Translate this page into:
Enhanced anti-fatigue and exercise performances due to Huáng qí polysaccharide supplementation in mice
⁎Corresponding author. pohsien0105@gmail.com (Po-Hsien Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In modern society, people have busy and competitive lives, with different stresses causing fatigue and overwork, which may ultimately harm their health. We investigated the beneficial effects of Astragalus polysaccharide (rAPS) on fatigue and exercise performance. Forty male Institute of Cancer Research (ICR) mice were randomly divided into four groups according to body weight (BW): control group (distilled water), 1-fold dose group (0.70 g rAPS/kg BW/day), 2-fold dose group (1.40 g rAPS/kg BW/day) and a 5-fold dose group (3.50 g rAPS/kg BW/day). In terms of exercise performance, rAPS supplementation significantly improved grip strength performance and time to exhaust-loaded swim times when compared with controls (p < 0.05). Moreover, rAPS supplementation significantly reduced fatigue-related biochemical parameters, including blood lactate and BUN levels, while significantly improve muscle strength and swimming endurance times t(p < 0.05). Hence, we recommend that rAPS elicit antifatigue and performance-enhancing health benefits.
Keywords
Dietary supplement
Astragalus polysaccharide (rAPS)
Anti-fatigue
Health
Stresses
1 Introduction
Huáng qí is the root of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge) Hsiao is a widely cultivated perennial plant, and the literature has reported evidence of its use as a medicinal herb in China, the United States, Japan, Korea, Iran, Russia, and other European countries (Chen et al., 2020; Du et al., 2022; Jia et al., 2019). In Traditional Chinese Medicine, Huáng qí has well-known anti-oxidant, anti-aging, and anti-fatigue properties that exert protective effects on the cardiovascular system and are often used as immunomodulators in mixed herbal medicines to treat common colds, diarrhea, fatigue, loss of appetite, and dizziness (Chen et al., 2022; Hao et al., 2015; Huang et al., 2016; Li et al., 2012; Yang et al., 2022). This treatment may be used by frail and elderly individuals, patients with chronic diseases accompanied by low-grade inflammation, and work-stressed individuals (Chen et al., 2020). Several studies have reported physiological activities of Astragalus polysaccharide (rAPS) such as immunomodulatory, antiviral, hepatoprotective, hematopoietic, neuroprotective, anti-inflammatory, organ protective, anti-insulin resistance, anti-tumor, anti-aging, expectorant, diuretic, improved post-stroke symptoms, and gut microbiota modulation (Chen et al., 2020; Du et al., 2022; Feng et al., 2021; Hao et al., 2015; Hu et al., 2022; Jia et al., 2019; Li et al., 2019; Li et al., 2023; Liu et al., 2017a; Liu et al., 2017b; Liu et al., 2019; Wang et al., 2021; Wu et al., 2019; Xu et al., 2021; Yang et al., 2022; Zengin et al., 2022).
rAPS are widely used in animal husbandry and aquaculture sectors to improve the animal immunity, concomitant with reduced antibiotic use (Chen et al., 2020; Farag & Alagawany, 2019; Liu et al., 2022; Yu et al., 2022; Zahran et al., 2014). Owing to these beneficial effects, rAPS has significant potential in the health food industry (Liu et al., 2017a). Many “over the counter” rAPS functional foods are available, such as liquid extracts (beverages and soups), powders (trail mix), capsules, and tea products (Chen et al., 2022; Chen et al., 2020; Xu et al., 2021; Zhang et al., 2011; Zhao et al., 2021). Notably, rAPS is used in the anti-aging field to inhibit mitochondrial swelling, anti-aging effects, and reduce endoplasmic reticulum stress (Tang & Huang, 2022). In vivo and in vitro studies have reported that rAPS suppresses transforming growth factors by inhibiting inflammatory responses, while improving oxidative stress, myocardial fibrosis, and ventricular hypertrophy via anti-reactive oxygen species mechanisms (Li et al., 2012; Ren et al., 2023). Additionally, 3 mg/mL rAPS for 20 days promoted antioxidant gene expression (dFOXO and 4E-BP) in Drosophila and modulated the insulin/insulin growth factor-1 signaling pathways to exert anti-aging activities (such as the activity of SOD and CAT is significantly increased, and improved exercise capacity and lipid metabolism are regulated) (Yang et al., 2021). Cycloastragenol from Huáng qí extracts also exerted stimulatory effects on telomerase activity and neuroprotective effects against 6-hydroxydopamine (6-OHDA) in a dose-dependent manner, and was therapeutically active against physiopathology-associated oxidative stress, telomere depletion, and protein loss (Yilmaz et al., 2022). Huáng qí also enhances endurance and acts as an anti-fatigue agent in mice (Yeh et al., 2014), while rAPS restores oxidative stress and low Sirt1 expression levels caused by excessive exercise, while promoting mitochondrial fusion, inhibiting fission processes, and reducing peroxisome proliferator-activated receptor-γ coactivator-1α expression (Huang et al., 2016; Luo et al., 2019). In recent years, rAPS has been developed as an injectable product (PG2) for treating cancer-related fatigue, with > 65 % of individuals experiencing at least 10 % improvement in fatigue in a single treatment cycle (Wang et al., 2019).
Therefore, this study investigated the anti-fatigue (fatigue mitigation or elimination) effects of rAPS by performing a 42-day gavage supplementation study in Institute of Cancer Research (ICR) mice. Animals were assessed for body weight (BW), dietary and water intake, forelimb grip strength, loaded or non-loaded swimming exhaustion activities (exercise performance, days 43––45), and post-exercise fatigue-related blood biochemical parameters (day 47). Blood was collected for biochemical analyses on the 51st day (after killing), while the liver and hind-leg muscles were analyzed for glycogen levels.
2 Materials and methods
2.1 Materials
Huáng qí (Astragalus membranaceus) polysaccharide [rAPS; 2-(chloromethyl)-4-(4-nitrophenyl) thiazole], MW 254.693)] was provided by Phytohealth Co. (Taipei, Taiwan). All chemicals were purchased directly from Sigma-Aldrich® (Merck KGaA, Darmstadt, Germany), unless otherwise noted.
2.2 Calculation of the dose
The rAPS recommended daily intake (RDI) is 3.4 g per day (0.057 g per kg BW per day) for adults weighing 60 kg BW. In addition, this study developed trials of dose effects, which were evaluated at one, two, and five folds of the recommended dose. Because the metabolic rates of humans and experimental animals differed, it was necessary to convert the human dose to the dose administered to the animals in the trial. In this study, doses were calculated according to the method published by the U.S. Food and Drug Administration (FDA; guidance for industry: estimate the maximum safe starting dose in initial clinical trials for therapeutics in healthy adult volunteers) (FDA, 2005). The conversion factor between humans and mice was 12.3 folds, which resulted in 0.70 g/kg BW/day for the 1-fold dose group (70 mg/mL), 1.40 g/kg BW /day for the 2-fold dose group (140 mg/mL), and 3.50 g/kg BW /day for the 5-fold dose group (350 mg/mL), while the control group was given an equal volume of distilled water (ddH2O). In addition, gavage was used in this study, mixed with feed or ddH2O, if necessary, within 10 mL/kg of BW or less; otherwise, multiple feedings were administered within 6 h. Different concentrations of rAPS (70, 140, and 350 mg/mL) were dissolved in ddH2O, to calculate the amount of gavage required in ratio to the BW of mice, and each mouse was gavaged once a day (10 mL/kg BW).
2.3 Experimental animal
A total of 40 male, six-weeks-old Bltw: CD1 (ICR) mice (BW, approximately 30 g) were purchased from BioLasco Co., Ltd. (Taipei, Taiwan) and randomly grouped by weight distribution order into control and treatment groups (low, medium, and high doses of rAPS) via two weeks of adaptation (eight weeks old), for a total of four groups of ten mice each (n = 10). The mice were housed under specific pathogen-free conditions (25 ± 2 °C, humidity of 65 ± 5 %, 12 h light/dark cycle, and lights on at 7p.m.) with a diet (5001 Rodent Laboratory Chow, Research Diets Inc., New Brunswick, NJ, USA) and water supplied ad libitum during the experiment. Food and drinking water were freshly prepared and replaced every morning. All animal procedures were conducted according to the standards outlined in the Guidelines for the Care and Use of Experimental Animals of the Committee for Control and Supervision of Experiments on Animals and the National Institutes of Health. The Committee on Animal Research, Providence University (code 20,191,211 A008) approved the protocol.
2.4 Design of animal experiments
The trial period was 51 days, during which BW, feed, and water intake were recorded. The samples were administered by gavage at 9:00 a.m. every day for six weeks. Then, the experiments were performed sequentially (forelimb grip strength on day 43, swimming endurance on day 45, swimming endurance and blood lactate analysis on day 47, and swimming endurance, BUN concentration, and CK analysis on day 49) with the fatigue-related biochemical index analysis (Fig. 1, while more detailed operation is described in the subsequent sections). In addition, the study followed the above guidelines for animal welfare, with the intensity and duration of the exercise tests ranging from low to high intensity and from short to long periods, and blood was collected for each test. Finally, on day 51, the animals were sacrificed and the liver, kidneys, and para-testicular fat were weighed, and each organ was observed visually. Additional histopathological sections will be organized for observation purposes where abnormalities such as abnormal color, enlargement, or hard masses are found.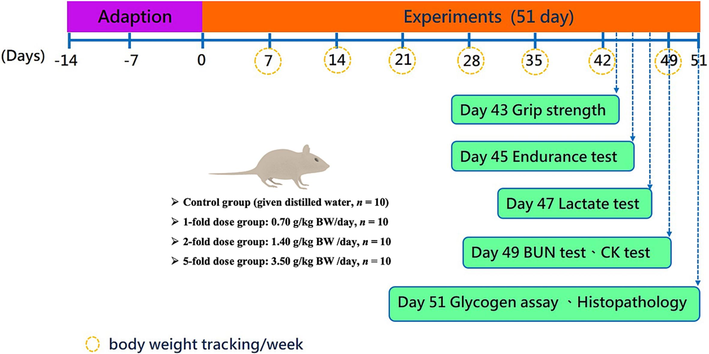
The study designs.
2.5 Effect of forelimb grip strength in mice
The forelimb grip strength of mice was measured as described by Harrigan et al. (2019), with some modifications. The effect of rAPS on forelimb grip strength in mice was evaluated using an animal forelimb grip strength meter (Cat. No. 47200, Ugo Basile® SRL, Gemonio, Italy) was used to analyze the change in strength between the groups. The forelimb grip force test was performed 30 min after feeding on day 43. The principle underlying this is that as the mouse's tail was dragged, it naturally grabbed the front grip bar to avoid involuntary backward movement. The pulling force of the operator exceeded the maximum grasping force. The instrument automatically recorded the maximum force while the mouse released the grasping bar. First, the mice were positioned on a platform with a force sensor grip bar in front of their head, and the height of the grip bar was configured. The operator grasped the mouse at 1/3 of the root of its tail, prompting the mouse to obtain the measurement device, and then pulled parallel to the opposite direction so that the mouse's body and the sensor were horizontal. The tail was pulled back until the mice released their grip, and this process was repeated three times to obtain the maximum value.
2.6 Swimming exhaustion trial
Enhanced exercise endurance performance was measured as described by Matsuoka et al. (2018) with slight modifications. The swimming exhaustion (with a 5 % BW load) test was designed to evaluate the effect of rAPS supplementation on enhancing exercise endurance performance. Therefore, the mice were acclimated to swimming (water temperature 27 ± 1 °C) one week before the official swimming exhaustion test (day 38, 30 min after feeding). On the 45th day, the swimming exhaustion test was carried out with loading (5 % of the BW of mice), fasting for 12 h before swimming, and 30 min after feeding the rAPS. Each time, the mouse swam in the cylinder (15 cm in diameter and water depth of 20 cm) at 27 ± 1℃. In addition, the mice were kept in motion during the trial; otherwise, mice floating on the water surface without moving limbs were forced to continue swimming by stirring with a stirring stick nearby. Swimming time was defined as the time mouse's head (mouth and nose) remaining completely submerged for 8 s continuously.
2.7 Variation of biochemical blood indicators concentration before and after exercise
The changes in biochemical blood indicators (lactate) before and after exercise, with the calculation of the lactate raised ratio, provide an opportunity to evaluate the effect of rAPS supplementation on post-exercise fatigue reduction. On day 47, for the swimming exhaustion test, 0.1 mL of blood was collected 30 min after feeding, followed by 10 min of swimming in water at 30 °C for 10 min without load. Next, 0.1 mL of blood was collected after swimming and resting for 20 min, at three time points for blood lactate analysis.
In addition, changes in BUN concentration in each group of mice after exercise were measured on the 49th day of the test. Similarly, after feeding rAPS for 30 min, the mice swam for 90 min (at 30 °C in water) without loading and rested for 60 min; 0.1 mL of blood was collected for BUN concentration analysis. Moreover, CK analysis was performed on some blood samples to investigate whether rAPS protects against muscle damage during prolonged exercise. Blood samples were examined using a biochemistry analyzer (7060, Hitachi Ltd., Tokyo, Japan).
2.8 Analysis of the weight and morphology of the liver, kidney, and para-testicular fat
The pathological sections were prepared as described by Jia et al. Jia et al. (2019) and Dai et al. (2023) with some modifications. In general, 10 % formalin was used to fix the liver, kidneys, and para-testicular fat. The sections were then embedded in paraffin and sectioned. After hematoxylin and eosin (H&E) staining, the tissue sections were analyzed by light microscopy.
2.9 Analysis of glycogen content in liver and muscle
All mice were rested for 2 days after the trial described in the above section. On the 51st day of the trial, after 30 min of feeding, all groups of mice were sacrificed, and blood was collected for analysis of liver damage indicators [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)]. In addition, the liver and hind leg muscles were collected, washed with saline, swabbed, and weighed. The tissues were cut at a similar location and frozen at −80 °C for subsequent analysis of muscle glycogen content.
The phenol–sulfuric acid method used to determine liver and muscle tissue glycogen levels was performed as described by Matsunaga et al. (2021), with modifications. The sample was homogenized by adding a 5-fold volume (w/v) of tissue homogenate (pH 7.4, tris buffer and saline) using a bullet blender (Next Advance, Cambridge, MA, USA). The tissue homogenate was dispensed into microcentrifuge tubes and centrifuged for 15 min (4 °C, 12,000 × g) using a microcentrifuge (22R, Beckman Coulter Inc., Brea, Calif., USA). The supernatant was mixed with phenol and sulfuric acid, followed by a reaction for 15 min, and the absorbance was measured at 490 nm using a spectrophotometer (U-2000, Hitachi Ltd.). A standard curve was prepared using glycogen, which, by interpolation, calculated the glycogen content in the liver and muscle tissues.
2.10 Serum biochemical analysis
Blood was collected from the heart immediately after the mice were sacrificed, followed by centrifugation at 12,000 × g for 10 min at 4 °C. The supernatants were analyzed for biochemical parameters of plasma using a biochemical analyzer, including glucose, total protein, albumin, triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), while the analysis operation was performed according to the manufacturer's instructions.
2.11 Statistical analysis
All values in this study are expressed as mean ± standard deviation (S.D.), while as the data were analyzed using SAS (ver. 9.0, SAS Institute Inc., Cary, NC, USA). The data were analyzed using one-way analysis of variance (ANOVA) to test for differences between groups. Duncan's multiple allometric tests were used to assess differences between groups for significance when p < 0.05, a significant difference was indicated.
3 Results and discussion
3.1 Variations in BW and water and dietary intake
During the trial, it was observed that all groups of mice were able to maintain their BW levels with increasing stability (Table 1). Additionally, no significant differences were observed in terms of the average daily diet and water intake (Table 1). Thus, supplementation with 1-, 2-, or 5-fold rAPS neither inhibited mouse growth nor affected dietary status, which is consistent with the results of Yeh et al. (2014). Animals were divided into four groups (n = 10): control, 1-, 2-, and 5-folds group. Values were expressed as the mean ± standard deviation. Different lowercase letters on the same line indicate significant differences (p < 0.05).
Characteristics
Control
1-fold
2-folds
5-folds
Body weight (g)
Initial (0 day)
33.15 ± 1.16a
33.17 ± 1.26a
33.19 ± 0.86a
33.26 ± 1.14a
Week
1st
33.66 ± 1.21a
33.71 ± 1.18a
33.58 ± 1.00a
33.55 ± 1.01a
2nd
34.64 ± 1.53a
34.47 ± 1.17a
34.40 ± 1.11a
34.48 ± 1.04a
3rd
35.64 ± 1.87a
35.26 ± 1.10a
35.52 ± 1.31a
35.27 ± 0.84a
4th
36.17 ± 1.93a
36.09 ± 1.07a
36.19 ± 1.50a
36.01 ± 1.10a
5th
36.99 ± 1.91a
36.98 ± 1.09a
37.07 ± 1.63a
36.96 ± 1.02a
6th
37.44 ± 1.90a
37.41 ± 0.94a
37.47 ± 1.63a
37.47 ± 1.05a
7th
37.89 ± 1.84a
37.85 ± 0.93a
37.93 ± 1.52a
37.99 ± 1.04a
Final
(51th day)38.45 ± 1.78a
38.38 ± 0.98a
38.38 ± 1.59a
38.40 ± 1.01a
Water intake (mL/mouse/day)
7.89 ± 0.19a
7.91 ± 0.19a
7.89 ± 0.19a
7.92 ± 0.18a
Diet intake (g/mouse/day)
6.60 ± 0.92a
6.61 ± 0.91a
6.60 ± 0.92a
6.60 ± 0.92a
Diet calories (Kcal/mouse/day)
(A)22.18 ± 3.11a
22.20 ± 3.05a
22.18 ± 3.08a
22.19 ± 3.09a
rAPS calories (Kcal/mouse/day)
(B)0.00 ± 0.00a
0.06 ± 0.00b
0.11 ± 0.01c
0.28 ± 0.01d
Total daily caloric intake (Kcal/mouse/day)(A) +
(B)21.18 ± 3.11a
22.25 ± 3.05a
22.30 ± 3.08a
22.47 ± 3.09a
Organ weight(g)
Liver
2.29 ± 0.10a
2.30 ± 0.07a
2.29 ± 0.12a
2.30 ± 0.08a
Kidney
0.62 ± 0.03a
0.62 ± 0.02a
0.62 ± 0.04a
0.62 ± 0.05a
Para-testicular fat
0.25 ± 0.02a
0.25 ± 0.03a
0.25 ± 0.03a
0.25 ± 0.02a
3.2 Assessing forelimb grip strength
To evaluate the effects of rAPS supplementation (different doses) on muscle strength, forelimb grip strength studies were performed after six weeks. Values in control, 1-, 2-, and 5-fold rAPS groups were 112.70 ± 6.06, 134.20 ± 9.53, 148.20 ± 9.22, and 155.40 ± 20.85 (g), respectively, which were significantly increased by 1.19- (p = 0.0006), 1.31- (p < 0.0001), and 1.38-folds (p < 0.0001) when compared with the controls (Fig. 2A). Additionally, considering the effects of BW on absolute forelimb grip strength in individual mice, relative forelimb grip strength (%) values were calculated to eliminate the effects of absolute grip strength by BW. Relative forelimb grip strength (%) values in the control, 1-, 2-, and 5-fold rAPS supplementation groups were 295.48 ± 27.54, 359.89 ± 32.56, 380.25 ± 25.74, and 407.04 ± 54.57 (%), respectively. Compared with controls, relative grip strength in the 1-, 2-, and 5-fold rAPS supplementation groups were significantly increased by 1.22– (p = 0.0004), 1.29- folds (p < 0.0001), and 1.38-folds (p < 0.0001), respectively (Fig. 2B). Thus, rAPS supplementation significantly increased forelimb grip strength after six weeks. Yeh et al. Yeh et al. (2014) reported that Huáng qí putatively contributed to physiological activity in mice, with dose-dependence improvement tendencies observed in exercise performances, consistent with our findings. Additionally, Chang et al. Chang et al. (2020) reported significant improvements in rat motor performances (grip strength and swimming time) when animals were supplemented with Danggui Buxue Tang (Huáng qí and Angelica sinensis (Oliv) Diels in a 5:1 ratio).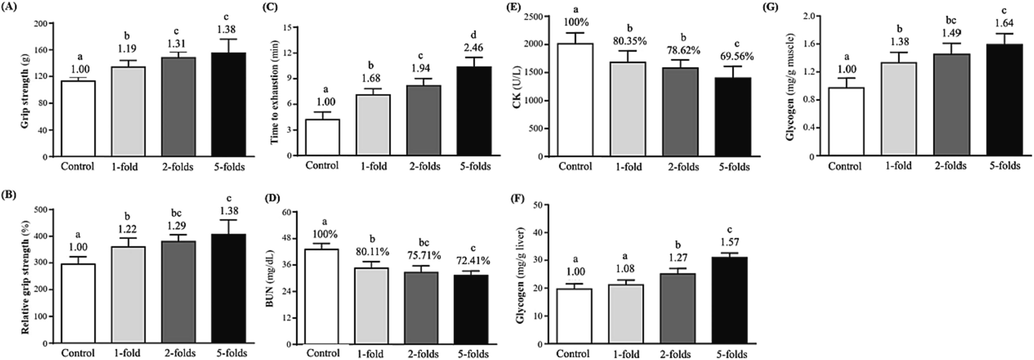
The effect of rAPS supplementation on (A) grip strength, (B) relative grip strength (%), (C) time to exhaustion in swimming tests, (D) blood urea nitrogen (BUN) concentrations after 60 min of rest, (E) blood creatine kinase (CK) activity after 60 min of rest, (F) liver glycogen levels, and (E) muscle glycogen levels.
3.3 Loading swimming exhaustion tests (exercise performance)
Exercise endurance tests are used to assess the effects of natural medicines on fatigue (Matura et al., 2018; Miao et al., 2018). Therefore, this study used an objective, user-friendly, and widely used forced-swimming test model to facilitate observations/measurements of exercise endurance times and muscle or organ tissues in animals (Tang et al., 2017). Exercise performances (5 % BW load) were 4.21 ± 0.89, 7.09 ± 0.73, 8.17 ± 0.83, and 10.35 ± 1.12 (min) in control, 1-, 2-, and 5-fold rAPS groups, respectively (p < 0.05) (Fig. 2C). Additionally, test durations were significantly improved by 1.68- (p < 0.0001), 1.94- folds (p < 0.0001), and 2.46-folds (p < 0.0001) in the 1-, 2-, and 5-fold groups, respectively, when compared with controls. Thus, rAPS supplementation for six weeks improved exercise performance in mice with dose-dependent and satisfactory exercise performance in the 5-fold dose group. In another study, mice swimming at a 3 % BW load after the fourth week of Huáng qí dietary supplementation showed dose-dependent and increased exercise endurance performance concomitant with increased supplementation (Yeh et al., 2014).
3.4 Biochemical blood indicators
3.4.1 Blood lactate concentrations before and after loaded-swimming exhaustion tests
During high-intensity exercise, muscles receive sufficient energy from anaerobic glycolysis, which generates high lactate levels and contributes to biochemical and physiological side effects in muscle contractions mediated by phosphofructokinase and calcium ion release (Hargreaves & Spriet, 2020; Wu et al., 2013). Therefore, biochemical markers, such as lactate, ammonia, glucose, and creatine kinase (CK) can be used to assess muscle fatigue after exercise (Wu et al., 2013; Yeh et al., 2014). Over-exercise can cause blood biochemical abnormalities, but supplementation with Buyang-huanwu Decoction [composed of Huáng qí (Astragali Radix), Angelicae Sinensis Radix, Paeoniae Radix Rubra, and Pheretima] can effectively correct these abnormal changes (Li et al., 2022). Since “a lack of energy” causes poor mental performance, exercise fatigue may directly affect nerve signal transduction (Li et al., 2022; Loy et al., 2018). Blood lactate concentrations were 3.01 ± 0.30––3.02 ± 0.27 (mmol/L) before swimming, with no significant differences across groups (Table 2). However, blood lactate concentrations in control, 1-, 2-, and 5-fold rAPS dose groups were 7.09 ± 0.44, 6.01 ± 0.41, 5.52 ± 0.42, and 5.26 ± 0.24 (mmol/L), respectively, after 10 min of swimming. We observed significant reductions of 15.23 % (p < 0.0001), 22.14 % (p < 0.0001), and 25.81 % (p < 0.0001) in blood lactate concentrations in the 1-, 2-, and 5-fold groups, respectively, compared with controls. Thus, during a six-week study, rAPS significantly reduced post-exercise blood lactate concentrations, with the 5-fold dose group showing good performance. Animals were divided into four groups (n = 10): control, 1-, 2-, and 5-fold groups. Values were expressed as the mean ± standard deviation. Different lowercase letters on the same line indicate significant differences (p < 0.05). Lactate production rate (B/A) = after swimming (B)/before swimming (A). Clearance rate [(B-C)/B)] = after swimming (B)- after a 20 min rest (C)/after swimming (B).
Period
Control
1-fold
2-folds
5-folds
Lactate (mmol/L)
Before swimming
(A)3.01 ± 0.38a
3.02 ± 0.27a
3.02 ± 0.20a
3.01 ± 0.30a
After
swimming
(B)7.09 ± 0.44a
6.01 ± 0.41b
5.52 ± 0.42c
5.26 ± 0.24c
After a 20 min rest (C)
6.03 ± 0.50a
4.80 ± 0.26b
4.37 ± 0.50c
3.98 ± 0.55c
Rate of lactate production and clearance
Lactate production rate = B/A
2.38 ± 0.28a
2.01 ± 0.24b
1.84 ± 0.19bc
1.77 ± 0.23c
Clearance rate= (B-C)
/B0.15 ± 0.06a
0.20 ± 0.07ab
0.21 ± 0.07ab
0.24 ± 0.10b
Lactate clearance rates calculated from blood lactate concentrations at two time points (before and after swimming) were 2.38 ± 0.28, 2.01 ± 0.24, 1.84 ± 0.19, and 1.77 ± 0.23 in the control, 1-, 2-, and 5-fold rAPS supplementation groups, respectively (Table 2). Clearance rates were significantly reduced by 15.55 % (p = 0.0011), 22.69 % (p < 0.0001), and 25.63 % (p < 0.0001) in the 1-, 2-, and 5-fold rAPS dose groups, respectively, compared with controls.
After 10 min of swimming and rest for 20 min, the blood lactate concentrations in the control, 1-, 2-, and 5-fold groups were 6.03 ± 0.50, 4.80 ± 0.26, 4.37 ± 0.50, and 3.98 ± 0.55 (mmol/L) (Table 2). Therefore, rAPS supplementation for six weeks effectively reduced blood lactate concentrations after exercise (20 min rest), which, when compared with controls, was significantly reduced by 20.40 % (p < 0.0001), 27.53 % (p < 0.0001), and 34.00 % (p < 0.0001), respectively. Blood lactate clearance rates were then calculated at two-time points after 10 min of swimming and 20 min of rest after 10 min of swimming and were 0.15 ± 0.06, 0.20 ± 0.07, 0.21 ± 0.07, and 0.24 ± 0.10 in control, 1-, 2-, and 5-fold groups, respectively. Notably, clearance rates in the 5-fold group increased significantly by 1.63-folds (p < 0.05) compared to the controls and showed good post-exercise and elevated blood lactate effects. Additionally, post-exercise recovery times depend on BUN and blood lactic acid production and elimination rate, while an anti-fatigue effect will follow if some substance inhibits that accumulation and accelerates its elimination (Miao et al., 2018).
3.4.2 BUN and CK concentrations before and after non-loading swimming exhaustion tests
BUN is the end-product of protein metabolism, an important fatigue indicator, and accumulates as exercise levels increase (Luo et al., 2019). Additionally, amino acid metabolism (liver urea cycle or glutamine synthesis), which produces ammonia, decreases endurance rates and causes fatigue (Coqueiro et al., 2018). In terms of BUN concentrations, after 90 min of swimming (non-loading) with rest for 60 min in all groups, 43.03 ± 2.77, 34.47 ± 3.05, 32.58 ± 2.95, and 31.16 ± 1.98 (mg/dL) BUN levels were observed in control, 1-, 2-, and 5-fold rAPS supplementation groups, respectively (Fig. 2D). Significant reductions of 19.89 % (p < 0.0001), 24.29 % (p < 0.0001), and 27.59 % (p < 0.0001) were observed in the supplementation groups compared with the control group, respectively. Thus, rAPS supplementation for six weeks significantly reduced post-exercise BUN concentrations. Li et al. (2014) reported that Huáng qí (3 g/kg−1 BW) facilitated rats recovery rates from fatigue by regulating glucose, lipid, and energy metabolism. Additionally, Miao et al. (2018) reported that fatigue levels in mice were confirmed by increased BUN and blood lactic acid levels and decreased SOD levels after exhaustion, and confirmed that Danggui Buxue Tang treatment decreased fatigue-related biochemical marker levels. Positive correlations were observed between BUN, blood lactic acid, and exercise endurance.
Moreover, muscle cell damage caused by metabolites (e.g., malondialdehyde) during biochemical responses to exercise-related fatigue may cause muscle cell dissolution, leading to increased CK levels in the blood (Luo et al., 2019). In our study, CK activities in control, 1-, 2-, and 5-fold rAPS dose groups were 2010.60 ± 195.88, 1679.80 ± 207.57, 1580.80 ± 144.60, and 1398.50 ± 209.55 (U/L), respectively (Fig. 2E). Compared with the controls, CK activity weas significantly reduced by 16.45 % (p = 0.0004), 21.38 % (p < 0.0001), and 30.44 % (p < 0.0001) in the rAPS supplementation groups, respectively. Thus, rAPS supplementation significantly reduced post-exercise CK activity, with the 5-fold dose groups performing the best. Yeh et al. (2014) reported that Huáng qí dietary supplementation increased serum glucose and decreased lactate, ammonia, and CK levels in post-exercise mice, with significant dose-dependent effects, consistent with our study.
3.5 Organ and tissue weight effects
This study also examined whether rAPS supplementation causes organ and tissue abnormalities in ICR mice. After sacrificed, the liver, kidney, and para-testicular fat samples were weighed and stained for histopathology. All tissues were similar across the groups (Table 1). No significant toxic effects or damage were identified in the histopathological sections (Fig. 3). Thus, six weeks of rAPS supplementation was not associated with organ or tissue damage in ICR mice. Yeh et al. (2014) reported that six weeks of Huáng qí treatment caused histological abnormalities in the liver, muscle, heart, kidney, lung, and testis of mice, consistent with our data.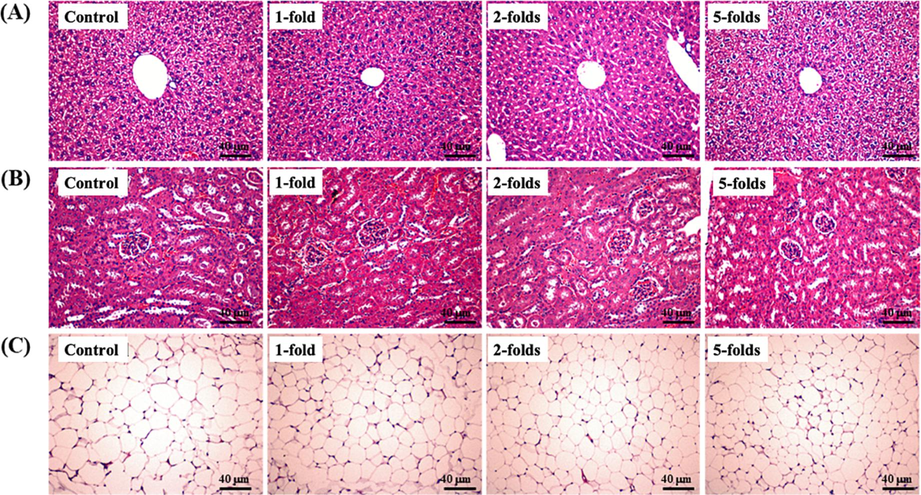
The effect of rAPS supplementation on histopathological sections from (A) the liver, (B) kidney, and (C) para-testicular fat in study mice. Scale bar = 40 μm.
3.6 Liver and leg muscle glycogen levels
Performance during aerobic exercise is influenced by intracorporeal tissues, which are glycogen storage sites (Chang et al., 2020; Hargreaves & Spriet, 2020; Matsunaga et al., 2021). Therefore, we collected liver and leg muscle tissues from animals after they were sacrificed, and glycogen levels were analyzed. In control, 1-, 2-, and 5-fold groups, levels were 19.68 ± 1.88, 21.20 ± 1.73, 25.09 ± 1.99, and 30.98 ± 1.67 (mg/g liver), respectively (Fig. 2F) (p < 0.05). Notably, liver glycogen levels were significantly increased by 1.27- (p < 0.0001) and 1.57- (p < 0.0001) in the 2- and 5- groups, respectively, when compared with controls. Moreover, glycogen levels in leg muscles were 0.97 ± 0.14, 1.34 ± 0.15, 1.45 ± 0.16, and 1.59 ± 0.16 (mg/g muscle) in control, 1-, 2-, and 5-fold groups, respectively (Fig. 2G). Significant fold increases of 1.38 (p < 0.0001), 1.49 (p < 0.0001), and 1.64 levels (p < 0.0001) in muscle glycogen were identified in 1-, 2-, and 5-fold groups, respectively, when compared with controls.
3.7 Clinical blood biochemical parameters
ALT and AST are predictors of liver cell and tissue damage and are typical biochemical responses to exercise fatigue (Luo et al., 2019). The clinical blood biochemical parameters, ALT and AST values, and albumin levels related to liver function remained unchanged across the four study groups (Table 3). Thus, rAPS supplementation for 6 weeks did not affect liver function. Moreover, no effects were observed on TG, TC, HDL, LDL, glucose, or total protein levels in blood. Thus, no abnormal physiological, metabolic, or nutritional parameters were observed. Animals were divided into four groups (n = 10): control, 1-, 2-, and 5-fold groups. Values were expressed as the mean ± standard deviation. Different lowercase letters on the same line indicate significant differences (p < 0.05).
Blood Biochemistry Index
Control
1-fold
2-folds
5-folds
AST (U/L)
114.00 ± 9.21a
113.40 ± 10.20a
115.00 ± 9.23a
114.00 ± 9.64a
ALT (U/L)
44.30 ± 7.97a
45.90 ± 6.05a
44.40 ± 8.25a
46.90 ± 4.58a
Albumin (g/dL)
3.27 ± 0.10a
3.26 ± 0.11a
3.27 ± 0.12a
3.27 ± 0.11a
HDL (mg/dL)
94.88 ± 11.55a
95.78 ± 13.94a
96.63 ± 8.64a
96.29 ± 9.15a
LDL (mg/dL)
15.69 ± 2.61a
15.58 ± 1.88a
15.71 ± 2.41a
15.85 ± 3.30a
TC (mg/dL)
128.20 ± 8.78a
128.10 ± 10.10a
128.20 ± 8.07a
127.80 ± 6.92a
TG (mg/dL)
132.50 ± 10.27a
130.90 ± 7.87a
130.80 ± 10.43a
132.20 ± 6.91a
Glucose (mg/dL)
199.90 ± 14.14a
200.90 ± 10.73a
200.40 ± 14.58a
202.40 ± 13.53a
Total protein (g/dL)
5.37 ± 0.18a
5.25 ± 0.20a
5.40 ± 0.24a
5.39 ± 0.25a
This study recommended that daily rAPS supplementation for six weeks significantly improved muscular endurance, time of swimming exhaustion during loading, blood lactate clearance rate, glycogen stock (in the liver and muscle), and exercise endurance while positively relieving fatigue.
4 Conclusions
This study examined the effects of six weeks of rAPS supplementation (1-, 2-, and 5-fold with respect to RDI) in mice and identified significantly increased exercise performance and improved muscle strength in loaded-swimming exhaustion time tests. Moreover, rAPS supplementation potentially reduced lactate, BUN concentrations, and CK activity in the blood after exercise, while it increased muscle glycogen levels, exercise endurance, and relieved fatigue, which suggesting anti-fatigue benefits. In future studies, more scientific and representative clinical trials should be conducted. Hence, our study provides a scientific basis and potential therapeutic strategy for the development of nutritional supplements with anti-fatigue potential for market positioning clarification to guide future development.
Acknowledgments
This research was financially supported by Phytohealth Co. (Taipei, Taiwan) (PU108-11150-A083). The authors also thank Phytohealth Co. (Taipei, Taiwan) for providing rAPS as an experimental material.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chinese herbal decoction (Danggui Buxue Tang) supplementation augments physical performance and facilitates physiological adaptations in swimming rats. Pharm. Biol.. 2020;58(1):545-552.
- [CrossRef] [Google Scholar]
- A reference-grade genome assembly for Astragalus mongholicus and insights into the biosynthesis and high accumulation of triterpenoids and flavonoids in its roots. Plant Communications. 2022;100469
- [CrossRef] [Google Scholar]
- Astragali Radix (Huangqi): A promising edible immunomodulatory herbal medicine. J. Ethnopharmacol.. 2020;258:112895
- [CrossRef] [Google Scholar]
- Effects of glutamine and alanine supplementation on central fatigue markers in rats submitted to resistance training. Nutrients. 2018;10(2):119.
- [CrossRef] [Google Scholar]
- HDAC6 promotes aggressive development of liver cancer by improving egfr mRNA stability. Neoplasia. 2023;35:100845
- [CrossRef] [Google Scholar]
- A critical review of Astragalus polysaccharides: From therapeutic mechanisms to pharmaceutics. Biomed. Pharmacother.. 2022;147:112654
- [CrossRef] [Google Scholar]
- The role of Astragalus membranaceus as immunomodulator in poultry. Worlds Poult. Sci. J.. 2019;75(1):43-54.
- [CrossRef] [Google Scholar]
- FDA, U. S. (2005). Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Retrieved from https://www.fda.gov/media/72309/download.
- Astragalus polysaccharide enhances the immune function of RAW264.7 macrophages via the NF-kappa B p65/MAPK signaling pathway. Exp Ther Med. 2021;21(1):20.
- [CrossRef] [Google Scholar]
- Astragalus polysaccharide suppresses excessive collagen accumulation in a murine model of bleomycin-induced scleroderma. Int J Clin Exp Med. 2015;8(3):3848-3854.
- [Google Scholar]
- Skeletal muscle energy metabolism during exercise. Nature Metabolism. 2020;2(9):817-828.
- [CrossRef] [Google Scholar]
- Assessing rat forelimb and hindlimb motor unit connectivity as objective and robust biomarkers of spinal motor neuron function. Sci. Rep.. 2019;9(1):16699.
- [CrossRef] [Google Scholar]
- Medicinal and edible plants in the treatment of dyslipidemia: advances and prospects. Chin. Med.. 2022;17(1):113.
- [CrossRef] [Google Scholar]
- Effects of Astragalus polysaccharides on dysfunction of mitochondrial dynamics induced by oxidative stress. Oxid. Med. Cell. Longev.. 2016;2016:9573291.
- [CrossRef] [Google Scholar]
- Antioxidant, immunomodulatory, oxidative stress inhibitory and iron supplementation effect of Astragalus membranaceus polysaccharide-iron (III) complex on iron-deficiency anemia mouse model. Int. J. Biol. Macromol.. 2019;132:213-221.
- [CrossRef] [Google Scholar]
- Comparative analysis of twenty-five compounds in different parts of Astragalus membranaceus var. mongholicus and Astragalus membranaceus by UPLC-MS/MS. J. Pharm. Anal.. 2019;9(6):392-399.
- [CrossRef] [Google Scholar]
- 1H NMR based metabolomic study of the antifatigue effect of Astragali Radix. Mol. Biosyst.. 2014;10(11):3022-3030.
- [CrossRef] [Google Scholar]
- Research progress of Astragalus membranaceus in treating peritoneal metastatic cancer. J. Ethnopharmacol.. 2023;305:116086
- [CrossRef] [Google Scholar]
- The antifatigue mechanism of buyang-huanwu decoction as revealed by serum metabolomics in an endurance swimming rat model. J. Med. Food. 2022;25(11):1038-1049.
- [CrossRef] [Google Scholar]
- Mitochondrial protection and anti-aging activity of Astragalus Polysaccharides and their potential mechanism. Int. J. Mol. Sci.. 2012;13(2):1747-1761.
- [CrossRef] [Google Scholar]
- Liu, P., Zhao, H., & Luo, Y. (2017). Anti-aging implications of Astragalus membranaceus (Huangqi): A well-known Chinese Tonic. Aging Dis, 8(6), 868-886. 10.14336/ad.2017.0816.
- Liu, P., Zhao, H., & Luo, Y. (2017). Anti-aging implications of Astragalus membranaceus (Huangqi): A well-known Chinese tonic. Aging and disease, 8(6), 868-886. 10.14336/ad.2017.0816.
- Astragalus polysaccharide from Astragalus Melittin ameliorates inflammation via suppressing the activation of TLR-4/NF-κB p65 signal pathway and protects mice from CVB3-induced virus myocarditis. Int. J. Biol. Macromol.. 2019;126:179-186.
- [CrossRef] [Google Scholar]
- Evaluation of the effects of Astragalus polysaccharides as immunostimulants on the immune response of crucian carp and against SVCV in vitro and in vivo. Comp. Biochem. Physiol. C: Toxicol. Pharmacol.. 2022;253:109249
- [CrossRef] [Google Scholar]
- Perceived fatigue and energy are independent unipolar states: Supporting evidence. Med. Hypotheses. 2018;113:46-51.
- [CrossRef] [Google Scholar]
- Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res.. 2019;148:104409
- [CrossRef] [Google Scholar]
- Effects of glucose ingestion at different frequencies on glycogen recovery in mice during the early hours post exercise. J. Int. Soc. Sports Nutr.. 2021;18(1):69.
- [CrossRef] [Google Scholar]
- Egg white hydrolysate improves fatigue due to short-term swimming load test in mice. Food Sci. Nutr.. 2018;6(8):2314-2320.
- [CrossRef] [Google Scholar]
- A systematic review of biological mechanisms of fatigue in chronic illness. Biol. Res. Nurs.. 2018;20(4):410-421.
- [CrossRef] [Google Scholar]
- Metabolomics analysis of serum reveals the effect of Danggui Buxue Tang on fatigued mice induced by exhausting physical exercise. J. Pharm. Biomed. Anal.. 2018;151:301-309.
- [CrossRef] [Google Scholar]
- Research progress of natural medicine Astragalus mongholicus Bunge in treatment of myocardial fibrosis. J. Ethnopharmacol.. 2023;305:116128
- [CrossRef] [Google Scholar]
- Extraction, structure, and activity of polysaccharide from Radix astragali. Biomed. Pharmacother.. 2022;150:113015
- [CrossRef] [Google Scholar]
- Structural characterization and antifatigue effect in vivo of Maca (Lepidium meyenii Walp) polysaccharide. J. Food Sci.. 2017;82(3):757-764.
- [CrossRef] [Google Scholar]
- Karnofsky performance status as a predictive factor for cancer-related fatigue treatment with Astragalus polysaccharides (PG2) injection—a double blind, multi-center, randomized phase IV study. Cancers. 2019;11(2):128.
- [CrossRef] [Google Scholar]
- Astragalus saponins improves stroke by promoting the proliferation of neural stem cells through phosphorylation of Akt. J. Ethnopharmacol.. 2021;277:114224
- [CrossRef] [Google Scholar]
- Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules. 2013;18(4):4689-4702.
- [CrossRef] [Google Scholar]
- The combination of Astragalus membranaceus and Angelica sinensis inhibits lung cancer and cachexia through its immunomodulatory function. J. Oncol.. 2019;2019:9206951.
- [CrossRef] [Google Scholar]
- Astragalus polysaccharides attenuate ovalbumin-induced allergic rhinitis in rats by inhibiting NLRP3 inflammasome activation and NOD2-Mediated NF-κB activation. J. Med. Food. 2021;24(1):1-9.
- [CrossRef] [Google Scholar]
- Chemical comparison of Astragali Radix by UHPLC/Q-TOF-MS with different growing patterns. Eur. Food Res. Technol.. 2022;248(9):2409-2419.
- [CrossRef] [Google Scholar]
- Extension of drosophila lifespan by Astragalus polysaccharide through a mechanism dependent on antioxidant and insulin/IGF-1 signaling. Evid. Based Complement. Alternat. Med.. 2021;2021:6686748.
- [CrossRef] [Google Scholar]
- Astragalus membranaceus Improves exercise performance and ameliorates exercise-induced fatigue in trained mice. Molecules. 2014;19(3):2793-2807.
- [CrossRef] [Google Scholar]
- The role of cycloastragenol at the intersection of NRF2/ARE, telomerase, and proteasome activity. Free Radic. Biol. Med.. 2022;188:105-116.
- [CrossRef] [Google Scholar]
- Effects of dietary Astragalus polysaccharides on growth, health and resistance to Vibrio harveyi of Lates calcarifer. Int. J. Biol. Macromol.. 2022;207:850-858.
- [CrossRef] [Google Scholar]
- Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol.. 2014;38(1):149-157.
- [CrossRef] [Google Scholar]
- Integration of in vitro and in silico approaches to assess three Astragalus species from Turkey flora: A novel spotlight from lab bench to functional applications. Food Biosci.. 2022;49:101858
- [CrossRef] [Google Scholar]
- New isoflavonoid glycosides and related constituents from Astragali Radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem.. 2011;59(4):1131-1137.
- [CrossRef] [Google Scholar]
- Natural plant extracts and compounds for rheumatoid arthritis therapy. Medicina. 2021;57(3):266.
- [CrossRef] [Google Scholar]







