin I), LDH (lactate dehydrogenase), lipid profile, and selected liver enzymes including ALP (alkaline phosphatase), ALT (alanine transaminase), AST (aspartate aminotransferase), BT (bilirubin total) and BD (direct bilirubin) were associated with 5-FU toxicity. After administration of the test compound at the mentioned doses, these biomarkers significantly decreased. Likewise, histological examination revealed 5-FU damaged the heart and hepatic tissues, which were also considerably recovered following administration of the test compound. Immunohistochemistry of heart tissue also revealed the low expression of COX-2 and TNF-α in Succ-5 treated groups compared to toxic group. Dose-response evaluation showed that a dose of 10 mg/kg provided better results than 5 mg/kg. The analysis of binding energy values computed via docking simulations showed that Succ-5 interacts with the human beta2-adrenergic G protein-coupled receptor with a slightly stronger affinity than calcium channel T-type. In conclusion, the histological and biochemical findings revealed that the test compound had significant cardioprotective, hepatoprotective, and lipolytic effects in the 5-FU-induced toxicity.
Keywords
Atenolol
Succinimide Derivative (Succ-5)
5-FU induced toxicity
Cardioprotective
Hepatoprotective
1 Introduction
Cancer treatment has advanced spectacularly in recent years, and many cases are now treatable and expected to be cured. Chemotherapy-induced cardiac and liver impairment is a profound side effect of cancer therapy (Saidi & Alharethi, 2011). Due to the cardiotoxicity and hepatotoxicity of these drugs, an increasing number of survivors are at high risk of developing cardiovascular and hepatic diseases (Payne & Nohria, 2017). Comorbid cardiac and hepatic dysfunctions occur in severe cardiac and hepatic diseases due to intricate cardio-hepatic linkages(Xanthopoulos, Starling, Kitai, & Triposkiadis, 2019). 5-Fluorouracil (5-FU) is an antimetabolite that was first developed as pyrimidine analogues as a rational synthetic anticancer drug (More, Lane, & Asnani, 2021). It is frequently used to treat different types of cancers, such as colorectal, breast, and skin cancers(Longley, Harkin, & Johnston, 2003). This drug competitively inhibits metabolites with similar structures to show anticancer effects (Jansman, Sleijfer, de Graaf, Coenen, & Brouwers, 2001).Cardiovascular toxicity, hepatotoxicity, and hyperlipidemia are also observed by this drug along with other side effects(Südhoff et al., 2004). The cytostatic agent 5-fluorouracil (5-FU) causes cardiotoxicity that has a serious influence on the patient's survival rate irrespective of the oncologic prognosis(Payne & Nohria, 2017). 5-fluorouracil (5-FU) causes cardiac side effects in 1.2–7.6 percent of patients (Macdonald, 1999). Reduced antioxidant properties lead to cardiac muscle peroxidation, which causes cell damage(Ray, Roy, & Sengupta, 2007). Incorporation of FdUTP, F-UTP, and 5-fluorocytosine into DNA, as well as F-UTP and 5-fluorocytosine incorporation into RNA, causes cytotoxicity. These metabolites are thought to interfere with calcium channel-dependent membrane function, alter contractile proteins, increase oxidative damage and the formation of vasoactive substances such as histamine and catecholamines, and trigger autoimmune responses. Furthermore, transmural ischemia causes severe cardiotoxicity such as arrhythmias, ventricular tachycardia, and cardiac arrest (Lim et al., 2012). Risk factors for cardiotoxicity have gotten a lot of attention, and new strategies for accurately managing chemotherapy-induced heart failure have emerged (An, Li, Shang, Gao, & Xing, 2019).
The heart and liver are linked via blood circulation. Therefore, damage to one organ can seriously affect the physiological functioning of the other. In this connection, liver cirrhosis can lead to both acute and chronic heart failure as well. Cirrhosis of the heart, also known as congestive hepatopathy, is a group of liver illnesses that are linked to right-sided heart failure (Xanthopoulos et al., 2019). Ischemic reperfusion injury results in hypoperfusion, hypoxia, and a reduction in oxygen supply to liver tissues, which may result in cell injury of the local hepatocytes (Bernardi, 2013). The microsomal enzyme system also extensively metabolises 5-fluorouracil in the liver, and the formation of a toxic intermediate can harm the liver. Inhibiting mitochondrial function may be a toxicity mechanism associated with this chemotherapeutic agent (Maring et al., 2003). Besides this, demographically, age, sex, the standard of living, obesity, diet, genetic background, drug exposure, and dosage all play a role in drug-induced liver impairment. Hepatotoxicity is a serious side effect that can occur during the development of new drugs. They are considered one of the most dangerous side effects of certain medications, including chemotherapeutic agents (Pandit, Sachdeva, & Bafna, 2012).It can result in a lack of immunological components needed to resist infectious microorganisms. Cholestasis, protein depletion such as albumin and fibrinogen, metabolic abnormalities, and body imbalances are examples of toxicities(Hunter, Smaill, & biology, 1988). Long-term drug use, in most cases, can impair the cardiovascular and hepatic systems. It is critical for drug research to investigate and seek out these adverse effects and see possible alternatives and/or protective measures (Schuster et al., 2005).
Hyperlipidemia and obesity are also on the rise, owing in part to the adoption of a sedentary lifestyle characterized by a high diet of carbohydrates and fats combined with a low energy utilization that has a known and serious link to an increased risk of liver and heart diseases. 5-fluorouracil has also been linked to the development of hyperlipidemia (Lim et al., 2012).The main focus of drug development is to search for a better option regarding therapeutic effectiveness, less harmfulness added with cost-effective and risk-free treatments.
Chemically, pyrrolidine-2,5-dione is a useful pharmacological moiety as an anticonvulsant drug (Sadiq & Nugent, 2020). Over the past, several approaches have been used to get easy access to succinimide synthesis (Sakkani & Nanda, 2022). One of the convenient approaches is a single step Michael addition to maleimide (Nugent et al., 2012).Like other organocatytic reactions using enolizable carbonyls, this approach is used to add different maleimide acceptors to produce new products with enhanced pharmacological activities (Ahmad et al., 2020; A. Bibi et al., 2019). The succinimide products belong to a well-known class of anticonvulsant drugs, and they have been used previously in neuropharmacological activities (Sadiq et al., 2015). Moreover, its close structural resemblance with thiazolidinedione makes it an effective antidiabetic agent (Huneif et al., 2022). Lactam can be synthesized by reducing pyrrolidione-2,5-dione. So, the structural features that are similar to beta lactams also proved it to be an efficient antimicrobial agent(Mahmood et al., 2017). The incorporation of suitable substitutions patterns on its C and N positions lead to structures that are suitable inhibitors of cyclo and lipoxygenase pathways (Mahmood et al., 2022; Sadiq et al., 2021). Keeping in view the unique structural features and potential pharmacological activities of succinimides, we have synthesized a new succinimide product 2-(1-benzyl-2,5-dioxopyrrolidin-3-yl)-3-(4-isopropylphenyl)-2-methylpropanal (Succ-5) for hepato and cardioprotective activities.
2 Materials and methods
2.1 Chemicals and reagents
Centronics (Germany) provided diagnostic kits for cardiac indicators, liver enzymes, and lipid profiles; an authorised distributor from Sigma Aldrich (St. Louis, MO, USA) provided dimethyl sulfoxide (DMSO), chloroform, formalin, 5-fluorouracil, and Atenolol. Surgical gloves, syringes, saline solution, and water for injection were obtained locally.
2.2 Research ethical approval and animal breeding
The albino rats were raised and kept in the animal house and bioassay laboratory at the Department of Pharmacy, University of Peshawar. They were kept at a comfortable temperature (22 °C) and underwent a 12-hour light–dark cycle. The animals were given standardized laboratory nutrition and given water ad libitum. Animals within the required or optimized range of weight and age were selected for experimental investigation. All experiments were performed after formal approval by the Ethical Research Committee of the Department of Pharmacy, University of Peshawar, referred to as form No. 412/EC/F.LIFE, UoP-2021; dated October 28, 2021; and in compliance with the UK Animal Scientific Procedures Act, 1986.
2.3 Experimental procedures
2.3.1 Synthesis of 2-(1-benzyl-2,5-dioxopyrrolidin-3-yl)-3-(4-isopropylphenyl)-2-methylpropanal (Succ-5)
The compound (Succ-5) was synthesised with our previously reported method with a minor modification by changing the Michael donor (Mahmood et al., 2022). The synthesis of 2-(1-benzyl-2,5-dioxopyrrolidin-3-yl)- −3-(4-isopropylphenyl) 2-methylpropanal (Succ-5) was initiated by adding 3-(4-isopropylphenyl) − 2-methylpropanal (1) (1.5 mmol, 0.46 ml) to a closed-cap 2 ml reaction vial. Catalytic amounts of O-tert-bu-l-threonine (3) and potassium hydroxide (4) (each 5 mol%) were added, diluted with dichloromethane (DCM 1 M, 1.0 ml), and the reaction was stirred at room temperature. After 2–3 min of stirring, N-benzylmaleimide(2) (1.0 mmol, 187 mg) was added and continued stirring. The reaction was monitored with TLC analysis visualised under UV light time-to-time. The reaction was considered completed after 5 min when no limiting reagent (N-benzylmaleimide) was visualised in the reaction. The reaction was quenched with water (10 ml). The crude reaction mixture was transferred to a small separating funnel with 10 ml of DCM. The organic layer was separated from the aqueous layer. The same procedure was repeated three times to get the maximum amount of the product. The combined organic layer was dried with anhydrous sodium sulphate and the solvent was evaporated with a low-pressure rotary evaporator. The structure of Succ-5 was confirmed by 1H and 13C NMR analyses.
2.3.2 Acute toxicity study
For the acute toxicity study, 24 mice (BALB/c) were used, considered with both genders (to minimize potential sex differences associated with the study results), with an accepted weight of 20–25 gm, 8–12 weeks old, maintained in a sequential manner of light and dark (12 h each), and retained at a feasible temperature (22 °C). The animals were divided into 3 groups, each having animals, n = 8. The acute toxicity of the test compound (Succ-5) was studied in rats after intraperitoneal (I.P) injections at dose levels of 50 mg/kg (group I), 150 mg/kg (group II), and 250 mg/kg (group III) (Bhat et al., 2019).
2.3.3 Pre-clinical observations and survival
In the preclinical phase, physical motor activity (movement), body appearance in various scenarios, such as sitting or standing, and cognitive and behavioral conversations were all observed (interactions with cage mates and nesting). These observations were made on a regular basis during acute experimental investigation (Kamil et al., 2021).
2.3.4 Cardioprotective study
Albino male/female, adult Rats (weighing 120–150 g) were chosen, housed in standard cages at room temperature, and fed a consistent basal diet. The rats were placed into five groups at random and given various treatments. The control group got saline alone (orally) every day for 8 days, while the 5-FU-treated group received saline orally for 8 days followed by a single dose of 5-FU (150 mg/kg B.W.I.P injection) on day 5 of the regimen. Two compound test preparations (5 mg/kg/day/IP and 10 mg/kg/day/IP) were given for eight days, followed by a single dose of 5-FU (150 mg/kg B.W.I.P.) on day five of the regimen. Twenty-four hours following the last treatment, blood samples were taken, and all rats were examined. Histopathological and biochemical tests were performed on the serum, heart, and liver tissues collected, including the evaluation of myocardial markers, liver enzymes, and lipid profiles (Mohamed, Safwat, & sciences, 2016).
2.3.5 Biochemical assay
After the provision of the corresponding treatment regimen to the respective group of animals for 8 days, blood samples were taken from each group on day 9; centrifuged at 3000 rpm for 10–15 min to separate serum parts; and kept at 4 °C until analysis. Finecare TM analyzers were used to measure troponin I, creatinine kinase-MB (CK-MB), and lactate dehydrogenase (LDH). An analytical kit from Centronics, Germany, was used to measure serum glutamate pyruvate transaminase (SGPT, also known as ALT), serum glutamate oxaloacetate transaminase (SGOT, also known as AST), serum glutamate pyruvate transaminase (SGPT, also known as ALP), and serum oxaloacetate transaminase (SG). An analytical kit from Centronics in Germany was used to assess total cholesterol (TC), triglycerides (TG), high-density lipids (HDL-c), low-density lipids (LDL-c), and very low-density lipids (VLDL-c). The nature and extent of the toxic and protective effects of 5-FU and the tested chemical (Succ-5) on heart and liver tissues were correlated using the results of these experiments.
2.3.6 Histological study
To investigate the part/area, the pattern of toxicity that occurs in the targeted tissue and the level of cell damage by inducing agents and subsequent protective effects of the test compound in each group, the heart and liver were isolated within 24 h of the last dose given to the animals. This assessment was utilised to compare the effects of the aforementioned effects to those of the toxic control group. The isolated organs of experimental animals (heart and liver) were promptly cleaned in normal saline and fixed in a 10 % buffered neutral formalin solution. Heart and liver tissues were processed and embedded in paraffin after fixing. Hematoxylin and eosin were used to stain the tissues of the aforementioned organs (H & E). The slides were studied with a light microscope fitted with a camera after staining (LABOMED LX400, iVu 3100, Auburn Court, Fremont, CA, USA). The photos were then analyzed for abnormal alterations. These lesions, which may be comprised of necrosis, inflammatory cell aggregation, steatosis, fibrosis, interstitial edema, hemorrhage, and myocyte degeneration, were graded as none, few, mild, moderate, and severe based on the pathologist's subjective judgment.
2.3.7 Immunohistochemistry of heart tissue
Heart tissue slides were put through a de-paraffinization procedure that starts with three separate xylene treatments for 10 min, followed by rehydration in a graded alcohol preparation (starting at 100 %, going down to 70 %, and washing for 5–10 min). After that, distilled water was used to clean these slides and remove any traces of ethanol. Proteinase K was used to treat the slides for antigen retrieval, and 0.1 M PBS (phosphate buffer solution) was used to wash them. A solution of diluted hydrogen peroxide (3 % methanolic solution) was used to stop peroxidase activity. The washed slides were then incubated with 5 % normal goat serum, containing 0.1 % Triton X-100 for a minimum of 1 h in a humidified chamber. After blocking, the slides were kept for overnight incubation at 4°Cwith primary antibodies against cyclooxygenase 2 (COX-2) and anti-tumor necrosis alpha (TNF-α). The next morning, after washing twice with 0.1 M PBS, they were incubated for 90 min with biotinylated secondary antibodies (dilution factor 1:0) in a humidified chamber. The slides were washed again and incubated for 1 h with ABC reagent in a humidified chamber. After staining in a DAB (3,3′-Diaminobenzidine) solution, the slides were washed with distilled water, dehydrated in graded ethanol, fixed in xylene, and cover-slipped with a mounting medium. Immuno-histochemical tagged image file format (TIF) images of slides were taken using a light microscope at a rate of three images per slide. The number of immune-positive cells expressing COX2 and TNF-α in heart tissues were counted using software (Image J) by first optimising the TIF image background according to threshold intensity and then analyzing the intensity for the number of immune-positive cells at the same intensity threshold for all groups, expressed in terms of relative integrated density compared to the control (Shah et al., 2019).
3 Docking studies
Docking studies were performed by using Molecular Operating Environment (MOE 2016.0802) (ULC, 2018). Docking studies were carried out on enzyme obtained from protein data bank (PDB) with 6KZP and 2RH1 accession codes, respectively. All the involved processes i.e. ligand and downloaded enzymes preparation, determination of binding site and docking simulations were performed by using our previously reported procedures 3 (M. Bibi et al., 2021; Biovia, 2017; Jan et al., 2020; Tanoli et al., 2019). 2-D interaction plots were analyzed by using discovery studio visualizer was used (Biovia, 2017).
4 Statistical analysis
One-way ANOVA was performed using Graph-Pad Prism software version 5.01 (Graph-Pad Software, Inc., San Diego, CA, USA). The mean, standard deviation (SD), and standard error of the mean (SEM) were used to express the data. Statistical significance was defined as a P-value of < 0.05.
5 Results and discussion
5.1 Chemistry of the synthesized compound
The synthesis scheme of compound 2-(1-benzyl-2,5-dioxopyrrolidin-3-yl)-3-(4-isopropylphenyl)-2-methylpropanal (Succinimide 5) or Succ-5 is provided in Scheme 1. The Succ-5 was isolated with 95 % yield as half white solid with Rf value of 0.42 (n-hexane 80 % and ethyl acetate 20 %). The 1H NMR of Succ-5 is shown in Fig. 1. 1H NMR (400 MHz, chloroform-d): 9.57 (s, 1H), 7.38 (d, J = 7.08 Hz, 2H), 7.33–7.24 (m, 3H), 7.15–7.11 (m, 2H), 7.06 (d, J = 6.85 Hz, 2H), 4.65 (d, J = 9.65 Hz, 2H), 3.08 (d, J = 12.43 Hz, 2H), 2.97–2.81 (m, 2H), 2.73 (dd, J = 9.96 and 18.19 Hz, 1H), 2.50 (dd, J = 5.73 and 18.21 Hz, 1H), 1.23 (d, J = 6.92 Hz, 6H), 1.06 (s, 3H). The 13C NMR of compound Succ-5 is shown in Fig. 2. 13C NMR (100 MHz, chloroform-d): 203.97, 203.37, 178.05, 177.77, 175.61, 175.46, 148.02, 147.82, 135.86, 135.77, 132.42, 131.99, 130.55, 128.81, 128.80, 128.76, 128.74, 128.04, 126.73, 126.67, 60.47, 52.94, 51.95, 43.20, 42.59, 42.17, 40.41, 39.88, 33.80, 32.30, 31.37, 24.08, 21.15, 16.81, 16.59 and 14.34.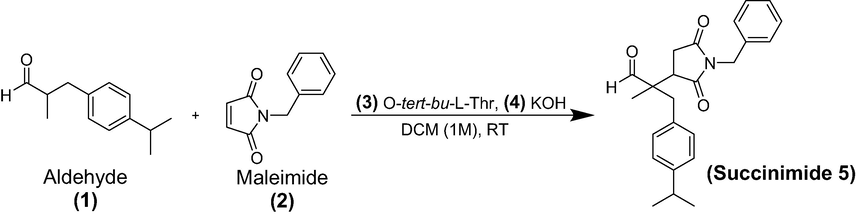
Synthesis of 2-(1-benzyl-2,5-dioxopyrrolidin-3-yl)-3-(4-isopropylphenyl)-2-methylpropanal (Succ-5).
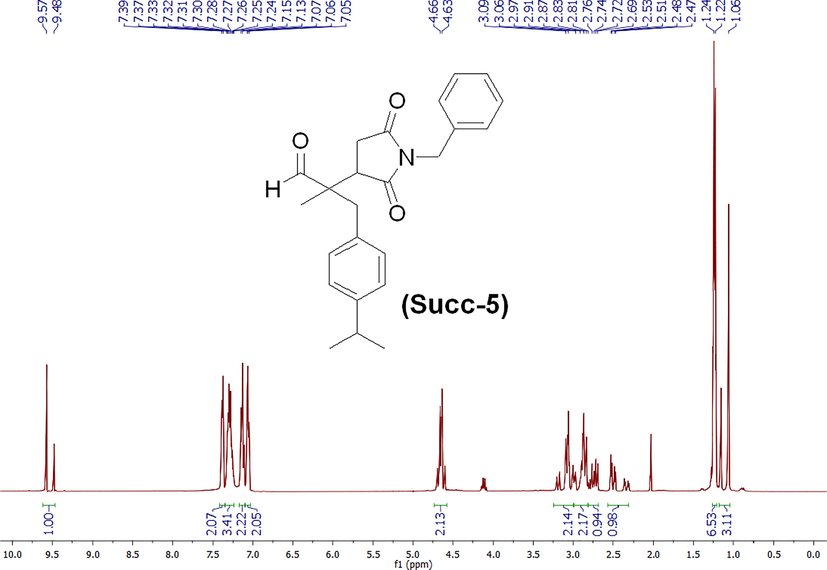
1H NMR spectrum of 2-(1-benzyl-2,5-dioxopyrrolidin-3-yl)-3-(4-isopropylphenyl)-2-methylpropanal (Succ-5).
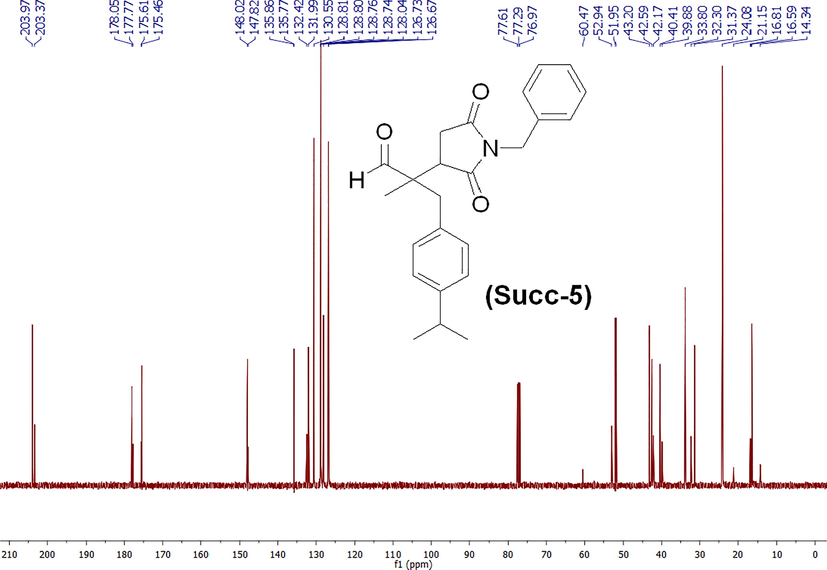
13C NMR spectrum of 2-(1-benzyl-2,5-dioxopyrrolidin-3-yl)-3-(4-isopropylphenyl)-2-methylpropanal (Succ-5).
5.2 Acute toxicity
Experimental animals in groups I (provided with a dose of 50 mg/kg) and II (150 mg/kg) did not die suddenly after the administration of the test compound. However, animals given a dose of 150 mg/kg of the test substance, died within 24 h of administration. Furthermore, the test compound (at a dose of 250 mg/kg) caused immediate death in group III animals. As a result, the lethal dose for the test compound in subjected animals was estimated to be greater than 50 mg/kg body weight.
5.3 Cardiac markers
Table 1 summarized the effects of the tested compound on cardiac enzyme levels and histopathological changes. The current preclinical investigation found that 5-FU-treated rats had significantly higher serum CK-MB and cTnI activity, as well as LDH levels than the control group. The mean value of CK-MB for control was 16.50 U/L, while it was 35.50 U/L for toxic control. In the treated rats, these values revealed a substantial difference as a result of the 5-FU treatment. For 5 mg/kg and 10 mg/kg doses of the test compound, CK-MB levels were 18.33 U/L and 13.50 U/L, respectively, indicating a highly significant reduction compared to the toxic group. Similarly, the mean value of cTnI in the control group was 0.0293 ng/ml, compared to 1.996 ng/ml for the toxic group (a significant increase in serum level) attributed by 5-FU-induced toxicity. cTnI values in Succ-5 treated groups were significantly reduced to 0.7213 ng/ml and 0.0365 ng/ml at the provided doses of 5 mg/kg and 10 mg/kg, respectively. LDH levels were 391.2 U/L in the control group. These values were remarkably increased to 1061 U/L in the toxic controlled group. However, by administering 5 mg/kg and 10 mg/kg dosages of the test agent, LDH levels were highly significantly reduced to 879 U/L and 288.2 U/L, respectively. Values are means ± S.D for each group (n = 6).Symbols represent statistical significance. *** P < 0.001.
Groups
CK-MB(U/L)
CTnI (ng/ml)
LDH (U/L)
Normal control
16.50 ± 0.6191***
0.02933 ± 0.001745***
391.2 ± 9.495***
Standard drug control (Atenolol 20 mg/kg)
17.83 ± 0.6540***
0.08567 ± 0.01731***
604.2 ± 19.58***
Toxic control (5-FU 150 mg/kg)
35.50 ± 1.928###
1.996 ± 0.04584***
1061 ± 22.20###
Succ-5 (5 mg/kg)
18.33 ± 1.022***
0.0365 ± 0.001821***
879.5 ± 34.62***
Succ-5 (10 mg/kg)
13.50 ± 1.803***
0.0365 ± 0.001821***
288.2 ± 45.39***
Elevated levels of diagnostic markers (used as indicative parameters) can be found in the myocardium. As a result of its impairment, it quickly releases its contents into the extracellular fluid (Panteghini, Pagani, & Cuccia, 1987). Because of its abundance in cardiac tissue and sensitivity, serum CK-MB isoenzyme activity testing is an essential diagnostic sign. Changes in plasma membrane integrity and permeability are reflected by its increased serum activity (Farvin et al., 2004). Cardiac troponin I (cTnI) is a cardiac tissue-specific intracellular structural protein. For acute myocardial infarction and drug-induced cardiotoxicity, it is considered the gold standard. Its significance stems from the presence of a highly sensitive and specific biochemical marker of cardiac cell death (Farvin et al., 2004).The lactate dehydrogenase (LDH) test checks the body for evidence of tissue damage. Because its job is to convert sugars to energy, when oxygen consumption drops, LDH levels rise (Hammond, Nadal-Ginard, Talner, & Markert, 1976). Many chronic diseases, such as cardiovascular disease, are thought to be exacerbated by oxidative stress (Liguori et al., 2018). Reactive oxygen and nitrogen species (ROS) are produced as a result of this situation (RNS). These substances cause cell death by interfering with and oxidizing biological molecules such as DNA, proteins, and membrane lipids (Forman & Zhang, 2021). Increased metabolism, resulting in ATP depletion, increased superoxide ions, and decreased antioxidant capacity; arterial vasoconstriction, and changed plasma levels of chemicals implicated in blood coagulation and fibrinolysis (Mohamed et al., 2016). 5-FU metabolites are thought to interfere with calcium channel-dependent membrane function, modify contractile proteins, cause oxidative damage and the production of vasoactive chemicals such as histamine and catecholamines, and initiate autoimmune pathways, among other things (Cianci et al., 2003; Parker, Cheng, & therapeutics, 1990). Excessive catecholamines cause cardiac muscle contractions to increase, which could lead to cardiotoxicity. Oxidative stress kills cells, causes coronary artery spasms, and reduces the oxygen-carrying capacity of red blood cells, culminating in myocardial ischemia, cardiac arrest, and sudden death (Polk, Vistisen, Vaage-Nilsen, Nielsen, & toxicology, 2014). 5-FU-induced oxidative stress also affects endothelial function, resulting in reduced NO levels and higher inflammatory cytokine release. This can result in atherosclerosis, which is a type of heart disease, as well as neurological issues (Leitão et al., 2007). 5-FU-induced cardiotoxicity is characterized by hemorrhagic infarctions, cardiac inflammatory response with interstitial fibrosis, arterial endothelial damage, and eventual thrombosis (Bertolini et al., 2001). Various antioxidant-rich natural or synthetic compounds have been found to reduce heart risk (Hamilton, sports, & exercise, 2007). In previous studies, succinimide derivatives have been shown to exhibit calcium channel blocking properties as well as the ability to scavenge free radicals in earlier studies (Ahmad et al., 2019). Furthermore, few studies have reported their anti-inflammatory and analgesic properties as well (Cieślak et al., 2021). The drug's likely action involves blocking the calcium channel, which causes vasodilatation and reduced contractility, improving blood flow. Calcium channel blocking ability may limit catecholamine release, resulting in less phosphorylation and proper membrane function. Docking study also reveals good affinity with beta 2 adrenergic receptors which also play an important role in vasodilatation.
5.4 Liver enzymes analysis
Serum analysis revealed that the 5-FU-treated group had higher levels of liver enzymes. In rats, injected with 5-fluorouracil (150 mg/kg BW I.P), the levels of ALP (alkaline phosphatase), ALT (alanine transaminase), AST (aspartate aminotransferase), BT (bilirubin total), and BD (direct bilirubin) were significantly higher than their corresponding levels in the control group. The mean ALP level in animals in the control group was 35.51 U/L, while it was 40.83 U/L in 5-FU-treated rats. However, Succ-5 treated groups showed significant reductions at doses of 5 mg/kg and 10 mg/kg, with mean values of 31.23 U/L and 24.02 U/L, respectively. Similarly, test compound treatment at doses of 5 mg/kg and 10 mg/kg reduced mean AST readings from 37.56 U/L to 25.50 U/L and 24.08 U/L, respectively, In comparison with the 5-FU-treated group, the test chemical had a significant effect on the liver enzymes ALT to reduce from 52.57 to 38.17 and 29.16, at the given doses of 5 mg/kg and 10 mg/kg, respectively. Similarly, Succ-5 led to significant reductions in mean BT values from 16.80 mg/dl to 15.02 mg/dl and 12.08 mg/dl at the doses of 5 and 10 mg/kg, respectively. Furthermore, mean values of BD were also reduced from 1.97 mg/dl to 1.527 mg/dl and 0.863 mg/dl which shows a significant effect of the test compound on both doses. In addition, the higher dose of 10 mg/kg of the test compound produced better results than the dose of 5 mg/kg, demonstrating the drug's dose-dependent effects, as indicated in Table 2. Values are means ± S.D for each group (n = 6). Symbols represent statistical significance.*P value 0.05, **P value 0.01, ***P value 0.001, and ns denotes not significant. ALP (alkaline phosphatase), ALT (alanine transaminase), AST (aspartate aminotransferase), BT (bilirubin total), BD (direct bilirubin).
Groups
ALP(U/L)
ALT(U/L)
AST(U/L)
BT (mg/dL)
BD (mg/dL)
Normal control
34.18 ± 1.213*
33.13 ± 1.504***
26.83 ± 0.8724***
7.142 ± 0.200***
0.218 ± 0.015***
Standard drug control
35.00 ± 0.9661 ns
38.68 ± 0.7800***
25.00 ± 1.155***
8.012 ± 0.195***
0.366 ± 0.022***
Toxic control
40.83 ± 1.815###
52.57 ± 1.235 ###
37.56 ± 1.262###
16.80 ± 0.173###
1.907 ± 0.142###
Succ-5 5 mg/kg
31.23 ± 1.505***
38.17 ± 0.7950***
25.50 ± 1.118***
15.02 ± 0.3017***
1.527 ± 0.1270*
Succ-5 10 mg/kg
24.02 ± 2.177***
29.16 ± 1.4057***
24.08 ± 0.6758***
12.80 ± 0.2343***
0.8633 ± 0.1059***
Biochemically, ALT-catalyzed processes can remodel the site and release glutamate and pyruvate; hence elevated AST levels suggest liver injury. As a result, ALT is thought to be a more specific indicator of liver disease than AST. Elevated serum concentrations of these enzymes indicate that the hepatic membrane has lost its functional integrity. Liver function is also affected by serum protein, total protein, ALP, and total bilirubin levels (Shehab, Abu-Gharbieh, Bayoumi, & medicine, 2015).
The hepatic artery system and portal vein supply 20 % of cardiac output to the liver under normal physiological circumstances. The buffered hepatic artery response occurs when portal flow declines and adenosine is released, causing the hepatic artery to dilate. If hypotension continues, visceral blood supply is severely decreased, resulting in severe hypoxia and necrosis, which can result in high bilirubin levels and alterations in alkaline phosphatase (ALP) (Henrion et al., 1994; Naschitz, Yeshurun, & Shahar, 1990).On the other hand liver is responsible for the majority of chemical metabolism highlights the organ's importance as well as vulnerability to drug-induced metabolic damage. Drug-induced liver injury is common, accounting for around half of all acute liver failure cases and mimicking all types of acute and chronic liver disease (Reid et al., 2005). They are considered one of the most dangerous side effects of certain medications including chemotherapeutic agents(Pandit et al., 2012). Most medicines and their metabolites linked to liver damage produce hepatotoxicity by interfering with cellular antioxidant mechanisms, resulting in the generation of free radicals and oxidative stress (Kaplowitz, 2001).These reactions result in impaired blood flow and a decrease in the removal of hazardous chemicals from the bloodstream(Hunter et al., 1988). 5-Fluorouracil hepatotoxicity is also a well-known occurrence (Benincasa et al., 2021). Inhibition of thymidylate synthase may be responsible for this impairment(Javot et al., 2011). Furthermore, 5-fluorouracil is extensively degraded by microsomal enzymes in the liver, and the generation of toxic intermediates may induce liver damage (Hoofnagle, 2013). Previous studies have reported that succinimide derivatives have antioxidant properties and can help to prevent oxidative stress by scavenging free radicals (Ahmad et al., 2019; Sadiq et al., 2015). Its derivatives interact with calcium channels, thereby letting the membrane fraction be normalized. Considering the above-stated mechanisms, succinimide and its derivatives are thought to have hepato-protective effects (V Krivoshein, 2016).
5.5 Lipid profile
Blood samples of the experimental animals were assessed for the determination of various lipid biomarkers as indicative parameters regarding the safety profile of the test compound. When compared to the control group, the 5-FU-treated group had an extensive rise in blood total cholesterol (TC), triglycerides (TG), very low-density lipids (VLDL-c), and low-density lipids (LDL-c) fractions, as well as a significant drop in high-density lipids HDL-c. However, treatment with the tested compound significantly decreased the TC, TAG, VLDL-c, and LDL-c levels while increasing HDL-c levels at a dose of 10 mg/kg. Nevertheless, a dose of 5 mg/kg had no significant effect on HDL-c to restore the damage done in the toxic control group of animals (Table 3). For each group (n = 6), the values are the means and standard deviations. Statistical significance is represented by symbols. *** P 0.001.ns stands for statistically insignificant.
Group
TC (mg/dl)
TG(mg/dl)
HDL-c (mg/dl)
LDL-c (mg/dl)
VLDL-c (mg/dl)
Normal Control (Saline)
27.95 ± 0.3425***
113.7 ± 1.404***
63.12 ± 1.608***
78.07 ± 0.9472***
24.27 ± 0.8417***
Standard control (Atenolol)
37.89 ± 0.9605***
133.0 ± 6.005***
68.66 ± 2.989***
81.44 ± 0.9891***
23.23 ± 0.8126***
Toxic control (5-FU)
63.43 ± 0.9452###
209.6 ± 4.037###
36.56 ± 1.626###
150.0 ± 1.891###
42.47 ± 0.6625###
Succ-5 (5 mg/kg)
19.87 ± 2.056***
165.9 ± 4.799***
37.22 ± 7.019 ns
114.1 ± 2.019***
29.30 ± 0.8120***
Succ5 (10 mg/kg)
14.34 ± 1.221***
140.2 ± 6.086***
65.97 ± 1.434***
70.76 ± 2.208***
24.25 ± 1.015***
Studies conducted before, have described the alteration in lipid profile by 5-fluorouracil (Abdel-Hamid, Soliman, Helaly, & Ragab, 2011; Mohamed et al., 2016). In this connection, chemotherapy-induced hyper-triglycemia can cause vascular lesions and severe pancreatitis, thus it's critical to keep this risk under control (Saito, Takekuma, Komatsu, & Sugawara, 2021). Thymidine phosphorylase is thought to be implicated in elevated triglyceride induction (Javot et al., 2011). Similarly, thymidine phosphorylase and thymidine kinase compete for thymidine and catalyze synthetic and catabolic processes involved in proliferation and angiogenesis, respectively (Brockenbrough et al., 2009). The function of oxidative alteration of LDL in the development of atherosclerosis has been demonstrated in animal models (Steinberg, 2009). Triglycerides and low-density lipids are produced as a result of lipid peroxidation, which can aggravate cardiovascular and liver disorders (Kasapović et al., 2010). Recent research also supports the theory of multiple LDL modification, claiming that LDL particles undergo a variety of modifications in blood flow and in the vessel wall that change their density, size, and chemical properties, with oxidation being the final step in the overall cascade that leads to atherosclerogenic properties (Poznyak et al., 2021). Lipid peroxidation is a major cause of elevated triglycerides. As previously stated, succinimides have antioxidant properties, which may lead to a reduction in reactive oxygen and an increase in high-density lipids. More research is needed to determine the benefits and processes of these succinimide derivatives in reducing oxidative stress and pathogenicity.
5.6 Histopathological study
5.6.1 Effect on cardiac muscles
The protective effect of the test chemical was also demonstrated in histopathological investigations of heart tissue. Rats in the control group had normal morphology in their cardiac sections. In the same way, heart tissues from rats given with 5-Fluorouracil added with Atenolol showed normal morphology. On the other hand, cardiovascular cells treated with 5-Fluorouracil alone showed generalized cardiac edema, perivascular mononuclear cell infiltration, hyaline degeneration, necrosis, and cellular invasion. These effects of 5-FU are in agreement with previous studies due to thrombosis and oxidative stress (Dechant et al., 2012)0.5-Fluorouracil and Succ-5 (at the dose of 5 mg/kg I.P) treated group showed degeneration and mononuclear cell infiltration, hyaline degeneration, and a few necroses. However, the 5-Fluorouracil added with the test compound (at the dose of 10 mg/kg I.P.) treatment group showed significant protection against 5-Florouracil-induced cardiac injury. Animals in this group showed mild mononuclear cell infiltration around normal blood vessels (see images increased, 10 × ). Fig. 3 and Table 4. 0-none,1- mild., 2- moderate, 3-severe.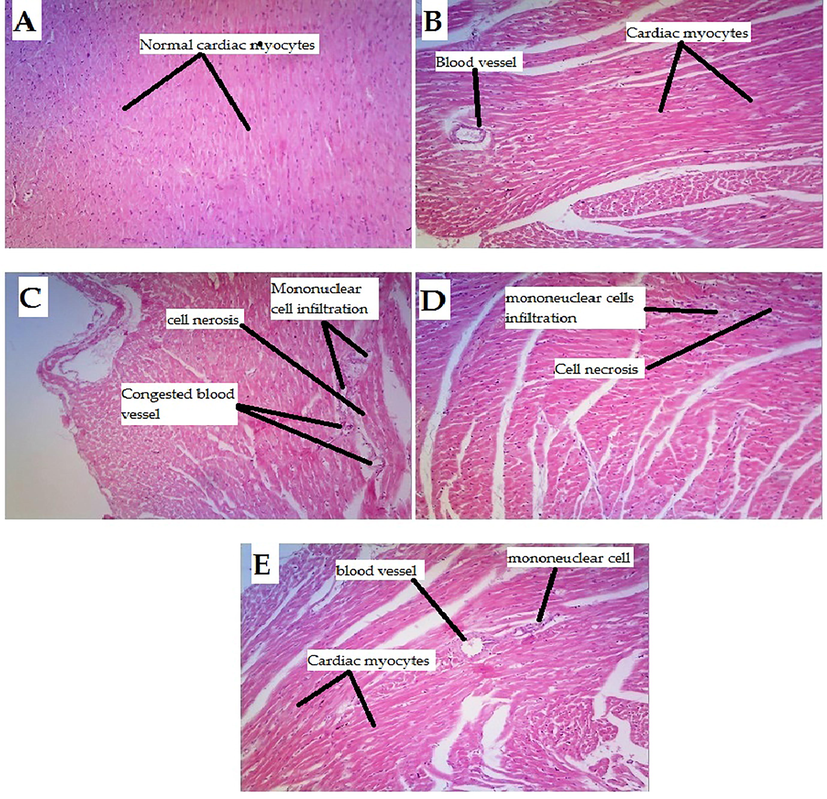
Histopathological changes in rat hearts induced by 5-Florouracil and protective effect of the test compound (A) Rat heart sections from the control group (normal saline) had normal morphology. (B) Heart sections of rats treated with 5-Florouracil + Athenol had normal morphology with no cellular infiltration and normal blood vessels. (C) Heart sections from rats treated with 5-Florouracil alone, showing generalized cardiac edema, perivascular mononuclear cell infiltration, hyaline degeneration, and necrosis. (D) 5-Florouracil + Succ-5 (5 mg/kg I.P) treated group showed degeneration and mononuclear cell infiltration, hyaline degeneration, and a few necrosis (E) 5-Florouracil + Succ-5 (10 mg/kg I.P) treated group against 5-Florouracil induced heart injury. The 5-Florouracil + Succ-5 (10 mg/kg I.P.) treatment group showed significant protection against 5-Florouracil induced cardiac injury as mild mononuclear cell infiltration was observed in and around normal blood vessels (Increased, 10 × ).
GROUPS
Normal Control (Normal saline)
Standard drug controle Atenolol + 5-FU treated group
Toxic Control 5-FU treated group
Succ-5 5 mg/kg Treated group
Succ-5 10 mg/kg Treated group
Cellular infiltration
0
0
2
1
1
Necrosis
0
0
2
1
0
arterial congetion
0
0
3
1
0
Fibrosis
0
0
2
1
0
Several researches in the past have revealed that succinimide compounds have the ability to inhibit calcium channels as well as scavenge free radicals. Consequently, they lower oxidative stress, preserving the cardiac tissues' infarction area(Ahmad et al., 2019).
5.6.2 Effect on liver tissues
Besides biochemical evaluation, the hepatoprotective effect of the test compound was assessed by histopathological examination as well. The typical histological anatomy of the hepatic lobe was observed in the control group, which included a normal central vein (CV) and regular hepatocytes. The 5-Fluorouracil-treated group, on the other hand, showed centrilobularhepatocellular necrosis with bleeding, mononuclear inflammatory cell infiltration, and fibrosis. It is also well established that5-FU-induced liver injury is characterized by hepatic necrosis, vacuolated cytoplasm, congested hepatic nuclei, congested hepatic sinusoids, and inflammatory cell infiltration (Abou-Zeid & Zoology, 2014). Hepatocyte degeneration, mononuclear inflammatory cell infiltration, and fibrosis were observed in the group treated with 5-Fluorouracil added with Succ-5 at a dose of 5 mg/kg I.P. However, the target tissues of the animals belong to the group treated with 5-Fluorouracil and Succ-5 at 10 mg/kg I.P., demonstrated significant protective effects through hepatocyte regeneration, moderate fibrosis, and normal central vein Fig. 4 and Table 5. 0 = None, 1 = Mild; 2 = Moderate; 3 = Severe.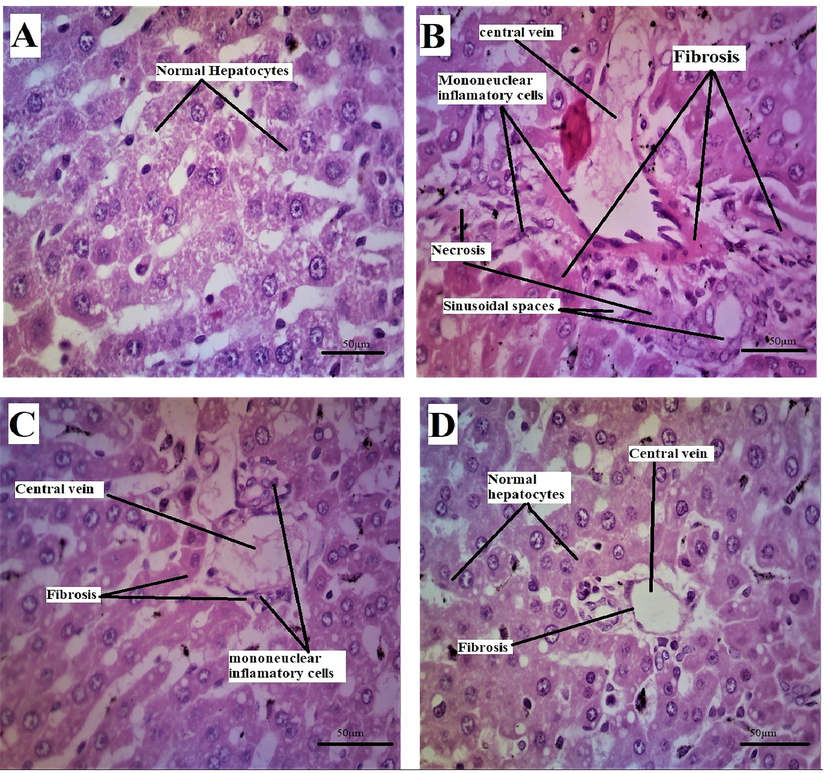
Rat liver (A) of the normal group showing the normal histological structure of the hepatic lobe with normal central vein (CV) and normal hepatocytes. (B) 5-Florouracil treated group showing centrilobular hepatocellular necrosis associated with hemorrhage and mononuclear inflammatory cell infiltration and fibrosis. (C) Group treated with 5-Florouracil + Succ-5 5 mg/kg I.P showing hepatocyte degeneration, mononuclear inflammatory cell infiltration and fibrosis with congested central vein. (D) Group treated with the formula 5-Florouracil + Succ-5 10 mg/kg I.P, showing hepatocyte regeneration and mild fibrosis (Enlargement, 40 × ).
GROUPS
NORMAL CONTROL (Normal saline)
TOXIC CONTROL 5-fu treated group
Succ-5 5 mg/kg Treated group
Succ-5 10 mg/kg Treated group
Sinusoidal spaces
0
2
1
1
Necrosis
0
3
1
0
hepatocyte degeneration
0
3
1
1
Fibrosis
0
3
2
1
It is possible to explain this protective effect by the fact that succinimides derivatives have free radical scavenging action (Ahmad et al., 2019).
5.7 Immunohistochemistry (IHC) of heart tissues
The major ROS-producing enzymes in the cardiovascular system include the Nox-based reduced nicotinamide-adenine dinucleotide (NADPH) oxidases, xanthine oxidase, the mitochondrial electron transport system, and, under certain circumstances, NO synthase. Diseases like hypertension, atherosclerosis, hypercholesterolemia, diabetes, and insulin resistance enhance the expression or activity of these enzymes, which increases the production of ROS [66]. This ROS activates pro-inflammatory cytokines COX-2 and TNF-α. Therefore, oxidative stress can lead to inflammation. IHC was used to evaluate the expression. Results of the IHC study revealed that, as compared to the normal control (saline) group, the5-FU(150 mg/kg) treated group's heart tissues had considerably higher levels of these markers. When compared to the 5-FU-treated group, the Atenolol (20 mg/kg) and Succ-5 (5 mg/kg and 10 mg/kg) groups had significantly lower COX-2 and TNF-α expression as shown in Figs. 5 and 7, respectively. Figs. 6 and 8 graphically represents the relative integrative density of IHC.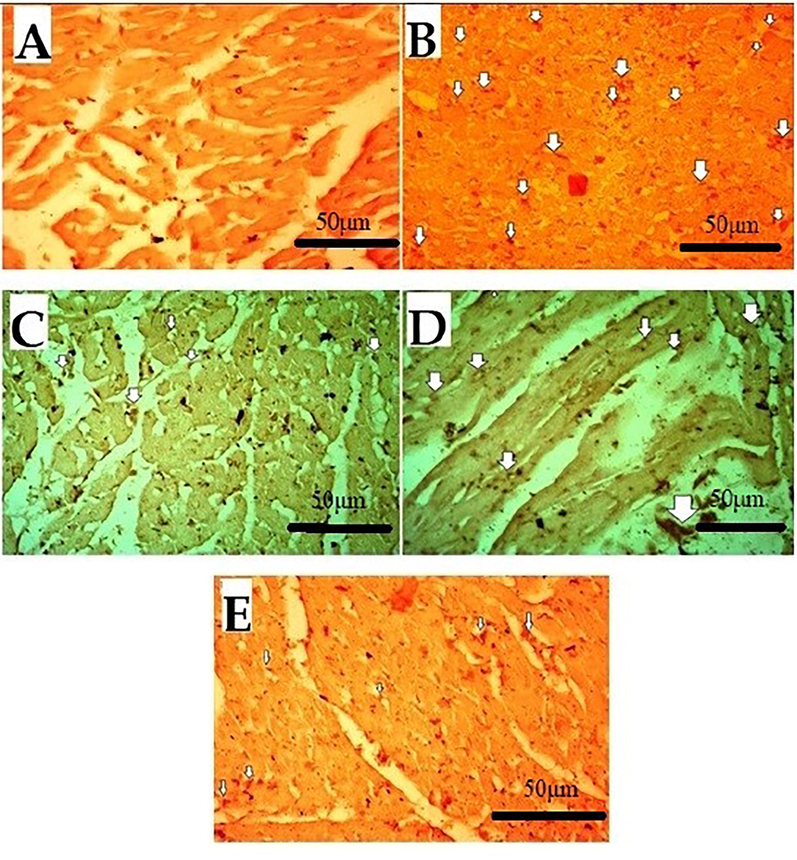
Effect of Succ-5 (at 5 mg/kg, 10 mg/kg doses) and Atenolol against immune-histochemical expression of cyclooxygenase-2 (COX-2) in 5-FU induced toxicity rats' heart tissue, using immunohistochemistry technique. A. saline group, B. 5-FU treated group, C. Atenolol treated group, D. Succ-5 at 5 mg/kg dose treated group, E.Succ-5 at 10 mg/kg dose treated group. White arrows indicate the expression of immune marker on cardiac cells. (Enlargement, 40 × ).
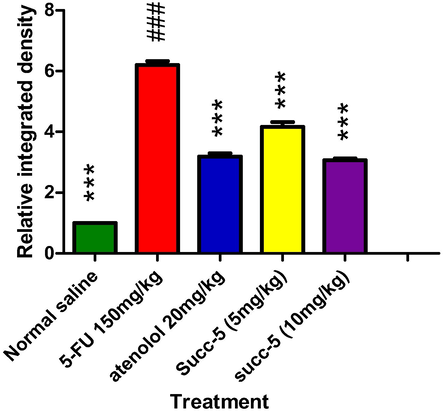
Graphical presentation of inhibitory effect by Succ-5 at 5 mg/kg, 10 mg/kg doses and Atenolol against cyclooxygenase-2 (COX-2) by in rat’s heart tissue, using immune-histochemical technique. Values expressed as mean ± SEM (n = 6). Where ***p < 0.001 vs 5-FU group.
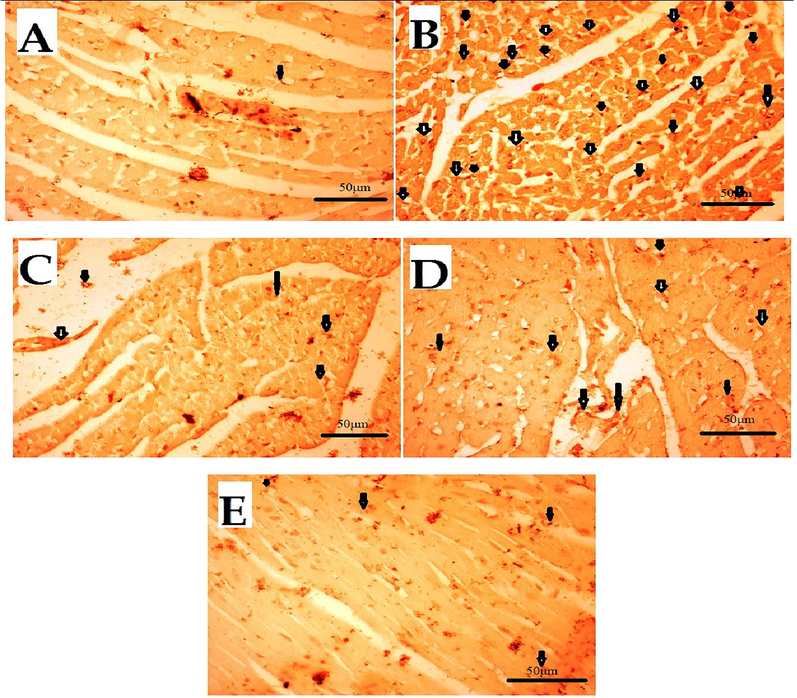
Effect of Succ-5 (at 5 mg/kg, 10 mg/kg doses) and Atenolol against immune-histochemical expression of tumour necrosis factor alpha (TNF-α) in 5-FU induced toxicity in rats' heart tissue, using immunohistochemistry technique. A. Saline group, B. 5-FU treated group, C. Atenolol treated group, D. Succ-5 (at 5 mg/kg dose) treated group, E.Succ-5 (at 10 mg/kg dose) treated group. Black arrows indicate the expression of immune marker on cardiac cells. (Enlargement, 40 × ).
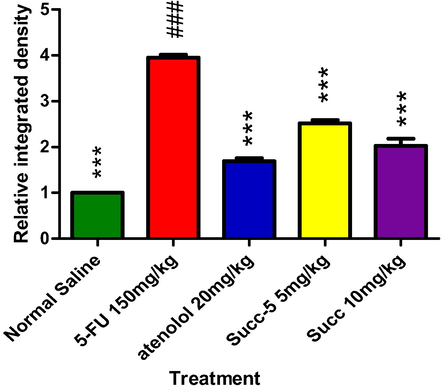
Graphical presentation of inhibitory effect bySucc-5(at 5 mg/kg, 10 mg/kg doses) and Atenolol against tumor necrosis factor alpha (TNF- α) in rat’s heart tissue, using immunohistochemical technique. Values expressed as mean ± SEM (n = 6). Where; ***p < 0.001 vs 5-FU treated group.
5.8 Docking studies
T-shape calcium channels are considered important in the regulation of blood pressure and cardiac function. We tried to explore the mechanism of action of our synthesized compound. We selected T-type calcium channels (Cav 3.1) for docking studies. Cryo-EM structure of antagonist-bound human Cav 3.1 with Protein data bank accession code 6KZP.
After validation of docking protocol by using redock method. Native ligand was re-docked into the binding site of 6KZP. The docking procedure with root mean square deviation (RMSD) value < 2.0 Å between experimental and redocked ligand was choose for docking simulations. Two-dimensional interaction plots of native ligand and synthesized compound are shown in Fig. 9. Hydrogen bond interactions with Leu920, Asn952 and Lys1462 (Fig. 9a).While Succ-5 interacts with Asn952 and Lys1462 via hydrogen bond interactions. Phe956 form π-π stacked interaction with phenyl ring (Fig. 9b). The computed binding energy values of native compound of the Succ-5 in the binding site of 6KZP are − 7.9834 kcal/mol and − 6.9618 kcal/mol respectively.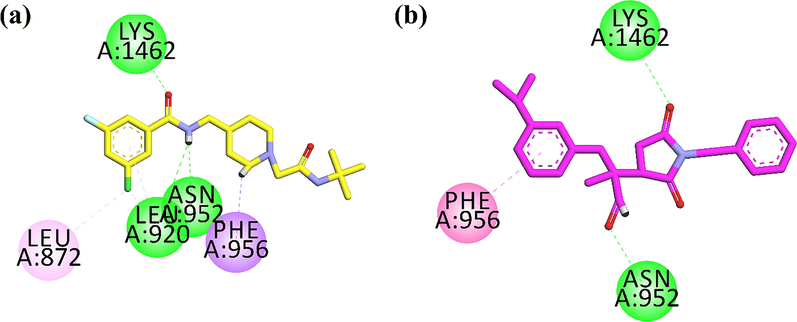
2D interaction plot of (a) native compound (yellow stick model) and (b) Succ-5(pink stick model) in the binding site of 6KZP.
Next, we docked Succ-5in the binding site of human beta2-adrenergic G protein-coupled receptor. 3D high resolution crystal structure with PDB code 2RH1 was obtained with (2S)-1-(9H-Carbazol-4-yloxy)-3-(isopropylamino) propan-2-ol (CAU) as native ligand. 2D interaction plots of native ligand and Succ-5are shown in Fig. 10. Native interacts with the active site residues Asp113, Ser203 and Asn312 via hydrogen bond interaction. Phe193, Tyr199 and Phe290 forms π-π stacked interaction. While, Val114 establishes π-σ type of interaction (Fig. 10a). Succ-5 forms hydrogen bond interactions with Asn312 and Tyr316. Phe289 and Phe290 interact with phenyl ring through π-π stacked interaction. While Val114 establishes π-σ type of interaction (Fig. 10b). The computed binding energy values of native compound of the Succ-5in the binding site of 2RH1 are − 8.2680 kcal/mol and − 7.5822 kcal/mol, respectively. Here we can conclude that Succ-5interacts toward human beta2-adrenergic G protein-coupled receptor (S = -7.5822 kcal/mol) with slight stronger affinity than calcium channel T-type (S = -6.9618 kcal/mol).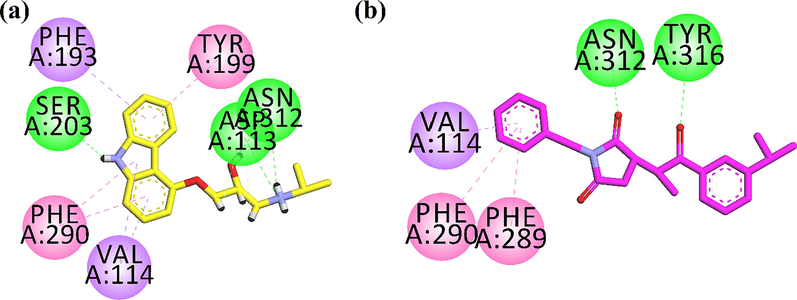
2D interaction plot of (a) native compound (yellow stick model) and (b) Succ-5(pink stick model) in the binding site of human beta2-adrenergic G protein-coupled receptor.
6 Conclusion
In this preclinical evaluation, it was concluded that the new succinimide derivative (Succ-5) was found to have significant cardioprotective, hepatoprotective, and lipids-lowering properties against 5-fluorouracil-induced toxicity. Furthermore, the tested compound's protective effects were greater at a dose of 10 mg/kg, I.P than at 5 mg/kg, I.P. The analysis of binding energy values computed via docking simulations showed that AS-a-5 interacts toward human beta2-adrenergic G protein-coupled receptor with slight stronger affinity than calcium channel T-type.
Acknowledgements
Authors would like to acknowledge the support of the Deputy for Research and Innovation- Ministry of Education, Kingdom of Saudi Arabia for this research through a grant ( NU/IF/INT/01/007) under the institutional Funding Committee at Najran University, Kingdom of Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cytotoxic potency and induced biochemical parameters in mice serum of new furan derivatives against liver cancer cell line. Acta Pol. Pharm.. 2011;68(4):499-505.
- [Google Scholar]
- Abou-Zeid, N. R. J. T. J. o. B., & Zoology, A. (2014). Ameliorative effect of vitamin C against 5-fuorouracil-induced hepatotoxicity in mice: A light and electron microscope study. 67(4), 109-118.
- Ahmad, A., Ullah, F., Sadiq, A., Ayaz, M., Rahim, H., Rashid, U., . . . Therapy. (2019). Pharmacological evaluation of aldehydic-pyrrolidinedione against HCT-116, MDA-MB231, NIH/3T3, MCF-7 cancer cell lines, antioxidant and enzyme inhibition studies. 13, 4185
- Ahmad, A., Ullah, F., Sadiq, A., Ayaz, M., Jan, M. S., Shahid, M., . . . Therapy. (2020). Comparative cholinesterase, α-glucosidase inhibitory, antioxidant, molecular docking, and kinetic studies on potent succinimide derivatives. 14, 2165
- Novel risk stratification for chemotherapy-induced heart failure. JACC Heart Fail.. 2019;7(4):368-369.
- [Google Scholar]
- Benincasa, G., Cuomo, O., Vasco, M., Vennarecci, G., Canonico, R., Della Mura, N., . . . Hepatology. (2021). Epigenetic-sensitive challenges of cardiohepatic interactions: clinical and therapeutic implications in heart failure patients. 33(10), 1247-1253
- Acute cardiotoxicity during capecitabine treatment: a case report. Tumori.. 2001;87(3):200-206.
- [Google Scholar]
- Bhat, M. A., Al-Omar, M. A., Khan, A. A., Alanazi, A. M., Naglah, A. M. J. D. d., development, & therapy. (2019). Synthesis and antihepatotoxic activity of dihydropyrimidinone derivatives linked with 1, 4-benzodioxane. 13, 2393.
- Bibi, M., Qureshi, N. A., Sadiq, A., Farooq, U., Hassan, A., Shaheen, N., . . . Khan, F. A. J. E. j. o. m. c. (2021). Exploring the ability of dihydropyrimidine-5-carboxamide and 5-benzyl-2, 4-diaminopyrimidine-based analogues for the selective inhibition of L. major Dihydrofolate reductase. 210, 112986.
- Bibi, A., Shah, T., Sadiq, A., Khalid, N., Ullah, F., & Iqbal, A. J. R. J. o. O. C. (2019). L-isoleucine-catalyzed michael synthesis of N-alkylsuccinimide derivatives and their antioxidant activity assessment. 55(11), 1749-1754
- Biovia, D. S. J. S. D., CA, USA. (2017). Discovery studio visualizer. 936.
- Brockenbrough, J. S., Morihara, J. K., Hawes, S. E., Stern, J. E., Rasey, J. S., Wiens, L. W., . . . Cytochemistry. (2009). Thymidine kinase 1 and thymidine phosphorylase expression in non-small-cell lung carcinoma in relation to angiogenesis and proliferation. 57(11), 1087-1097
- Cianci, G., Morelli, M., Cannita, K., Morese, R., Ricevuto, E., Di Rocco, Z., . . . Ficorella, C. J. B. j. o. c. (2003). Prophylactic options in patients with 5-fluorouracil-associated cardiotoxicity. 88(10), 1507-1509
- Cieślak, M., Napiórkowska, M., Kaźmierczak-Barańska, J., Królewska-Golińska, K., Hawrył, A., Wybrańska, I., & Nawrot, B. J. I. j. o. m. s. (2021). New Succinimides with Potent Anticancer Activity: Synthesis, Activation of Stress Signaling Pathways and Characterization of Apoptosis in Leukemia and Cervical Cancer Cells. 22(9), 4318
- Dechant, C., Baur, M., Böck, R., Czejka, M., Podczeck-Schweighofer, A., Dittrich, C., & Christ, G. J. C. R. i. O. (2012). Acute reversible heart failure caused by coronary vasoconstriction due to continuous 5-fluorouracil combination chemotherapy. 5(2), 296-301
- Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol. Res.. 2004;50(3):231-236.
- [Google Scholar]
- Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov.. 2021;20(9):689-709.
- [Google Scholar]
- Hamilton, K. L. J. M., sports, s. i., & exercise. (2007). Antioxidants and cardioprotection. 39(9), 1544-1553.
- Myocardial LDH isozyme distribution in the ischemic and hypoxic heart. Circulation. 1976;53(4):637-643.
- [Google Scholar]
- Henrion, J., Descamps, O., Luwaert, R., Schapira, M., Parfonry, A., & Heller, F. J. J. o. h. (1994). Hypoxic hepatitis in patients with cardiac failure: incidence in a coronary care unit and measurement of hepatic blood flow. 21(5), 696-703
- LiverTox: a website on drug-induced liver injury. In: Drug-Induced Liver Disease. Elsevier; 2013. p. :725-732.
- [Google Scholar]
- Huneif, M. A., Alshehri, D. B., Alshaibari, K. S., Dammaj, M. Z., Mahnashi, M. H., Majid, S. U., . . . Pharmacotherapy. (2022). Design, synthesis and bioevaluation of new vanillin hybrid as multitarget inhibitor of α-glucosidase, α-amylase, PTP-1B and DPP4 for the treatment of type-II diabetes. 150, 113038
- Hunter, P. J., Smaill, B. H. J. P. i. b., & biology, m. (1988). The analysis of cardiac function: a continuum approach. 52(2), 101-164
- Jan, M. S., Ahmad, S., Hussain, F., Ahmad, A., Mahmood, F., Rashid, U., . . . Sadiq, A. J. E. J. o. M. C. (2020). Design, synthesis, in-vitro, in-vivo and in-silico studies of pyrrolidine-2, 5-dione derivatives as multitarget anti-inflammatory agents. 186, 111863
- Management of chemotherapy-induced adverse effects in the treatment of colorectal cancer. Drug Saf.. 2001;24(5):353-367.
- [Google Scholar]
- Javot, L., Spaëth, D., Scala-Bertola, J., Gambier, N., Petitpain, N., & Gillet, P. J. B. j. o. c. (2011). Severe hypertriglyceridaemia during treatment with capecitabine. 104(7), 1238-1239
- Toxicological evaluation of novel cyclohexenone derivative in an animal model through histopathological and biochemical techniques. Toxics. 2021;9(6):119.
- [Google Scholar]
- Kaplowitz, N. J. D. s. (2001). Drug-induced liver disorders. 24(7), 483-490.
- Antioxidant status and lipid peroxidation in the blood of breast cancer patients of different ages after chemotherapy with 5-fluorouracil, doxorubicin and cyclophosphamide. Clin. Biochem.. 2010;43(16–17):1287-1293.
- [Google Scholar]
- V Krivoshein, A. J. C. P. D. (2016). Antiepileptic drugs based on the α-substituted amide group pharmacophore: from chemical crystallography to molecular pharmaceutics. 22(32), 5029-5040.
- Leitão, R., Ribeiro, R., Bellaguarda, E., Macedo, F., Silva, L., Oriá, R., . . . pharmacology. (2007). Role of nitric oxide on pathogenesis of 5-fluorouracil induced experimental oral mucositis in hamster. 59(5), 603-612
- Liguori, I., Russo, G., Curcio, F., Bulli, G., Aran, L., Della-Morte, D., . . . Bonaduce, D. J. C. i. i. a. (2018). Oxidative stress, aging, and diseases. 13, 757
- Lim, S. S., Vos, T., Flaxman, A. D., Danaei, G., Shibuya, K., Adair-Rohani, H., . . . Andrews, K. G. J. T. l. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. 380(9859), 2224-2260.
- Longley, D. B., Harkin, D. P., & Johnston, P. G. J. N. r. c. (2003). 5-fluorouracil: mechanisms of action and clinical strategies. 3(5), 330-338
- Mahmood, F., Jan, M. S., Ahmad, S., Rashid, U., Ayaz, M., Ullah, F., . . . Aasim, M. J. F. i. c. (2017). Ethyl 3-oxo-2-(2, 5-dioxopyrrolidin-3-yl) butanoate derivatives: anthelmintic and cytotoxic potentials, antimicrobial, and docking studies. 5, 119
- Mahmood, F., Khan, J. A., Mahnashi, M. H., Jan, M. S., Javed, M. A., Rashid, U., . . . Bungau, S. J. M. (2022). Anti-Inflammatory, Analgesic and Antioxidant Potential of New (2 S, 3 S)-2-(4-isopropylbenzyl)-2-methyl-4-nitro-3-phenylbutanals and Their Corresponding Carboxylic Acids through In Vitro, In Silico and In Vivo Studies. 27(13), 4068
- Maring, J., Piersma, H., van Dalen, A., Groen, H. J., Uges, D. R., De Vries, E. G. J. C. c., & pharmacology. (2003). Extensive hepatic replacement due to liver metastases has no effect on 5-fluorouracil pharmacokinetics. 51(2), 167-173
- Mohamed, E. T., Safwat, G. M. J. B.-S. u. j. o. b., & sciences, a. (2016). Evaluation of cardioprotective activity of Lepidium sativum seed powder in albino rats treated with 5-fluorouracil. 5(2), 208-215
- 5-FU cardiotoxicity: vasospasm, myocarditis and sudden death. Curr.Cardiol. Rep.. 2021;23(3):1-8.
- [Google Scholar]
- Nugent, T. C., Sadiq, A., Bibi, A., Heine, T., Zeonjuk, L. L., Vankova, N., & Bassil, B. S. J. C. A. E. J. (2012). Noncovalent bifunctional organocatalysts: powerful tools for contiguous quaternary‐tertiary stereogenic carbon formation, scope, and origin of enantioselectivity. 18(13), 4088-4098
- Pandit, A., Sachdeva, T., & Bafna, P. J. J. A. P. S. (2012). Drug-induced hepatotoxicity: a review. 2(5), 233-243
- Panteghini, M., Pagani, F., & Cuccia, C. J. C. c. (1987). Activity of serum aspartate aminotransferase isoenzymes in patients with acute myocardial infarction. 33(1), 67-71
- Parker, W. B., Cheng, Y. C. J. P., & therapeutics. (1990). Metabolism and mechanism of action of 5-fluorouracil. 48(3), 381-395
- Payne, D. L., & Nohria, A. J. C. h. f. r. (2017). Prevention of chemotherapy induced cardiomyopathy. 14(5), 398-403
- Polk, A., Vistisen, K., Vaage-Nilsen, M., Nielsen, D. L. J. B. p., & toxicology. (2014). A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. 15(1), 1-11
- Poznyak, A. V., Nikiforov, N. G., Markin, A. M., Kashirskikh, D. A., Myasoedova, V. A., Gerasimova, E. V., & Orekhov, A. N. J. F. i. P. (2021). Overview of OxLDL and its impact on cardiovascular health: focus on atherosclerosis. 2248.
- Ray, S., Roy, K., & Sengupta, C. J. A. P. P. (2007). In vitro evaluation of protective effects of ascorbic acid and water extract of Spirulina plantesis (blue green algae) on 5-fluorouracil-induced lipid peroxidation. 64(4), 335-344
- Reid, A. B., Kurten, R. C., McCullough, S. S., Brock, R. W., Hinson, J. A. J. J. o. P., & Therapeutics, E. (2005). Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. 312(2), 509-516
- Sadiq, A., Mahnashi, M. H., Alyami, B. A., Alqahtani, Y. S., Alqarni, A. O., & Rashid, U. J. B. c. (2021). Tailoring the substitution pattern of Pyrrolidine-2, 5-dione for discovery of new structural template for dual COX/LOX inhibition. 112, 104969
- Sadiq, A., & Nugent, T. C. J. C. (2020). Catalytic access to succinimide products containing stereogenic quaternary carbons. 5(38), 11934-11938
- Sadiq, A., Mahmood, F., Ullah, F., Ayaz, M., Ahmad, S., Haq, F. U., . . . Jan, M. S. J. C. C. J. (2015). Synthesis, anticholinesterase and antioxidant potentials of ketoesters derivatives of succinimides: a possible role in the management of Alzheimer’s. 9(1), 1-9
- Management of chemotherapy induced cardiomyopathy. Curr. Cardiol. Rev.. 2011;7(4):245-249.
- [Google Scholar]
- Saito, Y., Takekuma, Y., Komatsu, Y., & Sugawara, M. J. J. o. O. P. P. (2021). Hypertriglyceridemia induced by S-1: a novel case report and review of the literature. 27(4), 1020-1025
- Sakkani, N., & Nanda, S. K. J. A. J. o. O. C. (2022). A Review on the Synthesis and Applications of α‐Alkylidene Succinimides. 11(4), e202200041
- Schuster, D., Laggner, C., & Langer, T. J. C. p. d. (2005). Why drugs fail-a study on side effects in new chemical entities. 11(27), 3545-3559.
- Shah, F. A., Li, T., Kury, L. T. A., Zeb, A., Khatoon, S., Liu, G., . . . Khan, A.-U. J. F. i. n. (2019). Pathological comparisons of the hippocampal changes in the transient and permanent middle cerebral artery occlusion rat models. 10, 1178.
- Shehab, N. G., Abu-Gharbieh, E., Bayoumi, F. A. J. B. c., & medicine, a. (2015). Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. 15(1), 1-12.
- Steinberg, D. J. J. o. l. r. (2009). The LDL modification hypothesis of atherogenesis: an update. 50, S376-S381.
- Südhoff, T., Enderle, M.-D., Pahlke, M., Petz, C., Teschendorf, C., Graeven, U., & Schmiegel, W. J. A. o. o. (2004). 5-Fluorouracil induces arterial vasocontractions. 15(4), 661-664.
- Tanoli, S. T., Ramzan, M., Hassan, A., Sadiq, A., Jan, M. S., Khan, F. A., . . . Mahmood, T. J. B. c. (2019). Design, synthesis and bioevaluation of tricyclic fused ring system as dual binding site acetylcholinesterase inhibitors. 83, 336-347
- ULC, C. J. W., Suite. (2018). Molecular Operating Environment (MOE), 2013.08, 1010 Sherbooke St. 910.
- Heart failure and liver disease: cardiohepatic interactions. JACC Heart Fail.. 2019;7(2):87-97.
- [Google Scholar]







