Translate this page into:
Design, synthesis, characterization, computational study and in-vitro antioxidant and anti-inflammatory activities of few novel 6-aryl substituted pyrimidine azo dyes
⁎Corresponding author. khushiazeez@yahoo.co.in (Aziz Unnisa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A series of 6-aryl substituted pyrimidine azodyes were synthesized by coupling of phenyl pyrimidine 2-amine with different aromatic amines. The synthetic compounds were screened for their in-vitro antioxidant and anti-inflammatory activities. The characterization of the synthesized compounds was carried out by IR, 1H NMR, 13C NMR and Mass spectrophotometry. Computational study of designed compounds was done by OCHEM, Molinspiration cheminformatics, Datawarrior, and Swiss ADME. DPPH assay was used to determine the antioxidant activity and heat hemolysis method for anti-inflammatory activity.
Keywords
Pyrimidine azo dyes
DPPH assay
Heat hemolysis
BOILED egg
1 Introduction
Azo dyes constitute one of the largest, versatile, and important classes of organic compounds with a range of uses in science and technology (Hamid, 2007; Zollinger, 2003). Of the diverse classes of dyes, azo dyes are one of the key class constituting a majority of the dyes used in the industry. The basic skeleton of Azo dyes is composed of Ar − N = N − Ar’, where Ar represents the aromatic groups. The unit containing the N = N is designated as an ‘azo group’. The color and polarity of the dyes depend on the nature of the aromatic substituents on the sides of the azo group.
Azo dyes are successfully employed as chemical sensors, LCD color filters, textile dyes, the chromophoric substrate for redox enzymes, optical polymers (Derkowska-Zielinska et al., 2019, 2020b), lasers, non-linear optics, and in many more specific applications, such as photovoltaic production(Derkowska-Zielinska et al., 2020a), as drug, cosmetic, food, and in organic synthesis.
Azo derivatives were effective biologic agents (Shridhar et al., 2012), possessing potent medicinal values. A Survey of the literature reveals their application as antidiabetics (Garg and Prakash, 1972), antitumor(Bradshaw et al., 2001; Kini et al., 2007; Racane et al., 2006), anti-inflammatory (Abadi et al., 2003; Venkatesh and N. Pandeya, 2009), anti-tuberculosis (Sah, 2010; Sah and Oneto, 2010), anti-neoplastic (Child et al., 1977; Yazdanbakhsh et al., 2012). Hence, studies have been conducted for the design, synthesis, purification, and use of azo dyes derived from different drug moieties to assess its antimicrobial properties (Koshti et al., 2008; Pathan and Borul, 2008; Rathod, 2011, 2010; Rathod and Thakre, 2013).
In the present study, we aim to design and synthesize novel azo compounds that can exhibit antioxidant and anti-inflammatory activities with lesser toxicity. The study will also be considering the influence of the three-dimensional nature of chemical structures on the ligand-receptor binding in the process of new drug development.
2 Materials and methods
The laboratory reagent grade chemicals were used without further purification. Melting points were determined in open glass capillaries using Tempo (6 0 0) melting point apparatus. IR spectra obtained from Bruker analyzers were established by Shimadzu FT-IR Spectrophotometer using the KBr pellets, Model No.8400S (Japan). 1H and 13C NMR spectra were documented on Bruker 400 MHz NMR spectrometer (Switzerland) using DMSO as a solvent. Shimadzu 1800 double beam UV–Visible spectrophotometer with a wavelength accuracy of 0.5 nm and a pair of 1 cm matched quartz cell was used for the measurement of optical density values. Centrifuge (REMI-C854/8) was used for centrifugation. TLC was run on silica gel-G plates using n-hexane: ethyl acetate (5:5) as a mobile phase to assess the reaction progression and purity of synthesized compounds.
2.1 General procedure for the synthesis of substituted chalcones (Palleros, 2004)
Equimoles of acetophenone (0.01 mol) and different aromatic benzaldehyde (0.01 mol) were taken into a mortar, NaOH was added and ground by pestle at room temperature by employing the friction method. Then the mixture was moistened with water. The reaction progression was checked by TLC, and all the reactions were observed to be completed in a time range of 15–45 min. The product was recrystallized from methanol and the purity was confirmed by melting point and TLC.
2.2 General procedure for the synthesis of substituted phenyl pyrimidine-2-amine derivatives (Kumar et al., 2017)
Equimoles of substituted Chalcones (0.01 mol) and Guanidine HCl (0.01 mmol) were refluxed for 4hrs in the presence of DMF by maintaining the temperature between 50 and 60 °C in a water bath. The mixture was moved into ice-cold water, the precipitate was filtered, dried, and recrystallized from methanol. The purity and progress of the reaction were confirmed by TLC and melting point.
2.3 General procedure for synthesis of azo compounds
Compounds 5a-5l (Table 1 & scheme 1) were prepared by the conventional method. The Phenyl pyrimidine-2-amine derivatives were dissolved in 2.5 ml conc. HCl and transferred into the beaker containing 2.5 ml (4 N) ice-cold solution of NaNO2 with stirring until the fumes disappear. The temperature of the reaction was maintained up to 0–5 °C. Diazonium salt solution prepared above was added dropwise to the acidic solutions of different aromatic amines. The reaction mixture was stirred for 10 min by maintaining the temperature between 5 and 10 °C. The dye products obtained were filtered, washed, and recrystallized from ethanol.
Compound
R1
R2
R3
Ar
Physical state
Colour
State
5a
H
NO2
H

orange
Solid
5b
H
NO2
H
Brown
Solid
5c
H
Cl
H
orange
Solid
5d
H
Cl
H

Brown
Solid
5e
H
Cl
H
yellow
Solid
5f
H
Cl
H
Yellow
Solid
5g
H
Cl
H
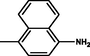
Brown
Solid
5h
OCH3
OCH3
OCH3

Brown
Solid
5i
OCH3
OCH3
OCH3
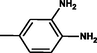
Brown
Solid
5j
OCH3
OCH3
OCH3
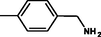
yellow
Solid
5k
OCH3
OCH3
OCH3
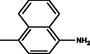
Yellow
Solid
5l
OCH3
OCH3
OCH3
Brown
Solid
Synthesis of Azodyes.
2.3.1 4-{(E)-[4-(4-nitrophenyl)-6-phenylpyrimidin-2-yl]diazenyl}aniline 5a
Molecular formula:C22H16N6O2,;Composition:C (66.66%) H (4.07%) N (21.20%) O (8.07%); Yield: 84.07%; M.P: 234℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.70 (s, 1H), 8.26 – 8.20 (m, 2H), 8.12 – 8.06 (m, 2H), 7.92 – 7.86 (m, 2H), 7.58 – 7.51 (m, 2H), 7.54 – 7.46 (m, 2H), 7.46 – 7.38 (m, 1H), 6.64 – 6.58 (m, 2H), 5.30 (s, 2H); 13C NMR (100 MHz, DMSO‑d6) δ 165.20, 157.68, (d, J = 11.9 Hz), 157.58, 150.43, 146.01, 137.45, 135.94, 129.49, 129.18, 128.99, 128.63, 125.89, 125.45, 124.60, 114.44, 114.07; FT-IR (KBr, v max (cm−1)): 3198 (Ar–CH), 1310 (C–N), 1657 (C = N), 1469 (weak, N = N), 1337 & 1480 (N = O), 1515 & 3080 (N-H); ESI-MS: 396.4 [M + H] + .
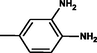
2.3.2 4-{(E)-[4-(4-nitrophenyl)-6-phenylpyrimidin-2-yl]diazenyl}benzene-1,2diamine 5b
Molecular formula:C22H17N7O2;Composition:C (64.23%), H (4.16%), N (23.83%), O(7.78%); Yield: 84.07%; M.P: 260℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.70 (s, 1H), 8.26 – 8.20 (m, 2H), 8.12 – 8.06 (m, 2H), 7.92 – 7.86 (m, 2H), 7.58 – 7.50 (m, 2H), 7.46 – 7.38 (m, 1H), 6.95 (dd, J = 7.7, 2.2 Hz, 1H), 6.62 (d, J = 7.7 Hz, 1H), 6.52 (d, J = 2.2 Hz, 1H); 13C NMR (100 MHz, DMSO‑d6) δ 165.20, 157.63 (d, J = 39.7 Hz), 146.01, 137.72, 137.45, 136.73, 135.94, 129.49, 129.18, 128.99, 128.63, 127.64, 124.60, 120.18, 119.48, 114.44, 111.83; FT-IR (KBr, v max (cm−1)): 3195 (Ar–CH), 1317 (C–N), 1656 (C = N), 1495 (weak,N = N), 1335& 1446 (N = O), 1513 & 3085 (N-H); ESI-MS: 411.4 [M + H] + .
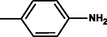
2.3.3 4-{(Z)-[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]diazenyl}aniline 5c
Molecular formula: C22H16ClN5;Composition:C (64.48%), H (4.18%), N (18.15%), Cl (9.19%); Yield: 77.28%; M.P: 218℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.70 (s, 1H), 7.94 – 7.86 (m, 4H), 7.55 6.58 (m, 2H), 5.30 (s, 2H); 13C NMR (100 MHz, DMSO‑d6) δ 165.20, 157.63 (d, J = 39.7 Hz), 150.43, 145.81, 137.45, 135.13, 132.88, 130.09, 129.49, 129.18, 128.76, 128.63, 125.45, 114.44, 114.07; FT-IR (KBr, v max (cm−1)): 3192 (Ar–CH), 1309 (C–N), 1654 (C = N), 1500 (weak,N = N), 1512 & 3079 (N-H), 1029 (C-Cl); ESI-MS: 385.8 [M + H] + .
2.3.4 4-{(Z)-[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]diazenyl}benzene-1,2-diamine 5d
Molecular formula: C22H17ClN6;Composition:C (65.92%), H (4.27%), N (2.96%), Cl (8.84%); Yield: 77.82%; M.P:256℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.70 (s, 1H), 7.94 – 7.86 (m, 4H), 7.58 – 7.50 (m, 2H), 7.46 – 7.38 (m, 1H), 7.33 – 7.27 (m, 2H), 6.95 (dd, J = 7.7, 2.0 Hz, 1H), 6.62 (d, J = 7.7 Hz, 1H), 6.52 (d, J = 2.0 Hz, 1H); 13C NMR (100 MHz, DMSO‑d6) δ 165.20, 157.63 (d, J = 11.9 Hz), 137.72, 137.45, 136.73, 135.13, 132.88, 130.09, 129.49, 129.18, 128.69 (d, J = 16.5 Hz), 127.64, 120.18, 119.48, 114.44, 111.83; FT-IR (KBr, v max (cm−1)): 3194 (Ar–CH), 1309 (C–N), 1656 (C = N), 1431 (weak,N = N), 1501 & 3075 (N-H), 1032 (C-Cl); ESI-MS: 400.86 [M + H] + .
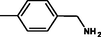
2.3.5 1-(4-{(Z)-[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]diazenyl}phenyl)methanamine 5e
Molecular formula: C23H18ClN5; Composition:C (69.08%), H (4.54%), N (17.51%), Cl (8.87%); Yield: 80.63%; M.P: 148℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.74 (t, J = 6.4 Hz, 2H), 8.70 (s, 1H), 7.94 – 7.84 (m, 6H), 7.58 – 7.50 (m, 2H), 7.46 – 7.38 (m, 1H), 7.36 – 7.27 (m, 4H), 4.13 – 4.07 (m, 2H); 13C NMR (100 MHz, DMSO‑d6) δ 165.20, 157.68, 157.58(d, J = 11.9 Hz), 149.04, 140.20, 138.54 – 122.36 (m), 114.44(d, J = 10.1 Hz), 45.59; FT-IR (KBr, v max (cm−1)): 3199 (Ar–CH), 2893 (Alif.–CH), 1309 (C–N), 1656 (C = N), 1445 (weak,N = N), 1518 & 3070 (N-H), 1032 (C-Cl); ESI-MS: 399.87 [M + H] + .
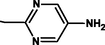
2.3.6 5-{(Z)-[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]diazenyl}pyrimidin-2-amine 5f
Molecular formula: C20H14ClN7; Composition:C (61.94%), H (3.64%), N (25.28%), Cl(9.14%); Yield: 78.03%; M.P: 242℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.50 (s, 1H), 8.28 (s, 2H), 7.94 – 7.86 (m, 4H), 7.58 – 7.50 (m, 2H), 7.46 – 7.38 (m, 1H), 7.33 – 7.27 (m, 2H), 6.61 (s, 2H); 13C NMR (100 MHz, DMSO‑d6) δ 169.92, 163.97, 160.65 (d, J = 2.5 Hz), 151.53, 139.89, 137.01 (d, J = 17.9 Hz), 132.30, 130.20 (d, J = 1.4 Hz), 128.95, 128.65 (d, J = 13.5 Hz), 115.68; FT-IR (KBr, v max (cm−1)): 3191 (Ar–CH), 1309 (C–N), 1656 (C = N), 1480 (weak,N = N), 1519 & 3072(N-H), 1032 (C-Cl); ESI-MS: 387.8 [M + H] + .
2.3.7 4-{(E)-[4-(4-chlorophenyl)-6-phenylpyrimidin-2-yl]diazenyl}naphthalen-1-amine 5g
Molecular formula: C26H18ClN5; Composition:C (71.64%), H (4.16%), N (16.07%), Cl (8.13%); Yield: 83.67%; M.P: 216℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.70 (s, 1H), 8.39 – 8.33 (m, 1H), 8.01 (dd, J = 7.7, 1.4 Hz, 1H), 7.94 – 7.86 (m, 5H), 7.63 (td, J = 7.5, 1.3 Hz, 1H), 7.58 – 7.50 (m, 2H), 7.46 – 7.38 (m, 2H), 7.33 – 7.27 (m, 2H), 6.93 (d, J = 7.7 Hz, 1H), 5.45 (s, 2H); 13C NMR (100 MHz, DMSO‑d6) δ 165.20, 157.63 (d, J = 11.9 Hz), 146.22, 144.30, 137.45, 135.13, 132.88, 132.19, 130.09, 129.49, 129.18, 128.69 (d, J = 16.5 Hz), 128.13, 123.90 (d, J = 1.9 Hz), 122.51, 120.51, 114.40 (d, J = 10.1 Hz), 109.01; FT-IR (KBr, v max (cm−1)): 3192 (Ar–CH), 1309 (C–N), 1655 (C = N), 1501 (weak,N = N), 1510 & 3072(N-H), 1032 (C-Cl); ESI-MS: 435.9 [M + H] + .
2.3.8 4-[(1E)-2-[4-phenyl-6-(3,4,5-trimethoxyphenyl)pyrimidin-2-yl]diazen-1-yl]aniline 5 h
Molecular formula: C25H23N5O3; Composition:C (68.01%), H (5.25%), N(15.86%), O (10.87%); Yield: 83.96%; M.P: 198℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.70 (s, 1H), 7.92 – 7.86 (m, 2H), 7.58 – 7.51 (m, 2H), 7.51 – 7.46 (m, 2H), 7.46 – 7.38 (m, 1H), 6.92 (s, 2H), 6.64 – 6.58 (m, 2H), 5.30 (s, 2H), 3.75 (s, 3H); 13C NMR (100 MHz, DMSO‑d6) δ 164.69, 157.94, 157.71, 153.44, 150.43, 141.39, 137.45, 130.30, 129.49, 129.18, 128.63, 125.89, 125.45, 114.99, 114.07, 111.16, 60.68, 56.26; FT-IR (KBr, v max (cm−1)): 3191(Ar–CH), 2894 (Alif.–CH), 1276 (C–N), 1655 (C = N), 1010 & 1276(C–O), 1489 (weak,N = N), 1503&3070(N-H); ESI-MS: 441.4 [M + H] + .
2.3.9 4-[(1E)-2-[4-phenyl-6-(3,4,5-trimethoxyphenyl)pyrimidin-2-yl]diazen-1-yl]benzene-1,2-diamine 5i
olecular formula: C25H24N6O3, Composition:C (65.78%), H (5.30%), N (18.41%), O (10.51%); Yield: 79.32%; M.P: 236℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.70 (s, 1H), 7.92 – 7.86 (m, 2H), 7.58 – 7.50 (m, 2H), 7.46 – 7.38 (m, 1H), 6.95 (dd, J = 7.7, 2.2 Hz, 1H), 6.92 (s, 2H), 6.62 (d, J = 7.7 Hz, 1H), 6.52 (d, J = 2.2 Hz, 1H), 3.87 (s, 6H), 3.75 (s, 3H); 13C NMR (100 MHz, DMSO‑d6) δ 164.69, 157.94, 157.71, 153.44, 141.39(d, J = 575.2 Hz), 138.86 – 135.66 (m), 133.97 – 124.13 (m), 119.83 (d, J = 339.7 Hz), 114.99, 111.83, 111.16, 60.68, 56.26(d, J = 1767.9 Hz); FT-IR (KBr, v max (cm−1)): 3191(Ar–CH), 2894 (Alif.–CH), 1318 (C–N), 1659 (C = N), 1017 & 1248(C–O), 1499 (weak, N = N), 1503&3072(N-H);ESI-MS: 456.4 [M + H] + .
2.3.10 1-{4-[(1E)-2-[4-phenyl-6-(3,4,5-trimethoxyphenyl)pyrimidin-2-yl]diazen-1yl] phenyl}methanamine 5j
Molecular formula: C26H25N5O3; Composition:C (68.56%), H (5.53%), N (15.37%), O (10.54%); Yield: 76.25%; M.P:142℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.77 – 8.68 (m, 3H), 7.88 (d, J = 9.2, 8.0, 1.5 Hz, 4H), 7.58 – 7.50 (m, 2H), 7.46 – 7.38 (m, 1H), 7.33 (dt, J = 7.7, 1.0 Hz, 2H), 6.92 (s, 2H), 4.10 (tt, J = 6.2, 1.0 Hz, 2H), 3.75 (s, 3H); 13C NMR (100 MHz, DMSO‑d6) δ 164.69, 157.94, 157.71, 153.44, 140.80 (d, J = 573.2 Hz), 137.45, 130.30, 129.49, 129.18, 129.12, 128.89, 128.63, 124.11, 114.99, 111.16, 60.68, 56.26, 45.59; FT-IR (KBr, v max (cm−1)): 3194(Ar–CH), 2892 (Alif.–CH), 1318 (C–N), 1658 (C = N), 1017 & 1247 (C–O), 1499 (weak,N = N), 1502 & 3074(N-H);ESI-MS: 455.5 [M + H] + .
2.3.11 4-[(1E)-2-[4-phenyl-6-(3,4,5-trimethoxyphenyl)pyrimidin-2-yl]diazen-1-yl]naphthalen-1-amine 5k
Molecular formula: C29H25N5O3; Composition:C (70.86%), H (5.13%), N (14.25%), O (9.76%); Yield: 82.36%; M.P: 238℃ ± 1; 1H NMR (400 MHz, DMSO‑d6) δ 8.70 (s, 1H), 8.39 – 8.33 (m, 1H), 8.01 (dd, J = 7.7, 1.2 Hz, 1H), 7.94 – 7.86 (m, 3H), 7.63 (td, J = 7.5, 1.3 Hz, 1H), 7.58 – 7.50 (m, 2H), 7.46 – 7.38 (m, 2H), 6.93 (d, J = 8.4 Hz, 3H), 5.45 (s, 2H), 3.75 (s, 3H); 13C NMR (100 MHz, DMSO‑d6) δ 170.42, 160.80, 158.40, 153.02, 146.25, 142.01, 139.74, 137.10, 130.16 (d, J = 6.0 Hz), 129.27, 128.95, 128.53 (d, J = 10.2 Hz), 127.93, 124.48 (d, J = 6.8 Hz), 122.76, 121.12, 116.55, 111.06, 108.15, 60.78, 56.26; FT-IR (KBr, v max (cm−1)): 3191(Ar–CH), 2894 (Alif.–CH), 1318 (C–N), 1658 (C = N), 1017 & 1247 (C–O), 1478 (weak,N = N), 1502 & 3073(N-H);ESI-MS: 491.5 [M + H] + .
2.3.12 5-[(1E)-2-[4-phenyl-6-(3,4,5-trimethoxyphenyl)pyrimidin-2-yl]diazen-1-yl]pyrimidin-2-amine 5l
Molecular formula: C23H21N7O3; Composition:C (62.29%), H (4.77%), N (22.11%), O (10.82%); Yield: 75.13%; M.P: 244℃ ± 1;1H NMR (400 MHz, DMSO‑d6) δ 8.28 (s, 2H), 7.92 – 7.86 (m, 2H), 7.58 – 7.50 (m, 2H), 7.46 – 7.38 (m, 1H), 6.92 (s, 2H), 6.61 (s, 2H), 3.75 (s, 3H); 13C NMR (100 MHz, DMSO‑d6) δ 164.02 (d, J = 537.9 Hz), 157.94, 157.71, 153.44, 148.53, 141.39, 137.45, 134.63 – 123.78 (m), 129.49, 129.18, 128.63, 114.99, 111.16, 60.68, 56.26(d, J = 2121.4 Hz); FT-IR (KBr, v max (cm−1)): 3192(Ar–CH), 2893 (Alif.–CH), 1318 (C–N), 1658 (C = N), 1017 & 1247(C–O), 1468 (weak, N = N), 1502&3071(N-H);ESI-MS: 443.4 [M + H] + .
2.4 Computational study
The activity of compounds against CYP450 subtypes (CYP2D6, CYP3A4, CYP2C9, CYP1A2, and CYP2C19) was predicted using OCHEM. Bioactivity scores were projected by online Molinspiration cheminformatics. The drug-likeness and toxicity properties were determined using the OSIRIS property explorer (Data warrior). The human brain permeability and intestinal absorption were predicted by the construction of a Brain Or IntestinaL EstimateD permeation(BOILED), -Egg model, using the Swiss-ADMET web tool.
2.5 Molecular docking:
Docking involved three steps i) protein preparation ii) ligand preparation iii) molecular docking. 2.5.1 Protein preparation:
Protein preparation was performed on the crystal structure of a complex formed between phospholipase A2 and aspirin (Pdb id: 3NT1) using protein preparation wizard (Schrödinger suite LLC) in which water molecules were removed, bond orders were assigned, hydrogens were added, creating zero bond orders to metals, creating disulfide bonds and deleting 5A distance water from the protein.
2.5.1 Ligand preparation
Ligand preparation was done using a pool of tools intended to produce high-quality 3D structures for a variety of drug-like structures beginning with 2D or 3D structures in SD or maestro format. The use of LIGPREP software produces single, low energy, 3D structure with correct chiralities for each of the planned structure. The structures of ligands were downloaded from the PubChem search beta (https://pubchem.ncbi.nlm.nih.gov/). Ligand structures were geometrically minimized using the OPLS_2005 force field by LIG PREP molecule of Maestro 11.1(Schrodinger LLC). The prepared ligands were used for docking
2.5.2 Molecular docking
docking was performed using Glide molecule version 11.1.011 in the Schrodinger suite. Induced fit docking was carried out using Schrodinger’s Glide.
2.6 Biological evaluation of the synthesized compounds 5a–5l
2.6.1 Anti-inflammatory activity
2.6.1.1 Preparation of RBCs suspension (Juvekar et al., 2009; Sadique et al., 1989)
Blood from the healthy human volunteer who has not taken any NSAIDs for two weeks before the experiment was conducted and was transferred into the centrifuge tubes which were then centrifuged at 3000 rpm for 10 min. The RBC’s suspension was washed thrice with an equal volume of Normal saline (NS). The volume of blood was measured and reconstituted to 10% v/v suspension with NS.
2.6.1.2 Heat-induced hemolysis (Shinde et al., 1999)
The mixture consisting of a 1 ml test sample in a concentration range of50 − 250 μg/ml and 1 ml of 10% RBCs suspension were taken into the centrifuge tubes. The tubes were incubated for 30 min on a water bath maintained at 56 °C which was then cooled under running tap water, the mixture was centrifuged at 2500 rpm for 5 min and the OD values of the supernatant were measured at 660 nm. Instead of a test sample, only saline was taken as the control. Aspirin was used as a reference drug.
The % inhibition of Haemolysis was calculated from the formula:
2.6.2 Anti-oxidant activity
DPPH Free Radical Scavenging Assay (Shridhar et al., 2016)
Sample stock solutions (1.0 mg/mL) were diluted to final concentrations of 2, 4, 6, 8, 10 g/mL, using D. water. One mL of a 0.1 mM DPPH ethanolic solution was added to 2.5 ml of sample solutions of different concentrations and allowed to react under room temperature. The OD values were recorded at 518 nm after 30 min and converted into the % antioxidant activity using the formula:
From the % Inhibition, the IC-50 values were determined.
3 Results and discussion
The titled compounds i.e. azodyes were synthesized in three steps via the formation of chalcones. Step-1 for chalcone preparation involved solvent-free condition, by frictional force applied through grinding various aldehydes with acetophenone in a mortar and a pestle. (Bathelemy et al., 2016). The observations showed that there was no difference in yield and purity. This method of preparation of chalcones reduced the cost of solvent, time, and usage of organic solvents such as alcohol. This method was more reliable in normal laboratory conditions.
step-2 involved the formation of substituted Phenyl pyrimidine-2-amine derivatives by cyclization of chalcones with guanidine hydrochloride at a temperature of 60 °C in presence of dimethylformamide. DMF is a polar aprotic hydrophilic solvent served as a good catalyst and a reagent. (Heravi et al., 2018) The free primary amino group was diazotized and coupled with various aromatic amines by conventional diazotization of the free amino group in step-3. The presence of azo group was characterized by IR spectroscopy at a frequency between 1500 and 1400 and by the absence of free NH-stretching.
The progress of the reaction and purity of the compounds was assessed by thin-layer chromatography and their melting points. Characterization was done by FTIR, proton and C-13 NMR spectroscopy done on Bruker at 400 and 100 MHZ
The present work also focused on computational tools used to predict the properties, toxicities, and activity of the compounds on various targets.
3.1 OCHEM
Cytochrome P450 and its subtypes are an important set of enzymes involved in the metabolism of various drugs. The present work involves the in-silico study of the synthesized drug candidates by these enzymes. OCHEM (online chemical modeling), a web-based tool (Sushko et al., 2011), was chosen to evaluate the activity of the synthesized azo dyes against the cytochromes P450 (CYPs). OCHEM predicts the ability of a chemical entity as an inhibitor or non-inhibitor of CYP450 subtypes (CYP3A4, CYP2C19, CYP2D6, CYP1A2, CYP2C9).
The synthesized compounds were established to be inhibitors of the subtypes of cytochrome P450. The compound 5j (inhibitors of CYP2D6), 5c, 5d, 5e, 5h, 5i, 5j (inhibitors of CYP2C19), 5a-5e, 5g-5l (inhibitors of CYP2C9), 5c, 5d, 5e, 5f, 5g (inhibitors of CYP1A2), 5a-5e, 5g-5l(inhibitors of CYP3A4) & remaining were non-inhibitors of subtypes which was shown in the Table 2.
Compound
AMES
CYP1A2
CYP2C9
CYP2C19
CYP2D6
CYP3A4
5a
active
–
+
–
–
+
5b
active
–
+
–
–
+
5c
active
+
+
+
–
+
5d
active
+
+
+
–
+
5e
inactive
+
+
+
–
+
5f
inactive
+
–
–
–
–
5g
inactive
+
+
–
–
+
5h
active
–
+
+
–
+
5i
active
–
+
+
–
+
5j
inactive
–
+
+
+
+
5k
inactive
–
+
–
–
+
5l
inactive
–
+
–
–
+
3.2 Molinspiration cheminformatics
Molinspiration cheminformatics (Nadeem et al., 2016) bioactivity score prediction for drug targets include GPCR ligands, kinase inhibitors, ion channel modulators, enzymes, and nuclear receptors. The larger is the bioactivity score, the higher is the probability of the compound to be active. Therefore, a molecule having a bioactivity score more than 0.00 is likely to possess biological activity, value −0.50 to 0.00 are moderately active and if the score is less than −0.50 it is presumed to be inactive
Compounds 5c-5l were active, 5a & 5b were moderately active on GPCR ligand, 5c-5g, 5i-5l were active, remaining were moderately active on enzyme inhibitor, all the compounds were active on kinase inhibitor, 5e was active, and remaining were moderately active on ion channel modulator, all of them were moderately active on nuclear receptor ligand & protease inhibitors, which was tabulated in Table 3 and have prospective for further structural alteration using QSAR studies.
S. No
compounds
GPCR ligand
Ion channel modulator
Kinase inhibitor
Nuclear receptor ligand
Protease inhibitor
Enzyme inhibitor
1
5a
−0.01
−0.07
0.13
−0.42
−0.24
−0.05
2
5b
−0.05
−0.11
0.15
−0.44
−0.27
−0.03
3
5c
0.12
−0.03
0.26
−0.39
−0.18
0.00
4
5d
0.08
−0.08
0.27
−0.41
−0.21
0.03
5
5e
0.17
0.00
0.28
−0.47
−0.04
0.01
6
5f
0.19
−0.04
0.36
−0.30
−0.20
0.07
7
5g
0.13
−0.01
0.21
−0.33
−0.25
0.03
8
5h
0.05
−0.10
0.24
−0.40
−0.21
−0.01
9
5i
0.01
−0.13
0.25
−0.42
−0.24
0.01
10
5j
0.09
−0.07
0.25
−0.47
−0.10
0.00
11
5k
0.06
−0.13
0.20
−0.35
−0.27
0.01
12
5l
0.11
−0.10
0.32
−0.32
−0.23
0.05
3.3 Open source program OSIRIS property explorer
Druglikeness, a qualitative concept used in drug design for how “drug-like” a substance is concerning bioavailability usually given a score based on the Lipinski rule of five concepts. The Insilco toxicity properties and drug-likeness were obtained from OSIRIS (Sander et al., 2015), property explorer.Compounds 5a, 5b, 5c, 5d, 5e, 5f, 5h, 5i, 5j, 5l were non mutagenic, 5b, 5d, 5e, 5g, 5i, 5j, 5k were non tumorigenic (Fig. 1) and 5a, 5c, 5d, 5f, 5k were non irritant(Fig. 2)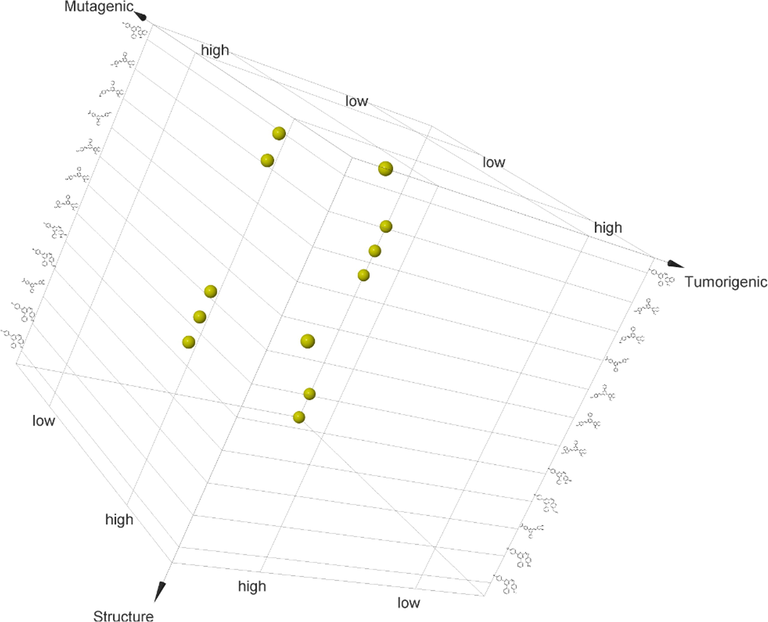
3D Graph- Structure vs Tumorigenic vs Mutagenic.
3D Graph- Structure vs Tumorigenic vs Mutagenic.
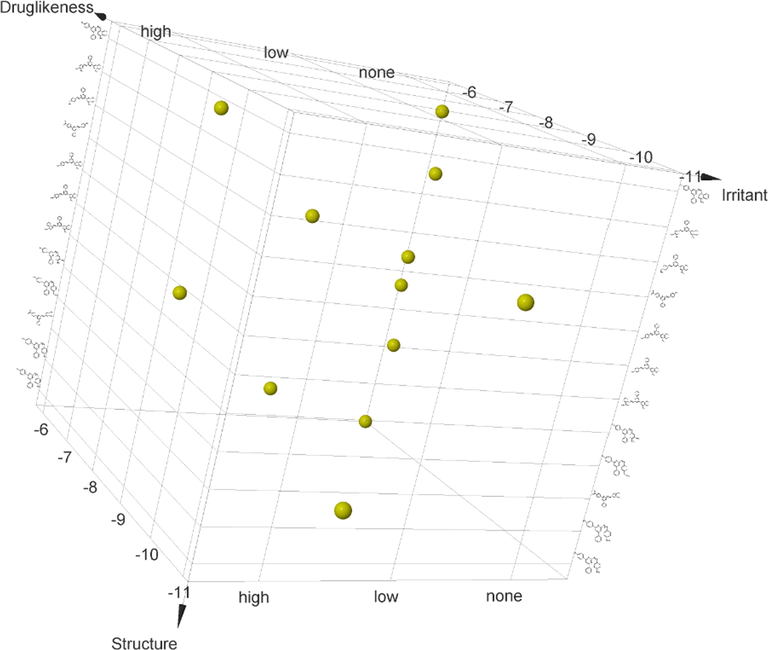
3.4 Human intestinal absorption(HIA) and brain permeability prediction
Brain permeability and HIA are two important pharmacokinetic parameters that contribute to the bioavailability and cell permeability to a considerable degree. To predict the HIA and brain permeability, we constructed a BOILED-Egg model on the online Swiss ADMET tool by the tendering SMILE notations as input (Daina et al., 2017), which resulted in the creation of the BOILED-Egg model with WLOGP Vs TPSA onx&y-axis respectively (Fig. 3). Molecules that fall in the yellow region depict the BBB permeation whereas the white eclipse region represents gastrointestinal absorption. Among all the analyzed molecules, Compounds 5c, 5e, 5f was well-absorbed but have no access to the brain. Compound 5j showed passive intestinal absorption. Compounds 5e, 5j were substrates for P-gp while the others are nonsubstrates of P-gp efflux. None of the produced compounds could cross the BBB. The conclusion drawn from these studies would be useful to advance further for the enhancement of their pharmacokinetic properties.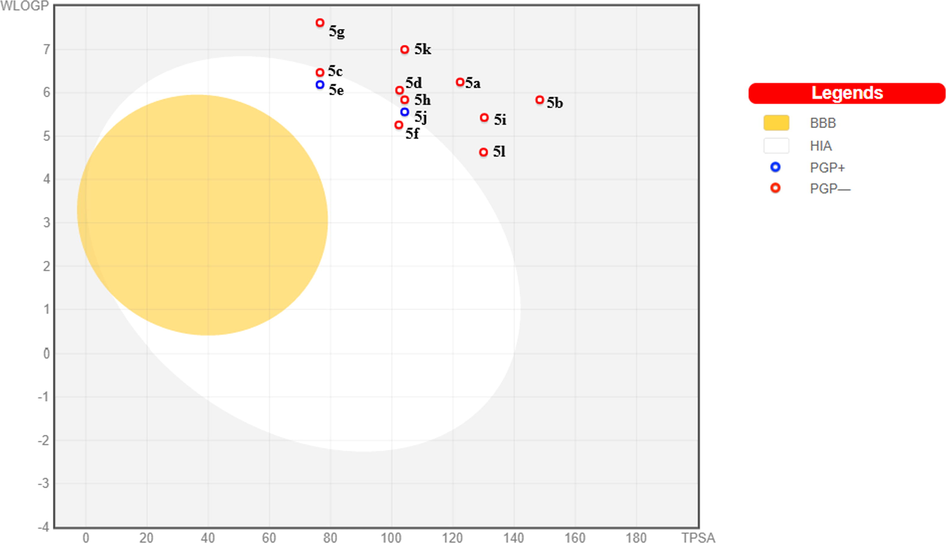
Graphical representation of Brain Or IntestinaLEstimateD permeation. HIA: Human Intestinal AsorptionBBB: Blood-Brain Barrier PGP: PermeabilityGlyco Protein.
3.5 Molecular docking
Inflammation common symptoms for various ailments and diseases occur due to various eicosanoids released by the action of COX enzymes on arachidonic acid due to cell or tissue damage. Though many classes of drugs were found to be the inhibitors for COX yet a perfect inhibitor was unknown. Several researchers are still working on COX inhibitors to bring out effective and safe drugs used in all types of inflammations. In this research, we used molecular docking to understand the interaction of azodyes with COX-2.GLIDE was used for docking studies. (Kumar et al., 2019). Amongst the synthesized compounds, it was observed that three compounds could dock into the COX-2 active site successfully. The G-Scores were of −6.23, −6.14, and −5.809 kcal/mol were obtained for 5c, 5d, and 5f respectively. The lower score was observed for 5c rationalizes the tighter binding of azodye analog into the COX-2 active site than that of the other two compounds. All the three compounds involved in the hydrogen bonding with a residue Tyr 355, which can be due to the bonding with azo nitrogen and pi interaction of phenyl ring with Tyr 355. The azo dye group was surrounded by residues Ser353, Leu 352, Val 349, Ala527, and Glu 524 of COX-2. Therefore using phenyl azo fragment of the molecule and Tyr 355 contributed to stabilizing the ligand–enzyme complex. Molecular docking studies further supported the strong inhibitory activity of 5c compared to 5d and 5f (Fig. 4).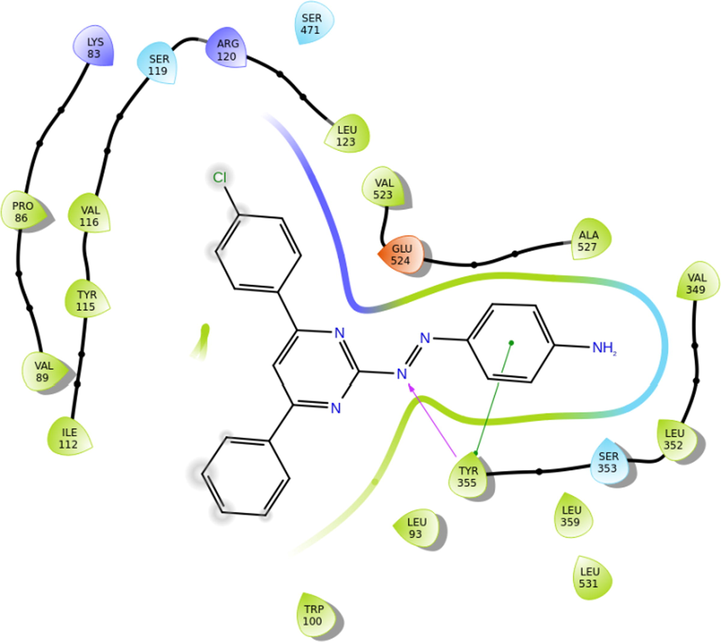
Molecular docking; The interaction of compound 5c with 3NT1.
3.6 Anti-inflammatory activity
The heat-induced hemolysis method was used to measure the anti-inflammatory activity of the synthesized compounds. The synthesized compounds 5b, 5c, 5e, 5h, and 5j exhibited membrane stabilization effect by inhibiting the lysis of the erythrocyte membrane. The percentage of membrane stabilization for synthesized compounds and standard were done at 50, 100, 150, 200, 250 µg/ml, and values were reported in the Table 4. Compound 5c, 5j showed maximum inhibition of 71.08%, 71.91% respectively which were closer to that of the standard aspirin (72.91%). The anti-inflammatory action of the synthesized compounds is completely concentration-dependent as seen from Fig. 5.
S. No
Compound
% inhibition
50 µg/ml
100 µg/ml
150 µg/ml
200 µg/ml
250 µg/ml
1
5a
39.32
44.94
52.19
63.31
70.53
2
5b
15.73
21.12
29.21
43.82
50.05
3
5c
24.49
26.74
33.11
40.22
49.21
4
5d
34.05
42.07
49.38
58.34
64.87
5
5e
19.24
23.48
29.59
35.59
42.80
6
5f
25.61
27.83
30.31
34.82
40.44
7
5g
33.23
40.28
46.42
57.23
66.58
8
5h
36.56
47.69
53.58
60.21
69.74
9
5i
26.54
32.85
42.81
49.95
52.91
10
5j
24.58
28.34
33.86
37.29
42.18
11
5k
27.24
32.89
40.47
49.57
55.23
12
5l
34.59
48.20
53.43
61.68
67.92
13
Aspirin
35.29
48.83
56.75
63.27
72.91
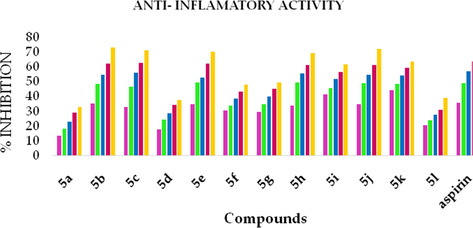
Concentration-dependent increase in the anti-inflammatory activity of Azo Dyes.
3.7 Antioxidant activity
DPPH assay is widely used to assess the compounds for their antioxidant activity because of its reliability and simple procedure. The results of the DPPH assay of the synthesized compounds are shown in Table 5. Lower the IC-50 value better than the DPPH activity. In the assay carried out the compounds 5c, 5f, 5j, 5l showed better activities. It was important to note that compounds 5c(3.13) has the IC-50 value closer to that of ascorbic acid (3.03). A concentration-dependent increase in the antioxidant activity of the tested compounds was observed as shown in Fig. 6.
S.No
Compound
IC50
% inhibition
2 µg/ml
4 µg/ml
6 µg/ml
8 µg/ml
10 µg/ml
1
5a
6.53
33.21
39.96
47.87
53.41
59.25
2
5b
5.82
39.25
36.21
41.14
48.62
53.54
3
5c
3.13
43.99
30.21
36.81
41.81
48.22
4
5d
7.66
30.41
34.26
40.31
44.59
51.19
5
5e
11.05
24.98
25.80
32.96
43.57
58.23
6
5f
3.61
42.63
48.21
53.57
58.92
57.14
7
5g
8.99
30.85
32.54
39.96
47.26
55.72
8
5h
10.19
28.64
37.44
43.80
51.62
57.92
9
5i
8.94
19.82
45.34
49.85
57.33
61.59
10
5j
3.35
43.01
52.31
55.88
59.67
63.29
11
5k
6.92
38.59
47.83
52.51
58.26
64.11
12
5l
3.91
40.96
42.56
47.16
50.81
57.33
13
Ascorbic acid
3.03
43.91
56.13
59.55
62.72
67.53
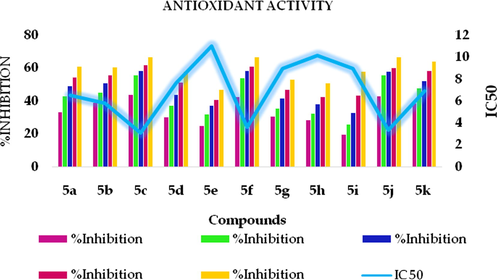
Graphical representation of compounds vs % inhibition for anti-inflammatory activity.
4 Conclusion
The results of the present study indicated that all the synthesized pyrimidine azo dyes exhibited antioxidant and anti-inflammatory activities with less toxicity. Among all the 12 compounds 5c and 5j were found to greatly influence the activities which may be due to the type of substituents present on the ring. The study carried out considered that the three-dimensional nature of chemical structures plays an important part in ligand-receptor binding and assists in providing an approach for further optimization and development of new leads.
Acknowledgement
This research has been funded by Scientific Research Deanship at the University of Ha’il – Saudi Arabia through project number RG-191339.
References
- Synthesis of novel 1,3,4-trisubstituted pyrazole derivatives and their evaluation as antitumor and antiangiogenic agents. Chem. Pharm. Bull. (Tokyo). 2003;51:838-844.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of antimicrobial properties of some chalcones. Br. J. Pharm. Res.. 2016;14:1-11.
- [CrossRef] [Google Scholar]
- Bradshaw, T.D., Stevens, M.F.G., Westwell, A.D., 2001. The discovery of the potent and selective antitumour agent 2-(4-amino-3-methylphenyl) benzothiazole (DF 203) and related compounds. Curr. Med. Chem. 8, 203–210. https://doi.org/https://doi.org/10.2174/0929867013373714.
- Child, R.G., Wilkinson, R.G., Tomcu-Fucik, A., 1977. Effect of substrate orientation of the adhesion of polymer joints, in: Chem. Abstr. p. 6031.
- SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep.. 2017;7:42717.
- [CrossRef] [Google Scholar]
- Photovoltaic cells with various azo dyes as components of the active layer. Sol. Energy. 2020;203:19-24.
- [CrossRef] [Google Scholar]
- All-optical poling and two-photon absorption in heterocyclic azo dyes with different side groups. J. Phys. Chem. C. 2019;123:725-734.
- [CrossRef] [Google Scholar]
- Photoresponsive behavior of heterocyclic azo polymers with various functional groups. J. Phys. Chem. C. 2020;124:939-944.
- [CrossRef] [Google Scholar]
- Potential antidiabetics. 11. Preparation of 4-arylazo-3,5-disubstituted-(2H)-1,2,6-thiadiazine 1,1-dioxides. J. Med. Chem.. 1972;15:435-436.
- [CrossRef] [Google Scholar]
- Characterization and thermal decompsiton of indolylidene aniline azo-dyes derivatives. Jordan J. Chem.. 2007;2:133-144.
- [Google Scholar]
- Beyond a solvent: triple roles of dimethylformamide in organic chemistry. RSC Adv.. 2018;8:27832-27862.
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata. Planta Med.. 2009;75:PJ178.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of novel benzothiazole derivatives against human cervical cancer cell lines. Indian J. Pharm. Sci.. 2007;69:46.
- [CrossRef] [Google Scholar]
- Koshti, S.M., Sonar, J.P., Sonawane, A.E., Pawar, Y.A., Nagle, P.S., Mahulikar, P.P., More, D.H., 2008. Synthesis of azo compounds containing thymol moiety.
- Kumar, D.T., Iyer, S., Christy, J.P., Siva, R., Tayubi, I.A., Doss, C.G.P., Zayed, H., 2019. A comparative computational approach toward pharmacological chaperones (NN-DNJ and ambroxol) on N370S and L444P mutations causing Gaucher’s disease, in: Advances in Protein Chemistry and Structural Biology. Elsevier, pp. 315–339. https://doi.org/https://doi.org/10.1016/bs.apcsb.2018.10.002.
- Synthesis and pharmacological screening of 4, 6-substituted di-(phenyl) pyrimidin-2-amines. Arab. J. Chem.. 2017;10:S877-S880.
- [CrossRef] [Google Scholar]
- Synthesis, spectral characterization and in vitro antibacterial evaluation and Petra/Osiris/Molinspiration analyses of new Palladium(II) iodide complexes with thioamides. Alexandria J. Med.. 2016;52:279-288.
- [CrossRef] [Google Scholar]
- Palleros, D.R., 2004. Solvent-free synthesis of chalcones. J. Chem. Educ. 81, 1345. https://doi.org/doi:10.1021/ed081p1345.
- Synthesis and antimicrobial activity of Azo compounds containing drug moiety. Orient. J. Chem.. 2008;24:1147-1148.
- [Google Scholar]
- Racane, L., Stojkovic, R., Tralic-Kulenovic, V., Karminski-Zamola, G., 2006. Synthesis and antitumor evaluation of novel derivatives of 6-amino-2-phenylbenzothiazoles. Molecules 11, 325–333. https://doi.org/https://doi.org/10.3390/11050325.
- Synthesis and antimicrobial activity of azo compounds containing resorcinol moiety. Asian J. Res. Chem.. 2011;4:734-736.
- [Google Scholar]
- Synthesis and antimicrobial activity of azo compounds containing paracetamol moiety. Orient. J. Chem.. 2010;26:1163.
- [Google Scholar]
- Synthesis and antimicrobial activity of azo compounds containing m-cresol moiety. Chem. Sci. Trans.. 2013;2:25-28.
- [CrossRef] [Google Scholar]
- The bioactivity of certain medicinal plants on the stabilization of RBC membrane system. Fitoterapia. 1989;60:525-532.
- [Google Scholar]
- The antitubercular activity of azo dyes. I. Bisazo dyes derived from tetrazotized bis(4-aminophenyl)-sulfone and various coupling components. Recl. des Trav. Chim. Pays-Bas. 2010;69:1407-1434.
- [CrossRef] [Google Scholar]
- The antitubercular activity of azo dyes. II. Monazo dyes derived from diazotized sulfa drugs and bis (4-aminophenyl)-sulfone and bisazo dyes from tetrazotized bis (4-aminophenyl)-sulfone and sulfa drugs. Recl. des Trav. Chim. des Pays-Bas. 2010;69:1435-1447.
- [CrossRef] [Google Scholar]
- DataWarrior: an open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model.. 2015;55:460-473.
- [CrossRef] [Google Scholar]
- Preliminary studies on the immunomodulatory activity of Cedrus deodara wood oil. Fitoterapia. 1999;70:333-339.
- [CrossRef] [Google Scholar]
- Synthesis of 1, 3, 4-oxadiazole incorporated azo dye derivatives as a potent Biological activity molecules. Int. J. Pharm. Pharm. Sci.. 2012;4:386-390.
- [Google Scholar]
- Synthesis and biological activities of Bis alkyl 1,3,4-oxadiazole incorporated azo dye derivatives. Arab. J. Chem.. 2016;9:S1643-S1648.
- [CrossRef] [Google Scholar]
- Online chemical modeling environment (OCHEM): web platform for data storage, model development and publishing of chemical information. J. Cheminform.. 2011;3:P20.
- [CrossRef] [Google Scholar]
- Venkatesh, P., N. Pandeya, S., 2009. Synthesis and Anti-inflammatory Activity of Some Novel 2,4-Diaryl-3,5-bis(arylimino)-1,2,4-thiadiazolidine Derivatives. E-Journal Chem. 6, 495–503. https://doi.org/10.1155/2009/645131.
- Synthesis, spectral characterization and antimicrobial activity of some new azo dyes derived from 4,6-dihydroxypyrimidine. J. Mol. Liq.. 2012;169:21-26.
- [CrossRef] [Google Scholar]
- Color chemistry: syntheses, properties, and applications of organic dyes and pigments. John Wiley & Sons; 2003.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.09.050.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







