Translate this page into:
Effect of the concentration of uronic acids in Opuntia mucilage on the removal of heavy metals and water quality of the Yautepec River, Mexico
⁎Corresponding author. frrodriguezg@ipn.mx (Francisco Rodríguez-González),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
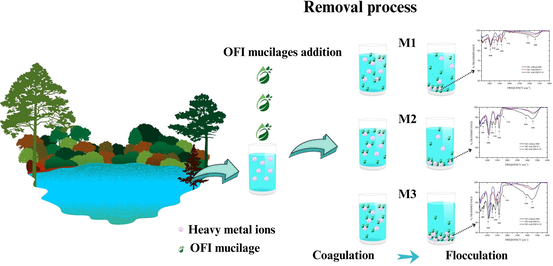
Abstract
The content of UA in OFI mucilages increases with the maturation age. UA in OFI mucilages presented the ability to remove HM in the water river. The highest content of UA removed up to 90.28% of HM.
Abstract
The purpose of this study was to analyze the effect of uronic acid (UA) content of Opuntia Ficus-indica (OFI) mucilages on the removal process of heavy metals (HM) present in water samples (WS) from the Yautepec River, Mexico. For this, mucilages from nopals with different maturation ages were extracted (20–35, 80–100 and 550–600 days, namely, M1, M2 and M3, respectively); and the UA content was determined by UV–vis spectrophotometry. Furthermore, ten WS were collected in different fluvial sites of the river; and the pH, electrical conductivity (EC) and turbidity were determined in situ; and the concentration of HM (cooper, cadmium, chromium, nickel, lead, zinc, iron, manganese, and arsenic) was quantified by atomic absorption spectrometry. Subsequently, the WS were used to evaluate the effect of UA content of M1, M2, and M3 on the removal of HM by the jar method; then, the pH, EC, turbidity, and total percentage concentration of HM removed (%TCHR) were determined. Also, the functional groups of OFI mucilages were identified by Fourier transform infrared spectroscopy (FTIR) before and after the HM removal process. The results show that the UA content increased with the maturation age of the nopals, and M3 exhibited the highest content with 2.22 mg/mL; the WS showed a high content of iron, manganese, and lead with 1546, 209 and 80.8 μg/L, respectively. After the HM removal process, the %TCHR was determined, and it was found that M3 achieved the highest percentage with 90.3 %. The concentration of UA in the mucilage was a factor that influenced in the removal of HM, as well as the initial pH conditions and the initial concentration of HM. Finally, the spectra of FTIR showed typical bands of pectic polysaccharides such as carboxyl, carbonyl, and hydroxyl, which interacted with HM by electrostatic interactions.
Keywords
Natural coagulant
Opuntia Ficus-indica mucilages
Functional groups
Removal
Heavy metals
Contaminated water
Morelos
- UA
-
uronic acid
- HM
-
heavy metals
- OFI
-
Opuntia Ficus-indica
- EC
-
electrical conductivity
- (TCHR)M1, (TCHR)M2, and (TCHR)M3
-
correspond to the total concentration of heavy metals removed after the removal process with OFI mucilages M1, M2 and M3, respectively
Abbreviations
1 Introduction
The presence of heavy metals (HM) in water bodies (rivers, lagoons, and lakes) has caused a decrease in their quality; it also represents a danger for human consumption due to their toxicity, non-biodegradability, and accumulation in the tissues and organs of living beings (Agarwal et al., 2020; El-Reash, 2016; Jadoun et al., 2023; Kumar et al., 2017; WHO, 2022). Contamination of water bodies by HM can be caused by human intervention and natural events, among others (Al-Asadi et al., 2020; Hasan et al., 2019). Also, the growth of the industrial sector and urban areas have caused an increase in contaminated wastewater, which is discharged into water bodies. However, these are used by some people to satisfy their daily needs, exposing themselves to contact and consumption of HM (Hasan et al., 2019; Owodunni and Ismail, 2021). Particularly, the Yautepec River is in the peri-urban area of the state of Morelos, central Mexico, this has been designated as a contaminated river according to the quality of the water and the presence of heavy metals, namely, iron, manganese, copper, cadmium, chromium, nickel, lead, zinc and arsenic; the contamination has been attributed to anthropogenic and natural activities because of the Popocatepetl volcano (Vargas-Solano et al., 2019). These HM in water are toxics at very low concentrations (compared to other ions), making it unsafe for ecosystems and human consumption (Kumar et al., 2019; Prasad et al., 2021). It is known that water quality is influenced by different physicochemical characteristics, namely, pH, EC, biochemical oxygen demand, chemical oxygen demand, total dissolved solids, minerals, and the concentration of HM. Likewise, water quality criteria are different for raw water and its different uses such as drinking water, agriculture, and recreation (Pohl, 2020; Zhang et al., 2023).
On the other hand, several strategies have been implemented for the treatment of contaminated water, among these are membrane filtration, chemical precipitation, ion exchange and coagulation-flocculation, the latter being the most widely used (Abujazar et al., 2022; Agarwal et al., 2020; Bolto, 1995; Jadoun et al., 2023; Kratochvil and Volesky, 1998; Owodunni and Ismail, 2021). To carry out the coagulation-flocculation process it is necessary to use coagulant materials, which can interact with metal ions and/or suspended particles present in the water samples (Abujazar et al., 2022; Bolto, 1995; Hosseinzadeh et al., 2019; Kalhori et al., 2022; Owodunni and Ismail, 2021). The interactions that occur during this process can be through 4 mechanisms, namely, (1) Compression of the double layer, this is carried out with the presence of salts which destabilize the diffuse layer, decreasing the repulsive forces while increasing the forces of Van der Waals attraction; (2) Sweep flocculation, this occurs when the coagulant material traps suspended particles while it settles; (3) Adsorption and charge neutralization, resulting from the adsorption of ions of opposite charge to the particles in suspension and (4) Adsorption and bridging between particles, which occurs when the particles in suspension bind to the coagulant material (polymer chain) without entering contact with each other (Chamani and Heshmati, 2008; El-taweel et al., 2023; Hosseinzadeh et al., 2019; Kalhori et al., 2022; Miller et al., 2008).
On the other hand, the most commonly used inorganic coagulant materials are hydrated aluminum sulfate salts [Al2(SO4)3], sodium aluminate (NaAlO2), and aluminum chloride (AlCl3); however, the drawback of these materials is the large amount of sludge and residue they leave behind after use, making these materials a health risk for consumers (Ma et al., 2017; Owodunni and Ismail, 2021). Therefore, other alternatives have been sought for the treatment of contaminated water, such as the use of natural coagulants, namely, cactus derivatives and mucilages, and agro-industrial wastes (Amari et al., 2020; Belbahloul et al., 2015; Dotto and McKay, 2020; Noli et al., 2019; Onditi et al., 2016; Vargas-Solano et al., 2022b). Natural coagulants are environmentally friendly, effective, inexpensive and do not generate large volumes of sludge, unlike inorganic coagulants. Moreover, these materials have functional groups in their molecules, namely carbonyl, carboxyl, hydroxyl, and amines, which increase their efficiency in the removal of heavy metals (Agarwal et al., 2020; Bouaouine et al., 2018; Bouaouine et al., 2019; Fox et al., 2012; Hopkins and Hawboldt, 2020; Nharingo et al., 2015; Vargas-Solano et al., 2022b). The efficiency of HM removal is affected by the concentration of these present in the water sample, as well as by the ions that are in the composition of the adsorbent material; since during the removal process, they compete for free sites of the adsorbent material (Chen et al., 2023; Miretzky et al., 2008).
The mucilage extracted from cacti Opuntia species have been used in the removal of suspended particles and HM present in polluted waters (Barka et al., 2013; Belbahloul et al., 2015; Bouaouine et al., 2018; Bouaouine et al., 2019; Choudhary et al., 2019; Fox et al., 2012; Miller et al., 2008; Onditi et al., 2016; Vargas-Solano et al., 2022b; Young et al., 2006). In addition, it has been reported that the trapping and/or adsorption mechanism is by adsorption and bridging; i.e., the suspended particles do not come into direct contact with each other, but are bound by the mucilage due to the physicochemical properties of the molecules that constitute it (Bouaouine et al., 2018; Choudhary et al., 2019; Fox et al., 2012; Onditi et al., 2016; Vargas-Solano et al., 2022b; Young et al., 2006). The mucilage extracted from Opuntia Ficus-indica (OFI) nopals is a polysaccharide composed of neutral carbohydrates such as L-arabinose, D-galactose, L-rhamnose, and D-xylose, as well as acid carbohydrates such as D-galacturonic acid and glucuronic acid (Trachtenberg and Mayer, 1981; Vargas-Solano et al., 2022a). Rodríguez-González et al. (2021) quantified the acid and neutral carbohydrates of OFI mucilage extracted from prickly pear cactus with different maturation ages (tender, young, mature), and observed an increase and reduction in the content of uronic acids (UA) and neutral carbohydrates, respectively, in mucilage extracted from mature prickly pear cactus. Young et al. (2006) studied the efficiency of three OFI mucilage fractions, namely, gelling (GE), non-gelling (NE), and a combination of both fractions in the removal of arsenic (As) and solid particles present in drinking water samples. Their results showed that GE had the fastest flocculation capacity compared to Al2(SO4)3. They concluded that the ability and efficiency shown by OFI mucilage to flocculate and remove arsenic was attributed to the amine functional groups (–NH2) present in the coagulant molecules. Miller et al. (2008) suggested that galacturonic acid is the active component that allows coagulation between OFI mucilage and solid particles present in contaminated water. Fox et al. (2012) evaluated the removal of arsenic (V) present in synthetic waters by fractions (GE and NE) of OFI mucilage. They found that GE removed a higher concentration of arsenic (V) with respect to NE due to a higher content of UA in the pectic compounds; furthermore, in both cases they evidenced the binding of arsenic (V) to ionizable functional groups in mucilage through hydrogen bonded binding. Onditi et al. (2016) studied the mechanism of adsorption of Pb2+ and Cd2+ ions present in synthetic waters by Opuntia mucilage; the results showed that the mechanism involved in the adsorption process was due to interactions between mucilage molecules and metal ions, i.e., adsorption and bridging mechanism. Vargas-Solano et al., (2022b) studied the capacity of OFI mucilage in the adsorption of HM present in river water samples, using different concentrations of mucilage (87.5, 175 and 350 mg/L). The results showed that the mucilage had an adsorption capacity greater than 90 % for iron and manganese, and greater than 60 % for chromium and arsenic. Finally, the FTIR spectra of OFI mucilage showed changes after adsorption, suggesting that carbonyl, carboxyl, and hydroxyl groups acted in the adsorption process of HM; these functional groups are associated to the UA of OFI mucilage. Therefore, the objective of the present investigation was to evaluate the effect of uronic acid content in OFI mucilage on the removal of heavy metals present in water samples from the Yautepec River, Mexico. In addition, during the development of this research, it was important and relevant to maintain the natural conditions of the water samples. This is because one of the focuses of the work consisted of obtaining and applying materials for removal with the least possible processing, which will allow it to be affordable and practical to apply in real conditions of contaminated waters.
2 Materials and methods
2.1 Extraction and drying of nopal mucilages
For the experimental work, cactus cladodes of the species Opuntia Ficus-indica (OFI) were collected in the town of San Juan Tlacotenco (altitude: 2378 m.a.s.L.; latitude: 19°01′73″ W and longitude: 99°09′28″ N) in the municipality of Tepoztlan, Morelos, Central Mexico. The conditions of the plots were: igneous-sandy soil of medium depth, pH 6.5 to 8.5, mean annual temperature of 18 °C, and mean annual rainfall of 750 mm. The nopals were harvested according to their age of maturation proposed by Rodríguez-González et al. (2021) to obtain different concentrations of uronic acids (UA) in the extracted mucilage. The choice and harvesting of the nopals was carried out randomly from different plants, after which they were taken to the laboratory. The prickles were removed from all the nopals, and these were washed with running water and neutral liquid soap (Hycel, Mexico) to remove dust and impurities, and then washed with distilled water. Subsequently, the nopals were cut into strips with a polyethylene grater with 3 mm openings to obtain a fine grating.
On the other hand, the mucilage extraction process of the nopals was carried out according to the methodology proposed by Rodríguez-González et al. (2021), it is important to mention that OFI mucilages were not purified; because we wanted to use and work with an inexpensive and quick-to-use coagulant materials for people. For this, grated nopals and distilled water in a 1:1 ratio (wt./v) were added in beakers and placed on a stirrer hot plate at a temperature of 40 ± 2 °C and left for agitation at 300 rpm for 4 h. Then, the cactus mucilage from each of the vessels was filtered separately through a 250 μm aperture mesh and stored at 4 °C for 18 h. Finally, the filtered mucilages were freeze-dried (Genesis 12 SE, model VirTis, U.S.A) under high vacuum (0.04 mbar) for 6 days to obtain the dry powdered OFI mucilages, namely M1, M2 and M3. These were classified according to the age of maturation: M1 for mucilage extracted from prickly pear cactus with 20–35 days of maturation, M2 for mucilage extracted from prickly pear cactus with 80–100 days of maturation, and M3 for mucilage extracted from prickly pear cactus with 550–600 days of maturation.
2.2 Quantification of uronic acids
Quantification of UA of OFI mucilages (M1, M2 and M3) was performed by the carbazole colorimetric method (Dische, 1947). For this purpose, 20 mg of mucilage were dispersed in 10 mL of distilled water at 40 °C for 1 h; then a 100 μL aliquot of the mucilage solutions was mixed with 6 mL of concentrated H2SO4 immersed in a stainless-steel vessel with 3 L of chilly water, and then the mixture was placed in water bath for 10 min. Subsequently, the mixture was cooled at room temperature and 0.4 mL of an alcoholic solution of carbazole (0.1 v/v%) was added. The absorbance of this mixture was measured in a UV–vis spectrophotometer (Shimadzu, model 160A, Japan) at λ = 530 nm after 30 min; and the content of UA was determined by a calibration curve using a galacturonic acid as standard (Sigma-Aldrich, Germany) (0.25–1 mg/mL solutions in distilled water were used). The above procedure was performed for each of the mucilages (M1, M2 and M3) separately.
2.3 Study area and sampling
The Yautepec River is in the state of Morelos, central Mexico (latitude 18°57′3″ to 18°34′10″ N and longitude 98°52′14″ to 99°11′14″ W), near the corridor the Chichinautzin biological park, the Iztaccíhuatl-Popocatepetl National Park (Popocatepetl volcano has been active since 1994), the Apatlaco, Cuautla, and Amacuzac river basins. The Yautepec River is 95 km long and has an average annual temperature of 21 °C; approximately 410,000 people live along the river (INEGI, 2018). This water is used for agricultural, livestock, recreational, public, and industrial activities (CONAGUA, 2018). The industries near the river belong to the pharmaceutical, textile, agrochemical, metallurgical, automotive, chemical, food, and paint areas (INEGI, 2018). Also, the Yautepec River water experiences pollutant deposition (cadmium, iron, manganese, lead, sulfates) due to ash and gases spewed by the Popocatepetl volcano (D’Addabbo et al., 2015; Shruti et al., 2018).
The water samples were collected at 10 different points (S1–S10) along the Yautepec River (Fig. 1) in December 2016. These points were selected based on the results obtained by Vargas-Solano et al. (2019). Water samples were stored in high-density polyethylene containers, that were previously washed with neutral detergent and deionized water, and then immersed in a 2 % ultrapure nitric acid solution (HNO3, Fermont, Mexico). Finally, the containers with water were kept at a temperature of 4 °C until processing in the laboratory (NMX-AA-14, 1980).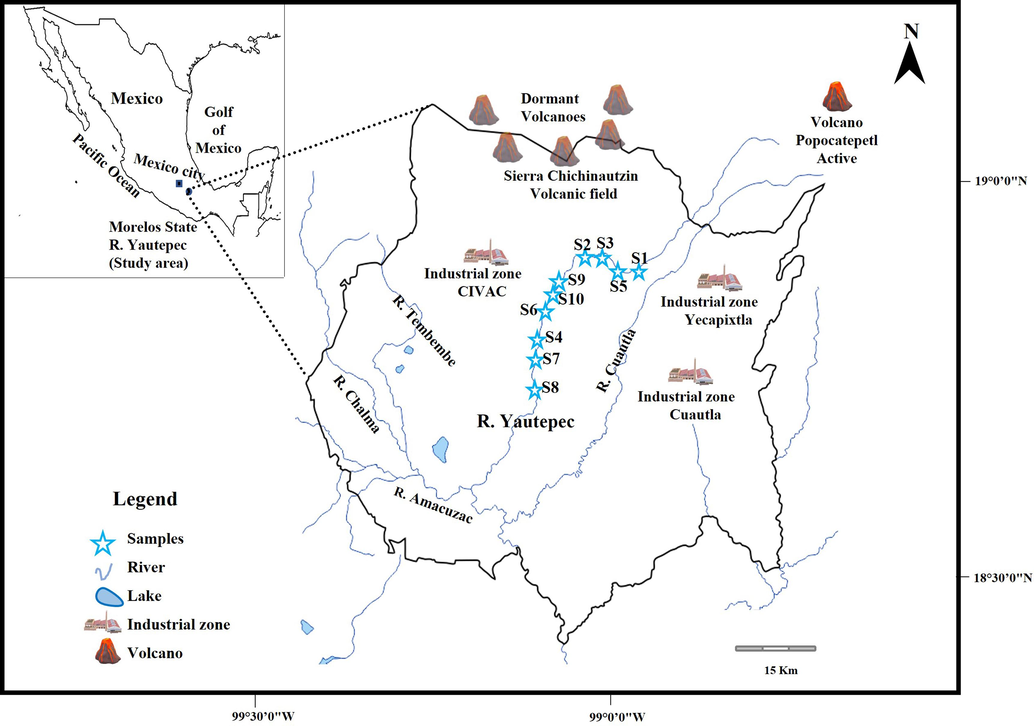
Map of the study area indicating the sampling sites of the Yautepec River, Morelos central Mexico.
2.4 Field and laboratory analysis
Turbidity, pH, and EC of the water samples were determined in situ using portable equipment, namely, turbidity meter (HANNAH instrument, model HI 93703, Romania), pH meter (Oakton, model pH Tester 30, Singapore), and conductivity meter (HM Digital, model aqua, China), respectively. The portable equipment was previously calibrated. Subsequently, the pH meter and conductivity meter were introduced into the water river for the determination of pH and EC, respectively; while, for the determination of turbidity, the water samples were placed in a glass cell of the equipment that allowed it to be read. On the other hand, the determination of the total initial concentration of HM in the water samples was performed in the same month of the collected of water as follows; the water samples (500 mL) were acidified with 2 mL of ultrex HNO3 (Fermont, Mexico), digested and analyzed using flame atomic absorption spectrometry (FAAS) (Perkin Elmer, model Analyst 100, U.S.A) for cooper, cadmium, chromium, nickel, lead, iron, zinc and manganese; and hydride generation for arsenic. Milli Q water was used as an internal control, and a standard reference material (high purity 1.605.428) was used to maintain the precision of analysis. Each of the measures was carried out in triplicate.
2.5 Heavy metal removal experiments
The heavy metal removal experiments in the 10 water samples of the Yautepec River using OFI mucilages (M1, M2 and M3) were carried out using the jar method (ASTM D2035-19, 2019). During the experiments, the water samples presented a pH between 5.9 and 8.5 and a temperature of 25 ± 1 °C. For these experiments, the water samples (500 mL) were shaken at 300 rpm for 1 min, and at that moment OFI mucilage in solution (3.5 mg/mL distilled water) was added; this concentration was used according to reported results (Vargas-Solano et al., 2022b). Afterwards, the water samples were shaken at 80 rpm for 30 min and rested for 60 min; then they were centrifuged at 10,000 rpm for 10 min (Hermle, model 323-K, Germany), and immediately the measurement of turbidity, pH and EC of the recovered water (supernatants) was carried out in the laboratory; also, the concentration of copper, cadmium, chromium, nickel, lead, iron, zinc, manganese and arsenic was determined using the equipment and methodology described above. In this part of the research, the three mucilages studied (M1, M2 and M3) were used in triplicate for each of the 10 river water samples. On the other hand, the initial total heavy metal concentration (IC) in the different water samples was calculated using Eq. (1) (Vargas-Solano et al., 2022b):
Finally, the total percentage of HM removed (%TCHR) from the water samples was calculated using Eq. (4):
2.6 Identification of functional groups in the different OFI mucilages
The identification of the functional groups in the different OFI mucilages (M1, M2, and M3) before and after the removal process was carried out by Fourier transform infrared spectroscopy (FTIR) using a spectrophotometer (Shimadzu, model IR-Affinity 1, Japan) operated in a frequency range of 4000–600 cm−1 and a resolution of 4 cm−1, using an Attenuated Total Reflection (ATR) plate. For this purpose, a dehydrated sample (1 mg) of each of the OFI mucilage powder was placed on the ATR plate; the experiments were performed in triplicate at room temperature.
2.7 Statistical analysis
Statistical analyzes of the UA content and the physicochemical characteristics of the studied water samples were carried out to calculate the mean values, standard deviation and statistical differences using one-way ANOVA and Tukey's test with a significance level of p < 0.05; as well as the correlation matrix between the physicochemical characteristics, the IC, as well as TCHR had a significance level of p < 0.05, 0.01 and 0.001. All tests were performed using Statistica software version 12.0 (2013) (StatSoft Inc.).
3 Results and discussion
3.1 Uronic acid content in OFI mucilages
The content of UA in OFI mucilages (M1, M2 and M3) obtained in the present work and in previous research is presented in Table 1; where it is reported that mucilage M3 had the highest content of UA with 2.22 ± 0.04 mg/mL; this value is statistically different (p < 0.05) to the content of these compounds quantified in mucilages M1 and M2, which presented 1.87 and 1.99 mg/mL, respectively. The content of UA quantified in mucilages M1 and M2 was similar to that reported by Rodríguez-González et al. (2021), who extracted mucilages from OFI with maturation ages of 20 and 80 days, with contents of 1.92 and 2.14 mg/mL, respectively; this result may be related to the same plant growth factor (variety and age of the cladode, type of soil and crop climate). Also, Table 1 shows that the content of UA increased with the age of maturation of the nopals of 20–600 days; this is consistent with reports of purified mucilage extracted from nopals of 80 and 600 days or more of maturation, and with different varieties of the OFI species (Ribeiro et al., 2010). The increase in UA content in the different mucilages has been related to an increased biosynthesis of carbohydrate precursors of homogalacturonan compounds in mucilaginous cells, which have the function of regulating water content in the plant (Baba, 2006; Ribeiro et al., 2010). Furthermore, the difference between the UA content in the OFI mucilage of the present work and the results obtained by other researchers (Ribeiro et al., 2010), can be attributed to the different varieties of Opuntia studied and the purification of the mucilage. Also, it has been mentioned that the UA content in OFI mucilage can differ due to the modifications made in the techniques used to determine the UA content, as well as in the maximum wavelength (λmax) used in the spectrometer (Li et al., 2007). The results of this section indicate that the change in UA content quantified in OFI mucilages is related to the age of maturation of nopals. Values represent mean ± standard deviation in mg/mL (n = 3). The results of this work in the rows labeled with different letters indicate significant differences for Tukey (p < 0.05). NR = Not reported.
Age (days)
Ribeiro et al. (2010)
Rodríguez-González et al. (2021)
This work
20–35
NR
1.92 ± 0.09
1.87 ± 0.02a
80–100
4.50 ± 0.00
2.14 ± 0.12
1.99 ± 0.05b
500 or more
7.95 ± 0.00
NR
2.22 ± 0.04c
3.2 Physicochemical characteristics of water samples
The values obtained for pH, EC, and turbidity of the river water samples before and after the HM removal process using OFI mucilages are presented in Table 2, where a decrease in pH with respect to its initial value is reported, except in water sample S1. In addition, water samples S2, S3, and S4 presented initial pH values < 8, and after the removal process, the pH decreased between 1.3 and 5.2 %. The other six water samples had initial pH values ≥ 8, and a pH decrease between 3.7 and 15.3 % after the removal process; water sample S10 showed the largest pH decrease. The decrease in pH in the water samples after the removal process did not show a linear trend with the initial pH values; however, it was observed that the water samples with initial pH values ≥ 8.1 showed the largest decreases. These results may be related to a higher or lower proportion of deprotonation of the functional groups present in the molecules that constitute OFI mucilages. That is, in water samples with pH ≈ 8, a deprotonation of the functional groups of the mucilage occurred when incorporated and solubilized in water; in this case, the H+ ions of the functional groups were released to water to produce a charge equilibrium. On the other hand, water samples with a pH < 7 presented a lower or slight deprotonation of the carboxyl, carbonyl, and hydroxyl functional groups than waters with pH ≈ 8. This could be due to the lower amount of H+ ions and the little deprotonation of the functional groups of the OFI mucilage, which slightly affected the pH of the waters (Choudhary et al., 2019; Nharingo et al., 2015; Onditi et al., 2016; Vargas-Solano et al., 2022b). Finally, Table 2 shows that the river water samples, except for water sample S1, are within the permissible pH limits, according to the international standard (WHO, 2022); the sample pH values are between 6.2 and 8.0. On the other hand, in water bodies, pH conditions can be modified by several factors, including pollution from recreational, domestic, and industrial activities (Nobel et al., 1992), as well as natural substances generated during the rainy season, CO2 emissions, and climate change (Rahmanian et al., 2015; Sarker et al., 2021). *In situ, NR = Not reported.
* Values before the removal process
Values after the removal process
M1
M2
M3
Samples
pH
EC (μs/cm)
Turbidity (NTU)
pH
EC (μs/cm)
Turbidity (NTU)
pH
EC (μs/cm)
Turbidity (NTU)
pH
EC (μs/cm)
Turbidity (NTU)
S1
5.9 ± 0.1
239 ± 0.1
0 ± 0
6.3 ± 0.1
332 ± 0.2
1.9 ± 0.2
6.3 ± 0.1
337 ± 0.3
1.9 ± 0.2
6.2 ± 0.1
346 ± 0.2
1.8 ± 0.1
S2
7.5 ± 0.1
1153 ± 0.1
0 ± 0
7.3 ± 0.1
1117 ± 0.6
1.1 ± 0.1
7.3 ± 0.2
1142 ± 0.1
0.8 ± 0.1
7.4 ± 0.2
1132 ± 0.1
1.1 ± 0.1
S3
7.7 ± 0.1
1245 ± 0.2
0 ± 0
7.3 ± 0.1
1201 ± 0.4
1.0 ± 0.1
7.5 ± 0.2
1213 ± 0.2
0.4 ± 0.1
7.5 ± 0.1
1191 ± 0.4
2.3 ± 0.2
S4
7.8 ± 0.1
1105 ± 0.3
3.4 ± 0.1
7.5 ± 0.2
1075 ± 0.1
0.9 ± 0.2
7.6 ± 0.1
1101 ± 0.1
0.7 ± 0.2
7.7 ± 0.1
1076 ± 0.6
0.9 ± 0.1
S5
8.0 ± 0
1236 ± 0.1
0 ± 0
7.1 ± 0.1
1192 ± 0.1
0.9 ± 0.1
7.5 ± 0.1
1219 ± 0.3
0.9 ± 0.1
7.6 ± 0.1
1119 ± 0.2
1.2 ± 0.1
S6
8.0 ± 0
2628 ± 0.3
0 ± 0
7.7 ± 0.1
2263 ± 0.2
0.9 ± 0.1
7.7 ± 0.1
2280 ± 0.6
0.4 ± 0.1
7.7 ± 0.1
2100 ± 0.2
0.9 ± 0.2
S7
8.1 ± 0.1
1119 ± 0.1
1.4 ± 0.1
7.6 ± 0.1
1109 ± 0.2
1.0 ± 0.3
7.8 ± 0.2
1103 ± 0.2
1.3 ± 0.4
7.8 ± 0.1
1085 ± 0.5
1.2 ± 0.4
S8
8.2 ± 0
1156 ± 0.1
1.5 ± 0.1
7.5 ± 0.2
1125 ± 0.3
1.1 ± 0.1
7.7 ± 0.2
1145 ± 0.1
1.1 ± 0.1
7.9 ± 0.1
1130 ± 0.1
1.2 ± 0.2
S9
8.3 ± 0
1127 ± 0.2
2.5 ± 0.1
7.5 ± 0.1
1076 ± 0.1
0.9 ± 0.1
7.8 ± 0.1
1109 ± 0.2
0.9 ± 0.1
7.8 ± 0.1
1096 ± 0.1
1.3 ± 0.1
S10
8.5 ± 0.1
1100 ± 0.5
1.0 ± 0
7.2 ± 0.1
1075 ± 0.1
0.9 ± 0.1
7.6 ± 0.1
1090 ± 0.2
0.9 ± 0.2
7.6 ± 0.1
1075 ± 0.2
0.9 ± 0.2
WHO (2022)
6.5 to 8.5
NR
< 5
SSA1 (2022)
6.5 to 8.5
NR
< 5
For EC values, Table 2 shows that this parameter exhibited a decrease in all water samples after the HM removal process with respect to its initial value, except for water sample S1. This decrease is between 4 and 528 μS/cm; however, water sample S1 presented an average increase of 99 μS/cm. The decrease in EC in the water samples may be related to a reduction in the concentration of HM present in them; this is attributed to the removal of HM from the water samples by the application of OFI mucilages as adsorbent material. In the case of water sample S1, the increase in EC may be related to the release of mineral salts such as Na2+, K+, Ca2+ from the mucilage into the water sample during the removal process (Choudhary et al., 2019; Rubio-Arias, et al., 2016; Vargas-Solano et al., 2022b). As seen in Table 2, the water samples collected in the Yautepec River present different values of electrical conductivity. The differences in electrical conductivity after the removal process would be given by the pH and the concentration of the total presence of ions in solution, which affect, to a greater or lesser degree, the hydrodynamics of the mucilage molecule. Also, columns 2 and 3 of Table 2 show the pH and EC of the water samples of the Yautepec River; as can be seen, these do not present a proportional relationship. In the measurement of EC, all the ions present in the water samples contribute to the magnitude of this physicochemical characteristic (Marandi et al., 2013); therefore, the EC values could be due to the composition of the water itself, as well as the presence of contaminants and chemical substances, which were able to ionize and modifying the electrical conductivity of the water samples.
For turbidity, Table 2 shows that after the HM removal process the values of this physicochemical characteristic changed, which are discussed in two scenarios: those related to an increase and a decrease in the turbidity of the water samples. Also, these values showed a dependence on the initial turbidity values. The first scenario was presented in the water samples with initial turbidity values <1 NTU, which after the removal process, had an increase in their final value, which was between 0.46 and 1.9 NTU. The second scenario emerged in the water samples with initial turbidity values between 1.0 and 3.4 NTU, which decreased turbidity between 0.74 and 1.27 NTU. The decrease in turbidity after the removal process was observed in all the water samples that presented turbidity ≥ 1 NTU. Furthermore, these coincide with initial values of pH ≥ 8.1 and pH = 7.8 (sample S4). The increase in this characteristic may be due to an excess of fine particles suspended or dispersed from the added mucilage (organic matter), which had no interaction with the heavy metals or other particles that generate turbidity, due to a low concentration of ions or absence of particles in the water (Belbahloul et al., 2015). Moreover, these findings establish an influence of the initial turbidity and pH values of the water samples; also, the results of the present work are consistent with those obtained by Zhang et al. (2006), who used nopal flour as a coagulant material for turbidity reduction of synthetic water samples with kaolinite; these had initial turbidity values between 56 and 104 NTU. The former authors found a higher coagulation activity of nopal flour in water samples with pH = 10 and nopal flour dosage between 30 and 60 mg/L; under these conditions they obtained removal efficiencies higher than 90 %. Miller et al. (2008) observed the same behavior using nopal flour to remove turbidity from water samples with kaolinite (0 to 125 NTU). They noted a higher coagulation activity of nopal flour in water samples with values of 8 ≤ pH ≤ 10. Finally, the results presented in Table 2 indicate that, after the HM removal process using OFI mucilages, all water samples had turbidity values below 5 NTU, which are within the values established by international and national standards (WHO, 2022; Zhang et al., 2006). The results of this section indicate that the water samples from the Yautepec River, except for water samples S1, after the removal process of HM present pH and turbidity values permitted for human use and consumption, according to the international standard.
3.3 Presence of heavy metals
The concentrations of cooper, cadmium, chromium, nickel, lead, zinc, iron, manganese, and arsenic, quantified in each of the river water samples before the removal process are presented in Table 3, as well as the permissible limits of the HM established in international and national standards (WHO, 2022; Zhang et al., 2006). The results reported in Table 3 show that each water sample presented differences in the concentrations of each of the HM studied, as well as in the initial total HM concentration (IC). Water sample S1 presented the lowest IC with 55.1 μg/L; while samples S7, S8 and S9 exhibited the highest ICs. It is important to mention that sample S1 was collected in a spring located 32 km from the active Popocatepetl volcano; while water samples S7, S8, and S9 were collected in places of the Yautepec River near agricultural, industrial, and urban areas, respectively (see Fig. 1). Sample S1 presented different concentrations of the different HM studied (Table 3); this can be attributed to the presence of volcanic ash with a surface coating of soluble salts, which could have caused an increase in acidity in the water sample (Stewart et al., 2006). Likewise, the high iron and lead values may be related to the presence of dissolved iron oxide from andesite rocks and geochemical elements in basaltic andesites, which dissolve in surface water (Chen et al., 2007). The presence of arsenic in this water sample can be attributed to volcanic emissions and hydrothermal fluids from the active volcano (Jiao et al., 2012; Vargas-Solano et al., 2019). IC: Initial total heavy metal concentration, n = 3 (mean ± STD), NR = Not reported.
Samples
Cooper
Cadmium
Chromium
Nickel
Lead
Zinc
Iron
Manganese
Arsenic
IC
S1
1.9 ± 0.1
0.0 ± 0
3.7 ± 0.1
0.0 ± 0
9.0 ± 0.1
5.1 ± 0.1
31.0 ± 0.1
2.3 ± 0.1
2.1 ± 0
55.1 ± 0.8
S2
5.2 ± 0.2
3.9 ± 0.1
6.6 ± 0.1
17.9 ± 0.1
54.6 ± 0.1
13.3 ± 0.1
388.9 ± 0.1
102.8 ± 0.2
4.2 ± 0.1
597.4 ± 1.5
S3
4.6 ± 0.1
3.7 ± 0.1
6.3 ± 0.2
18.8 ± 0.1
57.1 ± 0.2
13.9 ± 0.1
123.8 ± 0.1
21.7 ± 0.1
4.1 ± 0.2
254.0 ± 1.7
S4
4.1 ± 0.2
3.5 ± 0.1
7.1 ± 0.1
20.5 ± 0.1
54.6 ± 0.1
10.9 ± 0.2
582.5 ± 0.1
95.7 ± 0.1
3.4 ± 0.1
782.3 ± 1.6
S5
4.6 ± 0.1
3.8 ± 0.1
6.5 ± 0.2
18.4 ± 0.1
53.8 ± 0.2
7.4 ± 0.1
128.8 ± 0.1
24.5 ± 0.1
2.0 ± 0.1
249.9 ± 1.5
S6
11.9 ± 0.1
9.5 ± 0.1
13.4 ± 0.1
44.1 ± 0.2
80.8 ± 0.1
12.5 ± 0.2
257.6 ± 0.1
50.0 ± 0.1
2.9 ± 0.2
482.7 ± 1.7
S7
4.3 ± 0.1
3.6 ± 0.1
6.2 ± 0.2
17.5 ± 0.1
54.0 ± 0.1
17.8 ± 0.1
472.2 ± 0.1
209.7 ± 0.1
2.7 ± 0.1
788.0 ± 1.4
S8
5.3 ± 0.1
4.6 ± 0.1
7.8 ± 0.1
23.4 ± 0.1
61.8 ± 0.1
19.6 ± 0.1
1546.0 ± 0.1
118.3 ± 0.1
9.1 ± 0.1
1795.9 ± 1.3
S9
5.0 ± 0.2
3.7 ± 0.1
5.7 ± 0.1
17.4 ± 0.1
50.3 ± 0.1
7.7 ± 0.1
587.4 ± 0.2
405.9 ± 0.1
2.7 ± 0.1
1085.8 ± 1.6
S10
4.1 ± 0.1
2.8 ± 0.1
5.6 ± 0.2
15.4 ± 0.1
45.6 ± 0.2
5.9 ± 0.1
513.8 ± 0.2
58.8 ± 0.1
2.5 ± 0.1
654.5 ± 1.7
WHO (2022)
2000
3
50
70
10
500
NR
NR
10
SSA1 (2022)
2000
5
50
NR
10
5000
300
150
50
On the other hand, the high concentration of HM presented in water samples S7, S8, and S9 may be related to the agricultural, industrial, and domestic activities that take place in the sites near the collection areas (Al-Asadi et al., 2020; Chen et al., 2007). Vargas-Solano et al. (2019) reported that some sugarcane crops near the sampling sites are treated with agrochemicals to eliminate pests, so chemical residues are carried to the Yautepec River by runoff during crop irrigation and rainfall. In turn, Nharingo et al. (2015) reported a high concentration of lead, cadmium, and copper in water samples from the Mukuvisi River, which was attributed to contaminated water discharged into the river by industries and residential houses. Table 3 shows that in some water samples the concentration of cadmium, lead, iron, and manganese exceeded the permissible limits established for human use and consumption; while other HM such as copper, chromium, nickel, zinc, and arsenic were present in low concentrations; these did not exceed the permissible limits for human use and consumption, as established in international and national standards (WHO, 2022; Zhang et al., 2006). The low concentrations of such HM in the river water samples may be due to the low solubility of such metals in water and the presence of metal ions that are associated with colloids suspended (sand, silt, and clay) by the adsorption process (Al-Asadi et al., 2020; Campillo-Cora et al., 2020; Mimba et al., 2017).
3.4 Removal of heavy metals
The IC quantified in the ten water samples studied before the removal process and the TCHR by OFI mucilages (M1, M2 and M3) are displayed in Fig. 2, which shows that the TCHR presented different HM removal values. The different letters at the top of the bars for each water sample establish significant differences at p < 0.05. Water samples S1, S3, and S5 (with pH ≤ 8.0) showed the lowest TCHR values, between 4.33 and 5.22 μg/L, 95.1 and 114.6 μg/L, and 98.1 and 121.9 μg/L, respectively. Whereas, the highest TCHR values were exhibited in samples S4, S7, S8, and S9 (with pH > 8.0, except sample 4), ranging between 590.8 and 639.8 μg/L, 586.1 and 625.8 μg/L, 1593.3 and 1621.3 μg/L, and 871.7 and 932.2 μg/L, respectively. The minimum and maximum values of all the above intervals of TCHR correspond to HM removal by mucilages M1 and M3, respectively; while intermediate values of the intervals are related to HM removal by mucilage M2 (see Fig. 3).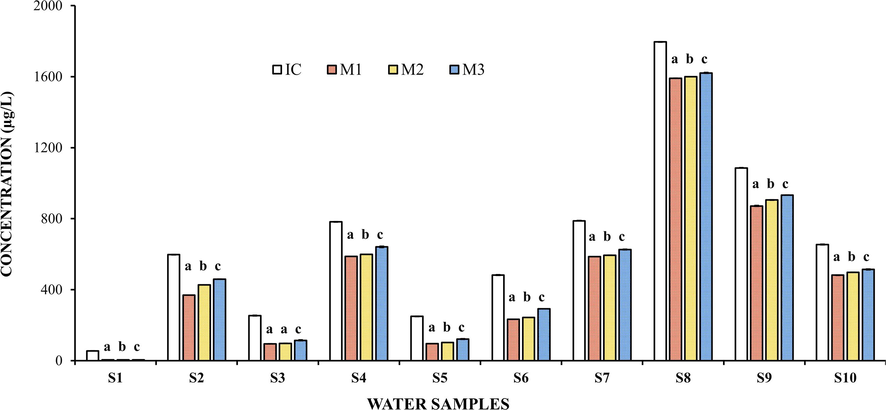
Concentration of total heavy metals determined in water samples from the Yautepec River before and after the removal process using OFI mucilages M1, M2 and M3. The results in the bars labeled with different letters indicate significant differences for Tukey (p < 0.05).
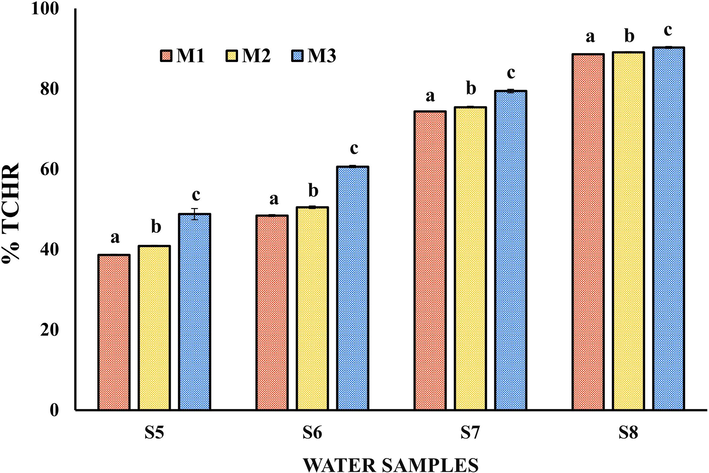
Percentage of heavy metals removed from the Yautepec River water samples with a pH of 8.1 ± 0.1 using OFI mucilages (M1, M2 and M3). The results in the bars labeled with different letters indicate significant differences for Tukey (p < 0.05).
The differences in TCHR values show a dependence on the mucilage used for removal; these differences can be attributed to the UA content of OFI mucilages due to the presence of the functional groups in the mucilage molecules (Barka et al., 2013; Fox et al., 2012; Nharingo et al., 2015; Vargas-Solano et al., 2022b). Fox et al. (2012) studied the removal of arsenic ions by GE and NE fractions of Opuntia mucilage; and showed that GE removed a higher percentage of arsenic, for a given pH and mucilage dose with respect to NE. This result was attributed to the UA content of pectic polysaccharides present in both fractions. On the other hand, the changes in TCHR values also presented a dependence on the initial pH and IC in each water sample (Barka et al., 2013; Nharingo et al., 2015; Onditi et al., 2016). The dependence of TCHR values on these parameters is analyzed in Sections 3.4.1 and 3.4.2, respectively.
On the other hand, the removal percentages of total heavy metals (%TCHR) in the 10 water samples of the Yautepec River by mucilages M1, M2 and M3 are presented in Table 4; %TCHR values were calculated using Eq. (4). In this Table, it can be seen that the lowest %TCHR values were obtained in all water samples evaluated with mucilage M1, with removal percentages from 7.88 % to 88.6 %, referring to samples S1 and S8, respectively. Meanwhile, when evaluating the water samples with the mucilages M2 and M3, an increase in the %TCHR was observed with respect to the increase in the UA content of the mucilages. In the case of mucilage M2, heavy metal removal percentages between 8.94 % and 89.1 % were obtained, and for mucilage M3, removal percentages between 9.47 % and 90.26 % were obtained, for water samples S1 and S8, respectively. The increases in %TCHR as the UA content of the mucilages used for the removal of HM present in the water samples increased, may be related to the increase in adsorption sites by the functional groups of the uronic acids of the OFI mucilages (Fox et al., 2012). On the other hand, considering the physicochemical characteristics, the removal process can be attributed to the adsorption mechanism due to opposite charges between the functional groups and the heavy metals, as well as the electrostatic interactions of these components and/or adsorption and bridging among the molecules of mucilage and solid particles present in water (Bouaouine et al., 2018; Choudhary et al., 2019; Chamani, 2006; Kalhori et al., 2022; Moosavi-Movahedi et al., 2003; Taheri et al., 2022). This result is consistent with what was reported by Qi et al. (2024), who worked with a carbon/iron compound derived from biomass (FexOy-BC (RM)) and carried out the adsorption of cadmium(II) present in wastewater, they observed that by increasing the dose of the adsorbent compound, an increase in the percentage of metal removal was obtained. n = 3 (mean ± STD).
Samples
(%TCHR)M1
(%TCHR)M2
(%TCHR)M3
S1
7.88 ± 0.15
8.94 ± 0.15
9.47 ± 0.17
S2
61.83 ± 0.31
71.43 ± 0.24
76.95 ± 0.37
S3
37.45 ± 1.05
38.22 ± 1.08
45.13 ± 1.79
S4
75.48 ± 0.35
76.18 ± 0.38
81.94 ± 0.79
S5
39.24 ± 1.01
40.66 ± 0.76
48.79 ± 1.41
S6
48.44 ± 0.22
50.47 ± 0.33
60.63 ± 0.27
S7
74.36 ± 0.39
75.43 ± 0.22
79.42 ± 0.47
S8
88.60 ± 0.11
89.10 ± 0.12
90.26 ± 0.26
S9
80.28 ± 0.43
83.38 ± 0.30
85.91 ± 0.28
S10
73.63 ± 0.57
76.01 ± 0.36
78.57 ± 0.71
3.4.1 Effect of initial pH
The initial total concentration of heavy metals (IC) of the ten water samples before the removal process and TCHR by OFI mucilages (M1, M2 and M3) were analyzed with a correlation matrix to relate the effect of IC and TCHRs with respect to the initial pH of the water samples; the results obtained are shown in Table 5. The correlation matrix was divided into two sections for analysis; for water samples with pH values < 8 and for pH ≥ 8, respectively. p < 0.05*, 0.01†, 0.001‡.
IC
TCHR(M1)
TCHR(M2)
TCHR(M3)
pH
pH < 8
IC
1.000
TCHR(M1)
0.977*
1.000
TCHR(M2)
0.991*†
0.991*†
1.000
TCHR(M3)
0.964*
0.997*†
0.987*
1.000
pH
1.000
pH ≥ 8
IC
1.000
TCHR(M1)
0.841*
1.000
TCHR(M2)
0.871*
0.997*†‡
1.000
TCHR(M3)
0.851*
0.995*†‡
0.995*†‡
1.000
pH
1.000
Table 5 shows that for water samples with pH < 8, a positive correlation was recorded between IC versus TCHRs using OFI mucilages (M1, M2, and M3); the correlation coefficients obtained were 0.977, 0.991 and 0.964, respectively, exhibiting significant differences (p < 0.05) among them. This result establishes a correlation between IC and TCHRs, regardless of the OFI mucilage used in HM removal. And it is consistent with that reported by other research groups (Barka et al., 2013; Fox et al., 2012; Nharingo et al., 2015; Onditi et al., 2016; Vargas-Solano et al., 2022b), who observed that HM such as cooper, cadmium, chromium, lead, zinc, iron and arsenic, present in water were removed by mucilages from and/or derived from cacti under weak acid and weak basic pH conditions (5 ≤ pH < 8). In this regard, the positive correlation shown in Table 5 (IC versus TCHRs) for water samples under conditions of 5.9 ≤ pH < 8, can be attributed to the removal of HM from water samples by the ionization effect of functional groups that are present in the uronic acids of the OFI mucilages (Fox et al., 2012; Nharingo et al., 2015; Onditi et al., 2016; Vargas-Solano et al., 2022b). On the other hand, for water samples with pH ≥ 8, a positive correlation was presented between IC versus TCHRs, which was shown for the three OFI mucilages studied. The correlation coefficients obtained were 0.841, 0.871 and 0.851, for M1, M2, and M3, respectively; these coefficients also presented significant differences (p < 0.05) among them. According to these results, the initial pH conditions in the water samples have a strong influence on the percentage of HM removal using mucilage and cacti derivatives as adsorbent materials. Particularly for acidic environments, Opuntia mucilages present a lower deprotonation in their ionizable groups and, in addition, a competition between hydrogen ions (H+) and HM for the negatively charged mucilage adsorption sites are exhibited, resulting in a low removal of HM. However, as the pH (weak basic) increases, the amount of H+ ions in the medium decreases and the number of negatively charged mucilage sites increases; this effect causes an increase in the removal of positively charged HM through the adsorption of ions of opposite charge and/or electrostatic interactions between heavy metals and functional groups present in the uronic acids of the OFI mucilages (Chamani, 2006; Fox et al., 2012; Barka et al., 2013; Moosavi-Movahedi et al., 2003; Nharingo et al., 2015; Onditi et al., 2016). Also, Table 5 shows in water samples with pH < 8 positive correlations between (TCHR)M1 versus (TCHR)M2, (TCHR)M1 versus (TCHR)M3, and between (TCHR)M2 versus (TCHR)M3; the correlation coefficients obtained were 0.991, 0.997 and 0.987, respectively, which have significant differences (p < 0.01, 0.05) among them.
For water samples with pH ≥ 8, positive correlations were presented between (TCHR)M1 versus (TCHR)M2, (TCHR)M1 versus (TCHR)M3, and between (TCHR)M2 versus (TCHR)M3; the correlation coefficients obtained were 0.997, 0.995 and 0.995, respectively, which have significant differences (p < 0.001, 0.01, 0.05) among them. These results show the correlation between TCHR and the different OFI mucilages used for HM removal, for an IC in water samples. The results obtained can be attributed adsorption of ions with opposite charge and/or electrostatic interactions between heavy metals and functional groups present in the OFI mucilage molecules for a specific pH condition (Chamani, 2006; Choudhary et al., 2019; Fox et al., 2012; Moosavi-Movahedi et al., 2003; Nharingo et al., 2015; Onditi et al., 2016; Vargas-Solano et al., 2022b). Therefore, the initial pH in water samples is a key factor in TCHRs by the OFI mucilages studied.
3.4.2 Effect of the initial HM concentration
Water samples S5, S6, S7, and S8 exhibited values of pH = 8.1 ± 0.1 and different IC values (see Tables 2 and 3), which allowed an analysis of the effect of IC versus %TCHR as a function of the different OFI mucilages used in HM removal. Fig. 3 shows an increase in %TCHR as IC increased in the water samples, regardless of the OFI mucilage used for HM removal. The %TCHR values are between 39.3 and 48.8 %, 48.4 and 66.7 %, 74.4 and 79.4 %, and 88.7 and 90.3 %, for the former water samples, respectively. These values may be related to the removal of the HM present in the water samples by the functional groups (–C=O, –COOH and –OH) that are present in the OFI mucilage molecules and pectic polysaccharide. On the other hand, the percentages of HM not removed can be attributed to a coiling of the mucilage molecules, which can be caused by a dissociation of ionic groups present in them towards water because of the dielectric constant (Fox et al., 2012; Onditi et al., 2016; Vargas-Solano et al., 2022b). The Fig. 3 shows that the %TCHR values exhibit a dependence on the OFI mucilage used in HM removal, the different letters at the top of the bars for each water sample establish significant differences at p < 0.05. The differences in %TCHR can be attributed to the concentration of UA present in each of the OFI mucilages (see Table 1) (Barka et al., 2013; Fox et al., 2012; Nharingo et al., 2015; Vargas-Solano et al., 2022b). This is consistent with that reported by Fox et al. (2012), who showed that an OFI mucilage fraction with a higher pectic polysaccharide content removed a higher percentage of arsenic relative to a fraction that had a lower content of such polysaccharides. Thus, the total percentages of heavy metals removed from the water samples for a given pH are related to IC, as well as to the concentration of UA present in OFI mucilages.
3.5 Study of mucilage with heavy metals by FTIR
The Fig. 4a, b, and c present the normalized FTIR spectra of OFI mucilages (M1, M2, and M3, respectively) before and after the HM removal process for water samples S1 and S10. In general, the spectra show the characteristic bands of the specific functional groups of pectic polysaccharides, i.e., carboxylic acid, carbonyl, and hydroxyl groups (Barka et al., 2013; Bayar et al., 2016; Choudhary et al., 2019; Fox et al., 2012; Nharingo et al., 2015; Vargas-Solano et al., 2022b). Also, the spectra exhibit some major features; the first is a broad band at 3290 cm−1 corresponding to the –OH stretching vibrational mode with hydrogen bonds of the hydroxyl groups. The second is a band located at 2930 cm−1 that concerns the C–H stretching vibration of the methyl ester group of galacturonic acid; this band is more pronounced in mucilage M3 with respect to mucilages M2 and M1 (see the FTIR spectra of the graphical abstract). This can be attributed to the higher concentration of UA in mucilage M3 (see Table 1). The third, is a region between 1716 and 1330 cm−1 that is related to the presence of carboxylic groups in OFI mucilages. The band corresponding to 1716 cm−1 is attributed to the stretching of the C=O bond, a non-ionized form of the carboxyl group. Two other bands were identified at 1590 cm−1 and 1395 cm−1 corresponding to symmetric and antisymmetric COO– stretching vibration, respectively.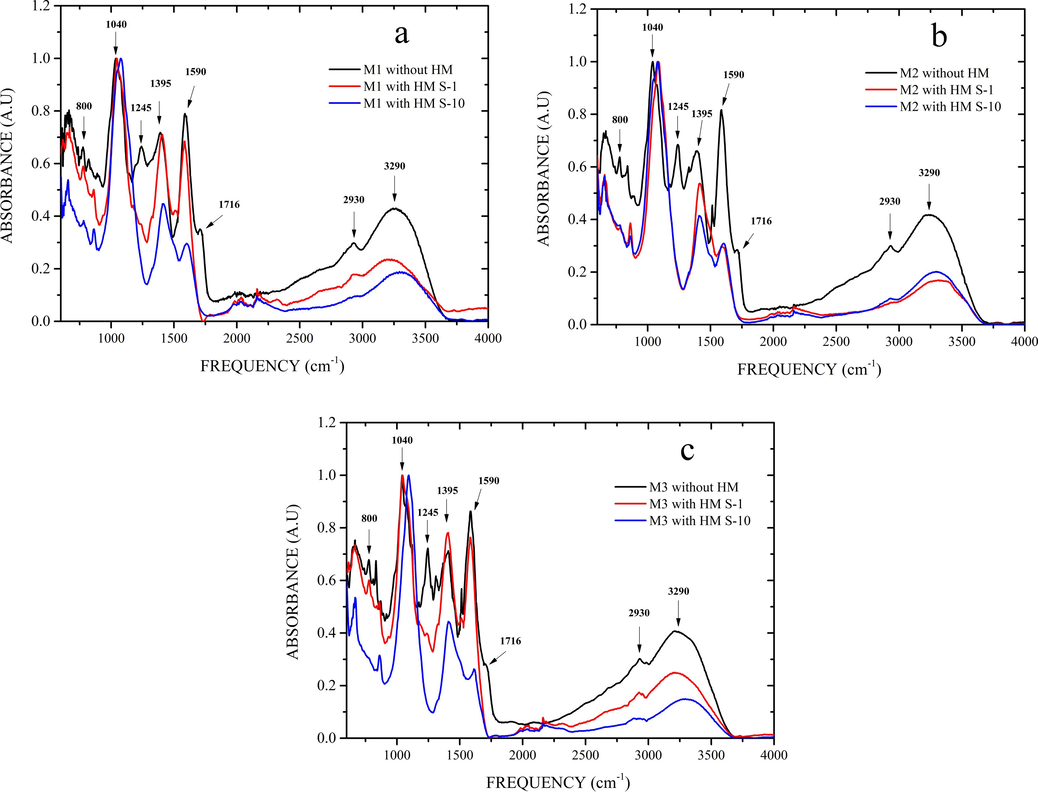
Normalized FTIR spectra of OFI mucilage before and after the heavy metal removal process from Yautepec River water samples from sites S1 and S10. (a) M1, (b) M2 and (c) M3.
The fourth feature is a band at 1245 cm−1, referred to the stretching mode of the ether group (C–O–C). Finally, the fingerprint region, the bands at 1200 cm−1 and 800 cm−1 are related to the presence of functional groups of the polysaccharides, namely bending of –OH, stretching and deflection of C–O–C, and CH3 groups, respectively. In addition, the presence of bands between 1120 cm−1 and 1040 cm−1, which are related to UA (Fox et al., 2012). The intensity of these bands can be correlated with the UA content; such effect is observed in the spectra of the studied mucilages, where the intensities of the bands are higher in the spectrum of mucilage M3 than the bands of M2 and M1 mucilages (see the FTIR spectra of the graphical abstract); which confirms the result shown in Table 1. On the other hand, when comparing the FTIR spectra of OFI mucilages before and after the HM removal process, some differences between them can be observed. The first one corresponds to the decrease in the intensity or disappearance of some bands of the spectrum after the removal process; particularly, a decrease was recorded in the bands corresponding to the frequencies of 3290 cm−1, 2930 cm−1, as well as in the region between 1716 and 1330 cm−1 and in the fingerprint region. Also, the disappearance of the bands referring to the frequencies of 1716 cm−1 and 1245 cm−1 is observed. This may be related to the interaction of HM and functional groups present in OFI mucilage molecules (Fox et al., 2012; Nharingo et al., 2015; Vargas-Solano et al., 2022b). The second difference concerns a phase shift of the FTIR spectra before and after the removal process in the bands corresponding to the frequencies of 1590 cm−1, 1512 cm−1, 1395 cm−1 and 1040 cm−1; these frequencies correspond to the participation of carboxylic acids and polysaccharides in the removal of HM (Barka et al., 2013; Bayar et al., 2016; Choudhary et al., 2019; Fox et al., 2012; Nharingo et al., 2015; Vargas-Solano et al., 2022b).
The results obtained are consistent with those reported by other researchers, who evaluated nopal mucilage in the removal of metal ions (Fox et al., 2012; Nharingo et al., 2015; Onditi et al., 2016). Nharingo et al. (2015) used OFI mucilage for Pb2+ removal and reported changes in the FTIR spectra of mucilage before and after removal; these changes were attributed to the electrostatic interactions between the functional groups present in the pectic molecules and compounds of the OFI mucilage with the Pb2+ ions. In the present study, the FTIR spectra of OFI mucilage before and after the HM removal process underwent changes, namely, dephasing, decrease in the intensity and disappearance of some bands; these changes result from the electrostatic interactions between the functional groups present in the mucilages and the HM.
4 Conclusions
The effect of the concentration of uronic acids (UA) in Opuntia Ficus-indica mucilage was evaluated on the removal of heavy metals present in water samples from the Yautepec River, Morelos (central Mexico). The water samples presented initial values of heavy metals from 1.9 to 1546.0 µg/L, depending on the metal species while cadmium, lead, iron, and manganese exceeded the permissible limits established for human use and consumption according to national and international standards. After the HM removal process, the pH (7.1–7.9) and turbidity (0.4–2.3 NTU) values of most river water samples (except for water sample S1), were within the permissible limits for human use, according to the international standard. The total percentages of HM removed in water samples were strongly affected by the uronic acid content in mucilages from 7.88 to 88.6 % for M1, from 8.94 to 89.1 % for M2 and from 9.46 to 90.26 % for M3. FTIR spectra of OFI mucilages after the removal of HM showed changes in intensities and displacements of bands due to electrostatic interactions between carboxyl, carbonyl, and hydroxyl functional groups of OFI mucilages and the metal ions present in water samples. An efficient, economical, and practical absorbent material was obtained from nopals with possible potential real applications in contaminated waters.
5 Author Agreement
Ethics approval: We would also like to state that the study does not involve human subjects.
Consent to participate: The authors declare that the study does not involve human materials.
Consent for publications: All authors have agreed to participate in the present publication.
Competing interests: The authors have no relevant financial or non-financial interests to disclose.
CRediT authorship contribution statement
Edgar González-Avilez: Investigation, Validation. Francisco Rodríguez-González: Conceptualization, Methodology, Investigation, Validation. Silvia Viridiana Vargas-Solano: Conceptualization, Methodology, Validation. Alex Osorio-Ruiz: Investigation, Validation. M.P. Jonathan: Conceptualization, Methodology, Investigation, Validation, Writing – review & editing. Lorena Elizabeth Campos-Villegas: Methodology, Validation.
Acknowledgments
This research was supported by Instituto Politécnico Nacional (SIP-IPN, 20220588, 20230817). EGA wishes to thank CONAHCyT (Mexico) for the research grant. AOR wishes to thank CONAHCyT (Mexico) for the postdoctoral fellowship. FRG and MPJ wish to express their gratitude to the Sistema Nacional de Investigadoras e Investigadores (SNII) and CONAHCyT, Mexico and also thank EDI & COFAA, IPN, Mexico for their support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: a review. J. Clean Prod.. 2022;345:131133
- [CrossRef] [Google Scholar]
- A review on valorization of biomass in heavy metal removal from wastewater. J. Water Process. Eng.. 2020;38:101602
- [CrossRef] [Google Scholar]
- Water quality and impacting factors on heavy metals levels in Shatt Al-Arab River, Basra, Iraq. Appl. Water Sci.. 2020;10:103.
- [CrossRef] [Google Scholar]
- Cactus material-based adsorbents for the removal of heavy metals and dyes: a review. Mater. Res. Exp.. 2020;7:012002
- [CrossRef] [Google Scholar]
- ASTM D2035-19 (American Society for Testing and Materials). Standard practice for coagulation-flcculation jar test of water, 2019. ASTM Annual Book of Standard, Edition. ASTM International, West Conshohocken. <https://www.astm.org/d2035-19.html>.
- Models of plant cell walls. In: Hayashi T., ed. The Science and Lore of the Plant Cell Wall: Biosynthesis, Structure and Function. FL: Brown Walker Press; 2006. p. :3-10.
- [Google Scholar]
- Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficus indica) cladodes. J. Environ. Chem. Eng.. 2013;1:144-149.
- [CrossRef] [Google Scholar]
- Extraction and characterization of three polysaccharides extracted from Opuntia ficus indica cladodes. Int. J. Biol. Macromol.. 2016;92:441-450.
- [CrossRef] [Google Scholar]
- Bioflocculants extraction from Cactaceae and their application in treatment of water and wastewater. J. Water Process. Eng.. 2015;7:306-313.
- [CrossRef] [Google Scholar]
- Soluble polymers in water purification. Prog. Polym. Sci.. 1995;20:987-1041.
- [CrossRef] [Google Scholar]
- Identification of functional groups of Opuntia ficus-indica involved in coagulation process after its active part extraction. Environ. Sci. Pollut. Res.. 2018;25:11111-11119.
- [CrossRef] [Google Scholar]
- Identification and role of Opuntia ficus indica constituents in the flocculation mechanism of colloidal solutions. Sep. Purif. Technol.. 2019;209:892-899.
- [CrossRef] [Google Scholar]
- Specific adsorption of heavy metals in soils: individual and competitive experiments. Agronomy.. 2020;10:1113.
- [CrossRef] [Google Scholar]
- Comparison of the conformational stability of the non-native α-helical intermediate of thiol-modified β-lactoglobulin upon interaction with sodium n-alkyl sulfates at two different pH. J. Colloid Interface Sci.. 2006;299:636-646.
- [CrossRef] [Google Scholar]
- Mechanism for stabilization of the molten globule state of papain by sodium n-alkyl sulfates: Spectroscopic and calorimetric approaches. J. Colloid Interface Sci.. 2008;322:119-127.
- [CrossRef] [Google Scholar]
- Assessing long-term environmental risks of trace elements in phosphate fertilizers. Ecotoxicol. Environ. Saf.. 2007;67:48-58.
- [CrossRef] [Google Scholar]
- Factors affecting the adsorption of heavy metals by microplastics and their toxic effects on fish. Toxics. 2023;11(6):490.
- [CrossRef] [Google Scholar]
- Evaluation of the potential application of cactus (Opuntia ficus-indica) as a bio-coagulant for pre-treatment of oil sands process-affected water. Sep. Purif. Technol.. 2019;209:714-724.
- [CrossRef] [Google Scholar]
- CONAGUA (Comisión Nacional del Agua), 2018. Resúmenes Mensuales de Temperaturas y Lluvia. México. <https://www.gob.mx/conagua/documentos/calidad-del-agua-184053?idiom=es> (Accessed on June 28, 2023).
- Ash leachates from some recent eruptions of Mount Etna (Italy) and Popocatépetl (Mexico) volcanoes and their impact on amphibian living freshwater organisms. Biogeosciences.. 2015;12:7087-7106.
- [CrossRef] [Google Scholar]
- A new specific color reaction of hexuronic acids. J. Biol. Chem.. 1947;167:189-198.
- [CrossRef] [Google Scholar]
- Current scenario and challenges in adsorption for water treatment. J. Environ. Chem. Eng.. 2020;8:103988
- [CrossRef] [Google Scholar]
- Magnetic chitosan modified with cysteine-glutaraldehyde as adsorbent for removal of heavy metals from water. J. Environ. Chem. Eng.. 2016;4:3835-3847.
- [CrossRef] [Google Scholar]
- A review of coagulation explaining its definition, mechanism, coagulant types, and optimization models; RSM, and ANN. Curr. Opin. Green Sustain. Chem.. 2023;6:100358
- [CrossRef] [Google Scholar]
- Fox, D.I., Pichler, T., Yeh, D.H., Alcantar, N.A., 2012. Removing heavy metals in water: The interaction of cactus mucilage and arsenate (As (V)). Environ. Sci. Technol. 46, 4553-4559. <https://doi: 10.1021/es2021999>.
- Water pollution in Bangladesh and its impact on public health. Heliyon.. 2019;5:e02145.
- [Google Scholar]
- Biochar for the removal of metals from solution: a review of lignocellulosic and novel marine feedstocks. J. Environ. Chem. Eng.. 2020;8:103975
- [CrossRef] [Google Scholar]
- Characterization of the structural changes of human serum albumin upon interaction with single-walled and multi-walled carbon nanotubes: spectroscopic and molecular modeling approaches. Res. Chem. Intermed.. 2019;45:401-423.
- [CrossRef] [Google Scholar]
- INEGI (Instituto Nacional de Estadística y Geografía), Calidad del agua, 2018. <http://cuentame.inegi.org.mx/territorio/agua/sobreexplota.aspx?tema=T> (Accessed on June 28, 2023).
- A review on adsorption of heavy metals from wastewater using conducting polymer-based materials. J. Environ. Chem. Eng.. 2023;11:109226
- [CrossRef] [Google Scholar]
- Environmental risks of trace elements associated with longterm phosphate fertilizers applications: a review. Environ. Pollut.. 2012;168:44-53.
- [CrossRef] [Google Scholar]
- Enzyme activity inhibition properties of new cellulose nanocrystals from Citrus medica L. pericarp: a perspective of cholesterol lowering. Luminescence.. 2022;37:1836-1845.
- [CrossRef] [Google Scholar]
- Advances in the biosorption of heavy metals. Trends Biotechnol.. 1998;16:291-300.
- [CrossRef] [Google Scholar]
- A tabulated review on distribution of heavy metals in various plants. Environ. Sci. Pollut. Res.. 2017;24:2210-2260.
- [CrossRef] [Google Scholar]
- Global evaluation of heavy metal content in surface water bodies: a meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere. 2019;236:124364
- [CrossRef] [Google Scholar]
- An improved methodology for the quantification of uronic acid units in xylans and other polysaccharides. Carbohydr. Res.. 2007;342:1442-1449.
- [CrossRef] [Google Scholar]
- Flocculation properties and kinetic investigation of polyacrylamide with different cationic monomer content for high turbid water purification. Sep. Purif. Technol.. 2017;182:134-143.
- [CrossRef] [Google Scholar]
- A new approach for describing the relationship between electrical conductivity and major anion concentration in natural waters. Appl. Geochem.. 2013;38:103-109.
- [CrossRef] [Google Scholar]
- Toward understanding the efficacy and mechanism of Opuntia spp. As a natural coagulant for potential application on water treatment. Environ. Sci. Technol.. 2008;42:4274-4279.
- [CrossRef] [Google Scholar]
- Seasonal hydrological inputs of major ions and trace metal composition in streams draining the mineralized lom basin, east cameroon: basis for environmental studies. Earth Syst. Environ.. 2017;1:22.
- [CrossRef] [Google Scholar]
- Experimental binding of lead to a low cost on biosorbent: Nopal (Opuntia streptacantha) Bioresour. Technol.. 2008;99:1211-1217.
- [CrossRef] [Google Scholar]
- Electrochemical evidence for the molten globule states of cytochrome c induced by N-Alkyl sulfates at low concentrations. J. Protein Chem.. 2003;22:23-30.
- [CrossRef] [Google Scholar]
- Exploring the use of cactus Opuntia ficus indica in the biocoagulation-flocculation of Pb(II) ions from wastewaters. Int. J. Environ. Sci. Technol.. 2015;12:3791-3802.
- [CrossRef] [Google Scholar]
- NMX-AA-14, 1980, NORMA MEXICANA. Secretaria de Comercio y Fomento Industrial. 1980. Cuerpos Receptores-Muestreo. México. <https://www.sinec.gob.mx/SINEC/Vista/Normalizacion/DetalleNMX.xhtml?pidn=dnltN3lkc2lrQmUxckw5RXFqMEx1dz09> (Accessed on May 02, 2022).
- Nobel, P.S., Cavelier, J., Andrade, J.L., 1992. Mucilage in Cacti: its apoplastic capacitance, associated solutes, and influence on tissue water relations. J. Exp. Bot. 43, 641–648. <https://www.jstor.org/stable/23694093>.
- Biosorption of uranium and cadmium using sorbents based on Aloe vera wastes. J. Environ. Chem. Eng.. 2019;7:102985
- [CrossRef] [Google Scholar]
- Removal of Pb2+ and Cd2+ from drinking water using polysaccharide extract isolated from cactus pads (Opuntia ficus indica) J. Appl. Polym. Sci.. 2016;133:43913.
- [CrossRef] [Google Scholar]
- Revolutionary technique for sustainable plant-based green coagulants in industrial wastewater treatment - a review. J. Water Process. Eng.. 2021;42:102096
- [CrossRef] [Google Scholar]
- Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water Air Soil Pollut.. 2020;231:503.
- [CrossRef] [Google Scholar]
- Chromium contamination and effect on environmental health and its remediation: a sustainable approaches. J. Environ. Manage.. 2021;285:112174
- [CrossRef] [Google Scholar]
- Biomass-derived carbon/iron composite (FexOy-BC (RM)) with excellent Cd(II) adsorption from wastewater–Red mud resource utilization. Arab. J. Chem.. 2024;17:105411
- [CrossRef] [Google Scholar]
- Analysis of physicochemical parameters to evaluate the drinking water quality in the State of Perak. Malaysia. J. Chem.. 2015;1:1-10.
- [CrossRef] [Google Scholar]
- Study of carbohydrates present in the cladodes of Opuntia ficus-indica (fodder palm), according to age and season. Ciênc. Tecnol. Aliment.. 2010;30:933-939.
- [CrossRef] [Google Scholar]
- Influence of age on molecular characteristics and rheological behavior of nopal mucilage. Food Sci. Nutr.. 2021;9:6776-6785.
- [CrossRef] [Google Scholar]
- Recreational water quality index (RWQI) for Colina Lake in Chihuahua. Mexico. Acta Univ.. 2016;26:14-22.
- [CrossRef] [Google Scholar]
- Surface and ground water pollution: causes and effects of urbanization and industrialization in South Asia. Sci. Rev.. 2021;7:32-41.
- [CrossRef] [Google Scholar]
- Metal concentrations in recent ash fall of Popocatepetl volcano 2016, Central Mexico: is human health at risk? Ecotoxicol. Environ. Saf.. 2018;162:324-333.
- [CrossRef] [Google Scholar]
- SSA1-2021., 2022. Agua para uso y consumo humano. Límites permisibles de la calidad del agua. México. <https://www.dof.gob.mx/nota_detalle.php?codigo=5650705&fecha=02/05/2022#gsc.tab=0> (Accessed on April 19, 2023).
- Contamination of water supplies by volcanic ashfall: a literature review and simple impact modelling. J. Volcanol. Geotherm. Res.. 2006;158:296-306.
- [CrossRef] [Google Scholar]
- Exploring the HSA/DNA/lung cancer cells binding behavior of p-Synephrine, a naturally occurring phenyl ethanol amine with anti-adipogenic activity: multi spectroscopic, molecular dynamic and cellular approaches. J. Mol. Liq.. 2022;368:120826
- [CrossRef] [Google Scholar]
- Composition and properties of Opuntia ficus-indica mucilage. Phytochemistry.. 1981;20:2665-2668.
- [CrossRef] [Google Scholar]
- Heavy metals in the volcanic and peri-urban terrain Watershed of the River Yautepec. Mexico. Environ. Monit. Assess.. 2019;191:187-201.
- [CrossRef] [Google Scholar]
- Chemical composition of pear cactus mucilage at different maturity stages. Agrociencia.. 2022;56:126-150.
- [Google Scholar]
- Removal of heavy metals present in water from the Yautepec River Morelos México, using Opuntia ficus-indica mucilage. Environ. Adv.. 2022;7:100160
- [CrossRef] [Google Scholar]
- WHO (World Health Organization), 2022. Guidelines for drinking-water quality: fourth edition incorporating the first and second addenda, pp. 237-248. <https://www.who.int/publications/i/item/9789240045064/> (Accessed on June 28, 2023).
- Young, K., Anzalone, A., Pichler, T., Picquart, M., Alcantar, N., 2006. The Mexican cactus as a new environmentally benign material for the removal of contaminants in drinking water. MRS Proceedings. 930, 0930-JJ01-01. <https://doi:10.1557/PROC-0930-JJ01-01>.
- Water quality degradation due to heavy metal contamination: Health impacts and eco-friendly approaches for heavy metal remediation. Toxics.. 2023;11:828.
- [CrossRef] [Google Scholar]
- A preliminary study on cactus as coagulant in water treatment. Process Biochem.. 2006;41:730-733.
- [CrossRef] [Google Scholar]







