Translate this page into:
Durable biobased hybrid compounds: Potential modifying agents for the development of functional cotton fabrics
∗Corresponding author. mihmondal@gmail.com (Md. Ibrahim H. Mondal),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Abstract
The advancement of antibacterial, stain-resistant, and easy-to-clean multifunctional cotton fabrics finds its scientific appeal and practical value due to their multidisciplinary uses in pharmacy, sanitation, clinics, etc. In this investigation, the cotton fabric was immersed in chitosan- vinyltriethoxy silane (Ch-VTES) and N-(2-hydroxy)propyl-3-trimethylammonium chitosan chloride- vinyltriethoxy silane (HTACC-VTES), prepared via the cost-effective sol–gel process to produce self-cleaning and antimicrobial cotton fabrics for end-uses. The Ch-VTES and HTACC-VTES modified cotton fabrics showed encouraging water contact angles of 102° and 139° respectively i.e. closer to superhydrophobicity as well as strong self-cleaning behavior without compromising the physicochemical properties of unmodified cotton fabric. Notably, the modified fabric demonstrated enchanting bacterial killing efficiency with a noticeable zone of inhibition against E. coli (17 mm for Ch-VTES and 21 mm for HTACC-VTES modified fabrics) and S. aureus (20 mm for Ch-VTES and 25 mm for HTACC-VTES modified fabrics) bacteria. Both modified cotton textiles showed an absorption peak at 1208 cm−1 (Si-O-C bending) in FTIR, suggesting that silane binds to the cotton substrate more firmly. The stability and longevity of the modified cotton fabrics with desired properties remain unchanged till 15 cycles of washing for the antibacterial test and the 20 cycles for the water contact angle. The fabricated textiles would be used for a wide range of uses, including medical applications as well as personal care products.
Keywords
Antibacterial
Easy-cleaning
Cotton textile
Quaternary chitosan
Silane
1 Introduction
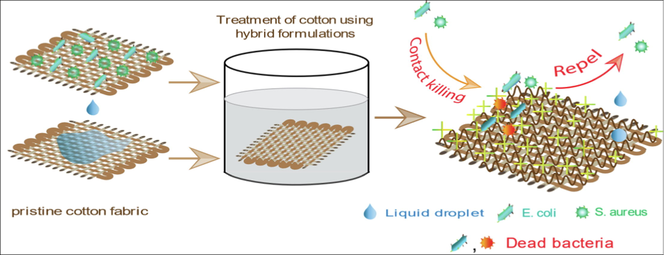
Cotton textiles have reached the pinnacle of the textile industry in modern society, opening up multiple applications in fields such as manufacturing, architecture, upholstery, medical, and so on, due to top-notch characteristics such as high comfort, air permeability, solid biodegradability, renewability, hygroscopicity, etc. Hygroscopicity i.e. hydrophilic behavior of cotton textiles owing to their hydroxyl (–OH) group present in cotton lessen its potential uses in medical garments, and personal care textiles which provides a proper environment for frequent growth and prosperity of microorganisms, as well as enhances the probability of stain on the cotton textile with liquids, soil, dust, and other contaminants. Some bacteria are indeed accountable for the discoloration and degradation of textile apparel, as well as the production of unpleasant odors (Bu et al. 2019, Chauhan et al. 2018, Mondal and Saha 2019). To resolve such deficiencies as bacterial proliferation, bad odor caused by bacteria, and staining with other pollutants on the cotton substrate, it is more dynamic to produce self-cleaning surfaces with an antimicrobial finish on cotton fiber for multifaceted applications (see Scheme 1).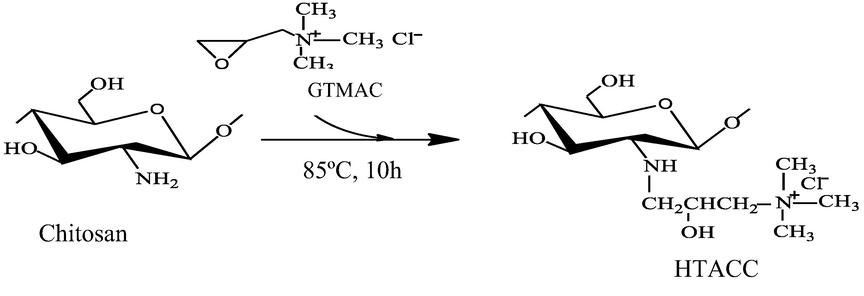
N-(2-hydroxy)propyl-3-trimethylammonium chitosan chloride (HTACC) from chitosan.
Several researchers have made an impressive endeavor to fabricate self-cleaning or hydrophobic antimicrobial cotton textiles in order to discover new areas of the application over the last few decades (Bu et al. 2019, Chauhan et al. 2018, Ghasemi et al. 2018, Shateri-Khalilabad and Yazdanshenas 2013). For instance, Ivanova et al. (2012) have used the spray coating process to establish superhydrophobic anti-bacterial textiles for biomedical applications modified by chitosan-based hydrophobic nanoparticles with negatively charged fluoro anions. But the fluorinated textile is not consumers health friendly. Chauhan et al. (2018) developed superhydrophobic cotton fabric in another study using a hexadecyltrimethoxysilane (HDTMS) solution that revealed self-cleaning and stain-resistant properties and it would also be an excellent medium to isolate oil from the mixture of oil and water. Another study shows the modified cotton fabric subsequently showed repellency to liquids whose surface tension exceeded 47mN/m and also showed potential antibacterial activity (Chauhan et al. 2018). Also using sol–gel coating, Tomsic et al. (2008) established multifunctional cotton fabrics displayed antimicrobial activity and consequently water and oil repellence properties that may indirectly influence antibacterial properties by impacting bacterial adhesion on cotton fibers because lower surface energy results in the decreased bacterial stain. In addition to getting a potential hydrophobic antibacterial cotton textile multiple nanoparticles have been used including Ag (Bu et al. 2019, Shateri-Khalilabad and Yazdanshenas 2013), ZnO (Ghasemi et al. 2018; Ren et al. 2018), TiO2 (Syafiq et al. 2019), SiO2 (Yazdanshenas and Shateri-Khalilabad 2013), Cu (Yang et al. 2017), etc. for imparting nanoscale roughness (Bu et al. 2019, Ghasemi et al. 2018) and also antimicrobial (Shateri-Khalilabad and Yazdanshenas 2013, Yang et al. 2017) activity on the cotton substrate with low surface energy forming materials. Earlier studies have used numerous methods for the preparation of hydrophobic antimicrobial cotton fiber, including sol–gel-based coating (Tomšič et al. 2008), dip coating (Ghasemi et al. 2018, Yang et al. 2019), spray coating (Lin et al. 2018), wet-chemical technique (Wang et al. 2017), solution-immersion coating (S. Li et al., 2008; Ren et al., 2018), and so-called froth, applicable in many fields, such as self-cleaning textiles (Chauhan et al. 2018), antibacterial textiles (Shateri-Khalilabad and Yazdanshenas 2013), oil–water separating substrate (Yang et al. 2019), UV blocking textiles (Wang et al. 2011), microfluidic transport (Xing et al. 2013).
However, different types of inorganic particles, including nano-sized particles, have been added to natural fiber as an important antimicrobial agent that carries harmful effects to natural fiber from the biodegradation point of view. Despite possessing excellent properties of inorganic particles, it induces slower biodegradation due to inhibition of the activity of the microbes responsible for biodegradation present in soil or water, as well as the killing of neighboring microbes through the mechanism of leaching of those particle species within the surrounding fibers, which eventually have a detrimental effect on the environment (Lazić et al. 2015, Tomsic et al. 2011). Furthermore addressing the treatment of fluorination onto cotton textiles to minimize surface energy poses considerable challenges due to the negative impact of fluorination on the human body (Ren et al. 2018). Previous findings found that little attention was paid to the compound extracted from natural waste as an enticing antimicrobial agent for the fabrication of hydrophobic cotton textiles with an antimicrobial activity using materials that have low surface energy. In this regard, non-leaching antimicrobials, such as quaternary chitosan, have the noticeable capability of maintaining the significant value of chitosan offers high durability and washing resistance as compared to leaching antimicrobials such as metal salts (e.g. Silver, copper, zinc, etc.), halogenated phenols (triclosan), in order to strong binding nature (Speier 1982, White and Monticello 2002).
Designing a promising hydrophobic antimicrobial cotton fabric using a compound extracted from natural fishing industry waste as an antimicrobial agent is, therefore, a well-timed challenge. Furthermore, The considerably decreased bacterial adhesion on the surfaces of superhydrophobic materials becomes one of the most prominent aspects of superhydrophobicity (Zhang et al. 2013). Superhydrophobicity may lessen the force of adhesion between bacteria and a clean cloth, allowing bacteria to be effectively cleaned before a thick biofilm forms on the cloth surface (Crick et al. 2011).
Naturally, the cotton fabric is hydrophilic, prone to microbial attack and easily stained with dirt as well. But when this fabric is modified with silane monomer like vinyltriethoxy silane, it gains superhydrophobicity which in turn provides anti-staining i.e. self-cleaning activity without having antimicrobial activity. Again, chitosan and quaternary chitosan impart antimicrobial activity to cotton fabrics but no self-cleaning properties. So, when silane monomer and chitosan or quaternary chitosan are blended together could impart both antimicrobial and self-cleaning properties on cotton fabric. There are no related research in literature on modification of cotton fabrics using chitosan or quaternary chitosan and silane coupling agents-based hybrid materials. In this study we have developed hybrid compounds like chitosan- vinyltriethoxysilane (Ch-VTES) and N-(2-hydroxy)propyl-3-trimethylammonium chitosan chloride-vinyltriethoxysilane (HTACC-VTES) by sol–gel method and in-situ applied on cotton fabric by simple immersion technique. Because of their incredibly hydrophobic properties as well as holding a positive charge, these textile surfaces repel and destroy bacteria and at the same time, they also possess anti-stain activity. Besides this study focused on comparing the properties of two-hybrid compounds modified fabric, with particular attention to antimicrobial and self-cleaning properties. Washing durability properties of two-hybrid compound modified textile have also been added.
2 Experimental
2.1 Materials
Plain weave poplin fabric (100% cotton) was collected from Akij Textile Ltd, Bangladesh. The shrimp shell was collected from Khulna region of Bangladesh, which is infamous for dumping waste from the nearest shrimp processing area. Chitosan (Degree of deacetylation- 86%) Vinyltriethoxysilane (VTES) (≥99%), glycidyltrimethylammonium chloride (≥90%), N-methyl-2-pyrrolidone (≥99%), iodomethane (≥98.5%), sodium hydroxide, sodium carbonate, ethanoic acid, ethanol, sodium chloride, potassium bromide (KBr), hydrochloric acid, acetone, etc. were procured from Sigma- Aldrich (Germany), BDH (England) Merck (Germany), Research Lab and SRL (India). All these reagents used were of analytical grade.
2.2 Formulation of chitosan
Various chemical treatments were used to prepare chitosan from collected shrimp shell waste. After washing with hot water, grinding with hammer mill, and drying with oven, the physically processed shrimp shell has been passed through a set of chemical treatments namely demineralization, deproteinization, decoloration of using the different chemical agents in definite proportion to produce chitin, and finally using the deacetylation process chitin is converted to chitosan which has greater commercial value (Li et al. 1992).
2.3 Preparation of quaternary chitosan
2.3.1 N-(2-hydroxy)propyl-3-trimethylammonium Chitosan chloride (HTACC)
Quaternization of chitosan (2 gm) was performed by adding 7.1 mL of glycedyl trimethyl ammonium chloride (GTMAC), a derivating agent, in three portions (2.37 mL each) at 2 h intervals. Whereas, chitosan was completely dispersed into distilled water in the reaction vessel, at that time a certain amount of GTAMC was added to the reaction vessel. After the reaction, 200 mL of cold acetone was added to the desired faint yellowish solution while stirring and kept in the refrigerator overnight. The gel-like product was obtained after decanting of remaining acetone. A certain amount of methanol was added to dissolve the gel-like product. The solution was precipitated (white product) using acetone-ethanol (v/v, 4:1). The filtration process was used to collect the white product which was crude HTACC and then washed with hot ethanol using a soxhlet extractor. After washing the crude HTACC, the ultimate final product was collected and dried at 60 °C overnight (Lim and Hudson 2004).
2.3.2 Preparation of chitosan-silane hybrid compounds
After dissolving chitosan or quaternary chitosan in DMSO for 12 h at 80 °C, vinyl triethoxy silane (VTES), a silane coupling agent, was added to the solution at room temperature and the mixture was stirred for 30 h. An aqueous 1% HCl solution was uniformly mixed with the above solution which acts as a catalyst. The solvent was evaporated using drying oven and the resulting hybrid semi-solid substance was washed with distilled water and dried under vacuum (Uragami et al. 2004).
2.4 Cotton fabric
The cotton fabrics were first treated with 1% Na2CO3 solution at 75 °C for 30 min in a fabric: liquor ratio of 1:50. The fabrics were then thoroughly washed with distilled water and then dried in a oven at 60 °C.
2.5 Modification of cotton textile with prepared hybrid compounds
A certain amount of cotton fabric was immersed in the prepared chitosan-silane or quaternary chitosan-silane hybrid compund solution and stirred continuously for 1.5 h at 65 °C temperatures. To keep the liquor's pH around 3.5–4, acetic acid was added. The wettability of the solution was improved by adding sodium lauryl sulfate and Triton-X. Thereafter the modified cotton was washed with deionized water and dried in the open air and then in an oven at 60 °C, to a constant weight. To complete the polycondensation reaction between the cotton fabric and functionalized silane, the fabric were dried for 30 min at 60 °C before being cured for 20 min at 120 °C. The fabrics were then conditioned for about 4 h at a temperature of 27 °C and relative humidity around 65 percent (Saif et al. 2015).
2.6 Assessment of physicochemical properties of modified fabrics
2.6.1 Add-on percentage
The cotton fabrics were mainly modified with Ch-VTES and HTACC-VTES. The fabric's weight increased as a result of this. The weight supplement was computed using the following formula (Ammayappan and Moses, 2009).
Where W1 and W2 are the weight of unmodified and modified fabrics respectively.
2.6.2 Fabric weight
Fabric weight per unit area was measured as per Standard ASTM-D 3776-96 (2002).
2.6.3 Moisture absorption study
At a constant humidity level, modified and unmodified (Sample size: 2.5 × 2.5 cm2) cotton fabrics were investigated for moisture absorption analysis. Both types of cotton fabric samples were stored in a humidity chamber at room temperature for 48 h, where humidity maintained at saturation level. Following that, the fabrics were analyzed using a moisture analyzer, to obtain the moisture content. The moisture content was determined using the following formula (Abbasipour et al. 2014).
Where Wf and Wi are the weight of the wet sample and dry sample respectively.
2.6.4 Water vapor permeability
The following formula was used to calculate water vapor permeability under the BS 7209 test (1990).
2.6.5 Tensile strength test
For rapid and reliable tensile strength measurement, the tests were carried out using a “Universal Tensile Tester” from Fanyuan Instrument (HF) Co. Ltd., China. The length of each specimen between the machine's jaws was 10 cm, and the cotton fabrics were cut into equal pieces of 25 × 5 cm2. After starting the machine, the breaking weight was steadily raised until the specimen was broken down. The machine came to a halt at the point of failure. The breaking load was shown in N on the tensile tester's scale (Nasreen et al. 2018).
2.7 Biodegradation study
The Swain procedure was used to conduct the soil depletion examination, with certain design changes. In three pots, loaded with nearly 75% soil and 25% cow dung, then modified or unmodified samples (Sample size: 2.5 × 2.5 cm2) were placed under soil beneath around six inches from the surface. Enthralling at daily intervals, 100 mL of water was introduced to the containers. Weight loss after every 10 days interval was used to assess how much the samples had eroded. The samples were carefully taken out of the dirt and gently cleaned with water before being dried in the sun. Weight loss was calculated according to the following equation (Swain et al. 2015).
where W1 is the original weight and W2 is the weight after-burial.
2.8 Swelling capacity
Water treatment was used to assess the swelling ability of both modified and unmodified cotton fabrics. For 72 h, both fabrics (2.5 × 2.5 cm2) were soaked in 100 mL of solvents at room temperature. With the aid of filter paper, the samples were extracted and the residual solvent was eliminated before going to take the final weight. The swelling in percentages was determined in the following way from the rise in original weight (Mondal et al. 2018, Singha and Thakur 2009).
Where Wf and Wi are the weight of swelled sample and dried sample respectively.
2.9 Fourier transform infrared spectrophotometer (FTIR)
The fabric samples and potassium bromide (KBr) were dried in an oven at 105 °C for 10 h. Using a mortar and pestle, the samples were blended with KBr pellets to make a powdered form in a mass proportion of 1:100 (1 mg sample and 100 mg KBr). Blended samples were dried again and the FTIR spectra of the sample was then obtained using a Shimadzu IR-8900 spectrophotometer (Shimadzu, Kyoto, Japan) with a scanning scale of 400–4000 cm−1 (Bagheri-Khoulenjani et al. 2009).
2.10 Scanning electron microscopy (SEM)
The microstructure and surface morphology were observed using a scanning electron microscope (SEM) (FEI Quanta Inspect, Model: S50, Netherlands) to confirm hybrid compounds (Ch-VTES and HTACC-VTES) attachment to fabric and orientation on the fabric sample. The samples were studied under 5000 magnifications and scanned at 5 kV (Hunt and James 1993).
2.11 Thermal analysis
Seiko-Exstar-6000, TG/DTA-6300 (Seiko Instruments Inc. Japan) was performed to analyze the thermal decomposition rate and thermal stability of the modified and unmodified fabrics. This study was carried out in a nitrogen atmosphere with a temperature range of 25–600 °C, a heating rate of 10 °C/min, and a nitrogen flow rate of 200 mL/min. As a function of temperature, the sample’s weight loss was constantly reported.
2.12 Contact angle measurements
At room temperature, static contact angles were measured using a self-developed smartphone-based contact angle measurement device to evaluate the surface-wetting properties of the modified and unmodified fabric (2.5 × 2.5 cm2) surfaces. In all of the calculations, the volume of a single water droplet was 5 µL. In a stationary condition all of the recorded contact angles were measured after 30 s of falling 5 µL water drop onto the fabric surface (Chen et al. 2018, Zimmermann et al. 2009).
2.13 Self-cleaning assessment
Self-cleaning tests were conducted to determine the modified cotton textile's easy-cleaning quality. To assess the modified cotton textile's easy-cleaning efficiency, solid and liquid contaminants such as coffee powder and Methylene blue solution (0.01%) were used (Yang et al. 2019) respectively and the fabric samples were cut into 2.5 × 2.5 cm2 size.
2.14 Antibacterial assay
The disc diffusion method was used to study the antibacterial activity of modified cotton fabrics. The fabrics were cut into discs of about 5 mm in diameter, and each type of bacteria and sample was placed in its own sterile petri dish. The E. coli and S. aureus were subcultured in nutrient broth for 24 h at 37 °C by disk diffusion method. Around, 10 mL of sterilized nutrient agar was poured into culture petri plates. Then the fabric disc was loaded on the media where the bacterial strain was spread uniformly and all the plates were incubated at 37 °C for 24 h to ensure bacterial proliferation.
The parallel streak approach was used to assess the inhibition zones of modified and unmodified samples according to the AATCC-147 guideline (2010). The inhibition zone was determined using the following formula (VI).
Where W is the clear zone of inhibition in width (mm), D is the diameter of the test specimen
in mm and T is the overall diameter of the test specimen and clear zone of inhibition in mm.
2.15 Antioxidant activity (DPPH Assay)
Antioxidant activity was assessed using the widely used 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging test, which measures the capacity of antioxidants to neutralize free radicals, which are known to play a role in the development of oxidative stress-related effect on bacteria (Jadav and Gowda 2017, Singh and Sheikh 2020). As the DPPH radical interacts with the hydrogen donor species, this approach provides information on the investigated compounds' antioxidant capacity. For 30 min, cotton samples (2.5 × 2.5 cm2) were exposed to a DPPH in 0.1 mM methanolic solution at 37 °C in the dark with vigorous shaking. The UV/VIS spectrophotometer was used to check the absorbance at 517 nm of reaction solutions. The following equation (7) was used to compute the antioxidant activity:
where Ac is the absorbance of blank DPPH solution and As is the absorbance of DPPH solution in the contact with functionalised cotton fabric.
2.16 Measurement of UV protection factor
The transmission through the modified and unmodified cotton fabrics (4.5 × 1 cm2) was measured in the ultraviolet radiation range of 280––400 nm with a 5 nm interval. The UPF was measured by UV spectrophotometer (Shimadzu 1650, Japan). The UPF was calculated according to the AATCC test method (AATCC, 2010) by the following equation (8):
where Eλ is erythemal spectral effectiveness, Sλ is solar spectral irradiancies, λ is the wavelength in nm, Tλ is the spectral transmission of the fabric and Δλ is the measured wavelength intervals in nm.
2.17 Wash durability test
The modified fabric (5 × 5 cm2) was subjected to washing by an industrial machine and the antibacterial activity of the washed sample was evaluated by the AATCC-100 (2010) and AATCC 124 (2010) test standards respectively.
2.18 Statistical analysis
All the experiments were carried out in triplicate and the data were expressed as mean ± standard deviation (SD, n = 3) for reproducibility using SPSS 22.0 for windows (SPSS, Chicago, IL, USA). One-way ANOVA was used to test differences among the results which were considered statistically significant at p-value<0.05 (p < 0.05).
3 Results and discussion
This study focuses on the functionalizations of pristine cotton-bleached fabric with as-prepared chitosan-VTES and HTACC-VTES hybrid composites and their enhanced properties. Aside from some characterizations, silane coupling agents and non-leaching antimicrobial agents, such as chitosan and HTACC, were applied to the fabric individually.
3.1 Physicochemical properties
3.1.1 Add-on percentage & fabric weight
Table 1 indicates that after modification of a cotton fabric with modifying agent, the weight add-on percentage rise, with hybrid materials onto cotton substrate exhibiting slightly more weight than when using a single modifying agent except the VTES. Consequently, the GSM (grams per square meter) of the modified fabric improved as well. Because VTES has a high affinity for cotton fabric, the add-on percent of VTES-modified fabric was higher than that of chitosan and quaternary chitosan i.e. HTACC modified fabric at the same concentration. That’s why VTES-modified fabric became higher in GSM weight and also thicker. Fig. 1 shows the photographic images of modified and unmodified cotton fabrics. The photographic images do not shows any remarkable changes. Values were recorded in three replicates (n = 3) and expressed as mean ± SD where ANOVA was performed to test the physicochemical properties of the samples significantly different at the level of 5% (p < 0.05). Identical uppercase letters of average values in the same column do not differ significantly.
Cotton fabric modified with
Weight add-on, (%)
Fabric weight
(g/m2)
Moisture Absorption (%)
Water vapor permeability (g/m2/day)
Swelling
Capacity (%)
Unmodified
0.0a
128 ± 2a
15.4 ± 0.80 d
891 ± 5.29 a
165 ± 2.08 d
VTES
8.6 ± 0.17e
140 ± 1d
4.6 ± 0.30 a
701 ± 3.51 a
57 ± 2.0 a
Chitosan (Ch)
5.1 ± 0.20c
133 ± 1b
13.2 ± 0.20c
819 ± 3.61c
178 ± 3.0 e
HTACC
4.5 ± 0.10b
132 ± 2ab
18.4 ± 0.30 e
878 ± 3.00 e
168 ± 2.0 ad
Ch-VTES
5.5 ± 0.40c
135 ± 2 bc
9.3 ± 0.30b
807 ± 2.52b
131 ± 1.0b
HTACC-VTES
7.9 ± 0.20d
138 ± 2cd
10.2 ± 0.30b
866 ± 4.00 d
148 ± 3.0c
Photographic images of (A) ummodified, (B) Ch-VTES modified and (C) HTACC-VTES modified cotton fabrics.
3.1.2 Moisture content
According to the moisture absorption test findings, unmodified cotton retained moisture from the atmosphere due to the hydroxyl groups in their structure that form hydrogen bonds. The hydrogen of hydroxyl groups in the silane-modified cotton fabric can be eliminated by oxane bond forming with a silane binding agent, resulting in a decrease in moisture absorption potential as compared to cotton fabric (Arkles 2006). On the point of attachment capability, HTACC outperformed chitosan. Finally, owing to the mixture of silane with chitosan or quaternary chitosan, which carries hydroxyl groups, cotton fabric modified with hybrid compounds retains moisture on average, but less than unmodified cotton and more than silane-modified fabric.
3.1.3 Water vapor premeability study
The capacity of a substance to permit the passage of a vapor (such as water vapor or any gas) is referred to as vapor permeability. Sweat is trapped in because moisture vapor cannot move into cotton fast enough, allowing heat to build up and cause discomfort. Even though silane-based treatment makes the fabric surface hydrophobic, it also allows for a high level of water vapor permeability (Arkles 2006). Quaternary chitosan on fabric (HTACC) has a higher hydroxyl group, which attracts water vapor and encourages it to permeate but narrows the pores due to the binding of the cotton fabric. However, as compared to other modified fabrics, the hybrid material HTACC-VTES modified fabrics exhibit endurable water vapor permeability (Table 1). In this case study, HTACC-VTES modified fabric had a marginal advantage over Ch-VTES modified substrate in terms of water vapor permeability (Nawalakhe et al. 2013).
3.1.4 Swelling study
The reflection of swelling behavior analysis showed a correlation between the voids of the polymeric backbone and the size of the solvent molecule for a given solvent (Singha and Thakur 2009). A review of swelling cotton fabric has a greater swelling potential due to the voids in its backbone and the presence of hydroxyl groups, which allow the substrate to swell up further (Table 1). Because of the hydrophobic pendant group instead of the hydroxyl group, cotton fabric with a silane coupling agent has a poorer swelling potential (Pulat and Isakoca 2006). In contrast to unmodified cotton, hybrid materials improved cotton yielded promising results.
3.1.5 Mechanical strength test
Likewise, because of the treatment of cotton fabrics for all hybrid components, the tensile strength i.e. breaking force and elongation at break of modified cotton fibric was stronger than that of unmodified cotton fabric (Table 2) according to the International Standard ISO 5081–1977 (E) (1977). Due to modifications the ruptures unmodified fabric surface were recovered which provide improved mechanical strength. Among the modified fabrics, HTACC-VTES modified cotton showed noticible performances due highest fibre reactivity. Values were recorded in three replicates (n = 3) and expressed as mean ± SD where ANOVA was performed to test the tensile strength of the samples significantly different at the level of 5% (p < 0.05). Identical uppercase letters of average values in the same column do not differ significantly.
Cotton fabric modified with
Warp Direction
Weft Direction
Breaking Force, N
Elongation at Break, %
Breaking Force, N
Elongation at Break, %
Unmodified
305 ± 4.0a
10.10 ± 0.3 a
730 ± 5.0 a
19.20 ± 0.3 a
VTES
395 ± 2.5b
10.90 ± 0.4 ab
780 ± 6.0b
26.30 ± 0.5 d
Chitosan (Ch)
410 ± 6.0c
10.50 ± 0.4 ab
810 ± 7.0c
22.10 ± 0.6b
HTACC
425 ± 4.0 d
10.80 ± 0.4 ab
880 ± 7.0 d
22.60 ± 0.2 bc
Ch-VTES
490 ± 4.0 e
11.20 ± 0.4b
910 ± 9.0 e
23.40 ± 0.6c
HTACC-VTES
520 ± 3.0f
11.50 ± 0.4b
940 ± 7.0f
25.50 ± 0.4 d
3.2 Biodegradability study
In terms of a sustainability, environmentally friendly greener society, the study of soil degradation carries cabalistic importance among numerous studies. The unmodified sample experienced the greatest weight loss as a result of soil deterioration. The involvement of microorganisms in the soil quickly infiltrated the unmodified fabric because it was not modified by any modifier that inhibited microbe assault. Thus, in every study, quaternary chitosan exhibits effective antimicrobial activity in the soil according to Fig. 2 although modified fabric with it is still biodegradable. In the presence of microorganisms, the modified fabric takes time to degrade because microorganisms that consume the natural cellulose portion and oxygen can invade the surface by producing oxides, hydroperoxides, peroxides, and so on. These oxides facilitate the scission of polymeric chains into small fragments, rendering the fabric vulnerable to microorganism attack (Peanasky et al. 1991). The soil degradation findings show that modified samples are already degradable and therefore environmentally sustainable, despite the fact that they withstand soil degradation only slightly. Among the modified fabrics, HTACC-VTES modified cotton showed the slowest weight loss i.e. degradation as it showed higher antibacterial activities due to having positively charged quaternary groups. The soil degradation in terms of weight loss (%) of unmodified, Ch-VTES and HTACC-VTES modified fabrics after 30 days was 28.4%, 17.2% and 10.8% respectively.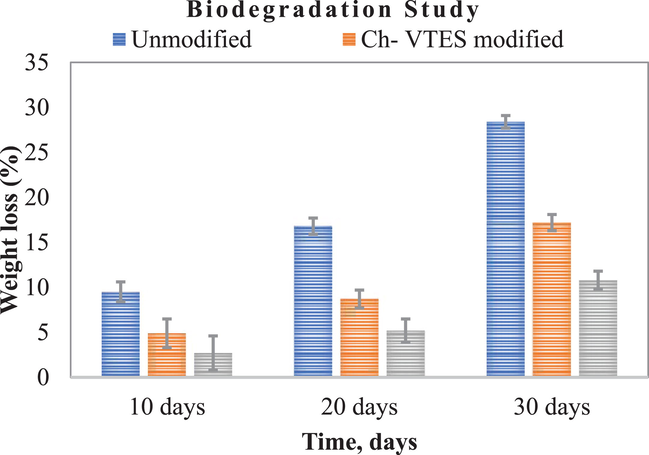
Biodegradation of unmodified, Ch-VTES modified and HTACC-VTES modified cotton fabrics at 10 days interval (10 days, 20 days and 30 days).
3.3 Water contact angle study
For demonstrating the surface wetting behavior of unmodified and processed cotton fabrics, contact angle measurements are immensely significant (Bu et al. 2019). In this experiment, a smartphone equipped with a macro lens was used to assess the contact angle of water droplets on a textile surface due to its significant advantages in terms of compact scale, portability, and versatility as compared to the efficiency of top measurement instruments. We captured the optical image with a smartphone equipped with a macro lens and analyzed it with software (Chen et al., 2017, 2018). Unmodified and modified textiles acted in opposite ways: unmodified fabric easily absorbed water droplets, but modified fabric could hold the water droplet in a spherical state. In this measurement cotton substrate modified with both Ch-VTES and HTACC-VTES hybrid materials revealed a water contact angle of 102° and 139° respectively as shown in Fig. 3. Since HTACC has a longer hydrocarbon chain than chitosan, we investigated that HTACC-VTES modified textiles had a higher contact angle. Various studies discovered that bacteria effectively attached to polymer surfaces at water contact angles between 40° and 70°, with a maximum adhesion of bacteria at a water contact angle of about 57° (Arima and Iwata 2007, Lee et al. 1998). It is possible to argue that the aforementioned hybrid compounds modified fabrics especially HTACC-silane-modified cotton is also well suited for bacterial antiadhesion (Table 1).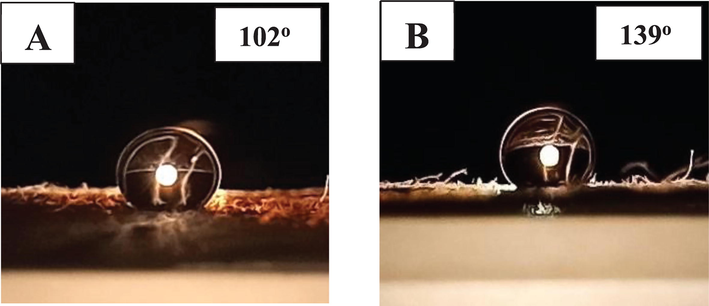
Contact angle measurements: Photographic images of water droplet on modified cotton fabric surface (A) Ch-VTES modified and (B) HTACC-VTES modified.
3.4 Self-cleaning study
The evaluation of self-cleaning properties is becoming more important in order to assess cloth performers such as water-repellent properties, stain-resistant properties, anti-dirt properties, and bacterial antiadhesion properties, among other things. A fabric surface can be stained by both solid and liquid dirt depending on the fabric’s nature, either it is hydrophilic or hydrophobic. As the cotton fabric is hydrophilic, it is easily stained with hygroscopic solid dirt and water-soluble colour compounds. For this, coffee dust as a solid pollutant and methylene blue solution as a liquid pollutant were used to analyze the efficacy of the modified cotton fabric's self-cleaning ability. From Fig. 4A, it can be seen that water droplets fell on the dirty surface of the original garment, coffee powders dispersed, diffused, and absorbed the fabrics, instantly wetting and staining the cotton. The coffee powders, on the other hand, were adhered to and absorbed by the water droplets that dropped on the modified fabric surface (Fig. 4B). On a dirty surface, the water droplet may maintain its spherical shape. Furthermore, when placed in aqueous solutions with liquid contaminants, the unmodified cotton easily absorbed the liquid and became polluted as shown in Fig. 4C. While the surface of the excellent hydrophobic textile stayed dry and tidy, it was getting closer to being superhydrophobic (Fig. 4D) Lei et al. 2017 and Shuhui et al. 2015 have reported similar observations. This demonstration elucidates the self-cleaning and anti-staining properties of the modified textile in great detail. Moreover, as compared to hydrophilic surfaces, a few studies have discovered that hydrophobic surfaces have reduced bacterial adhesion (Quirynen et al. 1989). Bacteria that have become stuck to a highly hydrophobic fabric surface, allowing increased flow or an air-bubble jet more efficient removing them (Scheuerman et al. 1998). This assertion opens up a new horizon in terms of bacterial antiadhesion for strongly hydrophobic textiles.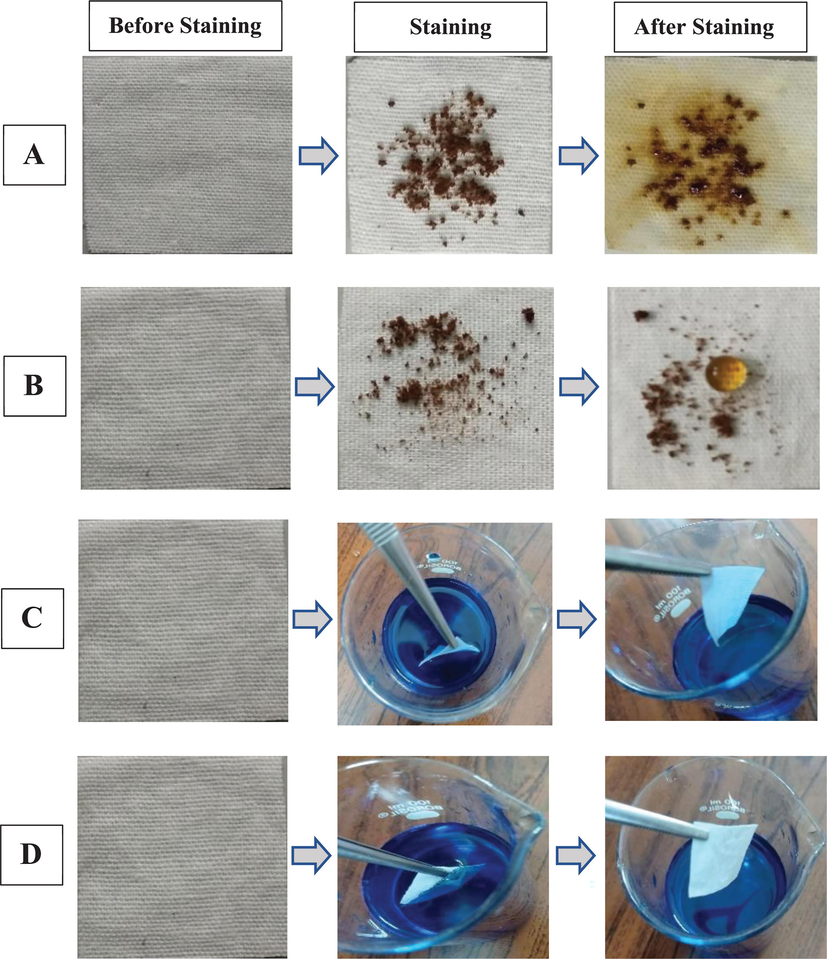
Optical photos demonstrated the wetting activity of (A) unmodified cotton fabric, (B) modified fabric against coffee dust (solid pollutant), and (C) unmodified fabric, (D) modified fabric against colored 0.01% methylene blue solution (liquid pollutant).
3.5 FTIR analysis
Fig. 5 shows the FTIR spectra of modified cotton fabric recorded with a spectrophotometer (Shimadzu IR-8900 spectrophotometer, Kyoto, Japan). Except for the latest additional peak in the modified textile, the FTIR spectra of unmodified and modified fabric were largely identical, as the absorption peaks were recorded in the spectra for the whole specimen. The absorption peak at 1280 cm−1 (Si-O-C bending) was revealed in FTIR by both modified cotton textiles, demonstrating the binding of silane coupling agents to the cotton substrate with hydrogen bonding or van der Waals forces. Cotton textiles modified with Ch-VTES and HTACC-VTES hybrid compounds reveal -Si-O-Si- linkages and -Si-O stretching at approximately 1070 cm−1 and 765 cm−1, respectively. Furthermore, at 957 cm−1 (CH2 wagging of Si-CH = CH2), both textiles with hybrid materials showed peaks at the same position in their spectra. Then, Ch-VTES and HTACC-VTES modified cotton indicated an additional peak at 2914 cm−1 and 2910 cm−1 respectively, which is evidence of N–H stretching of chitosan amine group in modified cotton fabric (Alagar et al. 2006, Rangel and Leal-García 2010, Singha and Thakur 2009).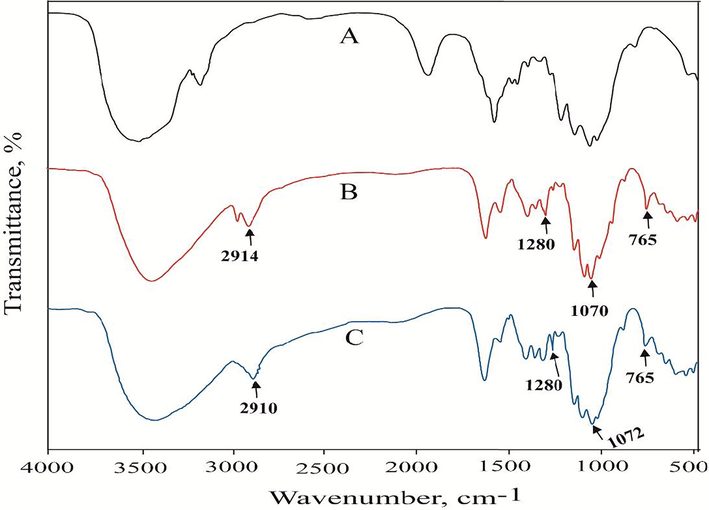
FTIR spectra of (A) Unmodified, (B) Ch-VTES modified and (C) HTACC-VTES modified cotton fabrics.
3.6 Surface morphology
The surface morphology of pristine and modified cotton textiles with hybrid substrates was analyzed using images taken with a scanning electron microscope (SEM). As seen in Fig. 6(A), a pristine cotton garment shows a significant number of protruding fibers (red circle marked) as well as micropores on the fabric surface. Very few protruding fibers, on the other hand, were observed onto the processed cotton textile. The fabric has a rough texture because of the protruding fibers (indicated by the red circle on Fig. 6(A)) (Naebe et al. 2016, Oktem 2003). Apart from that, micropores between fibers in fabrics were filled with a modified substrate that shows a minor irregularity or microstructure roughness (indicated by red circle on Fig. 6(B) & (C)), which aids in the roll-off of different liquid contaminants from the textile surface. Conglomerated granules were clearly apparent on the fiber surface, indicating that the applied substrate had adhered to the fiber. The microscale roughness of both modified fabrics is represented in both Figures.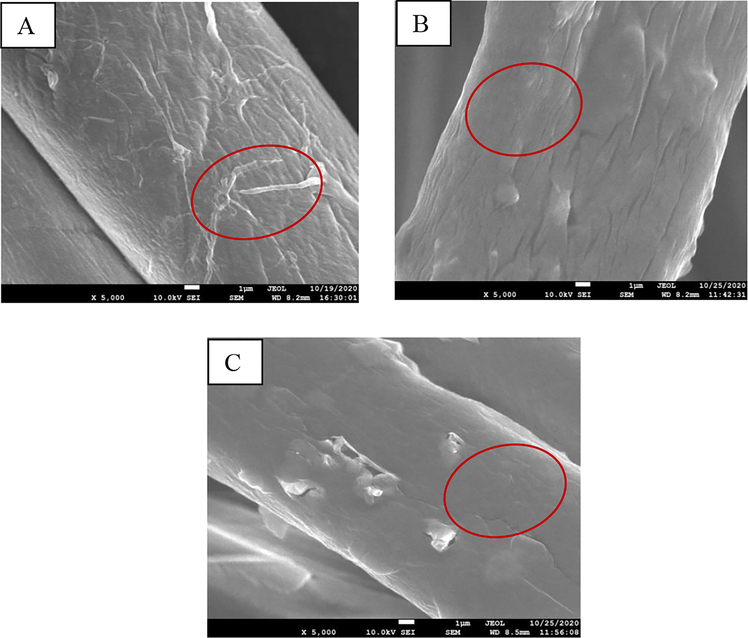
SEM images showing the surface morphology of the pristine cotton textile (A), Ch-VTES modified fabric (B), and HTACC-VTES modified fabric (C).
3.7 Thermal stability study
Thermogravimetric analysis (TGA), is a type of thermal analysis that involves calculating the mass of a sample over time as the temperature increases. Unmodified and modified cellulosic cotton fabrics degraded with thermal treatment, as seen by the TGA thermogram (Fig. 7), which indicates three levels of decomposition routes. The original decomposition temperature (Ti) of unmodified fabric is about 310 °C, 270 °C for Ch-VTES modified fabric and 250 °C for HTACC-VTES modified fabric. Unmodified, Ch-VTES and HTACC-VTES modified cotton fabrics loss 69%, 53% and 55% of their weight at 358 °C, 350 °C and 355 °C respectively. Temperatures of 300–400 °C for unmodified fabric, 270–380 °C for Ch-VTES and 250–400 °C for HTACC-VTES modified cotton fabrics resulted in drastic weight loss. The thermal stability of such modified fabrics can be ordered in the following manner based on Ti: HTACC-VTES modified < Ch-VTES modified < Unmodified cotton.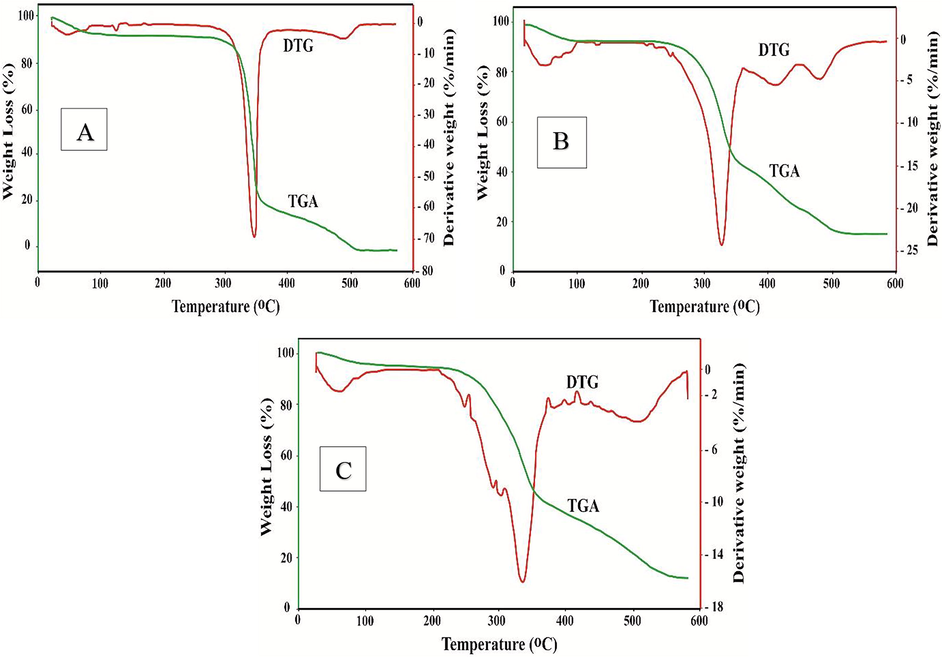
TGA/DTG curve of unmodified (A), Ch-VTES modified (B) and HTACC-VTES modified (C) cotton fabrics.
Besides, the DTG thermogram reflects the rate of decomposition at varying temperature scales. According to the study, it may be stated that Ch-VTES and HTACC-VTES fabrics are less thermally stable than unmodified fabrics. This may be attributed to the modifying agent being bound together with the surface of the cotton fibres.
3.8 Antioxidant activity
DPPH is a valuable reagent for determining a compound's ability to scavenge free radicals. This approach involves converting an alcoholic DPPH solution into a non-radical DPPH-H in the presence of an antioxidant and then measuring the color change over time. Fig. 8 shows the DPPH-radical scavenging properties of Ch-VTES and HTACC-VTES-modified cotton textiles at highest add-on percentage. The HTACC-VTES-modified cotton fabric showed the highest scavenging ability against DPPH-radical with respect to ascorbic acid reference. The results showed that the scavenging effects were 54 ± 1.1% and 69 ± 1.3% at 15% (WoF) of Ch-VTES and HTACC-VTES-modified cotton fabrics respectively. The DPPH radical's interaction with active hydrogen in quaternary chitosan derivatives to form a more stable macromolecule radical might explain the scavenging activity. To put it another way, quaternized groups are a crucial component that determines DPPH radical scavenging activity. Table 3 represents the comparision of antioxidant activity of present study with variously reported chitosan-modified cotton fabrics.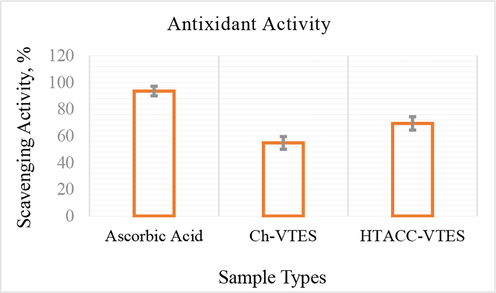
Antioxidant activity of modified cotton fabrics.
Fabric Modified with
Scavenging Activity (%)
Ref.
Chitosan/
sodium lignin sulphonate93.4 ± 0.41
(Safi et al., 2020)
Chitosan/AgNPs
96.8
(Shahid-ul-Islam et al., 2019)
Chitosan oligosaccharides
45%
(Lan et al., 2023)
Chitosan
59 ± 0.63
(Shahid-ul-Islam et al., 2020)
Chitosan-VTES
54 ± 1.1
Present work
HTACC-VTES
69 ± 1.3
Present work
3.9 UV shielding activity
The effectiveness of the modified textiles for ultra-violet protection (UPF) was assessed. The UPF values of unmodified, Ch-VTES and HTACC-VTES-modified fabrics are shown in Fig. 9. Cotton textiles enhanced with functional hybrid compounds were found to have higher UPF ratings than unmodified cotton fabrics. As a result, it should be noted that all of the tested samples provide UV protection to cotton textiles, which was beneficial in modified fabrics.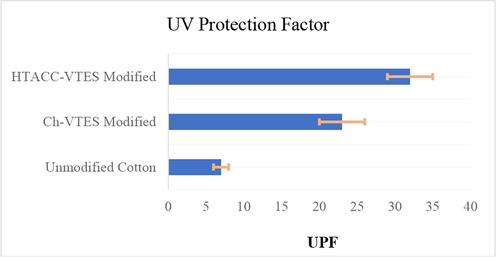
UV protection factor of unmodified, Ch-VTES modified and HTACC-VTES modified cotton fabrics.
3.10 Antibacterial activity
Antibacterial activities of the resulting fabrics toward gram-positive S. aureus and gram-negative E. coli bacteria were evaluated for antibacterial cotton textiles as shown in Fig. 10. The bactericidal or bacteriostatic effect of the garments on bacteria was investigated using a widely understood disc diffusion test. Samples including antibiotic (Chlorampehnical, 30 µg) disc were put on bacteria-spreading agar media to assess antibacterial efficacy. In interaction with both E. coli and S. aureus, a distinct and noticeable zone of inhibition (clear areas with no bacterial growth) was found for both modified samples (17 mm for Ch-VTES and 21 mm for HTACC-VTES modified fabrics against E. coli) and (20 mm for Ch-VTES and 25 mm for HTACC-VTES modified fabrics against S. aureus), whereas unmodified cotton had no antibacterial activity. In the case of both modifier hybrid compounds, antimicrobial activity is attributed to chitosan and quaternary chitosan (HTACC). In the course of this inquiry, the existence of the inhibition zone specifically suggested that the fabric's biocidal activity was mediated by non-leaching type chitosan and quaternary chitosan with a positive charge. Most fungi and bacteria have negatively charged residues on their cell surfaces, and this positive charge (N+) interacts with them. This electrostatic attraction leads to the leakage of cell membrane and causes cell fluid out. The interaction also causes protein denaturation which in term hinders the replica of DNA. In this way microorganisms natural metabolism is inhibited, which eventually leads to their destruction (Huang et al. 2013, Kannan er al. 2021a & 2021b).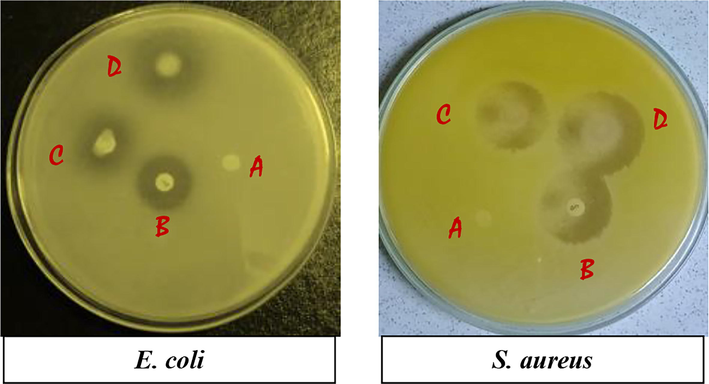
Antibacterial plate photos of the (A) unmodified, (B) Antibiotic (Chloramphenical), (C) Ch-VTES modified and (D) HTACC-VTES modified cotton fabrics against E. coli and S. aureus.
The investigation reveals that, HTACC-VTES modified cotton fabric had better antibacterial efficacy than Ch-VTES-modified cotton fabric against both S. aureus and E. coli bacteria. As the modified fabrics showed superhydrophobicity (especially HTACC-VTES modified fabric), bacteria can not easily be attached and proliferated on the fabric surface. The results reveal that the modified fabrics can be used for hygiene as well as healthcare purposes.
3.11 Wash durability study
In the consideration of wash durability both modified fabrics exhibited significant durability against antibacterial assessment as well as water contact angle. The stability and durability of the modified cotton fabric with desired properties remain till the 15th cycles of washing for the antibacterial test and the 20th cycles for water contact angle. After these washing cycles both the antimicrobial activity and water contact angle decrease sharply. Between the modified fabrics HTACC-VTES modified showed better performances than Ch-VTES modified cotton as higher reactivity of HTACC towards the cellulosic fabric.
4 Conclusion
In this study, sol–gel approach was used to prepare quaternary chitosan-silane based hybrid compounds (Ch-VTES and HTACC-VTES) and applied on cotton fabric using the immersion technique at a cost-effective and environmentally safe condition. Various physicochemical properties and instrumental analyses like FTIR, TGA and SEM were carried out to confirm the attachment of hybrid compounds to cotton fabrics. Modified cotton fabrics showed effective antibacterial activity with respect to antibiotics against S. aureus and E. coli. The hybrid compounds on cotton fabric dramatically increased the modified fabric's water contact angle i.e. hydrophobicity. That’s why, modified fabrics showed excellent self-cleaning properties against both solid (coffee dust) and liquid (methylene blue) pollutants. Furthermore, the excellent water vapor permeability and biodegradability makes these modified fabrics more feasible in terms of comfort and environmental considerations. After 15th cycles of washing for antibacterial test and 20th cycles for water contact angle, also the modified fabrics essentially retained the desired mechanical and physical properties. Thus the work has aided in the development of environmentally sustainable, antimicrobial and self-cleaning cotton textile products that would be used in healthcare as well as medical settings.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Coated cotton gauze with Ag/ZnO/chitosan nanocomposite as a modern wound dressing. J. Eng. Fibers Fabr.. 2014;9:124-130.
- [CrossRef] [Google Scholar]
- Studies on thermal, thermal ageing and morphological characteristics of EPDM-g-VTES/LLDPE. Eur. Polym. J.. 2006;42(2):336-347.
- [CrossRef] [Google Scholar]
- Study of antimicrobial activity of aloevera, chitosan, and curcumin on cotton, wool, and rabbit hair. Fibers Polym.. 2009;10(2):161-166.
- [CrossRef] [Google Scholar]
- Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials. 2007;28(20):3074-3082.
- [CrossRef] [Google Scholar]
- Hydrophobicity, Hydrophilicity and Silanes. Paint and Coatings Industry. 2006;22(10):114.
- [Google Scholar]
- An investigation on the short-term biodegradability of chitosan with various molecular weights and degrees of deacetylation. Carbohydr. Polym.. 2009;78(4):773-778.
- [CrossRef] [Google Scholar]
- BS 7209, 1990. Specification for water vapour permeable apparel fabrics. British Standards Institution.
- Fabrication of durable antibacterial and superhydrophobic textiles via in situ synthesis of silver nanoparticle on tannic acid-coated viscose textiles. Cellul.. 2019;26(3):2109-2122.
- [CrossRef] [Google Scholar]
- Self-cleaning, stain-resistant and anti-bacterial superhydrophobic cotton fabric prepared by simple immersion technique. J. Colloid Interface Sci.. 2018;535:66-74.
- [CrossRef] [Google Scholar]
- Surface tension measurement with a smartphone using a pendant drop. Colloids Surf A Physicochem Eng Asp. 2017;533:213-217.
- [CrossRef] [Google Scholar]
- Contact angle measurement with a smartphone. Rev. Sci. Instrum.. 2018;89(3):035117
- [CrossRef] [Google Scholar]
- An investigation into bacterial attachment to an elastomeric superhydrophobic surface prepared via aerosol assisted deposition. Thin Solid Films. 2011;519(11):3722-3727.
- [CrossRef] [Google Scholar]
- D 3776-96, 2002. Standard test methods for mass per unit area (weight) of fabric. ASTM International, 5. https://doi.org/10.1520/D3776-96R02http://www.teycsa.com/files/astmnorma.pdf.
- Imparting superhydrophobic and antibacterial properties onto the cotton fabrics: Synergistic effect of zinc oxide nanoparticles and octadecanethiol. Cellul.. 2018;25:4211-4222.
- [CrossRef] [Google Scholar]
- Antibacterial activity evaluation of quaternary chitin against Escherichia coli and Staphylococcus aureus. Int. J. Biol. Macromol.. 2013;52:85-91.
- [CrossRef] [Google Scholar]
- Hunt, B. J. (Barry J., James, M. I., 1993. Polymer characterisation, edited by Hunt, B.J. and James, M.I.. (1st ed.), Springer-Science+Business Media,BV., The Netherlands. https://doi.org/10.1007/978-94-011-2160-6.
- International Standard ISO 5081-1977 (E), 1977. Determination of breaking strength and elongation (Strip Method). International Organization for Standardization.
- Superhydrophobic chitosan-based coatings for textile processing. Appl. Surf. Sci.. 2012;263:783-787.
- [CrossRef] [Google Scholar]
- Antioxidant property of cotton fabric dyed with natural dye extracted from bark peel of araucaria columnaris. IOSR Journal of Polymer and Textile Engineering (IOSR-JPTE). 2017;4(2):21-26.
- [CrossRef] [Google Scholar]
- Y3+ and Sm3+ co-doped mixed metal oxide nanocomposite: Structural, electrochemical, photocatalytic, and antibacterial properties. Applied Surface Science Advances. 2021;4:100085
- [CrossRef] [Google Scholar]
- Photocatalytic and antimicrobial properties of microwave synthesized mixed metal oxide nanocomposite. Inorg. Chem. Commun.. 2021;125:108429
- [CrossRef] [Google Scholar]
- Antibacterial and antiviral chitosan oligosaccharide modified cellulosic fibers with durability against washing and long-acting activity. Int. J. Biol. Macromol.. 2023;231:123587
- [CrossRef] [Google Scholar]
- Negative influence of Ag and TiO2 nanoparticles on biodegradation of cotton fabrics. Cellul.. 2015;22(2):1365-1378.
- [CrossRef] [Google Scholar]
- Interaction of different types of cells on polymer surfaces with wettability gradient. J. Colloid Interface Sci.. 1998;205(2):323-330.
- [CrossRef] [Google Scholar]
- Durable superhydrophobic cotton fabric for oil/water separation. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2017;533:249-254.
- [CrossRef] [Google Scholar]
- Applications and properties of chitosan. J. Bioact. Compat. Polym.. 1992;7(4):370-397.
- [CrossRef] [Google Scholar]
- Fabrication of superhydrophobic cellulose-based materials through a solution-immersion process. Langmuir. 2008;24(10):5585-5590.
- [CrossRef] [Google Scholar]
- Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res.. 2004;339(2):313-319.
- [CrossRef] [Google Scholar]
- Durably antibacterial and bacterially antiadhesive cotton fabrics coated by cationic fluorinated polymers. ACS Appl. Mater. Interfaces. 2018;10(7):6124-6136.
- [CrossRef] [Google Scholar]
- Modification of cotton fibre with functionalized silane coupling agents vinyltriethoxysilane and aminopropyltriethoxysilane. Journal of Textile Science & Engineering. 2018;08(03):361.
- [CrossRef] [Google Scholar]
- Antimicrobial, UV resistant and thermal comfort properties of chitosan- and aloe vera-modified cotton woven fabric. J. Polym. Environ.. 2019;27:405.
- [CrossRef] [Google Scholar]
- Investigation of chitosan adsorption onto cotton fabric with atmospheric helium/oxygen plasma pre-treatment. Cellul.. 2016;23(3):2129-2142.
- [CrossRef] [Google Scholar]
- Development and characterization of three-dimensional woven fabric for ultra violet protection. International Journal of Clothing Science and Technology. 2018;30(4):536-547.
- [CrossRef] [Google Scholar]
- Novel atmospheric plasma enhanced chitosan nanofiber/gauze composite wound dressings. J. Appl. Polym. Sci.. 2013;129(2):916-923.
- [CrossRef] [Google Scholar]
- Surface treatment of cotton fabrics with chitosan. Rev. Prog. Color. Relat. Top.. 2003;119:241-246.
- [Google Scholar]
- Percolation effects in degradable polyethylene-starch blends. J Polym Sci B. 1991;29(5):565-579.
- [CrossRef] [Google Scholar]
- Chemically induced graft copolymerization of vinyl monomers onto cotton fibers. J. Appl. Polym. Sci.. 2006;100(3):2343-2347.
- [CrossRef] [Google Scholar]
- The influence of surface free-energy on planimetric plaque growth in man. J. Dent. Res.. 1989;68(5):796-799.
- [CrossRef] [Google Scholar]
- Spectroscopy analysis of chemical modification of cellulose fibers. J. Mex. Chem. Soc.. 2010;54:192-197.
- [Google Scholar]
- A simple way to an ultra-robust superhydrophobic fabric with mechanical stability, UV durability, and UV shielding property. Journal of Colloid and Interface Science. 2018;522:57-62.
- [CrossRef] [Google Scholar]
- Multifunctional modification of cotton using layer-by-layer finishing with chitosan, sodium lignin sulphonate and boric acid. Int. J. Biol. Macromol.. 2020;158:903-910.
- [CrossRef] [Google Scholar]
- An eco-friendly, permanent, and non-leaching antimicrobial coating on cotton fabrics. The Journal of The Textile Institute. 2015;106(9):907-911.
- [CrossRef] [Google Scholar]
- Effects of Substratum Topography on Bacterial Adhesion. J. Colloid Interface Sci.. 1998;208(1):23-33.
- [CrossRef] [Google Scholar]
- Facile synthesis of chitosan-silver nanoparticles onto linen for antibacterial activity and free-radical scavenging textiles. Int. J. Biol. Macromol.. 2019;133:1134-1141.
- [CrossRef] [Google Scholar]
- Green chemistry based in-situ synthesis of silver nanoparticles for multifunctional finishing of chitosan polysaccharide modified cellulosic textile substrate. Int. J. Biol. Macromol.. 2020;152:1135-1145.
- [CrossRef] [Google Scholar]
- Fabrication of superhydrophobic, antibacterial, and ultraviolet-blocking cotton fabric. The Journal of The Textile Institute. 2013;104(8):861-869.
- [CrossRef] [Google Scholar]
- Robust flower-like TiO2@Cotton fabrics with special wettability for effective self-cleaning and versatile oil/water separation. Adv. Mater. Interfaces. 2015;2:1500220.
- [CrossRef] [Google Scholar]
- Cleaner functional dyeing of wool using Kigelia Africana natural dye and Terminalia chebula bio-mordant. Sustain. Chem. Pharm.. 2020;17:100286
- [CrossRef] [Google Scholar]
- Synthesis and characterizations of silane treated Grewia optiva fibers. Int. J. Polym. Anal. Charact.. 2009;14(4):301-321.
- [CrossRef] [Google Scholar]
- Development of an organosilicone antimicrobial agent for the treatment of surfaces. Journal of Coated Fabrics. 1982;12(1):38-45.
- [CrossRef] [Google Scholar]
- Biodegradation studies of chitosan-polycaprolactone (PCL) nanocomposite in soil burial test. Middle-East J. Sci. Res.. 2015;23(2):253-258.
- [CrossRef] [Google Scholar]
- Study on self-cleaning performance and hydrophobicity of TiO2 /silane coatings. Pigment & Resin Technology ahead-of-p 2019
- [CrossRef] [Google Scholar]
- AATCC Test Method: TM 183-2004, 2010. Transmittance or blocking of erythemally weighted ultraviolet radiation through fabric. AATCC Technical Manual, Research Triangle Park, NC, USA, 85, 318-321.
- Test Method AATCC: TM 124–2004, 2010. Smoothness appearance of fabrics after repeated home laundering. AATCC Technical Manual, Research Triangle Park, 85, 195–198. https://members.aatcc.org/store/tm124/533/.
- Test Method AATCC: TM 147–2004, 2010. Determination of zone of inhibition by qualitative method. AATCC Technical Manual, Research Triangle Park, 85, 251–252.
- Influence of antimicrobial finishes on the biodeterioration of cotton and cotton/polyester fabrics: Leaching versus bio-barrier formation. Polym. Degrad. Stab.. 2011;96(7):1286-1296.
- [CrossRef] [Google Scholar]
- Sol–gel coating of cellulose fibres with antimicrobial and repellent properties. J. Sol-Gel Sci. Technol.. 2008;47(1):44-57.
- [CrossRef] [Google Scholar]
- Dehydration of an ethanol/water azeotrope by novel organic-inorganic hybrid membranes based on quaternized chitosan and tetraethoxysilane. Biomacromolecules. 2004;5(4):1567-1574.
- [CrossRef] [Google Scholar]
- Superhydrophobic and ultraviolet-blocking cotton textiles. ACS Appl. Mater. Interfaces. 2011;3(4):1277-1281.
- [CrossRef] [Google Scholar]
- Durable, self-healing, superhydrophobic fabrics from fluorine-free, waterborne, polydopamine/alkylsilane coatings. RSC Adv.. 2017;7(54):33986-33993.
- [CrossRef] [Google Scholar]
- Antimicrobial performance of medical textiles. Midland, USA: IFAI Expo; 2002.
- Interfacial microfluidic transport on micropatterned superhydrophobic textile. Lab Chip. 2013;13(10):1937-1947.
- [CrossRef] [Google Scholar]
- Facile construction of robust superhydrophobic cotton textiles for effective UV protection, self-cleaning and oil-water separation. Colloids Surf A Physicochem Eng Asp. 2019;570:172-181.
- [CrossRef] [Google Scholar]
- Lasting superhydrophobicity and antibacterial activity of Cu nanoparticles immobilized on the surface of dopamine modified cotton fabrics. Surf. Coat. Technol.. 2017;309:149-154.
- [CrossRef] [Google Scholar]
- One-step synthesis of superhydrophobic coating on cotton fabric by ultrasound irradiation. Ind. Eng. Chem. Res.. 2013;52(36):12846-12854.
- [CrossRef] [Google Scholar]
- Superhydrophobic surfaces for the reduction of bacterial adhesion. RSC Adv.. 2013;3(30):12003-12020.
- [CrossRef] [Google Scholar]
- Water shedding angle: A new technique to evaluate the water-repellent properties of superhydrophobic surfaces. Text. Res. J.. 2009;79:1565-1570.
- [CrossRef] [Google Scholar]







