Translate this page into:
Electrochemical detection of dihydronicotinamide adenine dinucleotide using Al2O3-GO nanocomposite modified electrode
⁎Corresponding author. moataz_mekawy@yahoo.com (Moataz M. Mekawy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
NADH plays a vital role in the electron transfer processes between metabolites in the cellular energetic reactions. Therefore, there is a crucial need to develop analytical techniques for detecting NADH levels with the metabolism of glucose. In the present study, a nanocomposite of alumina (Al2O3) nanoparticles confined graphene oxide (GO) sheet acts as a modifier for carbon paste electrode (CPE) for a sensitive detection of NADH level in a mediator-less detection scheme. Our findings after optimization of experimental conditions reveal that, there is a remarkable enhancement in the direct electron transfer through the Al2O3-GO nanocomposite surface with high electrocatalytic activity towards NADH oxidation. Results show that, there is a linear increase in NADH detection from 30 µM to 330 µM, together with linear regression coefficient of 0.98 and LOD 4.5 µM. These results confirm that, the developed Al2O3-GO based CPE electrode is a promising electrode for real NADH level detection in practical enzymatic applicability.
Keywords
Al2O3-GO nanocomposite
NADH
Modified electrodes
Electrochemical detection
1 Introduction
Dihydronicotinamide adenine dinucleotide (NADH) plays a very important role in the enzymatic reactions. NADH and its oxidized form NAD+ handle hydrogen and electron transfer between metabolites in the cellular redox energetic reactions. Moreover, they are the coenzymes for numerous dehydrogenase enzymes and components of biomarker systems. Several studies showed that, NADH is essential for the regeneration of glutathione following its oxidation where the levels of glutathione changed dramatically based on NADH levels (Limoges et al., 2006). Furthermore, NADH behaves as a crucial co-factor of other enzymes in biological, clinical and pharmaceutical samples (Wei et al., 2008, Wu et al., 2007). Thus, there is a high demand to develop suitable analytical techniques that helps in sensitive screening and detecting NADH level along with the metabolism of glucose, which considered among the important detections of diabetic and cancer cells (Moradi et al., 2013). However, the direct oxidation of NADH occurs associated with high overpotential along with non-desired oxidation products. That leads to surface fouling of the used sensor and hence decreases the detection sensitivity.
So far, many research trials focused on NADH detection, including electrochemical investigations (Serban and Murr, 2004, Lee and Compton, 2013), analytical methods based on colorimetric (Liu et al., 2012), photo-electrochemical (Li et al., 2014, Wang et al., 2009), enzymatic assay (Ricci et al., 2007) and chemiluminescence techniques (Downey and Nieman, 1992, Devadoss et al., 2012). Among those techniques, electrochemical methods received significant considerations due to their high sensitivity, fast response, ease of sample preparation and lower cost (Chen and Shah, 2013). Much research effort directed towards new materials which can help to develop and improving the electrochemical biosensors based on composite electrode for more sensitive and rapid response results (Kimmel et al., 2012).
In recent years, design of graphene based nanomaterials received much interest towards their applicability as electro-catalysts for NADH oxidation without the aid of redox mediators (Li et al., 2014). Zhang and co-workers (Zhang et al., 2011) reported about the non-mediated use of screen printed carbon electrode (SPCE) which was modified using graphene oxide to detect NADH in a neutral solution with the concentration ranging from 0.8 to 500 μM and the detection limit of 0.10 μM. On the other hand, Lin and co-workers (Lin et al., 2014) reported about the detection of NADH using graphene oxide and multi-walled carbon nanotube (GO- MWCNT) composites via hybridization of poly-luminol and poly-neutral red as electron mediators with a linear range from 1.33 × 10−8 to 1.95 × 10−4 M and a detection limit of 1.33 × 10−8 M. Most recently, Roushani and co-workers (Roushani et al., 2016) reported about using the ternary composite of metallic Pt, Fe3O4 metal oxide and graphene oxide for NADH detection using a glassy carbon electrode with a detection limit of 5 nM. However, to date, there are no reports for NADH detection using non-mediated Al2O3/GO nanocomposite modified carbon paste electrodes.
Graphene oxide (GO) consists of a 2D single layer of sp2-hybridized carbon atoms and possesses unique features such as high surface area, superior charge transport at room temperature, outstanding mechanical stability, easiness of mass production and functionalization. However, some of these unique features can be deteriorated due to its ease of aggregation and poor solubility which hinder its real applicability in electrochemical biosensors. To overcome these limitations, the oxide containing groups in GO can be used for surface anchoring of noble metals and/or metal oxide particles to enhance the electrochemical sensing ability (Novoselov et al., 2012). In other words, it is necessary to modify GO surface to have a multifunctional composite which possesses both superior properties of graphene as well as its functionalizing materials (Shao et al., 2010).
Nano-structured metal oxides have been extensively applied for the energy and environmental applications due to their high surface area, good biocompatibility and catalytic activity, chemical stability and relatively low toxicity (Pratima et al., 2011). Although, some metal oxides possess moderate electrical conductivity, having them as a combined nanocompsite electrochemical biosensor with GO is expecting to help reducing the produced overpotential from the investigated analyte and decreasing the current density. In this regard, several research works have been done by our group and others using metal oxide/graphene oxide nanocomposites (MO/GO) for the non-enzymatic detection of H2O2 (Salih et al., 2016, Li et al., 2010, Huang and Li, 2013), and monitoring of the bioelectrochemical response from the stimulated living microbial cells (Rabeay et al., 2017).
The aim of present study is to reveal the real applicability of novel Al2O3-GO nanocomposite modified carbon paste electrode (CPE) to be used in the real detection of NADH level in a mediator-less detection scheme. To the best of our knowledge, there are no reports in literature on the detection of NADH using Al2O3-GO nanocomposite. Advantages of this innovative method include (i) an easy and fast synthesis strategy; (ii) formation of uniform Al2O3 nanoparticles-GO nanocomposite, where GO has thick layer, and (iii) enhancement of the direct electron transfer through the composite surface groups, where Al2O3-GO nanocomposite shows high electrocatalytic activity and stability for NADH oxidation without any need for additional surface modification. This exhibits an excellent performance for the sensitive detection of NADH.
2 Experimental
2.1 Materials
Graphite flakes, sulfuric acid (H2SO4), potassium permanganate (KMnO4), Alumina (Al2O3), phosphoric acid (H3PO4), hydrochloric acid (HCl), ethanol, alcohol dehydrogenase from Saccharomyces cerevisiae and NAD+ were purchased from Sigma-Aldrich., Tris-Base and Tris-HCl were purchased from Fisher Scientific. Miller Lite Highlife beer was purchased from local liquor store in Riverside, California, USA.
2.2 Synthesis of graphene oxide (GO)
GO was synthesized using modified Hummer's method (Marcano et al., 2010) where 75 ml of a concentrated mixture H2SO4/H3PO4 (4:1 vol ratio) was added to 3.0 g of purified natural graphite in a round bottom flask. 10.5 ml KMnO4 were added to the flask under temperature preservation at 4 °C. The mixture was subjected to control stirring for 12 h while increasing slowly the temperature to 60 °C. Afterwards, 100 ml of deionized water was added to the mixture, followed by further addition of 5 ml of 30% H2O2. The yield precipitate of GO was collected, washed thoroughly with 500 ml of 1.0 M HCl using vacuum filtration and subjected to additional washing with ethanol and deionized water. Finally, the suspended GO was collected by centrifugation at 6000 rpm for 30 min and kept for drying at 80 °C.
2.3 Synthesis of Al2O3-GO nanocomposite
1.0 g of commercially available α-Al2O3 powder (99.99% purity, average particle size: 200 nm) was soaked in 100 ml of deionized water and sonicated for 1 h followed by addition of 100 or 500 mg of GO under vigorous stirring for 30 min. The resulting light brownish 10% or 50% (w/w) Al2O3-GO nanocomposite particles were separated from the solution under moderate vacuum filtration and kept for drying at 100 °C.
2.4 Electrode fabrication and electrochemical characterization
The Al2O3-GO nanocomposite modified carbon paste electrodes were prepared by mixing 0.1 g of the 10% or 50% Al2O3-GO nanocomposite powder with 0.9 g of synthetic carbon powder and 0.3 ml paraffin oil using a mortar and pestle. The prepared paste was packed into the tip of the electrode assembly with a surface area of 0.5 cm2. Hereafter, we name it as a working electrode. Regeneration of the working electrode surface was carried out by polishing with a wet smooth filter paper until a shiny electrode surface was obtained. Prior to electrochemical measurements, the working electrode was electrochemically activated in phosphate buffer solution (0.1 M, pH 7.0) by repeating 10 cyclic scans from −0.2 to +1.0 V at a scan rate of 50 mV/s. In addition, the working electrode assessment screening was examined in NADH detection using cyclic voltammetry. The electrochemical signal arises from the NADH oxidation was recorded and compared with the signals arise from unmodified CPE electrode and modified CPE electrode with a series of modifiers such as Ag-NPs, Au-NPs, SWCNTs, MWCNTs and Au-GO. Moreover, optimization of electrochemical parameters such as Al2O3-GO nanocomposite concentration, pH and tools was carried out where we examined the effect of Al2O3-GO nanocomposite concentration on the peak current using electrochemical impedance spectroscopy (EIS) vs. Ferricyanide and the suitable working pH was estimated. Finally, the calibertaion curve of NADH oxidation was established with the optimized experimental parameters using in CV and DPV electrochemical tools where NADH concentration varied from 30 µM to 330 µM. Thus, the LOD and the linear regression coefficient of Al2O3-GO nanocomposite modified CPE electrochemical biosensor were estimated.
2.5 Alcohol-dehydrogenase immobilization and electrochemical testing
In a trial to establish a real electrochemical enzymatic biosensor model for NADH and ethanol detection, adequate amounts of chitosan as a biocompatible cross linker and alcohol dehydrogenase enzyme (ADH; stock solution of 3 U/μl) were thoroughly mixed in 1% w/v ratio. 10 μl of the mixture (Chitosan/ADH) were drop-casted to the surface of the working electrode forming a thin layer and allowed to dry at 4 °C. After drying, the chitosan/ADH/working electrode biosensor was placed into the electrochemical cell that contains 1.0 mM of NAD+ and 1.0 mM of absolute ethanol and the electrochemical oxidation of the enzymatically produced NADH was recorded.
2.6 Determination of alcohol concentration in beer
For the real applicability of enzymatic reactions to determine the alcohol concentration within forensic and foodstuffs, we followed the same above mentioned detecting methodology while using commercially available beer. In a typical protocol, 100 μl of beer was added in the electrochemical cell followed by two consecutive standard additions of the standard ethanol (450 μl each, 1.0 mM) the reaction rate was recorded using Shimadzu UV–vis spectrophotometer UV-3501 against the change in NADH absorbance at 340 nm (εNADH = 6220 M−1 cm−1). The standard ethanol calibration assay was performed in a range of ethanol concentration varied between 0 and 13.68 mM with 1.5 mM NAD+, 50 µg/mL alcohol dehydrogenase (ADH) in 20 mM Tris-HCl buffer solution at pH 8.5. 100 times diluted Miller Lite Highlife beer sample was used to estimate the unknown ethanol concentration under the same reaction conditions as the standard ethanol calibration assay, which contains 1.5 mM NAD+, 50 µg/mL alcohol dehydrogenase (ADH) in 20 mM Tris-HCl buffer at pH 8.5. All reactions were started by the addition of ADH while monitoring the absorbance at 340 nm for 8 min at 25 °C. The reaction rates for the standard ethanol calibration assay were estimated and plotted versus the ethanol concentration in a Michaelis-Menton plot and a double-reciprocal Lineweaver-Burk plot.
3 Results and discussion
3.1 General screening of the CPE electrode modifiers
It was reported that, nanomaterials play an important role in the direct electron transfer of the electro- and bioelectro- chemical systems (Rabeay et al., 2014). In this regard, the direct electron transfer of NADH oxidation was tested using several nanomaterials which were used as CPE electrode modifiers. A series of Ag-NPs, Au-NPs, SWCNTs, MWCNTs, Au-GO and Al2O3-GO nanocomposites were incorporated individually into the matrix of CPE as sensor modifiers, then the electrochemical response of each electrode was recorded using the cyclic voltammetry for monitoring the electrocatalytic oxidation of NADH. In comparison to the unmodified CPE electrode sensor, the nano-materials modified CPE electrode sensor exhibited a great enhancement in the oxidation of NADH. Among the investigated materials, Al2O3-GO-based electrode sensors (10%, w/w) exhibited the highest oxidation current along with the sharpest peak (Fig. 1). Therefore, the Al2O3-GO nanocomposite was selected for the desired purpose of NADH detection. Consequently, the electrochemical characterizations for the nanocomposite modified CPE electrode sensor were conducted.
Cyclic voltammetric responses of the nano-materials-based sensors and the blank CPE in 0.1 M PBS (pH 7.4) with 300 µM NADH at v = 50 mV/s.
4 The electrochemical characterizations
4.1 Electrochemical impedance spectroscopy (EIS) analysis
EIS was used to determine the impact of the Al2O3-GO nanocomposite and its elements on the electrical conductivity of modified CPE elecrode. As shown in Fig. 2A, Nyquist and Bode Plots revealed that, the modified electrode surface with either Al2O3 or GO has lower resistance than the unmodified CPE electrode. However, Al2O3-GO-based electrode exhibited the lowest Impedimetric signal.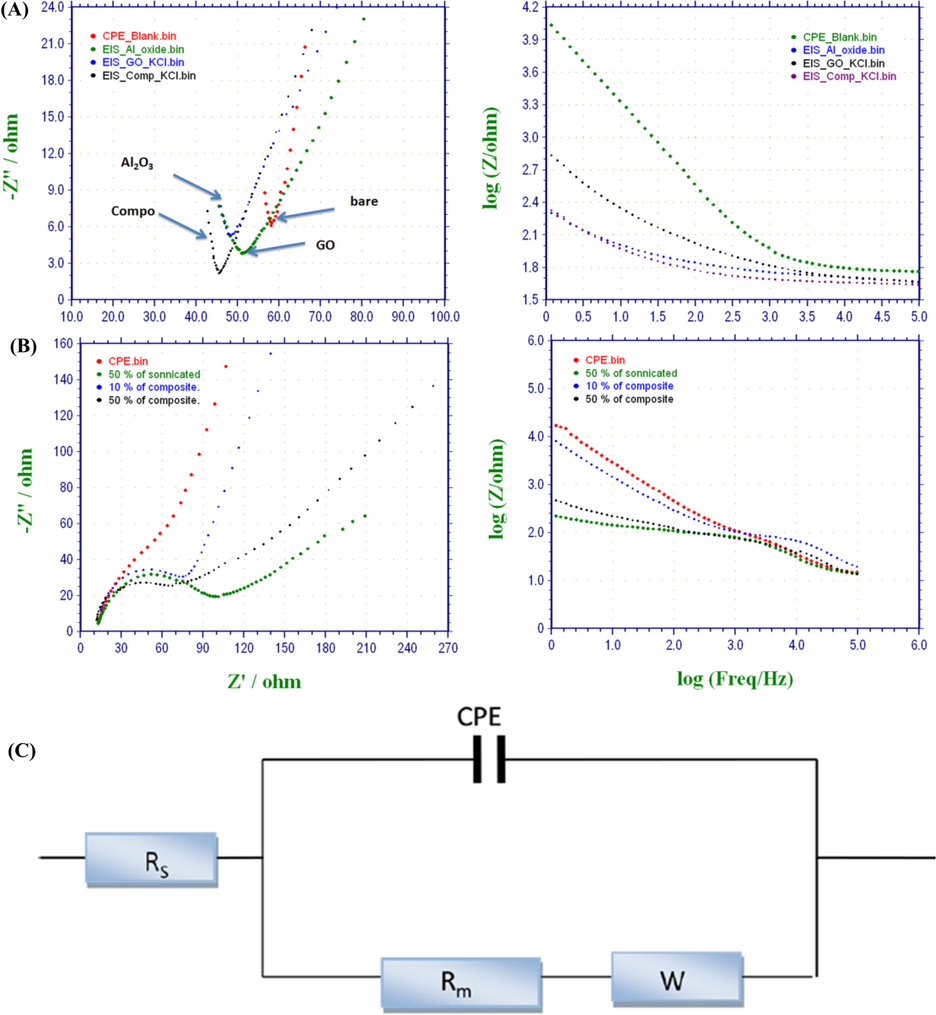
(A) Nyquist and Bode Plots for the modified electrodes with Al2O3, GO, or Al2O3-GO composite in comparison with the bare-electrode. (B) Nyquist and Bode Plots for the modified electrodes with different composite concentrations. (C) Randles model is the appropriate equivalent circuits (EC) used in PBS solution.
Consequently, two different concentrations of nanocomposite (10% and 50%) were used and the EIS measurements were recorded. Our results show a significant decrease of resistance while increasing the composite concentration. In particular, the 50% nanocomposite (after sonication) demonstrated the lowest resistance as shown in Fig. 2B. Therefore, this concentration will be used for NADH determination. On the other hand, the impedance spectra were fitted to an appropriate equivalent circuit (EC). Our results confirmed that, Randles Equivalent Circuit (Fig. 2C) was matched perfectly.
4.1.1 Characterization using ferricyanide (FCN)
Al2O3-GO nanocomposite was selected as the best modifier for the potential CPE based sensor; we examined its response towards the redox reactions of ferricyanide (FCN). As shown in Fig. 3, cyclic voltammograms (CVs) of the unmodified CPE electrode and the Al2O3-GO-based CPE electrodes were demonstrated. The peak currents (oxidation/reduction peaks of FCN) located at around 0.46 V (vs. Ag/AgCl) were 8 orders of magnitude higher than the unmodified electrode. This could be attributed to the acceleration of electron-transfer and the enhancements of the electro-catalytic functions of the modified sensor.![(A, B) Cyclic voltammogram for 0.1 mM K4[Fe(CN)6] on a unmodified and modified CPE electrode with Al2O3-GO nanocomposite and its elements at 40 mV/s in 0.1 M KCl. (C) Cyclic voltammogram of NADH (300 µM) in phosphate buffer solution at pH 7.4 using unmodified or modified electrode (50% w/w) and its elements.](/content/184/2018/11/6/img/10.1016_j.arabjc.2018.03.017-fig3.png)
(A, B) Cyclic voltammogram for 0.1 mM K4[Fe(CN)6] on a unmodified and modified CPE electrode with Al2O3-GO nanocomposite and its elements at 40 mV/s in 0.1 M KCl. (C) Cyclic voltammogram of NADH (300 µM) in phosphate buffer solution at pH 7.4 using unmodified or modified electrode (50% w/w) and its elements.
Similar to the mentioned EIS investigations, we examined two different concentrations of the nanocomposite (10% and 50%) modified CPE sensor while measuring the FCN redox reactions. The results showed that, there is a significant increase of electrochemical signals while increasing the composite concentration from 10% to 50% as shown in Fig. 3B. This could be attributed to the more increase of the number of free electrons that enhance the electron transfer process leading to higher electro-catalytic functionality of the CPE modified sensor.
4.2 Scan rate and pH effects
We examined the influence of pH on the oxidation peak current of NADH over a wide range of pH values. Our results shown in Fig. 4A indicated that, the oxidation current increased while increasing the pH reaching maximum activity at pH = 7.5. Further increase beyond this value leads to reaching a steady-state showing a reverse decay in the peak current at pH 9.5. Therefore, pH 8.5 was recognized as an optimal working pH value. In addition, the effect of scan rate as one of the important electrochemical parameters which might affect the rate of electron transfer as well as the sensitivity of the detection. Thus, different scan rates were applied while the oxidation peak currents of a single concentration of NADH were analyzed. As a result, plots of oxidation peak current (ip) vs. the square root of the scan rate (ν1/2) were established according to the principle of Randles-Sevcik (Gowda and Nandibewoor, 2014, Prashanth et al., 2011). Our results shown in Fig. 4B indicated that, the peak current was increasing linearly as a function of the square root of the scan rate confirming that, the electrochemical process is a diffusion controlled process.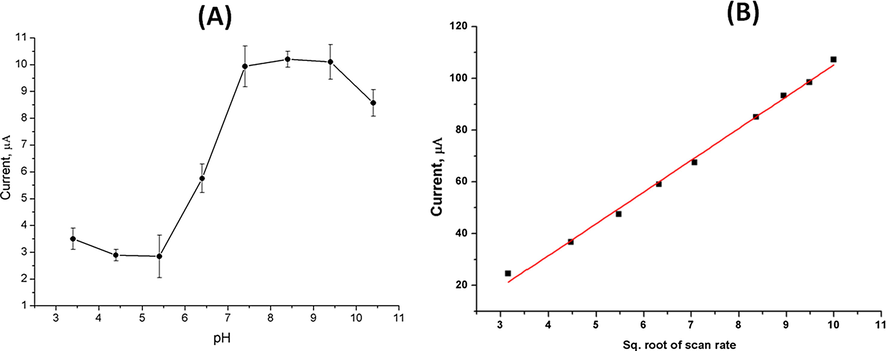
(A) Effect of pH changes on the oxidation peak current of NADH (300 µM). (B) The effect of scan rate on the changes of oxidation peak current of NADH (300 µM).
4.3 Calibration curve
After defining and obtaining the optimal experimental detection conditions, the Differential Pulse Voltammetry (DPV) and Cyclic Voltammery (CV) were used for monitoring the changes of oxidation peak current while changing the NADH concentrations. As shown in Fig. 5A, a higher and continues increase of peak current with increasing the NADH concentration was obtained when the DPV was the tool of measurements. However, while using the CV as a measurement tool, the peak current was declined after increasing the NADH concentration over 180 µM putting limitations to the Al2O3-GO modified CPE biosensor electrode beyond this concentration of NADH however, this behavior was not noticed while using DPV. Thus, DPV measurement is considered to be more sensitive and better recommended for the current assay detection.
(A) Calibration curve of NADH using DPV (B) Calibration curve of NADH using CV and (C) Extracted current outputs of both techniques.
On the other hand, from calibration curve values, a remarkable linear increase was obtained from 30 µM to 330 µM, while the linear regression coefficient and LOD were estimated as 0.98 and 4.5 µM, respectively.
5 Determination of alcohol concentration in beer
The standard ethanol calibration assay was performed and the reaction rates were recorded at 340 nm using UV–vis spectrophotometer where NADH was oxidized to NAD+ as shown in Scheme 1. The estimated reaction rates for the standard ethanol calibration assay were plotted vs. the ethanol concentration in a Michaelis-Menton plot as shown in Fig. 6A. Moreover, the reciprocal of the reaction rate is plotted vs. the reciprocal of ethanol concentration in a double-reciprocal Lineweaver-Burk plot as shown in Fig. 6B.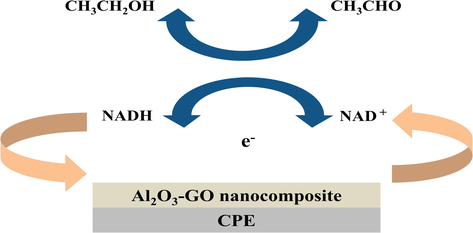
Schematic representation for the bioelectrocatalytic sensing of ethanol through the catalytic oxidation of NADH using Al2O3-GO nanocomposite modified CPE electrode.
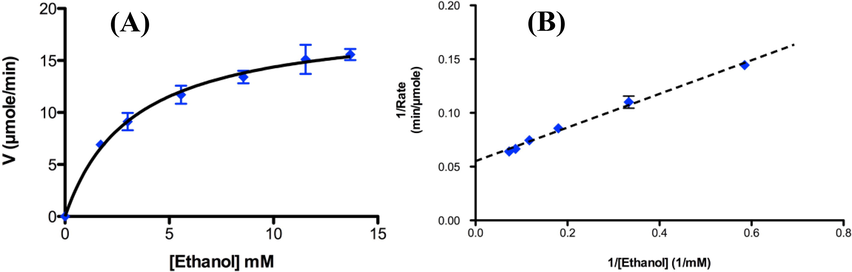
(A) Michaelis-Menton plot and (B) double-reciprocal Lineweaver-Burk plot of reaction rates with ethanol concentrations.
Using Michaelis-Menten equation;
From Fig. 6A, we can estimate the reaction rate V as 12.62 µmol/min and from Fig. 6B we can estimate Km/Vmax and 1/Vmax values form the slope and interception to be 0.1564 and 0.0552, respectively.
Therefore, Eq. (1) can be rewritten as:
Consequently, [S] which represents the 100 times diluted ethanol concentration is estimated as 6.52 µM. Thus, the concentration from Miller Lite Highlife beer is 652.0 µM. Taking into consideration the density of absolute ethanol as 0.789 g/ml, we can estimate the% ethanol concentration as ca. 3.807% (v/v).
6 Conclusion
We developed a sensitive bioelectrochemical electrode sensor for the detection of NADH level using Al2O3-GO nanocomposite modified CPE electrode. The newly developed modified electrode was electrochemically characterized and experimental conditions were optimized for the successful direct detection of NADH level in the linear range between 30 µM and 330 µM with LOD of 4.5 µM and linear regression coefficient of 0.98. We could successfully apply our Al2O3-GO nanocomposite modified CPE electrode to estimate the real ethanol concentration in an enzymatic redox reaction based beer model. The estimated ethanol concentration was 6.52 µM which is within our electrode detection limit. This interesting finding reveals that, the Al2O3-GO nanocomposite modified CPE electrode is a promising electrode sensor to provide a sensitive bioelectrochemical screening of vital enzymes once the detecting experimental parameters are optimized. More applicable attentions of Al2O3-GO nanocomposite modified CPE electrode is to be considered for more sensitive and selective level detections of several bio-matrices.
Acknowledgements
Authors acknowledge the financial support received from the Science and Technology Department Fund (STDF, Egypt) for funding the running research project (code 11929). AM acknowledges the financial support from W. Ruel Johanson Chair in Environmental Engineering.
Declaration of conflicting interests
The authors declare that, there is no conflict of interest.
References
- Electrochemical sensing and biosensing based on square wave voltammetry. Anal. Methods. 2013;5:2158-2173.
- [Google Scholar]
- Highly sensitive detection of NADH using electrochemiluminescent nanocomposites. Electrochem. Comm.. 2012;19:43-45.
- [Google Scholar]
- Chemiluminescence detection using regenerable tris(2,2'- bipyridyl)ruthenium(II) immobilized in Nafion. Anal. Chem.. 1992;64:261-268.
- [Google Scholar]
- Electrochemical behavior of paclitaxel and its determination at glassy carbon electrode. Asian J. Pharm. Sci.. 2014;9(1):42-49.
- [Google Scholar]
- Electrocatalytic performance of silica nanoparticles on graphene oxide sheets for hydrogen peroxide sensing. J. Electroanal. Chem.. 2013;690(2013):8-12.
- [Google Scholar]
- Electrochemical detection of NADH, cysteine, or glutathione using a caffeic acid modified glassy carbon electrode. Electroanalysis. 2013;25(7):1613-1620.
- [Google Scholar]
- A novel nonenzymatic hydrogen peroxide sensor based on MnO2/graphene oxide nanocomposite. Talanta. 2010;82:1637-1641.
- [Google Scholar]
- Facilitation of high-rate NADH electrocatalysis using electrochemically activated carbon materials. ACS Appl. Mater. Interfaces. 2014;6:6687-6696.
- [Google Scholar]
- Electrochemistry of immobilized redox enzymes: kinetic characteristics of NADH oxidation catalysis at diaphorase monolayers affinity immobilized on electrodes. J. Am. Chem. Soc.. 2006;128:2084-2092.
- [Google Scholar]
- A highly sensitive NADH sensor based on a mycelium-like nanocomposite using graphene oxide and multi-walled carbon nanotubes to co-immobilize poly(luminol) and poly(neutral red) hybrid films. Analyst. 2014;139:3991-3998.
- [Google Scholar]
- Sensitive colorimetric visualization of dihydronicotinamide adenine dinucleotide based on anti-aggregation of gold nanoparticles via boronic acid–diol binding. Biosens. Bioelectron.. 2012;35:443-446.
- [Google Scholar]
- Synthesis and application of FePt/CNTs nanocomposite as a sensor and novel amide ligand as a mediator for simultaneous determination of glutathione, nicotinamide adenine dinucleotide and tryptophan. PCCP. 2013;15:5888-5897.
- [Google Scholar]
- Electrochemical oxidation of an immunosuppressant, mycophenolate mofetil, and its assay in pharmaceutical formulations. Int. J. Electrochem.. 2011;2011:1-7.
- [Google Scholar]
- Nanomaterials-based microbial sensor for direct electrochemical detection of Streptomyces Spp. Sens. Actuators B. 2014;203:848-853.
- [Google Scholar]
- Monitoring of microbial cell viability using nanostructured electrodes modified with Graphene/Alumina nanocomposite. Biosens. Bioelectron.. 2017;91:857-862.
- [Google Scholar]
- A probe for NADH and H2O2 amperometric detection at low applied potential for oxidase and dehydrogenase based biosensor applications. Biosens. Bioelectron.. 2007;22:854-862.
- [Google Scholar]
- Electrocatalytic oxidation behavior of NADH at Pt/Fe3O4/reduced-graphene oxide nanohybrids modified glassy carbon electrode and its determination. Mater. Sci. Eng. C. 2016;67:237-246.
- [Google Scholar]
- Synthesis, characterization and electrochemical-sensor applications of zinc oxide/graphene oxide nanocomposite. J. Nanostruct. Chem.. 2016;6(2):137-144.
- [Google Scholar]
- Synergetic effect for NADH oxidation of ferrocene and zeolite in modified carbon paste electrodes: New approach for dehydrogenase based biosensors. Biosens. Bioelectron.. 2004;20:161-166.
- [Google Scholar]
- Graphene based electrochemical sensors and biosensors. Electroanalysis. 2010;22(10):1027-1036.
- [Google Scholar]
- Dopamine sensitized nanoporous TiO2 film on electrodes: photoelectrochemical sensing of NADH under visible irradiation. Biosens. Bioelectron.. 2009;24:2494-2498.
- [Google Scholar]
- Adsorption behavior of dinucleotides on bare and Ru-modified glassy carbon electrode surfaces. Langmuir. 2008;24:12375-12384.
- [Google Scholar]
- Detection of NADH and ethanol based on catalytic activity of soluble carbon nanofiber with low overpotential. Anal. Chem.. 2007;79:453-458.
- [Google Scholar]
- Electrocatalytic oxidation of NADH on graphene oxide and reduced graphene oxide modified screen-printed electrode. Int. J. Electrochem. Sci.. 2011;6:819-829.
- [Google Scholar]







