Translate this page into:
Designing and immobilizing gold nanoparticles over a lignosulfonate/starch composite for Suzuki-Miyaura coupling reactions, and investigation of its anti-pancreatic and anti-hepatitis cancer effects
* Corresponding authors: E-mail addresses: guiminhou@outlook.com (G. Hou), llxxff17@163.com (X. Feng)
-
Received: ,
Accepted: ,
Abstract
The current research explored a green approach for the fabrication and in situ immobilization of gold nanoparticles over a polymeric composite of starch combined with sodium lignosulfonate (ST-LS/Au NPs). It also assessed their catalytic activity and anti-cancer effects. The mixed polymeric ST-LS composite functioned as a reducing-stabilizing agent to cap the gold nanoparticles. Field-Emission Scanning Electron Microscopes (FE-SEM), Fourier Transform Infrared Spectroscopy (FT-IR), Energy Dispersive X-ray Electron Spectroscopy (EDX)-elemental mapping, Transmission Electron Microscopy (TEM), and X-ray diffraction (XRD) were used to examine the synthesized ST-LS/Au NPs. The powdered XRD analysis of ST-LS/Au NPs nanoparticles revealed their crystallinity, with distinct peaks observed at 2θ values of 38.4°, 47.6°, 67.2°, and 77.2°. These peaks indicate the presence of pure gold nanoparticles in an fcc lattice. According to JCPDS No. 65-2870, the prominent reflection, consistent with the (111) plane at 38.4, confirms the formation of nanocrystals. TEM images revealed that the ST-LS/Au NPs nanoparticles exhibited a globular shape, averaging approximately 10 nm in size. Additionally, we chose to utilize Suzuki-Miyaura coupling (SMC) reactions to determine and evaluate the catalytic performance/efficacy of the gained ST-LS/gold nanoparticles composite. We could easily utilize this catalyst over 9 times in the catalytic testing period. This catalyst exhibits great applicability and extension for different aryl halides as well. The antioxidant activity was assessed by investigating its potential biological applications through the DPPH (diphenyl-1-picrylhydrazyl) radical scavenging assay, which gave promising results. Additionally, the study expanded to incorporate the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide], aiming to evaluate its effect on the inhibition of growth of human pancreatic and hepatoma cancer cell lines (MIA PaCa2 and HepG2). The results highlighted significant cytotoxic effects.
Keywords
Gold nanoparticles
Hepatoma cancer
Pancreatic
Starch-lignosulfonate composite
Suzuki–Miyaura
1. Introduction
Gold nanoparticles (AuNPs) have numerous applications owing to their distinctive characteristics [1,2]. AuNPs have intrigued researchers due to their applications across various fields, including engineering, pharmacology, medicine, biotechnology, energy, and environmental sectors, in contrast to their bulky counterparts [3-5]. These materials find applications in catalysis, biomedical fields, recording media, electronics, and sensing technologies [2,3]. AuNPs have garnered the attention of scientists due to their antioxidant, UV filter, antifungal, and antibacterial properties among metal oxides. Various chemical methods, such as gold reaction with hydrothermal synthesis, precipitation method, steam, and transfer alcohol, have been proposed for AuNP formulation [2-5].
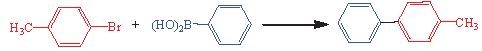
Cross-mating reactions are important in synthetic organic chemistry for C-C bond formation [6-9]. Among these, the Suzuki-Miyaura mating reactions for creating stable C–C bonds are pivotal for biphenyl formative and discrete syntheses [10]. Suzuki-Miyaura reactions generally occur in similar conditions in the presence of a phosphine ligand and a palladium accelerator. Such situations display privileged practice and pickup [11]. Nonetheless, the costly reagents, lengthy reaction times, and extreme temperatures appear to be critical and demand profound attention in the field of industrial application [9-11].
Nanobiotechnology, which combines nanoscience with biology, has been a hopeful and encouraging field arena for medical studies in the last few years [12-16]. Nanoscience plays a crucial role in multiple diagnostic methods, drug delivery vesicles, clinical practices, and cancer treatments [17-20]. The structural attributes and biological effects of nanoparticles have attracted considerable attention from researchers for applications in pharmaceutical science [21-23].
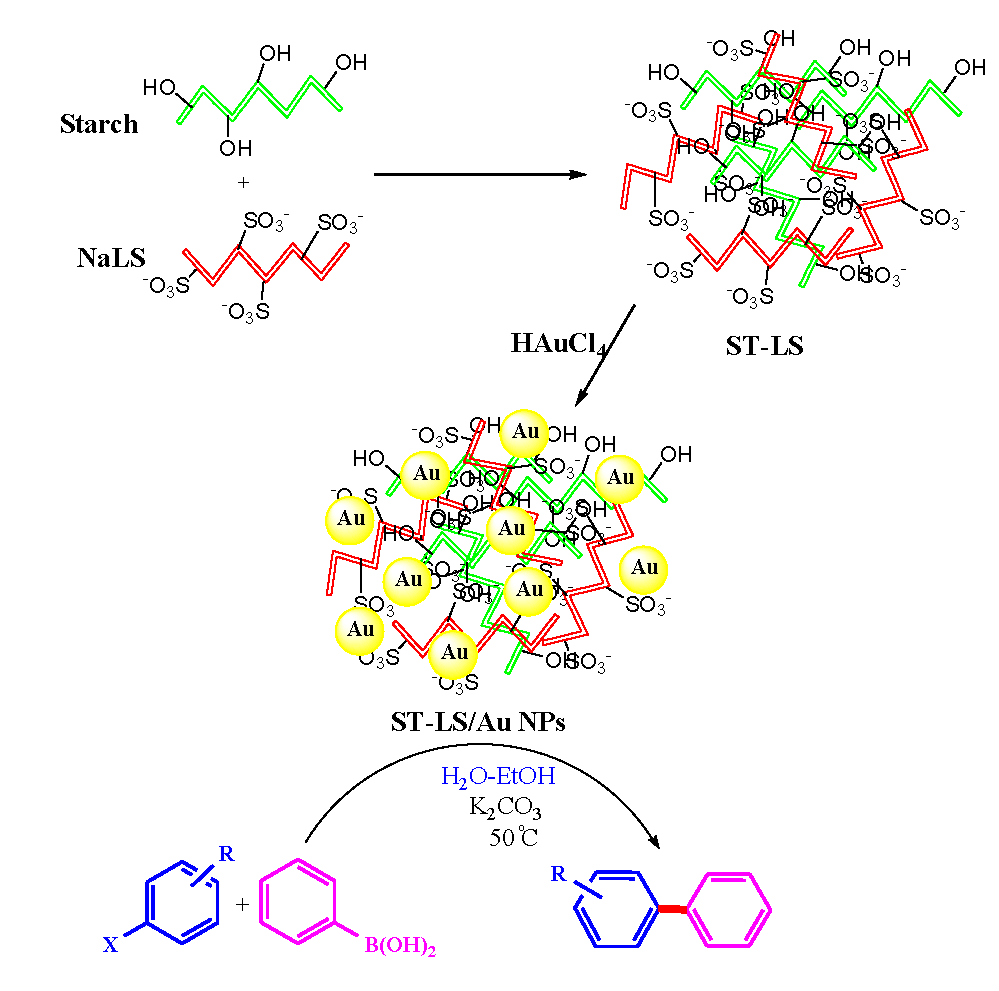
Knowing the excellent biomedical properties and associative abilities of these two biopolymers (sodium lignosulfonate combined starch), we opted to design a polymeric composite of starch combined with sodium lignosulfonate (ST-LS) combo-polymer in our research for the bio-inspired synthesis of ST-LS/Au NPs (Scheme 1). Furthermore, the ST-LS/Au NPs nanocomposite was explored for its possible biological applications, specifically focusing on its antioxidant potential using the 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) radical scavenging assay. Additionally, its ability to inhibit the growth (proliferation) of pancreatic cancer cell lines such as MIA PaCa2, as well as hepatocellular carcinoma cells like HepG2, was evaluated by assessing cytotoxicity in terms of cell viability percentage, using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay.
- Schematic production of ST-LS/Au NPs nanocomposite and its usage in the SMC reactions.
2. Materials and Methods
2.1. Materials
Sodium lignosulfonate (NaLS), starch, HAuCl4, CH3COOH, NaOH, DI H2O, and ethanol were purchased from Sigma Aldrich. UV-Vis spectrometric analysis was performed using an Agilent Cary 100 spectrophotometer. The cell line, MTT reagent, and DPPH were obtained from Fieser Ltd., USA. fourier transformed infrared spectroscopy (FT-IR) spectra were recorded using a Bruker VERTEX 80V spectrophotometer. Field emission scanning electron microscopy (FE-SEM) was performed with a TESCAN MIRA3 microscope, equipped with an Energy dispersive x-ray spectroscopy (EDX) analyzer. Transmission electron microscopy (TEM) images were provided using a Philips CM10 microscope, operating at 200 kV. For x ray diffraction (XRD) studies, Co Kα radiation (wavelength 1.78897 Å) was employed, with a power capacity of 40 keV and 40 mA. Diffraction data were recorded within a 2θ range of 10° to 80°.
2.2. Fabrication of the ST-LS/Au NPs
In a round bottom flask, Lignosulfonate (LS) and Starch (ST) (0.1 g each) were added to 50 mL DI-H2O and sonicated for 2 hs at 60°C. Then, a solution of HAuCl4 (20 mg in 10 mL water) was added dropwise under the same conditions while sonicating for 30 mins. The reduction of Au3+ to Au NPs was confirmed by the color change of the solution from yellow to dark wine. The obtained solid (ST-LS/Au NPs) was isolated using a centrifuge, rinsed in de ionized (DI) water, and dehumidified in a vacuum furnace. The amount of Au in the final composite calculated by the Inductively coupled plasma-optical emission spectroscopy (ICP-OES) technique was 0.058 mmol/g.
2.3. General way for Suzuki-Miyaura coupling
We added the ST-LS/Au NPs catalyst (10 mg, 0.02 mol%) to the mixture of phenylboronic acid (1 mmol), aryl halide (1 mmol), and K2CO3 (2 mmol) in 3 mL H2O-EtOH (1: 1), warmed it up mildly at 50°C for a specific time period. After consuming the original material (as corroborated by thin layer chromatography (TLC) (n-hexane/acetone; 4:1), we infused 10 mL of EtOAc into the flask and separated the catalyst over a centrifuge. Finally, we purified the organic mixture, dehumidified Na2SO4, and refined it using perpendicular chromatography to give the correlated biphenyl that was discerned by 1H NMR and 13C NMR.
2.4. Investigating of the antioxidant effects of ST-LS/Au NPs
The DPPH method, originally developed by Brand-Williams, is a widely used technique for assessing the antioxidant capacity of a substance by measuring its ability to neutralize the DPPH free radical. This colorimetric method functions by scavenging free radicals in the process of which the purple DPPH solution loses its color by accepting a hydrogen atom or free electron. This change in color results in a decrease in the absorption value of DPPH free radicals at 517 nm. To prepare the working solution, 2 mg of the DPPH powder was dissolved in 30 mL of ethanol. To assess the ability of ST-LS/Au NPs to inhibit free radicals, 0.5 mL of various concentrations of the nanoparticles, prepared through serial dilution, were placed in microtubes. Each microtube was then filled with equivalent volumes of DPPH. Butylated hydroxytoluene (BHT), a synthetic antioxidant, served as the positive control, and ethanol was used as the negative control. The free radical inhibition was quantified using the IC50 value, which represents the concentration of the substance required to inhibit 50% of the free radicals. This index can be calculated using the following formula:
A0= absorbance of reference, As = Absorbance of samples
2.5. Cytotoxic effects of ST-LS/Au NPs
In vitro cytotoxicity studies were conducted using the MTT colorimetric assay on several standard hepatocellular and pancreatic carcinoma cell lines, including MIA PaCa2, and HepG2, to evaluate the effects of the ST-LS/Au NPs nanocomposite on inhibiting abnormal cell growth. The cell lines were first cultured. An appropriate culture medium was selected, comprising 10% RPMI (Roswell Park Memorial Institute Medium), fetal bovine serum (FBS), and antibiotics (streptomycin and penicillin) to maintain optimal growth conditions of humidified incubation with 5% CO2 at 37°C. Once the cells reached approximately 80% confluence, they were washed with fresh medium and centrifuged at 3000 rpm for 5 mins. The cell pellet was resuspended in 5 mL of culture medium and incubated under the same conditions (5% CO2, 37°C, and adequate humidity). Live cell counting and contamination checks were performed using an inverted microscope. The cells were detached by adding trypsin to the plate and then counted using trypan blue dye, followed by washing with phosphate-buffered saline (PBS). After 24 hrs of incubation, the cells were treated with various concentrations of ST-LS/Au NPs (ranging from 1 to 1000 µg/mL) and incubated with 20 µL of MTT dye for 4 h. This reaction produced purple formazan crystals, which were then dissolved in 200 µL of dimethyl sulphoxide (DMSO). Absorbance was measured using an enzyme-linked immunosorbent assay (ELISA) reader, and the percentage of viable cells was calculated using the following Eqs. (1) and (2):
2.6. Statistical analysis
The SPSS24 software was used for statistical analysis, and one-way analysis of variance (ANOVA) and least significant difference (LSD) tests (p<0.05) were then performed. There were three duplicate analyses.
3. Results and Discussion
3.1. Structural analysis of the ST-LS/Au NPs
In the present work, a novel nanomaterial named ST-LS/Au NPs has been synthesized by immobilizing AuNPs over the polymeric composite of LS biopolymer combined with ST as capping/reducing and stabilizing polymeric template (Scheme 1). The prepared nanocomposite was fully characterized using various techniques.
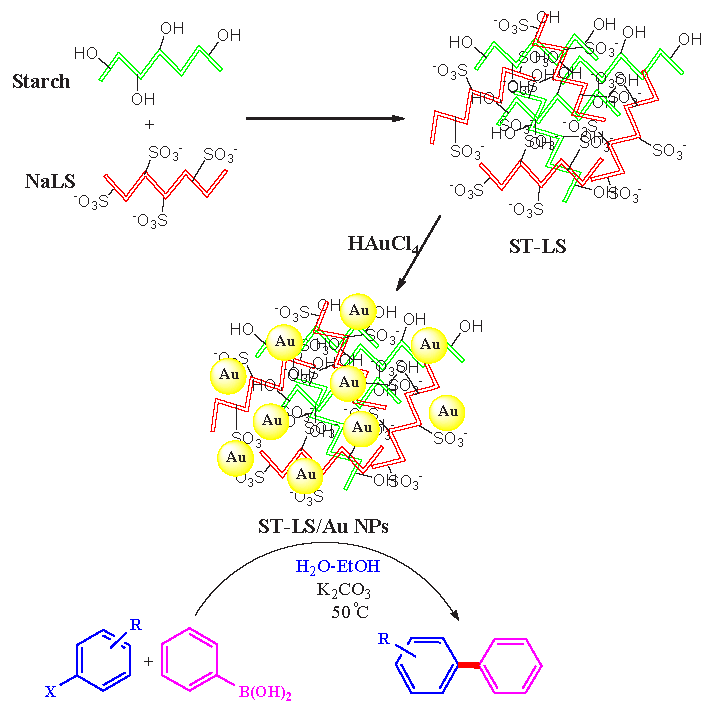
Successful preparation of the desired nanocomposite was well confirmed by FT-IR data. Figure 1 presents the FT-IR perspective of LS, Starch (ST), ST-LS hydrogel, and the prepared ST-LS/Au NPs material. The LS shown the characteristic bonds at 3419 cm-1 (O-H bond), 2926 cm-1 (C-H), 1620 cm-1 (C=O), 1498 cm-1 (COO-), 1421 cm-1 (C-H), 1043 cm-1 (SO3-), and 1050-1350 cm-1 due to C=C of alkenyl bonds (Figure 1a). Similarly, starch showed the signals at 3421 cm-1, 1083 cm-1, and 1649 cm-1, allocated to the expansion of O-H, C–O, and O–H flexing vibrations (Figure 1b). The preparation of the ST-LS composite was detected by studying the FT-IR spectrum in Figure 1(c), which shows common peaks of both components with just a slight shift. The resulting spectrum of ST-LS/Au NPs is also same as Figure 1(c), including some small shifts due to interactions of gold nanoparticles with the functional groups of ST-LS composite (Figure 1d). The results agreed well with the Changxu Wanyan et al report that designed and introduced the Cu NPs/CS-Starch bio-composite with the same characteristic peaks [24].
- FT-IR perspective of (a) LS, (b) ST, (c) ST-LS, and (d) ST-LS/AuNPs.
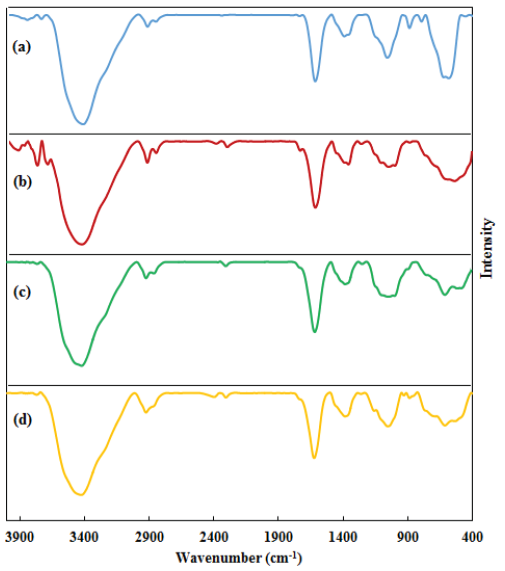
The FE-SEM and TEM have been investigated to study the morphology, form, and dimensions of ST-LS/Au NPs. The results have been presented in Figures 2, 3, and 4. The SEM image shows the pseudo-spherical particles (Figure 2). The capping and stabilizing functional group ST-LS can effectively coat the synthesized AuNPs, forming a stabilizing layer and thereby producing particles with nano sizes [25]. Next, an inherent structural morphological analysis was conducted using TEM (Figures 3 and 4). It indicated that the mono-dispersed AuNPs had a spherical shape with similar sizes (10 ± 5 nm). The same composite has been introduced for silver nanoparticles encapsulated by chitosan/starch mixed hydrogel, which acted as a reducing/stabilizing template that was applied as a novel nano-drug [25].
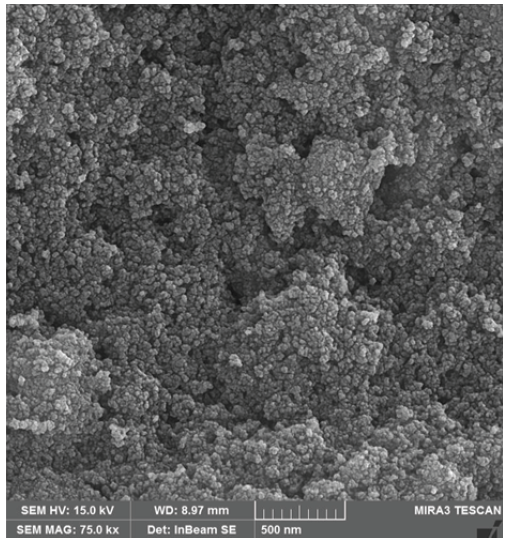
- FE-SEM of the ST-LS/ AuNPs
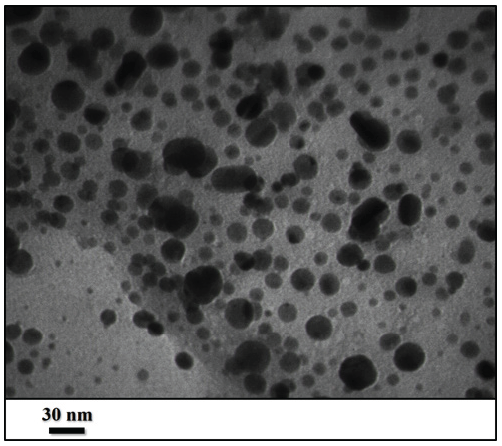
- TEM of the ST-LS/AuNPs (scales bar 150 and 60 nm.
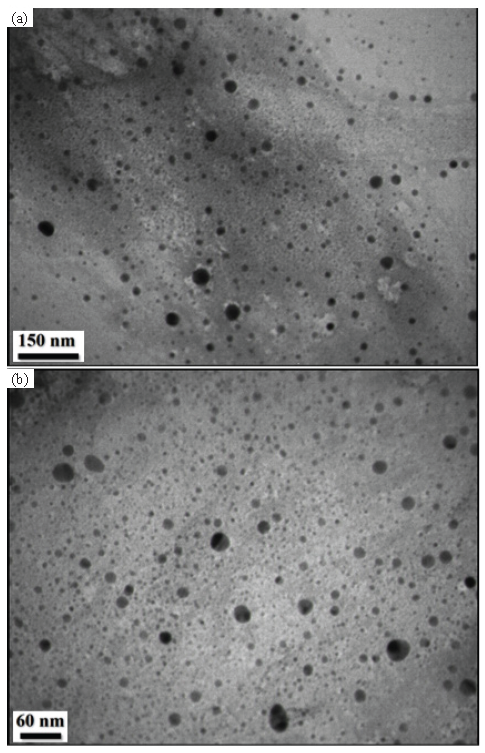
- TEM of the ST-LS/AuNPs with scale bar (a) 150 nm, (b) 30 nm.
The elemental constitution of the synthesized NaLS-Starch/AuNPs nanomaterial was justified by EDX studies. The profile, as displayed in Figure 5, shows a main peak at 2.21 keV ascribed to the Au element. In addition, there were C, O, Na, and S as compositional elements attributed to the NaLS-Starch composite. The elemental mapping study confirmed the EDX data and showed homogenous distribution of C, O, Na, and S elements over the matrix (Figure 6). Karmakar et al. [25] also described similar data for AuNPs synthesized using corn starch as a natural bio-reducing and capping agent.
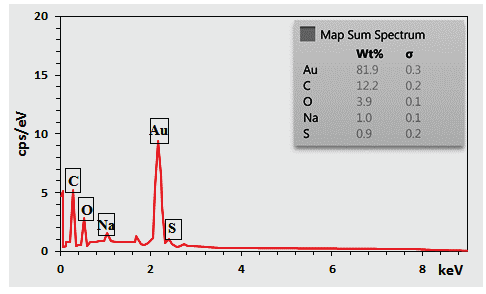
- EDX of the ST-LS/Au NPs.
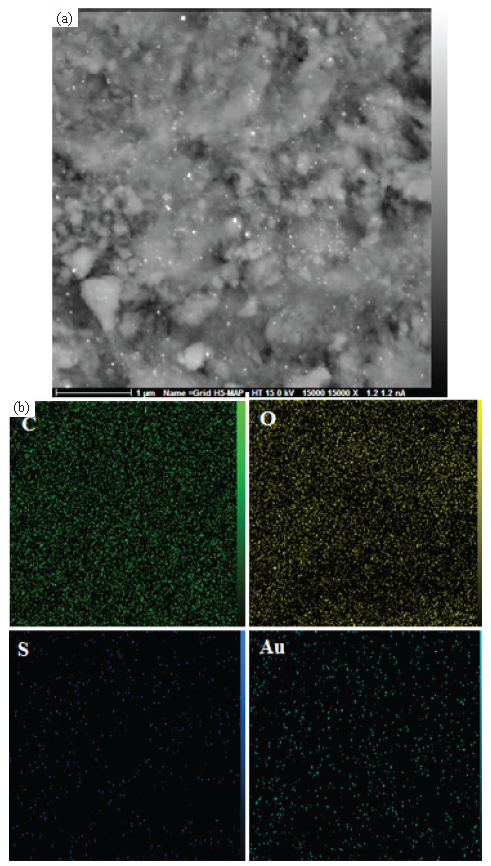
- (a) X ray scanning of the FE SEM image of ST-LS/Au NPs, (b) elemental mapping of the ST-LS/AuNPs.
Next, the crystalline structure of ST-LS/Au NPs was studied using XRD. The XRD pattern (Figure 7) shows a characteristic wide bond assigned to the ST-LS polymeric phase observed around 10-20° (002). The other arranged signals peak at (111), (200), (220), and (311) crystal planes, supported the polycrystalline nature and were well-matched with JCPDS no: 81-0792 of the Au cubic crystal [26].
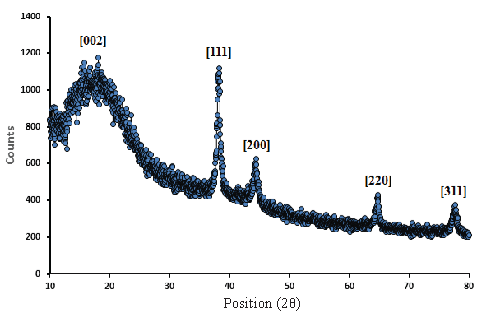
- XRD pattern of the ST-LS/Au NPs.
3.2. Catalytic application of ST-LS/Au NPs nanocatalyst
The research aimed to employ the ST-LS/Au NPs accelerator catalytically according to its bimolecular structural synthesis and exact delineation of specific character. We studied the material in the C-C bond shaping using the Suzuki-Miyaura coupling (SMC) reaction (Scheme 1). Originally, despite being crucial to standardizing the reaction requirements by accepting a pattern reaction, including 4-bromotoluene and phenylboronic acid, Table 1 is a documentary picture of the findings concerning stabilization decreeing various provisions, containing solvent, kind of base, temperature, and accelerator over the reaction. The inquiry into the aforesaid elements implies that the pattern reaction resulted in an enormous yield of necessary biphenyl in presence 10 mg of ST-LS/Au NPs (0.02 mol% Au amount) and K2CO3 as the proper base through employing EtOH-H2O (1:1) as solvent at 50°C in air (Table 1, entry 5).
| Entry | Catalyst (Au mol%) | Solvent | Base | T (°C) | Time (h) | Output(%)b |
|---|---|---|---|---|---|---|
| 1 | 0.02 | EtOH | K2CO3 | 50 | 2 | 62 |
| 2 | 0.02 | H2O | K2CO3 | 50 | 2 | 45 |
| 3 | 0.02 | CH3CN | K2CO3 | 50 | 1 | 45 |
| 4 | 0.02 | DMF | K2CO3 | 50 | 1 | 70 |
| 5 | 0.02 | H2O-EtOH (1:1) | K2CO3 | 50 | 1 | 98 |
| 6 | 0.02 | H2O-EtOH (1:1) | Et3N | 50 | 2 | 72 |
| 7 | 0.02 | H2O-EtOH (1:1) | Na2CO3 | 50 | 2 | 68 |
| 8 | 0.02 | H2O-EtOH (1:1) | – | 50 | 5 | 0 |
| 9 | 0.01 | H2O-EtOH (1:1) | K2CO3 | 50 | 2 | 65 |
| 10 | 0.03 | H2O-EtOH (1:1) | K2CO3 | 50 | 1 | 98 |
| 11 | 0.00 | H2O-EtOH (1:1) | K2CO3 | 50 | 3 | 0 |
| 12 | 0.02 | H2O-EtOH (1:1) | K2CO3 | 25 | 3 | 70 |
After we had obtained strong and consistent reaction requirements, we could display their appropriate application and different adaptation capacity to numerous substrates. Table 2 demonstrates the wide variety of layers used in the coupling reaction, affirming our findings that the major components were greatly compliant with the reaction factors. As a result of their feeble leaving valency, the chloroarenes were remarkably slower in their responses than the pertaining bromo or iodo (Table 2, entries 3, 6, 9). It should also be noted that despite the effect of other organic possibilities, similar electron-giving (CH3, OCH3, OH) or withdrawing groups (COCH3) helped to supply excellent yield just in 1-3 hrs.
| Entry | RC6H4X | R | X | Time (h) | Yield (%)b |
|---|---|---|---|---|---|
| 1 | H | H | I | 1 | 98 |
| 2 | H | H | Br | 2 | 98 |
| 3 | H | H | Cl | 10 | 40 |
| 4 | 4-Me | H | I | 1 | 98 |
| 5 | 4-Me | H | Br | 2 | 98 |
| 6 | 4-Me | H | Cl | 10 | 40 |
| 7 | 4-COMe | H | I | 1 | 95 |
| 8 | 4-COMe | H | Br | 2 | 95 |
| 9 | 4-COMe | H | Cl | 10 | 35 |
| 10 | 4-MeO | H | I | 1 | 90 |
| 11 | 4-MeO | H | Br | 2 | 90 |
| 12 | 4-OH | H | I | 1 | 90 |
| 13 | 4-OH | H | Br | 2 | 80 |
Having completed a modern category of the pattern response, we detached /isolated the heterogeneous ST-LS/AuNPs nanocomposite by a centrifuge entirely cleaned with EtOH and dehumidified at 50°C to discover its reusability. We also appraised stability and efficiency of the material through reusing additional deals of substance, and surprisingly, the catalyst had adequate potency to continue operating for nine more successive consecutive periods (Figure 8). The strength of the nanocatalyst was confirmed through a hot filtration test in which the catalyst was separated in the middle of the reaction using a centrifuge following half of the experiment. Afterwards, we went on to fulfill the rest of the work in catalyst-free situations. Amazingly, the reaction yield did not show any additional progress, illustrating lack of active sorts and varieties, and when removed in certain conditions, it would not be catalytically active either.
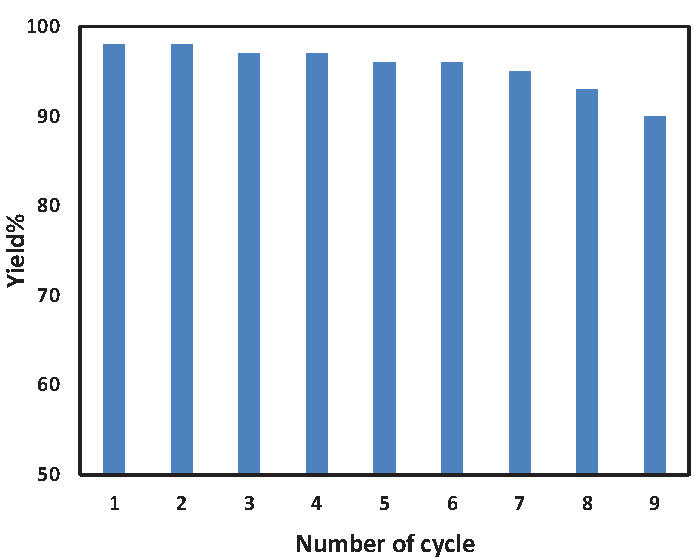
- Reusability of the ST-LS/Au NPs catalyst.
3.3. Evaluation of antioxidant capacity and cytotoxicity studies of ST-LS/Au NPs nanocomposite
Several studies have highlighted the remarkable antioxidant properties of Au NPs and their nanocomposite derivatives, which are currently under in vitro investigation. AuNP-based antioxidant materials are in high demand in the healthcare industry for their potential to alleviate oxidative stress. Additionally, materials exhibiting strong anti-tumor activity and the ability to induce cell death in cancerous cells have been reported to possess considerable antioxidant properties. In this study, we utilized the well-established DPPH assay to assess the antioxidant potential of the ST-LS/Au NPs nanocomposite (Figure 9). The DPPH solution (150 µL, 0.04 mg/mL in ethanol) was combined with the antioxidant sample at six different concentrations (31.25, 62.5, 125, 250, 500, and 1000 µg/mL). Upon radical neutralization, the purple color of the DPPH solution faded to a pale yellow. The antioxidant capacity of the material was quantified through UV absorption measurements, and the percentage inhibition was calculated using the following equation (Eq. 1). The IC50 value for ST-LS/Au NPs nanocomposite against DPPH free radicals was found to be 139 µg/mL in the antioxidant assay (Figure 9).

- Antioxidant potential of ST-LS/Au NPs nanocomposite.
The cytotoxicity of the ST-LS/Au NPs nanocomposite was evaluated using the MTT assay on pancreatic carcinoma and hepatocellular cell lines, including MIA PaCa2 for pancreatic carcinoma and HepG2 for hepatocellular carcinoma, across different concentrations. The results, presented in Figure 10, clearly indicate that the ST-LS/AuNPs nanocomposite exhibits considerable cytotoxic effects on both cell lines, with toxicity increasing as the concentration of the nanocomposite rises. As cell viability is inversely related to toxicity, a decrease in cell viability was observed with higher concentrations of the material. The calculated IC50 values were 272 and 228 µg/mL for MIA PaCa2 and HepG2 cell lines, respectively. Remarkably, the highest inhibition was observed for the HepG2 cell line. Furthermore, cytotoxicity assays conducted on the normal human cell line human umbilical vein endothelial cells (HUVEC) demonstrated minimal effects, suggesting that the ST-LS/AuNPs nanocomposite is relatively safe for human cells (Figure 11).
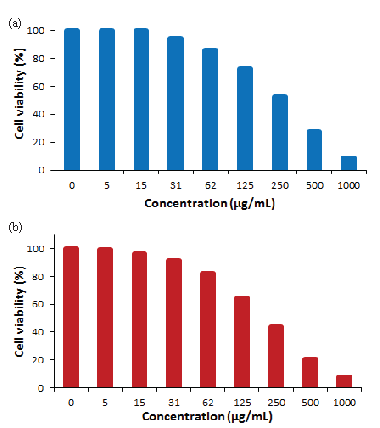
- In vitro toxicity analysis of ST-LS/Au NPs on (a) MIA PaCa2 cell and (b) HepG2 cell.
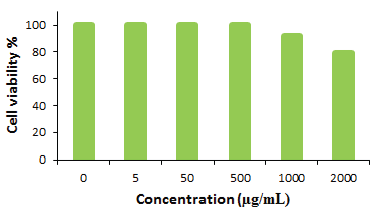
-
In vitro toxicity analysis of ST-LS/Au NPs on HUVEC cell.
4. Conclusions
ST-LS/Au NPs, a novel nanomaterial for catalytic and bioapplication, was synthesized. By using ST-LS polymeric composite as a green capping-reducing template and decorating Au NPs into the ST-LS composite, a mild procedure was introduced. The generated ST-LS/Au NPs were fully characterized using various advanced analytical methods. The images obtained from FE-SEM and TEM indicate that the particles are predominantly spherical and exhibit a uniform size of approximately 10 ± 5 nm. In addition, the AuNPs are applied as a splendid catalyst for the Suzuki-Miyaura mating reaction. The NiO NPs/Pistacia nanocomposite was investigated for its anticancer potential against hepatocellular and pancreatic carcinoma cell lines. The potential of the ST-LS/Au NPs nanocomposite as an anticancer agent was explored through its effects on pancreatic and hepatocellular carcinoma cell lines. To begin, the antioxidant properties of the material were evaluated using the DPPH assay, which yielded notable IC50 values, indicating strong antioxidant activity. Following this, the nanocomposite was tested for its cytotoxic effects on two cancer cell lines— MIA PaCa2 (pancreatic carcinoma) and HepG2 (hepatocellular carcinoma). The results revealed promising IC50 values, demonstrating the compound’s potential as an effective anti-cancer agent. These findings point to the ST-LS/AuNPs nanocomposite as a promising candidate for further preclinical in vivo investigations, with the potential to serve as a novel therapeutic approach for treating pancreatic and hepatocellular cancers in humans.
Acknowledgement
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through the Large Research Project under grant number RGP2/128/46.
CRediT authorship contribution statement
Hui Zhang, Aixiang Liu, Yong Hu: Visualization, Writing original draft, Formal analysis, Guimin Hui, Attalla F. El-kott, Kareem Morsy, Ali S. Alshehri: Funding acquisition, Methodology, Supervision. Mingyi Zhang, Xielin Feng: Writing original draft, Formal analysis, Writing-review and editing. All authors reviewed the manuscript.
Declaration of competing interest
The authors report no conflicts of interest in this work.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- The molecular mechanism of action of bactericidal gold nanoparticles on escherichia coli. Biomaterials. 2012;33:2327-2333. https://doi.org/10.1016/j.biomaterials.2011.11.057
- [Google Scholar]
- In-vitro hepatoprotective activity of Moringa oleifera mediated synthesis of gold nanoparticles. Journal of Chemical and Pharmaceutical Research. 2015;7:781-788.
- [Google Scholar]
- Effect of iron oxide and gold nanoparticles on bacterial growth leading towards biological application. Journal of nanobiotechnology. 2011;9:34. https://doi.org/10.1186/1477-3155-9-34
- [Google Scholar]
- Size-dependent antimicrobial properties of sugar-encapsulated gold nanoparticles synthesized by a green method. Nanoscale Research Letters. 2012;7:623. https://doi.org/10.1186/1556-276X-7-623
- [Google Scholar]
- Analytical detection and biological assay of antileukemic drug 5-fluorouracil using gold nanoparticles as probe. International Journal of Pharmaceutics. 2007;337:275-281. https://doi.org/10.1016/j.ijpharm.2006.12.027
- [Google Scholar]
- Viral templates for gold nanoparticle synthesis. Journal of Materials Chemistry. 2005;15:749-753. https://doi.org/10.1039/B413074J
- [Google Scholar]
- Introduction to cross-coupling reactions. In: Miyaura N., ed. Cross-Coupling Reactions. Topics in Current Chemistry. Vol vol 219. Berlin, Heidelberg: Springer; 2002. https://doi.org/10.1007/3-540-45313-X_1
- [Google Scholar]
- Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chemical Reviews. 1995;95:2457-2483. https://doi.org/10.1021/cr00039a007
- [Google Scholar]
- Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chemical Reviews. 20022002;102:1359-1470. https://doi.org/10.1021/cr000664r
- [Google Scholar]
- Palladium-catalyzed Suzuki-Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Accounts of Chemical Research. 2008;41:1461-1473. https://doi.org/10.1021/ar800036s
- [Google Scholar]
- Copper-catalyzed Suzuki cross-coupling using mixed nanocluster catalysts. Journal of the American Chemical Society. 2002;124:11858-9. https://doi.org/10.1021/ja027716+
- [Google Scholar]
- Synthesis and application of a novel multifunctional nanoprodrug for synergistic chemotherapy and phototherapy with hydrogen sulfide gas. Journal of Medicinal Chemistry. 2025;68:3197-3211. https://doi.org/10.1021/acs.jmedchem.4c02426
- [Google Scholar]
- Platinum–Iron nanoparticles for oxygen-enhanced sonodynamic tumor cell suppression. Inorganics. 2024;12:331. https://doi.org/10.3390/inorganics12120331
- [Google Scholar]
- Precisely tailoring molecular structure of doxorubicin prodrugs to enable stable nanoassembly, rapid activation, and potent antitumor effect. Pharmaceutics. 2024;16:1582. https://doi.org/10.3390/pharmaceutics16121582
- [Google Scholar]
- Fabrication of a porous metal-organic framework with polar channels for 5-fu delivery and inhibiting human osteosarcoma cells. Journal of Chemistry. 2018;2018:1-7. https://doi.org/10.1155/2018/1523154
- [Google Scholar]
- Liquid chromatography/Mass spectrometry analysis and hepatoprotective effect of steamed platycodi radix on acute alcohol-induced liver injury. International Journal of Pharmacology. 2018;14:952-962. https://doi.org/10.3923/ijp.2018.952.962
- [Google Scholar]
- Inhibitory effect of semen litchi drug serum on the proliferation of human hepatoma hepG2 cells and expression of VEGF and MMP-9. Journal of the College of Physicians and Surgeons--Pakistan: JCPSP. 2019;29:532-6. https://doi.org/10.29271/jcpsp.2019.06.532
- [Google Scholar]
- Drug metabolism and transport mediated the hepatotoxicity of Pleuropterus multiflorus root: A review. Drug Metabolism Reviews. 2024;56:349-358. https://doi.org/10.1080/03602532.2024.2405163
- [Google Scholar]
- A multi-omic analysis reveals that gamabufotalin exerts anti-hepatocellular carcinoma effects by regulating amino acid metabolism through targeting STAMBPL1. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2024;135:156094. https://doi.org/10.1016/j.phymed.2024.156094
- [Google Scholar]
- Stimulus responsive nanocarrier for enhanced antitumor responses against hepatocellular carcinoma. International Journal of Nanomedicine. 2024;19:13339-13355. https://doi.org/10.2147/IJN.S486465
- [Google Scholar]
- Simultaneous quantification of mirabegron and vibegron in human plasma by HPLC-MS/MS and its application in the clinical determination in patients with tumors associated with overactive bladder. Journal of Pharmaceutical and Biomedical Analysis. 2024;240:115937. https://doi.org/10.1016/j.jpba.2023.115937
- [Google Scholar]
- Effect of metformin on hepatocellular carcinoma patients with type II diabetes receiving transarterial chemoembolization: A multicenter retrospective cohort study. International Journal of Surgery. 2025;111:828-838.
- [Google Scholar]
- Unlocking the full potential of memory T cells in adoptive T cell therapy for hematologic malignancies. International Immunopharmacology. 2025;144:113392. https://doi.org/10.1016/j.intimp.2024.113392
- [Google Scholar]
- Design and evaluation of a novel nano copper/chitosan–starch bio-composite on antimicrobial property and wound-healing efficacy. Inorganic Chemistry Communications. 2022;140:109433. https://doi.org/10.1016/j.inoche.2022.109433
- [Google Scholar]
- Sonochemical synthesis of gold nanoparticles mediated by potato starch: Its performance in the treatment of esophageal cancer. Open Chemistry. 2024;22:20230193. https://doi.org/10.1515/chem-2023-0193
- [Google Scholar]
- Paclitaxel-loaded pluronic P123/F127 mixed polymeric micelles: Formulation, optimization and in vitro characterization. International Journal of Pharmaceutics. 2009;376:176-185. https://doi.org/10.1016/j.ijpharm.2009.04.030
- [Google Scholar]







