Translate this page into:
Design, synthesis, characterization of zirconium (IV), cadmium (II) and iron (III) complexes derived from Schiff base 2-aminomethylbenzimidazole, 2-hydroxynaphtadehyde and evaluation of their biological activity
⁎Corresponding author at: Chemistry Department, College of Sciences, Qassim University, Qassim, Saudi Arabia. Alhakemi10@yahoo.com (Ahmed N. Al-Hakimi), A.Alhakimi@qu.edu.sa (Ahmed N. Al-Hakimi),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
(E)-1-((((1H-benzo[d]imidazol-2-yl)methyl)imino)methyl)naphthalen-2-ol, ligand was synthesized by condensation of (1H-benzo[d]imidazol-2-yl) methanamine, with 2-hydroxynaphthaldehyde. The ligand and the metal complexes were characterized by various spectroscopic techniques. Interpretation of spectra confirmed that the ligand adopted either a neutral tridentate or a monobasic tridentate mode, bonded to the metal ions through the nitrogen atom of the heterocyclic benzimidazole, azomethine nitrogen atom and deprotonated phenolic oxygen atom forming either a square planer geometry. X-ray powder diffraction analysis of complexes indicated that the complexes were crystalline in nature with triclinic structures. The antibacterial and the antifungal activities of the ligand and its complexes were examined against Escherichia coli, Bacillus subtilis and Aspergillus niger by well- diffusion method. The results showed that Cd (II) complex exhibits the highest antifungal and antibacterial activities among the tested complexes. The in vitro antitumor activityies of the different complexes were tested and the results revealed an important cytotoxicity of cadmium complex against MCF-7, Hep G2 and HCT 116 cell. Iron complex showed also a strong toxicity towards Hep G2 and HCT cell whereas moderate activity against MCF-7. The bioassay study of different complexes against mosquito larvae demonstrated strong activity of zirconium complex with 0.172 values of LC50.
Keywords
Schiff base complexes
2-Aminomethylbenzimidazole
Potential anticancer colon agents
Antimicrobial
Insecticide agents
1 Introduction
Target benzimidazole compounds represent one of the heterocyclic classes owning a multitude of biological activity and clinical therapy. Benzimidazole derivatives are used for bioactivity in the treatment for various diseases including antibacterial (Eman et al., 2020), antifungal (Chandrika et al.; 2016, Leila et al., 2020), anti-tuberculous (Vatsal et al., 2020; Gill et al., 2008), antimalarial (Rudolf et al., 2020), analgesic and anti-inflammatory (Maghraby et al., 2020; Monika et al., 2014), anti-amoebic (Balasubramanian et al., 2012), and antihistamine (Wang et al., 2012). Some benzimidazole was employed as antiplasmodial agents (Rylands et al., 2019) and have an insecticidal activity (Arfa et al., 2015).
In addition, current progress on substituted benzimidazoles exhibited diverse heterocycles at 2-position to generate potent anticancer agents at multiple carcinoma cell lines. These cover pyrimidine derivatives (Yadav et al., 2016), pyrazoline derivatives (Shaharyar et al., 2010), and thiazole derivatives (Luo et al., 2011). Furthermore, 2-substituted benzimidazoles possessed chloro or carboxy group at 5-position linked with 4-amino-thioxothiazole, 4-oxothiazolidine, 4-fluorobenzylidene and cycloalkylidene reviewed as potent antitumor agents (Refaat, 2010).
Later, all surveys motivated scientists to discover a new molecular architecture possessing significant biological activity (Osipyan et al.). Indeed, the benzimidazoles have numerous uses, especially, widely applied as ligands in the preparation of metal complex. A variety of studies on benzimidazole complexes that revealed a diverse and important biological activity including antiamoebic (Neelam et al., 2000), inhibition of protein tyrosine phosphatases (Ying et al., 2011), antiproliferative agents (Ying et al., 2011), antibacterial (Fahd, 2020; Lotfi, 2020), antimalarial (Patricia et al., 2013), and anticancer (Ülku et al., 2019).
On the other hand, complexes derived from 2-aminomethylbenzimidazole were well documented which represent one of the most target compounds that have multiple biological activities in medicinal and pharmacological fields (Abeer et al., 2015). Miscellaneous reports mentioned the good efficiency of 2-aminomethylbenzimidazole complexes against cancer cell in particular cytotoxic activity on MCF-7 cell line (Fatma et al., 2003), colon carcinoma (HCT116) and larynx carcinoma (HEP2) (Ahmed, 2011), hepatocellular carcinoma (HepG2) and neuroblastoma (SHSY5Y) (Maurício et al., 2019).
Recently, Naeema et al. described the synthesis Schiff base metal complexes of Co(II), Ni(II), Cu(II) and Zn(II) derived from 2-aminomethylbenzimidazole and 2-hydroxynaphtaldehyde (Naeema et al., 2017).
This article describes the synthesis of Schiff base from 2-aminomethyl benzimidazole and 2-hydroxynaphthaldehyde as a ligand. Three different metal chlorides were prepared, including ZrCl4, CdCl2·6H2O, and FeCl3·6H2O This work aims to assess the biological activity of synthesized compounds. First, all complexes were tested against colon cancer cells. Second, the antibacterial activity was assayed against Escherichia coli and Bacillus subtilis. The antifungal activity using Fungus (Aspergillus niger) was reported here. Finally, the ligand and its complexes were also tested as insecticide agents.
2 Experimental section
2.1 Materials and methods
FT-IR spectroscopy was recorded using Thermo-Nicolet-6700 FT-IR spectrometer by the KBr disc technique in the wavenumber range of 4000–400 cm−1. Electronic absorption spectral was carried out in DMF on a UV-2102 PC Shimadzu spectrophotometer using 1 cm matched quartz cell in the wavelength range 200–900 nm. The 1H NMR and 13C NMR were recorded on Bruker NMR at 400 MHz and 100 MHz, respectively. The chemical shifts were measured with reference to TMS as internal standard in deuterated DMSO as the solvent. Simultaneous TGA and DTA analyses were performed employing a Shimadzu DTG-60 instrument using a heating rate of 10 °C / min in air atmosphere. The average samples weight was 10 mg α-Al2O3 and used as a reference material in the DTA measurements. The X-ray powder diffraction patterns of the compounds were recorded using XRD diffractometer Model PW 1710 control unit the (Philips). The anode material was Cu Kα (λ = 1.54180 Å) and operated at 40 K.V and 30 M.A. The mass spectra of the different complexes were registered in a positive mode using the DART-TOf-MS. The most intense fragment ions resulting from cleavage possible from the compounds detected were chosen and analyzed. The high-resolution mass spectrometer instrument used was AccuTOF LC-Plus from JEOL (Japan). All reagents employed in the synthesis of different complexes were commercially available without purification. 2-aminomethylbenzimidazole was purchased from Sigma-Aldrich.
2.2 Preparation of ligand (H2L) (1).
The ligand was prepared by common method (Naeema et al., 2017). Yield (75%). m.p. 126 °C. EI-MS spectra m/z = 301. IR (KBr), ν (cm−1): 3420 (s, br), 3255(s), 1631(s), 1548(m), 1320(m). 1H NMR (300 MHz, DMSO‑d6) δ (ppm): δ: 5.07(s, 2H, CH2), 6.77–8.09(d, m, 10H, Ar-H), 9.29 (s, 1H, N⚌CH), 12.66 (s, 1H, NH imidazole), 14.16 (d, 1H, ArOH). 13C NMR (125 MHz, DMSO‑d6) δ (ppm): 175.68, 161.46, 151.06, 138.01,134.61, 134.17, 129.48, 128.76, 126.3, 124.57, 123.39,119.25, 119.03, 112.02, 106.99, 50.09, UV–Vis (DMF); λmax (nm): 272, 318, 420.
2.3 Preparation of the metal complexes
Metal complexes were prepared by mixing a hot ethanolic solution of the metal salts: ZrCl4, CdCl2·6H2O and FeCl3·6H2O with a suitable amount of a hot ethanolic solution of the ligand in molar ratio 1 M:1 L (metal:ligand). The reaction mixture was refluxed 3 h under reflux. At the end of reaction, the reaction mixture was filtered. The formed precipitates were washed with ethanol and then with diethyl ether and finally dried under vacuum over anhydrous CaCl2.
2.3.1 Zirconium complex Zr(IV) (2)
Orange precipitate; Mp: 310 °C, yield (78%), UV–Vis (DMF); λmax [nm]: 282; 350. IR (KBr, υ /cm−1): =, 3260 (m), 1600, (m), 1554 (m), 1325 (m), 661(m), 551 (s), 450 (m). HRMS (DART-TOf-MS, positive mode) m/z = 434.25890, 386.22732, 330.16139, 296.1904, 267.1574, 211.09619, 189.13845.
2.3.2 Cadmium complex Cd(II) (3)
Red precipitate, yield (80%), Mp: 190 °C, UV–Vis (DMF), λmax (nm): 282. IR (KBr), ν (cm−1): = 3261 (m), 1615 (m), 1550(m), 1307 (s), 594 (s), 547(m), 454 (m). HRMS (DART-TOf-MS, positive mode) m/z = 448.19515, 395.19103, 370.17865, 336.21198, 314.13062, 293.15230, 261.10118, 283.13390, 186.09159, 161.11262, 133.07561, 108.04600.
2.3.3 Iron complex Fe(III) (4)
Dark precipitate, yield (75%), Mp: 280 °C, UV–Vis (DMF), λmax (nm): 282. IR (KBr), ν (cm−1): = 3430(s), 3290 (m), 1620(m), 1590 (m), 1300 (s), 661 (s), 580(m), 440 (s). HRMS (DART-TOf-MS, positive mode) m/z = 393.17835, 314.13699, 261.10030, 238.13376, 211.09752, 196.08782.
2.4 Cell culture
Cell lines, human breast adenocarcinoma (MCF-7), hepatocellular carcinoma (HepG-2) and colorectal adenocarcinoma (HCT 116) were received from the American Type Culture Collection (ATCC). Cells were preserved in RPMI-1640 supplemented with (100 μg/mL), penicillin (100 units/ mL) and heat-inactivated fetal bovine serum (10% v/v) in a humidified, 5% (v/v) CO2 atmosphere at 37 °C (Ali Mokhtar et al., 2012; Sabrin et al., 2016).
2.5 Cytotoxicity assay
Cells were treated with six various concentrations of each complexes (0.01, 0.1, 1, 10, 100 and 1000 µg); untreated cells (control) were added. The cells were incubated with each concentration for 72 h and finally fixed with TCA (10% w/v) for 1 h at 4 °C. Cells were washed several times and stained by 0.4% (w/v) SRB solution for 10 min in a dark place. The excess of stain was extracted and eliminated with 1% (v/v) glacial acetic acid. The SRB-stained cells were dried overnight, then dissolved with Tris-HCl and the color intensity was analyzed in a microplate reader at 540 nm. The relation between viability percentage of each tumor cell line and compound concentrations was analyzed to get the IC50 (dose of the drug which reduces survival to (50%) using Sigma Plot 12.0 software (Jeong-Chae et al., 2002).
2.6 In-vitro antimicrobial and antifungal activity
All tests of antimicrobial and antifungal activities for the compounds (ligand and complexes) were achieved in the Botany Department, Laboratory of microbiology. The antimicrobial activities of the compound were studied by Well Diffusion Method (Abdulrahman et al., 2018). The antibacterial activities were examined using E. coli and B. subtilis, while the antifungal activity was achieved using Fungus (A. niger) in DMSO, and poured disc was used as a negative control. The resulting wells are sites for tested compounds of known concentration. For comparison, a standard antibacterial drug (tetracycline), an antifungal drug (nystatin) as a positive control. A solution of metal salts was also examined under similar conditions. Areas of growth inhibition around the holes were observed, which indicates that the tested compound inhibits the growth of microorganisms. The zone of inhibition was carefully measured in millimeters. All determinations were made in triplicate for each compound. An average of the two independent readings for each compound was recorded.
2.7 Rearing of mosquitoes
The mosquitoes in the south of the house (Aedes aegypti) were transferred to the Dengue Mosquito Station, Jeddah, for rearing. During the rearing, the mosquito larvae produced were introduced into plastic enamel trays containing tap water. The rearing conditions were fixed at (27 ± 2 °C) and 75–78% RH with photoperiod (14:10 light and dark) respectively. A special diet for mosquitoes was prepared from brewer's yeast, dog biscuits and algae taken from pond water in a ratio of 3: 1: 1, respectively Gerald (Gerald et al., 1996).
2.8 Larvicidal activity of four chemical compounds
Amid initial screening with the lab experiment, A. aegypti mosquito's larvae were collected, identified, from the rearing cage at dengue station, King Abdulaziz University, Jeddah. From the stock solution with the use of dechlorinated tap water 1000 mg/l extract was prepared. This material was received from the World health organization with slight modifications (Abdul et al., 2000).
3 Results and discussion
3.1 Chemistry
Our approach started with the synthesis of the ligand by reacting one equivalent ethanolic solution of 2-aminomethylbenzimidazole (AMBI) with one equivalent ethanolic solution of 2-hydroxynaphthaldehyde according to Fig. 1. The synthesis of the complexes were achieved by reacting one equivalent ethanolic solution of Ligand (H2L) with one equivalent of metal chloride salt (ZrCl4, FeCl3,·6H2O and CdCl2·6H2O) inside the same solvent ethanol (Fig. 2). The reaction initiated by reacting 8.4 m.mol of metal halides solution with 8.4 m.mol solution of Ligand (H2L) at room temperature. The mixture was heated gradually and left under reflux for 3 h (Table 1). All obtained complexes were characterized by spectroscopic data of FT-IR, high resolution mass spectrometry (HRMS), UV–Visible electronic absorption, thermal analysis and X-ray powder diffraction studies.![Synthesis of ligand (H2L) (E)-1-((((1H-benzo[d]imidazol-2-yl)methyl)imino)methyl)naphthalen-2-ol.](/content/184/2020/13/10/img/10.1016_j.arabjc.2020.08.014-fig1.png)
Synthesis of ligand (H2L) (E)-1-((((1H-benzo[d]imidazol-2-yl)methyl)imino)methyl)naphthalen-2-ol.
Structures of complexes Co (II), Cd(II) and Fe(III).
Compound
Colour
Yield (%)
Mp(°C)
λmax [nm]
ν(Cm−1)
Assignment
1
C19H15ON3
Yellow
80
272
36,764
π → π*
318
31,446
n → π*
420
23,809
(υ2) 3A2g(F)→3T1g(F)
2
C19H14ON3ZrCl3
Orange
85
340
282
35,460
π → π*
350
29,411
LMCT
3
C19H14ON3CdCl
Red brick
80
280
282
35,460
π → π*
390
25,641
n → π*
4
C19H15ON3FeCl3
Dark Broun
75
190
530
18,867
6A1g→4T2g
480
20,833
6A1g→4T1(G)
410
24,390
6A1g→4 Eg(G)
282
35,460
π → π*
3.1.1 Mass spectra
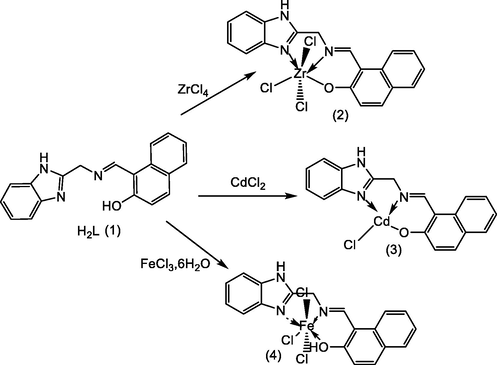
The mass spectrum of the zirconium complex illustrated in Fig. 3, manifest the absence of molecular peak. This structure was proved essentially by ion peak situated at m/z = 434,2589 (calcd.: 433,91658) corresponding to methyl-5-vinylimidazolmethylene azanylidene methylphenol linked to zirconium metal and three chlorine atoms. The fragment at m/z = 386.22732 (calcd. 385,91658) was attributed to hydroxybenzylideneamino-N-methyl acetimidamide connected to zirconium metal and three chlorine atoms. Furthermore, the spectra revealed the presence of basic peak at m/z = 211,09619 (calcd. 210,95692) assigned to 2-aminomethylphenol linked to zirconium metal. These data confirmed that the zirconium is linked with ligand via oxygen atom and coordinated with the azomethine group of both benzimidazole group and Schiff base motif (Fig. 3).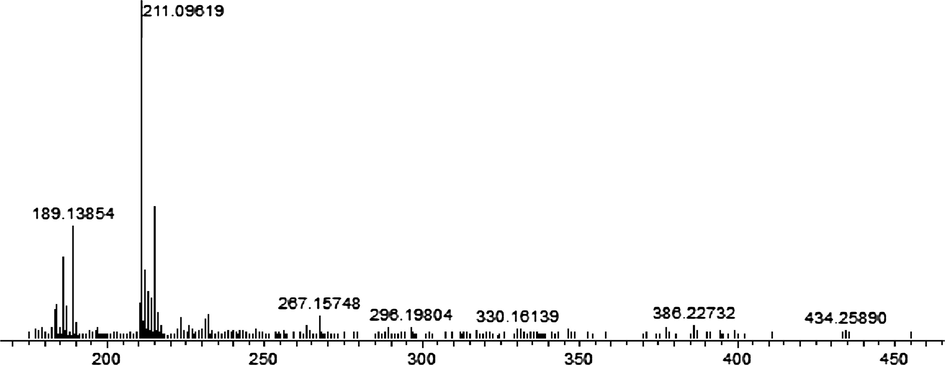
Mass Spectra of Zr (IV) complex.
Regarding the iron complex, the mass spectrum illustrated in Fig. 4, manifest the absence of molecular ion peak. The structure was also confirmed by the fragment at m/z = 393,17835 (calcd. 393,03258) corresponding to the loss of two chlorine atoms from the molecular mass ion. The fragment at m/z = 314,13699 (calcd. 314,09503) was attributed to benzimidazol methylaminomethylcyclohexanol connected to iron metal. The basic fragment at m/z = 261,10030 (calcd. 261,05591) was associated with 4,5-dihydroimidazol methylaminomethylphenol joined with iron metal. The structure was also confirmed with the fragment at m/z = 238,13376 (calcd. 237,98289) related to aminomethylbenzimidazole linked with iron metal and a chlorine atom. These results proved the formation of the complex and possess a hexagonal structure described above proposes that the coordination of the iron was provided by the hydroxyl OH group and also with both azomethine groups (Fig. 2).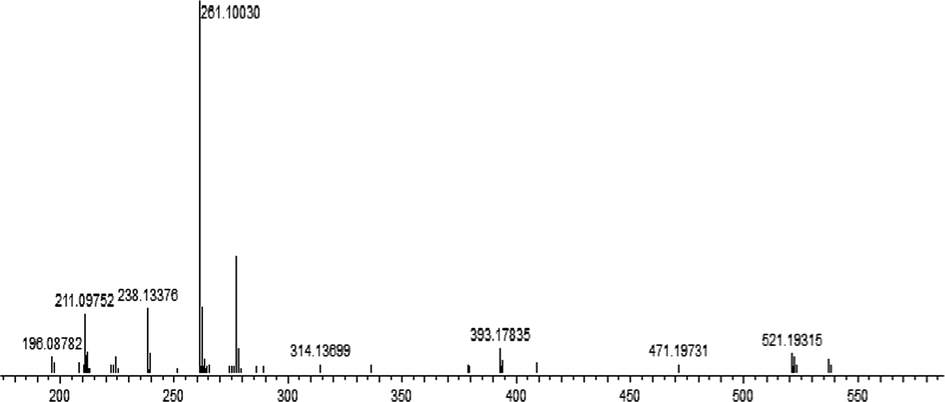
Mass Spectra of Fe (III) complex.
The structure of the Cd (II) complex was established by mass spectrometry data and suggests tetrahedral coordination with ligand. The mass spectrum of the cadmium complex, reported in Fig. 5, affirmed the presence of a molecular ionic peak (M−H)+ at m/z = 448,19515 (calcd. 447,97752). The structure was proved by the basic fragment at m/z = 261,10118 (calcd. 260,98246) corresponding to 2-aminomethylbenzimidazole linked with cadmium. The fragment at m/z = 283,31390 (calcd. 282,95557) was associated to (2-hydroxynaphthalen-1-yl) methaniminium attached with cadmium metal. The peak fragment at m/z = 238,13390 (calcd. 238,00286) was attributed to hexahydro-1H-benzoimidazolium connected to cadmium metal. The fragment at m/z = 108,04600 (calcd. 108,06820) was assigned to o-phenyldiamine motif.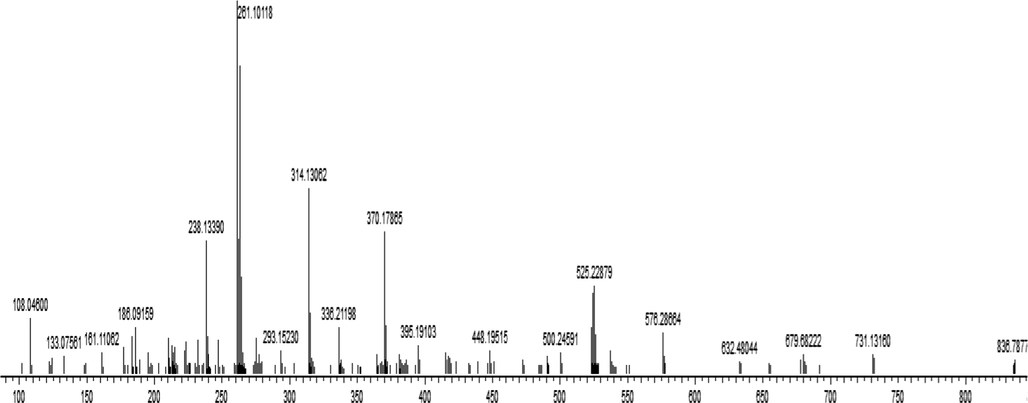
Mass Spectra of Cd (II) complex.
These data, in the case of the cadmium complex, confirmed that the metal is linked by the hydroxyl group of ligand and the coordination of cadmium metal was established by the azomethine groups.
3.1.2 Electronic spectra
The Ultraviolet–visible (UV–Vis) absorption of ligand and its metal complexes were achieved in DMF at room temperature and the results were written in Table 1. Free ligand (H2L) revealed, respectively, three transitions at 272 nm attributed to π → π* band, the second transition at 318 nm relatively to n → π* band and the third transition at 420 nm associated to (υ2) 3A2 g(F)→3T1g(F). The energy of the ligand indicates that the difference between HOMO and LUMO is called the HOMO-LUMO gap. In our case, this difference in energy between these two boundary orbitals referred to predict the strength and stability of transition metal complexes and the created bonds in compounds. The numerous of double and single bonds as well as nonbinding electrons represent an electron density in the ligand. This electron density makes the ligand effective for the correlation, interaction or very high vital. zirconium (IV) complexes (2) do not show d-d transitions. The bands observed in the 282, 350 nm may be due to intra-ligand transition and (LMCT) (Har and Jangbhadur, 2013).
Moreover, the cadmium (II) complex revealed the absence of d-d transition and except two transitions at 390 and 282 nm, it is assumed to π → π* and n → π* bands that confirmed the formation of the complex via the coordination of ligands to metal.
The iron (III) complex (3) provided four bands at 520, 460, 400 and 282 nm which assigned to 6A1g→4T2g, 6A1g→4T1(G), 6A1g→4 Eg(G) and 6A1g→4A1g(G), transitions. These bands characterize the octahedral iron (III) complex (El-Tabl et al., 2008a, 2008b).
3.1.3 IR spectra
Ligand has three possible sites for binding to the metallic element, namely the nitrogen of the azomethine group, oxygen of the hydroxyl group, and the nitrogen atom of the benzimidazole moiety. Therefore, the ligand can act as neutral or mono basic tridentate ion or non-ionic tridentate. In complexes (2) and (3) the ligand acts as a mono basic tridentate chelator forming two successive six and five rings. This behavior was confirmed by the negative shift in the position of the both azomethine and benzimidazole and the disappearance of the absorption peak of the hydroxyl group. Whereas in complex (4), the ligand behavior a neutral tridentate with metal forming six and five rings. This behavior was confirmed by the negative shift in the position of the both the azomethine, benzimidazole and the hydroxyl group.
The IR spectrum of the ligand H2L was employed as reference in particular to compare the spectra of metal complexes. As presented in Table 2, the IR spectrum of the ligand exhibited broad and strong features at 3420 cm−1 attributed to the stretching vibration of the phenolic hydroxyl group. Inversely, the spectra of complexes (2) and (3) display the absence of the OH stretching vibration which corresponding to the deprotonation of the hydroxyl group in the formation processes of the complexes (2) and (3) whereas the medium band at 3255 cm−1 may be assigned to υ(NH) group. The relatively strong and medium bands which located at 1631 and 1570 cm−1 corresponded to the azomethine group (Ahmed, 2020; El-Tabl et al., 2008a, 2008b). In complexes (2) and (3) showed the ligand H2L acts as a mono-negative tridentate O,N,N ligand coordinating via azomethine nitrogen (C⚌N) and deprotonated phenolic hydroxyl group. This mode of bonding is supported by the disappearance of the band characteristic to hydroxyl group ν(OH) with simultaneous positive shift of ν(C-O phenolic) band; the negative shift of azomethine group ν(C⚌N) band. This mode of chelation is supported by the appearance of new bands in the (661–584) cm−1, (551–547) cm−1 and (450–444) cm−1 assigned to ν(M ← O), ν(M ← N) and (M ← Cl) respectively (Table 2).
No.
Ligand/complexes
υ(OH)
υ(NH)
υ(C⚌N)
υ(C-O)ph
υ(M−O)
υ(M−N)
υ(M−Cl)
1
C19H15N3O (H2L)
3420
3255
1631, 1570
1320
–
–
2
C19H14ON3ZrCl3
---
3260
1600,1550
1325
661
551
450
3
C19H14ON3CdCl
----
3361
1630,1555
1327
584
547
444
4
C19H15ON3FeCl3
3425 (br)
3290
1626,1560
1320
661
580
440
On the other hand, complex (4) manifest an absorption band at 3425 cm−1 attributed to the stretching vibration of the phenolic hydroxyl group coordinated with iron (III) ion. The absorption bands of the coordinated nitrogen in both benzimidazole and azomethine group is located at 3290 cm−1 and 1626 cm−1, respectively. This mode of chelation also is supported by the appearance of new bands in the 661 cm−1, 580 cm−1 and 440 cm−1 assigned to ν(M ← O), ν(M ← N) and (M ← Cl), respectively.
3.1.4 Thermal analyses (DTA and TGA)
The DTA and TGA curves in the temperature range 27–600 °C for the complexes (2)-(4) emphasized that these complexes were thermally stable up to 100 °C. The change happened when the temperature rose to 150 °C, here the chlorine was released in the form of hydrochloride.
Complex (2) showed the decomposition peak at (250–300) °C with a mass loss 21.50% (Calcd. 21.38%), corresponding to the loss of three hydrochloride. However, the spectra of complex (2) revealed a peak at 550 °C with mass loss 32.00% (Calcd. 31.46%), referring to the formation of ZrO2. Complex (3) exhibited an endothermic peak at 230 °C with a mass loss of 8.20% (Calcd. 8.15%), denoting the loss of one hydrochloride. In addition to the above, the observed peak at 550 °C with a mass loss 31.01% (Calcd. 31.21) referred to the formation of CdO.
The decomposition of iron complex (4) occurred at 270 °C with a mass loss 23.20% (Calcd. 23.07%) and this attributed to the elimination of three chloride ions. Also, the observed peak at 580 °C with a mass loss 49.21% (Calcd. 49.08) pointed to the formation of Fe2O3.
3.1.5 XRD analysis
The X-ray powder diffraction patterns of the three complexes are shown in Figs. 6-8. The output data from XRD measurements were treated and refined using some software. There are many computer codes that have been used to analyze the X-ray line profile. The residual-peak fitting (PeakFit) software was used. This program helps to detect, separate, quantify hidden peaks that standard instrumentation misses and background correction. PeakFit also includes different nonlinear spectral application line shapes (Singh et al., 2011).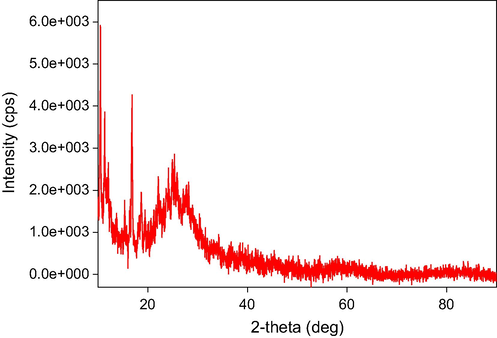
XRD Spectra of Zr (IV) complex.
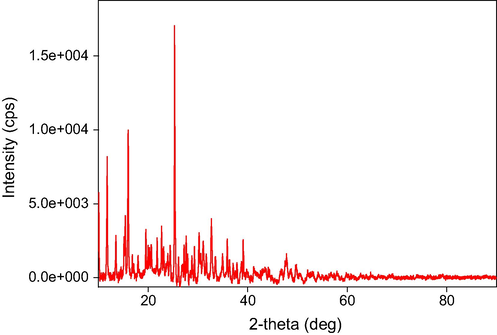
XRD Spectra of Cd (II) complex.
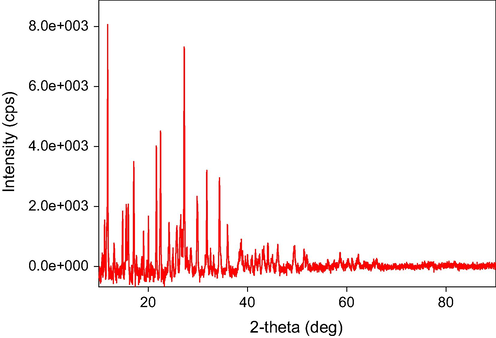
XRD Spectra of Fe (III) complex.
A standard cell parameter was used for the three complexes as starting values for “CHEKCELL” program to identify the Bragg reflections (Karelle et al., 2018). CHEKCELL program is a powder Indexing software. The crystal lattice parameters were computed with the aid of the computer program CHEKCELL (Laugier et al., 2004). The crystal data of these complexes were observed and fitted well with the triclinic crystal system. The unit cell parameters of the complexes are given in Table 3. The different values of the calculated unit cell volumes are due to the sizes of impurities entered the base material. So, the large atom/molecule size will increase the cell unit volume and this leads to deformation in bonds as well as changing the properties of the product material.
Lattice and crystal parameters Zr(IV) complex
a(Å)
b(Å)
c(Å)
α(o)
β(o)
γ(o)
Cell Volume(Å)
Crystal size nm
9.945
18.311
43.955
93.27
98.83
90.06
8255.73
22.99
Crystal type
Triclinic
Space group
P1
Lattice and crystal parameters Cd(II) complex
a(Å)
b(Å)
c(Å)
α(o)
β(o)
γ(o)
Cell Volume(Å)
Crystal size nm
7.869
14.695
13.756
89.93
102.36
90.54
1553.771
42.5
Crystal type
Triclinic
Space group
P1
Lattice and crystal parameters Fe(II) complex
a(Å)
b(Å)
c(Å)
α(o)
β(o)
γ(o)
Cell Volume(Å)
Crystal size nm
7.909
14.545
13.7568
90.44
102.01
90.22
1548.042
35.4
Crystal type
Triclinic
Space group
P1
Furthermore, using the diffraction data, the mean crystallite sizes of the complexes, D, were determined according to the Scherer equation: (D = 0.9λ/(βcosθ), where λ is X-ray wavelength (1.54180 Å), θ is Bragg diffraction angle, and β is the full width at half maximum of the strong diffraction peak of the samples (Miranda and Sasaki, 2018). The Fig. 7 show the crystalline nature of the three complexes and the peak sharpness in the XRPD patterns indicates the existence of the nano-sized complex. The average crystallite sizes of the three complexes are given in Table 3. The values of crystallite sizes indicate that Cd-complex is more crystalline than the others.
3.2 Biology
3.2.1 In vitro antitumor activities
The in vitro cytotoxic activities of the tested compounds were determined using SRB assay towards MCF-7, HepG2 and HCT 116 tumor cell lines over concentration range of 0.01 to 1000 μg. Tested compounds showed variable cytotoxic activity against tumor human cells. It was observed that the most significant toxicity was with cadmium complex against MCF-7, HepG2 and HCT 116 cells with IC50s 5.1, 1.1 and 1.3, respectively. While the iron complex exhibited strong toxicity towards liver cancer (HepG2) among all compounds with IC50 0.4 μg. The iron complex was having potent toxicity against HCT 116 with IC50 1.4 μg and moderate effect towards breast cancer MCF-7 with IC50 19.5 μg (Fig. 9).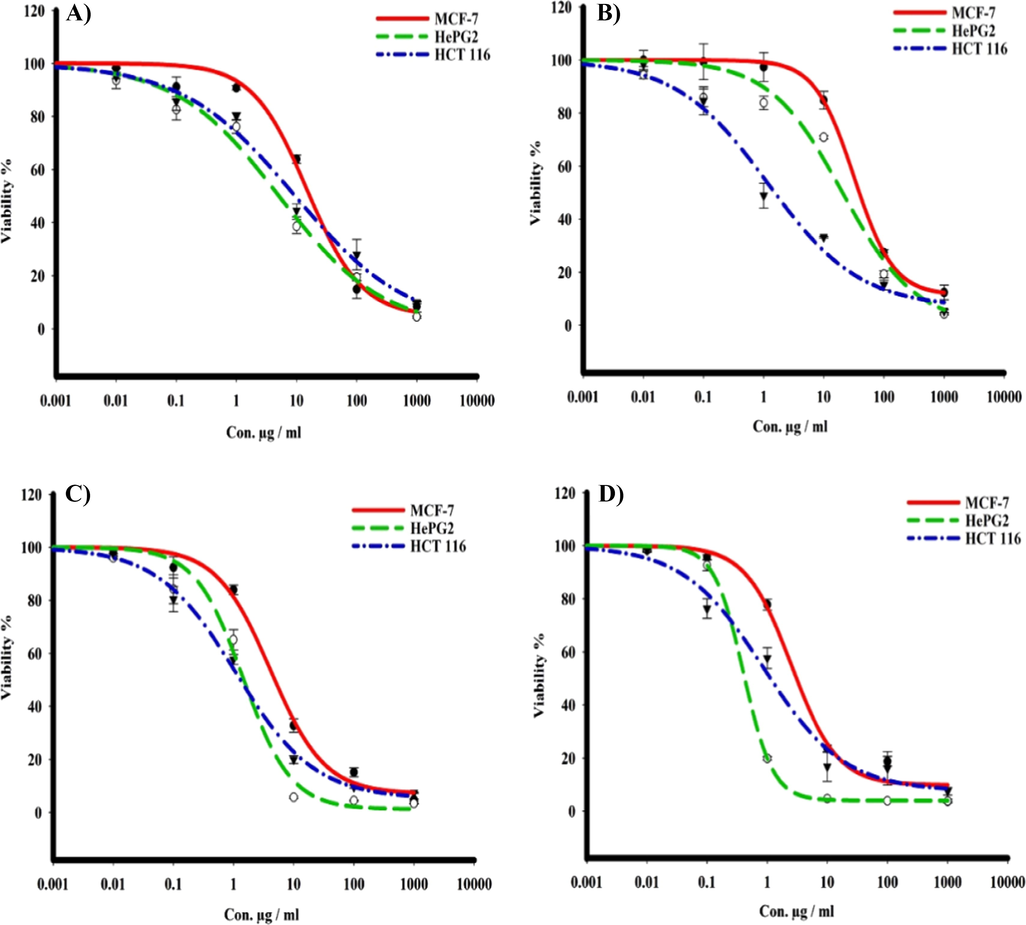
The dose response curves of the cytotoxicity of different compounds towards MCF-7, HepG2, and HCT 116 tumor cell lines. Cells were exposed to ligand and its metal complexes with different concentrations for 72 h. Cell viability was determined by SRB stain.
In addition, the ligand C19H15ON3 (H2L) revealed a slight toxicity effect towards MCF-7 tumor cells with IC50 17.6 and moderate on HepG2 and HCT 116 cells with IC50s 5.7 and 10.2 μg, respectively. On the other hand, compound Zr(IV) Complex was weaker to kill cells of MCF-7, moderate with HepG2, and having a significant effect on HCT 116 with IC50s 45.6, 21.6 and 1.6 μg, respectively (Table 4).
Compound
IC50 (μg)
MCF-7
HepG2
HCT 116
C19H15ON3 (H2L)
17.6 ± 1.5
5.7 ± 0.5
10.2 ± 1.3
C19H14ON3ZrCl3
45.6 ± 0.9
21.6 ± 1.1
1.6 ± 0.2
C19H14ON3CdCl
19.5 ± 1
0.4 ± 0.1
1.4 ± 0.1
C19H15ON3FeCl3
5.1 ± 0.4
1.1 ± 1.4
1.3 ± 0.2
3.2.2 Antimicrobial activity
The biological activity of the ligand and its metal complexes were examined in vitro against two types of bacteria (E. coli), (B. subtilis) and one type of fungus (A. niger). The antimicrobial activities were expressed as a percentage of inhibition versus the standard drug, (Table 5). The results indicate a high efficacy of samples on bacteria and fungi. The ligand demonstrated high activity against all types of organisms. On the contrary, all-metal complexes showed promising activity against E. coli, and B. subtilis. On the other hand, the metal complexes demonstrated moderate activity against A. niger. Metal complexes were lower activity than the ligand due to increasing the polarity in metal ion (Fig. 10).
Compounds
Inhibition zone in mm
A. niger
E. coli
B. subtilis
DMSO
0
0
0
Nystatin
32
–
–
Tetracyclene
–
35
38
1
25
30
32
2
22
19
26
3
29
30
32
4
24
19
15
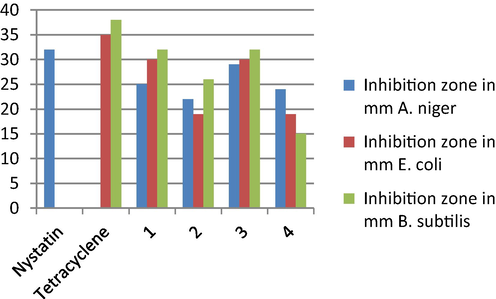
Evaluation of biological activities of the ligand and its metal complexes against bacteria and fungus.
The fungicidal activity order of the complexes against A. niger was as follows: nystatin > Cd (II) complex > ligand (H2L) > Fe (III) complex > Zr(IV) Complex.
From another standpoint, the order of antibacterial activity for the different complexes against B. subtilis was as follows: Tetracycline > Cd (II) complex = ligand (H2L) > Zr(IV) Complex > Fe (III) complex, and against E. coli was: Tetracycline > Cd (II) complex = ligand (H2L) > Zr(IV) Complex = Fe (III) complex.
3.2.3 Bioassay against mosquito larvae
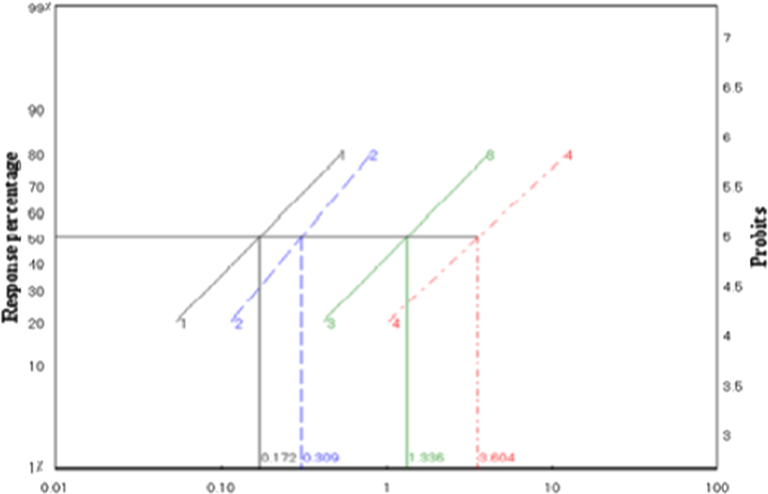
The results of the ligand (H2L) and their metal complexes manifested high larvicidal activity with low concentrations as stated in Tables 6 and 7. The relation between different concentrations and larvae death of these four compounds is illustrated in Fig. 11. The ligand (H2L) and cobalt complex revealed a high mortality rate of A. aegypti larvae at a concentration of 1 ppm. The cadmium and iron complex showed the highest activity at a concentration of 10 ppm and the mortality of larvae was 97.9 and 80.2, respectively. a: Five replicates, 20 larvae each. b: Tabulated χ2 = 7.8, When tabulated χ2 larger than calculated at 0.05 level of significance indicates the homogeneity of results.
Insecticide Used
Conc. (ppm)
Mortality (%)a
LC50 (ppm)
(LCL-UCL)
LC90 (ppm)
(LCL-UCL)
χ2 b
slope
C19H15ON3(H2L)
0.1
39.6
0.1717
0.9873
9.42
1.687
0.3
59.4
0.5
74.0
(0.056–0.237)
(0.77–3.939)
0.8
85.4
1
96.6
C19H14ON3ZrCl3
0.1
17.7
0.3093
1.372
2.2853
1.981
0.3
49
0.5
62.5
(0.262–0.357)
1.092–1.873)
0.8
77.1
1
88.5
C19H14ON3CdCl
1
46.9
1.3356
7.6675
8.93
1.688
3
63.5
5
82.3
(0.369–1.787)
(5.665–26.548)
8
89.6
10
97.9
C19H15ON3FeCl3
1
21.9
3.6043
23.8079
3.2515
1.563
3
39.6
5
59.4
(2.986–4.278)
(16.816–40.174)
8
67.7
10
80.2
No
Line name
LC50
Lower limit
Upper limit
RR
1
C19H15ON3 (H2L)
0.172
0.056
0.237
1
2
C19H14ON3ZrCl3
0.309
0.263
0.357
1.797
3
C19H14ON3CdCl
1.336
0.369
1.787
7.767
4
C19H15ON3FeCl3
3.604
2.986
4.277
20.953
Regression lines for insecticides used against the Aedes aegypti larvae using dipping technique.
The LC50 value of the ligand (H2L), Zr(IV), cadmium (II), and iron (III) complexes were respectively 0.172, 0.309, 1.336, and 3.604 ppm, as illustrated in Fig. 11. These results proposed that ligand (H2L) and cobalt complex possess the ability to kill larvae of A. aegypti with low concentration. While at the same concentration for cadmium and iron complex, larvae were survived.
A comparative study was carried out and evaluated using five compounds against A. aegypti. The Given values of LC50 (concentration: which kills 50 ٪ of mosquito larvae) showed that the mosquito larvae of A. aegypti were more sensitive to Pesguard FG 161 (0.046 ppm) than to Actikil 50 ٪ (0.061 ppm) and Bacilod (0.142 ppm), respectively. The results exhibited that Pesguard FG 161 was more effective against A. aegypti larvae than Actikil 50 ٪ and Bacilod about 1.3 and 3.1 times, respectively (Deepak et al., 2014).
The comparative study of the larvicidal activity for the insecticides was tested against the larvae of A. Aegypti, and it demonstrated that the bacterial insecticide Bactilarvae was the most effective compound followed by OP Safroten, Temephos and Solfac (Samuel et al., 2012). Plant extracts showing larvicidal activity against Culex quinquefasciatus (Javaid et al., 2018).
Conclusion: In this paper, the synthesis of Schiff base elaborated from 2-aminomethylbenzimidazole and 2-hydroxynaphthylldehyde was reported. The spectral data confirmed that the ligand adopted either a neutral tridentate or a monobasic tridentate mode, bonded to the metal ions through the nitrogen atom of the heterocyclic benzimidazole, azomethine nitrogen atom and deprotonated phenolic oxygen atom forming either a square planer geometry. X-ray powder diffraction analysis of complexes indicated that the complexes are crystalline in structure with the triclinic system as well as having nan-sized dimensions. The antibacterial as well as the antifungal activities of the ligand and its complexes were evaluated against (E. coli), (B. subtilis) and A. niger by well-diffusion method. The results showed that cadmium (II) complex exhibited the highest antifungal activity and antibacterial activity among the tested complexes. The In vitro antitumor activities of different complexes were tested and the results revealed an important cytotoxicity of cadmium complex against MCF-7, Hep G2 and HCT 116 cell. Iron complex demonstrated also a strong toxicity towards Hep G2 and HCT cell but moderate activity against MCF-7. The bioassay study of the different complexes against mosquito larvae exhibited that Zr(IV) Complex showed significant activity with 0.172 values of LC50.
Acknowledgment
The authors extends their appreciation Department of chemistry, College of Science, Qassim University and to the Deanship of Scientific Research at King Khalid University for funding this work through General Research Project under grant number (G.R.P-206-39).
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- Mixed Ligand Complex Formation of Cetirizine Drug with Bivalent Transition Metal (II) Ions in the Presence of 2-Aminomethylbenzimidazole: Synthesis, Structural, Biological, pH-Metric and Thermodynamic Studies. J. Solution Chem.. 2015;44:1673-1704.
- [CrossRef] [Google Scholar]
- Anti-helicobacter, antitubercular and cytotoxic activities of scalaranes from the red sea sponge hyrtios erectus. Molecules. 2018;23:978-989.
- [CrossRef] [Google Scholar]
- Effect of Feronia limonia on mosquito larvae. Fitoterapia. 2000;71:553-555.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of some potential antitumor palladium (II) complexes of 2-aminomethylbenzimidazole and amino acids. J. Coord. Chem.. 2011;64:2035-2055.
- [CrossRef] [Google Scholar]
- Synthesis, Characterization and Microbicides Activities of N-(hydroxy-4-((4-nitrophenyl)diazenyl)benzylidene)-2-(phenylamino)acetohydrazide metal complexes. Egyp. J. Chem.. 2020;63
- [CrossRef] [Google Scholar]
- Anti-cancer characteristics of mevinolin against three different solid tumor cell lines was not solely p53-dependent. J. Enzyme Inhibition Med. Chem.. 2012;27:673-679.
- [CrossRef] [Google Scholar]
- Antimalarial and insecticidal activities of newly synthesized derivatives of Benzimidazole. Pak. J. Pharm. Sci.. 2015;28:2179-2184.
- [Google Scholar]
- Benzimidazole: a medicinally important heterocyclic moiety. Med. Chem. Res.. 2012;21:269-283.
- [CrossRef] [Google Scholar]
- Synthesis and investigation of novel benzimidazole derivatives as antifungal agents. Bioorg. Med. Chem.. 2016;24:3680-3686.
- [CrossRef] [Google Scholar]
- Larvicidal Activity of Cassia occidentalis (Linn.) against the Larvae of Bancroftian Filariasis Vector Mosquito Culex quinquefasciatus. J. Parasit. Res.. 2014;2014
- [CrossRef] [Google Scholar]
- Spectroscopic characterization and biological activity of metal complexes with an ONO trifunctionalized hydrazone ligand. J. Coord. Chem.. 2008;61:2380-2401.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic characterization and biological activity of the metal complexes of the Schiff base derived from phenylaminoacetohydrazide and dibenzoylmethane. Spectrochim. Acta Part A: Mol. Biomol. Spec.. 2008;71:90-99.
- [CrossRef] [Google Scholar]
- Development of benzimidazole-based derivatives as antimicrobial agents and their synergistic effect with colistin against gram-negative bacteria. Eur. J. Med. Chem.. 2020;186:111850-111854.
- [CrossRef] [Google Scholar]
- Synthesis, Design and Biological Evaluation of Antibacterial Activity of Novel Mixed Metal Complexes Derived from Benzoimidazolphenylethanamine and 6-Amino-N, N-dimethyluracil. Lett. Org. Chem.. 2020;17
- [CrossRef] [Google Scholar]
- Synthesis, cytotoxic activity on MCF-7 cell line and mutagenic activity of platinum (II) complexes with 2-substituted benzimidazole ligands. Eur. J. Med. Chem.. 2003;38:473-480.
- [CrossRef] [Google Scholar]
- Practical Medical Microbiology (14th ed.,). New York: Churchill Livingstone:; 1996. p. :978.
- Clubbed [1, 2, 3] triazoles by fluorine benzimidazole: a novel approach to H37Rv inhibitors as a potential treatment for tuberculosis. Bioorg. Med. Chem. Lett.. 2008;18:6244-6247.
- [CrossRef] [Google Scholar]
- Synthesis of New Zirconium(IV) Complexes with Amino Acid Schiff Bases: Spectral, Molecular Modeling, and Fluorescence Studies. J. Inorg. Chem. Inter. 2013
- [CrossRef] [Google Scholar]
- Evaluation of larvicidal efficacy of indigenous plant extracts against Culex quinquefasciatus (Say) under laboratory conditions. Turk. J. Agricul. Forestry. 2018;42:207-215.
- [CrossRef] [Google Scholar]
- Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J. Agr. Food Chem.. 2002;50:6490-6496.
- [CrossRef] [Google Scholar]
- Nuclear magnetic resonance spectroscopy investigations of naphthalene-based 1, 2, 3-triazole systems for anion sensing. Magnetochemistry. 2018;4:15-33.
- [CrossRef] [Google Scholar]
- Laugier, J., Bochu B., Chekcell: Graphical powder indexing cell and space group assignment software, 2004. http://www.ccp14.ac.uk/tutorial/lmgp/achekcelld.htm.
- Nano-SnCl4. SiO2, an efficient catalyst for synthesis of benzimidazole drivatives as antifungal and cytotoxic agents. Res. Pharma. Sci.. 2020;14:504-514.
- [CrossRef] [Google Scholar]
- Novel Mixed Complexes Derived from Benzoimidazolphenylethanamine and 4-(Benzoimidazol-2-yl)aniline: Synthesis, characterization, antibacterial evaluation and theoretical prediction of toxicity, Asian. J. Chem.. 2020;32:1266-1272.
- [CrossRef] [Google Scholar]
- Synthesis and in vitro cytotoxic evaluation of some thiazolylbenzimidazole derivatives. Eur. J. Med. Chem.. 2011;46:417-422.
- [CrossRef] [Google Scholar]
- Novel class of benzimidazole-thiazole hybrids: The privileged scaffolds of potent anti-inflammatory activity with dual inhibition of cyclooxygenase and 15-lipoxygenase enzymes. Bioorg. Med. Chem.. 2020;28:115403-115413.
- [CrossRef] [Google Scholar]
- Oxidative assets toward biomolecules and cytotoxicity of new oxindolimine-copper (II) and zinc (II) complexes. Inorganics. 2019;7:12-29.
- [CrossRef] [Google Scholar]
- The limit of application of the Scherrer equation. Acta Crystall Sect A: Found. Adv.. 2018;74:54-65.
- [CrossRef] [Google Scholar]
- Benzimidazole: An emerging scaffold for analgesic and anti-inflammatory agents. Eur. J. Med. Chem.. 2014;76:494-505.
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic and DNA binding ability of CoII, NiII, CuII and ZnII complexes of Schiff base ligand (E)-1-(((1H-benzo [d] imidazol-2-yl) methylimino) methyl) naphthalen-2-ol. X-ray crystal structure determination of cobalt (II) complex. Mat. Sci. Eng.: C. 2017;75:1059-1067.
- [CrossRef] [Google Scholar]
- Synthesis and antiamoebic activity of new cyclooctadiene ruthenium (II) complexes with 2-acetylpyridine and benzimidazole derivatives. Bioorg. Med. Chem. Lett.. 2000;10:2243-2245.
- [CrossRef] [Google Scholar]
- Organometallic benzimidazoles: Synthesis, characterization and antimalarial activity. Inorg. Chem. Commun.. 2013;35:126-129.
- [CrossRef] [Google Scholar]
- Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives. Eur. J. Med. Chem.. 2010;45:2949-2956.
- [CrossRef] [Google Scholar]
- Lerisetron Analogues with Antimalarial Properties: Synthesis, Structure-Activity Relationship Studies, and Biological Assessment. ACS Omega. 2020;5:6967-6982.
- [CrossRef] [Google Scholar]
- Structure-activity relationship studies of antiplasmodial cyclometallated ruthenium (II), rhodium (III) and iridium (III) complexes of 2-phenylbenzimidazoles. Eur. J. Med. Chem.. 2019;161:11-21.
- [CrossRef] [Google Scholar]
- Integracides HJ: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Fitoterapia. 2016;112:161-167.
- [CrossRef] [Google Scholar]
- Screening of twenty five plant extracts for larvicidal activity against Culex quinquefasciatus Say (Diptera: Culicidae) Asian Pacific J. Tropical. Biomed.. 2012;2:1130-1134.
- [CrossRef] [Google Scholar]
- Pyrazoline bearing benzimidazoles: search for anticancer agent. Eur. J. Med. Chem.. 2010;45:114-119.
- [CrossRef] [Google Scholar]
- Singh T. B., Rey L., Gartia R. Applications of PeakFit software in thermoluminescence studies, 2011. http://nopr.niscair.res.in/handle/123456789/11596.
- Synthesis and evaluation of anticancer properties of novel benzimidazole ligand and their cobalt (II) and zinc (II) complexes against cancer cell lines A-2780 and DU-145. Inorg. Chimica Acta. 2019;495:118977-118983.
- [CrossRef] [Google Scholar]
- N-Mannich bases of benzimidazole as a potent antitubercular and antiprotozoal agents: Their synthesis and computational studies. Synt. Commun.. 2020;50:858-878.
- [CrossRef] [Google Scholar]
- Synthesis, biological evaluation and SAR studies of benzimidazole derivatives as H1-antihistamine agents. Chin. Chem. Lett.. 2012;23:707-710.
- [CrossRef] [Google Scholar]
- Perspectives of Benzimidazole Derivatives as Anticancer Agents in the New Era. Anti-cancer Agents Med. Chem.. 2016;16:1403-1425.
- [Google Scholar]
- Potent inhibition of protein tyrosine phosphatases by copper complexes with multi-benzimidazole derivatives. Biometals. 2011;24:993-1004.
- [CrossRef] [Google Scholar]







