Translate this page into:
Eco-dyeing and functional finishing of wool fabric based on Portulaca oleracea L. as colorant and Musa basjoo as natural mordant
⁎Corresponding author at: College of Textile and Garments, Hebei University of Science and Technology, NO.26 Yuxiang Street, Yuhua District, Shijiazhuang 050018, China. weizhang@hebust.edu.cn (Wei Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Biomass energy is the most acknowledged renewable resource due to its universality, richness, and renewability. This study utilized a Portulaca oleracea L. plant as a natural colorant for wool fabric dyeing with a high color yield at optimum extraction and dyeing conditions. To evaluate the dyeing mechanism and feasibility of the extracted dyes, we analyzed and characterized the molecular structure and nano-level particle size. The dyeing kinetics and the morphology of dyed fabrics were integratedly explored; the adsorption process of wool fabric on natural colorant molecules was increasingly in line with the pseudo-second-order kinetic adsorption model. Further, the dyeing effects of wool fabrics were compared to that of Musa basjoo mordant and synthetic dyes to confirm the superior color depth (K/S value 23.53), biological function as anti-ultraviolet (UPF value 253.47), and anti-bacterial activity (antibacterial rate of Staphylococcus aureus/Escherichia coli was 71.3%/37%). Our findings provide a feasible scheme for providing deep color and biological activity to wool fabrics. This has broad application prospects in the field of eco-friendly textile materials.
Keywords
Green dyeing
Nano-scale colorant
Wool materials
Color depth
Adsorption kinetics
Biological activity
- WF
-
Wool Fabric
- NF
-
Naturally dyed fabric
- MF
-
Mordant dyed fabric
- SF
-
Synthetic dyed fabric
- Nd
-
Natural dyes
- Md
-
Mordant dyes
- Sd
-
Synthetic dyes
Abbreviations
1 Introduction
With the advancement of global living standards, consumers focus on style and color, environmental protection as well as health when acquiring wearable textiles (Shahid et al., 2013; Zhou and Tang, 2017). Natural dyes are extensively used in antibacterial finishing, UV protection, and other fields due to their non-toxic, environmental protection, and multi-functional properties including antibacterial, anti-ultraviolet, and insect repellent (Safapour et al., 2018, Mr et al., 2021; Tang et al., 2016). Due to its unique appearance style, i.e. soft feel, bright color, plump plush and excellent warmth retention, wool fabric is the first choice for high-end suits and coats (Luo et al., 2018). Considering ecological security and protection, scientists in the textile field are constantly searching for novel plants as natural dyes to produce high value-added wool fabrics exhibiting anti-insect and anti-bacterial properties (Baliarsingh et al., 2012, Ibrahim et al., 2015).
Portulaca oleracea L. is a dual-purpose herb for medicine and food. It is rich in fatty acids, vitamins and other nutrients. It is widely used in biology, medicine and other fields. It has anti-aging, analgesic, anti-inflammatory and neuroprotective effects. In addition, Portulaca oleracea L. has strong inhibitory effects on Escherichia coli, Staphylococcus aureus and other bacteria, and has strong biological activities in antibacterial, anti-inflammatory, anti allergic and anti-oxidation (Aludatt et al., 2020). Boris Nemzer et al. extracted 184 compounds in Portulaca oleracea L., including phenolic acids, organic acids, flavonoids, and alkaloids, among them, α-linolenic acid, quercetin, p-coumaric acid, catechin, kaempferol, and tannic acid were the primary active ingredients of the observed medicinal properties of leaves (Fig. 1) Petropoulos et al., 2016; Lm et al., 2019; Nemzer et al., 2020). These components have strong biological properties such as anti-bacterial, anti-inflammatory, anti-allergy, and prevention of diabetic complications (Hosseinzadeh et al., 2021; Oh et al., 2021; Ye et al., 2021). At present, researches on Portulaca oleracea L. are focused on the physical and chemical properties and antioxidant activity of its seed, leaf and stem, as well as its application in food and biomedicine (Desta et al., 2020; Seif et al., 2019; Wang et al., 2020).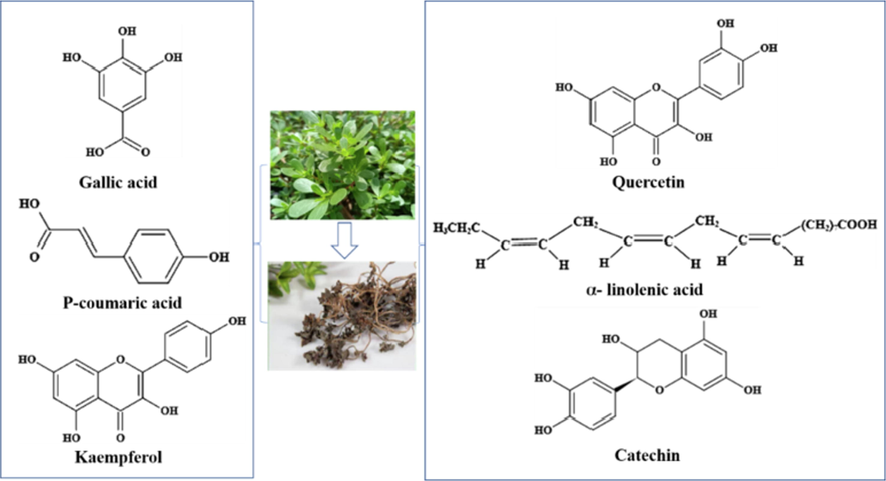
The main active ingredients in Portulaca oleracea L.
The traditional health care fabrics are made by dyeing with acid and reactive dyes and finishing with antibacterial and anti-inflammatory additives (Eid and Ibrahim, 2020; Kenawy et al., 2007). The process is complicated, complex, high cost, and has a great stimulating effect on the skin. Natural dyes are organic macromolecules, and their intermolecular binding ability (intermolecular force or hydrogen bond) with nature fibers is general. In order to improve the color fastness, metal mordants are widely used, especially chromium mordants. However, the chromium-containing mordants will cause chromium pollution to the environment, which is a very serious problem. It is necessary to searching for an ecotype material as an alternative to a metal medium. Natural mordants are usually the substances containing tannins and ionic structuresin plants (Ylj et al., 2021). Aline Pereira et al found that Musa basjoo contains anthraquinone, flavonoids, coumarins, phenols, cyanide, amine, coumarin and other chemicals, which can simultaneously interact with certain dyes and fabric macromolecules (Pereira and Maraschin, 2015). The leaves, bark and fruit rinds of Musa basjoo have been used for dyeing of textiles as mordanting agent. The cell sap of Musa basjoo contain a considerable amount of tannin, which could stain the textile in almost dark black color (Mathur and Gupta, 2003). Tannin compounds are water-soluble polyphenols, which is composed of phenolic groups and aromatic rings. These active agents have the advantages of environmentally-friendly and safe to use, as well as antioxidant and antimicrobial capacity (Cano et al., 2021). Therefore, the tannin in Musa basjoo leaves can be used as a mordant to realize ecological dyeing and endow wool fabrics with functionality at the same time.
Herein, the wool fabric was considered a research object, and the colorant of purslane was extracted using the water-boiling method. The optimal colorant extraction and the dyeing processes were determined through the Statistical Product and Service Solutions (SPSS) design. The molecular structure and particle size of the extracted dyes were analyzed and characterized to investigate the dyeing mechanism and feasibility. Additionally, we comprehensively characterized the dyeing effect, functional and biological properties of the dyed wool fabric based on the color characteristic, rubbing fastness, anti-bacterial, anti-oxidation, and anti-ultraviolet properties. We are confident that purslane can become an eco-friendly, biologically active finishing agent and effective colorants for wool fabrics.
2 Experimental
2.1 Materials and chemicals
The Portulaca oleracea L. plant grows in Anhui Province, China. Wool fabric was purchased from Jiangsu Lugang Wool Textile Printing and Dyeing Co., Ltd. with the following specifications: Warp and weft: 17.2 × 17.2 tex; warp density: 216 threads per 10 cm; weft density: 184 threads per 10 cm, and weight: 224 g/m2. Fresh Musa basjoo leaves were produced in Deqing County, Guangdong Province.
Staphylococcus aureus CMCC (b) 26,003 and E. coli CMCC (B) 44,102 were provided by the National Center for Medical Culture Collections. The nutritional broth was purchased from Beijing Aoboxing Biotechnology Co. Ltd.
Synthetic dyes including cerulein golden CE-01, cerulein blue 3G, cerulein red 6G were provided by Tianjin Sanhuan Chemical Co., Ltd. 1,1-diphenyl-2-trinitrophenylhydrazine was purchased from Fuzhou Feijing Biotechnology Co., Ltd. Acetic acid (HAC) was used for pH adjustment. The analytical reagents including anhydrous sodium carbonate (Na2CO3), anhydrous ethanol (C2H5OH), ammonium sulfate ((NH4)2SO4), were obtained from Tianjin Yongda Chemical Reagent Co. Ltd., China.
2.2 Methodology
2.2.1 Extraction of Portulaca oleracea L. colorant
The natural colorant of Portulaca oleracea L. was extracted by water bath method, and the absorbance of the extracted dye was determined by UV–vis spectrophotometry in the wavelength range of 320–400 nm. We analyzed the effects of different extraction conditions on the extraction efficacy of Portulaca oleracea L., including temperature (60–100 °C), time (0.5 h–2.5 h), solid–liquid bath ratio 2.5–5 g/100 mL H2O (25.0–50.0 g/L). With temperature, time, and bath ratio as influencing factors, SPSS design was used to explore the optimal extraction process of Nd. The obtained filtrate was evaporated at room temperature, and the extracted dye powder was stored at low temperature (4 °C) for further analysis of components and functional groups.
2.2.2 Dyeing WF with natural dyes
The single factor optimization was used to explore the dyeing parameters, including pH (2–6), temperature (60–100 °C), dye concentration (1:20–1:40), and dyeing time (30–90 min). The dyed wool fabric (WF) was named naturally dyed fabric (NF).
2.2.3 Adsorption dynamics
Wool fabric was dyed at 80 °C, 90 °C, and 100 °C, respectively for a certain period to explore the adsorption kinetics of Nd on WF (Rather et al., 2017; Schaumlffel et al., 2021).
The isotherm method was used to dye the wool fabric at 80 °C, 90 °C, and 100 °C for a certain period to detect the adsorption kinetics of the Nd on the wool fabric.
2.2.4 Mordant dyeing WF with Musa basjoo
Naturally dyed fabric samples were dyed with 3.5% Musa basjoo leaf natural mordant to obtain MF (Mordant dyed fabric) (Kang et al., 2019; Zhang et al., 2021). The dye discharge under the optimum dyeing conditions (pH, temperature, dye concentration, and dyeing time) was measured at the maximum absorption wavelength of the Nd solution. The exhaustion rate was calculated as follows (Mathur and Gupta, 2003):
2.2.5 Dyeing WF with synthetic dyes
The synthetic dyes are used to compare the dyeing strength and functional properties of fabrics with that of natural dyed fabrics to evaluate the advantages of eco-dyeing and functional finishing of Portulaca oleracea L. as natural dyes. In order to obtain the same color as NF and MF, synthetic dye (Sd) baths were prepared with 1.28% CE-01, 0.17% CE-blue 3G and 0.23% CE-6G, and WF was dyed based on the process shown in Fig. 2 to obtain synthetic dyed fabric (SF) samples. The assistant comprised two drops of ring coeven B, 2% ammonium sulfate, and 0.15 mL HAc.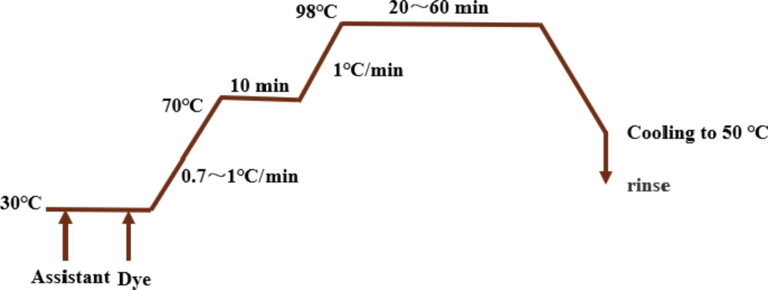
Dyeing process flow chart of WF with synthetic dyes.
2.3 Characterization
The visible spectrophotometer (V-5600, Shanghai Yuanshi Instrument Co., Ltd., China) was used for spectral scanning of Nd at a wavelength range of 320–400 nm. The chemical groups of extracted Nd were analyzed using the Infrared-Raman spectrometer (Nicolet 6700/FT-Raman modules, Thermo Fisher, USA); the infrared spectra of WF, NF, and MF were collected at the wavelength range of 600 cm−1–4000 cm−1 by Thermo Nicolet Is10 Fourier transform infrared spectrometer. Transmission electron microscopy (FEI Talos F200s, USA) was used to observe the size and shape of Nd particles. The surface morphology of WF, NF, and MF and the distributed elements on the surface was recorded using an electron microscope analyzer (Bruker nano Berlin, Germany) equipped with XFlash 6T|30 detectors. The K/S and L*, a*, b*, c*, ho values of wool fabrics at 340 nm wavelength under different dyeing processes were measured by computer color matching instrument (Color i 5D, American Datacolor). The wash fastness of dyed woolen yarn samples was measured in Digi wash SSTM (Launder-o-meter) as per the ISO 105-C06:1994 (2010) specifications. The wet and dry rubbing fastness were tested based on ISO 105-X16-2002. The colorfastness to sunlight was rated according to ISO 105-B02-2013. The ultraviolet protection factor (UPF) and ultraviolet transmittance (UVA and UVB) were measured on the textile ultraviolet protection performance tester (YG909-III, Wenzhou Fangyuan Instrument Co., Ltd.).
The antibacterial properties of WF, NF, MF, and SF samples were tested using S. aureus and E. coli as test strains following the GB/T 20944.3–2008 “textile antibacterial performance evaluation method”. The bacteriostatic rate was calculated based on Formula (3):
In vitro, antioxidant activity was evaluated by the DPPH radical scavenging assay based on Ivan Best with modifications. Briefly, 50 μL of Nd, mordant dye, synthetic dyes extract was mixed with 950 μL DPPH (65 μmol/L in ethanol), then shaken for 10 min to completely dissolve DPPH. The wool fabric was placed in the mixture and reacted in dark for 30 min. Then, a small amount of DPPH ethanol solution was transferred into a 10 mm colorimetric dish (Ren et al., 2021). The absorbance was measured by a UV spectrophotometer at 517 nm. The free radical scavenging efficacy of DPPH was calculated using Formula (4) (Iba et al., 2020):
3 Results and discussion
3.1 Chemical composition of Nd powder
The major active components in Portulaca oleracea L. include α-linolenic acid, quercetin, p-coumaric acid, catechin, and tannic acid. Generally, quercetin (flavonols) is largely gray-yellow. In the quercetin molecules, the introduction of —OH and —OCH3 and other auxochromes at the 7-site and 4-site promotes electron transfer and rearrangement, resulting in the darker color of the compound as brownish-yellow (Fig. 3) (Yang et al., 2021). Fig. 3 shows the FT-IR infrared spectra of Nd in the range of 4000–500 cm−1, where 3418.6 cm−1 and 2969.8 cm−1 are broad absorption peaks with moderate intensity. The —OH stretching vibration of tannin molecules contained in phenolic aldehyde and aliphatic structure is at 3418.6 cm−1, and the small peak at 2969.8 cm−1 originated from the —CH stretching vibration in aromatic methoxy and the side chain of methyl and methylene (Chupin et al., 2013). The carbonyl absorption peak is in the region of 1900–1600 cm−1. Due to the stretching vibration of C⚌O and the influence of hydrogen bond, the C⚌O absorption in carboxyl group moves to a low wave. The peaks at 1621.8 cm−1 and 1384.6 cm−1 are attributed to C⚌C stretching vibration of α-linolenic acid, —C—OH stretching vibration of phenolic acid, C⚌O stretching vibration of carboxyl group and —CH deformation in flavonoid aromatic ring system. The peak at 1318.5 cm−1 was attributed to the out-of-plane bending vibration of C-H of olefin compounds. The peak at 1075.1 cm−1 and 619.5 cm−1 corresponded to the stretching vibration of —CO and the plane bending vibration of the C—O bond of tannins (Ricci et al., 2015).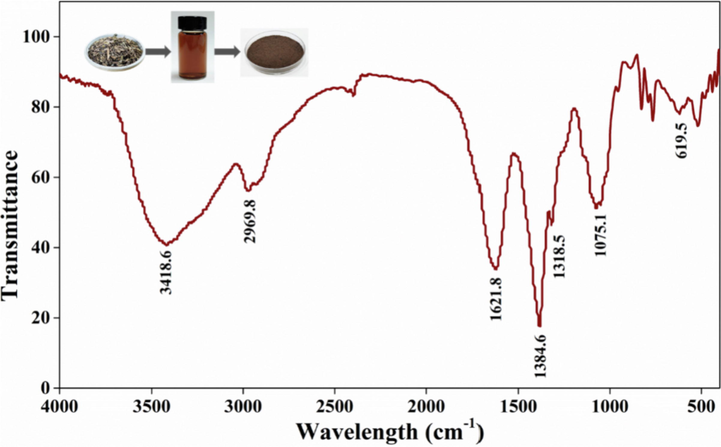
FT-IR spectra of Nd powder.
3.2 Extract processes of Nd colorant
The parameters such as temperature, time, and bath ratio of wool fabric were optimized by a single factor experiment and the results are shown in Fig. 4. As shown, the absorbance increased with increasing temperature, and reached the maximum value at extraction temperature of 90 °C, however, it decreased with further increasing temperature. This is because the α-linolenic acid molecules primarily comprising ω-3 in Portulaca oleracea L. are easy to decompose under high temperature, which potentially disrupts the conjugated system of Nd and absorbance, thereby producing a deepening effect. Nonetheless, if the temperature was too high, it would destroy the structure of Portulaca oleracea L. and reduce absorbance (Ye et al., 2021). Therefore, the extraction temperature can be defined as 90 °C.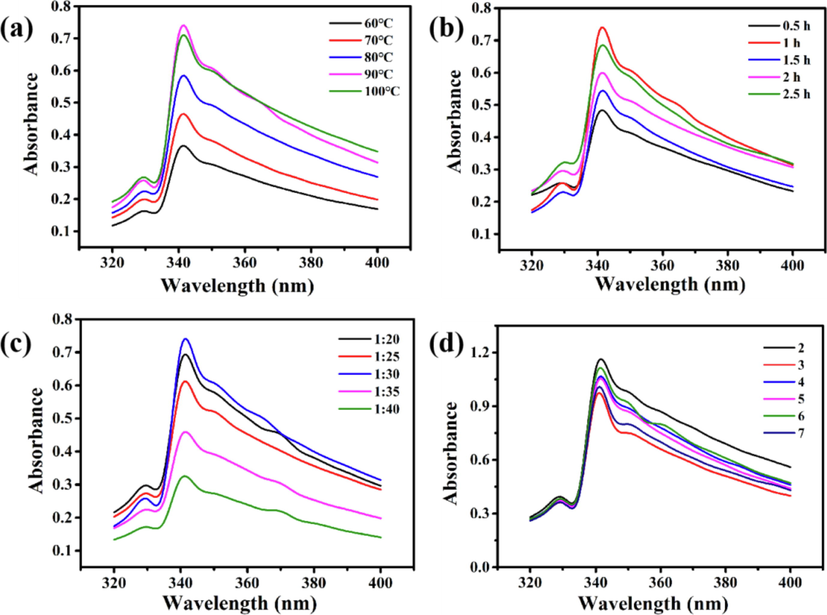
UV–vis spectral analysis for the absorbance of Nd extracted by the conditions: (a) different temperatures under 1 h and 1:30; (b) different times under 1 h and 90 °C; (c) different bath ratios under 1 h and 90 °C. (d) Stability of extracted Nd solution under acidic conditions.
The absorbance increased with the extension of extract time within 1 h, whereas decreased with a further prolonged time. When the time is within 1 h and the time is short, the extraction of Portulaca oleracea L. is not sufficient. If the time exceeds 1 h, it may cause the decomposition of colorant, which is not conducive to the extraction. The time should be controlled within a certain range. Under this condition, it is more appropriate to select the extraction time of about 1 h. The absorbance of Nd at a bath ratio of 1:20–1:30 was higher than that of a large bath ratio due to the higher Nd and more tannins obtained per unit mass of deionized water at a low bath ratio.
Through single factor experiment, SPSS was designed for the factors that have a great impact on Portulaca oleracea L. pigment, namely extraction temperature, extraction time and solid–liquid ratio. The test scheme is shown in Table 1.
Tem. A (°C)
T. B (h)
Ratio. C
1
80 °C
1 h
1:20
2
90 °C
1.5 h
1:25
3
100 °C
2 h
1:30
Through single factor experiment, the better three levels under each factor were selected for three factor and three level SPSS design to screen the best extraction conditions of Portulaca oleracea L. The extraction effect is analyzed according to the value of each factor, as shown in Table 2. The results show that the best extraction conditions are: temperature 100 °C, time 1.5 h and bath ratio 1:20. Note: K is the sum of the test results of each factor level. For example, K1 is the sum of the absorbance corresponding to level 1, that is, K1 = 0.76 + 0.57 + 0.43 = 1.76; k is the mean value of the sum of factor test results; R is the range of K value under each factor.
Tem. (°C)
Time (h)
Ratio
Absorbance
1
1
1
1
0.76
2
1
2
2
0.57
3
1
3
3
0.43
4
2
1
2
0.51
5
2
2
3
0.43
6
2
3
1
0.52
7
3
1
3
0.79
8
3
2
1
1.26
9
3
3
2
0.79
K1
1.76
2.06
2.54
K2
1.45
2.25
1.86
K3
2.84
1.74
1.64
k1
0.58
0.69
0.85
k2
0.48
0.75
0.62
k3
0.95
0.58
0.55
R
0.46
0.17
0.30
Fig. 4(d) is mainly used to test the acid resistance stability of the extracted oleracea L. solution. The initial pH value of nature dye solution was recorded as 7. First of all, the absorption wavelength of the solution does not change with the change of pH, but always maintains the same position. Second, at the λmax of 340 nm, the absorbance corresponding to each pH fluctuates regularly, and the absorbance value fluctuates around 1. It indicate that the acid resistance stability of the solution is very good and shows that acidic medium will not change the chemical composition and chromogenic group of Portulaca oleracea L., but promote the increase of release by improving the permeability of solvent and damage to plant tissue (Shelembe et al., 2014).
3.3 Dyeing conditions and exhaustion rate
As shown in Fig. 5(a) and Table 3, the color intensity of wool fabric decreasedwith increased pH value when the pH of the dyeing bath was below the isoelectric point of wool of 4.2–4.8 due to the existing coulomb force and Van der Waals force. In contrast, the dye uptake decreased with a further increased pH value when the pH was higher than 4.8 (Zhou et al., 2020). As shown in Fig. 5(b), the maximum exhaustion rate was 35.67% at 90 °C. The increase in temperature promotes the swelling of the wool fiber; this is conducive to the further diffusion of Nd into the wool fiber. When the temperature exceeds 60 °C, the disulfide bond in the macromolecular structure of wool begins to open, and the reaction activity between the colorant and wool fabric is simultaneously enhanced. A reasonable increase in dyeing time would help in the increase of Nd exhaustion rate (Fig. 5(c)); the dyeing rate cannot significantly increase when the dyeing time is higher than 75 min. Fig. 5(d) shows that the increase of bath ratio would increase the number of Nd molecules in dye solution, which will improve the binding rate with wool fabric. When the liquor ratio is 1:35, the dye exhaustion rate reaches the maximum. Nevertheless, the adsorbed capacity of remaining Nd in dye solution would decrease after adequate adsorption on the surface of wool fabric. Therefore, if continue to increase the bath ratio and dye content, the adsorption capacity of the wool fabric will decrease instead.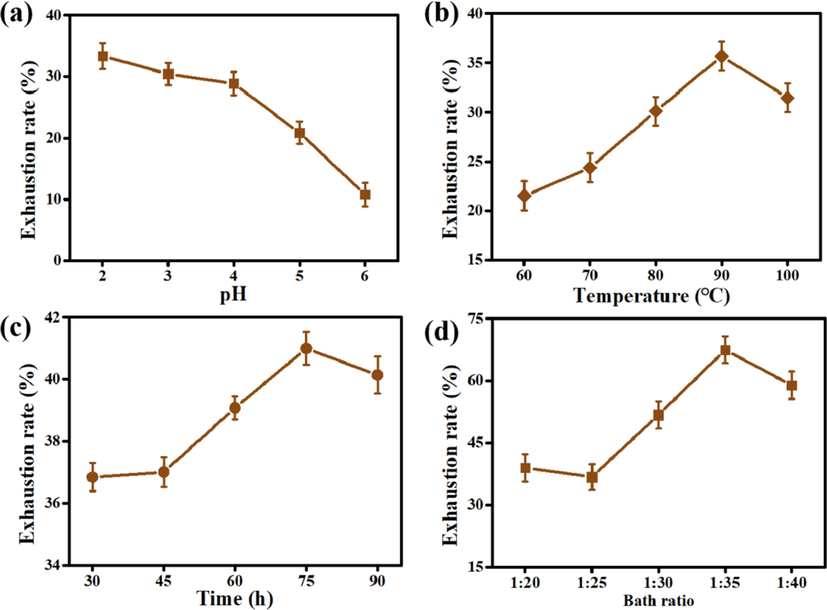
The effect of conditions on dye exhaustion rate:(a) pH values with other conditions: 90 °C, 1 h and 1:20; (b) Temperatures with other conditions: pH 2, 1 h and 1:20; (c) Times with other conditions: pH 2, 90 °C and 1:20; (d) Bath ratio with other conditions: pH 2, 90 °C, 75 min.
pH
K/S
Tem.(°C)
K/S
T(min)
K/S
Ratio
K/S
pH = 2
15.85
60
12.352
30
17.464
1:20
13.68
pH = 3
14.122
70
16.022
45
17.881
1:25
13.102
pH = 4
13.898
80
16.808
60
17.601
1:30
16.376
pH = 5
12.984
90
21.635
75
20.379
1:35
17.262
pH = 6
11.873
100
16.376
90
18.318
1:40
16.256
Through single factor experiment, the factors that have great influence on dyeing were designed, namely dyeing pH, temperature, time and solid–liquid ratio. The test scheme is shown in Table 4.
pH. A
Tem. B (°C)
T. C (h)
Ratio. D
1
2
80 °C
60 min
1:30
2
3
90 °C
75 min
1:35
3
4
100 °C
90 min
1:40
The dyeing effect was analyzed based on the value of each factor, and SPSS design was applied (Table 5). The findings show the optimum dyeing process of pH = 2, temperature 100 °C, time 1.5 h, solid–liquid bath ratio 1:35, and the dye uptake of up to 43.79%. Note: K is the sum of the test results of each factor level. For example; k is the mean value of the sum of factor test results; R is the range of K value under each factor.
pH
Tem. (°C)
Time(h)
Ratio
Exhaustion rate (%)
1
1
1
1
1
28.42
2
1
2
2
3
28.56
3
1
3
3
2
43.79
4
2
1
2
2
4.19
5
2
2
3
1
14.44
6
2
3
1
3
22.82
7
3
1
3
3
7.27
8
3
2
1
2
9.86
9
3
3
2
1
6.38
K1
94.2
39.88
61.10
49.24
K2
41.45
52.86
39.13
51.27
K3
23.51
69.90
58.93
58.65
k1
31.40
13.293
20.367
16.413
k2
13.817
17.62
13.043
17.09
k3
7.837
23.30
19.643
19.55
R
23.563
10.007
7.324
2.460
3.4 Adsorption dynamics
Based on the single factor experimental results of natural dye dyeing, a suitable adsorption system was selected, and the temperatures were regulated at 80 °C, 90 °C, and 100 °C. Fig. 6(a) reveals that the adsorption capacity of the wool fabric significantly increases within the first 40 min. After dyeing for 40 min, the adsorption rate decreased from 1.3205 kg/g/min to 7.828 × 10−2 kg/g/min, and the equilibrium time was about 120 min. To comprehensively understand the adsorption kinetics, the adsorption kinetics equation, pseudo-first-order, and pseudo-second-order models were utilized to further analyze the adsorption features and influencing factors (Shen et al., 2014).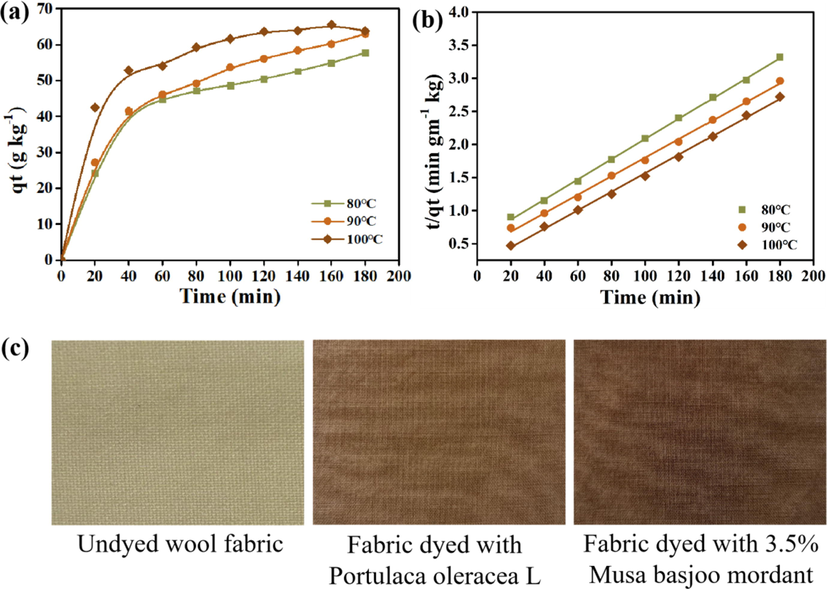
(a) Kinetic curves of dyeing wool fabric with Nd at different temperatures; (b) pseudo-second-order fitting line; (c) Comparison of wool fabrics dyed with different methods.
For pseudo-first-order dynamics, the linearized rate equation was calculated using the Formula (5):
In the equation, K2 (kg/g/min) is the equilibrium rate constant of the pseudo-second-order process. The linear changes of ln (qe-qt) and
verses t (min) was analyzed by nonlinear least square regression, which confirms the applicability and fitting of the above two dynamic models. The rate constant (k2) and equilibrium adsorption capacity (qe) were calculated using the slope and intercept of the linearized Equation (Kayodé et al., 2012). In addition, the adsorption rate and half-staining time (t1/2) are calculated using Formulas (7) and (8):
Generally, the pseudo-first-order kinetic model is suitable for describing the initial stage of adsorption, while the pseudo-second-order kinetic model describes the whole adsorption process. Table 5 demonstrates that the R2 of the pseudo-second-order kinetic model is above 0.997, indicating that the adsorption process of Nd on WF is increasingly in line with the pseudo-second-order kinetic adsorption model. Besides, it illustrates that the adsorption process is primarily regulated by chemical adsorption accompanied by electrostatic force, hydrogen bond, and ion–dipole interaction (Zhang et al., 2017). As shown in Table 6, when the temperature is further increased from 90 °C to 100 °C, the adsorption capacity increases from 25.991 g/kg to 44.067 g/kg, suggesting that the equilibrium is immediately attained at 100 °C compared to 80 °C and 90 °C. The initial adsorption rate ri (g/kg/min) increases with increasing temperature, resulting in the decrease of t1/2 (min).
Pseudo-first-order model
Pseudo-second-order model
Tem (°C)
qe1 (g/Kg)
R2
k1 (kg/g/min)
qe2 (g/Kg)
R2
k2 (kg/g/min)
ri (g/kg/min)
t1/2 (min)
80
15.141
0.9653
3.208 × 10−1
10.746
0.9991
1.526 × 10−2
1.762
6.098
90
25.991
0.9732
3.501 × 10−1
13.345
0.9975
1.4 × 10−2
2.493
5.352
100
44.067
0.9040
3.543 × 10−1
20.791
0.9979
1.402 × 10−2
6.060
3.431
As shown in Table 7, the sequence of color intensity (K/S value) of different dyeing methods is MF > NF > SF > WF. According to the literature, the use of mordant improves the combination of dye and fiber; the fiber can attain a stable color (Vankar et al., 2008). The higher K/S value and darker color of the sample are attained after dyeing with natural mordant as shown in Fig. 6(c).
Item
Colorfastness to
K/S
L*
a*
b*
C*
ho
Wash
Dry rubbing
Wet rubbing
Light
WF
3–4
12.9
82.4
1.75
2.67
3.19
56.7
NF
4
4
3–4
3–4
21.1
49
9.63
23.05
24.98
67.33
MF
4
4–5
3–4
4
23.5
43
8.75
20.74
22.51
67.12
SF
4–5
4–5
4–5
4–5
21.1
49.24
9.82
22.97
25.11
67.37
3.5 Chemical analysis of fabrics
The FT-IR spectrums of WF, NF, and MF were significantly similar (Fig. 7(a)), indicating that the overall structure of wool fabric dyed with Nd and Md remained undamaged. Notably, near 3280 cm−1 in the infrared spectrum is the coupling of - NH stretching vibration and amide stretching vibration, which is related to the change of force caused by hydrogen bond breaking between molecular chains. When the hydrogen bond between molecular chains breaks, the electron cloud density of C in C—H decreases, causing a shift in the stretching vibration band of C—H. To highlight the small differences that are difficult to detect, NF and MF infrared spectra were subtracted from WF infrared spectra, respectively (Fig. 7(b)) to eliminate the interference of background spectra and similar conditions. In the difference spectrum, COO– and —OH in natural dyes are combined with —NH2 or —CO—NH on protein fibers to enhance the hydrogen bond between natural dyes and wool. Among them, 1631 cm−1 and 1647 cm−1 are carboxyl stretching vibration regions, and 3750–3000 cm−1 are O—H and N—H stretching vibration regions. Therefore, the corresponding characteristics of the absorption peak are gradually enhanced (Yrab et al., 2019).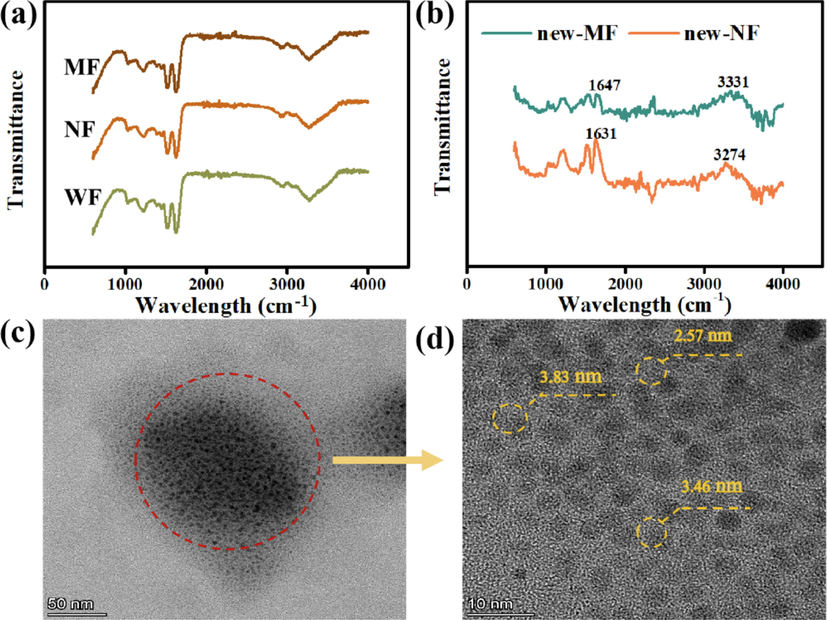
(a) Infrared spectrum of three dyed fabric samples; (b) Infrared difference spectrum of MF and NF; (c) and (d): TEM images of Nd solution.
The dye colorants exist in black spot-like aggregates with a diameter of 100 nm–150 nm (Fig. 7b). Unlike other natural dyes with the particle size of 2–4 μm (Lbkma et al., 2021; Li et al., 2021; Zhang et al., 2019), the dye aggregates comprise dozens to hundreds of nanoscale particles and the particle size of about 2.98 ± 0.54 nm (Fig. 7(c)); this is similar to the size of quantum dots. Thus, the colorant particles could be effectively and evenly dispersed in water and diffused into the wool fabric to attain a high exhaustion rate and color intensity (K/S).
The SEM images in Fig. 8(a-c) show the surface morphology of different wool fabrics without an apparent difference, suggesting that the dyeing process would not alter the surface morphology of the fabrics. The smooth fiber with a clear scale layer structure is shown on the surface of WF (Fig. 8(d)). However, the images in Fig. 8(e) and (f) show that the scale layer of wool fiber is destroyed, which would promote the diffusion of dye molecules into the fiber and significantly improve the efficiency of dyeing. Fig. 8(g-i) shows the cross-sectional of WF, NF, and MF. Of note, the material is solid and will not lose its capillary structure in the fiber due to expansion. These capillary structures ensure satisfactory diffusion characteristics.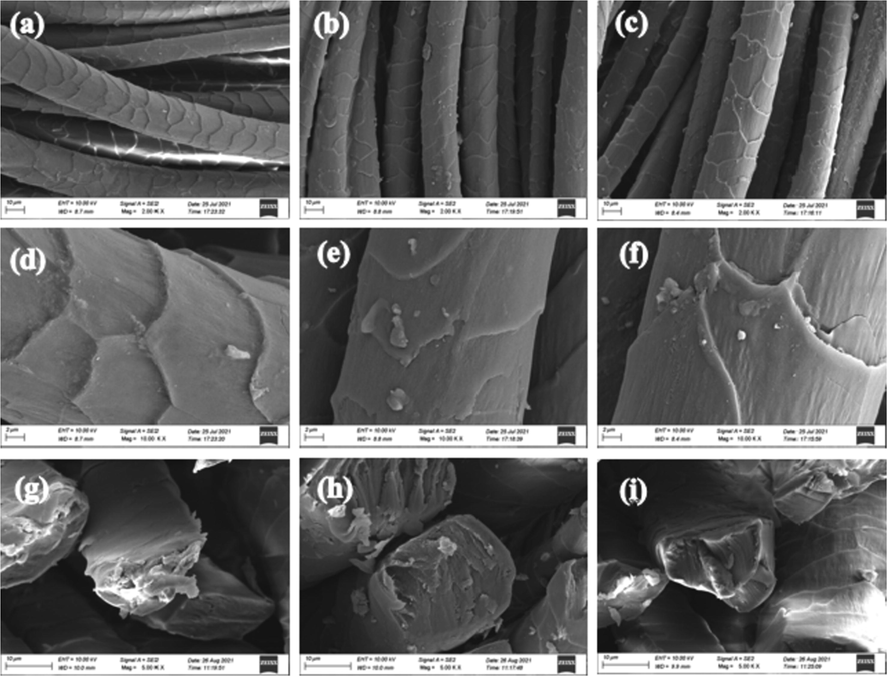
SEM images of fabric samples :(a) WF × 2 K; (b) NF × 2 K; (c) MF × 2 K; (d) WF × 10 K; (e) NF × 10 K; (f) MF × 10 K; (g), (h) and (i): Cross section electron microscope of wool WF, NF and MF.
Fig. 9 shows the distribution of elements in undyed and dyed WFs. Because of the presence of Se in Portulaca oleracea L., K in Musa basjoo leaves, Se and K appearing in Fig. 9(b) and (c) further verifies that natural dyes and mordants have been successfully dyed on WFs. The carbon–oxygen ratios (C/O) of WF, NF, and MF are 2.49, 2.67, and 2.85, respectively. MF samples have higher C/O and higher color intensity. The weight percentage of carbon and oxygen confirms the presence of hydroxylated flavonoids and catechin derivatives, which in turn verifies that the natural mordants rich in tannins cause the adsorption of fibers and dyes, and the excellent C/O improves the bioactivity of dyed fabrics (Hosseinzadeh et al., 2021; Rather et al., 2021).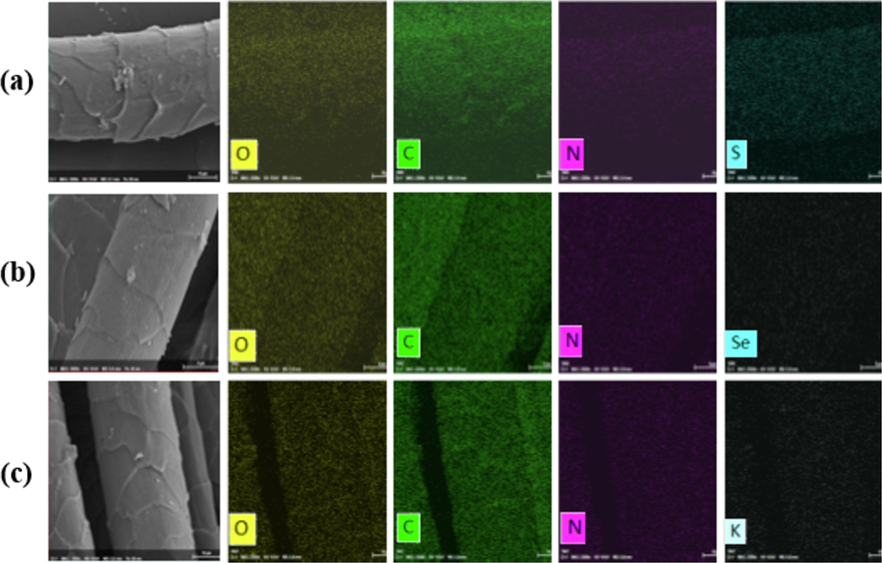
EDS analysis of fabrics: (a) WF; (b) NF; (c) MF.
3.6 UV protection and antioxidant properties
Due to the presence of quercetin, the number of functional groups including phenolic groups on the surface of fabric increased after Nd dyeing, thereby enabling NF and MF samples to have a better antioxidant capacity (Lu et al., 2018; Diao et al., 2021). The transmittance of UVA and UVB radiation depends on the type and degree of chemical interaction between wool fabric and natural dyes. As shown in Table 8, the UPF of WF is 153.29; this is classified as excellent. The dyed fabrics demonstrated lower transmittance (blocking ultraviolet radiation) and higher UPF values than that of the control samples, hence determining the superior UV resistance (Shabbir et al., 2018).
Transmittance
UPF values
Antioxidant
Error
T (UVA)
T (UVB)
WF
0.84
0.62
153.29
19.2%
4.2%
NF
0.47
0.43
229.05
25.3%
4.8%
MF
0.42
0.39
253.47
32.0%
5.1%
SF
0.41
0.40
248.92
24.8%
6.4%
3.7 Antibacterial properties
S. aureus and E. coli colonies in the dyed fabric were lower than that in the blank control group; S. aureus was significantly reduced on the MF in Table 9. This is because α-linolenic acid, quercetin, coumarin, catechin, and other components exhibit certain antibacterial abilities. Quercetin inhibits DNA and RNA synthesis in bacterial cells and interferes with cell membrane activity (Yl et al., 2021). Coumarinic acid exhibits effective antibacterial activity against E. coli and S. aureus; it inhibits cell proliferation resulting in apoptosis (Lu et al., 2018; Ren et al., 2021). The phenolic hydroxyl groups in the catechins molecule are combined with peptide bonds, amino and carboxyl groups in bacterial cell membrane proteins, resulting in membrane protein denaturation and further bacterial death (Ben et al., 2015; Ren et al., 2016). The antibacterial rate of wool fabric increased after mordant dyeing, specifically for S. aureus (71.3%); this confirms that the chemical reaction of Nd has a significant effect on its antibacterial activity.
S. No.
S. aureus
E. coli
Antibacterial rates against S. aureus / E. coli
Error
WF
–/–
–/–
NF
47.8%/12.5%
6.8%/4.5%
MF
71.3%/37.0%
5.6%/3.9%
SF

25.2%/11.8%
7.2%/4%
4 Conclusion
This study explored the high color yield and bioactive finishing of wool fabric with Portulaca oleracea L. as a natural dye, evaluated its dyeing properties and dyeing kinetics, and understood its dyeing mechanism. Natural dye molecules have good stability in acid and high temperature. Due to the nano particle size, they are evenly dispersed in the aqueous solution, which further improves the dyeing strength and fastness. In addition, the adsorption process of wool fabric on natural colorant molecules conforms to the quasi second-order kinetic adsorption model. In addition, the dyeing effect of wool fabric was compared with that of Musa basjoo mordant and synthetic dye. After the natural mordant added to the dye solution, the mordant and the dye migrate to the fiber surface and are adsorbed, and then dynamically diffuse to the fiber for bonding. The results showed that Portulaca oleracea L. dye and natural mordant worked together to make the fabric have superior color depth (K/S value 23.53) and biological functions, such as UV resistance (UPF value 253.47) and antibacterial activity (antibacterial rate of S. aureus/E. coli is 71.3%/37%). As a functional natural dye, Portulaca oleracea L. is a potential substitute in textile and garment industry.
Acknowledgment
We acknowledge financial support from the National Youth Natural Science Fund Project (Grant No. 22005083) and the Funding of Hebei Education Department (Grant No. ZD2020112).
Declaration of Competing Interest
The authors declare no known competing financial interests or personal relationships that could have appeared to affect the study.
References
- Herbal yield, nutritive composition, phenolic contents and antioxidant activity of purslane (Portulaca oleracea L.) grown in different soilless media in a closed system. Ind. Crops Prod.. 2020;141:111746
- [Google Scholar]
- Exploring sustainable technique on natural dye extraction from native plants for textile: identification of colourants, colourimetric analysis of dyed yarns and their antimicrobial evaluation. J. Cleaner Prod.. 2012;37:257-264.
- [Google Scholar]
- Accelerated solvent extraction of phenolic compounds from sorghum brans. J. Cereal Sci.. 2013;58:305-312.
- [Google Scholar]
- Mixture approach for optimizing the recovery of colored phenolics from red pepper (Capsicum annum L.) by-products as potential source of natural dye and assessment of its antimicrobial activity. Ind. Crops Prod.. 2015;70:34-40.
- [Google Scholar]
- Using tannins as active compounds to develop antioxidant and antimicrobial chitosan and cellulose based films. Carbohydr. Polym. Technol. Appl.. 2021;2:100156
- [Google Scholar]
- Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind Crops Prod.. 2013;49:897-903.
- [Google Scholar]
- Characterization of physico-chemical properties and antioxidant activity of oil from seed, leaf and stem of purslane (Portulaca oleracea L.) Biotechnol. Rep.. 2020;27:e00512
- [Google Scholar]
- Enhanced cytotoxicity and antioxidant capacity of kaempferol complexed with α-lactalbumin. Food Chem. Toxicol.. 2021;153:112265
- [Google Scholar]
- Recent developments in sustainable finishing of cellulosic textiles employing biotechnology. J. Cleaner Prod.. 2020;284:12470.
- [Google Scholar]
- Application of manure and biofertilizer to improve soil properties and increase grain yield, essential oil and ω3 of purslane (Portulaca oleracea L.) under drought stress. Soil Tillage Res.. 2021;205:104633
- [Google Scholar]
- Phytochemical screening and DPPH radical scavenging activity of three morphotypes of Mauritia flexuosa L.f. from Peru, and thermal stability of a milk-based beverage enriched with carotenoids from these fruits. Heliyon.. 2020;6:e05209
- [Google Scholar]
- New finishing possibilities for producing durable multifunctional cotton/wool and viscose/wool blended fabrics. Carbohydr. Polym.. 2015;119:182-193.
- [Google Scholar]
- Natural pigment during flora leaf senescence and its application in dyeing and UV protection finish of silk and wool – a case study of Cinnamomum Camphora. Dyes Pigm.. 2019;166:114-121.
- [Google Scholar]
- Extraction of antioxidant pigments from dye sorghum leaf sheaths. LWT-Food Sci. Technol.. 2012;46:49-55.
- [Google Scholar]
- The chemistry and applications of antimicrobial polymers: a state-of-the-art review. Biomacromolecules. 2007;8:1359-1384.
- [Google Scholar]
- Adding value to lignocellulosic byproducts by using acetate and p-coumaric acid as substrate in a microbial fuel cell. Ind. Crops Prod.. 2021;171:113844.
- [Google Scholar]
- Tuning the morphology of micro- and nano-spheres from bamboo shoot shell acetosolv lignin. Ind. Crops Prod.. 2021;171:113860
- [Google Scholar]
- The anti-inflammatory potential of Portulaca oleracea L. (purslane) extract by partial suppression on NF-κB and MAPK activation. Food Chem.. 2019;290:239-245.
- [Google Scholar]
- Composition and antioxidant, antibacterial, and anti-HepG2 cell activities of polyphenols from seed coat of Amygdalus pedunculata Pall. Food Chem.. 2018;265:111-119.
- [Google Scholar]
- Novel sustainable synthesis of dyes for clean dyeing of wool and cotton fibres in supercritical carbon dioxide. J. Cleaner Prod.. 2018;199:1-10.
- [Google Scholar]
- Use of natural mordant in dyeing of wool. Indian J. Fibre Text. Res.. 2003;28:90-93.
- [Google Scholar]
- Facile technique for wool coloration via locally forming of nano selenium photocatalyst imparting antibacterial and UV protection properties. J. Ind. Eng. Chem.. 2021;101:153-164.
- [Google Scholar]
- Phytochemical composition and nutritional value of different plant parts in two cultivated and wild purslane (Portulaca oleracea L.) genotypes. Food Chem.. 2020;320:126621
- [Google Scholar]
- Quercetin and its ester derivatives inhibit oxidation of food, LDL and DNA. Food Chem.. 2021;364:130394
- [Google Scholar]
- Banana (Musa spp) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol.. 2015;160:149-163.
- [Google Scholar]
- Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci. Technol.. 2016;55:1-10.
- [Google Scholar]
- Colorful and semi durable antioxidant finish of woolen yarn with tannin rich extract of Acacia nilotica natural dye. Dyes Pigm.. 2017;139:812-819.
- [Google Scholar]
- Valorization of agro-industrial waste from peanuts for sustainable natural dye production: focus on adsorption mechanisms, ultraviolet protection, and antimicrobial properties of dyed wool fabric. ACS Food Sci. Technol.. 2021;1:427-442.
- [Google Scholar]
- Clean dyeing of acrylic fabric by sustainable red bacterial pigment based on nano-suspension system. J. Cleaner Prod.. 2021;281:125295
- [Google Scholar]
- Effect of dye bath ph on dyeing and functional properties of wool fabric dyed with tea extract. Dyes Pigm.. 2016;134:334-341.
- [Google Scholar]
- Application of Fourier Transform Infrared (FTIR) spectroscopy in the characterization of tannins. Appl. Spectrosc. Rev.. 2015;50:407-442.
- [Google Scholar]
- Extraction, dyeing, and antibacterial properties of crataegus elbursensis fruit natural dye on wool yarn. Fibers Polym.. 2018;19:1428-1434.
- [Google Scholar]
- Vegetable tannins-based additive as antioxidant for biodiesel. Fuel. 2021;292:120198
- [Google Scholar]
- Hepato-renal protective effects of egyptian purslane extract against experimental cadmium toxicity in rats with special emphasis on the functional and histopathological changes. Toxicol. Rep.. 2019;6:625-631.
- [Google Scholar]
- Economically viable UV-protective and antioxidant finishing of wool fabric dyed with Tagetes erecta flower extract: valorization of marigold. Ind. Crops Prod.. 2018;119:277-282.
- [Google Scholar]
- Recent advancements in natural dye applications: a review. J. Cleaner Prod.. 2013;53:310-331.
- [Google Scholar]
- Effect of acidic condition on phenolic composition and antioxidant potential of aqueous extracts from sorghum (sorghum bicolor) bran. J. Food Biochem.. 2014;38:110-118.
- [Google Scholar]
- The effect of tris(2-carboxyethyl)phosphine on the dyeing of wool fabrics with natural dyes. Dyes Pigm.. 2014;108:70-75.
- [Google Scholar]
- Bioactive and UV protective silk materials containing baicalin - The multifunctional plant extract from Scutellaria baicalensis. Georgi. Mater. Sci. Eng. C.. 2016;67:336-344.
- [Google Scholar]
- Ecofriendly sonicator dyeing of cotton with Rubia cordifolia Linn. using biomordant. Dyes Pigm.. 2008;76:207-212.
- [Google Scholar]
- Antioxidant activity of purslane extract and its inhibitory effect on the lipid and protein oxidation of rabbit meat patties during chilled storage. J. Sci. Food Agric.. 2020;2:100156
- [Google Scholar]
- A new skeleton flavonoid and a new lignan from Portulaca oleracea L. and their activities. Fitoterapia. 2021;153:104993
- [Google Scholar]
- Formulation and evaluation of a α-linolenic acid and vitamin E succinate microemulsion with low surfactant content and free of co-surfactant for use as a nutritional supplement. Food Chem.. 2021;364:130433
- [Google Scholar]
- Antiviral activity of portulaca oleracea L. extracts against porcine epidemic diarrhea virus by partial suppression on myd88/NF-κb activation in vitro. Microb. Pathog.. 2021;154:104832
- [Google Scholar]
- The condensed tannin chemistry and astringency properties of fifteen Vitis davidii Foex grapes and wines. Food Chem.: X.. 2021;11:100125
- [Google Scholar]
- Dyeing cotton with tea extract based on in-situ polymerization: an innovative mechanism of coloring cellulose fibers by industrial crop pigments - ScienceDirect. Ind. Crops Prod.. 2019;142:111863
- [Google Scholar]
- Aqueous extraction of buckwheat hull and its functional application in eco-friendly dyeing for wool fabric. Text. Res. Journal. 2019;90:1-14.
- [Google Scholar]
- Adsorption kinetics of Cu2+ on nano alumina supported on activated carbon. Filtr. Sep.. 2017;27(03)
- [Google Scholar]
- Agricultural waste of Eriobotrya japonica L. (Loquat) seeds and flora leaves as source of natural dye and bio-mordant for coloration and bio-functional finishing of wool textile. Ind. Crops Prod.. 2021;169:113633
- [Google Scholar]
- Environmental friendly bioactive finishing of wool textiles using the tannin-rich extracts of Chinese tallow (Sapium sebiferum L.) waste/fallen leaves. Dyes Pigm.. 2020;176:108230
- [Google Scholar]
- Natural flavonoid-functionalized silk fiber presenting antibacterial, antioxidant, and UV protection performance. ACS Sustain. Chem. Eng.. 2017;5:10518-10526.
- [Google Scholar]







