Translate this page into:
Efficient biogas production from food waste utilizing Ag@ZnONPs catalyst and inoculum immobilization within anaerobic digestion reactors
* Corresponding author: E-mail address: aalalawy@ut.edu.sa (Adel I. Alalawy)
-
Received: ,
Accepted: ,
Abstract
Food waste presents a major opportunity for bioenergy production. Anaerobic digestion (AD) is effective but limited by low biogas yields. This study enhances biogas and biomethane generation by immobilizing anaerobic sludge (AS) within a polyvinyl alcohol/chitosan (PVA/Ch) cryogel matrix and incorporating silver doped zinc oxide nanoparticles (Ag@ZnONPs) as a catalyst. The highest biogas and biomethane production were achieved in R4-(FW+cryogel+NPs) at 534 mL/gVS and 252 mL/gVS, respectively, showing a 56.6% increase in biogas yield compared to R1-(FW). The methane content improved from 40.64% (R1) to 60.15% (R4), while substrate degradation efficiency also increased, with total solids (TS) reduction reaching 66.34% and volatile solids (VS) reduction at 74.8% in R4. The synergistic effect of cryogel and nanoparticles (NPs) enhanced microbial retention, improved substrate biodegradability, and stabilized the digestion process. These findings demonstrate the potential of advanced AD techniques for efficient waste-to-energy conversion and sustainable waste management.
Keywords
Anaerobic digestion
Biogas production
Food waste
Hydrolysis
Inoculum immobilization
ZnO/Ag NPs
1. Introduction
Many nations are implementing the strategy of recycling organic waste as an alternative energy source in response to severe environmental challenges like climate change and energy scarcity [1]. The world is prioritizing meeting the growing demand for food and energy to support a projected population of over 10 billion by 2050 while reducing environmental harm. This includes reducing reliance on conventional energy sources, fossil fuel emissions, and solid waste. The daily 23.7 million tons of agricultural produce stresses ecosystems, affecting public health and ecosystem resilience [2]. The two main organic waste sources that need proper processing are food waste (FW) and sewage sludge (SS). In China, food waste accounts for approximately 30% to 60% of the entire municipal solid waste (MSW). Meanwhile, in Japan, Europe, and the United States, the proportion of food waste within the total MSW stands at around 23%, 15-25%, and 12%, respectively [3]. Global waste management is increasingly challenged by economic activities and population growth, necessitating innovative solutions. Current methods include landfill disposal and incineration, but effectiveness is being evaluated. Biological processes like anaerobic digestion, aerobic composting, bioethanol fermentation, and feed fermentation are being explored [4,5].
Due to its effectiveness in degrading complex organic elements, Anaerobic digestion (AD) appears as a particularly suited technology for the treatment of FW. Additionally, the biogas produced by AD may be easily separated and put into a variety of applications [6]. The largest potential for FW recycling is one of the benefits that AD provides, along with a simplified process flow, increased reactor efficiency, less pollutant load, and significant economic benefits. For AD applications, FW is regarded as a high-quality feedstock [5,7]. FW organic material undergoes AD conversion, primarily methane, which can be utilized as energy, but lag periods and low methane generation rates limit its use [8]. AD is a multistep biochemical process involving acidogenesis, acetogenesis, and methanogenesis, which hydrolyzes complex organic materials. The initial acidification phase converts organic waste into volatile fatty acids (VFAs), methane, or a mixture of both. The methanogenesis phase converts hydrogen and VFAs into methane, while the first phase involves acidogenic and acetogenic bacteria [9].
AD is an effective technology for food waste treatment and biogas production. However, challenges such as slow hydrolysis, low methane yield, and process instability hinder its efficiency. Various strategies, including bioaugmentation, enzymatic hydrolysis, co-digestion, and thermal pretreatment have been explored to enhance AD performance. Several techniques, including mechanical, thermal, chemical, and biological pretreatment methods, have been developed to improve the effectiveness of methane fermentation using FW substrates [10]. By raising the specific surface area, mechanical pretreatment, for instance, enhances the anaerobic process and increases the substrate’s accessibility for microbial activity [11]. Thermal pretreatment in FW degrades organic content, causing protein breakdown stagnation and increased gas generation, but may require substantial financial investments for specialist facilities and therapies [12]. Ethanol pre-fermentation, a biological pretreatment technique, enhances methane output by stimulating methanogenic bacteria development and reducing fermentation lag periods [13].
Recent developments in waste valorization and bioenergy recovery have investigated several bioconversion methods to extract high-value biomolecules and biofuels from organic waste. A possible method involves the extraction of arabinoxylans, a category of hemicelluloses sourced from agro-industrial byproducts, which have garnered interest for their use in food formulations and biofuel generation [2]. Research has shown that arabinoxylans can be efficiently extracted via enzymatic and physicochemical methods, presenting potential uses in prebiotic development and fermentation-based bioenergy systems (Arabinoxylans: A review on protocols for their recovery, functionalities, and roles in food formulations). These biomolecules provide fermentable sugars, facilitating microbial metabolism and enhancing biohydrogen and biomethane production [14]. Concurrently, dark fermentation has arisen as a feasible method for hydrogen (H₂) generation from organic waste streams. This approach utilizes anaerobic microbial consortia to transform carbohydrates into hydrogen (H₂) and volatile fatty acids (VFAs), hence reducing dependence on fossil fuels. Recent studies indicate that undiluted tequila vinasse, concentrated wastewater from ethanol distillation, can function as a substrate for hydrogen fermentation without requiring external nutrient supplementation [15]. Additionally, the use of NPs in anaerobic fermentation has been seen to augment microbial electron transfer and increase biofuel production. Iron (magnetite) NPs have demonstrated the ability to enhance direct interspecies electron transfer (DIET), thereby expediting hydrogen and methane production from lignocellulosic hydrolysates [16].
AD operations face challenges like complex substrate characteristics, insufficient productivity, ineffective biodegradability, and poor stability, hindering the generation of biogas and biomethane [17]. Immobilization methods provide a viable way to overcome these difficulties and produce advantageous outcomes in many areas of contemporary biocatalysis [18]. To address the aforementioned issues and drawbacks, a variety of techniques involving the immobilization of microorganisms have been devised to transform pre-treated lignocellulose waste into a variety of products [19]. Anaerobic sludge immobilization using carrier materials has been unsuccessful due to premature biodegradation and physicochemical deterioration, primarily due to pressure from gaseous metabolites [20]. Polyvinyl alcohol (PVA) cryogel, a macroporous matrix, can be used to immobilize anaerobic sludge cells, preventing carrier breakdown under anaerobic conditions. It resists excess pressure from gases produced by anaerobic microbes, such as CO2 and H2, and facilitates nutrient passage into producer cells without hindering the overall biofuel production process [21].
A trend that is rapidly developing in the field of wastewater treatment is the use of NPs in AD processes to increase biogas production [22]. Similar benefits have been shown in accelerating substrate degradation, improving AD performance, and speeding up the bioconversion process [23]. These advantages result from nanoparticles’ particular properties, which include their large surface area, profusion of active sites, specificity, and increased reactivity [24]. Recent studies reveal NPs additions significantly impact anaerobic digesting procedures [25]. Ag@ZnONPs’ catalytic properties, due to their large surface area and reactivity, accelerate the breakdown of complex organic molecules during anaerobic digestion, increasing biogas output [26].
This study introduces a new method for AD to degrade pollutants and extract bioenergy from food waste. It uses Ag@ZnONPs and cryogel matrix to improve digestion efficiency. The main goal is to increase biogas production and improve waste material breakdown in AD reactors. This innovative approach aims to revolutionize waste management by utilizing energy recovery techniques for a sustainable and efficient AD system.
2. Materials and Methods
2.1. Materials
Soluble starch, silver nitrate (ACS reagent, ≥99.0%), high molecular weight chitosan (Ch), and sodium hydroxide were purchased from Merck KGaA, Darmstadt, Germany. Zinc acetate dihydrate was purchased from Molekula GmbH, Schwarzmannstr. 8, München, 80798, Germany. Polyvinyl alcohol (PVA, Mw 89,000-98,000, 87-89% hydrolyzed) was purchased from Sigma-Aldrich (USA). Glutaraldehyde (25% (v/v), aqueous) was purchased from Thermo Fisher Scientific Inc.
2.2. Methods
2.2.1. Preparation of Ag@ZnONPs
ZnONPs were formed and followed by the preparation of AgNPs. For this preparation, 1 g of soluble starch was dissolved in 50 mL of H2O with continuous stirring for 10 mins. Then, 2 g of zinc nitrate in 10 mL of H2O was added to the dissolved soluble starch. After that, NaOH (2 g/40 mL H2O) was dropwise added to the previous solution. The prepared white precipitate was attributed to the formation of zinc hydroxide, which converted to ZnONPs upon calcination at 550°C for 2.5 hrs. On the other side, AgNPs were prepared using soluble starch, in which, soluble starch plays the dual role of reductant and stabilizing agent. The preparation was performed by grinding 0.5 g of soluble starch with 0.05 g of NaOH and 0.05 g of AgNO3. The grinding was carried out using a ball milling device for 20 mins at 50 rpm. The color of soluble starch changed from white to brown at the end of the AgNO3 addition. The stabilized AgNO3 (soluble starch-coated AgNPs) was mechanically grinded with ZnONPs using a ball milling device. AgNPs (0.1 g) was added to 0.5 g of ZnONPs and mechanically grinded for 15 mins. After completing the two processes of addition and mechanical grinding, it was noticed that the dark color of AgNPs was reduced, and this is due to the presence of white ZnONPs.
2.2.2. Preparation of PVA/chitosan cryogel
Ch (1 g) was dissolved in 100 mL of 1% acetic acid solution while being magnetically stirred to create a Ch solution (1% w/v). For 3 hrs, PVA solution (1% w/v) was dissolved in deionized water at 80°C with continuous mechanical stirring. The PVA and Ch solutions were blended in order to produce an admixture with a volume ratio of 1:0.5 using mechanical stirring. After complete dissolution and the formation of a homogeneous solution, 50 mL of the Ch/PVA admixture was obtained. Under continuous mechanical stirring, a crosslinking agent (glutaraldehyde, 1 mL) was gradually added to the formed mixture. After being placed in the freezer, the Ch/PVA admixture solution was incubated for 24 hrs. The frozen solution was then freeze-dried at -80°C for 48 hrs.
2.2.3. Characterization
Transmission electron microscopy (TEM, JEOL, 2100, Japan) was used to study the particle shape of Ag@ZnONPs. Prior to TEM analysis, Ag@ZnONPs sample solution was sonicated for 15 mins, during which, 0.01 g of the prepared NPs were dispersed in 10 mL of H2O. Field emission scanning electron microscopy (FESEM, Quanta FEG 250, Czech) was used to evaluate the surface morphology of Ag@ZnONPs and the Ch/PVA cryogel. Field emission scanning electron microscopy (FESEM) was combined with energy dispersive X-ray (EDX) to do the elemental analysis of the formed NPs.
2.2.4. Food waste feedstock and inoculums
In Saudi Arabia, fruit stores and restaurants provided the FW for the tests. First, the pericarps of FW, non-biodegradable materials, and inactive things (including seashells and bones) were obtained. The FW was uncontrolled and included cooked meat and other proteins along with carbs such as cooked rice, legumes, plant roots crops, and fruits. The FW was left for several days for drying at 60°C. After that, the dried FW was manually prepared by using a sharp knife to chop it into small fragments. After that, the chipped FW was mixed using an electric mixer to reduce the particle diameter and make it easier for the anaerobic microbe to break down the FW. Prior to blending, 100 g of pretreatment FW was mixed with 1 L of distilled water (DW) to create a FW slurry. This was accomplished by diluting FW with 10% (w/v) distilled water. Chemical pretreatment was carried out on the FW by H2SO4 (1 %, w/v). The mixtures were then autoclaved at 121ᵒC for 15 mins. The seeding anaerobic sludge (AS) was taken from a full-scale anaerobic treatment unit in Municipal wastewater treatment plant, KSA. The harvested AS was initially sieved to remove the debris and unwanted materials, and then stored in closed bottles for a week to stimulate anaerobic conditions. The main characteristics of the sludge have been presented in Table 1. The FW was then mixed equally with AS (adding ratio: 1-1 v/v in the four groups of AD experiments), where 250 mL of FW slurry was blended with 250 mL of AS to be utilized as inoculum in anaerobic digestion reactors.
| SN | Code | Specifications |
|---|---|---|
| 1 | R1-(FW) | AD reactor 1 contains 500 mL of pretreated FW slurry which mixed with seeding AS (mixture FW slurry) with in volumetric ratio of 1:1. |
| 2 | R2-(FW+cryogel) | AD reactor 2 contains 500 mL of pretreated FW slurry which mixed with immobilized seeding AS in cryogel material with in ratio of 1:1. |
| 3 | R3-(FW+NPs) | AD reactor 3 contains 500 mL of mixture FW slurry which mixed and incorporated with 50 ppm of bimetallic Ag@ZnONPs. |
| 4 | R4-(FW+cryogel+NPs) | AD reactor 4 contains 500 mL of pretreated FW slurry which mixed with immobilized seeding AS in cryogel material and incorporated with 50 ppm of bimetallic Ag@ZnONPs. |
2.2.5. AD fabrication and operational conditions
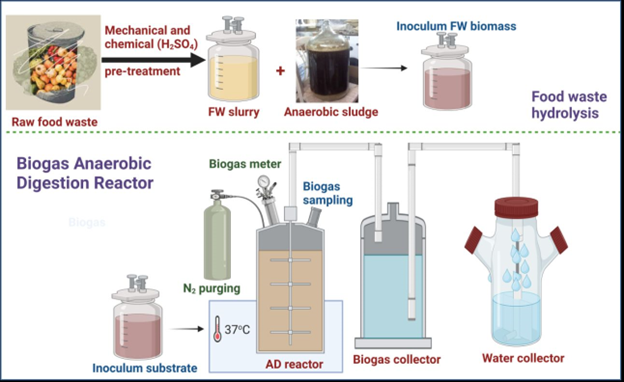
As laboratory-scale AD for the generation of biogas, graded serum bottles were used in this investigation. The total volume capacity of the four different AD units, each having a capacity of 500 mL, was operated in batch mode. Each AD had a section of its top 50 mL purposefully left empty to provide room for by-product reactions. A digestive chamber, intake and output tubes, a mechanical stirring mechanism, and a biogas collecting tube were all part of each AD unit’s specific construction. Seeding AS was immobilized inside a cryogel matrix in an attempt to improve the productivity of biogas production in the AD reactor. This immobilization technique shields the methanogenic bacteria against adverse circumstances such as low pH, high temperatures, and the presence of poisonous substances. The seeding AS suspension was used to immerse disc-shaped cryogel carriers during immobilization. This was followed by a week-long incubation phase at 37°C under anaerobic conditions. During this time, the methanogenic bacteria developed a protected and hospitable environment inside the small pores of the cryogel carriers, enabling prolonged biogas and biomethane production. Figure 1 shows an illustration of the experimental procedure’s schematic depiction and water displacement method for biogas measurement.
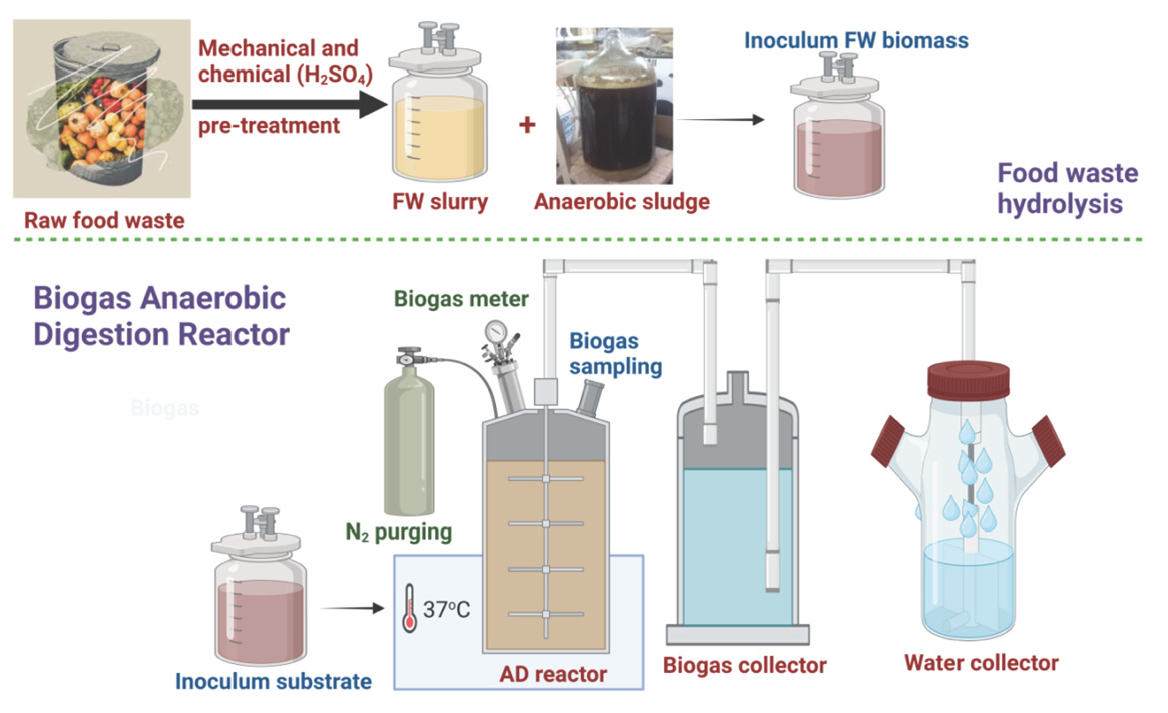
- Schematic sketch for food waste pretreatment and the biogas measuring method using water displacement system in biogas AD reactor.
To assess the biogas generation from the fermentation of FW, which was infected with bimetallic Ag@ZnONPs and integrated inside cryogel matrices, a series of batch anaerobic test procedures were rigorously carried out in triplicate. The (AD) reactors used had a coding system, which has been described in Table 1. The AD reactors were hermetically sealed and kept at a constant temperature of 37°C while being agitated at a speed of 60 rpm before the tests began to get rid of any extra air. For these trials, the hydraulic retention time (HRT) lasted up to 40 days. A set of regular sample intervals was developed, and different critical parameters were monitored during these times. The water displacement method was used to calculate daily biogas generation. It is noteworthy that the gases were produced quickly, which might be related to the increased biodegradability of the feedstock used in the tests.
To ensure uniform contents within the digesters and confirm even temperature distribution throughout the digestion chamber, mechanical stirrers were installed in each digester. Additionally, a heated water bath was employed externally to maintain a constant digestion temperature of 37°C ± 2.
2.3. Analytical methods
The study monitored the physicochemical characteristics of the pretreated FW slurry, seeding AS, and mixture of FW slurry with seeding AS samples in AD reactors. These characteristics included pH, total solid (TS), total volatile solid (TVS), total chemical oxygen demand (tCOD), and soluble COD (sCOD) [27]. The TSs remaining after evaporation were measured using TS measurement. The oxygen required to oxidize organic matter in food waste was estimated using tCOD. Standard protocols by American Public Health Association (APHA) were used to ascertain Soluble COD, total phosphorus (TP), ammonia, and total Kjeldahl nitrogen (TKN) [28].
2.4. Biogas output determination
Measurements using the gas displacement technique were used consistently to quantify the output of biogas. The contents of the produced biogas, which included methane, carbon dioxide, and trace gases such as oxygen and hydrogen sulfide, were measured using a portable gas analyzer (BIOGAS 5000, GeoTech).
2.5. Statistical analysis
The results of the anaerobic digestion experiment were presented together with the mean values and standard error (SE) after three sets of observations. The statistical significance of the experimental components was assessed using a one-way multifactor analysis of variance (ANOVA).
3. Results and Discussion
Fermentation plays a fundamental role in bioenergy production and waste valorization, particularly in the transformation of organic substrates into high-value products such as biofuels, bioactive compounds, and functional ingredients. Traditionally, fermentation has been widely applied in the food industry, contributing to food preservation, flavor enhancement, and nutritional improvements [29]. However, its application has expanded to waste valorization and biogas enhancement, where microbial fermentation facilitates the conversion of complex organic waste into biohydrogen, methane, and other bioproducts [30].
TEM for Ag@ZnONPs has been demonstrated in Figure 2. It was observed that Ag@ZnONPs formed with two different phases (different colors; faint and deep black). The two different phases may be attributed to the presence of AgNPs coated with ZnONPs. The NPs were formed with small size as proven from the TEM scale. In addition, the faint color can be related to the stabilizing agent (soluble starch). The formation of particles in a nanoform demonstrated the capability of soluble starch for reducing Ag ions and acting as a stabilizing agent for the formed ZnONPs coated AgNPs.
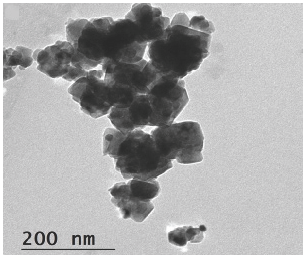
- TEM image of AgNPs-doped ZnONPs.
From the SEM images shown in Figure 3, both AgNPs and ZnONPs were well distributed on the surface of soluble starch. Also, using different magnifications of SEM image it was found that the size is considered small to some extent, which confirms that the process of preparing the catalyst was successful.
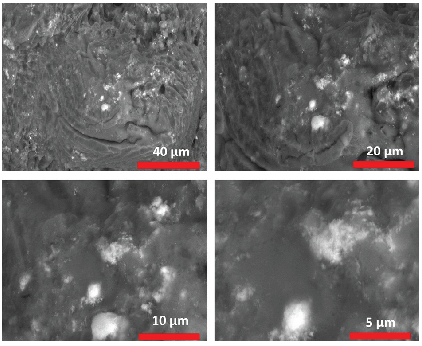
- SEM images of AgNPs-doped ZnONPs at four different magnifications.
To confirm the presence of NPs loaded soluble starch and the distribution of these NPs, the material as a whole was analyzed using EDX and mapping for all elements of the material, and the results obtained have been recorded in Figure 4. From Figure 4(a), it is clear that the material in its elemental composition consisted of carbon, oxygen, zinc, and silver. The percentage of each element has been represented in the table attached to the EDX image. The presence of carbon and oxygen may be due to the presence of soluble starch. The elements of zinc and silver are mainly due to the presence of zinc oxide and silver nanoparticles. In Figure 4(b), the distribution of each element for the sample evaluated by SEM-EDX analysis has been illustrated. As shown in Figure 4(b), it was ensured that all the elements of the material that will be used as a catalyst are well distributed and that the preparation process and the use of soluble starch to prepare NPs is considered effective material and has the power to prepare both AgNPs and ZnONPs.
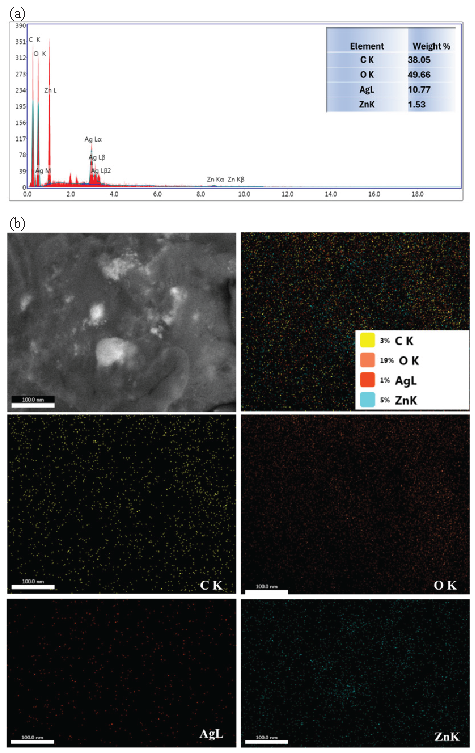
- (a) EDX and (b) mapping SEM of AgNP-coated ZnONPs.
Transitioning to the assessment of the cryogel generated from a combination of PVA and Ch through SEM analysis (Figure 5), it becomes evident that the resultant cryogel manifests a porous structural composition. These porous structures of PVA/Ch cryogel were formed owing to the effect of crosslinking agent and freeze-drying step. As shown, the surface structure of PVA/Ch was demonstrated at four different magnifications (Figures 5a-d). The interconnected network was formed due to the interaction between PVA and Ch.
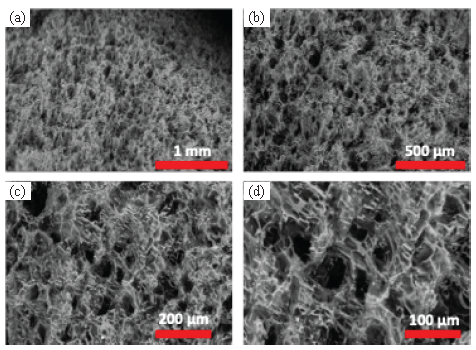
- (a-d) SEM of PVA/Ch cryogel at four different magnifications.
3.1. Characteristics of feedstock used for anaerobic digestion
To assess the potential biogas yield from the substrates intended for anaerobic digestion, the fundamental attributes of the pre-treated FW slurry, the AS used as inoculum, and the composite mixture of FW biomass inoculum were investigated and have been documented in Table 2. The pre-treated FW slurry exhibited distinctive characteristics, featuring a notably low pH of 3.3 and elevated values of total chemical oxygen demand (tCOD) at 21752 mg/L, total solids (TS) at 13600 mg/L, total volatile solids (TV) at 5421 mg/L, total phosphorus (TP) at 2531 mg/L, and total Kjeldahl nitrogen (TKN) at 4592 mg/L. In contrast, the pH of the FW biomass-inoculum mixture registered a higher value of 7.8, potentially attributed to its elevated NH4+ content at 1325 mgN/L. Conversely, the AS exhibited a neutral pH of 6.8 and a comparatively lower COD content of 3525 mg/L in comparison to the FW slurry. As delineated in the tabulated data, these three waste materials—FW slurry, seeding AS, and FW biomass-inoculum mixture—manifest discernibly distinct attributes. However, the mixing of materials with varying characteristics can synergistically address deficiencies present in one constituent by supplementing it with complementary attributes from other constituents. Specifically, the equimolar mixture of FW slurry and seeding AS strategically provides a balanced nutrient composition, leveraging the AS’s reservoir of anaerobic methanogenic microorganisms. This harmonized nutrient balance within the digested materials collectively contributes to enhanced biogas production yields.
| Parameters | Unit | FW slurry | Seeding AS | Mixture FW biomass |
|---|---|---|---|---|
| pH | 3.3±0.42 | 6.8±0.85 | 7.8±0.62 | |
| Total solids (TS) | mg/L | 13600±215 | 528±75 | 921±152 |
| Volatile solids (VS) | mg/L | 5421±124 | 72.6±59 | 247±92 |
| Total dissolved solids (TDS) | mg/L | 4265±351 | 1251±195 | 2982±251 |
| Total phosphorus (TP) | mg/L | 2531±165 | 735±85 | 1692±127 |
| Total Kjeldahl nitrogen (TKN) | mgN/L | 4592±351 | 1825±154 | 2482±231 |
| Ammonia (NH4-N) | mgN/L | 1074±121 | 1892±248 | 1325±148 |
| Total COD (tCOD) | mgO3/L | 21752±410 | 3525±268 | 16521±317 |
| Soluble COD (sCOD) | mgO3/L | 4375±135 | 958±94 | 3621±115 |
Higher organic load generally leads to increased biogas production. The organic matter in the feedstock serves as a substrate for the anaerobic microorganisms that produce methane as a metabolic byproduct [31,32]. The value of TS, VS, and COD are good parameters to indicate the ability of biodegradation of the substrate; the higher the initial VS and COD, the more organic matter in it. Thereby, the bacteria were more accessible to the substrate; the hydrolysis degree was enhanced, resulting in high solubility organic matter converted into biogas production under anaerobic conditions [33]. The efficiency of organic matter conversion to methane is influenced by the organic load [34]. The increasing the organic load from 2.5 to 15 g VS/L (volatile solids per liter) resulted in increased biogas production. However, at organic loads greater than 15 g VS/L, the methane production rate decreased due to process inhibition [35].
3.2. Specific biogas production and methane potential
The findings of the experiment presented the biogas production outcomes for different configurations of anaerobic digestion reactors (R1 to R4). These reactors were operated with varying combinations of substrates and materials, namely FW, cryogel, and NPs, to evaluate their impact on biogas generation. The results highlight that the reactor labeled as R4, which employed a combination of FW, cryogel, and NPs, exhibited the highest biogas output among all configurations. In this reactor, the biogas produced reached 534 mL/gVS. Following R4-(FW+cryogel+NPs), the next highest biogas outputs were observed in R3-(FW+ NPs) with 489 mL/gVS, R2-(FW+cryogel) with 415 mL/gVS, and R1-(FW) with 341 mL/gVS (Figure 6a). Additionally, a consistent pattern emerged across all the AD reactors regarding their biogas production behavior. As shown in Figure 6(b), the cumulative biogas production represented the total volume of biogas collected throughout the 40-day digestion period. The total amount of biogas produced over time indicates how well the AD process can convert organic feedstock into biogas. The digestive process and the microbial population impact this process, which progressively converts complex organic compounds into simpler gases. The efficiency is shown by trends in the rate of biogas generation and cumulative output; variations may point to changes in the composition of the substrate and microbial dynamics.
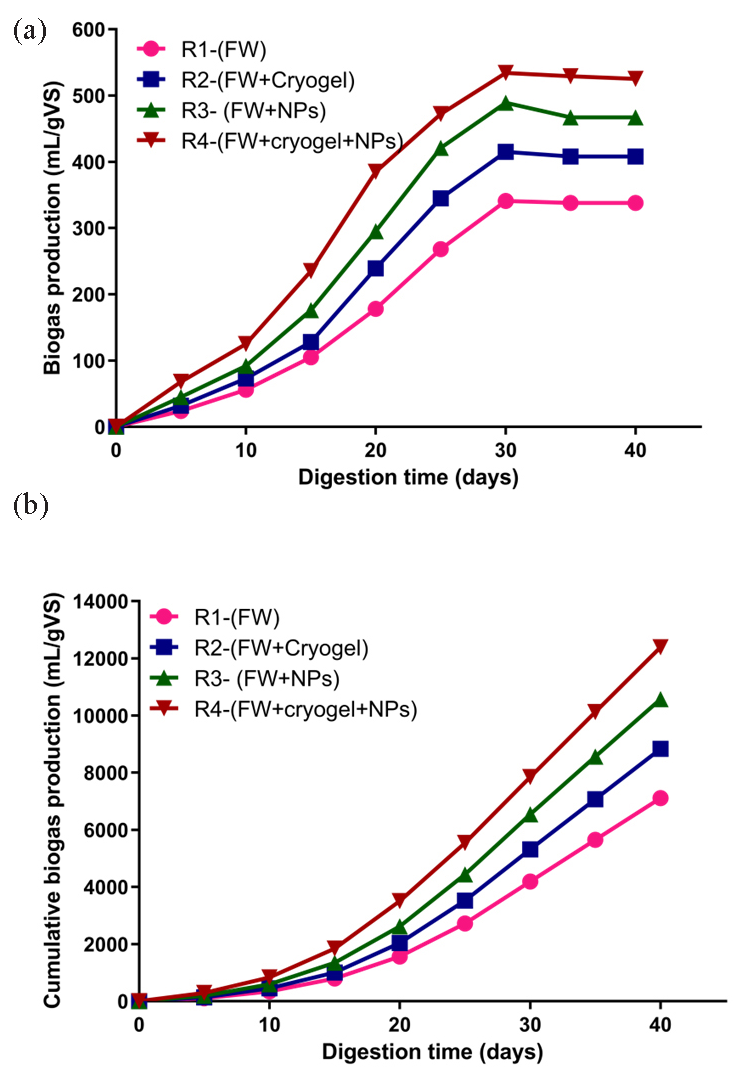
- (a) Biogas production rate and (b) cumulative biogas production in four AD reactors over a 40-day digestion period at a constant temperature of 37°C.
Recent studies have demonstrated that fermentation-based technologies can enhance the recovery of valuable nutrients from food and seafood waste while improving substrate biodegradability for energy production. For instance, the fermentation of seafood processing by-products has been explored as an efficient strategy to extract proteins, lipids, and bioactive peptides [36]. Concurrently, strengthening waste digestibility for biogas and biomethane generation is being done (Implementing fermentation technology for the complete valorization of seafood processing by-products: A critical evaluation on recovering important nutrients and optimizing use). The data indicate that incorporating fermentation into anaerobic digestion systems may substantially improve substrate hydrolysis, augment microbial activity, and elevate methane production [37].
The shift toward renewable energy production has increasingly relied on biotechnological systems, particularly microbial fermentation and AD, to convert organic waste into biofuels such as biogas and biohydrogen (H₂). Dark fermentation has emerged as a promising approach for biohydrogen production, offering the advantage of substrate flexibility and low energy input. However, several challenges, such as low hydrogen yields, process inhibition, and inefficient electron transfer, limit its large-scale feasibility. Recent studies have highlighted lactate metabolism as a crucial factor influencing dark fermentative hydrogen production, as it serves as both an intermediate and an inhibitor depending on microbial interactions and operational conditions [38]. Despite the progress in biogas and biohydrogen production, several drawbacks persist in biotechnological energy systems. These include slow hydrolysis rates, poor substrate conversion efficiency, and reactor instability due to fluctuations in pH and intermediate accumulation. Additionally, traditional anaerobic digestion processes suffer from low methane content and long retention times, limiting their commercial viability [39]. To address these limitations, nanoparticle-assisted AD and microbial immobilization have gained attention for their ability to enhance substrate degradation, electron transfer, and microbial stability. Studies show that incorporating metallic NPs can accelerate microbial metabolism and improve hydrogen and methane yields, while cryogel matrices provide a protective environment for microbial retention, ensuring process efficiency and stability.
Data represented in Figure 7(a) displays the amount of generated methane produced by the reactors under investigation as 161.6, 188.5, 205.5, and 252 mL/gVS, for R1-(FW), R2-(FW+cryogel), R3-(FW +NPs), and R4-(FW+cryogel+NPs), respectively. The cumulative value of biomethane pointed out the sum of methane gas generated at each time point during the 40-day period, showcasing the overall methane yield over the entire duration of the experiment. It serves as a crucial metric for assessing the effectiveness of the AD process and the efficiency of methane production from the given substrates under the specified conditions (Figure 7b).
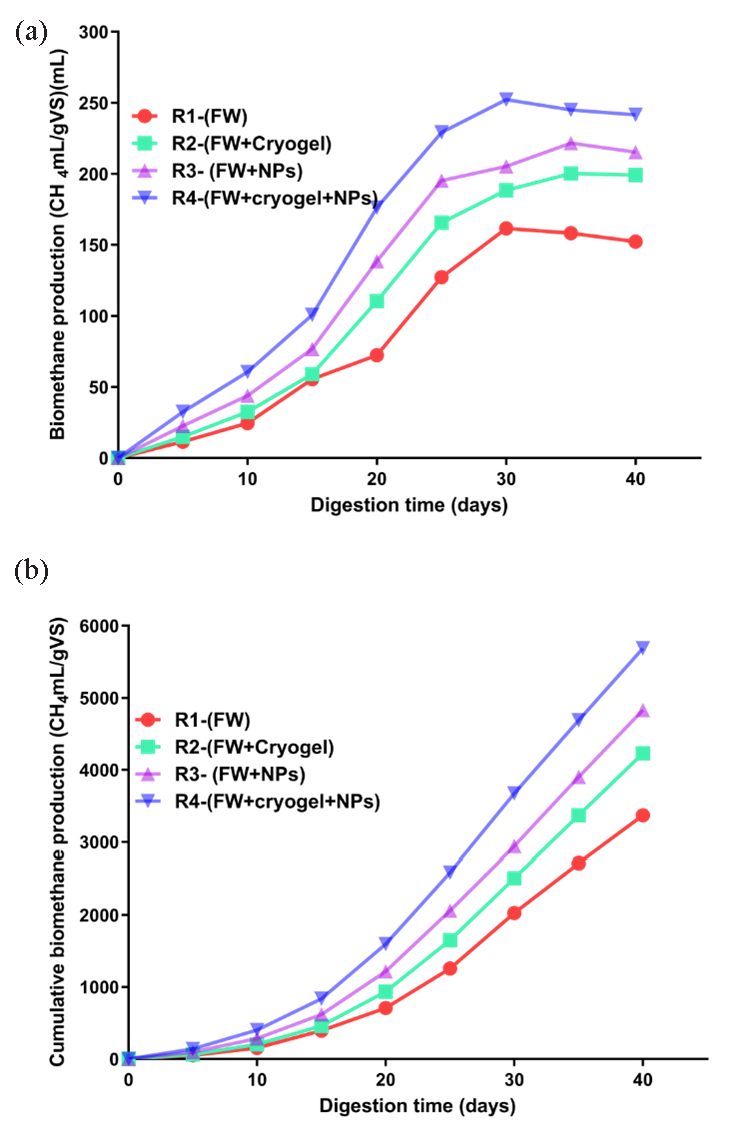
- (a) Biomethane production rate and (b) cumulative biomethane production in four AD reactors over a 40-day digestion period at a constant temperature of 37°C.
Specifically, the highest production rate of biogas and biomethane was consistently observed around the 30-day mark since the start of the operation. The digestion process takes time to optimize, leading to increased biogas generation after initial adaptation. A 10-day adjustment period is crucial for the microbial community to adjust to the digestion environment, promoting efficient biogas production. The presence of methanogens accelerates the breakdown of complex organic compounds, releasing methane and carbon dioxide gases as byproducts, thereby increasing biogas production. Methanogenic bacteria collaborate with other microbial communities involved in the degradation of organic substrates, fostering a balanced ecosystem within the reactor [40]. Moreover, bioaugmentation techniques involving the deliberate introduction of enriched methanogenic cultures can be employed to enhance the performance of AD reactors. This approach ensures that the necessary microorganisms are present in sufficient numbers to effectively carry out the methane-producing reactions. As a result, the AD process becomes more robust and capable of generating higher volumes of biogas from the same input materials [41].
The effects of nanoparticles on the performance and stability of AD for biogas production were investigated. Some of the nanoparticle materials utilized include metal iron, copper, ZnO, cobalt, silver, and nickel [42]. These NPs can influence the microbial community structure and metabolism within the AD system. Some studies suggest that NPs can stimulate the growth and activity of certain microorganisms involved in methanogenesis, the process of methane production. This can lead to more efficient methane generation and improved overall biogas production [43]. In the current study, the bimetallic Ag and ZnO nanoparticles were applied as catalysts, accelerating the breakdown of complex organic molecules in the substrate. They provide active sites on their surfaces that facilitate the enzymatic reactions carried out by microorganisms during anaerobic digestion. This catalytic effect speeds up the degradation process and enhances the release of biogas precursors. The combination of bimetallic AgNPs and ZnONPs may lead to synergistic effects, where their individual properties work together to create a more conducive environment for biogas production. For instance, ZnONPs could assist in promoting substrate degradation, while AgNPs might exhibit antimicrobial properties, potentially reducing unwanted microbial competition [44]. Due to the conductive nature of metal NPs, their incorporation could potentially be utilized to improve DIET, thereby promoting the proliferation of biofilms within the AD system. Recent studies have indicated that the incorporation of electrically conductive materials can elevate DIET among electroactive microorganisms [45].
Furthermore, immobilizing the inoculum within a cryogel matrix helps to retain the microbial population within the anaerobic digestion system. The cryogel matrix provides a supportive structure that prevents the loss of microorganisms during the operation of the reactor. This enhanced microbial retention ensures a stable and consistent microbial community, promoting the resilience and efficiency of the anaerobic digestion process. The cryogel matrix acts as a physical barrier that protects the microorganisms from shear forces and changes in environmental conditions, such as pH fluctuations or temperature variations. This protection helps to maintain a more stable microbial activity, reducing the risk of process imbalances and potential system failures. Overall, immobilizing the inoculum within a cryogel matrix in the anaerobic digestion process can provide benefits, such as improved microbial retention, enhanced process stability, increased reactor performance, extended operational lifespan, and scalability. These advantages contribute to the optimization and efficiency of anaerobic digestion, promoting sustainable waste management and bioenergy generation [46].
3.3. Biogas composition
The valorization of agro-industrial by-products through fermentation has gained increasing attention in biofuel production and circular economy strategies. Among these, brewery spent grain (BSG), a major by-product of the brewing industry, represents a valuable feedstock for biohydrogen, carboxylic acids, and methane production via microbial fermentation and anaerobic digestion [47].
Recent studies have demonstrated that BSG hydrolysis releases fermentable sugars and organic acids, which can serve as precursors for biohydrogen production through dark fermentation, followed by methane generation from residual biomass (Brewery spent grain valorization through fermentation: Targeting biohydrogen, carboxylic acids, and methane production). This two-stage bioconversion process enhances overall energy recovery and optimizes substrate utilization [48].
The maximum yield of biogas and biomethane produced from each reactor and biogas composition are illustrated in Figure 8. As shown in Figure 8(a), the biogas and biomethane production was found to be in R4-(FW+cryogel+NPs) followed by R3-(FW+NPs), and R2-(FW+cryogel). The lowest production rate was recorded for R1-(FW). Furthermore, in the reactor nominated R1-(FW), results displayed that the biogas composition in this reactor consists of approximately 40.64% methane, 28.5% carbon dioxide, 17.5% nitrogen, and 13.35% other gases. This indicates that methane is the predominant component of the biogas, followed by carbon dioxide. The presence of nitrogen and other gases suggests the presence of impurities or non-methane gases. Likewise, In R2-(FW+cryogel) reactor, the biogas composition shows an increase in methane content compared to R1. It contains around 47.83% methane, 19% carbon dioxide, 18.2% nitrogen, and 14.97% other gases. The addition of the cryogel matrix might have contributed to improved methane production, resulting in a higher methane percentage in the biogas. The biogas composition in R3-(FW+NPs) reactor exhibits a further increase in methane content. It consists of approximately 54.25% methane, 12.5% carbon dioxide, 14% nitrogen, and 19.25% other gases. The introduction of Ag@ZnONPs might have enhanced the anaerobic digestion process, leading to a higher methane yield. On the other hand, the notable biogas and biomethane production could be found in R4-(FW+cryogel+NPs), where this reactor shows the highest methane content among all the reactors. The biogas composition (Figure 8b) includes about 60.15% methane, 9.5% carbon dioxide, 11% nitrogen, and 19.35% other gases. The combined effects of the cryogel matrix and Ag@ZnONPs in R4 likely contributed to the significant increase in methane production compared to the other reactors.
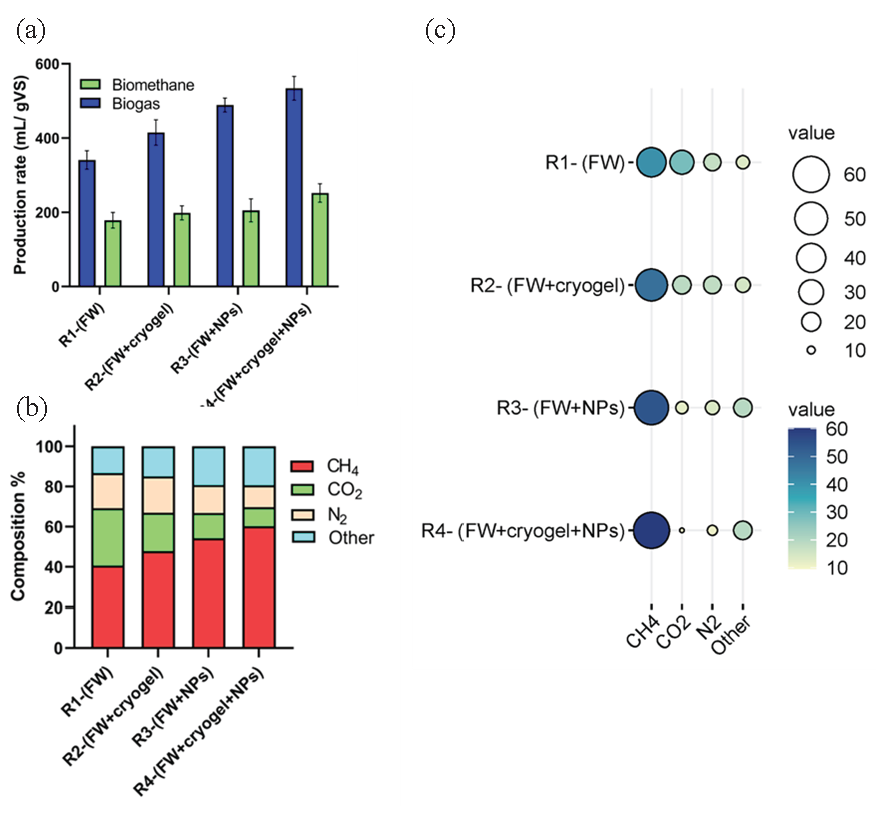
- (a) Maximum biogas and biomethane yield in four reactors, (b) biogas composition analysis at four reactors (gas produced other than the methane, carbon dioxide, and nitrogen are included in the “others gases), (c) Balloons size of biogas compositions %.
As shown in Figure 8(c), the biogas composition can be represented using balloon diagrams, where the size of each balloon corresponds to the percentage of a specific gas in the biogas mixture. The larger the balloon size indicated, the higher the percentage of that gas. The balloon size of methane had the largest percentage compared with other gases. Generally, the biogas composition analysis reveals that the addition of the cryogel matrix and Ag@ZnONPs in the reactors has a positive impact on methane production. The highest methane content is observed in R4, where the cryogel matrix and NPs are used together. These findings suggest that the utilization of these additives can enhance the anaerobic digestion process and improve the quality of biogas generated, which is beneficial for bioenergy production.
The research highlights an interesting result: Ag@ZnONPs and the PVA/Ch cryogel matrix work in concert to improve biogas output during anaerobic digestion. The research demonstrates the increased biogas output from this combination, although it did not precisely explain the precise mechanism behind their synergistic impact. This phenomenon likely arises from the combined attributes of Ag@ZnONPs and the cryogel matrix, which individually possess unique characteristics. Ag@ZnONPs exhibit catalytic properties that potentially accelerate the breakdown of recalcitrant compounds present in the substrate, thereby enhancing biogas yield. Meanwhile, the PVA/Ch cryogel matrix might serve as a supportive environment for the immobilized anaerobic sludge, promoting microbial activity and providing a favorable microenvironment for digestion processes. The intricate interplay between these components, potentially involving increased surface area for catalytic reactions, improved substrate accessibility, and enhanced microbial activity within the cryogel matrix, could contribute to the observed synergistic effect. However, further research and detailed investigations are warranted to precisely delineate the underlying mechanisms driving this synergy and its precise contributions to the augmented biogas production observed in the anaerobic digestion process.
Nanotechnology has transformed several scientific and industrial fields by allowing exact manipulation of material characteristics at the nanoscale. This methodology has markedly enhanced procedures in medical, electronics, environmental research, food safety, building, energy conversion, harvesting, storage, and biofuel generation. Progress in nanomaterials and nanostructures has improved efficiency, functionality, and sustainability in these domains, fostering innovation and broadening applications in renewable energy, bioprocessing, and environmental remediation [49].
AgNPs play a significant role in fermentative biohydrogen production by enhancing substrate utilization, bacterial metabolism, and acidogenesis. Studies have shown that AgNPs can significantly increase hydrogen yields and reduce the lag phase during synthesis [50]. Zhao et al. [51] found that 20 nmol/L Ag NPs in an anaerobic batch reactor with Clostridium butyricum increased H₂ production by 67.6% (2.48 mol H₂/mol glucose) and accelerated microbial adaptation. Further, Khan et al. [52] compared AgNPs synthesized from henna and DU-C2 actinomycete with Clostridium beijerinckii, where henna-assisted NPs increased biohydrogen yield (1.71 mol H₂/mol glucose), while DU-C2-assisted NPs had a negative effect. Yildirim et al. [53] examined silver oxide nanoparticles synthesized using Chlorella sp. microalgae. When applied to Clostridium sp., 400 mg/L silver oxide nanoparticles boosted H₂ production by 17% (2.44 mol H₂/mol glucose). Likewise, AgNPs improve hydrogen production efficiency by stimulating metabolic pathways, supporting microbial activity, and enhancing enzyme immobilization, making them a promising tool for biohydrogen generation [54].
3.4. Degradation rate of FW
The percentage of reduction in various physicochemical parameters after 40-day digestion period in different AD reactors is presented as reduction efficacy values. Results illustrated in Figure 9(a-d) indicated that the reduction percentages of TS were 51.6% (R1), 57.67% (R2), 61.9% (R3), and 66.34% (R4). Similarly, reductions in VS were 44.16% (R1), 55.8% (R2), 73.6% (R3), and 74.8% (R4). TP exhibited reductions of 56.2% (R1), 62.17% (R2), 66.8% (R3), and 78.02% (R4). Further, TKN showed reductions of 49.31% (R1), 61.2% (R2), 67.3% (R3), and 69.3% (R4). Likewise, the ammonia content decreased by 73.2% (R1), 78.4% (R2), 80.37% (R3), and 83.9% (R4). Additionally, the reductions in tCOD were 72.69% (R1), 76.6% (R2), 79.71% (R3), and 82.06% (R4), whereas sCOD) reductions were 64.56% (R1), 68.13% (R2), 71.6% (R3), and 74.8% (R4) (Figure 9a-d).
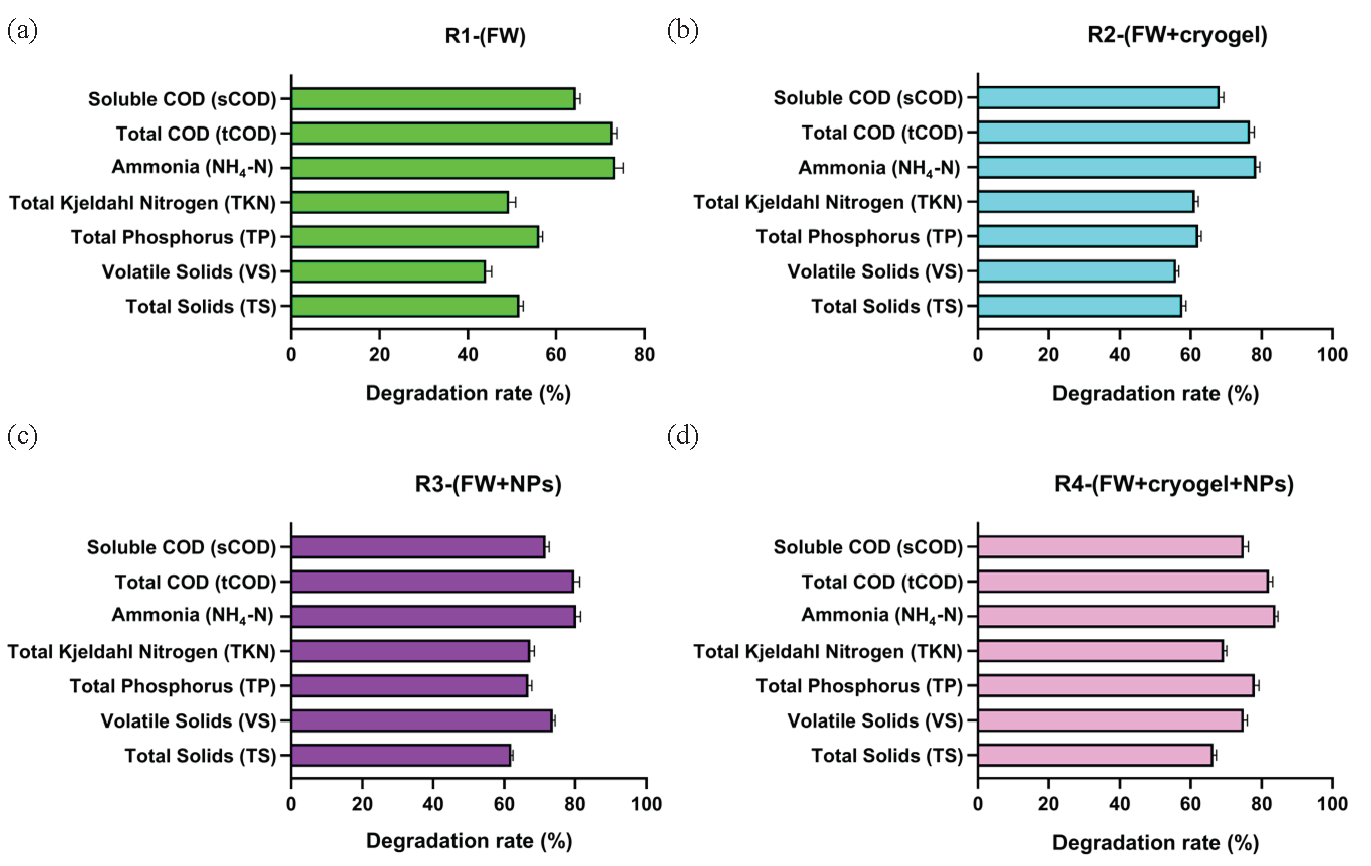
- Reduction efficacy (%) for some physicochemical parameters in mixture FW substrate after inoculation in four AD reactors (a) R1-(FW), (b) R2-(FW+cryogel), (c) R3-(FW +NPs), and (d) R4-(FW+cryogel+NPs) after digestion time of 40 days.
The reduction efficacy data demonstrates the performance of the AD reactors in diminishing the physicochemical properties of the food waste substrate during a 40-day digesting period. The findings indicate that including cryogel, NPs, and their combination into the FW substrate in the anaerobic digestion reactors enhanced reduction effectiveness for most parameters relative to the reactor using only food waste (R1).
Reduced waste volume, enhanced biogas generation potential, nutrient recycling, and process stability are all indicated by the decrease in total solids and volatile solids during anaerobic digestion. These reductions are crucial for effective waste management, renewable energy production, and sustainable resource recovery from organic waste. Volatile solids are the principal contributors to biogas production during anaerobic digestion. The reduction in VS signifies the effective conversion of organic matter into biogas, which typically consists of methane and carbon dioxide (CO2). Biogas can be utilized as a renewable energy source for electricity generation, heat production, or as a vehicle fuel. Therefore, a higher reduction in VS indicates a higher potential for biogas production, leading to energy recovery and reduced reliance on non-renewable energy sources [55]. The reduction in TS and VS is an indicator of the stability and efficiency of the anaerobic digestion process. A higher reduction signifies effective microbial activity and degradation of organic matter. Process stability is crucial to maintain optimal operating conditions, prevent process inhibition, and avoid the accumulation of volatile fatty acids or other intermediates that could hinder the digestion process. The reduction in TS and VS indicates a well-functioning anaerobic digestion system capable of sustaining the degradation of organic waste over an extended period [56].
Stacked bar charts can be an effective graphical representation to illustrate the reduction percentages of various physicochemical parameters in conjunction with biogas and biomethane production data from the reactors. These physicochemical parameters can include TS, VS, COD, pH, NH3-N, phosphorus, etc. The percentage reduction or change in the values of the physicochemical parameters and production of biogas and biomethane have been represented in Figure 10(a). Each bar in the chart represents a reactor or a specific condition being studied. The height of the bar corresponds to the reduction percentage achieved for a particular parameter. By using a stacked bar chart, the reduction percentages of various physicochemical parameters can be visually compared and analyzed alongside biogas and biomethane production data. This graphical representation allows for a comprehensive understanding of the relationship between process performance, reduction efficiencies, and biogas/biomethane production in the studied reactors.
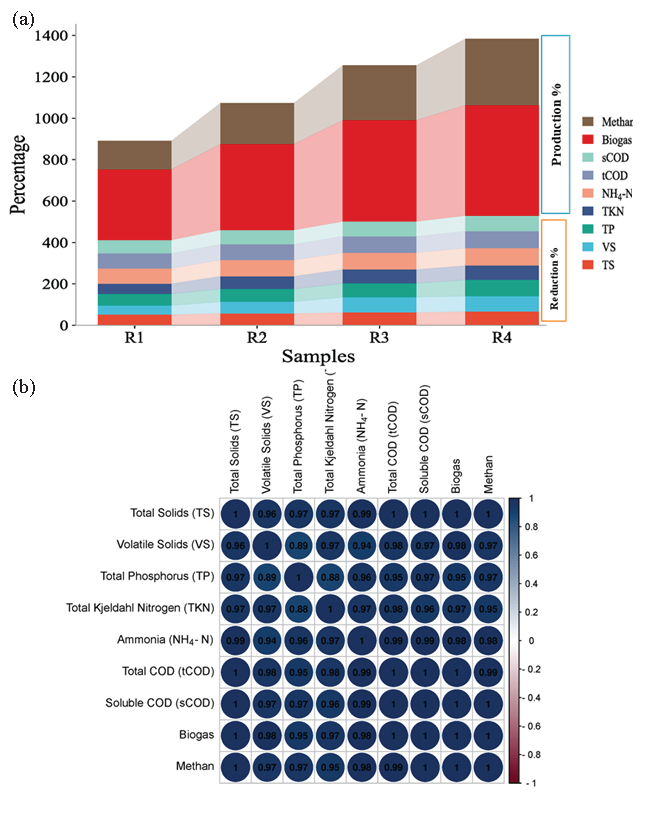
- (a) Stacked bars representing the reduction percentages of various physicochemical parameters alongside biogas and biomethane production from the reactors. This visual representation enables a comparative analysis of reduction efficiencies and gas production across different experimental conditions, (b) a correlation coefficient plot, illustrating the relationship between the physicochemical parameters and the generated biogas and methane. This plot aids in understanding the degree of association or interdependence between these factors, providing insights into their potential influence on gas production within the reactors.
Figure 10(b) indicates a strong correlation between the efficacy of AD reactors in degrading organic compounds and biogas production, suggesting that as the degradation of organic compounds improves, there is a corresponding increase in biogas production. This finding implies that the efficiency of the AD process in breaking down organic compounds directly influences the generation of biogas. The correlation coefficient provides a quantitative measure of the strength of this relationship. A high positive correlation coefficient value close to +1 would indicate a strong positive correlation between organic compound degradation and biogas production.
The observed synergistic impact of Ag@ZnONPs and the cryogel matrix in augmenting biogas generation may be ascribed to several mechanistic interactions during the anaerobic digestion process. Ag@ZnONPs serve as electron mediators, promoting DIET between syntrophic bacteria and methanogenic archaea, thereby enhancing methane synthesis. Furthermore, ZnONPs augment the enzymatic activity by facilitating hydrolytic and fermentative enzymes, hence enhancing the degradation of complex organic materials into accessible substrates. AgNPs preferentially inhibit non-methanogenic bacteria, diminishing microbial competition and promoting methanogenic communities, hence enhancing the efficiency of the biogas conversion process. Moreover, the elevated surface area of Ag@ZnONPs facilitates microbial adhesion and promotes substrate accessibility, hence augmenting the efficiency of organic matter breakdown. The cryogel matrix enhances this process by maintaining microbial retention, providing a safe habitat for methanogens, and inhibiting biomass waste. Collectively, these processes enhance biogas output, methane yield, and the overall efficacy of anaerobic digestion in the R4-(FW+cryogel+NPs) reactor.
4. Conclusions
This study demonstrates the potential of food waste as a viable bioenergy source through advanced AD techniques. The integration of Ag@ZnONPs catalyst and cryogel-immobilized AS significantly improved biogas and biomethane production, with the highest yield observed in R4-(FW+cryogel+NPs). The synergistic effect of NPs and cryogel enhanced substrate degradation, microbial retention, and process stability, leading to higher methane content and better conversion efficiency. The findings suggest that this approach can be scaled for industrial applications, particularly in municipal waste treatment plants and agro-industrial facilities, where enhanced AD performance can contribute to sustainable waste management and renewable energy generation. These advancements align with global efforts to develop environmentally friendly waste-to-energy solutions and promote circular economy principles.
Acknowledgment
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number (0146-1443-S).
CRediT authorship contribution statement
Adel Alalawy, Mohamed Sakran, Nahla Zidan, Fahad Alzuaibr, and Mahmoud Abdelaziz: Conceptualization, Methodology, Data curation, Formal analysis, writing original draft, validation, Investigation, Writing - review & editing, Visualization.
Declaration of competing interest
The authors declare no conflict of interest.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Food waste management for biogas production in the context of sustainable development. Energies. 2022;15:6268. https://doi.org/10.3390/en15176268
- [Google Scholar]
- Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Global Ecology and Conservation. 2020;22:e00902. https://doi.org/10.1016/j.gecco.2020.e00902
- [Google Scholar]
- Enhancement of methane production in anaerobic digestion process: A review. Applied Energy. 2019;240:120-137. https://doi.org/10.1016/j.apenergy.2019.01.243
- [CrossRef] [Google Scholar]
- Anaerobic co-digestion of food waste and sewage sludge under mesophilic and thermophilic conditions: Focusing on synergistic effects on methane production. Bioresource Technology. 2020;301:122765. https://doi.org/10.1016/j.biortech.2020.122765
- [CrossRef] [PubMed] [Google Scholar]
- Turning food waste to energy and resources towards a great environmental and economic sustainability: An innovative integrated biological approach. Biotechnology Advances. 2019;37:107414. https://doi.org/10.1016/j.biotechadv.2019.06.013
- [CrossRef] [PubMed] [Google Scholar]
- Anaerobic biorefinery: Current status, challenges, and opportunities. Bioresource Technology. 2016;215:304-313. https://doi.org/10.1016/j.biortech.2016.03.074
- [CrossRef] [PubMed] [Google Scholar]
- Concise review on ethanol production from food waste: Development and sustainability. Environmental Science and Pollution Research International. 2018;25:28851-28863. https://doi.org/10.1007/s11356-018-2972-4
- [CrossRef] [PubMed] [Google Scholar]
- Towards effective management of digester dysfunction during anaerobic treatment processes. Renewable and Sustainable Energy Reviews. 2019;116:109424. https://doi.org/10.1016/j.rser.2019.109424
- [CrossRef] [Google Scholar]
- Enhanced anaerobic digestion of food waste by thermal and ozonation pretreatment methods. Journal of Environmental Management. 2014;146:142-9. https://doi.org/10.1016/j.jenvman.2014.07.042
- [CrossRef] [PubMed] [Google Scholar]
- Biogas production from anaerobic co-digestion of waste activated sludge: Co-substrates and influencing parameters. Reviews in Environmental Science and Bio/Technology. 2019;18:771-793. https://doi.org/10.1007/s11157-019-09515-y
- [CrossRef] [Google Scholar]
- A critical review of volatile fatty acids produced from waste activated sludge: Enhanced strategies and its applications. Environmental Science and Pollution Research International. 2019;26:13984-13998. https://doi.org/10.1007/s11356-019-04798-8
- [CrossRef] [PubMed] [Google Scholar]
- Effects of thermal pretreatment on degradation kinetics of organics during kitchen waste anaerobic digestion. Energy. 2017;118:377-386. https://doi.org/10.1016/j.energy.2016.12.041
- [CrossRef] [Google Scholar]
- Effect of ethanol pre-fermentation and inoculum-to-substrate ratio on methane yield from food waste and distillers’ grains. Applied Energy. 2015;155:846-853. https://doi.org/10.1016/j.apenergy.2015.04.081
- [Google Scholar]
- Arabinoxylans: A review on protocols for their recovery, functionalities and roles in food formulations. International Journal of Biological Macromolecules. 2024;259:129309. https://doi.org/10.1016/j.ijbiomac.2024.129309
- [CrossRef] [PubMed] [Google Scholar]
- Dark fermentation and microalgae cultivation coupled systems: Outlook and challenges. The Science of the Total Environment. 2023;865:161136. https://doi.org/10.1016/j.scitotenv.2022.161136
- [CrossRef] [PubMed] [Google Scholar]
- Strategies and applications of enhancing extracellular electron transfer in anaerobic digestion for wastewater resource recovery:A critical review. Environmental Functional Materials 2025 [Article in Press]. https://doi.org/10.1016/j.efmat.2025.01.001
- [CrossRef] [Google Scholar]
- Enhancing and upgrading biogas and biomethane production in anaerobic digestion: A comprehensive review. Frontiers in Energy Research. 2023;11:1170133. https://doi.org/10.3389/fenrg.2023.1170133
- [CrossRef] [Google Scholar]
- Improvement of aspergillus flavus saponin hydrolase thermal stability and productivity via immobilization on a novel carrier based on sugarcane bagasse. Biotechnology Reports (Amsterdam, Netherlands). 2017;17:55-62. https://doi.org/10.1016/j.btre.2017.12.007
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SFF) Bioresource Technology. 2019;272:1-9. https://doi.org/10.1016/j.biortech.2018.09.143
- [CrossRef] [PubMed] [Google Scholar]
- Fast anaerobic sludge granulation at elevated salinity. Water Research. 2018;128:293-303. https://doi.org/10.1016/j.watres.2017.10.038
- [CrossRef] [PubMed] [Google Scholar]
- Optimization of the use of His6-OPH-based enzymatic biocatalysts for the destruction of chlorpyrifos in soil. International Journal of Environmental Research and Public Health. 2017;14:1438. https://doi.org/10.3390/ijerph14121438
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Enhanced dark hydrogen fermentation by addition of ferric oxide nanoparticles using enterobacter aerogenes. Bioresource Technology. 2016;207:213-9. https://doi.org/10.1016/j.biortech.2016.02.009
- [CrossRef] [PubMed] [Google Scholar]
- High-solid anaerobic digestion: Reviewing strategies for increasing reactor performance. Environments. 2021;8:80. https://doi.org/10.3390/environments8080080
- [Google Scholar]
- Application of nano-structured materials in anaerobic digestion: Current status and perspectives. Chemosphere. 2019;229:188-199. https://doi.org/10.1016/j.chemosphere.2019.04.193
- [CrossRef] [PubMed] [Google Scholar]
- Review of impact of nanoparticle additives on anaerobic digestion and methane generation. Fuel. 2020;277:118234. https://doi.org/10.1016/j.fuel.2020.118234
- [CrossRef] [Google Scholar]
- Antibacterial effect of low-concentration znO nanoparticles on sulfate-reducing bacteria under visible light. Nanomaterials (Basel, Switzerland). 2023;13:2033. https://doi.org/10.3390/nano13142033
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Total solids content drives high solid anaerobic digestion via mass transfer limitation. Bioresource Technology. 2012;111:55-61. https://doi.org/10.1016/j.biortech.2012.01.174
- [CrossRef] [PubMed] [Google Scholar]
- Standard Methods for the Examination of Water and Wastewater. American Public Health Association; 2017.
- Turning waste into value: Extraction and effective valorization strategies of seafood by-products. Waste Management Bulletin. 2024;2:84-100. https://doi.org/10.1016/j.wmb.2024.06.008
- [CrossRef] [Google Scholar]
- Valorization of fruits and vegetables waste byproducts for development of sustainable food packaging applications. Waste Management Bulletin. 2024;2:21-40. https://doi.org/10.1016/j.wmb.2024.08.005
- [CrossRef] [Google Scholar]
- Methane productivity evaluation of an invasive wetland plant, common reed. Biomass Conversion and Biorefinery. 2020;10:689-695. https://doi.org/10.1007/s13399-019-00451-z
- [CrossRef] [Google Scholar]
- Responses of anaerobic digestion of food waste to coupling effects of inoculum origins, organic loads and pH control under high load: Process performance and microbial characteristics. Journal of Environmental Management. 2021;279:111772. https://doi.org/10.1016/j.jenvman.2020.111772
- [CrossRef] [PubMed] [Google Scholar]
- Life cycle assessment of common reed (Phragmites australis (Cav) trin. ex steud) cellulosic bioethanol in jiangsu province, China. Biomass and Bioenergy. 2016;92:40-7. https://doi.org/10.1016/j.biombioe.2016.06.002
- [CrossRef] [Google Scholar]
- Simultaneous enhancement of methane production and methane content in biogas from waste activated sludge and perennial ryegrass anaerobic co-digestion: The effects of pH and c/N ratio. Bioresource Technology. 2016;216:323-330. https://doi.org/10.1016/j.biortech.2016.05.100
- [CrossRef] [PubMed] [Google Scholar]
- Methane production characteristics of an anaerobic co-digestion of pig manure and fermented liquid feed. Molecules (Basel, Switzerland). 2022;27:6509. https://doi.org/10.3390/molecules27196509
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Valorization and repurposing of seafood waste to next-generation carbon nanofertilizers. Bioresource Technology. 2025;416:131783. https://doi.org/10.1016/j.biortech.2024.131783
- [CrossRef] [PubMed] [Google Scholar]
- A critical review for the impact of anaerobic digestion on the sustainable development goals. Journal of Environmental Management. 2024;349:119458. https://doi.org/10.1016/j.jenvman.2023.119458
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of industrial waste black cumin (Nigella sativa) for biohydrogen production without pretreatment. Environment, Development and Sustainability. 2022;24:12182-12202. https://doi.org/10.1007/s10668-021-01939-3
- [Google Scholar]
- A review on process modeling and design of biohydrogen. International Journal of Hydrogen Energy. 2022;47:30404-30427. https://doi.org/10.1016/j.ijhydene.2022.06.317
- [CrossRef] [Google Scholar]
- Bioaugmentation with marine sediment-derived microbial consortia in mesophilic anaerobic digestion for enhancing methane production under ammonium or salinity stress. Bioresource Technology. 2023;376:128853. https://doi.org/10.1016/j.biortech.2023.128853
- [CrossRef] [PubMed] [Google Scholar]
- Effect of bioaugmentation on psychrotrophic anaerobic digestion: Bioreactor performance, microbial community, and cellular metabolic response. Chemical Engineering Journal. 2023;455:140173. https://doi.org/10.1016/j.cej.2022.140173
- [CrossRef] [Google Scholar]
- Effects of silver nanoparticles on performance of anaerobic digestion of sewage sludge and associated microbial communities. Renewable Energy. 2021;171:1014-1025. https://doi.org/10.1016/j.renene.2021.02.127
- [CrossRef] [Google Scholar]
- Application of nanomaterials in anaerobic digestion processes: A new strategy towards sustainable methane production. Biochemical Engineering Journal. 2022;188:108694. https://doi.org/10.1016/j.bej.2022.108694
- [CrossRef] [Google Scholar]
- Effects of different materials on biogas production during anaerobic digestion of food waste. Sustainability. 2023;15:5698. https://doi.org/10.3390/su15075698
- [CrossRef] [Google Scholar]
- The impact of different types of high surface area brush fibers with different electrical conductivity and biocompatibility on the rates of methane generation in anaerobic digestion. The Science of the Total Environment. 2021;787:147683. https://doi.org/10.1016/j.scitotenv.2021.147683
- [CrossRef] [PubMed] [Google Scholar]
- Anammox sludge immobilized in polyvinyl alcohol (PVA) cryogel carriers. Bioresource Technology. 2012;114:231-240. https://doi.org/10.1016/j.biortech.2012.03.077
- [CrossRef] [PubMed] [Google Scholar]
- Brewery spent grain valorization through fermentation: Targeting biohydrogen, carboxylic acids and methane production. Process Safety and Environmental Protection. 2024;191:206-17. https://doi.org/10.1016/j.psep.2024.08.071
- [Google Scholar]
- Insights into biohydrogen production through dark fermentation of food waste: Substrate properties, inocula, and pretreatment strategies. Energies. 2024;17:6350. https://doi.org/10.3390/en17246350
- [CrossRef] [Google Scholar]
- Nanomaterials as catalysts for CO2 transformation into value-added products: A review. The Science of the Total Environment. 2023;868:161547. https://doi.org/10.1016/j.scitotenv.2023.161547
- [CrossRef] [PubMed] [Google Scholar]
- Application of nanotechnology in the production of biohydrogen: A review. Chemical Engineering & Technology. 2023;46:218-233. https://doi.org/10.1002/ceat.202000565
- [CrossRef] [PubMed] [Google Scholar]
- Enhancement effect of silver nanoparticles on fermentative biohydrogen production using mixed bacteria. Bioresource Technology. 2013;142:240-5. https://doi.org/10.1016/j.biortech. 2013.05.042
- [CrossRef] [PubMed] [Google Scholar]
- Comparative effect of silver nanoparticles (AgNPs) derived from actinomycetes and henna on biohydrogen production by Clostridium beijerinckii (KTCC1737) International Journal of Energy Research. 2021;45:17269-78. https://doi.org/10.1002/er.6076
- [Google Scholar]
- Effect of green synthesized silver oxide nanoparticle on biological hydrogen production. International Journal of Hydrogen Energy. 2022;47:19517-19525. https://doi.org/10.1016/j.ijhydene.2021.11.176
- [CrossRef] [Google Scholar]
- Factors affecting biohydrogen production: Overview and perspectives. International Journal of Hydrogen Energy. 2023;48:27513-27539. https://doi.org/10.1016/j.ijhydene.2023.04.001
- [CrossRef] [Google Scholar]
- The environmental sustainability of anaerobic digestion as a biomass valorization technology. Bioresource Technology. 2012;121:396-403. https://doi.org/10.1016/j.biortech.2012.06.109
- [CrossRef] [PubMed] [Google Scholar]
- An overview of biogas production from anaerobic digestion and the possibility of using sugarcane wastewater and municipal solid waste in a south african context. Applied System Innovation. 2023;6:13. https://doi.org/10.3390/asi6010013
- [CrossRef] [Google Scholar]







