Translate this page into:
Developments in small molecule antiviral drugs against hepatitis B and C viruses: FDA approved therapies and new drugs in clinical trials
⁎Corresponding author at: Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, Ajman University, Ajman, P.O. Box 346, United Arab Emirates. s.boddu@ajman.ac.ae (Sai H.S. Boddu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Given the continuous emergence of hepatitis and the associated consequences, developing effective antiviral strategies has become a major public health concern. Current antiviral strategies for the treatment of hepatitis, especially for hepatitis B virus (HBV) and hepatitis C virus (HCV), and those under discovery and development are broadly classified into two categories, i.e., biologics and small molecules. These therapeutic classes include small molecule modulators, peptides, nucleic acid polymers, small interfering RNA (siRNA), antisense oligonucleotides, monoclonal antibodies, and vaccines. To date, several small molecule antiviral agents targeting HBV and HCV have advanced into different phases of clinical investigation for monotherapy and/or combination therapy. In ongoing clinical investigations, researchers are testing small molecule-based antiviral therapies that mainly target complex molecular interactions between virus and host cells involved in viral replication. An up-to-date comprehensive analysis is required to examine the current state of antiviral drug discovery and understand the new trends in antiviral approaches. In the present compilation, we have highlighted the FDA-approved therapies for HBV and HCV with a special focus on the ongoing clinical developments of small molecules for the treatment of hepatitis.
Keywords
Antivirals
Hepatitis B
Hepatitis C
Small molecule
Clinical trials of drugs
1 Introduction
Globally infections due to viral diseases remain an important healthcare problem. Hepatitis is a viral disease characterized by inflammation of the liver. The five main types of hepatitis include hepatitis A, hepatitis B, hepatitis C, hepatitis D, and hepatitis E. Despite having similar symptoms, hepatitis A, hepatitis B, and hepatitis C spread in different ways and with different treatment options. Hepatitis viruses are pathogens that cause acute and chronic liver diseases such as fulminant liver failure, cirrhosis, and hepatocellular carcinoma. In particular, types B and C lead to chronic diseases such as liver cirrhosis and cancer. The most common chronic blood-borne pathogen is the hepatitis C virus (HCV). About 70% of all cases of chronic viral hepatitis are caused by HCV and close to 71 million people worldwide are infected. HCV causes both acute and chronic hepatitis with an incubation period of 0.5–6 months. Transmission of hepatitis B virus (HBV) occurs through bodily fluids, while hepatitis C transmits through blood-to-blood contact (Jacobi et al., 2007; Pietschmann and Brown, 2019).
HBV contains a DNA genome that multiplies through an RNA intermediate with the help of an active viral reverse transcriptase (RT)/polymerase enzyme, while HCV is an RNA virus with no RT activity and multiplies on the cellular membrane through RNA replication (H. C. Li et al., 2021; Szabó et al., 2004). There are 3 types of viral-associated particles of HBV found in serum: the virion, spherical subviral particles, and filamentous subviral particles. While the HBV virion is infectious, both spherical particles and filaments are devoid of the HBV genome (Warner and Locarnini, 2012). The HBV virion has a diameter of 42 to 47 nm with an outer envelope consisting of three envelope proteins surrounding the nucleocapsid containing the hepatitis B core protein. According to the Centers for Disease Control and Prevention (CDC), in 2020, around 2,157 cases of acute hepatitis B were reported with an overall incidence rate of 0.7 cases per 100,000 population (2020 Viral Hepatitis Surveillance Report | CDC, n.d.). World Health Organization (WHO) estimated that around 296 million people worldwide are infected with HBV in 2019 regardless of having an effective vaccine (WHO, 2021). HBV virus mainly invades the liver cells (hepatocytes) and relies on the machinery of cells to replicate within it. HBV virion binds to the hepatocytes with the help of preS domain of the viral surface antigen. The virus is then taken by the cells through the endocytosis process. Following infection, the relaxed circular DNA gets converted inside the nucleus of host cells into a plasmid-like covalently closed circular DNA (cccDNA). The cccDNA further acts as a template for viral mRNAs transcription. The formation of cccDNA is an indicator of successful initiation of HBV infection (Seeger and Mason, 2000). HBV is more infectious (5–10 times) and stable than HCV. Further, chronic hepatitis B patients are at higher risk of dying from liver-related complications compared to those infected with hepatitis C.
The genomic RNA of HCV is single-stranded with a positive polarity, which is enclosed by a lipid bilayer containing two viral glycoproteins (E1 and E2) to form the virion. HCV is categorized as the most widespread blood-borne disease in the United States with an estimated 2.7–3.9 million people having chronic HCV infection. WHO estimated that around 58 million people worldwide are infected with HCV in 2019 (WHO, 2021). No vaccine is currently available for HCV treatment. This is partly a reason why hepatitis C attracts more attention and research funding than hepatitis. HCV infection is considered a leading cause of liver complications such as cirrhosis, liver failure, and hepatocellular cancer (Li and Lo, 2015). Immunizations against hepatitis A and B viruses are available, but not for HCV. The lifecycle of HCV involves receptor attachment, endocytosis, uncoating, translation, polyprotein processing, RNA replication, virion assembly, maturation and release. The symptoms associated with HCV include scarring, dry eyes and mouth, peripheral neuropathy with leg numbness/weakness, seizures, cirrhosis and liver cancer, and in advanced cases may lead to death. Various extrahepatic manifestations such as insulin resistance, type 2 diabetes mellitus, glomerulopathies and oral manifestations are associated with HCV infections (Hayes et al., 2022; Mack et al., 2012; Salama et al., 2022; Songtanin and Nugent, 2023). The cellular receptors such as a high-density lipoprotein receptor, scavenger receptor class B type I, tetraspanin CD81, tight junction protein claudin-1, and occludin are responsible for initiating the attachment step of HCV infection. HCV replication involves cyclophilin A and microRNA-122. The presence of HCV in the blood and blood products is considered the main source of infection. HCV transmission routes might be different depending on the circumstances, such as blood transfusion of unscreened products, the transfusion of clotting factors or other blood products, sexual transmission, organ transplantation, reuse of medical instruments, hemodialysis, endoscopy and intravenous injections (Parsons, 2022; Sassi et al., 2007). The high prevalence of HCV infection occurs in males, the middle age population, low to middle socioeconomic status and those residing in rural areas. Dental treatment and therapeutic injections with reusable syringes/needles are also the most common sources for the spread of HCV infections (Mahajan et al., 2018). Apart from HBV and HCV monoinfections, HBV-HCV coinfection is considered more complex and could be transmitted through blood transfusion, intravenous drug use, and vertical transmission. Superinfection is the most common mechanism of development of HBV-HCV coinfection. Furthermore, HCV superinfection is observed more frequently as compared to HBV superinfection (Liu and Hou, 2006; Mavilia and Wu, 2018). Treatment in coinfections is far more complex due to the potential for reactivation of either virus with antiviral therapy directed against a single virus. Pegylated interferon alfa-2a based therapy has shown some antiviral activity against both HBV and HCV. The readers can refer to a recent publication by Mavilia et al. (Liu and Hou, 2006; Mavilia and Wu, 2018). for more information on monoinfection and coinfection with HBV and HCV along with disease management.
The complexity of HCV has generated a great deal of interest in developing new pharmacological therapies to control the disease. A few drugs have been approved by the United States Food and Drug Administration (FDA) that are either direct-acting antivirals (DAAs) to target a specific viral protein or target cellular processes that are essential for the replication of one of several viruses. For instance, the replication of HCV is catalyzed by the NS5B protein. DAAs are found to be effective in inhibiting NS5B RNA-dependent RNA polymerase, while the nucleotide analogue, sofosbuvir, specifically inhibits RNA polymerase enzyme, leading to the inhibition of HCV replication (Alves et al., 2013). Medications available for viral infections also include acyclovir, ganciclovir, famciclovir, ledipasvir, daclatasvir and valacyclovir (Bryan-Marrugo et al., 2015; Mohamed et al., 2009). Pegylated interferon with ribavirin is reported to be the standard drug of choice for HCV infections. Boceprevir and telaprevir as nonstructural 3/4A protease inhibitors of shorter duration of therapy are FDA-approved choice drugs for HCV infections. Double and triple therapies to attain a sustained virological response are also being studied for HCV infections. The treatment challenges for HCV infections involve drug resistance, suboptimal activity against certain HCV genotypes, and high cost of therapy (Hayes et al., 2022; Liu and Kao, 2023; Timm and Roggendorf, 2007).
With advancements in the drug discovery process, several antiviral drugs have been identified in recent years and many drug molecules are currently undergoing clinical trials. The landscape of approved antiviral drugs is quite dynamic. On the one hand, new drug molecules with shorter treatment durations are entering the market, but on the other hand less competitive antivirals are losing popularity and are discontinued. An up-to-date comprehensive analysis is required to examine the current state of antiviral drug discovery and understand the new trends in antiviral approaches. This review aims to highlight the FDA-approved antiviral medications and the small molecule antiviral agents in clinical development to treat HBV and HCV. Antivirals were categorized based on the mechanisms of action and promising (yet to be approved) therapeutic approaches for treating HBV and HCV are discussed. The information on emerging therapeutics and current trends in the development of small molecule antiviral agents against HBV and HCV, was gathered from online scientific databases and clinicaltrials.gov website. (“Clinical Research and Drug Information | CenterWatch,” n.d., “Home - AdisInsight,” n.d., “Home - ClinicalTrials.gov,” n.d.).
2 Treatment approaches and FDA-approved antiviral agents against HBV and HCV infections
The development of effective antiviral strategies has become a major public health concern due to the high emergence of hepatitis. The main goal of ongoing therapeutic research for chronic HBV and HCV is to achieve a functional cure after completion of a defined course of therapy. Therapeutic modalities used for the treatment of HCV and HBV are under development. They are broadly classified in two therapeutic classes: (i) biologics and (ii) small molecules. These agents usually target complex molecular interactions between virus and host cells involved in viral replication. Treatment modalities with biologicals are further classified into different subclasses i.e. peptides, nucleic acid polymers, small interfering RNA (siRNA), antisense oligonucleotides, monoclonal antibodies, and vaccines. Since the early discovery of interferons, different treatment modalities to treat HBV and HCV have been approved by FDA. In this section, FDA-approved monotherapies and combination therapies for the treatment of HBV and HCV are listed (Fig. 1). The information on FDA-approved antiviral agents targeting HBV and HCV was obtained from the FDA drug database (Table 1). Furthermore, structures of small molecule antivirals against HBV and HCV are shown in Fig. 2. and Fig. 3. (Drugs@FDA: FDA-Approved Drugs, n.d.; Fanning et al., 2019; Lee and Banini, 2019; Westin et al., 2020; Zając et al., 2019). In terms of treatment, HBV mono-infection is treated using a nucleotide analogue of an antiviral such as lamivudine, entecavir, or tenofovir. Along with them, Pegylated interferon is also used in HBV patients. Drugs like lamivudine has been incorporated inside the single walled carbon nanotubes (SWCNTs) by using first-principles van der Waals density functional (vdW-DF) calculations (Rezvani et al., 2013). When it comes to HCV patients, they are generally treated with DAAs such as elbasvir, grazoprevir, sofosbuvir, and velpatasvir. For HBV-HCV co-infection treatment, the predominance of one virus over another, the presence of liver cirrhosis, hepatocellular carcinoma (HCC), and/or HIV infection and comorbidities in coinfected patients emphasize the importance of best choice for the treatment. In most virological conditions, Pegylated interferon therapy is shown to be effective in suppressing the viral load within patients. The antiviral regime is usually selected based on the virus predominance, viremia levels and serological indexes, which include treatment with interferon and ribavirin, interferon plus lamivudine, adefovir plus entecavir, and liver transplantation. Furthermore, DAA regimens have become the treatment of choice even in HBV-HCV coinfection with a predominance of HCV as DAAs are found to be effective and well tolerated in around 95% of coinfection cases (Abdelaal et al., 2019; Mavilia and Wu, 2018; Sagnelli et al., 2017; Torres Ibarra, 2006; Zarębska-Michaluk et al., 2020).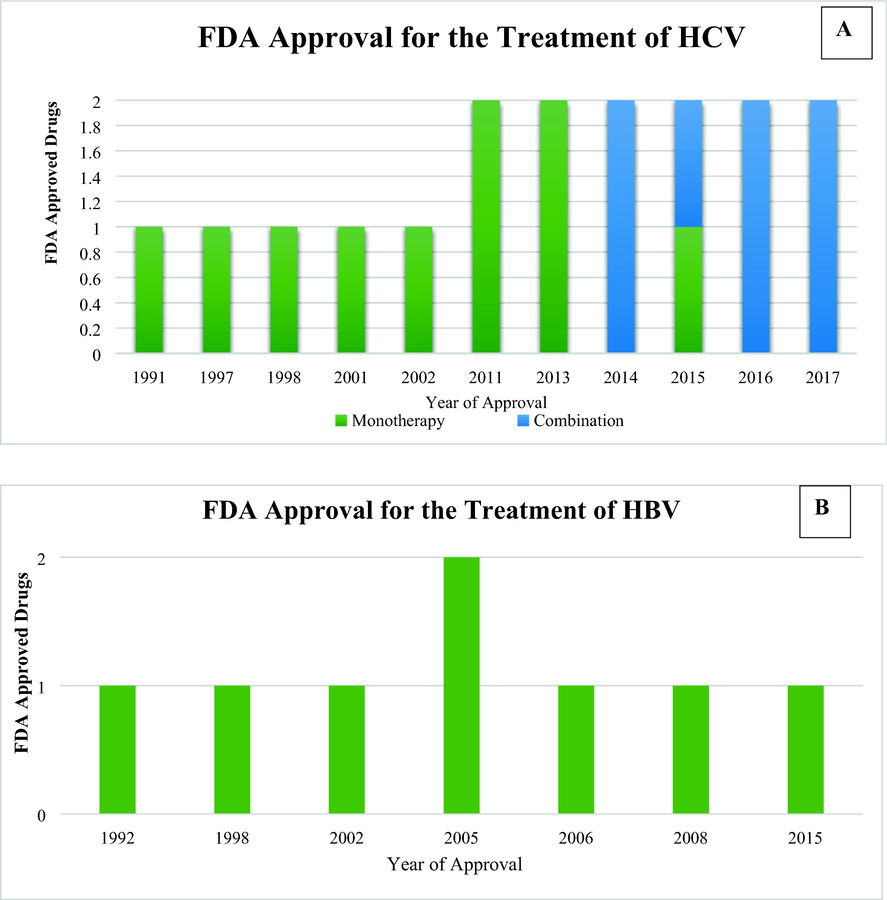
FDA-approved monotherapies and combination regimens for the treatment of HCV (A) and HBV (B).
Virus
Year of Approval
Name of Drug
Trade Name
Target
Therapy
HCV1991
Interferon alfa-2b
Intron A
Host
Monotherapy
1997
Interferon alfacon-1
Infergen
Host
Monotherapy
1998
Ribavirin
Rebetol
Host
Monotherapy
2001
Pegylated interferon alfa-2b
Pegintron/
SylatronHost
Monotherapy
2002
Pegylated interferon alfa-2a
Pegasys
Host
Monotherapy
2011
Boceprevir*
Victrelis
NS3/4A protease
Monotherapy
2011
Telaprevir*
Incivek
NS3/4A protease
Monotherapy
2013
Simeprevir*
Olysio
NS3/4A protease
Monotherapy
2013
Sofosbuvir
Sovaldi
NS5B polymerase
Monotherapy
2014
Ledipasvir/Sofosbuvir
Harvoni
NS5A/NS5B polymerase
Combination
2014
Dasabuvir/Ombitasvir/Paritaprevir /Ritonavir*
Viekira Pak
NS5B polymerase/NS5A/NS3/4A protease/Protease
Combination
2015
Daclatasvir*
Daklinza
NS5A
Monotherapy
2015
Ombitasvir/Paritaprevir /Ritonavir*
Technivie
NS5A/NS3/4A protease/Protease
Combination
2016
Elbasvir/Grazoprevir
Zepatier
NS5A/NS3/4A protease
Combination
2016
Sofosbuvir/Velpatasvir
Epclusa
NS5B Polymerase/NS5A
Combination
2017
Sofosbuvir/Velpatasvir/Voxilaprevir
Vosevi
NS5B Polymerase/NS5A
/NS3/4A proteaseCombination
2017
Glecaprevir/Pibrentasvir
Mavyret
NS3/4A protease/NS5A
Combination
HBV
1992
Interferon alfa-2b
Intron A
Host
Monotherapy
1998
Lamivudine
Epivir-HBV
Polymerase
Monotherapy
2002
Adefovir dipivoxil
Hepsera
Polymerase
Monotherapy
2005
Entecavir
Baraclude
Polymerase
Monotherapy
2005
Pegylated interferon alfa-2a
Pegasys
Host
Monotherapy
2006
Telbivudine*
Tyzeka
Polymerase
Monotherapy
2008
Tenofovir disoproxil
Viread
Polymerase
Monotherapy
2016
Tenofovir alafenamide
Vemlidy
Polymerase
Monotherapy
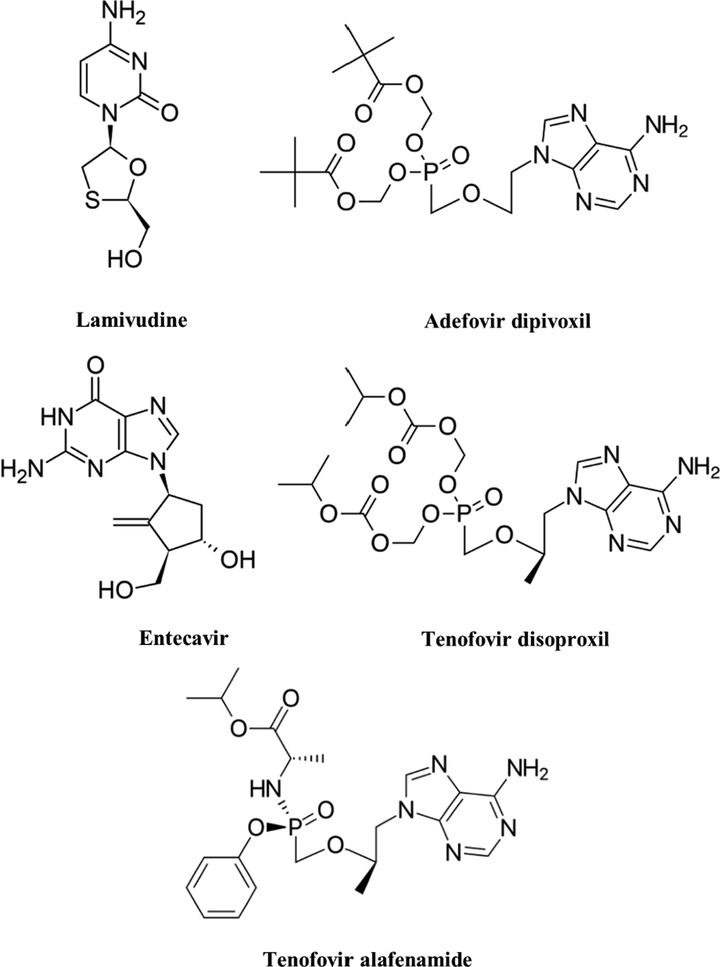
Chemical structures of FDA approved agents for the treatment of HBV.
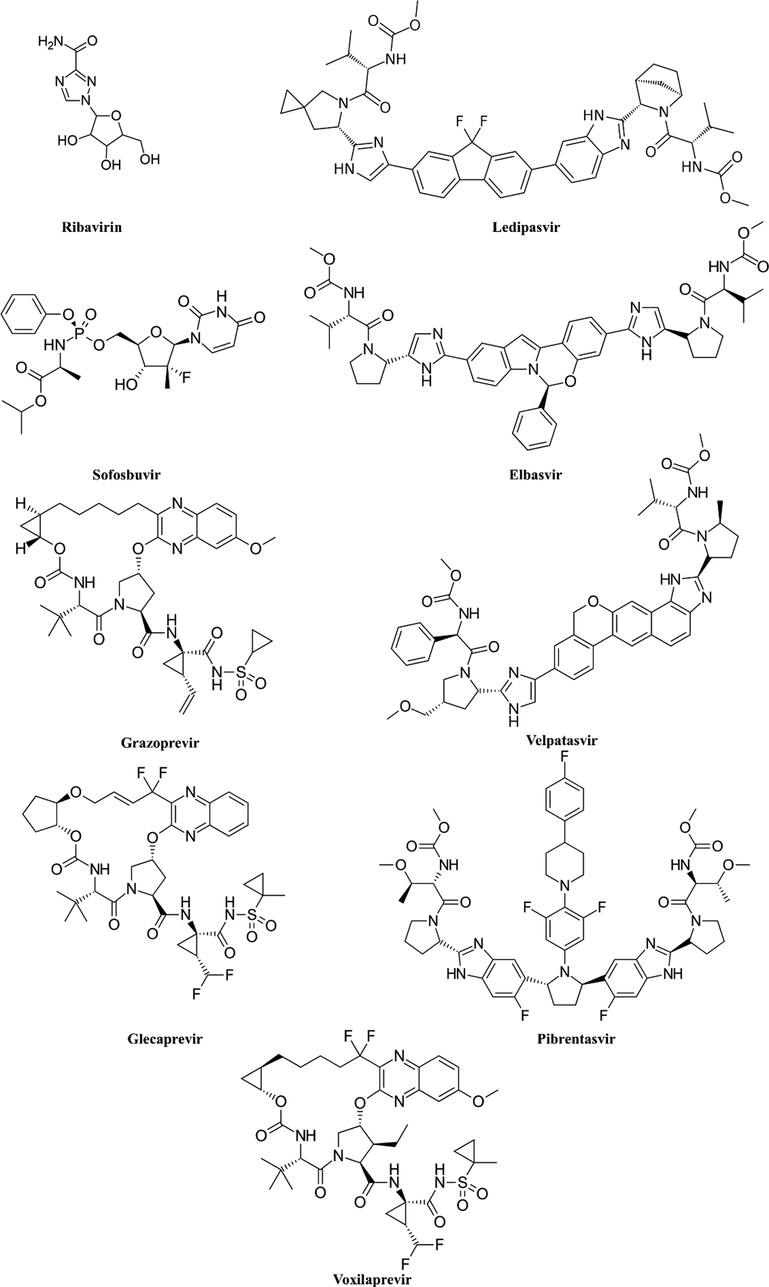
Chemical structures of FDA approved agents for the treatment of HCV.
3 Antiviral drugs undergoing clinical trials for the treatment of HCB and HCV infections
Every step in the lifecycle of HCV such as receptor binding of virus and endocytosis, fusion and uncoating, translation and polyprotein processing, RNA replication, virion assembly and virion release, can be considered as targets in the development of new anti-HCV drugs. Predominantly drugs targeting two major steps of lifecycle have reached clinical development. These include (a) blocking of viral replication through inhibition of NS5A viral protein, and (b) inhibitors of the HCV NS3/4A protease that block polyprotein processing (Parlati et al., 2021). The treatment for chronic HBV infection mainly suppresses replication without eliminating the virus due to the persistence of its cccDNA. With the broader understanding of the HBV life cycle, new antiviral approaches focusing on immune modulation, interference with viral life cycle and spread, and cccDNA targeted therapy are underway. Table 2 summarizes the list of drugs that are in clinical trials (Fanning et al., 2019).
Compound
Developer
Mode of Action
Phase of Development
Monotherapy/
Combination
Development status
Indications
Clinical trial Identifier
Ravidasvir/PPI-668
Presidio Pharmaceuticals; Pharco International Pharmaceutical Company
NS5A inhibitor
Phase II/III
+ Sofosbuvir
Recruiting
Non-cirrhotic chronic hepatitis C
NCT04885855
Phase II/III
+ Sofosbuvir
Active, not recruiting
Chronic hepatitis C
NCT02961426
DAG181/
YimitasvirDongguan HEC TaiGen Biopharmaceuticals;
HEC PharmNS5A inhibitor
Phase III
+ Sofosbuvir
Completed
Chronic hepatitis C
NCT03487107
Furaprevir/
TG-2349Dongguan HEC TaiGen Biopharmaceuticals; TaiGen Biotechnology
NS3/4A inhibitor
Phase III
+ DAG 181
+ RibavirinCompleted
Chronic hepatics C genotype 1
NCT04155515
Kangdaprevir/
HEC84048NaSunshine Lake Pharma
NS3/4A inhibitor
Phase I
–
Completed
Chronic
hepatitis CNCT03811678
SH229/ Holybuvir
Nanjing Sanhome Pharmaceutical, Co. Ltd.
NS5B polymerase inhibitor
Phase II/III
+ Daclatasvir
Unknown
Chronic hepatitis C
NCT04070235
Selgantolimod
Gilead Sciences
Toll-like receptor 8 agonist
Phase II
Combination therapies
RecruitingChronic hepatitis B
NCT04891770
Phase II
–
Not yet recruiting
Chronic hepatitis B and HIV
NCT05551273
AL-034; JNJ-4964; JNJ-64794964; TQ-A3334
Alios BioPharma; Chia Tai Tianqing Pharmaceutical Group
Toll-like receptor 7 agonist
Phase I
–
Completed
Hepatitis B
NCT03285620
HRS9950
Jiangsu Hengrui Medicine Co.
Toll-like receptor 8 agonist
Phase I
–
Unknown
Chronic hepatitis B
NCT04464733
RO7020531; RG 7854
Roche
Toll-like receptor 7 agonist
Phase II
Combination therapies
Recruiting
Chronic hepatitis B
NCT04225715
Vonafexor
EYP001aEnyo Pharma
Farnesoid X-activated receptor agonist
Phase II
+ Pegylated interferon alpha-2a
± EntecavirCompleted
Chronic hepatitis B
NCT04365933
ASC 42
Ascletis Pharma Inc
Farnesoid X-activated receptor agonist
Phase II
+ Entecavir and Pegylated interferon alpha-2a
Recruiting
Chronic hepatitis B
NCT05107778
GLS-4;
MorphothiadinSunshine Lake Pharma Co. Ltd.
Capsid assembly modulator
Phase II
+ Ritonavir and Entecavir vs (in Comparison) Entecavir
Unknown
Chronic hepatitis B
NCT04147208
RO7049389; RG 7907
Hoffmann-La Roche
Capsid assembly modulator
Phase I
± Midazolam
Completed
Chronic hepatitis B
NCT02952924
Phase II
Multiple combination therapies
Recruiting
Chronic hepatitis B
NCT04225715
QL-007
Qilu Pharmaceutical
Capsid assembly modulator
Phase II
+ Entecavir or Tenofovir
Unknown
Chronic hepatitis B who has received nucleoside (Acid) tsherapy
NCT04157699
Phase II
+ Tenofovir
Unknown
Chronic
Hepatitis BNCT04157257
EDP-514
Enanta Pharmaceuticals
Capsid assembly modulator
Phase I
–
Completed
Chronic hepatitis B
NCT04470388
ALG-000184
Aligos Therapeutics
Capsid assembly modulator
Phase I
–
Recruiting
Chronic hepatitis B
NCT04536337
GST-HG141
Fujian Cosunter Pharmaceutical
Capsid assembly modulator
Phase I
–
Completed
Chronic hepatitis B
NCT04868981
HRS5091
Jiangsu Hengrui Medicine Co.
Capsid assembly modulator
Phase I
–
Active, not recruiting
Chronic
hepatitis BNCT04480294
AB-836
Baruch S. Blumberg Institute; Drexel University College of Medicine; Enantigen Therapeutics; Arbutus Biopharma
Capsid protein inhibitor; Hepatitis B virus replication inhibitor
Phase I
–
Recruiting
Chronic
hepatitis BNCT04775797
Nitazoxanide
Romark Laboratories; Chugai Pharmaceutical
EIF-2 kinase modulator; HN protein inhibitor; Oxidoreductase inhibitor; Receptor protein-tyrosine kinase modulator
Phase II
–
Active, not recruiting
Hepatitis B
NCT03905655
JNJ-56136379;
JNJ-6379; BersacapavirJanssen Sciences Ireland UC
Capsid assembly modulator
Phase II
+ JNJ-73763989, Nucleos(t)ide Analogs, and Pegylated interferon alpha-2a
Active, not recruiting
Chronic hepatitis B
NCT04667104
ZM-H1505R
Zhimeng Biopharma
Capsid assembly modulator
Phase I
–
Completed
Chronic hepatitis B
NCT05470829
Phase II
+ Entecavir
Not yet recruiting
Chronic hepatitis B
NCT05484466
JNJ-440
Alios BioPharma
Capsid assembly modulator
Phase I
–
Completed
Chronic hepatitis B
NCT03439488
Pradefovir
Ligand Pharmaceuticals;
Valeant Pharmaceuticals International; Xian Xintong Pharmaceutical ResearchNucleos(t)ide reverse transcriptase inhibitor
Phase III
–
Recruiting
Chronic hepatitis B
NCT04543565
HS-10234; Tenofovir amibufenamide
Jiangsu Hansoh Pharmaceutical
Nucleos(t)ide reverse transcriptase inhibitor
Phase III
–
Active, not recruiting
Hepatitis B
NCT03903796
ATI-2173
Emory University;
Antios TherapeuticsNucleos(t)ide reverse transcriptase inhibitor
Phase II
+ Tenofovir Disoproxil Fumarate
± AB-729Active, not recruiting
Chronic hepatitis B
NCT04847440
GST-HG131
Fujian Cosunter Pharmaceutical Co. Ltd.
Hepatitis B surface antigen expression inhibitor
Phase I
–
Unknown
Hepatitis B
NCT04499443
LP 128;
NWP-1080Newave Pharmaceuticals;
Guangzhou Lupeng Pharmaceutical Co. Ltd.Hepatitis B surface antigen expression inhibitor; Hepatitis B virus replication inhibitor
Phase I
–
Recruiting
Chronic
hepatitis BNCT05130567
CKD 388
Chong Kun Dang
–
Phase I
–
Not yet recruiting
Hepatitis B
NCT04676893
Phase I
–
Completed
Chronic hepatitis B
NCT05189288
PA1010
Zhejiang Palo Alto Pharmaceuticals
–
Phase I/II
–
Recruiting
Chronic
hepatitis BNCT05019040
DA-2803
Dong-A ST
–
Phase I
–
Completed
Hepatitis B
NCT04906109
APG-1387;
SM-1387Ascentage Pharma; Southern Medical University
Inhibitor of apoptosis
Phase II
+ Entecavir
Recruiting
Chronic hepatitis B
NCT04568265
HEC 121120
Sunshine Lake Pharma Co. Ltd.
–
Phase I/II
–
Recruiting
Chronic hepatitis B
NCT04536532
3.1 Nonstructural protein 5A (NS5A) inhibitors
The HCV-encoded NS5A is a zinc-binding multifunctional phosphoprotein having a pivotal role in viral RNA replication, for assembling of mature virion particles and complex interactions with cellular functions (Gitto et al., 2017; Ivanenkov et al., 2017; Macdonald and Harris, 2004). The NS5A has approximately 447 amino acids and its cytoplasmic moiety comprises three distinct domains separated by two relatively disordered segments. The N-terminal domain I possess a conserved zinc-binding motif and an amphipathic N-terminal helix, which are essential for viral RNA replication and membrane association, respectively. The domain I was confirmed to be an alternative dimeric form by crystallography. The domain II interacts with the host cell protein, cyclophilin A and stimulates the RNA binding ability of NS5A, which in turn enhances viral RNA replication. The C-terminal domain III regulates viral packaging and assembly. Domain II and domain III remain unfolded during an examination in isolation (Reghellin et al., 2014)(Hamatake et al., 2012)(Foster et al., 2011)(Belda and Targett-Adams, 2012)(Love et al., 2009). NS5A is expressed in phosphorylated (p56) and hyperphosphorylated (p58) forms. During in vivo and in vitro studies, it was found that NS5A directly interacts with RdRp (RNA-dependent RNA polymerase), and this interaction stimulates RdRp-catalyzed synthesis of the negative RNA strand (Quezada and Kane, 2009)(Pawlotsky, 2013). Although NS5A lacks any enzymatic activity, it plays a crucial role in viral packaging and assembly during the HCV life cycle. Hence, it is regarded as a potential target for antiviral therapy (Belda and Targett-Adams, 2012). To date, several small molecule NS5A inhibitors were developed possessing excellent antiviral potency with pan-genotypic activity. They are divided into two classes, namely first-generation and second-generation NS5A inhibitors. Daclatasvir is the first FDA-approved NS5A inhibitor followed by the discovery of ledipasvir and ombitasvir. However, these first generation NS5A inhibitors have broad genotype coverage with a low barrier to viral resistance. The development of second generation NS5A inhibitors exhibited improved potency against the resistant variants (Nakamoto et al., 2014). Yimitasvir (DAG181), a novel NS5A inhibitor displayed a favorable safety profile in Chinese healthy volunteers and HCV-infected patients during phase I and II studies. The combination of yimitasvir and sofosbuvir displayed 100% of sustained virologic response [HCV RNA less than the lower limit of quantification (LLOQ)] for 12 weeks (SVR12) (Zhang et al., 2018)(Guan et al., 2021). Furthermore, yimitasvir was investigated in combination with sofosbuvir in a phase III clinical investigation in patients having chronic genotype 1 HCV for 12 weeks (NCT03487107). The study investigated the safety, tolerability and efficacy in approximately 360 adult subjects with a dosage of 100 mg for yimitasvir and 400 mg for sofosbuvir, once daily for 12 weeks. This study concluded that 12 weeks of treatment with yimitasvir-sofosbuvir is highly effective and safe in patients with HCV genotype 1b infection without cirrhosis (Rao et al., 2020).
Ravidasvir, a pan-genotypic NS5A inhibitor was investigated in various phase II and phase III studies. Ravidasvir was investigated in combination with other anti-viral agents, including ritonavir, danoprevir, ribavirin and sofosbuvir (Esmat et al., 2018)(Kao et al., 2018)(Xu et al., 2019). It is approved for the treatment of genotype 1 hepatitis C treatment in Egypt (Gomaa et al., 2021). In phase II/III trials (STORM-C-1), ravidasvir in combination with sofosbuvir was investigated in patients having chronic hepatitis C infection with no or compensated cirrhosis for the duration of 12 or 24 weeks, respectively. In an interim analysis, this once daily combination (ravidasvir − 200 mg + sofosbuvir − 400 mg) regimen was found efficacious in patients with HCV genotype 1a, 1b or 3 infections. Furthermore, no deaths or discontinuation of treatment due to major adverse effects were observed during the study and the combination was found to be safe and effective during the HCV treatment in 97% of patients (Andrieux-Meyer et al., 2021). Recently in June 2021, Pharmangia registered ravidasvir with the Malaysian National Pharmaceutical Regulatory Agency for the treatment of Hepatitis C (Cheong et al., 2021).
3.2 Nonstructural 3/4A (NS3/4A) protease inhibitors
The NS3/4A serine protease is an essential non-structural protein that plays a critical role in the HCV replication cycle. The NS3 protein is a bifunctional macromolecule comprising of a N-terminal serine protease and a C-terminal RNA helicase. The binding of the NS4 peptide co-factor to NS3 facilitates polyprotein maturation (McCauley and Rudd, 2016)(Bakulin et al., 2014). The NS3/4A complex is accountable for the proteolytic cleavage between non-structural proteins at NS3/4A, NS4A/4B, NS4B/5A and NS5A/5B sites and is important for HCV replication and production of infectious viral particles. Thus, NS3/4A has regarded as a prime target of intervention for anti-HCV treatment (Rupp and Bartenschlager, 2014)(Bartenschlager et al., 2013)(Ejeh et al., 2021a). Based on binding mode analysis and structural characteristics, NS3/4A inhibitors are divided into three groups as follows; covalent (reversible) inhibitors, non-covalent inhibitors and macrocyclic inhibitors (Rupp and Bartenschlager, 2014)(Abuelizz et al., 2020). Kangdaprevir, a NS3/4A inhibitor was evaluated in a phase I trial to study the tolerability and pharmacokinetics in healthy volunteers (NCT03811678). In a phase III open-labelled 12-week treatment study, efficacy and safety of TG2349 (furaprevir) was assessed in combination with DAG181 and ribavirin in the patients suffering from HCV genotype 1 infection (NCT04155515).
3.3 Nonstructural protein 5B (NS5B) polymerase inhibitors
The HCV NS5B RdRp is a non-structural multifunctional RNA binding protein having an important role in virus replication via synthesis of the double-stranded RNA from a single-stranded genomic viral RNA, serving as a template (Ejeh et al., 2021b)(Shan et al., 2016). The NS5B is comprised of approximately 590 amino acids and its Gly317-Asp318-Asp319 (GDD) motif, among various common motifs of RdRp has furnished the first indication for its function. From three independent NS5B structure determination by crystallography, it was found that the NS5B has a shape like the right hand of a human with characteristic subdomains of palm, fingers and thumb. The HCV NS5B was reported to be crucial for RNA synthesis activity in a cooperative manner (Sofia et al., 2012)(Zając et al., 2019)(Moradpour et al., 2007)(Clemente-Casares et al., 2011). Thus, NS5B holds special importance for the treatment of HCV. NS5B inhibitors are categorized into two classes, which are nucleoside/nucleotide polymerase inhibitors and non-nucleoside polymerase inhibitors. Sofosbuvir, the first NS5B polymerase inhibitor, is a nucleotide polymerase approved in 2013. In a phase II/III trial, SH229 (holybuvir) was given in combination with daclatasvir dihydrochloride once daily to evaluate efficacy and safety in patients with chronic hepatitis C. The combination displayed high efficacy and safety in patients with HCV genotype 1, 2, 3 or 6 during a phase II open labelled 12-week treatment study (Hua et al., 2020).
3.4 Toll-like receptor agonists
Toll-like receptors belong to the family of pattern recognition receptors (PRRs), which recognize pathogen-associated molecular patterns (PAMPs) of invading microbial pathogens, thus initiating the innate immune responses (O’Neill et al., 2010)(Carty and Bowie, 2010)(Zhou et al., 2021). They are highly conserved type-I transmembrane protein receptors having molecular architecture, with an N-terminal ligand recognition domain, a single transmembrane-spanning helix, and a C-terminal cytoplasmic Toll/IL-1 receptor (TIR) domain that initiate downstream signaling pathway (Botos et al., 2011)(Kawasaki and Kawai, 2014). The family of TLRs is divided into two subclasses based on their localization i.e., cell surface TLRs (TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10) and intracellular TLRs (TLR3, TLR7, TLR8, TLR9, TLR11, TLR12, and TLR13). Among different TLRs, TLR1, TLR2, TLR3, TLR4, TLR7, TLR8 and TLR9 have been involved in responses to viral infection (Saeed and Piracha, 2016)(Carty and Bowie, 2010). Given the critical roles in numerous host immune defenses, including induction of an antiviral state through the production of type I interferons, TLRs hold special significance as targets for the treatment of viral diseases (Mifsud et al., 2014)(Horscroft et al., 2012)(Patel et al., 2014). Various TLR7, TLR8 and TLR9 agonists are under clinical investigation. Selgantolimod (GS-9688), a TLR-8 agonist is under clinical investigation for the treatment of hepatitis B. In a phase I investigation, it was found to be safe and well-tolerated in both viremic and virally suppressed chronic hepatitis B patients (Gane et al., 2021). Preliminary results from a phase II study of GS-9688 have exhibited a modest decline in hepatitis B surface antigen (HBsAg) and induced dose-dependent cytokine responses after 24 weeks of treatment in virally suppressed chronic Hepatitis B patients (Gane et al., 2020). It is currently in a phase II clinical trial as combination therapy for the treatment of chronic hepatitis B (NCT04891770). RO7020531 is a TLR7 agonist that has demonstrated safety and acceptable tolerability in healthy Chinese volunteers in a phase I clinical trial. RO7020531 displayed a dose-dependent increase in biomarkers of TLR7 pathway activation at doses of 100 mg and higher (Luk et al., 2020). Furthermore, it is currently being assessed in a phase II investigation that evaluates the safety and efficacy of new molecular entity multiple combination therapies in patients with chronic hepatitis B (NCT04225715). In a Phase I clinical trial, AL-034 (JNJ-64794964), a TLR7 agonist was found to be safe and well tolerated at single oral doses of 0.2 to 1.8 mg in healthy adults. It induced cytokines/IFN-stimulated genes (ISGs) and displayed dose-proportional PK (Gane et al., 2019). HRS9950 is a TLR8 agonist with the potential to stimulate adaptive immunity response and treat chronic hepatitis B. It is currently in a phase I evaluation to assess safety, tolerability, PK, PD and food effect in healthy volunteers (NCT04464733) (Lin and Li, 2021; Pipeline-Jiangsu Hengrui Pharmaceuticals Co., Ltd., n.d.).
3.5 Farnesoid X receptor (FXR) agonists
FXR belongs to the superfamily of nuclear receptors and is highly expressed in the intestine, kidney, liver and adipose tissues. Bile acids are endogenous ligands for FXR and are responsible for its activation. FXR is involved in cholesterol, bile acid and glucose homeostasis (Fiorucci et al., 2007; Jiao et al., 2015; Li et al., 2020). There are two known genes of FXR in mammals, namely FXRα and FXRβ (Jiang et al., 2021). Furthermore, FXR plays a crucial role in the replication and transcription of hepatitis B virus via viral gene regulation. The proviral activities of FXRα include the involvement of viral covalently closed circular DNA (cccDNA) formation and maintenance during the early stage of the replication cycle. HBV replication is altered by the modulation of FXR activity by ligands. During in vivo and in vitro studies, it was found that treatment with FXR agonist inhibited the proviral effect on cccDNA and hepatitis B viral X protein (HBx) dependent pregenomic and precore RNA transcription and viral DNA secretion (Niu et al., 2011)(Mouzannar et al., 2019). In a phase Ib double-blinded, placebo-controlled study, EYP001a (Vonafexor), a FXR agonist was evaluated as a monotherapy or in combination with pegylated interferon alpha-2a. In EYP001a monotherapy, a significant decline of hepatitis B core-related antigen (HBcrAg) was observed at a 400 mg/day dose, whereas a significant decrease in pregenomic RNA (pgRNA) and HBcrAg was observed when given in combination with pegylated interferon alpha-2a (Fig. 4). Pruritus was the most common adverse event observed with twice-daily compared with once-daily regimens (Erken et al., 2021). It is currently being evaluated in a phase II clinical trial in combination with pegylated interferon alpha-2a alone and with entecavir for safety and anti-viral efficacy in patients with chronic hepatitis B (NCT04365933).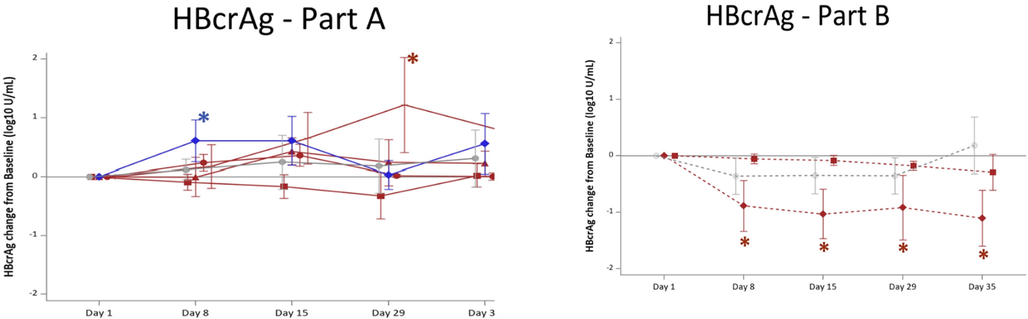
Change in circulating HBcrAg in patients after a 4-week Vonafexor treatment. Mean changes (SD) from day 1 baseline to day 35 of HBcrAg (log10 U/mL). Groups were treated with Vonafexor (red lines), placebo (grey, straight line, circle), or entecavir (blue, straight line, rhombus). Vonafexor 100 mg once daily (straight line square); Vonafexor 200 mg once daily (straight line, triangle); Vonafexor 400 mg once daily (straight line, circle); Vonafexor 200 mg twice daily (straight line, cross); Vonafexor 300 mg daily combined with pegylated interferon alpha-2a (dotted line, rhombus); Vonafexor 150 mg twice daily combined with pegylated-interferon alpha-2a (dotted line, square); placebo with pegylated interferon alpha-2a (dotted line, circle). HBcrAg, hepatitis B core related antigen. Significance: * indicates p < 0.05 for change from baseline. # indicates p < 0.05 vs. placebo. o indicates p < 0.05 for change from baseline and vs. placebo. Reproduced with permission from Erken et al (Erken et al., 2021).
In another phase IIa double-blinded, placebo-controlled investigation, patients with chronic hepatitis B were randomized to receive EYP001a or placebo, combined with entecavir and tenofovir disoproxil. This study is under evaluation to determine the safety and anti-viral effect of EYP001a in combination with entecavir and tenofovir disoproxil (NCT04465916). ASC42 is an investigational molecule with FXR agonist activity (“Ascletis Announces Dosing of the First Cohort of Healthy Subjects in the FXR Agonist ASC42 Bridging Study for Chronic Hepatitis B Indication in China | BioSpace,” n.d.). Ascletis Pharmaceuticals has registered for phase II clinical investigation of ASC42 or placebo in combination with entecavir and pegylated interferon alpha-2a. It is a single blinded, placebo-controlled investigation to assess safety and efficacy of ASC42 in patients with chronic hepatitis B (NCT05107778).
3.6 Capsid assembly modulators
The HBV core protein is a polypeptide of 183 amino acids with a globular N-terminal assembly domain (1–149 amino acid residues) and a nucleic acid binding arginine rich C-terminal domain (150–183 amino acid residues) (Schlicksup et al., 2018). The assembly of pre-genomic RNA and viral DNA polymerase into HBV nucleocapsid is the most characterized function of the core protein (Guo et al., 2017). Furthermore, the HBV core protein has a pleiotropic role in the HBV life cycle, including formation and secretion of the virion, transport and regulation of viral reverse transcription, amplification and maintenance of cccDNA and interaction with HBx protein (Mak et al., 2017)(Ligat et al., 2020)(Zlotnick et al., 2015). Hence, HBV core protein is regarded as a promising target for the treatment of HBV. Drugs acting on the core protein of HBV, known as capsid assembly modulators (CAMs) or core protein allosteric modulators are subdivided into class-I and class-II compounds. Class-I compounds (heteroaryldihydropyrimidines) produce aberrantly assembled nucleocapsids by misdirecting capsid assembly, while class-II compounds (e.g., phenylpropenamides) produce nucleic acid-free empty capsids, thereby preventing encapsidation of pregenomic RNA (pgRNA) (Yan et al., 2019). Several class I CAMs include BAY 41–4109, HAP-R10, GLS4 (morphothiadin) and RO7049389 (RG7907) and class II CAMs include AT-130, AB-423, NVR 3–778, BSBI-62083, GLP-26, JNJ-56136379 (bersacapavir) and ABI-H0731 (vebicorvir) (Kim et al., 2021). GLS4 (morphothiadin) is currently in a phase II clinical investigation to assess its antiviral efficacy in combination with ritonavir and entecavir compared with entecavir alone in patients with chronic HBV with HBeAg positive (NCT04147208). The results obtained from an interim analysis at the 12th week of the treatment showed that the combination therapy displayed superior antiviral efficacy as compared to entecavir monotherapy. The alanine aminotransferase activity (ALT) normalization rate after 12 months was 85.7% and 100% for the monotherapy and combination therapy groups, respectively with a P value of 0.0019. Similarly, the interferon-gamma secretion by HBsAg showed an increasing trend of combination therapy compared to mono-treatment (Fig. 5) (Yeh et al., 2020).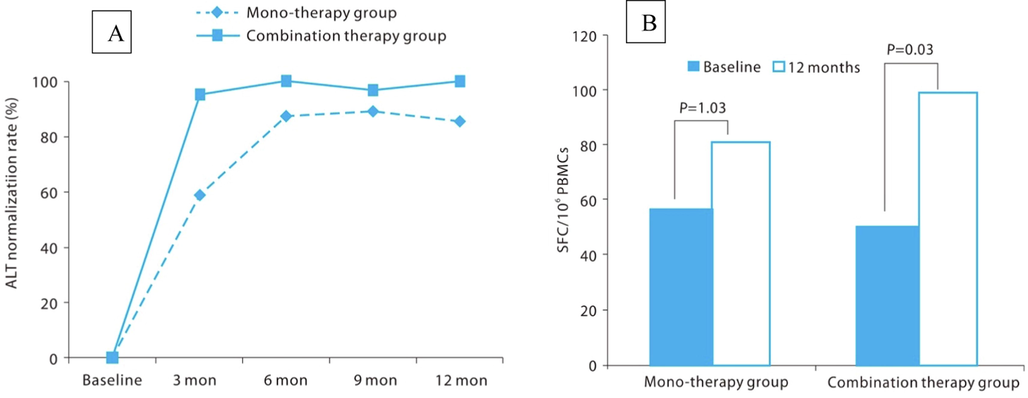
(A) ALT normalization rate during follow up period. Serum ALT normalization rates after 3, 6, 9-month dosing were statistically significantly higher in the combination therapy group than mono-therapy group. ALT, alanine aminotransferase activity, (B) Changes of interferon-gamma during treatment period. Lymphocyte interferon-gamma secretion activity was increase in both combination and mono-therapy group. Combination therapy group showed a significant increase in the 12-month treatment compared to pretreatment. Reproduced with permission from Jun et al (Jun et al., 2013).
In a phase I clinical trial RO7049389 demonstrated safety and good tolerability with a daily dose ranging from 200 mg to 1000 mg in 28 days of treatment. It displayed a substantial decline in HBV RNA and HBV DNA in both HBeAg-negative and HBeAg-positive patients (Yuen et al., 2021). Furthermore, a phase II investigation (Piranga) evaluating the antiviral efficacy and safety of RO7049489 in multiple combinations with nucleos(t)ide (NUC), TLR7 agonist (RO7020531), siRNA (RO7445482) and pegylated interferon is currently underway (NCT04225715). QL-007 is another CAM and is currently undergoing a phase II clinical investigation in combination with tenofovir in naïve patients with chronic hepatitis B (NCT04157699). Furthermore, another phase II clinical trial is underway to evaluate the safety and efficacy of QL-007 in combination with entecavir or tenofovir for the treatment of chronic hepatitis B patients who have received nucleoside therapy (NCT04157257). EDP-514, a-class-II CAM modulator was evaluated in a phase I trial to assess safety, tolerability, pharmacokinetics and antiviral effect in viremic chronic hepatitis B patients not currently on treatment (NCT04470388). Furthermore, it was also evaluated in a randomized, double-blinded, placebo-controlled phase I study to evaluate tolerability, safety, and pharmacokinetics of single and multiple ascending doses in healthy subjects and antiviral efficacy in nucleos(t)ide reverse transcriptase inhibitor (nuc)-suppressed chronic hepatitis B patients (NCT04008004). After 28 days, EDP-514 displayed the best antiviral effect in chronic hepatitis B patients (Enanta Pharmaceuticals Reports Positive Data from Phase 1b Study of EDP-514, a Hepatitis B Virus (HBV) Core Inhibitor, in Viremic Chronic HBV Patients | Business Wire, n.d.). ALG-000184 is a class II CAM and is currently being investigated in a phase I trial to assess safety, tolerability, pharmacokinetics and pharmacodynamics in healthy volunteers and chronic hepatitis B patients (NCT04536337) (Zhang et al., 2020). Another CAM, GST-HG141 displayed good tolerability, safety and pharmacokinetic profiles in a double-blinded, randomized, placebo-controlled single-ascending-dose and a multiple-ascending-dose a phase I study in healthy Chinese subjects (C. Li et al., 2021). HRS5091 and AB836 are capsid inhibitors and are currently being evaluated in phase I clinical trials (Pipeline-Jiangsu Hengrui Pharmaceuticals Co., Ltd., n.d.)(AB-836 (Capsid Inhibitor) | Pipeline | Arbutus Biopharma, n.d.). Furthermore, ZM-H1505R and JNJ-440 were evaluated in phase I clinical investigations (NCT04220801, NCT03439488).
3.7 Hepatitis B virus surface antigen (HBsAg) inhibitors
HBsAgs are different sized (small, medium, and large) multi-transmembrane envelope proteins encoded by the S genome (Jia et al., 2015)(Kiruthika et al., 2021). A high level of HBsAg is one of the classic hallmarks of HBV infection and the serum level of HBsAg may reach to 400 μg/mL in patients with chronic infection (Mohebbi et al., 2018). The high level of sub-viral particles (SVPs) bearing HBsAg plays a crucial role in suppressing HBV-specific immune response by directly modulating the functions of monocytes, dendritic cells and natural killer cells (Yu et al., 2011). Therefore, reducing the elevated level of HBsAg is regarded as a promising strategy to restore the exhausted immune response induced by SVPs (Schluep et al., 2017). GST-HG131, a novel HBsAg inhibitor, is currently recruiting subjects for a phase I clinical investigation (NCT04499443) (“Guangshengtang hepatitis B treatment of the global innovative drug HBsAg inhibitor GST-HG131 was approved by the Clinical Trial Ethics Committee.,” n.d.). Another HBsAg inhibitor, LP-128 is being evaluated in a phase I clinical trial (NCT05130567) (Pipelines - Lupeng, n.d.).
3.8 Nucleos(t)ide reverse transcriptase inhibitors
The HBV genome is replicated by a specialized viral polymerase, reverse transcriptase (Clark and Hu, 2015). It is a multifunctional protein possessing RNA- and DNA-dependent DNA polymerase functions that play a pivotal role in viral replication (Fung et al., 2011). Nucleos(t)ide reverse transcriptase inhibitors (NRTIs) act by inhibiting the HBV polymerase activity and thus result in decreased viral DNA synthesis. However, prolonged anti-viral treatment with nucleos(t)ide analogues leads to viral resistance because of the emergence of NRTI-resistant HBV mutant strains (Papatheodoridis et al., 2002)(Higashi-Kuwata et al., 2021). In a phase 1B investigation, HS-10234 (a prodrug of tenofovir), was found to be safe, well-tolerated, and as effective as tenofovir disoproxil fumarate during a treatment course of 28 days (Zhang et al., 2021). HS-10234 is currently being evaluated in phase III trials in treatment-naïve and treatment-experienced patients with chronic HBV infection. The study is planned to compare the safety and efficacy of HS-10234 vs tenofovir disoproxil fumarate (NCT03903796). Pradefovir, a prodrug of adefovir, is currently being evaluated in a phase III clinical trial for safety and efficacy in HBeAg-positive or HBeAg-negative chronic hepatitis B patients in a treatment course of 144 weeks. The interim analysis of the study will be carried out after completion of 48 weeks (NCT04543565). ATI-2173, a prodrug of clevudine monophosphate, is under investigation in a phase II clinical trial. It is being evaluated in combination with tenofovir disoproxil fumarate and with/without AB-729 in patients with chronic HBV infection and patients with hepatitis D virus coinfection (NCT04847440).
4 Conclusion
Elimination of HBV and HCV infections are recognized as achievable targets. Implementation of programs such as HBV vaccination in babies and blood safety programs have significantly reduced HBV and HCV incidence and morbidity. Currently, prophylactic and/or therapeutic vaccines against HCV using different platforms, such as proteins, adenovirus, DNA, or modified vaccinia virus Ankara (MVA) are being developed. Several small molecules possessing excellent antiviral potency with pan-genotypic activity are also being developed. For example, the development of second-generation NS5A inhibitors exhibited improved potency against the resistant variants. DAAs are generally used to treat HCV infection in most patients. Clinical trials are being conducted on compounds that directly inhibit the actions of viral proteins or cell factors essential for viral replication. It is hoped that clinical trials of pangenotypical drugs with high genetic barriers will demonstrate their effectiveness against all HCV types. TLRs hold special significance as targets for the treatment of viral diseases. TLR-7 and TLR-8 agonists are under clinical investigation for the treatment of hepatitis B. Furthermore, HBsAg inhibitors, capsid assembly modulators, farnesoid X receptor agonists, nucleos(t)ide reverse transcriptase inhibitors also hold special importance as targets for HBV treatment with various agents in different phases of clinical trials.
Overall, antiviral strategies appear to target the inhibition of viral DNA polymerase for the treatment of DNA virus infections. Combination therapies are considered a potential hope to eradicate/control HCV. Though the ideal oral combination for universal HCV cure has not been found yet, one can sensibly expect substantial development in this direction over the next 5 years. It has been also observed that many drugs in clinical development are associated with specific side effects and raise issues related to drug–drug interactions. The improved understanding of the viral life cycle and its interaction with the liver microenvironment and host immune responses, together with the development of new study models will provide the right impetus for upfront research in this field. The better knowledge and dimension of the major clinical end points will provide guidance for the preclinical and early clinical evaluation of treatment fundamentals, which may translate into improved treatment outcomes in the future. Future technologies to rapidly detect, diagnose and treat HBV and HCV carriers could also achieve the obliteration of diseases.
Consent for publication: Authors give consent for information to be published in the Arabian Journal of Chemistry.
The authors would like to acknowledge Ajman University for their support towards Article Processing Charges.
Ethical Approval: Not applicable.
Consent to Participate: Not applicable.
Competing Interests: None.
Availability of data and materials: Not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 2020 Viral Hepatitis Surveillance Report | CDC [WWW Document], n.d. URL https://www.cdc.gov/hepatitis/statistics/0surveillance/index.htm (accessed 5.15.23).
- AB-836 (Capsid Inhibitor) | Pipeline | Arbutus Biopharma [WWW Document], n.d. URL http://www.arbutusbio.com/portfolio/capsid-inhibitors.php (accessed 2.1.22).
- HBV/HCV Coinfection in the Era of HCV-DAAs. Clin. Liver Dis.. 2019;23:463-472.
- [CrossRef] [Google Scholar]
- Investigation of some benzoquinazoline and quinazoline derivatives as novel inhibitors of HCV-NS3/4A protease: Biological, molecular docking and QSAR studies. RSC Adv.. 2020;10:35820-35830.
- [CrossRef] [Google Scholar]
- Dry eye disease caused by viral infection: review. Arq. Bras. Oftalmol.. 2013;76:129-132.
- [CrossRef] [Google Scholar]
- Efficacy and safety of ravidasvir plus sofosbuvir in patients with chronic hepatitis C infection without cirrhosis or with compensated cirrhosis (STORM-C-1): interim analysis of a two-stage, open-label, multicentre, single arm, phase 2/3 trial. Lancet Gastroenterol. Hepatol.. 2021;6:448-458.
- [CrossRef] [Google Scholar]
- Ascletis Announces Dosing of the First Cohort of Healthy Subjects in the FXR Agonist ASC42 Bridging Study for Chronic Hepatitis B Indication in China | BioSpace [WWW Document], n.d. URL https://www.biospace.com/article/releases/ascletis-announces-dosing-of-the-first-cohort-of-healthy-subjects-in-the-fxr-agonist-asc42-bridging-study-for-chronic-hepatitis-b-indication-in-china/ (accessed 1.9.22).
- NS3 protease inhibitors for treatment of chronic hepatitis C: Efficacy and safety. World J. Hepatol.. 2014;6:326-339.
- [CrossRef] [Google Scholar]
- The molecular and structural basis of advanced antiviral therapy for hepatitis C virus infection. Nat. Rev. Microbiol. 2013
- [CrossRef] [Google Scholar]
- Small molecule inhibitors of the hepatitis C virus-encoded NS5A protein. Virus Res.. 2012;170:1-14.
- [CrossRef] [Google Scholar]
- History and progress of antiviral drugs: From acyclovir to direct-acting antiviral agents (DAAs) for Hepatitis C. Med. Univ.. 2015;17:165-174.
- [CrossRef] [Google Scholar]
- Recent insights into the role of Toll-like receptors in viral infection. Clin. Exp. Immunol.. 2010;161:397-406.
- [CrossRef] [Google Scholar]
- Ravidasvir: equitable access through an alternative drug development pathway. Lancet Glob. Heal.. 2021;9:e1496-e1497.
- [CrossRef] [Google Scholar]
- Hepatitis B Virus Reverse Transcriptase – Target of Current Antiviral Therapy and Future Drug Development. Antiviral Res.. 2015;123:132.
- [CrossRef] [Google Scholar]
- De Novo polymerase activity and oligomerization of hepatitis C virus RNA-dependent RNA-polymerases from genotypes 1 to 5. PLoS One. 2011;6:e18515.
- [Google Scholar]
- Clinical Research and Drug Information | CenterWatch [WWW Document], n.d. URL https://www.centerwatch.com/ (accessed 1.4.23).
- Drugs@FDA: FDA-Approved Drugs [WWW Document], n.d. . FDA. URL https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021588%0Ahttps://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020905%0Ahttps://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?eve (accessed 1.13.23).
- Computer-aided identification of a series of novel ligands showing high potency as hepatitis C virus NS3/4A protease inhibitors. Bull. Natl. Res. Cent.. 2021;45:1-15.
- [CrossRef] [Google Scholar]
- Computational insight to design new potential hepatitis C virus NS5B polymerase inhibitors with drug-likeness and pharmacokinetic ADMET parameters predictions. Futur. J. Pharm. Sci.. 2021;7:1-13.
- [CrossRef] [Google Scholar]
- Enanta Pharmaceuticals Reports Positive Data from Phase 1b Study of EDP-514, a Hepatitis B Virus (HBV) Core Inhibitor, in Viremic Chronic HBV Patients | Business Wire [WWW Document], n.d. URL https://www.businesswire.com/news/home/20210622006023/en/Enanta-Pharmaceuticals-Reports-Positive-Data-from-Phase-1b-Study-of-EDP-514-a-Hepatitis-B-Virus-HBV-Core-Inhibitor-in-Viremic-Chronic-HBV-Patients (accessed 1.30.22).
- Farnesoid X receptor agonist for the treatment of chronic hepatitis B: A safety study. J. Viral Hepat.. 2021;28:1690-1698.
- [CrossRef] [Google Scholar]
- Effectiveness of ravidasvir plus sofosbuvir in interferon-naïve and treated patients with chronic hepatitis C genotype-4. J. Hepatol.. 2018;68:53-62.
- [CrossRef] [Google Scholar]
- Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat. Rev. Drug Discov. 2019
- [CrossRef] [Google Scholar]
- Targeting farnesoid X receptor for liver and metabolic disorders. Trends Mol. Med.. 2007;13:298-309.
- [CrossRef] [Google Scholar]
- Cyclophilin A Interacts with Domain II of Hepatitis C Virus NS5A and Stimulates RNA Binding in an Isomerase-Dependent Manner. J. Virol.. 2011;85:7460-7464.
- [CrossRef] [Google Scholar]
- Nucleoside/nucleotide analogues in the treatment of chronic hepatitis B. J. Antimicrob. Chemother.. 2011;66:2715-2725.
- [CrossRef] [Google Scholar]
- Safety, Pharmacokinetics, and Pharmacodynamics of the Oral TLR8 Agonist Selgantolimod in Chronic Hepatitis B. Hepatology. 2021;74:1737-1749.
- [CrossRef] [Google Scholar]
- FRI-198-A Phase, double-blind, randomized, placebo-controlled, first-in-human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of oral JNJ-64794964, a toll-like receptor-7 agonist, in healthy adults. J. Hepatol.. 2019;70:e478.
- [Google Scholar]
- Efficacy and safety of 24 weeks treatment with oral TLR8 agonist, selgantolimod, in virally-suppressed adult patients with chronic hepatitis B: a phase 2 study. J. Hepatol.. 2020;73:S52.
- [CrossRef] [Google Scholar]
- NS5A inhibitors for the treatment of hepatitis C infection. J. Viral Hepat.. 2017;24:180-186.
- [CrossRef] [Google Scholar]
- Ravidasvir hydrochloride for genotype 1 hepatitis C treatment. Drugs of Today. 2021;57:199-208.
- [CrossRef] [Google Scholar]
- Population Pharmacokinetic Analysis of Yimitasvir in Chinese Healthy Volunteers and Patients With Chronic Hepatitis C Virus Infection. Front. Pharmacol.. 2021;11:617122
- [CrossRef] [Google Scholar]
- Guangshengtang hepatitis B treatment of the global innovative drug HBsAg inhibitor GST-HG131 was approved by the Clinical Trial Ethics Committee. [WWW Document], n.d. URL https://topic.echemi.com/a/guangshengtang-hepatitis-b-treatment-of-the-global-innovative-drug-hbsag-inhibitor-gst-hg131-was-approved-by-the-clinical-trial-ethics-committee_93906.html (accessed 3.9.22).
- HBV core protein allosteric modulators differentially alter cccDNA biosynthesis from de novo infection and intracellular amplification pathways. PLOS Pathog.. 2017;13:e1006658.
- [Google Scholar]
- Hamatake, R., Maynard, A., Kazmierski, W.M., 2012. HCV Inhibition Mediated Through the Nonstructural Protein 5A (NS5A) Replication Complex, in: Annual Reports in Medicinal Chemistry. Elsevier Inc., pp. 331–345. https://doi.org/10.1016/B978-0-12-396492-2.00022-9.
- Road to elimination of HCV: Clinical challenges in HCV management. Liver Int.. 2022;42:1935-1944.
- [CrossRef] [Google Scholar]
- Higashi-Kuwata, N., Hayashi, S., Kumamoto, H., Ogata-Aoki, H., Das, D., Venzon, D., Hattori, S. ichiro, Bulut, H., Hashimoto, M., Otagiri, M., Takamune, N., Kishimoto, N., Davis, D.A., Misumi, S., Kakuni, M., Tanaka, Y., Mitsuya, H., 2021. Identification of a novel long-acting 4’-modified nucleoside reverse transcriptase inhibitor against HBV. J. Hepatol. 74, 1075–1086. https://doi.org/10.1016/J.JHEP.2020.12.006/ATTACHMENT/EB9554ED-8F20-4C86-A4E4-6289094775E9/MMC3.PDF.
- Home - AdisInsight [WWW Document], n.d. URL https://adisinsight.springer.com/ (accessed 1.11.23).
- Home - ClinicalTrials.gov [WWW Document], n.d. URL https://clinicaltrials.gov/ (accessed 12.30.22).
- Antiviral applications of Toll-like receptor agonists. J. Antimicrob. Chemother.. 2012;67:789-801.
- [CrossRef] [Google Scholar]
- A phase 2, open-label study of pan-genotype regimen of SH229 plus daclatasvir in Chinese patients with chronic hepatitis C virus infection, in. Journal of Hepatology. 2020:S342.
- [CrossRef] [Google Scholar]
- Small-molecule inhibitors of hepatitis C virus (HCV) non-structural protein 5A (NS5A): a patent review (2010–2015) Expert Opin. Ther. Pat.. 2017;27:401-414.
- [CrossRef] [Google Scholar]
- Hepatitis C and Ocular Surface Disease. Am. J. Ophthalmol.. 2007;144:705-711.e1.
- [CrossRef] [Google Scholar]
- Recent advance of the hepatitis B virus inhibitors: a medicinal chemistry overview. Future Med. Chem.. 2015;7:587-607.
- [Google Scholar]
- Farnesoid X receptor (FXR): Structures and ligands. Comput. Struct. Biotechnol. J.. 2021;19:2148-2159.
- [CrossRef] [Google Scholar]
- Farnesoid X receptor: A master regulator of hepatic triglyceride and glucose homeostasis. Acta Pharmacol. Sin.. 2015;36:44-50.
- [CrossRef] [Google Scholar]
- Efficacy and safety of entecavir plus carnitine complex (GODEX®) compared to entecavir monotherapy in patient with ALT elevated chronic hepatitis B: randomized, multicenter open-label trials. The GOAL study. Clin. Mol. Hepatol.. 2013;19:165-172.
- [CrossRef] [Google Scholar]
- Twelve-week ravidasvir plus ritonavir-boosted danoprevir and ribavirin for non-cirrhotic HCV genotype 1 patients: A phase 2 study. J. Gastroenterol. Hepatol.. 2018;33:1507-1510.
- [CrossRef] [Google Scholar]
- Current Progress in the Development of Hepatitis B Virus Mode-of-Action and Efficacy. Molecules. 2021;26:7420.
- [Google Scholar]
- Kiruthika, S., Bhat, R., Dash, R., Rathore, A.S., Vivekanandan, P., Jayaram, B., 2021. A novel piperazine derivative that targets hepatitis B surface antigen effectively inhibits tenofovir resistant hepatitis B virus. Sci. Reports 2021 111 11, 1–13. https://doi.org/10.1038/s41598-021-91196-1.
- Updates on Chronic HBV: Current Challenges and Future Goals. Curr. Treat. Options Gastroenterol.. 2019;17:271-291.
- [CrossRef] [Google Scholar]
- Hepatitis C virus: Virology, diagnosis and treatment. World J. Hepatol.. 2015;7:1377-1389.
- [CrossRef] [Google Scholar]
- Farnesoid X Receptor Agonists as Therapeutic Target for Cardiometabolic Diseases. Front. Pharmacol.. 2020;11:1247.
- [CrossRef] [Google Scholar]
- Safety, Tolerability, and Pharmacokinetics of the Novel Hepatitis B Virus Capsid Assembly Modulator GST-HG141 in Healthy Chinese Subjects: a First-in-Human Single- and Multiple-Dose Escalation Trial. Antimicrob. Agents Chemother.. 2021;65
- [CrossRef] [Google Scholar]
- Li HC, Yang CH, Lo SY. Hepatitis C Viral Replication Complex. Viruses. 2021 Mar 22;13(3):520. https://doi.org/10.3390/v13030520. PMID: 33809897; PMCID: PMC8004249.
- Targeting Viral cccDNA for Cure of Chronic Hepatitis B. Curr. Hepatol. Reports. 2020;19:235-244.
- [CrossRef] [Google Scholar]
- Analysis of Clinical Trials of New Drugs for Liver Diseases in China. Drug Des. Devel. Ther.. 2021;15:3181.
- [CrossRef] [Google Scholar]
- Hepatitis B virus (HBV) and hepatitis C virus (HCV) dual infection. Int. J. Med. Sci.. 2006;3:57-62.
- [CrossRef] [Google Scholar]
- Acute hepatitis C virus infection: clinical update and remaining challenges. Clin. Mol. Hepatol. 2023
- [CrossRef] [Google Scholar]
- Crystal structure of a novel dimeric form of NS5A domain I protein from hepatitis C virus. J. Virol.. 2009;83:4395-4403.
- [CrossRef] [Google Scholar]
- A Single and Multiple Ascending Dose Study of Toll-Like Receptor 7 Agonist (RO7020531) in Chinese Healthy Volunteers. Clin. Transl. Sci.. 2020;13:985-993.
- [CrossRef] [Google Scholar]
- Hepatitis C virus NS5A: tales of a promiscuous protein. J. Gen. Virol.. 2004;85:2485-2502.
- [CrossRef] [Google Scholar]
- NASPGHAN Practice guidelines: Diagnosis and management of hepatitis c infection in infants, children, and adolescents. J. Pediatr. Gastroenterol. Nutr.. 2012;54:838-855.
- [CrossRef] [Google Scholar]
- Clinical profile of hepatitis C virus infection in a developing country: India. J. Gastroenterol. Hepatol.. 2018;33:926-933.
- [CrossRef] [Google Scholar]
- Hepatitis B core protein as a therapeutic target. Expert Opin. Ther. Targets. 2017
- [CrossRef] [Google Scholar]
- HBV-HCV coinfection: Viral interactions, management, and viral reactivation. J. Clin. Transl. Hepatol.. 2018;6:296-305.
- [CrossRef] [Google Scholar]
- Hepatitis C virus NS3/4a protease inhibitors. Curr. Opin. Pharmacol.. 2016;30:84-92.
- [CrossRef] [Google Scholar]
- TLR agonists as modulators of the innate immune response and their potential as agents against infectious disease. Front. Immunol.. 2014;5:79.
- [CrossRef] [Google Scholar]
- Mohamed, S.M., Hassan, E.M., Ibrahim, N.A., 2009. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. https://doi.org/10.1080/14786410902906959 24, 1395–1402. https://doi.org/10.1080/14786410902906959.
- An overview of hepatitis B virus surface antigen secretion inhibitors. Front. Microbiol.. 2018;9:662.
- [CrossRef] [Google Scholar]
- Farnesoid X receptor-α is a proviral host factor for hepatitis B virus that is inhibited by ligands in vitro and in vivo. FASEB J.. 2019;33:2472-2483.
- [CrossRef] [Google Scholar]
- Hepatitis C virus NS5A inhibitors and drug resistance mutations. World J. Gastroenterol.. 2014;20:2902-2912.
- [CrossRef] [Google Scholar]
- Molecular mechanism for the involvement of nuclear receptor FXR in HBV-associated hepatocellular carcinoma. Acta Pharm. Sin. B. 2011;1:73-79.
- [CrossRef] [Google Scholar]
- Targeting Toll-like receptors: Emerging therapeutics? Nat. Rev. Drug Discov.. 2010;9:293-307.
- [CrossRef] [Google Scholar]
- Nucleoside analogues for chronic hepatitis B: antiviral efficacy and viral resistance. Am. J. Gastroenterol.. 2002;97:1618-1628.
- [CrossRef] [Google Scholar]
- Treatment of hepatitis C virus infection. Clin. Res. Hepatol. Gastroenterol.. 2021;45:101578
- [CrossRef] [Google Scholar]
- Hepatitis C: epidemiology, transmission and presentation. Prescriber. 2022;33:20-23.
- [CrossRef] [Google Scholar]
- Novel drugs targeting Toll-like receptors for antiviral therapy. Future Virol.. 2014;9:811-829.
- [CrossRef] [Google Scholar]
- NS5A inhibitors in the treatment of hepatitis C. J. Hepatol.. 2013;59:375-382.
- [CrossRef] [Google Scholar]
- Pipeline-Jiangsu Hengrui Pharmaceuticals Co., Ltd. [WWW Document], n.d. URL https://www.hengrui.com/en/pipeline.html (accessed 2.1.22).
- Pipelines - Lupeng [WWW Document], n.d. URL http://www.lupengbio.com/PRODUCT.html (accessed 3.9.22).
- The Hepatitis C Virus NS5A Stimulates NS5B During In Vitro RNA Synthesis in a Template Specific Manner. Open Biochem. J.. 2009;3:39-48.
- [CrossRef] [Google Scholar]
- Rao, H., Yang, X., Tan, Y., Ning, Q., Yang, Daokun, Wang, J., Yang, Y., Zheng, S., Yang, Dongliang, Hou, J., Xie, Q., Zhao, C., Zhang, L., Mao, X., Sun, T., Bai, L., Zhang, F., Jin, J., Zhao, Y., Wang, M., Xie, W., Ma, Y., Quan, J., Yan, X., An, P., Lin, F., Jia, J., Hu, X., Gong, Z., Wu, J., Chen, Y., Jia, Z., Lin, M., Wang, G., Zhu, Y., Zhang, Y., Xie, H., Luo, L., Ren, Q., Huang, R., Wei, L., 2020. Efficacy and Safety of All-oral Emitasvir and Sofosbuvir in Patients with Genotype 1b HCV Infections without Cirrhosis. http://www.xiahepublishing.com/ 8, 255–261. https://doi.org/10.14218/JCTH.2020.00031.
- NS5A inhibitors impair NS5A-phosphatidylinositol 4-kinase III∝ complex formation and cause a decrease of phosphatidylinositol 4-phosphate and cholesterol levels in hepatitis C virus-associated. Antimicrob. Agents Chemother.. 2014;58:7128-7140.
- [CrossRef] [Google Scholar]
- Encapsulation of lamivudine into single walled carbon nanotubes: A vdW-DF study. Phys. E Low-Dimensional Syst. Nanostructures. 2013;52:27-33.
- [CrossRef] [Google Scholar]
- Targets for antiviral therapy of hepatitis C. Semin. Liver Dis.. 2014;34:9-21.
- [CrossRef] [Google Scholar]
- Bridging the importance of Toll like receptors in human viral infections. Asian Pacific J. Trop. Dis.. 2016;6:573-580.
- [CrossRef] [Google Scholar]
- An update on the treatment options for HBV/HCV coinfection. Expert Opin. Pharmacother.. 2017;18:1691-1702.
- [CrossRef] [Google Scholar]
- Impact of direct-acting antiviral regimens on hepatic and extrahepatic manifestations of hepatitis C virus infection. World J. Hepatol.. 2022;14:1053-1073.
- [CrossRef] [Google Scholar]
- Sassi, A. Ben, Harzallah-Skhiri, F., Bourgougnon, N., Aouni, M., 2007. Antiviral activity of some Tunisian medicinal plants against Herpes simplex virus type 1. http://dx.doi.org/10.1080/14786410701589790 22, 53–65. https://doi.org/10.1080/14786410701589790.
- Hepatitis B virus core protein allosteric modulators can distort and disrupt intact capsids. Elife. 2018;7:e31473.
- [Google Scholar]
- Safety, Tolerability, and Pharmacokinetics of ARC-520 Injection, an RNA Interference-Based Therapeutic for the Treatment of Chronic Hepatitis B Virus Infection, in Healthy Volunteers. Clin. Pharmacol. Drug Dev.. 2017;6:350-362.
- [CrossRef] [Google Scholar]
- New Non-Nucleocide NS5B Protein Inhibitors for the Treatment of Chronic Hepatitis C Infection. Curr. Top. Med. Chem.. 2016;16:1392-1401.
- [CrossRef] [Google Scholar]
- Nucleoside, nucleotide, and non-nucleoside inhibitors of hepatitis C virus NS5B RNA-dependent RNA-polymerase. J. Med. Chem.. 2012;55:2481-2531.
- [CrossRef] [Google Scholar]
- Burden, Outcome, and Comorbidities of Extrahepatic Manifestations in Hepatitis C Virus Infection. Biology (Basel).. 2023;12:23.
- [CrossRef] [Google Scholar]
- Similarities and differences in hepatitis B and C virus induced hepatocarcinogenesis. Pathol. Oncol. Res.. 2004;10:5-11.
- [CrossRef] [Google Scholar]
- Sequence diversity of hepatitis C virus: implications for immune control and therapy. World J. Gastroenterol.. 2007;13:4808-4817.
- [CrossRef] [Google Scholar]
- Replication of Hepatitis B Virus. Zakim and Boyer’s Hepatology. 2012:86-96.
- [CrossRef] [Google Scholar]
- Management of hepatitis B virus infection, updated Swedish guidelines. Infect. Dis. (Auckl). 2020
- [CrossRef] [Google Scholar]
- WHO, 2021. Global Health Sector Strategies on HIV, Viral Hepatitis and the sexually transmitted infections [WWW Document]. URL https://www.who.int/publications/i/item/9789240027077 (accessed 5.15.23).
- Efficacy and safety of all-oral, 12-week ravidasvir plus ritonavir-boosted danoprevir and ribavirin in treatment-naïve noncirrhotic HCV genotype 1 patients: Results from a phase 2/3 clinical trial in China. J. Clin. Transl. Hepatol.. 2019;7:213-220.
- [Google Scholar]
- Direct Inhibition of Hepatitis B e Antigen by Core Protein Allosteric Modulator. Hepatology. 2019;70:11-24.
- [CrossRef] [Google Scholar]
- Yeh, M.-L., Chen, C., Cheng, P.-N., Pai, M.-C., Chen, J.-J., Lo, C., Tai, C.-M., Tsai, C.-Y., Tseng, K.-C., Chen, C.-H., Hung, C.-H., Huang, J.-F., Dai, C.-Y., Chuang, W.-L., Yu, M.-L., Zhang, M., Zhang, Jiming, Tan, Y., Xin, Y., Gao, H., Zheng, S., Yi, Y., Zhang, Jie, Wu, C., Zhao, Y., Jin, Z., Gao, Z., Mao, X., Wang, M., Hu, P., Rao, H., Jia, Z., Hou, J., Chen, L., Yang, X., Lu, J., Han, T., Chen, Yongping, Ning, Q., Yang, D., Shang, J., Jiang, J., He, Q., Chen, Yunfu, Ren, Q., Luo, L., Zhou, Q., Zhang, Y., Kong, F., Pan, Y., Ding, Y., Niu, J., 2020. Efficacy and safety of GLS4/ritonavir combined with entecavir in HBeAg-positive patients with chronic hepatitis B: interim results from phase 2b, multi-center study, in: Journal of Hepatology. Elsevier, pp. S878–S880. https://doi.org/10.1016/S0168-8278(20)32197-8.
- Yu, W., Goddard, C., Clear, E., Mills, C., Xiao, T., Guo, H., Morrey, J.D., Motter, N.E., Zhao, K., Block, T.M., Cuconati, A., Xu, X., 2011. Design , Synthesis , and Biological Evaluation of Triazolo-pyrimidine Derivatives as Novel Inhibitors of Hepatitis B Virus Surface Antigen (HBsAg) Secretion 5660–5670.
- Safety, pharmacokinetics, and antiviral activity of RO7049389, a core protein allosteric modulator, in patients with chronic hepatitis B virus infection: a multicentre, randomised, placebo-controlled, phase 1 trial. Lancet Gastroenterol. Hepatol.. 2021;6:723-732.
- [CrossRef] [Google Scholar]
- Hepatitis C - New drugs and treatment prospects. Eur. J. Med. Chem. 2019
- [CrossRef] [Google Scholar]
- Management of hepatitis B and hepatitis C coinfection: an expert review. Expert Rev. Anti. Infect. Ther.. 2020;18:1033-1044.
- [CrossRef] [Google Scholar]
- Zhang, Q., Vendeville, S., Serebryany, V., Welch, M., Liu, J., Williams, C., Debing, Y., Jekle, A., Stevens, S., Deval, J., Lin, T.-I., Misner, D., Chanda, S., Schinazi, R.F., Raboisson, P., Symons, J., Blatt, L., Beigelman, L., Smith, D., 2020. ALG-000184, a prodrug of capsid assembly modulator ALG-001075, demonstrates best-in-class preclinical characteristics for the treatment of chronic hepatitis B, in: Journal of Hepatology. Elsevier B.V., pp. S880–S881. https://doi.org/10.1016/s0168-8278(20)32200-5.
- Clinical evaluation of efficacy, tolerability and pharmacokinetics of yimitasvir phosphate in patients infected with hepatitis C virus. J. Pharm. Pharmacol.. 2018;70:855-864.
- [CrossRef] [Google Scholar]
- Randomised clinical trial: safety, efficacy and pharmacokinetics of HS-10234 versus tenofovir for the treatment of chronic hepatitis B infection. Aliment. Pharmacol. Ther.. 2021;53:243-252.
- [CrossRef] [Google Scholar]
- Viral proteins recognized by different TLRs. J. Med. Virol.. 2021;93:6116-6123.
- [CrossRef] [Google Scholar]
- Core protein: a pleiotropic keystone in the HBV lifecycle. Antiviral Res.. 2015;121:82.
- [CrossRef] [Google Scholar]







