Translate this page into:
Electrochemical behavior of PbO2/PbSO4 electrode in the presence of surfactants in electrolyte
⁎Corresponding author. zerroual@yahoo.fr (L. Zerroual)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The electrochemical behavior of PbO2/PbSO4 electrode is investigated in 4.5 M H2SO4 in presence of three surfactants, Sodium Dodecyl Sulfate (SDS), Cetyltrimethylammonium bromide (CTAB) and Sodium tripolyphosphate (STPP), using cyclic voltametry, electrochemical spectroscopy impedance and galvanostatic discharge as techniques. The micro morphology of the surface of the modified PbO2 electrodes is examined by scanning electron microscopy. The results show that SDS and CTAB when added in the electrolyte could refine the coating particles and change the roughness of the surface of the electrode leading to a thin film of PbO2 with amorphous character. In addition, SDS and CTAB shift the hydrogen evolution potential towards more negative values, improve the discharge capacity of the anodic layer and accelerate the charge transfer. Under cathodic polarization, CTAB presents the lowest value of the charge transfer resistance Rct. In the contrary, STPP shifts the oxygen evolution potential towards more positive values, passivates the surface of the electrode and inhibits completely the reaction of PbO2 formation.
Keywords
Lead dioxide
Surfactants
Cyclic voltammetry
Galvanostatic discharge
Electrochemical impedance spectroscopy
1 Introduction
The lead/acid battery derives its power from the electrochemical energy released during the conversion of (α- and β-PbO2 to PbSO4, on the positive plate, and Pb to PbSO4, on the negative plate. The major factor contributing to the decline in capacity of the positive plate during charge/discharge cycling was considered to be a progressive decrease in the degree of electrical contact between the PbO2 particles in the plate and between these particles and the grid. The change in conductivity is mainly due to the combined effects of grid corrosion and a gradual softening of the plate mass and shedding of the PbO2 particles. The life of lead acid battery positive plates depends on both grid corrosion and the softening and shedding of PbO2 active material. During charging, the positive grid of the lead acid battery is oxidized and thus a boundary layer is formed between the grid and the active mass. The growth of the oxide layer is due to the diffusion of oxygen through the layer. Previous works (Pohl and Shendler, 1984; Bullock and Butler, 1986; Pavlov and Mohanov, 1993) proposed different mechanisms. The oxygen generated at the electrolyte interface diffuses into the layer in the form of atoms or radicals and oxidizes the underlying lead to tetragonal PbO which further gives intermediate oxides PbOx and finally these non-stoichiometric oxides are converted to α-PbO2. The composition of the anodic layer depends on the potential and the quantity of electricity that has passed through the electrode. It has been found that the anodic layer formed after oxidation of Pb-Sb-Sn electrodes contains in addition of tet-PbO and PbO2 substituted oxides. Recently, a new concept has been proposed for the structure of lead dioxide (Mohanov and Pavlov, 1987).
According to this concept, the lead dioxide active mass consists of crystal zones and hydrated zones designated by PbO(OH)2 with amorphous character. The characteristics of the layer have an influence on the cycling behaviour and cycle life of the battery. That is why the growth mechanism and the properties of the boundary layer are of great interest. Since the early start of manufacturing lead/acid cells, the use of additives, and more specifically the selection of these compounds, has been conducted by empirical rules and experience.
During the last decades the lead/acid cell has been the subject of many studies, which have increased enormously the knowledge of the effects of additives and of the manufacturing process. PbO2 electrode is an attractive and promising anode material due to its low cost, ease synthesis, high oxygen evolution potential and corrosion resistance (Liu and Liu, 2008; Tan et al., 2011). In addition of its high electrical performance as positive active material for lead-acid batteries, it exhibits a strong mineralization capacity for recalcitrant organic compounds. The effect of some additives on the capacity of the PbO2 electrode and its efficiency in wastewater treatment was widely investigated in the literature (Comninellis, 1994; Panizza and Cerisola, 2009; Niu et al.2016; Xiaoliang et al., 2017; Boudieb et al., 2015).
In a previous work (Ghaemi et al., 2006) demonstrated that the non-ionic surfactant t-octyl phenoxy polyethoxyethanol (Triton X-100) improves the charge/discharge of the modified PbO2 electrode and increases the overpotential for oxygen evolution. (Darabizad et al., 2015) synthesized nanostructured PbO2 by electrodeposition at constant current on Ti substrate in presence of polyvinylpyrrolidone (PVP). The results revealed that the particle size and morphology of PbO2 can be controlled by altering PVP concentration. In addition, the charge transfer resistance significantly decreased in the presence of PVP. In their study on the effects of surfactants on sulfation of negative active material in lead acid battery under PSOC condition (Ghavami et al., 2016) showed that amongst all tested surfactants, the cell with anionic sodium dodecyl sulfate surfactant (SDS) exhibits the longest cycle life with the least overcharge and fine PbSO4 crystals but the cell with cationic cethyl trimethyl ammonium bromide (CTAB) surfactant shows opposite effects. (Boudieb et al., 2016) published a paper on the effect of a phosphonate surfactant (PS) on the electrochemical behavior of the lead-acid battery positive plate and concluded that the use of surfactant at low concentration substantially changes the morphology of the lead sulfate crystals and increases the capacity and cycle life of the positive plate. Recently, Fu et al. (2018) prepared various structured PbO2 thin-film electrodes by surfactant assisted anodic electro-deposition method. The electrochemical performance of PbO2 electrodes was found to be strongly dependant on their morphology and structure. Compared to other structures studied, PbO2-PEG electrode shows excellent electro-catalytic activity, stability, and service life. Similarly, Duan et al. (2018) fabricated a hydrophobic PbO2 electrode modified by sodium dodecyl benzene sulfonate (SDBS-PbO2) and tested it as anode for electrochemical degradation of nitrobenzene in aqueous solution. The results showed that the electro-catalytic activity for nitrobenzene degradation obtained with SDBS-PbO2 electrode was much higher than that of pure PbO2 electrode.
In the present work, different structured PbO2 electrodes were prepared by surfactant-assisted anodic polarization method. We investigated the effect of SDS, CTAB and STPP as surfactants added in the electrolyte (4.5 M H2SO4) on the electrochemical behavior of Pb-Sb-Sn alloy. Cyclic voltametry, electrochemical spectroscopy impedance and galvanostatic discharge were used as techniques. The effect of the surfactants on the micro morphology of the surface and structure of the modified PbO2 electrodes were examined by scanning electron microscopy.
2 Experimental
The working electrode, a Pb-Sb-Sn alloy, was polished with emery paper (600, 800, 1200 mesh) under water and etched with a mixture (80% CH3COOH and 20% H2O2). Cleaning was done by shaking the electrode for approximately 20 s. Afterwards, the working electrode was rinsed with double-distilled water and ethanol. This procedure was repeated until the electrode appeared shiny. The electrochemical behavior of Pb-Sb-Sn alloy in 4.5 M H2SO4 containing SDS (0.12), CTAB (0.024), and STPP (1.20%), was investigated using cyclic voltammetry. After a rest of 1 min in the electrolyte, the electrodes were cathodically polarized at −1.3 V for 30 min then anodically polarized at 1.7 V for another 30 min. Cyclic voltammograms were obtained up to 100 cycles between 0.7 and 1.7 V versus Hg/Hg2SO4 reference electrode at a sweep rate of 10 mV·s−1. The potential current density curves were recorded. The capacity expressed in mAh·cm−2 is determined by integrating the surface area of the cathodic peak corresponding to the reduction of PbO2 to PbSO4. Impedance measurements were performed in the frequency range from10 mHz to 100 kHz at a signal amplitude of 10 mV. The impedance spectra of pure and modified PbO2 electrodes were recorded at open circuit potential (1077 mV) and under cathodic polarization (1050, 850 and 750 mV). The charge transfer resistance was plotted against the cathodic polarization value. A thin layer of PbO2 was formed after polarization of each electrode at 1.7 V for 30 min and the micro morphology and crystal structure of the electrodes were characterized by scanning electron microscopy (SEM, JEOL, JSM-6390A).
The different electrodes were then discharged at a constant cathodic current of 1 mA, the potential-time curves were recorded using Hg/Hg2SO4/saturated K2SO4 reference electrode. All experiments were carried out at room temperature.
3 Results and discussion
3.1 Oxygen evolution
Fig. 1 shows the linear sweep voltammetry of Pb-Sb-Sn alloy in 4.5 M H2SO4 in the range potential of oxygen evolution in presence and absence of the three surfactants. During the potential sweep, we observe an increase in the current density and the appearance of an anodic peak at 1.42 V corresponding to the oxidation of PbSO4 to PbO2. The presence of SDS and CTAB in the electrolyte compared to the blank test seems to facilitate the oxidation reaction of lead sulfate crystals with a slight shift of oxygen evolution potential towards negative values. In contrast, when STPP is added to the solution, a low and constant current density is recorded. The STPP covers the surface of the electrode with a passive film and inhibits completely the oxidation reaction of PbSO4 crystals with a shift of oxygen evolution potential towards more positive values.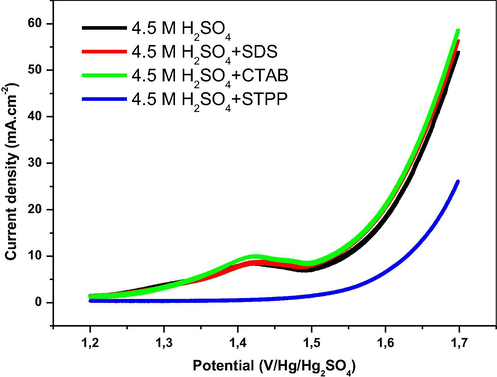
Linear sweep voltammograms of Pb-Sb-Sn alloy at a sweep rate of 50 mV·s−1 in 4.5 M H2SO4 without and with SDS, CTAB and STPP as additives (anodic polarization).
3.2 Hydrogen evolution
Fig. 2 illustrates the linear sweep voltammetry of Pb-Sb-Sn alloy in 4.5 M H2SO4 in the range potential of hydrogen evolution in presence and absence of the three surfactants. During the potential sweep, we record a progressive increase in the current density at −1.4 V. This is followed by an important gasing due to the hydrogen evolution at the surface of the electrode. The hydrogen evolution potential is shifted to more negative values when SDS and CTAB are present in the electrolyte. In fact surfactants may strongly adsorb, suppress and/or modify the structure of the electrode so that its surface may be less accessible to water molecules and water and/or protons discharge is inhibited. Consequently, both oxygen and hydrogen evolutions at the positive and negative electrode are respectively shifted to more positive and negative potentials. As STPP does not influence the reduction of H+ and inhibits the oxidation of PbSO4, the study is restricted to CTAB and SDS.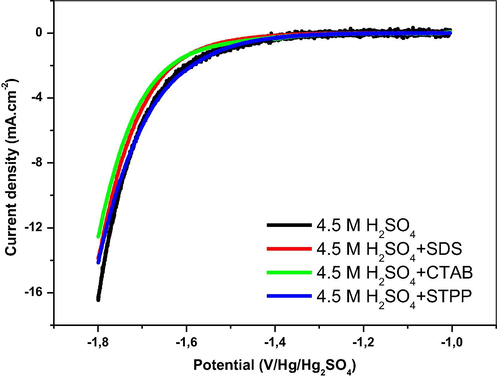
Linear sweep voltammograms of Pb-Sb-Sn alloy at a sweep rate of 50 mV·s−1 in 4.5 M H2SO4 without and with SDS, CTAB and STPP as additives (cathodic polarization).
3.3 Micro morphology of PbO2 electrodes
Fig. 3 shows the SEM images of the different PbO2 electrodes. From Fig. 3a, it could be observed that the morphology of PbO2 electrode was coarse and uneven. Typical crystalline structures were observed on the electrode surface. Larger and clearly pronounced individual PbO2 particles with a number of micro-pores are grouped in small agglomerates, which coalesce into an aggregate. As shown in Fig. 3b and c, smaller and closer interconnected PbO2 particles in agglomerates with individual nanosized particles still distinguished are observed in the SEM images of the modified PbO2 electrodes after addition of SDS and CTAB. The surfactants change the roughness of PbO2 films, consequently smooth and hydrated layers with amorphous structure are obtained in presence of SDS and CTAB as additives in H2SO4 solutions.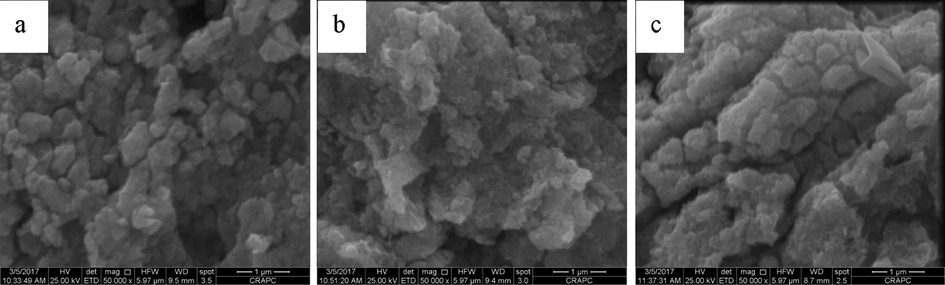
SEM images of the different PbO2 electrodes obtained after anodic polarization of Pb-Sb-Sn alloy in: (a) 4.5 M H2SO4, (b) 4.5 M H2SO4 + SDS, (c) 4.5 M H2SO4 + CTAB.
3.4 Cyclic voltammetry
The cyclic voltammetry of Pb-Sb-Sn alloy electrode in 4.5 M H2SO4 performed in the potential range (1.5–0.5 V vs Hg/Hg2SO4/K2SO4 reference electrode), in presence and absence of the different surfactants, are presented in Fig. 4. Both SDS and CTAB present almost the same voltammograms as that of the blank test (see Fig. 4a). During discharge, we observe a cathodic peak at around 1 V which corresponds to the reduction of PbO2 to PbSO4. The peak current density increases with the increase of the cycle number. High current densities are recorded for the solutions containing respectively SDS and CTAB (see Fig. 4a and b). The capacity versus the number of cycles for the different electrodes is illustrated in Fig. 4d. We can see that the increase in capacity for PbO2 prepared in presence of SDS and CTAB respectively is more important compared to that of the blank test. Consequently the presence of the surfactants in the electrolyte seems to facilitate the production of active PbO2. In the anodic branch, it is clearly seen that the peak that corresponds to the oxidation of PbSO4 to PbO2 is completely masked by oxygen evolution.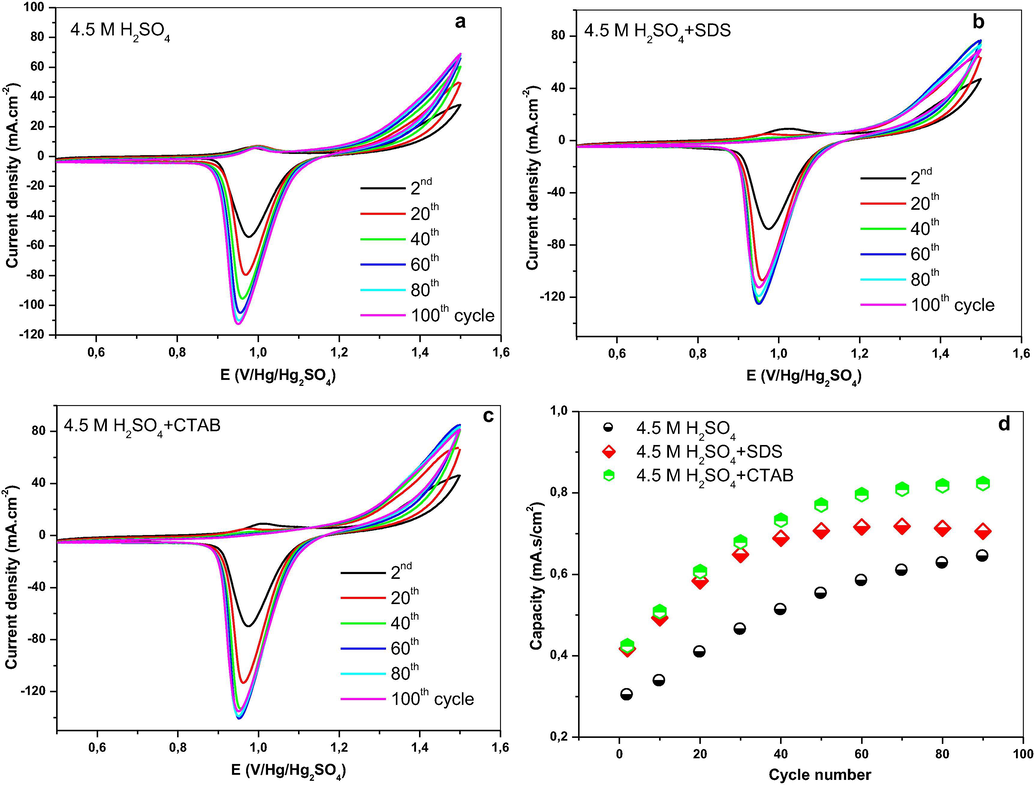
Cyclic voltammograms of Pb-Sb-Sn alloys at a sweep rate of 10 mV·s−1 in: (a) 4.5 M H2SO4, (b) 4.5 M H2SO4 + SDS, (c) 4.5 M H2SO4 + CTAB, and (d) discharge capacity versus cycle number.
3.5 Electrochemical impedance spectroscopy
The Nyquist plots of the impedance at open circuit potential (OCP) and under cathodic polarization of Pb-Sb-Sn alloy in the different electrolytes are shown in Fig. 5. They consist of a semicircle and a straight line. The semicircle is related to charge transfer resistance and double layer capacitance formation. It can be observed that the diameter of semicircle that is related to charge transfer resistance, and capacitance value are increased with the increase of the cathodic polarization (see Fig. 5a–d). Fig. 6 represents the variation of the charge transfer resistance with the cathodic polarization applied to PbO2 prepared in presence and absence of SDS and CTAB as additives to H2SO4 solution. The different electrodes show an increase of the charge transfer resistance with the increase of the overpotential value. This could be explained by the fact that under polarization amorphous particles of PbO2 are partially converted to PbSO4 leading to higher resistance. Small values of charge transfer resistance are obtained when SDS and CTAB are added to H2SO4 solution.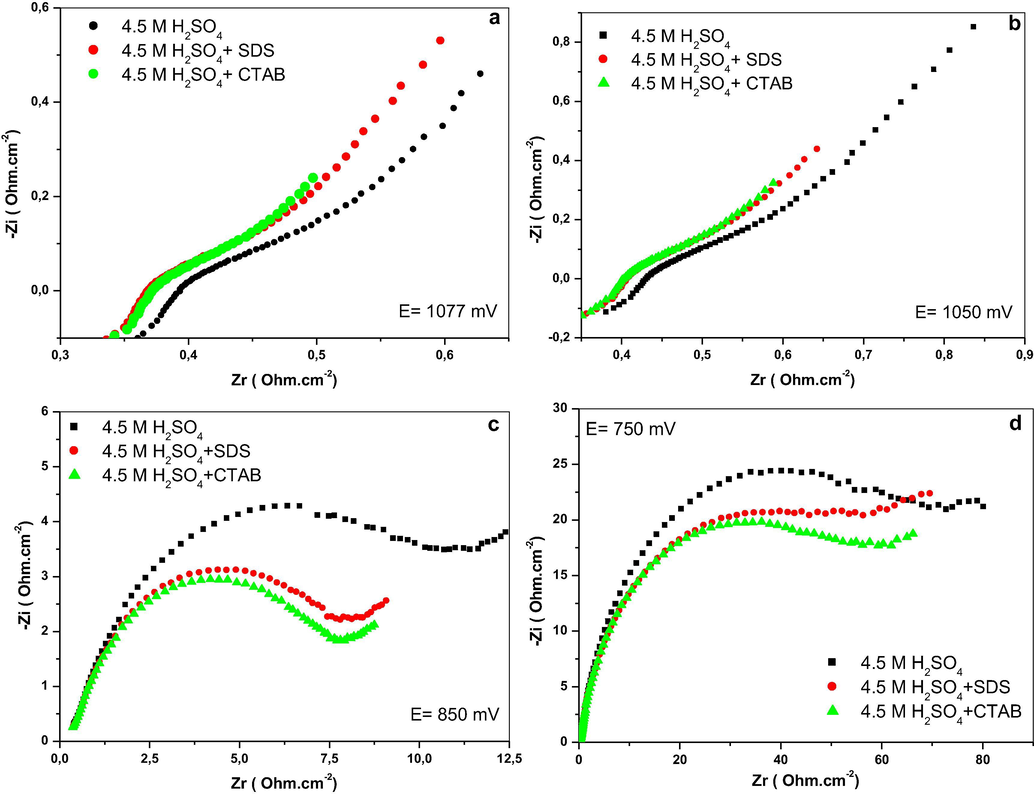
Nyquist plots of the different PbO2 electrodes in 4.5 M H2SO4: (a) E = 1077 mV, (b) E = 1050 mV, (c) E = 850 mV, (d) E = 750 mV.
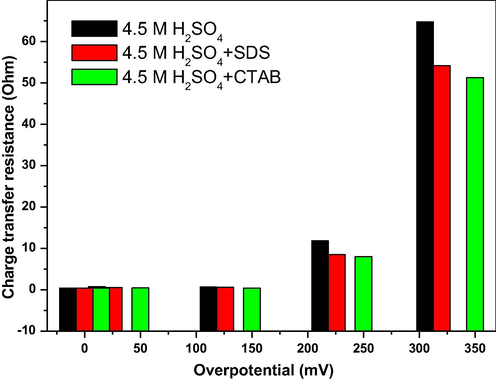
Variation of the charge transfer resistance of the different PbO2 electrodes in 4.5 M H2SO4 with overpotential.
3.6 Galvanostatic discharge
The discharge curves of thin layers of PbO2 formed after polarization of the electrodes at 1.7 V for 30 min in electrolytes containing SDS and CTAB as additives are compared in Fig. 7. We report the change in potential versus discharge time expressed in seconds. We can see that PbO2 formed in H2SO4 solutions containing SDS and CTAB presents an open circuit potential slightly higher than that of PbO2 prepared in absence of additives. Consequently, this yields to a potential plateau that corresponds to the reduction of PbO2 to PbSO4 slightly higher. We notice that the discharge times recorded for the thin layers of PbO2 formed in H2SO4 solutions containing SDS and CTAB are much higher than that of the blank test (i.e. PbO2 obtained in H2SO4 solutions without additives). This is due to the amorphous structure of PbO2 layers as clearly shown by the SEM images of the modified electrodes.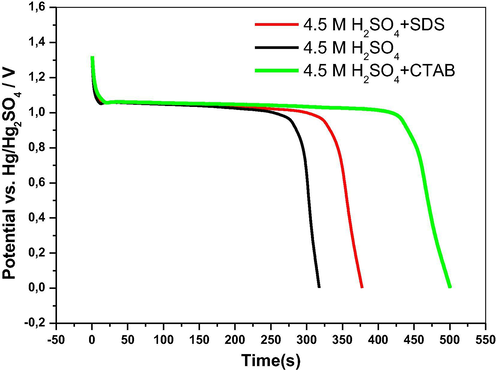
Galvanostatic discharge curves of the different PbO2 electrodes in 4.5 M H2SO4.
4 Conclusion
The study of the behavior of Pb-Sb-Sn alloys in 4.5 M H2SO4 in presence of three surfactants SDS, CTAB, and STPP are carried out. The electrodes are anodically polarized to form thin layers of PbO2. The morphology and electrochemical properties of the prepared PbO2 electrodes are characterized to investigate the effects of the surfactants when added in H2SO4 solutions. The examination of the surface of the PbO2 modified electrodes shows that in presence of SDS and CTAB smooth thin films of PbO2 with amorphous character are obtained. The experimental results show among these surfactants STPP inhibits completely the reaction of the formation of the anodic layer of PbO2. In addition, it does not affect the discharge of H+. Whereas CTAB seems to improve its discharge capacity, electrical performance and increase hydrogen evolution potential. Under cathodic polarization, low value of charge transfer resistance is obtained in presence of this additive. Further investigations on lead-acid battery pastes are in progress to understand this controversy between the different surfactants.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Proc. – Soc. Behav. Sci.. 2015;195:1618.
- Int. J. Electrochem. Sci.. 2016;11:215.
- J. Electrochem. Soc.. 1986;133:1085.
- Electrochim. Acta. 1994;39:1857.
- Mater. Chem. Phys.. 2015;156:121.
- Electrochim. Acta. 2018;282:662.
- Mater. Chem. Phys.. 2018;220:155.
- J. Power Sources. 2006;157:550.
- J. Storage Mater.. 2016;7:121.
- Electrochim. Acta. 2008;53:5077.
- J. Appl. Electrochem.. 1987;218:135.
- Chemosphere. 2016;146:526.
- Chem. Rev.. 2009;109:6541.
- J. Electroanal. Chem.. 1993;23:1244.
- J. Power Sources. 1984;13(101)
- Chem. Eng. J.. 2011;166:15.
- J. Alloy. Compd.. 2017;718:386.







