Translate this page into:
Efficient removal of organic pollutant by activation of persulfate with magnetic Co3O4/CoFe2O4 composite
⁎Corresponding author at: College of Earth and Environmental Sciences, Lanzhou University, Lanzhou 730000, PR China. chengxw@lzu.edu.cn (Xiuwen Cheng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, magnetic spinel Co3O4/CoFe2O4 composite were synthesized by the mechanical mixing of both powdered pristine samples. Then the catalyst was characterized by TEM, SEM, XRD, BET, XPS and VSM measurement. Next, Co3O4/CoFe2O4 composite was applied to degrade rhodamine B (RhB) in water by activating persulfate. Results showed that Co3O4/CoFe2O4 composite exhibited high efficiency for removal of RhB, and 95.59% of it could be degraded in 45 min. Besides, the effects of parameters, such as initial pH, PS dosage, Co3O4/CoFe2O4 composite dosage, initial concentration of RhB and temperture were studied. Also, the effects of coexisting anions on RhB degradation were observed and explained. Furthermore, we conducted the quenching experiment and found that sulfate radical and hydroxyl radicals were the main active radicals in the degradation process. Finally, recycle experiments proved that Co3O4/CoFe2O4 had a good stability for RhB degradation. In short, Co3O4/CoFe2O4 composite is a promising catalyst for wastewater treatment.
Keywords
Co3O4/CoFe2O4 composite
Persulfate
Rhodamine B
Sulfate radicals
Hydroxyl radicals
1 Introduction
Today, water environment pollution is one of the major environmental problems in the world. Among various types of polluted water, printing and dyeing wastewater, as one of the hard-degradable wastewater, is gradually receiving more and more attention (Hou et al., 2016; He et al., 2018). Due to the processing of chemical fiber and other spinning products, the printing and dyeing wastewater contains a large amount of dyes, oils, starch alkalis, resins and inorganic salts, etc. It is very difficult to deal with. Most of the dyes are easily dissolved in water in form of cationic (rhodamine B, malachite green, methylene blue, and methyl violet) and anionic (Congo red, methyl orange, and rose Bengal) (Nafiey et al., 2017).
Advanced oxidation technology is a new technology which can efficiently remove difficult-to-degrade pollutants from water. In this method, highly active radicals (HO•, SO4•−, etc.) can be generated through various routes to degradate pollutants, including Fenton method (Wang et al., 2009), photocatalytic oxidation method (Cheng et al., 2015), electrochemical oxidation method (Li et al., 2013), etc. Among these approaches, SO4•−-based technology has received increasing attention due to its higher redox potential (2.5–3.1 V) based on the activation methods compared with HO• (Lutze et al., 2015; Ghauch and Tuqan, 2012). Apart from these, SO4•− has higher selectivity and longer half-life over HO• in some cases (Ahmed et al., 2012). It can be generated by persulfate (PS) and peroxymonosulfate (PMS) activation through heat (Antoniou et al., 2010; Huang et al., 2002), ultraviolet (Chu et al., 2016; Sharma et al., 2016; Sharma et al., 2015), ultrasound (Gayathri et al., 2010; Chakma et al. 2017; Babu et al. 2017; Neppolian et al., 2010), electron beam (Criquet and Leitner, 2011; Furman et al., 2010); electrochemical (Ahmadi and Ghanbari, 2016; Ghanbari and Martinez-Huitle, 2019; Popat et al., 2019); zero valent metal (Khatri et al., 2018; Devi et al., 2016) and transition metal ions (Xie et al., 2016; Rastogi et al., 2009; Ayoub and Ghauch, 2014; Senthilnathan and Nachiappana, 2015; Sarath et al., 2014; Nidheesh and Rajan, 2016; Asha et al., 2017). Particularly, potassium persulfate (K2S2O8), as one of the common source of SO4•−, attached much attention due to its moderate cost, high stability, high solubility in aqueous solution, and it exists in the form of solid at room temperature (Zacharias et al., 2016; Lin et al., 2011). In view of economy and operation, choosing transition metal to activate PS is a good choice because it doesn't require energy. Furthermore, the Co2+ was proven to be most effective transition metal ions (Anipsitakis and Dionysiou, 2003). However, Co2+ is highly soluble in water environments, which could induce health problems (Huang and Huang, 2009; Yang et al., 2009; Ji et al., 2016). In order to overcome this shortcoming, heterogeneous Co catalysts such as CoO, CoO2, Co2O3, and Co3O4, have been used to activate PS. Among them, Co3O4, as one of the P-type metal oxide semiconductors, attracted wide attention due to its magnetic (Wang et al., 2015). Besides, owing to its low valence band maximum (VBM), Co3O4 has a superior oxidation capacity (Bautista et al., 2008). What's more, spinel ferrites (MFe2O4 (M = Fe, Cu, Co, Ni, etc.)) have high catalytic activity, stable crystalline structure, extremely low solubility and good magnetic in terms of activating PS in water environment. So they have been taken as very promising candidates (Yang et al., 2009; Zhang et al., 2013; Deng et al., 2013). In particular, spinel cobalt ferrite (CoFe2O4) has been confirmed to be one of the efficient catalyst which can activate PMS to degrade organic compounds due to its excellent magnetic property and surface active Co2+ (Ren et al., 2015; Liu et al., 2015). Sun et al. (2016) fabricated an octahedral CoFe/CoFe2O4 submicron composite by a flexible one-pot hydrothermal, which can efficient activate PMS to decompose methyl orange. Li et al. (2017) successfully prepared polypyrrole/CNTs-CoFe2O4 magnetic nanohybrid (CNTs-CoFe2O4@PPy), which was used as a superior adsorbent and catalyst to remove anionic and cationic dyes according activating PMS. However, at present, there is less research on activating PS with CoFe2O4 to produce sulfate radicals for degradation of pollutants. If combine Co3O4 with CoFe2O4, the cobalt leaching will reduce and magnetic will strengthen, thus catalysts can be better recycled.
In this study, we first prepared Co3O4 by calcination, then syntheized CoFe2O4 by a simple one-step hydrothermal method. Subsequently, we combined the two of catalysts by mechanical stirring. Next, the physicochemical properties of Co3O4/CoFe2O4 composite were analyzed. After this, we evaluated the catalytic activity of Co3O4/CoFe2O4 by degradation of RhB via the formation of sulfate radicals and hydroxyl radicals by activating PS. Finally, we proposed the possible reaction mechanism towards the degradation of RhB.
2 Materials and methods
2.1 Materials
Ferric nitrate (Fe(NO3)3·6H2O), cobalt nitrate (Co(NO3)2·6H2O), and potassium persulfate(K2S2O8) were purchased from Tianjin Kemiou Chemical Reagent Co. LTD. Cobalt chloride (CoCl2·6H2O), urea (CO(NH2)2) and ascorbic acid (C6H8O6) were provided by Sinopharm Chemical Reagent Co. Ltd. Ultra-pure water was used throughout the whole experiment.
2.2 Synthesis of Co3O4 powder
In a typical experiment, 5 g of Co(NO3)2·6H2O was placed in a closed ceramic crucible and heated in a muffle furnace at around 400 °C for 4 h. After the furnace cooling to room temperature, Co3O4 powder was obtained. All chemicals were of analytical reagent grade and used without any further purification. Ultra-pure water was used throughout this experiment.
2.3 Synthesis of Co2Fe2O4 powder
CoFe2O4 were synthesized by a simple one-step hydrothermal method. 3.9975 mmol Fe(NO3)3·9H2O, 1.995 mmol CoCl2·6H2O, 4.26 mmol C6H8O6 and 15 mmol CO(NH2)2 were slowly added to the 100 mL breaker which contained 60 mL distilled water. Afterwards, it was stirred under magnetic for 30 min until dissolved. Subsequently, the mixture was transferred into an autoclave and treated at 160 °C for 6 h. After centrifuging and washing with water and ethanol for several times, the black precipitate was dried at 80 °C for 3 h. Finally, it was calcined in static air at 500 °C for 4 h.
2.4 Synthesis of Co3O4/Co2Fe2O4 composite
The Co3O4/CoFe2O4 composite was fabricated by the mechanical mixing method. In detail, a certain amount of Co3O4 and CoFe2O4 powders were dispersed in absolute ethanol and stirred for 2 h under magnetic. After, the powders were dried at 70 °C for 4 h.
2.5 Characterization
In this paper, the surface morphology of Co3O4/CoFe2O4 composite catalyst was observed by scanning electron microscopy (SEM) at 20 kV. Transmission electron microscopy (TEM) was conducted on a FEI Tecnai F20 transmission electron microscope at an acceleration voltage of 200 kV. The crystal structure, composition and lattice constant of catalysts were measured on a Rigaku D/MAX III-3B X-ray diffractometer (XRD) with Cu kα (λ = 0.15418 nm) radiation. And the accelerating voltage and applied current were held at 40 kV and 200 mA, respectively. The specific surface area and pore size distribution of samples were measured on an AUTOSORB-1 (Quantachrome Instruments) at 77 K. X-ray photoelectron spectroscopy (XPS) analysis of the catalysts obtained were conducted on PHI-5700 ESCA apparatus with an Al Kα X-ray source to explore the elements of the surface, and the binding energies were referenced to adventitious carbon for 284.62 eV. Lakershore Model 7304 instrument was used to measure the magnetic properties of catalysts. Magnetic measurement was performed at room temperature by using a physical property measurement system (PPMS, 730 T, LAKESHORE, USA)
2.6 Pollutants degradation experiment
As for the organic pollutant degradation tests, RhB was selected to be a typical model contaminant. In each run, a certain amount of catalysts above was added into 50 mL of RhB solution (10 mg·L−1) under stirring. The initial pH value of RhB solution solution was about 5.49 without adjustment. Before to reaction, the suspension was kept in dark for 20 min to ensure the establishment of adsorption/desorption equilibrium between catalysts and rhodamine B. Next, a fixed amount of PS was added to initiate the catalytic reaction. At given time intervals, the collected samples after centrifugation and filtration (through a 0.22 μm membrane) were measured at the characteristic peak of 550 nm by using a T6 ultraviolet-visible spectrophotometer.
3 Results and discussion
3.1 SEM and TEM analysis
The morphological observations clearly indicated the successful synthesis of Co3O4/CoFe2O4 composite. As shown in Fig. 1a, a large amount of nanoparticles gathered together. However, it was difficult to distinguish Co3O4 and CoFe2O4 since both of them were spherical particles. From the TEM image (Fig. 1b), a very serious aggregation phenomenon was also exhibited. Besides, EDXA elemental mapping and the selected energy dispersive spectrum (EDS) were also analyzed in order to see the element dispersion of the Co3O4/CoFe2O4 composite. As displayed in Fig. 1c–f, the composite was composed of Co, Fe and O elements.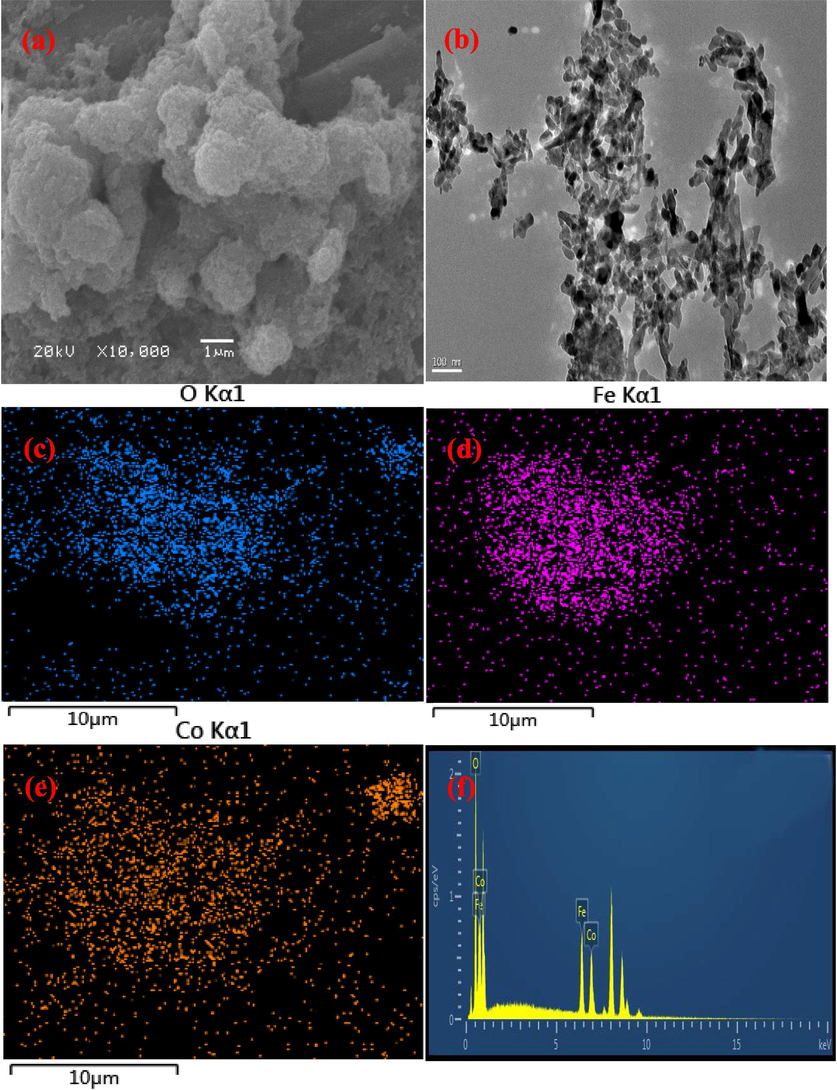
TEM image (a), SEM image (b), EDXA elemental mapping (Co, Fe and O elements (c–e)) and EDS spectroscopy (f) of Co3O4/CoFe2O4 composite.
3.2 XRD analysis
XRD patterns further indicated the crystal structure of the catalysts. Seen from Fig. 2, The diffraction peaks of pure Co3O4 appearing at 18.9°, 31.2°, 36.8°, 45.2°, 59.6° and 65.2° could be perfectly assigned to the (1 1 1), (2 2 0), (3 1 1), (4 0 0), (5 1 1) and (4 4 0) crystal planes of Co3O4 respectively. For single CoFe2O4, the peaks at 30.2°, 35.5°, 42.3°, 57.2°, and 62.9° were detected, which could be indexed to the cubic spinel structure of cobalt ferrite (Li et al., 2011). As for pure Co3O4/CoFe2O4 catalyst, all the characteristic peaks belonging to Co3O4 didn’t shift. However, due to the lower doping content of CoFe2O4 nanoparticles, only the peak at 35.5° could be observed, which could be attributed to the (3 1 1) crystal plane of cobalt ferrite, the other diffraction peaks of CoFe2O4 were not be directly detected.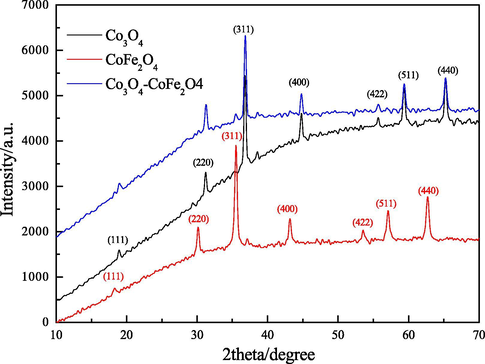
XRD patterns of Co3O4, CoFe2O4 and Co3O4/CoFe2O4 samples.
3.3 Nitrogen adsorption-desorption
It is well known that the catalytic properties of materials are also closely related to their specific surface area and porosity. We studied the structural properties of pure Co3O4 and Co3O4/CoFe2O4 composites by nitrogen adsorption analysis. As shown in Fig. 3, according to the IUPAC classification, the isotherms of pure Co3O4 and Co3O4/CoFe2O4 were type IV, characteristic for mesoporous structures, with relatively similar H2 hysteresis loops. The specific surface area of Co3O4/CoFe2O4 was calculated to be 13.894 m2·g−1, which was larger than that of pure Co3O4 (12.143 m2·g−1). For pure Co3O4, the BJH pore size analysis on the desorption branch exhibited only a peak centered at 35.04 nm. The pore size distribution of Co3O4/CoFe2O4 composite also with one peak at 36.09 nm. Both of the samples had the good homogeneity of the pores. Similarly, the Co3O4/CoFe2O4 composite had a bigger pore volume (0.146 m3·g−1) compared with pure Co3O4 (0.132 m3·g−1). Large accessible surface area and numerous pores made the catalyst to provide more reactive sites, thus enhanced the catalytic performance.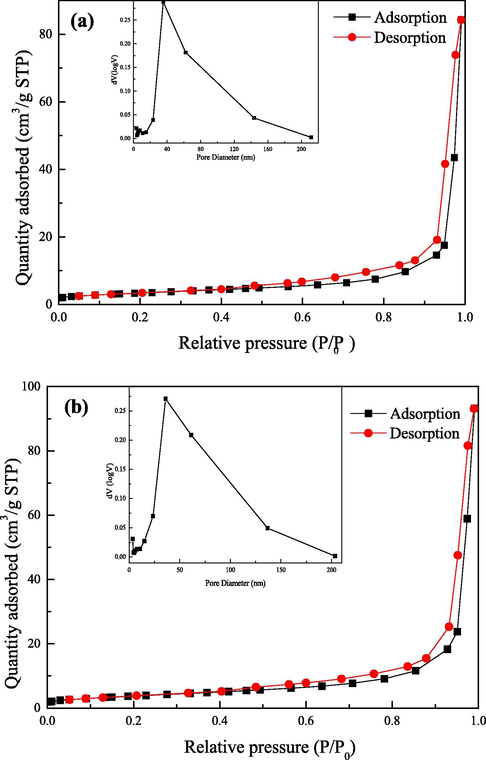
N2 adsorption and desorption isotherm and BJH pore-size distribution of Co3O4 (a) and Co3O4/CoFe2O4 (b).
3.4 XPS measurement
The XPS spectra of the Co3O4/CoFe2O4 composite were recorded and given in Fig. 4. As shown in Fig. 4a, the de-convolution of the Co 2p peak in CoFe2O4 exhibited four peaks. From CoFe2O4, the peak at 780.7 eV was attributed to Co 2p3/2, while the peak at 795.65 eV was caused by Co 2p1/2. What's more, the peaks of the satellites at about 785.75 eV and 802.67 eV were two shake-up type peaks of Co at the high binding energy side of the Co 2p3/2 and Co 2p1/2 edges. However, when Co3O4 was combined with CoFe2O4, the de-convolution of the Co 2p peak in Co3O4/CoFe2O4 composite exhibited six peaks. The peaks at 779.59 eV and 794.36 eV were assigned to Co3+, whereas the peaks at 781.07 eV and 795.97 eV were attributed to Co2+ (Li et al., 2011). Besides, the peaks at 788.97 eV and 803.99 eV belonged to the shake-up satellite peaks (Deng et al., 2016). This suggested that the Co species existed in the form of Co2+ and Co3+ in the Co3O4/CoFe2O4 composite. In addition, from the peak area, it could be determined that the content of Co3+ was much more than that of Co2+, which also indicated that the content of Co3O4 was much higher than that of CoFe2O4, which was consistent with the results of SEM and TEM. The Fe 2p XPS spectra of the CoFe2O4 and the Co3O4/CoFe2O4 samples were presented in Fig. 4b. Two peaks at a binding energy of 710.75 eV and 724 eV, corresponding to Fe 2p3/2 and Fe 2p1/2, respectively, which suggested the presence of Fe3+. Furthermore, the peak at 717.35 eV and 732.66 were assigned to shake-up satellite peaks (Salunkhe et al., 2015). However, it could be observed that the binding energy of Fe 2p1/2 shifted from 724 eV of the CoFe2O4 to 722.35 eV of the Co3O4/CoFe2O4 composite. Seen from Fig. 4c, the high resolution XPS spectrum of O1s (CoFe2O4) included two peaks with binding energy at 529.59 and 531.31 eV, corresponding to the surface lattice oxygen species (Olatt) and the surface adsorbed oxygen species (Oads), respectively. But it had a slightly decrease in Co3O4/CoFe2O4. Compared with pure CoFe2O4, the varied binding energy values of Co 2p, Fe 2p and O 1s for the Co3O4/CoFe2O4 composite meant the rapid transfer of electrons.
High-resolution XPS spectrum of Co 2p (a), Fe 2p (b) and O 1 s (c) for CoFe2O4 and Co3O4/CoFe2O4 samples.
3.5 VSM analysis
As is known to all, pulverous catalysts were difficult to separate and recycle from the slurry system, which caused the practical bottleneck of applications. In this paper the magnetic property of the Co3O4/CoFe2O4 composite was confirmed by the M−H analysis. As given in Fig. 5, the saturation magnetization (MS) value of Co3O4/CoFe2O4 composite was 6.13 emu·g−1, which was lower than that of pure Co3O4/CoFe2O4 sample (59.62 emu·g−1). This decrease could be explained that the introduced Co3O4 sample was non-magnetic. Despite this, Co3O4/CoFe2O4 composite still performed good magnetic property.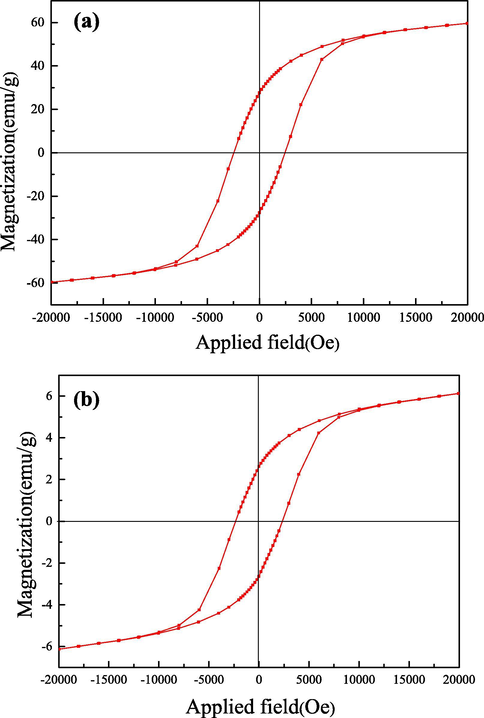
Hysteresis loop diagram of CoFe2O4 (a) and Co3O4/CoFe2O4 (b) sample.
3.6 Degradation performance
3.6.1 Activation of PS by Co3O4/CoFe2O4 composite for RhB degradation
Fig. 6 exhibited the degradation of RhB under different conditions. As seen from Fig. 6, the catalyst alone did not substantially remove RhB, only 6.61%, 2.09% and 8.08% of RhB was removed by CoFe2O4, Co3O4 and Co3O4/CoFe2O4 composite, respectively. The low removal efficiencies might be due to the weak adsorption capacity of these materials. Besides, 32.15% RhB could be removed by PS in the absence of activator owing to the high oxidation potential of PS (2.01 V). Also, 36.56% of RhB was removed in 45 min in CoFe2O4/PS system, suggesting that CoFe2O4 alone could hardly efficiently activate PS. However, the degradation efficiency of RhB was greatly enhanced in the presence of both Co3O4 spinel and PS. The Co3O4/CoFe2O4 composite performed best degradation of RhB, which could almost completely remove the RhB (95.59%), which was attributed to the coupling effect of Co3O4 and CoFe2O4.
Removal curves of RhB under different reaction conditions. Experimental conditions: catalyst = 0.5 g·L−1, PS = 0.5 g·L−1, pH = 5.49 (natural) and RhB = 10 mg·L−1.
3.6.2 Effects of persulfate concentration, catalyst dosage, solution pH and reaction temperature
The degradation of RhB in Co3O4/CoFe2O4/PS system with different PS doses was investigated and exhibitied in Fig. 7a. The removal efficiency of RhB increased from 8.08% to 96.64% with the PS dosage increasing from 0 to 0.9 g·L−1, respectively. This showed that PS provided a source of SO4•− and RhB removal was indeed caused by oxidative degradation, rather than a simple adsorption by the catalyst. However, excessive persulfate would cause self-quenching of SO4•− (Liang et al., 2004) (reaction (1) and (2)), the degradation efficiencies of RhB were similar when the amount of PS was 0.5 g·L−1 and 0.9 g·L−1. In addition, the reaction stoichiometric efficiency (RSE) was used to evaluate the utilization efficiency of PS according previous studies (Jaafarzadeh et al., 2017; Amasha et al., 2018). The calculated RSE% was the largest when the PS concentration was 0.5 g·L−1. Thus 0.5 g·L−1 was selected as the optimal concentration of PS and used in later experiments.

Influence of initial pH (a) amount of persulfate (b) amount of catalyst (c) initial temperature (d) on RhB degradation. Experimental conditions: catalyst = 0.5 g·L−1, PS = 0.5 g·L−1, pH = 5.49 (natural) and RhB = 10 mg·L−1.
Fig. 7b showed the degradation of RhB under different Co3O4/CoFe2O4 dosages (0–0.9 g·L−1). Generally speaking, higher the Co3O4/CoFe2O4 dosage resulted in higher RhB degradation efficiency, which could be attributed to the increased number of active sites. As exhibited in Fig. 6b, the degradation efficiency of RhB increased as the amount of catalyst increased to 0.5 g·L−1, and then hardly increased. Although a large number of catalysts could provide more active sites to activate PS, it would also capture the active radicals (Wang et al., 2018). In addition, high concentrations of catalysts would gather together, thus reduced the available amount of active sites.
Due to the wide range of pH in natural water bodies, it was very important to study the optimal reaction conditions for the removal of refractory organic pollutants using Co3O4/CoFe2O4/PS system. In this study, the effects of initial solution pH on RhB removal were tested in a broad pH ranging from 3.0 to 11.0 at PS dosage of 0.5 g·L−1 and the Co3O4/CoFe2O4 dosage of 0.5 g·L−1(Fig. 7c). Among them, 5.49 was the original pH of the RhB solution formulated. It was noted that both extremely acidic (pH = 3) and alkaline (pH = 11) conditions were unfavorable for the degradation process. Co3O4/CoFe2O4 composite was easily dissolved to release metal ions under acidic solution, as a result, the activity of the catalyst was greatly reduced (Wang et al., 2011), which only degraded 64.28% of RhB (pH = 3). In addition, hydrogen ion might scavenge SO4•− and •OH (reaction (3) and (4)) (Ahmadi et al., 2017; Ghanbari et al., 2019). The best degradation of RhB was achieved at pH of 5 in a Co3O4/CoFe2O4/PS system, then started to fall. The kapp values of RhB degradation with initial pH of 3, 5, 5.49, 7, 9 and 11 were 2.07 × 10−2, 6.89 × 10−2, 6.65 × 10−2, 5.80 × 10−2, 4.94 × 10−2 and 3.82 × 10−3 min−1, respectively. According to the literature, the pHpzc of CoFe2O4 and Co3O4 was 5.96 and 8.5, respectively (Du et al., 2016; Zhang et al., 2016). When the Co3O4/CoFe2O4 composite was dispersed in water (pH < 5.96), the surface of the catalyst was positively charged, which would increase the coverage of the hydroxyl group (–OH) from H2O, which was beneficial to the formation of active radicals. At higher pH values, the electrostatic repulsion between the S2O82− and the Co3O4/CoFe2O4 surface might limit the contact of the PS onto the catalyst surface. Besides, SO4•− could be converted into •OH by hydrolysis. For aromatic compounds, SO4•− reacted with them by means of electron transfer, and SO4•− was more prone to electron transfer reactions than •OH (Neta et al., 1977; Buxton et al., 1988). So only 49.95% of RhB was removed when pH was 11.
What's more, the effect of temperature on RhB degradation was also evaluated by varying the reaction temperatures, which was exhibited in Fig. 7d. When the temperature was increased from 25 °C to 55 °C, it could be observed that the degradation efficiency of RhB was greatly improved. When the temperature was 55 °C, the degradation efficiency of RhB could reach nearly 100% within 30 min. These results indicated that the degradation of RhB depended on the temperature. A higher temperature could accelerate the decomposition of PS and the movement of oxidant, intermediates, RhB molecules (Chen et al., 2014). According to the Arrhenius equation (5):
Among them, A is the Arrhenius constant, Ea is the apparent activation energy (kJ·mol−1), R is the universal gas constant (8.314 × 10−3 kJ·mol−1·K−1), and T is the absolute temperature (K). In this experiment, the activation energy of the reaction was calculated as 25.32 kJ·mol−1, which implied the intrinsic chemical reaction of the Co3O4/CoFe2O4/PS system. Furthermore, compared with other similar materials (Deng et al., 2018; Rida et al., 2018), the Co3O4/CoFe2O4 in this article exhibited the lower activation energy, indicating it could more easily activate PS.
3.6.3 Effect of co-existing ions
In practice, a large amount of salts were usually added in various dyeing processes and might affect the active radical chain reaction in SO4•− based AOP (Li et al., 2015). In this paper, the effects of Cl−, NO3– and HCO3– that commonly occurred in real dye wastewater on RhB degradation were evaluated. As shown in Fig. 8, different inorganic anions exhibited different inhibiting effects on the RhB degradation. As seen from Fig. 8a, chloride ion inhibited the degradation of RhB, and the degradation efficiency of RhB decreased with increasing chloride ion concentration. Compared to the case where Cl− was not added, only 88.18% of RhB was degraded in the existence of 40 mM Cl−. It had been reported that chloride ions could act as sinks for HO• and SO4•− to produce less reactive chlorine species Cl2, HOCl•−, Cl• and Cl2•− (reaction (6), 7 and 8), thereby severely inhibiting the degradation process of RhB (Du et al., 2016; Sharma et al., 2015; Feng et al., 2017). What’s more, NO3– also had an apparently inhibition effect towards the removal of RhB. This was mainly because NO3– can react with the catalyst, resulting in a decrease in catalytic efficiency (Li et al., 2013; Gordon et al., 2013). It was worth noting that the most significant decrease was observed after adding HCO3– to the reaction system. In fact, HCO3– was an inorganic radical scavenger, which could react with HO• (k = 8.5 × 106 M−1·s−1) and SO4•− (k = 1.6 × 106 M−1·s−1) quickly, thus producing less reactive carbonate radicals (reaction (9), 10, 11 and 12) (Lin and Wu, 2014; Zuo et al., 1999).
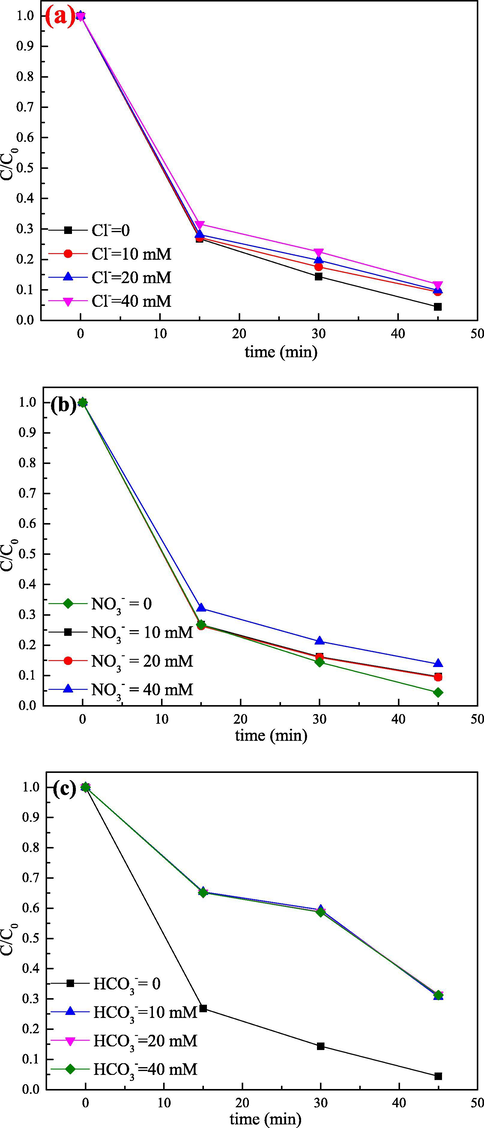
Effect of (a) Cl−, (b) NO3– and (c) HCO3– on the degradation of RhB. Experimental conditions: catalyst = 0.5 g·L−1, PS = 0.5 g·L−1, pH = 5.49 (natural) and RhB = 10 mg·L−1.
3.6.4 Free radical quenching studies
In order to reveal the activation mechanism, we need to identify the main radical species in the Co3O4/CoFe2O4/PS system for the degradation of RhB. It had been reported that activation of PS mediated by transition metals or metal oxides produced two main types of active radicals, namely SO4•− and •OH. As we all know, Methanol (MeOH) is an effective scavenger both for OH (4.6–9.7 × 108 M−1·s−1) and SO4•− (1.1–2.5 × 107 M−1·s−1). Tert-butanol (t-BuOH) could quickly capture •OH (k•OH = (3.8–7.6) × 108 M−1·s−1) (Chen et al., 2005). However, it is reported that isopropanol (IPA) could react with both free and adsorbed hydroxyl radicals (Rivas et al., 2015), which had a more significant inhibiting effect for the degradation of pollutant. In this study, we choose isopropanol and methanol as scavengers to conduct quenching experiments. As seen in Fig. 9, when IPA and MeOH were added, the degradation efficiency of RhB were only 82.46% and 57.23%, respectively. Clearly, the inhibition of MeOH was significantly higher than that of IPA, which indicated that SO4•− and •OH were the main radical in this system, and the contribution of SO4•− was larger than •OH.
Effects of different scavengers on PS oxidation for RhB degradation. Experimental conditions: catalyst = 0.5 g·L−1, PS = 0.5 g·L−1, pH = 5.49 (natural) and RhB = 10 mg·L−1 (The inset shows pseudo-first-order rate constants).
3.7 Mechanism analysis
Fig. 10a and b presented the XPS of Co 2p and O 1s of the catalysts before and after oxidation process. After PS reacted with Co3O4/CoFe2O4 composite, the position of the Co 2p peak had a little shift and the area of peaks decreased, indicating that the fraction of Co2+/Co3+ changed after used. From the deconvolution of Co 2p envelop (Fig. 10a), the proportion of Co2+ dropped from 47.96% to 45.27% of Co specie, which implied that the valence state of cobalt increased from Co2+ to Co3+ due to transferring electrons to PS to generate SO4•−. Fortunately, the binding energy of the two main peaks was basically unchanged, which indicated that the catalyst was well regenerated. In the case of O 1s spectra (Fig. 10b), the Oads/Olatt ratio had changed and the proportion of Oads had increased from 31.17% to 35.09% and the proportion of Olatt reduced from 68.83% to 64.91%, indicating that both of them are involved during the degradation process. The increase of Oads concentration might be ascribed to the formation of Co-OH and Fe-OH groups adsorbed on the Co3O4/CoFe2O4 surface, which might contribute to the enhancement of the PS activation process.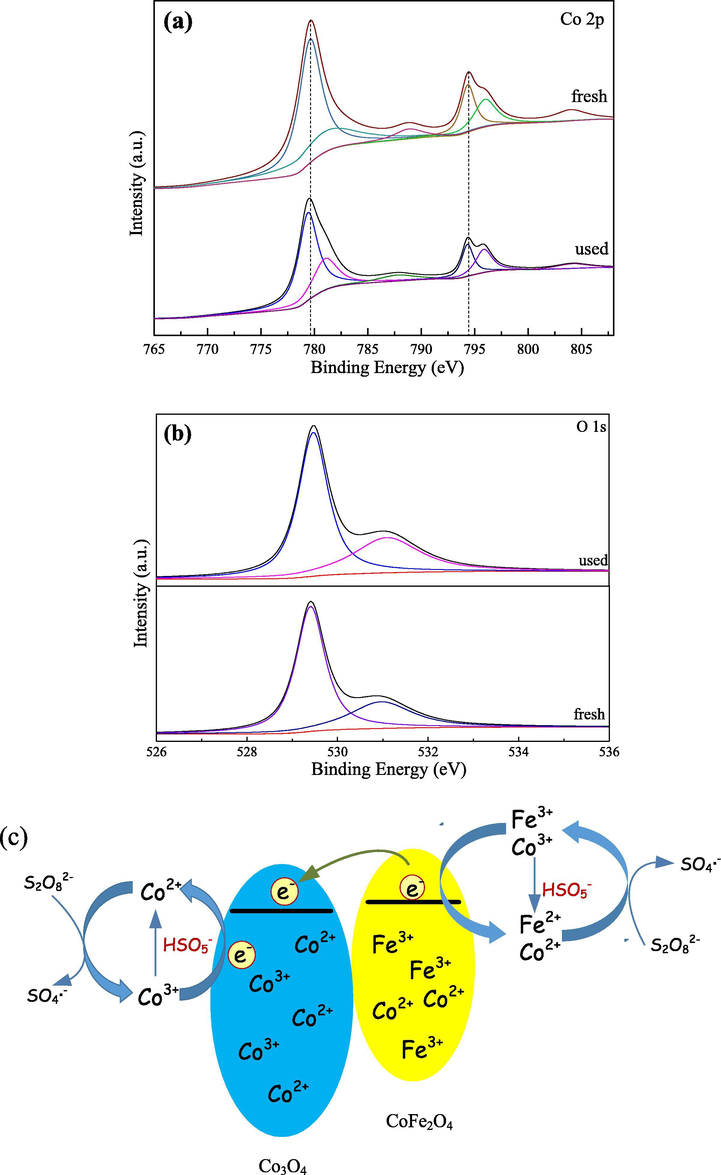
(a) Co 2p and (b) O 1s XPS envelop of the fresh and used Co3O4/CoFe2O4 catalyst; (c) Mechanism for RhB degradation by Co3O4/CoFe2O4/PS system.
Based on XPS analysis and other findings, the catalytic mechanism of PS activation by Co3O4/CoFe2O4 composite was proposed. As shown from Fig. 10c, Co2+ and Fe3+ were main active centers in Co3O4/CoFe2O4/PS system. Generally speaking, PS could react with Co2+ and Fe2+ to produce SO4•−, and simultaneously generate Co3+ and Fe3+ (reaction (13) and (14)). In addition, the conduction band of CoFe2O4 was more negative than the Co3O4. When Co3O4 combined with CoFe2O4, the heterojunction was rapidly formed and electrons were more easily transferred from CoFe2O4 to Co3O4, which promoted the regeneration of Co2+ (reaction (15)), which was consistent with the XPS analysis. However, due to the transfer of electron from CoFe2O4 to Co3O4, only a few portion of Fe3+ could be converted to Fe2+ to activate PS (reaction (16)). Fortunately, S2O82− could produce HSO5− by hydrolysis under acidic condition, therefore Co3+ and Fe3+ could react with HSO5− to achieve the regeneration of Co2+ and Fe3+ (reactions (17), (18) and (19)). In addition, SO4•− could also react with H2O to produce HO• (reaction (20)). Ultimately, all these radicals generated further degraded RhB.
3.8 Stability of the catalyst
The repeatability and stability of catalysts are key factors in actual application of persulfate activation. In this study, the recycling experiment was conducted by the sample recovery method of centrifugation and drying. Fig. 11a exhibited the RhB removal in successive four cycles with recycled Co3O4/CoFe2O4 composite. It was clearly that the Co3O4/CoFe2O4 composite maintained its performance among the four cycles. Co3O4/CoFe2O4 composite catalyst could still degrade 85.49% RhB after 4 cycles. In addition, we tested the XRD pattern of the fresh and used Co3O4/CoFe2O4 composite, which were exhibited in Fig. 11b. Obviously, the used Co3O4/CoFe2O4 composite maintained the stable crystal structure without phase transition. These indicated that the Co3O4/CoFe2O4 composite catalyst as-prepared had a good practical potential, which could provide the possibility of practical application in future.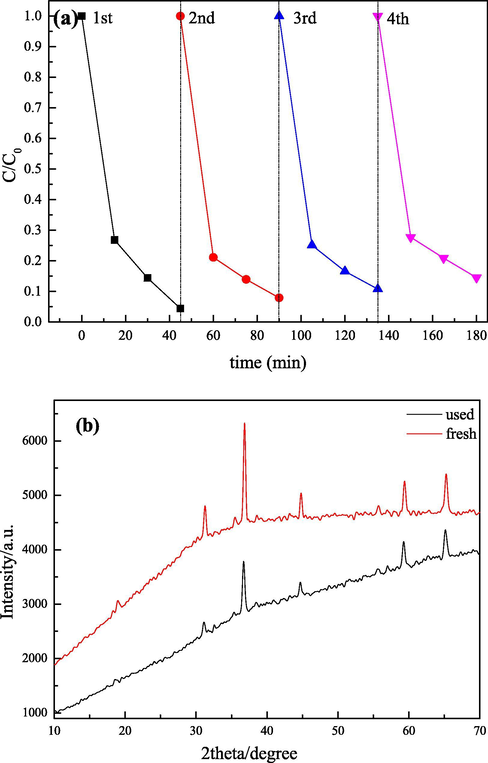
(a)Reusability of Co3O4/CoFe2O4 composite for RhB degradation. Experimental conditions: catalyst = 0.5 g·L−1, PS = 0.5 g·L−1, pH = 5.49 (natural) and RhB = 10 mg·L−1; (b) XRD patterns of fresh and used Co3O4/CoFe2O4.
4 Conclusion
In the present study, a novel magnetic catalyst was synthesized, which was applied for PMS activation to achieve effective RhB degradation. It was found that the degradation of RhB was highly pH-dependent with a higher degradation efficiency near the weakly acidic pH. The maximum degradation efficiency was obtained at pH of 5, where 96.05% of RhB was removed (the persulfate concentration of 0.5 g∙L−1, catalyst dosage of 0.5 g∙L−1 and reaction temperature of 25 °C). What’s more, naturally occurring species (Cl−, NO3− and HCO3−) exhibited inhibitory effects on RhB degradation, especially at higher concentrations. Quenching experiment showed that sulfate radicals were the main active species. Furthermore, Co3O4/CoFe2O4 composite performed good magnetic property, which could be easily separated through external magnetic and recycled from the aqueous solution. In addition, recycle experiments was conducted, in which the degradation efficiency of RhB could still reach 85.49% after 4 cycles. Finally, the enhanced mechanism was proposed. Overall, this paper provided a viable strategy for the fabrication of high-efficient recyclable catalysts which could be used in the treatment of printing and dyeing wastewater.
Acknowledgment
This work was kindly funded by National Natural Science Foundation of China (51978319), Fundamental Research Funds for the Central Universities (lzujbky-2017-it98), National College student innovation and Entrepreneurship training program of Lanzhou University and Key Laboratory of Comprehensive and Highly Efficient Utilization of Salt Lake Resources, Qinghai Institute of Salt Lake, Chinese Academy of Sciences.
References
- Optimizing COD removal from greywater by photoelectro-persulfate process using Box-Behnken design: assessment of effluent quality and electrical energy consumption. Environ. Sci. Pollut. Res.. 2016;23:19350-19361.
- [Google Scholar]
- UV-LEDs assisted peroxymonosulfate/Fe2+ for oxidative removal of carmoisine: the effect of chloride ion. Korean J. Chem. Eng.. 2017;34:2154-2161.
- [Google Scholar]
- Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem. Eng. J.. 2012;197:440-447.
- [Google Scholar]
- A comparative study of the common persulfate activation techniques for the complete degradation of an NSAID: the case of ketoprofen. Chem. Eng. J.. 2018;350:395-410.
- [Google Scholar]
- Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol.. 2003;37:4790-4797.
- [Google Scholar]
- Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e(-) transfer mechanisms. Appl. Catal. B. 2010;96:290-298.
- [Google Scholar]
- Treatment of stabilized leachate by ferrous-activated persulfate oxidative system. J. Hazard. Toxic Radioact. Waste. 2017;21:04016012.
- [Google Scholar]
- Assessment of bimetallic and trimetallic iron-based systems for persulfate activation: application to sulfamethoxazole degradation. Chem. Eng. J.. 2014;256:280-292.
- [Google Scholar]
- Ultrasound-assisted mineralization of organic contaminants using a recyclable LaFeO3 and Fe3+/persulfate Fenton-like system. Ultrason. Sonochem.. 2017;34:924-930.
- [Google Scholar]
- An overview of the application of Fenton oxidation to industrial wastewaters treatment. J. Chem. Technol. Biotechnol.. 2008;83:1323-1338.
- [Google Scholar]
- Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O) in aqueous solution. J. Phys. Chem. Ref. Data. 1988;17:513-531.
- [Google Scholar]
- Mechanistic investigations in sono-hybrid (ultrasound/Fe2+/UVC) techniques of persulfate activation for degradation of Azorubine. Ultrason. Sonochem.. 2017;38:652-663.
- [Google Scholar]
- Sulfate radical-induced degradation of Acid Orange 7 by a new magnetic composite catalyzed peroxymonosulfate oxidation process. J. Hazard. Mater.. 2014;279:476-484.
- [Google Scholar]
- Role of primary active species and TiO2 surface characteristic in UV-illuminated photodegradation of Acid Orange 7. J. Photochem. Photobiol., A. 2005;172:47-54.
- [Google Scholar]
- Construction of high-efficient photoelectrocatalytic system by coupling with TiO2 nano-tubes photoanode and active carbon/polytetrafluoroethylene cathode and its enhanced photoelectrocatalytic degradation of 2,4-dichlorophene and mechanism. Chem. Eng. J.. 2015;279:264-272.
- [Google Scholar]
- Effects of UV/PS and UV/H2O2 pre-oxidations on the formation of trihalomethanes and haloacetonitriles during chlorination and chloramination of free amino acids and short oligopeptides. Chem. Eng. J.. 2016;301:65-72.
- [Google Scholar]
- Electron beam irradiation of aqueous solution of persulfate ions. Chem. Eng. J.. 2011;169:258-262.
- [Google Scholar]
- CoFe2O4 magnetic nanoparticles as a highly active heterogeneous catalyst of oxone for the degradation of diclofenac in water. J. Hazard. Mater.. 2013;262:836-844.
- [Google Scholar]
- Cd doped porous Co3O4 nanosheets as electrode material for high performance supercapacitor application. Electrochimca Acta. 2016;196:316-327.
- [Google Scholar]
- Activation of peroxymonosulfate by metal (Fe, Mn, Cu and Ni) doping ordered mesoporous Co3O4 for the degradation of enrofloxacin. RSC Adv.. 2018;8:2338-2349.
- [Google Scholar]
- Heterogeneous advanced photo- Fenton process using peroxymonosulfate and peroxydisulfate in presence of zero valent metallic iron: a comparative study with hydrogen peroxide photo-Fenton process. J. Water Process Eng.. 2016;13:117-126.
- [Google Scholar]
- Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard. Mater.. 2016;308:58-66.
- [Google Scholar]
- Rapid selective circumneutral degradation of phenolic pollutants using peroxymonosulfate-iodide metal-free oxidation: role of iodine atoms. Environ. Sci. Technol.. 2017;51:2312-2320.
- [Google Scholar]
- Mechanism of base activation of persulfate. Environ. Sci. Technol.. 2010;44:6423-6428.
- [Google Scholar]
- Sonochemical degradation of textile dyes in aqueous solution using sulphate radicals activated by immobilized cobalt ions. Ultrason. Sonochem.. 2010;17:566-571.
- [Google Scholar]
- Electrochemical advanced oxidation processes coupled with peroxymonosulfate for the treatment of real washing machine effluent: a comparative study. J. Electroanal. Chem.. 2019;874:113182.
- [Google Scholar]
- Heterogeneous activation of peroxymonosulfate via nanocomposite CeO2-Fe3O4 for organic pollutants removal: The effect of UV and US irradiation and application for real wastewater. Sep. Purif. Technol.. 2019;228:115732.
- [Google Scholar]
- Oxidation of bisoprolol in heated PS/H2O systems: kinetics and products. Chem. Eng. J.. 2012;183:162-171.
- [Google Scholar]
- Reduction of nitrite and nitrate on nano-dimensioned FeS. Orig. Life Evol. Biospheres. 2013;43:305-322.
- [Google Scholar]
- Synthesis of MnO2 nanosheets on montmorillonite for oxidative degradation and adsorption of methylene blue. J. Colloid Interface Sci.. 2018;510:207-220.
- [Google Scholar]
- Ascorbic acid enhanced activation of oxygen by ferrous iron: a case of aerobic degradation of rhodamine B. J. Hazard. Mater.. 2016;308:67-74.
- [Google Scholar]
- Kinetics of heat-assisted persulfate oxidation of methyl tert-butyl ether (MTBE) Chemosphere. 2002;49:413-420.
- [Google Scholar]
- Behavioral evidence of the dominant radicals and intermediates involved in Bisphenol A degradation using an efficient Co2+/PMS oxidation process. J. Hazard. Mater.. 2009;167:418-426.
- [Google Scholar]
- Integration of coagulation and electro-activated HSO5− to treat pulp and paper wastewater. Sustain. Environ. Res.. 2017;27:223-229.
- [Google Scholar]
- Cobalt catalyzed peroxymonosulfate oxidation of tetrabromobisphenol A: kinetics, reaction pathways, and formation of brominated by-products. J. Hazard. Mater.. 2016;313:229-237.
- [Google Scholar]
- Advanced oxidation processes based on zero-valent aluminium for treating textile wastewater. Chem. Eng. J.. 2018;348:67-73.
- [Google Scholar]
- Preparation and characterization of palladium/polypyrrole/foam nickel electrode for electrocatalytic hydrodechlorination. Chem. Eng. J.. 2013;225:489-498.
- [Google Scholar]
- Efficient removal of organic pollutants from aqueous media using newly synthesized polypyrrole/CNTs-CoFe2O4 magnetic nanocomposites. Chem. Eng. J.. 2017;316:893-902.
- [Google Scholar]
- Simultaneous removal of nitrogen and phosphorus from wastewater by means of FeS-based autotrophic denitrification. Water Sci. Technol.. 2013;67:2761-2767.
- [Google Scholar]
- Role of inorganic ions and dissolved natural organic matters on persulfate oxidation of acid orange 7 with zero-valent iron. RSC Adv.. 2015;5:99935-99943.
- [Google Scholar]
- Preparation of magnetic CoFe2O4-functionalized graphene sheets via a facile hydrothermal method and their adsorption properties. J. Solid State Chem.. 2011;184:953-958.
- [Google Scholar]
- Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate-thiosulfate redox couple. Chemosphere. 2004;55:1213-1223.
- [Google Scholar]
- Feasibility study of ultraviolet activated persulfate oxidation of phenol. Chemosphere. 2011;82:1168-1172.
- [Google Scholar]
- UV/S2O82 process for degrading polyvinyl alcohol in aqueous solutions. Chem. Eng. Process. Process Intensif.. 2014;85:209-215.
- [Google Scholar]
- Metal-free carbonaceous materials as promising heterogeneous catalysts. ChemCatChem. 2015;7:2765-2787.
- [Google Scholar]
- Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter. Environ. Sci. Technol.. 2015;49:1673-1680.
- [Google Scholar]
- Reduced graphene oxide decorated with Co3O4 nanoparticles (rGO-Co3O4) nanocomposite: a reusable catalyst for highly efficient reduction of 4-nitrophenol, and Cr(VI) and dye removal from aqueous solutions. Chem. Eng. J.. 2017;322:375-384.
- [Google Scholar]
- Sonochemical oxidation of arsenic (III) to arsenic (V) using potassium peroxydisulfate as an oxidizing agent. Water Res.. 2010;44:3687-3695.
- [Google Scholar]
- Rate constants and mechanism of reaction of SO4· with aromatic compounds. J. Am. Chem. Soc.. 1977;99:163-164.
- [Google Scholar]
- Removal of rhodamine B from a water medium using hydroxyl and sulphate radicals generated by iron loaded activated carbon. RSC Adv.. 2016;6:5330-5340.
- [Google Scholar]
- Mixed industrial wastewater treatment by combined electrochemical advanced oxidation and biological processes. Chemosphere. 2019;237:124419.
- [Google Scholar]
- Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B. 2009;85:171-179.
- [Google Scholar]
- Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M = Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water. Appl. Catal. B. 2015;165:572-578.
- [Google Scholar]
- Magnetic CoFe2O4 nanoparticles supported on graphene oxide (CoFe2O4/GO) with high catalytic activity for peroxymonosulfate activation and degradation of rhodamine B. RSC Adv.. 2018;8:1351-1360.
- [Google Scholar]
- Photocatalytic elimination of aqueous 2-methyl-4-chlorophenoxyacetic acid in the presence of commercial and nitrogen-doped TiO2. Int. J. Environ. Sci. Technol.. 2015;12:513-526.
- [Google Scholar]
- Asymmetric supercapacitors using 3D nanoporous carbon and cobalt oxide electrodes synthesized from a single metal-organic framework. ACS Nano. 2015;6:6288-6296.
- [Google Scholar]
- Removal of reactive magenta-MB from aqueous solution by persulphate-based advanced oxidation process. Desalin. Water Treat.. 2014;57:11872-11878.
- [Google Scholar]
- Treatment of pharmaceutical effluent using novel heterogeneous fly ash activated persulfate system. J. Environ. Chem. Eng.. 2015;3:2229-2235.
- [Google Scholar]
- Oxidative removal of Bisphenol A by UV-C/peroxymonosulfate (PMS): Kinetics, influence of co-existing chemicals and degradation pathway. Chem. Eng. J.. 2015;276:193-204.
- [Google Scholar]
- Mechanistic study of photo-oxidation of Bisphenol-A BPA) with hydrogen peroxide (H2O2) and sodium persulfate (SPS) J. Environ. Manage.. 2016;166:12-22.
- [Google Scholar]
- One-pot hydrothermal synthesis of octahedral CoFe/CoFe2O4 submicron composite as heterogeneous catalysts with enhanced peroxymonosulfate activity. J. Mater. Chem. A. 2016;4:9455-9465.
- [Google Scholar]
- 3D-hierarchically structured MnO2 for catalytic oxidation of phenol solution by activation of peroxymonosulfate: structure dependence and mechanism. Appl. Catal. B. 2015;164:159-167.
- [Google Scholar]
- Degradation of rhodamine B in aqueous solution by using swirling jet-induced cavitation combined with H2O2. J. Hazard. Mater.. 2009;169:486-491.
- [Google Scholar]
- Enhanced superoxide radical production for ofloxacin removal via persulfate activation with Cu-Fe oxide. Chem. Eng. J.. 2018;354:473-480.
- [Google Scholar]
- Effects of chloride ions on bleaching of azo dyes by Co2+/oxone regent: kinetic analysis. J. Hazard. Mater.. 2011;190:1083-1087.
- [Google Scholar]
- Formation of halogenated disinfection by-products in cobalt-catalyzed peroxymonosulfate oxidation processes in the presence of halides. Chemosphere. 2016;154:613-619.
- [Google Scholar]
- Iron–cobalt mixed oxide nanocatalysts: heterogeneous peroxymonosulfate activation, cobalt leaching, and ferromagnetic properties for environmental applications. Appl. Catal. B. 2009;88:462-469.
- [Google Scholar]
- Removal of cibacron black commercial dye with heat- or iron-activated persulfate: statistical evaluation of key operating parameters on decolorization and degradation by-products. Desalination Water Terat.. 2016;57:2616-2625.
- [Google Scholar]
- Activation of persulfate by Co3O4 nanoparticles for orange G degradation. RSC Adv.. 2016;6:758-768.
- [Google Scholar]
- Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: efficiency, stability, and mechanism. Environ. Sci. Technol.. 2013;47:2784-2791.
- [Google Scholar]
- Reinvestigation of the acid-base equilibrium of the (bi)carbonate radical and pH dependence of its reactivity with inorganic reactants. Radiat. Phys. Chem.. 1999;55:15-23.
- [Google Scholar]







