Translate this page into:
Elemental composition of Cyrtanthus obliquus and Lippia javanica used in South African herbal tonic, Imbiza
⁎Corresponding author. Tel.: +27 31 260 7325; fax: +27 31 260 3091. jonnalagaddas@ukzn.ac.za (Sreekanth B. Jonnalagadda)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Imbiza is a herbal tonic, which has gained popularity amongst South Africans as an immune booster and for the treatment of many minor and chronic illnesses. Main ingredients of Imbiza are extracts from two medicinal plants namely Cyrtanthus obliquus and Lippia javanica. The concentrations of various elements in these two plants are examined. The total and water extractable elemental concentrations were determined in both the plants. The elemental concentrations in the water extracts more closely represent concentrations in the herbal tonic. Concentrations of selected elements in C. obliquus bulbs collected from eight market sites around the KwaZulu-Natal province were investigated. The levels of the elements in decreasing order were Ca > Mg > Fe > Zn > Mn > Cu ≈ Se > Pb > Cr for total concentrations; and Ca > Mg > Fe > Zn > Mn for water extractable forms. Elemental levels in C. obliquus bulbs were dependent on the source, which suggested the importance of collection sites on elemental levels. A high percentage of Zn (77.5–91.5%) was found in water extracts.
The levels of selected elements in L. javanica leaves collected from ten different locations around the KwaZulu-Natal province were also investigated. The levels of the elements were found to be in the decreasing order of Ca > Mg > Fe > Zn > Mn > Cu > Se > Cr > Pb > Co > Cd for total concentrations and Ca > Mg > Fe > Zn > Cu > Cr > Pb for water extractable forms. It was evident from the study that the herbal tonic was a good source of Ca and Mg, thus accredited as a viable immune booster and toxic metals were below the recommended levels in the investigated samples.
Keywords
Cyrtanthus obliquus
Lippia javanica
Imbiza
Elemental composition
Herbal preparation
1 Introduction
Traditional, complementary and alternative medicines are quite prevalent, and herbal and traditional medicines such as Ayurveda (India), Siddha (South Asia), Unani (Arabic), traditional Chinese medicine, homoeopathy, acupuncture, chiropractic etc. are practiced and utilized by millions across the globe (Boedeker et al., 2005). China and India have standardized their own indigenous medicine and pharmacopoeia yet countries in Africa, despite the pressures of disease and the abundance of plant species, have not followed suit. Most of the existing texts on traditional medicine in Africa deal only with medicinal plants and their uses, ignoring chemical and pharmacological studies (Sofowora, 1993). Studies indicate that almost 80% of Africans utilize and rely on traditional medicine for their healthcare needs (Gqaleni et al., 2007; Goggin et al., 2009). Many South Africans regard traditional medicine as a safe, cheaper and desirable alternative to treating their health problems (Mander et al., 2007). Studies in South Africa have shown a link between traditional medicine and diseases such as AIDS (Green et al., 1995; King et al., 2009; Richter, 2003; UNAIDS, 2002). This is because a variety of herbal tonics that are purported to treat and cure these ailments are prepared by traditional healers. One such well-known herbal tonic is Imbiza. Imbiza is a decoction prepared from a variety of medicinal plants that are collected from wild populations or bought from informal plant traders or traditional healers (Mander, 1998). Imbiza has been used to treat ailments such as colds, chest infections, skin infections, diabetes, tuberculosis, cancer and symptoms of HIV and AIDS (UNAIDS, 2006; Ndhlala et al., 2009). Although this decoction has claimed medicinal value, it is also known to cause poisoning if the administered dosage is too high (Zikulu et al., 2012).
Cyrtanthus obliquus (Umathunga), of the plant family Amaryllidaceae, is found mostly in the Eastern Cape, South Africa. The bulbs of C. obliquus are used to treat diseases such as scrofula, leprosy and chronic coughs (Watt and Breyer-Brandwijk, 1962). Lippia javanica (uMsuzwane) of the plant family Verbenaceae is found in grasslands and woodlands, throughout the eastern and central parts of Southern Africa (Roberts, 1990; van Wyk et al., 2009). The leaves of L. javanica are commonly used to fight colds and flu and they are also used to make a caffeine-free tea (Shikanga et al., 2010). A study done by Palgrave et al. (2003) reported that the tea infusions of the leaves are used by patients in KwaZulu-Natal to treat common symptoms associated with HIV and AIDS including the treatment of lung infections and diarrhoea. C. obliquus and L. javanica are two of the five medicinal plants used to prepare the herbal tonic, Imbiza.
Since Imbiza is widely consumed by the South African population, the elemental distribution in these two plants was examined to assess their benefits and potential elemental toxicities. The study considered site location as a potential variable in elemental distribution in the plants. Since preparation of the herbal tonic, Imbiza involves boiling of the plant parts in water, the water extractable concentrations of the elements were also determined. Eleven elements were analysed which include Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Pb, Se and Zn.
2 Materials and methods
2.1 Sample collection
C. obliquus bulbs were purchased from traders at eight different market sites in KwaZulu-Natal. The chosen market sites were A1-Durban, A2-Umzinto, A3-Eshowe, A4-Verulam, A5-Stanger, A6-Tongaat, A7-KwaNkalokazi and A8-Ixopo (Fig. A.1). L. javanica leaves were collected from ten different sites in KwaZulu-Natal. The chosen sites were B1-Amandawe, B2-Eshowe, B3-Ntumeni, B4-Mangeti, B5-Maphumulo, B6-Stanger, B7-Ndwedwe, B8-Ixopo, B9-KwaNkalokazi and B10-Highflats (Fig. A.2). Plant and soil samples were collected at the beginning of April 2012; the average temperature of the five day sampling period was 27 °C with no rain or wind but sunshine during this time. All plant samples were washed with double distilled water. Bulb samples were cut into smaller pieces with a stainless steel knife. All samples (both plant and soil) were then dried in an oven at 50 °C, overnight. Dried samples were crushed into a fine powder using a food processor and stored in labelled polyethylene bags until analysed.
2.2 Reagents and chemicals
All chemicals used were supplied by Merck and Sigma Chemical Companies and were of analytical-reagent grade. Double distilled water was used throughout the experiments. Working standards were made up with double distilled water and 10 mL of 70% HNO3 to match the sample matrix. To minimize the risk of contamination all glassware and other equipment were cleaned with 6.0 M HNO3 and rinsed off with double distilled water.
2.3 Extraction of water soluble metals
In accordance with the conventional method provided by the traditional healers, C. obliquus bulbs (0.375 g) were placed into a 200 mL beaker containing 50 mL of deionised water and brought to a boil (at medium heat) for 10 min on a hotplate. The resulting solution was filtered by gravity into a 50 mL volumetric flask and made up to the mark with distilled water. The solution was then transferred into polyethylene bottles. L. javanica leaves (0.200 g) were prepared in a similar manner and the resultant solution was stored in polyethylene bottles. All extractions were done in quintuplicate (n = 5).
2.4 Elemental analysis
The bulb and leaf samples were digested prior to analysis using the microwave-assisted closed vessel technique. Digestions were performed using the CEM MARS (CEM Corporation, USA) microwave reaction system with patented Xpress technology. Five replicates of each sample were digested to improve accuracy and precision. Plant samples (0.5 g) were accurately weighed into 50 mL liners to which 10 mL of 70% HNO3 was added. For digestion, the power was set at 100% at 1600 W and the temperature was ramped to 180 °C (ramp time 15 min) where it was held for 15 min. Digested samples were removed from the liners, transferred into 50 mL volumetric flasks, diluted to the mark with double distilled water and stored in polyethylene bottles for elemental analysis.
All plant and soil samples were analysed for the following elements As, Ca, Cd, Co, Cr, Cu, Fe, Mg, Mn, Ni, Pb, Se and Zn. Elemental analysis was carried out by Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) using the Perkin Elmer Optima 5300 DV. The analysis of a sample was done in triplicate. The accuracy of the elemental determination was measured by the use of the certified reference material (CRM), lyophilized brown bread (BCR 191), from the Community Bureau of Reference of the Commission of the European Communities. All samples were analysed in quintuplicate (n = 5).
3 Results and discussion
3.1 Elemental composition of C. obliquus bulbs
The accuracy of the method for elemental analysis was measured by comparing results obtained with certified results (Table B.1). Values for Cu, Fe, Mn and Zn are certified and those for Ca and Mg are indicative so no standard deviations were provided. Measured values compared well with certified results.
Fig. A.3 shows the total and the water extractable concentrations of the elements detected in C. obliquus bulbs. High concentrations of Ca and Mg were found in the bulbs. Both these elements are said to be amongst the most abundant in plants (Broadley et al., 2008; Sedaghathoor et al., 2009). Calcium in C. obliquus bulbs ranged from 3022 to 4617 μg g−1, which is comparable to typical Ca concentrations in plants (5000 μg g−1) (Epstein, 1994). Magnesium concentrations in the bulbs ranged from 506 to 1316 μg g−1. Bulbs from site A1 had the highest concentration of Ca and those from site A6 had the highest total concentration of Mg. The exact location from which the bulbs were obtained in the Eastern Cape is not known, as these were purchased from traders who obtained them from suppliers. However, the variance in concentrations in the bulbs from diverse market sites suggests that the bulbs were obtained from sites with differing soil properties.
The minor elements that were investigated were Cr, Cu, Fe, Mn, Pb, Se and Zn. Of the minor elements studied, Fe concentrations were high in the bulbs. Iron in plants is reportedly stored by a specialized protein, ferritin which helps plants cope with high levels of the element (Connolly and Guerinot, 2002; Briat et al., 2010). Sites A3, A4 and A8 had high concentrations of Fe in the bulbs, while sites A5 and A6 had lower concentrations (Fig. A.3). The distribution of Cr, Cu, Se and Pb (Table B.4) in the bulbs was somewhat similar at all sites except for sites A5, A6 and A1, where no Cr or Pb was detected. The concentrations of Mn and Zn varied at the different sites. Manganese concentrations were highest at site A1 and lowest at site A5. The variation in Mn concentration was not as significant as that in Zn where Zn concentrations at the different sites ranged from 8.2 to 42.2 μg g−1. Site A3 had the highest concentration of Zn. The concentrations of the elements in C. obliquus bulbs were, typically, in the decreasing order of Ca > Mg > Fe > Zn > Mn > Cu = Se > Pb > Cr.
Concentrations of elements Ca, Fe, Mg, Mn and Zn in C. obliquus bulbs were compared to concentrations of these elements in the water extracts which more closely represents concentrations in the herbal tonic, Imbiza (Fig. A.3). The extraction percentages of the elements were also determined to ascertain what fraction of the elements from the bulbs was extracted into solution. If present in the water extracts, Cr, Cu, Pb and Se were below the instrument detection limits. Calcium concentrations extracted from the bulbs at all sites were relatively low. The extraction percentages ranged from 9.2% to 31.4% (Table B.2). The extraction percentage for Mg ranged from 17.7% to 77.6% with relatively high extraction percentages at most sites. Although the extraction percentages for Mg were high, the concentration of Mg in the solution was still lower than Ca. The concentrations of Ca and Mg extracted are within acceptable limits for human consumption. Extraction percentages of Fe at all sites ranged from 35.8% to 81.4% with more Fe being extracted from bulbs obtained at sites A4 and A8. The percentage of Mn extracted was between 11.0% and 49.3%. The percentage of Zn extracted was relatively high compared to the other elements with extraction percentages ranging from 30.9% to 91.5%. For most sites, extracted Zn more closely resembled bulb concentrations (Fig. A.3). The concentrations of extracted Zn are within acceptable limits for human consumption.
The extraction percentages give an indication of what fraction of the elements from the bulbs is transferred to the solution used to make the herbal tonic, Imbiza. For the detected elements, the concentrations of elements in the water extracts were, generally, in the decreasing order of Ca > Mg > Fe > Zn > Mn.
3.2 Elemental composition of L. javanica leaves
Total concentrations of selected elements in L. javanica leaves and water extracts from ten different sites in KZN are shown in Fig. A.4. The plant was located in the forest edges, where it is known to grow abundantly. The climate was humid and subtropical. The soil was generally sandy or loamy in texture. The total concentration of Ca in the leaves ranged from 2856 to 9225 μg g−1 and Mg ranged from 1598 to 5619 μg g−1. The ratio of Ca to Mg in the leaves at most sites was 1:3. According to Sedaghathoor et al. (2009), Ca and Mg are amongst the most abundant elements in tea plants.
Concentrations of the minor elements were dependant on site location. Iron in the leaves at Site B7 was relatively high compared to the other sites. Lead in the leaves from different sites was within a small range of variation and between 0.38 and 1.18 μg g−1. Selenium was detected in leaves at seven sites with concentrations ranging from 0.86 to 4.96 μg g−1 (Table B.5). Zinc in the leaves ranged from 15.6 to 27.3 μg g−1. For the detected elements, the concentrations of elements in L. javanica leaves were typically in the decreasing order of Ca > Mg > Fe > Zn > Mn > Cu > Se > Cr > Pb > Co > Cd.
The elements extracted into water from L. javanica leaves were Ca, Cr, Cu, Fe, Mg, Pb and Zn. If present in solution, Cd, Co, Mn, and Se were below the instrument’s detection limits. Calcium concentrations in L. javanica leaves were high, but the amount extracted was considerably lower. The extraction percentage was between 16.2% and 23.4% (Table B.3), which was similar to that obtained for C. obliquus bulbs. Even though the extraction percentages are similar, the concentrations of the element in the plants differ significantly with higher Ca concentrations in L. javanica leaves than C. obliquus bulbs. The extraction percentage for Mg was between 31.4% and 37.3% in the leaves which was also similar to that obtained for C. obliquus bulbs. Again, the concentrations of the element in the plants differ significantly with higher Mg concentrations in L. javanica leaves than C. obliquus bulbs.
The extraction percentage for Cr ranged from 71.8% to 93.9%, which is relatively high. This indicates that more than 70% of Cr from the leaves will be extracted into solution and consumed. However, Cr is considered a safe nutrient as there are no observed adverse effects even with intakes 300 times the acceptable limit and vomiting is likely to expel Cr before any toxicity damage can occur (Dourson, 1994). Copper concentrations in the leaves were relatively low and for the very low concentrations Cu was not extracted by water (Fig. A.4). When Cu was extracted, the percentage ranged from 21.5% to 48.8%.
The extraction percentage for Fe from L. javanica leaves was between 23.1% and 28.7% which was lower than that obtained for C. obliquus bulbs (44.6% to 61.5%). The extraction percentage estimated for Pb was 37.0% to 68.8%; a very wide range was obtained probably due to the extremely low concentrations in the leaves. For most sites, Zn in leaves was more than double the extracted amount and the extracted percentage for Zn ranged from 29.1% to 89.1%.
For the detected elements, the concentrations of elements in the water extracts were, generally, in the decreasing order of Ca > Mg > Fe > Zn > Cu > Cr > Pb.
4 Conclusion
The elemental composition of C. obliquus bulbs was determined. The study revealed that the concentration of elements Ca, Cr, Cu, Fe, Mg, Mn, Pb, Se, and Zn was controlled and variations according to site location were moderate. A high concentration of Ca and Mg as well as high percentage of Zn was extracted into water from the bulbs.
Concentrations of elements in L. javanica leaves and growth soil were determined. Concentrations of elements, Cd, Cr, Co, Cu, Fe, Mn, Pb, and Zn present in leaves were moderate. A high concentration of Ca and Mg, similar to C. obliquus bulbs, and a high percentage of Cr were extracted into water from the leaves.
This study shows that a fair amount of essential elements are extracted into the herbal tonic, Imbiza from C. obliquus bulbs and L. javanica leaves. Those concentrations are acceptable, when consumed can contribute positively to human health. Furthermore, only low levels of toxic metals were found in the herbal tonic.
Acknowledgements
The authors would like to thank the University of KwaZulu-Natal (UKZN) and the National Research Foundation (NRF) for financial support.
References
- WHO global atlas of traditional, complementary and alternative medicine. WHO Kobe Center; 2005.
- Shoot calcium and magnesium concentration differ between subtaxa, are highly heritable and associate with potentially pleiotropic loci in Brassica oleracea. Plant Physiol.. 2008;146:1707-1720.
- [Google Scholar]
- The chromium reference doses (RfD) In: Mertz W., Abernathy C.O., Olin S.S., eds. Risk Assessment of Essential Elements. Washington, DC: ILSI Press; 1994. p. :207-212.
- [Google Scholar]
- The role of South African health practitioners in HIV/AIDS prevention and treatment. In: Pope C., White R., Malow R., eds. HIV/AIDS Global Frontiers in Prevention and Intervention. New York: Routledge Taylor and Francis; 2009.
- [Google Scholar]
- Harrison S., Bhana R., Ntuli A., eds. Traditional and Complementary Medicine. South African Health Review; 2007. p. :175-185.
- The experience of an AIDS prevention programme focused on South African traditional healers. Soc. Sci. Med.. 1995;40:503.
- [Google Scholar]
- Developing pathways and partnership. In: Marlink R.G., Teitelman S.J., eds. From the Ground up: The Role of Traditional Healers in Comprehensive HIV/AIDS Prevention and Care in Africa: Untapped Opportunities. Washington, DC: Elizabeth Glaser Pediatric AIDS Foundation; 2009. p. :301-323.
- [Google Scholar]
- Marketing of Indigenous Medicinal Plants in South Africa, A Case Study in KwaZulu-Natal. Rome: Food and Agriculture Organization of the United Nations; 1998.
- Harrison S., Bhana R., Ntuli A., eds. Economics of the Traditional Medicine Trade in South Africa. South African Health Review; 2007. p. :189-200.
- In vitro pharmacological effects of manufactured herbal concoctions used in KwaZulu-Natal, South Africa. J. Ethnopharmacol.. 2009;122:117-122.
- [Google Scholar]
- Trees of Southern Africa (third ed.). Cape Town: Struik Publishers; 2003.
- Richter, M., 2003. Traditional Medicine and Traditional Healers in South Africa. Discussion Paper Prepared for the Treatment Action Campaign and AIDS Law Project, 4–47.
- Yields and quality of response of tea plants to fertilizers. Afr. J. Agric. Res.. 2009;4:568-570.
- [Google Scholar]
- South African Lippia herbal infusions: total phenolic content, antioxidant and antibacterial activities. S. Afr. J. Bot.. 2010;76:567-571.
- [Google Scholar]
- Medicinal Plants and Traditional Medicine in Africa. New York: John Wiley Ltd.; 1993. pp 289
- United Nations Programme on HIV/AIDS, 2002. Collaboration with traditional healers in HIV/AIDS prevention and care in Sub-Saharan Africa. A literature review, Geneva, Switzerland.
- United Nations Programme on HIV/AIDS, 2006. AIDS epidemic update, pp. 1–69.
- Medicinal plants of South Africa. Pretoria: Briza; 2009.
- The Medicinal and Poisonous Plants of Southern and Eastern Africa. London: E. and S. Livingston Ltd.; 1962.
Appendix A
See Figs. A.1–A.4.
Map of eight chosen market sites in KwaZulu-Natal, South Africa, from which C. obliquus bulbs were purchased. Sites: A1-Durban, A2-Umzinto, A3-Eshowe, A4-Verulam, A5-Stanger, A6-Tongaat, A7-KwaNkalokazi and A8-Ixopo.
Map of ten chosen sites in KwaZulu-Natal, South Africa, from which L. javanica leaves and soil samples were collected. Sites: B1-Amandawe, B2-Eshowe, B3-Ntumeni, B4-Mangeti, B5-Maphumulo, B6-Stanger, B7-Ndwedwe, B8-Ixopo, B9-KwaNkalokazi and B10-Highflats.
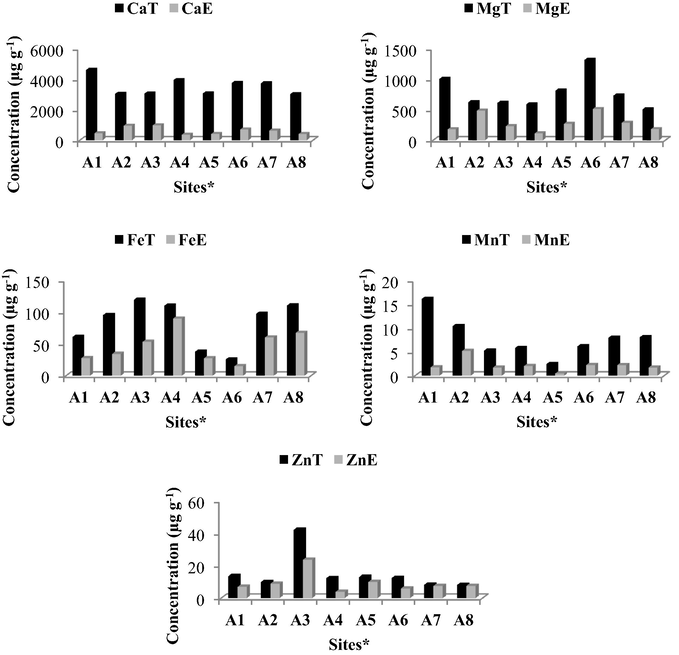
Total (T) concentration of Ca, Mg, Fe, Mn and Zn in bulbs compared to concentration in water extract (E) Sites∗: A1-Durban, A2-Umzinto, A3-Eshowe, A4-Verulam, A5-Stanger, A6-Tongaat, A7-KwaNkalokazi and A8-Ixopo.
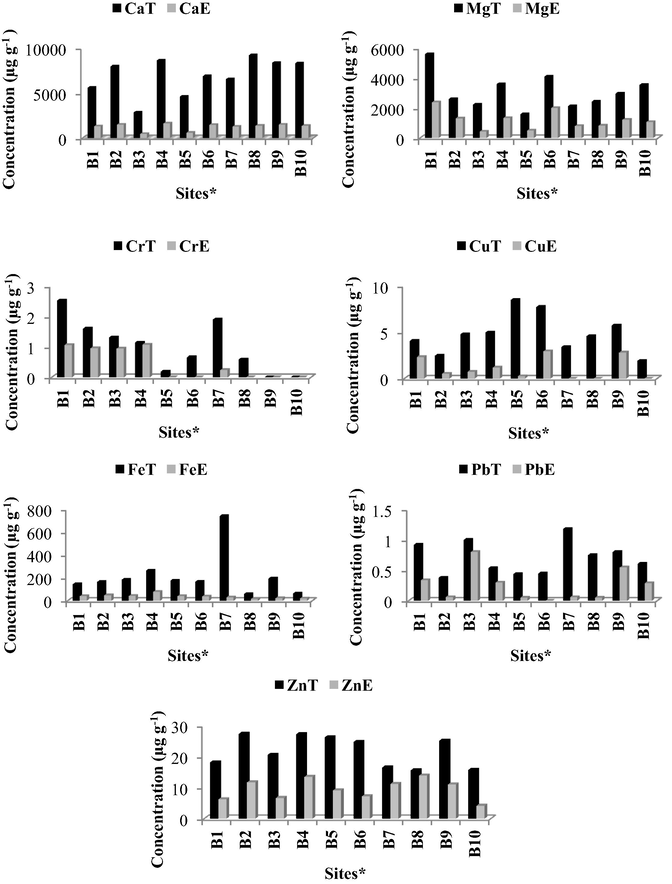
Total (T) concentration of Ca, Mg, Cr, Cu, Fe, Pb and Zn in leaves compared to concentration in water extract (E). Sites∗: B1-Amandawe, B2-Eshowe, B3-Ntumeni, B4-Mangeti, B5-Maphumulo, B6-Stanger, B7-Ndwedwe, B8-Ixopo, B9-KwaNkalokazi and B10-Highflats.
Appendix B
See Tables B.1–B.5. ND: not determinable. ND: not determinable.
Element
Wavelength/nm
Concentration⁎
Certified⁎⁎
Measured⁎⁎
Cu
324.76
2.6 ± 0.1 μg g−1
2.8 ± 0.2 μg g−1
Fe
259.93
40.7 ± 2.3 μg g−1
40.6 ± 1.9 μg g−1
Mn
257.61
20.3 ± 0.7 μg g−1
20.1 ± 0.6 μg g−1
Zn
260.20
19.5 ± 0.7 μg g−1
19.0 ± 0.5 μg g−1
Ca
317.93
0.41 mg g−1
0.41 ± 0.02 mg g−1
Mg
279.07
0.5 mg g−1
0.5 ± 0.01 mg g−1
Element
Sitea
[WE]/[B] %
Ca
A 1
9.9
A 2
31.4
A 3
15.8
A 4
9.2
A 5
13.3
A 6
18.8
A 7
17.0
A 8
13.4
Fe
A 1
45.6
A 2
35.8
A 3
44.6
A 4
81.4
A 5
72.9
A 6
58.5
A 7
61.5
A 8
60.8
Mg
A 1
17.8
A 2
77.6
A 3
37.8
A 4
19.2
A 5
33.1
A 6
38.8
A 7
39.3
A 8
36.0
Mn
A 1
11.0
A 2
49.3
A 3
32.8
A 4
35.0
A 5
18.7
A 6
36.8
A 7
28.1
A 8
21.3
Zn
A 1
51.1
A 2
89.9
A 3
55.9
A 4
30.9
A 5
77.5
A 6
47.6
A 7
91.5
A 8
85.2
Element
Sitea
[WE]/[L] %
Ca
B 1
23.4
B 2
18.8
B 3
16.2
B 4
18.9
B 5
12.9
B 6
21.5
B 7
19.5
B 8
14.9
B 9
17.9
B 10
16.4
B 1
42.1
Cr
B 2
59.4
B 3
71.8
B 4
93.9
B 5
–
B 6
–
B 7
12.6
B 8
–
B 9
–
B 10
–
Cu
B 1
57.4
B 2
21.5
B 3
15.5
B 4
24.1
B 5
3.1
B 6
37.9
B 7
–
B 8
–
B 9
48.8
B 10
–
Fe
B 1
27.9
B 2
28.7
B 3
23.0
B 4
29.6
B 5
23.1
B 6
23.1
B 7
4.1
B 8
27.5
B 9
11.7
B 10
31.7
Mg
B 1
42.6
B 2
50.2
B 3
19.4
B 4
37.3
B 5
31.4
B 6
49.1
B 7
37.3
B 8
34.1
B 9
41.3
B 10
30.2
Pb
B 1
37.0
B 2
15.8
B 3
80.0
B 4
55.6
B 5
11.4
B 6
–
B 7
5.1
B 8
6.7
B 9
68.8
B 10
47.5
Zn
B 1
34.6
B 2
42.9
B 3
32.5
B 4
49.6
B 5
34.7
B 6
29.1
B 7
67.9
B 8
89.1
B 9
43.8
B 10
26.8
Elements
Sitesa
Concentration/μg g−1
Cr
A 1
0.202 ± 0.005
A 2
0.340 ± 0.055
A 3
0.640 ± 0.055
A 4
0.140 ± 0.055
A 5
ND
A 6
ND
A 7
0.360 ± 0.055
A 8
0.110 ± 0.022
Cu
A 1
4.06 ± 0.09
A 2
2.68 ± 0.29
A 3
3.84 ± 0.11
A 4
2.16 ± 0.21
A 5
2.26 ± 0.15
A 6
1.76 ± 0.27
A 7
1.30 ± 0.07
A 8
3.62 ± 0.15
Mn
A 1
16.12 ± 0.41
A 2
10.44 ± 0.48
A 3
5.24 ± 0.21
A 4
5.80 ± 0.22
A 5
2.46 ± 0.44
A 6
6.14 ± 0.11
A 7
7.96 ± 0.09
A 8
8.06 ± 0.45
Pb
A 1
ND
A 2
0.54 ± 0.05
A 3
0.66 ± 0.09
A 4
0.42 ± 0.08
A 5
0.42 ± 0.08
A 6
0.46 ± 0.05
A 7
0.56 ± 0.05
A 8
0.36 ± 0.05
Se
A 1
2.68 ± 0.24
A 2
3.24 ± 0.24
A 3
1.06 ± 0.21
A 4
4.18 ± 0.18
A 5
2.42 ± 0.26
A 6
1.90 ± 0.27
A 7
0.90 ± 0.07
A 8
3.90 ± 0.34
Elements
Sitesa
Concentration/μg g−1
Cd
B 1
0.39 ± 0.03
B 2
0.64 ± 0.06
B 3
0.56 ± 0.06
B 4
ND
B 5
ND
B 6
ND
B 7
ND
B 8
ND
B 9
ND
B 10
ND
Co
B 1
0.20 ± 0
B 2
0.10 ± 0
B 3
0.20 ± 0
B 4
0.20 ± 0
B 5
ND
B 6
0.26 ± 0.02
B 7
0.19 ± 0.01
B 8
ND
B 9
ND
B 10
ND
Mn
B 1
18.9 ± 0.9
B 2
15.1 ± 1.0
B 3
45.1 ± 1.0
B 4
58.4 ± 1.6
B 5
13.6 ± 0.3
B 6
60.4 ± 0.4
B 7
34.1 ± 1.4
B 8
57.4 ± 3.1
B 9
64.2 ± 1.4
B 10
33.7 ± 0.5
Se
B 1
3.34 ± 0.37
B 2
4.96 ± 0.30
B 3
1.68 ± 0.17
B 4
1.58 ± 0.11
B 5
3.40 ± 0.16
B 6
2.20 ± 0.16
B 7
0.86 ± 0.09
B 8
ND
B 9
ND
B 10
ND







