Efficient separation of Cinnamomum camphora leaf essential oil and in vitro evaluation of its antifungal activity
⁎Corresponding author. zaizhiliu@hotmail.com (Zaizhi Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cinnamomum camphora leaf essential oil (CEO) was extracted using enzymatic-ultrasound pretreatment followed by microwave assisted extraction (EUP-MAE) method and simultaneously studied as a mycelial growth inhibitor against five important pathogens which cause potato dry rot. The optimum EUP-MAE conditions with a real CEO yield of 19.23 ± 0.12 mg/g were obtained through Plackett–Burman design and Box–Behnken design as follows: 3 % of enzyme dosage, 2 h of pretreatment time, 5 of pH, 210 W of ultrasound power, 50 °C pretreatment temperature, 16 mL/g of water to solid ratio, 30 min of microwave time and 500 W of microwave power. Compared to the reference methods, EUP-MAE possessed a highest CEO yield than these of ultrasound-microwave assisted extraction (U-MAE) and traditional hydrodistillation (HD). Gas chromatography-mass spectrometry (GC–MS) analysis demonstrated that eucalyptol, camphor, and α-terpineol were the three main constituents of CEO. Results from in vitro antifungal activity assay revealed that the mycelial growths of all the five tested Fusarium solani, Fusarium culmorum, Fusarium trichothecioides, Fusarium sporotrioides, and Fusarium avenaceum were apparently affected by CEO. These findings not only provide a potential paradigm for the separation of plant essential oil, but also guarantee a promising utilization of the CEO for potato protection to control the Fusarium spp.
Keywords
Cinnamomum camphora
Essential oil
Separation
Optimization
Antifungal
1 Introduction
Essential oils extracted from plant materials have been widely used in diversified fields, including food, agriculture, cosmetic and pharmaceutical (Zhang et al., 2020a,b). In recent years, as there is growing awareness of the potential harm to human body and the environment caused by the use of chemical synthesizers, plant essential oils from natural sources have been considered as a promising substitute for traditional antibacterial agents owing to their excellent antibacterial properties (Siddiqui et al., 2017). Cinnamomum camphora (L.) Presl., belonging to Lauraceae family, is an evergreen tree natively cultivated in southern China, as a landscape tree and Chinese herbal medicine (Tian et al., 2020; Zhang et al., 2020a,b). C. camphora leaf essential oil (CEO) gives off a strong aroma, which exhibits strong insecticidal, antimicrobial, antioxidant, anticancer properties (Luo et al., 2021), and anti-inflammatory activities (Chen et al., 2020), and thus CEO was used as a valuable raw material in food, pharmaceutical and cosmetics industries (Ni et al., 2021). Guleria and Kumar (2006) found that CEO can inhibit the growth of Alternaria alternata, Curvularia lunata, and Candida sp. Yu et al. (2019) reported that CEO has the effect of relieving pain and clearing heat, refreshing the brain. Xiao et al. (2021a,b) pointed out that CEO demonstrated strong anti-inflammatory activity and has great potential to be developed as a new and natural therapeutic agent for inflammatory skin conditions. Herein, an effective method to isolate high-value-adding essential oil from C. camphora leaf with high yield and better quality is urgently required.
Generally speaking, traditional hydrodistillation (HD) is a common method to extract plant essential oils, but long-time extract is easy to cause negative effects on some heat sensitive components of essential oils (Fiorini et al., 2020; Ozturk and Gonzalez-Miquel, 2019), so the development of economic and eco-benign technology for plant essential oil extraction is urgently needed. There are many improved methods, such as ultrasound assisted, solvent-free microwave assisted, ultrasound-microwave assisted extraction (U-MAE) and aqueous enzymatic assisted method, that indeed enhance the quality and extraction efficiency of essential oils and have been widely used in extraction of different plant essential oils (Fiorini et al., 2020; Liu et al., 2018; Singla and Sit, 2021; Zhang et al., 2019). Nevertheless, mechanisms of these methods diverse from each other. Ultrasound irradiation works by acoustic cavitation, which damages the stromal cell walls of plants, thereby helping to release bioactive compounds (Singla and Sit, 2021). Enzymatic hydrolysis pretreatment could hydrolyze plant cell wall components and degrades protein networks, thereby enhancing the dissolution of intracellular materials (Chari et al., 2013; Domínguez-Rodríguez et al., 2020; Vishwakarma and Malik, 2022). Additionally, under the mild ultrasound irradiation, the collision between enzyme and substrate could improve mass transfer efficiency, so as to enhancing the enzyme activity and the enzymatic hydrolysis reaction in the enzymolysis-ultrasound treatment process (Khadhraoui et al., 2021; Singla and Sit, 2021). Due to the molecular vibration and instantaneous thermal effect induced by microwave irradiation, the release of essential oil components in cells was facilitated, thus improving the extraction efficiency (Pimentel-Moral et al., 2018; Wang et al., 2019). Therefore, it is of our great interest on the extraction of CEO using a multi-technology fusion of enzymatic-ultrasound pretreatment followed by microwave assisted extraction (EUP-MAE) method as a substitute method to HD for the efficient extraction of CEO.
Potato dry rot caused by Fusarium spp. was a devastating postharvest disease worldwide (Tiwari et al., 2020a). It severely affected potato tuber storage and seed pieces after planting, causing big losses to the food processing industry and consumers (Tiwari et al., 2020b). The pathogenic fungi species of potato dry rot vary from planting areas and seasonal environmental conditions (Tiwari et al., 2021). More than thirteen species of Fusarium strains were the pathogenic factors of potato dry rot worldwide (Bojanowski et al., 2013; Cullen et al., 2005). In the northern of China, potato dry rot mainly caused by Fusarium solani, Fusarium avenaceum, Fusarium trichothecioides, Fusarium sporotrioides and Fusarium culmorum (Du et al., 2012; Min et al., 2010). What's more notable is that some fusarium pathogens also produce toxins, such as enniatins A, enniatins B, butylpicolinic acid and moniliformin, which are harmful to human and animals (Wang et al., 2020). Currently, chemical fungicides (e.g., thiabendazole, flusilazole, difenoconazole, 2-aminobutane), are considered as the main strategy and widely used in the control of potato dry rot (Ren et al., 2021). Nevertheless, the development of antimicrobial resistance and increasing consumer awareness of the safety of food and agricultural products are increasingly prompting researchers to find an alternative that are nontoxic to human health and eco-benign (Li et al., 2012; Nicosia et al., 2016). From this point of view, biological control of plant fungal diseases using natural substances has been caught great attention. Various alternatives, such as borates (Li et al., 2012), sulfur-containing salts (Kolaei et al., 2012), chitosan (Cullen et al., 2005), and plant essential oils (Xiao et al., 2021a,b) have been proposed for the control of Fusarium spp. As plant essential oils possess the natural origin, biodegradability, and low toxic activity to the environment and they are generally recognized as safe (GRAS) (Cabral et al., 2013). Previous studies reported that the essential oils separated from Origanum vulgare and Cinnamomum cassia have shown inhibitory activities against some Fusarium spp. in vitro (Dimitra et al., 2003; Li et al., 2012). Thus, using essential oils to control these diseases has the most promising prospect and corresponds well with the concept of sustainable agriculture. Therefore, it is of our great interest to investigate the in vitro antifungal activity of CEO on Fusarium spp. of potato dry rot.
The objective of this study is to develop an efficient EUP-MAE approach for the efficient separation of CEO and the evaluation of its antifungal activity against the five dominant pathogens caused potato dry rot. Herein, EUP-MAE was firstly proposed for obtaining CEO and the influential parameters of the extraction process was optimized by Plackett–Burman design (PBD) and Box-Behnken design (BBD). U-MAE and HD were performed as the reference methods to make a comparison with the proposed EUP-MAE. The chemical components of the obtained CEOs were analyzed by GC–MS. In order to promote the comprehensive utilization of CEO and provide the scientific data on the potential use of CEO as natural antifungal agents, the in vitro antifungal activity of the obtained CEO was investigated against five pathogenic fungi of F. solani, F. culmorum, F. trichothecioides, F. sporotrioides, and F. avenaceum.
2 Materials and methods
2.1 Plant materials and chemicals
Fresh C. camphora leaves were gathered from Jiangxi Normal University Yaohu campus (Nanchang, China) and identified by Professor Ronggen Deng (College of Life Science, Jiangxi Normal University, China). The C. camphora leaves were dried in the shade at 25 ± 2 °C for 7 days, then smashed with a grinder, screened through 40 mesh, and stored in a refrigerator.
F. avenaceum, F. sporotrioides, F. culmorum were purchased from China General Microbiological Culture Collection Center (CGMCC, Beijing, China). F. solani were purchased from China Center for Type Culture Collection (CTMCC, Wuhan, China). F. trichothecioides were obtained from Prof. Fenglan Li (Northeast Agricultural University, China). Potato was bought from the supermarket. Neutral protease (purity ≥ 95 %, enzyme activity ≥ 100 U/mg), cellulase (purity ≥ 95 %, enzyme activity ≥ 50 U/mg), hemicellulase (purity ≥ 95 %, enzyme activity ≥ 20 U/mg), pectinase (purity ≥ 95 %, enzyme activity ≥ 500 U/mg), Tween 80, nystatin, and d-Glucose were obtained from Shanghai Yuanye Biotechnology Co., ltd. (Shanghai, China). Agar Powder was gained from Beijing Aoboxing Bio-Tech Co., ltd (Beijing, China). A Milli-Q water purification system (Millipore, Billerica, MA, USA) was used to provide deionized water and used in the whole study.
2.2 CEO extraction
EUP-MAE consisted of two steps, namely enzymatic-ultrasound pretreatment and microwave assisted extraction procedure for the separation of CEO. C. camphora leaves powder (50 g) was mixed with a certain amount of enzyme solution (individual enzyme or enzyme mixture), and then the suspension was placed into KH-250DE ultrasonic bath (Kunshan Leibo Instrument Equipment Co., ltd., Kunshan, China) for enzymatic-ultrasound pretreatment. After the pretreatment, the suspension was transferred to a microwave oven for the extraction of CEO. The effects of eight factors were pre-tested to ascertain the appropriate ranges for the subsequent process optimization, and the corresponding results are shown on Table 1. The obtained CEO was collected in amber bottles after drying with anhydrous sodium sulphate and then placed in a 4 °C refrigerator for the latter analysis. CEO yields were calculated as the milligram of CEO per gram of raw materials.
| Variable code | Variable name | low level (−1) | Central level (0) | high level (+1) |
|---|---|---|---|---|
| A | Enzyme dosage (%) | 1 | 2 | 3 |
| B | Pretreatment time (h) | 1.0 | 1.5 | 2.0 |
| C | pH | 3 | 4 | 5 |
| D | Ultrasound power (W) | 150 | 200 | 250 |
| E | Pretreatment temperature (°C) | 30 | 40 | 50 |
| F | Water to solid ratio (mL/g) | 8 | 12 | 16 |
| G | Microwave time (min) | 20 | 30 | 40 |
| H | Microwave power (W) | 230 | 385 | 540 |
U-MAE and HD were conducted to make comparisons with EUP-MAE for CEO extraction. U-MAE process was carried out as the same as EUP-MAE optimal conditions without loading any enzymes. As for HD, samples (50 g) were mixed with 900 mL of deionized water, and then heated 240 min by an electric jacket at a heating power of 1000 W. The essential oil was collected in amber bottles, dried with anhydrous sodium sulphate, and then stored at 4 °C before the subsequent analysis.
2.3 Experimental design
In order to optimize the extraction conditions of EUP-MAE, PBD coupled with BBD of RSM were employed. The independent or interactive effects of factors may determine the extraction efficiency, so multivariate analysis can identify the interaction effects between parameters and provide scientific experiment design. Among the experimental design, PBD technology was firstly adopted to screen out the relatively important factors from eight independent variables (A: enzyme dosage, %; B: pretreatment time, h; C: pH; D: ultrasound power, W; E: pretreatment temperature, °C; F: water to solid ratio, mL/g; G: microwave time, min; H: microwave power, W). The whole PBD design consisted of 12 runs with combinations of eight independent variables at high level (+) or low level (−) as shown in Table 2. Then BBD was used to further optimize the yield of CEO, and the effects of four relatively important independent parameters selected from PBD on the yield were investigated. The BBD tested three predicted responses (yield of CEO) in different combinations of four parameters, namely enzyme dosage (%, A), ultrasound power (W, D), microwave time (min, G) and microwave power (W, H) and three levels (−1, 0 and +1). A total of 29 different combinations, including 5 repetitions of the central point, were performed to estimate experimental errors and demonstrate the applicability of the model. The actual values of coding levels and parameters obtained from the pre-experiment are given in Table 1. Through the application of “Design Expert” software version 8.0 (Stat-Ease, Minneapolis, USA), the experimental design was structured and the actual results were analyzed.
| Runa | A | B | C | D | E | F | G | H | Yield (mg/g) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Actual | Predicted | |||||||||
| 1 | −1 | 1 | −1 | −1 | −1 | 1 | −1 | 1 | 12.75 | 13.12 |
| 2 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | 14.59 | 14.38 |
| 3 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | 14.05 | 14.26 |
| 4 | 1 | 1 | −1 | −1 | 1 | −1 | 1 | 1 | 17.42 | 17.21 |
| 5 | −1 | 1 | 1 | 1 | 1 | −1 | −1 | −1 | 10.65 | 10.65 |
| 6 | 1 | 1 | −1 | 1 | −1 | 1 | 1 | −1 | 13.03 | 12.87 |
| 7 | 1 | −1 | 1 | −1 | 1 | 1 | −1 | 1 | 16.08 | 15.92 |
| 8 | 1 | −1 | −1 | 1 | 1 | 1 | −1 | −1 | 11.3 | 11.46 |
| 9 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 11.02 | 10.65 |
| 10 | 1 | 1 | 1 | 1 | −1 | −1 | −1 | 1 | 13.52 | 13.52 |
| 11 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | −1 | 14.5 | 14.87 |
| 12 | −1 | 1 | 1 | −1 | 1 | 1 | 1 | −1 | 14.45 | 14.45 |
| ANOVA | |||||||||
| Source | Sum of squares | Degree of freedom | Mean square | F Value | P value | Inferencec | |||
| Model | 44.55 | 8 | 5.57 | 27.27 | 0.0101 | * | |||
| Residual | 0.61 | 3 | 0.20 | ||||||
| Cor Totalb | 45.16 | 11 | |||||||
| Regression data | ||||||||||
| Term | Effect | Coefficient | Standard error | T value | F value | P value | Inference | |||
| A | 1.39 | 0.70 | 0.13 | 5.38 | 28.39 | 0.0129 | * | |||
| B | 0.05 | 0.02 | 0.13 | 0.18 | 0.032 | 0.8694 | ns | |||
| C | 0.70 | 0.35 | 0.13 | 2.69 | 7.27 | 0.0740 | ns | |||
| D | −1.51 | −0.76 | 0.13 | −5.85 | 33.65 | 0.0102 | * | |||
| E | 0.76 | 0.38 | 0.13 | 2.92 | 8.41 | 0.0625 | ns | |||
| F | 0.17 | 0.09 | 0.13 | 0.67 | 0.44 | 0.5539 | ns | |||
| G | 2.12 | 1.06 | 0.13 | 8.15 | 66.04 | 0.0039 | ** | |||
| H | 2.24 | 1.12 | 0.13 | 8.62 | 73.95 | 0.0033 | ** | |||
***Significant at p ≤ 0.001.
2.4 GC–MS analysis
GC–MS analysis of CEOs was operated on a Thermo Trace-1300 ISQ-mass chromatograph (Thermo Fisher Scientific Inc., Waltham, MA, USA) coupled with a HP-5MS capillary column (30 m × 0.25 mm, 0.25 mm i.d., 0.2 μm film thickness). The carrier gas was helium. A 10 μL of the diluted CEOs was injected with a split ratio of 1:2 at a flow rate of 1 mL/min. The heating process was started at 60 °C and kept for 5 min, followed by a constant rate of 10 °C/min to 120 °C and maintained for 5 min, then increased to 200 °C and held for 5 min, and finally enhanced to 280 °C for 8 min. The temperature presets for the sampler and detector were 230 °C and 280 °C, respectively. The MS was run at a voltage of 70 eV in EI mode with a scanning range of 50–500 amu. The volatile compound content was determined by peak area normalization. The chemical constituents of CEO were recognized by comparing with the mass spectrum and retention index (RI) in NIST02 Mass Spectral Library.
2.5 In vitro antifungal activity assays of the CEO
CEO was tested for its antifungal activities against five pathogenic fungi of F. solani, F. culmorum, F. trichothecioides, F. sporotrioides and F. avenaceum. Antifungal activity of CEO was investigated using in vitro contact assays (Ben Ghnaya et al., 2016). CEO was diluted in 5 % of Tween 80 and then mixed with potato dextrose agar (PDA) at 50 °C to a volume of 10 mL. Round mycelial disks with a 5 mm diameter were cut from the circumference of 7 days old cultures and then inoculated in the center of each PDA plates (90 mm diameter) at 25 ± 2 °C for 7 days. PDA plates loaded with 5 % of Tween 80 solution and nystatin to replace CEO solution were recorded as negative and positive controls, respectively. The mycelium growth inhibition rate was calculated using the formula as Eq. (1) follows,
2.6 Statistical analysis
Design Expert 8.0 software (Stat Ease Inc., Minneapolis, MN, USA) was used to perform process design, analyze regression models, and draw response surface plots. All tests were in triplicate. The CEO yields were shown as the mean value ± SD and the significance of their differences were calculated by the ANOVA test using OriginPro 2018 software (OriginLab Corporation, Northampton, MA, USA).
3 Results and discussion
3.1 The selection of enzyme species
Enzyme type could affect the extraction efficiency in the essential oil separation process involving enzymatic treatment (Hu et al., 2020). Therefore, the effects of four commercial enzymes (neutral protease, cellulase, hemicellulase and pectinase) and their mixtures (hemicellulase/pectinase, cellulase/pectinase, cellulase/hemicellulase, and cellulase/hemicellulase/pectinase) on CEO yield using EUP-MAE were studied. As shown in Fig. 1a, higher essential oil yields were achieved with enzymatic pretreatment compared to the control set (without enzymatic treatment). Cellulase possessed the highest essential oil yield compared to other individual enzymes and the control, which is similar to the previous studies (Jiao et al., 2012; Rashed et al., 2018). As cellulase, hemicellulase, and pectinase has the relatively higher CEO yield than neutral protease and the control group, and therefore these three enzymes were mixed to study the enzyme combinations on the essential oil yield in EUP-MAE process. The results in Fig. 1b indicated that the highest CEO yield was obtained by using the mixture of cellulase/hemicellulase/pectinase (1/1/1, w/w/w). This result was in consistent with previous studies, in which an equal quality ratio of cellulase/hemicellulase/pectinase provided the highest yield for the extraction of Cyperus esculentus seed oil and Lavandula angustifolia essential oil (Hu et al.,2020; Rashed et al., 2017). Plant cellular structures determine the enzyme type in the plant essential oil extraction process. Generally, cellulose, hemicellulase, and pectin are the three dominant components of plant cell cytoderm. Once these components were degraded, plant essential oil can be easily released into the solvent (Gil-Chavez et al., 2013). In the EUP-MAE extraction process, the mixed enzymes of cellulase/hemicellulase/pectinase had the higher essential oil yield thanks to their action on the decomposition of C. camphora leaves cell structures. Additionally, cellulase, hemicellulase, and pectinase have similar pH values and temperature ranges (Hu et al., 2020), which is conducive to the synergistic effect of their mixing and improve enzymatic hydrolysis during the extraction process (Nadar et al., 2018). Therefore, an enzyme mixture of cellulase/hemicellulase/pectinase (1/1/1, w/w/w) was selected for further optimization experiments.
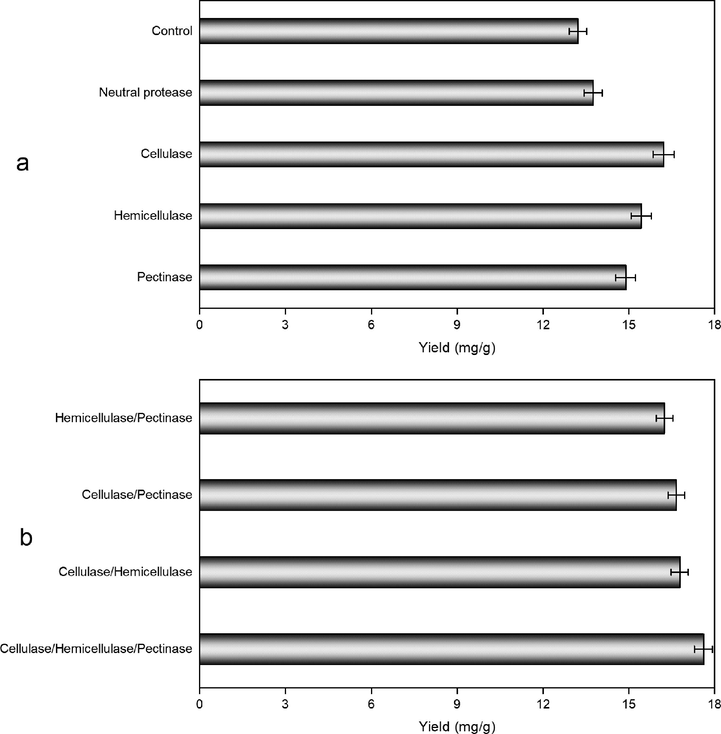
- The selection of enzyme species on CEO extraction using enzymolysis-ultrasound pretreatment and followed by microwave assisted extraction method. Control set: without enzymatic treatment.
3.2 Optimization of CEO extraction conditions by EUP-MAE
3.2.1 PBD
For EUP-MAE process, there were numerous variables that affect the final essential oil yield. Whereupon, PBD was firstly used to select the variables that had a significant impact on the essential oil yield from eight factors (A: enzyme dosage, %; B: pretreatment time, h; C: pH; D: ultrasound power, W; E: pretreatment temperature, °C; F: water to solid ratio, mL/g; G: microwave time, min; H: microwave power, W). The first-order model equation developed by PBD for the essential oil yield was expressed as Eq. (2) following,
The design and results of PBD were presented on Table 2. The influence of each variable on CEO yield can be illustrated by the magnitude of its T value and P value, and the corresponding positive or negative sign of T value represents the positive or negative influence on oil production, respectively. As represented on Table 2 and Fig. 2, all the factors had positive effects on CEO yield except for ultrasound power, which had a negative effect. Enzyme dosage (A), ultrasound power (D), microwave time (G) and microwave power (H) demonstrated significant effects on CEO separation in EUP-MAE procedure, so they were further investigated by BBD. Pretreatment time (B), pH (C), pretreatment temperature (E), and water to solid ratio (F) had no significant effect on the EUP-MAE process. According to the results of pre-experiment and PBD, these insignificant factors were maintained at 2 h for pretreatment time, 5 for pH, 50 °C for pretreatment temperature, and 16 mL/g for water to solid ratio.
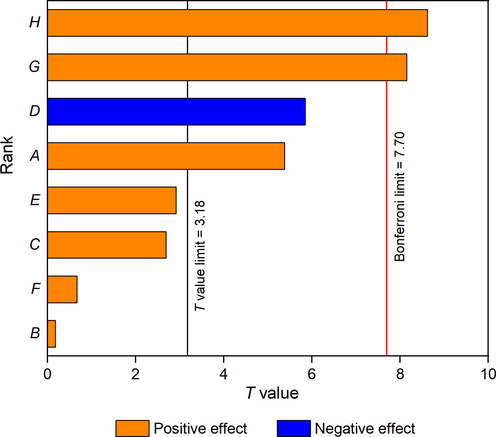
- Pareto chart of PBD for CEO extraction. (A) enzyme dosage; (B) pretreatment time; (C) pH; (D) ultrasound power; (E) pretreatment temperature; (F) water to solid ratio; (G) microwave time; (H) microwave power.
3.2.2 Regression coefficients and the model fitting of BBD
BBD was applied to further optimize the significant influence variables in EUP-MAE process. The complete BBD design matrix combined with the determined and predicted values of experimental yields, which consisted of 24 factorial tests and 5 center tests as given on Table 3. Probability P (P value) and Fisher test (F value) were employed to determine the significance of each coefficient and the corresponding results are presented on Table 4 to verify the analysis of variance (ANOVA) of the entire experimental design. Larger absolute F values and smaller P values could better reflect the significant influence of the relevant variables. The higher model F value (45.58) and the associated lower P value (P < 0.0001) manifested the significance of the developed model and demonstrated that the regression equation could explain the most of the variation presented in the response. The quality of the developed model was evaluated based on the correlation coefficient (R2) and lack of fit values. As given on Table 4, the ANOVA results indicated that the prediction model reasonably represented the determined values as evidenced by the R2 of 0.9785, lacking fitting F value of 0.26, and P value of 0.0569. The high values of correlation coefficient (R2 = 0.9785), adjusted (R2 = 0.9571), and predicted (R2 = 0.8824) clearly exhibited that 95.71 % of the response value variation and 97.85 % of the response variation could be explained by the selection factor variation. Hence, the selected form of the developed model reflected a good correlation between independent parameters and responses, and the RSM model established in this study can be applied to predict the yield of CEO using EUP-MAE. Linear terms of A, G, H and second-order terms of A2 and D2 were observed to be extremely significant; interaction term of AH and quadratic term of G2 were highly significant; interaction terms of AG, DG, DH and quadratic term H2 of were significant. Using the predicted second-order polynomial equation, a mathematical model was found to predict the optimal conditions to maximize the CEO yield and to evaluate the combined relationship between process parameters and response. The second-order predictive model of coding parameters was given as Eq. (3) follows,
| Runa | A | D | G | H | Yield (mg/g) | |
|---|---|---|---|---|---|---|
| Actual | Predicted | |||||
| 1 | 1 | 0 | 0 | 1 | 18.76 | 18.71 |
| 2 | 0 | 1 | 1 | 0 | 16.09 | 16.67 |
| 3 | 1 | 0 | −1 | 0 | 17.58 | 17.98 |
| 4 | 0 | −1 | −1 | 0 | 16.05 | 15.57 |
| 5 | 0 | 1 | −1 | 0 | 14.75 | 14.30 |
| 6 | −1 | 0 | 1 | 0 | 14.48 | 14.13 |
| 7 | 0 | −1 | 0 | −1 | 15.68 | 15.29 |
| 8 | 0 | 0 | 0 | 0 | 17.73 | 17.60 |
| 9 | 0 | 0 | 0 | 0 | 17.65 | 17.60 |
| 10 | 1 | 1 | 0 | 0 | 17.37 | 17.23 |
| 11 | 0 | 1 | 0 | −1 | 14.18 | 14.04 |
| 12 | 0 | 0 | 0 | 0 | 17.32 | 17.60 |
| 13 | 0 | 1 | 0 | 1 | 17.13 | 17.56 |
| 14 | 0 | 0 | −1 | 1 | 17.44 | 17.25 |
| 15 | 1 | 0 | 1 | 0 | 18.44 | 17.99 |
| 16 | 1 | 0 | 0 | −1 | 17.77 | 17.88 |
| 17 | 0 | 0 | 0 | 0 | 17.86 | 17.60 |
| 18 | 0 | 0 | 0 | 0 | 17.47 | 17.60 |
| 19 | −1 | 0 | −1 | 0 | 11.25 | 11.74 |
| 20 | 1 | −1 | 0 | 0 | 17.29 | 17.43 |
| 21 | 0 | −1 | 1 | 0 | 15.05 | 15.59 |
| 22 | −1 | 1 | 0 | 0 | 12.57 | 12.29 |
| 23 | 0 | −1 | 0 | 1 | 16.3 | 16.49 |
| 24 | −1 | 0 | 0 | −1 | 11.14 | 11.29 |
| 25 | 0 | 0 | −1 | −1 | 14.33 | 14.55 |
| 26 | −1 | −1 | 0 | 0 | 12.27 | 12.27 |
| 27 | 0 | 0 | 1 | −1 | 16.04 | 16.09 |
| 28 | 0 | 0 | 1 | 1 | 18.48 | 18.11 |
| 29 | −1 | 0 | 0 | 1 | 15.21 | 15.20 |
| Source | Sum of squares | Degrees of freedom | Mean square | F value |
P value Prob > F |
Inference | |
|---|---|---|---|---|---|---|---|
| Modela | 126.16 | 14 | 9.01 | 45.58 | <0.0001 | *** | |
| A | 76.53 | 1 | 76.53 | 387.12 | <0.0001 | *** | |
| D | 0.025 | 1 | 0.025 | 0.13 | 0.7275 | ns | |
| G | 4.29 | 1 | 4.29 | 21.71 | 0.0004 | *** | |
| H | 16.75 | 1 | 16.75 | 84.75 | <0.0001 | *** | |
| AD | 0.012 | 1 | 0.012 | 0.063 | 0.8051 | ns | |
| AG | 1.40 | 1 | 1.40 | 7.11 | 0.0185 | * | |
| AH | 2.37 | 1 | 2.37 | 11.96 | 0.0038 | ** | |
| DG | 1.38 | 1 | 1.38 | 6.96 | 0.0195 | * | |
| DH | 1.36 | 1 | 1.36 | 6.86 | 0.0202 | * | |
| GH | 0.11 | 1 | 0.11 | 0.57 | 0.4624 | ns | |
| A2 | 13.41 | 1 | 13.41 | 67.82 | <0.0001 | *** | |
| D2 | 12.05 | 1 | 12.05 | 60.95 | <0.0001 | *** | |
| G2 | 3.23 | 1 | 3.23 | 16.35 | 0.0012 | ** | |
| H2 | 1.02 | 1 | 1.02 | 5.14 | 0.0397 | * | |
| Residual | 2.77 | 14 | 0.20 | ||||
| Lack of fit | 2.58 | 10 | 0.26 | 5.53 | 0.0569 | ns | |
| Pure error | 0.19 | 4 | 0.047 | ||||
| Cor totalb | 128.93 | 28 | |||||
| Std. Dev.c | Mean | C.V.d % | Press | R2 | Adjusted R2 | Predicted R2 | Adequate precision |
| 0.44 | 15.99 | 2.78 | 15.16 | 0.9785 | 0.9571 | 0.8824 | 23.19 |
3.2.3 Response surfaces analysis
The interaction influences of enzyme dosage, ultrasound power, microwave time, and microwave power on CEO yield were illustrated by using the three-dimension profile of multivariate nonlinear regression model. Fig. 3 emphasized the behavior of essential oil yield under the interactions influences of two parameters. In each figure, the other two variables were fixed at a central value. Fig. 3a described the interactive influences of enzyme dosage (A) and microwave time (G) on CEO yield with an ultrasound power (D) of 200 W and a microwave power (H) of 385 W. It can be concluded that increasing of enzyme dosage and microwave time was helpful to significantly enhance essential oil yield. Additionally, when the essential oil reach to the peak, the optimum value of the two parameters were 3.0 % of enzyme dosage and 34 min as microwave time. The reason for this phenomenon may be owing to that the increases of enzyme dosage and microwave time were beneficial to the deconstruction of plant cell walls, thus improving the essential oil yield (Hou et al., 2019). As there is no change on the substrate concentration, further increase in enzyme dosage did not contribute the enhancement of CEO yield, but improved the cost. Fig. 3b showed strong interactive influences between enzyme dosage (A) and microwave power (H) on CEO yield. On the basis of the literature (Gligor et al., 2019), microwave irradiation at the initial stage may contribute a significant increase in extracted yield by making essential oil more solubilization, whereas a further increase in microwave power may lead to the destruction of enzyme or plant substrate, due to decreased essential oil yield at the later phase. As exhibited in Fig. 3c, the interactive influences were depicted between microwave time (G) and ultrasound power (D) on the CEO yield. It was found that the CEO yield improved with the increases of microwave time and ultrasound power, and then decreased gradually with the further increase of these two variables. Fig. 3d showed the interactive influences of ultrasound power (D) and microwave power (H) on CEO yield. The results suggested that CEO yield increased with the increase of microwave power and ultrasound power, and the maximum CEO yield was obtained when the microwave time is about 34 min and the ultrasound power approximately is 190 W. This result may be due to the fact that the increase in ultrasound power produced many cavitation effects in the extracted matrix which promoted hydrolysis of the cell wall by the mixed enzyme, and the enhancement of microwave power accelerated the thermal motion of molecules in plant materials, resulting in active ingredient dissolves faster and improved essential oil yield (Rashed et al., 2017). Whereas, it worth to note that exorbitant ultrasound power and microwave power can degrade or isomerize some heat-sensitive components of target product (Bachtler and Bart, 2021), resulting in decline of the CEO yield.
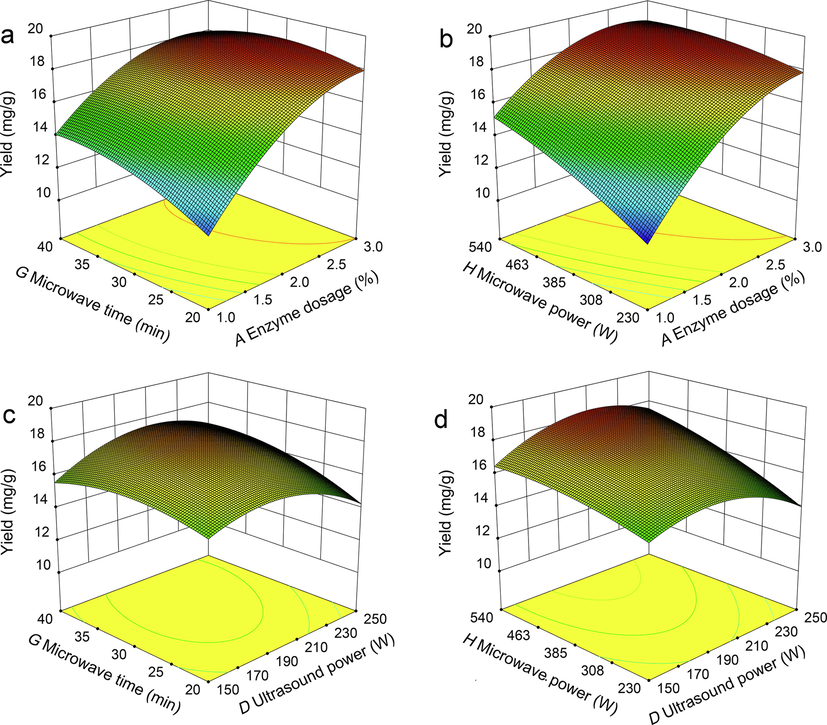
- The three-dimensional and contour surface plots from BBD. (a) The interactive influences of enzyme dosage (A) and microwave time (G) on CEO yield; (b) The interactive influences of enzyme dosage (A) and microwave power (H) on CEO yield; (c)The interactive influences of microwave time (G) and ultrasound power (D) on CEO yield; (d) The interactive influences of ultrasound power (D) and microwave power (H) on CEO yield.
3.2.4 Verification of the model
In consideration of operational efficiency, experimental feasibility and reduction in energy consumption, BBD, and RSM were used in this study to ensure the maximum CEO yield by optimizing the extraction conditions of EUP-MAE. BBD results demonstrated that the optimal EUP-MAE conditions with a theoretical CEO yield of 18.96 mg/g were: 3 % of enzyme dosage, 210 W of ultrasound power, 32 min of microwave time and 521 W of microwave power. The verification study was carried out with slightly modifications of the obtained conditions in EUP-MAE procedure to verify the accuracy and acceptability of the developed model. An average CEO yield of 19.23 ± 0.12 mg/g was calculated based on actual experimental data, which demonstrated that optimal conditions could be calculated using mathematical optimization of response surface methodology in a practical and reliable manner.
3.3 Comparison of EUP-MAE with reference methods
As illustrated in Table 5, the comparison of the essential oil yield of the developed EUP-MAE technology with other reference technologies (U-MAE and HD) was carried out under optimal conditions. EUP-MAE (19.23 ± 0.12 mg/g) exhibited a significantly higher CEO yield than those of traditional HD (16.37 ± 0.10 mg/g) and U-MAE (17.18 ± 0.15 mg/g). This phenomenon could be attribute to three reasonable respects. Firstly, according to the characteristics of plant matrix cell walls, the mixed enzymes pretreatment of plant materials can fully degrade plant cell walls and significantly increase the separation efficiency (Chari et al., 2013; Domínguez-Rodríguez et al., 2020; Vishwakarma and Malik, 2022). Secondly, ultrasound waves can propagate through any medium as mechanical waves by generating phases of expansion and compression, and induce the formation, development and implosion of cavitation bubbles in the liquid medium, thus accelerating cell collapse and promoting mass transfer behavior (Singla and Sit, 2021); and it is interesting to note that low-intensity ultrasound has been proved to improve the activity of several enzymes, such as cellulase and pectinase, by increasing the binding of the enzyme active site to the substrate (Khadhraoui et al., 2021), thus enhancing the enzymatic hydrolysis reaction and promoting the release of components in the plant matrix. Thirdly, after the high frequency electromagnetic wave produced by microwave can penetrate the water medium and reach the interior of the material, it can be quickly converted into heat, heating the water in the cells and evaporating, so as to rapidly increase the temperature and pressure inside the cells (Rashed et al., 2017). When the intracellular pressure exceeds the pressure the cell wall can withstand, the cell wall ruptures and the plant intracellular components are leached more quickly into the surrounding solvents (Suktham et al., 2021). In summary, EUP-MAE offers an obvious advantage over U-MAE and HD in enhancing the yield of plant essential oils thanks to the synergistic effects of enzymatic, ultrasound, and microwave radiation. Therefore, EUP-MAE is a promising separation technology of plant essential oils.
| No1 | Compounds | Retention time (min) | CAS number | Molecular formula | Relative peak area (%) | RI2 | Identification | ||
|---|---|---|---|---|---|---|---|---|---|
| EUP-MAE | U-MAE | HD | |||||||
| 1 | α-Phellandrene | 6.98 | 00099–83-2 | C10H16 | 1.23 | 1.46 | ND5 | 966 | RI3, MS4 |
| 2 | α-Pinene | 7.08 | 02437–95-8 | C10H16 | 0.64 | 0.78 | 1.65 | 975 | RI, MS |
| 3 | Sabinene | 7.18 | 03387–41-5 | C10H16 | ND | ND | 0.70 | 977 | RI, MS |
| 4 | α-Terpinen | 8.08 | 00099–86-5 | C10H16 | ND | 0.52 | ND | 995 | RI, MS |
| 5 | Camphene | 8.31 | 00079–92-5 | C10H16 | 0.62 | 0.69 | 0.72 | 999 | RI, MS |
| 6 | d-Limonene | 8.58 | 00138–86-3 | C10H16 | 2.01 | 2.33 | 1.91 | 1020 | RI, MS |
| 7 | Eucalyptol | 9.30 | 00470–82-6 | C10H18O | 32.53 | 31.00 | 31.46 | 1028 | RI, MS |
| 8 | 3-Carene | 9.68 | 13466–78-9 | C10H16 | 0.70 | 0.83 | 1.15 | 1031 | RI, MS |
| 9 | trans-4-Thujanol | 10.15 | 17699–16-0 | C10H18O | 0.37 | ND | ND | 1043 | RI, MS |
| 11 | Terpinolene | 10.50 | 00586–62-9 | C10H16 | 0.67 | 0.70 | 0.68 | 1080 | RI, MS |
| 12 | Linalool | 10.89 | 00078–70-6 | C10H18O | 1.15 | 1.38 | 0.86 | 1095 | RI, MS |
| 13 | Terpinen-4-ol | 13.10 | 00562–74-3 | C10H18O | 2.72 | 2.82 | 2.93 | 1157 | RI, MS |
| 14 | Camphor | 13.31 | 00076–22-2 | C10H16O | 32.54 | 29.29 | 19.55 | 1163 | RI, MS |
| 15 | α-Terpineol | 13.61 | 00098–55-5 | C10H18O | 11.26 | 10.97 | 11.61 | 1170 | RI, MS |
| 16 | Caryophyllene | 19.39 | 00087–44-5 | C15H24 | 2.07 | 2.26 | 4.50 | 1395 | RI, MS |
| 17 | β-Farnesene | 20.21 | 77129–48-7 | C15H24 | 0.49 | 0.64 | 1.08 | 1402 | RI, MS |
| 18 | Humulene | 20.64 | 06753–98-6 | C15H24 | 1.43 | 1.48 | 4.55 | 1434 | RI, MS |
| 19 | Germacrene D | 21.47 | 23986–74-5 | C15H24 | 0.67 | 0.76 | 1.06 | 1442 | RI, MS |
| 22 | Eremophilene | 21.63 | 10219–75-7 | C15H24 | 1.14 | 1.44 | ND | 1486 | RI, MS |
| 23 | δ-Guaiene | 21.70 | 03691–11-0 | C15H24 | 1.22 | 1.43 | 2.27 | 1489 | RI, MS |
| 24 | α-Farnesene | 21.81 | 00502–61-4 | C15H24 | 0.43 | ND | 0.78 | 1496 | RI, MS |
| 25 | β-Germacrene | 22.13 | 15423–57-1 | C15H24 | 2.44 | 2.81 | 4.18 | 1506 | RI, MS |
| 26 | Methyleugenol | 22.62 | 00093–15-2 | C11H14O2 | 0.66 | 0.78 | ND | 1508 | RI, MS |
| 27 | δ-Cadinene | 22.67 | 00483–76-1 | C15H24 | ND | ND | 0.63 | 1541 | RI, MS |
| 28 | Nerolidol | 23.43 | 07212–44-4 | C15H26O | 2.04 | 2.21 | 4.72 | 1561 | RI, MS |
| 29 | β-Spathulenol | 24.63 | 77171–55-2 | C15H24O | 0.63 | 0.88 | 0.91 | 1582 | RI, MS |
| Total (%) | 99.66 | 97.46 | 97.90 | ||||||
| Oxygenated compounds (%) | 83.90 | 79.33 | 72.04 | ||||||
| Terpene hydrocarbons (%) | 15.76 | 18.13 | 25.86 | ||||||
| Yield (mg/g) | 19.23 ± 0.12a | 17.18 ± 0.15b | 16.37 ± 0.10c | ||||||
Values with the different letter are significantly different at the 5 % (P < 0.05) level according to ANOVA.
3.4 Compositions of CEOs obtained by different methods
Chemical components of CEOs isolated by EUP-MAE, U-MAE and HD were presented on Table 5. Based on the peak area normalization method for GC peaks, the contents of each component are calculated and listed in accordance with GC (HP-5MS) elution order. A total of 26 compounds were detected by GC–MS analysis. 23 compounds accounting for 99.66 % were detected on CEO extracted by EUP-MAE. As these for U-MAE and HD, 21 compounds were detected on the essential oils with the total proportions of 97.46 % and 97.90 %, respectively. The CEOs obtained by the three methods have the same main components but different contents. Three main ingredients were detected in CEO were eucalyptol, camphor and α-terpineol, which content of were approximately to those reported in Sriramavaratharajan et al. (2016). In the present study, it was worthy to note that camphor took the second highest proportion in CEO, which was different from the previous research of our group (Liu et al., 2018). This difference may be due to genetic variation or chemical variety (Cano et al., 2008; Lota et al., 2000). Sriramavaratharajan et al. (2016) have also pointed out that there were differences on camphor content in CEOs distributed in adjacent areas.
Notably, among the identified chemical components of CEO obtained by different methods, the proportion of oxygenated compounds was obviously more than that of sesquiterpene hydrocarbons. The content of oxygenated compounds in the CEO extracted from EUP-MAE was significantly higher than that of the essential oil extracted from U-MAE and HD (83.90 % of EUP-MAE, 79.33 % of U-MAE, 72.04 % of HD), while the proportion of sesquiterpene hydrocarbons was evidently lower than that of U-MAE and HD (15.76 % of EUP-MAE, 18.13 % of U-MAE, 25.86 % of HD). The reason behind the above results may be that oxygenated compounds with high dipole moments interact more strongly with microwave radiation and can be extracted more easily than sesquiterpene hydrocarbons with low dipole moments (Filly et al., 2014). Furthermore, the high proportion of oxygenated compounds detected in CEO by EUP-MAE possibly was attributed to the fact that thermal degradation and hydrolysis are reduced resulting from the shortening of distillation time under the combined influence of enzymolysis - ultrasound pretreatment and microwave radiation (Reis et al., 2020). Studies (Ferhat et al., 2006) suggested that oxygenated compounds intensify the aroma and value of essential oils. Therefore, the CEO extracted by our developed EUP-MAE method are more fragrant than these extracted by U-MAE and HD.
3.5 In vitro antifungal activity
Previous studies have reported that essential oil appears to be safer for controlling fungal strains than pure chemical bacteriostatic agents (Derbalah et al., 2011; Masyita et al., 2022). Therefore, the in vitro antifungal activity of CEO on inhibiting mycelial growth of the five fungal pathogens of potato dry rot were investigated. The minimum inhibitory concentration (MIC, mg/mL) and the effective concentration resulting in 50 % inhibition of mycelial growth (EC50, mg/mL) were calculated graphically by linear regression using OriginPro 2018 software, in which CEO concentration and inhibition rate were taken as vertical axis and horizontal axis, respectively. As given on Table 6, the MIC and EC50 values of CEO against F. solani, F. culmorum, F. trichothecioides, F. sporotrioides, and F. avenaceum varied in the range of 2.5–3 mg/mL and 0.74–1.54 mg/mL, respectively. Fig. 4 exhibited the inhibition patterns of CEO (A2-E2) and nystatin (A3-E3) at EC50 values against five Fusarium strains. Compared with the negative control sets (PDA plates with only 5 % of Tween 80 solvent added) in Fig. 4A1-4E1, CEO could inhibit mycelial growths of all tested pathogen strains and exhibited the potential antifungal activity against these strains. Of particular interest was the fact that CEO had obvious influence on the colony color of the tested strains in comparison with nystatin.
| Fungal strain | MICa (mg/mL) | EC50b (mg/mL) |
|---|---|---|
| F. solani | 3.0 | 1.37 |
| F. culmorum | 2.5 | 1.29 |
| F. trichothecioides | 2.5 | 1.18 |
| F. sporotrioides | 2.5 | 0.74 |
| F. avenaceum | 2.5 | 1.54 |
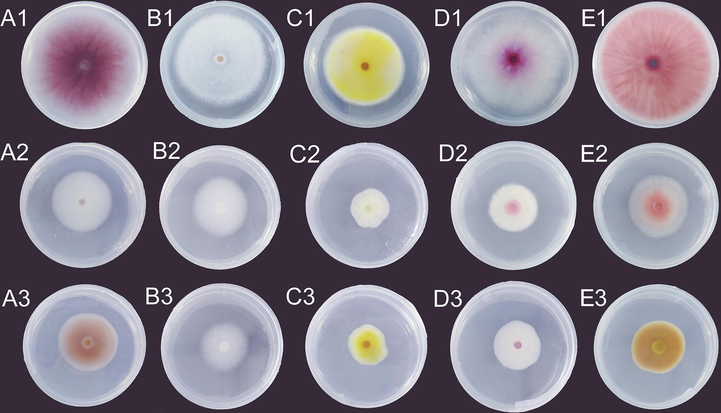
- The inhibition patterns for the mycelial growths of F. solani (A), F. culmorum (B), F. trichothecioides (C), F. sporotrioides (D), and F. avenaceum (E) after treated with CEO (A2-E2) and nystatin (A3-E3). Negative control sets (A1-E1) showed the PDA plates with only 5% of Tween 80 solvent added.
Based on the previous study (Stević et al., 2014), CEO could be considered as high antifungal activity against the tested fungi. It is considered that plant essential oils possess an antibacterial property that could be a result of a single compound or of a combination of compounds acting synergistically or antagonistically (Lopes-Lutz et al., 2008; Tariq et al., 2019). Pragadheesh et al. (2013) suggested that the lipophilicity of volatile oils helps the components to penetrate the fungal plasma membrane, resulting in cytoplasmic coagulation and mycelial dissolution. Jeldi et al. (2022) pointed out that in the case of antifungal activity, the mechanism of action of essential oils appears to involve disturbing the cell wall and plasma membrane, leading to cell lysis and leakage of intracellular compounds, and ultimately cell death. Conner and Beuchat (1984) put forward the point of view that inhibitory activity was caused by interaction of essential oils in the synthesis of microbial cellular structural units and in enzyme systems associated with energy production. A metabolomics analysis exhibited that CEO has antibacterial activity by interfering with cellular metabolism, especially alanine, aspartic and glutamic acid metabolic pathways (Chen et al., 2020). Based on Chaturvedi et al. (2018), the efficacy of CEO could also be attributed to activity of oxygenated monoterpenes, eucalyptol, camphor and α-terpineol. Polec et al. (2019) has pointed out that the antibacterial activity of essential oil contained the high proportions of oxygen-containing monoterpenes, such as eucalyptol, a terpene that can influence sterols, a component of fungal cell membrane, to produce antibacterial effects. CEO has been observed on controlling the mycelial growth of Choanephora cucurbitarum thanks to the high content of camphor and eucalyptol, which was speculated to play a synergistic role with minor components (Viljoen et al., 2003). Zhou et al. (2014) have pointed out that the antifungal property of α-terpineol against Geotrichum citri-aurantii was ascribed to the destruction of the fungi cell membrane and thus leading to the subsequent leakage of intracellular compounds. Moreover, α-terpineol has been proved to possess the ability to destroy or penetrate into lipid structures (Kararli et al., 1995).
4 Conclusions
EUP-MAE technology was used to extract essential oil from the leaves of C. camphora. A mixture of cellulase/hemicellulase/pectinase with an equal proportion were identified as suitable enzyme combinations for the essential oil extraction process. PBD, BBD and RSM were successfully carried out for investigation of simultaneous influences of four independent parameters (enzyme dosage, ultrasound power, microwave time and microwave power) on the yield of essential oil and for obtaining the optimal extraction conditions (3 % of enzyme dosage, 2 h of pretreatment time, 5 of pH, 210 W of ultrasound power, 50 °C pretreatment temperature, 16 mL/g of water to solid ratio, 30 min of microwave time and 500 W of microwave power) with a yield of 19.23 ± 0.12 mg/g. By comparison with U-MAE, EUP-MAE (individual enzyme) and traditional HD, optimized EUP-MAE (mixed enzyme) showed higher extraction yield and higher quality essential oils. Moreover, CEO exhibited inhibitory activities against the five tested pathogenic fungus, which could be a promising alternative to chemical fungicides for the control of potato dry rot or other applications on agriculture or food industrial in consideration of safety requirements and meeting consumer demand.
Acknowledgement
The authors thank the financial supports by National Natural Science Foundation of China, China (31860189), Natural Science Foundation of Jiangxi Province, China (20202BAB213024), National Natural Science Foundation of China, China (31760099), and Natural Science Foundation of Jiangxi Province, China (20202BAB203022).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Increase the yield of bioactive compounds from elder bark and annatto seeds using ultrasound and microwave assisted extraction technologies. Food Bioprod. Process.. 2021;125:1-13.
- [Google Scholar]
- Tetraclinis articulata (Vahl.) Masters essential oil from Tunisia: chemical characterization and herbicidal and antifungal activities assessment. Ind. Crop. Prod.. 2016;83:113-117.
- [Google Scholar]
- Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol.. 2013;166(1):1-14.
- [Google Scholar]
- Bioactive compounds in different citrus varieties. Discrimination among cultivars. J. Food Compos. Anal.. 2008;21(5):377-381.
- [Google Scholar]
- Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe) Food Chem.. 2013;139:509-514.
- [Google Scholar]
- Chemical composition, genetic diversity, antibacterial, antifungal and antioxidant activities of camphor-basil (Ocimum kilimandscharicum Guerke) Ind. Crop. Prod.. 2018;118:246-258.
- [Google Scholar]
- Metabolomics analysis to evaluate the antibacterial activity of the essential oil from the leaves of Cinnamomum camphora (Linn.) Presl. J. Ethnopharmacol.. 2020;253:112652
- [Google Scholar]
- Effects of essential oils from plants on growth of food spoilage yeasts. J. Food Sci.. 1984;49:429-434.
- [Google Scholar]
- Use of quantitative molecular diagnostic assays to investigate Fusarium dry rot in potato stocks and soil. Phytopathology. 2005;95(12):1462-1471.
- [Google Scholar]
- Efficacy and safety of some plant extracts against tomato early blight disease caused by Alternaria solani. Plant Pathol. J.. 2011;10(3):115-121.
- [Google Scholar]
- The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. Michiganensis. Crop Prot.. 2003;22(1):39-44.
- [Google Scholar]
- Enzyme-assisted extraction of bioactive non-extractable polyphenols from sweet cherry (Prunus avium L.) pomace. Food Chem.. 2020;339:128086
- [Google Scholar]
- Characterization of Fusarium spp. causing potato dry rot in China and susceptibility evaluation of Chinese potato germplasm to the pathogen. Potato Res.. 2012;55(2):175-184.
- [Google Scholar]
- An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J. Chromatogr. A. 2006;1112(1–2):121-126.
- [Google Scholar]
- Solvent-free microwave extraction of essential oil from aromatic herbs: from laboratory to pilot and industrial scale. Food Chem.. 2014;150:193-198.
- [Google Scholar]
- Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design. Ind. Crop. Prod.. 2020;154:11268.
- [Google Scholar]
- Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr. Rev. Food Sci. F.. 2013;12(1):5-23.
- [Google Scholar]
- Enzyme-assisted extractions of polyphenols - a comprehensive review. Trends Food Sci. Tech.. 2019;88:302-315.
- [Google Scholar]
- Antifungal activity of some Himalayan medicinal plants using direct bioautography. J. Cell Mol. Biol.. 2006;5:95-98.
- [Google Scholar]
- Aqueous enzymatic pretreatment ionic liquid-lithium salt based microwave-assisted extraction of essential oil and procyanidins from pinecones of Pinus koraiensis. J. Clean. Prod.. 2019;236:117581
- [Google Scholar]
- Oil extraction from tiger nut (Cyperus esculentus L.) using the combination of microwave-ultrasonic assisted aqueous enzymatic method design, optimization and quality evaluation. J. Chromatogr. A. 2020;1627:461380.
- [Google Scholar]
- Chemical composition, antifungal and antioxidant activities of wild and cultivated Origanum compactum essential oils from the municipality of Chaoun, Morocco. S. Afr. J. Bot.. 2022;147:852-858.
- [Google Scholar]
- Enzyme-assisted microwave hydro-distillation essential oil from Fructus forsythia, chemical constituents, and its antimicrobial and antioxidant activities. Food Chem.. 2012;134(1):235-243.
- [Google Scholar]
- Enhancement of transdermal transport of azidothymidine (AZT) with novel terpene and terpene-like enhancers: in vivo-in vitro correlations. J. Control. Release. 1995;34(1):43-51.
- [Google Scholar]
- Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem.. 2021;76:105625
- [Google Scholar]
- Antifungal activity of sulfur-containing salts against the development of carrot cavity spot and potato dry rot. Postharvest Biol. Tec.. 2012;63(1):55-59.
- [Google Scholar]
- Antifungal effect of borates against Fusarium sulphureum on potato tubers and its possible mechanisms of action. Postharvest Biol. Tec.. 2012;74:55-61.
- [Google Scholar]
- Optimization of solvent-free microwave assisted extraction of essential oil from Cinnamomum camphora leaves. Ind. Crop. Prod.. 2018;12:353-362.
- [Google Scholar]
- Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69(8):1732-1738.
- [Google Scholar]
- Chemical variability of peel and leaf essential oils of mandarins from Citrus reticulata Blanco. Biochem. Syst. Ecol.. 2000;28(1):61-78.
- [Google Scholar]
- Leaf morphological and photosynthetic differences among four chemotypes of Cinnamomum camphora in different seasons. Ind. Crop. Prod.. 2021;169:113651
- [Google Scholar]
- Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X. 2022;13:100217
- [Google Scholar]
- Identification of species and pathogenicity of the Fusarium on potato in Heilongjiang Province. Plant Protect.. 2010;36(4):112-115.
- [Google Scholar]
- Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: a review. Food Res. Int.. 2018;108:309-330.
- [Google Scholar]
- Integrating GC-MS and ssRNA-Seq analysis to identify long non-coding RNAs related to terpenoid biosynthesis in Cinnamomum camphora. Ind. Crop. Prod.. 2021;171:113875
- [Google Scholar]
- Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Tec.. 2016;114:54-61.
- [Google Scholar]
- Alkanediol-based deep eutectic solvents for isolation of terpenoids from citrus essential oil: experimental evaluation and COSMO-RS studies. Sep. Purif. Technol.. 2019;22:115707
- [Google Scholar]
- Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. J. Pharmaceut. Biomed.. 2018;156:313-322.
- [Google Scholar]
- The influence of terpinen - 4 - ol and eucalyptol - The essential oil components- on fungi and plant sterol monolayers. BBA – Biomembranes. 2019;1861(6):1093-1102.
- [Google Scholar]
- Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind. Crop. Prod.. 2013;49:628-633.
- [Google Scholar]
- Isolation of essential oil from Lavandula angustifolia by using ultrasonic-microwave assisted method preceded by enzymolysis treatment, and assessment of its biological activities. Ind. Crop. Prod.. 2017;100:236-245.
- [Google Scholar]
- Enhancement of mass transfer intensification for essential oil release from Lavandula pubescence using integrated ultrasonic-microwave technique and enzymatic pretreatment. ACS Sustain. Chem. Eng.. 2018;6(2):1639-1649.
- [Google Scholar]
- Enzyme extraction by lab-scale hydrodistillation of ginger essential oil (Zingiber officinale Roscoe): chromatographic and micromorphological analyses. Ind. Crop. Prod.. 2020;146:112210
- [Google Scholar]
- Chitosan is an effective inhibitor against potato dry rot caused by Fusarium oxysporum. Physiol. Mol. Plant P.. 2021;113:101601
- [Google Scholar]
- Chemical composition and antifungal properties of the essential oil and various extracts of Mikania scandens (L.) Willd. Arab. J. Chem.. 2017;10:S2170-S2174.
- [Google Scholar]
- Application of ultrasound in combination with other technologies in food processing: a review. Ultrason. Sonochem.. 2021;73:105506
- [Google Scholar]
- Leaf essential oil of Cinnamomum agasthyamalayanum from the Western Ghats, India—a new source of camphor. Ind. Crop. Prod.. 2016;86:259-261.
- [Google Scholar]
- Antifungal activity of selected essential oils against fungi isolated from medicinal plant. Ind. Crop. Prod.. 2014;55:116-122.
- [Google Scholar]
- Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia L. (Rubiaceae): Errata and review of technological development and prospects. Sep. Purif. Technol.. 2021;256:117844
- [Google Scholar]
- A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathogenesis. 2019;134:103580
- [Google Scholar]
- Terpinene and β-pinene acting as signaling molecules to improve Cinnamomum camphora thermotolerance. Ind. Crop. Prod.. 2020;154:112641
- [Google Scholar]
- Continuous and emerging challenges of silver scurf disease in potato. Int. J. Pest Manage.. 2020;68(1):89-101.
- [Google Scholar]
- Potato dry rot disease: current status, pathogenomics and management. 3 Biotech.. 2020;10:503.
- [Google Scholar]
- Impact of Fusarium dry rot on physicochemical attributes of potato tubers during postharvest storage. Postharvest Biol. Tec.. 2021;181:111638
- [Google Scholar]
- Osmitopsis astericoides (Asteraceae)-the antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J. Ethnopharmacol.. 2003;88:137-143.
- [Google Scholar]
- Partial enzymatic cell wall disruption of Oocystis sp. for simultaneous cultivation and extraction. Sep. Purif. Technol.. 2022;293:112107
- [Google Scholar]
- Research progress on potato dry rot disease in China and its control measures. China Vegetables. 2020;4:22-29.
- [Google Scholar]
- Optimization of Microwave-Ultrasound-assisted enzymatic hydrolysis extraction of iodine amino acids in laminaria by high performance liquid chromatography with a photodiode array detector. Algal Res.. 2019;39:101452
- [Google Scholar]
- Improved method to obtain essential oil, asarinin and sesamin from Asarum heterotropoides var. mandshuricum using microwave-assisted steam distillation followed by solvent extraction and antifungal activity of essential oil against Fusarium spp. Ind. Crop. Prod.. 2021;162:113295
- [Google Scholar]
- The anti-inflammatory potential of Cinnamomum camphora (L.) J. Presl essential oil in vitro and in vivo. J. Ethnopharmacol.. 2021;267:113516
- [Google Scholar]
- Extraction of Cinnamomum camphora chvar. Borneol essential oil using neutral cellulase assisted-steam distillation: optimization of extraction, and analysis of chemical constituents. Ind. Crop. Prod.. 2019;141:111794
- [Google Scholar]
- Effect of simultaneous ultrasonic and microwave assisted hydrodistillation on the yield, composition, antibacterial and antibiofilm activity of essential oils from Citrus medica L. var. sarcodactylis. J. Food Eng.. 2019;244:126-135.
- [Google Scholar]
- Antitumor activity of nanoemulsion based on essential oil of Pinus koraiensis pinecones in MGC-803 tumor-bearing nude mice. Arab. J. Chem.. 2020;13:8226-8238.
- [Google Scholar]
- Ethanol extracts from Cinnamomum camphora seed kernel: potential bioactivities as affected by alkaline hydrolysis and simulated gastrointestinal digestion. Food Res. Int.. 2020;137:109363
- [Google Scholar]
- Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control. 2014;37:277-283.
- [Google Scholar]







