Translate this page into:
Effects of baicalein and fangchinoline on abemaciclib metabolism in vivo and in vitro and molecular docking analysis
⁎Corresponding authors. ysxurenai@hotmail.com (Ren-ai Xu), shilu199004@163.com (Lu Shi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
The primary objective of this study was to investigate the effects of baicalein and fangchinoline on the metabolism of abemaciclib. We hypothesized that these two natural compounds could significantly affect the metabolism of abemaciclib by inhibiting the activity of the CYP3A4 enzyme, thus potentially increasing its concentration in the body. In vitro, rat liver microsomes (RLM) and human liver microsomes (HLM) were employed to explore the inhibitory effects and mechanisms of baicalein and fangchinoline on abemaciclib. In vivo, twelve healthy male Sprague-Dawley (SD) rats were randomly assigned to three groups: Group A (control group), Group B (baicalein), and Group C (fangchinoline). The concentrations of abemaciclib and its metabolite N-desethylabemaciclib (M2) were evaluated using ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). Finally, molecular docking method was employed to understand the interaction between abemaciclib and baicalein. It was indicated by the in vitro findings that both baicalein and fangchinoline inhibited abemaciclib metabolism in RLM through a mixed mechanism of competitive and non-competitive inhibition pathway. In HLM, baicalein inhibited abemaciclib metabolism by employing a hybrid mechanism of uncompetitive and non-competitive inhibition, while fangchinoline exhibited its inhibition in a competitive manner. In vivo, pharmacokinetic experiments revealed significant increases for AUC(0-t) and AUC(0-∞) of abemaciclib in Group B and Group C when compared to Group A, while the plasma clearance (CLz/F) of abemaciclib exhibited significant reductions. Moreover, molecular docking studies showed that both abemaciclib and baicalein docked to the active pocket of CYP3A4. This study demonstrated that the co-administration of baicalein or fangchinoline significantly affected the metabolism of abemaciclib, providing valuable insights for its clinical application.
Keywords
Abemaciclib
UPLC-MS/MS
Metabolism
Pharmacokinetics
Molecular docking
1 Introduction
Globally, breast cancer is recognized as the predominant form of malignant neoplasm and constitutes the primary cancer-related mortality factor in females (Harbeck and Gnant, 2017; Kolak et al., 2017; Liang et al., 2020). Previous studies have shown metastatic heterogeneity in breast cancer, with bone, liver, lung and brain being the main organs targeted for breast cancer metastasis (Medeiros and Allan, 2019; Tahara et al., 2019; Liang et al., 2020). In metastatic breast cancer, endocrine therapy and combination therapy, as well as the development of targeted therapies for HER2, have made it increasingly possible to control the long-term disease of metastatic breast cancer (Cardoso et al., 2014; Lee et al., 2017; Swain et al., 2020; Hurvitz et al., 2023).
In recent years, significant advances have been made in the fields of drug properties, mechanisms of action, and drug interactions, particularly in cancer treatment and antimicrobial drug applications. (Ahmad et al., 2023; Farheen et al., 2023; Singh et al., 2023). Abemaciclib, an oral antineoplastic agent and a dual inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), interrupts the growth and reproduction of malignant tumor cells by inhibiting the phosphorylation of retinoblastoma (RB) protein and the activation of the E2F transcription factor, and blocking the cycle transition from G1 phase to S phase (Goel et al., 2017; Praveenkumar et al., 2021; Huang et al., 2023). Abemaciclib was first approved by the US Food and Drug Administration (FDA) in 2017 combined with endocrine therapy (ET) for advanced or metastatic breast cancer patients with positive hormone receptors (HR) and negative human epidermal growth factor receptor 2 (HER2), and as a monotherapy for adult metastatic breast cancer patients with disease progression following endocrine therapy or chemotherapy (Kim, 2017; Royce et al., 2022). Abemaciclib has shown significant clinical efficacy and safety as a monotherapy or in conjunction with non-steroidal aromatase inhibitors (Dickler et al., 2017; Sledge et al., 2017; Johnston et al., 2020). Although the safety of abemaciclib has been confirmed, there are still many adverse reactions, such as diarrhea, fatigue, neutropenia, infection, thrombocytopenia, venous thromboembolism, etc. (Patnaik et al., 2016; Rugo et al., 2022).
Abemaciclib is widely metabolized by CYP3A4 in the human body, and forms three metabolites with similar potency and protein binding compared to abemaciclib, namely N-desethylabemaciclib (M2), hydroxyabemaciclib (M20) and hydroxy-N-desethylabemaciclib (M18) (Martínez-Chávez et al., 2021). Among these, M2 and M20 are the most abundant active metabolites, with their area under the curve (AUC) accounting for 39 % and 77 % of the parent drug, respectively. M18 is further metabolized from M2 and M20 through CYP3A4 (Martínez-Chávez et al., 2021).
In recent years, traditional Chinese medicine has aroused people's interests because of its curative effects on human diseases. For instance, flavonoids are being more and more frequently used as chemotherapeutic agents and dietary chemoprophylaxis agents (Ullah et al., 2020). Baicalein belongs to flavonoids and is one of the active components in the root of Scutellaria baicalensis Georgi (SBG). Moreover, an accumulating amount of in vivo and in vitro experiments have demonstrated that the anti-tumor effects of baicalein (Liu et al., 2016; Morshed et al., 2023). Furthermore, baicalein has a promising potential in inhibiting breast cancer metastasis, with its mechanism possibly associated with the downregulation of SATB1 and the Wnt/β-catenin pathway (Ma et al., 2016). Besides, fangchinoline is another promising drug for metastasis treatment of breast cancer. It is extracted from the roots of S. tetrandra, which is classified as a bisbenzylisoquinoline alkaloid (Wang et al., 2020). It exhibits inhibitory effects on the proliferation of breast cancer cells MDA-MB-231 and MCF-7 by inducing of apoptosis, arrest of G1 cycle and inhibition of migration (Xing et al., 2013). Radix Stephaniae Tetrandrine (RST), a compound characterized by a structural and pharmacological profile similar to that of fangchinolin, derived from the dried root of Stephania tetrandra S.Moore, has been successfully applied in clinical trials of traditional Chinese medicine and in preclinical trials of anti-breast cancer, to show that the active ingredient of RST has significant therapeutic potential against breast cancer (Guo et al., 2020).
Nowadays, the application of Nuclear Magnetic Resonance Spectroscopy (NMR) in metabolomics has made significant progress, particularly in metabolite identification and reaction mechanism studies. Improvements in sensitivity and resolution provide powerful support for in-depth analysis of drug metabolism and drug interactions (Emwas et al., 2019; Kijewska et al., 2021).
As we know, there have been no prior studies investigating the interaction between abemaciclib and baicalein or fangchinoline. In this study, we characterized the influences of baicalein and fangchinoline on the metabolism of abemaciclib in both rat liver microsomes (RLM) and human liver microsomes (HLM). Furthermore, we investigated the pharmacokinetic changes of abemaciclib in rats following the administration of abemaciclib combined with baicalein or fangchinoline by quantifying abemaciclib levels in rat plasma using ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). In addition, we performed molecular docking studies, which revealed the binding interactions between abemaciclib, baicalein, fangchinoline, and CYP3A4, providing insights into their inhibitory mechanisms. This study has significance in evaluating the clinical safety of abemaciclib.
2 Materials and methods
2.1 Chemical and reagents
Abemaciclib, its metabolite N-desethylabemaciclib (M2), alpelisib (internal standard, IS), baicalein and fangchinoline were supplied by Shanghai Canspec Scientific Instruments Co., Ltd. (Shanghai, China). The HLM was from iPhase Pharmaceutical Services Co., Ltd. (Jiangsu, China). Nicotinamide Adenine Dinucleotide Phosphate (NADPH) was offered by Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Additionally, all remaining chemical reagents and solvents employed in the experiment were of analytical grade. The chemical structures of abemaciclib, baicalein and fangchinoline are shown in Fig. 1.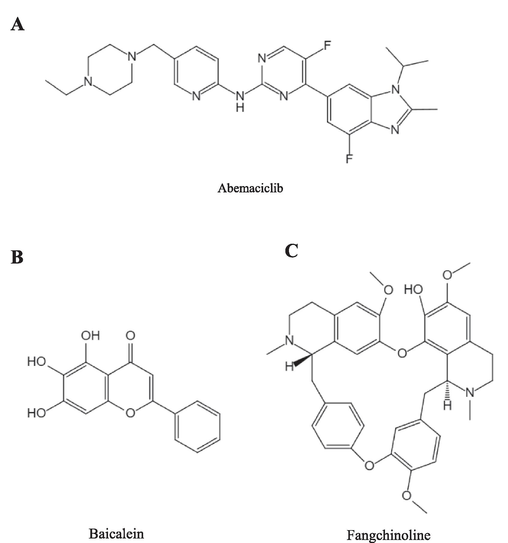
Chemical structures of abemaciclib (A), baicalein (B) and fangchinoline (C).
2.2 Conditions of ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS)
UPLC-MS/MS was employed to measure the concentrations of abemaciclib and M2, which was equipped with a Waters Acquity UPLC BEH C18 column (2.1 mm × 50 mm, 1.7-μm particle size; Waters Corp., Millipore, Bedford, MA, USA). The autosampler rack and column temperatures were set at 10 °C and 40 °C, respectively. The mobile phase was comprised 0.1 % formic acid (solution A) and acetonitrile (solution B), employing a gradient elution with the rate of 0.40 mL/min. The procedure of gradient elution included the following stages: 0–0.5 min (10 % B), 0.5–1.0 min (10–90 % B), 1.0–1.4 min (90 % B), 1.4–1.5 min (90–10 % B), and 1.5–2.0 min (10 % B). Quantitation was carried out using the Waters XEVO TQS Triple Quadrupole Mass Spectrometer, equipped with an electrospray ionization source (ESI) which was in positive mode. The ion transitions used were m/z 507.09 → 393.04 for abemaciclib, m/z 479.05 → 393.00 for M2 and m/z 442.02 → 327.97 for alpelisib, respectively.
2.3 Method for obtaining RLM
Male SD rats were fasted overnight and then killed by cervical dislocation. Each liver was removed, washed with cold saline solution (0.9 % NaCl by weight/volume), weighed, and homogenized in an ice-cold 0.01 mM cold PBS buffer containing 0.25 mM sucrose solution. After homogenization, the mixture was centrifuged at 11,000 rpm at 4 °C for 15 min. After that, the precipitation was discarded, and the supernatant was transferred to a fresh tube and centrifuged once again at 11,000 rpm at 4 °C for 15 min. The clear liquid above was centrifuged again at 24,860 rpm at 10 °C for 120 min. The solid part that settled was mixed back into an ice-cold 0.01 mM cold PBS buffer containing 0.25 mM sucrose solution and kept frozen at −80 °C until it was needed. The protein content of RLM was measured using the Bradford Protein Assay Kit (Thermo Scientific, Waltham, MA, USA) (Kielkopf et al., 2020).
2.4 The inhibitory effects of baicalein and fangchinoline on abemaciclib in RLM and HLM
RLM and HLM were used to examine the inhibitory effects of baicalein and fangchinoline on abemaciclib in vitro. The formation of M2 was served as an indicative marker of the inhibitory effects. The 200 μL incubation system was comprised of potassium phosphate buffer (PBS, 100 mM, pH = 7.4), NADPH (1 mM), 0.3 mg/mL of RLM or HLM, abemaciclib, and inhibitor (baicalein or fangchinoline). At first, various concentrations of abemaciclib (0.1, 1, 2, 5, 10, 20, and 50 μM for RLM; 0.1, 1, 2, 5, 10, 15, and 20 μM for HLM) were added into the reaction buffer to establish the Michaelis constant (Km) values. Subsequently, different concentrations of baicalein or fangchinoline, ranging from 0, 0.01, 0.1, 1, 10, 25, 50 to 100 μM, were employed to determine the corresponding half-maximal inhibitory concentration (IC50) values. At last, to uncover the inhibitory mechanisms of baicalein and fangchinoline on abemaciclib, a variety concentration of baicalein were generated, guided by the IC50 values (RLM: 0, 3.99, 7.98 and 15.95 μM; HLM: 0, 4.60, 18.38, and 36.76 μM). The concentrations of fangchinoline were also prepared based on IC50 values (RLM: 0, 0.50, 1.01, and 2.02 μM; HLM: 0, 3.06, 6.12, and 12.24 μM). Meanwhile, a set of abemaciclib concentrations were established based on the Km values (RLM: 0.32, 0.64, 1.28, and 2.56 μM; HLM: 0.90, 1.80, 3.60, and 7.20 μM). The reaction was initiated by adding 10 μL of NADPH after pre-incubation at 37 °C for 5 min. After incubating for 30 min at 37 °C and terminating at −80 °C, 20 μL of IS working solution (500 ng/mL) was added, as well as 400 μL of acetonitrile. The samples were spun at 13,000 rpm for 10 min to obtain 100 μL of the supernatant for UPLC-MS/MS analysis.
2.5 Pharmacokinetic interactions of baicalein and fangchinoline with abemaciclib in rats
Sprague–Dawley (SD) male rats (200 ± 20 g) were sourced from the Animal Experimental Center of the First Affiliated Hospital of Wenzhou Medical University (Zhejiang, China). The day before the experiment, the SD rats underwent a 12 h fasting period, during which water was unrestricted but food was restricted. After the fasting period, 12 SD rats were randomly distributed into three groups (n = 4), group A (control group) and group B, C (experimental group). Groups B and C were received an oral administration of baicalein (50 mg/kg), and fangchinoline (50 mg/kg), respectively (Lai et al., 2003; Shi et al., 2018; Wu et al., 2019), and Group A was given an equal volume of solvent. After 30 min, all rats were received an oral administration of 30 mg/kg of abemaciclib. Blood samples (300 μL each) were collected from the tail vein at 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 36, and 48 h after administration of abemaciclib, and were promptly centrifugated at 8,000 rpm for 8 min. For sample preparation, 10 μL of the IS working solution (500 ng/mL) was added to 100 μL of collected plasma. After that, 300 μL of acetonitrile was added, and the mixture was thoroughly vortexed before centrifugation at 13,000 rpm for 10 min. Then, we collected 100 μL of the supernatant to quantify the concentration of abemaciclib by UPLC-MS/MS.
2.6 Molecular docking simulations
Molecular docking simulations were conducted with resources from the Protein Data Bank (PDB, https://www.rcsb.org/pdb). X-ray crystal structures of human CYP3A4 (PDB ID: 5ET8) were accessed, and 3D sdf format files of baicalein and abemaciclib were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/). Initially, the 3D protein conformation was optimized using PyMOL, which involved the removal of the protein crystal water and the original ligand, and hydrogen atoms were added using Auto Dock Tools 1.5.6. A grid box measuring 40 Å × 40 Å × 40 Å with a grid spacing of 0.375 Å was set. The molecular docking process was conducted using the AutoDock 4.2.6 program, employing the Lamarckian Genetic Algorithm (LGA) for ligand tethering of targeted protein, producing a maximum output of 50 gestures (Shivanika et al., 2022). Subsequently, further visualization of the best scoring conformation was performed using PyMOL.
2.7 Statistical analysis
The kinetic parameters of abemaciclib in both RLM and HLM, including Km and IC50, were analyzed. Lineweaver-Burk graphs and mean plasma concentration–time curves were produced by GraphPad Prism 9.0. Pharmacokinetic parameters, including AUC(0-t), AUC(0-∞), time to peak plasma concentration (Tmax), maximal plasma concentration (Cmax), plasma clearance (CLz/F), and elimination half-life (t1/2) were calculated with Drug and Statistics (DAS) 3.0 software. Statistical analysis was performed using SPSS 23.0 software, employing a one-way analysis of variance (ANOVA) with Dunnett's test to assess differences in dynamic parameters among the three rat groups. All parameters from experiments were presented as mean ± standard deviation (SD). P < 0.05 was considered to have statistical significance.
3 Results
3.1 Evaluation of the influences of baicalein and fangchinoline on abemaciclib metabolism in vitro
The Michaelis-Menten curves and Km values of abemaciclib in RLM and HLM are depicted in Fig. 2 and Table 1. Ultimately, the Km values of abemaciclib were determined to be 1.28 μM for RLM and 3.60 μM for HLM, respectively. Fig. 3 and Table 1 illustrate that baicalein had inhibitory effect on abemaciclib metabolism, exhibiting IC50 values of 15.95 μM in RLM and 18.38 μM in HLM, respectively. As shown in Table 2, fangchinoline also displayed inhibition on abemaciclib metabolism, showing IC50 values of 2.02 μM in RLM and 12.24 μM in HLM, respectively. These results suggested that baicalein exhibited a weak inhibition on abemaciclib metabolism in both RLM and HLM. Besides, fangchinoline demonstrated a weak inhibition of abemaciclib in HLM, and a moderate inhibition in RLM, respectively. For a more comprehensive understanding of how baicalein and fangchinoline inhibited abemaciclib, Figs. 4 and 5 present the Lineweaver-Burk plots. In RLM, both baicalein and fangchinoline inhibited abemaciclib metabolism through a mixed mechanism of competitive and non-competitive inhibition, exhibiting Ki values of 10.22 and 1.84, and α values of 2.73 and 3.90, respectively. In addition, in HLM, baicalein inhibited the metabolism of abemaciclib through a mixed way of uncompetitive and non-competitive mechanism with Ki and α values of 27.24 and 0.18, while fangchinoline exhibited its inhibition in a competitive way with Ki value of 6.52.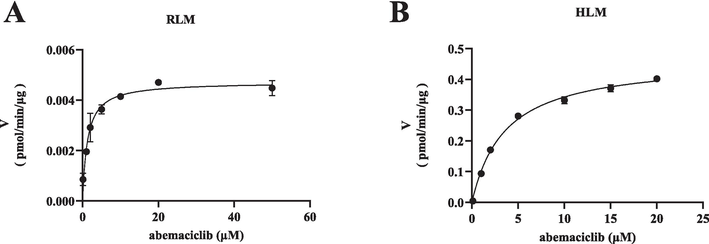
Michaelis-Menten plots of abemaciclib in RLM (A) and in HLM (B). Data are expressed as mean ± SD, n = 3.
IC50 values (μM)
Km (μM)
Inhibition type
Ki (μM)
αKi (μM)
α
RLM
15.95
1.28
Mixed inhibition
10.22
27.88
2.73
HLM
18.38
3.60
Mixed inhibition
27.24
4.79
0.18
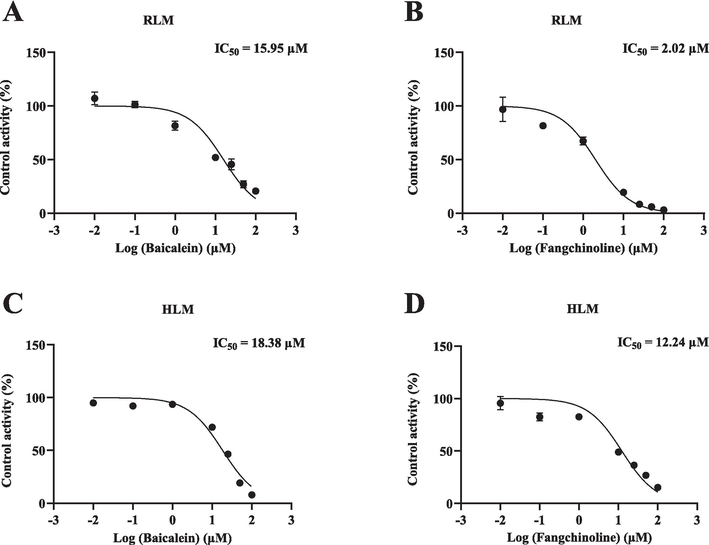
IC50 plots of the effects of baicalein and fangchinoline on abemaciclib in RLM (A, B) and HLM (C, D). Data are expressed as mean ± SD, n = 3.
IC50 values (μM)
Km (μM)
Inhibition type
Ki (μM)
αKi (μM)
α
RLM
2.02
1.28
Mixed inhibition
1.84
7.17
3.90
HLM
12.24
3.60
Competitive inhibition
6.52
/
/
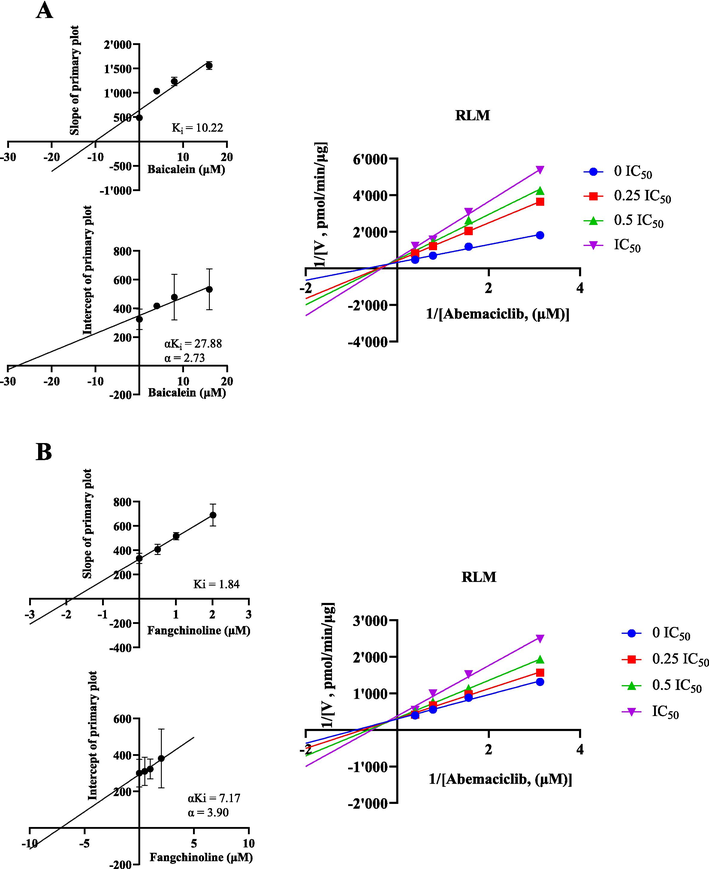
In RLM, Lineweaver-Burk plots, secondary diagrams of Ki and secondary diagrams of αKi inhibiting abemaciclib metabolism at different concentrations of baicalein (A) and fangchinoline (B). Data are expressed as mean ± SD, n = 3.
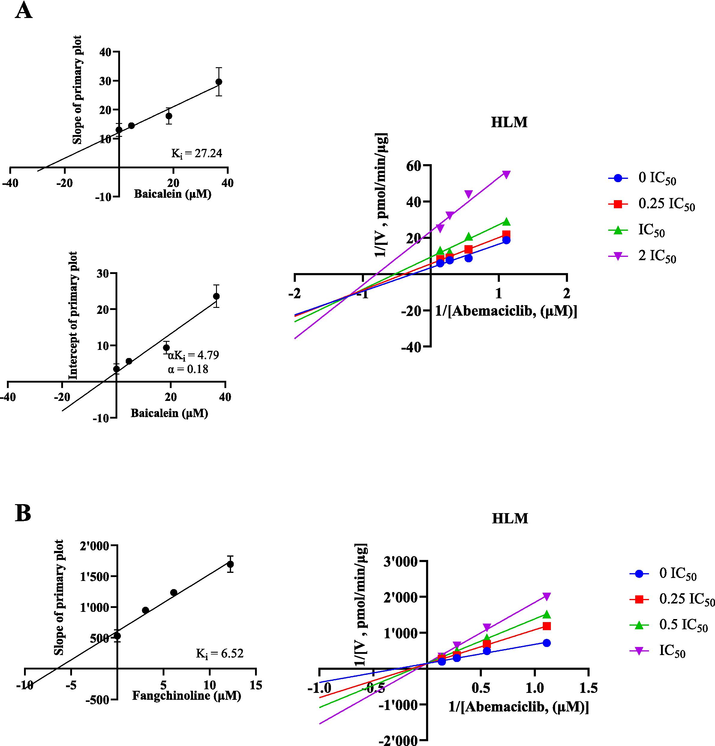
In HLM, Lineweaver-Burk plots, secondary diagrams of Ki and secondary diagram of αKi inhibiting abemaciclib metabolism at different concentrations of baicalein (A) and fangchinoline (B). Data are expressed as mean ± SD, n = 3.
3.2 Evaluation of the influences of baicalein and fangchinoline on abemaciclib pharmacokinetics in vivo
The pharmacokinetic parameters of abemaciclib were determined in Group A (30 mg/kg abemaciclib alone), Group B (50 mg/kg baicalein + 30 mg/kg abemaciclib), and Group C (50 mg/kg fangchinoline + 30 mg/kg abemaciclib). The results of the statistical tests are displayed in Table 3. Fig. 6 illustrates the mean plasma concentration–time curves of abemaciclib in different groups. Following pretreatment with a single dose of baicalein, the AUC(0-∞) and AUC(0-t) of abemaciclib were 1.99-fold and 1.84-fold of the control group accompanied by a 48.90 % reduction in CLz/F. Moreover, fangchinoline significantly elevated the AUC(0-∞) of abemaciclib to 1.54-fold, with the decreasing CLz/F of 37.63 %. However, the parameters of Tmax, Cmax and t1/2 in both Groups B and C did not show significant differences. These findings suggested that both baicalein and fangchinoline could inhibit the metabolism of abemaciclib in rats. Compared with Group A, *P < 0.05.
Parameters
Group A
Group B
Group C
AUC(0-t) (ng/mL*h)
3366.66 ± 659.02
6192.14 ± 927.26*
5186.52 ± 1220.69*
AUC(0-∞) (ng/mL*h)
3529.86 ± 606.72
7030.12 ± 1635.16*
6154.86 ± 2428.65
t1/2z (h)
9.81 ± 6.45
15.33 ± 7.10
19.57 ± 15.59
Tmax (h)
10.50 ± 9.00
21.00 ± 17.32
24.00 ± 0.00
CLz/F (L/h/kg)
8.69 ± 1.49
4.44 ± 1.00*
5.42 ± 1.88*
Cmax (ng/mL)
164.88 ± 40.33
201.11 ± 14.26
224.48 ± 82.59
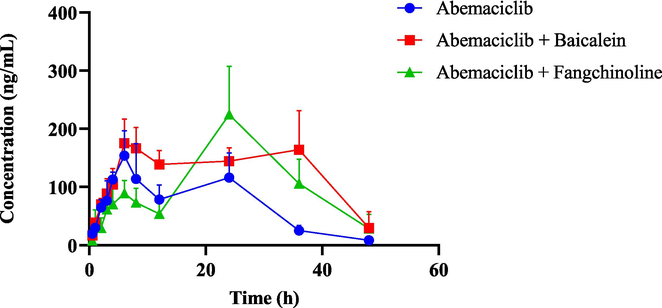
The average plasma concentration–time curves of abemaciclib in the single group (abemaciclib alone) and the combined groups (abemaciclib with baicalein or fangchinoline). Data are expressed as mean ± SD, n = 4.
3.3 Molecular docking studies of abemaciclib and baicalein on CYP3A4
To further understand the interactions between abemaciclib and baicalein, both compounds were co-docked into the active catalytic cavity of the target enzyme CYP3A4. As illustrated in Fig. 7, it was observed that abemaciclib established a hydrogen bond interaction with HEM-601 (3.2 Å) of CYP3A4, presenting a binding energy of −9.34 kcal/mol. Additionally, with the binding energy of −5.8 kcal/mol, baicalein interacted with the active amino acid residues ALA-305 and SER-119 of enzyme through conventional hydrogen bonds at distances of approximately 2.2Å and 2.7 Å, respectively. Importantly, baicalein can also undergo hydrogen bonds of 2.5 Å and 3.5 Å linking with HEM-601. These results indicated a significant overlap in the binding sites of abemaciclib and baicalein with CYP3A4, with HEM-601 being a shared binding site. Therefore, when abemaciclib and baicalein are administered together, they may compete for the identical binding site and spatial position. However, the binding sites are not exactly coincident, which is essentially consistent with the mixed inhibition pattern of baicalein against abemaciclib.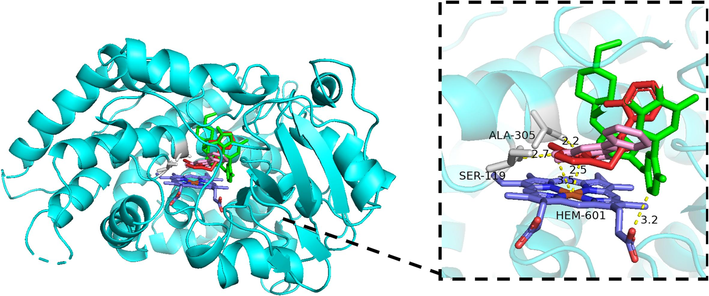
Stereo views of molecular docking results of CYP3A4 with abemaciclib and baicalein. The structures of abemaciclib, baicalein and the reference ligand are shown in green, pink, and red respectively, and the amino acid residues interacting with them are shown in gray. Hydrogen bonds are indicated by yellow dash lines.
4 Discussion
The global impact of breast cancer is rapidly increasing, with notable differences among countries. Specifically, China has witnessed a sharp rise in both the incidence and mortality rates of breast cancer (Lei et al., 2021; Xia et al., 2022).
In 2017, the FDA granted its first approval for abemaciclib, to be employed alongside ET for patients diagnosed with metastatic or advanced breast cancer who are HR+ and HER2− (Kim 2017). Additionally, it was approved as a monotherapy treatment in adult patients with metastatic breast cancer who had experienced disease progression after endocrine therapy or chemotherapy (Royce et al., 2022). Abemaciclib undergoes extensive metabolism in the human body primarily by CYP3A4 (Martínez-Chávez et al., 2021).
In recent years, traditional Chinese medicine has been a hot spot due to its efficacy in treating various human diseases. Baicalein is classified as a flavonoid. It is possibly applied for treatment of metastatic breast cancer because of the downregulation of SATB1 and the Wnt/β-catenin pathway (Ma et al., 2016). Fangchinoline belongs to bisbenzylisoquinoline alkaloids (Wang et al., 2020). It demonstrates an inhibitory effect on the proliferation of breast cancer cells MDA-MB-231 and MCF-7 by inducing apoptosis, arresting the G1 cell cycle, and inhibiting migration (Xing et al., 2013).
The research findings indicated a growing trend in the utilization of herbal remedies by patients undergoing cancer treatment. It aims to reduce the side effects of chemotherapy and improve their overall health (Yin et al., 2017). Therefore, it is crucial to evaluate potential herb-drug interactions.
To explore the impacts of baicalein and fangchinoline on the in vitro metabolism of abemaciclib, enzyme kinetic studies were performed using both RLM and HLM. Our findings indicated that baicalein demonstrated a weak inhibition of abemaciclib in both RLM and HLM. Fangchinoline functioned as a weak inhibitor in HLM, and it moderately inhibited the metabolism of abemaciclib in RLM. Fangchinoline appeared to have a stronger inhibitory effect in RLM than in HLM. Significant biological and physiological differences exist between rats and humans, including variations in the expression and activity levels of liver metabolic enzymes. These differences may contribute to the different responses in drug metabolism between the two species. Further investigation confirmed the inhibitory type of baicalein and fangchinoline on abemaciclib. The Lineweaver-Burk plots indicated that the set of straight lines met in the second quadrant, suggesting that both baicalein and fangchinoline inhibited abemaciclib metabolism through a mixed way of competitive and non-competitive inhibition in RLM. Additionally, in HLM, baicalein and fangchinoline inhibited abemaciclib metabolism through mixed inhibition and competitive inhibition, respectively. These results implied that baicalein and fangchinoline may exhibit mixed inhibition through multiple pathways.
Abemaciclib exhibited significant metabolic differences across species, particularly in the generation of its metabolites. For example, the CYP enzymes in humans and rats demonstrated different metabolic pathways and the formation of isomers when producing abemaciclib metabolites (Thakkar and Kate, 2020; Martínez-Chávez et al., 2021). Consequently, baicalein and fangchinoline showed varying inhibition types in RLM and HLM, most likely due to structural differences in the CYP enzymes across species. These differences led to distinct binding modes of the inhibitors at the active sites and thus affected their inhibition mechanisms in RLM and HLM.
To comprehend the potential influences of baicalein and fangchinoline on the in vivo pharmacokinetics of abemaciclib, SD rats were divided into control and co-treatment groups. In the presence of baicalein, there were significant changes in the AUC(0-∞), AUC(0-t) and CLz/F of abemaciclib compared to those of the control group. After pretreatment with fangchinoline, significant changes were observed in AUC(0-t) and CLz/F of abemaciclib compared to the control group. However, there were no significant differences in the Tmax, Cmax and t1/2 in both Groups B and C. The in vivo findings were consistent with the in vitro study, suggesting that both baicalein and fangchinoline had inhibitory effects on abemaciclib metabolism.
Based on in vivo results, baicalein had relatively a stronger inhibitory effect than fangchinoline. Moreover, a molecular docking analysis was performed to model the binding and possible docking poses of abemaciclib and baicalein to the active cavity of the human CYP3A4 enzyme. The molecular docking results revealed the binding mechanism of baicalein and abemaciclib with CYP3A4. The rigid benzene ring and hydroxyl groups of baicalein are key structural features that enhance its binding stability. These hydroxyl groups (5-, 6-, and 7-hydroxyl groups) form stable hydrogen bonds with key residues SER-119 and ALA-305 in the CYP3A4 active cavity at 2.2 Å and 2.7 Å, respectively, as well as two hydrogen bonds at the HEM-601 site at 2.5 Å and 3.5 Å. This hydroxyl group distribution and its precise docking within the active cavity significantly strengthen the enzyme-ligand interaction while competitively preventing the binding of other substrate molecules. In comparison, abemaciclib primarily binds to CYP3A4 through a hydrogen bond at the HEM-601 site with a bond length of 3.2 Å. Since both baicalein and abemaciclib bind to the HEM-601 site, a competitive mechanism may exist between the two, which leads to baicalein inhibiting the catalytic activity of CYP3A4 on abemaciclib. Thus, it was concluded that baicalein may increase the plasma exposure of abemaciclib by inhibiting the enzymatic activity of CYP3A4.
In a study involving the screening of 50 individual herbal preparations using HLM to assess CYP3A4 activity for in vitro probe reactions, Huang Qin (Scutellaria baicalensis Georgi) had been demonstrated to exhibit significant inhibitory effects on the metabolism of CYP3A4 (Pao et al., 2012). Additionally, it had been demonstrated that baicalein significantly increased the bioavailability of nimodipine in rats, possibly due to its inhibition of CYP3A4 (Cho et al., 2011). Moreover, baicalein increased the oral bioavailability of tamoxifen, mainly due to its inhibitory effect on CYP3A4-mediated metabolism of tamoxifen, occurring either in the small intestine or in the liver (Li et al., 2011). Consequently, the suppressive impact of baicalein on the metabolism of abemaciclib could be ascribed to its inhibition of CYP3A4. However, there are no prior reports of fangchinoline interactions with other herbs or drugs.
Abemaciclib inhibited tumor cell proliferation by targeting CDK4/6, but over time, tumor cells may develop resistance. Common resistance mechanisms included alterations in the RB1 gene, overexpression of Cyclin E, and activation of the PI3K/AKT/mTOR pathway (Pandey et al., 2019; Huang et al., 2022; Zhu and Zhu, 2023). Co-administration of baicalein and fangchinoline could inhibit CYP3A4 enzyme activity, then slowing the metabolism of abemaciclib, and helping to decrease the risk of resistance associated with prolonged high-dose exposure. This could delay resistance mechanisms such as RB1 gene loss or Cyclin E overexpression (Pandey et al., 2019; Huang et al., 2022; Zhu and Zhu, 2023). Additionally, abemaciclib therapy often caused untoward effects such as diarrhea, fatigue, and hematological toxicity (Rugo et al., 2022; Tong et al., 2024). By reducing the necessary dose through metabolic inhibition, baicalein and fangchinoline may help to mitigate these adverse effects and thereby improve patient tolerance.
So far, no previous studies have investigated the interaction between abemaciclib and baicalein or fangchinoline. Our results suggested that if baicalein or fangchinoline is taken together with abemaciclib, it might increase abemaciclib levels in the body, and adjusting abemaciclib dosage may be necessary.
5 Conclusion
Our findings in this study indicated that both baicalein and fangchinoline not only significantly inhibited abemaciclib metabolism in vitro, but also demonstrated inhibitory effects in rats. Besides, molecular docking studies simulated the interaction of two small molecules with CYP3A4. Future research may involve investigating potential interactions between other flavonoids or alkaloid compounds from traditional Chinese medicine and abemaciclib. Considering abemaciclib is processed differently in various species, further studies in cell-based models or disease-specific rat models will be essential to deepen our understanding of how baicalein and fangchinoline impact on abemaciclib metabolism. These findings could then inform subsequent human studies, providing a foundation for medical applications.
CRediT authorship contribution statement
Xiaohai Chen: Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. Fengsheng Hong: Writing – original draft, Visualization, Methodology, Investigation. Hualu Wu: Writing – review & editing, Conceptualization. Yuxin Shen: Writing – review & editing. Hailun Xia: Methodology, Investigation, Formal analysis. Ren-ai Xu: Writing – review & editing, Validation, Supervision, Software, Resources, Project administration, Conceptualization. Lu Shi: Writing – review & editing, Validation, Supervision, Software, Resources, Project administration.
Acknowledgement
This work was supported by Wenzhou City Science and Technology Bureau Project(Y20220193).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Natural oils enhance the topical delivery of ketoconazole by nanoemulgel for fungal infections. ACS Omega. 2023;8:28233-28248.
- [CrossRef] [Google Scholar]
- ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Breast. 2014;23:489-502.
- [CrossRef] [Google Scholar]
- Effects of the antioxidant baicalein on the pharmacokinetics of nimodipine in rats: a possible role of P-glycoprotein and CYP3A4 inhibition by baicalein. Pharmacol. Rep.. 2011;63:1066-1073.
- [CrossRef] [Google Scholar]
- MONARCH 1, a phase II study of Abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin. Cancer Res.. 2017;23:5218-5224.
- [CrossRef] [Google Scholar]
- Surface-modified biobased polymeric nanoparticles for dual delivery of doxorubicin and gefitinib in glioma cell lines. ACS Omega. 2023;8:28165-28184.
- [CrossRef] [Google Scholar]
- CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471-475.
- [CrossRef] [Google Scholar]
- Radix stephaniae tetrandrine: an emerging role for management of breast cancer. Curr. Pharm. Des.. 2020;26:25-36.
- [CrossRef] [Google Scholar]
- SETDB1 modulates degradation of phosphorylated RB and anticancer efficacy of CDK4/6 inhibitors. Cancer Res.. 2023;83:875-889.
- [CrossRef] [Google Scholar]
- CDK4/6 inhibitor resistance mechanisms and treatment strategies (Review) Int. J. Mol. Med.. 2022;50
- [CrossRef] [Google Scholar]
- Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401:105-117.
- [CrossRef] [Google Scholar]
- Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE) J. Clin. Oncol.. 2020;38:3987-3998.
- [CrossRef] [Google Scholar]
- Bradford assay for determining protein concentration. Cold Spring Harb. Protoc.. 2020;2020:102269
- [CrossRef] [Google Scholar]
- Lossen rearrangement of p-toluenesulfonates of N-oxyimides in basic condition, theoretical study, and molecular docking. Front. Chem.. 2021;9:662533
- [CrossRef] [Google Scholar]
- Primary and secondary prevention of breast cancer. Ann. Agric. Environ. Med.. 2017;24:549-553.
- [CrossRef] [Google Scholar]
- Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J. Pharm. Pharmacol.. 2003;55:205-209.
- [CrossRef] [Google Scholar]
- Fulvestrant for hormone-sensitive metastatic breast cancer. Cochrane Database Syst. Rev.. 2017;1:Cd011093
- [CrossRef] [Google Scholar]
- Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. (Lond.). 2021;41:1183-1194.
- [CrossRef] [Google Scholar]
- Effects of baicalein on the pharmacokinetics of tamoxifen and its main metabolite, 4-hydroxytamoxifen, in rats: possible role of cytochrome P450 3A4 and P-glycoprotein inhibition by baicalein. Arch. Pharm. Res.. 2011;34:1965-1972.
- [CrossRef] [Google Scholar]
- Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol.. 2020;60:14-27.
- [CrossRef] [Google Scholar]
- The fascinating effects of baicalein on cancer: a review. Int. J. Mol. Sci.. 2016;17
- [CrossRef] [Google Scholar]
- Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. Drug Des. Devel. Ther.. 2016;10:1419-1441.
- [CrossRef] [Google Scholar]
- Simultaneous quantification of abemaciclib and its active metabolites in human and mouse plasma by UHPLC-MS/MS. J. Pharm. Biomed. Anal.. 2021;203:114225
- [CrossRef] [Google Scholar]
- Molecular mechanisms of breast cancer metastasis to the lung: clinical and experimental perspectives. Int. J. Mol. Sci.. 2019;20
- [CrossRef] [Google Scholar]
- Baicalein as promising anticancer agent: a comprehensive analysis on molecular mechanisms and therapeutic perspectives. Cancers (Basel). 2023;15
- [CrossRef] [Google Scholar]
- Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: a review. Int. J. Cancer. 2019;145:1179-1188.
- [CrossRef] [Google Scholar]
- Herb-drug interaction of 50 Chinese herbal medicines on CYP3A4 activity in vitro and in vivo. Am. J. Chin. Med.. 2012;40:57-73.
- [CrossRef] [Google Scholar]
- Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov.. 2016;6:740-753.
- [CrossRef] [Google Scholar]
- Selective CDK4/6 inhibition of novel 1,2,3-triazole tethered acridinedione derivatives induces G1/S cell cycle transition arrest via Rb phosphorylation blockade in breast cancer models. Bioorg. Chem.. 2021;116:105377
- [CrossRef] [Google Scholar]
- FDA approval summary: abemaciclib with endocrine therapy for high-risk early breast cancer. J. Clin. Oncol.. 2022;40:1155-1162.
- [CrossRef] [Google Scholar]
- Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: safety and patient-reported outcomes from the monarchE study. Ann. Oncol.. 2022;33:616-627.
- [CrossRef] [Google Scholar]
- Baicalein attenuates monocrotaline-induced pulmonary arterial hypertension by inhibiting vascular remodeling in rats. Pulm. Pharmacol. Ther.. 2018;48:124-135.
- [CrossRef] [Google Scholar]
- Molecular docking, validation, dynamics simulations, and pharmacokinetic prediction of natural compounds against the SARS-CoV-2 main-protease. J. Biomol. Struct. Dyn.. 2022;40:585-611.
- [CrossRef] [Google Scholar]
- Challenges and opportunities in the crusade of BRAF inhibitors: from 2002 to 2022. ACS Omega. 2023;8:27819-27844.
- [CrossRef] [Google Scholar]
- MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol.. 2017;35:2875-2884.
- [CrossRef] [Google Scholar]
- Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol.. 2020;21:519-530.
- [CrossRef] [Google Scholar]
- Bone metastasis of breast cancer. Adv. Exp. Med. Biol.. 2019;1152:105-129.
- [CrossRef] [Google Scholar]
- Update on metabolism of abemaciclib: in silico, in vitro, and in vivo metabolite identification and characterization using high resolution mass spectrometry. Drug Test. Anal.. 2020;12:331-342.
- [CrossRef] [Google Scholar]
- Comparative efficacy and safety of CDK4/6 inhibitors combined with endocrine therapies for HR+/HER2-breast cancer: systematic review and network meta-analysis. Heliyon.. 2024;10:e31583
- [CrossRef] [Google Scholar]
- Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25
- [CrossRef] [Google Scholar]
- Bioactive bisbenzylisoquinoline alkaloids from the roots of Stephania tetrandra. Bioorg. Chem.. 2020;98:103697
- [CrossRef] [Google Scholar]
- Fangchinoline ameliorates diabetic retinopathy by inhibiting receptor for advanced glycation end-products (RAGE)-nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway in streptozotocin (STZ)-induced diabetic rats. Med. Sci. Monit.. 2019;25:1113-1121.
- [CrossRef] [Google Scholar]
- Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J. (Engl.). 2022;135:584-590.
- [CrossRef] [Google Scholar]
- Fangchinoline induces G1 arrest in breast cancer cells through cell-cycle regulation. Phytother Res.. 2013;27:1790-1794.
- [CrossRef] [Google Scholar]
- Phytochemicals approach for developing cancer immunotherapeutics. Front. Pharmacol.. 2017;8:386
- [CrossRef] [Google Scholar]
- Differences in metabolic transport and resistance mechanisms of Abemaciclib, Palbociclib, and Ribociclib. Front. Pharmacol.. 2023;14:1212986
- [CrossRef] [Google Scholar]







