Translate this page into:
Effect of gambogenic acid in attenuating diethylnitrosamine (DEN)-induced hepatocellular carcinoma in rat model
⁎Corresponding author. zheng_sheng523@sina.com (Sheng Zheng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Liver cancer, specifically hepatocellular carcinoma has been a widespread problem among general population. This study aims to investigate the modulating mechanism of gambogenic acid, a phenolic xanthonoid, in diethylnitrosamine (DEN)-induced liver cancer in rats. Male Wistar albino rats were clustered into four groups (n = 6). Group I served as control treated with normal saline. Hepatocellular carcinogenesis was induced in rats by single intraperitoneal (i.p.) administration of DEN in saline (200 mg/kg b.w.) for groups II and III. Group III received oral administration of gambogenic acid (20 mg/kg b.w.) one hour post DEN administration, whereas group IV received oral administration of gambogenic acid (20 mg/kg b.w.) alone. Rats were sacrificed after 16 weeks to determine the levels of hepatic biomarkers, oxidative stress markers, hematological profile and histopathological changes. Gambogenic acid significantly ameliorated the expressions of oxidative stress markers TBARS, GSH (P < 0.05), enzymatic antioxidants GPx, CAT, SOD, GST (P < 0.05), apoptosis mediators (P < 0.05), and serum biomarkers for liver damage and tumor formation (P < 0.05) compared with DEN-induced model group. Hepatocellular levels of 8-OHdG were significantly diminished (P < 0.05) by gambogenic acid against the damage incurred by DEN. Liver histopathological derangements caused by DEN were reversed by gambogenic acid. The results clearly impacted the effect of gambogenic acid in attenuating DEN-induced hepatocellular carcinoma in rats mediated through NF-kβ pathway and hepatocellular oxidative damage.
Keywords
Hepatocellular carcinoma
Phenolic acid
Oxidative stress
Immunohistochemistry
Histopathology
1 Introduction
Liver cancer is prevalent among large population of the world and is a common cause of mortality. Hepatocellular carcinoma being the prominent type of liver cancer, has a high risk of mortality and is widely studied as to identify the possible causes and underlying mechanisms (Yang et al, 2019). Factors like hormone exposure, high alcoholism, hepatitis, diethylnitrosamine (DEN), aflatoxin B1, and haemochromatosis were detected to be the major causes for hepatocellular carcinoma. DEN is a cancer causing compound that triggers the formation of carcinogenic activity in organs, being a hepatocarcinogen and also a substrate of hepatic cytochrome CYP2E1 that incurs the generation of intracellular reactive oxygen species (ROS) (Verma et al., 2017). Oxidative stress due to elevated ROS levels are defined to be a paradigm for cancer progression. Therefore, DEN is applied as a model to induce hepatocellular carcinoma in animal studies (Kurma et al., 2021). Patients diagnosed with hepatocellular carcinoma have a normal survival time of <5 years as the disease normally progresses into a secondary metastasis without early detection. High recurrence rate of hepatocellular carcinoma and progression of metastasis reflects the non-curative methods of treatment given to patients (Singh et al, 2018). Consequently, the quality of life of the patients is conceded throughout the treatment period and the survival rate declines. Even though chemotherapy and radiotherapy are the common methods to counter cancer progression, they are associated with several adverse reactions that could worsen the health condition of patients.
Considering the factors in current treatment methods of hepatocellular carcinoma, an alternative treatment should be considered for the betterment of patient’s wellbeing. Medicinal plants and herbs have long been considered as potential remedies for cancer and numerous compounds have been identified with anti-cancer properties in pre-clinical studies (Lodhi et al., 2020; Sayed-Ahmed et al., 2010). Many of the commercial anti-cancer drugs like etoposide, cisplatin, vinblastine, vincristine, paclitaxel, doxorubicin, irinotecan, topotecan, were discovered and derived from medicinal plants. Plant-derived anti-cancer compound could increase the therapeutic efficacy in modulating hepatocellular carcinoma. Garcinia hanburyi is a gamboge producing tree, rich in cytotoxic xanthonoids used in traditional Chinese medicine for the treatment of tape worms in humans (Li et al., 2022). Among a few active compounds isolated from gamboge, gambogenic acid has been studied and known to possess anti-cancer properties as reported to exert anti-proliferative and apoptosis in hepatoma cells, lung cancer cells, breast cancer and colorectal cancer in animal models (Liu et al., 2021; Wang et al., 2021; Shen et al., 2020; Huang et al., 2019; Yu et al., 2016; He et al., 2015; Yan et al., 2011). Gambogenic acid was reported to have modulated the liver biotransformation enzyme CYP2E1 and has also been reported with hepato-protective effect through regulating PI3K/Akt and NF-kβ pathways (Ding et al., 2021; Sun et al., 2018). Considering the effectiveness of gambogenic acid as a potent anti-cancer agent, this research was designed to learn the effect on DEN-induced hepatocellular carcinoma in rat model.
2 Materials and methods
2.1 Chemicals and reagents
Gambogenic acid (purity ≥ 95 %), DEN, commercial assay kits, xylazine, ketamine, phosphate buffer, hematoxylin, eosin, primary and secondary antibodies were purchased from Sigma (St Louis, US). All reagents were of analytical grade (AR) or in the purest form.
2.2 Animals and study design
Male Wistar albino rats of 8–10 weeks (150–180 g) used in this study were acclimatized for a week under standard conditions (25 ± 2 °C; 12:12 h light/dark cycle) before the experiment with standard feed and water ad libitum. The experimental design was sanctioned by the Institutional Animal Ethics Committee (IAEC) of The Third People's Hospital of Yunnan Province Kunming City, Yunnan Province. Briefly, 24 rats were divided into four groups consisting six rats in each. Group I received oral administration of normal saline (0.1 N) for 16 weeks, group II received a single intraperitoneal (i.p.) injection of DEN (200 mg/kg b.w.) in buffer on the first day of experiment, group III was administered with a single intraperitoneal (i.p.) injection of DEN (200 mg/kg b.w.) in buffer on the first day of experiment followed by oral administration of gambogenic acid (20 mg/kg b.w.) for 16 weeks, and group IV was treated with oral administration of gambogenic acid (20 mg/kg b.w.) only for 16 weeks following the methods of Singh et al. (2018). Body weight of rats were monitored every day for 16 weeks. Rats were euthanized through cervical dislocation on the last day of week 16 under deep anesthesia with ketamine/xylazine injection. Liver tissues were extracted, cleaned, blotted with filter paper and weighed. Blood samples were obtained and stored for serum analysis. Liver sections were stored in 10 % formaldehyde for histopathological investigations. Liver tissues were homogenized in phosphate buffer (pH 7.4) and RIPA buffer with protease inhibitor cocktail and centrifuged at 12,000 g for 20 min to obtain supernatants for biochemical assays. Protein concentrations in the supernatant were estimated using BCA protein assay kit (Beyotime Biotechnology, China).
2.3 Serum biochemical analysis
Serum aspartate aminotransferase (AST), total bilirubin, alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH) were quantified as liver damage markers using colorimetric assay kits (R&D Systems, Minneapolis, US) following the manufacturer’s guidance and results were displayed as IU/L. Carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) were quantified as tumor markers using ELISA assay kits (R&D Systems, Minneapolis, US) following the manufacturer’s guidance and the results were displayed as ng/mL.
2.4 Liver oxidative stress assay: TBARS and GSH
Supernatant of liver tissues were subjected to lipid peroxidation and reduced glutathione (GSH) analysis following the protocols of You et al. (2021). Thiobarbituric acid reactive substances (TBARS) were analyzed in the supernatant to measure malondialdehyde (MDA) formation using spectrophotometer at 532 nm. The results were given as nmol MDA/mg protein. Reaction of GSH with dinitrobenzoic acid (DTNB) were assayed using spectrophotometer at 412 nm. The results were given as nmol/mg protein.
2.5 Liver enzymatic antioxidant assays
Activities of enzymatic antioxidants in the liver supernatant were measured following the protocols of Ma et al (2021). Glutathione peroxidase (GPx), catalase (CAT), superoxide dismutase (SOD), and glutathione transferase (GST) were measured using standard colorimetric assay kits (Sigma, US) following the methods given by manufacturer. The results were given as U/mg protein.
2.6 Apoptosis marker analysis
Apoptosis marker proteins were examined using western blot analysis using primary antibodies (Bax, Bcl-2; 1:500) and HRP-conjugated secondary antibodies according to the methods described by Ding et al. [16], with GAPDH as internal control. Enhanced chemiluminescent substrate and Image J software (NIH, US) were used to visualize and quantify the protein expressions.
2.7 Oxidative damage and tumor indication analysis
Liver oxidative damage were analyzed using immunohistochemical analysis on deparaffinized liver tissue sections by incubating with primary antibodies against 8-OHdG (1:1000), p65 (1:200), p-p65 (1:200) stained with DAB (EnVision kit, Dako, Denmark) and counterstained with hematoxylin following the method of Xu et al. (2021).
2.8 Histopathological analysis
Liver tissues fixed in formaldehyde were dehydrated, embedded in paraffin, sectioned (5 µm) and stained with hematoxylin and eosin (H&E) to be imaged under light microscope. Pathological changes in liver tissues were observed and verified by unbiased pathologist who was not aware of the experimental groups.
2.9 Statistical analysis
The experimental results were mentioned as mean ± SEM of six animals. The statistical analysis was conducted with SPSS 21.0 software using one-way ANOVA and Tukey-Kramer multiple comparisons analysis. Values denoting significance were fixed at P < 0.05.
3 Results
3.1 Effects of gambogenic acid on body, liver weight and tumor formation
Average body weight of rats that received DEN alone (group II) was reduced (P < 0.05) compared to control group I as displayed in Table 1. Rats treated with gambogenic acid (groups III and IV) exhibited relatively lower liver weight (P < 0.05) against DEN-induced hepatocarcinoma. Higher liver weight was observed in DEN treated group II, with 100 % incidence of tumor formations compared to group I. Tumor incidence in gambogenic acid treated group III were 33.3 % whereas gambogenic acid alone treated group IV did not show any signs of tumor formation. Values expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by Tukey-Kramer multiple comparisons test. The symbol ‘a’ represents significance (P < 0.05) as compared to the control group, whereas the symbol ‘b’ represents significance (P < 0.05) as compared to the DEN alone treated group.
Groups
Initial body weight (g)
Final body weight (g)
Liver weight (g)
Tumor incidence
Control
158.3 ± 13.2
253.1 ± 2.4
10.57 ± 0.60
0
DEN
158.1 ± 12.1
205.3 ± 14.4 a
14.12 ± 1.56 a
13 ± 2.76 a
DEN + GA
161.6 ± 11.6
246.7 ± 2.5b
11.24 ± 1.12b
4 ± 1.32b
GA
160.5 ± 10.1
256.4 ± 4.3
10.57 ± 0.62b
0
3.2 Effects of gambogenic acid on serum liver markers
Liver injury markers ALP, AST, ALT, LDH, total bilirubin and tumor indicators CEA, AFP were significantly increased (P < 0.05) in DEN alone treated group II compared to control and gambogenic acid treated groups. Gambogenic acid significantly suppressed the liver and tumor markers as shown in Fig. 1. Gambogenic acid alone treated group IV showed similar findings as the control group.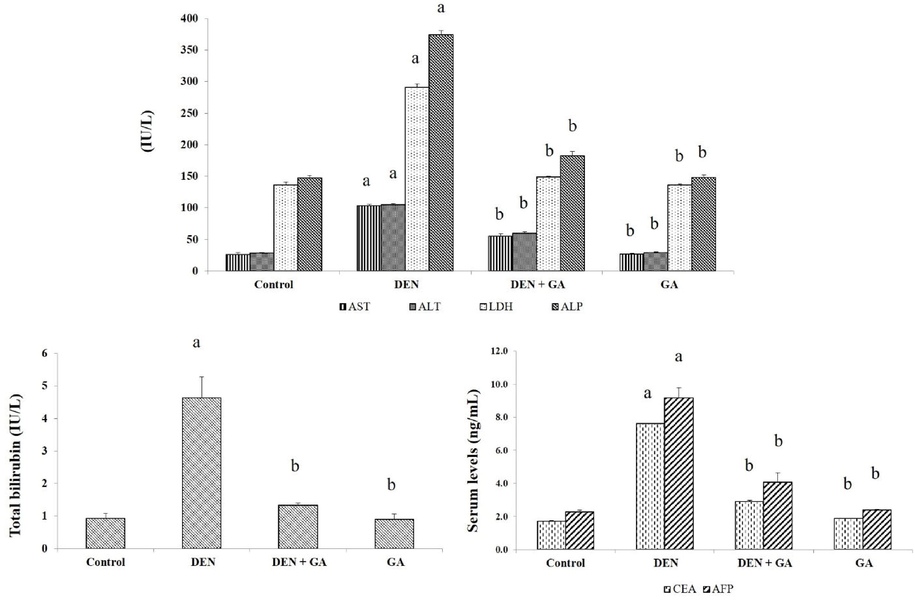
Effect of gambogenic acid on liver and tumor biomarkers in DEN-induced rats. Values expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by Tukey-Kramer multiple comparisons test. The symbol ‘a’ represents significance (P < 0.05) as compared to the control group, whereas the symbol ‘b’ represents significance (P < 0.05) as compared to the DEN alone treated group.
3.3 Gambogenic acid reverses oxidative stress
Tissue lipid peroxidation levels were high in DEN alone treated model group II (P < 0.05) indicating oxidative stress, whereas levels of lipid peroxidation in liver tissues were reduced by gambogenic acid (P < 0.05) treatment. Moreover, GSH levels were strongly elevated in gambogenic acid treated groups (P < 0.05). Antioxidant enzyme activities were significantly suppressed (P < 0.05) in DEN only treated group II compared to control group I. Gambogenic acid treatment restored the activities of GPx, CAT, SOD and GST (P < 0.05) in contrast with DEN treatment. All results for oxidative stress related markers are shown in Fig. 2.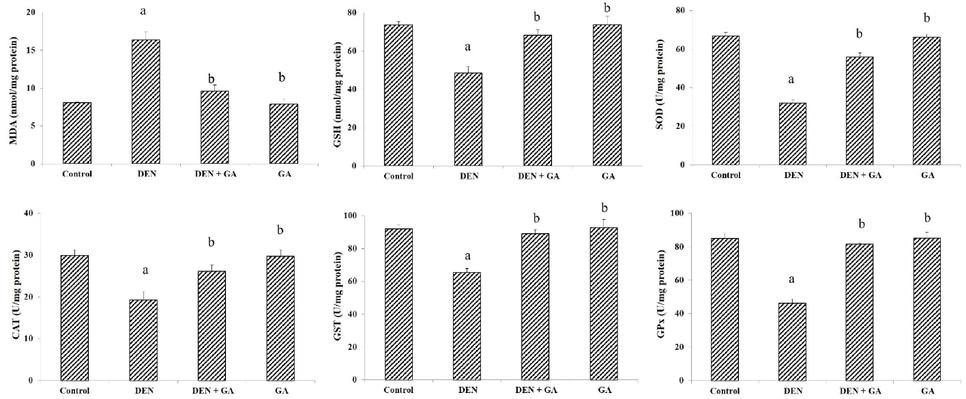
Effect of gambogenic acid on liver enzymes, GSH and MDA in DEN-induced rats. Values expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by Tukey-Kramer multiple comparisons test. The symbol ‘a’ represents significance (P < 0.05) as compared to the control group, whereas the symbol ‘b’ represents significance (P < 0.05) as compared to the DEN alone treated group.
3.4 Effects of gambogenic acid on apoptosis markers
The effects of gambogenic acid on apoptosis markers Bcl-2 and Bax were examined using western blot analysis. Apoptosis formation was apparent (P < 0.05) in DEN only treated group II as the anti-apoptotic Bcl-2 expression was prominent whereas Bax was suppressed compared to the control group I. Gambogenic acid reversed the effect of DEN by reducing the anti-apoptosis incidents in hepatocellular carcinoma as shown in Fig. 3. Gambogenic acid alone treated group IV showed similar findings as control group I.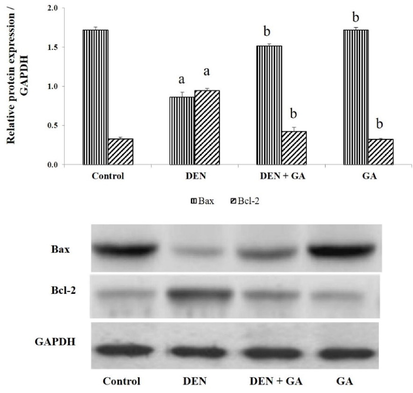
Effect of gambogenic acid on apoptosis markers in DEN-induced rats. Values expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by Tukey-Kramer multiple comparisons test. The symbol ‘a’ represents significance (P < 0.05) as compared to the control group, whereas the symbol ‘b’ represents significance (P < 0.05) as compared to the DEN alone treated group.
3.5 Gambogenic acid regulates NF-kβ pathway and prevented oxidative damage
Immunohistochemical analysis on translocation of NF-kβ subunit p-p65 into hepatic nuclei was clearly observed in DEN alone treated group II as compared to control group I. The effect of gambogenic acid treatment was prevalent when nuclear translocation of p-p65 was significantly prevented. Similarly, increased expression of 8-OHdG in DEN alone treated group II compared to control group I indicates the mutation caused by DEN. The images are shown in Fig. 4. Gambogenic acid prevented the oxidative damage caused by DEN by hence the expression of 8-OHdG is relatively minimal in group III. Expressions in gambogenic acid alone treated group IV is comparable to control group I.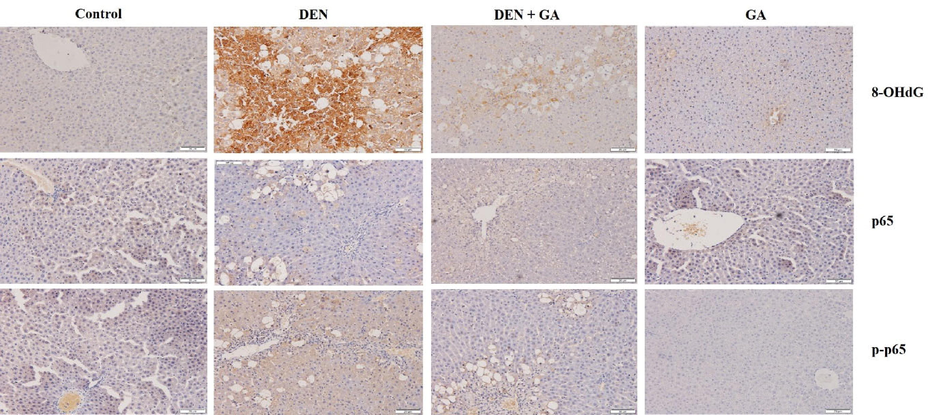
Effect of gambogenic acid on expressions of 8-OHdG and p-p65 in DEN-induced rats. Immunohistochemical analysis shows the oxidative damage marker 8-OHdG and NF-kβ p65 inflammatory mediator in DEN-induced liver tissues. Gambogenic acid administration exhibits remarkable prevention of the 8-OHdG and p-p65 expressions in liver tissues. 100 × magnification.
3.6 Histopathological alterations caused by DEN in rat liver
The extent of histopathological alterations caused by DEN in rat liver is shown in Fig. 5. DEN alone treated group II exhibited severe changes such as enlarged sinusoidal spaces, ballooning degeneration, inflammatory infiltrates, focal nodular hyperplasia compared to the normal arrangement of hepatocytes in control group I. The changes were significantly reversed by gambogenic acid compared to DEN alone treated group by restructuring the hepatocellular architecture. Gambogenic acid alone treated group IV showed similar hepatocyte arrangements as control group I.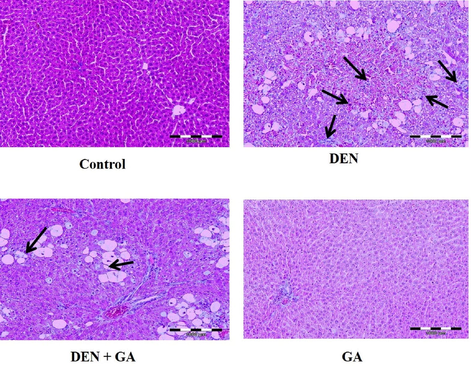
Effect of gambogenic acid on histopathological changes in DEN-induced rats. Black arrows indicate loss of cellular boundaries, infiltration of inflammatory mediators, ballooning degeneration, and focal nodular hyperplasia in liver tissues of DEN alone treated group and gambogenic acid treated group. 100 × magnification.
4 Discussion
Past research on DEN-induced hepatocellular carcinoma indicates the development of carcinogenesis were caused by ROS formations and oxidative damage characterized with depletion of antioxidants, diminished antioxidant enzyme activities, and inflammation (Abdu et al., 2022). The pathogenesis of cancer in liver tissues are associated with prevalence of oxidative stress biomarkers in serum due to occurrence of injury at the tissues and cells. The results of serum biomarkers represented similar findings in this study whereby DEN administration in rats increased the liver injury markers AST, ALT, ALP, LDH, and total bilirubin. Similar findings were reported by Ahmed et al. (2022) and You et al. (2021). The elevation of serum biomarkers were significantly reduced by oral administration of gambogenic acid as shown in the results. Gambogenic acid provides antioxidant defense to prevent ROS from developing oxidative stress. Significant decrease in body weight and increase in liver weight of rats treated with DEN were indicating the extent of liver injury caused as these preliminary indicators describe the prevalence of liver damage. Gambogenic acid reversed the effects of DEN as an elevation of the body weight and reduced liver weight were observed, which indicates the improvement of liver tissues. The antioxidant enzymes are regulators of antioxidants and are responsible for the expelling of ROS and other free radicals from cells and tissues (Gnanaraj et al., 2016). DEN alone treated rats exhibited increased MDA formations and reduced activities of enzymatic antioxidants and GSH levels. Enzymatic antioxidants SOD, GPx, GST, CAT and non-enzymatic antioxidant GSH were enhanced in gambogenic acid treated rats due to the antioxidant nature of the compound. Liver tissue morphology were protected by gambogenic acid against DEN-induced oxidative injury as lipid peroxidation levels were markedly reduced.
Tumor formation on liver tissues is the hallmark for DEN triggered hepatocellular carcinoma model in animals. Tumor occurrence were recorded heavily in DEN alone treated rat liver and also mildly in a gambogenic acid treated rat liver. CEA and AFP are two important biomarkers for tumor formation and metastases of cancer in the organ at an early stage. Elevated levels of these proteins indicates clear carcinogenesis triggered by DEN. Similar results were reported by (Gokuladhas et al., 2015) whereby DEN induced the tumor formations and the expressions of CEA and AFP were increased. Gambogenic acid administration remarkably reduced the tumor incidences and also reduced the expressions of CEA and AFP. This shows gambogenic acid was able to prevent the progression of tumor as CEA levels are specific for liver metastasis. Liver oxidative damage marker 8-OHdG was evidenced to be elevated in DEN alone treated rats, thus proving the hepatocellular damage linked to oxidative stress and lipid peroxidation. Expressions of 8-OHdG resembles the oxidative damage incurred by hepatic tissues at cell cytoplasm and nuclear levels. Gambogenic acid treatment was able to minimalize the expression of 8-OHdG in the immunohistochemical results as evidenced by the reduction of lipid peroxidation and reversal of oxidative stress. The expression of NF-kβ p65 is an inflammatory response triggered by ROS and DEN is well-known to induce inflammation. Translocation of p-p65 into nucleus triggers the activation of NF-kβ pathways which is also an underlying prognosis of hepatocellular carcinoma (Chang et al., 2016). DEN treatment caused the increased expressions of p-p65 in DEN alone treated rat liver tissues, which projects the involvement of inflammatory response in the development of hepatocellular carcinoma. The prevention of inflammation by gambogenic acid was clearly evidenced by the suppression of p-p65 at hepatocellular levels.
The histopathological derangements of rat liver caused by DEN were strongly evidenced with the biochemical findings with the loss of cellular boundaries, infiltration of inflammatory mediators, ballooning degeneration, and focal nodular hyperplasia. The results are in accordance to previous reports by Kurma et al. (2021) and Singh et al. (2018). Incidence of apoptosis in cancer cells triggered by plants or plant derived compounds are supported in recent studies as a potential approach to inhibit the progression of the disease (Hamza et al., 2020; Gupta & Sarwat, 2022). Apoptosis is commonly regulated by a few pathways and one of the prominent pathway is mTOR/PI3K/AKT. Past reports mention that the anti-apoptosis Bcl-2 gene is overexpressed whereas expression of pro-apoptosis Bax gene is normally decreased in numerous cancer cells (Ahmed et al., 2022; You et al., 2021). Similar findings were observed in DEN alone treated rats, where the protein expression of Bcl-2 was stronger than Bax. Gambogenic acid reversed the protein expressions of the anti-apoptosis and pro-apoptosis proteins thus promoting apoptosis in the carcinogenic liver cells. Therefore, gambogenic acid could be promoting the regulation of mTOR/PI3K/AKT, along with the prevention of NF-kβ pathway activation to suppress inflammation and restore enzymatic and non-enzymatic antioxidant activities in preventing ROS induced oxidative stress.
5 Conclusion
In conclusion, this study confirms the attenuating effect of gambogenic acid on DEN-induced hepatocellular carcinoma in rat liver. Gambogenic acid remarkably inhibited oxidative stress, prevented hepatocellular damage supported by histopathological changes and progression of tumor incidences, regulated apoptosis through elevation of Bax and suppression of Bcl-2, and inhibited inflammation through inactivation of NF-kβ p65 against DEN induced hepatocellular carcinoma. Hence, gambogenic acid can be recommended for further investigation as potential drug candidate for the treatment of liver cancer.
6 Funding statement
This research was funded by Yunnan Province high-level Health and Family planning Personnel Training Project (Yunwei Science and Education Development [2017] No. 14) and Basic Research Joint Special General Project of Yunnan Provincial Local Universities (part) (No.: 2018FH001-076, 2018FH001-080).
Author contribution
RZ & TW equally contributed in writing the manuscript; JY, ML, JL & CM performed the experiments and data acquisition; XYM and GX performed data analysis and edited the manuscript; SZ conceptualized the experiment and funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Therapeutic effects of crocin alone or in combination with sorafenib against hepatocellular carcinoma: in vivo & in vitro insights. Antioxidants. 2022;11(9):1645.
- [CrossRef] [Google Scholar]
- Protective effects of Persea americana fruit and seed extracts against chemically induced liver cancer in rats by enhancing their antioxidant, anti-inflammatory, and apoptotic activities. Environ. Sci. Pollut. Res.. 2022;29(29):43858-43873.
- [CrossRef] [Google Scholar]
- Protective effects of celastrol on diethylnitrosamine-induced hepatocellular carcinoma in rats and its mechanisms. Eur. J. Pharmacol.. 2016;784:173-180.
- [CrossRef] [Google Scholar]
- Role of gambogenic acid in regulating PI3K/AKT/NF-kβ signaling pathways in rat model of acute hepatotoxicity. Biosci. Biotech. Bioch.. 2021;85(3):520-527.
- [CrossRef] [Google Scholar]
- Hepatoprotective effects of Flagellaria indica are mediated through the suppression of pro-inflammatory cytokines and oxidative stress markers in rats. Pharm. Biol.. 2016;54(8):1420-1433.
- [CrossRef] [Google Scholar]
- Exploring the potential role of chemopreventive agent, hesperetin conjugated pegylated gold nanoparticles in diethylnitrosamine-induced hepatocellular carcinoma in male Wistar albino rats. Indian J. Clin. Biochem.. 2015;31:171-184.
- [CrossRef] [Google Scholar]
- Protective effects of plant-derived natural products against hepatocellular carcinoma. Herbal Medicines. 2022;609–627
- [CrossRef] [Google Scholar]
- Dandelion prevents liver fibrosis, inflammatory response, and oxidative stress in rats. JoBAZ. 2020;81:43.
- [CrossRef] [Google Scholar]
- Gambogenic acid alters chemosensitivity of breast cancer cells to Adriamycin. BMC Complement. Altern. Med.. 2015;15:181.
- [CrossRef] [Google Scholar]
- Gambogenic acid inhibits the proliferation of small–cell lung cancer cells by arresting the cell cycle and inducing apoptosis. Oncol. Rep.. 2019;41:1700-1706.
- [CrossRef] [Google Scholar]
- DEN-induced rat model reproduces key features of human hepatocellular carcinoma. Cancers. 2021;13(19):4981.
- [CrossRef] [Google Scholar]
- Gambogenic acid protects against high glucose-induced damage of renal tubular epithelial cells by inhibiting pyroptosis through regulating the AMPK–TXNIP pathway. Qual. Assur. Saf. Crops Foods. 2022;14(2):40-46.
- [CrossRef] [Google Scholar]
- Gambogenic acid induces endoplasmic reticulum stress in colorectal cancer via the aurora A pathway. Front. Cell Dev. Biol.. 2021;9
- [CrossRef] [Google Scholar]
- Protective effects of luteolin on injury induced inflammation through reduction of tissue uric acid and pro-inflammatory cytokines in rats. J. Tradit. Complement. Med.. 2020;10:60-69.
- [CrossRef] [Google Scholar]
- Targeting PI3K/AKT/NRF2 pathway by glabridin alleviates acetaminophen-induced hepatic injury in rats. Arab. J. Chem.. 2021;14(2):102968
- [CrossRef] [Google Scholar]
- Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid. Med. Cell. Longev.. 2010;3(4):254-261.
- [CrossRef] [Google Scholar]
- Gambogenic acid exerts anticancer effects in cisplatin-resistant non-small cell lung cancer cells. Mol. Med. Rep.. 2020;21:1267-1275.
- [CrossRef] [Google Scholar]
- Attenuation of diethylnitrosamine (DEN) – induced hepatic cancer in experimental model of Wistar rats by Carissa carandas embedded silver nanoparticles. Biomed. Pharmacother.. 2018;108:757-765.
- [CrossRef] [Google Scholar]
- Effect of gambogenic acid on cytochrome P450 1A2, 2B1 and 2E1, and constitutive androstane receptor in rats. Eur. J. Drug Metab. Pharmacokinet.. 2018;43:655-664.
- [CrossRef] [Google Scholar]
- Triterpenoids principle of Wedelia calendulacea attenuated diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-KB pathway. Inflammopharmacology. 2017;26(1):133-146.
- [CrossRef] [Google Scholar]
- Oral delivery of gambogenic acid by functional polydopamine nanoparticles for targeted tumor therapy. Mol. Pharm.. 2021;18(3):1470-1479.
- [CrossRef] [Google Scholar]
- Phosphorylation of NF-κbp65 drives inflammation-mediated hepatocellular carcinogenesis and is a novel therapeutic target. J. Exp. Clin. Cancer Res.. 2021;40(1):253.
- [CrossRef] [Google Scholar]
- Gambogenic acid mediated apoptosis through the mitochondrial oxidative stress and inactivation of Akt signaling pathway in human nasopharyngeal carcinoma CNE-1 cells. Eur. J. Pharmacol.. 2011;652:23-32.
- [CrossRef] [Google Scholar]
- A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol.. 2019;16(10):589-604.
- [Google Scholar]
- Phyllanthin prevents diethylnitrosamine (DEN) induced liver carcinogenesis in rats and induces apoptotic cell death in HepG2 cells. Biomed. Pharmacother.. 2021;137:111335
- [CrossRef] [Google Scholar]
- Gambogenic acid induces proteasomal degradation of CIP2A and sensitizes hepatocellular carcinoma to anticancer agents. Oncol. Rep.. 2016;36:3611-3618.
- [CrossRef] [Google Scholar]







