Translate this page into:
Emerging electrocatalysts for green ammonia production: Recent progress and future outlook
⁎Corresponding author. shahidiqbal@hzu.edu.cn (Shahid Iqbal),
⁎⁎Corresponding author at: Nanomaterials Research Center, Department of Chemistry, College of Science, Mathematics, and Technology, Wenzhou-Kean University, Wenzhou 325060, Zhejiang Province, China. abahadur@wku.edu.cn (Ali Bahadur)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In the Haber-Bosch process (HBp), which uses elevated pressure and temperature to produce more concentration of nitrogen and hydrogen gases, 90% of the 175 million metric tons of NH3 generated worldwide in 2016 were manufactured in this process. According to the road plan for sustainable ammonia production sustainably, using water as a reducing agent is the most effective way to fix nitrogen in close-quarters ambiance. A complete explanation of theoretical and practical work on electrocatalytic nitrogen reduction is provided in this article, with special attention paid to the low selectivity of nitrogen reduction to ammonia in comparison to protons to hydrogen. Since they are essential for accurately achieving high nitrogen production and Faradaic efficiency (FE), their information outlines electrocatalysts, electrolyte selection criteria, and managed experiment design. Under diverse conditions, the evolution of theory and experiment is examined. Finally, feedback is given on this field’s present issues and prospects.
Keywords
Nitrogen reduction
Electrochemical reaction
Electrocatalyst
Nitrogen production rate
Faradaic efficiency
1 Introduction
Ammonia production has contributed to population growth and has been employed as fertilizer throughout the past century. Nitrogen sustains around 27% of the world's population and is provided via ammonia fertilizers. In 1908, a German scientist named Fitz Haber developed a device to fulfill the demand using recycled hydrogen and nitrogen under high pressure and temperature. This technique, known as the HBp, was carried out commercially by Carl Bosch in 1911. In 1918, Fritz Haber's efforts in producing NH3 earned him the Nobel Prize in Chemistry. This incredible approach showed a novel ammonia production method combining nitrogen and hydrogen. The following reaction: N2 + 3H2 → 2NH3 occurs when H2 and N2 are mixed at high pressures of 20 to 40 MPa and temperatures of 400 to 600 °C using an iron-based catalyst. Low temperatures are necessary for this exothermic reaction to produce ammonia (ΔHo298K= −44.91 KJ/mol, ΔGo298K= −16.3 KJ/mol, Keq = 749.9). The nitrogen-nitrogen bond is exceptionally strong. According to equilibrium calculations, 99% of NH3 decomposes into nitrogen and hydrogen at pressures of 0.1 MPa and temperatures above 400 °C. Higher temperatures are required to overcome this bond strength barrier, and NH3 decomposes into nitrogen and hydrogen. Therefore, the HBp, which produces ammonia, operates at a high pressure of 20–40 MPa. A single reaction synthesizes approximately 15% of ammonia, and the remaining nitrogen and hydrogen can be recycled back into the reaction chamber, producing an approximate 97% yield of ammonia (Qing et al., 2020; Foster et al., 2018). This procedure is used to produce fertilizer on a massive scale, and about 80% (136 megatons in 2011) of ammonia production is utilized for this purpose, according to the survey (Kandemir et al., 2013). In the field of agriculture, the HBp has brought a revolution. Nitrogen in gaseous form (N2) is present in the environment at 78% by volume. It is not helpful in gaseous form for plants. Soil having nitrogen benefits agricultural productivity, but nitrogen depletes each crop very quickly (Wang et al., 2018). The Haber-Bosch method, which produces NH3 as fertilizer, is frequently used to increase agricultural productivity·NH3 is only of the world's most widely formed industrial compounds, and 89.9% of it is produced using the Haber-Bosch method. The productivity of NH3 increased from 117 million metric tons in 1996 to 175.0 million metric tons in 2016 (Qing et al., 2020; Zhang et al., 2016).
According to a 2016 survey, about 88% of ammonia consumption in the USA was for fertilizer. The fertilizer forms of ammonia were urea, ammonium phosphates, anhydrous ammonia, ammonium nitrate, and other nitrogen compounds for direct use. Ammonia residue makes several chemical compounds, plastics, resins, industrial refrigerants, explosives, and synthetic fibers (Wang et al., 2018; Chen et al., 2023; Xu et al., 2024). The consumption of ammonia drastically changes the landscape of our planet. Since 1950, the amount of N2 consumed has quadrupled. According to estimates, the HBp for NH3 provides around 40% of the world's nutritional protein source. Synthetic ammonia is terrible for the environment because it requires a lot of energy and fossil fuels (Wang et al., 2024). Another disadvantage is that capturing N2 requires low temperatures, whereas hydrogen produced from fossil fuels undergoes highly endothermic reactions (Gui et al., 2024). Low-temperature procedures are typically necessary to obtain nitrogen feedstock from the environment. This is because synthesizing H2 from fossil fuels comprising coal, natural gas, petroleum, and oil involves highly endothermic reactions. Natural gas accounts for almost 70% of the hydrogen in ammonia synthesis (Zhen et al., 2024). Desulfurization, steam reforming, and water–gas shift reactions are all components of the multi-stage natural gas hydrogen production process (Qing et al., 2020; Ercolino et al., 2015).
Steam reforming:
Water-gas shift reaction:
Steam reforming is an energy-intensive process due to its endothermic nature. When factoring in the cost of natural gas, it becomes evident that around 75% of the overall ammonia production expenses are attributed to the production of H2 (Chen et al., 2022). The remaining 25% of the cost pertains to the separation and synthesis of ammonia, which necessitates 485 kJ/mol per mole of energy. The production of industrial ammonia consequences in the release of approximately 300 million metric tons of carbon dioxide (CO2), contributing to roughly 3–5% of the worldwide natural gas consumption and over 1% of yearly energy production (Chen et al., 2022; Shipman and Symes, 2017; Licht et al., 2014). If the current pace of consumption holds, fossil fuels may run out in the next 100 years. Releasing greenhouse gases like CO2 into the atmosphere quickly alters the climate. About 400 ppm of CO2 is present in the air, more than double the industrial standard (280 ppm) (Blasing et al., 2016). Extreme weather, arctic permafrost melting, and widespread coral bleaching are all believed to be effects of man-made climate change (Wallace et al., 2014; Schuur et al., 2015; Hughes et al., 2017). Authors and international organizations have recently advocated for ammonia as a carbon-free energy carrier for renewable sources. Ammonia is documented as a viable and environmentally friendly fuel with global potential for future utilization (Valera-Medina et al., 2021; Khan et al., 2024; Mahmood et al., 2024). Ammonia's consumption in heavy transportation, energy production, and distributed energy storage are all growing rapidly. Ammonia can replace a sizeable amount of the liquid fuel now used if it is produced on a big scale (MacFarlane et al., 2020; Wang et al., 2019; Mahmood et al., 2024). This chemical is vital to the fertilizer industry, producing 150 million tons globally annually.
In addition, ammonia is considered a viable option for transporting chemical energy owing to its high hydrogen gravimetric density (17.8 wt%) (Iqbal and Iqbal, 2013). Although it is currently challenging to transport and store hydrogen, it is an excellent fuel for zero-emission vehicles since, in a fuel cell, it reacts with oxygen to produce just water (Eberle et al., 2012). Since hydrogen has a boiling point of −253 °C at atmospheric pressure, it can only be stored under either high pressure or low temperature (Irfan et al., 2021). On the other hand, ammonia has a vapor pressure of 0.86 MPa at 20 °C and a boiling point of −33 °C at atmospheric pressure, allowing it to liquefy in benign conditions. Also, a solid infrastructure is in place for transporting and storing large volumes of liquid ammonia. Ammonia can be converted into hydrogen at the application site through thermal decomposition or electrolysis (Ju et al., 2017; Little et al., 2015). The performance of solid oxide fuel cells is comparable to that of hydrogen gas because they permit the direct use of ammonia as fuel (Molouk et al., 2015). Ammonia is also fuel for power plants and internal combustion engines (Xiao et al., 2016; Mahmood et al., 2024). This review focuses on the techno-economic analysis that researchers have done for the technological challenges and advances in the fixation of the electrochemical conversion of N2 to NH3 (Irfan et al., 2020; Mahmood et al., 2024; Yan et al., 2024). Contemporary research focuses on developing new, highly engaged catalysts for ammonia synthesis by employing ruthenium, cobalt, nickel, promoted iron, and metal nitrides to discover their industrial application. Moreover, the efficiency of the HBp can be enhanced by optimizing the conversion rate through the utilization of lower temperatures and pressures.
Over the past decade, several attempts have been made to design catalysts that can fix nitrogen reduction under favorable reaction conditions to produce ammonia and other beneficial nitrogen-containing compounds. These processes include low-temperature thermal catalysis, non-thermal plasma catalyst supports, enzymatic catalysis, photocatalysis, and electrochemical catalysis (Shi et al., 2020; Shen et al., 2023; Liu et al., 2023). Innovative catalysts for ammonia synthesis, which employ oxynitride hydrides, nitrides, amides, hydrides, and oxide supports, are promising substitutes for traditional Ru, Fe, Ni, and Co-based catalysts. There has been substantial research dedicated to exploring the connection between catalyst morphology and carriers, indicating that, while these new catalysts hold great potential, there is room for further improvements in activity. The presence of anionic oxygen and nitrogen vacancies in promoters or co-catalysts dramatically increases the stability and activity of catalysts used in ammonia synthesis. Developing catalysts that are resistant to oxygenate is crucial for enhancing the overall efficiency of HBp, particularly in the context of small-scale green ammonia production. This advancement also simplifies the gas treatment procedures involved. These innovative catalysts must undergo a rigorous stability study for industrial applications under varied operating settings with various contaminants in the precursor gases. Novel catalysts must carefully consider the cost to compete with less expensive fused iron catalysts. The advantages of increased catalytic activity cannot be lost due to the more expensive catalyst if the aim is to replace the current fused iron catalysts (Humphreys et al., 2021).
Although ammonia production is exothermic and can attain more significant thermodynamic conversion at reduced temperatures, the present catalysts need higher temperatures to generate reasonable rates (He et al., 2019; Pan et al., 2018; Liu et al., 2018). In designing future catalysts, it should be considered that they operate at a low temperature to optimize conversion in the reactor loop. Nitride-supported nickel-based and hydride-supported nickel-based catalysts have been demonstrated to offer low-temperature activity using a chemical cycle process. Therefore, the possible solution for low-temperature ammonia synthesis is nickel-based catalysts with specifically designed support materials. The activity of the Ru-supported CaFH catalyst at this low temperature is due to weak ionic bonding in solid solution, which again presents an important area for investigation. This catalyst has effectively produced ammonia at the extremely low temperature of 50 °C (Humphreys et al., 2021). Oxygenates in the feed gas stream contaminate the catalysts for ammonia production and significantly decrease their activity. As a result, ammonia synthesis plants typically use various purifying techniques. The capital and operating costs of these purification methods significantly increase in large-scale ammonia synthesis plants, which restricts the production of small-scale green ammonia and makes these technologies unfeasible. Therefore, oxygen and anion vacancies in the catalyst may contribute to developing ammonia-production catalysts resistant to oxygenation (Humphreys et al., 2021). These purification procedures raise the capital and operational expenses of large-scale ammonia synthesis facilities, limiting the availability of green ammonia synthesis on a smaller scale and making these technologies expensive. Oxygen and anion vacancies in the catalyst could open the way for oxygenate-tolerant ammonia synthesis catalysts.
Scientists have invented contemporary techniques to reduce the energy needed for ammonia manufacturing and carbon dioxide emissions. Lowering the operating temperature and pressure of an ammonia plant may lead to a substantial reduction in both construction and running expenses. A heterogeneous catalyst based on ruthenium (Ru) is essential. The KBR Advanced Ammonia Process (KAAP), which produces ammonia at 10 MPa, uses Ru-based catalysts (Brown et al., 2014). It is also reported that ternary intermetallic compounds such as transition metal-LiH composites (Wang et al., 2017), La-Co-Si (Gong et al., 2018), Ru/Ba-Ca(NH2)2 Ru/C12A7:e-, (Gong et al., 2018), and Ru/Pr2O3 (Sato et al., 2017) exhibit exceptional catalytic efficiency for ammonia production from nitrogen and hydrogen at 400 °C and 1 MPa pressure. Ru-based catalysts are rarely used in commercial processes due to excessive cost and poor stability (Foster et al., 2018). As mentioned, two significant limitations are the high energy requirements for producing hydrogen from natural gas and the CO2 emissions from ammonia synthesis. The electrolysis of water using electricity generated by wind or solar sources to produce hydrogen is promising (Energy, 2018). Unexpectedly, the cost of producing renewable energy is falling dramatically, and substantial advancements have been made in the research of electrocatalysts for processes that include the production of oxygen and hydrogen (Qing et al., 2020; Jin et al., 2018; Zou and Zhang, 2015). As a result, it is possible and profitable to produce ammonia using renewable energy. Renewable electricity is intermittent, which is the main issue in combining renewable hydrogen production with a Haber-Bosch reactor because the Haber-Bosch reaction is continuous and requires a constant hydrogen supply. Therefore, the installation cost of renewable ammonia plants will significantly enhance if more energy or H2 storage infrastructure is built to fill hydrogen's supply and demand gap. The main benefit of electrochemical nitrogen reduction technology is the ability to connect sporadic renewable electricity generation with ammonia production directly.
To achieve this, the researchers concentrate on creating transition metal-nitrogen complexes that allow nitrogen to be reduced to ammonia under benign reaction circumstances (Nishibayashi, 2015; Zhao et al., 2022). Another strategy to achieve environmentally friendly ammonia synthesis is the employment of semiconductors with sunlight to generate photocatalytic reactions (Brown et al., 2016; Zhao et al., 2017). The ideal scenario involves photo-generating valence band holes from water and N2 as precursors, oxidizing water, and reducing N2 to ammonia with conduction band electrons (Hirakawa et al., 2017). The major barriers to effective photocatalytic ammonia production are high-energy reaction intermediates and poor N2 binding to the catalyst surface (Li et al., 2017). Manufacturing nitric oxide OR ammonia from nitrogen is possible using the plasma formed by ionizing gases (Patil et al., 2015). H2 and N2 molecules first dissociate on the catalyst's surface in the HBp, then recombine as free radicals in the plasma (Hessel et al., 2013). It has been demonstrated that, at atmospheric pressure, the generation of ammonia from nitrogen and hydrogen is enhanced by plasma and catalysts (Qing et al., 2020; Iwamoto et al., 2017). In recent years, the effective synthesis of ammonia using electrocatalysts from the N2 reduction reaction has recently attracted much attention. This method could replace the Haber-Bosch method, which has problems with noteworthy energy utilization and high carbon dioxide production. Consequently, the number of studies focused on the electro-catalytic reduction of nitrates has dramatically expanded (Wang et al., 2021). Nitrogen is recycled by the electrochemical process of converting nitrate into ammonia, which is more valuable than nitrogen (Wang et al., 2020). However, this method is still not mature, and needs to comprehend the mechanisms of materials for heterogeneous electrocatalytic reduction of nitrates and efficiently compare performance of various electrocatalysts (Wang et al., 2021; Wu et al., 2024). Extensive research has been conducted on electrocatalysts, including transition metals (such as bi-transition metals, mono-transition metals, and transition metal composites) and non-metals, due to their crucial role in nitrate reduction. These materials exhibit excellent electrochemical performance and economic efficiency.
Due to the interest in fuel cells and electrolyzers, extensive research is being done on electrocatalyst materials for the evolution and oxidation of hydrogen and oxygen. However, even though experimental trials were described in 1960, the electrochemical generation of ammonia has just become the subject of significant study. At the electrode surface of an electrochemical cell, N2 is reduced to form ammonia. The following summary of the electrode procedure: Protons are supplied to create ammonia, and electrons from an external circuit lower the N2 molecules that must stick to the electrode's surface. Electrochemical cells offer numerous potential benefits for ammonia production. Ammonia can be synthesized electrochemically under gentle circumstances. Due to its utilization of electricity instead of heat to initiate the N2 molecules, it exhibits significantly higher efficiency than the Haber-Bosch technique. An extensive investigation has been conducted to better understand the electrochemical production of ammonia across a broad temperature range, from room temperature to 800 °C (Zhen et al., 2024). Second, manufacturing ammonia using renewable electricity is possible with the electrochemical synthesis of ammonia (Foster et al., 2018). Large-scale operations (usually 1000–1500 tons/day) have been required for ammonia manufacturing to achieve economies of scale. The electrochemical synthesis method enables ammonia production on a small scale and in specific locations, utilizing renewable energy from wind or solar sources. Water can be used as a source of H2 in the electrochemical production of ammonia. Natural gas reserves are usually considered for the location of modern ammonia plants to help synthesize H2. Since water is the most prevalent liquid on Earth, there is no such restriction when using it as the H2 source for the electrochemical generation of ammonia. In this case, the catalytic reduction of nitrogen and oxidation of water in the electrochemical cell enables the generation of ammonia without the requirement for fossil energy as an H2 source. Furthermore, advancements in membrane electrode assembly methods for water electrolyzers and fuel cells can potentially improve the electrochemical production of ammonia (Qing et al., 2020; Wang et al., 2019; Greenlee et al., 2018; Rouwenhorst et al., 2020; Liu et al., 2021; Zhang et al., 2021).
The effectiveness of the electrochemical synthesis process is contingent upon the efficiency of the water electrolysis step, hydrogen pressurization stage, and ammonia synthesis step. Each of these steps' efficiencies is a function of several other factors, such as pressure, temperature, recycle rate, ammonia separation, and others. In addition to liquid electrolytes like ionic liquids, aqueous acidic and alkaline electrolytes, and more practical molten alkali metal hydroxides or chloride salts, the electrochemical synthesis of ammonia has also been done using solid electrolytes like PEM8, AEM, and high-temperature proton and oxygen conductive solids oxide membranes (Gao et al., 2017; Ni et al., 2023). Because electrolysis in acidic electrolytes releases ammonium ions into the solvent and changes the solution's acidity, this approach is used for electrocatalyst screening (Suryanto et al., 2018). Nitrogen's poor solubility makes electrolysis in solutions even more difficult. Therefore, a suitable gas diffusion electrode design is crucial for productivity (Soloveichik, 2019). Numerous electrocatalysts, such as supported and unsupported noble metals, metal oxides, and metal nitrides, have been used for electrochemical NH3 production, depending on the pH and physical state of the electrolyte (solid, polymer, or liquid). Studies have also been done on catalysts devoid of transition metals, such as black phosphorus and carbon, doped with nitrogen and BN. Building effective and specific catalysts for nitrogen reduction reaction (NRR) is sought since NRR will produce more ammonia (Wang et al., 2019; Soloveichik, 2019).
New reaction systems and electrodes were invented due to the rising interest of scientists and researchers in the electrochemical reduction of nitrogen, and they have made substantial progress toward achieving a higher ammonia production rate in the past several years. Consequently, a wide range of readers should be interested in thoroughly examining this subject. Considerable research efforts have been directed toward exploring electrode materials for electrocatalytic nitrogen reduction reactions (ENRR). This research has encompassed a wide array of materials, including supported and unsupported noble metals, nitrides, phosphides, metal carbides, metal oxides, as well as non-metallic materials. The primary objective of these investigations is to boost electrochemical proficiency, a critical factor in the context of ENRR. The role of electrocatalysts is of paramount importance as they significantly influence the performance and catalytic processes involved in the electrochemical production of ammonia (NH3). Nevertheless, to make a logical choice for a novel system that can efficiently carry out the electrocatalytic reduction of N2, it is crucial to fully understand the underlying mechanisms of the electrode materials, which are currently not fully developed. Numerous evaluations have emphasized the progress made in electrolytes for ENRR, but evaluating different most recent electrocatalyst materials has been neglected. Hence, an immediate assessment of the latest advancements in this domain is vital.
In this review, we examine the latest advancements in electrocatalyts used for ENRR, focusing on the production of ammonia (NH3) (Fig. 1). This review aims to offer insights into the latest advancements in this field while also providing essential foundational information. This review follows the following structure: In Section 2, the reaction pathway for the electrolytic reduction of nitrogen is briefly examined. This section delves into the electrolytic production of ammonia, covering topics related to reaction kinetics and thermodynamics. A discussion of the challenges of nitrogen reduction in selectivity and solutions to improve selectivity is suggested. Section 3 discusses the relationship between electrocatalyst performance and structure from a theoretical and experimental pint of view. It provides an overview of current developments in electrocatalysts NRR. Section 3 also provides an overview of non-metal compound-based electrocatalyst and their performance in NRR. Finally, the state of the field is summarized in Section 4, which also extends plans on existing concerns and future developments.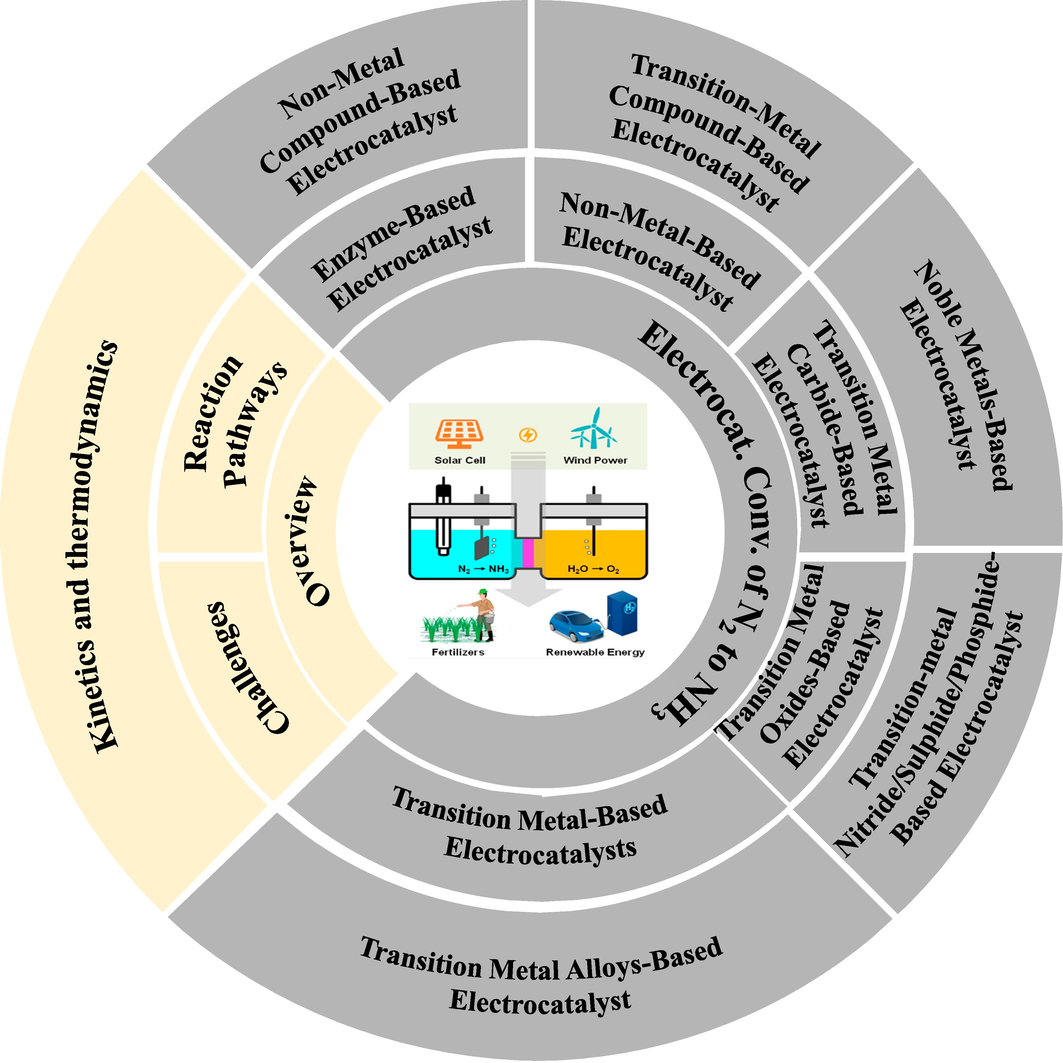
A Schematic diagram illustrating the recent advancements in electrocatalysts employed for the ENRR with a specific focus on ammonia (NH3) production is presented below.
2 Fundamental aspects of catalytic nitrogen reduction
2.1 Reaction pathways
Nitrogen is obtained from atmospheric N2 molecules in the electrochemical process for ammonia production in an aqueous electrolyte. Water electrolysis produces protons, which are subsequently transported to the surface of the electrocatalyst employing a voltage (Van der Ham et al., 2014). This technique usually uses a three-electrode setup consisting of two chambers divided by a membrane, especially for proton exchange. The cathode supports the reduction process known as NRR, which depends on the electrolyte's pH. At the same time, the anode promotes the oxidation process known as the oxygen evolution reaction (OER). The production of ammonia takes place through the generation of protons at the counter electrode under acidic conditions. These protons are subsequently transported across a proton exchange membrane to the cathode. Thus, Eqs. (4) and (5) explain the anodic and cathodic reactions, respectively.
Anodic reaction:
Cathodic reaction:
Under conditions that are either basic or alkaline, nitrogen molecules undergo direct combination with water and electrons at the cathode, resulting in the production of ammonia. This process is distinct from what occurs under acidic conditions (Hou et al., 2020). The working electrode generates hydroxyl (OH–) ions sent to the cathode via an anion exchange membrane. As depicted in the equations (6) to (8), these ions undergo oxidation at the cathode to yield oxygen (O2).
Anodic reaction:
Cathodic reaction:
The overall reaction:
The process underlying the electrochemical conversion of N2 to NH3 is not completely understood owing to its complex character. Examining the reaction mechanisms associated with the ammonia synthesis process is essential, but comprehending the pathways is important for attaining electrochemical ammonia production. The formation process involves associative and dissociation mechanisms (Xu et al., 2020; Ma et al., 2021; He et al., 2024). The nitrogen reduction process's reaction pathways depend on the arrangements in which N2 molecules are adsorbed. During the nitrogen reduction reaction, alternating and distal channels allow for the end-on adsorption of nitrogen, as seen in Fig. 2.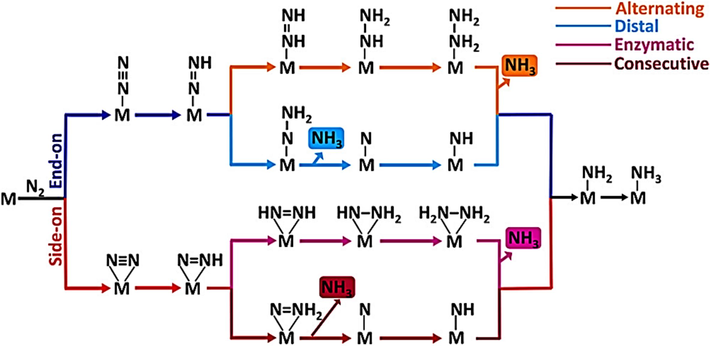
The diagram illustrates the process of N2 reduction to NH3 using different pathways. Used by permission (Ma et al., 2019). Copyright 2019, American Chemical Society.
The electron acceptor–donor mechanism involving the vacant d-orbitals of transition metals and nitrogen's empty π* orbitals plays a crucial role in activating nitrogen molecules for the NRR (Zhang et al., 2021; Paul et al., 2023). Transition metals have vacant or partially filled d-orbitals that can participate in bonding interactions with other molecules. These d-orbitals can accept electrons from or donate electrons to reactant molecules, making them highly versatile catalysts. N2 molecules have a strong triple bond (N≡N), which makes them chemically inert under standard conditions. The π* (antibonding) orbitals of N2 are typically empty and play a key role in its activation. When N2 approaches the surface of a transition metal catalyst, it gets adsorbed onto the metal surface. This adsorption is the first step in the activation process. The N2 molecule aligns itself such that its π* orbitals are close to the metal’s d-orbitals. The transition metal can donate electrons from its d-orbitals into the empty π* orbitals of N2. This electron donation weakens the triple bond in N2, making it more reactive. Additionally, this procedure may include back-donation, in which electrons from the occupied π-bonding orbitals of N2 may be moved to the unoccupied d-orbitals of the metal. This creates a synergistic effect that further diminishes the strength of the N≡N bond. An electron transfer occurs, resulting in the creation of an activated N2 complex on the surface of the catalyst. This complex is characterized by a partially broken N≡N bond, making it more susceptible to subsequent chemical reactions. Once N2 is activated, it can undergo further reduction steps, adding additional electrons and protons to the system. These steps eventually lead to the formation of ammonia (NH3). The specific pathway can vary depending on the catalyst and reaction conditions, but the initial activation through electron transfer is a common and critical step.
Enzymatic and sequential pathways are involved in the nitrogen reduction reaction, which leads to side-on nitrogen adsorption (Ma et al., 2019). The HBp employs a dissociative pathway to reduce nitrogen into ammonia. Before undergoing hydrogenation, the triple bonds within the adsorbed N2 molecule are broken, leading to the presence of N atoms on the surface of the catalyst. These N atoms are then subjected to hydrogenation, resulting in the production of ammonia (Wang et al., 2019). This dissociative pathway prevents the electrochemical reduction of nitrogen to ammonia under normal circumstances since it requires a large amount of energy. The adsorbed nitrogen molecules with two nitrogen centers are protonated in an associative alternating pathway awaiting the final bond between N-N is dissociated. The process of N2 reduction to NH3 using different pathways are shown in Fig. 2 (Shipman and Symes, 2017). The initial ammoflow nia molecule is primarily formed by the dissociation of the last N-N bond, while the remaining ammonia molecules are generated through sequential proton addition. The first ammonia molecule is produced through consecutive hydrogenation reactions within the associative distal pathway, specifically targeting the N atoms farthest from the catalyst. Following this, the second ammonia molecule is created by gradually introducing protons onto the catalyst's surface, where they interact with the captured nitrogen atom. From a thermodynamic perspective, the electrochemical reduction of N2 to yield NH3 is notably favored through distal and alternate pathways involving associative mechanisms under ambient circumstances (Yang et al., 2020; Paul et al., 2023). First, ammonia molecules are formed by dissociating the last N-N bond, and the progressive addition of protons produces second and subsequent ammonia molecules. The first ammonia is produced in the associative distal pathway by a sequence of subsequent hydrogenation events directed at the N atoms farthest from the catalyst. Following this, the second ammonia molecule is produced by gradually introducing protons onto the catalyst's surface, where they interact with the trapped nitrogen atom. This process contributes to the overall synthesis of ammonia.
2.2 Reaction kinetics and thermodynamics
Nitrogen gas is frequently utilized to establish an inert atmosphere and serve as a carrier gas in reactive environments due to the remarkable stability of the nitrogen molecule. Despite a strong triple bond between nitrogen-nitrogen with a prominent dissociation energy of 941.0 kJ mol−1, this feature alone does not explain the inertness of nitrogen. Surprisingly, compounds such as carbon monoxide (CO, 1070.0 kJ mol−1) and acetylene (HCCH, 963.0 kJ mol−1) exhibit much greater reactivity with even higher triple bond dissociation energies than nitrogen. Several factors contribute to nitrogen's inertness. To begin with, the nitrogen molecule has a lower proton affinity than methane and no stable dipole (493.8 kJ mol−1). Its negative electron affinity is −1.9 eV, its ionization potential is 15.83 eV, and a notable energy gap of 10.82 eV between its lowest unoccupied and highest occupied molecular orbitals. When combined, these properties obstruct electron transfer reactions, which makes it difficult to activate nitrogen atoms even in extremely acidic settings, where quick protonation is not always possible (Van der Ham et al., 2014; Jia and Quadrelli, 2014). The process of hydrogenation of nitrogen to produce ammonia does not happen spontaneously (with a standard enthalpy change of formation of −92.22 kJ mol−1 and a standard Gibbs free energy change of formation of −16.48 kJ mol−1), but it is thermodynamically favored. The formation enthalpies of diazene (N2H2, ΔHf° = 212.90 kJ/mol for (Z)-diazene) and hydrazine (N2H4, ΔHf° = 95.34 kJ mol−1) exhibit very high values. Due to the extremely high energy of the predicted intermediates, producing ammonia via a homogenous (non-catalytic) method is currently impossible (Jia and Quadrelli, 2014).
Moreover, these intermediates are part of the reaction pathway but can divert the process from the desired ammonia production, reducing overall yield. Understanding and controlling the formation of these intermediates is crucial for optimizing the NRR. Ammonia conversion efficiency can be improved by: (i) Design heterogeneous catalysts that favor the formation of NH3 over other intermediates. Transition metals such as Fe and Mo have shown promise in selectively reducing N2 to NH3. By tuning the electronic properties of these catalysts, one can enhance selectivity (Adalder et al., 2023); (ii) Adding promoters such as alkali metals (e.g., K, Na) can improve the electron density at the active sites, promoting the selective reduction of N2 to NH3. Surface functionalization with specific groups can create an environment that stabilizes the desired intermediates leading to NH3, thereby suppressing the formation of N2H₄ (Wang et al., 2020); (iii) Fine-tuning the applied potential during electrochemical NRR can help in controlling the energy landscape of the reaction pathway, promoting NH3 production and suppressing other intermediates (Paul et al., 2023); (iv) Using in-situ techniques like in-situ X-ray absorption spectroscopy (XAS) and in-situ infrared spectroscopy (IR) to monitor the formation of intermediates can provide insights into the reaction mechanism. This allows real-time adjustments to reaction conditions to favor NH3 formation (Zaera, 2021), and (v). Using density functional theory (DFT) calculations to predict the most favorable reaction pathways and design catalysts that selectively reduce N2 to NH3 (Yao et al., 2020). Computational studies can help identify the binding energies of intermediates and guide experimental efforts. By implementing these strategies, we can enhance the efficiency of ammonia conversion in the NRR, suppress the formation of unwanted intermediates like hydrazine, and improve the overall yield of NH3 (He et al., 2022). These approaches provide a deeper understanding of the reaction mechanisms and pave the way for designing more effective catalysts and reaction systems for ammonia synthesis.
Heterogeneously catalyzed ammonia synthesis involves the formation of chemisorbed intermediate complexes, which offer an alternate pathway for the reaction with much-reduced energy barriers that can be surmounted by thermal energy. Six protons and six electrons must be transferred to convert nitrogen into ammonia through a multi-step electrochemical process similar to the catalytic reduction of nitrogen. It is important to note that under acidic and alkaline circumstances, the equilibrium potentials of the half-reactions leading to nitrogen reduction and hydrogen evolution are different (Deng et al., 2018; Cui et al., 2018). The electrode's suitable negative potential should accelerate the N2 reduction process at both atmospheric pressure and room temperature. The equilibrium potentials for reactions (9)–(12), which produce hydrogen, are like those for processes which reduce N2. Conversely, in a solitary half-reaction, the H2 evolution mechanism necessitates only two electrons to produce one hydrogen. In contrast, the E° for the 6-electron reduction of nitrogen to ammonia is determined by averaging the values across multiple individual steps.
Reactions (13)–(15) offer the thermodynamic parameters (E° values) for further two-electron reductions from N2 to NH3. Unless explicitly mentioned otherwise, these E° values are determined through calculations based on empirically derived ΔGf° (298K). It's crucial to remember that other values have been published in study articles and investigations since the source and that the origin of some E° figures may not be clear. When possible, references to ΔGf° (298.0K) values mitigate potential misinterpretation. In most cases, the ΔGf° (298.0K) values utilized in calculating E°, unless specified otherwise, have been sourced from the National Bureau of Standardization database (Wagman et al., 1982).
To overcome the difficulty of activating the inert molecule, the hybrid electrochemical loop (HETL) approach combines the electrochemical step with the thermochemical step to convert N2 to NH3 and CO2 to C (Singh and Rohr, 2017; Luc et al., 2018). Lithium (Li) is the sole metal capable of reacting with nitrogen gas (N2) under normal room circumstances. This unique property positions Li as a promising option for selectively activating N2. McEnaney et al. have just published a study on a lithium-mediated high-entropy transition metal nitride method for the synthesis of ammonia. The procedure commenced by employing an electrochemical technique to generate lithium metal from lithium hydroxide (LiOH) within an electrochemical cell utilizing molten salt at a reaction temperature ranging from 600 to 700 K (McEnaney et al., 2017). The cycle was completed when the resultant metallic Li interacted with N2 to create lithium nitride (Li3N), which was then exposed to water to create NH3. (Fig. 3) (Siddiqui and Dincer, 2018; Jiao and Xu, 2019).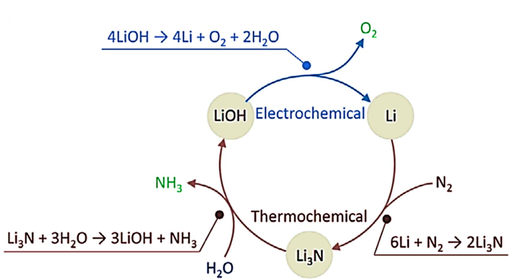
Schematic diagram of ammonia synthesis via electrochemical and thermochemical looping method.
Given that quantifiable yields were obtained at all thermochemical stages, the NH3 process had a FE of 88.5%, much greater than that of the SOA-N2 electrolysis process (FE, 10%). Furthermore, the LiOH electrolysis approach demonstrated an impressive current density surpassing 500 mA cm−2. This value exceeds the current density achieved through the state-of-the-art SOA-N2 electrolysis method by two orders of magnitude. Nevertheless, the conversion of Li+ to metallic Li through electrical reduction necessitates a cell potential exceeding −3.1V. Consequently, the overall energy expenditure for ammonia production using the HETL method amounts to 1.08 MJ mol−1 NH3. Li-mediated looping costs 0.93 MJ mol−1 NH3 under ideal conditions, suggesting limited potential for enhancement (Jiao and Xu, 2019). Fig. 4 illustrates a comparative analysis of the energy expenditure associated with several techniques of ammonia production.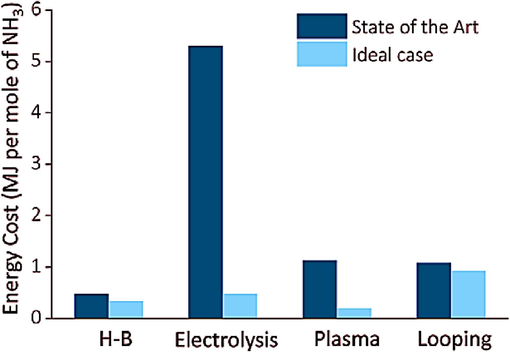
A comparison of the energy cost for the NH3 synthesis through the Haber–Bosch process (H-B), Electrolysis, Plasma, and Looping methods (McEnaney et al., 2017; Mehta et al., 2018).
The thermodynamic method has also established an energetic relationship known as the “scale relationship” among the mediates in catalytic interactions encompassing multiple intermediate steps (Koper, 2013). When examining oxygen evolution and reduction reactions, it becomes evident that the adsorption energies of the intermediates OH and UN consistently exhibit a 3.2 ± 0.2 eV variance. This discrepancy exceeds the 2.46 eV difference typically observed in an ideal catalyst. This energy difference can be attributed to the fact that both intermediates share an identical coordination on the catalyst surfaces. Therefore, oxygen evolution and reduction reactions need a minimum overvoltage of 0.25 to 0.35 V (Koper, 2013; Man et al., 2011). The binding energies of NH2 and N2H are interdependent in the N2 reduction reaction, leading to an unfavorable energy scaling between these reaction intermediates. This unfavorable scaling leads to a minimum overvoltage of 0.4 V (Van der Ham et al., 2014). It is necessary to overcome scale relationships to create better electrocatalysts. One potential avenue involves discovering reaction pathways that do not necessitate the participation of one of the intermediates. Alternatively, an alternative approach is to design catalysts capable of binding the two intermediates in distinct and unique ways (Brown et al., 2014; Koper, 2013). The performing temperature of the electrochemical cell significantly influences the thermodynamics of N2 reduction. Illustrated in Fig. 5 are the thermodynamic voltages essential for electrolysis of ammonia production and water splitting, spanning a temperature range from 25 to 900 °C. In this temperature range, negative values signify that synthesizing ammonia from N2 and H2 (illustrated by red squares) occurs spontaneously based on thermodynamic principles, particularly at a pressure of 0.1 MPa. Conversely, the two half-reactions essential to produce ammonia from N2 and H2O (depicted by green circles) are non-spontaneous, necessitating an external energy source to drive them. For instance, at a temperature of 25 °C, a minimum voltage of 1.17 V is involved to facilitate these reactions. At temperatures below approximately 175 °C, a somewhat lower minimum voltage is necessary for water splitting, as indicated by the blue triangles (Qing et al., 2020).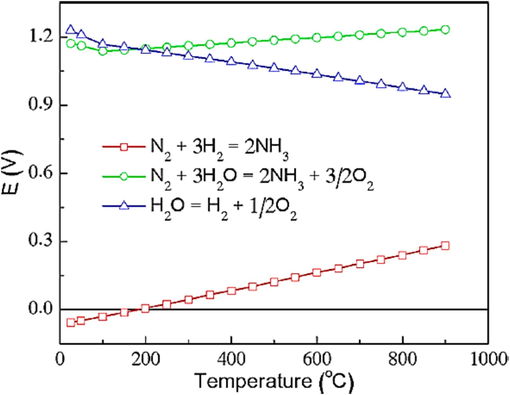
The graph illustrates the thermodynamic electrolysis energies for various processes, including water splitting (indicated by blue triangles), ammonia construction from N2 and H2O (denoted by red squares), and the reactions involving N2 and H2O (represented by green circles) (Qing et al., 2020). Copyright 2020 American Chemical Society.
2.3 Challenges for electrocatalytic nitrogen reduction to ammonia
Significant advancements have recently been yielded in the electrochemical production of ammonia in aqueous solutions under standard conditions. Nevertheless, the inadequate rates of ammonia generation and the low Faraday efficiency significantly restrict its practical utility (Kibsgaard et al., 2019; Suryanto et al., 2019). The leading constraints of the catalytic electrochemical performance are: (i) Due to the little overpotential, the FE is impeded by a major side reaction known as hydrogen evolution reaction (HER) (Montoya et al., 2015). (ii) In the process of N2 conversion, the N2 molecule experiences chemical adsorption onto the active site of the catalyst. Subsequently, the catalyst provides electrons to initiate the activation process (Kuang et al., 2020). With the elevated dissociation energy (941 kJ/mol) and a little proton attraction of N2 molecules owning to triple bonds, it is hard for nitrogen molecules to adsorb on the catalyst’s active site and activate, resulting in low electrochemical performance of the catalyst for ammonia production (Chen et al., 2023). (iii) The constrained solubility and suboptimal mass transport of nitrogen in aqueous electrolytes significantly impact the electrocatalytic efficiency of the catalyst. To attain a heightened rate of ammonia synthesis, improving nitrogen transfer can be achieved by enhancing nitrogen's solubility in the electrolyte and increasing the catalyst's surface area. (iv) The primary parameter for evaluating the electrocatalyst's performance in ammonia production is the measurement of the quantity of ammonia generated. Various techniques are employed for including ion-selective electrode detection, ammonia detection, ion chromatography, isotope detection and spectrophotometry (Cui et al., 2018). (v) The amplification process from lab-scale H-type cells to industrial-scale flow cells significantly impacts catalyst requirements. Industrial-scale NRR processes demand catalysts with enhanced durability, scalability, and efficiency, capable of operating under continuous flow conditions. Developing such catalysts requires a comprehensive understanding of material properties, reaction mechanisms, and reactor design. Addressing these challenges through innovative research and development is crucial for advancing sustainable and efficient nitrogen reduction technologies at an industrial level.
Spectrophotometry primarily relies on using Nessler’s reagent and the indophenol blue techniques. These approaches are widely favored for ammonia detection owing to their cost-effectiveness and remarkable sensitivity. The indophenol blue method stands out because it provides low detection limits (10 × 10−6 mol L−1), excellent color representation, and outstanding reproducibility. On the other hand, the Nessler reagent method offers advantages in minimizing reagent consumption and efficiently utilizing time. However, its color reagent, mercury, does not effectively safeguard the environment. Nevertheless, the presence of metal ions in the solution can result in imprecise results. A control experiment has been proposed to remove any potential sources of interference (Tang and Qiao, 2019). The ion-selective electrode method is a rapid and straightforward technique capable of detecting a broad spectrum of concentrations, ranging from 0.01 to 17,000 ppm. Maintaining precise control over the temperature and stirring velocity is crucial throughout the experimental procedure. Conversely, ion chromatography is renowned for its exceptional accuracy, reproducibility, and strikingly low 3 × 10−7 mol L−1 detection limit. The electrolytes that include Na+ and Li+ are not appropriate for this function because of their short retention times, and organic electrolytes are also not useful (Cui et al., 2018). Furthermore, experiments with the 15N2 isotope are also necessary to determine the ammonia's source, namely the nitrogen-containing catalyst responsible for the nitrogen reduction reaction. Using at least two different detection techniques to accurately identify the quantity of goods is advisable since this will provide more compelling results (Hao et al., 2019).
3 Electrocatalytic conversion of nitrogen into ammonia
In response to the persistent need for high-efficiency electrochemical energy conversion and storage devices, many catalysts have been extensively investigated to evaluate their suitability as economical and effective electrode materials for various electrochemical reactions. Catalytic performance is profoundly affected by factors like crystallinity, shape, particle size, and the abundance of surface-active sites, widely recognized as pivotal considerations in this sphere. The discovery of new electrocatalysts via a mix of theoretical understanding and experimental innovation has been crucial to the progress in the electrochemical reduction of N2 to NH3 (Ghorai et al., 2021). These electrocatalysts have played a vital role in enabling these substantial achievements. Most of the experimental research has been on developing catalysts composed of noble metals, transition metals, and non-metals to address the kinetic limitations of the nitrogen reduction reaction and enhance selectivity under normal circumstances. Recently, various attempts have been made to obtain varying ammonia production rates and FE values, ranging from less than one percent to several tens of percent, employing an electrocatalyst under normal conditions (Table 1). This section examines the correlation between the performance and structure of electrocatalysts, considering both theoretical and experimental perspectives. This article comprehensively summarises the latest electrocatalyst advancements used for nitrogen reduction processes. The ammonia formation from NO3– and NO2– can be given by the reactions (16)–(18):
Electrocatalyst
Ammonia production rate (mol·s−1 cm−2/µg mg–1cat. h−1/Sμg/h cm−2)
Faradaic Efficiency (FE)
Electrolyte
Ref.
TiO2/Ti
9.16 × 10−11
2.50 %
0.1 M Na2SO4
(Zhang et al., 2018)
MoS2/CC
8.08 × 10−11
1.17 %
0.1 M Na2SO4
(Zhang et al., 2018)
MoO3 nanosheet
4.80 × 10−10
1.9 %
0.1 M HCl
(Han et al., 2018a)
Mo nanofilm
3.09 × 10−11
0.72 %
0.01 M H2SO4
(Yang et al., 2017)
Ru/C
3.43 × 10−12
0.28 %
0.1 M HCl
(Kordali et al., 2000)
PEBCD/C
2.58 × 10−11
2.85 %
0.5 M Li2SO4
(Chen et al., 2017)
Fe2O3-CNT
3.58 × 10−12
0.15 %
KHCO3
(Chen et al., 2017)
Au nanorod
2.69 × 10−12
4 %
0.1 M KOH
(Bao et al., 2017)
CP2TiCl2
9.50 × 10−10
0.23 %
H2O
(Jeong et al., 2017)
MnO/TM
1.11 × 10–10
8.02 %
0.1 M Na2SO4
(Wang et al., 2019)
Mo nanofilm
3.09 × 10–11
0.72 %
0.01 M H2SO4
(Yang et al., 2017)
Mo2N
4.60 × 10−10
4.5 %
0.1 M HCl
(Ren et al., 2018)
VN/TM
8.40 × 10–11
2.25 %
0.1 M HCl
(Suryanto et al., 2018)
Ag nanosheet
4.62 × 10−11
4.8 %
0.1 M HCl
(Huang et al., 2018)
γ-Fe2O3
0.212
1.9 %
0.1 M KOH
(Kong et al., 2017)
Bi4V2O11/CeO2
23.21
10.16 %
0.1 M HCl
(Lv et al., 2018)
N-doped porous carbon
23.8
1.4 %
0.05 M H2SO4
(Liu et al., 2018)
Au nanorods
1.648
3.88 %
0.1 M KOH
(Bao et al., 2017)
B4C
26.57
15.95 %
0.1 M HCl
(Qiu et al., 2018)
Nb2O5 nanofiber
43.6
9.26 %
0.1 M HCl
(Han et al., 2018b)
Hollow Cr2O3 microspheres
25.3
6.78 %
0.1 M Na2SO4
(Zhang et al., 2018)
TiO2-rGO
15.13
3.3 %
0.1 M Na2SO4
(Zhang et al., 2018)
Fe2O3 nanorods
15.9
0.94 %
0.1 M Na2SO4
(Xiang et al., 2018)
Defect-rich MoS2 nanoflower
29.28
8.34 %
0.1 M Na2SO4
(Li et al., 2018)
Cr2O3/CPE
28.13
8.56 %
0.1 M HCl
(Du et al., 2018)
TA-reduced Au/TiO2
21.40
8.11 %
0.1 M HCl
(Shi et al., 2017)
α-Au/CeOx-RGO
8.31
10.1 %
0.1 M HCl
(Li et al., 2017)
Au flowers
25.57
6.05 %
0.1 M HCl
(Wang et al., 2018)
β-FeOOH nanorods
23.32
6.7 %
0.5 M LiClO4
(Zhu et al., 2018)
AuHNCs
3.90
30.2 %
0.5 M LiClO4
(Nazemi et al., 2018)
Ag-Au@ZIF
0.61
18 %
THF-based
(Lee et al., 2018)
Ru/Ti
7.34
−
0.5 M H2SO4
(Kugler et al., 2015)
MoN
0.06
1.15 %
0.1 M HCl
(Zhang et al., 2018)
Fe3O4/Ti
0.012
2.6 %
0.1 M Na2SO4
(Liu et al., 2018)
3.1 Transition metal-based electrocatalysts
Transition metals are the predominant nanoparticles, exhibiting either crystalline or amorphous structures, characterized by their extremely small dimensions, large surface area, and numerous active edge sites. Transition metals are predominantly utilized in catalysis due to the relative simplicity of synthetic methods involved in their production, resulting in the majority of nanomaterials employed in this field. Both theoretical and practical investigations have demonstrated that the utilization of transition metal catalysts enhances the performance of nitrogen reduction reactions. It is important to emphasize that the performance of these transition metal catalysts is significantly elevated in both alkaline and acidic environments (Geng et al., 2018; Liu et al., 2018; Han et al., 2019). Sclafani et al. used an iron cathode and a stainless-steel anode to study the electrochemical synthesis of ammonia in a 6 M aqueous KOH solution. The experiment continued until an equilibrium state was achieved, as confirmed by the absence of ammonia in the liquid, indicating a stable rate of NH3 formation. Remarkably, they attained an impressive ammonia generation rate of 5.3 × 10−14 mol s−1 cm−2 at a voltage of −1.06 V, with a Faraday efficiency of approximately 1%. The cathode voltage, relative to the saturated calomel electrode (SCE), ranged from −0.92 to −1.21 V. The rate of ammonia synthesis experienced a decline when the cathodic potential intersected with the hydrogen evolution region, attributable to the competition between the two processes. In a subsequent experiment by Grayer et al., a Faraday efficiency of up to 5.4% was discovered (Grayer and Halmann, 1984). Most of the ammonia, according to the scientists, was created by the electrolyte's elimination of N2-containing contaminants.
In their experiment, Geng et al. utilized Ru SAs/N-C, which refers to single Ru atoms scattered on nitrogen-doped carbon, demonstrating excellent Faraday efficiency for N2 electroreduction (Geng et al., 2018). Tao et al. (2019) used a monatomic Ru catalyst and nitrogen-doped porous carbon to achieve outcomes comparable to Geng et al. (2018). The first instance of catalysts with a single Fe site was presented by Lü et al. (2019). The hydrothermal technique was used to create frameworks composed of bimetallic Fe/Zn zeolite. After carbonization and etching processes, the framework was converted into individual iron sites connected to nitrogen-doped carbon frameworks. These specific iron sites are denoted as ISAS-Fe/NC. The distribution of Fe, N, and C elements was mapped using EDX spectroscopy to analyze their composition. The results illustrated a uniform dispersion of iron (Fe) and nitrogen (N) elements within the framework. The atomic arrangement of the Fe sites was elucidated via XANES. During the NRR testing, a notably high Faraday efficiency of 18.6 ± 0.8% was achieved, employing a 0.1 M phosphorus-saline buffer solution (PBS). This solution is pondered as a more ecologically sustainable choice compared to acidic or alkaline alternatives. The rate of ammonia production was 62.9 g per hour per square centimeter. Single-atom catalysts provide the highest recorded Faraday efficiency and ammonia-generating rate for Fe, while also demonstrating good selectivity for NRR.
In their study, Cai et al. (Cai et al., 2022) examined the electrocatalytic performance of noble metal catalysts for the reduction of N2 in aqueous solutions under normal circumstances. The study employed commercially available catalysts, including nanoparticles of noble metals (Pt, Pd, Rh, Au) that were uniformly dispersed on carbon support. The particle sizes varied between 1.5 and 7 nm. The assessments were conducted in an H-type cell utilizing 0.1 M HCl as the electrolyte. The obtained outcomes were then compared to a lithium-mediated approach in nonaqueous electrolytes. Surprisingly, the noble metal catalysts showed little to no ammonia production despite their known catalytic properties. This discrepancy was attributed to the presence of nitrogen-containing contaminants in the catalysts. To obtain reliable results, the study demonstrated the importance of removing these contaminants by consecutive cyclic voltammetry (CV) scans. In contrast, the lithium-mediated system produced significant ammonia, highlighting its effectiveness for nitrogen reduction.
In a groundbreaking approach to electrochemical ammonia synthesis, Davide et el. (Ripepi et al., 2021) have introduced an innovative catalyst design that separates the activation of hydrogen and nitrogen, facilitating the production of ammonia at ambient conditions (Fig. 6). This unconventional electrochemical setup utilizes a dense metallic hydrogen-permeable electrode, primarily composed of nickel. This electrode is a pivotal component in this system, allowing for the independent activation of hydrogen and nitrogen. In the proposed system, a two-compartment setup is created, with the hydrogen activation occurring on one side (in contact with the electrolyte) and the nitrogen activation on the opposite side (in contact with gaseous nitrogen). The electrolyte facilitates the electrochemical water oxidation and reduction reactions, driving the production of atomic hydrogen within the electrode lattice. This atomic hydrogen is then allowed to permeate through the nickel electrode, ensuring its controlled access to the nitrogen activation sites while blocking the ingress of unwanted species like oxygen and water. The process of adsorbed N2 interacting with diffusing atomic hydrogen produces ammonia. The critical insight is that atomic hydrogen effectively circumvents the need for available active sites on the catalyst surface for hydrogen and nitrogen dissociation, overcoming a major challenge in ammonia synthesis. Additionally, this setup offers the advantage of directly producing ammonia in the gas phase, making product separation easier and preventing ammonia from diffusing back to the anode surface. In addition, the researchers discovered that active N2 on the nickel surface obstructs the process of activating gaseous hydrogen. This emphasizes the advantages of utilizing permeating atomic hydrogen for nitrogen hydrogenation.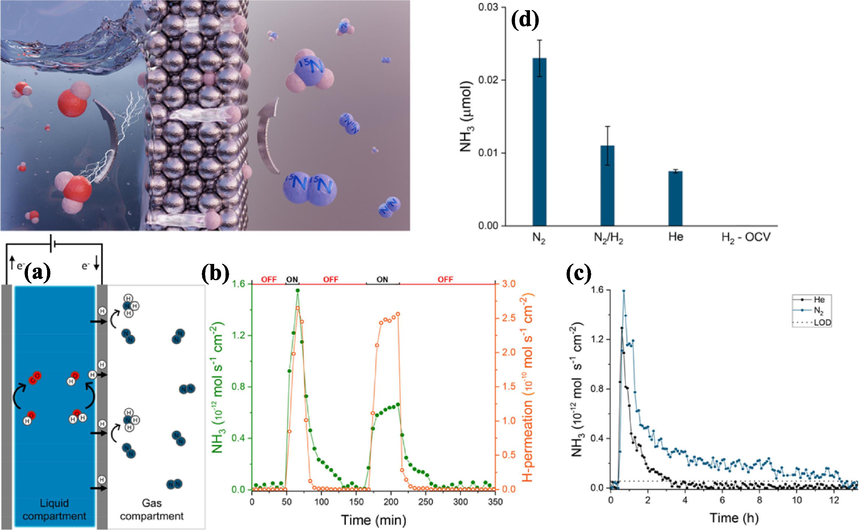
The diagram provides a conceptual representation of the proposed setup for direct ammonia production through electrochemically generated and permeating atomic hydrogen. Within this configuration, a slender and compact metallic cathode is employed to facilitate the passage of atomic hydrogen. This system comprises two distinct active interfaces: one interacts with the electrolyte to enable hydrogen electrochemical activation (situated on the liquid compartment side), while the other interfaces with the gas compartment to initiate dinitrogen activation and hydrogenation processes. The direct interaction of adsorbed nitrogen with the atomic hydrogen that seeps through it produces ammonia within the gas compartment. Using a 0.0125 mm thick Ni-electrode, the rate of ammonia synthesis (solid green symbols) and the electrochemical penetration of hydrogen (open orange symbols) are tracked over time in the accompanying graph (b). When switching from open circuit circumstances (OFF) to cathodic charging, data is gathered (ON). The release of atomic hydrogen from the gas compartment and the interaction of hydrogen with nitrogen to generate NH3 are the two processes that lead to hydrogen gas (H2). The long-term rate of ammonia generation in N2 (blue) and He atmospheres is investigated in panel (c) (black). An increased rate of synthesis and an extended duration of ammonia (NH3) production up to nine hours are seen in the nitrogen gas (N2) environment. The point at which detection becomes feasible is known as the limit of detection (LOD) threshold, and the black dotted line shows it. Lastly, after 12 h of electrochemical hydrogen permeation under normal circumstances, panel (d) compares the amount of ammonia generated in the nitrogen (N2), helium (He), and combination of nitrogen and hydrogen (N2:H2, 96:4%) atmospheres. Compared to He, the quantity of NH3 is around three times more when N2 is present. Notably, ammonia did not show up when pure H2 was utilized in the absence of electrochemical hydrogen permeation. The figures' error bars show the standard deviation computed from three or more measurements using recently taken samples (Ripepi et al., 2021). Copyright 2021 American Chemical Society.
The Co−Mo−CNF catalyst, featuring molybdenum and cobalt within a carbon nanofiber matrix, has been meticulously designed to amplify NRR selectivity towards NH3. Cobalt, known for its exceptional water dissociation capabilities, augments the local proton supply around molybdenum, a critical site for NRR hydrogenation. This groundbreaking catalyst demonstrates remarkable ammonia (NH3) production, attaining a 72.72 μg h−1 mg−1 rate and an exceptional Faraday efficiency of 34.5% at a voltage of −0.5 V vs. RHE (Chung et al., 2022). An innovative approach emerges by creating hybrid Cu2O-CeO2-C nanorods on a copper mesh scaffold, achieved using a metal–organic framework template technique. This breakthrough converts a copper-loaded cerium-based metal–organic framework (Ce-MOF) into heterojunctions consisting of copper oxide (Cu2O) and cerium oxide (CeO2), which contain interfacial structures of copper-oxygen-cerium (Cu-[O-X]-Ce). These structures are embedded inside a carbon matrix. Theoretical analysis emphasizes the reduced establishment energy of oxygen vacancies within Cu-[O-X]-Ce complexes, in contrast to standalone Cu2O or CeO2 phases, indicating their advantageous characteristics. The existence of oxygen-deficient Cu-[O-X]-Ce structures promotes the creation of Cu(I) species abundant in electrons at their interfaces, significantly enhancing the electrocatalytic N2 conversion. This pioneering catalyst showcases an impressive NH3 generation rate of 6.37 × 10−3 µg per second per square centimeter and a commendable FE of 18.21% at −0.3 V compared to the reversible H2 electrode. The significance of charge distribution manipulation in Cu-based electrocatalysts for enhancing nitrogen reduction activity is emphasized by this study (Jing et al., 2022).
The performance of a novel electrocatalyst designed for nitrogen reduction, comprising a vanadium single-atom dimer (V-O-V) on N-doped carbon (O-V2-NC), is exceptionally remarkable. The oxygen-spanned metal atom dimer acts as the electron-deficient active center for N2 reduction in this catalyst, which has a vanadium dimer coordinated with N2 and bridged by oxygen. Theoretical simulations unveil a dynamic structural transformation process during the reaction, with the V-O-V core undergoing protonation, resulting in a temporary disintegration into V-O and V species. However, it subsequently reassembles into its original configuration upon the complete release of all nitrogen species. The O-V2-NC structure exhibits outstanding electrochemical NRR performance, characterized by a notable Faraday efficiency of 77% and a yield of 9.97 µg mgcat.−1 h−1 at 0 V (versus the reversible hydrogen electrode, RHE). Additionally, it demonstrates substantial ammonia production, generating 26 µg mgcat.−1 h−1 with a 4.6% Faraday efficiency at −0.4 V (versus RHE) (Wang et al., 2023). The research centers on creating Ag-doped Cu nanosheets supported on carbon paper, denoted as Ag-Cu-NS/CP, as highly effective electrocatalysts designed for NRR. The presence of Ag causes the creation of Cu sites that lack electrons in the Ag-Cu-NS/CP system. The altered copper sites effectively inhibit the HER and enhance the adsorption of N2 in neutral conditions, resulting in a substantial improvement in NRR activity. When operated at −0.4 V relative to the reversible H2 electrode, the Ag-Cu-NS/CP catalyst demonstrates an impressive Faraday efficiency of 20.9% and an NH3 generation rate of 61.5 µg mgcat.−1 h−1 within a 0.1 M Na2SO4 electrolyte. This research introduces a straightforward yet highly effective method for producing potent and selective electrocatalysts tailored for NRR (Qu et al., 2022).
The researchers Xue et al. have presented a single-atom catalyst (SAC) called Mo@C9N4, which consists of a single Mo atom attached to the C9N4 substrate. This catalyst demonstrates remarkable efficiency in the conversion of N2 into NH3. The Mo@C9N4 structure has proven to be highly effective in catalyzing the activation and reduction of gaseous N2 to NH3 on its surface, as revealed through density functional theory (DFT) simulations. The NRR predominantly occurs at the Mo center, following a favored distal pathway with a limiting potential of 0.40 V, which is advantageous. Significantly, the Mo@C9N4 catalyst demonstrates remarkable structural durability and a notable preference for NRR (Xue et al., 2021). Interlayer strain compression within bismuth (Bi) nanocrystals has emerged as a pivotal aspect for enhancing the selectivity and activity in NH3 electrosynthesis via NO3– reduction. The lattice compression leads to shorter Bi-Bi bonds, which in turn broadens the 6p electron bandwidth, resulting in improved electron delocalization. This has been confirmed through extensive spectroscopic studies and theoretical calculations. As a result, it increases the chemical attractions of nitrogen compounds in between, making it easier to activate NO3– and lowering the energy needed for better performance. Furthermore, it reduces the release of *NO2, inhibiting nitrite production. The Bi electrocatalyst, when subjected to strain-compressive conditions, demonstrates an outstanding FE of 90.6% and a substantial NH3 production rate of 46.5 g gcat.−1 h−1, even at current densities as elevated as 300 mA cm−2, which can be easily implemented on an industrial scale. This innovation shows significant potential for the effective and specific synthesis of NH3 (Zhang et al., 2022).
A highly dispersed amorphous Cu catalyst demonstrated exceptional working in nitrate-reduction to ammonia, achieving an impressive ammonia yield rate of 1.42 mol gcat.−1h−1 and an extraordinary FE of 95.7%. This exceeds the capabilities of Cu catalysts that have undergone crystallization. The effectiveness of amorphous Cu can be ascribed to its higher quantity of catalytic sites, enhanced strength of NO3– adsorption with flat adsorption topologies, and greater facilitation of the potential deciding step of *NO protonation to *NHO. Significantly, the shapeless Cu catalyst has strong electrochemical durability at −0.3 V, while crystalline competitors show reduced effectiveness at more negative potentials (Shen et al., 2023). The electrocatalytic capability for ammonia production experiences a substantial boost thanks to a distinctive Ni foam catalyst crafted using an active H2 bubble template-supported electrodeposition method. The distinctive structure of the catalyst's foam enhances its efficiency. This process achieves an outstanding Faraday efficiency of over 95% for ammonia synthesis while operating within a low potential range of −0.1 to −0.3 V versus RHE. The only by-product of nitrate reduction is hydrogen. Remarkably, electrolysis only converts nitrate to ammonia without producing other nitrogen-containing compounds, such as NO, N2O, or N−2, demonstrating a 100% selectivity (Liu and Wang, 2022).
3.2 Transition metal alloys-based electrocatalyst
There has been a growing interest in the use of renewable electrical energy for the ambient electrochemical reduction of N2 to NH3. Because it uses less energy and has a greater energy conversion efficiency than current techniques, this environmentally friendly electrochemical N2 reduction process has a lot of promise as an ammonia manufacturing alternative (Lv et al., 2018; Cui et al., 2017). To facilitate electrochemical nitrogen reduction, there is an ongoing demand for active metallic catalysts that exhibit both exceptional activity and selectivity in ammonia production. The alloying of numerous transition metals produces synergistic effects that make it possible to create bimetallic nanostructures with much higher electrocatalytic activity than monometallic ones. Moreover, the incorporation of transition metals into alloys might reduce the utilization of precious metals. Transition metal alloys are advantageous in numerous applications due to their exceptional catalytic activity and cost-effectiveness. Xiang et al. (2018) produced a Pd0.2Cu0.8/RGO bimetallic catalyst by combining tannic acids, Cu, Pd, and graphene oxide precursors reduced using NaBH4. The Pd0.2Cu0.8/RGO catalyst exhibited an exceptional NH3 production rate of 2.80 g mgcat.−1 h−1. The Faraday efficiency is exceptionally high, reaching 4.6% when measured at 0.0 V compared to the RHE. A Faraday efficiency of 0.6% was observed in a nitrogen-saturated solution of 0.1 M aqueous KOH. Wang et al. synthesized Pd-Ru alloy catalysts featuring tripod and nanorod architectures. The tripod made of a RuPd alloy demonstrated a FE of 1.85% and a rate of ammonia generation of 37.23 g mgcat.−1 h−1 (1.94 × 10−10 mol s−1 cm−2) (Wang et al., 2019; Wang et al., 2019). Compared to Pd-Ru nano dendrites or pure Pd nanoparticles, a higher yield of ammonia was observed over the RuPd alloy tripod with higher structural stability. The formation of Pd-Ru nanorods was detailed to produce NH3 at an impressive rate of 34.2 g mgcat.−1 h−1 (equivalent to 5.62 × 10−11 mol s−1 cm−2) with an FE of 2.4%, based on the electrochemically working surface area. Given the NH3 generation per unit area, the nano-rod assembly structure may still be chosen even though tripod structures produce more NH3 per milligram of the catalyst.
Shi et al. introduced an innovative electrochemical flow-through cell to significantly advance sustainable wastewater treatment and ammonia fertilizer synthesis (Fig. 7). This system incorporates a porous working FeNi alloy cathode and a membrane extraction unit of hollow polypropylene fibers. It allows for the simultaneous electrochemical reduction of nitrate (NO3–) to ammonia and the in-situ generation of acid/base to facilitate NH3 extraction in the form of ammonium sulfate salts ((NH4)2SO4). The study's findings were impressive: The system demonstrated exceptional performance over 14 h, removing 150 mM NO3– from synthetic wastewater with a cathodic current density of 30 mA/cm2. It also obtained a 98 percent ammonia selectivity, 93 percent Faraday efficiency, and 97 percent total ammonia nitrogen (TAN) recovery. Of significant note, the NH3 recovery flux reached 2050 g-(NH4)2SO4/m2/d, and the specific energy consumption was measured at 11 kWh/kg-(NH4)2SO4, surpassing the performance of many existing processes. This research highlights the central role of direct electron transfer in electrochemical NO3– reduction to NH3. The analysis of interfacial reactions underscores the reactivity and specificity of the NiFe2O4 (3 1 1)-Ni site on the thermally working FeNi alloy surface, rendering it highly efficient for electrochemical NO3– reduction to NH3, surpassing the competitive processes of N2 or H2 generation (Shi et al., 2023).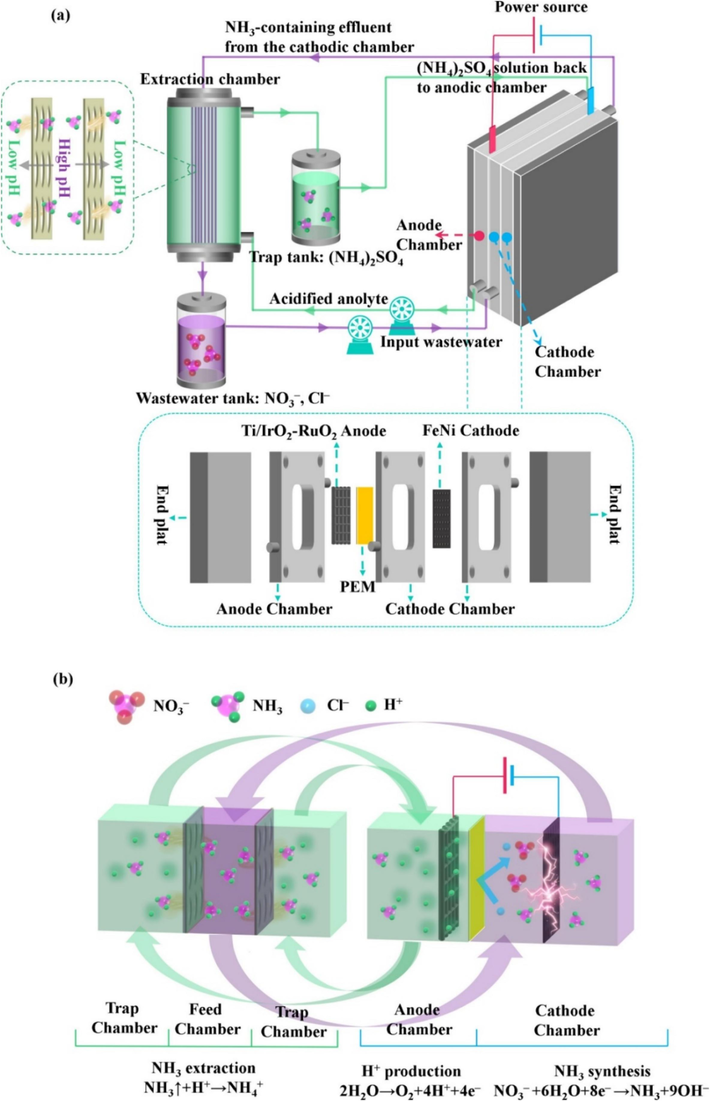
The schematic diagram illustrates the flow-through electrolysis cell that was employed, along with its operational principles (Shi et al., 2023). Copyright 2023, Elsevier.
3.3 Transition metal oxides-based electrocatalyst
There is a growing attention in the electrochemical synthesis of ammonia due to its energy-efficient nature and utilization of water as a hydrogen source. However, developing cost-effective materials and high performance for catalyzing N2 reduction reactions has been a bottleneck in enabling scalable ammonia production. While precious-metal-based electrocatalysts have shown promise in electrochemical ammonia synthesis, their high cost and low FE fall short of meeting industrial demands. To address this, the prospect of designing a transition metal oxide-based array architecture for ammonia electrosynthesis appears promising. The goal is to boost the production rate of NH3 and enrich FE. Transition metal oxide-based array architecture may offer a viable solution to overcome the limitations associated with current electrocatalysts in ammonia synthesis, making it more aligned with industrial requirements. Wang and colleagues devised a method for creating porous Fe2O3 nanorods on carbon cloth (p-Fe2O3/CC) through a combination of hydrothermal processes and high-temperature calcination, as illustrated in Fig. 8a. The resulting porous structure of p-Fe2O3/CC presented an abundance of active sites and open channels, facilitating nitrogen adsorption and its conversion into ammonia. Remarkably, the p-Fe2O3/CC catalyst demonstrated remarkable stability in the electrochemical conversion of N2 to ammonia, achieving a peak NH3 production rate of 13.56 g mgcat.−1 h−1 and a Faraday efficiency of 7.69% in a 0.1 M Na2SO4 solution at −0.4V vs. RHE. Notably, the ammonia production rate and Faraday efficiency declined when operating at a lower potential. This effect was assigned to the adsorption of H2 on the surface of the p-Fe2O3/CC catalyst, which blocked active sites, as illustrated in Fig. 8b (Wang et al., 2019).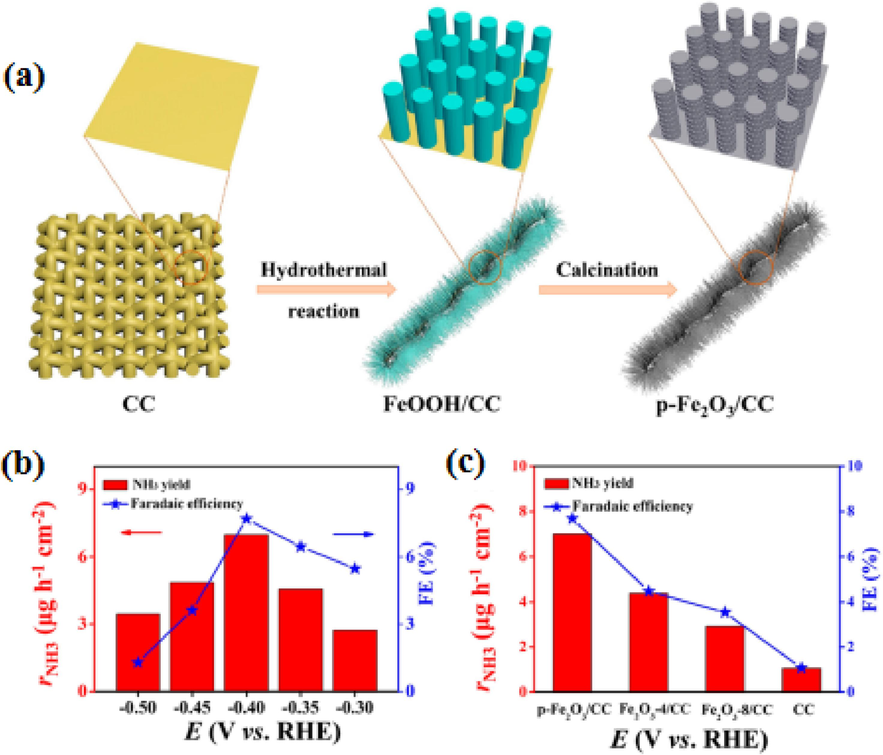
The study investigates the electrochemical nitrogen reduction using the p-Fe2O3/CC catalyst. The text contains a visual representation of the catalyst preparation process and data regarding the NH3 generation rate and Faradaic efficiency observed with the p-Fe2O3/CC catalyst under various potentials. Furthermore, it compares the NH3 generation rate and Faradaic efficiency between the p-Fe2O3/CC catalyst and the Fe2O3-4/CC, Fe2O3-8/CC, and CC catalysts. This content has been shared with permission (Wang et al., 2019). Copyright 2019, American Chemical Society (ACS).
Additionally, the influence of the duration of hydrothermal synthesis on the efficiency of the p-Fe2O3/CC catalyst was also examined. The study revealed that the p-Fe2O3/CC catalyst, synthesized hydrothermally for 6 h, had the maximum NH3 generation rate and FE. The exceptional performance of the p-Fe2O3/CC catalyst can be attributed to its notably higher double-layer capacitance (Cdl) value, measuring 46 mF cm−2. In contrast, the Cdl values for Fe2O3-8/CC and Fe2O3-4/CC stand at 21 mF cm−2 and 28 mF cm−2, correspondingly. This variance in Cdl values indicates the electrochemically working surface area (ECSA) for the catalyst, providing a multitude of active sites essential for the nitrogen reaction, as demonstrated in Fig. 8c. Additionally, the influence of the duration of hydrothermal synthesis on the efficiency of the p-Fe2O3/CC catalyst was also examined.
The study revealed that the p-Fe2O3/CC catalyst, synthesized hydrothermally for 6 h, had the maximum NH3 generation rate and FE The exceptional performance of the p-Fe2O3/CC catalyst can be attributed to its significantly higher double-layer capacitance (Cdl) value, which stands at 46 mF cm-2, a marked contrast to the values of 21 mF cm−2 for Fe2O3-8/CC and 28 mF cm−2 for Fe2O3-4/CC. This Cdl value indicates the electrochemically working surface area (ECSA), providing numerous active sites crucial for the nitrogen reaction, as illustrated in Fig. 7c. Hu et al. used a similar procedure in another investigation to create a Fe/Fe3O4 catalyst: they first oxidized the Fe foil and then reduced it electrochemically. This catalyst was subsequently utilized for the electrochemical conversion of N2 to NH3 under standard atmospheric conditions. Notably, the nitrogen reduction reaction demonstrated a substantial improvement in working the Fe/Fe3O4 catalyst compared to pure Fe foil. The FE of the Fe/Fe3O4 catalyst surpassed that of Fe foil by a remarkable 120-fold. This catalyst reached its peak ammonia production rate at 0.19 µg cm−2 h−1, accompanied by a FE of 8.29%, in a 0.1 M phosphate buffer solution at −0.3V versus RHE. Furthermore, the influence of oxidation temperature was investigated, revealing that the catalyst's performance can be accredited to the varying ratios of Fe to Fe3O4. In summary, the Fe/Fe3O4 catalyst displayed superior performance in the nitrogen reduction reaction when compared to Fe, Fe2O3, and Fe3O4 nanoparticles, emphasizing the efficiency of the Fe/Fe-oxide composite as a highly effective electrocatalyst (Hu et al., 2018). Liu et al. utilized the hydrothermal synthesis and prepared efficient Fe3O4 nanorods on Ti mesh (Fe3O4/Ti) electrocatalyst for electrocatalytic reduction from nitrogen to ammonia with long-term electrochemical stability. The Fe3O4/Ti electrocatalyst realized an NH3 generation rate of 5.6 × 10−11 mol s−1 cm−2 and FE of 2.6% in 0.1 M Na2SO4 at −0.4 V vs RHE. It showed a negligible loss in the electrochemical performance after its stability test 6 times (Liu et al., 2018).
Zhang and their team developed a TiO2 nanosheet array on a Ti plate (TiO2/Ti) through hydrothermal synthesis. They harnessed it as an exceptionally well-organized electrocatalyst for the electrochemical generation of ammonia from N2 in typical environmental conditions. The TiO2/Ti electrocatalyst achieved an impressive peak ammonia production rate, reaching 9.16 × 10−11 mol·s−1 cm−2 within a 0.1 M Na2SO4 solution at a potential of −0.7 V versus RHE. It is worth noting that while the TiO2/Ti electrocatalyst exhibited a peak FE of 3.34% for NH3 generation at −0.6V, this efficiency slightly decreased to 2.50% at −0.7V due to competing H2 evolution processes. Considering the rate of NH3 generation, the potential of −0.7V was identified as the most favorable, surpassing that of most electrocatalysts documented for the N2 reduction reaction under normal conditions (as indicated in Table 1). The exceptional performance of the TiO2/Ti electrocatalyst in NH3 synthesis can be ascribed to the creation of O2 vacancies, which significantly enhance the adsorption and activation of nitrogen during the electrochemical tests. The TiO2/Ti electrocatalyst demonstrated exceptional electrochemical durability in NH3 synthesis. After the 10-cycling test, the NH3 production rate and FE remained at 90.2% and 88.8% of their initial values, respectively. The electrode's decay effect during extended electrolysis had a minor impact on the losses (Zhang et al., 2018).
In a study by Zhang and colleagues, TiO2-rGO was synthesized using a hydrothermal technique and then applied to carbon paper (CP) to produce the TiO2-rGO/CP electrocatalyst. This electrocatalyst demonstrates remarkable selectivity in the electrochemical reduction of N2 to NH3. Specifically, the TiO2-rGO/CP electrocatalyst exposed a peak ammonia production rate of 15.13 µg mgcat.−1 h−1 and a high FE of 3.3% in a 0.1 M Na2SO4 solution at a potential of −0.90 V vs RHE. To assess the performance of various electrocatalysts during 2-hour electrolysis at −0.9V, a comparison was made involving different materials, namely CP, TiO2/CP, rGO/CP, and TiO2-rGO/CP, as depicted in Fig. 9(a) and 9(b). When CP was employed as an electrocatalyst in the nitrogen reduction procedure, it yielded significantly lower performance. However, the TiO2-rGO/CP electrocatalyst outperformed both the TiO2/CP and rGO/CP electrocatalysts due to the well-distributed TiO2 nanoparticles on rGO, providing an optimal contact of nitrogen-rich working sites for efficient adsorption and reduction into ammonia. In addition, TiO2-rGO/electrochemical CP's stability was thoroughly assessed using six consecutive recycling experiments at a potential of −0.9V (Fig. 9(c)). Because there were no changes in the NH3 production rate or FE throughout the testing, the findings showed the TiO2-rGO/CP electrocatalyst's exceptional electrochemical stability (Zhang et al., 2018).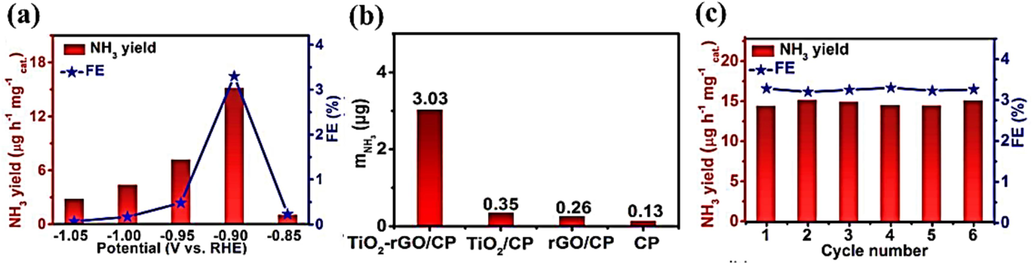
Here are the results from electrochemical nitrogen reduction using the TiO2-rGO catalyst: (a) The NH3 production rate and Faradaic efficiency of the TiO2-rGO/CP catalyst at various potentials in a 0.1 M Na2SO4 solution. (b) The amount of NH3 generated after two hours of electrolysis at −0.90 V under ambient conditions. (c) The NH3 production rate and Faradaic efficiency during six recycling tests at a potential of −0.90 V. The figures and data are presented with permission from the source (Zhang et al., 2018). Copyright 2018, Royal Society of Chemistry (RSC).
In a study conducted by Wu and colleagues, Mn3O4 nanocubes were synthesized through hydrothermal methods. Subsequently, these Mn3O4 nanocubes were modified on a carbon paper (CP) substrate, creating an exceptionally competent electrocatalyst for converting N2 to NH3 under normal conditions. The Mn3O4/CP electrocatalyst showcased a peak NH3 generation rate of 11.6 µg mgcat.−1 h−1 and achieved a FE of 3% in a 0.1 M Na2SO4 solution, all occurring at a potential of −0.8V vs RHE. In contrast, the unmodified CP demonstrated minimal electrochemical performance when subjected to the N2 conversion reaction, further underscoring the effectiveness of the Mn3O4/CP electrocatalyst in this context. After subjecting the Mn3O4/CP electrocatalyst to 10 cycles of recycling in a nitrogen reduction reaction, the NH3 generation rate and FE showed negligible fluctuations, indicating the high recycling stability of the electrocatalyst (Wu et al., 2018).
Zhang and colleagues used a hydrothermal synthesis approach for effective electrochemical nitrogen reduction to NH3 under standard circumstances to generate an MnO nanoparticle catalyst on a Ti mesh (MnO/Ti). Their findings were based on density functional theory (DFT) calculations, which showed that the adsorption energy of N on the Mn atom of the MnO (2 0 0) surface was −2.20 eV. This is less than the adsorption energy of H on the O atom for the same surface (−1.56 eV). This shows the great selectivity of the MnO/Ti catalyst for nitrogen, indicating that the *N2 determines the N2 reduction process on the MnO (2 0 0) surface to the *N2H transformation. The MnO/Ti catalyst exhibited a remarkable ammonia generation rate of 1.11 × 10−10 mol s−1 cm−2 and a FE of 8.02% in a 0.1M Na2SO4 solution at a potential of −0.39 V versus RHE. Moreover, it demonstrated exceptional structural and electrochemical stability, as no decline in NH3 generation rate or FE was observed after ten consecutive cycles at −0.39V (Wang et al., 2019). Han and colleagues employed a hydrothermal method to synthesize MoO3 nanosheets and utilized electrospinning to fabricate Nb2O5 nanofibers. The electrochemical conversion of N2 to NH3 using MoO3 nanosheets as electrocatalysts achieved a maximum NH3 generation rate of 4.80 × 10−10 mol s−1 cm−2 and an FE of 0.8% at −0.50V versus RHE in a 0.1 M HCl solution. DFT simulations indicated that MoO3 nanosheets, with Mo atoms located on their outermost surface, served as active sites for N adsorption, facilitating its reduction to NH3. The performance of Nb2O5 nanofibers as a catalyst in the electrochemical conversion of N2 to NH3 was exceptional. The catalyst achieved a maximum NH3 generation rate of 43.6 μg mgcat.−1h−1 and an FE of 9.26% in 0.1M HCl at −0.55V versus RHE. DFT studies revealed that the presence of niobium-edge atoms on the surface of Nb2O5 (1 8 1) played a significant role in diverging and stimulating N2 molecules, leading to their reduction into ammonia. Notably, during the recycling test, the Nb2O5 nanofibers exhibited exceptional electrochemical stability (Han et al., 2018a,2018b).
The synthesis of multishell hollow Cr2O3 microspheres (MHCMs) as efficient electrocatalysts for N reduction to NH3 employed a straightforward process. In a 0.1 M Na2SO4 solution at −0.9V vs. RHE, these MHCMs electrocatalysts achieved an impressive maximum ammonia production rate of 25.3 g per milligram of catalyst per hour, accompanied by a FE of 6.78%. DFT simulations were conducted to gain insights into the mechanism occurring on the surface of the metal-heteroatom-carbon materials (MHCMs) electrocatalyst during the nitrogen conversion reaction. The results of these calculations revealed the sequence of events on the MHCM electrocatalyst surface. Initially, N2 was absorbed on the Cr3+ species of MHCMs, monitored by hydrogenation, involving the addition of an (H + e-) pair. This process indicated the involvement of both distant associative and partially alternative pathways in the nitrogen reduction reaction over the MHCMs electrocatalysts (Zhang et al., 2018). The employment of Cr2O3 nanofibers as an electrocatalyst for the reduction of N2 to NH3 has been documented, showcasing its remarkable efficacy. The Cr2O3 nanofiber displayed a notable NH3 generation rate of 28.13 µg mgcat.−1 h−1 and a FE of 8.56% in a 0.1M HCl solution at −0.7V vs RHE. Moreover, it exhibited outstanding electrochemical robustness, sustaining its performance through 6 cycles of electrolysis (Du et al., 2018).
Bi4V2O11 and CeO2 were combined by Lv et al. to create a hybrid catalyst using the electrospinning technique and air-conditioning calcination. This catalyst was created to reduce N2 to NH3 electrochemically in ambient situations. The molar ratio of Ce to Bi substantially impacted the crystalline structure of Bi4V2O11 in the hybrid catalyst. A mixture of crystalline CeO2 and amorphous Bi4V2O11 was seen in the BVC-A hybrid catalyst when the molar ratio of Bi to Ce was 2:1. Maintaining the molar ratio of Bi to Ce at 4:1 resulted in the creation of a BVC-C hybrid catalyst consisting of both crystalline CeO2 and crystalline Bi4V2O11 (Fig. 10(a)). The BVC-A hybrid catalyst outperformed the BVC-C hybrid catalyst and the pristine Bi4V2O11 and CeO2 catalysts in terms of ammonia generation rate (Fig. 10(b)). By using the BVC-A hybrid catalyst in 0.1M HCl at −0.2V vs. RHE, it was possible to achieve the highest NH3 production rate of 23.33 µg mgcat.−1 h−1 and a FE of 10.17%. The remarkable performance of the BVC-A hybrid catalyst is attributed to the presence of amorphous Bi4V2O11 species, which offer numerous defective locations and a minimal energy barrier for the electrocatalytic reduction of N2 to NH3. Due to conflicting processes linked to H2 development, the rate of ammonia generation and the FE reduced at larger negative potentials (Fig. 10(c)). (Lv et al., 2018).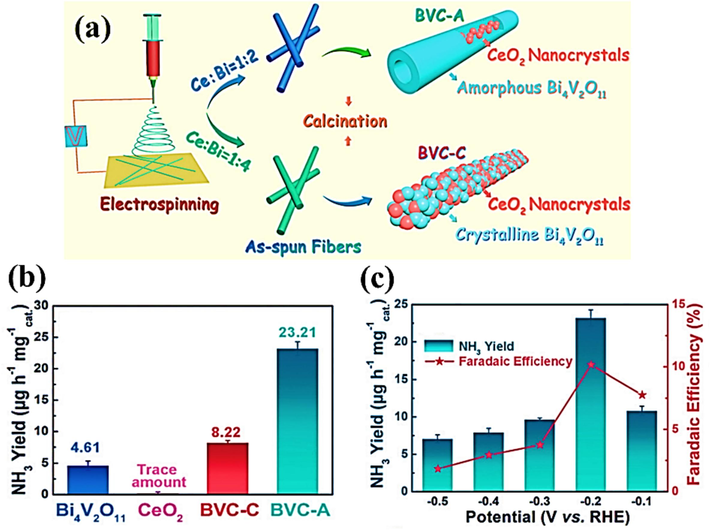
The electrochemical conversion of N2 using a hybrid catalyst consisting of Bi4V2O11 and CeO2 is illustrated. The text comprises (a) a schematic diagram depicting the construction process of BVC-A and BVC-C catalysts, (b) a comparative analysis of the electrochemical performance of Bi4V2O11, CeO2, BVC-A, and BVC-C at −0.2 V vs. RHE, and (c) data presenting the NH3 formation rate and Faradaic efficiency of the BVC-A catalyst in N2-saturated 0.1 M HCl at diverse potentials. Reproduced with permission (Lv et al., 2018). Copyright 2018 Wiley-VCH.
Zheng et al. developed a SnO2/CC electrocatalyst for the room-temperature electrocatalytic conversion of nitrogen to NH3. They achieved this by synthesizing cubic sub-micron SnO2 particles on a carbon cloth (CC) using hydrothermal synthesis. In a 0.1M Na2SO4 electrolyte, the SnO2/CC electrocatalyst demonstrated the highest FE of 2.17% at −0.7V, surpassing all other tested catalysts. Additionally, it reached the maximum ammonia generation rate at −0.8V, 4.03 µg mgcat.−1 h−1. It is possible to attribute the decrease in ammonia generation rate and FE at larger negative potentials to the electrode surface's strong selectivity for the hydrogen evolution process over the nitrogen reduction reaction. The electrochemical stability of the SnO2/CC electrocatalyst was noteworthy (Zhang et al., 2018). To create a highly efficient electrocatalyst for the electrochemical conversion of N into NH3, Y2O3 nanosheets were synthesized using the hydrothermal synthesis method. Characterization results revealed that a 2:3 atomic ratio of Y and O could produce uniformly distributed Y2O3 nanosheets. Utilizing Y2O3 nanosheets, NH3 was formed at a maximum rate of 1.06 × 10−10 mol s−1 cm−2 with an FE of 2.53% in a 0.1M Na2SO4 aqueous solution at a voltage of −0.9V compared to the RHE. It was noted that the hydrogen evolution reaction had a significant impact, occurring at a much lower potential, which led to a notable formation in the rate of NH3 construction and the overall electrochemical efficiency. DFT calculations identified the hydrogenation of *N2 to *N2H as the crucial step influencing the reaction potential, with a free energy of 2.19 eV (Li et al., 2018).
3.4 Transition metal nitrides-based electrocatalyst
Transition metal nitrides (ZrN, VN, MoN, and NbN) have been predicted to provide high activity in nitrogen electroreduction and hydrogen evolution suppression (Abghoui et al., 2015). The nitrogen electroreduction activity of MoN and VN nanostructures coated on substrates with a large surface area was tested experimentally to reveal active sites and enhance the passage of gas molecules and electrolyte particles (Suryanto et al., 2018; Zhang et al., 2018; Zhang et al., 2018). Due to the likelihood of ammonia being created during the reductive breakdown of transition metal nitrides, it is imperative to carry out control tests with labeled 15N N2 to synthesize ammonia electrochemically successfully. Thermal nitriding produced a collection of VN nanosheets on VN nanowires on carbon cloth (VN/CC) alongside V2O5 nanowires, a Ti grid (VN/TM), in an atmosphere with flowing ammonia. Under the conditions of a nitrogen-saturated solution of 0.1 M hydrochloric acid at an applied voltage of −0.50 V vs RHE, the VN/TM catalysts exhibited an atypical catalytic behavior, resulting in an NH3 formation rate of 8.40 × 10−11 mol s−1 cm−2. A FE of 2.25% accompanied this. Furthermore, the catalyst consistently maintained a value of −0.50 V relative to RHE during the recycling test (Zhang et al., 2018).
A series of MoN2 nanosheets were synthesized on a carbon fabric substrate, denoted as MoN2/CC, employing a method similar to that used for VN nanostructures (Zhang et al., 2018). An investigation was carried out to evaluate the electrocatalytic efficiency of MoN2 nanorods in the reduction of N2 in a nitrogen-saturated 0.1 M HCl solution. This process yielded an NH3 production rate of 78.4 µg mgcat.−1 h−1. When operating MoN nanorods at −0.3 V versus the RHE, a Faraday efficiency of 4.5% was achieved. Notably, it was observed that the nitrogen particles within the Mo2N catalyst disappeared during electrolysis under an argon atmosphere. According to these results, the Mars Van Krevelen (MvK) mechanism was used for N2 reduction on the MoN2 catalyst. Although the study did not encompass the reduction 15N2, the formation of NH4+ was observed because of the MoN2 catalyst's activity (Jiao and Xu, 2019). The investigation of Fe-N-C and Nb-N-C electrocatalysts demonstrates that, when subjected to the electrochemical conditions of an H-cell in an alkaline environment, these catalysts do not display significant reactivity towards NRR. The electrochemical research demonstrates that the Faradaic processes initiate earlier, and the current density increases when nitrogen (N2) is present, as opposed to an argon (Ar) atmosphere. Although the higher capacitance in N2 indicates the presence of N at the catalyst surface, it seems that the metal centers (Fe and Nb) in these catalysts are inefficient in catalyzing their conversion to ammonia (Sveinbjörnsson et al., 2022).
3.5 Transition metal carbide-based electrocatalyst
Numerous studies have focused on non-noble metal catalysts commonly used for hydrogen evolution, rendering them unsuitable for nitrogen reduction. Nevertheless, extensive research has demonstrated that with precise adjustments to their composition and structure, non-noble metal catalysts can effectively catalyze the electroreduction of nitrogen into NH3 under normal conditions. Cheng et al. employed a molten salt synthesis approach to craft a Mo2C/C catalyst. This innovative catalyst was created by integrating Mo2C nanodots onto ultra-thin carbon nanosheets with particle sizes ranging from 2 to 3 nm (illustrated in Fig. 11(a)) (Cheng et al., 2018). The performance of the Mo2C/C catalyst was assessed in 0.5 M Li2SO4 aqueous solutions with pH values of 2 and 3, while being supported on both hydrophilic and hydrophobic carbon cloth. The outcomes are depicted in Fig. 11(b) and 11(c). Employing a hydrophobic carbon fabric with an electrolyte characterized by a higher pH was anticipated to mitigate hydrogen evolution by limiting proton accessibility at the electrode interface and amplifying the FE. The experimental results confirmed the prediction, as evidenced by the data presented in Fig. 8. The FE reached its peak at 7.8%, a significant improvement from the initial value of 1.6%, as the availability of protons decreased, as illustrated in Fig. 10(b). Nevertheless, as depicted in Fig. 11(c), the rise in FE coincided with a decrease in the rates of ammonia formation. This indicates that although effectively reducing hydrogen evolution can enhance the efficiency of the faradic process, other measures are required to get a substantial rate of ammonia production. This is because the N2 reduction reaction directly entails the participation of protons. These findings emphasize the challenges in attaining a substantial rate of ammonia generation and optimal faradic efficiency at an electrode surface.
Electrochemical reduction of nitrogen was achieved using Mo2C nanodots entrenched in ultra-thin Mo2C/C. The text includes (a) a design of the construction process of Mo2C/C through the molten salt method, (b) assessments of the Mo2C/C catalyst's Faradaic efficiency in 0.5 M Li2SO4 aqueous solutions with pH levels of 2 and 3 on both hydrophilic and hydrophobic carbon cloth, and (c) the rate of NH3 formation. Reproduced with permission (Cheng et al., 2018). Copyright 2018 Wiley-VCH.
3.6 Transition metal sulfide-based electrocatalyst
In recent times, there has been considerable interest in transition metal sulfide because of its commendable catalytic activity in hydrodesulfurization (Oshikiri et al., 2016; Cherkasov et al., 2015), HER (Hou et al., 2013; Kyriakou et al., 2017), and CO2 or O2 reduction reactions (Bao et al., 2017). The substance's inherent catalytic activity greatly inhibits the nitrogen reduction process through its ability to reduce water. However, given the anticipated reactivity of the transition metal sulfide towards NRR. A MoS2 nanosheet was cultivated on MoS2/CC using the hydrothermal technique. When MoS2/CC was used as an electrocatalyst in a solution of 0.1 M Na2SO4 at a voltage of 0.5 V compared to the RHE, it resulted in the production of NH3 at a maximum rate of 8.07 × 10−11 mol s−1 cm−2 and with a FE of 1.17% (Zhang et al., 2018). Suryanto et al. utilized MoS2 as a carrier and produced a Ru/MoS2 catalyst for nitrogen reduction. Research has observed that MoS2 polymorphs (1T-MoS2 and 2H-MoS2) exert control over each other by suppressing the reduction of H+ ions. The 1T-MoS2 polymorph exhibited metallic properties, whereas the 2H-MoS2 polymorph displayed semiconductor characteristics. The HER potential was suggested to reduce the competition between HER and 2H-MoS2, owing to its higher energy level in the conduction band compared to 2H-MoS2. This resulted in an increased NH3 formation rate and FE. The NH3 construction rate was 6.7 × 10−11 mol cm−2 s−1 for Ru/2H-MoS2 and 9.6 × 10−12 mol cm−2 s−1 for Ru/1T-MoS2 when measured at −0.2 V versus RHE. The Ru/2H-MoS2 catalyst demonstrated the highest NH3 synthesis rate (9.1 × 10−11 mol cm−2 s−1) and FF (12.2%) at a potential of −0.15 V compared to the RHE. The impact of the catalyst support was examined by studying the Ru/2H-MoS2 under various temperatures. It was found that the maximum rate of NH3 construction and FE occurred at a temperature of 50 °C. The reaction resulted in an NH3 generation rate of 1.14 × 10–10 mol cm−2 s−1 and a FE of 17.6% (Suryanto et al., 2018).
3.7 Transition metal phosphide-based electrocatalyst
The electrocatalyst made from transition metal phosphide has excellent performance, highlighting the remarkable capabilities of transition metals and related compounds in the nitrogen reduction reaction. This discovery opens new possibilities for systematically designing catalysts and studying their mechanisms. Guo et al. (Guo et al., 2018) utilized a ZIF-67 nanocrystal as a precursor through a cobalt layered-double-hydroxide (Co-LDH) intermediate and prepared CoP hollow nanocage (CoP HNC) catalyst for the electrochemical reaction of nitrogen to NH3 (Fig. 12(a–d)). CoP HNC catalyst provided a surface with rich active sites due to a 3D hierarchical structure (nanoparticle, nanosheet, nanocage) for nitrogen adsorption and reducing ammonia. When employed as a catalyst in the electrochemical conversion of N, CoP HNC demonstrated a remarkable Faraday efficiency of 7.36% even at low overpotentials, specifically at 0 V vs. RHE. Moreover, the rate of ammonia production exhibited an exponential increase, ultimately reaching 10.78 µg mgcat.−1 h−1 at a more negative potential of −0.4 V vs. RHE, all achieved under an ambient environment without the generation of hydrazine.
(a) The synthesis technique of CoP HNC is illustrated in a schematic. (b) An image obtained by HIM, (c) A TEM image of CoP, (d) An HRTEM image showing the edge of the nanosheet in CoP HNC. Reproduced with permission (Guo et al., 2018). Copyright 2018 Wiley-VCH.
3.8 Noble metal-based electrocatalyst
Numerous studies have been employed to construct gold-based electrocatalysts, for instance, tetrahexahedral Au nanorods (Bao et al., 2017), Au subnanoclusters embedded in TiO2 (Shi et al., 2017), Au nanoparticles deposited on N-doped nanoporous graphite carbon membrane (Wang et al., 2018), Au nanoparticles anchored on CeOx-RGO (Li et al., 2017), hollow Au nanocages (Nazemi et al., 2018), Au dispersed carbon nitride (Li et al., 2018), and Au supported on N-doped carbon (Qin et al., 2018), 3D dealloyed nanoporous gold (NPG) (Ma et al., 2021) for nitrogen electroreduction, attaining astoundingly high FE. Tetrahexahedral Au nanorods were produced through the seeded growth method, resulting in a structure characterized by a stepped (7 3 0) face and a multi-step face comprising (2 1 0) and (3 1 0) subfaces (as illustrated in Fig. 13(a) and (b)). These Au nanorods exhibited impressive performance, achieving a maximum rate of NH3 synthesis at 1.648 g h−1 cm−2 and a FE of 3.879% when employed in the electroreduction of nitrogen in a 0.1 M KOH solution at a potential of −0.2V versus RHE. Simultaneously, a quantity of 0.102 g per hour per square centimeter of hydrazine hydrate was generated, resulting in an elevation of the FE to 4.02%.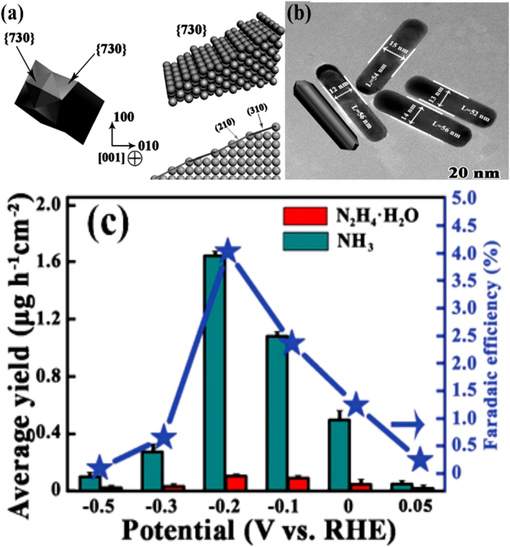
Au THH NR's atomic-level surface structures were investigated through (a) geometric representations displaying Au nanorods with 24(7 3 0) facets. Gold (Au) nanorods captured in a TEM picture (b). (c) In a nitrogen-saturated 0.1 M KOH solution, the rates of hydrazine hydrate and ammonia production, as well as the Au nanorods' Faradaic efficiency, at different potentials. It's important to note that the (7 3 0) facet comprises the (2 1 0) and (3 1 0) sub-facets on Au nanorods. Reprinted with permission (Bao et al., 2017). Copyright 2016 Wiley-VCH.
The rise in potential from −0.2V vs RHE (Fig. 13(c)) resulted in a dominance of H2 evolution in the electrode reaction, indicating a decline in both the rate of ammonia generation and the FE. The exceptional activity of Au nanorods is attributed to the substantial presence of many high-index (2 1 0) and (3 1 0) facets on the catalysts. These facets offer abundant active sites for nitrogen reduction. The Au nanorods experienced gradual corrosion and aggregation throughout multiple NH3 production trials, slightly decreasing their catalytic efficiency. The concurrent generation of ammonia and hydrazine offers compelling evidence for the electroreduction of N2 on Au nanorods organized in an alternating and distal configuration. DFT simulations reveal that N2 electroreduction on Au nanorods tends to prefer an associative alternating route over an associative distal one, supported by this co-production. Introducing Au nanoparticles into various substrates to create a low-coordination metal center environment has proven to be a valuable strategy for reducing the dependence on expensive noble metals and enhancing catalytic performance (Bao et al., 2017).
To enhance catalytic effectiveness and reduce costs, Au nanoparticles have been utilized as a substitute for expensive noble metals by being supported on substrates. A set of TiO2 catalysts incorporating Au were synthesized and characterized for the electroreduction of nitrogen in a 0.1 M aqueous HCl solution. The size of the Au particle was effectively modified from about 0.5 to approximately 4 nm and subsequently to 37 nm utilizing the photoreduction method in conjunction with tannic acid (TA) and NaBH4 reducing agents. The TA-reduced Au/TiO2 catalyst achieved a significant ammonia generation rate of 21.4 µg mgcat.−1 h−1 during N2 electroreduction. Additionally, the FE at −0.2 V vs. RHE reached 8.11%. The FE at greater negative potentials and the rate of NH3 formation were significantly reduced in the presence of the H2 evolution process (Fig. 14(a)). The electrocatalytic efficacy of the Au/TiO2 catalyst reduced by tannic acid (TA) exceeded that of the NaBH4− and photo-reduced Au/TiO2 catalysts. This superiority could be attributed to the existence of Au-O-Ti bonding and the incorporation of Au+ in the TA-reduced Au/TiO2 catalyst, distinguishing it from the other two catalysts (as shown in Fig. 14(b)). These unique features likely played a role in promoting N2 electro-reduction (Shi et al., 2017).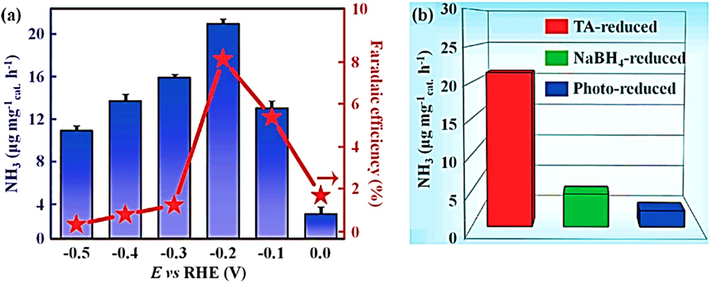
In electrochemical nitrogen reduction using the TA-reduced Au/TiO2 catalyst, various measurements were taken. (a) The rate of NH3 synthesis and the FE of the TA-reduced Au/TiO2 catalyst at different potentials in a nitrogen-soaked 0.1 M HCl solution, and (b) a comparison of NH3 synthesis rates among the Au/TiO2 catalysts that underwent photo-reduction, TA-reduction, and NaBH4-reduction at −0.2 V versus the RHE. This content is shared with permission (Shi et al., 2017). Copyright 2017 Wiley-VCH.
A nanoporous graphitic carbon membrane (NCM) was doped with nitrogen and functionalized with gold nanoparticles to form an Au NPs/NCM catalyst. This catalyst was designed for the electrochemical transformation of N to NH3 in ambient conditions. Compared to the NCM substrate, the Au NPs/NCM substrate exhibited a substantial improvement in the rate of NH3 construction, from 0.08 to 0.36 g m−2 h−1, and a substantial improvement in FE, from 5.2 to 22%, in 0.1 M HCl. Electrons were anticipated to move from NCM to Au at the interface of Au NPs/NCM due to the larger Ef value of NCM compared to Au. This led to the nitrogen surface becoming positively charged and exhibiting a strong affinity for nitrogen, resulting in a more efficient reduction of ammonia (Wang et al., 2018).
An uncomplicated co-reduction technique was employed, utilizing NaBH4 as a reducing agent to deposit Au and CeOx onto RGO. This resulted in the formation of an Au/CeOx-RGO composite, which serves as an electrocatalyst for the electrochemical reduction of N to NH3. Amorphous Gold (Au) nanoparticles with a particle size of approximately 5 nm were formed because of CeOx, which was disseminated and attached to the RGO substrate in the electrocatalyst. The Au/CeOx-RGO electrocatalyst outperformed the Au/CeOx and Au/RGO electrocatalysts in NH3 generation. The enhanced performance may be attributed to the existence of amorphous Au on the substrate, which offers several unsaturated coordination sites (Fig. 15(a)). The Au/CeOx-RGO catalyst, with a 1.32 wt% loading of Au in 0.1 M HCl, achieved a maximum NH3 synthesis rate of 8.3 μg mgcat.−1 h−1 and a FE of 10.10% at −0.2V versus RHE. At greater negative potentials, there was a noteworthy reduction in the rate of NH3 generation and the FE. This was attributed to the counteractive occurrence of H2 evolution, as depicted in Fig. 15(b) (Li et al., 2017).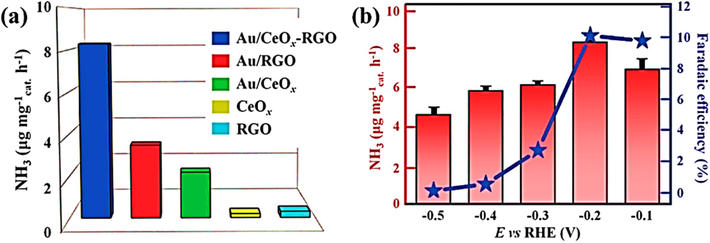
Electrochemical N conversion involving the Au/CeOx-RGO composite was investigated, and the results are presented as follows: (a) NH3 synthesis rates were determined for diverse catalysts at a potential of −0.2 V versus RHE, and (b) the NH3 synthesis rate and FE of the Au/CeOx-RGO composite were examined in a 0.1 M HCl solution at varying potentials. This content has been shared with permission (Li et al., 2017). Copyright 2017 Wiley-VCH.
To synthesize an electrocatalyst for the electrochemical conversion of N2 to NH3 under moderate environments, a hollow gold nanocage (AuHNC) was created using cubic silver nanoparticles as a template through the galvanic replacement method. When employing the AuHNC electrocatalyst, the most notable ammonia formation rate and FE were recorded at 3.9 µg cm−2 h−1 and 30.2%, respectively. These measurements were taken in a solution containing 0.5 M LiClO4, with the electrode potential set at −0.5 V versus the RHE for ammonia production rate and −0.4 V versus RHE for FE. As the negative potential increased, the NH3 production rate and FE experienced a decrease, indicating the prevalence of the HER over the N reduction reaction at the cathode. The study also investigated the influence of operating temperature and AuHNC concentration on the performance of the AuHNC electrocatalyst in the nitrogen reduction reaction. At −0.4 V versus RHE, an increase in operating temperature from 20 to 50 °C led to an enhancement in the NH3 synthesis rate (from 2.35 to 2.82 µg cm−2 h−1) and FE (from 30.2% to 40.55%). This was attributed to the increased rate of mass transport at higher temperatures. Likewise, elevating the concentration of AuHNC on the substrate from 0.9 to 1.8 µg mL−1 resulted in an improvement in both the NH3 synthesis rate (from 1.88 to 3.98 µg cm−2 h−1) and FE (from 3.12% to 14.8%) at −0.5 V versus RHE. Additionally, the AuHNC electrocatalyst outperformed Au nanospheres (AuNSs), Au nanorods (AuNRs), and Au nanocubes (AuNCs) in the nitrogen reduction reaction, thanks to its high surface area, minimal presence of capping materials within the cavity, and the enhanced propensity for nitrogen to interact with the Au surface inside the cavity (Nazemi et al., 2018).
The reduction process involved using HAuCl4 and H2 to prepare Au atoms dispersed individually on a carbon nitride catalyst (Au1/C3N4). A NaBH4 reduction method was also used to create Au nanoparticles supported on a carbon nitride catalyst (Au NPs/C3N4). These catalysts were then used for the electrochemical conversion of N to ammonia at room temperature. The Au1/C3N4 catalyst exhibited superior electrochemical performance in reducing nitrogen to ammonia compared to the Au NPs/C3N4 catalyst, as shown in Fig. 16(a). The FE of C3N4 was found to be very low, suggesting that the active sites of the Au1/C3N4 catalyst were the Au single atoms (Fig. 16(b)). Thus, the Au1/C3N4 catalyst can attain a high rate of ammonia synthesis by the efficient usage and widespread distribution of Au atoms. The Au1/C3N4 catalyst had a much higher maximum rate of ammonia generation (1305 μg mgAu−1 h−1) than the Au NPs/C3N4 catalyst. This rate increase was nearly 22.5 times greater. Additionally, the Au1/C3N4 catalyst had a FE of 11.1% in 5 mM H2SO4 at −0.1V versus RHE (Li et al., 2018). Qin et al. (Qin et al., 2018) prepared Au single sites supported on an N-doped carbon catalyst and achieved a maximum NH3 production rate of 2.32 μg h−1 cm−2 and FE of 12.3% in 0.1 M HCl electrolyte at −0.2V vs RHE. This work used surface-enhanced infrared absorption spectroscopy to investigate the intermediate species generated on an Au thin film electrode placed on a silicon prism during the nitrogen reduction process. This investigation was motivated by the remarkably high FE observed in ammonia production using gold-based catalysts. According to the analysis findings, an associative process was followed for the electrochemical reduction of N to ammonia on the Au surface. In this mechanism, the triple bond in nitrogen tended to cleave simultaneously with the introduction of hydrogen (Yao et al., 2018).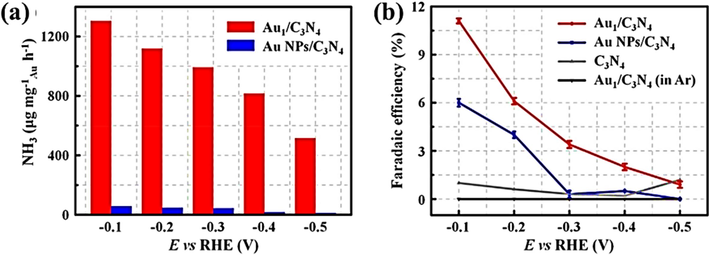
The electrochemical reduction of nitrogen with the Au NPs/C3N4 catalyst is illustrated by the following figures: (a) The NH3 production rate for both the Au1/C3N4 and Au NPs/C3N4 catalysts at various electrode potentials. (b) The Faradaic efficiency data for the Au1/C3N4, Au NPs/C3N4, and C3N4 catalysts at different potentials, along with that of the Au/C3N4 catalyst under various electrode potentials. These figures have been reproduced with the necessary permissions (Li et al., 2018). Copyright 2018 Science China Press.
A highly efficient and durable electrocatalyst, 3D dealloyed nanoporous gold (NPG), has demonstrated remarkable performance in ammonia production under normal atmospheric pressure. In a 0.1 M nitrogen-saturated aqueous KOH solution, the electrode underwent a potential shift from 0.4 to −0.5 V vs. RHE, resulting in significant ammonia production at a rate of 45.7 g mgcat.−1 h−1. It is thought that the Au ligament surface of NPG has many surface steps and kinks that serve as active sites for the NRR, which makes it a very efficient NRR electrocatalyst. It displays a remarkable FE of 3.41% at 0.10 V compared to the RHE. This study represents a significant advancement in utilizing ambient NRR for NH3 synthesis. It lays the foundation for effective electrocatalyst design for ambient NRR as well as other chemical and electrochemical processes (Ma et al., 2021).
3.9 Non-metal-based electrocatalyst
Extensive experimental and theoretical research on nitrogen electroreduction has utilized transition metal catalysts. However, the bulk of transition metals weakly binds N2 molecules, barely activating them, resulting in low Faraday efficiency owing to competing hydrogen reactions (Lv et al., 2018). Though electrocatalytic nitrogen reduction depends on transition metal-based electrocatalysts, allowing substantial N2 activation on metal-free catalysts remains a grand challenge. They may present new opportunities for developing high-performance N2 reduction electrocatalysts with low H2 evolution activity due to the low selectivity of transition metal-free catalysts for H2 evolution (Mukherjee et al., 2018; Song et al., 2018). Carbon nanostructures can provide many open, active sites and enhance mass transfer due to their vast surface area and porous structure. Doping with heteroatoms (such as nitrogen and boron) modifies the carbon framework’s electronic design and results in defect formation and charge polarization. Therefore, employing carbon nanomaterials with vacancy defects is anticipated to improve nitrogen adsorption and the conversion of nitrogen to ammonia. N-doped carbon compounds are prone to deterioration and ammonia release during electrolysis into the electrolyte.
Mukherjee and their team utilized a one-step thermal activation process to produce nitrogen-doped nanoporous carbon from ZIF-8. Under typical circumstances, this special material acted as an electrocatalyst for the electrochemical nitrogen reduction process, which produced ammonia. The developed electrocatalyst exhibited impressive rates of NH3 construction and FE, reaching 3.4 × 10−6 mol cm−2 h−1 and 10.2%, respectively, at −0.3 V vs. (RHE using a 0.1 M potassium hydroxide (KOH) electrolyte. The ammonia synthesis rate was positively correlated with temperature, peaking at 7.3 × 10−6 mol cm−2 h−1 at 60 °C. Importantly, the N-doped carbon electrocatalyst established exceptional durability over an uninterrupted 18-hour test, maintaining a consistent ammonia generation rate. Furthermore, the study explored the influence of introducing iron (Fe) into the N-doped nanoporous carbon on catalytic activity. Fe doping dramatically reduced the ammonia generation rate by blocking the electrocatalyst's active sites and speeding up the HER (Mukherjee et al., 2018). The researchers utilized a similar approach to fabricate an electrocatalyst of N-doped nanoporous carbon derived from ZIF-8. This electrocatalyst exhibited an ammonia production rate of 2.6 × 10−10 mol s−1 cm−2 and a FE of 0.9% when operated at −1.1 V vs. RHE with a 0.05 M H2SO4 electrolyte (Liu et al., 2018). A thermal treatment method was utilized to create polymeric carbon nitride (PCN) for electrocatalytic nitrogen reduction to ammonia (NH3). The PCN catalyst exhibited N-vacancy defects in the N2C site, where two nitrogen atoms are coordinated. This PCN electrocatalyst demonstrated outstanding performance, achieving an NH3 synthesis rate of 8.09 μg mgcat.−1 h−1 and a FE of 11.59% in a 0.1 M HCl electrolyte at −0.2 V versus RHE, as illustrated in Fig. 17(a and b). According to DFT calculations, the nitrogen molecule was absorbed by the PCN catalyst's N-vacancy, which caused the bond length of the adsorbed N2 to significantly elongate from 1.10 Å (the bond length of the free nitrogen molecule) to 1.26 Å. As seen in Fig. 17, this elongation resulted from electron transfer between the adsorbed N2 molecule and nearby carbon atoms (c). N2 may be reduced into NH3 more easily since its bond length is between that of hydrazine (1.47 Å) and diazene (1.20 Å) (Lv et al., 2018).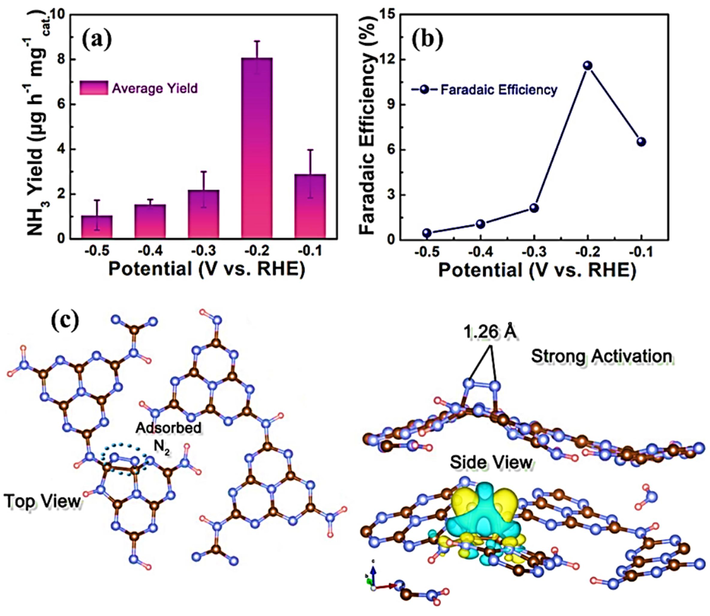
An investigation on the electroreduction of N2 utilizing metal-free polymeric carbon nitride (PCN) produced the following findings: The rate at which NH3 forms, the FE of PCN with N-vacancies in nitrogen-saturated 0.1 M HCl at different potentials, and the charge density difference of the nitrogen-adsorbed PCN with N-vacancies as well as the geometry of nitrogen adsorption on PCN with N-vacancies are the three variables that need to be considered. The yellow and blue surfaces in the charge density difference graphs stand for charge buildup and depletion in the space, respectively. With permission, these numbers are shown (Lv et al., 2018). Copyright 2018 Wiley-VCH.
Song and colleagues employed the CVD technique to design N-doped carbon nano-spikes (CNS), which they used as a highly efficient electrocatalyst for NRR conversion. The CNS electrocatalyst demonstrated impressive results, with a peak ammonia formation rate of 97.18 μg h−1 cm−2 and an FE of 11.56%. These results were achieved in an aqueous solution having 0.25 M LiClO4 at a voltage of −1.19 V vs. RHE. The remarkable electrocatalytic performance of the CNS catalyst in NRR can be attributed to its distinctive tip morphology, which intensifies the electric field and significantly influences the energy levels of the molecular orbitals involved in the N2 molecule (Song et al., 2018). Yu and colleagues employed a thermal reduction process to synthesize a boron-doped graphene electrocatalyst using boric acid and graphene oxide. This process retained the conjugated planar structure and sp2 hybridization of the graphene framework while introducing electron deficiency through the incorporation of boron atoms (as depicted in Fig. 18(a) and (b)). The positively charged boron atoms served as active sites for nitrogen adsorption. They prevented proton binding, resulting in exceptional electrocatalytic activity and high FE during the reduction of nitrogen to ammonia. Among the various boron doping sites, such as B4C, BC2O, and BCO2, DFT simulations showed that the BC3 structure of boron-doped graphene had the lowest energy barrier (Fig. 18(a)). This BC3 structure facilitated nitrogen adsorption and subsequent reduction to ammonia. The boron-doped graphene, with a boron content of 6.2%, achieved impressive electrocatalytic performance under ambient conditions. It exhibited a peak ammonia generation rate of 9.7 μg h−1 cm−2 and an FE of 10.8% in a 0.05 M electrolyte at −0.5 V vs. RHE, as illustrated in Fig. 18(c) (Yu et al., 2018). A boron carbide (B4C) nanosheet electrocatalyst was synthesized using a liquid-phase exfoliation method to enable efficient electrocatalytic conversion of N to NH3. This B4C nanosheet displayed exceptional selectivity and demonstrated high electrochemical stability. It achieved a peak ammonia production rate of 26.57 μg mg–1cat h−1 and an FE of 15.95% in a 0.1 M HCl solution, operating at −0.75 V vs. RHE (Qiu et al., 2018).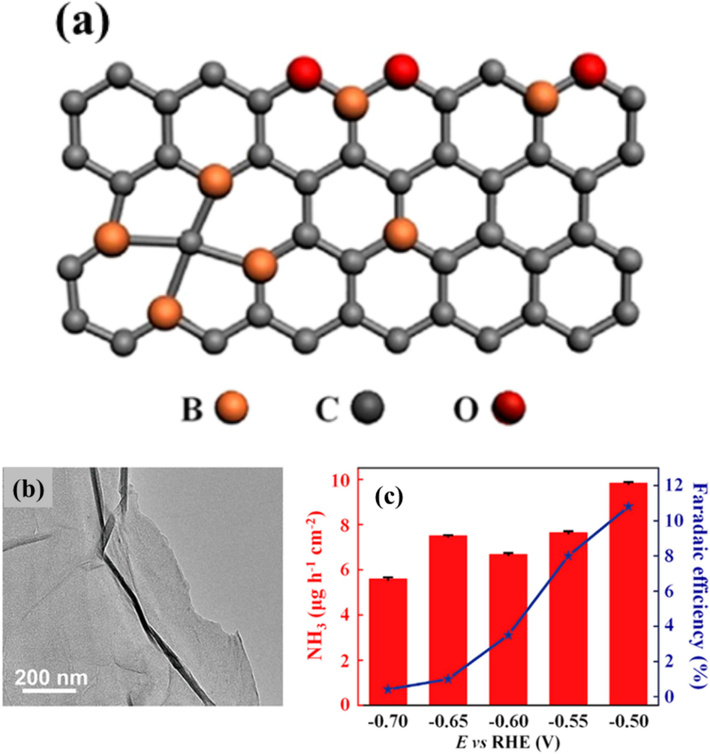
The electrochemical nitrogen reduction of the boron-doped graphene electrocatalyst was investigated through various means: (a) utilizing a schematic illustration to depict the boron-doped graphene structure, (b) capturing a TEM image of the boron-doped graphene, and (c) evaluating the ammonia synthesis rate and Faradaic efficiency of the boron-doped graphene at different electrode potentials. These results were obtained and are presented with permission (Yu et al., 2018). Copyright 2018 Elsevier Inc.
A defect-rich carbon cloth was made appropriate for the electrochemical nitrogen reduction process in ambient settings using a simple heat treatment technique. The untreated, pristine commercial carbon cloth exhibited a comparatively low NH3 formation rate of 5.6 × 10−11 mol cm−2 s−1 with a FE of 1.29%. This was observed in a solution comprising 0.1 M Na2SO4 and 0.02 M H2SO4 at an operating potential of −0.8V vs. RHE. The thermal treatment of carbon fabric at numerous temperatures (250 °C, 350 °C, and 450 °C) introduced defects that significantly enhanced nitrogen adsorption and its subsequent reduction to ammonia. Furthermore, this treatment improved the electrocatalytic stability of the carbon cloth. Notably, the carbon cloth treated at 450 °C exhibited outstanding electrocatalytic performance, achieving a peak ammonia formation rate of 2.59 × 10−10 mol cm−2 s−1 with a FE of 6.92%. At an operating potential of −0.3V vs. RHE, this was seen in a solution of 0.1 M Na2SO4 and 0.02 M H2SO4. This research emphasizes the significance of adding potentially active defect sites to non-metallic electrodes to increase ammonia production (Li et al., 2018).
3.10 Transition-metal compound-based electrocatalyst
According to Van Tamelen and Akermark (1968) and Van Tamelen and Seeley (1969), NH3 was synthesized using electrolysis in a cell equipped with an aluminum anode and a nichrome cathode, utilizing titanium tetraisopropoxide, naphthalene, tetrabutylammonium chloride, and aluminum isopropoxide. The electrolysis process was carried out at a voltage of 40 V with continuous nitrogen gas flow, and naphthalene was utilized as an electron carrier. Naphthalene underwent conversion to naphthalide at the cathode, following the reaction Np + e− → Np−. The reverse reaction, converting naphthalide back to naphthalene, was facilitated by titanium-derived nitrogen atoms through the reaction 6Np− + Ti(II) N2 → 6Np + [Ti(II) N2]6−. An electrolyte was provided by the reaction of aluminum isopropoxide with a nitrogen-reduced titanium compound (2Al(III) + [Ti(II) N2]6− → 2Al(III) N3– + Ti(II)). This interaction released Ti(II), which was capable of binding with additional molecular nitrogen (Ti(II) + N2 → Ti(II) N2). Subsequently, the solution underwent treatment with an aqueous NaOH solution to hydrolyze aluminum nitride and release ammonia. The aluminum cathode was consumed and transformed into aluminum nitride, which was then hydrolyzed. Approximately 99% of the electrons entering the anode were contributed by the dissolution of aluminum. Based on the NH3/Ti molar ratio, this initial experimental investigation achieved an impressive ammonia yield of 61.1%. However, it should be noted that this procedure is not suitable for prolonged or continuous use. The Ti(OH)3-Mo(III) system, created by electroreducing TiCl4 and MoCl5 in methanol using a mercury electrode, subsequently reported on nitrogen reduction catalyzed by Gorodisky and colleagues. To facilitate the flow of electrons from the anode to the N2 molecule, it has been suggested that clusters of Mo(III) and Ti(III) hydroxides must stick to the cathode. As a result, at an appropriate negative potential, a complex with Mo(III) forms. However, a complete explanation of the precise process is still pending. Notably, the highest yields of ammonia and hydrazine were observed when H2O was added to the electrolyte, up to 3%, suggesting that water played a crucial role as the proton source in the process (Gorodyskii et al., 1979).
3.11 Non-metal compound-based electrocatalyst
Pospil and colleagues conducted a unique study on nitrogen reduction to NH3 utilizing a metal-free combination, specifically fullerene cyclodextrin as an intermediary. While free and unmodified C60 fullerene can be eased to a highly reactive redox state and is soluble in protic solvents, it cannot be directly employed as a mediator for nitrogen conversion in aqueous solutions. They created a hydrophilic compound to address this limitation by encapsulating C60 within a cyclodextrin cavity. When introduced alongside 0.31 mM of 0.1 M KCl to a mercury pool electrode, the complex successfully enabled the conversion of N and H2O into ammonia. This process is considered electrocatalytic since 3 µmol of the complex undergoes electrolysis, producing 30 µmol of ammonia. A plausible mechanism has been proposed: the cathode generates the highly reactive C60 dianion by transferring two electrons to N2, which can undergo further reduction at the same site. The further reduction of nitrogen intermediates to ammonia utilizing electrons from the cathode or the dianion of the C60 complex is made possible by these latter phases, which are comparatively simpler than the first activation phase. Sadly, Pospil's method was only around for so long. The contact of the ammonia product with the cyclodextrin cavity would have caused the complex to dissociate or occupy active nitrogen activation sites, which would have been the most plausible explanation for the stop of ammonia synthesis. Slowly accumulating unreacted fullerene after electrolysis provided proof of the complicated dissociation (Pospíšil et al., 2007).
3.12 Enzyme-based electrocatalyst
Within the realm of natural environments, the nitrogenase family stands as the exclusive group of enzymes endowed with the capacity to drive the conversion of nitrogen into ammonia. This family of enzymes consists of two pivotal components: The Fe protein and the MoFe protein, although other variants include FeFe and VFe proteins. The Fe protein takes on the crucial role of orchestrating the reduction of the MoFe protein, employing a series of energy-intensive electron transfer reactions propelled by ATP hydrolysis. In turn, the MoFe protein harnesses these received electrons to effectuate the transformation of N2 into NH3 (Milton et al., 2016; Milton et al., 2017). The intriguing and continuous process of nitrogen reduction to ammonia transpires through the supply of electrons to the MoFe protein, and notably, this occurs independently of ATP. The extensive polypeptide shell typically serves as an insulating layer, which can hinder the direct flow of electrons between the protein and the electrode, leading to a deceleration of electron transfer. This obstruction, in turn, hampers the effective communication between the redox-active cofactor and the surface of the electrode (Hickey et al., 2018). Consequently, reversible electron mediators are used to expedite the passage of electrons from the surfaces of electrodes to enzymes. The process of chemical cross-linking is used to attach it onto the surface of a glassy carbon electrode, as shown in Fig. 19 (a). This technique facilitated the reduction of protons to H2, nitrite (NO2–) to ammonia using bioelectrocatalysis, and azide (N3) to ammonia, where cobaltocene served as an electron mediator within a HEPES buffer at a pH of 7.4. Conditional on the specific reaction mechanism, the Faraday efficiency for reducing N3− to ammonia ranged from 35% to 46%, while it reached 100% for reducing NO2– to ammonia. Nonetheless, the excessive voltage of cobaltocene hindered the electrochemical nitrogen reduction process, mainly due to its role in promoting the reduction of protons into H2, which, in turn, acted as an inhibitor for the MoFe protein (Milton et al., 2016). To circumvent this limitation, the scientists fixed the MoFe protein onto a carbon electrode by using a pyrene-modified linear poly(ethyleneimine) platform. This novel technique facilitated the production of ammonia from N2 without the need for artificial redox mediators or ATP. It achieved this by establishing a direct bioelectrochemical link between the MoFe protein and the surface of the electrode. (Hickey et al., 2018).
Enzymatic conversion of nitrogen into ammonia was achieved by employing the MoFe protein immobilized within a chemically cross-linked poly(vinylamine) substrate, acting as a catalyst for reducing protons to H2, azide (N3−) to NH3, and nitrite (NO2−) to NH3. In this bioelectrocatalytic process, cobaltocene was utilized as an electron mediator, as depicted in Fig. 17(a) (Milton et al., 2016). Copyright 2016 The Royal Society of Chemistry, (b) This enzymatic fuel cell exhibits the capacity to yield NH3 from N2 and H2, all while generating electrical power. This unique process incorporates the use of MV as an electron mediator (Milton et al., 2017). Copyright 2017 Wiley-VCH, and (c) In a unified reactor, the concurrent reduction of N2 and CO2 to solid biomass is achieved by merging electrocatalytic water splitting with Xanthobacter autotrophicus, a hydrogen-utilizing bacterium. This microorganism uniquely harnesses hydrogen as its primary energy source to transform nitrogen and carbon dioxide into biomass. The diagram illustrates carbon cycling through the red paths and N2 cycling through the blue pathways (Liu et al., 2017). Copyright 2017 The Authors.
The glassy carbon may exhibit slightly less activity than carbon paper due to its smooth surface, and the substrate's uniformity and stability can yield more dependable and consistent outcomes. However, carbon paper's large surface area and efficient mass transport capabilities can enhance catalytic activity by facilitating greater accessibility of reactants to the catalyst sites. The carbon paper's porous structure can experience mechanical degradation over extended periods of usage, particularly in severe electrochemical conditions. Glassy carbon's chemical stability and resilience enhance its durability for long-term NRR research. The permeable characteristics of carbon paper enable a substantial catalyst loading and enhanced utilization of the catalyst particles. While glassy carbon may have a lower amount of catalyst used than carbon paper, its even distribution guarantees that the catalyst particles are well utilized without forming large clusters. Both carbon paper and glassy carbon substrates possess distinct advantages and can impact the NRR performance in varying manners. The selection between them is contingent upon the particular demands of the experiment, such as the necessity for elevated surface area and mass transportation (carbon paper) as opposed to the necessity for consistency and durability (glassy carbon). Researchers can enhance the effectiveness and dependability of their NRR experiments by carefully choosing a suitable substrate.
Milton et al. conducted a separate investigation where they constructed an enzymatic fuel cell capable of generating electricity and ammonia by utilizing N2 and H2 (Milton et al., 2017). Fig. 19(b) illustrates using methyl viologen (MV) as an electron intermediary. In this setup, the hydrogenase enzyme catalyzed the oxidation of H2, leading to the reduction of MV2+ to MV•+, while the Fe and MoFe proteins within the nitrogenase system promoted the oxidation of MV•+ back to MV2+. The fuel cell exhibited a peak current of 48.0 µA cm−2 and accomplished an extreme power density of 1.50 µW cm−2. It further showed a 228-mV open circuit potential. Ammonia was produced when the fuel cell was discharged with a FE of 26.4% and a voltage of 10 millivolts. It is important to mention that nitrogenases are highly susceptible to oxygen, requiring anaerobic reaction conditions. According to Liu and colleagues, an effective way to convert CO2 into solid biomass and simultaneously reduce nitrogen is to combine electrocatalytic water splitting with the H2-oxidizing bacteria Xanthobacter autotrophicus in a single reactor (Liu et al., 2017). Water splitting was achieved by employing cobalt oxide phosphate (CoPi) catalysts for the oxygen evolution reaction and biocompatible cobalt-phosphorus (Co-P) alloy catalysts for the HER, as illustrated in Fig. 19(c). The bacteria can exclusively produce H2 through electrochemical means with low oxygen levels (5% O2). This ability enables them to utilize N2 and CO2 as energy sources to produce biomass. When the formation of biomass (route 1 in Fig. 19(c)) is inhibited, ammonia may escape into the extracellular environment (pathway 2 in Fig. 19(c)), where it can be utilized as a biofertilizer to enhance crop yields.
One strategy to tackle the challenge of hydrogen production dominating nitrogen reduction on most electrode surfaces involves decoupling the nitrogen reduction process from the subsequent protonation to create ammonia. This approach's effectiveness has been demonstrated by employing mediators like complexes, enzymes, and lithium metal. For instance, lithium metal can chemically reduce N2 at standard room temperature, forming lithium nitride. This compound can then react with a medium rich in protons (such as water or ethanol) to yield ammonia. This technology offers the advantage of achieving a high Faraday efficiency, nearing 90%, and rapid response kinetics. Two drawbacks of using mediators to synthesize ammonia may prevent this approach from being used for extensive energy storage applications: a high reduction potential and extremely high energy costs. Other issues encompass the perilous characteristics of lithium production and the limited durability of complex chemicals and enzymes in the short term. Nevertheless, we believe that at this early stage in the development of nitrogen electrochemical reduction technology, all viable options are worthwhile, and we remain optimistic that an efficient and stable indirect approach may be implemented. Moreover, the stability of electrocatalysts directly impacts their practical viability and economic feasibility. An in-depth evaluation of electrocatalyst stability entails analyzing its long-term performance, structural and electrochemical stability, and ability to withstand chemical degradation. Implementing thorough and methodical stability testing protocols, along with comprehensive post-reaction analysis, is crucial to guarantee the practical usability and economic feasibility of electrocatalysts for nitrogen reduction reactions in real-world applications. This comprehensive approach offers valuable insights into the durability and reliability of the electrocatalysts, which helps in the advancement of more resilient and effective systems for sustainable ammonia production.
4 Conclusion and outlook
Potentially providing a way to store renewable energy in chemical compounds, nitrogen electroreduction is set to replace the antiquated HBp in an ecologically responsible manner. Since 2015, significant endeavors have been undertaken by the scientific community to accelerate and improve the nitrogen electroreduction process. This overview explores the sequence of chemical reactions, fundamental principles of energy transformation, and the detailed mechanism by which nitrogen is converted into a reduced form. Theoretical studies reveal that these surfaces require increasingly more negative potentials, resulting in significant overpotentials compared to the hydrogen evolution process. Some monoatomic electrocatalysts, transition metal substances, including alloys, oxides, nitrides, carbides, sulfides, and phosphides as electrocatalysts and non-metal electrocatalysts are expected to have a far higher ammonia selectivity than hydrogen gas. Transition metal nanostructures, which may include single atoms and non-metal electrocatalysts, have been documented to attain remarkably high FE (>10%) in aqueous electrolytes under normal circumstances. Even though ammonia produced electrochemically can degrade at elevated temperatures, necessitating a quick withdrawal of the ammonia from the reactor, increasing the reaction temperature can be employed to get even faster kinetic reactions. Despite notable improvements in FE and the yield of ENRR, the industrial-scale implementation of ENRR remains a considerable challenge. Several other substantial factors must also be considered, as shown in Fig. 20.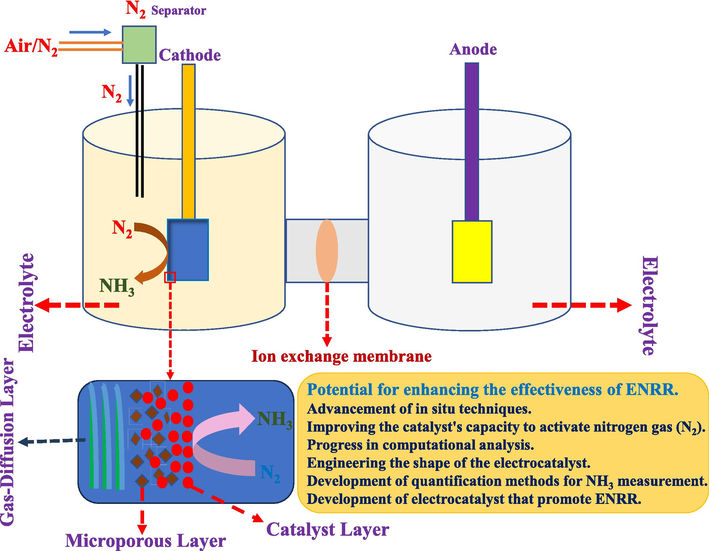
Perspectives for enhancing ENRR.
Several recommendations for more research in this area may be made based on the achievements in the nitrogen electroreduction process. An appropriate control experiment and recording of the findings must be conducted. Much past research has shown that false positives can occur without control tests. The background levels of ammonia for the electrochemical production of ammonia can be influenced by the experimental apparatus, the chemicals (including gases), and even the experimenter. Therefore, careful control tests are needed to identify ammonia produced electrochemically. It is highly suggested that 15N-labeled N2 be used in control trials. However, 15N2 may be too costly for work at higher pressures, even if we strongly encourage utilizing it for testing at atmospheric pressure. Researchers should publish their work with full disclosure of all information regarding control trials and both positive and negative outcomes to aid the scientific community in better understanding the potential directions in terms of catalyst and electrocatalytic cell construction. Although the scientific community has made great progress in developing catalysts with high Faraday efficiencies, the pace at which ammonia is produced has not increased much, especially regarding nitrogen electroreduction in ambient environments. The tactics used to increase selectivity to ammonia by slowing down either the proton transfer rate or the electron transfer rate are thought to cause extraordinarily low ammonia production rates.
Most experimental studies have demonstrated that ambient ammonia generation occurs at rates less than 10−9 mol s−1 cm−2. More research into ways to speed up ammonia production will be beneficial once a workable nitrogen reduction electrocatalyst is discovered. The catalyst's stability is also crucial, just as critical as its activity and selectivity. Over time, electrochemical reactions may cause the degradation of electrocatalysts and other cell components, or active regions may become poisoned or inactive. This process may be incredibly sluggish and barely perceptible during electrochemical studies that run anywhere between a few hours and many days. Therefore, it is advised that the characteristics of new, highly active nitrogen reduction electrocatalysts be determined over an extended period of testing. The key to understanding this subject is the development of electrocatalysts capable of arbitrarily and effectively catalyzing the nitrogen reduction process. Recent research has demonstrated that searching for novel ultra-high-performance catalysts can benefit from both practical (such as nanomaterials and defect engineering) and theoretical (such as DFT simulations) strategies. The significant progress in DFT calculations in recent years is expected to contribute to developing improved catalysts by offering fresh insights into the nitrogen reduction process at the electrode.
The performance of the electrolyzer is significantly impacted by factors such as the electrocatalyst, electrolyte, electrochemical cell design (including both liquid and gas phases), and reaction conditions. Additionally, the competing hydrogen evolution process can be effectively avoided by nitrogen reduction by employing mediators. Yet, new intermediate technologies that are more cost-effective and secure are preferred because handling highly reactive metal poses a safety risk and consumes much energy. It is anticipated that the advancement of in situ technology will provide new perspectives on the dynamics of catalyst surface changes during electrochemical reactions and the nitrogen reduction process. These recent advancements in nitrogen electroreduction research demonstrate the growing interest and notable progress in this field. However, the present circumstance is inadequate for both practical and monetary reasons. Through collaboration, chemists, electrochemists, chemical engineers, and materials scientists have the potential to achieve significant advancements in this interdisciplinary domain. The ammonia industry's energy consumption and carbon dioxide emission may be significantly reduced if this technology assumes the function of the HBp, and it may also provide a practical way to store renewable energy.
Author contributions
The manuscript was written with contributions from all authors. All authors have approved the final version of the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Sajid Mahmood: Writing – original draft, Investigation. Shahid Iqbal: Writing – original draft, Supervision, Conceptualization. Zeping Wang: Visualization, Validation, Software. Muhammad Ammar: Writing – review & editing, Validation, Software. Muhammad Javed Iqbal: Resources, Methodology, Investigation. Ali Bahadur: Writing – review & editing, Writing – original draft, Resources. Nasser S. Awwad: Writing – review & editing, Funding acquisition. Hala A. Ibrahium: Writing – review & editing, Software, Funding acquisition.
Acknowledgments
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work under grant number RGP2/144/45. This research was supported by grants from the International Collaborative Research Program of Wenzhou-Kean University (ICRP2023008) and Student Partnering with Faculty/Staff Research Program of Wenzhou-Kean University (WKUSPF2023036) awarded to Dr. Ali Bahadur.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Enabling electrochemical reduction of nitrogen to ammonia at ambient conditions through rational catalyst design. PCCP. 2015;17:4909-4918.
- [Google Scholar]
- Controlling the metal–ligand coordination environment of manganese phthalocyanine in 1D–2D heterostructure for enhancing nitrate reduction to ammonia. ACS Catal.. 2023;13:13516-13527.
- [Google Scholar]
- Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle. Adv. Mater.. 2017;29:1604799.
- [Google Scholar]
- Blasing, T., Smith, K., 2016. Recent greenhouse gas concentrations.
- The genesis and development of the commercial BP doubly promoted catalyst for ammonia synthesis. Catal. Lett.. 2014;144:545-552.
- [Google Scholar]
- Light-driven dinitrogen reduction catalyzed by a CdS: nitrogenase MoFe protein biohybrid. Science. 2016;352:448-450.
- [Google Scholar]
- Evaluation of electrocatalytic activity of noble metal catalysts toward nitrogen reduction reaction in aqueous solutions under ambient conditions. ChemSusChem. 2022;15:e202102234.
- [Google Scholar]
- Ammonia electrosynthesis with high selectivity under ambient conditions via a Li+ incorporation strategy. J. Am. Chem. Soc.. 2017;139:9771-9774.
- [Google Scholar]
- Fundamental understanding and optimization strategies for dual-ion batteries: a review. Nano-Micro Lett.. 2023;15(1):121.
- [Google Scholar]
- Key difference between transition state stabilization and ground state destabilization: increasing atomic charge densities before or during enzyme-substrate binding. Chem. Sci. 2022
- [CrossRef] [Google Scholar]
- Electrocatalytic synthesis of ammonia at room temperature and atmospheric pressure from water and nitrogen on a carbon-nanotube-based electrocatalyst. Angew. Chem.. 2017;129:2743-2747.
- [Google Scholar]
- Molybdenum carbide nanodots enable efficient electrocatalytic nitrogen fixation under ambient conditions. Adv. Mater.. 2018;30:1803694.
- [Google Scholar]
- A review of the existing and alternative methods for greener nitrogen fixation. Chem. Eng. Process.. 2015;90:24-33.
- [Google Scholar]
- Local proton source enhanced nitrogen reduction on a combined cobalt-molybdenum catalyst for electrochemical ammonia synthesis. Angew. Chem. Int. Ed.. 2022;61:e202212676.
- [Google Scholar]
- A review of electrocatalytic reduction of dinitrogen to ammonia under ambient conditions. Adv. Energy Mater.. 2018;8:1800369.
- [Google Scholar]
- Electrochemical synthesis of ammonia directly from N 2 and water over iron-based catalysts supported on activated carbon. Green Chem.. 2017;19:298-304.
- [Google Scholar]
- Cr 2 O 3 nanofiber: a high-performance electrocatalyst toward artificial N 2 fixation to NH 3 under ambient conditions. Chem. Commun.. 2018;54:12848-12851.
- [Google Scholar]
- Fuel cell electric vehicles and hydrogen infrastructure: status 2012. Energ. Environ. Sci.. 2012;5:8780-8798.
- [Google Scholar]
- I.G. Energy, 2018. CO2 Status Report 2017. International Energy agency. 2017c, in ed, 2018.
- Performance evaluation and comparison of fuel processors integrated with PEM fuel cell based on steam or autothermal reforming and on CO preferential oxidation or selective methanation. Appl. Energy. 2015;143:138-153.
- [Google Scholar]
- Barium hydride-mediated nitrogen transfer and hydrogenation for ammonia synthesis: a case study of cobalt. ACS Catal.. 2017;7:3654-3661.
- [Google Scholar]
- Achieving a record-high yield rate of 120.9 for N2 electrochemical reduction over Ru single-atom catalysts. Adv. Mater.. 2018;30:1803498.
- [Google Scholar]
- Scalable production of cobalt phthalocyanine nanotubes: efficient and robust hollow electrocatalyst for ammonia synthesis at room temperature. ACS Nano. 2021;15:5230-5239.
- [Google Scholar]
- Ternary intermetallic LaCoSi as a catalyst for N2 activation. Nat. Catal.. 2018;1:178-185.
- [Google Scholar]
- Electrocatalytic properties of the Ti (OH) 3− Mo (III) system in the reduction of molecular nitrogen. React. Kinet. Catal. Lett.. 1979;11:337-342.
- [Google Scholar]
- Electrochemical and photoelectrochemical reduction of molecular nitrogen to ammonia. J. Electroanal. Chem. Interfacial Electrochem.. 1984;170:363-368.
- [Google Scholar]
- The use of controls for consistent and accurate measurements of electrocatalytic ammonia synthesis from dinitrogen. ACS Publications 2018:7820-7827.
- [Google Scholar]
- One-step electrodeposition synthesis of NiFePS on carbon cloth as self-supported electrodes for electrochemical overall water splitting. J. Colloid Interface Sci.. 2024;673:444-452.
- [Google Scholar]
- Hierarchical cobalt phosphide hollow nanocages toward electrocatalytic ammonia synthesis under ambient pressure and room temperature. Small Methods. 2018;2:1800204.
- [Google Scholar]
- MoO 3 nanosheets for efficient electrocatalytic N 2 fixation to NH 3. J. Mater. Chem. A. 2018;6:12974-12977.
- [Google Scholar]
- Ambient N2 fixation to NH3 at ambient conditions: Using Nb2O5 nanofiber as a high-performance electrocatalyst. Nano Energy. 2018;52:264-270.
- [Google Scholar]
- Atomically dispersed molybdenum catalysts for efficient ambient nitrogen fixation. Angew. Chem.. 2019;131:2343-2347.
- [Google Scholar]
- Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water. Nat. Catal.. 2019;2:448-456.
- [Google Scholar]
- Advancing the electrochemistry of gas-involved reactions through theoretical calculations and simulations from microscopic to macroscopic. Adv. Funct. Mater.. 2022;32:2208474.
- [Google Scholar]
- Donor-site-acceptor covalent organic frameworks enable spontaneous nitrogen dissociation for boosted photoelectrochemical ammonia synthesis. Adv. Funct. Mater. 2024
- [Google Scholar]
- Identification of FeN4 as an efficient active site for electrochemical N2 reduction. ACS Catal.. 2019;9:7311-7317.
- [Google Scholar]
- Energy, catalyst and reactor considerations for (near)-industrial plasma processing and learning for nitrogen-fixation reactions. Catal. Today. 2013;211:9-28.
- [Google Scholar]
- Pyrene hydrogel for promoting direct bioelectrochemistry: ATP-independent electroenzymatic reduction of N 2. Chem. Sci.. 2018;9:5172-5177.
- [Google Scholar]
- Photocatalytic conversion of nitrogen to ammonia with water on surface oxygen vacancies of titanium dioxide. J. Am. Chem. Soc.. 2017;139:10929-10936.
- [Google Scholar]
- Angew. Chem. Int. Ed. Engl. 2013;52:3621-3625.
- Recent advances in catalysts, electrolytes and electrode engineering for the nitrogen reduction reaction under ambient conditions. Nanoscale. 2020;12:6900-6920.
- [Google Scholar]
- Ambient electrochemical ammonia synthesis with high selectivity on Fe/Fe oxide catalyst. ACS Catal.. 2018;8:9312-9319.
- [Google Scholar]
- Donor–acceptor type blends composed of black phosphorus and C₆₀ for solid-state optical limiters. Chem. Commun. 2018
- [Google Scholar]
- Development and recent progress on ammonia synthesis catalysts for Haber-Bosch process. Adv. Energy Sust. Res.. 2021;2:2000043.
- [Google Scholar]
- Fe3C/CdS as Noble-Metal-Free Composite Photocatalyst for Highly Enhanced Photocatalytic H2 Production under Visible Light.. 2020;603:117768
- Synthesis of Stable and Highly Luminescent Beryllium and Magnesium Doped ZnS Quantum Dots Suitable for Design of Photonic and Sensor Material.. 2013;134:739-746.
- Co 3 C as a Promising Cocatalyst for Superior Photocatalytic H 2 Production Based on Swift Electron Transfer Processes.. 2021;9(9):3145-3154.
- Ammonia synthesis on wool-like Au, Pt, Pd, Ag, or Cu electrode catalysts in nonthermal atmospheric-pressure plasma of N2 and H2. ACS Catal.. 2017;7:6924-6929.
- [Google Scholar]
- Electrochemical ammonia synthesis mediated by titanocene dichloride in aqueous electrolytes under ambient conditions. ACS Sustain. Chem. Eng.. 2017;5:9662-9666.
- [Google Scholar]
- Mechanistic aspects of dinitrogen cleavage and hydrogenation to produce ammonia in catalysis and organometallic chemistry: relevance of metal hydride bonds and dihydrogen. Chem. Soc. Rev.. 2014;43:547-564.
- [Google Scholar]
- Electrochemical ammonia synthesis and ammonia fuel cells. Adv. Mater.. 2019;31:1805173.
- [Google Scholar]
- Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev.. 2018;118:6337-6408.
- [Google Scholar]
- Formation of interfacial Cu-[OX]-Ce structures with oxygen vacancies for enhanced electrocatalytic nitrogen reduction. Small. 2022;18:2201200.
- [Google Scholar]
- Mesoporous Ru/MgO prepared by a deposition-precipitation method as highly active catalyst for producing COx-free hydrogen from ammonia decomposition. Appl Catal B. 2017;211:167-175.
- [Google Scholar]
- The Haber-Bosch process revisited: on the real structure and stability of “ammonia iron” under working conditions. Angew. Chem. Int. Ed.. 2013;52:12723-12726.
- [Google Scholar]
- Fe–CuP nanocubes for nitrate-to-ammonia conversion. New J. Chem.. 2024;48:6933-6942.
- [Google Scholar]
- The difficulty of proving electrochemical ammonia synthesis. ACS Energy Lett.. 2019;4:2986-2988.
- [Google Scholar]
- Electrochemical synthesis of NH3 at low temperature and atmospheric pressure using a γ-Fe2O3 catalyst. ACS Sustain. Chem. Eng.. 2017;5:10986-10995.
- [Google Scholar]
- Theory of multiple proton–electron transfer reactions and its implications for electrocatalysis. Chem. Sci.. 2013;4:2710-2723.
- [Google Scholar]
- Electrochemical synthesis of ammonia at atmospheric pressure and low temperature in a solid polymer electrolyte cell. Chem. Commun. 2000:1673-1674.
- [Google Scholar]
- Interface engineering in transition metal carbides for electrocatalytic hydrogen generation and nitrogen fixation. Mater. Horiz.. 2020;7:32-53.
- [Google Scholar]
- Galvanic deposition of Rh and Ru on randomly structured Ti felts for the electrochemical NH 3 synthesis. PCCP. 2015;17:3768-3782.
- [Google Scholar]
- Favoring the unfavored: selective electrochemical nitrogen fixation using a reticular chemistry approach. Sci. Adv.. 2018;4:eaar3208.
- [Google Scholar]
- Amorphizing of Au nanoparticles by CeOx–RGO hybrid support towards highly efficient electrocatalyst for N2 reduction under ambient conditions. Adv. Mater.. 2017;29:1700001.
- [Google Scholar]
- Solar water splitting and nitrogen fixation with layered bismuth oxyhalides. Acc. Chem. Res.. 2017;50:112-121.
- [Google Scholar]
- Enabling electrocatalytic N2 reduction to NH3 by Y2O3 nanosheet under ambient conditions. Ind. Eng. Chem. Res.. 2018;57:16622-16627.
- [Google Scholar]
- Boosted electrocatalytic N2 reduction to NH3 by defect-rich MoS2 nanoflower. Adv. Energy Mater.. 2018;8:1801357.
- [Google Scholar]
- Nitrogen-free commercial carbon cloth with rich defects for electrocatalytic ammonia synthesis under ambient conditions. Chem. Commun.. 2018;54:11188-11191.
- [Google Scholar]
- Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science. 2014;345:637-640.
- [Google Scholar]
- Electrolysis of liquid ammonia for hydrogen generation. Energ. Environ. Sci.. 2015;8:2775-2781.
- [Google Scholar]
- Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun.. 2018;9:1-9.
- [Google Scholar]
- Ambient nitrogen reduction cycle using a hybrid inorganic–biological system. Proc. Natl. Acad. Sci.. 2017;114:6450-6455.
- [Google Scholar]
- Ambient nitrogen reduction cycle using a hybrid inorganic–biological system. Proc. Natl. Acad. Sci.. 2017;114:6450-6455.
- [Google Scholar]
- Facile ammonia synthesis from electrocatalytic N2 reduction under ambient conditions on N-doped porous carbon. ACS Catal.. 2018;8:1186-1191.
- [Google Scholar]
- Insights into tuning of Mo-based structures toward enhanced electrocatalytic performance of nitrogen-to-ammonia conversion. Adv. Energy Sust. Res.. 2022;3:2100179.
- [Google Scholar]
- Solvent-in-gas system for promoted photocatalytic ammonia synthesis on porous framework materials. Adv. Mater.. 2023;35:2211730.
- [Google Scholar]
- Ambient N 2 fixation to NH 3 electrocatalyzed by a spinel Fe 3 O 4 nanorod. Nanoscale. 2018;10:14386-14389.
- [Google Scholar]
- Recent advances in strategies for highly selective electrocatalytic N2 reduction toward ambient NH3 synthesis. Curr. Opin. Electrochem.. 2021;29:100766
- [Google Scholar]
- Nitrogen-coordinated single Fe sites for efficient electrocatalytic N2 fixation in neutral media. Nano Energy. 2019;61:420-427.
- [Google Scholar]
- Carbon dioxide splitting using an electro-thermochemical hybrid looping strategy. Energ. Environ. Sci.. 2018;11:2928-2934.
- [Google Scholar]
- An amorphous noble-metal-free electrocatalyst that enables nitrogen fixation under ambient conditions. Angew. Chem.. 2018;130:6181-6184.
- [Google Scholar]
- Defect engineering metal-free polymeric carbon nitride electrocatalyst for effective nitrogen fixation under ambient conditions. Angew. Chem.. 2018;130:10403-10407.
- [Google Scholar]
- Electroreduction of nitrogen to ammonia on nanoporous gold. Nanoscale. 2021;13:1717-1722.
- [Google Scholar]
- Computational evaluation of electrocatalytic nitrogen reduction on TM single-, double-, and triple-atom catalysts (TM= Mn, Fe Co, Ni) based on graphdiyne monolayers. J. Phys. Chem. C. 2019;123:19066-19076.
- [Google Scholar]
- Theoretical screening of the transition metal heteronuclear dimer anchored graphdiyne for electrocatalytic nitrogen reduction. J. Energy Chem.. 2021;54:501-509.
- [Google Scholar]
- Enhancing selective nitrate-to-ammonia electrocatalysis with high-performing Ni2P embedded nitrogen phosphide doped carbon (NPC) deposited on CP: Unprecedented performance and stability. Int. J. Hydrogen Energy. 2024;70:315-324.
- [Google Scholar]
- Recent progress and prospects of electrolytes for electrocatalytic nitrogen reduction toward ammonia. Chin. Chem. Lett.. 2024;35:108550
- [Google Scholar]
- Pt-modified Fe3O4 supported on Ni foam nanocomposite for electrocatalytic nitrate reduction to ammonia. Electrocatalysis. 2024;15:159-170.
- [Google Scholar]
- Highly selective and stable electrochemical reduction of nitrate to ammonia using VO2-x/CuF catalyst with oxygen vacancies. J. Power Sources. 2024;608:234644
- [Google Scholar]
- Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem. 2011;3:1159-1165.
- [Google Scholar]
- Ammonia synthesis from N 2 and H 2 O using a lithium cycling electrification strategy at atmospheric pressure. Energ. Environ. Sci.. 2017;10:1621-1630.
- [Google Scholar]
- Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energ. Environ. Sci.. 2017;10:1621-1630.
- [Google Scholar]
- Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat. Catal.. 2018;1:269-275.
- [Google Scholar]
- Nitrogenase bioelectrocatalysis: heterogeneous ammonia and hydrogen production by MoFe protein. Energ. Environ. Sci.. 2016;9:2550-2554.
- [Google Scholar]
- Bioelectrochemical Haber-Bosch process: an ammonia-producing H2/N2 fuel cell. Angew. Chem. Int. Ed.. 2017;56:2680-2683.
- [Google Scholar]
- Electrochemical and catalytic behaviors of Ni–YSZ anode for the direct utilization of ammonia fuel in solid oxide fuel cells. J. Electrochem. Soc.. 2015;162:F1268.
- [Google Scholar]
- The challenge of electrochemical ammonia synthesis: a new perspective on the role of nitrogen scaling relations. ChemSusChem. 2015;8:2180-2186.
- [Google Scholar]
- Metal-organic framework-derived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N2 and H2O in alkaline electrolytes. Nano Energy. 2018;48:217-226.
- [Google Scholar]
- Enhancing the rate of electrochemical nitrogen reduction reaction for ammonia synthesis under ambient conditions using hollow gold nanocages. Nano Energy. 2018;49:316-323.
- [Google Scholar]
- Deciphering electrolyte selection for electrochemical reduction of carbon dioxide and nitrogen to high-value-added chemicals. Adv. Funct. Mater.. 2023;33:2212483.
- [Google Scholar]
- Recent progress in transition-metal-catalyzed reduction of molecular dinitrogen under ambient reaction conditions. Inorg. Chem.. 2015;54:9234-9247.
- [Google Scholar]
- Selective dinitrogen conversion to ammonia using water and visible light through plasmon-induced charge separation. Angew. Chem. Int. Ed.. 2016;55:3942-3946.
- [Google Scholar]
- Fe2P nanoparticles as highly efficient freestanding co-catalyst for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy. 2018;43(10):5337-5345.
- [Google Scholar]
- Dual metal site-mediated efficient C-N coupling toward electrochemical urea synthesis. J. Mater. Chem. A. 2023;11:13249-13254.
- [Google Scholar]
- Progress of electrocatalytic urea synthesis: strategic design, reactor engineering, mechanistic details and techno-commercial study. Mater. Chem. Front.. 2023;7:3820-3854.
- [Google Scholar]
- Strengthening the metal center of Co–N4 active sites in a 1D–2D Heterostructure for nitrate and nitrogen reduction reaction to ammonia. ACS Sustain. Chem. Eng.. 2023;11:6191-6200.
- [Google Scholar]
- Electrochemical conversion of dinitrogen to ammonia mediated by a complex of fullerene C 60 and γ-cyclodextrin. Chem. Commun. 2007:2270-2272.
- [Google Scholar]
- Single-site gold catalysts on hierarchical N-doped porous noble carbon for enhanced electrochemical reduction of nitrogen. Small Methods. 2018;2:1800202.
- [Google Scholar]
- Recent advances and challenges of electrocatalytic N2 reduction to ammonia. Chem. Rev.. 2020;120:5437-5516.
- [Google Scholar]
- High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst. Nat. Commun.. 2018;9:1-8.
- [Google Scholar]
- Tailoring electronic structure of copper nanosheets by silver doping toward highly efficient electrochemical reduction of nitrogen to ammonia. Chem. Eng. J.. 2022;433:133752
- [Google Scholar]
- Electrochemical N 2 fixation to NH 3 under ambient conditions: Mo 2 N nanorod as a highly efficient and selective catalyst. Chem. Commun.. 2018;54:8474-8477.
- [Google Scholar]
- Ammonia synthesis at ambient conditions via electrochemical atomic hydrogen permeation. ACS Energy Lett.. 2021;6:3817-3823.
- [Google Scholar]
- Ammonia production technologies. Techno-Economic Challenge. Green Ammon. Energy Vector 2020:41-84.
- [Google Scholar]
- A low-crystalline ruthenium nano-layer supported on praseodymium oxide as an active catalyst for ammonia synthesis. Chem. Sci.. 2017;8:674-679.
- [Google Scholar]
- Highly distributed amorphous copper catalyst for efficient ammonia electrosynthesis from nitrate. J. Hazard. Mater.. 2023;445:130651
- [Google Scholar]
- N, O trans-coordinating silver single-atom catalyst for robust and efficient ammonia electrosynthesis from nitrate. Appl. Catal. B. 2023;331:122687
- [Google Scholar]
- Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions. Adv. Mater.. 2017;29:1606550.
- [Google Scholar]
- Upcycling wastewater nitrate into ammonia fertilizer via concurrent electrocatalysis and membrane extraction. Chem. Eng. J.. 2023;455:140959
- [Google Scholar]
- The journey toward low temperature, low pressure catalytic nitrogen fixation. Adv. Energy Mater.. 2020;10:2000659.
- [Google Scholar]
- Recent progress towards the electrosynthesis of ammonia from sustainable resources. Catal. Today. 2017;286:57-68.
- [Google Scholar]
- A review and comparative assessment of direct ammonia fuel cells. Therm. Sci. Eng. Prog.. 2018;5:568-578.
- [Google Scholar]
- Energy Environ. Sci.. 2017;10:1621-1630. 7, 706–709; b)
- Electrochemical synthesis of ammonia as a potential alternative to the Haber-Bosch process. Nat. Catal.. 2019;2:377-380.
- [Google Scholar]
- A physical catalyst for the electrolysis of nitrogen to ammonia. Sci. Adv.. 2018;4:e1700336.
- [Google Scholar]
- MoS2 polymorphic engineering enhances selectivity in the electrochemical reduction of nitrogen to ammonia. ACS Energy Lett.. 2018;4:430-435.
- [Google Scholar]
- Rational electrode–electrolyte design for efficient ammonia electrosynthesis under ambient conditions. ACS Energy Lett.. 2018;3:1219-1224.
- [Google Scholar]
- Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal.. 2019;2:290-296.
- [Google Scholar]
- Rational electrode-electrolyte design for efficient ammonia electrosynthesis under ambient conditions. ACS Energy Lett.. 2018;3:1219-1224.
- [Google Scholar]
- Demonstration of no catalytical activity of Fe-N-C and Nb-N-C electrocatalysts toward nitrogen reduction using in-line quantification. SusMat. 2022;2:476-486.
- [Google Scholar]
- How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem. Soc. Rev.. 2019;48:3166-3180.
- [Google Scholar]
- Nitrogen fixation by Ru single-atom electrocatalytic reduction. Chem. 2019;5:204-214.
- [Google Scholar]
- Review on ammonia as a potential fuel: from synthesis to economics. Energy Fuel. 2021;35:6964-7029.
- [Google Scholar]
- Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev.. 2014;43:5183-5191.
- [Google Scholar]
- Electrolytic reduction of molecular nitrogen. J. Am. Chem. Soc.. 1968;90:4492-4493.
- [Google Scholar]
- Catalytic fixation of molecular nitrogen by electrolytic and chemical reduction. J. Am. Chem. Soc.. 1969;91:5194.
- [Google Scholar]
- Erratum: The NBS tables of chemical thermodynamic properties. Selected values for inorganic and C1 and C2 organic substances in SI units [J. Phys. Chem. Ref. Data 11, Suppl. 2 (1982)] J. Phys. Chem. Ref. Data. 1989;18:1807-1812.
- [Google Scholar]
- Breaking scaling relations to achieve low-temperature ammonia synthesis through LiH-mediated nitrogen transfer and hydrogenation. Nat. Chem.. 2017;9:64-70.
- [Google Scholar]
- Electrocatalytic hydrogenation of N2 to NH3 by MnO: experimental and theoretical investigations. Adv. Sci.. 2019;6:1801182.
- [Google Scholar]
- Recent progress towards mild-condition ammonia synthesis. J. Energy Chem.. 2019;36:25-36.
- [Google Scholar]
- A general strategy to glassy M-Te (M= Ru, Rh, Ir) porous nanorods for efficient electrochemical N2 fixation. Adv. Mater.. 2020;32:1907112.
- [Google Scholar]
- Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem.. 2018;10(6):667-672.
- [Google Scholar]
- Ambient electrochemical synthesis of ammonia from nitrogen and water catalyzed by flower-like gold microstructures. ChemSusChem. 2018;11:3480-3485.
- [Google Scholar]
- Ambient electrosynthesis of ammonia: electrode porosity and composition engineering. Angew. Chem. Int. Ed.. 2018;57:12360-12364.
- [Google Scholar]
- One-pot synthesis of bi-metallic PdRu tripods as an efficient catalyst for electrocatalytic nitrogen reduction to ammonia. J. Mater. Chem. A. 2019;7:801-805.
- [Google Scholar]
- Direct fabrication of bi-metallic PdRu nanorod assemblies for electrochemical ammonia synthesis. Nanoscale. 2019;11:5499-5505.
- [Google Scholar]
- Oxygen-bridged vanadium single-atom dimer catalysts promoting high faradaic efficiency of ammonia electrosynthesis. ACS Nano. 2023;17:7406-7416.
- [Google Scholar]
- Fast reversion of hydrophility-superhydrophobicity on textured metal surface by electron beam irradiation. Appl. Surf. Sci.. 2024;669:160455
- [Google Scholar]
- Enhanced nitrate-to-ammonia activity on copper–nickel alloys via tuning of intermediate adsorption. J. Am. Chem. Soc.. 2020;142:5702-5708.
- [Google Scholar]
- Electrocatalytic nitrogen reduction to ammonia by Fe2O3 nanorod array on carbon cloth. ACS Sust. Chem. Eng.. 2019;7:11754-11759.
- [Google Scholar]
- Integrated selective nitrite reduction to ammonia with tetrahydroisoquinoline semi-dehydrogenation over a vacancy-rich Ni bifunctional electrode. J. Mater. Chem. A. 2021;9:239-243.
- [Google Scholar]
- Mn3O4 nanocube: an efficient electrocatalyst toward artificial N2 fixation to NH3. Small. 2018;14:1803111.
- [Google Scholar]
- Efficient electrocatalytic reduction of nitrate to ammonia over Mo2CTx micro-foam with rich edge sites. Chem. Eng. J.. 2024;479:147602
- [Google Scholar]
- Ammonia synthesis from electrocatalytic N2 reduction under ambient conditions by Fe2O3 nanorods. ChemCatChem. 2018;10:4530-4535.
- [Google Scholar]
- Study on reduced chemical mechanisms of ammonia/methane combustion under gas turbine conditions. Energy Fuel. 2016;30:8701-8710.
- [Google Scholar]
- Electrochemical hydrogen storage materials: state-of-the-art and future perspectives. Energy Fuel. 2024;38(9):7579-7613.
- [Google Scholar]
- Electrochemical ammonia synthesis through N2 and H2O under ambient conditions: theory, practices, and challenges for catalysts and electrolytes. Nano Energy. 2020;69:104469
- [Google Scholar]
- Anchoring Mo on C9N4 monolayers as an efficient single atom catalyst for nitrogen fixation. J. Energy Chem.. 2021;57:443-450.
- [Google Scholar]
- Facilitating reactant accessibility to heterometallic phosphide with superhydrophilic interfaces for efficient electrochemical production of ammonia and lactic acid. Appl. Catal. B: Environ. Energy. 2024;358:124390
- [Google Scholar]
- Electrochemical reduction of aqueous nitrogen (N 2) at a low overpotential on (110)-oriented Mo nanofilm. J. Mater. Chem. A. 2017;5:18967-18971.
- [Google Scholar]
- Electrochemical synthesis of ammonia in molten salts. J. Energy Chem.. 2020;43:195-207.
- [Google Scholar]
- Density functional theory study of single metal atoms embedded into MBene for electrocatalytic conversion of N2 to NH3. ACS Appl. Nano Mater.. 2020;3:9870-9879.
- [Google Scholar]
- A spectroscopic study on the nitrogen electrochemical reduction reaction on gold and platinum surfaces. J. Am. Chem. Soc.. 2018;140:1496-1501.
- [Google Scholar]
- In-situ and operando spectroscopies for the characterization of catalysts and of mechanisms of catalytic reactions. J. Catal.. 2021;404:900-910.
- [Google Scholar]
- Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 catalyst: theoretical and experimental studies. Adv. Mater.. 2018;30:1800191.
- [Google Scholar]
- Efficient electrochemical N2 reduction to NH3 on MoN nanosheets array under ambient conditions. ACS Sustain. Chem. Eng.. 2018;6:9550-9554.
- [Google Scholar]
- A novel aluminum-graphite dual-ion battery. Adv. Energy Mater.. 2016;6(11):1502588.
- [Google Scholar]
- TiO 2 nanoparticles–reduced graphene oxide hybrid: an efficient and durable electrocatalyst toward artificial N 2 fixation to NH 3 under ambient conditions. J. Mater. Chem. A. 2018;6:17303-17306.
- [Google Scholar]
- Highly efficient electrochemical ammonia synthesis via nitrogen reduction reactions on a VN nanowire array under ambient conditions. Chem. Commun.. 2018;54:5323-5325.
- [Google Scholar]
- High-performance electrohydrogenation of N2 to NH3 catalyzed by multishelled hollow Cr2O3 microspheres under ambient conditions. ACS Catal.. 2018;8:8540-8544.
- [Google Scholar]
- Ambient NH 3 synthesis via electrochemical reduction of N 2 over cubic sub-micron SnO 2 particles. Chem. Commun.. 2018;54:12966-12969.
- [Google Scholar]
- Enabling effective electrocatalytic N2 conversion to NH3 by the TiO2 nanosheets array under ambient conditions. ACS Appl. Mater. Interfaces. 2018;10:28251-28255.
- [Google Scholar]
- Governing interlayer strain in bismuth nanocrystals for efficient ammonia electrosynthesis from nitrate reduction. ACS Nano. 2022;16:4795-4804.
- [Google Scholar]
- Multi-site electrocatalysts boost pH-universal nitrogen reduction by high-entropy alloys. Adv. Funct. Mater.. 2021;31:2006939.
- [Google Scholar]
- Constructing Cu-CuO heterostructured skin on Cu cubes to promote electrocatalytic ammonia production from nitrate wastewater. J. Hazard. Mater.. 2022;439:129653
- [Google Scholar]
- Layered-double-hydroxide nanosheets as efficient visible-light-driven photocatalysts for dinitrogen fixation. Adv. Mater.. 2017;29:1703828.
- [Google Scholar]
- Synergistic integration of anammox and endogenous denitrification processes for the simultaneous carbon, nitrogen, and phosphorus removal. Environ. Sci. Tech.. 2024;58(24):10632-10643.
- [Google Scholar]
- Efficient and durable N 2 reduction electrocatalysis under ambient conditions: β-FeOOH nanorods as a non-noble-metal catalyst. Chem. Commun.. 2018;54:11332-11335.
- [Google Scholar]
- Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev.. 2015;44:5148-5180.
- [Google Scholar]







