Translate this page into:
Documentation of bioactive principles of the exudate gel (EG) from the stem of Caralluma retrospiciens (Ehrenb) and in vitro antibacterial activity – Part A
⁎Corresponding author at: Department of Pharmaceutics, Faculty of Pharmacy, Jazan University, P.O. Box 114, Postal Code 45142, Jazan, Saudi Arabia. smoni@jazanu.edu.sa (Sivakumar Sivagurunathan Moni)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Caralluma retrospiciens (Ehrenb) is a desert plant widely distributed in the hilly semi-desert regions of southern part of Saudi Arabia. The exudate gel (EG) from the stem of plant is occasionally used for wound healing by the people of the southern part of Saudi Arabia. This study investigated the phytochemical composition, FT-IR, GC–MS spectral analysis and in vitro antibacterial activity of the EG from the stem of C. retrospiciens (Ehrenb). The plant C. retrospiciens (Ehrenb.) was collected from the hills of Rijal Almaa, a heritage village of Saudi Arabia. The EG was isolated from the stem of C. retrospiciens (Ehrenb.). Physical parameters such as viscosity and zeta potential (ZP) were determined. Phytochemical analysis, FT-IR and GC–MS spectroscopy analysis were performed to determine the bio active constituents. The antibacterial activity of the isolated gel was performed by in vitro agar well diffusion technique. The study demonstrated that the viscosity and ZP of EG influenced the efficacy of antibacterial spectral properties. The FT-IR spectroscopy of the EG showed various functional groups at 3278.29, 2951.16, 2840.44, 2527.55, 2161.67, 1647.40, 1450.06, 1406.7, 1286, 1108.34, 5536.63 cm−1. Various pharmaceutically important chemical compounds were identified using GC–MS analysis. The bioactive compounds are “Sorbic Acid”, “Rhodopsin”, “1-Heptatriacotanol”, “Oxiraneundecanoic acid, 3-pentyl-, methyl ester, trans”, “Cholestan-3-ol, 2-methylene-, (3á,5à)”, “Benzoic acid”, “3-pentyl-, methyl ester trans”, “Hexanoic acid, 2-ethyl-, oxybis (2,1-ethanediyloxy-2,1-ethanediyl) ester”, etc. The antibacterial effect of the EG showed a wide spectrum of activity against the screened human pathogenic bacteria. The results demonstrate the bioactive principles of EG from C. retrospiciens (Ehrenb.) exerts the antibacterial properties in vitro.
Keywords
Desert plant
Caralluma retrospiciens (Ehrenb.)
Phytochemicals
Antibacterial effect
Gram-positive and Gram-negative bacteria
1 Introduction
Medicinal plants are important and prominent sources of bioactive substances. The importance of medicinal plants as therapeutic agents has been known to mankind since early human civilization. Studies have revealed that several plants are used in traditional health care worldwide as agents for treating chronic diseases (Alghamdi et al., 2018; Roumita et al., 2018; Zamawe et al., 2018; Rukshana et al., 2017). The usage of medicinal plants is common in Saudi Arabia where it has been estimated that the usage of herbal medicine to treat various ailments accounts for about 8–76% (Aati et al., 2019; Mushabab et al., 2018; Alrowais and Alyousefi, 2017). Traditional systems of medicine are involved in natural remedies using various herbs. Indeed, several researchers have demonstrated that herbs are classical resources for many potential novel drug molecules (Hosseinkhani et al. 2017). Plants like Aloe vera has been used in folk medicine for its wound healing properties for more than 4000 years (Moriyama et al. 2016). The World Health Organization (WHO) recognizes that the usage of herbal medicines and phytonutrients have rapidly expanded worldwide (WHO, 2004) and in fact approximately 25% of drugs prescribed worldwide originated from plants (Rates, 2001). Therefore, several research efforts in the past 4 decades have been focused on plants/herbs to scientifically document their efficacies as pharmaceutical agents for treating various ailments.
C. retrospiciens (Ehrenb.) is the synonym of Desmidorchis retrospiciens (Ehrenb.) belong to the Apocynaceae family and widely distributed succulent taxon found in the dry regions of the world (Nasser et al., 2017; Sharawy et al., 2015). The plant C. retrospiciens (Ehrenb.) is mainly distributed in the semi-desert regions of southern Saudi Arabia and parts of Africa. Traditionally, the people of Saudi Arabia have been using the EG from C. retrospiciens (Ehrenb.)for wound healing. However, there is no scientific evidence for the wound-healing properties of this plant or its antimicrobial activity. Consequently, the present study was aimed at investigating the antibacterial properties of the stem EG of C. retrospiciens (Ehrenb.).
2 Materials and methods
2.1 Study area, plant collection, and identification
The study area was Rijal Almaa region located in Asir province in the Southern region of Saudi Arabia (Fig. 1). The region of Rijal Almaa is located 45 km west of the city of Abha, with coordinates N 2015840.65 and E 211634.87. It is a mountainous area bordered on the East by Al-Souda Center, on the North by Muhayil, Asir province, and on the South by Al-Darb, Jizan province. C. retrospiciens (Ehrenb.) (Fig. 2A) was collected from the hilly regions of Rijal Almaa during November 2018 by simply plucking it from the soil. The stem of the plants was used for this research work. The plant materials were packed in perforated polyethylene bags and transported to the laboratory. The stem part was thoroughly washed with normal water, and thereafter with distilled water, to remove impurities, after which it was air-dried. The plant was identified by an herbarium curator and a voucher specimen (reference number = JAZUH 1623) was deposited in the herbarium of Jazan University.
The study area was Rijal Alma region, Asir province which is located 45 km away from Abha city in western side that coordinates N 2015840.65 and E 211634.87. The Region Rijal Alma is a mountainous area bordered to the east by Al-Souda Center, and from the North Muhayil of Asir province.
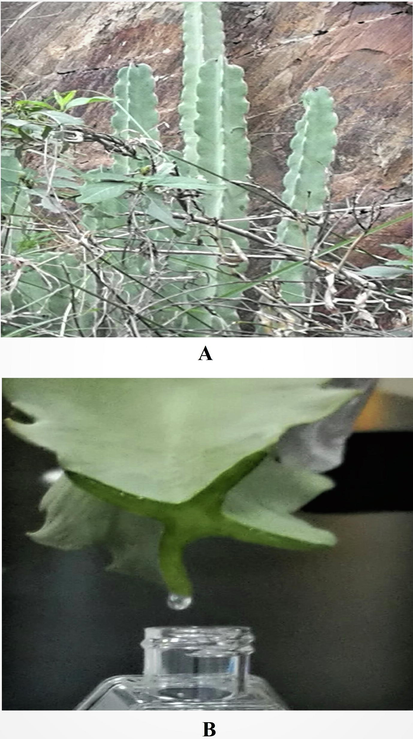
Photographic representation of the plant Caralluma retrospiciens (Ehrenb.) in the hilly region of Rijal Alma, Saudi Arabia. (A) Plant at collecting spot with shrubs on the rocks was robust, densely branched, cactus-like, succulent which was plucked out from the rock soil, the stem grown erect 300–400 cm tall, 2–5 cm width of angular fleshy stems, smooth surface, pale green in colour (B) The Collection of exudate gel (EG) from the stem by cutting with sterile blade and collected in a sterile plastic container by dropwise. The EG was highly viscous, pale yellow in colour and homogenous in texture by visual appearance.
2.2 Collection and Physical characterization of EG
2.2.1 Collection
The exudate from C. retrospiciens (Ehrenb.) (Fig. 2B) was collected from the stem of the plant by cutting with a sterile blade and holding the stem in a slanting position. The exudate was characterized physically as gel and designated as exudate gel (EG). The sample was collected in a 50 mL sterile screw-cap glass reagent bottle and immediately stored in refrigerator at 2–8° C. EG was utilized for spectral characterization and antibacterial studies.
2.2.2 Organoleptic characteristics
The organoleptic characteristics were determined from physical appearance, colour, texture, and homogeneity. Homogeneity was determined by visual inspection after EG was put in a clean glass beaker. The EG was observed visually for appearance and the presence of any aggregates.
2.2.3 Determination of viscosity
The rheological properties of the EG samples were determined by using Brookfield digital viscometer (Model LVDV-E, USA). The viscosity of the samples was determined using spindle S63. 25 ml of EG was placed in a clean, kept the sample holder and allowed to settle for 5 min. Then the viscosity was measured at a rotating speed of 30 rpm at room temperature.
2.2.4 Determination of zeta potential
The zeta potential (ZP) of EG was determined and the 100% v/v EG was subjected to ZP analysis followed by EG was diluted at predetermined concentration 50, 10, 5, 2.5, 1.25% v/v with Millipore water. The ZP analysis was performed by using Zetasizer Nano ZS, Malvern Instruments, UK (Moni et al., 2018).
2.3 Identification of phytochemical compounds
2.3.1 Preliminary phytochemical analysis
The EG was subjected to phytochemical analysis through standard protocols for the identification of carbohydrates, proteins, amino acids, alkaloids, flavonoids, tannins, steroids and saponins (Trease and Evans, 1989; Harbone, 1998; Kokate, 2000).
2.3.2 Fourier transform infrared spectroscopy studies (FT-IR Studies)
The EG was subjected to FT-IR studies for functional group analysis. The sample was placed in silicon tube and the spectra were recorded in transmission mode in the spectral region range of 4000 to 400 cm−1.
2.3.3 Gas chromatography–mass spectrometry (GC–MS) analysis
The GC-MS analysis was performed using Thermo Scientific GC-MS equipped with AS 3000 autosampler, trace ultra GC and ISQ detector. The sample was diluted in methanol in a volume ratio of 1:10. The mass spectra were interpreted using xcalibur software and the fragmentation patterns of mass spectra were compared with those stored in the spectrometer database using NIST, MAINLIB and REPLIB built-in libraries. The percentages of constituents were measured based on peak areas.
2.4 Assessment of antibacterial activity of EG
2.4.1 Bacterial strains used and standardization of bacterial cultures
The bacterial strains used in the study were Staphylococcus aureus, Streptococcus pyogenes, Bacillus subtilis, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa. Twenty-four-hour cultures were prepared and standardized using gradient dilution from 10−1 to 10−7 with nutrient broth. The viability of bacterial culture was determined by assessing the colony-forming unit in 1 ml (CFU/ml).
2.4.2 Determination of minimum inhibitory concentration
The minimum inhibitory concentration was determined using the broth tube dilution method. Predetermined concentrations of EG 50, 10, 5, 2.5 and 1.25% (v/v) were prepared by diluting the sample 1 in Millipore water. The minimum inhibitory concentration was determined based on the visibility of bacterial growth in the broth.
2.4.3 Preparation of samples for antibacterial studies
Based on MIC study, two different concentrations of the EG samples were used to determine the potential antibacterial effect against the screened human pathogenic bacteria.
Sample 1
The collected EG was not diluted in Millipore water since the focus was to demonstrate the direct effect of the EG against human pathogenic bacteria. Therefore, the concentration of each sample was considered as 100% (v/v).
Sample 2
A 50% (v/v) of EG sample was prepared by dissolving in an equal volume of Millipore water since the exudate was highly viscous.
2.4.4 Determination of antibacterial susceptibility
The antibacterial susceptibility test was performed according to the method of Moni et al. 2018. Muller Hinton (MH) agar plates were prepared for evaluating the antibacterial study. The bacterial cultures were sub-cultured from the stock culture, and after 24 h incubation, the cultures were subjected to antibacterial studies. A spread plate technique was used for growing the organisms. Agar well diffusion technique was employed for the samples and standard ciprofloxacin at the concentration of 50 mcg/ml (Cappuccino and Sherman, 2014). The inoculation was done by dipping a sterile cotton swab into the standardized (CFU/ml) individual cultures and streaking on MH agar plates by rotating the petri dish to distribute the culture evenly. The inoculation procedure was repeated twice for better growth of the culture. The plates were allowed to dry for about 10 min before the administration of samples. The agar well diffusion technique was performed by punching holes on inoculated MH agar plates using standard sterile stainless-steel borer. The diameter of the well was 10 mm, the samples and standard were placed in separate wells of individual plates. The plates were incubated at 37 °C for 24 h and the antibacterial spectrum was assessed through the appearance of inhibitory zones after 24 h of incubation. The spectrum of activity is directly proportional to the diameter of the zones of inhibition. The diameter of the zone was measured and recorded.
3 Results and discussion
3.1 Physical characteristics
The colour of EG was pale yellow, highly viscous and homogeneous, with a uniform smooth texture. Physiochemical characteristics such as viscosity grade, surface ZP studies are essential for assessing the diffusion properties of gels into the cell. The ZP analysis showed the physical characterization of the EG. The dilution factor also influenced the zeta potential. The ZP also changed as a function of viscosity i.e. it decreased with decrease in viscosity (Table 1). The purpose of this study was to demonstrate the actual antibacterial effect of the EG against various human pathogenic bacterial organisms. The study showed that the spectrum of activity was increased when the gel was diluted to 50% v/v in Millipore water when compared to 100% undiluted EG. The antibacterial effect is directly proportional to the diffusion property of the EG. The viscosity of the gel was also influenced by the dilution factor, and it declined from 100% to 1.25%, and the consistency of decline in viscosity was seen in the gel. The R2 value of EG was 0.9707 and confirm the linearity in the viscosities of the EG. The viscosity study showed the pseudoplastic behaviour of the gel. Similar results have been observed on formulated antibacterial creams and gels by Mei et al. (2016). On the other hand, the ZP of the EG was observed in the range between − 4.97 to − 0.715 mV. Studies have suggested that ZP value less than −10 mV is associated with good stability due to the presence of high repulsion between particles of the gel which prevent particle aggregation (Mora-Huertas et al., 2010). Therefore, these results confirm that the EG was highly unstable. However, the ZP of sample 1 and sample 2 of EG could not be determined due to high viscosity (Table 1). The viscosities of the EG was linearly influenced by dilution factor (Fig. 3), and had a pseudoplastic behaviour as expected. Therefore, the gels complied with the non-Newtonian system.
Concentration (% v/v)
Physical parameters
Physical appearance
Texture
Homogeneity
Viscosity (mpos)*
Zeta potential (mV)**
100
Opaque
Smooth
Yes
649
–
50
Opaque
Smooth
Yes
356
–
10
Less Opaque
Smooth
Yes
187.5
− 4.97
5
Transparent
Smooth
Yes
142
− 3.07
2.5
Transparent
Smooth
Yes
53
− 2.27
1.25
Transparent
Smooth
Yes
37
− 0.715
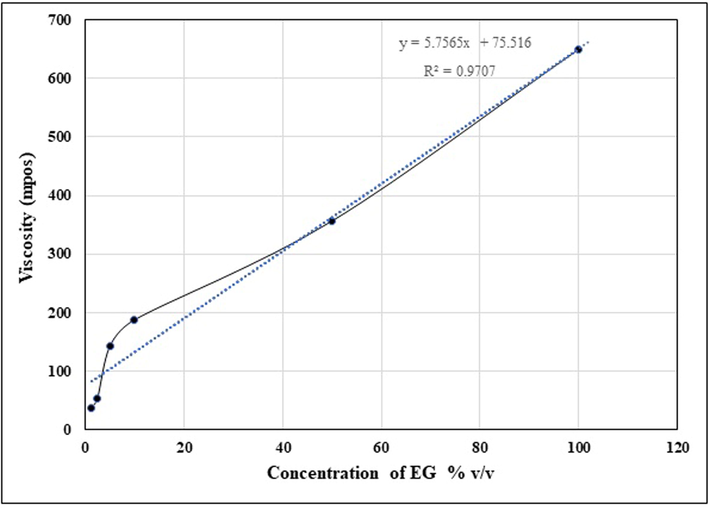
Viscosity curve of sample analyte the exudate gel (EG) from the stems of Caralluma retrospiciens (Ehrenb.)
3.2 Phytochemical analysis
The present study revealed that the EG contained various phytoconstituents such as carbohydrates, alkaloids, flavonoids, tannins, steroids, and saponins. An earlier report on the phytochemical analysis of Caralluma fimbriata depicted various compounds such as carbohydrates, tannins, fats, amino acids, proteins, sterols, saponins, gum and mucilage (Packialakshmi and Naziya, 2014). In contrast, the present study revealed the absence of amino acids and proteins in the EG of C. retrospiciens (Ehrenb.). Kiranmayee et al., 2015 studied the phytochemical composition of Caralluma attenuata, and reported the presence of saponins, steroids, flavonoids, and glycosides. However, the report also showed the absence of alkaloids and cardiac glycosides in Caralluma attenuata. An earlier report showed that a new pregnane glycoside derivative retrospinoside (1) was isolated from the aerial parts of Caralluma retrospiciens (Ehrenb.) and chemically characterised (Elsebai & Ietidal, 2015).
3.2.1 FT-IR analysis
The chemical groups in the EG were determined using FT-IR spectra by recording in the fingerprint region of 400–4000 cm−1 (Table 2). The FT-IR spectroscopy showed the presence of many peaks at various fingerprint regions for EG. Spectral analysis of EG showed unique peaks that confirmed the presence of functional groups and their respective compounds (Fig. 4A). The FT-IR spectral studies of EG showed the bell-shaped cure at 3278.29 cm−1 showed the existence of various functional groups such as hydroxy (OH) group with stretching frequency representing the presence of glycosides, flavonoids and saponins.
Wave number (cm−1)
Intensity
Functional groups
Phytocompounds
3278.29
Strong
O-H str. (hydroxyl)
Glycosides, Flavonoids, Saponins, hexose sugars
2951.16
Strong
CH2 str. (Asymmetrical)
Aliphatic compounds
Steroids, Saponins, Flavonoids
2840.44
Medium
CH2 str. (Symmetrical)
Aliphatic compounds
Steroids, Saponins, Flavonoids
2523
Strong
O-H str (Carboxylic acid)
Glycosides, Flavonoids
2161.67
Strong
N=N=N str (azide)
Flavonoids
1647.40
Medium
C=O str
C=C str (Olefinic)Steroidal glycosides, Flavonoids
1450.06
Medium
CH2 bend (Alkane)
Aliphatic compounds
Steroids, Saponins, Flavonoids
1406.7
Medium
S=O (Sulfonyl chloride)
Tannins
Cutin
1286
Weak
C-O Stretching (phenols)
Tannins
Cutin
1108.34
Strong
C-O-C Str (Ether, Ester)
Glycosides
556.63
Weak
S-S (disulfides)
Glycosides
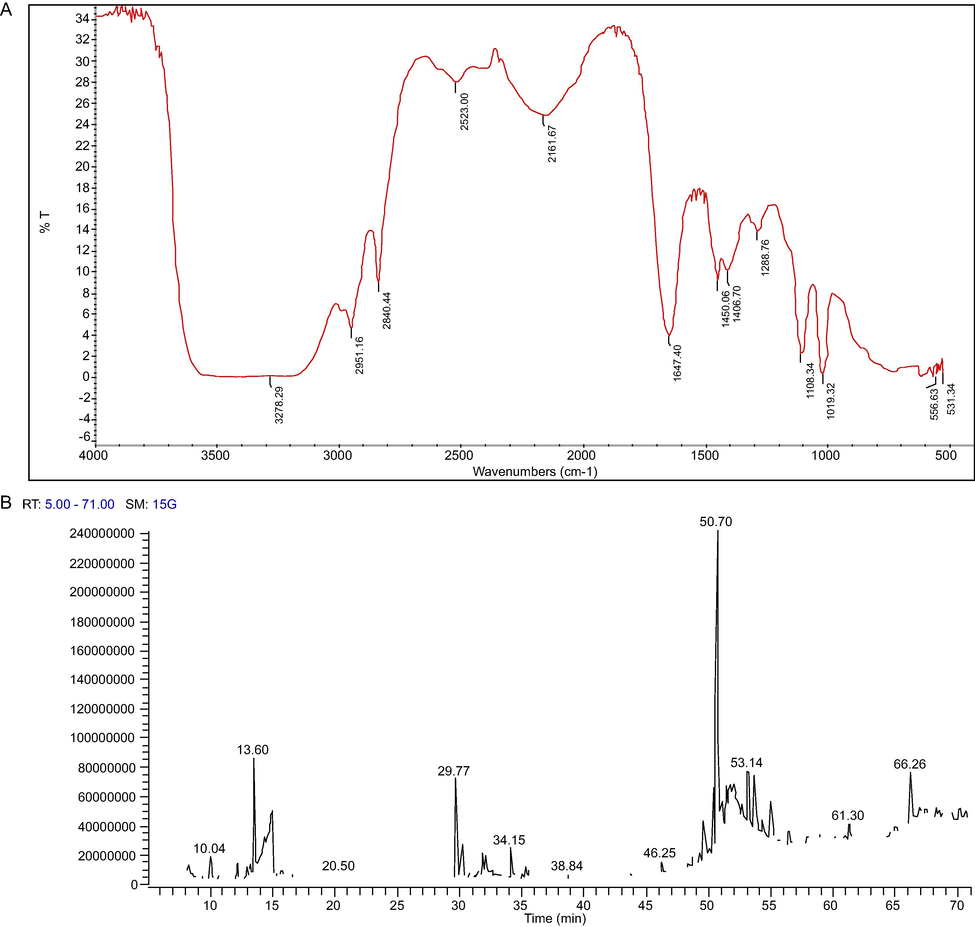
Spectral characterization of the exudate gel (EG) of Caralluma retrospiciens (Ehrenb.) (A) Finger print region of EG at 400– 4000 cm−1 (B) GC–MS Chromatogram of the EG of Caralluma retrospiciens (Ehrenb.) showing various peaks.
The frequencies at 2951.16 and 2840.44 cm−1 represents the aliphatic compounds with CH2 stretching vibrations and depicting the presence of steroids, tannins, and saponins. Steroids and saponins were the most abundant compounds. The finger print region at 2527.55, 2161.67, 1647.40, 1450.06 cm−1 exhibited the presence of glycosides, flavonoids, steroidal glycosides and saponins. The frequencies at 1406.7 and 1286 cm−1 displayed S=O (Sulfonyl chloride) and C-O (Phenols) indicated the presence of tannins and cutin. Earlier report suggested that the FT-IR spectrum showed the presence of alkyne (C-H), methylene (C-H), isocyanate (-N=C=O), organic nitrates, aliphatic nitro compounds, ammonium ion and aromatic nitro groups revealed the presence of phenolic compounds, flavonoids, saponins, steroids, terpenoids, proteins, quinones, coumarins and phytosterols (Asha et al., 2014; Showkat and Surender, 2015; Kavipriya and Chandran, 2018).
3.2.2 GC–MS analysis
The GC–MS analysis of the EG showed the presence of phytochemical chemical compounds. The GC-MS chromatogram exhibited various peaks for the compounds, as shown in Fig. 4B. The results indicated the presence of various chemical compounds such as unsaturated fatty acids, phenolic compounds, steroidal compounds, glycosides, saponins, and imidazole derivatives. GC–MS study revealed the presence of many bioactive constituents, molecular formula, retention time and their molecular weights (Table 3). The structure of bioactive constituents is represented in Fig. 5. The analysis showed the presence of various pharmaceutically important compounds in the EG. Similar results have been reported in Caralluma geniculata by Asha et al. (2014). The GC–MS study showed the presence of steroids, saponins, and flavonoids in EG. Kalimuthu et al. (2013) reported the presence of pharmaceutically important compounds in Caralluma diffusa. Presence of sorbic acid was observed in the EG in the current study. Earlier studies have shown that sorbic acid as potassium sorbate and sodium sorbate can be used as food preservatives and also it was shown that it has antimicrobial properties (Dániel et al., 2020). Rhodopsin is a photopigment very rarely present in plants (Ruiz-Gonzalez and Marin, 2004). The pigment was identified in the EG of C. retrospiciens (Ehrenb.) which was not reported in any Caralluma species. 1-Heptatriacotanol was identified in the EG and similar compound was observed in Aloe aspera (Dey et al., 2017). Earlier study suggested that the ethanolic extract of Trilepisium madagascariense DC contained 1-Heptatriacotanol as one of the constituents and was also reported to have antibacterial properties (Olufunmiso et al., 2018).
Sample ID
Bioactive compound
Molecular formula
Retention time (Minutes)
Molecular weight
1
Sorbic acid
C6H8O2
10.67
112
2
Rhodopsin
C40H58O
48.10
554
3
1-Heptatriacotanol
C37H76O
48.56
536
4
Oxiraneundecanoic acid, 3-pentyl-, methyl ester, trans
C19H36O3
48.72
312
5
Cholestan-3-ol, 2-methylene-, (3á,5à)
C28H48O
39.44
400
6
Hexanoic acid, 2-ethyl-, oxybis(2,1-ethanediyloxy-2,1-ethanediyl) ester
C24H46O7
35.08
446
7
2-Octadecenoic acid, methyl ester
C19H36O2
30.72
296
8
Uridine, 2′-deoxy-3-methyl-3′,5′-di-O-methyl
C12H18N2O5
30.16
270
9
3,4-Altrosan
C6H10O5
24.35
162
10
á-D-Glucopyranose, 1,6-anhydro
C6H10O5
23.66
162
11
D-Allose
C6H12O6
23.66
180
12
Glucosamine, N-acetyl-N-benzoyl
C15H19NO7
25.60
325
13
D-Ribo-hexose, 2,6-dideoxy-3-O-methyl
C7H14O4
15.74
162
14
Benzoic acid
C7H6O2
14.09
122
15
Imidazole-4-carboxylic acid, 2-fluoro-1-methoxymethyl-, ethyl ester
C8H11FN2O3
11.21
202
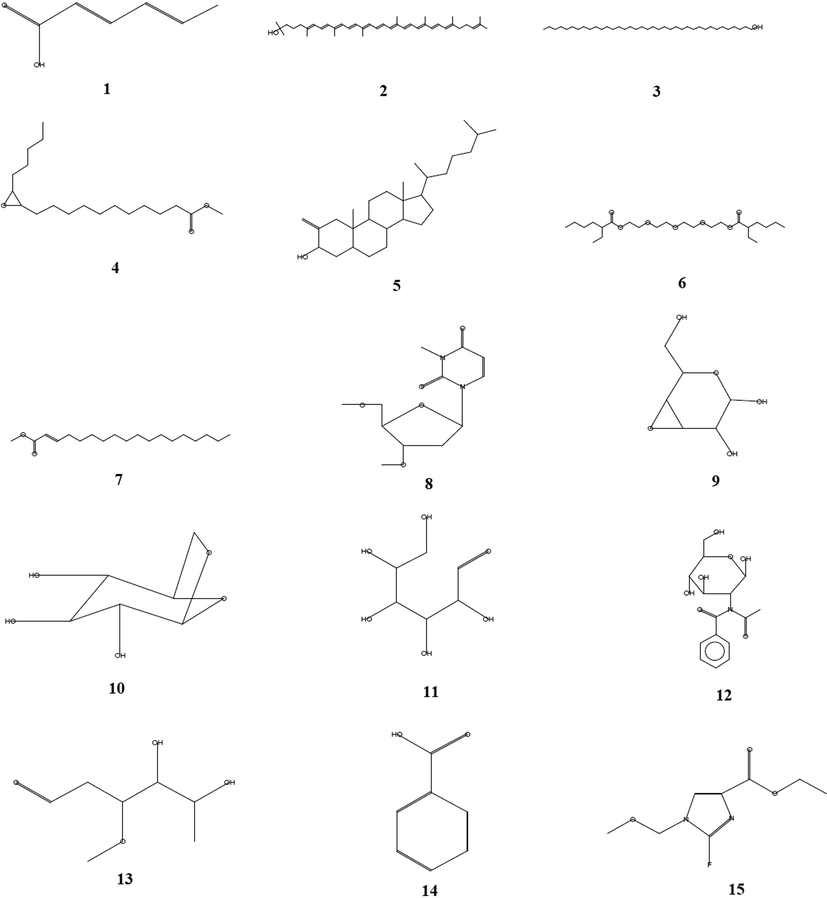
The structure of bioactive compounds of the exudate gel (EG) from the stem of Caralluma retrospiciens (Ehrenb.) through GC–MS analysis.
Oxirane undecanoic acid, 3-pentyl-, methyl ester, trans was present in EG. The compound was reported earlier in methanolic leaf extract of Frankenia aucheri Jaub et Spach (Huda Jasim, 2018). Cholestan-3-ol, 2-methylene-, (3á,5à), a steroidal molecule, was detected in EG. Several studies have suggested that the molecule exhibits many pharmacological and biological activities such as antimicrobial, anticancer, antiarthritic, anti-inflammatory, anti-asthmatic, and hepatoprotective activities (Al-Rubaye et al., 2017; Sindhu and Neelamegam, 2015). In this study Hexanoic acid, 2-ethyl-, oxybis(2,1-ethanediyloxy-2,1-ethanediyl) ester, 2-Octadecenoic acid, methyl ester, Uridine, 2′-deoxy-3-methyl-3′,5′-di-O-methyl, D-Ribo-Hexose, 2,6-dideoxy-3-O-methyl, a á-D-Glucopyranose, 1,6-anhydro, Glucosamine, N-acetyl-N-benzoyl and D-Allose were observed in EG of C. retrospiciens. 3,4-Altrosan was detected in the EG with unique retention time. Jadhav et al. (2014) reported the 3,4-Altrosan exhibited fungicidal and bacteriostatic properties. Benzoic acid is a well-known compound that has been identified in this study. The compound was reported for its antibacterial effect (Arokiyaraj et al., 2018; Eun-Soo Park, 2001). Imidazole-4-carboxylic acid, 2-fluoro-1-methoxymethyl-, ethyl ester also have been detected in the EG of C. retrospiciens (Ehrenb.) in this study. The compund is a well known for the antibacterial activity (Haider Mashkoor Hussein et al., 2016).
3.3 Antibacterial activity screening study
The MIC varied with the screened organisms (Table 4). The results indicate that the inhibitory concentration was highly influenced by the viscosity. The MICs of the EG were found to be 50% (v/v), respectively. Initially, sample 1, a 100% gel was used in the determination of antibacterial effect since the focus was to demonstrate their antibacterial effects against the tested bacteria. However, from the MIC study, the concentrations of the EG were fixed and their efficacies were affected. From the results, it is obvious that the viscosities of the EG influenced the antibacterial effect since 50% (v/v) of EG produced better antibacterial activities than lower concentrations. In general, the spectrum of activity against Gram-negative bacteria was wider than that against Gram-positive bacteria (Table 5). Besides, the spectrum of antibacterial activities in EG was found to be promising. Muhammad Imran Khan et al. (2018) demonstrated the antibacterial activity of saponin fractions from green tea. An earlier report showed that saponins exerted an inhibitory effect on Gram-positive bacteria but not on Gram-negative bacteria (Soetan et al. 2006). In contrast, the present study demonstrated that C. retrospiciens (Ehrenb.) exudate showed antibacterial activity against both Gram-positive and Gram-negative bacteria, however the antibacterial effect was more against Gram-negative bacteria than Gram-positive bacteria. (+) Presence of bacterial growth; (−) No bacterial growth. # Each value is the mean of 6 batches with standard deviation. The values of EG (Both 100% and 50%) were significantly lesser than standard ciprofloxacin at p < 0.001. **Extremely significant when compared to EG (100%) at p < 0.01. ns is non-significant when compared to EG (100%) at p greater than 0.05; $ EG was employed in the screening directly without dilution, designated as 100% v/v gel, $$ EG was employed in the screening by diluting with equal volume of Millipore water.
Bacterial organisms
Concentration of 24 h culture
CFU /mLMinimum inhibitory concentration of exudate gel (EG) (% v/v)
100
50
10
5
2.5
1.25
Bacillus subtilis
2 × 10−4
–
–
+
+
+
+
Staphylococcus aureus
2 × 10−5
–
–
+
+
+
+
Streptococcus pyogenes
2 × 10−4
–
–
+
+
+
+
Escherichia coli
2 × 10−6
–
–
+
+
–
+
Pseudomonas aeruginosa
2 × 10−3
–
–
+
+
+
+
Klebsiella pneumoniae
2 × 10−3
–
–
+
+
+
+
Organisms
Zone of inhibition (mm) of EG Analyte
Concentration
CFU#/mLEG (100%) $
EG (50%) $$
Ciprofloxacin (50 µg/ml)
Bacillus subtilis
2 × 10−4
17.8 ± 1.7
18.83 ± 0.87 ns
25.2 ± 1.2
Staphylococcus aureus
2 × 10−5
16.5 ± 1.04
20.8 ± 1.2**
24.6 ± 1.2
Streptococcus pyogenes
2 × 10−4
16.6 ± 1
20.3 ± 1.2**
24.16 ± 0.4
Escherichia coli
2 × 10−6
20.6 ± 1
21.8 ± 0.75 ns
26 ± 2.1
Pseudomonas aeruginosa
2 × 10−3
20.3 ± 1.2
22.3 ± 0.2 ns
26.5 ± 1.04
Klebsiella pneumoniae
2 × 10−3
22.16 ± 1.16
24.5 ± 0.83 ns
28.8 ± 1.3
4 Conclusion
The present study has documented scientific evidence of the antibacterial properties of the exudate gel (EG) from the stem of C. retrospiciens (Ehrenb.). The results showed the presence of various bioactive compounds that might be beneficial in the development of new drugs for treating various diseases in future. Furthermore, the EG was showing good spectrum of activity against screened Gram-positive and Gram-negative bacteria. However, further studies are needed, especially in vivo investigations, to confirm the antibacterial activity of EG.
Acknowledgment
The authors are thankful to Dr. Remesh Moochikkal, herbarium curator for the identification of seaweed and maintaining the samples in the herbarium of Jazan University (JAZUH) and also special thanks to Dr.S.Jayapraksh, Mr. Rahimullah Siddiqui, Mr. Mohamed Eltaib Elmobark for their help during the study.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomed.. 2019;15:1-9.
- [CrossRef] [Google Scholar]
- Phytochemical screening of methanolic leaves extract of Malva sylvestris. Int. J. Pharmacognosy Phytochem. Res.. 2017;9:537-552.
- [CrossRef] [Google Scholar]
- Herbal medicine use by Saudi patients with chronic diseases: a cross-sectional study (experience from Southern Regio of Saudi Arabia) J. Health Spec.. 2018;6:77-81.
- [CrossRef] [Google Scholar]
- The prevalence extent of complementary and alternative medicine (CAM) use among Saudis. Saudi Pharm J.. 2017;25:306318
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant activity and antibacterial mechanism of action from Marsilea minuta leaf hexane: methanol extract. Chem. Cent. J.. 2018;12(1):105.
- [CrossRef] [Google Scholar]
- Phytochemical and FT-IR spectral analysis of Caralluma geniculata Grev. et Myur. An endemic medicinal plant. J. Chem. Pharm. Res.. 2014;6(7):2083-2088.
- [Google Scholar]
- Microbiology – A Laboratory Manual. USA: Pearson Education Inc.; 2014.
- Comparative biocompatibility and antimicrobial studies of sorbic acid derivates. Eur. J. Pharm. Sci.. 2020;143:105162
- [CrossRef] [Google Scholar]
- Variation in phytochemical composition reveals distinct divergence of Aloe vera (L.) Burm.f. from other Aloe Species: Rationale behind selective preference of Aloe vera in nutritional and therapeutic Use. J. Evid Based Complementary Altern Med.. 2017;22(4):624-631.
- [CrossRef] [Google Scholar]
- New pregnane glycoside derivative from Caralluma retrospiciens (Ehrenb) Nat. Prod. Res.. 2015;29(15):1426-1431.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of phenol and benzoic acid derivatives. Int. Biodeterior. Biodegrad.. 2001;47:209-214.
- [CrossRef] [Google Scholar]
- Antimicrobial activity and spectral chemical analysis of methanolic leaves extract of Adiantum capillus-veneris using GC-MS and FT-IR Spectroscopy. Int. J. Pharmacognosy Phytochem. Res.. 2016;8(3):369-385.
- [Google Scholar]
- Phytochemical methods: A guide to modern techniques of plant analysis (2nd Ed.). Londn, UK: Chapman A & Hall; 1998.
- An evidence-based review on wound healing herbal remedies from reports of traditional Persian medicine. J. Evid. Based Complementary Altern. Med.. 2017;22(2):334-343.
- [CrossRef] [Google Scholar]
- Phytochemical analysis of Frankenia Aucheri Jaub. Et Spach (Frankeniaceae) by GC-MS and FT-IR Techniques. Plant Archives. 2018;18(2):2263-2269.
- [Google Scholar]
- GC-MS analysis of bioactive compounds in methanolic extract of Holigarna grahamii (wight) Kurz. Int. J. Health Med.. 2014;2(2014):35-39.
- [Google Scholar]
- FT-IR and GC-MS analysis of bioactive phytocompounds in methonalic leaf extract of Cassia Alata. Biomed. Pharmacol. J.. 2018;11:141-147.
- [CrossRef] [Google Scholar]
- Practical Pharmacognosy. Delhi, India: Vallabh Prakashan; 2000.
- GC-MS analysis of Caralluma diffusa (Wight) NEBr. Asian J. Plant Sci. Res.. 2013;3:130-133.
- [Google Scholar]
- Phytochemical investigation of Caralluma attenuata (Wight) Roots. Int. J. Pharmacognosy Phytochem. Res.. 2015;7:1120-1124.
- [Google Scholar]
- Formulation and evaluation of antibacterial creams and gels containing metal ions for topical application. J. Pharm (Cairo). 2016;2016:1-10.
- [CrossRef] [Google Scholar]
- Potency of nano-antibacterial formulation from Sargassum binderi against selected human pathogenic bacteria. Braz J. Pharm. Sci.. 2018;54(4):e17811
- [CrossRef] [Google Scholar]
- Polymer-based nano capsules for drug delivery. Int. J. Pharm.. 2010;385:113-142.
- [CrossRef] [Google Scholar]
- Beneficial effects of the genus Aloe on wound healing, cell proliferation, and differentiation of epidermal keratinocytes. PLoS ONE. 2016;11:1-15.
- [CrossRef] [Google Scholar]
- Green tea seed isolated saponins exerts antibacterial effects against various strains of Gram-positive and Gram-negative bacteria, a comprehensive study in vitro and in vivo. Evid Based Complement Alternat Med.. 2018;2018:1-12.
- [CrossRef] [Google Scholar]
- Herbal medicine use by Saudi patients with chronic diseases: A cross-sectional study (experience from Southern Region of Saudi Arabia) J. Health Spec.. 2018;6(2):77-81.
- [CrossRef] [Google Scholar]
- Nasser, A.A.A., Al Saeed Salah, S., Ahmed, G., Sirajudheen, A., Al-Karani, K., Al-Khulaidi, A., 2017. Ethnopharmacological survey of medicinal plants in Albaha Region, Saudi Arabia. Pharmacognosy Res. 9, 401-407. 10.4103/pr.pr_11_17.
- Bioactive compounds and in vitro antimicrobial activities of ethanol stem bark extract of Trilepisium madagascariense DC. Int. J. Pharmacol.. 2018;14:901-912.
- [CrossRef] [Google Scholar]
- Screening of antibacterial and phytochemical analysis of Caralluma fimbriata. Pharma. Innovation J.. 2014;3(6):65-69.
- [Google Scholar]
- New insights into the evolutionary history of type 1 rhodopsins. J. Mol. Evol.. 2004;58:348-358.
- [CrossRef] [Google Scholar]
- Roumita, S.S., Namrita, L., Bianca, F., Analike Blom van, S., Fawzi, M., 2018. Antibiotic-potentiating activity, phytochemical profile, and cytotoxicity of Acalypha integrifolia Willd. (Euphorbiaceae) J. Herb Med. 11, 53-59. https://doi.org/10.1016/j.hermed.2017.03.005.
- Rukshana, M.S., Doss, A., Kumari Pushpa Rani, T.P., 2017. Phytochemical screening and GC-MS analysis of leaf extract of Pergularia daemia (Forssk) Chiov. Asian J Plant Sci Res., 7(1), 9-15.
- A systematic revision on Caralluma species of Saudi Arabia based on karyological and molecular data. Pak. J. Bot.. 2015;47(3):937-950.
- [Google Scholar]
- Evaluation of phytochemical profile in the ethanol extracts of Malachra capitate (L) L by GC-MS analysis. World J. Pharm. Res.. 2015;4(8):1342-1353.
- [Google Scholar]
- Evaluation of the antimicrobial activity of saponins extract of Sorghum Bicolor L. Moench. Afr. J. Biotechnol.. 2006;5(23):2405-2407.
- [Google Scholar]
- FT-IR Spectroscopic analysis of Holoptelea integrifolia (Roxb.) plant seed extracts and their antibacterial activity. Res. J. Medicinal Plant. 2015;9:417-426.
- [CrossRef] [Google Scholar]
- Pharmacognosy (13th ed.). London: Bailliere Tindall; 1989.
- World Health Organization (2004). WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. World Health Organization, Geneva, Switzerland. https://apps.who.int/iris/handle/10665/43034.
- Effectiveness and safety of herbal medicines for induction of labour: a systematic review and meta-analysis. BMJ Open.. 2018;8(10):e022499
- [CrossRef] [Google Scholar]







