Translate this page into:
Elucidation of potential anticancer, antioxidant and antimicrobial properties of some new triazole compounds bearing pyridine-4-yl moiety and cyclobutane ring
⁎Corresponding author. akifevren@hotmail.com (Akif Evren Parlak),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Thiosemicarbazides (2a–e) were obtained by the interaction of pyridine-4-carboxylic acid hydrazide (1) with five different isothiocyanate (RNCS) derivatives. By addition of KOH to the reaction medium, ethyl, allyl, phenyl and benzyl, p-tolyl substituted 1,2,4-triazoles (3a–e) were obtained. 3a–e were dissolved in dry acetone containing K2CO3 in the presence of 2-chloro-1-(3-methyl-3- mesitylcyclobutyl) ethanone (4) to give 3,4,5-trisubstituted 1,2,4-triazole sulfanyl compounds containing a cyclobutane ring (5a–e). The structures of the final compounds were confirmed by elemental analyses, FT-IR, 1H NMR and 13C NMR. Due to many activities regarding pharmacology, Compounds such as 1,2,4-triazole attract high attention in the fields of chemistry, pharmacology, and biology. All the newly synthesised compounds were screened for their Antioxidant, antimicrobial, anti-cancer activities. The measurement of the antioxidant feature of the compounds was performed by DPPH radical scavenging activity technique. It was observed that the activity of some of the compounds was close to the standard antioxidant BHT. Furthermore, the anticancer effects of compounds were investigated against HT29 human colon adenocarcinoma cells. It has been noted that all -compounds inhibited cancerous cells in a statistically significant compared to the control. For the measurement of antimicrobial activity Agar well diffusion method was preferred. For the antimicrobial study, S. aureus, E. faecalis, P. aeruginosa, E. coli, and a single fungi C. albicans have been used. They could also be stated as ATCC 29213, 29212, 27853, 2592, and 10231 respectively. The resulting range of MIC values for the chemicals was between 15.625 - >125 µM. In conclusion; all compounds have shown various and important bioactivities at different levels, such as being antioxidant, antimicrobial, and anticancer.
Keywords
1,2,4-Triazole
Anticancer
Antioxidant
Antimicrobial
Biological activities
1 Introduction
In recent years, a significant increase has been experienced in terms of the number of compounds with biological activities (Del-Toro-Sánchez et al., 2021). However, the use of these caused to search for alternatives having a high rate of drug resistance, toxicity risk, and difficulties of application. In addition, the observation of undesirable side effects was limited due to lack of activity and lack of pharmacokinetics. This necessitates the continual synthesis of new agents in scientific work in recent years. The main structure of most compounds that are used today as drugs, especially triazole, thiadiazoles, and oxadiazole, contains a five-membered heterocyclic ring structure. According to the arrangement of heteroatoms in the ring, there are various isomers of these rings. However, the most biologically active isomers among these isomers are as follows; 1,3,4-thiadiazole, 1,3,4- oxadiazole, and 1,2,4-triazole. The fact that triazole derivatives have potential application areas such as the ability to form multimolecular ligands, to be used as drug active ingredients, pesticides, artificial acceptor (Zhou and Wang, 2012; Sathish Kumar and Kavitha, 2013; Pereira et al., 2021) makes these substances a very attractive field of research. A triazole ring having three nitrogen atoms and a system enriched in terms of electrons is significant as a heterocyclic compound having five members. When we consider its characteristics, it has aromatic features as well. Derivatives of triazole has the ability to bond enzymes and receptors in the biological system. While performing such binding, it uses coordination linkage, ion–dipole force, cations-π, π-π, Van der Waals bonds, and hydrophobic effect. As a result, these compounds reveal various biological effects (Mi et al., 2008, Zhou and Wang, 2012; Küçükgüzel and Çıkla-Süzgün, 2015). Another characteristic of triazole compounds is the ability to form multimolecular structures (Rodriguez-Fernandez et al., 2005; Liu et al., 2011). Therefore, special attention is paid to triazole compounds, especially during new multi-molecule drug development (Zhou et al., 2009; Zhou et al., 2010; Castro et al., 2012; Parchenko et al., 2012).
Moreover, triazole ring could also be preferred as a tool for binding in forming bi-functional drug molecules and for combining different pharmacophore moieties. Thus, it provides a usable and efficacy way to improve different biologically active and practical compounds.
Triazoles are used in the pharmaceutical industry, as an anti-tuberculosis, in the paint industry, production of agricultural chemicals, obtaining photographic materials, and anti-corrosion materials, but one of the most important areas of their use is undoubtedly the area of chemotherapy. These compounds containing five-membered rings have a very comprehensive active biological spectrum and are particularly useful in anticancer activity, also as an antifungal, anti-HIV, antitumor, antibacterial, antiviral, antidepressant, anti-inflammatory, anti-tuberculosis, analgesic, diuretic substances (Tozkoparan et al., 2000; Turan-Zitouni et al., 2001; Cottineau et al., 2002; Olesen et al., 2003; Mocharla et al., 2005; Shiradkar et al., 2007; Sztanke et al., 2008; Bakunov et al., 2010; Khan et al., 2010; Menendez et al., 2011; Patpi et al., 2012; Khan et al., 2014).
Cyclobutane derivatives are widely used (Omer et al., 2022). For example, phenyl cyclobutane carboxylic acid is one of the esters of estrogenic prostaglandins. According to the literature, a study (Roger et al., 1977) has found that compounds of naphthalene and its derivatives containing cyclobutane are biologically active compounds in the structure. These compounds have been used against neurological diseases. It has also started to be used instead of widely used reserpine medicine. It is fact that cyclobutane carboxylic acid derivatives shows the sedative characteristics of antidepressants (Roger et al., 1977). Also, cyclobutane derivatives are known to have anti-inflammatory and anti-depressant activities (Dehmlow and Schmidt, 1990).
It has been noted that pyridine compounds are useful as being anti-cancer (Abbas et al., 2015; Mosaad et al., 2012) anti-microbial (Butnariu et al., 2007; Gaonkar et al., 2007; Kandile et al., 2009; Bhardwaj et al., 2016) and anti-fungal (Rajput and Sharma, 2011), anti-inflammatory and analgesic (Dogruer et al., 2007; Hosni and Abdulla, 2008; Gökçe et al., 2009), anti- hiv (Ali et al., 2007), anti-plasmodial (Kumar et al., 2008), anti-tubercular (Lourenco et al., 2007), antibacterial (Sharma and Jain, 2008), anticonvulsant (Rubat et al., 1990; Rastkari and Sharifzadeh, 2004), COX inhibitor (Chintakunta et al., 2002), antidiabetic (Rathish et al., 2009), antihypertensive (Barbaro et al., 2001; Joule et al., 2020), anticancer (Malinka et al., 2004; Brana et al., 2005; Atanasova et al., 2007; Karki et al., 2010), platelet aggregation inhibitors, anxiolytic (Griebel et al., 1999; Sotelo et al., 2002), antioxidant (Wermuth et al., 1989; Chen et al., 1997; Zhang et al., 1999; Çalişkan-Ergün et al., 2008), antiviral and antitumor (Blanco et al., 1999; Graf-Christophe et al., 2000). To illustrate, isoniazid and amlodipine contain pyridine in terms of being anti-tuberculosis and anti-hypertensive. Furthermore, antiviral, antineoplastic, antiproliferative effects of derivatives of the cyclobutane ring with other biologically active compounds and the contribution of the cyclobutane group to the enrichment of these properties are being tried to be explained (Blanco et al., 1999; Graf-Christophe et al., 2000). Based on the above findings, the pyridine structure is regarded as a distinctive structure and consequently attracted the general and continuous interest of synthetic organic chemists.
Because of these biological and pharmacological effects, it is envisaged that compounds containing cyclobutane, triazole, and pyridine in the structure will be used especially in the pharmaceutical industry. For this purpose, pyridine based 1,2,4-triazole compounds, which are not found in the literature, have been synthesized and investigated with biological potential.
Recently, cancer has become one of the most alerting diseases for human beings. and the prevalence of cancer has been increasing and definite treatment for cancer has not been found yet. New cancer drugs are being developed with billions of dollars spent on cancer treatment, but some of them are out of use due to serious side effects and durability. For this reason, new drug compounds with high therapeutic efficacy and low side effects are still under study for molecular synthesis and have become a focus of interest for scientists. The prevalence of colon cancer is seen highly among gastrointestinal diseases. Nearly, 10% of the cases of cancer seen in males and females is to be colorectal cancer (Jemal et al., 2009). These cancers are also caused by mitochondrial mutations as well as genomic mutations (Aral and Özer, 2007; Gürbüz et al., 2011). Chemotherapy is a very important and basic treatment for colon cancer (Segal and Saltz, 2009).
In recent years, research in chemistry and biochemistry has been accelerated to explain behaviors (platinum group metal complexes) as cytotoxic markers, speeding up the search for platinum and various transition metals as new antitumor markers, and continuing to study new metal complexes that are not cisplatin but have properties of antitumor compounds. Cisplatin is used in the treatment of neoplastic diseases and its effect depends on its interaction with Dna (Stubbert et al., 2010). Cis-platinum, carboplatin, oxaplatin, nedaplatin, and lobaplatin compounds are known as the main compounds of important compounds. However, clinical applications have shown serious side effects of cisplatin anti-carcinogens (Khan et al., 2011). For this purpose, new searches are ongoing. New quests are underway for this purpose. Alternative searches continue because side effects of compounds used in cancer therapy limit their practice.
Various studies regarding the anticancer effect of 1,2,4-triazole ringed derivatives in the literature are inconsistent with the results of our study. It was emphasized in the study that compounds having 1,2,4-triazole pyridine rings are potent anti-cancer agents due to in vitro activities (Wang et al., 2013, Qin et al., 2014). It has been noted that 2-(4-(2-(dimethylamino) ethyl)-4H-1,2,4-triazole-3-pyridine derivatives were revealed in-vitro cytotoxic activity for five different types of cancer cells including MKN-45, H460, HT-29, A549, and U87MG in addition to normal ones (Qin et al., 2014). In another study, triazole derivative compounds were reported to be potent anti-cancer as the result of using the method of MTT. This technique has been frequently preferred as a test for enzymes in terms of the evaluation of cytotoxicity, in which cytotoxic effects were examined in HCT-116, U-87-MG, and MCF-7 cancer cells. The researchers have noted compounds having 1,2,4-triazole pyridine rings were considered antiproliferative as a result of in vitro activity and that they were potent anti-cancer agents (Wang et al., 2013). In a study, it was determined that HT29 cells were more than 75% cell viability after 3 days when 4 μM (12 μg /ml) dose of cisplatin was administered (de Mattos et al., 2008). In another study conducted by Suzuki et al., it has been reported the IC50 value when cisplatin was administered to DLD1 cells, the colorectal cancer cell line, was 78.4 μM (Suzuki et al., 2010). Another study found that when cisplatin was administered to HT29 cells for 72 h, the dose of IC50 corresponding to the dose required to kill half of the cells in 75 µM concentration was reached (Gürbüz et al., 2011).
The heterocyclic compounds such as cyclobutane derivatives, 1,2,4-triazole, and pyridine rings have shown satisfactory antioxidant and cytotoxicity. It seems that the hybridization of these skeletons would also create more effective agents. In this work, we focused on the design, synthesis, and evaluation of new cyclobutane containing triazoles derivatives (5a-e) as anticancer, antimicrobial, and antioxidant agents.
The purpose of the study is to perform the synthesis of five new compounds by using methods found in academic reviews as cyclobutane derivative 1,2,4-triazole series (5a-e) with the pyridine ring. The process of synthesis was performed on the compounds and featured experimentally via IR, 1H NMR, 13C NMR methods. In addition, antioxidant, antimicrobial, and anticancer properties of synthesized compounds were examined.
2 Materials and methods
2.1 Chemicals properties
The hydrogen nuclear magnetic resonance (1H NMR) and 13-carbonnuclear (13C NMR) were performed on Bruker ascend 400 nuclear magnetic resonance spectrometers at the speed of 400 MHz for 1H and the speed of 100 MHz for 13C. Deuterated dimethyl sulfoxide (DMSO‑d6) was used for dissolving the NMR compound. The examination in terms of elemental was carried out on a LECO-CHNS-938. The chemicals used were supplied from Merck. Thiosemicarbazides compounds (2a–e), 4-(aryl–alkyl)-5- (2-furanyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (3a–e) and 2-chloro-1-(3-Methyl-3-mesityl-cyclobutyl)-ethanone (4) were synthesized according to the general procedure described earlier (Koparir et al., 2011; Koparir, 2019). These five test compounds (5a-e) are given in Table 1 and Supplemental File (IR, 1H NMR and 13C NMR). The synthesis and mechanism of test compounds are given in Scheme 1.
Code of Compounds
Nomenclature of compounds
Description
5a
1-(3-Methyl-3-mesityl)-cyclobutyl-2-{(5-(pyridine-4-yl)-4-ethyl-4H-[1,2,4]triazol-3-yl)sulfanyl}-ethanone
Yield 67%; m. p. 150–152 °C; FT-IR (KBr, cm−1, ʋ): 2945–2982 (Ar-H), 2870–2930 (C-H), 1701 (C = O), 1432 (C = C), 1386 (C = N), 1125 (C-N), 1042 (N-N), 713 (C-S); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 1.26 (t, 3H, N-CH2-CH3, J = 7.1 Hz), 1.50 (s, 3H, –CH3 (cyclobutane)), 2.14 (s, 9H, –CH3 (mesityl)), 2.42–2.54 (m, 4H, –CH2- (cyclobutane)), 3.58 (p, 1H, –CH- (cyclobutane), J = 8.80 Hz), 4.09 (q, 2H, N-CH2-CH3, J = 6.9 Hz), 4.35 (s, 2H, S-CH2-), 6.70 (s, 2H, Ar-H (mesityl)), 7.68 (d, 2H, Ar-H, J = 4.5 Hz, C = CH aromatic (pyridine)), 8.76 (d, 2H, Ar-H, J = 4.4 Hz, N = CH aromatic (pyridine)). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 15.4, 20.5, 21.4, 25.2, 39.1, 40.3, 41.2, 122.7, 130.5, 134.4, 143.7, 150.6, 151.7, 153.1, 205.3. Calcd. for 434.59 (C25H30N4OS). (%):C, 69.09; H, 6.96; N, 12.89; S, 7.38; found (%): C, 69.01; H, 6.90; N, 12.85; S, 7.45.
5b
1-(3-Methyl-3-mesityl)-cyclobutyl-2-{(5-(pyridine-4-yl)-4-allyl-4H-[1,2,4]triazol-3-yl)sulfanyl}-ethanone
Yield 72%; m. p. 115–117 °C; FT-IR (KBr, cm−1, ʋ): 2950–2990 (Ar-H), 2862–2914 (C-H), 1710 (C = O), 1435 (C = C), 1395 (C = N), 1126 (C-N), 1013 (N-N), 705 (C-S); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 1.49 (s, 3H, –CH3 (cyclobutane)), 2.14 (s, 9H, –CH3 (mesityl)), 2.39–2.50 (m, 4H, –CH2- (cyclobutane)), 3.57 (p, 1H, –CH- (cyclobutane), J = 8.70 Hz), 4.33 (s, 2H, S-CH2-), 4.74 (m, 2H, -N-CH2-CH-CH2), 4.86 (d, 1H, -N-CH2-CH-CH2(trans) J = 17.2 Hz), 5.25 (d, 1H, N-CH2-CH-CH2, Jcis = 9.8 Hz), 5.96–6.02 (m, 1H, N-CH2-CH-CH2), 6.70 (s, 2H, Ar-H (mesityl)), 7.64 (d, 2H, Ar-H, J = 4.2 Hz, C = CH aromatic (pyridine)), 8.75 (d, 2H, Ar-H, J = 4.4 Hz, N = CH aromatic (pyridine)). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 20.5, 21.4, 25.2, 39.1, 41.3, 47.1, 117.7, 122.4, 130.4, 132.6, 134.4, 134.9, 143.7, 150.9, 152.5, 153.6, 205.3. Calcd. for 446.6 (C26H30N4OS). (%):C, 69.92; H, 6.77; N, 12.54; S, 7.18 (%): C, 69.85; H, 6.72; N, 12.50; S, 7.25.
5c
1-(3-Methyl-3-mesityl)-cyclobutyl-2-{(5-(pyridine-4-yl)-4-phenyl-4H-[1,2,4]triazol-3-yl)sulfanyl}-ethanone
Yield 66%; m. p. 145–147 °C; FT-IR (KBr, cm−1, ʋ): 2941–3046 (Ar-H), 2863–2937 (C-H), 1709 (C = O), 1432 (C = C), 1334 (C = N), 1125 (C-N), 1035 (N-N), 699 (C-S); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 1.50 (s, 3H, –CH3 (cyclobutane)), 2.14 (s, 9H, –CH3 (mesityl)), 2.40–2.55 (m, 4H, –CH2- (cyclobutane)), 3.57 (p, 1H, –CH- (cyclobutane), J = 8.60 Hz), 4.30 (s, 2H, S-CH2-), 6.71 (s, 2H, Ar-H, (mesityl)), 7.29 (d, 2H, Ar-H, J = 4.4 Hz, C = CH aromatic (pyridine)) 7.46–7.48 (m, 2H, Ar-H), 7.59–7.63 (m, 3H, Ar-H), 8.55 (d, 2H, Ar-H, J = 4.4 Hz, N = CH aromatic (pyridine)). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 20.5, 21.4, 25.2, 39.0, 40.8, 121.9, 127.9, 130.5, 130.7, 131.0, 133.8, 134.2, 134.4, 134.9, 143.7, 150.6, 152.6, 153.4, 205.0. Calcd. for 482.6 (C29H30N4OS). (%):C, 72.17; H, 6.27; N, 11.61; S, 6.64; (%): C, 72.25; H, 6.32; N, 11.55; S, 6.57.
5d
1-(3-Methyl-3-mesityl)-cyclobutyl-2-{(5-(pyridine-4-yl)-4-benzyl-4H-[1,2,4] triazol-3-yl)sulfanyl}-ethanone
Yield 79%; m. p. 120.9 °C; FT-IR (KBr, cm−1, ʋ): 2969–3042 (Ar-H), 2854–2945 (C-H), 1716 (C = O), 1423 (C = C), 1363 (C = N), 1117 (C-N), 1036 (N-N), 698 (C-S); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 1.49 (s, 3H, –CH3 (cyclobutane)), 2.14 (s, 9H, –CH3 (mesityl)), 2.48–2.55 (m, 4H, –CH2- (cyclobutane)), 3.55 (p, 1H, –CH- (cyclobutane), J = 8.80 Hz), 4.32 (s, 2H, S-CH2-), 5.38 (s, 2H, N-CH2-C6H5), 6.70 (s, 2H, Ar-H, (mesityl)), 7.00–7.02 (m, 2H, Ar-H), 7.25–7.34 (m, 3H, Ar-H), 7.57 (d, 2H, Ar-H, J = 4.8 Hz, C = CH aromatic (pyridine)), 8.68 (d, 2H, Ar-H, J = 4.8 Hz, N = CH aromatic (pyridine)). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 20.5, 21.4, 25.2, 39.0, 41.2, 48.1, 122.5, 126.7, 128.4, 129.4, 130.5, 134.4, 134.7, 134.9, 135.5, 143.7, 150.9, 152.8, 153.7, 205.3. Calcd. for 496.7 (C30H32N4OS). (%):C, 72.55; H, 6.49; N, 11.28; S, 6.46; (%): C, 72.40; H, 6.55; N, 11.35; S, 6.55.
5e
1-(3-Methyl-3-mesityl)-cyclobutyl-2-{(5-(pyridine-4-yl)-4-(p-tolyl)-4H-[1,2,4]triazol-3-yl)sulfanyl}-ethanone
Yield 71%; m. p. 145–147 °C; FT-IR (KBr, cm−1, ʋ): 2947–3043 (Ar-H), 2865–2918 (C-H), 1694 (C = O), 1428 (C = C), 1333 (C = N), 1121 (C-N), 1035 (N-N), 694 (C-S); 1H NMR (400 MHz, DMSO‑d6, δ, ppm): 1.51 (s, 3H, –CH3 (cyclobutane)), 2.15 (s, 9H, –CH3 (mesityl)), 2.40 (s, 3H, C6H5-CH3), 2.42–2.56 (m, 4H, –CH2- (cyclobutane)), 3.57 (p, 1H, –CH- (cyclobutane), J = 8.70 Hz), 4.28 (s, 2H, S-CH2-), 6.71 (s, 2H, Ar-H, (mesityl)), 7.31 (d, 2H, Ar-H, J = 4.7 Hz, C = CH aromatic (pyridine)), 7.32–7.40 (m, 4H, Ar-H), 8.57 (d, 2H, Ar-H, J = 4.5 Hz, N = CH aromatic (pyridine)). 13C NMR (100 MHz, DMSO‑d6, δ, ppm): 20.5, 21.3, 21.4, 25.2, 39.0, 40.0, 40.8, 121.9, 127.7, 130.5, 131.2, 134.3, 134.4, 134.9, 140.8, 143.7, 150.6, 152.6, 153.4, 205.1. Calcd. for 496.6 (C30H32N4OS). (%):C, 72.55; H, 6.49; N, 11.28; S, 6.46 (%): C, 72.40; H, 6.55; N, 11.40; S, 6.39.
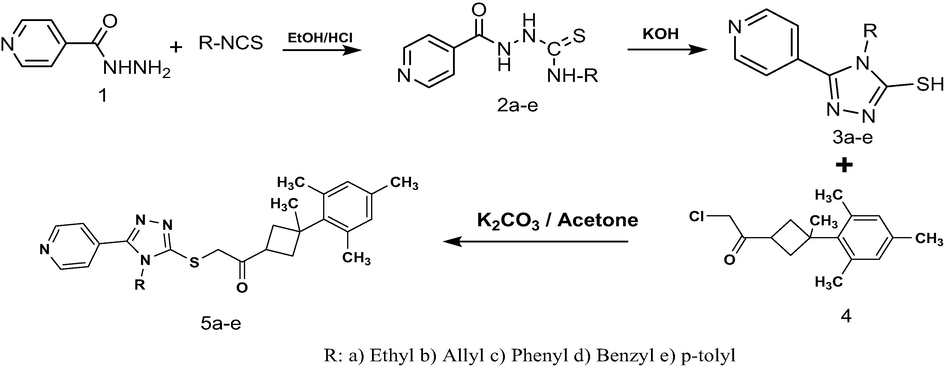
The synthesis scheme of test compounds (5a-e).
General synthesis of 1-(3-Methyl-3-mesityl)- cyclobutyl-2-(5-pyridine-2yl-4-(aryl–alkyl)-4H-[1,2,4]triazol-3-ylsulfanyl)-ethanone (5a-e): A solution of 0.445 g (2 mmol) of 2-chloro-1-(3-Methyl-3- mesityl-cyclobutyl)-ethanone (4) was dissolved in dry acetone (30 mL) containing (2 mmol) K2CO3. To this solution, (2 mmol) of 4-(aryl–alkyl)-5-(2-pyridine)-2,4-dihydro-3H1,2,4-triazole-3-thione (3a–e) in dry acetone (20 mL) was added dropwise over a 1 h period at room temperature with stirring. The resulting solid was collected by filtration, dried and recrystallized from ethyl alcohol.
2.2 Biological properties
2.2.1 Anticancer activity
The human colon adenocarcinoma cell line HT29 was used to determine anticancer activity. The frozen HT29 human colon adenocarcinoma cells which were taken from the bank of cell culture (Sigma, USA) have been thawed at room temperature. It was then taken to a flask of 75 mL. DC5 (25 mL) was already in the flask. Then the flask was put in a 5% CO2 incubator a Nuaire brand (Playmouth, MN, USA). The condition of cells was examined by means of a microscope SoFi brand (Soif Optical Inc., China) daily. A new DC5 was put instead of the previous one on the third day. This procedure was carried out for three days by specific intervals.
The number of cells has increased moving on top of each other and then they covered the surface of the flask. On the 15th day, the medium was removed, and a new 3 mL of trypsin was added and put into the incubator. Shaking was performed for the flasks nearly for 2–3 min in order to make cells separated from the basis attached. DC5 of 12 mL was added to the flask when all the cells were removed from the surface. The cells were distributed homogeneously into the solution by carefully triturating (the separation process by pulling the suspension into the pipette and emptying it).
Hemocytometer was used for cell counting. In each flask, a suspension of 1x105 was placed. Then, DC5 was mixed with them. When the volume reached 25 mL, the flasks were moved to the incubator. The cultivating, nutrition and experiments of the cells were carried out in Class II Laminar flow (Biolaf, Ankara) (Offiong and Martelli, 1997; Ferrari et al., 1999).
Centrifugation for HT29 human colon adenocarcinoma cell suspension was performed for 5 min at 2000 rpm. Hemocytometer was used for cell counting. The number of cells was identified as 1 × 105 / ml for the HT29 human colon adenocarcinoma cell experiments. Pre-trials were performed to determine the doses and the up-and-down technique was preferred. One ml was removed from the cell suspension and the agents were mixed at concentrations of 15, 30, 60 μM. Serum having the same amount was mixed in negative control tubes. DMSO was also added to the physiological vehicle tubes. They were exposed to incubation. DMSO was about 1% in cell suspensions. 24 h later, incubation was stopped and pulverized. 100 cells were taken in a random manner in 1:1 (v/v) and then counted with 0.4% trypan blue in hematocytometer. The percentage was used as showing the value of cell viability. The same procedure applied after 48 h, and the test has been terminated (Kumamoto et al., 1990).
2.2.2 DPPH radical scavenging activity
There was not a major change found in the antioxidant activity of the compounds by using Blois method including 1,1-Diphenyl 2-picrylhydrazyl (DPPH) (Blois, 1958). This technique majorly focuses on free colorful radical 1,1-Diphenyl 2-picrylhydrazyl radical (DPPH) eliminations via free radical scavengers. DPPH has a red color, and it is a free radical. When free radicals are scavenged by antioxidant compounds, the color turns from red to yellow. There was a reduction in terms of absorption mixture at 517 nm. This has indicated an increase in antioxidant activity in the free radical scavenging. Reagents used are: 0.1 mM DPPH; Butyl Hydroxy toluene (BHT) (1 mg/ml).
The compounds synthesized in the work were dissolved in ethyl alcohol as 1 mg/ml. The standard was dissolved in ethanol, again at 1 mg/ml. Test tubes were used to put test compound and standards in the amount of 50, 100, 250 μg/ml respectively. In order to form a total volume of 3 mL, DMSO was used for the completion. 1 mL of the stock DPPH solution was put into the experimental tubes. The incubation process was initiated at room temperature and the room was kept dark. The duration was about 30 min. After incubation absorbance of (UV–Vis Spectrophotometer: Shimadzu UV-1700 Spectrophotometer), the ethanol-containing mixture was found to be 517 nm. Ethanol ve DPPH solutions were used in the amount of 3 mL, and 1 mL respectively. The decreased absorbance has revealed the scavenging activity of free radicals for the remaining 1,1-Diphenyl 2-picrylhydrazyl radical (DPPH) solution. The calculation for DPPH radical scavenging activity in the reaction medium was performed in accordance with the formulation presented below. % DPPH Free Radical Scavenging Activity = (A0–A1/A0) × 100.
A0: Absorbance of the control reaction.
A1: Absorption of the sample or standard.
2.2.3 Determination of minimum inhibitory concentrations (MICs) of antimicrobial agents
After the chemicals were synthesized, the anti-microbial activities of these chemicals were examined. The method of microdilution broth was preferred with a slight change (Eloff, 1998), two gr-positive bacteria Staphylococcus aureus (ATCC 29213) and Enterococcus faecalis (ATCC 2921), two gr-negative bacteria Pseudomonas aeruginosa (ATCC 27853) and Escherichia coli (ATCC 25922) and single fungal strain Candida albicans (ATCC 10231) were used in order to determine this activity. Each chemical agent was dissolved in DMSO (5000 µM) and then the dilution process was performed as1/10 in sterile distilled water. Distilled water was poured into the wells consisting of 96-well microtiter plates. The amount of the water was 50 µl. The following step was pouring 50 μl of the chemical compounds into the wells. Then two-fold dilutions were performed serially in a 96-well plate. The concentration of chemical compounds in wells ranged from 125 µM to 0.48 µM. MHB (Mueller Hinton Broth, Accumix® AM1072) and SDB (Saboraud Dekstroz Broth, Himedia ME033) were preferred for diluting bacteria and Candida cultures. The size of the final inoculum was found to be 5 x105 CFU/mL at bacteria and 0.5–2.5 × 103 CFU/mL at Candida. For the positive and negative controls, gentamicin and fluconazole and preferred DMSO were used respectively. The incubation was performed for microtiter plates at the temperature of 37 °C for bacteria and at the temperature of 35 °C for Candida for about 16–24 h. Then, the wells were filled with 50 µl 2 mg/ml 2,3,5-Triphenyl tetrazolium chloride (TTC) (Meck, Germany) in terms of microbial growth. Then they were once again exposed to incubation at 37 °C for 2 h. A decrease was observed in the red color of formazan after the incubation process. This was considered to be an acceptable MIC value (Fig. 1). Findings obtained as a result of the study are given in Table 3.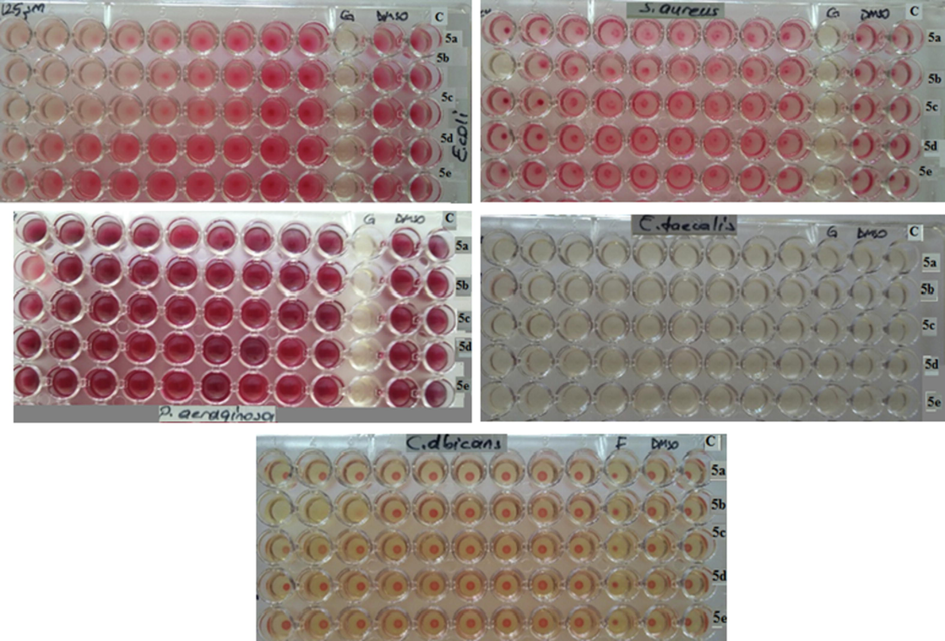
Determination of minimum inhibitory concentrations (MICs) of antimicrobial agents for compounds (5a-e).
2.2.4 Statistical analysis
Mean ± standard error was used for presenting the data in the study. The analysis of the data was conducted by using One-way ANOVA and Tukey test for the anti-tumor. SPSS/PC package program was used for the analysis of the study.
3 Results and discussion
The 3,4,5-trisubstituted 1,2,4-triazoles obtained in the first step in this work are reacted with 2-chloro-1-(3-methyl-3- mesityl-cyclobutyl) ethanone in dry acetone containing K2CO3 to obtain the 4,5-trisubstituted 1,2,4-triazole sulfanyl compounds. 3,4,5-trisubstituted 1,2,4-triazoles reacted with 2-chloro-1-(3-methyl-3-mesityl-cyclobutyl)-ethanone in the presence of K2CO3 via the SN2 reaction mechanism to yield the sulfanyl compounds containing the triazole and cyclobutane ring (Scheme 1). Efficient yields range from 66% to 79%. When the IR spectra of 5a–e in the 3,4,5-tri substituted 1,2,4-triazole sulfanyl compounds are examined, the most characteristic peak is the C=O peak. The C=O stretching vibration occurs in the range of 1694–1710 cm−1. The C-S-C strain peculiar to the structure is around 694–716 cm−1. Around 2941–3046 cm−1 Ar-H, around 2860–2920 cm-1C-H, around 1160 cm-1C-C peaks for the cyclobutane ring and C=C bands for the mesityl ring at 1600 cm−1 were obtained. When the proton NMR spectrum is examined, one of the characteristic peaks in the 3,4,5-trisubstituted 1,2,4-triazole sulfanyl is signalled by nine –CH3 protons in the mesityl group. This signal appeared as a singlet at approximately 2.14 ppm and was found to be equivalent to nine hydrogen signals. The signal of –CH3 in the cyclobutane ring appeared as a singlet at about 1.50 ppm. Four hydrogens in the cyclobutane ring were distinctly resonant as -cis and -trans. These four protons occurred as a multiplet at 2.45–2.55 ppm. Data for all compounds are given in Table 1.
In this study, we applied five compounds encoded as 5a-e to ht 29 human colon adenocarcinoma cells, findings of our compounds' anticancer properties have been shown in Table 1. according to the results, it was found that all of our test substances destroyed the cancerous cells in a statistically significant manner when we compared them with the control group. compounds were observed to kill ht 29 human colon adenocarcinoma cells, in a statistically significant (p < 0.05) way when we compared with the control group. the study was conducted on three different concentrations (15 μm, 30 μm, 60 μm).
When Table 2 and Fig. 1 of 5a-e coded test compounds were examined, it was determined that there were different statistical results between the groups at these concentrations (p < 0.05). According to the results obtained, it can be said that the higher concentrations of triazole compounds we use are more effective in destroying cancerous cells. Living cell results of HT 29 human colon adenocarcinoma cells exposed to substances were presented in Table 2 and Fig. 2. a‐d The difference between the groups with different letters on the same line is statistically significant (P < 0.05).
Groups
24 h
15 μM
24 h
30 μM
24 h
60 μM
48 h
15 μM
48 h
30 μM
48 h
60 μM
Control
93.14 ± 0.78a
93.14 ± 0.78a
93.14 ± 0.78a
96.64 ± 0.16a
96.64 ± 0.16a
96.64 ± 0.16a
5a
79.33 ± 1,80b
69.54 ± 1,60b
47.40 ± 1,96c
74.75 ± 0.85b
60.25 ± 0.48c
39.75 ± 0.18c
5b
76.15 ± 1.75b
63.60 ± 2.04b
38.33 ± 1.44c
70.25 ± 1.44b
59.75 ± 1.25c
39.50 ± 0.65c
5c
68.67 ± 1.03c
57.93 ± 0.91c
32.42 ± 0.63c
65.25 ± 1.03c
43.00 ± 0.41d
27.25 ± 1.11d
5d
56.75 ± 1.89c
44.82 ± 1.19c
30.02 ± 0.41c
52.50 ± 1.32c
37.75 ± 1.10d
22.25 ± 0.85d
5e
53.52 ± 1.55c
37.03 ± 1.49c
24.85 ± 1.08c
50.25 ± 1.49c
20.75 ± 0.85e
10.75 ± 0.35e
Cisplatin
84.70 ± 1,06b
77.70 ± 0,37b
70.80 ± 0,26b
81.90 ± 1,12b
77.50 ± 033b
70.42 ± 0,9b
Groups
E. coli
S. aureus
P. aeruginosa
E. faecalis
C. albicans
5a
15.625
>125
>125
>125
>125
5b
15.625
125
125
>125
31.25
5c
15.625
>125
>125
>125
>125
5d
>125
>125
>125
>125
>125
5e
>125
>125
>125
>125
>125
Fluconazole
>125
>125
>125
>125
>125
Gentamicin
>125
>125
>125
>125
>125
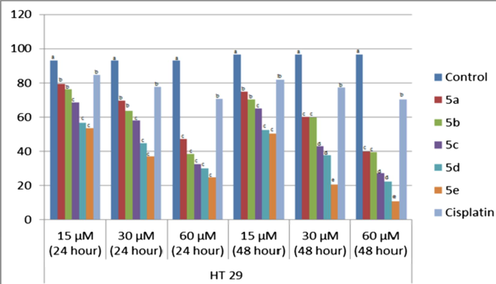
Cell viability, %, rates relative to doses and time in HT29 cell lines after treatment with triazole compounds (5a-e).
Fig. 2, was also showing the results accordingly. It was noted that the difference among the groups having different substituents on triazole ring on the same line was statistically significant.
For the antimicrobial study, bacteria S. aureus, E. faecalis, P. aeruginosa, and E. coli and a single fungal strain C. albicans were used. The results were shown in Table 3. As shown in Table 2, antimicrobial activities had different variations. MIC values of the chemicals were between 15.625 - >125 µM. The 5b was found the most effective chemical which it was effective on E. coli, S. aureus, P. aeruginosa, E. faecalis and C. albicans. E. coli was noted to be more sensitive compared to S. aureus, P. aeruginosa, E. faecalis, and C. albicans against the 5a and 5c chemicals. MIC values for the synthesized compounds were found as > 125 µM except for 5b, 5a, and 5c.
In vitro DPPH free radical scavenging activities were shown in Table 4. It has been noted that 5c and 5e of the compounds showed activity close to the standard antioxidant BHT. On the other hand, 5d test compounds have been found to have a moderate activity, and 5a and 5b test compounds have a low activity (Fig. 3). Data are presented as mean ± SD a-d; the difference between groups with different letters on the same line is statistically significant at P < 0.05.
Groups
50 µg/mL
100 µg/mL
250 µg/Ml
5a
18.90 ± 2,12 a
29.33 ± 1.02c
25,18 ± 2,02c
5b
20.05 ± 0,14 a
22.16 ± 1.59ab
24.66 ± 0,46 bc
5c
24.48 ± 0,88b
48.20 ± 0.19 d
46.75 ± 1,67 d
5d
22.48 ± 2,02 bc
39.33 ± 1.02 cd
38.44 ± 2,50c
5e
26.50 ± 1,11c
42.33 ± 0.22 d
48.50 ± 0.19 d
BHT
30.2 ± 0,22c
45.2 ± 0.99 d
50.3 ± 0.38 d
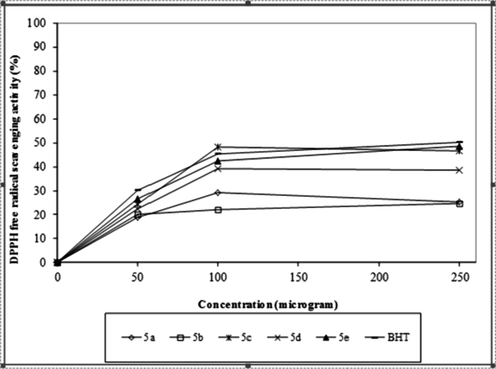
Graphical representation of % DPPH free radical scavenging activity of synthesis compounds and standard antioxidant compound BHT and at the concentrations (50–100–250 µg/mL) for (5a-e).
In this study, it was demonstrated that after 15 μM cisplatin administration, it is above 80% of the viability of HT29 cells (Fig. 2).
These results suggest that colon cancer cell lines and indirectly cancerous tissues may be resistant to cisplatin at a certain concentration. It is therefore thought to be useful to overcome the cisplatin resistance observed in the treatment of colon cancer with newly synthesized compounds and provide alternative treatment options. The anticancer activities of the test compounds in the 24- and 48-hour period are shown as 5e > 5d > 5c > 5b > 5a respectively. Test compounds were found to be highly effective in 48 h and high doses compared to the control group and cisplatin. It should be investigated whether there are different side effects of these compounds which are more effective than cisplatin. It is also planned to investigate the toxicity of these compounds in the future.
When we look at the substituents on compounds that exhibit good antimicrobial activity from the test compounds, the presence of allyl-, phenyl- and ethyl cyclobutane substituents is evident. Although there are very similar structures on all compounds, it is interesting that they exhibit very different antimicrobial activities. Perhaps the electron-donating property of these compounds can be a reason for their antimicrobial activity. Generally, similar materials in terms of structure have been synthesized and antimicrobial effects are observed at different degrees (Ahmedzade et al., 2003). The skeletons of the compounds are the same, but their substituents are different. Therefore, the idea for the cause is the emergence of antimicrobial activity values at different levels. So, there should be more exhaustive studies to learn the plausible mechanisms related to the inhibition of the growth of antimicrobial organisms.
According to the results of antioxidant results, it has been noted that 5c and 5e of the compounds showed activity close to the standard antioxidant BHT. On the other hand, 5d test compounds have been found to have a moderate activity and 5a and 5b test compounds have low activity. Probably these results can show that our test compounds 5c and 5e have good activity as hydrogen donor because antioxidants on radical scavenging effects is considered to be originated from its hydrogen donor capabilities. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) is accepted to be a stable free radical. It becomes a stable molecule when an electron or hydrogen radical is accepted (Baumann, 1979; Soare et al., 1997).
4 Conclusion
In conclusion, when the results obtained from this study are evaluated, it has been found some of the compounds which were exposed to synthesis have anti-tumor and anti-bacterial effects. We believe that the results obtained in this study will lead to the synthesis of different substituted derivatives of this material and investigation of its different activities in future studies.
Acknowledgements
We kindly thank Dr. Metin Koparir for synthesis of compounds and Assoc. Prof. Dr. Sevgi Durna Dastan for his laboratory support during this study.
References
- Synthesis and biological evaluation of new pyridines containing imidazole moiety as antimicrobial and anticancer agents. Turk. J. Chem.. 2015;39:334-346.
- [Google Scholar]
- Synthesis and antimicrobial activity of new Thiazole-2 (3 H)-thiones containing 1, 1, 3-trisubstituted Cyclobutane. S. Afr. J. Chem.. 2003;56:21-24.
- [Google Scholar]
- Synthesis and anti-HIV activity of N'-nicotinoyl-3-(4'-hydroxy-3'-methylphenyl)-5-[substituted phenyl]-2-pyrazo-lines. Acta Pol Pharm.. 2007;64:423-428.
- [Google Scholar]
- QSAR analysis of 1, 4-dihydro-4-oxo-1-(2-thiazolyl)-1, 8-naphthyridines with anticancer activity. Eur. J. Med. Chem.. 2007;42:1184-1192.
- [Google Scholar]
- Synthesis and antiprotozoal activity of cationic 1, 4-diphenyl-1 H-1, 2, 3-triazoles. J. Med. Chem.. 2010;53:254-272.
- [Google Scholar]
- Synthesis, biological evaluation, and pharmacophore generation of new pyridazinone derivatives with affinity toward α1-and α2-adrenoceptors. J. Med. Chem.. 2001;44:2118-2132.
- [Google Scholar]
- Prostaglandin synthetase inhibiting O_2-radical scavenging properties of some flavonoids and related phenolic compounds. Naunyn-Schmiedebergs Arch Pharmacol.. 1979;308:27-32.
- [Google Scholar]
- Synthesis, and antimicrobial evaluation of new pyridine imidazo [2, 1b]-1, 3, 4-thiadiazole derivatives. J. Saudi Chem. Soc.. 2016;20:S406-S410.
- [Google Scholar]
- Synthesis and antiviral and antineoplastic activities of some novel carbocyclic guanosine analogues with a cyclobutane ring. Chem. Pharm. Bull.. 1999;47:1314-1317.
- [Google Scholar]
- Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199-1200.
- [Google Scholar]
- Pyrazolo [3, 4-c] pyridazines as novel and selective inhibitors of cyclin-dependent kinases. J. Med. Chem.. 2005;48:6843-6854.
- [Google Scholar]
- Pyridazine and phthalazine derivatives with potential antimicrobial activity. J. Heterocycl. Chem.. 2007;44:1149-1152.
- [Google Scholar]
- Çalişkan-Ergün, B., şüküroğlu, M., Coban, T., et al., 2008. Screening and evaluation of antioxidant activity of some pyridazine derivatives. J. Enzyme Inhibition Med. Chem. 23, 225–229.
- Synthesis and biological evaluation of substituted 2-aryl-1, 8-naphthyridin-4 (1H)-ones as antitumor agents that inhibit tubulin polymerization. J. Med. Chem.. 1997;40:3049-3056.
- [Google Scholar]
- 3-O-Substituted benzyl pyridazinone derivatives as COX inhibitors. Eur. J. Med. Chem.. 2002;37:339-347.
- [Google Scholar]
- Synthesis and hypoglycemic evaluation of substituted pyrazole-4-carboxylic acids. Bioorg. Med. Chem. Lett.. 2002;12:2105-2108.
- [Google Scholar]
- FOXO3a mediates the cytotoxic effects of cisplatin in colon cancer cells. Mol. Cancer Ther.. 2008;7:3237-3246.
- [Google Scholar]
- Dehmlow, E.V., Schmidt, S., 1990. Synthese von stereoisomeren 3‐substituierten Cyclobutancarbonsäure‐Derivaten. Liebigs Annalen der Chemie. 1990, 411–414.
- Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv.. 2021;45:e15765
- [Google Scholar]
- Synthesis of 2-[5, 6-diphenyl-3 (2H)-pyridazinone-2-yl] acetamide and 3-[5, 6-diphenyl-3 (2H)-pyridazinone-2-yl] propanamide derivatives as analgesic and anti inflamematory agents. Turk. J. Pharm. Sci.. 2007;4:57-70.
- [Google Scholar]
- A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med.. 1998;64:711-713.
- [Google Scholar]
- Synthesis, structural characterization and biological activity of helicin thiosemicarbazone monohydrate and a copper (II) complex of salicylaldehyde thiosemicarbazone. Inorg. Chim. Acta. 1999;286:134-141.
- [Google Scholar]
- Synthesis of novel 3-[5-ethyl-2-(2-phenoxy-ethyl)-pyridin]-5-substituted isoxazoline libraries via 1, 3-dipolar cycloaddition and evaluation of antimicrobial activities. Med. Chem. Res.. 2007;15:407-417.
- [Google Scholar]
- Synthesis and analgesic and anti-inflammatory activity of 6-phenyl/(4-methylphenyl)-3 (2H)-pyridazinon-2-propionamide derivatives. Arzneimittelforschung.. 2009;59:357-363.
- [Google Scholar]
- Synthesis and antiproliferative activity of benzocyclobutacarbazol derivatives. A new class of potential antitumor agents. Bioorg. Med. Chem. Lett.. 2000;10:2589-2591.
- [Google Scholar]
- Differences in anxiolytic-like profile of two novel nonbenzodiazepine BZ (ϖ) receptor agonists on defensive behaviors of mice. Pharmacol. Biochem. Behav.. 1999;62:689-694.
- [Google Scholar]
- Gürbüz, V., Yilmaz, A., Gökçe, Ö., et al., 2011. The apoptotic effects of cisplatin on human colon cancer cell line (HT29) İnsan kolon kanser hücre hattında (HT29) sisplatin'in apoptotik etkisi. 24, 100–105.
- İnsan kolon kanser hücre hattında (ht29) sisplatin'in apoptotik etkisi. Marmara Med. J.. 2011;24:100-105.
- [Google Scholar]
- Hosni, H.M., Abdulla, M.M., 2008. Anti-inflammatory and analgesic activities of some newly synthesized pyridinedicarbonitrile and benzopyranopyridine derivatives. Acta Pharmaceutica 58, 175–186.
- Jemal, A., Siegel, R., Ward, E., et al., 2009. Cancer statistics, 2009. CA: A Cancer J. Clin. 59, 225–249.
- Joule, J.A., Mills, K., Smith, G.F., 2020. Heterocyclic Chemistry, third ed. CRC Press, London, 1995.
- Novel pyridazine derivatives: synthesis and antimicrobial activity evaluation. Eur. J. Med. Chem.. 2009;44:1989-1996.
- [Google Scholar]
- Synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure–activity relationship study of hydroxylated 2, 4-diphenyl-6-aryl pyridines. Bioorg. Med. Chem.. 2010;18:3066-3077.
- [Google Scholar]
- Synthesis, antioxidant activities and urease inhibition of some new 1, 2, 4-triazole and 1, 3, 4-thiadiazole derivatives. Eur. J. Med. Chem.. 2010;45:5200-5207.
- [Google Scholar]
- Synthesis, characterization and anticancer studies of mixed ligand dithiocarbamate palladium (II) complexes. Eur. J. Med. Chem.. 2011;46:4071-4077.
- [Google Scholar]
- Active compounds from a diverse library of triazolothiadiazole and triazolothiadiazine scaffolds: Synthesis, crystal structure determination, cytotoxicity, cholinesterase inhibitory activity, and binding mode analysis. Bioorg. Med. Chem.. 2014;22:6163-6173.
- [Google Scholar]
- Synthesis, antioxidant and antitumor activities of some of new cyclobutane containing triazoles derivatives. Phosphorus Sulfur Silicon Related Elements. 2019;194:1028-1034.
- [Google Scholar]
- Synthesis and in-vitro antimicrobial activity of novel aminophosphinic acids containing cyclobutane and 1, 3-thiazole. Phosphorus Sulfur Silicon Related Elements. 2011;186:2368-2376.
- [Google Scholar]
- Recent advances bioactive 1, 2, 4-triazole-3-thiones. Eur. J. Med. Chem.. 2015;97:830-870.
- [Google Scholar]
- Effect of 2, 4-dihydro-3H-1, 2, 4-triazole-3-thiones and thiosemicarbazones on iodide uptake by the mouse thyroid: the relationship between their structure and anti-thyroid activity. Chem. Pharm. Bull.. 1990;38:2595-2596.
- [Google Scholar]
- Antiplasmodial activity of [(aryl) arylsulfanylmethyl] pyridine. Antimicrob. Agents Chemother.. 2008;52:705-715.
- [Google Scholar]
- The coordination chemistry of Zn (II), Cd (II) and Hg (II) complexes with 1, 2, 4-triazole derivatives. Dalton Trans.. 2011;40:8475-8490.
- [Google Scholar]
- Evaluation of anti-tubercular activity of nicotinic and isoniazid analogues. Arkivoc.. 2007;15:181-191.
- [Google Scholar]
- New derivatives of pyrrolo [3, 4-d] pyridazinone and their anticancer effects. Il Farmaco.. 2004;59:457-462.
- [Google Scholar]
- Synthesis and biological activities of triazole derivatives as inhibitors of InhA and antituberculosis agents. Eur. J. Med. Chem.. 2011;46:5524-5531.
- [Google Scholar]
- Progress in anti-tumor agents: triazoles. West China J. Pharm. Sci.. 2008;23:84-86.
- [Google Scholar]
- In situ click chemistry: enzyme-generated inhibitors of carbonic anhydrase II. Angew. Chem.. 2005;117:118-122.
- [Google Scholar]
- Design, synthesis and cancer cell line activities of pyrazolo [3, 4-b] pyridine derivatives. 2012;2:78-88.
- Stereochemistry and antitumour activity of platinum metal complexes of 2-acetylpyridine thiosemicarbazones. Transition Met. Chem.. 1997;22:263-269.
- [Google Scholar]
- Synthesis and in vitro characterization of 1-(4-aminofurazan-3-yl)-5-dialkylaminomethyl-1 H-[1, 2, 3] triazole-4-carboxylic acid derivatives. A new class of selective GSK-3 inhibitors. J. Med. Chem.. 2003;46:3333-3341.
- [Google Scholar]
- Characterization and inhibitor activity of two newly synthesized thiazole. J. Bio- Tribo-Corrosion.. 2022;8:1-12.
- [Google Scholar]
- Synthesis, physical and chemical properties of some derivatives 1, 2, 4-triazolo-(3, 4-b)-1, 3, 4-thiodiazine with residue of fragments of furan. Intellectual Archive.. 2012;1:63-72.
- [Google Scholar]
- Design, synthesis, and structure–activity correlations of novel dibenzo [b, d] furan, dibenzo [b, d] thiophene, and N-methylcarbazole clubbed 1, 2, 3-triazoles as potent inhibitors of mycobacterium tuberculosis. J. Med. Chem.. 2012;55:3911-3922.
- [Google Scholar]
- Recent advances in bioactive flavonoid hybrids linked by 1, 2, 3-triazole ring obtained by click chemistry. Molecules. 2021;27:230.
- [Google Scholar]
- Design and synthesis of novel 2-(4-(2-(dimethylamino) ethyl)-4H-1, 2, 4-triazol-3-yl) pyridines as potential antitumor agents. Eur. J. Med. Chem.. 2014;81:47-58.
- [Google Scholar]
- Synthesis of new pyridine derivatives as potent antifungal agents. Int. J. Pharma Bio Sci.. 2011;2:200-209.
- [Google Scholar]
- Rastkari, N., M. Sharifzadeh, 2004. Anticonvulsant activities of new 1, 4dihydropyridine derivatives containing 4-nitroimidazolyl substituents. 12, 81–86.
- Synthesis and blood glucose lowering effect of novel pyridazinone substituted benzenesulfonylurea derivatives. Eur. J. Med. Chem.. 2009;44:2673-2678.
- [Google Scholar]
- Thiourea, triazole and thiadiazine compounds and their metal complexes as antifungal agents. J. Inorg. Biochem.. 2005;99:1558-1572.
- [Google Scholar]
- Synthesis and structure activity ons of 3-substituted cyclobutane carboxylic acids. Eur. J. Med. Chem. Chem-Ther.. 1977;12:501-509.
- [Google Scholar]
- Anticonvulsant activity of 3-oxo-5-substituted benzylidene-6-methyl-(4H)-2-pyridazinylacetamides and 2-pyridazinylacetylhydrazides. Chem. Pharm. Bull.. 1990;38:3009-3013.
- [Google Scholar]
- Synthesis and biological applications of triazole derivatives–a review. Mini-Rev. Org. Chem.. 2013;10:40-65.
- [Google Scholar]
- Synthesis and in vitro antibacterial activity of some novel N-nicotinoyl-1-ethyl-6-fluoro-1, 4-dihydro-7-piperazin-1-yl-4-oxoquinoline-3-carboxylates. Acta Pol Pharm.. 2008;65:551-556.
- [Google Scholar]
- Synthesis of new S-derivatives of clubbed triazolyl thiazole as anti-Mycobacterium tuberculosis agents. Bioorg. Med. Chem.. 2007;15:3997-4008.
- [Google Scholar]
- Antioxidant activities of some extracts of Thymus zygis. Free Radical Res.. 1997;26:469-478.
- [Google Scholar]
- Pyridazines. Part XXIX: synthesis and platelet aggregation inhibition activity of 5-substituted-6-phenyl-3 (2H)-pyridazinones. Novel aspects of their biological actions. Bioorg. Med. Chem.. 2002;10:2873-2882.
- [Google Scholar]
- Decreased transcription-coupled nucleotide excision repair capacity is associated with increased p53-and MLH1-independent apoptosis in response to cisplatin. BMC cancer.. 2010;10:1-10.
- [Google Scholar]
- Novel combination treatment for colorectal cancer using Nek2 siRNA and cisplatin. Cancer Sci.. 2010;101:1163-1169.
- [Google Scholar]
- Synthesis, determination of the lipophilicity, anticancer and antimicrobial properties of some fused 1, 2, 4-triazole derivatives. Eur. J. Med. Chem.. 2008;43:404-419.
- [Google Scholar]
- 6-benzylidenethiazolo [3, 2-b]-1, 2, 4-triazole-5 (6h)-onessubstituted with ibuprofen: synthesis, characterizationand evaluation of anti-inflammatory activity. Eur. J. Med. Chem.. 2000;35:743-750.
- [Google Scholar]
- Synthesis of some triazolyl-antipyrine derivatives and investigation of analgesic activity. Eur. J. Med. Chem.. 2001;36:685-689.
- [Google Scholar]
- Synthesis and anticancer activity evaluation of a series of [1, 2, 4] triazolo [1, 5-a] pyridinylpyridines in vitro and in vivo. Eur. J. Med. Chem.. 2013;67:243-251.
- [Google Scholar]
- 3-Aminopyridazine derivatives with atypical antidepressant, serotonergic and dopaminergic activities. J. Med. Chem.. 1989;32:528-537.
- [Google Scholar]
- Substituted 2-thienyl-1, 8-naphthyridin-4-ones: their synthesis, cytotoxicity, and inhibition of tubulin polymerization. Abstracts Am. Chem. Soc.. 1999;218 171-MEDI
- [Google Scholar]
- Zhou, C.H., Wang, Y., 2012. Recent researches in triazole compounds as medicinal drugs. Curr. Med. Chem. 19, 239–280.
- Review on supermolecules as chemical drugs. Sci. China, Ser. B Chem.. 2009;52:415-458.
- [Google Scholar]
- Recent researches in metal supramolecular complexes as anticancer agents. Anti-Cancer Agents Med. Chem. (Formerly Curr. Med. Chem.-Anti-Cancer Agents). 2010;10:371-395.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103957.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







