Translate this page into:
Efficient kefiran production by Lactobacillus kefiranofaciens ATCC 43761 in submerged cultivation: Influence of osmotic stress and nonionic surfactants, and potential bioactivities
⁎Corresponding author at: Institute of Bioproduct Development (IBD), Universiti Teknologi Malaysia (UTM), 81310 UTM Skudai, Malaysia. henshasy@ibd.utm.my (Hesham A. El Enshasy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Kefiran is a water soluble polysaccharide produced by Lactobacillus kefiranofaciens ATCC 43761. It has wide potential applications in food, pharmaceutical and nutraceutical industries. To the best of our knowledge, there have been no previous reports on the effect of osmotic stress and ionic surfactants on kefiran production by L. kefiranofaciens ATCC 43761. Accordingly, the current work aimed at optimizing kefiran production as affected by osmotic stress and nonionic surfactants in submerged cultivation system. Afterwards, the work was extended to investigate cytotoxic as well as antioxidant potentials of kefiran. Firstly, different osmolarities, different ionic surfactants (Triton X-100, Tween 20, Tween 80) as well as their concentrations and addition time were evaluated. The kinetics of cell growth and kefiran production were evaluated before and after the addition of surfactants. Results clearly demonstrated that osmotic stress and surfactant addition had a stimulatory effect on kefiran production. Using the optimal medium osmolality, 550 mOsmol.kg−1, kefiran production was enhanced from 1.29 to about 1.38 g.L−1. Furthermore, Triton X-100 was found to be the best surfactant stimulating kefiran production when added at a concentration of 1.0 g.L−1 at the onset of cultivation process (0 h). This increased kefiran production from 1.38 g.L−1 to 1.62 g.L−1. To summarize, the maximal kefiran production can be enhanced using 550 mOsmol.kg−1 and by adding 1.0 g.L−1 of Triton X-100 at 0 h. The new optimized medium showed an increase of about 25.6% in kefiran production (1.29 up to 1.62 g.L−1). After this step, the process was further optimized in 16-L stirred tank bioreactor. Maximal kefiran production reached 2.32 g.L−1 and 1.87 g.L−1 in bioreactor under control and un-controlled pH conditions, respectively, corresponding to 72.9 and 45.0% increase from the initial production titer, respectively. The produced kefiran exhibited promising anticancer activity against breast cancer (MCF-7) cells, with an IC50 value of 193.89 μg.mL−1. Also, kefiran showed 96.58% radical scavenging activity at 100 μg/mL, with an ED50 recorded of 12.29 ± 0.98 μg.mL−1.
Keywords
Lactobacillus kefiranofaciens
Kefiran
Osmotic stress
Surfactants
Submerged cultivation
1 Introduction
Kefiran, exopolysaccharides (EPS) present in the kefir grains, was first discovered in 1967 (Dailin et al., 2015, 2016). Certain lactic acid bacteria colonizing the kefir grains produce kefiran as a water soluble polysaccharide (Kandler and Kunath, 1983). Kefiran is a polymer with repeating unit of approximately equal amounts of D-glucose and D-galactose residues (Kooiman, 1968; Micheli et al., 1999). Recently, kefiran gained increased interest in food industries due to its potential in providing required rheological properties for the dairy products (Vuyst et al., 2001). When added to food products, polysaccharides such as kefiran could function as thickeners (Rimada and Abraham, 2006), gelling agents, (Zavala et al., 2015) and water binding agents (Piermaria et al., 2011). It was also reported to have anti-cancer (Elsayed et al., 2017; Sharifi et al., 2017), anti-oxidant (Chen et al., 2015), anti-bacterial (Blandón et al., 2016), anti-fungal (Cevikbas et al., 1994), anti-inflammatory (Furuno and Nakanishi, 2012) and cicatrizing agents (Rodrigues et al., 2005), and have been used to treat a variety of infections (Dailin et al., 2016). Recent studies show that kefiran has a potential application in nanotechnology fields (Esnaashari et al., 2014).
During their growth, bacterial cells are subjected to different types of stress, i.e. physical, chemical or biological (Le Marrec, 2011). Therefore, for their survival, cells must develop a certain response towards different stresses such as osmotic, pressure, pH, temperature and nutrient limitation (Santivarangkna et al., 2008). The literature contains scarce information about the effect of osmotic stress on microbial cell growth and polysaccharide production. In one study, it was reported that osmotic stress is an important factor for cell growth and EPS production by Bacillus agaradhaerens C9 (Liu et al., 2015). They concluded that cell resistance can be enhanced using NaCl as Na+/H+ antiporter. Furthermore, the water activity of lactic acid bacteria decreased by the addition of salts, which consequently affects cellular growth ((Robert et al., 2000). Surfactants have wide applications in biotechnological processes (Sheng et al., 2013). They have been applied in the fermentative production processes of various biomolecules such as Tween-40 for welan production (Xu et al., 2012), ween 80, Tween 40 and Triton X-100 for gellan production (Arockiasamy and Banik, 2008), sodium dedecyl sulfate, cetyltrimethyl ammonium bromide, polyoxyethylene lauryl ether, and Tween80 for glutathione production (Wei et al., 2003), Tween 20, Tween 40, Tween 80 and Triton X-100 for xanthan production (Janas et al., 2003), Tween 80, Tween 40 and Triton X-100 for levan production (Devi and Alamu, 2013) and Triton X-100, CHAPS, Tween-80 and sodium taurocholate for pullulanase and β-amylase production (Seenayya et al., 1999). Surfactants can be classified according to their properties, i.e. chemical structure, charge, hydrophilic-lipophilic balance (HLB), critical micelle concentration (CMC) and source (Ward et al., 2006). Generally, different microbial strains respond differently towards the same surfactants (Koch et al., 2007). Accordingly, it is worthwhile to determine the most suitable surfactant affecting the production bioprocess of interest. Surfactants play a great role in increasing the microbial production of polysaccharides (Liu and Wu, 2012). They function by increasing cell membrane permeability, which works as a natural barrier for extracellular substrate transport and product secretion (Wu et al., 2008). Authors reported that increased membrane permeability enhances membrane fluidity and thus allowing more uptake of extracellular substrates and increased secretion of intracellular products, i.e. their produced PGA.
Therefore, the objective of the current study was to investigate the effect of different osmotic stress values on kefiran production. Additionally, three nonionic surfactants, i.e; Triton X-100 (nonaethylene glycol octylphenol ether), Tween 20 (polyoxyethylene sorbitan monolaurate) and Tween 80 (polyoxyethylene sorbitan monooleate) were tested for their influence kefiran production in submerged cultivation. Moreover, work was extended to evaluate the production process in stirred-tank bioreactor under controlled and uncontrolled pH conditions. Finally, the anticancer and antioxidant activities of the produced kefiran were examined.
2 Materials and methods
2.1 Microorganism
The strain used throughout this study is Lactobacillus kefiranofaciens ATCC 43761 obtained in lyophilized form from American Type Culture Collection (ATCC, University Boulevard, Manassas, VA 20110 USA). The lyophilized cells were first activated in Man-Ragosa-Sharpe (MRS) broth medium consisting of (g.L−1): peptone casein, 30; meat extract, 10; yeast extract, 6.0; glucose, 2.0; triammonium citrate, 2.0; CH3COONa, 5.0; MgSO4·7H2O, 0.2; MnSO4·5H2O, 0.05; K2HPO4, 2.0. The pH was adjusted to 5.5 before sterilization. The working microbial cell bank was prepared by inoculating MRS broth medium with cells grown on MRS agar medium and incubating cells for 24 h at 30 °C and 200 rpm in a rotary shaker (Innova 4080, New Brunswick Scientific, NJ, USA). Grown cells were harvested, washed with saline buffer, centrifuged (Eppendorf, NJ, USA) and then frozen in 2 mL cryovials using 50% glycerol solution, and stored at −78 °C.
2.2 Inoculum preparation and kefiran production medium
Inoculum was prepared by inoculating 250 mL Erlenmeyer flasks containing 50 mL of MRS liquid medium with 0.5 mL of the frozen working cell bank cultures. The inoculated flasks were incubated at 200 rpm and 30 °C for 24 h. At a concentration of 5% (v/v), grown cells were used to inoculate the kefiran production medium, which composed of (g.L−1): Lactose, 50.0; yeast extract, 12.0; K2HPO4, 0.25; CH3COONa, 5.0; triammonium citrate, 2.0; MgSO4·7H2O, 0.2; MnSO4·5H2O, 0.05. Lactose was sterilized separately at 110 °C for 20 min and added to the medium before inoculation. Inoculated flasks were incubated at 200 rpm and 30 °C on rotary shaker (Innova 4080, New Brunswick, NJ, USA).
2.3 Effect of surfactants
Different surfactants of high reagent grade including Tween 20 (polyoxyethylene sorbitan monopalmitate), Tween 80 (polyoxyethylene sorbitan monooleate) and Triton X-100 (nonaethylene glycol octylphenol ether), were screened for their possible effect on cell growth and kefiran production throughout this part of the work. Each surfactant was added to the cultivation medium at the beginning of the cultivation time at a concentration of 1.0 g.L−1. Subsequently, different concentrations (0.0–2.0 g.L−1) of the most suitable surfactant were tested. Finally, the effect of different addition times (0.0–48.0 h) of the most promising concentration to the production medium was evaluated. The inoculated flasks were incubated on a rotary shaker (Innova 4080, New Brunswick, NJ, USA) at 200 rpm and 30 °C for 72 h.
2.4 Bioreactor cultivation
Bioreactor cultivations were conducted using semi-industrial scale-16-L stirred tank bioreactor (BioEngineering, Wald, Switzerland) having a working volume of 8 L. Sterilization was performed in situ at 121 °C for 20 min. After which, the bioreactor was cooled and inoculated at a 5% ratio of inoculum to cultivation medium. Bioreactor cultivations were run at the same conditions (pH, temperature) as the shake flask ones. However, agitation speed was adjusted to 400 rpm throughout the cultivation, and filtered sterile air was supplied continuously to the bioreactor at a rate of 1.0 v v−1 min−1. The bioreactor stirrer is equipped with two 6-bladded rushton turbine impellers (di(impeller diameter) = 85 mm; dt(tank diameter) = 214 mm, didt-1 = 0.397). Silicon-based antifoam grade A (Sigma-Aldrich Inc., MO, USA) was supplied to the bioreactor to suppress foam generation. During the cultivation process, pH and dissolved oxygen concentration were determined using liquid filled pH electrode and DO polarographic electrodes (Ingold, Mettler-Toledo, Switzerland), respectively. During controlled-pH cultivations, the initial medium pH was adjusted to 6.0 by cascading the pH controller with acid/base feeding peristaltic pumps connected to 2.0 M HCl and 2.0 M NaOH, respectively.
2.5 Analysis
2.5.1 Sample preparation and cell dry weight determination
Samples in the form of two flasks containing 50 mL of cultivation medium, or 50 mL in case of bioreactor cultivations, were withdrawn at different time intervals throughout the cultivation process. Immediately after collection, the fermentation broth was centrifuged at 9000 rpm (Eppendorf, NJ, USA) for 15 min, and the precipitated cells were separated. The supernatant was used for determining the concentration of kefiran. Cell pellets were washed with saline solution, re-centrifuged, and the supernatant was discarded. Cell pellets were dried at 65 °C for 48 h until a constant cell weight was obtained.
2.5.2 Determination of osmotic stress
The cells of L. kefiranofaciens were cultivated in the production medium having different osmolality values. Sodium chloride was added to the production medium to create different osmotic stress conditions. Different initial osmolalities (449–750 mOsmol.kg−1) of production media were tested. The required osmotic values of the medium were adjusted using digital freezing point osmometer (K-7400S, Knauer Wissenschaftliche Geräte GmbH, Berlin, Germany). Inoculated flasks were incubated on a rotary shaker (Innova 4080, New Brunswick, NJ, USA) at 200 rpm and 30 °C for 72 h.
2.5.3 Kefiran determination
Kefiran was extracted from the cultivation broth and was used to determine its concentration as described in our previous work (Dailin et al., 2016). Extraction proceeded by the addition of cold absolute ethanol at 4 °C at a ratio of 1:1 (v/v), and the mixture was left overnight to ensure complete precipitation. The mixture was then centrifuged at 9000 rpm for 15 min to separate the resulting precipitate, which was then dissolved in hot distilled water and re-precipitated with cold absolute ethanol. The final step was repeated at least three times to obtain pure kefiran, which was then dried at 65 °C for 48 h, and weighed.
2.5.4 Determination of anticancer and antioxidant activities of kefiran
Human breast cancer cells, MCF-7, as well as normal non-tumorigenic human breast cells, MCF-10A cells, (Sigma-Aldrich Chemical Company, St. Louis, MO, USA) were used. Cells maintained on Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% antibiotic/antimycotic solution and 3.6 g.L−1 NaHCO3. Cell cultivation and preparation was performed as per our previously developed protocol (Elsayed et al., 2015a). The in vitro cytotoxic effects of the produced kefiran was investigated using standard MTT assay (Elsayed et al., 2016a). The method depends on the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) by mitochondrial dehydrogenases in living cells. After being trypsinized and washed, 100 μL of cell suspension were seeded into 96-well plates to give a concentration of 104 cells/well. Plates were then incubated at standard conditions for 24 h. After cell adherence, medium was exchanged with fresh medium containing serial dilutions of LLE extract, and then incubated for another 24 h. Afterwards, MTT was added (10 μL/well, 5 mg.mL−1 in PBS), and plates were placed into the incubator for 4 h. Supernatants were aspirated, and DMSO (200 μL) was added. The absorbance of the developed formazan was read at 550 nm using a microplate reader (Thermo Scientific, Waltham, MA, USA) and was correlated with the percentages of viable cells. IC50 value were calculated from the linear regression of the calibration curve.
The antioxidant activity of the produced kefiran was determined using standard DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay (Mousa et al., 2019). Briefly, 50 μg of the produced kefiran was added to 5 mL of 0.004% ethanol solution of DPPH. The mixture was incubated at room temperature for 30 min. The absorbance was measured at 517 nm against blank using a UV–Vis spectrophotometer (Pharmacia Biotech, Cambridge, England). Ascorbic acid was used as a reference standard. The scavenging activity of the DPPH radical was expressed as inhibition percentage I (%), which was calculated as follows: in which AS is the absorbance of the tested sample, and AC is the absorbance of the control reaction (contains all reagents except the tested sample).
2.6 Statistical analysis
The obtained results were statistically analyzed using the SPSS software 19. Analysis of variance was performed by one-way ANOVA procedures. Data were presented as means ± SD, and significant differences between means were determined by the least significant digit (LSD) multiple range test at a level of P < 0.05.
3 Results and discussion
3.1 Effect of osmotic stress on cell growth and kefiran production
Osmolality plays an important role in polysaccharide production where cells produce polysaccharides to protect themselves from the adverse environment (Zeidan et al., 2017). Different initial medium osmolality ranged between 449 and 750 mOsmol.kg−1 were tested. Fig. 1 shows the effect of different osmolality on cell growth, kefiran production, cell productivity and pH. Generally, it can be noticed that increasing initial medium osmolality above certain limit, significantly had an inhibitory effect on cell growth of L. kefiranofaciens. As shown, increasing osmolality from 449 to 500 mOsmol.kg−1 slightly increased cell dry weight from 2.04 to 2.10 g.L−1. However, further increase of osmolality above 500 mOsmol.kg−1 greatly reduced cell growth by about 44.5% (1.17 at 750 mOsmol.kg−1). In addition, the kefiran production was enhanced by about 11.3% when osmolality was increased from 449 to 550 mOsmol.kg−1, where maximal kefiran production obtained increased from 1.24 up to 1.38 g.L−1, respectively. On the other hand, further increase of medium osmolality greatly inhibited kefiran production, where it gradually decreased from 1.38 at 550 mOsmol.kg−1 and reached a minimal of 0.475 g.L−1 at 750 mOsmol.kg−1. Accordingly, cell productivity reached its maximum (0.67 g kefiran.g−1 cells) at 550 mOsmol.kg−1, and then decreased gradually mainly due to decrease in cell mass and production. Therefore, an initial osmolality of 550 mOsmol.kg−1 was selected for the following experiments.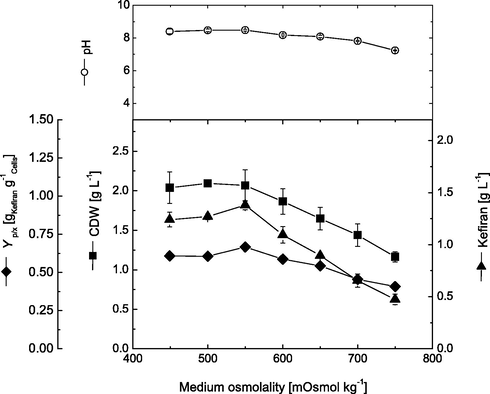
Effect of different medium osmolality on cell growth and kefiran production by L. kefiranofaciens. Data were taken after submerged cultivations for 72 h.
Despite the presence of various reports about the effect of different environmental factors on the production of exopolysaccharides, including kefiran, however, there still no published data concerning the effect of osmotic stress on kefiran production. To the best of our knowledge, this is the first report showing the effect of osmotic stress and surfactant addition on kefiran production. However, similar trends were observed for other polysaccharide producing organisms, where osmolality significantly increased the production of polysaccharides (Liu et al., 2015). It has been reported that exopolysaccharide biosynthesis increased at elevated osmotic stress in case of Z. mobilis EPS fermentation, and β-glucan production by Sclerotium rolfsii (Sarilmiser et al., 2015). This can be attributed firstly to the fact that produced polysaccharides result in culture flocculation/aggregation, which can protect cells against increased stress. In addition, under elevated osmotic stress, bacterial cells start to accumulate solutes in the cytoplasm in order to increased internal solute pressure, hence ensuring higher internal solute pressure and continuous flow of water molecules into the cells (Kets et al., 1997; Glaasker et al., 1998; Le Marrec, 2011). Furthermore, lactic acid bacteria (LAB) have been found to counteract the effect of increased osmotic stress by using intracellular metabolic protectants as glycine betaine and choline to replace the increased solutes (Le Marrec, 2011). Also, LAB have been reported to modulate types and concentrations of their amino acid pools as a secondary protective mechanism against osmotic stress (Tsakalidou and Papapdimitriou, 2011). LAB were also found to use their own metabolic pool of sugars to encounter increased osmotic stress conditions (Sunny-Roberts and Knorr, 2008). On the other hand, our results indicated that higher values of applied osmotic stress drastically decreased both cell growth and kefiran production. These results are in good agreement with those reported by Hutkins et al. (1987), who found that different Lactobacillus strains cannot survive NaCl concentrations higher than 0.3–0.6 M. Additionally, higher osmotic stress conditions were found to reduce growth rates of L. lactis and Z. mobilis by 30–50 and 78%, respectively (Kilstrup et al., 1997; Sootsuwanet al., 2013).
3.2 Effect of different type of surfactants on cell growth and kefiran production
The effect of surfactants on the production of exopolysaccharides is strain dependent (Xu et al., 2015). Different polysaccharide producing microbial strains have been found to respond differently towards the same surfactant used (Liu and Wu, 2012). Application of certain amount of surfactants increased significantly the production of EPS (Zhang and Cheung, 2011; Xu et al., 2012; Elsayed et al., 2016b). However, the effect of surfactant on cell growth and kefiran production has not yet been reported. Therefore, the effect of different surfactants, i.e. Triton X-100, Tween 20 and Tween 80 on cell growth and kefiran production was investigated. Fig. 2 shows the effect of different types of surfactants on cell dry weight, kefiran production, cell productivity and pH. As shown, highest cell dry weight of about 2.90 g.L−1 was obtained when Tween 80 was added into the fermentation medium. This was followed by Triton X-100, Tween 20 and finally the control experiment, where the final cell dry weight recorded were 2.75, 2.17 and 2.10 g.L−1, respectively. On the other hand, of the three detergents investigated, Triton X-100 was found to be the most suitable for improving kefiran production, where the highest volumetric production of about 1.62 g.L−1 was obtained. In contrast, Tween 20 and Tween 80 had an inhibitory effect on kefiran production, where its production decreased from the control experiment (1.27 g.L−1) by about 24.8 and 19.3%, respectively, and reached about 0.955 and 1.025 g.L−1, respectively. Furthermore, highest cell productivity of about 0.59 g kefiran.g−1 cells was recorded upon adding Triton X-100 to the production medium, which was almost the same as cell productivity in control experiments (0.58 g kefiran.g−1 cells). On the other hand, Tweens 20 and 80 afforded lower cell productivities about 0.44 g.L−1 and 0.35 g.L−1, respectively.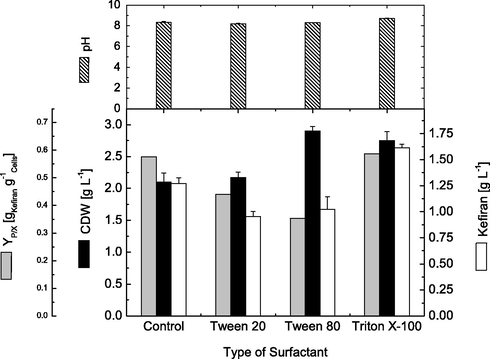
Effect of different types of surfactant on cell growth and kefiran production by L. kefiranofaciens. Data were taken after submerged cultivations for 72 h.
Obtained results showed that Triton X-100 was the most suitable for enhancing cellular growth and kefiran production (Fig. 2). Moreover, addition of other surfactants (Tween 20 and 80) greatly supported higher cellular growth and decreased kefiran production. Exopolysaccharide production has been reported to be significantly affected with the addition of surfactants (Seenayya et al., 1999; Arockiasamy and Banik, 2008; El Enshasy et al., 2011). Xanthan production was improved upon addition of Triton X-100, Tweens 20 and 80 (Devi and Alamu, 2013). Recently, Triton X-100 was found to greatly enhance cell growth as well as EPS production (Xu et al., 2015). The promoting effects of surfactants on cell growth and EPS production is mainly attributed to surfactant-cell wall interactions (Zeidan et al., 2017). Due to the amphipathic characteristics of surfactants they can diffuse through cell membrane, increase cell membrane permeability, and increase nutrient uptake efficiency, hence enhancing cellular growth and substrate consumptions rates and product secretion (Arockiasamy and Banik, 2008; Wu et al., 2008; Chen et al., 2010). Furthermore, surfactants are reported to decrease medium heterogeneity and facilitate nutrient and microbial oxygen transfer rates (Kumar et al., 2007; Sheng et al., 2013). Also, surfactants have been suspected to change the microbial physiology and certain membrane-associated enzymatic functions (El Enshasy et al., 2011).
Our obtained results revealed that the addition of Tweens 20 and 80 reduced kefiran production and increased cell biomass than the control experiment. These results are also in good agreement with those reported earlier (Hsieh et al., 2008). Authors found that Tween 80 increased cellular growth on the expense of EPS production, and they explained their results suggesting that Tween 80 is degraded by microbial lipases into oleic acid, which is used to enhance cellular growth than EPS production. However, our results showed that Tween 80 supported cell growth of L. kefiranofaciens. It has been found that there is no clear correlation between maximal cell growth and maximal exopolysaccharide production in different bacteria (Zeidan et al., 2017). Authors explained that cells compete for the available nutritional sources and direct them towards production pathways, on the expense of cellular growth pathways. They concluded that some bacterial strains were found to produce their maximal EPS under conditions favoring optimal cell growth, while others produced optimal EPS concentrations under suboptimal growth conditions (Sheng et al., 2016).
3.3 Effect of different concentrations of Triton X-100 on cell growth and kefiran production
Previous experiment showed that Triton X-100 was the most favorable surfactant for kefiran production. Due to the scarce of information regarding the effect of surfactants on kefiran production, therefore, different concentrations of Triton X-100 are expected to affect both cell growth and polysaccharide production. Accordingly, different concentrations of Triton X-100 ranging from 0.0 to 2 g.L−1 were evaluated. Results presented in Fig. 3 show that increasing Triton X-100 concentration up to 1.0 g.L−1 increased cell growth by about 28.5% (from 2.14 to 2.75 g.L−1) as well as kefiran production by about 34.4% (from 1.22 to 1.64 g.L−1). Furthermore, increase in Triton X-100 concentration above 1.0 g.L−1 decreased both cell growth and kefiran production drastically. Addition of 2.0 g.L−1 Triton X-100 significantly reduced cell growth by 61.9% (1.05 g.L−1) and kefiran production by 74.7% (0.42 g.L−1) from the maximal results obtained when 1.0 g.L−1 Triton X-100 was added. On the other hand, maximal cell productivity of 0.66 g kefiran g1 cells was obtained at 0.5 g.L−1 Triton X-100. However, this increase in specific cell productivity at 0.5 g.L−1 is mainly due to lower cell growth obtained at that concentration, and not due to increased cell productivity. This is because, at 0.5 and 1.0 g.L−1, the obtained cell dry weights were 2.315 and 2.74 g.L−1, respectively.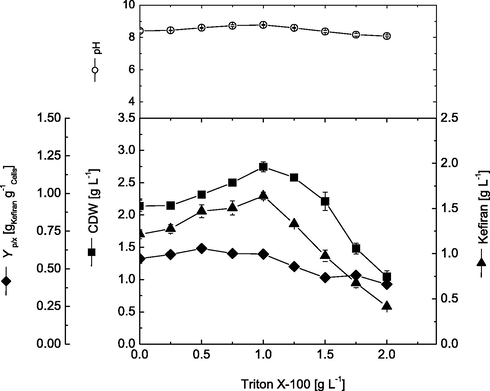
Effect of different Triton X-100 concentrations on cell growth and kefiran production by L. kefiranofaciens. Data were taken after submerged cultivations for 72 h.
The effect of different surfactants on EPS production by bacteria has been found to act in a dose-dependent manner, showing an optimal concentration, above which, growth and production decrease significantly (Janas et al., 2003; Liang et al., 2018). These findings correlate well with our reported results concerning kefiran production. Higher surfactant concentration might affect membrane integrity and interact with other cellular components, which in turn decreases cell growth and metabolic activities (Hsieh et al., 2008; Xu et al., 2015). Moreover, many surfactants are known to show bacteriostatic effects at higher concentrations (Masaki et al., 1990).
3.4 Effect of different Triton X-100 addition time on cell growth and kefiran production
Effect of the addition time of the most suitable concentration of Triton X-100 was investigated, where it was added at different time intervals ranging from 0.0 to 48 h of cultivation. Results presented in Fig. 4 clearly revealed that addition of Triton X-100 after the start of the cultivation process had an inhibitory effect on both cell growth and kefiran production. In other words, for optimum production, Triton X-100 should be added at the beginning of the cultivation (0 h), where maximal cell growth (2.72 g.L−1) and kefiran production (1.62 g.L−1) were obtained. On the other hand, later addition of Triton X-100 gradually decreased both cell growth and kefiran production, where minimal values (2.1 g.L−1 and 1.19 g.L−1 for cell mass and kefiran, respectively) were obtained when it was added after 48 h of cultivation. Concerning specific cell productivity, results showed that highest cell productivity was obtained of about 0.60 g kefiran.g−1 cells was obtained when Triton X-100 was added at 0 h. However, there was no noticeable change in cell productivities with later addition of Triton X-100, indicating that the decrease of volumetric kefiran production with later addition results mainly from decreased cell growth, since the obtained kefiran production decreased gradually from 1.59 g.L−1 obtained at 0 h addition and reached a minimum of 1.22 g.L−1 when Triton X-100 was added after 48 h.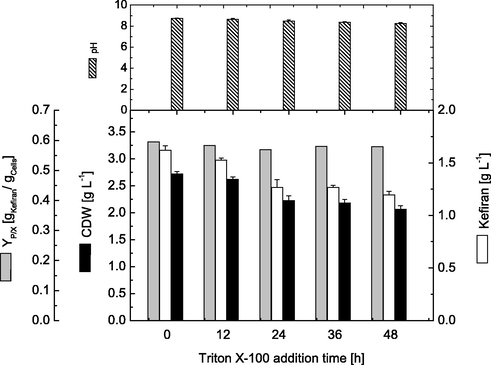
Effect of different Triton X-100 addition time on cell growth and kefiran production by L. kefiranofaciens. Data were taken after submerged cultivations for 72 h.
Other reports showed that addition of triton X-100 at the beginning of fermentation increased hypocrellins and EPS production, and when added lately, the production decreases significantly, and well with those reported earlier (Bautista et al., 2009; Xu et al., 2015). This is mainly due to that early addition increases cellular viability and enzyme secretion, as well as membrane permeability. Surfactants have also been found to be used as a carbon source by many microbial strains upon there addition in the initial growth phase (Açikel et al., 2011; Cai et al., 2011). Accordingly, Triton X-100 might enhance kefiran production by increasing substrate consumption and production rates and subsequently enhancing product secretion.
3.5 Kinetics of cell growth and kefiran production in medium before and after optimization
Shake flask cultivations were performed to investigate the kinetics of cell growth and kefiran production by L. kefiranofaciens cultivated at the initial un-optimized medium in comparison to those cultivated under optimized conditions, i.e. osmolality, 550 mOsmol.kg−1; Triton X-100, 1.0 g.L−1 added at 0 h. Periodic samples were withdrawn from cultivations every 12 h, and analyzed during cultivation process. Results presented in Fig. 5 show that in both cultivations cells grew exponentially during the first 60 h and showed similar patterns of cell growth and kefiran production. However, it can be clearly noticed that optimized conditions favoured cell growth and kefiran production than un-optimized conditions. Under optimized conditions, cells grew with an average growth rate of 0.043 g h−1, which is 34.4% higher than the average growth rate obtained under un-optimized conditions (0.032 g h−1). Accordingly, maximal cell growth reached 2.70 and 1.92 g.L−1 in optimized and un-optimized medium, respectively. After the exponential growth phase, cell growth started to cease and remained more or less constant in both cultivations. The superior cellular growth in case of optimized conditions was reflected on kefiran production. Under optimized conditions, cells produced kefiran with a production rate of 0.031 g.L−1h−1 and produced a maximal of 1.62 g.L−1 at 72 h. On the other hand, cells cultivated under un-optimized conditions produced a maximal of as 1.29 g.L−1 by the end of the cultivation. Also, the average production under un-optimized conditions (0.021 g.L−1.h−1) was lower by about 47.6% than that obtained under optimized conditions, which recorded 0.032 g.L−1.h−1. Although maximal kefiran production increased by about 25.6% under optimized conditions (1.62 g.L−1), however, yield coefficient calculations [YP/X] were used to better understand kefiran production in relation to cell performance. Results showed that maximal production yield in case of optimized conditions (0.63 g kefiran.g−1 cells) was slightly lower than that obtained under un-optimized conditions (0.67 g kefiran.g−1 cells). This can be attributed mainly due to the increase in cell biomass rather than the higher cell performance, where maximal cell dry weights obtained were 2.02 and 2.70 g.L−1 for un-optimized and optimized medium, respectively. The pH behaviour of both cultivations was similar, where the pH started to increase with cultivation time, until the stationary phase, where they reached their maximal by the end of cultivation.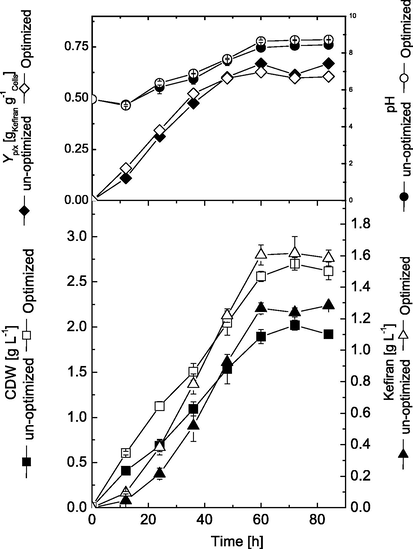
Kinetics of cell growth and kefiran production by L. kefiranofaciens in shake flask cultivations before and after optimization.
Our results showed that optimization of cultivation medium and conditions significantly improved process parameters, resulting in increased cellular growth and kefiran production. These results are in accordance with our reported previously for kefiran production (Dailin et al., 2015, 2016). Optimal medium composition and cultivation conditions play an important role in providing growing cells with essential nutrients at optimal concentrations (Elsayed et al., 2013). Hence, cellular activities reach their maximal status and cell mass and production increases with cultivation progress due to better physiological states of the cells. However, our results showed that the pH increases with cultivation progress, which may affect process performance. Previous reports on EPS production, i.e. pullulan, showed that pH 6 is mostly suitable for better growth and production, and increasing pH to 7.0, 8.0 and 9.0 greatly reduced EPS production by 13.3, 41.2 and 81.4%, respectively (Liu and Wu, 2012). Accordingly, the final optimization step in our production process was designed to investigate the effect of controlling pH throughout the cultivation course in 16-L stirred tank bioreactor.
3.6 Effect of controlled and uncontrolled pH conditions on cell growth and kefiran production
The cultivation process was further optimized by transferring it into the semi-industrial production scale in 16-L stirred bioreactor. Additionally, different bioreactor runs were conducted to compare the effect of controlling pH on the kinetics of cell growth and production of kefiran. Two parallel sets of experiments were performed using working volume of 8-L in the bioreactor under controlled (pH 6.0) and uncontrolled pH. Results obtained in Fig. 6 indicate that cells in both runs grew exponentially directly after inoculation without significant lag phase, however with different growth rates. Under uncontrolled pH, cells grew with an average growth rate of 0.052 g.h−1 reaching a maximal cell growth of 2.9 g.L−1 at 56 h. On the other hand, cells cultivated under controlled pH grew with an average growth rate of 0.074 g.h−1 (higher by about 42.3%), where maximal cell biomass reached 3.28 g.L−1 at 48 h (corresponding to 13% increase in cell biomass). For kefiran production, under uncontrolled pH, kefiran was produced with an average production rate of 0.039 g.L−1.h−1 and reached its maximal volumetric production (1.87 g.L−1) at 52 h. On the other hand, controlled pH improved kefiran production rate by about 43.6% (0.056 g.L−1.h−1) and maximal production by about 24.1% to record 2.32 g.L−1 at 60 h. It can also be noticed, that higher growth rates in controlled pH cultivations was also accompanied with higher lactose consumption rates (−1.12 g.L−1.h−1) as well as higher oxygen uptake rate (−6.34 DO%.h−1) than in case of uncontrolled pH cultivation (0.94 g.L−1.h−1 and −6.1 DO%.h−1, respectively). Such higher growth rate (0.074 g.h−1) and lactose consumption rate in (−1.12 g.L−1.h−1) controlled pH cultivation resulted in faster and earlier depletion of carbon source from the medium (after 44 h, where lactose was almost depleted from the medium), while in uncontrolled pH cultivation lactose was available in the cultivation medium until 60 h (0.1 g.L−1). Therefore, cell entered the stationary phase earlier in the pH controlled cultivation. Specific kefiran production was also calculated to explain cell performance and kefiran production in both cultivations. As shown in Fig. 6, the maximal kefiran yield coefficient of 0.72 g kefiran.g−1 cells was obtained in controlled pH cultivation, which was higher by about 7.5% than kefiran yield in uncontrolled pH cultivation (0.67 g kefiran.g−1 cells). Therefore, it can be concluded that such higher volumetric kefiran production is mainly due to higher cell performance rather than increase in cell biomass (where cell dry weights were 3.165 and 2.67 g.L−1, respectively). Additionally, kefiran production yield based on consumed lactose (YP/S) was also improved under pH controlled cultivation (0.046 g kefiran.g−1 consumed lactose) than that obtained in uncontrolled cultivation (0.038 g kefiran.g−1 consumed lactose).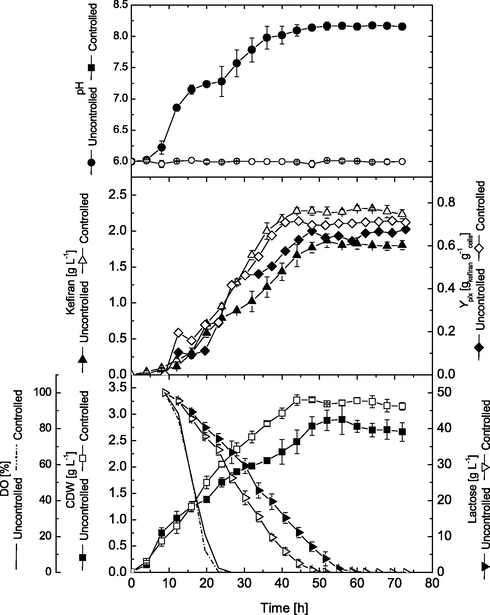
Cell dry weight and kefiran production in 16-L stirred tank bioreactor under uncontrolled and controlled pH conditions.
Obtained results clearly revealed that bioreactor cultivations were superior to those performed in shake flasks in terms of better growth and production rates. Moreover, the cells grew in bioreactor much faster without any noticeable lag phases. This is mainly attributed to the suitable cultivation conditions afforded in bioreactor, in terms of better oxygenation, culture mixing and homogeneity, nutrient distribution and availability (Elsayed et al., 2013, 2015b, 2016c; El Enshasy and Elsayed, 2017). Generally, it can be seen that controlled pH conditions in the bioreactor not only significantly improved cell growth and kefiran production, but also shortened the cultivation time required to obtain maximal growth and production. pH is an important factor influencing cell growth and metabolic activities in submerged fermentation of Lactobacilli, but little work has been done to examine this parameter. Similar results also reported that controlled pH conditions provide better nutrient assimilation leading to higher biomass and polysaccharide production (Cheirsilp et al., 2018). Controlling pH throughout the cultivation course has been used as an accepted strategy to optimize different biotechnological production processes (Elsayedet al., 2014; El Enshasy and Elsayed, 2017).
Furthermore, previous reports on EPS production found that pH conditions close to pH 6.0 are necessary to achieve higher process performance (Zhu et al., 2011). Our results also confirmed that controlled pH provides higher substrate consumption rates. According to Gassem et al. (1997), the elevated lactose consumption under controlled pH was correlated with substantial galactose production, which in turns accumulates in cultivation medium and serves as an additional substrate. It was believed that L. kefiranofaciens have a LacS transporter (lactose/galactose exchange activity of the transporter) that fosters galactose accumulation during growth on lactose (Cheirsilp et al., 2001). In addition, maintaining controlled pH conditions lengthens the stationary phase, accordingly peptidoglycan and teichoic acid syntheses decreases resulting in increased polysaccharide production (Prathima et al., 2014).
Finally, Table 1 presents different kinetic parameters obtained of cell growth, lactose consumption and kefiran production effects before and after optimization of production media and during different pH submerged cultivations of L. kefiranofaciens under controlled and uncontrolled conditions. It can be seen that each optimization step gradually increased process parameters, where final cultivation in 16-L stirred tank bioreactor under controlled pH conditions using the optimized medium, in terms of osmotic stress and surfactant addition, produced a maximal of 2.32 g.L−1 kefiran with a specific productivity of 0.72 g cells.g−1 cells. This increase in volumetric productivity corresponds to an increase of about 45.0% from the initial production process.
Shake-flask cultivation
Bioreactor cultivation
Parameters
Before optimization
After optimization
Uncontrolled pH
Controlled pH
Growth parameters
Xmax [g L−1]
1.92
2.70
2.90
3.28
dx/dt [g h−1]
0.032
0.043
0.0528
0.074
μ [h−1]
0.021
0.025
0.031
0.069
QLac [g L−1h−1]
n.d.
n.d.
0.94
1.12
Production parameters
Pmax [g L−1]
1.290
1.62
1.87
2.32
Qp [g L−1h−1]
0.021
0.032
0.039
0.056
YP/X [g g−1]
0.670
0.601
0.677
0.717
YP/S [g g−1]
n.d.
n.d.
0.039
0.046
3.7 Determination of antioxidant and anticancer potentials of produced kefiran
The performed DPPH radical scavenging assay revealed that the produced kefiran showed a dose-dependent scavenging percentage of DPPH radical from 10 to 100 μg.mL−1. Kefiran produced about 96.58% radical scavenging activity at 100 μg.mL−1, where the obtained ED50 recorded 12.29 ± 0.98 μg.mL−1. On the other hand, the standard antioxidant used (ascorbic acid) recorded 1.78 ± 0.58 μg.mL−1. These results showed that kefiran can exhibit a satisfactory degree of antioxidant potential, that can help overcoming oxidative stress by scavenging free radicals that can lead to the development of cancer diseases (Mousa et al., 2019). Microbial polysaccharides are known for their potential antioxidant activities through scavenging reactive oxygen species responsible for membrane damage (Elsayed et al., 2017).
Furthermore, anticancer activity of the produced kefiran was investigated against MCF-7 breast cancer cell line. Results (Fig. 7) showed that kefiran affected cell viability of MCF-7 in a concentration depend manner within the tested concentration range (0.0–1000 μg.mL−1). The highest toxicity against MCF-7 cells (71.96 ± 2.189%) was obtained at 1000 μg.mL−1, where cell viability decreased to 28.34%. Furthermore, the IC50 concentration required to inhibit 50% of viable cells recorded 193.89 μg.mL−1. Additionally, the results also revealed that kefiran had no toxicity towards normal MCF-10A breast cells, which impose little or no toxicity towards normal cells. We have previously reported on the cytotoxic activities of microbial kefiran again human hepatic (HepG2) and cervical (HeLa) carcinomas (Elsayed et al., 2017). Our previous results showed that microbial kefiran potentially inhibited cell growth of both HepG2 and HeLa cells at IC50 values of 413.5 ± 1.05 and 358.8 ± 1.65 μg.mL−1, respectively. However, the lowered IC50 value of the current work (193.89 μg.mL−1) can be attributed to the fact that different cancer cells react differently towards the same effector compound due to their inherent differences in membrane structure and organization. Kefiran has been reported to exhibit its cytotoxic effects due to the presence of various exopolysaccharide, i.e. kefiran, lentinan, viilian. β-1, 3-Glucans with 1, 6-glucopyranoside branching (Yamada et al., 1984; Adachi, 1992).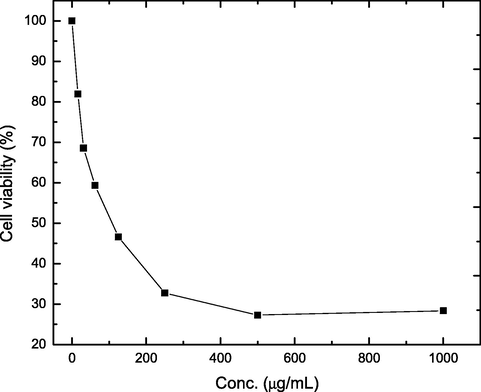
Effect of different concentrations of kefiran on the viability of MCF-7 breast cancer cells.
4 Conclusion
To the best of our knowledge, no reports are found about the effect of osmotic stress and surfactants on kefiran production by L. kefiranofaciens. Our obtained results clearly showed that osmotic pressure and surfactants enhanced kefiran production. Kefiran production was optimized by growing cells at 550 mOsmol.kg−1 and adding 1 g.L−1 of Triton X-100 at the beginning of cultivation. Such optimization increased kefiran production by about 25.6% than the preliminary un-optimized medium. Furthermore, controlling pH of the cultivation in stirred tank bioreactor proved to be an important variable for maximal kefiran production compared to uncontrolled pH condition. Controlled pH cultivation greatly enhanced both cell growth and kefiran production 13.0 and 24.0%, respectively. Furthermore, cellular growth, kefiran production and lactose consumption rates were greatly improved under controlled pH cultivation. Finally, the prepared kefiran showed promising antioxidant and anticancer potentials.
Acknowledgment
The Authors are thankful for Research Management Centre, Universiti Teknologi Malaysia, for supporting this work through the project Q.J130000.2609.06J04. The authors also would like to extend their appreciation for King Saud University, Riyadh, KSA, for funding the work through Researchers Supporting Project (Project No. RSP-2020/52).
Author contributions
Conceptualization, H.E, D.D. and D.S.; methodology, D.D. and R.M., E.A, and S.R.; validation, E.A and R.Z. and S.Z.; investigation, D.S.; resources, H.E., S.Z. and E.A.; writing—original draft preparation, S.S, R.M, and H.E.; writing—review and editing, D.D., H.E. and E.A.; supervision, H.E.; project administration, H.E., D.D. and R.M; funding acquisition, H.E. and E.A. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The effects of the composition of growth medium and fermentation conditions on the production of lipase by R. delemar. Turkish J. Biol.. 2011;35:35-44.
- [Google Scholar]
- Adachi, S., 1992. Lactic acid bacteria and the control of tumors. In: Wood, B.J.B. (Ed.). The lactic acid bacteria. Volume 1: The lactic acid bacteria in health and disease. Elsevier Sci UK, pp. 233-261.
- Optimization of gellan gum production by Sphingomonas paucimobilis ATCC 31461 with nonionic surfactants using central composite design. J. Biosci. Bioeng.. 2008;105:204-210.
- [Google Scholar]
- Effect of different non-ionic surfactants on the biodegradation of PAHs by diverse aerobic bacteria. Int. Biodeterior. Biodegr.. 2009;63:913-922.
- [Google Scholar]
- Kefiran-alginate gel microspheres for oral delivery of ciprofloxacin. Coll. Surf. B: Biointerfaces. 2016;145:706-715.
- [Google Scholar]
- Induction of hypocrellin production by Triton X-100 under submerged fermentation with Shiraia sp SUPER-H168. New Biotechnol.. 2011;28:588-592.
- [Google Scholar]
- Antitumoral, antibacterial and antifungal activities of kefir and kefir grain. Phytother. Res.. 1994;8:78-82.
- [Google Scholar]
- Modeling and optimization of environmental conditions for kefiran production by Lactobacillus kefiranofaciens. Appl. Microbiol. Biotechnol.. 2001;57:639-646.
- [Google Scholar]
- Co-production of functional exopolysaccharides and lactic acid by Lactobacillus kefiranofaciens originated from fermented milk, kefir. J. Food Sci. Technol.. 2018;55:331-340.
- [Google Scholar]
- The use of additives as the stimulator on mycelial biomass and exopolysaccharide productions in submerged culture of Grifola umbellate. Bioprocess Biosys. Eng.. 2010;33:401-406.
- [Google Scholar]
- Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Int. Dairy J.. 2015;43:15-21.
- [Google Scholar]
- Bioprocess development for kefiran production by Lactobacillus kefiranofaciens in semi industrial scale bioreactor. Saudi J. Biol. Sci.. 2016;23:495-502.
- [Google Scholar]
- Development of cultivation medium for high yield kefiran production by Lactobacillus kefiranofaciens. Inter. J. Pharm. Pharm. Sci.. 2015;7:159-163.
- [Google Scholar]
- Production of biopolymer levan by Bacillus subtilis using non-ionic surfactants. Asian J. Pharm. Technol.. 2013;3:149-154.
- [Google Scholar]
- Enhanced xanthan production process in shake flasks and pilot scale bioreactors using industrial semi-defined medium. Afr. J. Biotechnol.. 2011;10:1029-1038.
- [Google Scholar]
- Kinetics of cell growth and invertase production by the biotherapeutic yeast, Saccharomyces boulardii. J. Sci. Ind. Res.. 2017;76:477-484.
- [Google Scholar]
- Improvement in natamycin production by Streptomyces natalensis with the addition of short-chain carboxylic acids. Process Biochem.. 2013;48:1831-1838.
- [Google Scholar]
- Improvement of cell mass production of Lactobacillus delbrueckii sp. bulgaricus WICC-B-02: A newly isolated probiotic strain from mother’s milk. J. Appl. Pharm. Sci.. 2014;4:8-14.
- [Google Scholar]
- In vitro evaluation of cytotoxic activities of essential oil from Moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 cell lines. Asian Pac. J. Cancer Preven.. 2015;16:4671-4675.
- [Google Scholar]
- Development of fed-batch cultivation strategy for efficient oxytetracycline production by Streptomyces rimosus at semi-industrial scale. Braz. Arch. Biol. Technol.. 2015;58:676-685.
- [Google Scholar]
- In vitro assessment of anticancer properties of Moringa peregrine essential seed oil on different cell lines. Pak. J. Zoo.. 2016;48:853-859.
- [Google Scholar]
- Bioprocess optimization of Xanthan production by Xanthomonas campestris using semi-defined medium in batch and fed-batch culture. Der Pharmacia Lettre. 2016;8:288-296.
- [Google Scholar]
- Optimization of fed-batch cultivation for extracellular α-amylase production by Bacillus amyloliquefaciens in submerged culture. J. Sci. Ind. Res.. 2016;75:480-486.
- [Google Scholar]
- In vitro and in vivo biological screening of kefiran polysaccharide produced by Lactobacillus kefiranofaciens. Biomed. Res.. 2017;28:594-600.
- [Google Scholar]
- Preparation and characterization of kefiran electrospun nanofibers. Int. J. Biol. Macromol.. 2014;70:50-56.
- [Google Scholar]
- Kefiran suppresses antigen-induced mast cell activation. Biol. Pharm. Bull.. 2012;35:178-183.
- [Google Scholar]
- Exopolysaccharide production from whey lactose by fermentation with Lactobacillus delbrueckii ssp. Bulgaricus. J. Food Sci.. 1997;62:171-173.
- [Google Scholar]
- Physiological response of Lactobacillus plantarum to salt and nonelectrolyte stress. J. Bacteriol.. 1998;180:4718-4723.
- [Google Scholar]
- Effect of plant oil and surfactant on the production of mycelial biomass and polysaccharides in submerged culture of Grifola frondosa. Biochem. Eng. J.. 2008;38:198-205.
- [Google Scholar]
- Betaine transport imparts osmotolerance on a strain of Lactobacillus acidophilus. Appl. Environ. Microbiol.. 1987;53:2275-2281.
- [Google Scholar]
- Effect of detergents on xanthan production during batch and continuous cultivation of Xanthomonas campestris NRRL B-1459. Technol. Alimen.. 2003;2:125-133.
- [Google Scholar]
- Lactobacillus kefir sp. Nov., a component of the microflora of kefir. Syst. Appl. Microbiol.. 1983;4:286-294.
- [Google Scholar]
- Choline and acetylcholine: novel cationic osmolytes in Lactobacillus plantarum. Appl. Microbiol. Biotechnol.. 1997;48:94-98.
- [Google Scholar]
- Induction of heat shock proteins Dank, GroEL, and GroES by salt stress in Lactococcus lactis. Appl. Environ. Microbiol.. 1997;63:1826-1837.
- [Google Scholar]
- Osmotic stress induced by salt increases cell yield, autolytic activity, and survival of lyophilization of Lactobacillus delbrueckii subsp. lactis. Int. J. Food Microbiol.. 2007;117:36-42.
- [Google Scholar]
- The chemical structure of kefiran, the water-soluble polysaccharide of the kefir grain. Carbohydr. Res.. 1968;7:200-211.
- [Google Scholar]
- Responses of lactic acid bacteria to osmotic stress. In: Tsakalidou E., Papadimitriou K., eds. Stress Responses of Lactic Acid Bacteria. New York: Springer; 2011. p. :67-90.
- [Google Scholar]
- Influence of Tween-80 on the production and structure of water-insoluble curdlan from Agrobacterium sp. Int. J. Biol. Macromol.. 2018;106:611-619.
- [Google Scholar]
- A novel bioflocculant produced by a salt-tolerant, alkaliphilic and biofilm-forming strain Bacillus agaradhaerens C9 and its application in harvesting Chlorella minutissima UTEX2341. Biochem. Eng. J.. 2015;93:166-172.
- [Google Scholar]
- Effects of Tween 80 and pH on mycelial pellets and exopolysaccharide production in liquid culture of a medicinal fungus. J. Ind. Microbiol. Biotechnol.. 2012;39:623-628.
- [Google Scholar]
- Effect of Tween 80 on the growth of Mycobacterium avium complex. Microbiol. Immunol.. 1990;34:653-663.
- [Google Scholar]
- Isolation and characterisation of a ropy Lactobacillus strain producing the exopolysaccharide kefiran. Appl. Microbiol. Biotechnol.. 1999;53:69-74.
- [Google Scholar]
- Lagerstroemia speciosa (L.) Pers leaf extract attenuates lung tumorigenesis via alleviating oxidative stress, inflammation and apoptosis. Biomolecules. 2019;9:971.
- [Google Scholar]
- Kefiran films plasticized with sugars and polyols: water vapor barrier and mechanical properties in relation to their microstructure analyzed by ATR/FT-IR spectroscopy. Food Hydrocoll.. 2011;25:1261-1269.
- [Google Scholar]
- Optimization of exopolysaccharide production by Lactococcus lactis NCDC191 by response surface methodology. Int. J. Curr. Microbiol. Appl. Sci.. 2014;3:835-854.
- [Google Scholar]
- Kefiran improves rheological properties of glucono-d-lactone induced skim milk gels. Int. Dairy J.. 2006;16:33-39.
- [Google Scholar]
- Glycine betaine, carnitine, and choline enhance salinity tolerance and prevent the accumulation of sodium to a level inhibiting growth of Tetragenococcus halophila. Appl. Environ. Microbiol.. 2000;66:509-517.
- [Google Scholar]
- Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents. 2005;25:404-408.
- [Google Scholar]
- Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J. Appl. Microbiol.. 2008;105:1-13.
- [Google Scholar]
- Effective stimulating factors for microbial levan production by Halomonas smyrnensis AAD6T. J. Biosci. Bioeng.. 2015;119:455-463.
- [Google Scholar]
- Enhanced production of thermostable β-amylase and pullulanase in the presence of surfactants by Clostridium thermosulfurogenes SV2. Process Biochem.. 1999;34:87-92.
- [Google Scholar]
- Understanding the influence of Tween 80 on pullulan fermentation by Aureobasidium pullulans CGMCC1234. Carbohydr. Polym.. 2016;136:1332-1337.
- [Google Scholar]
- Mechanism study of Tween 80 enhancing the pullulan production by Aureobasidium pullulans. Carbohydr. Polym.. 2013;97:121-123.
- [Google Scholar]
- Sorbitol required for cell growth and ethanol production by Zymomonas mobilis under heat, ethanol, and osmotic stresses. Biotechnol. Biofuels. 2013;6:180.
- [Google Scholar]
- Evaluation of the response of Lactobacillus rhamnosus VTT E-97800 to sucrose-induced osmotic stress. Food Microbiol.. 2008;25:183-189.
- [Google Scholar]
- Stress Responses of Lactic Acid Bacteria. New York: Springer; 2011.
- Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J.. 2001;11:687-707.
- [Google Scholar]
- Physiological aspects Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv.. 2006;24:604-620.
- [Google Scholar]
- Effect of surfactants on extracellular accumulation of glutathione by Saccharomyces cerevisiae. Process Biochem.. 2003;38:1133-1138.
- [Google Scholar]
- Improvement of poly (γ-glutamic acid) biosynthesis and redistribution of metabolic flux with the presence of different additives in Bacillus subtilis CGMCC 0833. Appl. Microbiol. Biotechnol.. 2008;79:527-535.
- [Google Scholar]
- Optimization of exopolysaccharide welan gum production by Alcaligenes sp. CGMCC2428 with Tween-40 using response surface methodology. Carbohydr. Polym.. 2012;87:1363-1368.
- [Google Scholar]
- Effect of chemicals on production, composition and antioxidantactivity of polysaccharides of Inonotus obliquus. Int. J. Biol. Macromol.. 2015;77:143-150.
- [Google Scholar]
- Structure and antitumor activity of an alkali-soluble polysacchardie from Cordyceps ophioglossoides. Carbohydr. Res.. 1984;125:83-91.
- [Google Scholar]
- Gelling ability of kefiran in the presence of sucrose and fructose and physicochemical characterization of the resulting cryogels. J. Food Sci. Technol.. 2015;52:5039-5047.
- [Google Scholar]
- Polysaccharide production by lactic acid bacteria: from genes to industrial applications. FEMS Microbiol. Rev.. 2017;41:S168-S200.
- [Google Scholar]
- A mechanistic study of the enhancing effect of Tween 80 on the mycelial growth and exopolysaccharide production by Pleurotus tuberregium. Bioresource Technol.. 2011;102:8323-8326.
- [Google Scholar]
- pH-Control modes in a 5-L stirred-tank bioreactor for cell biomass and exopolysaccharide production by Tremella fuciformis spore. Bioresource Technol.. 2011;102:9175-9178.
- [Google Scholar]







