Translate this page into:
Documentation of bioactive principles of the flower from Caralluma retrospiciens (Ehrenb) and in vitro antibacterial activity – Part B
⁎Corresponding author at: Department of Pharmaceutics, Faculty of Pharmacy, Jazan University, P.O. Box 114, 45142 Jazan, Saudi Arabia. smoni@jazanu.edu.sa (Sivakumar Sivagurunthan Moni)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
The purpose of this study was to explore the biocomponents of flowers from Caralluma retrospiciens (Ehrenb). The study investigated the FT-IR and GC–MS spectral analysis of flower from C. retrospiciens (Ehrenb). A flower concentrate (FC) was prepared and the functional groups were determined by FT-IR spectroscopy study. FT-IR analysis showed the presence of significant compounds at frequency 3287.45, 2850.32, 2595.91, 2524.69, 2041.07 cm−1. The GC–MS study demonstrated the presence of unique pharmaceutically important compounds. The extract was characterized by the presence of unique compounds such as “7,8-Epoxylanostan-11-ol, 3-acetoxy”, “Pregnan-20-one, 5,6-epoxy-3-hydroxy-, (3á,5á,6á)-”, “Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester”, “Hexadecanoic acid, methyl ester”, “Dasycarpidan-1-methanol, acetate”, etc. The antibacterial effect of the FC showed a wide spectrum of activity against the screened human pathogenic bacteria. The activity was predominantly against Gram-negative bacteria.
Keywords
Desert plant
Caralluma retrospiciens (Ehrenb)
Flowers
Phytochemicals
Spectral analysis
Antibacterial studies
1 Introduction
Natural products are unique sources of bioactive substances possessing high molecular diversity and promising pharmacological activities (Moni et al., 2019). Traditional systems of medicine are involved in natural remedies using various herbs. It has been demonstrated by innumerable researchers that herbs are established resources for numerous prospective novel drug molecules (Hosseinkhani et al., 2017). Several studies have reported that the medicinal plants are used traditionally across the world as a treatment for many chronic diseases (Vikrant et al., 2020; Anoma and Fereidoon, 2018; Rukshana et al., 2017). In Saudi Arabia, desert occupies 30% of land and is the habitat of numerous unique plants (Plant diversity of Saudi Arabia, 2019; Yaseen et al., 2015). The diversity of plants has a major role in maintaining the ecosystem across the world (Aati et al., 2019).
Many flowering plants identified indigenous to Saudi Arabia, are widespread in Jazan, Asir, Hejaz, Al Baha, and the northern regions (Jou, 2015). Flower based therapeutic formulations currently in use are essential oils, flower infusions, flower juice, flower petals, and aromatherapy to heal the mind and body (Babar Ali et al., 2015; Christensen et al., 2010). Several flowers were used in the past as a therapeutic agent for the treatment of diseases by medical practitioners (Vikrant et al., 2020; Mlcek et al., 2011). C. retrospiciens (Ehrenb) N.E.Br belongs to Apocynaceae family and a widely distributed succulent taxon found in dry regions of the world (Sharawy et al., 2015). It is one of the unique plants of the southern region of Saudi Arabia. In continuation of our earlier work (Makeen, et al., 2020), the present study was aimed at investigating the bioactive components of the flower of C. retrospiciens (Ehrenb) through phytochemical, FT-IR, GC–MS spectral analysis, and antibacterial studies.
2 Materials and methods
2.1 Study area, plant collection, and identification
The study area was Rijal Almaa region located in Assir province in the Southern region of Saudi Arabia. The region is a natural corridor that links Yemen and the Levant to Makkah and Madinah, a feature that makes it an important regional commercial center. The plant materials were packed in perforated polyethylene bags and transported to the laboratory. The plant was identified by an herbarium curator and a voucher specimen (reference number = JAZUH 1623) was deposited in the herbarium of Jazan University.
2.2 Processing of flowers
The flowers of C. retrospiciens (Ehrenb) were plucked out from the plant and were thoroughly washed with tap water and distilled water. The washed flowers were cut into small pieces, crushed by adding few drops of Millipore water to get a gel consistency and was designated as flower concentrate (FC).
2.3 Physical characterization of the FC
2.3.1 Organoleptic characteristics
The organoleptic characters were determined by physical appearance, colour, texture, and homogeneity. The homogeneity was determined by visual inspection after the FC sample was placed in a clean glass beaker and observed for the presence of any aggregates.
2.3.2 Determination of viscosity
The rheological properties of the FC samples were determined by using Brookfield digital viscometer (Model LVDV-E, USA). The viscosity of the samples was determined using spindle S63. The sample (25 ml) was placed in the sample holder and allowed to settle for 5 min, after which the viscosity was measured at a rotating speed of 30 rpm at room temperature.
2.3.3 Determination of zeta potential
The zeta potential of FC was determined by diluting to 10% (v/v) with Millipore water. The diluted samples were subjected to zeta potential analysis using Zetasizer Nano ZS, Malvern Instruments, UK (Moni et al., 2018).
2.4 Determination of bioactive constituents using spectral analysis
2.4.1 Fourier transform infrared spectroscopy studies (FT-IR Studies)
The functional groups of the samples were analyzed by using Nicolet iS10 FT-IR spectrophotometer. The extract samples (2 drops) were placed in silicon tube and the spectra were recorded in transmission mode in the spectral region range of 4000–400 cm−1.
2.4.2 Gas chromatography-mass spectrometry (GC–MS) analysis
The GC–MS analysis was performed using Thermo Scientific GC–MS equipped with AS 3000 autosampler, trace ultra GC, and ISQ detector. Thermo Scientific TR 5MS column with dimensions of 30 m × 0.25 mm (internal diameter) × 0.25 µm (film thickness) was used for separation of the components. Helium, at a flow rate of 1.2 ml/min (constant flow mode), was used as carrier gas. A volume of 2 µL of sample extracts was injected in spitless mode. The injection port was set at 320 °C and the temperature of the oven was initially set at 70 °C for 5 min. The oven temperature was subsequently ramped to 205 °C at the rate of 5 °C/min for 5 min, 280 °C at the rate of 5 °C/min for 5 min, 290 °C at the rate of 5 °C/min for 5 min, and finally to 300 °C at the rate of 5 °C/min for 5 min. The maximum oven temperature was set at 320 °C. The mass spectrometer was operated in an electron ionization (EI) mode within the mass range of 60–900 amu with 0.6 scan times (min). The MS transfer line temperature and ion source temperature were kept at 320 °C and 350 °C respectively with an electron multiplier voltage of 1 Kv. The mass spectra were interpreted using Xcalibur software and the fragmentation patterns of mass spectra were compared with those stored in the spectrometer database using NIST, MAINLIB, and REPLIB built-in libraries. The percentages of constituents were measured based on peak areas. The components were identified through comparison with those available in the inbuilt library (NIST and Willey) attached to the GC–MS instrument.
2.5 Assessment of antibacterial potentiality of flower concentrate (FC)
Briefly, 24 h culture of Staphylococcus aureus, Streptococcus pyogenes, Bacillus subtilis, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa was prepared. The cultures were standardized by gradient dilution from 10−1 to 10−7 with nutrient broth. The viability of bacterial culture was identified by assessing the colony-forming unit in 1 ml (CFU/ml).
2.5.1 Determination of minimum inhibitory concentration
The minimum inhibitory concentration was performed by the broth tube dilution method. The inhibitory concentration at the minimum level was determined using the FC in a predetermined concentration. The gradient concentrations of samples were prepared by diluting 100% gel in Millipore water to get the predetermined concentration 50, 10, 5, 2.5 & 1.25% v/v. The minimum concentration of inhibiting the bacterial growth was determined based on the visibility of bacterial growth in the broth.
2.5.2 Determination of antibacterial susceptibility
The antibacterial susceptibility test was performed as established by Moni et al., 2018. Briefly, Muller Hinton (MH) agar plates were prepared for performing the antibacterial study. The bacterial cultures were subcultured from the stock culture and after 24 h incubation, the culture was subjected to antibacterial studies. The spread plate technique was followed for growing the organisms. Agar well diffusion technique was employed for the samples and standard ciprofloxacin at the concentration of 50 mcg/ml (Cappuccino and Sherman, 2014). The inoculation was done by dipping a sterile cotton swab into the standardized (CFU/ml) culture individually with various organisms and streaked on the MH agar plate by rotating the petri dish to distribute the culture evenly. The inoculating procedure was repeated twice for the better growth of the culture. The plates were allowed to dry for about 10 min before the administration of sample analytes. The agar well diffusion technique was performed by punching holes on the inoculated MH agar plates using standard sterile stainless-steel borer. The diameter of the well was 10 mm, the sample analytes were placed in the respective wells. The plates were incubated at 37 °C for 24 h and the antibacterial spectrum was assessed by the development of inhibitory zones after 24 h of incubation. The spectrum of activity is directly proportional to the diameter of the zones of inhibition.
3 Results and discussion
The present study revealed that the FC contained various phytoconstituents such as carbohydrates, alkaloids, flavonoids, tannins, steroids, and saponins. The organoleptic properties such as physical appearance, colour, homogeneity, and viscosity of FC were determined. The gel prepared from the crushed flower was black in appearance, non-homogeneous, non-uniform texture and viscous (Table 1). The viscosity of the gel 649 mpos immediately after the collection. It was found to be highly viscous as it cannot diffuse easily through the MH agar media in antibacterial spectral studies. Therefore, in this study, the viscosity was reduced by gradient concentration from 100 to 1.25% v/v, and the evaluation of their minimum inhibitory concentration was performed. The zeta potential analysis was showing the physical characterization of FC. The dilution factor also influenced the zeta potential of FC. Zeta potential also changed and found to be in reducing patterns from higher viscosity to the lower viscosity (Table 1). However, in this study, we could not determine the zeta potential for 100% and 50% gel of FC since the viscosity was high. The viscosity of FC was influenced by the dilution factor, the linear trend was observed (Fig. 1) and had a pseudoplastic behavior as expected. Therefore, the FC samples were complying with the non-Newtonian system.
Concentration (% v/v)
Physical appearance
Texture
Homogeneity
Viscosity (mpos)
Zeta potential (mV)
100
Opaque
Rough
No
16.5
–
50
Opaque
Rough
No
10.6
–
10
Less Opaque
Rough
No
2.5
−36.4
5
Less Opaque
Smooth
Yes
1.9
−32.9
2.5
Transparent
Smooth
Yes
1.1
−30.8
1.25
Transparent
Smooth
Yes
0.87
−29.3
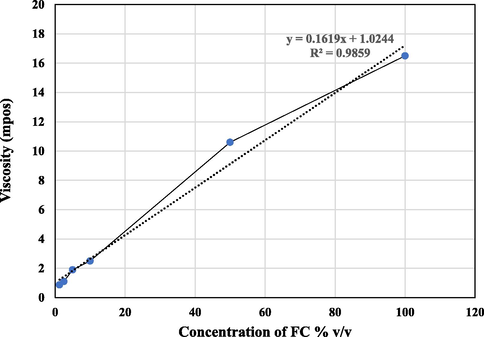
Viscosity curve for the flower concentrate (FC) from the flower of Caralluma retrospiciens (Ehrenb)
The FT-IR spectroscopy showed the presence of many peaks at various fingerprint regions (Fig. 2A) indicating the presence of various functional groups such as glycosides, flavonoids, steroids, tannins, and saponins. Steroids and saponins were the most abundant compounds (Table 2). The large parabola-shaped peak observed at 3287.45 cm−1 having stretching vibrations indicated the presence of phenolic O-H group which corresponds to the presence of tannins, glycosides, steroids, flavonoids, and saponins. Other strong intensity peaks observed at 2524.69, 2041.07 cm−1 indicated the presence of glycosides and flavonoids. Weak peaks at 2850.32 cm−1 with CH2 stretching and 2595.91 cm−1 with OH stretching indicated the presence of glycosides and flavonoids. Therefore, the FT-IR spectral study revealed that the presence of the phenolic group, glycosides, steroids, flavonoids, and saponins (Asha et al., 2014; Kumar et al., 2015; Showkat and Surender, 2015; Kavipriya and Chandran, 2018). Besides, the GC–MS chromatogram revealed the presence of many active constituents (Fig. 2B), as well as their structures and their molecular weights. The analysis showed the presence of various pharmaceutically important compounds (Table 3 & Fig. 3). Similar kinds of results were reported in Caralluma geniculata plant (Asha et al., 2014). Kalimuthu et al. (2013) reported the presence of various pharmaceutically important compounds in Caralluma diffusa. 7,8-Epoxylanostan-11-ol, 3-acetoxy, an alcoholic compound identified in FC that exhibited maximum retention time 58.19 min, was suggested in earlier reports to exhibit antimicrobial and anti-inflammatory effects (Wafaa Hassan et al., 2014).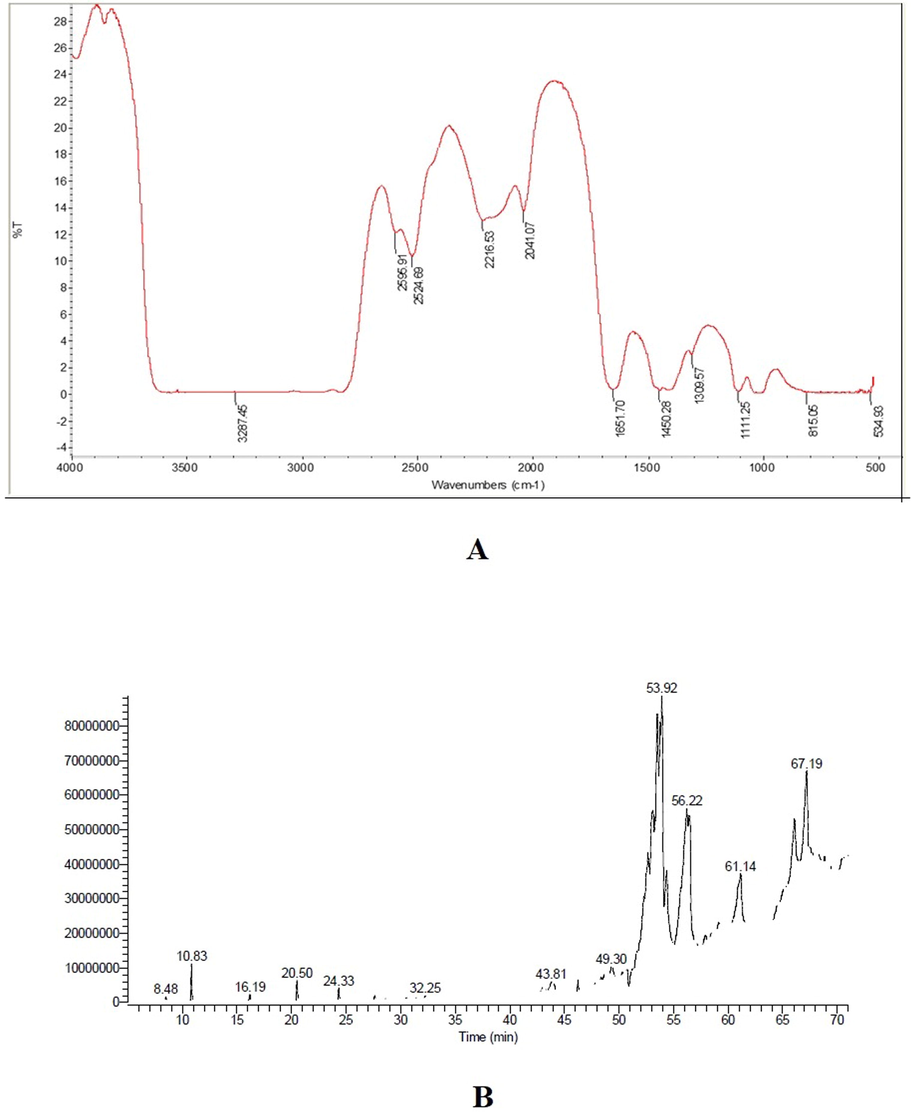
Spectral Characterization of the flower concentrate (FC) from the flower of Caralluma retrospiciens (Ehrenb) (A) FT-IR Fingerprint region of the FC at 400–4000 cm−1; (B) GC–MS Chromatogram of the FC showing various peaks
Wavenumber (cm−1)
Intensity
Functional groups
Probable Compounds
3287.45
Strong
O—H str. hydroxyl)
Glycosides, Tannins, Flavonoids, Saponins
2850.32
Weak
CH2 str. (Symmetrical)
Aliphatic compounds, Steroids, Saponins, flavonoids
2595.91
Weak
O—H str (Carboxylic acid)
Glycosides, Flavonoids
2524.69
Strong
O—H str (Carboxylic acid)
Glycosides, Flavonoids
2216.53
Weak
C⚌N (Nitriles)
Flavonoids
2041.07
strong
C≡C (Alkynes)
Glycosides
1651.70
Medium
C⚌O str
C⚌C str (Olefinic)Steroidal glycosides, Flavonoids
1450.28
Medium
CH2 bend (Alkane, Asymmetrical)
Aliphatic compounds
Steroids, Saponins, flavonoids
1309.57
Weak
CH2 bend (Alkane, Symmetrical)
Steroidal glycosides, Flavonoids, Saponins, hexose sugars
1111.25
Medium
C—C—C bend
Flavonoids
534.93
Weak
S—S (disulfides)
Glycosides
S. no
Bioactive compound
Molecular formula
Retention time (min)
Molecular weight
1
7,8-Epoxylanostan-11-ol, 3-acetoxy
C32H54O4
58.19
502
2
Diisooctyl phthalate
C24H38O4
46.24
390
3
Pregnan-20-one, 5,6-epoxy-3-hydroxy-, (3á,5á,6á)-
C21H32O3
42.69
332
4
Benzene propanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester
C18H28O3
31.61
292
5
Hexadecanoic acid, methyl ester
C17H34O2
31.48
270
6
Heptadecane, 9-hexyl-
C23H48
27.39
324
7
Dasycarpidan-1-methanol, acetate (ester)
C20H26N2O2
27.07
326
8
Eicosane, 2-cyclohexyl
C26H52
25.76
364
9
Phenol, 2,4-bis(1,1-dimethylethyl)
C14H22O
22.37
206
10
Octadecane, 3-ethyl-5-(2-ethylbutyl)
C26H54
21.90
324
11
Tetra tetracontane
C44H90
21.47
618
12
Nonadecane
C19H40
19.36
268
GC–MS detection of bioactive compounds of the flower concentrate (FC) from the flower of Caralluma retrospiciens (Ehrenb)
Diisooctyl phthalate is chemically 1,2-benzenedicarboxylic acid, diisooctyl ester which is a phthalate ester that was detected with unique retention time. Generally used as plasticizers that cause human health and environmental hazards (Azadeh et al., 2014). Madepalli and Thiyagaraj (2018) reported that di-butyl phthalate isolated from Begonia malabarica showed antibacterial effect against screened Gram-positive and Gram-negative bacteria. Pregnan-20-one, 5,6-epoxy-3-hydroxy-, (3á,5á,6á)-, a steroidal derivative showed a unique retention time. Earlier reports demonstrated that 3alpha-hydroxy-5beta-pregnan-20-one exhibited antineophobic effect and thus termed as a neuroactive steroid (Higgs and Cooper, 1998). Mahmoud Fahmi & Ietidal EL-Tahir (2015) reported a new pregnane glycoside derivative from the aerial part of C. retrospiciens (Ehrenb).
Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester a unique compound was detected at 31.61 min retention time and not reported earlier in C. retrospiciens (Ehrenb). Another novel compound hexadecanoic acid, methyl ester otherwise called palmitic acid a saturated fatty acid was detected at 31.48 min retention time but not reported in any Caralluma species. However, earlier studies suggested that hexadecanoic acid, methyl ester was observed in Aloe vera (Mansoor and Taiebeh, 2013). It has been reported that hexadecanoic acid, methyl ester as an antioxidant, anticholesteremic, and anti-inflammatory properties (Maghdu and Palaniyappan 2015). Hexadecanoic acid, methyl ester was reported to have antitumor, immunostimulant properties (Eman and Shiboob, 2017; Al-Rubaye et al., 2017). Heptadecane, 9-hexyl- is a unique hydrocarbon compound that was identified in the FC but not yet reported early for their presence in C. retrospiciens (Ehrenb). However, heptadecane, 9-hexyl- have been identified in Lepidagathis cristata and reported for its antifungal property (Maghdu and Palaniyappan 2015). Dasycarpidan-1-methanol, acetate is an alkaloidal compound that was identified in the FC but not yet reported as a constituent in C. retrospiciens (Ehrenb). However, the compound was reported in the recent study on Artemisia judaica a desert plant widespread in Saudi Arabia (Eman Ramadan and Shiboob, 2017). The compound was reported to have anti-inflammatory, anti-bacterial, anti-fungal, and anti-cancer (Al-Rubaye et al., 2017). Phenol, 2,4-bis(1,1-dimethylethyl) was identified in the FC which was reported for anti-biofilm formation in the uropathogen Serratia marcescens (Padmavathi et al., 2014). Furthermore, phenol is a well known antibacterial agent. Octadecane, 3-ethyl-5-(2-ethylbutyl) was detected in FC at 21.90 min of retention time. An earlier study suggested that the presence of octadecane, 3- ethyl-5-(2-ethylbutyl)- in Aloe vera (Mansoor and Taiebeh, 2013). Tetratetracontane and nonadecane are long-chain hydrocarbons that were detected in FC with 21.47 and 19.36 min of retention time. Tetratetracontane has been reported in the composition of wax compounds of Ziziphus nummularia(Alfarhan et al., 2020). Nonadecane has been identified in the essential oil obtained from Tunisian Allium nigrum L and exhibited antibacterial property (Rouis-Soussi et al., 2014).
The potential antibacterial effect of FC was determined by accessing minimum inhibitory concentration (MIC) for FC. The MIC was varying with the screened organisms (Table 4). The results indicating that the inhibitory concentration is highly influenced by the viscosity grade of FC because of diffusion property into the bacterial cell (Kai et al., 2019; Maja et al., 2012). The MIC of FC was 2.5% v/v. The results were showing a good antibacterial spectrum of activity (Table 5). From the results, it is obvious that the viscosity of the FC was influenced by the antibacterial effect since 50% v/v of FC determining better antibacterial activity. In general, the spectrum of activity against Gram-negative bacteria was found to more significant when compared to Gram-positive bacteria. FC - Flower concentrate from the flower of Caralluma retrospiciens (Ehrenb); (+) Presence of bacterial growth; (−) No bacterial growth # CFU- Colony Forming unit. Each value is the mean of 6 batches with standard deviation, * P < 0.05 significant (lesser) when compared to standard ciprofloxacin. *** P < 0.001 extremely significant (lesser) when compared to standard ciprofloxacin. ns- non significant at P < 0.01 when compared to standard ciprofloxacin; #ns - non significant at P < 0.05 when compared to FC 50%; FC - Flower concentrate from the flower of Caralluma retrospiciens (Ehrenb)
Bacterial organisms
Concentration of 24 h culture CFU /mL
Minimum inhibitory concentration of FC (% v/v) (Presence of visible growth)
100
50
10
5
2.5
1.25
Bacillus subtilis
2 × 10−5
−
−
−
−
+
+
Staphylococcus aureus
3 × 10−5
−
−
−
−
+
+
Streptococcus pyogenes
2 × 10−6
−
−
−
−
+
+
Escherichia coli
4 × 10−5
−
−
−
−
+
+
Pseudomonas aeruginosa
2 × 10−5
−
−
−
−
−
+
Klebsiella pneumoniae
2 × 10−5
−
−
−
−
−
+
Organisms
Zone of inhibition (mm) of sample analytes
Concentration CFU#/mL
FC (100%)
FC (50%)
Ciprofloxacin (50 µg/ml)
Bacillus subtilis
2 × 10−5
19 ± 1.2***
22.1 ± 0.3*
24.6 ± 1.2
Staphylococcus aureus
3 × 10−5
17.5 ± 0.8 ***
23.5 ± 1.6 ns
24.3 ± 1.3
Streptococcus pyogenes
2 × 10−6
20.6 ± 1.2***
24.16 ± 1.1 ns
25.6 ± 0.8
Escherichia coli
4 × 10−5
22.3 ± 0.5#ns, ***
25 ± 1 ns
26 ± 2.1
Pseudomonas aeruginosa
2 × 10−5
21.6 ± 1.4 #ns, ***
22.5 ± 1***
27.6 ± 0.7
Klebsiella pneumoniae
2 × 10−5
22.5 ± 0.5***
23.3 ± 0.3***
27.8 ± 1.3
4 Conclusion
The study demonstrated the presence of various bioactive molecules present in the flower of Caralluma retrospiciens. The GC–MS studies revealed the presence of unique molecules 7,8-Epoxylanostan-11-ol, 3-acetoxy, Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester, Hexadecanoic acid, methyl ester, Dasycarpidan-1-methanol, acetate, Heptadecane, 9-hexyl-, Phenol, 2,4-bis(1,1-dimethylethyl) and steroidal derivatives. These molecules are significant in the development of new drugs and pharmaceutical prospects. Further studies are under process for the identification of various pharmaceutical activity to develop a better novel pharmaceutical.
Acknowledgment
The authors are thankful to Dr. Remesh Moochikkal, herbarium curator for the identification of Caralluma retrospiciens and maintaining the samples in the herbarium of Jazan University (JAZUH) and also special thanks to Mr. Rahimullah Siddiqui, Mr. Mohamed Eltaib Elmobark for their help during the study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomed.. 2019;15:1-9.
- [CrossRef] [Google Scholar]
- Phytochemical screening of methanolic leaves extract of Malva sylvestris. Int. J. Pharmacogn. Phytochem. Res.. 2017;9:537-552.
- [CrossRef] [Google Scholar]
- Analysis of the cuticular wax composition and ecophysiological studies in an arid plant - Ziziphus nummularia (Burm.f.) Wight & Arn. Saudi J. Biol. Sci.. 2020;27:318-323.
- [Google Scholar]
- Herbal beverages: Bioactive compounds and their role in disease risk reduction - A review. J. Tradit. Complement. Med.. 2018;68:451-458.
- [CrossRef] [Google Scholar]
- Phytochemical and FT-IR spectral analysis of Caralluma geniculata Grev. et Myur. An endemic medicinal plant. J. Chem. Pharm. Res.. 2014;6(7):2083-2088.
- [Google Scholar]
- Presence of phthalate derivatives in the essential oils of a medicinal plant Achillea tenuifolia. DARU J. Pharm. Sci.. 2014;22(1):78.
- [CrossRef] [Google Scholar]
- Essential oils used in aromatherapy: a systemic review. Asian Pac. J. Trop. Biomed.. 2015;5:601-611.
- [Google Scholar]
- Microbiology - A Laboratory Manual. USA: Pearson Education Inc; 2014.
- Identification of bioactive compounds from flowers of black elder (Sambucus nigra L.) that activate the human peroxisome proliferator-activated receptor (PPAR) gamma. Phytother. Res.. 2010;24S:S129-S132.
- [Google Scholar]
- Antioxidant activity of phenolic and alkaloid fractions accumulated in Artemisia judaica and Artemisia Herba Alba. J. Nat. Med.. 2017;17:154-164.
- [Google Scholar]
- Antineophobic effect of the neuroactive steroid 3alpha-hydroxy-5beta-pregnan-20-one in male rats. Pharmacol. Biochem. Behav.. 1998;60:125-131.
- [Google Scholar]
- An evidence-based review on wound healing herbal remedies from reports of traditional persian medicine. J. Evid. Based Complement Alternat. Med.. 2017;22:334-343.
- [Google Scholar]
- Jou, P., 2015. 10 Endemic Flowers of Saudi Arabia. Destination: https://destinationksa.com/10-endemic-flowers-of-saudi-arabia/2015.
- GC-MS analysis of Caralluma diffusa (Wight) N.E.Br. Asian J. Plant Sci. Res.. 2013;3:130-133.
- [Google Scholar]
- Fabrication and physicochemical and antibacterial properties of ethyl cellulose structured cinnamon oil oleogel: relation between ethyl cellulose viscosity and oleogel performance. J. Sci. Food Agric.. 2019;99(8):4063-4071.
- [Google Scholar]
- FT-IR and GC-MS analysis of bioactive phytocompounds in methonalic leaf extract of Cassia Alata. Biomed. Pharmacol. J.. 2018;11:141-147.
- [Google Scholar]
- Synthesis of new O-alkyl and alkyne–azide cycloaddition derivatives of 4′-methoxy licoflavanone: a distinct prenylated flavonoid depicting potent cytotoxic activity. Med. Chem. Res.. 2015;24:669-683.
- [Google Scholar]
- Antibacterial activity of di-butyl phthalate isolated from Begonia malabarica. J. Appl. Biotechnol. Bioeng.. 2018;5:101-104.
- [Google Scholar]
- In vitro antifungal potentials of bioactive compounds heptadecane, 9- hexyl and ethyl iso-allocholate isolated from Lepidagathis cristata Willd. (Acanthaceae) leaf. Brit. Biomed. Bull.. 2015;3:336-343.
- [Google Scholar]
- New pregnane glycoside derivative from Caralluma retrospiciens (Ehrenb) Nat. Prod. Res.. 2015;29:1426-1431.
- [Google Scholar]
- Makeen, H.A., Santhosh J.M., Sivakumar, S.M., Saad Saeed, A., Zia ur, R., Md Shamsher., A., Syam, M., Mohammed, A., 2020. Documentation of bioactive principles of the exudate gel (EG) from the stem of Caralluma retrospiciens (Ehrenb) and in vitro antibacterial activity – Part A. Arab. J. Chem. 31, 6672–6681. https://doi.org/10.1016/j.arabjc.2020.06.022.
- Viscosity dictates metabolic activity of Vibrio ruber. Front. Microbiol.. 2012;3:255.
- [CrossRef] [Google Scholar]
- Identification of phytochemical components of aloe plantlets by gas chromatography-mass spectrometry. Afr. J. Biotechnol.. 2013;12:6876-6880.
- [Google Scholar]
- Fresh edible flowers of ornamental plants- A new source of nutraceutical foods. Trends Food Sci. Technol.. 2011;22:561-569.
- [Google Scholar]
- Potency of nano-antibacterial formulation from Sargassum binderi against selected human pathogenic bacteria. Braz. J. Pharm. Sci.. 2018;54(4):e17811
- [CrossRef] [Google Scholar]
- Moni, S.S., Sultan, M.H., Makeen, H.A., Jabeen, A., Sanobar, Siddiqui, S., Ur Rehman, Z., Alam, M.S., Ahmad, S., Elmobark, M.E., Moochikkal, R., 2019. Phytochemical and spectral analysis of the methanolic extracts of leaves of Murraya koenigii of Jazan, Saudi Arabia. Nat. Prod. Res. 10.1080/14786419.2019.1679137
- Phenol, 2,4-bis(1,1-dimethylethyl) of marine bacterial origin inhibits quorum sensing mediated biofilm formation in the uropathogen Serratia marcescens. Biofouling. 2014;30:1111-1122.
- [Google Scholar]
- Plant diversity of Saudi Arabia, 2019. http://www.plantdiversityofsaudiarabia.info/index.htm.
- Chemical composition and antibacterial activity of essential oils from the Tunisian Allium nigrum L. Excli. J.. 2014;13:526-535.
- [Google Scholar]
- Phytochemical screening and GC-MS analysis of leaf extract of Pergularia daemia (Forssk) Chiov. Asian J. Plant Sci. Res.. 2017;7:9-15.
- [Google Scholar]
- A systematic revision on Caralluma species of Saudi Arabia based on karyological and molecular data. Pak. J. Bot.. 2015;47(3):937-950.
- [Google Scholar]
- FT-IR Spectroscopic analysis of Holoptelea integrifolia (Roxb.) plant seed extracts and their antibacterial activity. Res. J. Med. Plant. 2015;9:417-426.
- [CrossRef] [Google Scholar]
- Phytopharmacological review on flowers: source of inspiration for drug discovery. Biomed. Prev. Nutr.. 2020;4:45-51.
- [Google Scholar]
- The chemical composition and antimicrobial activity of the essential oil of Lavandula coronopifolia growing in Saudi Arabia. J. Chem. Pharm.. 2014;6:604-615.
- [Google Scholar]
- Ethnobotany of medicinal plants in the Thar Desert (Sindh) of Pakistan. J Ethnopharmacol.. 2015;163:43-59.
- [Google Scholar]







