Translate this page into:
Electrospun zeolitic imidazolate framework-8/poly(lactic acid) nanofibers for pipette-tip micro-solid phase extraction of carbamate insecticides from environmental samples
⁎Corresponding author.at: Iranian Research and Development Center for Chemical Industries, ACECR, Tehran, Iran ghambarian.m@gmail.com (Mahnaz Ghambarian)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
In the present study, electrospun zeolitic imidazolate framework-8/poly(lactic acid) nanofibers were successfully synthesized and characterized as a potential nanosorbent for the pipette-tip micro-solid phase extraction of chlorpropham, pirimicarb, carbaryl, and methiocarb carbamate insecticides from environmental water samples. The extraction procedure was followed by gas chromatography/mass spectrometry separation and determination of the target analytes. All the effective parameters of the extraction procedure were optimized through the one variable at-a-time method. Thanks to the very simple extraction procedure as well as the application of electrospun nanofibers with high surface area, the four analytes were efficiently extracted with as lowest extraction times as practicable. Under the optimal conditions, the calibration plots of the analytes were obtained within broad linear dynamic ranges of 0.5 – 150 ng mL−1 for chlorpropham and pirimicarb plus 1.0 – 175 ng mL−1 for carbaryl and methiocarb, respectively. Besides, limits of detection as low as 0.2 and 0.15 ng mL−1 for chlorpropham and pirimicarb, respectively, as well as 0.5 ng mL−1 for carbaryl and methiocarb indicate the favorable sensitivity of the analytical procedure. The applicability of the developed method was evaluated by quantitative determination of the target analytes in four different environmental water samples. Relative recoveries higher than 88.0% shows the acceptable accuracy of the method in the quantitative determination of the four carbamate insecticides.
Keywords
Micro-solid phase extraction
Zeolitic imidazolate framework-8
Gas chromatography/mass spectrometry
Environmental water samples
1 Introduction
One of the major concerns in all the analytical procedures is to prepare the target sample in a form that is compatible to analyze with the analytical instrument (Pawliszyn and Lord, 2010). To this end, numerous sample pretreatment techniques have been introduced during the past decades for the quantitative analysis of different complex matrices from environmental to biological and industrial ones (de Koning et al., 2009). Classically, sample preparation dealt with liquid–liquid extraction (LLE) and solid phase extraction (SPE) as the most convenient and cheapest techniques capable of extraction various analytes from different matrices. Although these techniques are still being used due to the simplicity, high efficiencies, and considerable applicability for various matrices, they require high amounts of the testing sample and consume high volumes of hazardous organic solvents as well (Dimpe and Nomngongo, 2016).
Miniaturized sample preparation techniques have dominated the classical LLE and SPE techniques during the past years. Liquid-phase microextraction (LPME) is a miniaturized LLE technique in which a few microliters of an organic solvent are used to extract the target analytes from a sample solution (Tajik et al., 2015). Similarly, solid phase microextraction (SPME) uses a few micrograms of a sorbent coated on a blade that can extract the target analytes from a sample solution (Reyes-Garcés et al., 2017). Micro-solid phase extraction (µ-SPE) is another miniaturized technique that uses tens of micrograms of a sorbent dispersed into the sample solution or packed inside a tube (Seidi et al., 2019). During the past years, numerous configurations of these miniaturized liquid and solid-phase techniques have been reported in the literature. To name a few, hollow fiber liquid phase microextraction (HF-LPME) application (Khan et al., 2020), thin-film solid phase microextraction (TF-SPME) (Vuckovic et al., 2010), dispersive micro-solid phase extraction (D-µ-SPE) (Tajik et al., 2017), spin-column micro-solid phase extraction (SC-µ-SPE) (Amini et al., 2020), and pipette-tip micro-solid phase extraction (PT-µ-SPE) (Fresco-Cala et al., 2018) have been the most applied miniaturized sample preparation techniques.
Along with the outstanding efficiency of the liquid-based microextraction techniques, these protocols lack acceptable selectivity resulting in the co-extraction of various undesired compounds. That is why solid-based techniques have been more utilized in the past years for the quantitative analysis of different analytes in various complex matrices (Reyes-Garcés et al., 2017). The main reason relates to the possibility of designing specific sorbents for different analytes of interest so that the cleanup values increase at times. For this purpose, different types of materials have been applied for the extraction and preconcentration of countless analytes from different matrices (Lashgari and Yamini, 2019).
Metal-organic frameworks (MOFs) are a new class of hybrid materials containing transition metal ions interconnected with organic ligands. By changing the metal cores and type of organic ligands, numerous possible MOFs with various properties can be synthesized (Gutiérrez-Serpa et al., 2019). Such hybrid materials have uniform nanoscale cavities, high surface areas, and considerably high thermostability. Thanks to such valuable physicochemical features, MOFs have incredibly attracted attentions during the past years. Hundreds of papers have been reported in the literature on the applications of MOFs as sorbents in SPE and SPME procedures (Rocío-Bautista et al., 2019).
Conventionally, MOFs can be simply synthesized using solvothermal reaction between metal ions and the organic linkers. Although these methods are quite straightforward for the synthesis of a variety of MOF compounds, the obtained particles accumulate randomly leading to the formation of MOFs with different particle sizes. Interestingly, electrospinning has recently been developed for the processing of MOFs to obtain nanoscale fibers. In this technique, a solution containing desired material is passed through a spinning needle while high potential is applied between the needle and a counter electrode to achieve the electrospray ionization of the dissolved material. Electrospun materials have incredibly higher surface area in comparison with the conventionally synthesized materials. Numerous electrospun sorbent have been reported in the literature for the extraction of different compounds from biological and environmental samples.
Zeolitic imidazolate framework-8 (ZIF-8) is one of the most widely used MOFs. Owing to its special tetrahedral skeleton structure, ZIF-8 not only has a high specific surface area and porosity but also has excellent thermal stability and chemical stability (Dai et al., 2018). Among the utilized polymers in electrospun fibers, poly(lactic acid) (PLA) is more environmentally friendly because it is biodegradable and its raw materials are renewable. Combining porous fibers with porous MOFs will be potentially applied in extraction due to their large surface areas.
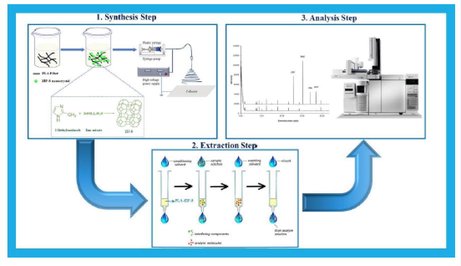
Herein, electrospun zeolitic imidazolate framework-8/poly(lactic acid) (ZIF-8/PLA) was successfully synthesized and utilized for the PT-µ-SPE of carbamate insecticides followed by gas chromatography/mass spectrometry (GC/MS) separation and quantitation. The procedure was optimized and utilized for the preconcentration and determination of chlorpropham (CPP), pirimicarb (PMC), carbaryl (CRL), and methiocarb (MTC) from environmental water samples.
2 Experimental
2.1 Chemicals and reagents
Analytical grades (>99%) Zn(NO3)2·6H2O, 2-methylimidazole, and polylactic acid (PLA), as well as reagent grade (37%) hydrochloric acid (HCl) were purchased from Sigma-Aldrich (Milwaukee, WI, USA). HPLC grades methanol, ethanol, and acetonitrile were obtained from Daejung Chemicals and Metals (Siheung, South Korea) ( www.daejungchem.co.kr). Carbamate insecticides comprising chlorpropham (CPP), carbaryl (CBL), pirimicarb (PMC), and methiocarb (MTC) were bought from Mina Tajhiz Aria (Tehran, Iran). The stock standard solutions of the carbamate insecticides were prepared in methanol at the concentration level of 1000 ng mL−1 and kept in a refrigerator at 4 °C. Working solutions of the carbamate compounds were freshly prepared by diluting appropriate volumes of the stock solutions with ultrapure water. The ultrapure water was also prepared by an Aqua Max-Ultra Youngling ultrapure water purification system (Dongan-gu, South Korea).
2.2 Instruments and apparatus
Morphological properties of the synthesized sorbent were evaluated using a Philips CM30T electron microscope (200 kV), equipped with an energy-dispersive X-ray analyzer (EDX) to obtain its chemical composition. A Thermo Scientific Nicolet IR100 (Madison, WI, USA) Fourier transform infrared (FT-IR) spectrometer was used to obtain specific functional groups of the synthesized sorbent. The N2 sorption analysis was conducted with an automated gas sorption analyzer (Autosorb-iQ-C) at 77 k applying the Brunauer-Emmett-Teller (BET) method for the specific surface area ZIF-8/ PLA nanofibers. X-ray diffraction (XRD) patterns of ZIF-8, PLA, and ZIF-8/PLA were recorded on an Rigaku diffractometer using Cu-Kα radiation in the range of 5 – 60°.
Separation and determination of the carbamates were performed using an Agilent 7890A (Wilmington, USA) GC system equipped with an Agilent MSD 5975C quadrupole mass spectrometer. A 30 m × 0.25 mm i.d., 0.25 μm film thickness, DB-5MS (5% phenyl–methylsiloxane) fused-silica column was used. Helium (99.999%) was the carrier gas, at a flow rate of 1.0 mL min-1. The gas chromatograph operated at the splitless mode and the temperature of the injection port was 270 °C. The initial oven temperature was set at 60 °C and held for 2 min. Then, an increase to 140 °C at 10 °C min−1, a further increase to 200 °C at 10 °C min−1, and lastly, an increase to 230 °C at 10 °C min−1, with the final temperature held for 3 min. The total analysis time for the insecticides was 22 min. The injection volume was 1 µL. Data acquisition was performed in the full scan mode (m/z in the range of 45 – 700) to confirm the retention times of analytes and in selected ion monitoring (SIM) mode for quantitative determination of the insecticides. The monitored ions (m/z) were 43 and 127 for chlorpropham, 72 and 166 for pirimicarb, 115 and 144 for carbaryl, and 153 and 168 for methiocarb.
2.3 Synthesis of ZIF-8 material
Synthesis of ZIF-8 was carried out based on a straightforward procedure reported in the literature. Briefly, 4.0 mmol Zn(NO3)2·6H2O and 0.4 mmol HCl were dissolved in 40 mL of 50% (v/v) ethanol solution while it was rigorously stirring. Afterwards, 80 mL of 50% ethanol containing 2-methylimidazole was added into the solution and stirred for a while. Then, the solution was transferred into an autoclave and heated up to 120 °C for 24 h. Finally, the obtained crystals were gently washed by pure ethanol and water several times and dried inside a vacuum at 50 °C for 12 h wang et al. (2018).
2.4 Electrospinning of ZIF-8/PLA nanofibers
The electrospun ZIF-8/PLA nanofibers were prepared based on a previous report in the literature (Salahi Chashami et al., 2020). In the nutshell, 5% (w/v) of the synthesized sorbent was added into a solution containing 10% (w/v) PLA in DMF. The electrospinning procedure was started by passing this solution through a syringe pump with the injection rate of 0.6 mL h−1, while the applied voltage between the needle of the syringe pump and a grounded electrode was set at 10 kV. The electrospun sorbent was collected at 12 cm of the needle by a collector (Lee et al., 2017). It should be noted that all these steps were carried out at room temperature. Finally, the obtained nanofibers were immersed in pure ethanol and dried at 60 °C in an oven for 12 h.
2.5 PT-µ-SPE procedure
PT-µ-SPE procedure was carried out by packing a few milligrams of the synthesized sorbent inside a pipette tip. To this end, 10 mg of the electrospun composite was packed between two small pieces of glass wool. Next, the tip was connected to a vacuum pump to be compressed. Afterwards, the tip was thoroughly washed by deionized water and acetonitrile to get the rid of any impurities of the sorbent and glass wool. The extraction procedure was carried out by passing 5 mL of the sample solution through the PT-µ-SPE device. Two repetitive aspiration/elution cycles of sample solution were carried out to ensure quantitative extraction of the analytes through the extraction procedure. Then, the tip was rinsed by 1 mL of deionized water followed by the elution of the sorbent three times by acetonitrile to desorb the extracted analytes (Mirzaee et al., 2020).
3 Results and discussions
3.1 Characterization of electrospun ZIF-8/PLA
The morphologies of ZIF-8 and electrospun ZIF-8/PLA nanofibers were evaluated using scanning electron microscopy (SEM). Fig. 1A illustrates the SEM image, where the cubic shapes of ZIF-8, which is a typical characteristic of MOF compounds. It should be noted that the small spherical particles on the surface of ZIF-8 crystals are palladium nanoparticles which were used prior to get the SEM images to make the particles conductive enough to obtain high-resolution SEM images. As well as this, the nanoscale electrospun ZIF-8/PLA fibers can be clearly seen in Fig. 1B. Based on the SEM image of the ZIF-8/PLA, the diameter of the fiber was in the range between 120 and 210 nm. Followed by SEM analysis, elemental composition of ZIF-8/PLA nanofibers were obtained by EDX spectroscopy. Table 1 shows the elemental composition of the electrospun nanofiber including C (73.6%), O (13.9%), N (11.8%), and Zn (0.7%), respectively, which shows the presence of ZIF-8 particles into the electrospun nanofibers.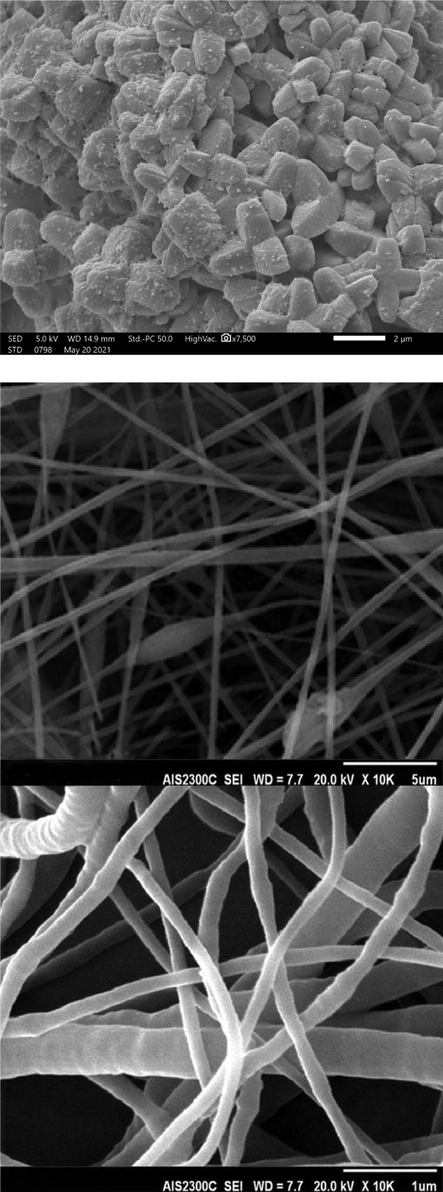
SEM images of (A) ZIF-8 metal-organic frameworks and (B) electrospun ZIF-8/PLA nanofibers at the specified magnifications.
Element
Measure (%)
C
73.6
O
13.9
N
11.8
Zn
0.70
Fig. 2 shows the XRD patterns of pure ZIF-8, pure PLA and the PLA/ZIF-8 nanofibers. The XRD spectra of ZIF-8 showed the characteristic peaks of ZIF-8 at about 7.4, 10.4, 12.8 and 18°. The characteristic peaks of ZIF-8 appear in XRD patterns of the ZIF-8/PLA nanofibers indicating the presence of ZIF-8 on the surface of the PLA fibers. As calculated by the isothermals, the specific surface areas of PLA and ZIF-8/PLA are 19.65, 25.81 m2/g, respectively. It is obvious that adding ZIF-8 increases the specific surface areas.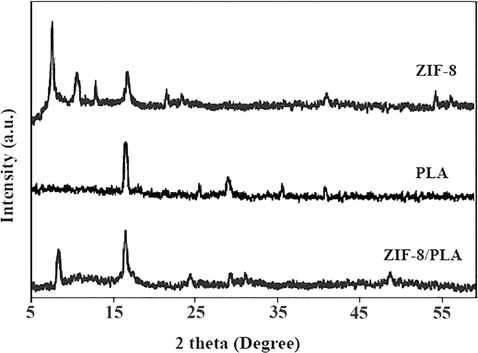
XRD spectra of (A) pure ZIF-8, (B) pure PLA, and (C) ZIF-8/PLA nanofibers.
FT-IR spectrometry was also carried out for further confirmation of the chemical structure of ZIF-8/PLA nanofibers. To this end, FT-IR spectra of ZIF-8, PLA, and electrospun ZIF-8/PLA were obtained. Fig. 3 shows the FT-IR spectra of these compounds. According to this figure, a sharp peak at 1680 cm−1 in both pure PLA and the synthesized composite corresponds to the C = O stretching, which confirms the presence of PLA into the electrospun ZIF-8/PLA composite (Mofokeng et al., 2012). Furthermore, the broad peak from 3200 to 3800 cm−1 in both ZIF-8 and the synthesized sorbent corresponds to the O-H bending in these structures, which is another indication for the successful synthesis of the electrospun ZIF-8/PLA nanofibers (Zhang and Jia, 2018).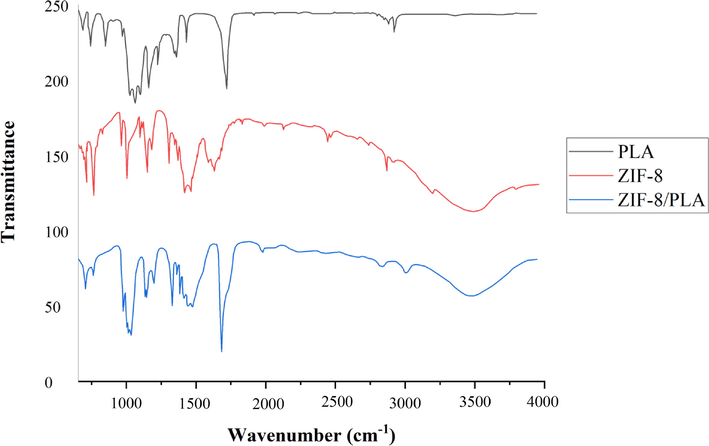
FT-IR spectra of PLA, ZIF-8, and the electrospun ZIF-8/PLA nanofibers.
3.2 Optimization of PT-µ-SPE procedure
To obtain the best performance of the analytical procedure, all the effective variables of the method was identified and optimized using the one variable at-a-time (OVAT) method. These parameters were sample pH, type of eluent, aspiration/elution cycles of the sample, the eluent volume, as well as the aspiration/elution cycles of the eluent.
Due to the presence of ionizable functional groups in the chemical structure of the carbamate insecticides, sample pH can affect the extraction efficiencies of these compounds considerably. To evaluate the effect of this parameter on the extraction efficiencies of the carbamate compounds, pH of the sample was changed in the range of 2.0 – 12.0. Fig. 4 shows how sample pH can change the analytical signals of the target analytes. As can be seen in this figure, neutral pHs can increase the extraction efficiencies of the analytes. The main reason for this phenomenon is that carbamate compounds contain amide functional groups that are neutral in pH values of 5 – 7. Also, owing to hydrophobicity, highly accessible pores, and great surface area of the ZIF-8/PLA nanofibers could achieve high extraction efficiencies of the target analytes.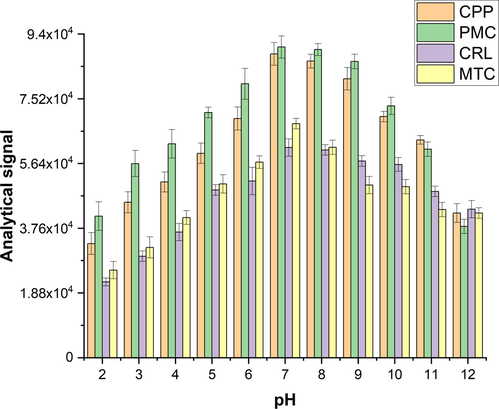
Influence of pH on the analytical signals of the four carbamate insecticides on the extraction efficiencies of the target carbamate insecticides.
To evaluate the effect of eluent type on the extraction efficiencies of the analytes, three different eluent comprising methanol, ethanol, and acetonitrile were chosen as potential eluents. Basically, an appropriate eluent should be capable of desorbing the whole analytes from the sorbent without any effect of the sorbent surface. Specific to the present work, the eluent should be compatible with the GC/MS instrument., as aqueous solutions cannot be injected into a GC system. According to the experiments, acetonitrile showed the best performance in the desorption of analytes from the synthesized sorbent.
The other effective parameter was the number of aspiration/elution cycles of the sample solution. It is quite understandable that the whole analytes cannot be extracted from the sample solution within a single aspiration of the sample. Thus, quantitative extraction of the analytes happens with several repetitive aspiration/elution cycles. To this end, aspiration/elution of the sample was repeated up to 5 times. As can be seen in Fig. 5, the analytical signals of the analytes did bot considerably changed after 2 aspiration/elution cycles of the sample. Therefore, this value was considered as the optimal value of this parameter in the further experiments.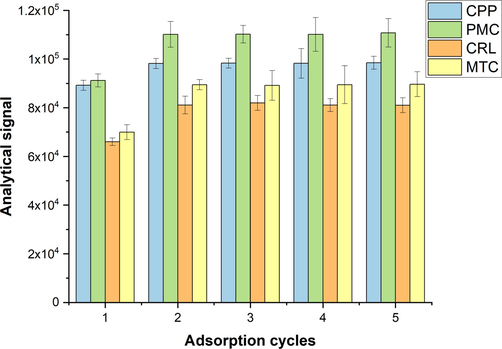
Influence of adsorption cycles on the extraction efficiencies of the target carbamate insecticides.
Eluent volume and the desorption cycles were the two parameters which were optimized, simultaneously. The main reason for the co-optimization of these two parameters is that it is quite possible for lower volumes of the eluent to be able to desorb the whole analyte with multiple desorption cycles. The whole desorption of the analytes can also be achieved with higher volumes, however, with the cost of lower extraction efficiencies. To this end, four eluent volumes of 50, 100, 150, and 200 µL were chosen and for each of them the analytes desorption was repeated for five cycles. Fig. 6 shows the effect of these two parameters on the average analytical signals of the four analytes. According to this figure, highest extraction efficiencies were obtained when the sorbent was eluted three times with 100 µL of acetonitrile.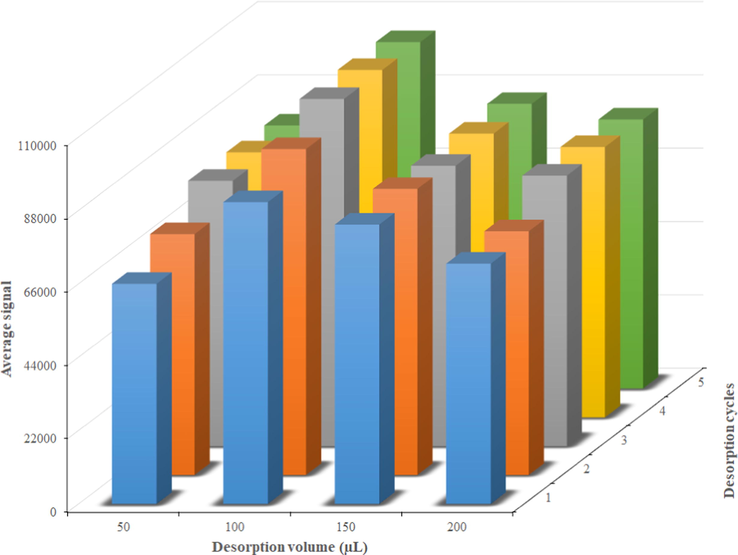
Effects of desorption cycles and the volume of the desorption solvent on the extraction efficiencies of the target carbamate insecticides.
3.3 Reusability of the sorbent
To evaluate the reusability of ZIF-8/PLA nanofibers, extraction efficiencies of the analytes were calculated after 5, 10, 20, 30, 40, and 50 times of a whole extraction procedure. According to the obtained results, extraction efficiencies were not considerably decreased up to 40 extraction experiments. Such results indicated the synthesized nanofiber had favorable reusability in the extraction of the target compounds.
3.4 Method validation
Analytical performance of the method in quantitative analysis of the carbamate insecticides was tested by the evaluation of figures of merit which were limits of detection (LODs), limits of quantitation (LOQs), linearity, preconcentration factors (PFs), precisions (inter- and intra-day relative standard deviations (RSDs)), as well as the extraction efficiencies (EE%). All the results were summarized in Table 2. LOD values of the method for each analyte were obtained by decreasing the concentrations of the spiked analytes into analyte-free aqueous samples to achieve the signal-to-noise ratio (S/N) of 3. LOQ values were simply obtained based on the lowest concentration after LODs that the linearities of the calibration curves were started. To this point, LODs and LOQs of the analytes were obtained in the range of 0.15 – 0.5 and 0.5 – 1.0 ng mL−1, respectively. Along with these results, linearity of the method for the analytes were evaluated by plotting the calibration curves for each analyte of interest. The calibration curves for CPP and PMC were linear (R2 > 0.9934) in the range of 0.5 – 150 ng mL−1. Also, the calibration curves for CRL and MTC were linear (R2 > 0.9953) in the range of 1.0 – 175 ng mL−1. PF of each analyte was calculated by dividing the slope of slope of the calibration curve obtained from the extraction of the respective analyte from the sample solution by the calibration curve obtained from the direct injection of the analyte. Given that, EE values were obtained according to the following equation:
Analyte
LOD (ng mL−1)
LOQ (ng mL−1)
LDR (ng mL−1)
Regression coefficient (R2)
PF
RSD% (n = 5)
Inter-day
Intra-day
CPP
0.20
0.50
0.50 – 150.0
0.9987
50.0
4.31
7.22
PMC
0.15
0.50
0.50 – 150.0
0.9934
50.0
5.78
6.64
CRL
0.50
1.00
1.00 – 175.0
0.9953
50.0
5.06
5.19
MTC
0.50
1.00
1.00 – 175.0
0.9991
50.0
5.52
7.31
According to the results shown in Table 2, PF values of 50 and EE% ∼ 100% for each analyte was obtained. Repeatability of the method in quantitative analysis of the analytes were evaluated by obtaining inter- and intra-day RSD% at two different concentrations (5.0 and 10.0 ng mL−1). As is shown in Table 1, inter- and intra-day RSD% were less than 5.78% and 7.31%, respectively, for the analytes of interest. To make a comparative viewpoint, figures of merit of this method were compared in Table 3 with some previous works reported in the literature. As can be seen in this table, figures of merit for this method were quite comparable with the similar studies. Besides, thanks to the utilization of PT-µ-SPE sample, sorbent, and organic solvent consumptions were noticeably low. Furthermore, as we used electrospun nanofibers with high specific area as a sorbent in the extraction procedure, EE% near to 100% for each analyte was obtained with the aid of a very fast extraction procedure. aMicro-solid phase extraction. bHigh-performance liquid chromatography with diode array detector. cLiquid–liquid extraction followed by ionic liquid–based dispersive liquid–liquid microextraction. dLiquid chromatography with mass spectrometry detection system. eUltrasound-assisted surfactant-enhanced emulsification microextraction.
Method
Analytical instrument
Analyte
LOD (ng mL−1)
LDR (ng mL−1)
RSD%
Ref.
µ-SPEa
HPLC-DADb
CRL
2.88
5.0 – 150.0
less than3.4
(Zhou and Fang, 2015)
HLLE-IL-DLLMEc
HPLC-DAD
PMC
CRL
MTC2.3
0.6
0.47.7 – 250.0
2.1 – 250.0
2.5 – 250.07.9
6.2
5.7(Anvar et al., 2020)
(Anvar et al., 2020)
(Anvar et al., 2020)
SPE
LC-MSd
CRL
MTC0.015
0.01–
-3.3
3.7(El Atrache et al., 2016)
(El Atrache et al., 2016)
DLLME
HPLC-DAD
PMC
CRL0.6
0.45.0 – 500.0
5.0 – 500.04.8
5.1(Wu et al., 2009)
(Wu et al., 2009)
UASEMEe
HPLC-DAD
PMC
CRL0.1
0.10.3 – 200.0
0.3 – 200.03.8
4.6(Wu et al., 2010)
(Wu et al., 2010)
PT-µ-SPE
GC–MS
CPP
PMC
CRL
MTC0.2
0.15
0.5
0.50.5 – 150.0
0.5 – 150.0
1.0 – 175.0
1.0 – 175.04.3
5. 8
5.1
5.5This work
This work
This work
This work
3.5 Real sample analysis
Applicability of the sorbent in the extraction of the analytes from water samples were evaluated by quantitative analysis of four different environmental water samples comprising tap water, wastewater, river water, and mineral spring. Table 4 summarizes the obtained results from the quantitative analysis of these real samples. Method accuracy was evaluated by the calculation of relative recoveries (RR%) for each analyte according to the following equation:
aNot Detected.
Sample
Analyte
Creal (ng mL−1)
Cadded (ng mL−1)
Cfound (ng mL−1)
RSD% (n = 3)
RR%
Tap water
CPP
n.d.a
5.0
4.5
6.9
90.0
10.0
9.6
6.7
96.0
20.0
19.4
6.5
97.0
PMC
n.d.
5.0
5.2
7.5
104.0
10
9.4
7.2
91.0
20.0
18.8
7.1
94.0
CRL
n.d.
5.0
4.7
6.2
94.0
10.0
9.6
6.0
96.0
20.0
20.0
5.7
100.0
MTC
n.d.
5.0
4.4
7.6
88.0
10.0
10.2
6.9
102.0
20.0
19.1
7.2
95.5
Wastewater
CPP
n.d.
5.0
4.8
7.1
96.0
10.0
9.7
6.9
97.0
20.0
19.9
6.7
99.5
PMC
n.d.
5.0
5.1
5.6
102.0
10.0
10.1
6.3
101.0
20.0
19.9
6.0
99.5
CRL
n.d.
5.0
4.5
6.7
90.0
10.0
9.7
6.4
97.0
20.0
19.8
6.3
99.0
MTC
n.d.
5.0
5.1
7.1
102.0
10.0
9.9
6.9
99.0
20.0
19.3
6.6
96.5
River water
CPP
n.d.
5.0
4.8
6.0
96.0
10.0
10.0
5.3
100.0
20.0
20.2
5.1
101.0
PMC
n.d.
5.0
4.9
7.4
98.0
10.0
9.8
7.0
98.0
20.0
19.8
6.9
99.0
CRL
n.d.
5.0
4.4
7.7
88.0
10.0
8.9
6.9
89.0
20.0
18.7
7.2
93.5
MTC
n.d.
5.0
4.7
6.1
94.0
10.0
10.2
7.0
102.0
20.0
20.2
6.4
101.0
Mineral spring
CPP
n.d.
5.0
4.7
6.3
94.0
10.0
9.1
6.1
91.0
20.0
19.2
5.9
96.0
PMC
n.d.
5.0
5.2
7.5
104.0
10.0
9.0
6.9
90.0
20.0
18.9
7.3
94.5
CRL
n.d.
5.0
4.8
6.8
96.0
10.0
9.2
6.5
92.0
20.0
19.6
6.2
98.0
MTC
n.d.
5.0
5.1
6.1
102.0
10.0
9.4
5.8
94.0
20.0
20.0
5.5
100.0
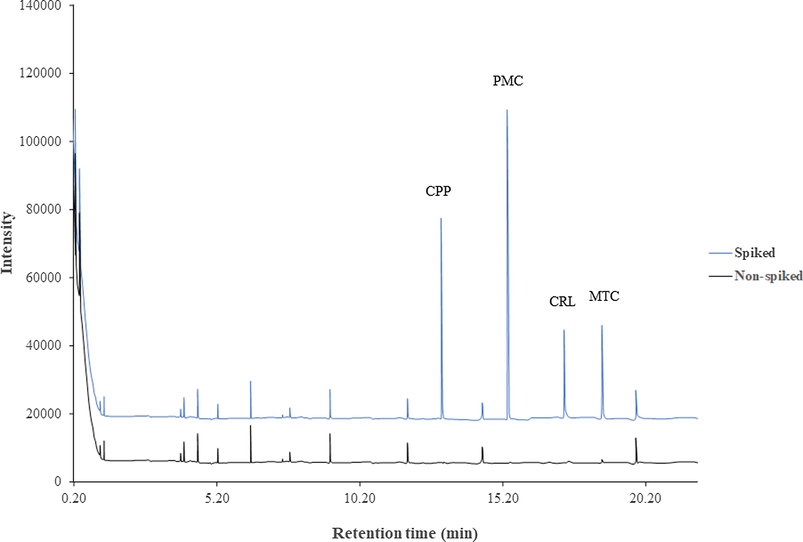
GC–MS chromatograms of a river water sample before and after spiking 20 ng mL−1 of each carbamate insecticides.
4 Conclusions
For the first time, to the best of our knowledge, electrospun ZIF-8/PLA composite nanofibers were utilized as an efficient sorbent for pipette-tip micro-solid phase extraction of carbamate insecticides from environmental water samples followed by GC/MS separation and detection. Thanks to the utilization of the electrospinning strategy for the preparation of the composite material, high surface areas of the ZIF-8/PLA nanofibers were obtained. This phenomenon resulted in favorable extraction efficiencies were obtained for the carbamate insecticides. Besides, PT-µ-SPE procedure brought about fast, labor-saving, and environmentally friendly sample pretreatment with as low organic solvent consumption as practicable. On top of this, the analytical performance of the method was acceptable with low LOD values along with broad linear dynamic ranges, as well as the desirable repeatability according to the low RSD% values.
Acknowledgement
The authors gratefully acknowledge the financial support of Iran University of Medical Sciences, Tehran, Iran. (Grant Number: 98-4-99-16848; Ethics Code: IR.IUMS.REC.1399.116)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Preparation of electrospun polyacrylonitrile/Ni-MOF-74 nanofibers for extraction of atenolol and captopril prior to HPLC-DAD. Microchim. Acta. 2020;187:1-12.
- [Google Scholar]
- Elevated temperature homogeneous liquid phase extraction coupled to ionic liquid–based dispersive liquid–liquid microextraction followed by high-performance liquid chromatography: application of water-miscible ionic liquids as extraction solvent in determ. Food Anal. Methods. 2020;13:1282-1291.
- [Google Scholar]
- Morphology controlled porous poly(lactic Acid)/zeolitic Imidazolate framework-8 fibrous membranes with superior PM2.5 capture capacity. Chem. Eng. J.. 2018;338:82-91.
- [Google Scholar]
- Modern methods of sample preparation for GC analysis. Chromatographia. 2009;69:33-78.
- [Google Scholar]
- Current sample preparation methodologies for analysis of emerging pollutants in different environmental matrices. TrAC Trends Anal. Chem.. 2016;82:199-207.
- [Google Scholar]
- Carbon nanotubes as solid-phase extraction sorbents for the extraction of carbamate insecticides from environmental waters. Int. J. Environ. Sci. Technol.. 2016;13:201-208.
- [Google Scholar]
- Carbon nanotube-modified monolithic polymethacrylate pipette tips for (micro) solid-phase extraction of antidepressants from urine samples. Microchim. Acta. 2018;185:127-133.
- [Google Scholar]
- Metal-organic frameworks as key materials for solid-phase microextraction devices—a review. Separations. 2019;6:47-75.
- [Google Scholar]
- Hollow fiber-based liquid phase microextraction followed by analytical instrumental techniques for quantitative analysis of heavy metal ions and pharmaceuticals. J. Pharm. Anal.. 2020;10:109-122.
- [Google Scholar]
- An overview of the most common lab-made coating materials in solid phase microextraction. Talanta. 2019;191:283-306.
- [Google Scholar]
- ZnO quantum dot-decorated carbon nanofibers derived from electrospun ZIF-8/PVA nanofibers for high-performance energy storage electrodes. Chem. Commun.. 2017;53:11441-11444.
- [Google Scholar]
- Pipette-tip SPE based on graphene/ZnCr LDH for Pb (II) analysis in hair samples followed by GFAAS. Anal. Biochem.. 2020;612:113949
- [Google Scholar]
- Comparison of injection moulded, natural fibre-reinforced composites with PP and PLA as matrices. J. Thermoplast. Compos. Mater.. 2012;25:927-948.
- [Google Scholar]
- J. Pawliszyn, H. L. Lord. Handbook of Sample Preparation, Wiley-Blackwell, Hoboken, NJ, USA (2010). doi:10.1002/9780813823621
- Advances in solid phase microextraction and perspective on future directions. Anal. Chem.. 2017;90:302-360.
- [Google Scholar]
- Salahi Chashami F, Khakbiz M, Zahedi P (2020) Investigation of Morphological and Mechanical Properties of Electrospun Nanofibers Nanocomposites Based on PLA/ZIF-8. The 11th International Chemical Engineering Congress & Exhibition (IChEC 2020).
- Micro solid-phase extraction (pipette tip and spin column) and thin film solid-phase microextraction: miniaturized concepts for chromatographic analysis. TrAC - Trends Anal. Chem.. 2019;118:810-827.
- [Google Scholar]
- On-line extraction and determination of two herbicides: comparison between two modes of three-phase hollow fiber microextraction. J. Sep. Sci.. 2015;38:649-655.
- [Google Scholar]
- Supercritical fluid extraction of papaverine and noscapine from poppy capsules followed by preconcentration with magnetic nano Fe3O4@Cu@diphenylthiocarbazone particles. New J. Chem.. 2017;41:7028-7037.
- [Google Scholar]
- Automated solid-phase microextraction and thin-film microextraction for high-throughput analysis of biological fluids and ligand–receptor binding studies. Nat. Protoc.. 2010;5:140-161.
- [Google Scholar]
- Magnetic solid-phase extraction based on magnetic zeolitic imazolate framework-8 coupled with high performance liquid chromatography for the determination of polymer additives in drinks and foods packed with plastic. Food Chem.. 2018;256:358-366.
- [Google Scholar]
- Application of dispersive liquid–liquid microextraction combined with high-performance liquid chromatography to the determination of carbamate pesticides in water samples. Anal. Bioanal. Chem.. 2009;393:1755-1761.
- [Google Scholar]
- Ultrasound-assisted surfactant-enhanced emulsification microextraction for the determination of carbamate pesticides in water samples by high performance liquid chromatography. J. Chromatogr. A. 2010;1217:1773-1778.
- [Google Scholar]
- Synthesis of zeolitic imidazolate framework-8 on polyester fiber for PM 2.5 removal. RSC Adv.. 2018;8:31471-31477.
- [Google Scholar]
- Graphene-modified TiO2 nanotube arrays as an adsorbent in micro-solid phase extraction for determination of carbamate pesticides in water samples. Anal. Chim. Acta. 2015;869:43-49.
- [Google Scholar]







