Translate this page into:
Determination of aromatic amines in environmental water samples by deep eutectic solvent-based dispersive liquid-liquid microextraction followed by HPLC-UV
⁎Corresponding author. mfaraji@standard.ac.ir (Mohammad Faraji)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study reports a deep eutectic solvent based dispersive liquid-liquid microextraction (DES-DLLME) to extract aromatic amines (4-chloroaniline, 3-nitroaniline, 2-naphtylamine) in environmental water samples before their HPLC-UV determination. The hydrophobic deep eutectic solvent (DES) was prepared by mixing bis(2-ethylhexyl) phosphate (BEHP) as a hydrogen bond acceptor and phenol as a hydrogen bond donor. Affecting factors on the extraction of the aromatic amines were investigated and optimized. Optimum conditions were: DES type: BHHP-Ph ratio: 1 to 2; pH of solution: 8.0; DES volume: 80 µL, salt amount: 10% (w/v). Under optimum conditions, the developed method showed a wide linear range of 0.2–200 µg L−1 (R2 ≥ 0.99) with satisfactory recoveries (≥90.0%). The limit of detections (LODs) and limit of quantifications (LOQs) were in the range of 0.07–0.17 µg L−1 and 0.2–0.5 µg L−1, respectively. The enrichment factors were 170, 180 and 190 for 4-chloroaninile, 3-nitroaniline, 2-naphtylamine, respectively. Based on obtained results, the proposed method is straightforward, efficient, sensitive, and eco-friendly for the extracting and determining of the aromatic amines in environmental water samples.
Keywords
Aromatic amines
Bis(2-ethylhexyl) phosphate
Deep eutectic solvents
Environmental water samples
Microextraction
1 Introduction
Aromatic amines (AAs) are important polar organic products and are widely used in many industrial fields such as pharmacy, manufacture of pesticides, and dyes (Katsumata et al., 2012). They can also be harmful products of diesel, rubber, combustion, etc. (Werner, 2020). Generally, this group of chemicals is considered hazardous to human health and involves inducing some types of cancers, including bladder cancer (Dasgupta, 1998). Furthermore, the presence of AAs in the water resources can seriously be hazardous for aquatic creatures and human health. Therefore, the allowable contaminant level of AAs in water samples should be less than 30 µg/mL (Werner, 2020). Therefore, it is vital to detect the content of AAs in water. Up to now, a variety of analytical methods, including gas chromatography (GC-FID) (Farajzadeh and Nouri, 2012; Han et al., 2013), gas chromatography-mass spectrophotometry (GC–MS) (Özkan et al., 2019; Zhang and Duan, 2019), high-performance liquid chromatography (HPLC-UV) (Werner, 2020; Wang et al., 2020), and liquid chromatography tandem mass spectrometry (LC-MS/MS) (Bie et al., 2017) have been applied to the analysis of AAs in aqueous matrices.
Due to the extremely low content of AAs in water samples and sometimes complexity of the studied matrices, a sample preparation method is required to enrich them and to reduce matrix effect.
Solid-phase extraction (SPE) (Hadjmohammadi et al., 2016), solid phase microextraction (SPME) (Zhang and Duan, 2019), dispersive liquid-liquid microextraction (DLLME) (Farajzadeh and Nouri, 2012; Zhou et al., 2010), deep eutectic solvent ultrasound assisted dispersive liquid-liquid microextraction (DES-UA-DLLME) (Werner, 2020), air-assisted liquid-liquid microextraction (AALLME) (Torbati et al., 2018), Dispersive micro-solid phase extraction (Jalilian et al., 2017), and monolith-based adsorbent/in-tip microextraction apparatus (MBA/ITMA) (Wang et al., 2020) are some of some pre-concentration methods adopting to AAs extraction from water samples.
Among the mentioned methods, the DLLME method is one of the most widely used micro-extraction methods in recent decades due to its simplicity, speed, low cost and high efficiency (Rezaee et al., 2010). One of the main trends in DLLME methods is to diversify the extraction solvents using solvents with features of multiple interactions, less toxicity, green and environmentally friendly. In this regard, DLLME techniques by using new generation green solvents including supramolecular solvents (SMSs), ferrofluid, ionic liquids (ILs), and deep eutectic solvents (DESs) are prevalent in the last decade due to their suitability for the rules of green analytical chemistry (ALOthman et al., 2020; Zhu et al., 2021; Makoś et al., 2020; Li et al., 2020; Afshar Mogaddam et al., 2021; Elik et al., 2021; Yuvalia et al., 2021; Yelmaz and Soylak, 2018; Faraji et al., 2020a, Faraji et al., 2020b; Faraji, 2019a, Faraji, 2019b).
DESs have emerged as a superior substitute for the ILs with similar properties but their less toxicity and cost as well as their easy preparation. DES solvents can be easily synthesized by mixing a hydrogen bond acceptor (HBA) and one or two hydrogen bond donors (HBD) (Noraee Nia and Hajmohammadi, 2021). The diversification of these solvents components and the possibility of creating various solvent interactions with target analytes have received considerable attention in recent years.
In this research, a novel DES was prepared by mixing bis(2-ethylhexyl) phosphate and phenol (BHEP-Ph) and used in DLLME method for pre-concentration of three aromatic amines (AAs) in water samples. The main factors affecting the current DES-DLLME procedure were investigated. Finally, the proposed method was coupled to HPLC-UV to analyze AAs in water samples and the satisfactory results proved the method’s performance.
2 Materials and methods
2.1 Chemical and reagents
4-Chloroaniline (4-CA, purity ≥ 99.0%), 3-nitroaniline (3-NA, purity ≥ 98.0%), 2-naphtylamine (2-NA, purity ≥ 99.0%), HPLC grade acetonitrile (ACN), bis(2-ethylhexyl) phosphate (BEHP, purity ≥ 97.0%), octanoic acid (OA, purity ≥ 99.0%), benzyltrimethylammonium chloride (BTEAC, purity ≥ 97.0%), phenol (Ph, purity ≥ 99.0%), ammonium acetate (purity ≥ 98.0%), and acetic acid (purity ≥ 99.7%) were purchased from Sigma-Aldrich (Steinhem, Germany). The chemical structure of the studied AAs is shown in Fig. 1a. A stock solution of the PAAs at concentration level of 2000 mg L−1 was prepared by dissolution of proper amounts of each AAs in methanol. The solution kept in the refrigerator and stable at least for 6 month. A mix working solution of AAs at a concentration level of 40 mg L−1 was also prepared in methanol by suitable diluting.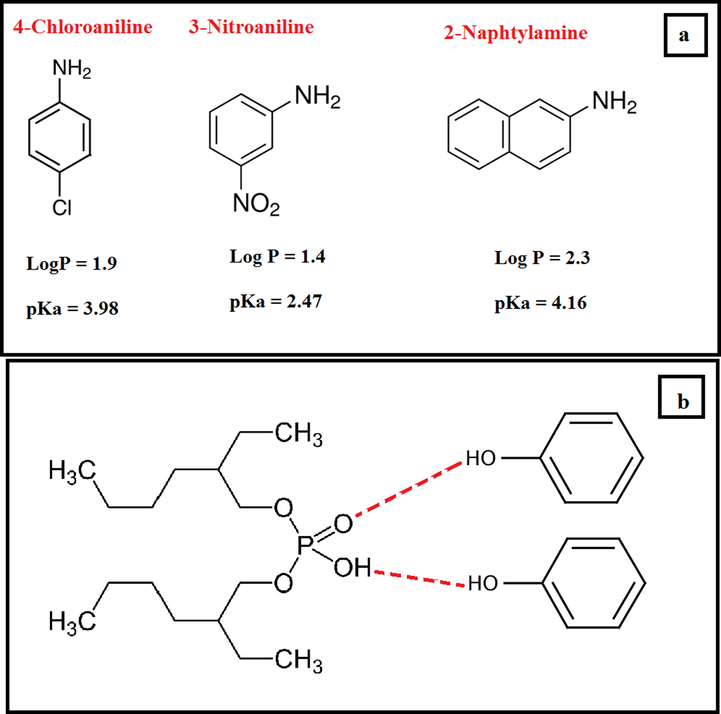
Chemical structure of PAAs (a). Suggested structure of the prepared DES (b).
2.2 Apparatus
Chromatographic separation was performed by EuroChrom model high-performance liquid chromatography (HPLC) from Knauer Germany. A Capital chromatography column with specifications of C8: 250 mm × 4.6 mm, 5 μm (Broxburn, UK) was used. The injection volume and detection wavelength was 20 µL and 235 nm, respectively. The elution of the analytes was done under isocratic elution by using a mobile phase of 0.1 mol L−1 ammonium acetate buffer (pH = 7.0) and ACN (65:35, %v/v) at a flow rate of 1.0 mL min−1.
Fourier transform infrared (FTIR) spectra were measured with a PerkinElmer RXI spectrometer in the range of 400 cm−1 to 4000 cm−1 using the KBr method. Samples were centrifuged by an Universal 320R centrifuge from Hettich Zentrifugen (Tuttlingen, Germany). Samples were vortexed by a vortex-2 Genie vortex mixer from Scientific Industries (Bohemia, NY, USA).
2.3 Deep eutectic solvent preparation
Two hydrophobic DESs (BTEAC-octanoic acid and BEHP-phenol) were prepared by mixing 10 mmol of BTEAC and BEHP as the acceptor of hydrogen bonds (HBA) with 30 mmol of the octanoic acid and phenol as the donor of hydrogen bonds (HBD) in a round-bottom flask, respectively. The mixtures were stirred at 50 °C until the mixtures became clear and then the solvents kept at the ambient temperature.
2.4 Samples and analytical procedure
Three types of water samples were collected and analyzed. Lake water sample was collected from the Persian Gulf Martyrs Lake (Tehran, Iran). A fish pond water sample was collected from a fish farm from Karaj (Alborz, Iran). Tap water sample was collected from Standard Research Institute (Karaj, Iran). The samples were filtered by filter paper of 0.45 μm and then kept in the refrigerator until before analysis. pH of the samples was adjusted and the extraction procedure was carried out.
For the microextraction of AAs from the samples via DES-DLLME method, 12.0 mL of sample was transferred to the falcon centrifuge tube. The optimization condition was conducted by adding 1.0 g sodium chloride (10%) to the sample and pH adjusted (pH = 6). Then, mixture of (80.0 µL of DES (BHEP-Ph) + 100 µL of methanol) was added to the solution as expeditiously as possible to disperse properly. The solution was vortexed vigorously for 1.0 min to get the complete dispersion of the solvent. the solution was centrifuged for 5.0 min (5000 rpm) to separate the extraction solvent from the aqueous phase. After centrifugation, two-phase were separated and DES phase was collected at the top of the tube. After withdrawn of the aqueous phase, due to having high viscosity of DES, most of the solvent stuck to the inner wall of the tube. For this reason, to collect all extraction solvent and complete separation of aqueous phase, tube again centrifuged for 1.0 min. Finally, the DES phase (30 ± 2 µL) was diluted with 20 µL methanol and injected into HPLC.
3 Results and discussions
3.1 Characterization of DES
FT-IR spectra of BEHP, Ph and its relevant DES (BEHP-Ph) were recorded to investigate structure of DES and its important functional groups. Based on the obtained results (Fig. 4), the appearance of characteristic peaks of each component of the solvent and the formation of a broad peak in the region of 3400 cm−1 proves the formation of hydrogen bond between components and as a result, demonstrate the DES formation. Stretching vibrations at 3200 cm−1, 2950 cm−1, 2868 cm−1, 1613 cm−1, 1432 cm−1 and 1241 cm−1, and 1082 cm−1 could be assigned to O-H, C-H aromatic, C-H aliphatic, C=C, and C-OH bonds of phenol (Stuart, 2004). Also, O-H, C-H aliphatic, P=O, P-O-C vibrations of BEHP are positioned at 3409 cm−1, 2979 cm−1, 1217 cm−1, and 1072 cm−1, respectively (Stuart, 2004).
Investigation of density, viscosity and melting point of the prepared DES showed that the physical properties of the solvent are favorable. The density and viscosity of the DES in 25 °C were 0.93 g mL−1 and 180 cP, respectively. Also, the DES melting point investigation showed that it has a very low melting point and does not freeze in the refrigerator (4 °C).
3.2 Optimization of DES-Dllme
3.2.1 Investigation of type and composition of DES
To have an efficient extraction with pinpoint accuracy, the selection of proper HBD and HBA in the structure of the DES is crucial because with targeted selection, it is possible to have strong and different interactions in the solvent structure at the same time, which leads to very efficient extractions. Based on the structure of the aniline compounds, two compositions of bis(2-ethylhexyl) phosphate-phenol (BEHP-Ph) and benzyltriethylammonium chloride-octanoic acid (BTEAC-OA) were investigated and tested. On the one hand, BEHP is used as a strong ion-pairing agent in extracting basic polar compounds (Hansen et al., 2020; Altunay and Gürkan, 2019). Also, phenol can present strong π-π interactions with the studied compounds. On the other hand, aniline compounds can also have good ion-pair interactions with carboxylic acids (Torbati et al., 2018). In addition, the presence of a benzene ring in the BTEAC structure can also cause π-π interactions. The results showed that BEHP-Ph composition shows better and higher efficiency in the extraction of AAs. The average recoveries for the AAs were 65% and 80% for BTEAC-OA and BEHP-Ph, respectively.
To acquire a better result, the effectiveness of various molar ratio has been considered; hence the combination of BEHP to phenol molar ratio (1:1, 1:2, 1:3, 1:4) was prepared and investigated. The obtained results (Fig. 2a) show that as the molar ratio increases, the extraction efficiency should increase slightly and become almost constant. Therefore, the 1:2 molar ratio of BEHP-Ph was chosen for subsequent studies.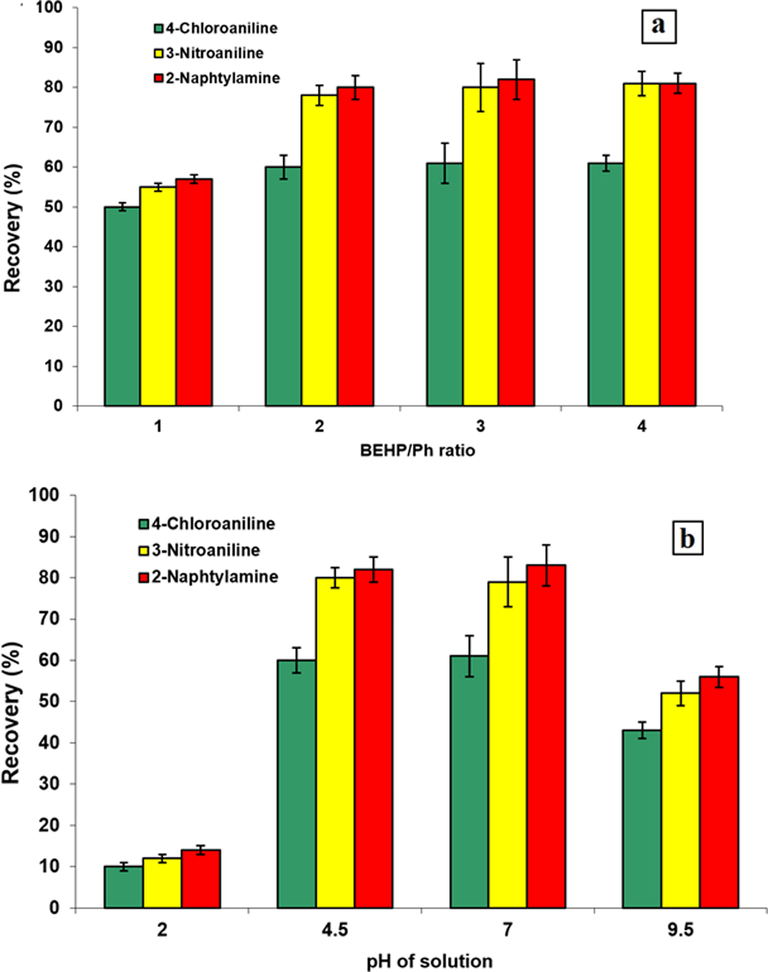
Effect of pH on the extraction of the PAAs (a), Effect of DES composition on extraction of PAAs (b).
3.2.2 Impact of pH
In this research, the pH was evaluated in the range of 2.0–9.5. According to Fig. 2b), the data display that enhancing pH from 2 to 4.5 can cause substantial enhancement in the extraction efficiency and the maximum value achieved in the range of 4.5–7.0. After that, the extraction efficiency decreased by increasing pH. These changes in the diagram can be attributed by pKa values of compounds in the solution. As the pKa values of 4-CA, 3-NA, and 2-naphtylamine are 10.02, 11.58 and 9.84, respectively, we may conclude that reduction in the extraction efficiency at pH above 7.0 can cause by deprotonation of aromatic amines in the solution which is not favorable for ion-pairing mechanism. In addition, at alkaline pHs, the solubility of BEHP in the aqueous phase can be increased dramatically (Hansen et al., 2020). On the other hand, at pH = 2.0, BEHP is not in deprotonated form (pKa = 1.9) which is also not favorable for the ion-pairing mechanism. Eventually, to acquire an acceptable result acetic acid/acetate buffer (10 mmol L−1) at pH = 4.5 was chosen as the optimum condition for the extraction.
3.2.3 Influence of DES volume
The effect of different volumes of the DES (60 µL, 80 µL, 100, 120 µL) was investigated. By enhancing DES volume from 60 to 80 µL, extraction efficiency rose. However, further increases in the volume of the DES lead to decreasing signal as a result of the dilution effect (Faraji, 2019a, Faraji, 2019b). Therefore, based on Fig. 3a, 80 µL was selected as an optimum DES volume for subsequent studies.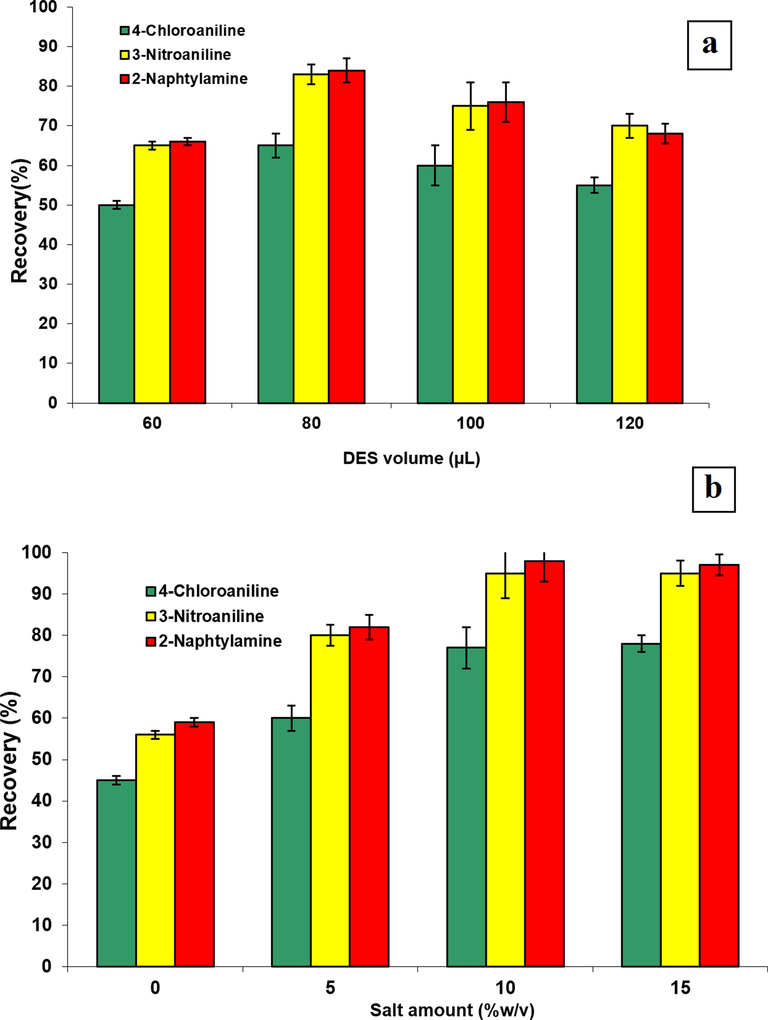
Effect of DES volume on extraction efficiency of aromatic amines (a), Effect of salt concentration on extraction of aromatic amines (b).
3.2.4 Influence of salt concentration
As the salt concentration increases, the extraction efficiency usually increases due to the salting-out phenomenon, this increasing effect can be offset by increasing the volume of the extraction phase. For this reason, the influence of the different amounts of sodium chloride was evaluated in the range of 0.0–15.0% (w/v). According to Fig. 3b, by increasing salt amount, the extraction recoveries of the AAs increase and then remains almost constant. Therefore, 10.0% NaCl was chosen for further studies.
3.2.5 Effect of vortex time
An effective solution vortex can accelerate the transfer of the analyte from the aqueous solution to the DES phase. Therefore, the effect of vortex time was investigated in the range of 1.0–3.0 min. According to obtained results; extraction efficiency was almost constant in the studied range. Therefore, 1.0 min vortex was chosen for further studies.
3.3 Analytical performance
Under optimal conditions, the performance of the developed method was evaluated by considering linear dynamic range (LDR), precision (intra-day and inter-day), the limit of detection (LOD), the limit of quantification (LOQ), and enrichment factor (EF). The figures of merit of the developed technique are presented in Table 1. The calibration curve was linear in the range of 0.5–200 μg L−1, 0.3–200 μg L−1, and 0.2–200 μg L−1, for 4-CA, 3-NA, and 2-naphtyl amine, respectively (R2 ≥ 0.99). Intra-day and inter-day RSD values are less than 6.0% at concentration levels of 5.0 and 25 µg L−1. The LOD values based on S/N = 3 were 0.17, 0.1 and 0.07 µg L−1 for 4-CA, 3-NA, 2-naphtylamine, respectively. Also, the LOQ values based on S/N = 10 were 0.5, 0.3 and 0.2 μg L−1 for 4-CA, 3-NA, 2-naphtylamine, respectively. The enrichment factors (EFs) were calculated as the ratio between analyte concentrations in the acceptor phase (DES phase) to the initial concentration in the sample. EFs were 170, 180 and 190 for 4-CA, 3-NA, and 2-naphtyl amine, respectively. RSD: Relative standard deviation; LOQ: Limit of quantification; LOD: Limit of detection; EF: Enrichment factor.
Analyte
RSD
LOD(µg L−1)
LOQ(µg L−1)
EF
Inter-days (n = 6)
Intra-day (n = 3)
5.0(µg L−1)
25(µg L−1)
5.0(µg L−1)
25(µg L−1)
4-Chloroaninile
5.8
4.7
1.9
2.1
0.17
0.5
170
3-Nitroaniline
3.8
3.2
3.5
2.2
0.1
0.3
180
2-Naphtylamine
3.7
3.8
2.1
1.8
0.07
0.2
190
3.4 Real sample analysis
The applicability of the developed method in real sample analysis was evaluated by analyzing some water samples under optimum conditions. The data are presented in Table 2. The concentration of the studied AAs in the water sample was below the LOD. Therefore, to verify the accuracy of the method, the samples were spiked at known concentration levels of PAAs. Good relative recoveries ranged from 90.0 to 98.0 with RSD less than 7.0% were obtained by analyzing three independent samples. Chromatogram of the lake water sample before and after the spike of level 1 (10, 5.0 and 5.0 µg L−1 respect to 4-CA, 3-NA, 2-naphtylamine, respectively) and level 2 (20, 10.0 and 10.0 µg L−1 respect to 4-CA, 3-NA, 2-naphtylamine, respectively) of mixture standard solution of the PAAs is shown in Fig. 4. N.D: not detected.
Sample
Analyte
Added (µg L−1)
Found (µg L−1)
Relative recovery (%)
RSD% (n = 3)
Tap water
4-Chloroaninile
–
N.D
–
5.7
10.0
9.2
92.0
4.2
20.0
19.4
97.0
3.8
3-Nitroaniline
–
N.D
–
4.1
5.0
4.7
94.0
3.9
10.0
9.8
98.0
2.5
2-Naphtylamine
–
N.D
–
4.5
5.0
4.9
98.0
4.1
10.0
9.7
97.0
3.7
Lake water
4-Chloroaninile
–
N.D
–
3.4
10.0
9.2
92.0
4.7
20.0
19.7
98.5
5.4
3-Nitroaniline
–
N.D
–
2.6
5.0
4.5
90.0
4.9
10.0
9.5
95.0
3.8
2-Naphtylamine
N.D
–
4.3
5.0
4.8
96.0
5.3
10.0
9.4
94.0
4.7
River water
4-Chloroaninile
–
N.D
–
2.9
10.0
9.3
93.0
5.3
20.0
19.2
96.0
4.7
3-Nitroaniline
–
N.D
–
2.5
5.0
4.6
92.0
4.3
10.0
9.3
93.0
2.8
2-Naphtylamine
–
N.D
–
4.4
5.0
4.8
96.0
5.1
10.0
9.9
99.0
2.8
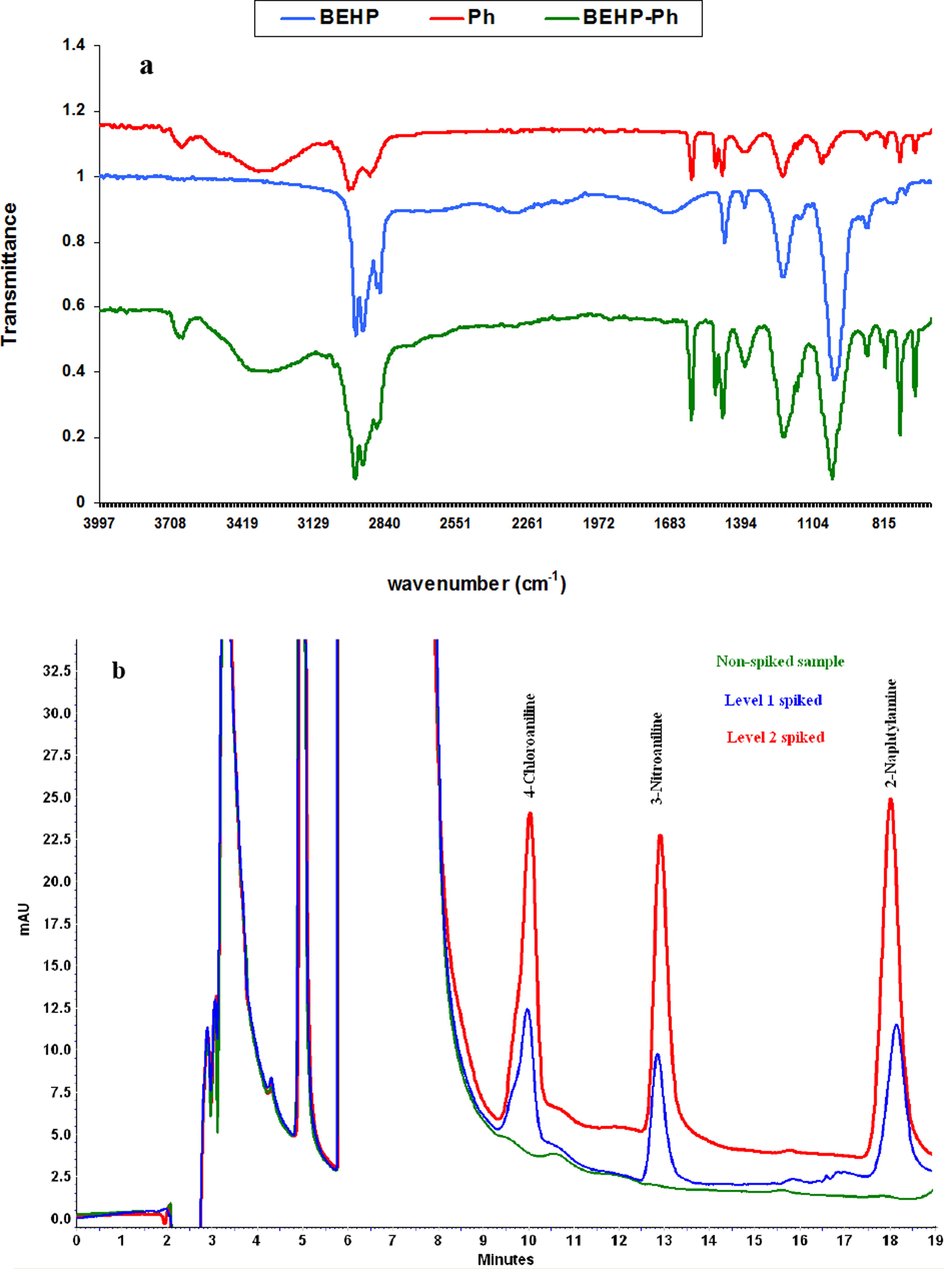
FT-IR spectrum of BEHP (blue), Ph (red) and BEHP-Ph (green) (a). Chromatograms of lake water before and after spike of level 1 (10, 5.0 and 5.0 µg L−1 respect to 4-CA, 3-NA, 2-naphtylamine, respectively) and level 2 (20, 10.0 and 10.0 µg L−1 respect to 4-CA, 3-NA, 2-naphtylamine, respectively) of the standard solutions of PAAs (b).
3.5 Comparison of proposed technique with previously published techniques
The proposed method was compared with some of the methods that recently reported in the literatures for the extraction and determination of AAs in aqueous matrices (Table 3). As it can be seen, the linearity, detection limit, repeatability and enrichment factor of the proposed method are better or comparable to the LC-MS (Bie et al., 2017) and GC–MS (Ozkan et al., 2019, Torbati at al., 2018; Zhang and Duan, 2019) methods. In particular, the cost, time, simplicity, and repeatability of the proposed method are better and more appropriate than the SPE (Jalilian et al., 2017). The extraction solvent used in our proposed method, same as other DES based methods (Torbati et al., 2018; Werner, 2020), is safer than classical solvent based DLLME methods (Ozkan et al., 2019; Farajzadeh and Nouri, 2012, Han et al., 2013; Zou et al., 2010). However, extraction solvent separation in DES based methods is a bit difficult and usually an additional centrifugation step is required to complete separation of DES phase because of higher viscosity of DES solvents compared to the classical solvents that can stick to the walls of the vials. It should be noted that the analysis of AAs by GC-FID (Farajzadeh and Nouri, 2012, Han et al., 2013; Zou et al., 2010) or GC–MS (Ozkan et al., 2019; Torbati et al., 2018) compared to LC-based methods (Wang et al., 2020, Jalilian et al., 2017, Werner, 2020, Bie et al., 2017), requires a polar column or a derivation step which complicates the method and reduces its greenness. However, GC-based methods offer higher enrichment factors than LC-based methods due to the greater compatibility of DLLME with GC techniques (Rezaee et al., 2010). Accessibility, simplicity and cost of reagents and instrument, the proposed methods are better than MS-based methods, but sensitivity, reliability and selectivity of MS-based methods are better than the proposed method. a: Linear range, b: Limit of detection, c: Relative standard deviation, d: Enrichment factor, e: In-tip microextraction apparatus monolith-based adsorbent, f: High performance liquid chromatography-Ultra violet detection g: Not reported, h: Dispersive liquid-liquid microextraction, i: Gas chromatography-mass spectrometry, j: Dispersive micro-solid phase extraction, k: Gas chromatography-flame ionization detector l: Micellar liquid chromatography, m: Solid phase microextraction, n: Ultrasound-assisted, o: Solidification of the aqueous phase, p: Air–assisted liquid–liquid microextraction, q: Liquid chromatography coupled with tandem mass spectrometry.
Analyte
Sample preparation
Instrumentation
Solvent/adsorbent
LRa (µg/L)
LODb (µg/L)
RSDc (%)
EFd
Ref.
Aniline, p-methylaniline, 2,4-dinitroaniline, 2-nitroaniline, 2-chloroaniline, diphenylamine, 3,4- dichloroaniline
MBA/ITMAe
HPLC-UVf
Monolith-based adsorbent
0.01–300
0.0021–0.026
0.85–11
2.7–8.1
Wang et al., 2020
4‑Aminobiphenyl, 4‑Chloro‑2‑methylaniline, Benzidine, 2‑Naphthylamine, 4‑Chloroaniline, 3,3′‑Dimethylbenzidine, p‑Cresidine, 4‑Aminoazobenzene
DLLMEh
GC-MSi
chloroform and 1,2‑dichloroethane
0.5–250
0.16–1.4
less than10
43–231
Özkan et al., 2019
3-Nitroaniline 4- chloroaniline 4- bromoaniline
3,4- dichloroanilineD-µ-SPEj
HPLC-UV
Multi-walled carbon nanotubes/Fe3O4@Poly(1,8-diaminonaphtalen)
0.25–500
0.1–0.25
3.4–5.6
40–65.7
Jalilian et al., 2017
Aniline, o-toluidine, 2-Chloroaniline, o-Anisidine, 4-Chloroaniline
DLLME
GC-FIDk
Butylchloroformate
10–10000
1–3
less than5.2
197–298
Farajzadeh and Nouri, 2012
Aniline, N,N-dimethylaniline, o-toluidine, m-toluidine and p-toluidine.
DLLME
GC-FID
Chlorobenzene
4–1000
0.2–3.4
1.2–7.9
207–4315
Han et al., 2013
o-nitroaniline, alpha-naphtylamine, o-chloroaniline
DLLME
HPLC-UV
Chlorobenzene
1–50
0.1–0.7
6.3–9.7
N/R
Zhou et al., 2010
4-nitroaniline, para toluidine, 4-chloroaniline, 2-nitroaniline, 3-nitroaniline, 3-chloroaniline, 4-bromoaniline
SPE
MLCl
C18 cartridge
3.1–125.0
1–4.5
5.8
N/R
Hadjmohammadi et al., 2016
Aniline, N-methylaniline, 2-methylaniline, 2,6-dimethylaniline, 2-methoxylaniline, 2-chloroaniline, 4-chloroaniline, 1-naphthylamine,
SPMEm
GC–MS
Polymeric ionic liquid fiber
10–10,000
0.67–4.29
2.1–8.3
N/R
Zhang and Duan, 2019
2-chloroaniline, 4-chloroaniline
DES-UAn-DLLME-SAPo
HPLC-UV
Trihexyl phosphonium chloride and decanol
N/Rg
0.07–0.11
2.9–6.2
116–121
Werner, 2020
Aniline, p-toluidine, p-chloroaniline, p-anisidine, 4-tert-butyl aniline
AALLMEp
GC–MS
Choline Chloride
n-butyric acidN/R
1.8–6
≤5.3
790–940
Torbati et al., 2018
o-toluidine (o-TOL), 2, 6-dimethylaniline (2, 6-DMA), o-anisidine (o-ASD), 1-naphthylamine (1-ANP), 2-naphthylamine (2-ANP), and 4-aminobiphenyl (4-AB
SPE
LC–MS/MSq
C18 cartridge
0.1–50
0.04–0.58 (ng/cig)
4.13–8.42
N/R
Bie et al., 2017
4-Chloroaniline, 3-Nitroaniline, 2-naphtylamine
DES-DLLME
HPLC-UV
Bis(2-ethylhexyl) phosphate- phenol
0.2–200
0.07–0.17
1.8–5.8
170–190
This work
4 Conclusion
In this study, a new hydrophobic DES solvent is developed to extract AAs from aqueous samples. The DES was prepared by mixing BEHP as HBA and phenol as HBD. According to the nature of the DES components, strong ion-pairing and π-π interactions could occur between target analytes and the solvent leading to superior extraction efficiencies (satisfactory recoveries within the range of 81.0–94.7% and high precision (RSD less than 6.7%)). Overall, the developed method presents an environmentally friendly and sensitive method for the extraction and enrichment of trace amounts of 4-CA, 3-NA, 2-naphtylamine in water samples. Meanwhile, the proposed method has great potential application value in water or other samples monitoring of PAAs.
Availability of data and material
The data which support the findings of this research are available from the corresponding author.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Organic solvent-free elevated temperature liquid–liquid extraction combined with a new switchable deep eutectic solvent-based dispersive liquid–liquid microextraction of three phenolic antioxidants from oil samples. Microchem. J.. 2021;168:106433.
- [Google Scholar]
- ALOthman, Z.A., Habila, M.A., Yilmaz, E., Alabdullkarem, E.A., Soylak, M., 2020. A novel deep eutectic solvent microextraction procedure for enrichment, separation, and atomic absorption spectrometric determination of palladium at ultra-trace levels in environmental samples. Measurement, 153, 107394.
- Ion pair vortex assisted-ionic liquid based dispersive liquid-liquid microextraction for selective separation and preconcentration of 4-methylimidazole from caramel colour drinks and foodstuffs prior to its spectrophotometric determination. Microchem. J.. 2019;147:999-1009.
- [Google Scholar]
- Bie, Z., Lu, W., Zhu, Y., Chen, Y., Ren, H., Ji, L., 2017. Rapid determination of six carcinogenic primary aromatic amines in mainstream cigarette smoke by two-dimensional online solid phase extraction combined with liquid chromatography tandem mass spectrometry. J. Chromatogr. A, 1482, 39-47.
- Dasgupta, A., 1998. Gas chromatographic-mass spectrometric identification and quantification of aniline after extraction from serum and derivatization with 2,2,2-trichloroethyl chloroformate, a novel derivative. J. Chromatogr. B 716, 345-358.
- Ionic hydrophobic deep eutectic solvents in developing air-assisted liquid-phase microextraction based on experimental design: application to flame atomic absorption spectrometry determination of cobalt in liquid and solid samples. Food Chem.. 2021;350:129237.
- [Google Scholar]
- Determination of some red dyes in food samples using a hydrophobic deep eutectic solvent-based vortex assisted dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. J. Chromatogr. A. 2019;1591:15-23.
- [Google Scholar]
- Novel hydrophobic deep eutectic solvent for vortex assisted dispersive liquid-liquid micro-extraction of two auxins in water and fruit juice samples and determination by high performance liquid chromatography. Microchem. J.. 2019;150:104130.
- [Google Scholar]
- Green, Fast and simple dispersive liquid-liquid microextraction method by using hydrophobic deep eutectic solvent for analysis of folic acid in fortified flour samples before liquid chromatography determination. Food Chem.. 2020;320:126486.
- [Google Scholar]
- Preparation of a ternary deep eutectic solvent as extraction solvent for dispersive liquid-liquid microextraction of nitrophenols in water samples. J. Environ. Chem. Eng.. 2020;8:103948.
- [Google Scholar]
- Simultaneous derivatization and dispersive liquid–liquid microextraction of anilines in different samples followed by gas chromatography–flame ionization detection. Talanta. 2012;99:1004-1010.
- [Google Scholar]
- Determination of aromatic amines in environmental water samples using solid-phase extraction modified with sodium dodecyl sulphate and micellar liquid chromatography. Int. J. Environ. Anal. Chem.. 2016;96:445-459.
- [Google Scholar]
- Determination of Anilines and Toluidines in Water by Salt-Assisted Dispersive Liquid-Liquid Microextraction Combined with GC-FID. Chromatographia. 2013;76:1747-1753.
- [Google Scholar]
- Electromembrane extraction of highly polar bases from biological samples – Deeper insight into bis(2-ethylhexyl) phosphate as ionic carrier. Anal. Chim. Acta. 2020;1115:23-32.
- [Google Scholar]
- Dispersive micro-solid phase extraction of aromatic amines based on an efficient sorbent made from poly(1,8-diaminonaphtalen) and magnetic multiwalled carbon nanotubes composite. J. Chromatogr. A. 2017;1499:38-47.
- [Google Scholar]
- Determination of aniline derivatives in water samples after preconcentration with oxidized multiwalled carbon nanotubes as solid-phase extraction disk. Front. Chem. Sci. Eng.. 2012;6:270-275.
- [Google Scholar]
- New low viscous hydrophobic deep eutectic solvents in vortex-assisted liquid-liquid microextraction for the determination of phthalate esters from food-contacted plastics. Food Chem.. 2020;309:125752.
- [Google Scholar]
- Hydrophobic deep eutectic solvents in microextraction techniques–A review. Microchem. J.. 2020;152:104384.
- [Google Scholar]
- Amino acids- based hydrophobic natural deep eutectic solvents as a green acceptor phase in two-phase hollow fiber-liquid microextraction for the determination of caffeic acid in coffee, green tea, and tomato samples. Microchem. J.. 2021;164:106021.
- [Google Scholar]
- Accurate and sensitive determination of harmful aromatic amine products of azo dyes in wastewater and textile samples by GC-MS after multivariate optimization of binary solvent dispersive liquid-liquid microextraction. Microchem. J.. 2019;145:84-89.
- [Google Scholar]
- Evolution of dispersive liquid-liquid microextraction method. J. Chromatogr. A. 2010;1217:2342-2357.
- [Google Scholar]
- Infrared Spectroscopy: Fundamentals and Applications. John Wiley & Sons; 2004.
- Simultaneous derivatization and air–assisted liquid–liquid microextraction based on solidification of lighter than water deep eutectic solvent followed by gas chromatography–mass spectrometry: An efficient and rapid method for trace analysis of aromatic amines in aqueous samples. Anal. Chim. Acta. 2018;1032:48-55.
- [Google Scholar]
- On-site sample preparation of trace aromatic amines in environmental waters with monolith-based multichannel in-tip microextraction apparatus followed by HPLC determination. Talanta. 2020;220:121423.
- [Google Scholar]
- Novel deep eutectic solvent-based ultrasounds-assisted dispersive liquid-liquid microextraction with solidification of the aqueous phase for HPLC-UV determination of aromatic amines in environmental samples. Microchem. J.. 2020;153:104405.
- [Google Scholar]
- A novel and simple deep eutectic solvent based liquid phase microextraction method for rhodamine B in cosmetic products and water samples prior to its spectrophotometric determination. Spectrochim. Acta A: Mol. Biomol. Spectrosc.. 2018;202:81-86.
- [Google Scholar]
- An environment-friendly and rapid liquid-liquid microextraction based on new synthesized hydrophobic deep eutectic solvent for separation and preconcentration of erythrosine (E127) in biological and pharmaceutical samples. Spectrochim. Acta A: Mol. Biomol. Spectroscopy. 2021;244:118842.
- [Google Scholar]
- A double-functionalized polymeric ionic liquid used as solid-phase microextraction coating for efficient aromatic amine extraction and detection with gas chromatography–mass spectrometry. Anal. Bioanal. Chem.. 2019;411:2209-2221.
- [Google Scholar]
- Dispersive liquid phase microextraction of aromatic amines in environmental water samples. Int. J. Environ. Anal. Chem.. 2010;90:1099-1107.
- [Google Scholar]
- Novel recyclable acidic hydrophobic deep eutectic solvents for highly efficient extraction of calcium dobesilate in water and urine sample. Talanta. 2021;233:122523.
- [Google Scholar]







