Translate this page into:
Effect of different molecular weights of polyethylene glycol as a plasticizer on the formulation of dry powder inhaler capsules: Investigation of puncturing size, morphologies, and surface properties
⁎Corresponding authors. r.ramezani@irancapsul.com (Ramin Ramezani Kalmer), mojganghanbari16@yahoo.com (Mojgan Ghanbari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Today, the principle of research and development is pulmonary drug delivery due to the potential for maximizing therapeutic effects for patients by direct drug targeting the pathology site in the lungs. Amongst the convenient delivery alternatives, the Dry Powder Inhaler (DPI) is the preferred device to remedy a variety of diseases. In this regard, the fabrication and development of a novel formulation for DPI capsules have been studied. We investigated the effects of various parameters, such as percentages of polyethylene glycol (PEG), propylene glycol (PG), glycerol (Gly), brittleness, test conditions, and particle release of manufactured DPI capsules. The efficacy of each parameter was evaluated in detail to understand and address the consequences of the mentioned factors. The results illustrated that the lower molecular weight of PEGs presented the better plasticizing capability of gelatin. Owing to the hygroscopicity of the utilized plasticizer, polyethylene glycol 400 (PEG 400) increased the capsule flexibility for a longer time and its stability under environmental conditions in the gelatin capsule formulation. Likewise, no particle release was observed in the gelatin/PEG capsule. The prepared gelatin/PEG400 capsules were compared with pure gelatin and HPMC capsules. The capsules were evaluated in terms of loss on drying (LOD), surface morphology, roughness, and puncture type. The results show that using PEG-400 can lead to the production of capsules that have low moisture content and minimal interaction with APIs. In addition, gelatin/PEG capsules have no particles due to the smooth surface after the punching process. The as-produced capsules are not blocked again after punching, allowing the patient to take the drug completely. In fact, the present research provided substantial insight regarding the development of DPI formulation in capsule investigations on an industrial scale.
Keywords
Capsule
Dry powder inhaler
Gelatin
Plasticizer
Poly Ethylene Glycol
Industrial scale
1 Introduction
Nowadays, respiratory diseases are increasing around the world (de Menezes et al., 2021; Ding et al., 2021). The utilization of dry powder inhalers (DPIs) is considered a beneficial strategy for drug delivery due to its straightforward usage and reasonable price. Undoubtedly, the DPI capsule is designed to transfer a dose of drug or composition of drugs substantially in a conventional dynamic area of particle sizes to provide therapy for systemic or positional pulmonary illnesses (de Boer & Thalberg, 2021; Rashid et al., 2021). The drug particles for dry powder inhalation must be so small at aerodynamic diameters of 1–5 μm to achieve the limited lung regions. In particular, whenever systemic particles smaller than 2 μm are demanded, topical drug delivery to the lungs is optimally at 2–5 μm (Islam & Gladki, 2008). There are two forms of DPIs noted: pre-metered DPIs and device-metered DPIs (Richardson, 2011). In addition, there are three types of DPI devices such, as blister-based dry powder inhalers, reservoir-based dry powder inhalers, and capsule-based dry powder inhalers. Capsule-based inhalers themselves are divided into three types, including Rotahalers, HandiHaler, and Aerolizers (Anderson, 2005).
To date, several practical reports have been published on the use of hard capsules for inhaled drugs, but none of the companies has provided a technical report on the formulations of these capsules (Benke et al., 2021; Buttini et al., 2021; de Boer et al., 2017; Lavorini et al., 2017; O’Connor, 2004). DPI capsules demonstrate many unique properties, including permanent formulation, cost efficiency, lack of propellants, and excellent stability. It is necessary to mention that the essential advantage of DPIs is to overcome metered-dose inhalers. Different kinds of substantial and crucial parameters, including dimensions, size, and moisture content of polymer type, powder retention, color, print, transparency, and capsule weight characteristics, should be considered to address the appropriate formulation for the DPI capsule (Al Sayyed, 2019). Moreover, alternative parameters that affect powder maintenance in the capsules, including capsule moisture, the roughness of the capsule's internal surface, and the amount of lubricant remaining, are also significant (Benque & Khinast, 2019; Ding et al., 2021). Each time the capsule shell is punched into the DPI device, the resulting holes should be arranged in shape. The employed material should not be too fragile to generate separate inhalable particles and should not be too elastic. One of the most important parameters in pharmaceutical capsules, especially hard gelatin capsules, is moisture content, which should be the minimum amount and not interact with APIs (Mei et al., 2006; Stegemann et al., 2014). Hence, it is noteworthy that the amount of moisture in the capsule is closely related to the type of employed plasticizer. Indeed, the water within the capsule shell acts as a kind of plasticizer, helping to sustain the indispensable plasticity, but it disappears over time and is not stable inside the formulation (Chong et al., 2016). Therefore, the selected plasticizers should be inherent in the DPI capsule formulation at low humidity.
Conventional gelatin capsules possess a standard moisture content of 13–16 % under representative manufacturing conditions. Since gelatin capsules are globally produced, it'll be very treasured to modify gelatin capsules for use in inhalation applications. An ideal plasticizer should provide the appropriate flexibility. Notably, considering the extent of plasticity of polymers is closely related to their chemical structure, molecular weight, miscibility with the host polymer, and concentration (Laohakunjit & Noomhorm, 2004; Zhu et al., 2021). Various materials such as glycerin (Gly), propylene glycol (PG), and polyethylene glycol (PEG) are used to plasticize gelatin capsules (Gullapalli & Mazzitelli, 2017; Langmaier et al., 2008; Park et al., 1994; Reich, 2004; Snejdrova & Dittrich, 2012).
PEGs have been extensively investigated as separation and purification assistance, food additives, lubricants in medical devices, and as anti-freezing agents (D’souza & Shegokar, 2016). PEG receives its appellation due to its high structural flexibility, amphiphilicity, biocompatibility, lack of any steric hindrances, and hydration magnitude (Knop et al., 2010). It is considered that the PEG100 to PEG700 are in the form of liquids at room temperature. PEGs with molecular weight (MW) values of between 1000 and 2000 are soft solids, and PEGs with MW > 2000 are hard crystalline solids with melting points of around 63 ℃ (Thomas et al., 2014). PEG has a high degree of polarity, which boosts hydrophilicity and improves water solubility, making it stand out among structural polymers of a similar kind. Most organic and inorganic solvents exhibit PEG's high solubility, and it forms a monolayer at the air–water interface (Pasut & Veronese, 2012). As a result, it is crucial for permeation and solubilization. PEGs have functional terminals that are extremely active and electrically neutral at all pH levels (Xi-Feng et al., 2013). Additionally, they have weak protein adsorption, low cell activation and adhesion, limited cellular uptake, and negligible inflammation in a biological context, making them inert. PEGs have a low redox potential and great thermosensitivity. This plasticizing effect is the basis for its mechanical properties (Tessmar & Göpferich, 2007). PEG has already been suggested as a suitable plasticizer in gelatin capsule production (Benke et al., 2021). In 2009, Cao et al. demonstrated that the appropriate molecular weight of PEG for gelatin films is 300 g/mol due to the shorter chain length, facile capability placed between the gelatin chains, and softening action in the gelatin structure (Cao et al., 2009).
The purpose of this research is to design and develop a novel and appropriate formulation for DPI capsules. Herein, we studied the effects of different molecular weights of PEGs and confirmed the initial test of this research in the form of gelatin film. Furthermore, we elucidated the puncturing force and hole morphology of hard capsules, which were punched with a pin using a commercially available DPI device. In particular, the present study was performed in the pilot industrial phase using a production machine, and the results were extracted. Following this, we aim to understand and address the effect of different crucial factors on DPI capsule properties on an industrial scale.
2 Materials and methods
2.1 Materials
All of the starting materials were used as received from valid companies without further purification. Gelatin type B was received from Henan Boom Gelatin Co. (China) and Lapi Gelatin Spa (Italy). Methylparaben and Propyl paraben were acquired from UENO Fine Chemical Industry (Japan), Sodium lauryl sulfate (SLS) was obtained from Godrej Industry (India). Polyethylene Glycol 400 (PEG 400) and Propylene glycol (PG) were purchased from Kimyagaran Emrooz Chemical Industries Co. (Iran), Colloidal Nano silicon dioxide (SiO2) was achieved from Evonik (Germany), Acetic acid glacial, and Zinc sulfate heptahydrate (ZnSO4·7H2O) were obtained from Dr. Mojallali Industrial Chemical Complex Co. (Iran) and Behansar (Iran), respectively.
2.2 Production procedure
2.2.1 Preparation of gelatin films containing various plasticizers
To select a suitable plasticizer for the formulation of DPI capsules and their effect on the brittleness and particle, various plasticizers, including glycerol (Gly), propylene glycol (PG), and different molecular weights of polyethylene glycols (PEGs), such as PEG-400, PEG-600, PEG-1500, and PEG-4000, with a weight ratio of 5 % to dry gelatin were added to the solutions. The mixture was stirred at 500 rpm at 55 ℃ for one hour. The exact formulation of these gelatin films is presented in Table 1.Table 2..
Sample
Gelatin (g)
Water (mL)
Glycerol (g)
PG (g)
PEG-400 (g)
PEG-600 (g)
PEG-1500 (g)
PEG-4000 (g)
Pure
20
44
—
—
—
—
—
—
F1
20
44
1.0
—
—
—
—
—
F2
20
44
—
1.0
—
—
—
—
F3
20
44
—
—
1.0
—
—
—
F4
20
44
—
—
—
1.0
—
—
F5
20
44
—
—
—
—
1.0
—
F6
20
44
—
—
—
—
—
1.0
F7
20
44
0.5
—
—
—
—
—
F8
20
44
0.5
0.5
—
—
—
—
F9
20
44
0.5
—
0.5
—
—
—
F10
20
44
0.5
—
—
0.5
—
—
F11
20
44
0.5
—
—
—
0.5
—
F12
20
44
0.5
—
—
—
—
0.5
Manufacturing machine parameters
Hard gelatin capsules
DPI capsules
Cap
Body
Cap
Body
Air conditioner pressure (Pa)
Kiln 1
43
19
60
92
Kiln 2
41
113
144
111
Kiln 3
64
109
395
346
Kiln 4
60
63
414
376
Kiln 5
27
29
294
253
Temperature of kilns (°C)
Kiln 1
22
23
23
23
Kiln 2
25
24
26
26
Kiln 3
26
26
27
27
Kiln 4
27
27
27.2
27.2
Kiln 5
21
22
23
22
Temperature of solution (°C)
51
51
50
49
Temperature of air conditioner (°C)
23
23.5
Humidity (%)
38.9
26.0
Speed of machine (Pin bar/min)
33
25
2.2.2 Preparation of melting solution for industrial production
The specified volume of Reverse Osmosis (RO) water) 77.0 L(was added to a 160.0-liters feed tank, and the melting process was performed inside the feed tank equipped with a mechanical stirrer containing a propeller blade to prepare the gelatin/PEG capsule. 104.0 g of acetic acid was added to the solution as the temperature increased, and 25.0 kg dry gelatin powder was added to the feed tank by increasing the temperature of the feed tank up to 60 °C. 1302.0 g nano silicon dioxide (SiO2) was added to the feed tank after the gelatin was completely melted. 173.0 g propylene paraben, 24.2 g methylparaben, and 55.3 g propylene glycol were dissolved in 10.0 L of PEG400 and added to the feed tank. Moreover, 73.2 g ZnSO4 and 30.4 g SLS were combined with melted gelatin, and the mixture was mixed for 30 min. Finally, a gelatin solution was prepared with a viscosity of 600 mPa.s and a total volume of 97.0 L.
2.3 Equipment's
The equipment and devices used in this research work include a homemade feed tank (160 Lit), mechanical stirrer with propeller blade, Brookfield Viscosel (VTE model, USA), manufacturing machine (size 3, HGCM, 03–06 380 V, 200Amps, 50 Hz, SCR 10KA, Technophar, Canada), quality control gauges (VSX136, Mitutoyo Japan), fan dryer (GB-121–3, GREENHECK Technophar, Canada), sorting machine (CI5S, Suzhou Sunny Pharmaceutical Machinery Co. ltd.), and hygrometer (MA35M-230 N, Sartorius Lab Instrument, Germany). The scanning electron microscopy (SEM) was recorded on a VEGA TESCAN to investigate the morphology and size of the as-prepared capsules and their puncturing holes diameter. The AFM device was the Ara Research Co Nano Experts model. All of the AFM investigations were performed in the tapping mode at room temperature. The capsule surfaces were measured in a scan size of 5 μm × 5 μm. The surface roughness parameters of the capsules, which are reflected in terms of the mean roughness (Sa), the root mean square of the Z data (Sq), and the mean difference between the highest peaks and lowest valleys (Sz) was achieved by Ara Research Co Nano Experts software for quantitating analysis of images.
3 Results and discussion
3.1 Optimizing plasticizer on laboratory scale
It is noteworthy that an ideal plasticizer should possess a series of beneficial parameters such as polarity, size, availability of polar groups, and shape of plasticizer. Table 1 depicts the formulation of samples F1 to F12 containing different plasticizers. As can be seen in this table, the effect of each plasticizer was studied to evaluate the brittleness of gelatin films. Various plasticizers, including Gly, PG, PEG-400, PEG-600, PEG-1500, and PEG-4000, were evaluated to select the suitable plasticizer for the formulation of DPI capsules. These materials were prepared as a film by combining different percentages (samples F1 to F12), and their brittleness was evaluated after 2.0 and 24.0 h of drying (Fig. 1). The results showed that the film containing Gly became very brittle and was highly susceptible to particle formation. This is because of the interaction type of Gly and gelatin that makes its structure hard and brittle. In other words, Gly changed the absorption features of gelatin films, increasing the amount of water at high relative humidities and reducing it at low relative humidities, proposing complicated polyol-matrix interactions (Díaz et al., 2011). Besides, the existence of PG reduces the tensile strength and elastic stiffness of the capsule, making it more brittle and weaker (Mei et al., 2006).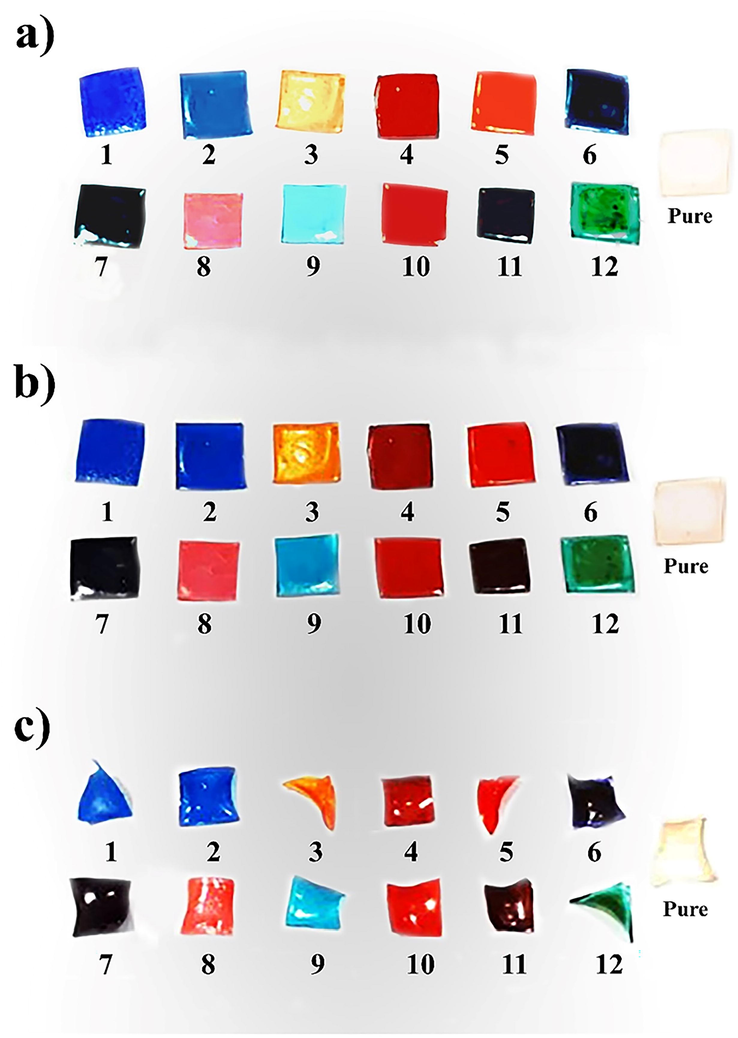
A schematic demonstration of (a) PEG molecular structure and investigating the effect of PEG on plasticizing of gelatin film (a) as-prepared samples (b) after drying (2.0 h) and (c) after drying (24.0 h).
The results showed that plasticizers containing hydroxyl functional groups demonstrate promising potential for protein-based materials because of their capability to decrease intermolecular hydrogen bonding whenever enhancing intermolecular spacing. Therefore, PEGs with various molecular weights, including 400, 600, 1500, and 4000 in four particular weight percentages, were added to the gelatin capsule formulation (Table 1). Plasticized gelatin films made of PEG with various molecular weights change in appearance. It was found that plasticizers tended to migrate to film surfaces for films plasticized by PEG that had a molecular weight of more than 1500. As a result, the films made of PEG1500 and PEG4000 had an opaque surface. However, films plasticized by PEG400 and 600 were transparent. The results revealed that the higher molecular weights of PEG were not a convenient choice for further considerations in DPI capsule formulation since the solution became two-phase with unfavorable parameters. According to the obtained results, the molecular weight of 400 exhibits an effectual effect during the capsule manufacturing process, in which its changes follow an exponential function. The outcome of these manual pining was approximately 80 DPI capsules. The results of these assessments illustrate that an increase in the PEG400 content in the gelatin formulation leads to a delay in the drying process. The capsules with 20 %wt PEG400 did not dry at a certain time. Drying of capsules with 15 %wt PEG400 was possible with a 50-minute delay, which is neither economically practical nor economical. Production of capsules with 10 %wt PEG400 was possible. Hence, this value (10 %wt PEG400) was selected and approved as the optimal amount of PEG400 and this percentage is optimized for industrial production (Fig. 2).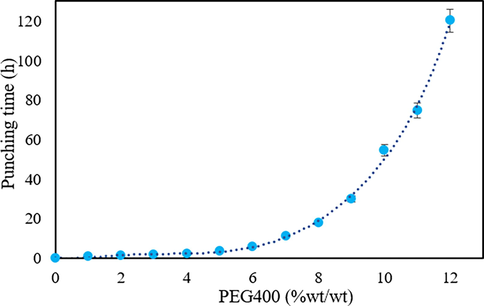
The effect of PEG400 content on different punching times in optimum formulation (n = 3).
3.2 Producing DPI capsules on an industrial scale
Based on previous knowledge, the parameters of the manufacturing machine must be fundamentally changed to produce DPI capsules (size 3) with a new melting formulation containing 10 %wt PEG400. Changing the speed of the air conditioners is essential to increase the capacity of the machine for manufacturing DPI capsules. Adjusting these parameters was necessary but not enough for complete drying. Therefore, several parameters were changed according to Table 3.
Samples
Types of PEG (molecular weight)
PEG (%wt)
Capsule Observations
Particle (min)
Brittleness (min)
F13
1500
0.05
Normal
45
35
F14
1500
0.55
Normal
45
40
F15
1500
1.11
Normal
45
40
F16
1500
1.67
Star dome
45
40
F17
600
0.55
Normal
45
40
F18
600
1.11
Normal
50
45
F19
600
1.67
Star dome
57
49
F20
4000
1.00
Normal
48
40
F21
4000
1.50
Opaque
55
39
F22
4000
2.00
Opaque
57
42
DPI capsules size 3 were produced by changing the parameters of the machine and the gelatin solution. 2 million capsules were manufactured on an industrial scale and were evaluated by the quality control unit. Several parameters, including the size, walls, domes, and shoulders of the prepared capsules, were reported. Concerning physical parameters, it is found that these manufactured capsules are in good agreement with standard capsule range parameters. Indeed, dimensional and physical appraisement of capsule parameters indicates that the manufactured capsules are within the global standard ranges (Fig. 3 and Table 4).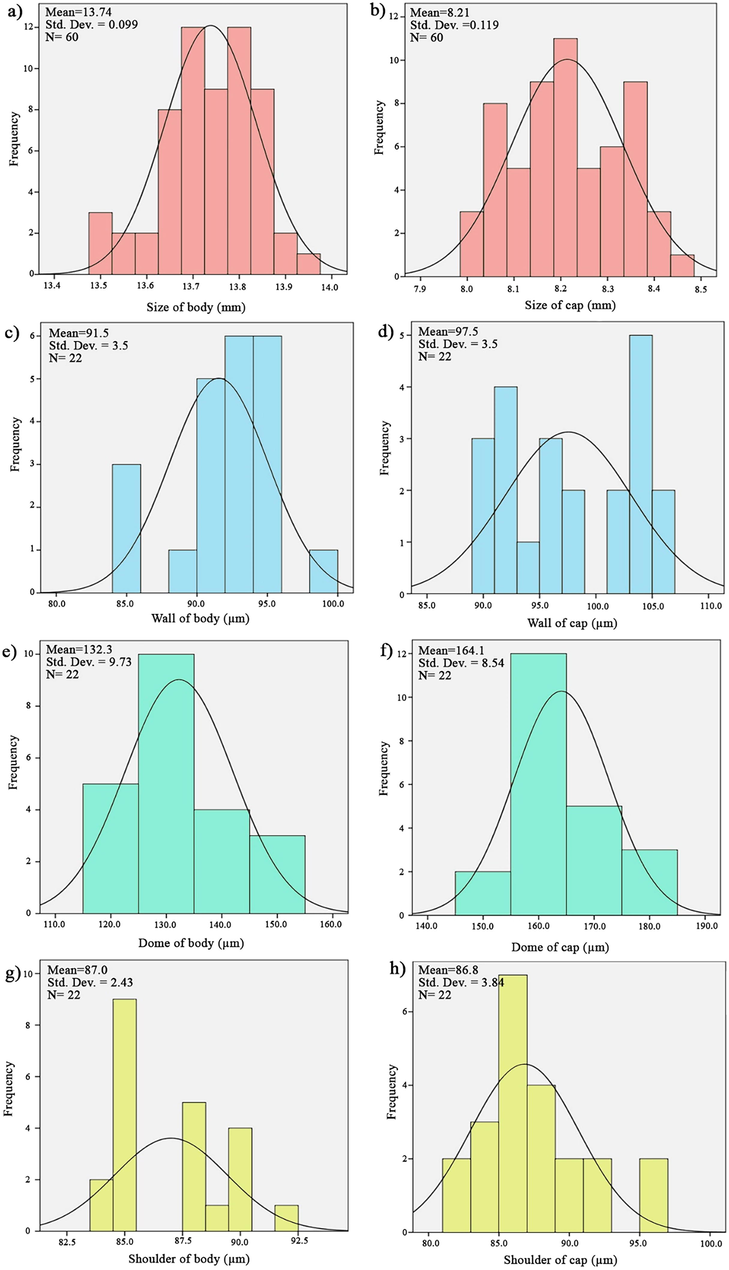
Normal graph distribution of produced DPI capsules (size 3).
Items
Cap
Body
Size (mm)
8.21 ± 0.1
13.74 ± 0.1
Wall (µm)
97.5 ± 5.0
91.5 ± 1.0
Dome (µm)
164.1 ± 8.0
132.3 ± 9.0
Shoulder (µm)
86.8 ± 4.0
87.0 ± 2.0
3.3 Comparing produced DPI capsules with commercial DPI capsules
The DPI capsules based on Gelatin/PEG400 that produced on an industrial scale in the gelatin capsule company (Iran) were compared with other commercial DPI capsules (HPMC and pure gelatin) available on the market. The results indicate (Fig. 4a) that gelatin/PEG-based DPI capsules lose some of their initial water content during the packaging process, but the moisture content stabilizes after 12.0 h. Due to the PEG used and its structure, this residual moisture prevents the capsule from becoming brittle.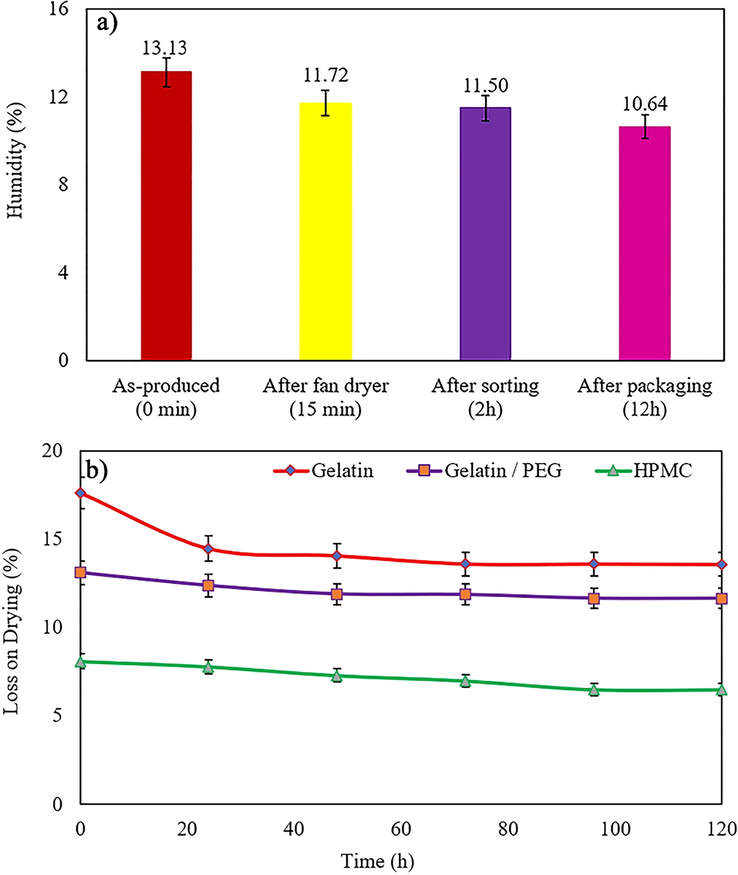
a) Moisture content in manufactured capsules in different steps, and b) loss on drying (%) of Gelatin, Gelatin/PEG and HPMC capsules in different intervals (n = 3).
Given that one of the essential and crucial parameters in capsules is moisture content. Loss on drying (LOD) of gelatin/PEG-based DPI capsules, pure gelatin, and HPMC capsules was evaluated and compared during 12.0 h (Fig. 4b). The results revealed that pure gelatin capsules have a higher moisture content than HPMC and gelatin/PEG capsules. Gelatin/PEG capsules have lower moisture than pure gelatin. The active pharmaceutical ingredient (API) must not aggregate and maintain its fine structure in the inhalation process. Therefore, it is better to use empty hard capsules with less moisture to fill these APIs. On the other hand, the lower moisture content can effectively prevent the API from reacting with the capsule shells. Although HPMC capsules have the most suitable moisture, since the machinery and equipment required for these capsules are cost-effective and not available in all countries, gelatin/PEG capsules are not only adequate in terms of moisture but also economically attainable.
Another important parameter of DPI capsules is the lack of particles in DPI capsules during the punching process. The rougher the surface of the capsule, the more particles are formed. Therefore, AFM analysis was used to evaluate and compare the roughness of pure gelatin, HPMC, and gelatin/PEG capsules. Fig. 5 shows 2D AFM photographs of gelatin (Fig. 5(a-c)), gelatin/PEG (Fig. 5(d-f)), and HPMC (Fig. 5(g-i)) capsules. The scan size of the images was 5 µm × 5 µm. The roughness parameters are shown in Table 5 based on the AFM image. The average roughness (Sa) of the capsules was estimated at 5, 2.5, and 7.5 µm for the gelatin, gelatin/PEG, and HPMC types, respectively. Indeed, when Sa is lower, the surface will be more uniform. It is noteworthy that the surface of the gelatin capsule has more roughness than the gelatin/PEG capsules and DPI capsules based on pure gelatin are prone to particle formation and brittleness.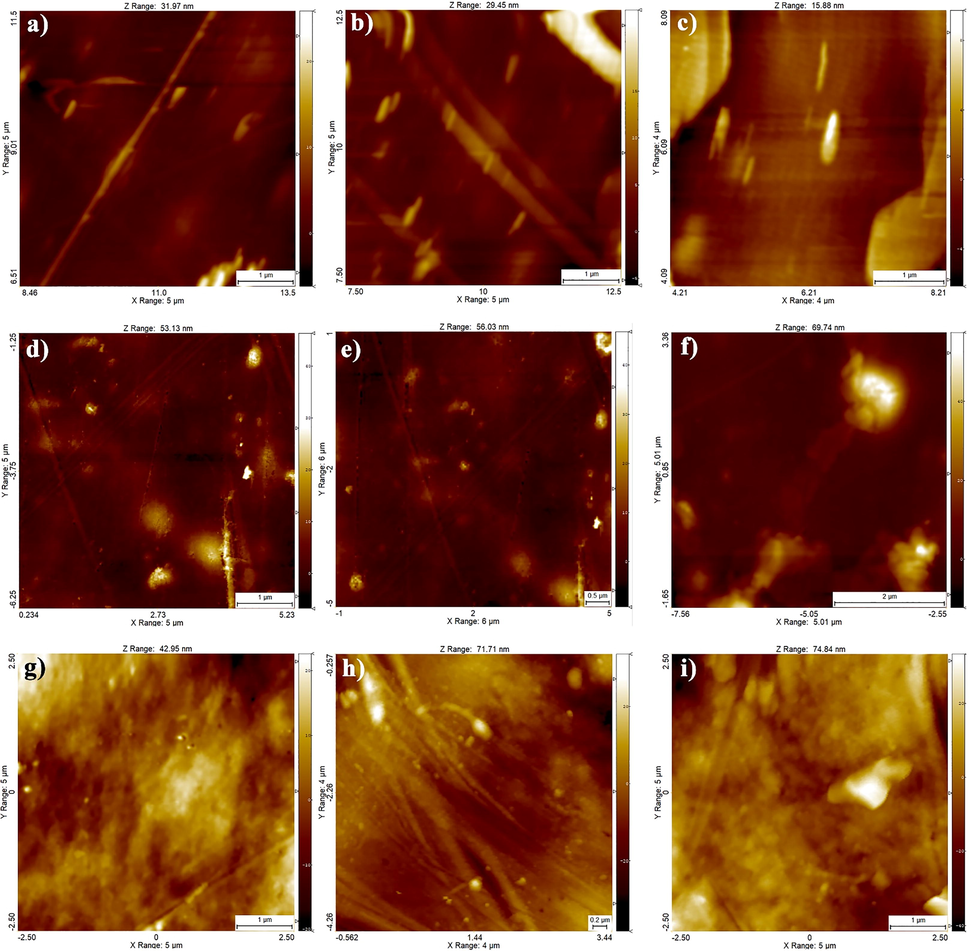
2D AFM surface plots of (a-c) gelatin (d-f) gelatin/PEG and (g-i) HPMC capsules.
Roughness
Type of capsuleSa
(nm)Sq
(nm)Spk
(nm)Sk
(nm)Sy
(nm)Sp
(nm)Smean
(nm)
Gelatin
5
10
22.5
5
12
55
5
Gelatin/PEG
2.5
5
10
3.5
5.5
22.5
2.5
HPMC
7.5
10
12.5
22
42
32
−2.5
In more detail, 3D AFM images of samples are illustrated in Fig. 6. As observed in Fig. 6, topographic images and roughness of the surfaces of the capsule samples can be seen with greater clarity and quality along different axes. It is worth noting that gelatin capsules with a rougher surface can have a greater potential for destruction (Fig. 6(a-c)). The higher roughness, the more brittleness the capsule. As a consequence, the utilization of PEG400 in capsule formulation diminishes the roughness or enhances the flexibility of the capsule against shrinkage and possible deformations (Fig. 6(d-f)). In fact, the flexibility of the manufactured capsule shells is remarkably enhanced due to the intermolecular interaction of gelatin with PEG400 (Scheme 1). The presence of plasticizing agent makes the gelatin chain very flexible and decreases related brittleness. Additionally, the presence of a plasticizing agent ensures that the capsule particles do not separate during punching.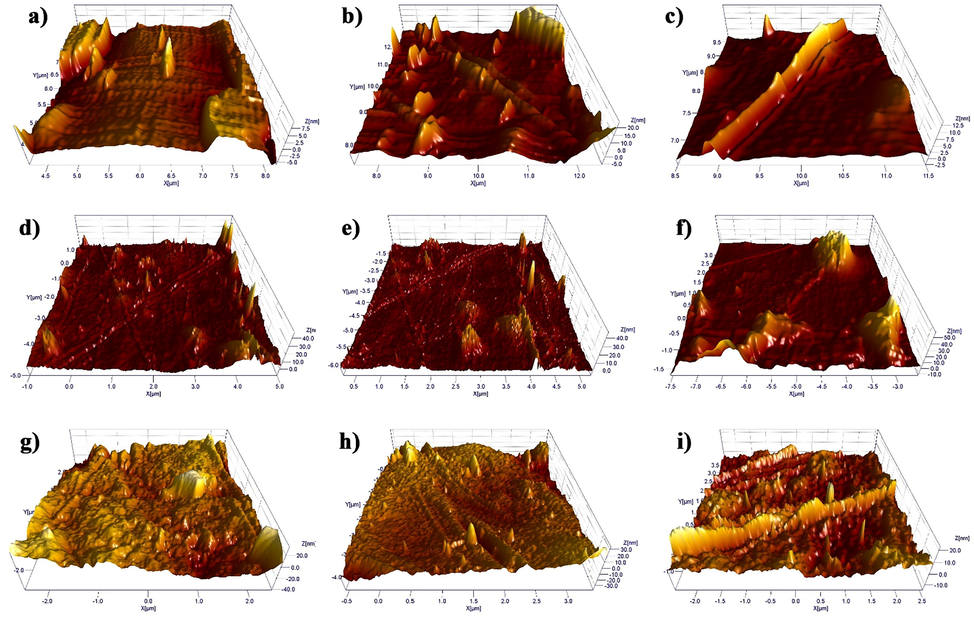
3D AFM images of (a-c) gelatin, (d-f) gelatin/PEG, and (g-i) HPMC capsules in tapping mode.
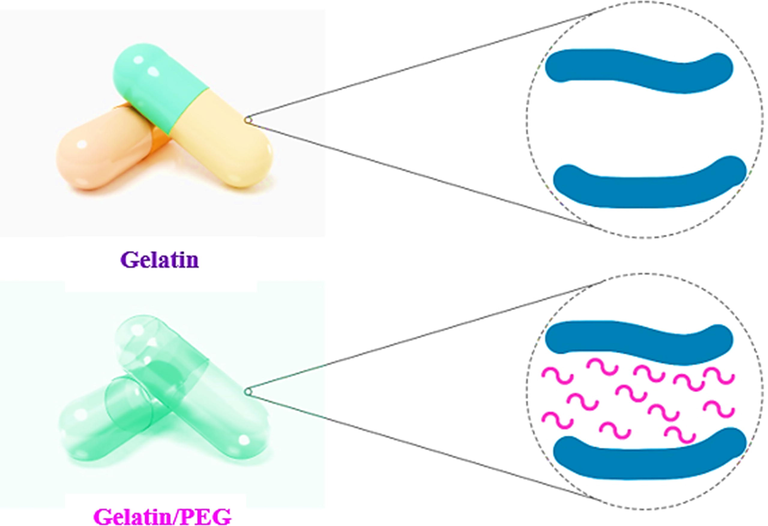
A schematic illustration of the intermolecular interaction of PEG with gelatin chains.
Given that the result of the punching process of DPI capsules is different from pure gelatin, HPMC, and gelatin/PEG (Scheme 2), the issue of not re-blocking the punched portion of the DPI capsule is another parameter to consider when developing DPI capsules. Therefore, SEM analysis was performed to evaluate this feature and determine the morphology and size of the punched part of DPI capsules. As observed in Fig. 7, the holes on the outer surfaces of capsules were monitored. The release of particles from the capsule into the API was identified from the SEM images of the gelatin capsule (Fig. 7a and 7b). In other words, due to punching, a negligible part of the capsule is inhaled with the drug and enters the patient's body. In more detail, the holes are circular in shape with a diameter of 2.19 mm. The SEM images of the gelatin/PEG capsule in Fig. 7c and 7d clearly show that no piece of the capsule is detached from its main body. Significantly, the addition of PEG400 to the DPI capsule formulation at a specific weight percentage gives the capsule the desired flexibility and does not release any particles easily. As shown in Fig. 7c and 7d, the addition of PEG400 to gelatin makes the properties and punching angle closer or quite similar to HPMC capsules. Conclusively, SEM results confirm that PEG400 is remarkably convenient and favorable for manufacturing DPI capsules. By comparing the SEM images of gelatin and gelatin/PEG, it is found that HPMC capsule holes are closer to gelatin/PEG capsules and smaller in size (1.84 mm) with no detached pieces (Fig. 7e and 7f), and the holes remained in regular shape.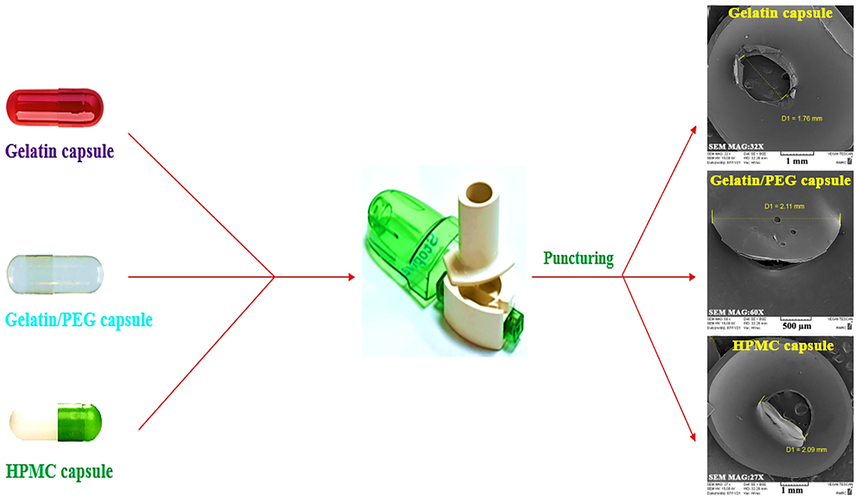
A schematic representation for the puncturing process of gelatin, gelatin/PEG and HPMC capsules and obtaining SEM images.
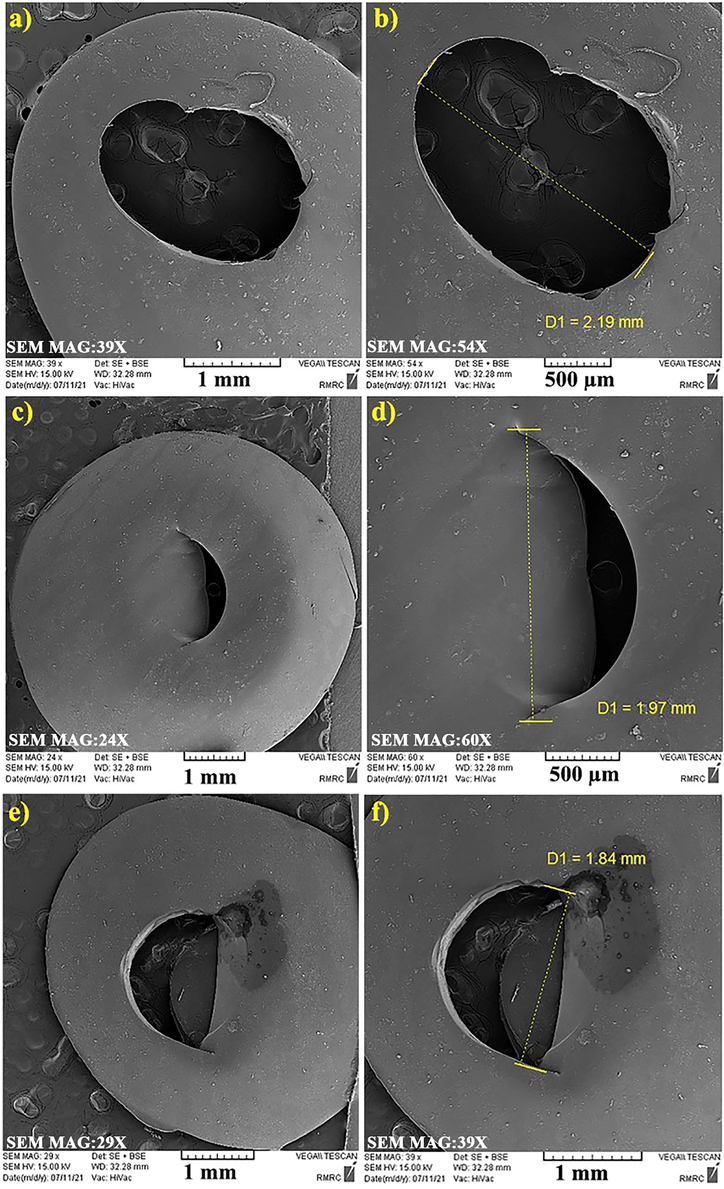
SEM images from outside of prepared Gelatin capsule (a,b), gelatin/PEG capsule (c,d) and HPMC capsules (e,f).
Furthermore, SEM techniques were taken from the inner surface of prepared capsules to figure out and address the internal surface characteristics, the size of the generated holes during punching, uniformity, and order of the holes. According to Fig. 8, it is evident that the gelatin capsule holes are not uniform in size. The size of the punched holes in the gelatin capsule was 1.76 mm. Fig. 8 displays the SEM images of the gelatin/PEG capsule inner surface. It is observed that the presence of PEG enhances the plasticity of this kind of capsule. This type of capsule does not show any particle release. It is conceivable that this behavior is due to the presence of PEG in capsule formulation and its plasticizing function.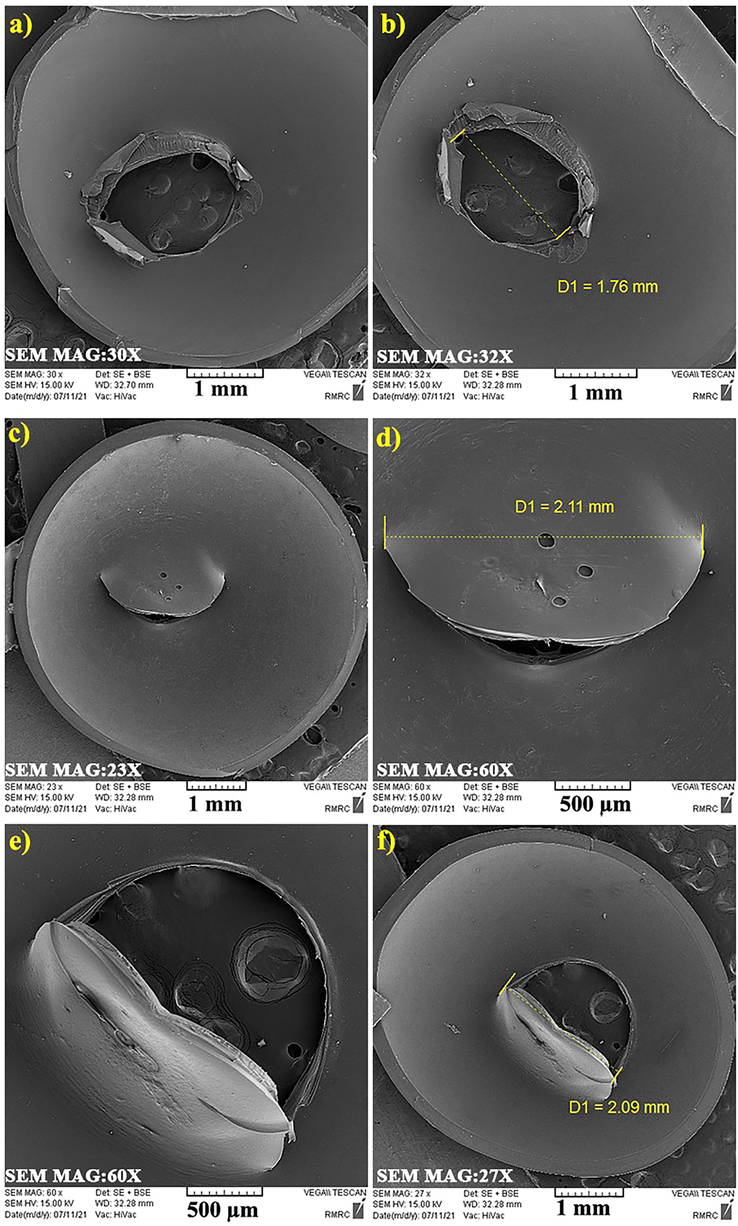
SEM images from inside of prepared gelatin/PEG capsule (a,b), Gelatin capsule (c,d) and HPMC capsules (e,f).
4 Conclusions
In this study, the design and development of gelatin/PEG capsules noted as DPIs was considered one of the main goals. The results and outputs of this work have entered the industrial and production phase after laboratory examinations and initial tests. All these results indicated that the utilization of PEG400 as a plasticizer displayed the best properties in the formulation of DPI capsules. It was found that the lower molecular weight of PEG resulted in better visual properties, a convenient plasticizing effect, a small amount of particle release, and low brittleness. It was well known that the gelatin/PEG capsule did not display any instability and preserved its regular holes. The prepared DPI capsules contained no particles and allowed all the medicine and API to reach the patient completely. According to the obtained information, gelatin/PEG capsules ensures that the capsules are not blocked after inhalation, and the patient can use all the medicine and active ingredient entirely. Based on the SEM images, it could be concluded that the gelatin/PEG capsules illustrated better results, and their residue remained on the outer surface of the capsule. Besides, the obtained results indicate that the present work is also appropriate and practical from an industrial viewpoint. According to the AFM study, the gelatin/PEG capsules have a smoother surface than gelatin and HPMC counterparts. Significantly, AFM results also confirm the effect of PEG400 on the roughness and corresponding flexibility of capsules. Overall, the optimum amount of plasticizing agent with unique characteristics leads to the best properties, including the convenient wall, dome, and shoulder properties in the formulation of DPI capsules, which are other advantages of this research work.
CRediT authorship contribution statement
Ramin Ramezani Kalmer: Conceptualization, Supervision, Project administration, Visualization. Afzal Karimi: Data curation, Validation. Mortaza Golizadeh: Methodology. Mohsen Mohammadi Haddadan: Investigation, Formal analysis, Methodology, Software. Maryam Azizi: Investigation, Formal analysis, Methodology, Writing – review & editing, Software. Hamed Ramezanalizadeh: Writing – original draft, Data curation, Validation, Resources. Mojgan Ghanbari: Writing – review & editing, Validation, Resources, Data curation.
Acknowledgment
Authors are grateful to the Iran Gelatin Capsule Mfg Co-IGCC for supporting this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Quality by design considerations for product development of dry-powder inhalers. In: Pharmaceutical Quality by Design. Elsevier; 2019. p. :173-192.
- [Google Scholar]
- History of aerosol therapy: liquid nebulization to MDIs to DPIs. Respiratory care. 2005;50(9):1139-1150.
- [Google Scholar]
- Stability and In Vitro Aerodynamic Studies of Inhalation Powders Containing Ciprofloxacin Hydrochloride Applying Different DPI Capsule Types. Pharmaceutics. 2021;13(5):689.
- [Google Scholar]
- Understanding the motion of hard-shell capsules in dry powder inhalers. Int. J. Pharm.. 2019;567:118481
- [Google Scholar]
- Understanding the Importance of Capsules in Dry Powder Inhalers. Pharmaceutics. 2021;13(11):1936.
- [Google Scholar]
- Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocolloids. 2009;23(3):729-735.
- [Google Scholar]
- Evaluating the sensitivity, reproducibility and flexibility of a method to test hard shell capsules intended for use in dry powder inhalers. Int. J. Pharm.. 2016;500(1–2):316-325.
- [Google Scholar]
- Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert opinion on drug delivery. 2016;13(9):1257-1275.
- [Google Scholar]
- Dry powder inhalation: past, present and future. Expert opinion on drug delivery. 2017;14(4):499-512.
- [Google Scholar]
- Current advances in drug delivery of nanoparticles for respiratory disease treatment. J. Mater. Chem. B. 2021;9(7):1745-1761.
- [Google Scholar]
- Effect of glycerol on water sorption of bovine gelatin films in the glassy state. Procedia Food Sci.. 2011;1:267-274.
- [Google Scholar]
- A quality by design framework for capsule-based dry powder inhalers. Pharmaceutics. 2021;13(8):1213.
- [Google Scholar]
- Gelatin and non-gelatin capsule dosage forms. J. Pharm. Sci.. 2017;106(6):1453-1465.
- [Google Scholar]
- Dry powder inhalers (DPIs)—a review of device reliability and innovation. Int. J. Pharm.. 2008;360(1–2):1-11.
- [Google Scholar]
- Poly (ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Ed.. 2010;49(36):6288-6308.
- [Google Scholar]
- Plasticizing collagen hydrolysate with glycerol and low-molecular weight poly (ethylene glycols) Thermochim Acta. 2008;469(1–2):52-58.
- [Google Scholar]
- Effect of plasticizers on mechanical and barrier properties of rice starch film. Starch-Stärke. 2004;56(8):348-356.
- [Google Scholar]
- Recent advances in capsule-based dry powder inhaler technology. Multidisciplinary respiratory medicine. 2017;12(1):1-7.
- [Google Scholar]
- Use of texture analysis to study hydrophilic solvent effects on the mechanical properties of hard gelatin capsules. Int. J. Pharm.. 2006;324(2):128-135.
- [Google Scholar]
- The ideal inhaler: design and characteristics to improve outcomes. Respir. Med.. 2004;98:S10-S16.
- [Google Scholar]
- Water vapor permeability and mechanical properties of grain protein-based films as affected by mixtures of polyethylene glycol and glycerin plasticizers. Transactions of the ASAE. 1994;37(4):1281.
- [Google Scholar]
- State of the art in PEGylation: the great versatility achieved after forty years of research. J. Control. Release. 2012;161(2):461-472.
- [Google Scholar]
- Puerarin dry powder inhaler formulations for pulmonary delivery: Development and characterization. PLoS ONE. 2021;16(4):e0249683.
- [Google Scholar]
- Formulation and physical properties of soft capsules. Pharmaceutical Capsules: Pharmaceutical Press, London; 2004. p. :201-212.
- Richardson, M. (2011). Impact of capsule selection on formulation stability in dry powder inhalers (DPIs). Inhaltion Mag.(April) www.inhahalationmag.com.
- Application of QbD principles for the evaluation of empty hard capsules as an input parameter in formulation development and manufacturing. AAPS PharmSciTech. 2014;15(3):542-549.
- [Google Scholar]
- Customized PEG-derived copolymers for tissue-engineering applications. Macromol. Biosci.. 2007;7(1):23-39.
- [Google Scholar]
- Beyond poly (ethylene glycol): linear polyglycerol as a multifunctional polyether for biomedical and pharmaceutical applications. Biomacromolecules. 2014;15(6):1935-1954.
- [Google Scholar]
- Surface modification of poly ethylene glycol to resist nonspecific adsorption of proteins. Chin. J. Anal. Chem.. 2013;41(3):445-453.
- [Google Scholar]
- Biobased Plasticizers from Tartaric Acid: Synthesis and Effect of Alkyl Chain Length on the Properties of Poly (vinyl chloride) ACS Omega. 2021;6(20):13161-13169.
- [Google Scholar]







