Translate this page into:
Design, synthesis, antibacterial and antiviral evaluation of chalcone derivatives containing benzoxazole
⁎Corresponding author. wxue@gzu.edu.cn (Wei Xue)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
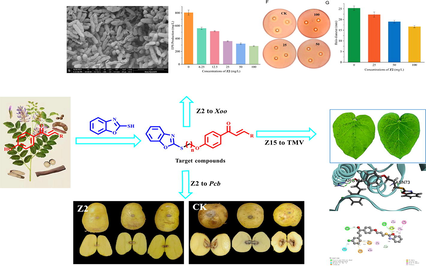
Abstract
In this study, 24 chalcone derivatives containing benzoxazole were designed and synthesized, and the structures of all target compounds were confirmed by NMR and HRMS. The results of antibacterial activity showed that the median effective concentration (EC50) value of Z2 against Xanthomonas oryzae pv.oryzae (Xoo) was 8.10 μg/mL, which was better than thiodiazole copper (60.62 μg/mL), the antibacterial activity of Z2 was verified by scanning electron microscopy (SEM). Antibacterial mechanism of Z2 on Xoo was preliminarily explored, and the results showed concentrations were 100, 50 mg/L, which had a good cell membrane permeability to Xoo and can inhibit the EPS content and the production of extracellular enzymes, affecting the formation of biofilm, preliminary validation of the antibacterial effect of Z2 on Xoo. At 200 mg/L, Z2 had a better protective activity on harvested potatoes, which was comparable with thiodiazole copper. The activity against tobacco mosaic virus (TMV) was determined with half leaf dry spot method. The EC50 value of curative activity of Z15 was 101.97 μg/mL, which was superior to ningnanmycin (NNM) 294.27 μg/mL, and the EC50 value of protective activity of Z16 was 104.05 μg/mL, which was superior to NNM 185.73 μg/mL. Molecular docking results showed Z15, Z16 had a significantly higher affinity with tobacco mosaic virus coat protein (TMV-CP) than NNM. The above results indicated that chalcone derivatives containing benzoxazole have the potential to become antibacterial and antivirial agents.
Keywords
Chalcone derivatives
Benzoxazole
Antibacterial activity
Antiviral activity
Mechanism of action
- 1H NMR
-
1H nuclear magnetic resonance
- 13C NMR
-
13C nuclear magnetic resonance
- 19F NMR
-
19F nuclear magnetic resonance
- HRMS
-
High-resolution mass spectrometry
- EC50
-
Median effective concentration
- DMSO
-
Dimethylsulfoxide
- Xac
-
Xanthomonas axonopodis pv. Citri
- Xoo
-
Xanthomonas oryzae pv.oryzae
- Psa
-
pseudomonas syringae pv.actinidiae
- Ac
-
Acidovorax citrulli
- Rs
-
Ralstonia solanacearum
- Pcb
-
Pectobacterium carotovorum subsp. Brasiliense
- Xcm
-
Xanthomonas campestris pv. mangiferae indicae
- SEM
-
Scanning electron microscopy
- TMV
-
Tobacco mosaic virus
- NNM
-
Ningnanmycin
- TMV-CP
-
Tobacco mosaic virus coat protein
- EPS
-
Exopolysaccharide
- SAR
-
Structure–activity relationship
Abbreviations
1 Introduction
Food is the basis for the survival and development of human beings, and pesticides are the most effective means to ensure food security (Song, 2020). All the time, plant diseases caused by bacteria and viruses have resulted in serious impact on the grain yield and quality of the world every year, causing huge economic losses (Cao et al., 2023; Hu et al., 2023). According to survey data from the UN Food and Agriculture Organization, the annual yield loss caused by plant pests accounts for about 40 % of the world's total annual grain production (Godfary et al., 2010). For example, the rice bacterial leaf blight (BLB) is one of the most serious bacterial diseases caused by Xoo, which can cause yield loss of the rice up to 50 % (Chen et al., 2020). Potato is the world’s most important non-grain food crop and is central to global food security and is one of the important food crops in China. It is widely planted in the northwest area, and its planting area and yield account for about 1/4 of China (Xu et al., 2011; Jiang et al., 2019). However, the damaged part caused by infection with Pectobacterium carotovorum subsp. Brasiliense (Pcb) accounts for about 15 %, causing serious economic losses (Naas, H., et al., 2018; Veltman, B., et al., 2023). Nowadays, in the current stage of agricultural production, the use of chemical control led by pesticide control is still the main method of plant disease control, which has made an indelible contribution to the world food security. However, with the progress of society, the public's requirements for pesticides are becoming higher and higher, and the resistance of traditional chemical pesticides and pesticide residues are becoming increasingly prominent (Su et al., 2021; Li et al., 2019). Therefore, screening out new, efficient, safe and environmentally friendly new pesticides is nowadays an important task in the field of pesticide research (Zhou et al., 2022).
Natural products, also known as secondary metabolites, have a wide range of sources such as flavonoids, phenols, terpenoids, and terpenes (Tang et al., 2020). Natural products are widely used in medicine, pesticides and other fields because of their significant biological activities, such as antibacterial, antiviral and anti-tumor (He et al., 2021). In recent years, natural products have played a very important role in the discovery of new drugs and lead compounds. Chalcone (Fig. 1) is a significant kind of natural products, which can be obtained by aldol condensation reaction of aromatic aldehydes and ketones. It is a vitial raw material for the synthesis of spices and drugs. Its most critical functional groups are α, β-unsaturated ketone (Xia et al., 2019). It is widely distributed in plants, such as licorice, safflower, and hops (Guo et al., 2020). In previous studies, used the natural product chalcone as the lead compound, the synthesis of a series of chalcone derivatives through the principle of active splicing (Fig. 2). Such as introduction of quinoreoline (Xia et al., 2019), thiophene sulfonate (Guo et al., 2019), 1,2,4-triazine (Tang et al., 2019), thioether triazole (Chen et al., 2020), indole (Hu et al., 2023), pyrazole oxime ethers (Hu et al., 2023), quinazolone (Zhou et al., 2023) etc., these drug molecules have excellent antibacterial activity in vitro against Xoo and Xanthomonas axonopodis pv citri (Xac). Introduction of purine (Wang et al., 2019; Fu et al., 2020), amide (Xiao et al., 2020), piperazine (Zhou et al., 2022), [1,2,4]-triazole-[4,3-b]-thiadiazole (Zhan et al., 2023), pyridazine (ketone) (Chen et al., 2023), pyrimidine (Zhan et al., 2023), etc., those derivatives show excellent antifungal activity in vitro against Sclerotiua sclerotiorum (S. sclerotiorum), Colletotrichum gloeosporioiles (C. gloeosporioides), Botrytis cinerea (B. cinerea). Futhermore, introduction of purines (Gan et al., 2017), 1,3,4-oxadiazole (Gan et al., 2017), trans ferulic acid (Gan et al., 2017), indanone (Sun et al., 2023) etc., those derivatives show excellent in vivo anti-TMV activity. In addition, chalcone have other biological activities, such as insecticidal (Zhang et al., 2010; Firmino et al., 2022), and anti-tumor (Saxena et al., 2007; Fathi et al., 2021), etc. Benzoxazole is a class of benzene heterocyclic compound containing O and N atoms, which were found to have a wide range of biological activities, with good antibacterial (Luo et al., 2018), anticancer (Padmini et al., 2021), insecticidal (Zhi et al., 2020), and anti-tumor (Zhang et al., 2007), etc. Because benzoxazole, as a very momentous heterocyclic compound, is widely used in medicine, pesticides and other multiple research fields, so the in-depth exploration of benzoxazole compounds has been favored by many chemists. Therefore, by introducing groups with antibacterial and antiviral activities into chalcones, the design and synthesis of new green pesticides with high activity, low toxicity and has become a hot spot in pesticide research and development in recent years.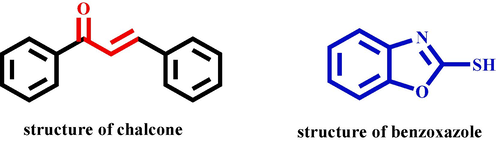
The structures of chalcone and benzoxazole.
The structures of some chalcone derivatives.
In order to obtain novel chalcone derivatives with better antiviral and bacterial inhibition, 24 chalcone derivatives containing benzoxazole were synthesized according to the principle of active sampling drug synthesis (Fig. 3 and Scheme 1). The in vitro antibacterial activities of all the target compounds were tested with a turbidity method, and anti-TMV activity of the target compounds was tested with the half leaf dry spot method. We found that Z2 had a good inhibiting effect on Xoo. The cell membrane permeability, exopolysaccharide, extracellular amylase and extracellular cellulase assays were performed between Z2 and Xoo, and the measurement results showed that Z2 could inhibit the content of Xoo biofilm to achieve the effect of inhibition. Otherwise, when the drug concentration is 200 and 100 mg/L, we tested the effect of Z2 on post-harvest potato and found that Z2 protected the potato from being infected with Pcb, which was never done in our previous work. The results of antiviral tests showed that the Z15, Z16 had excellent curative, protective activities against TMV. In this study, chalcone derivatives with certain antibacterial and antiviral activity were found, which provided a certain theoretical basis for the research, development and design of new pesticides.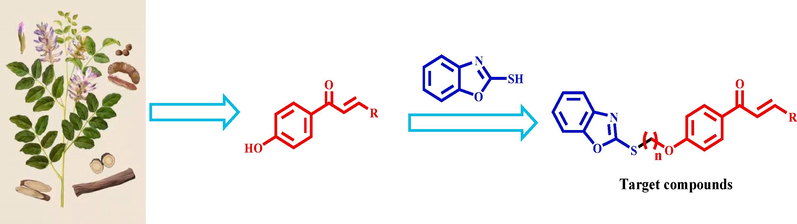
Design of target compounds.
Synthetic route of compounds Z1-Z24.
2 Materials and methods
2.1 Instruments and chemicals
2.1.1 Instruments
The melting points were determined by using an XT-4 binocular microscope (Beijing Taiji Instrument Co., Ltd.) and uncorrected. 1H, 13C, and 19F nuclear magnetic resonance (NMR) spentra of the target compounds were determined by 500 NMR Spectrometer (Bruker Corporation, Germany). Highresolution mass spectrometry (HRMS) was conducted by using a Thermo Scientific Q Exactive (Thermo Scientific, Missouri, USA). Scanning electron microscopy (SEM) data were obtained on FEI Nova Nano 450 (Hillsboro, OR, U.S.A.).
2.1.2 Chemicals
P-hydroxyacetenone, Formaldehyde with different substituents, amino phenol, carbon disulfide, thiodiazole copper, and ningnanmycin were all purchased from Shanghai Titan Technology Co., Ltd. (Shanghai, China); the solvents such as absolute ethanol and acetonitrile are commercially available.
2.2 Synthesis
The synthetic route of the target compounds Z1-Z24 is shown in Scheme 1.
2.2.1 Synthesis of intermediates 1–2
The synthesis of intermediates 1–2 were realized according to the method of the literature (Zhan et al., 2023).
2.2.2 Synthesis of the intermediate 3
The synthesis of intermediate 3 was realized according to the method of the literature (Niranjan, et al., 2018). 3.00 g (27.49 mmol) o-aminophenol was added, 1.54 g (27.49 mmol) KOH and 100 mLC2H5OH was added and kept for room temperature for 15 min, and then 3.31 mL (54.98 mmol) CS2 was added and heated to 80℃, refluxed. The reaction process was monitored by TLC (PE: EA = 3:1, v / v). After the raw materials completely reacted and concentrated under reduced pressure. After 250 mL of ice-cold water was added, pH in the system was adjusted to 1–2 with 20 % HCl. The system was constantly stirring until a large number of solids precipitated. After it was filtered, the filtrate was discarded. The filter cake was dried to get intermediate 3.
2.2.3 Synthesis of the target compounds Z1-Z24
Intermediate 3 (3.31 mmol), K2CO3 (9.92 mmol), and 40 mL of CH3CN were added to a 100 mL round bottom flask and kept stirring at room temperature for 30 min. The intermediate 2 (4.96 mmol) was added to the reaction system, kept stirring the reaction for 36 h, and the reaction process was monitored by TLC. (PE: EA = 1:1, v / v). After the reaction completed, the reaction system under pressure was concentrated to remove most of the solvent. 300 mL of ice water was poured in the system, stirred until a large amount of solids, filtered. The filter cake was washed with petroleum ether 2 ∼ 3 times and dried. Finally, the target compounds Z1-Z24 were purified by column chromatography (PE: EA = 4:1, v / v).
3 Activity test methods
3.1 Anti-bacterial activity test
According to the method reported in the previous literature (Tang et al., 2022), in vitro antibacterial activities of all the target compounds against seven plant bacteria were measured at 100 μg/mL. Furthermore, EC50 values were screened by two-fold dilution for compounds with some antibacterial activities. Thiodiazole copper was used as a control agent and each measurement was repeated three times.
3.2 The antibacterial mechanism of Z2 on Xoo
3.2.1 Effect of Z2 on the growth rate of Xoo
According to the method reported in the previous literature (Feng et al., 2022), at concentrations of 6.25, 12.5, 25, 50, 100 mg/L, Z2 to the Xoo growth rates were determined, while with 1 % DMSO as a negative control. Each concentration was repeated three times. OD595 was measured every 3 h for 30 h.
3.2.2 Scanning electron microscopy
SEM experiments were performed on the bacterial inhibition of Z2, all methods were referred to previous literature (Peng et al., 2021).
3.2.3 Cell membrane permeability assay of Z2 to Xoo
The experimental method was modified with reference to the method reported in the previous literature (Wang et al., 2022; Ye et al., 2005).
3.2.4 Effect of Z2 on the Xoo exopolysaccharide (EPS)
According to the method reported in the literature, the weighing method was employed to study the effect of Z2 against Xoo exopolysaccharides (Pan et al., 2018), with 1 % DMSO as a negative control. Each concentration was repeated three times.
3.2.5 Effect of Z2 on the extracellular enzyme of Xoo
According to the method reported previously (Li et al., 2022), the effect of Z2 against the Xoo extracellular enzyme (extracellular amylase and extracellular cellulases) was studied after some slight modifications of the experimental procedure.
3.3 In vivo inhibitory Pcb activity of Z2
The experimental method was modified with reference to the method reported in the previous literature (Li et al., 2016), in vivo experiments were performed on harvested potatoes at drug concentrations of 200 and 100 mg/L, with thiodiazole copper as a control drug. These experiments were repeated 3 times.
3.4 In vivo antiviral activity assay of Z1-Z24 against TMV
According to the method reported previously in the literature (Cao et al., 2023), the in vivo antiviral activity against TMV (curative and protective activity) was tested. The commercial drug ningnanmycin (NNM) was a control agent, and each experiment was repeated three times.
3.5 Molecular docking of the target compounds on the TMV
Molecular docking of compounds Z15, Z16 and NNM with TMV-CP was performed with the method reported in the literature (Liu, et al., 2022). Molecular docking of the TMV-CP (PDB: 1EI7) with the structure of the selected compounds was performed on the Discovery Studio 2019 software.
4 Results and discussion
4.1 Chemistry
The Z1-Z24 were synthesized according to Scheme 1. The structures of the desired target compounds were preliminarily characterized by using NMR and HNMR and the detailed data are provided for the Supporting Materials.
4.2 Antibacterial activity of Z1-Z24
The inhibitory activities of the synthetic target compounds against Xac, Xoo, Pseudomonas syringae pv. actinidiae (Psa), Acidovorax citrulli (Ac), Ralstonia solanacearum (Rs), Pcb, and Xanthomonas campestris pv. mangiferae indicae (Xcm) were determined at the concentration of 100 μg/mL. The results are shown in Table 1. Some of the target compounds showed inhibitory activity against seven plant bacteria. At the concentration of 100 μg/mL, Z1, Z2 showed 72.3, 68.2 % inhibition of Xac, respectively, which were better than thiodiazole copper (64.2 %); Z2 showed 93.0 % inhibition of Xoo, which was superior than thiodiazole copper (90.7 %). Z1, Z3, Z4, Z6, Z7, Z10 showed 86.2, 78.5, 81.2, 75.5, 73.0, 81.6 % inhibition of Ac, respectively, which were better than thiodiazole copper (47.4 %). Z2, Z6, Z7, Z12, Z17, Z20 showed 79.5, 72.9, 73.5, 72.9, 70.9, 78.5 % inhibition of Rs, respectively, which were higher than thiodiazole copper (43.5 %). Z2, Z3, Z4 and Z7 showed 84.6, 77.1, 72.2 and 75.9 % inhibition of Pcb, respectively, which were better than thiodiazole copper (63.0 %). Z2, Z10, Z11, Z17, Z19 showed 66.4, 65.7, 64.0, 78.6, 68.8 % inhibition of Xcm, respectively, which were superior than thiodiazole copper (40.9 %).
Compounds
Inhibition rates(%)a (100 μg/mL)
Xac
Xoo
Psa
Ac
Rs
Pcb
Xcm
Z1
72.3 ± 5.0
47.1 ± 2.9
44.9 ± 1.3
86.2 ± 5.6
47.9 ± 1.2
38.5 ± 4.0
14.8 ± 4.5
Z2
68.2 ± 2.7
93.0 ± 0.6
46.8 ± 2.4
67.8 ± 2.2
79.5 ± 3.4
84.6 ± 1.1
66.4 ± 3.8
Z3
23.2 ± 1.3
20.7 ± 0.7
37.0 ± 3.4
78.5 ± 2.7
68.6 ± 6.9
77.1 ± 4.5
52.0 ± 3.4
Z4
55.6 ± 4.6
21.2 ± 1.1
30.9 ± 3.6
81.2 ± 2.5
58.9 ± 4.4
72.2 ± 6.1
56.5 ± 2.3
Z5
48.5 ± 3.4
59.6 ± 3.5
8.7 ± 0.7
53.3 ± 1.9
34.1 ± 3.2
22.4 ± 3.2
36.7 ± 0.9
Z6
49.5 ± 6.4
11.3 ± 7.1
36.7 ± 1.3
75.5 ± 2.8
72.9 ± 1.7
61.6 ± 2.8
55.1 ± 1.8
Z7
30.3 ± 4.2
23.1 ± 2.6
23.4 ± 2.8
73.0 ± 2.4
73.5 ± 1.7
75.9 ± 4.3
49.6 ± 6.2
Z8
31.3 ± 6.7
24.7 ± 1.8
59.6 ± 0.9
45.1 ± 0.6
34.2 ± 3.0
43.7 ± 3.2
49.7 ± 2.2
Z9
22.9 ± 1.6
15.1 ± 1.7
30.6 ± 2.1
9.5 ± 3.6
30.5 ± 2.0
10.6 ± 3.9
57.7 ± 5.1
Z10
53.7 ± 3.4
11.0 ± 2.6
46.4 ± 6.9
81.6 ± 4.3
58.0 ± 6.6
43.7 ± 3.6
65.7 ± 2.5
Z11
56.6 ± 3.0
18.7 ± 4.6
54.5 ± 5.6
60.1 ± 3.2
62.1 ± 5.5
50.7 ± 5.8
64.0 ± 3.9
Z12
40.4 ± 3.8
33.2 ± 3.1
30.3 ± 2.6
15.7 ± 4.4
72.9 ± 5.8
41.3 ± 2.6
42.3 ± 3.2
Z13
61.2 ± 3.7
18.2 ± 2.6
47.0 ± 2.7
54.0 ± 6.3
35.8 ± 4.5
36.8 ± 2.1
38.2 ± 3.4
Z14
53.1 ± 4.6
23.7 ± 4.9
54.6 ± 2.9
60.6 ± 1.5
56.9 ± 3.5
59.7 ± 4.6
32.1 ± 5.6
Z15
42.9 ± 1.6
50.2 ± 4.1
49.1 ± 0.4
68.9 ± 3.0
49.1 ± 5.1
46.7 ± 1.7
63.3 ± 2.3
Z16
40.6 ± 4.7
19.5 ± 1.9
37.5 ± 2.5
38.8 ± 3.7
34.8 ± 2.7
41.1 ± 3.8
54.3 ± 2.6
Z17
40.2 ± 2.3
24.9 ± 5.7
18.9 ± 1.2
67.7 ± 5.7
70.9 ± 3.8
52.0 ± 5.7
78.6 ± 4.7
Z18
35.2 ± 1.8
5.2 ± 0.3
38.4 ± 1.4
37.9 ± 4.2
27.6 ± 5.3
18.2 ± 4.3
45.1 ± 4.2
Z19
60.9 ± 6.6
42.2 ± 5.6
40.5 ± 1.9
23.1 ± 4.7
54.1 ± 1.8
58.0 ± 6.2
68.8 ± 0.3
Z20
37.5 ± 2.6
49.3 ± 1.3
44.7 ± 4.2
21.1 ± 3.3
78.5 ± 2.6
55.2 ± 2.7
61.0 ± 2.3
Z21
32.0 ± 4.5
45.5 ± 1.2
10.7 ± 1.0
24.7 ± 4.7
24.6 ± 1.5
26.5 ± 2.1
1.5 ± 3.5
Z22
21.3 ± 2.0
27.4 ± 0.9
21.3 ± 3.9
9.7 ± 1.3
26.9 ± 3.3
29.1 ± 5.1
20.2 ± 1.1
Z23
15.2 ± 3.7
29.4 ± 0.2
14.9 ± 1.1
17.9 ± 2.3
23.5 ± 2.5
16.3 ± 4.1
11.7 ± 8.4
Z24
43.1 ± 9.2
9.9 ± 5.5
17.9 ± 3.0
18.6 ± 5.6
19.2 ± 2.7
10.0 ± 3.7
12.2 ± 2.7
TCb
64.2 ± 4.2
90.7 ± 6.7
48.2 ± 1.1
47.4 ± 4.1
43.5 ± 4.2
63.0 ± 1.8
40.9 ± 3.7
To further confirm the results of the preliminary screening, the EC50 values of some target compounds were tested, and the results are shown in Table 2. As shown in Table 2, the EC50 values for the compounds ranged from 8.10 to 67.88 μg/mL. Notably, the EC50 value of Z2 to Xoo was 8.10 μg/mL, which was better than thiodiazole copper (60.62 μg/mL).
Bacteria
Compounds
n
R
Toxic Regression equation
R2
EC50 (μg/mL)a
Xac
Z1
3
Ph
y = 0.5744x + 0.6620
0.9731
48.24
TC b
–
–
y = 2.1114x + 1.0540
0.9917
73.94
Xoo
Z2
4
Ph
y = 2.2563x + 2.9507
0.9884
8.10
TC b
–
–
y = 2.3436x + 0.8222
0.9966
60.62
AC
Z1
3
Ph
y = 2.1309x + 1.6366
0.9933
37.88
Z3
3
4-CH3-Ph
y = 1.8989x + 1.9037
0.9623
42.71
Z4
4
4-CH3-Ph
y = 1.8265x + 1.9016
0.9706
49.70
Z6
3
4-F-Ph
y = 1.7750x + 1.8810
0.9837
57.17
Z7
4
4-F-Ph
y = 1.8224x + 1.9062
0.9732
49.84
Z10
4
3-CH3-Ph
y = 1.8848x + 1.9187
0.9577
43.14
TC b
–
–
y = 1.5562x + 2.0569
0.9814
77.84
RS
Z2
4
Ph
y = 1.8888x + 1.9093
0.9100
43.28
Z12
4
3-OCH3-Ph
y = 1.7857x + 1.8605
0.9904
57.30
Z20
4
2-CH3-Ph
y = 1.7587x + 1.8596
0.9861
61.05
TC b
–
–
y = 1.2851x + 2.0590
0.9947
194.38
Pcb
Z2
4
Ph
y = 2.5152x + 1.5627
0.9872
23.26
Z3
3
4-CH3-Ph
y = 1.8894x + 1.8137
0.9847
48.58
Z4
4
4-CH3-Ph
y = 1.9397x + 1.8189
0.9691
43.65
Z7
4
4-F-Ph
y = 1.7332x + 1.9945
0.9608
54.22
TC b
–
–
y = 1.1341x + 2.8194
0.9933
83.70
Xcm
Z2
4
Ph
y = 1.7126x + 1.9006
0.9830
64.54
Z10
4
3-CH3-Ph
y = 1.9402x + 1.8484
0.9779
42.11
Z11
3
3-OCH3-Ph
y = 1.7300x + 1.8020
0.9840
70.56
Z15
3
3-Cl-Ph
y = 1.6907x + 1.9900
0.9689
59.21
Z17
3
3-Br-Ph
y = 1.7532x + 1.8853
0.9814
59.78
Z19
3
2-CH3-Ph
y = 1.6664x + 1.9476
0.9840
67.88
Z20
4
2-CH3-Ph
y = 1.7303x + 1.9163
0.9852
60.56
TC b
–
–
y = 1.3586x + 1.9534
0.9903
174.74
4.3 Structure-activity relationship (SAR) analysis of the antibacterial activity
According to the bacterial inhibiting activities shown in Table 1 and Table 2, SAR analysis showed that the compounds with certain antibacterial activities have some commonalities.
(1). At the concentration of 100 μg/mL, when the substituent was Ph, the inhibiting activity was relatively best, this indicated that when R was a small steric hindrance substituent, it can enhance the antibacterial activity of the compound. Such as Z1 (n = 3, R = Ph), Z2 (n = 4, R = Ph) showed that the inhibitory activities against Xac were 72.3, 68.5 %, respectively. Z2 (n = 4, R = Ph) showed the inhibitory activity against Xoo was 93.0 %. Z1 (n = 3, R = Ph) showed the inhibitory activity against Ac was 86.2 %. Z2 (n = 4, R = Ph) showed the inhibitory activity against Rs was 79.5 %. Z2 (n = 4, R = Ph) showed the inhibitory activityagainst Pcb was 84.6 %, which was higher than those of other substituents.
(2). When R was electron-giving group, it can enhance the inhibiting rate of the compounds against Ac and Rs, such as the inhibiting activity against Ac was Z3 (n = 3, R = 4-CH3-Ph, 78.5 %) > Z6 (n = 3, R = 4-F-Ph, 75.5 %) > Z21 (n = 3, R = 2-Furan, 24.7 %); the inhibiting activity against Rs was Z3 (n = 3, R = 4-CH3-Ph, 77.1 %) > Z6 (n = 3, R = 4-F-Ph, 72.9 %) > Z21 (n = 3, R = 2-Furan, 24.6 %).
(3). It is worth noting that when the number of carbon atoms of dibromine that replaces alkanesis was 4 and the substituent was Ph, it showed relatively significant inhibiting activity. For example, the EC50 values of Z2 (n = 4, R = Ph) against Xoo, Rs, Pcb were 8.10, 43.28, 23.26 μg/mL, respectively, which were superior to those of other substituents.
(4). We noticed that the substituent introduced in the early stage was relatively single, so the heterocyclic substituent (such as thiophene, furan) was introduced. The preliminary screening results of the activity test showed that the four synthesized compounds containing heterocyclic substituents did not have a prominent inhibiting effect on the seven plant bacteria.
In short, this result indicated that when the number of carbon atoms of dibromine alkanesis was 4 and the substituent was Ph, it can improve the antibacterial activities of the compounds against bacteria to some extent, especially the inhibition of Xoo was obviously enhanced.
4.4 Results of Z2 antibacterial mechanism
4.4.1 Growth rate plot of Z2 against Xoo
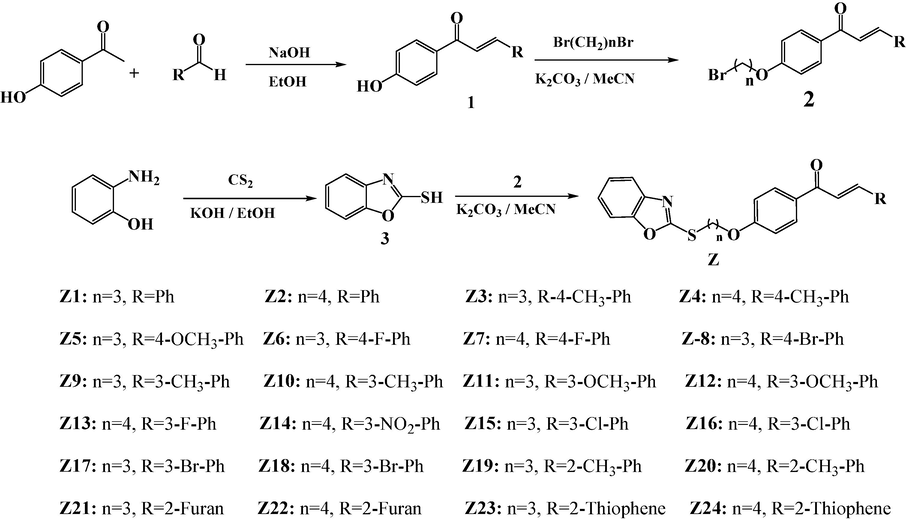
According to the results of the growth rate of Z2 against Xoo shown in Table 3 and Fig. 4. The test results showed that when Z2 concentration was 100, 50, 25 mg/L, respectively, it can effectively inhibit Xoo growth. When the concentration was 12.5, 6.25 mg/L, respectively, the inhibiting effect of Xoo is significantly worse, and the OD595 values increased significantly over time, which showed that the inhibition of Z2 against Xoo in a concentration-dependent way. This result was consistent with the screening results of EC50 value. The OD595 value represent average of three replicates. Different letters indicate that the means are significantly different at p ≤ 0.05.
Times (h)
Cell growth (OD595)
0 mg/L
6.25 mg/L
12.5 mg/L
25 mg/L
50 mg/L
100 mg/L
0
0.11 ± 0.08
0.10 ± 0.05
0.10 ± 0.04
0.10 ± 0.03
0.10 ± 0.04
0.10 ± 0.01
3
0.13 ± 0.10a
0.12 ± 0.07ab
0.12 ± 0.04ab
0.11 ± 0.05ab
0.11 ± 0.01ab
0.11 ± 0.05b
6
0.17 ± 0.06a
0.16 ± 0.09b
0.15 ± 0.05c
0.10 ± 0.04bc
0.13 ± 0.05c
0.11 ± 0.04d
9
0.21 ± 0.11a
0.19 ± 0.03b
0.17 ± 0.06c
0.14 ± 0.07 cd
0.15 ± 0.03d
0.12 ± 0.05e
12
0.28 ± 0.09a
0.25 ± 0.10b
0.21 ± 0.11c
0.18 ± 0.08d
0.16 ± 0.08d
0.11 ± 0.08e
15
0.37 ± 0.10a
0.25 ± 0.08b
0.22 ± 0.10c
0.19 ± 0.06d
0.18 ± 0.08d
0.12 ± 0.06e
18
0.43 ± 0.18a
0.29 ± 0.19b
0.23 ± 0.08c
0.22 ± 0.07c
0.19 ± 0.10d
0.14 ± 0.10e
21
0.46 ± 0.15a
0.29 ± 0.17b
0.24 ± 0.09c
0.24 ± 0.05c
0.18 ± 0.06d
0.15 ± 0.08e
24
0.55 ± 0.19a
0.31 ± 0.09b
0.26 ± 0.12c
0.25 ± 0.11d
0.19 ± 0.07e
0.15 ± 0.07f
27
0.65 ± 0.14a
0.33 ± 0.15b
0.29 ± 0.07c
0.26 ± 0.02d
0.20 ± 0.09e
0.15 ± 0.09f
30
0.70 ± 0.16a
0.38 ± 0.14b
0.34 ± 0.10c
0.28 ± 0.11d
0.19 ± 0.10e
0.15 ± 0.10f
Growth curves of Xoo treated with Z2.
4.4.2 Effect of Z2 on the hyphae morphology of Xoo
The mechanism of action of Z2 on Xoo was initially investigated by SEM. As shown in Fig. 5, the cells in the CK group without drug treatment were homogeneous and full. After Z2 treatment, at 50 μg/mL, some of the cells had a flattened morphology and cell membrane rupture; at 100 μg/mL, the degree of cell rupture increased significantly, showing contraction, collapse and deformation. SEM analysis showed that the greater the concentration of the drug, the greater the degree of cell membrane damage, which initially indicated that Z2 could destroy the cell membrane.
SEM of the hyphae of Xoo with or without compound Z2 treatment. (A) CK groups (DMSO); (B) Z2 at 50 µg/mL and (C) Z2 at 100 µg/mL.
4.4.3 Cell membrane permeability assay of Z2 to Xoo
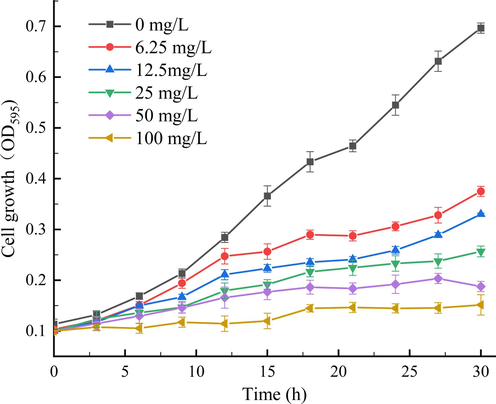
Cell membranes are important for maintaining the integrity of cell structure and normal life activities, and the permeability of cell membranes can be evaluated by relative conductivity. To verify the above results combined with the result of screening for the SEM, the effect of Z2 on the membrane permeability of Xoo cells was determined, and the results are shown in Fig. 6. It indicated that Z2 increased the cell membrane permeability in a concentration-dependent manner. At the concentrations of 0, 6.25, 12.5, 25, 50 and 100 mg/L for 22.5 h, the relative conductivities of the cell membrane gradually increased, and the relative conductivities were 5.9, 8.1, 8.6, 11.9, 15.9, and 18.0 %, respectively. In particular, when the concentration of Z2 was 50 and 100 mg/L, it can be seen that the conductivity of Xoo cells treated with Z2 increased significantly, which indicated that when the target compound was at 50, 100 mg/L, it had obvious cell membrane permeability against Xoo, which caused extensive damage to the Xoo cells membrane, leading to the rupture of the cell membrane, and the components in the membrane were released, affecting the integrity of the Xoo cell membrane, causing cells death, resulting in an increased relative conductivity. When the drug concentration was 6.25, 12.5, 25 mg/L, it can be seen that the relative conductivity does not change much, indicating that the permeability of the three concentrations weakens with the lower concentration, and the permeability of the Xoo cell membrane was significantly worse, so the destruction of the Xoo cell membrane was reduced to inhibit Xoo, which was consistent with the EC50 value determination results.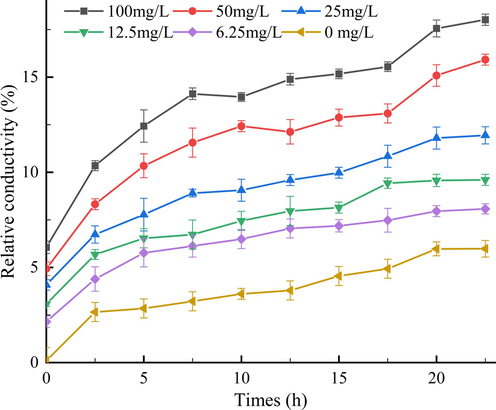
Relative conductance of Z2 treated Xoo cells at concentrations of 0, 6.25, 12.5, 25, 50, 100 mg/L.
4.4.4 Effect of Z2 on the Xoo exopolysaccharide (EPS)
During the establishment of bacterial colonies and the formation of biofilms, bacteria secrete extracellular polysaccharides (EPS) to protect themselves from adverse environments(Yun et al., 2006). Bacterial EPS is not only the primary substrate required for biofilm formation but also helps bacteria to resist host plant defense responses (Milling et al., 2011). At the same time, it also creates resistance to some antibiotics, which leads to the deterioration of drug resistance problems (Kakkar et al., 2015). Therefore, the effect of Z2 on Xoo EPS production was investigated at drug concentrations of 0, 6.25, 12.5, 25, 50, 100 mg/L, respectively. As shown in Table 4 and Fig. 7. The result showed that when the drug concentrations were 0, 6.25, 12.5, 25, 50 and 100 mg/L, respectively, the EPS contents of Xoo were 805.3, 560.0, 515.7, 360.3, 320.3, 283.3 mg/L, respectively, which suggested that the Z2 affects EPS production in a concentration-dependent manner, when EPS production decreased significantly with increasing compound concentration, especially the EPS content was at 100 and 50 mg/L. Thus, Z2 can significantly reduce bacterial biofilm formation and EPS production.
Concentration of Z2 (mg/L)
EPS Conduction
Repetition 1
Repetition 2
Repetition 3
Average content
0
848
768
800
805.3
6.25
556
580
544
560.0
12.5
524
515
508
515.7
25
360
352
369
360.3
50
308
333
320
320.3
100
284
276
290
283.3
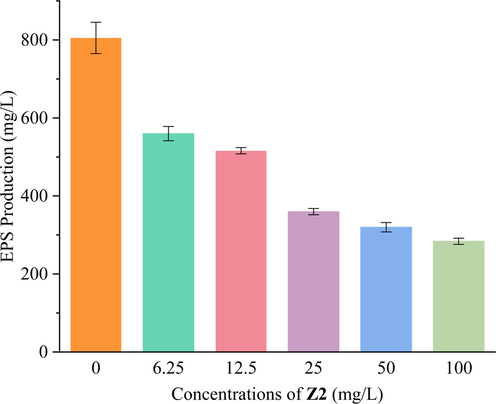
Quantification of EPS production triggered by Z2 at various concentrations ranging from 0 to 100 mg/L.
4.4.5 Effect of Z2 on the extracellular enzyme of Xoo
Most bacteria will produce and secrete various extracellular proteolytic enzymes during the growth stage, such as amylase, cellulase, pectinase, etc. These enzymes help to degrade extracellular macromolecules and obtain nutrients to meet nutritional needs of bacteria, improving adaptability of bacteria to the environment, and facilitating the colonization and infection in the plant body (Sinha et al., 2021; Huang et al., 2022). Therefore, the effects of Z2 on Xoo extracellular amylase and extracellular cellulase were explored at the concentrations of 25, 50, 100 mg/L, respectively, and the experimental results are as follows.
4.4.5.1 Effect of Z2 on extracellular amylase of Xoo
The results of the effect of Z2 on the extracellular amylase activity of Xoo are shown in Table 5 and Fig. 8. In the starch plate of incubation after 3 d, treatment with I2/KI solution and 70 % ethanol decorating treatment, a clear hydrolysis coil was observed in the control group with an average diameter of 26.5 mm. After treatment with Z2 at the concentrations of 25, 50, 100 mg/L, the average diameter of the transparent hydrolysis circle treated was 24.8, 22.3 20.3 mm, respectively and the diameter of the hydrolysis circle was reduced by 6.4, 15.7, and 23.3 %, respectively. The results showed that Z2 has some inhibiting activity against Xoo extracellular amylase.
Concentration of Z2 (mg/L)
Halo dianeter(mm)
Repetition 1–1
Repetition 1–2
Repetition 2–1
Repetition 2–2
Repetition 3–1
Repetition 3–2
Average diameter
0
26
27
25
27
27
27
26.5
25
24
25
24
25
25
26
24.8
50
22
22
22
23
22
23
22.3
100
21
20
21
21
20
19
20.3
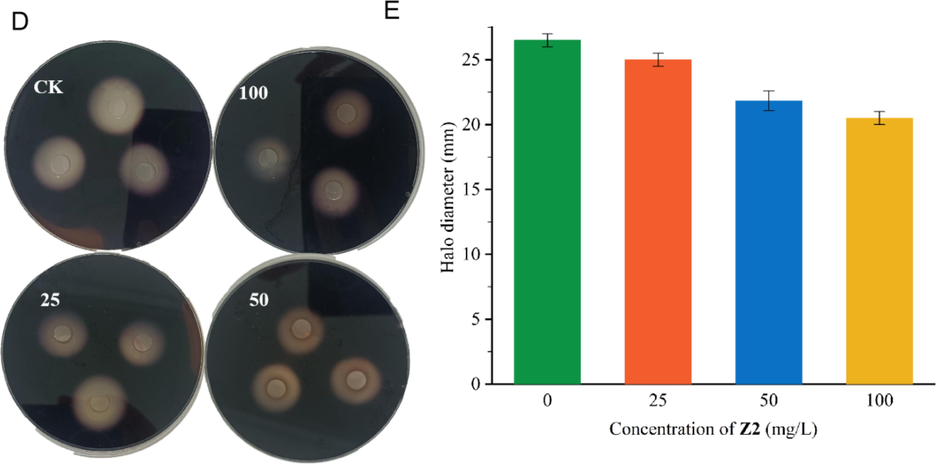
Impacts of Z2 on Xoo Extracellular cellulase (D and E).
4.4.5.2 Effect of Z2 on the extracellular cellulases of Xoo
The effect of Z2 on extracellular cellulase of Xoo was obtained as shown in Table 6 and Fig. 9, and the test results of Z2 on extracellular cellulase were the same as that of extracellular amylase. The cellulose plates had been incubated for 3 d, stained with 0.1 % Congo red solution and decolorized with the 1 mol/L NaCl solution. A transparent circle was clearly visible in the control group, with a mean diameter of 25.3 mm. In group treated with Z2 at the concentration of 25, 50, 100 mg/L, respectively. The average diameters of the hydrolysis circles were 22.3, 19.0 and 16.7 mm, which result in reducing the corresponding extracellular cellulases by 11.8, 25.0 and 34.2 %, respectively. The test results showed Z2 has some inhibiting effect on the extracellular cellulase of Xoo.
Concentration of Z2 (mg/L)
Halo dianeter(mm)
Repetition 1–1
Repetition 1–2
Repetition 2–1
Repetition 2–2
Repetition 3–1
Repetition 3–2
Average diameter
0
24
25
25
26
25
27
25.3
25
23
22
21
21
23
24
22.3
50
18
20
19
19
19
19
19.0
100
16
17
17
17
16
17
16.7
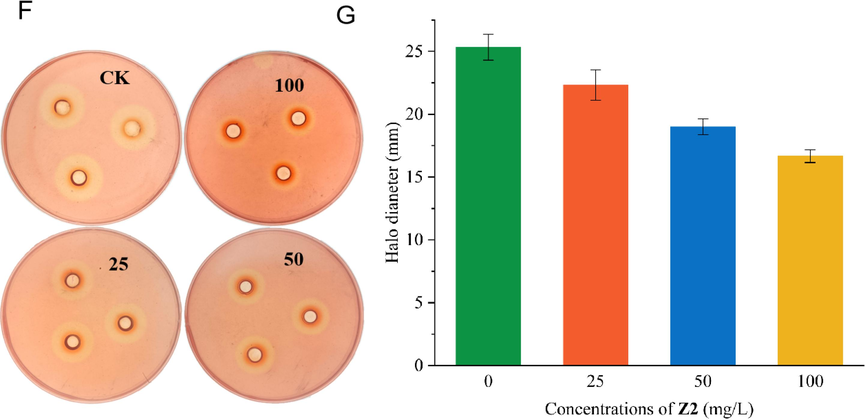
Impacts of Z2 on Xoo Extracellular amylase (F and G).
In conclusion, at the concentration of 100 mg/L of Z2, on the one hand, Z2 can change the permeability of Xoo cell membrane and destroy the integrity of the cell membrane. On the other hand, it can affect the content of EPS and extracellular amylase and extracellular cellulase of Xoo cell, and block Xoo biofilm to obtain external nutrients, thus affecting the growth rate of Xoo cells and achieving the purpose of inhibiting Xoo.
4.5 Effect of Z2 on harvested potato
The antibacterial activity (protection and curation) effect on harvested potato of Z2 was tested at the drug concentration of 200 and 100 mg/L, respectively, and the test results are shown in Figs. 10, 11. According to Fig. 10, at the concentration of 200 mg/L, as a negative control, the CK group inoculated with Pcb after only water, the potato turned black and soft. After incision, the interior was almost soft and decayed, with numerous water stains, and Z2 showed better results of protection and curation of the potato. Especially, the protective activity was even more significant, there were few lesions of the epidermis and interior of the potato (with an effect close to thiodiazole copper). According to Fig. 11, at 100 mg/L, the protective and curative effect of Z2 was significantly reduced, the reduction in the curative effect was even more distinct, the potato was partially decomposed, but the lesion was still less than that of CK. This result indicated that Z2 has a certain protective effect on the potato. However, it can be seen from Fig. 11 that Z2 has a poor curative effect on potato at the drug concentration of 100 mg/L, and the extent of the lesion was comparable to the CK.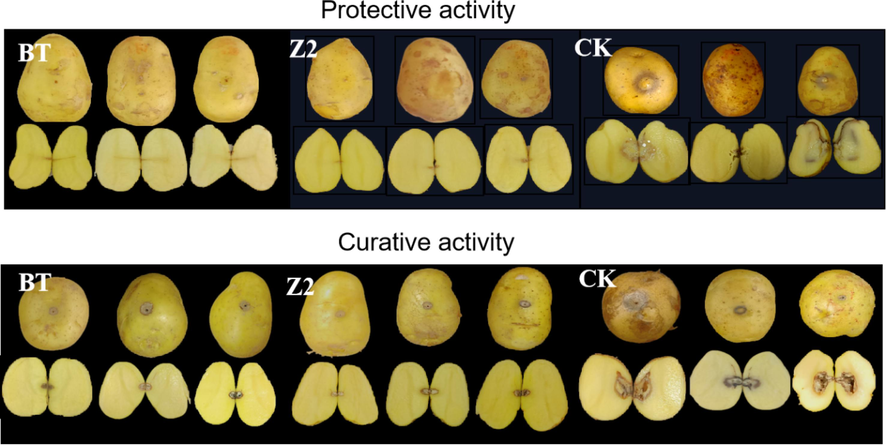
Effect of BT, Z2, CK on post-harvest potato at a concentration of 200 mg/L.
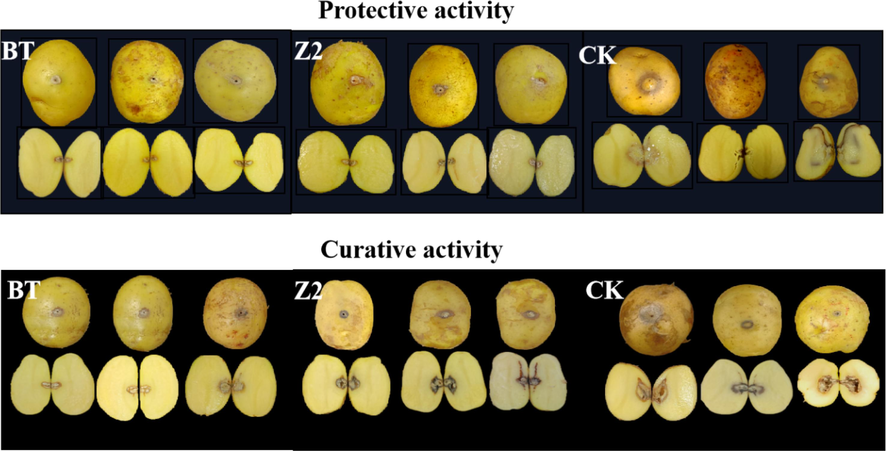
Effect of BT, Z2, CK on post-harvest potato at a concentration of 100 mg/L.
In conclusion, at high concentration, Z2 showed better protective activity of potatoes, but showed less curative activity of potatoes infected with Pcb.
4.6 Antiviral activity of Z1-Z24 against TMV in vivo
At a drug concentration of 500 μg/mL, the anti-TMV activities of the target compounds Z1-Z24 were tested by the half-leaf dry spot method, and the test results are shown in Table 7, most of the compounds showed some anti-TMV activities. It is worth noting that the curative activities of Z2, Z4, Z5, Z6, Z15, Z16, Z18 and Z19 were 76.1, 76.0, 74.2, 78.0, 84.0, 73.7, 80.4, 73.7 %, respectively, which were superior to NNM (57.4 %); the protective activities of Z1, Z4, Z5, Z6, Z7, Z14, Z15, Z18 were 73.0, 72.8, 77.2, 71.8, 72.7, 70.7, 76.8, 73.0 %, respectively, which were higher than NNM (65.0 %). Among them, the tobacco leaf morphology of Z15 and NNM against TMV in vivo is shown in Fig. 12. a Average of three replicates, b Ningnanmycin (NNM).
Compound.
n
R
Curation (%)a
Protection(%)a
Z1
3
Ph
60.0 ± 3.9
73.0 ± 3.9
Z2
4
Ph
76.1 ± 0.7
69.2 ± 4.9
Z3
3
4-CH3-Ph
59.9 ± 0.4
62.7 ± 3.1
Z4
4
4-CH3-Ph
76.0 ± 0.8
72.8 ± 3.6
Z5
3
4-OCH3-Ph
74.2 ± 4.9
77.2 ± 1.5
Z6
3
4-F-Ph
78.0 ± 3.5
71.8 ± 1.8
Z7
4
4-F-Ph
64.1 ± 3.0
72.7 ± 3.8
Z8
3
4-Br-Ph
48.8 ± 2.8
55.7 ± 3.8
Z9
3
3-CH3-Ph
62.3 ± 4.5
52.0 ± 1.7
Z10
4
3-CH3-Ph
57.0 ± 5.6
60.1 ± 0.7
Z11
3
3-OCH3-Ph
55.1 ± 1.8
63.4 ± 1.4
Z12
4
3-OCH3-Ph
67.4 ± 3.3
63.1 ± 1.2
Z13
4
3-F-Ph
68.0 ± 4.9
66.3 ± 4.9
Z14
4
3-NO2-Ph
66.5 ± 3.2
70.7 ± 4.0
Z15
3
3-Cl-Ph
84.0 ± 2.3
76.8 ± 4.2
Z16
4
3-Cl-Ph
73.7 ± 4.1
75.6 ± 2.4
Z17
3
3-Br-Ph
58.3 ± 1.4
62.9 ± 1.8
Z18
4
3-Br-Ph
80.4 ± 4.7
73.0 ± 5.1
Z19
3
2-CH3-Ph
73.7 ± 3.8
66.2 ± 1.0
Z20
4
2-CH3-Ph
51.0 ± 4.4
52.5 ± 2.0
Z21
3
2-Fruan
56.3 ± 4.0
60.1 ± 6.2
Z22
4
2-Fruan
56.1 ± 5.0
54.5 ± 3.9
Z23
3
2-Thioen
62.2 ± 3.0
62.3 ± 2.8
Z24
4
2-Thioen
39.4 ± 3.6
48.9 ± 0.8
NNMb
–
–
57.4 ± 1.8
65.0 ± 5.1
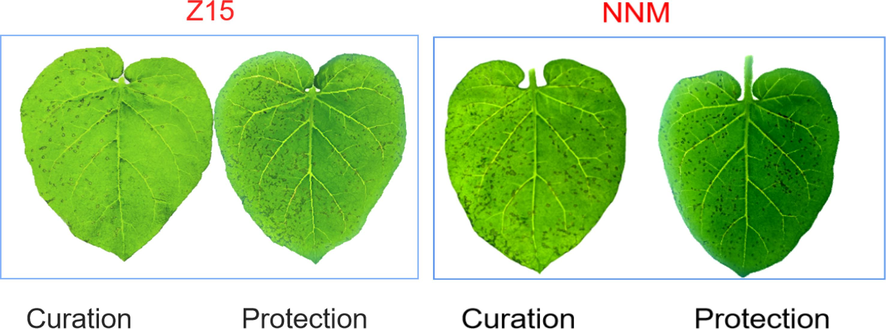
Anti-TMV activities of Z15 and NNM in vivo.
Based on the results of the initial screening, the EC50 values of some compounds against TMV were further tested and the results are shown in Table 8. The EC50 values of curative activities of Z15, Z16, and Z18 were 101.97,106.56, and 108.21 μg/mL, respectively, which were better than NNM which was 294.27 μg/mL. The EC50 values of protective activities of Z15, Z16, and Z18 were 105.26, 104.05 and 119.81 μg/mL, respectively, which were superior than NNM (185.73 μg/mL). a Average of three replicates, b Ningnanmycin (NNM).
Compounds
n
R
Toxic Regression
R2
EC50 (μg/mL)a
Curation
Z4
4
4-CH3-Ph
y = 1.3716x + 1.8734
0.9735
190.34
Z5
4
4-OCH3-Ph
y = 1.1376x + 24749
0.9781
165.83
Z6
3
4-F-Ph
y = 1.7490x + 2.5044
0.9824
133.07
Z15
3
3-Cl-Ph
y = 1.3837x + 2.2209
0.9725
101.97
Z16
4
3-Cl-Ph
y = 1.1810x + 2.6054
0.9707
106.56
Z18
4
3-Br-Ph
y = 1.1934x + 2.5723
0.9874
108.21
NNMb
–
–
y = 1.1888x + 2.0648
0.9974
294.27
Protection
Z4
4
4-CH3-Ph
y = 1.0554x + 2.7497
0.9979
135.57
Z5
4
4-OCH3-Ph
y = 1.8142x + 0.9455
0.9840
171.74
Z6
3
4-F-Ph
y = 1.402x + 2.6069
0.9639
125.56
Z15
3
3-Cl-Ph
y = 1.2312x + 2.5102
0.9912
105.26
Z16
4
3-Cl-Ph
y = 1.2984x + 2.3808
0.9881
104.05
Z18
4
3-Br-Ph
y = 1.2205x + 2.4632
0.9917
119.81
NNMb
–
–
y = 0.9067x + 2.9428
0.9821
185.73
The structure–activity relationship (SAR) was analyzed according to the anti-TMV activities shown in Table 7 and Table 8.
When the number of C-atoms of brominated alkane was 3 and R was an electron-withdrawing phenyl group with a better activity against TMV. For example, the curative activity situations were Z15 (n = 3, R = 3-Cl-Ph, 84.0 %) > Z9 (n = 3, R = 3-CH3-Ph, 62.3 %) > Z23 (n = 3, R = 2-Thiophene, 62.2 %) > Z21 (n = 3, R = 2-Furan, 56.3 %) > Z11 (n = 3, R = 3-OCH3-Ph, 55.1 %). The protective activity situations were Z15 (n = 3, R = 3-Cl-Ph, 76.8 %) > Z11 (n = 3, R = 3-OCH3-Ph, 63.4 %) > Z23 (n = 3, R = 2-Thiophene, 62.3 %) > Z21 (n = 3, R = 2-Furan, 60.1 %) > Z9 (n = 3, R = 3-CH3-Ph, 52.0 %).
When the number of C-atoms of brominated alkanes was n = 4 and R was an electron-withdrawing phenyl group with a better activity against TMV. For example, the curative activity situations were Z16 (n = 4, R = 3-Cl-Ph, 73.7 %) > Z12 (n = 4, R = 3-OCH3-Ph, 67.4 %) > Z10 (n = 4, R = 3-CH3-Ph, 57.0 %) > Z22 (n = 4, R = 2-Furan, 56.1 %) > Z24 (n = 4, R = 2-Thiophene, 39.4 %). The protective activity situations were Z16 (n = 4, R = 3-Cl-Ph, 75.6 %) > Z12 (n = 4, R = 3-OCH3-Ph, 63.1 %) > Z10 (n = 4, R = 3-CH3-Ph, 60.1 %) > Z22 (n = 4, R = 2-Furan, 54.5 %) > Z24 (n = 4, R = 2-Thiophene, 48.9 %).
We noticed that the substituent groups introduced in the early stage were relatively single, so we introduced heterocyclic substituents (thiophene, furan) and tested these target compounds for their activities against TMV, but the test results showed that the activities of the target compounds did not change significantly after the introduction of the heterocyclic substituents. For example, the curative activity situations were Z21 (n = 3, R = 2-Furan, 56.3 %), Z22 (n = 4, R = 2-Furan, 56.1 %), Z23 (n = 3, R = 2-Thiophene, 62.2 %), Z24 (n = 4, R = 2-Thiophene, 39.4 %); the protective activity situations were Z21 (n = 3, R = 2-Furan, 60.1 %), Z22 (n = 4, R = 2-Furan, 54.5 %), Z23 (n = 3, R = 2-Thiophene, 62.3 %), Z24 (n = 4, R = 2-Thiophene, 48.9 %).
In summary, Z15 (n = 3, R = 4-Cl-Ph) and Z16 (n = 4, R = 4-Cl-Ph) showed significant inhibiting activities against TMV in terms of curative, protective and inactivated activities, when n = 3, R was halogen-substituted phenyl, the protective and curative activities of the target compound against TMV were enhanced.
4.7 Molecular docking of compounds Z15, Z16 and NNM with TMV-CP
According to the screening results of the activities of some compounds against TMV, in Table 7, Z15 and Z16 have some curative and protective activities against TMV. Therefore, we explored the similarities and differences of binding modes of Z15, Z16, NNM and TMV-CP by molecular docking and the results are shown in Fig. 13. Molecular docking results revealed that Z15, Z16 and NNM are capable of hydrogen bonding with TMV-CP, and the common amino acid residues between Z15, Z16 and TMV-CP are ARG-A-134, TYR-A-139. Z15 formed two hydrogen bonds with TMV-CP, in which the benzoxazole nitrogen atom of Z15 formed a hydrogen bond with residue TYR-A-73(2.77 Å) of TMV-CP, and the chalcone-based oxygen atom formed a hydrogen bond with residue ARG-A-134 (2.85 Å). Z16 formed two hydrogen bonds with TMV-CP, in which the benzoxazole nitrogen atom of Z16 formed a hydrogen bond with residue TYR-A-139 (2.85 Å) of TMV-CP. The chalcone-based oxygen atom formed a hydrogen bond with residue ARG-A-134 (2.50 Å). Similarly, NNM forms hydrogen bonds with amino acid residues ARG-A-134 (1.91 Å) and TYR-A-139 (2.89 Å). Through molecular docking, the results showed that the better anti-TMV activities of Z15 and Z16 may be related to the amino acid residue TYR-A-139, the strength of hydrogen bonds of Z15, Z16, and NNM with the amino acid residue TYR-A-139 are as follows, Z15 > Z16 > NNM.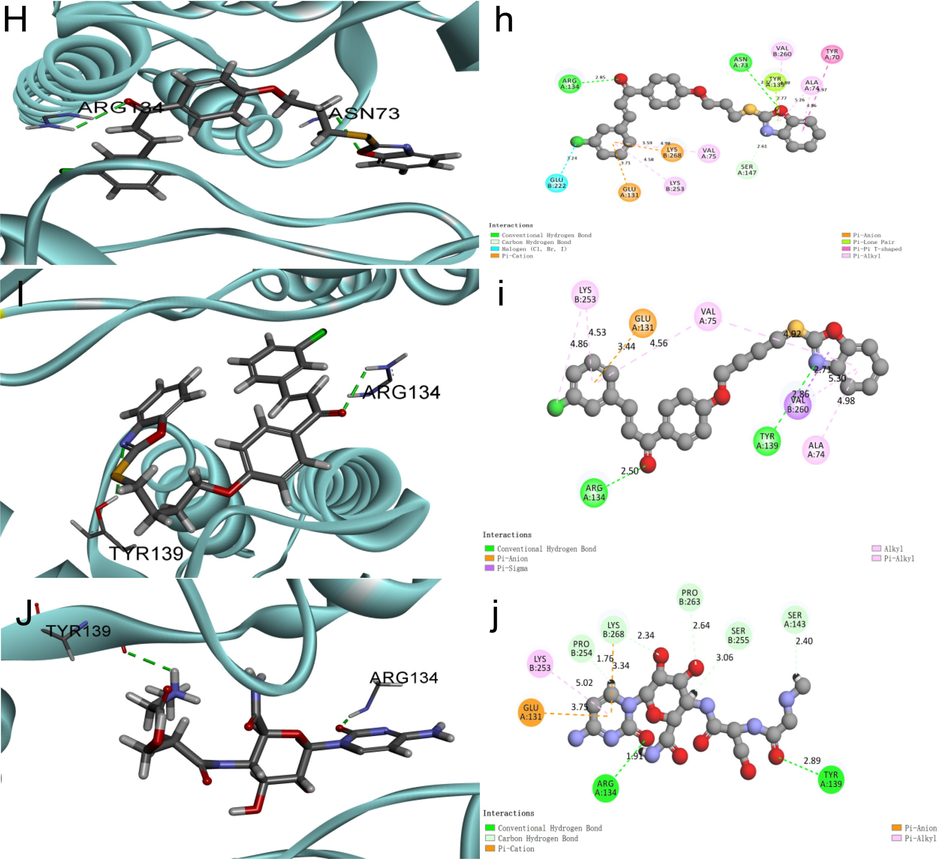
Molecular docking results of Z15 (H, h), Z16 (I, i), NNM (J, j).
In addition, Z15, Z16, and NNM formed 10, 8, 8 hydrophobic bonds with other amino residues of TMV-CP, respectively. Such as Pi-Alkyal, Pi-Pi, Pi-amion, Pi-sigma, and Alkyal. Due to the formation of these hydrophobic bonds provides a certain intermolecular force, it is likely to weaken the interaction of two subunits of TMV-CP, and prevents the self-assembly of TMV particle, as well as the binding capability with TMV-CP (Tang et al., 2019). Thus, the effect against TMV was achieved. The molecular docking results are that Z15 and Z16 have some anti-TMV activity, and Z15 > Z16 > NNM, which is in line with the data obtained from the preliminary screening data.
5 Conclusions
In this study, a series of chalcone derivatives containing benzoxazole were synthesized. The results of biological assays showed that some of the target compounds had excellent antibacterial and antiviral activities. The inhibiting activity of the Z2 against Xoo was tested with turbidity method, and the test results showed that Z2 had excellent inhibiting effect on Xoo. To study the mechanism of its inhibition, the cell membrane permeability, the content of EPS, the content of extracellular amylase and extracellular cellulase assays were performed between Z2 and Xoo, and the measurement results showed that Z2 could inhibit the content of Xoo biofilm to achieve the effect of inhibition. Moreover, when the drug concentrations were at of 200 and 100 mg/L, we tested the effect of Z2 on post-harvest potato and found that Z2 have a good protective activity for potatoes. Finally, Z15 and Z16 surpassed the commercial drug NNM in terms of both curative and protective activities. Molecular docking assay of Z15 and Z16 was performed, and the results of mechanism of action were consistent with the preliminary screening data. Consequently, this study lays the foundation for the application of chalcone derivatives in pesticides.
Acknowledgement
The authors gratefully acknowledge the National Nature Science Foundation of China (No. 32072446), the Science Foundation of Guizhou Province (No. 20192452).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Design, synthesis, bioactivity and mechanism of action of novel myricetin derivatives containing amide and hydrazide[J] Arab. J. Chem.. 2023;16(4):104588
- [Google Scholar]
- Design, synthesis and bioactivity of myricetin derivatives for control of fungal disease and tobacco mosaic virus disease[J] RSC Adv.. 2023;13(10):6459-6465.
- [Google Scholar]
- Synthesis and antibacterial activity of chalcone derivatives containing thioether triazole[J] J. Heterocycl. Chem.. 2020;57(3):983-990.
- [Google Scholar]
- Novel sulfone derivatives containing a 1,3,4-oxadiazole moiety: design and synthesis based on the 3D-QSAR model as potential antibacterial agent[J] Pest Manag. Sci.. 2020;76(9):3188-3198.
- [Google Scholar]
- Design, synthesis and biological activity of chalcone derivatives containing pyridazine[J] Arab. J. Chem.. 2023;16(7):104852.
- [Google Scholar]
- Design, synthesis, in silico and in vitro anticancer activity of novel bis-furanyl-chalcone derivatives linked through alkyl spacers[J] Chemistry Select. 2021;6(24):6202-6211.
- [Google Scholar]
- Fabrication of isopropanolamine-decorated coumarin derivatives as novel quorum sensing inhibitors to suppress plant bacterial disease[J] J. Agric. Food Chem.. 2022;70(20):6037-6049.
- [Google Scholar]
- Synthesis, molecular structure, thermal and spectroscopic analysis of a novel bromochalcone derivative with larvicidal activity[J] Crystals. 2022;12(4):440.
- [Google Scholar]
- New chalcone derivatives: synthesis, antiviral activity and mechanism of action[J] RSC Adv.. 2020;10(41):24483-24490.
- [Google Scholar]
- Novel trans-ferulic acid derivatives containing a chalcone moiety as potential activator for plant resistance induction[J] J. Agric. Food Chem.. 2017;65(22):4367-4377.
- [Google Scholar]
- Synthesis and antiviral evaluation of novel 1,3,4-oxadiazole/thiadiazole-chalcone conjugates[J] Bioorg. Med. Chem. Lett.. 2017;27(18):4298-4301.
- [Google Scholar]
- Design, synthesis, and antiviral activity of novel chalcone derivatives containing a purine moiety[J] Chin. J. Chem.. 2017;35(5):665-672.
- [Google Scholar]
- Food security: the challenge of feeding 9 billion people[J] Science. 2010;327(5967):812-815.
- [Google Scholar]
- Biological activity evaluation and action mechanism of chalcone derivatives containing thiophene sulfonate[J] RSC Adv. 2019;9(43):24942-24950.
- [Google Scholar]
- Synthesis, biological activity and action mechanism study of novel chalcone derivatives containing malonate[J] Chem. Bio.. 2020;195:2123-2130.
- [Google Scholar]
- Synthesis and antibacterial activity of novel myricetin derivatives containing sulfonyl piperazine[J] Chem. Pap.. 2021;75(3):1021-1027.
- [Google Scholar]
- Design, synthesis and biological activity of novel chalcone derivatives containing indole[J] Arab. J. Chem.. 2023;16(6):104776
- [Google Scholar]
- Synthesis and biological activities of novel chalcone derivatives containing pyrazole oxime ethers[J] Fitoterapia. 2023;166:105458
- [Google Scholar]
- Production of extracellular amylase contributes to the colonization of Bacillus cereus 0–9 in wheat roots[J] BMC Microbiol.. 2022;22(1):1-13.
- [Google Scholar]
- First report of bacterial soft rot of potato caused by Pectobacterium carotovorum subsp. brasiliense in Guangdong Province, China[J] Plant Dis.. 2019;103:363-364.
- [Google Scholar]
- Xanthomonas campestris cell–cell signalling molecule DSF (diffusible signal factor) elicits innate immunity in plants and is suppressed by the exopolysaccharide xanthan[J] J. Exp. Bot.. 2015;66(21):6697-6714.
- [Google Scholar]
- Cloning and analysis of characteristic sequence of StSnRK2. 4 gene in potatoes[J] J. Gansu. Agric. Uni.. 2016;49(2):77-83.
- [Google Scholar]
- Synthesis and biological activity of quinolorel myetin derivatives[J] J. Chem. High. Edu.. 2019;40(5):909-917.
- [Google Scholar]
- Increasing structural diversity of prenylated chalcones by two fungal prenyltransferases[J] J. Agric. Food Chem.. 2022;70(5):1610-1617.
- [Google Scholar]
- Design, synthesis, biological activity evaluation and mechanism of action of myricetin derivatives containing thioether quinazolinone[J] Arab. J. Chem.. 2022;15:104019
- [Google Scholar]
- Synthesis, antifungal activities and molecular docking studies of benzoxazole and benzothiazole derivatives[J] Molecules. 2018;23(10):2457.
- [Google Scholar]
- Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants[J] PLoS One. 2011;6(1):15853.
- [Google Scholar]
- Pectobacterium carotovorum subsp. Brasiliense and Pectobacterium carotovorum subsp. carotovorum as causal agents of potato soft rot in Algeria[J] Eur. J. Plant Pathol.. 2018;151(4):1027-1034.
- [Google Scholar]
- Synthesis and aharacterization of two novel thiofibrates bearing 1,3-benzoxazole moiety[J] Int. J. Chem. Tech. Res.. 2018;11(8):309-313.
- [Google Scholar]
- Synthesis of benzoxazole derivatives by Mannich reaction and in vitro cytotoxic, antimicrobial and docking studies[J] Chem. Data Collect.. 2021;31:100628
- [Google Scholar]
- Screening and characterization of Xanthomonas oryzae pv. oryzae strains with resistance to pheazine-1-carboxylic acid[J] Pestic. Biochem. Physiol.. 2018;145:8-14.
- [Google Scholar]
- Antibacterial and antiviral activities of 1,3,4-oxadiazole thioether 4H-chromen-4-one derivatives[J] J. Agric. Food Chem.. 2021;69:11085-11094.
- [Google Scholar]
- Synthesis of chalcone derivatives on steroidal framework and their anticancer activities[J] Steroids. 2007;72(13):892-900.
- [Google Scholar]
- Differential response of Brn1 at varied thermal regimes and its relation with virulence and extracellular release of cellulase and amylase in Alternaria brassicicola[J] J. Plant Pathol.. 2021;103(3):777-786.
- [Google Scholar]
- Green prevention and control help the high-quality development of ecological agriculture [J] China Sci. Found.. 2020;34(4):373.
- [Google Scholar]
- Design, synthesis, and antibacterial activity of novel myricetin derivatives containing sulfonate[J] Monatshefte fuer Chemie. 2021;152(3):345-356.
- [Google Scholar]
- Design, synthesis, and bioactivity of chalcone derivatives containing indanone[J] ACS Omega. 2023;8(2):2556-2563.
- [Google Scholar]
- Novel chalcone derivatives containing a 1,2,4-triazine moiety: design, synthesis, antibacterial and antiviral activities[J] RSC Adv.. 2019;9(11):6011-6020.
- [Google Scholar]
- Synthesis and antiviral activity of novel myricetin derivatives containing ferulic acid amide scaffolds[J] New J. Chem.. 2020;44(6):2374-2379.
- [Google Scholar]
- Synthesis of novel antibacterial and antifungal quinoxaline derivatives[J] RSC Adv.. 2022;12(4):2399-2407.
- [Google Scholar]
- Genetically engineered bacterial strains constructed as a whole-cell biosensor for specific volatiles identification of infected potato tubers with a soft rot disease[J] Sens. Actuators B: Chem.. 2023;387:133788
- [Google Scholar]
- Synthesis and antibacterial evaluation of novel chalcone derivatives containing a benzothiazole scaffold[J] Monatshefte fuer Chemie. 2019;150(6):1147-1154.
- [Google Scholar]
- Discovery of novel rost-4-ene derivatives as potential plant activators for preventing phytopathogenic bacterial infection: Design, synthesis and biological studies[J] Pest Manag. Sci.. 2022;78(8):3404-3415.
- [Google Scholar]
- Antimicrobial evaluation and action mechanism of chalcone derivatives containing quinoxaline moiety[J] Monatshefte fuer Chemie. 2019;150(7):1325-1334.
- [Google Scholar]
- Synthesis of chalcone derivatives and studies on their inhibitory activity and molecular docking[J] Chin. J. Org. Chem.. 2020;40:1704-1715.
- [Google Scholar]
- Effect of the surface activity on the antibacterial activity of octadecanoyl acetal sodium sulfite series[J] Colloids Surf. A Physicochem. Eng. Asp.. 2005;268(1):85-89.
- [Google Scholar]
- Xanthan induces plant susceptibility by suppressing callose deposition[J] Plant Physiol.. 2006;141(1):178-187.
- [Google Scholar]
- Zhan, W.L., Zhou, R., Mao, P., Yuan, C.M., Zhang, T., Liu, Y., Tian, J., Wang, H., Xue, W., 2023. Synthesis, antifungal activity and mechanism of action of novel chalcone derivatives containing 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole[J]. Mol. Diversity. DOI: org/10.1007/s11030-022-10593-4.
- Design, synthesis and antiviral activities of chalcone derivatives containing pyrimidine[J] J. Saudi Chem. Soc.. 2023;27(1):101590
- [Google Scholar]
- Synthesis and antitumor activity of oxazol-2-yl, benzoxazol-2-yl and-1,3,4-oxadiazol-2-yl-sulfur ethers[J] Chinese J. Org. Chem.. 2007;27(11):1342-1347.
- [Google Scholar]
- Insecticidal activities of chalcone derivatives against spodoptera- litura[J] J. Trop. Crop.. 2010;31(10):1821-1824.
- [Google Scholar]
- Semisynthesis and insecticidal bioactivities of benzoxazole and benzoxazolone derivatives of honokiol, a naturally occurring neolignan derived from Magnolia officinalis[J] Bioorg. Med. Chem. Lett.. 2020;30(9):127086
- [Google Scholar]
- Design, synthesis, and antifungal activity of novel chalcone derivatives containing a piperazine fragment[J] J. Agric. Food Chem.. 2022;70(4):1029-1036.
- [Google Scholar]
- Design, synthesis and antifungal activity of novel 1,4-penta-di-ene-3-one containing quinazolinone[J] Int. J. Mol. Sci.. 2023;24(3):2599.
- [Google Scholar]
Appendix A
Supplementary material
The Supporting Information includes the characterization data, HNMR and HRMS spectrogram for the target compounds. Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105368.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







