Translate this page into:
Effect of hydrophobically modified SiO2 nanoparticles on the stability of water-based SDS foam
⁎Corresponding authors. sunshuangqing%20@upc.edu.cn (Shuangqing Sun), songqinghu@upc.edu.cn (Songqing Hu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present study, SiO2 nanoparticles were first hydrophobically modified and then added into anionic surfactant sodium dodecyl sulfate (SDS) stabilized water-based foam to improve the foam stability. The foam stability was experimentally evaluated by measuring surface tension, Zeta potential and half-life of the foam. The foam stabilizing mechanism was also studied from a micro perspective by molecular dynamics simulation through analyzing the equilibration configuration and MSD curve of both SDS surfactant and water molecules. The results show that foam exhibits an optimal stability when SiO2 concentration is 0.35 wt% under a specific surfactant concentration (0.5 wt%) in this work. The addition of SiO2 nanoparticles with suitable concentration could improve the adsorption between SDS molecules and nanoparticles, thus limiting the movement of SDS and restricting the movement of surrounding water molecules, which is beneficial to enhance the foam stability.
Keywords
Foam stability
Surfactant
SiO2 nanoparticles
1 Introduction
Foams have a wide range of applications both in our daily life (Yang and Yang, 2010) and in many industrial fields such as mineral flotation (Paulson and Pugh, 1996), electromagnetic interference shielding (Yang, 2005), enhanced oil recovery (Farajzadeh, 2009), etc. However, the instability of foam usually limits its application. The drainage of liquid (Jun, 2012), the coalescence of foam and the rupture of foam film are the main factors affecting the stability of foam (Hansen and McCarlie, 2004; Attia, 2013; Samanta and Ghosh, 2011), so it begins to evolve as soon as it appears (Herzhaft, 2002). Surfactants can efficiently improve foam stability by reducing surface tension. However, in the actual application of foam, for example, when it comes to foam flooding, the foam is very easy to be destroyed under some harsh conditions such as high temperature and high salinity.
Solid nanoparticles are larger than surfactant molecules, and can be adsorbed on the air-liquid interface to reduce the contact area of air and liquid, thus greatly reducing the possibility of disproportionation reaction, making them a new type of effective foam stabilizer. Manan et al. studied the effects of nanoparticle types on foam flooding (Manan, 2015). They found that low concentration of nanoparticles can improve foam stability regardless of their type. Rafati et al. investigated the stability and rheological properties of aqueous foam in the presence of different solid particles (Rafati, 2016). It was found that different density, shape, and size of nanoparticles can impact the foam stability and structure. Among these nanoparticles, SiO2 has gradually developed its application advantages due to its excellent performance and wide adaptability (Yekeen, 2019; Yang, 2019; Llamas, 2019; Zhu, 2019). Adding appropriate SiO2 nanoparticles into the foam system can synergize with surfactant and make it strongly adsorb on the foam surface. At the same time, a three-dimensional network structure is formed between the foam surface and the continuous phase to prevent the coalescence and divergence of foam and achieve the effect of foam stabilization (Zhang, 2008). There have been some studies on improving foam stability by using both surfactant and SiO2 nanoparticles. Wang et al. studied the effect of hydrophilic nanoparticles on the stability of foams stabilized by different surfactants (Wang, 2018). The interfacial properties indicated that a synergistic effect occurred between the hydrophilic nanoparticles and the zwitterionic surfactant under acidic conditions. Li et al. examined the synergistic effect of hydrophilic SiO2 nanoparticles and cationic surfactant CTAB on the stability of CO2 foam (Li, 2017). With the increase of the SiO2 concentration, the synergistic stabilization effect of CTAB/SiO2 dispersion first increased and then decreased. Binks et al. confirmed the above conclusion and proposed that CTAB/SiO2 could increase the foam stability only at proper concentration ratio (Binks, 2008). Also, Ravera et al. found that the surface of nanoparticles changed from hydrophilic to hydrophobic as CTAB concentration increased (Ravera, 2008). In addition to ionic surfactants, Li et al. studied the properties of CO2 foam stabilized by SiO2 and nonionic surfactants C12E23 (Li, 2019). The experimental results showed that they attached to each other through van der waals forces and hydrogen bonds, which increased the hydrophobicity of the nanoparticles. More relevantly to this work, Sun et al. found that SiO2 nanoparticle had a synergetic effect with anionic surfactant SDS on foam stability at proper concentrations based on an experimental study (Sun, 2015). Although the research work mentioned above has studied the synergetic effect of nanoparticles and surfactant on the foam stability from the perspective of experiments, the further understanding on the microscopic mechanism especially those from the molecular or atomic level is still needed.
In this paper, the effect of the addition of hydrophobic modified SiO2 on the stability of water-based SDS-stabilized nitrogen-in-water foam was studied. First, SiO2 nanoparticles were hydrophobically modified. Then the effect of its size and concentration were studied through half-life, size and zeta potential of foam. In combination with experiments, the stabilizing mechanism of foam from the molecular level was also investigated using molecular dynamics simulation by calculating the mean square root displacement of SDS and water molecules. This study provides some further understanding on the effect of SiO2 nanoparticles on the foam stability.
2 Experimental and simulation details
2.1 Materials
Material
Property
Company
Sodium Dodecyl Sulfate
99.9%
Aladdin Biochemical Technology Co., Ltd
N-octyltriethoxysilane
97%
Aladdin Biochemical Technology Co., Ltd
SiO2 nanoparticles
99.5%
Diameter of 10 ± 5 nm, 30 ± 5 nm, 50 ± 5 nm, 100 ± 5 nm, 200 ± 5 nmMcLean Biochemical Technology Co., Ltd
Nitrogen
99.999%
Shandong Hongda Biological Technology Co., Ltd
Anhydrous ethanol
99.5%
McLean Biochemical Technology Co., Ltd
Deionized water
18.25 MΩ·cm
Laboratory preparation
2.2 Foam preparation and evaluation
The used SDS concentration for the current study is 0.5 wt% well above its critical micelle concentration (CMC) which is 0.23 wt%. Based on this SDS concentration, modified SiO2 nanoparticles with different size (10–200 nm) and different concentrations (0.1–0.45 wt%) were added into the foam solution to study the influence on foam stability. The nitrogen adsorption isotherm and pore diameter distribution of SiO2 nanoparticles used in this study are shown in Fig. 1. It can be seen that the adsorption isotherm (Fig. 1a) belongs to type II, reflecting the typical physical adsorption process on non-porous or macroporous adsorbent. According to the pore diameter distribution (Fig. 1b), it can be further determined that SiO2 nanoparticles we used are non-porous. Dodecyl triethoxysilane was used to modified SiO2 nanoparticles. Details about the hydrophobization and characterization of SiO2 nanoparticles were given elsewhere (Sun, 2019). In addition, the experimentally measured modified SiO2 potential is 32 mV.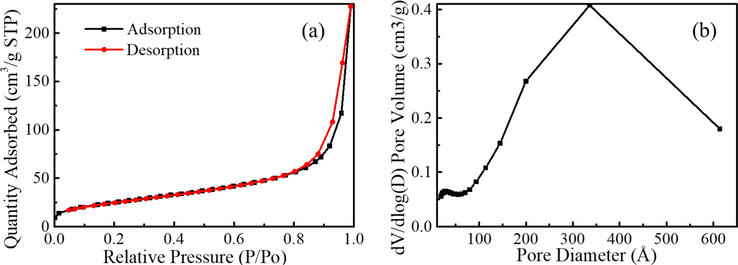
(a) Nitrogen adsorption isotherm and (b) pore size distribution of SiO2 nanoparticles.
The Zeta potential of SDS/SiO2 dispersion foam systems with different concentrations of SiO2 nanoparticles was measured (Sun, 2015; Yekeen, 2017) using a Zeta potentiometer (Zetasizer Nano) provided by Malvern Instruments Ltd. Each set of solution was measured three times to improve the data accuracy. Details about the method of foam preparation and the shooting of foam morphology were given elsewhere (Sun, 2019).
2.3 Molecular dynamics simulation
In order to analyze the stabilizing mechanism of foams containing different concentrations of SiO2 nanoparticles from the molecular level, MD simulations were performed to study the foam configuration change and molecular interaction using a software named Materials Studio (Accerly). For both of the energy minimization and dynamics steps, the COMPASS force field (Sun, 1998) was employed for all of molecules. Firstly, the initial configurations (Fig. 2) were minimized with smart minimizer algorithm which combines steepest descent and conjugate gradient algorithms, and the convergence criteria were set at 0.5 kcal/(mol·Å). Then, a 5 ns dynamics simulation for every model was accomplished at constant volume and temperature (NVT ensemble). The Nose thermostat is applied to control the simulation temperature at 298 K. A fixed time step of 1 fs is used, and data is collected every 5000 steps.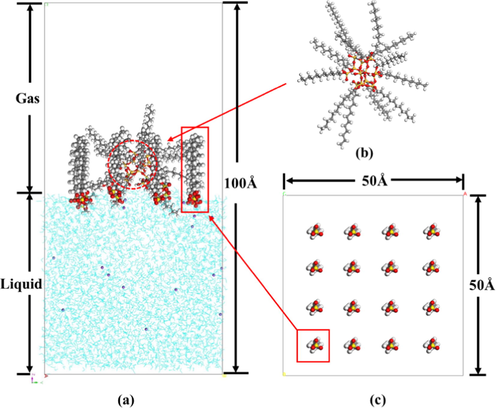
(a) Initial configuration; (b) SiO2 nanoparticle; (c) Surfactant layer. Atoms: Na in purple, Si in yellow, O in red, H in white, and C in gray.
The initial configuration mainly consists of two layers as shown in Fig. 2. The first layer (bottom) is a water box with a size of 5 × 5 × 5 nm, containing 4100 water molecules, which is calculated according to the volume and the density of water phase. The second layer (top) mainly consists of 16 SDS molecules according to the area/molecule at surface saturation (Rosen and Milton, 1989)and a certain number of modified SiO2 nanoparticles (1 to 4 respectively; which was based on the consideration of model size and the conversion of the relative concentration of SDS and SiO2 in the experiment). Correspondingly, 16 counterions (Na+) were added into the aqueous phase to make the system keep electrically neutral. Finally, a vacuum with the thickness of 10 nm was added to avoid the impact of periodic boundaries on the system.
In order to explore the effect of modified SiO2 nanoparticles on the stability of foam stabilized by SDS, the root mean square displacement (MSD) curves of SDS and water molecules in different systems were extracted from computational results to investigate the influence of adding nanoparticles on the movement and diffusion of different molecules in the foam system and further illustrate the stabilizing mechanism.
3 Results
3.1 Effect of SiO2 size
In order to study the effect of SiO2 nanoparticles size on the foam stability, SDS surfactant and fully modified SiO2 nanoparticles with different sizes were compounded at a mass concentration ratio of 1:1. The relationship between foam half-life and SiO2 nanoparticles size in different foam systems is shown in Fig. 3a. It can be seen that, within the scope of our research, regardless of the SiO2 concentration, the half-life of foam always presents the same trend with the change of the size of SiO2 nanoparticles, that is, the larger the size of added SiO2 nanoparticles, the shorter the foam half-life. According to above experimental results, we finally selected SiO2 nanoparticles with a size of 10 nm to be used for the subsequent experiments.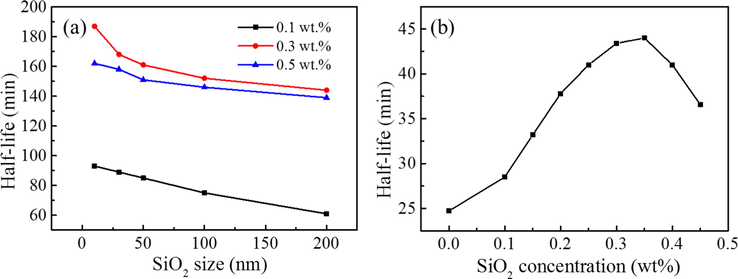
(a) The relationship between foam half-life and SiO2 size in mixed systems with different SiO2 concentrations; (b) Foam half-life varies with SiO2 (30 nm) concentration with 0.5 wt% SDS.
3.2 Effect of SiO2 concentration
3.2.1 Half-life, foam size and morphology
Fig. 3b shows the half-life of foam with different SiO2 concentrations. It can be seen that the half-life of the foam increases first and then decreases. Although half-life value of the system containing both SDS and SiO2 has been generally improved compared with the single SDS surfactant foam, excessive SiO2 nanoparticles will make the foam stability decrease. The optimum compounding concentrations of SiO2 nanoparticles was 0.35 wt%.
The time-dependent average foam size in each system is shown in Fig. 4. First, the foam size was found to increase with time for all four foam systems. Second, it can be seen that the average size of foams of pure SDS system was bigger than that of the system compounding with SiO2 during the whole measurement period of time. Third, the addition of SiO2 nanoparticles obviously decreases the foam size. In particular, when the SiO2 concentration is 0.3 wt%, the average foam size is the smallest among all four foam systems. The foam size change could be also found in the images of foam captured at different time after the generation of foam (Fig. 5).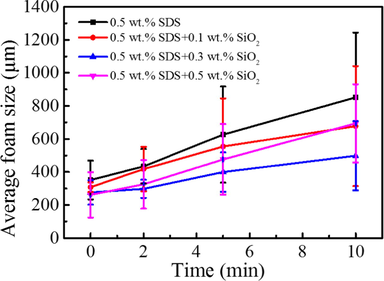
Time-dependent average foam size of different SiO2 concentration in 0.5 wt% SDS.
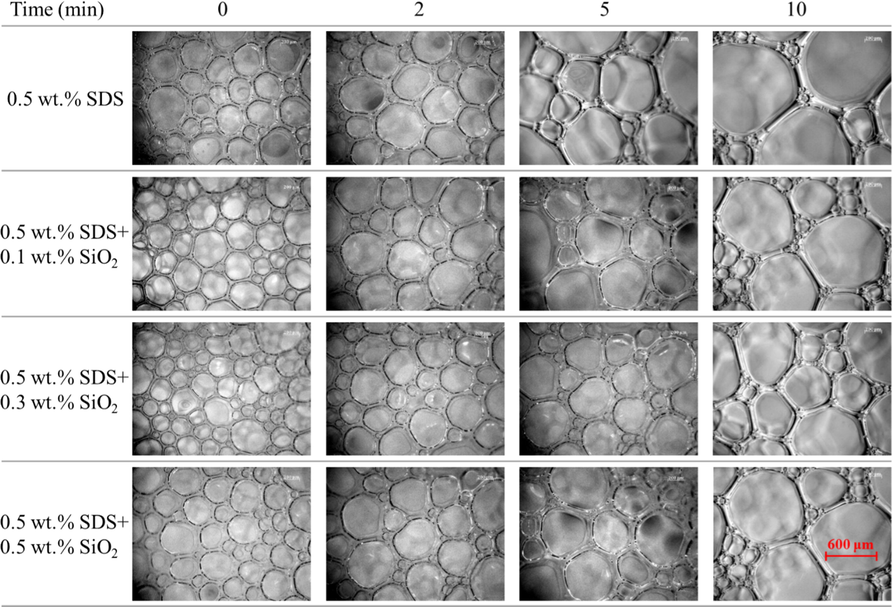
Time-dependent bubble state of foam systems.
3.2.2 Zeta potential of SDS/SiO2 dispersion
The evolution of the dispersion potential with the increase of SiO2 nanoparticles concentration is shown in Fig. 6. The SDS molecules were negatively charged and the modified SiO2 nanoparticles were positively charged. It can be seen that with the increase of SiO2 concentration, the potential of the system decreases from negative to positive, and its absolute value first decreases and then increases.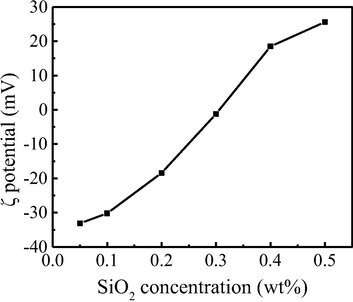
Solution potential changes with the concentration of SiO2 nanoparticles.
3.2.3 Microscopic configuration of foam system
The top view of equilibrium configurations of foam system with the addition of different concentrations of SiO2 is shown in Fig. 7. SiO2 nanoparticles and sulfur atoms in SDS head group (big ball in yellow) are displayed in ball-and-stick model while SDS molecules are displayed in line model to vividly show the configurations. It can be seen that there are the same number of SDS molecules in the foam system, and the number of SDS molecules adsorbed around SiO2 increases first and then decreases with the increase of the number of SiO2 nanoparticles in the system (SDS molecules those are not adsorbed by SiO2 nanoparticles are in the red circle). In particular, the foam system containing 3 SiO2 nanoparticles adsorbs the most SDS molecules. In addition, it also can be concluded that the number of SDS molecules adsorbed on the surface of a single SiO2 nanoparticle decreases gradually with the increase of the number of SiO2 nanoparticles.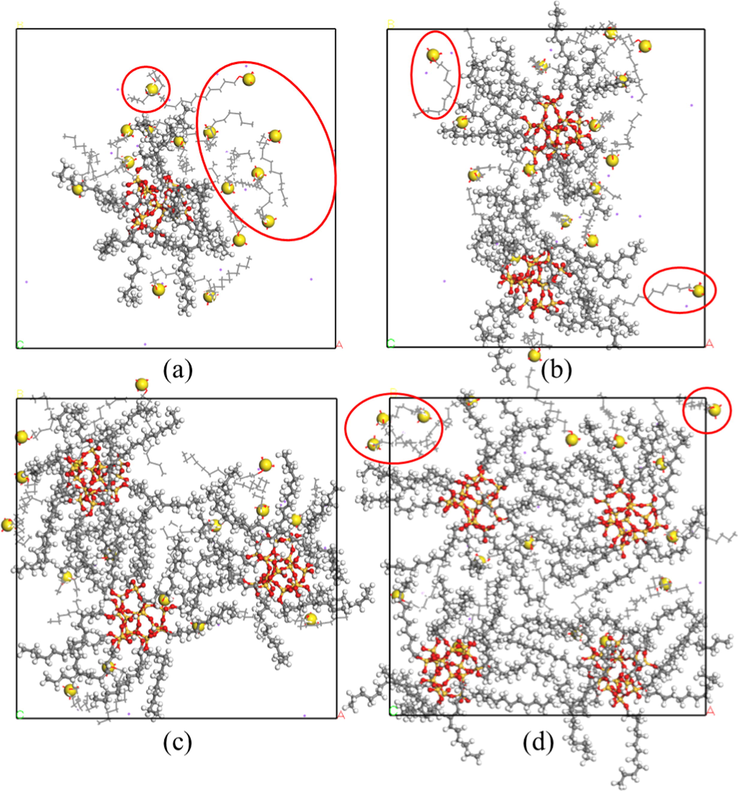
Top view of the equilibrium configuration of foam system with the addition of different concentrations of SiO2: (a) 1 SiO2; (b) 2 SiO2; (c) 3 SiO2; (d) 4 SiO2.
3.2.4 MSD curves of SDS and water molecules
We first investigated the influence of nanoparticles on SDS molecules by extracting the MSD curve of SDS (Fig. 8a), and then investigated the influence of SDS on water molecules by extracting the MSD curve of water (Fig. 8b). In this way, the effect of nanoparticles on the motion of water molecules is indirectly investigated. The higher the slope of the MSD curve, the less restricted the molecular motion. It can be seen that the MSD curves of water and SDS were consistent, that is, as the number of added SiO2 nanoparticles increases, the slope decreases first and then increases. In addition, in both figures, the slope of MSD curve contained 3 SiO2 systems is minimal.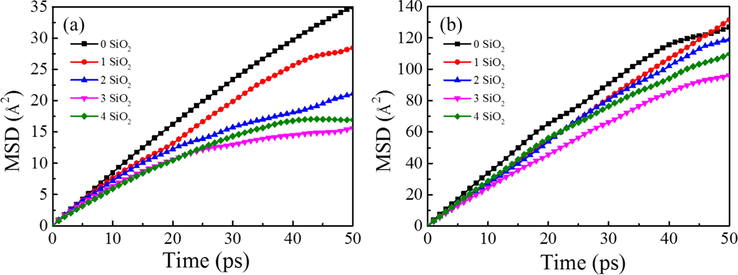
MSD of SDS molecules (a) and water molecules (b) in the first hydration layer of SDS head groups.
4 Discussion
In this work, we find that in the foam system containing both SDS surfactant and SiO2 nanoparticles (0.1, 0.3 and 0.5 wt%), the larger the size of added SiO2 nanoparticles, the shorter the foam half-life (Fig. 3a). According to the above experimental results, SiO2 nanoparticles with large size was not beneficial to the foam stability. This is because that under the same weight, small size means the larger number and larger surface area of nanoparticles, which could facilitate the uniform dispersion of nanoparticles in liquid phase. (Hariz, 2012) Furthermore, the addition of SiO2 nanoparticles could effectively improve the foam stability compared with single SDS system and the foam stability increases first and then decreases with the increase of SiO2 concentration. We speculated that the addition of SiO2 nanoparticles could enhance the foam stability mainly because of the following two aspects.
First, the addition of SiO2 nanoparticles could affect the adsorption of SDS molecules. When the low concentration of hydrophobic SiO2 nanoparticles were added into the foam system, hydrophilic SDS molecules could adsorb on the whole surface of SiO2 nanoparticles (Fig. 9b). At this level of SiO2 concentration, the surface of SiO2 nanoparticles changes from hydrophobic to hydrophilic, so they stay close to the gas-liquid interface. Although the nanoparticles are not exactly located at the gas-liquid interface, the presence of SiO2 nanoparticles could act as a framework support, increasing the strength of the foam and improve the foam stability. (Zhang, 2008) With the increases of SiO2 concentration, there are more SDS molecules adsorbed to the surface of SiO2 nanoparticles (Fig. 9c) until they are dissipated at the interface. With the further increase of SiO2 nanoparticles, SDS molecules are not enough to completely cover the surface of SiO2 nanoparticles, so the partially hydrophobic nanoparticles could stay at the gas-liquid interface (Fig. 9d), leading to a most stable foam system. However, if the SiO2 concentration was further increased, the gas-liquid interface could not accommodate all the SiO2 nanoparticles, excessive SiO2 nanoparticles will aggregate and sink into the liquid phase (Fig. 9e). At the same time, some SDS molecules originally stay at the gas-liquid interface will also sink into the aqueous phase to decrease the hydrophobicity of SiO2, so the total number of SiO2 and SDS molecules adsorbed at the gas-liquid interface decreases and the foam stability will decrease (Fig. 9e). The above discussion could be confirmed by the fact that zeta potential varies with SiO2 concentration (Fig. 6). We know that SDS molecules were negatively charged and modified SiO2 nanoparticles were positively charged. At low SiO2 concentration, there are relatively enough SDS molecules adsorbed on its surface to neutralize the positive charge, and the remaining SDS molecules make the solution potential negative. With the addition of SiO2 nanoparticles till SDS molecules in the foam system all attach on the SiO2 surface, the Zeta potential could change from negative to zero. However, when there are too many SiO2 nanoparticles in the foam system, zeta potential will become positive, and the absolute value of zeta potential first decreases and then increases (Fig. 6).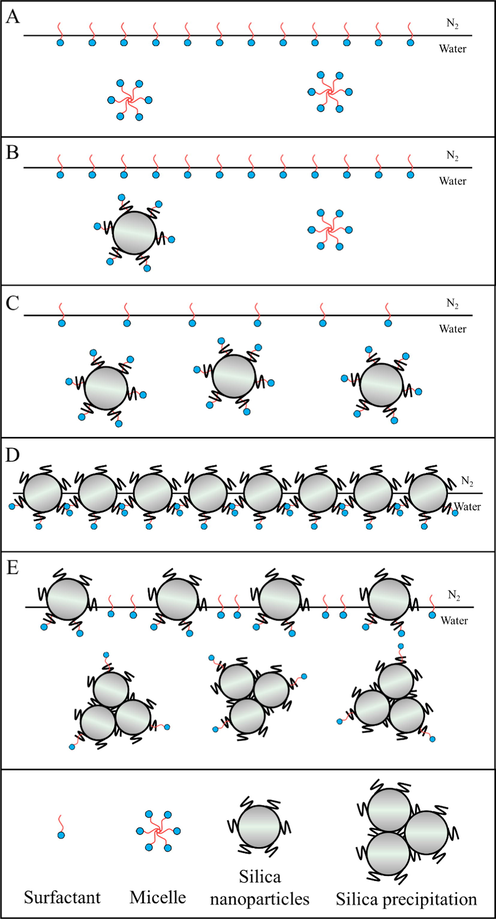
Adsorption of SDS and SiO2 nanoparticles at gas-liquid interface: (a) simple SDS system; (b) - (e) represent the gradual increase in the amount of SiO2.
Second, the addition of SiO2 nanoparticles could limit the movement of SDS molecules. According to equilibrium configurations of the foam system with different concentrations of SiO2 nanoparticles shown in Fig. 7, it can be seen that in the foam system containing the same number of SDS molecules, the number of SDS molecules adsorbed around SiO2 increases first and then decreases with increasing the number of SiO2 nanoparticles in the system. In particular, the foam system containing 3 SiO2 nanoparticles adsorbed the most SDS molecules. Therefore, it could be proposed that the movement of SDS molecules are restricted because of the addition of a certain number of SiO2 nanoparticles. This is confirmed by MSD curves of SDS molecules in different foam systems (Fig. 8a). In addition, from Fig. 8b, it can be seen that the slope of MSD curve of water molecules surrounding SDS head groups in the foam systems also first decreases and then increases with the increase of the number of SiO2 nanoparticles. In particular, for the foam system containing 3 SiO2 nanoparticles, the motion diffusion capacity of water molecules in this foam system is minimal, which indicates that the water molecules are greatly constrained by the head of the surfactant in this system. Therefore, in this foam system water molecules are not easy to be lost in the bubble discharge process and can reduce the effusion rate of foam, leading to the best foam stability.
5 Conclusions
The stability of aqueous foams decreases with the increase of SiO2 size, and it has the best stability when the SiO2 size is 10 nm.
The optimal concentration of SiO2 contributing to the best foam stability is 0.35 wt% at which SiO2 nanoparticles could adsorb all SDS molecules and stay at the gas-liquid interface to enhance the foam stability. Excessive addition of SiO2 nanoparticles could not be accommodated by the gas-liquid interface and finally sink into the liquid phase, leading to the decrease of foam stability.
According to MSD curves of SDS and water molecules, the slope of SDS surfactant and surrounding water molecules decreases first and then increases with the increase of SiO2 molecules, which indicate that the addition of SiO2 nanoparticles in the foam system also affect their movement and finally affect the stability of foam system.
Acknowledgements
This work was supported by the “National Natural Science Foundation of China” (51874331), the “PetroChina Innovation Foundation” (2018D-5007-0213), the “Shandong Provincial Natural Science Foundation” (ZR2017MEE028), the “Fundamental Research Funds for the Central Universities” (17CX05023 and 19CX05001A), the “Graduate innovation project of China University of Petroleum (East China)” (YCX2019074), and the “Natural Science Foundation of Shandong Province” (ZR2019MEM054).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Colloids Surf., A. 2013;436:1000.
- Soft Matter. 2008;4(12):2373.
- Ind. Eng. Chem. Res.. 2009;49(4):1910.
- J. Chem. Educ.. 2004;81(11):1581.
- Hariz, T. R., 2012.
- J. Colloid Interface Sci.. 2002;247(2):412.
- Langmuir: ACS J. Surf. Colloids. 2012;28(12):5323.
- Energy Fuels. 2017;31(2):1478.
- Energy Fuels. 2019;33(JUN.):5043.
- Colloids Surf., A. 2019;575:299.
- Liquid Fuels Technol.. 2015;33(12):1286.
- Langmuir: ACS J. Surf. Colloids. 1996;12(20):4808.
- Colloids Surf., A. 2016;509:19.
- Colloids Surf., A. 2008;323(1–3):99.
- Chem. Eng. Res. Des.. 2011;89(11):2344.
- Comput. Theor. Polym. Sci.. 1998;8(1–2):229.
- Colloids Surf., A. 2015;471:54.
- J. Ind. Eng. Chem. 2019
- Energy Fuels. 2018;32(3):3709.
- Nano Lett.. 2005;5(11):2131.
- Ind. Eng. Chem. Res. 2019
- J. Phys. Chem. B. 2010;114(31):10066.
- J. Petrol. Sci. Eng.. 2017;159:115.
- J. Petrol. Sci. Eng.. 2019;179:814.
- Colloids Surf., A. 2008;317(1–3):406.
- Colloids Surf., A. 2019;572:88.







