Translate this page into:
Enhanced knee joint treatment using a hybrid hyaluronic acid-alginate filler reinforced with hydroxyapatite-titanium nanoparticles for sports-related injuries
⁎Corresponding author. yangfeng@huas.edu.cn (Feng Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A novel composition of hyaluronic acid filler composed with Ti-NP. The crystalline properties of the porous scaffolds were investigated by XRD analysis. SEM images were used to show the scaffold structure. Presence of composite scaffolds increases the growth and proliferation of osteoblast cells. Hyaluronic acid/hydroxyapatite scaffolds containing 15 wt% of Ti-NP have successfully acted as bone fillers.
Abstract
Sports injuries, particularly bone injuries, have become increasingly prevalent in recent years. As athletes push themselves harder and become more competitive, the risk of injury rises. Additionally, the aging population has contributed to the increased prevalence of bone injuries, including those related to the knee joint. As a result, significant research efforts have focused on developing new techniques for knee joint repair. In this study, a novel hyaluronic acid-Alginate filler composition containing hydroxyapatite-titanium nanoparticles (HA/Ti-NPs) was prepared through freeze-drying. The crystalline properties of the porous scaffolds were analyzed using X-ray diffraction (XRD), while scanning electron microscope (SEM) images were used to visualize the scaffold structure. The morphological investigation demonstrated that adding 15 wt% Ti-NP to the scaffold reduces percentages of porosity to about 76.5 ± 5, while increasing the elastic modulus. Furthermore, the presence of nanoparticles decreased the rate of degradation, increased crystallinity, reduced pore size, and promoted calcium release, creating a favorable environment for bone tissue growth. The scaffold's degradation pH is close to neutral, which is ideal for bone tissue treatment. Adding 15 wt% Ti-NP reduces the contact angle from 120° to 93°. The composite scaffolds promote the proliferation and growth of osteoblast cells, and after 48 h, degraded material improved cell viability, growth, and proliferation. The three-component hyaluronic acid-Alginate/HA scaffolds with 15 wt% Ti-NP exhibited suitable morphological, mechanical, physical, and chemical properties for bone applications, as well as high potential for joint bonding. These porous scaffolds have the potential to be used as bone fillers of sport injuries and further research is needed to confirm their efficacy.
Keywords
Hyaluronic acid
Sport injuries
Titanium nanoparticles
Knee Joint
Scaffold morphology
Osteoblast cell proliferation
1 Introduction
Sports injuries can have a significant impact on athletes and sports enthusiasts. Among the injuries that are common among athletes are joint injuries, including knee joint injuries. One of the treatment methods for these types of injuries is the use of hyaluronic acid-based fillers with hydroxyapatite-titanium nanoparticles (HA-TiNPs) (Ali et al., 2022; Khan et al., 2021; Al-Musawi and Ali, 2023). These fillers exist as one-third mineral substance in bones and teeth and are used to strengthen joints. The use of hyaluronic acid fillers with HA-TiNPs enhances the ability of the filler to be absorbed and fixed in the damaged area, resulting in improved pain and knee joint-related issues. These fillers are known as a natural substitute for the treatment of sports-related joint injuries. Due to the greater capabilities of hyaluronic acid fillers with HA-TiNPs, this treatment method is recognized as an effective treatment for sports-related joint injuries (Abdal-hay et al., 2017; Khandan et al., 2015; Dave and Rahatgaonkar, 2016; Bhattacharjee et al., 2015). Additionally, hyaluronic acid fillers with HA-TiNPs have benefits such as pain reduction, improvement of joint function, and a decrease in the need for other treatments over time. The use of hyaluronic acid fillers with HA-TiNPs is therefore an effective treatment method for sports-related joint injuries. Bone damage can occur due to various factors such as trauma, infection, and inherited diseases. Osteoarthritis, a chronic joint disorder, results in several problems for patients, including disability, clinical symptoms, complaints, and limited joint movement, particularly in the knee joint.
Although non-steroidal anti-inflammatory drugs are widely used, their side effects can limit their effectiveness (Martinez-Mondragon et al., 2023; Oh et al., 2011; Rasan and Farhan, 2023; Xu et al., 2022). Corticosteroid injections, while effective and well-tolerated, must be restricted to avoid joint damage. Therefore, hyaluronic acid, which has fewer side effects, is a recommended treatment. In treating bone injuries, including those affecting the knee joint, traditional approaches such as autografts or animal products have limitations, such as the risk of disease transmission and donor shortage (Dudek and Goryczka, 2016; Sharma et al., 2016; Roh et al., 2017; Lin et al., 2013). Bone scaffolds and fillers are two different methods used in the reconstruction of sports injuries, including fractures and painful injuries. Bone scaffolds are structures with physical and chemical properties similar to natural bone and are highly effective in bone tissue repair. Fillers, on the other hand, are used in the treatment of joint and soft tissue injuries due to their ability to fill empty volumes and restore soft tissue. The common soft tissues injured are muscles, tendons, and ligaments. The common soft tissue injuries occur during sports and exercise activities, but sometimes everyday activities can cause an injury (Lu et al., 2023; Tang et al., 2021). In some cases, bone scaffolds are combined with fillers to improve the rehabilitation of sports injuries. Fillers can act as a filler, reduce inflammation, and hold bone scaffolds in place. This combination improves the physical and chemical characteristics of bone scaffolds and increases their resistance and stability. Researchers are currently exploring alternative methods to enhance bone damage repair (Dudek and Goryczka, 2016; Martinez-Mondragon et al., 2023; Oh et al., 2011; Rasan and Farhan, 2023; Roh et al., 2017; Sharma et al., 2016; Xu et al., 2022), yet the development of an ideal bone replacement with a complex structure remains a challenge. An ideal bone filler or scaffold should not only possess excellent mechanical properties but also promote osteogenesis and foster a suitable porous structure for cell growth. One proposed microstructure is a composite of HA nanoparticles, leading researchers to focus on developing bio-nanocomposites and nano-hydroxyapatite (n-HA) scaffolds for bone replacement and improvement (Maji et al., 2018; Dennis et al., 2017; Subramaniam et al., 2016; Bjursten et al., 2010; Ramírez-Cedillo et al., 2019; Al-hamdani and Yas, 2023). Due to its bioactivity, n-HA can bind to living tissue without forming a collagen layer, and the attachment of HA nanoparticles enhances mesenchymal cell differentiation into bone (Tomczykowa and Plonska-Brzezinska, 2019; Kadhim and Mahmood, 2023; He et al., 2019; Al-Taweel and Saud, 2016; Smith et al., 2012; Mishra et al., 2019; Zhang et al., 2020).
With similar chemical and mineral phases as bone, n-HA is widely used in dental and bone applications, particularly for its bone conduction and bone induction properties (Wiedmer et al., 2018; Hasan and Fatalla, 2023; Alnajm and Shukri, 2023; Kim et al., 2017; Hasan et al., 2023; Shuai et al., 2017). Another promising biomolecule for tissue engineering is hyaluronic acid (HLA), which is a linear polysaccharide with high molecular weight composed of repeating units of 2-acetamide-2-deoxy-beta-di-glucose and beta-di-glucuronic acid with (3–1) and (1–4) glucoside bonds. HLA plays various roles throughout the body, particularly in connective tissues, such as regulating tissue regeneration, cell movement and proliferation, and immune and inflammatory responses (Cui et al., 2020; Kopec et al., 2007). It can also accelerate healing by stimulating angiogenesis, yet its rapid degradation is a disadvantage. However, when combined with n-HA, the degradation rate of HLA can be balanced (Li et al., 2020; Gao et al., 2017; Liu et al., 2004; AL-Anezi et al., 2023; Rahman et al., 2009).
One of the promising materials for bone repair is titanium nanoparticles (Ti-NP) due to their biocompatibility and ability to conduct bone formation, as evidenced by studies demonstrating bone formation upon contact with bone tissue (AL-Anezi et al., 2023; Rahman et al., 2009; Lison et al., 2008). Moreover, Ti-NP has been shown to induce favorable chemical and biological properties in scaffolds and does not cause inflammatory reactions or fibrosis when injected subcutaneously into mice. The scaffolds are typically produced using techniques such as fuzzy foaming, fuzzy separation, freeze-drying, and sintering, which create a porous matrix structure that supports cell adhesion and positioning (Yang et al., 2023; Sanapalli et al., 2022; Bian and Xing, 2022). During that period, the utilization of Hyaluronic Acid (HA) and Alginate composites reinforced with Hydroxyapatite-Titanium Nanoparticles (HA/Alginate/HA-TiNPs) represented an innovative and promising approach in the field of tissue engineering. HA and Alginate have been extensively studied and employed as biocompatible and biodegradable materials for tissue engineering applications, particularly in the development of scaffolds for various tissues such as bone, cartilage, and skin. These materials create favorable environments that support cell adhesion, proliferation, and differentiation. By incorporating Hydroxyapatite, a bioactive material that closely resembles the mineral composition of natural bone, along with Titanium Nanoparticles known for their exceptional strength and stiffness, into the composite scaffold, the mechanical properties and osteogenic potential of the scaffold are enhanced. Hydroxyapatite promotes bone regeneration and facilitates integration with the surrounding tissues, while Titanium Nanoparticles contribute to the scaffold's mechanical integrity. This combined approach demonstrates the potential to advance tissue engineering strategies for various applications. The ideal scaffold should match the multi-scale, interconnected porosity of natural bone, as well as its external shape and composition, to promote cell functionality and tissue growth. The scaffold should be honeycomb in structure and have interconnected pores that allow for nutrient and oxygen transfer. In this study, the optimal percentage of hyaluronic acid and HA solutions are first determined, and an antibacterial enhancer of Ti-NP is added to the hyaluronic acid-Alginate/HA bone filler composition before freeze-drying to create a three-dimensional bone scaffold. The mechanical properties and biological tests, including bioactivity, biocompatibility, and toxicity, are then examined. By incorporating Hydroxyapatite, a bioactive material resembling natural bone composition, along with Titanium Nanoparticles known for their strength and stiffness, into the composite scaffold, we improve the scaffold's integrity and promote bone regeneration. Additionally, we optimize the composition of the scaffold and introduce an antibacterial enhancer of Ti-NP to further enhance its performance. Our study not only addresses the mechanical aspects of the scaffold but also evaluates its bioactivity, biocompatibility, and toxicity, providing comprehensive insights for potential tissue engineering applications.
2 Materials and methods
In this study we develop a new filler material using a combination of hyaluronic acid polymer powder, HA, and titanium nanoparticles (Ti-NP) with glutaraldehyde as a crosslinker. Hyaluronic acid (HLA) is a naturally occurring biopolymer that is widely used in the cosmetic industry as a dermal filler due to its biocompatibility, biodegradability, and ability to retain water. The HA polymer powder used in this study was obtained from Sigma-Aldrich, the USA, and had a molecular weight of 848.8 g/mol. HA and titanium nanoparticles (Ti-NP) were used in combination with HA polymer powder, with an initial amount of HA set at a constant value of 0.5 g, to enhance the mechanical properties of the filler material. HA is a well-known biocompatible and bioactive ceramic that is commonly used in bone tissue engineering applications due to its similarity to the mineral phase of natural bone. The hybrid filler was prepared by incorporating HLA and Alginate as the key components. HA, a biocompatible and bioactive ceramic, was chosen for its similarity to the mineral phase of natural bone and its ability to support bone tissue engineering. Alginate, a biopolymer derived from seaweed, was selected for its biocompatibility and previous use in tissue engineering. To enhance the structural support and promote bone regeneration, Hydroxyapatite (HA), a calcium phosphate ceramic resembling the mineral component of natural bone, was included in the hybrid filler. Additionally, Titanium Nanoparticles (Ti-NP), renowned for their excellent mechanical properties and biocompatibility, were employed as reinforcement in the hybrid filler. Ti-NP, on the other hand, is known for its excellent mechanical properties, including high strength, stiffness, and toughness, making it a promising additive for enhancing the mechanical properties of composites. In this study, glutaraldehyde was selected as a crosslinker because of its water solubility and its effectiveness in crosslinking a broad range of materials across various temperature and pH conditions. Additionally, glutaraldehyde is recognized for its ability to react with amino acid-based groups, hydroxyl groups, and thiol groups that are present in cell membranes, walls, and cytoplasm. These properties make it an ideal crosslinker for HA polymer powder, HA, and titanium nanoparticles. To formulate the filler material, a specific ratio of HA polymer powder, HA, and TiNPs were mixed, and glutaraldehyde was added as the crosslinker. The resulting mixture was allowed to crosslink, and the resulting material was characterized using a variety of analytical techniques. In summary, the use of a combination of HA polymer powder, HA, and titanium nanoparticles with glutaraldehyde as a crosslinker has demonstrated significant promise as a novel filler material for both cosmetic and medical applications. However, additional studies are necessary to optimize the properties of this composite material and assess its biocompatibility and safety for in vivo use. The research article proposes two hypotheses. Hypothesis 1 suggests that incorporating hydroxyapatite-titanium nanoparticles (Ti-NPs) into a hyaluronic acid-alginate filler will enhance the mechanical properties, degradation rate, and calcium release of the scaffold, thereby creating a favorable environment for bone tissue growth. Hypothesis 2 posits that the utilization of hyaluronic acid-based fillers containing hydroxyapatite-titanium nanoparticles (HA-TiNPs) will lead to improvements in pain and knee joint-related issues associated with sports-related joint injuries, providing an effective and natural alternative for treatment.
2.1 Preparation of polymer solution
This article develop a porous nanocomposite scaffold using gelatin, hyaluronic acid, and Ti-NP via the freeze-drying method to enhance the speed and quality of current treatment methods. Additionally, Ti-NP with a particle size less than 50 nm is utilized to accelerate the antibacterial properties of bone filler, reduce the risk of infection, and expedite the treatment process. The resulting scaffold exhibits favorable chemical and biological properties. Specifically, in this study, a homogeneous solution of hyaluronic acid and HA is prepared by stirring HA in deionized water at 450 rpm for 12 h at a temperature of 45 ± 0.5 °C. The hyaluronic acid purchased from Sigma USA with a molecular weight of 848.8 g/mol for high purity is used, along with HA nanoparticles from a density of 0.038 g/ml and Ti-NP with a density of 4.23 g/cm3 and a molar mass of 79,866 g/mol from Sigma Company. The resulting solution is divided into four containers and different amounts of Ti-NP (0 wt%, 5 wt%, 10 wt%, and 15 wt% labeled S1, S2, S3, and S4, respectively) are added and magnetically stirred at 450 rpm for 2 h to obtain a homogeneous solution while maintaining the functional groups of the polymers at a temperature of 45 ± 0.5 °C. The solution was initially frozen at −80 °C in the refrigerator before being transferred to the freezer dryer where it was left to prepare the scaffold under a pressure of 0.5 tor for 24 h. Once the scaffold was prepared, a series of tests were conducted to characterize it, which were divided into three main sections.
2.2 XRD analysis
The crystal structure and chemical composition were determined using X-ray diffraction (XRD) with a BRUKER D8-Advance model from Germany in which the CuKα beam was utilized to prepare diffraction patterns with a scanning speed of 0.0058°/s in the range of 10–90°. The identification of the patterns was accomplished by employing phase-detection reference cards from JCPDS.
2.3 FTIR analysis
To study the chemical agents and bonds in the samples, an infrared spectrum was taken in the wavelength range of 400–4000 cm−1. To investigate, the powdered samples were mixed with KBr in a ratio of 1:100 and then the infrared spectrum was taken from them. The obtained numbers are collected in Excel software and their diagrams are drawn and then the comparison between the diagrams is done.
2.4 SEM analysis
The microstructure of the samples is examined by SEM. For this purpose, a coating of gold is created on the samples and then the microstructure may be seen by a scanning electron device. The porosity of the samples may be evaluated using Image-J software. In this study, 20 porosities are measured from SEM images and the porosity is reported as an average. Porosity analysis involves quantifying the void spaces or pores within the scaffold, which is a critical parameter impacting cell infiltration, nutrient transport, and waste removal capacity. Porosity, typically expressed as a percentage, can be determined through methods such as SEM or micro-CT image analysis or liquid displacement techniques. Conducting a morphology study and porosity analysis provides researchers with valuable insights into the scaffold's physical structure, pore characteristics, and overall architecture. This information is vital for understanding how the scaffold interacts with cells, tissues, and biofluids, and evaluating its suitability for cell attachment, proliferation, differentiation, and the diffusion of nutrients and oxygen. Morphology and porosity analyses offer quantitative and qualitative data that contribute to the comprehensive assessment of scaffold performance, assisting researchers in optimizing scaffold design and fabrication techniques specific to tissue engineering applications.
2.5 Compressive strength test
The Hounsfield machine is employed for measuring compressive strength. The compressive strength test is carried out on the sample by applying pressure and tension at a rate of 0.2 mm/min. After placing the samples in the machine, pressure is exerted, and the resulting data is recorded in Excel, followed by graphing. The determination of tensile and compressive strength is crucial for bone scaffolds and can be calculated. The mechanical properties of bone scaffolds are significantly influenced by various factors, including the chemical structures, presence of additives, radiographic materials, porosity, sterilization methods, and monomer and polymer mixing techniques (Martínez-Vázquez et al., 2010).
2.6 Biodegradability test
To investigate the biodegradability of scaffolds, a methodology was employed wherein the porous scaffolds were submerged in a solution of phosphate buffer saline for a period of four weeks. The phosphate buffer saline solution was prepared by dissolving 0.988 g of the powder in 100 ml of water. The biodegradability of the samples was evaluated by measuring their dry weight (W1), followed by immersing them in a falcon containing 30 ml of phosphate buffer saline and placing it in a water bath set at 37 °C and 50 rpm. After a week, the scaffolds were removed from the solution, and their weight was measured again (W2). Subsequently, the samples were re-immersed in the same PBS solution. The biodegradability of the scaffolds was determined using Eq. (1).
2.7 pH concentration changes
The experiment analyze the pH fluctuations that occur during the degradation process of the samples. The samples were incubated at 37 °C in phosphate buffer saline and their solutions were subsequently measured for pH after removal from the incubator. The pH measurements were used to plot graphs that illustrate the pH changes for each group over the specified period. In the pH experiment, a specific pH value (e.g., pH 7.4) will be utilized. The scaffolds will be incubated at this pH for a designated incubation time (e.g., 24 h) to evaluate their performance under physiological conditions. By including these details, we can provide a clearer understanding of the experimental setup and ensure reproducibility.
2.8 Release of calcium ions
This investigate the degradation of hyaluronic acid/HA samples containing varying amounts of Ti-NP, with a focus on measuring and comparing the release of calcium ions into the surrounding environment and its potential impact on the healing process. The samples were incubated in phosphate buffer saline at a weight-to-volume ratio of 2.5 mg/ml and at 37 °C to carry out the investigation. The concentration of calcium ions was measured using an inductively coupled plasma atomic emission spectroscopy (ICP-AES), and the data was plotted over time to obtain a concentration curve. Furthermore, the study examined the formation of a HA layer on the scaffolds by immersing five samples in simulated body fluid (SBF) for 14–21 days, with weekly solution changes. The scaffolds were then analyzed using a TESCAN SEM machine to examine their microstructure and morphology, with a focus on the formation of two types of pores in the bone scaffold: large cavities with a diameter of more than 1 mm and small cavities with a diameter between 0.1 and 1 mm. The scaffolds were incubated in a soluble SBF solution for two weeks with a pH maintained between 7.42 and 7.45. After each stage of removal, the samples were washed with deionized water and dried in a vacuum oven.
2.9 Contact angle test (hydrophilicity)
A crucial aspect of this study was to investigate and compare the hydrophilicity of scaffolds that incorporated hyaluronic acid and HA with differing weight percentages of Ti-NP. To achieve this objective, four samples of each scaffold were cut to dimensions of 3 × 3 cm2. A droplet of water was deposited on the surface of each scaffold, and after 30 s, a photograph was taken using a device designed by the Faculty of Medical Engineering at Advance Research Center. The average contact angle of water on each scaffold was then calculated and recorded.
2.10 Cell survival assay
Measurement of viability and cell proliferation form the basis of many methods for studying the cellular response to external factors. At present, tetrazolium salt reduction is a safe and low-cost method for studying cell proliferation and analyzing the effects of substance toxicity on cells. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), Tetrazolium yellow, is regenerated by metabolically viable cells and dehydrogenases to form regenerative equivalences such as nicotinamide adenine dinucleotide (NADH), a pro-oxidant into NADPH. Intracellular purple formazan can be measured by spectrophotometer. In this work, the source of the cells prepared from Pasteur cell Bank as mesodermal progenitors were passaged when 80–90 % confluence was reached, until the 5th passage. To investigate ossification and toxicity, osteoblasts and toxicology cells are used. Using these cells, the effects of hyaluronic filler with HA-TiNPs on bone formation and its toxicity can be investigated. In this work, the culture medium was Dulbecco's Modified Eagle Medium (DMEM) containing 10 % fetal bovine serum, 1 % antibiotic–antimycotic which purchased from Adeb Knowledge based company. In this way, the rate of cell proliferation can be measured, and conversely, when metabolic events leading to programmed cell death and necrosis, the slow rate of cell proliferation can be measured. In this method, the number of work steps to increase the test speed is minimized.
This study investigate the effectiveness of UV radiation and 70 % EtOH for sterilizing porous scaffolds, albeit with an incomplete sterilization outcome. The MTT reagent is utilized for accurately measuring cell proliferation rate by taking advantage of the linear relationship between cell numbers and generated signals. It is worth noting that various methods are available for measuring cell proliferation rate, and in this study, the MTT test is conducted using human osteoblast cells. The test procedure involves specific steps for cell suspension preparation and incubation. The presence of purple spot dots is examined using an inverted microscope, and if observed, Dimethylsulfoxide (DMSO) solution is added to dissolve the sediment. The absorption of wells is measured using a spectrophotometer, and the results are recorded to compare cell growth.
In this study, we assessed the impact of composite scaffolds on cell viability and proliferation over time. Osteoblast cells were cultured and seeded onto scaffolds composed of a hybrid hyaluronic acid-alginate filler with hydroxyapatite-titanium nanoparticles. After sterilization, the cell-seeded scaffolds were incubated for cell attachment. Experimental groups with osteoblast-seeded scaffolds and control groups without cells were compared. Cell viability assays were conducted at 24, 48, and 72 h using MTT or resazurin assays, and absorbance or fluorescence intensity measurements were obtained to assess cell viability and proliferation. Statistical analyses including one-way ANOVA or t-test were performed to determine significant differences between the experimental and control groups. Graphical representations were generated using GraphPad Prism software. This study provide a comprehensive understanding of how the composite scaffolds influence cell behavior over time, offering valuable insights into their potential to promote cell viability and proliferation.
2.11 Statistical analysis
In this study, the data and results were presented as mean standard deviation (SD), with all tests being repeated at least thrice to ensure accuracy. Statistical significance of the results was determined using SPSS software and one-way analysis of variance, with a p-value of less than 0.05 being considered significant. The freeze-drying method was chosen as the scaffold fabrication technique due to its cost-effectiveness, widespread use and simplicity for incorporating Ti-NP in tissue engineering.
3 Results and discussion
Freeze drying is a commonly used method to produce porous three-dimensional structures by freezing a liquid or gel-like material and then removing the frozen solvent through sublimation. In this study, the researchers employed freeze drying to fabricate composite scaffolds consisting of hyaluronic acid, alginate, and hydroxyapatite-titanium nanoparticles (HA/Alginate/HA-TiNPs). The resulting freeze-dried scaffolds demonstrated favorable morphological, mechanical, physical, and chemical properties suitable for applications in bone tissue engineering. In contrast, electrospinning, an alternative fabrication technique not specifically utilized in this study, involves the use of an electric field to create fine jets of polymer solution or melt that solidify into ultrafine fibers upon solvent evaporation or cooling. Electrospinning enables the production of nanofibrous scaffolds with a high surface area-to-volume ratio, mimicking the structure of the natural extracellular matrix (ECM) and facilitating cell adhesion and proliferation. Both freeze drying and electrospinning offer distinct advantages and characteristics. Freeze drying is well-suited for generating porous scaffolds with interconnected pores, allowing for cell infiltration, nutrient diffusion, and the incorporation of nanoparticles into the scaffold matrix. On the other hand, electrospinning excels in producing nanofibrous scaffolds with a large surface area. The choice between freeze drying and electrospinning depends on the specific requirements of the desired scaffold structure and properties. Although freeze drying was selected as the fabrication method in this study, future investigations may explore the potential of electrospinning to create scaffolds with different material combinations or desired characteristics.
In sports, joint injuries can arise from various causes such as improper joint alignment, severe impacts, or overuse, resulting in pain, swelling, restricted movement, joint locking, and decreased quality of life (Yang et al., 2022). Several treatment methods are available to address these injuries, including injecting synthetic hyaluronic acid gel into the joint, which mimics a natural substance found in joints and can help alleviate joint damage symptoms. Hyaluronic acid gel injections can reduce joint inflammation, pain, and swelling while enhancing joint stability and function. Additionally, soft bracing can be used to treat joint injuries in sports. This type of bracing, made of elastic bands or fabric, is applied to support and apply pressure on the joint, thereby reducing joint stress during exercise. This method can assist in reducing joint swelling and pain while allowing the joint to rest for faster recovery. Fig. 1 illustrates the key materials and cells that have been found to be beneficial in repairing bone fractures resulting from sports injuries. The figure is divided into four main categories: porous scaffolds, growth factors (GFs), stem and progenitor cells, and mechanical simulation. The first category, scaffolds, includes three types of materials that have shown potential in bone fracture repair: ceramics, polymers, and nanocomposites. Ceramics are known for their biocompatibility and excellent mechanical properties, making them an attractive choice for bone tissue engineering. Polymers are another class of materials widely used in medical applications due to their versatility and ease of processing. Cellular behavior in hard tissue plays a crucial role in tissue regeneration and repair processes. The interaction between cells and biomaterials used in tissue engineering applications is a key factor in determining the success of the treatment. In the context of knee joint repair, understanding the cellular response to biomaterials is essential for developing effective strategies to enhance tissue healing and improve patient outcomes. The cellular study of the “Hyaluronic Acid-Alginate Filler” in this research provides valuable insights into its potential for promoting cellular behavior in hard tissue. Hyaluronic acid and alginate are biocompatible and biodegradable materials commonly used in tissue engineering due to their favorable properties, such as high water retention capacity and ability to mimic the extracellular matrix. The incorporation of a filler in this study enhances the mechanical properties of the scaffold, which is crucial for load-bearing applications such as knee joint repair. The results of the cellular study showed that the composite scaffold supported cell attachment, viability, and proliferation. The incorporation of the hyaluronic acid-alginate filler, along with the potential reinforcement of hydroxyapatite-titanium nanoparticles, may have contributed to the enhanced cellular response observed. The findings suggest that the scaffold has the potential to promote cell-mediated tissue regeneration and repair in the context of knee joint injuries. It is important to note that the cellular behavior observed in this study represents a preliminary step in evaluating the efficacy of the hyaluronic acid-alginate filler scaffold. Further investigations are warranted to assess other aspects of cellular behavior, including differentiation potential, extracellular matrix production, and the expression of specific markers related to tissue regeneration.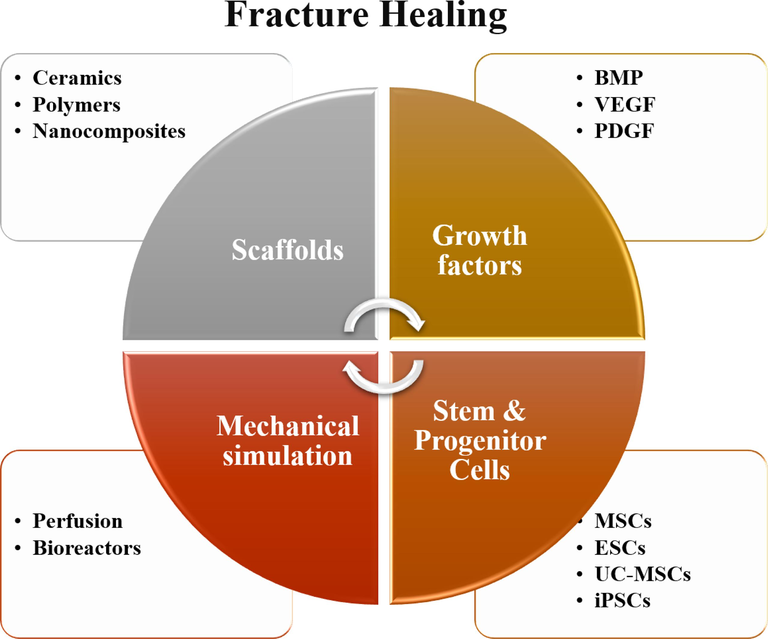
Materials and cells that are useful in the repair of bone fractures caused by sports injuries.
3.1 Materials characterization
3.1.1 X-ray diffraction spectrum analysis
The X-ray diffraction (XRD) spectrum was used to evaluate the crystalline and amorphous phases of the samples. Fig. 2(a-b) illustrates the XRD results of pure HA samples composite, which contain HLA-Alginate/-HA and various amounts of Ti-NP. The peaks at 26.93°, 31.37°, 39.81°, 45.15°, and 51.01° in the HA diagram correspond to the crystal plates 002, 211, 310, 222, and (3 2 0), respectively. As it is known, HA exhibits a crystalline phase. The sample containing 5 wt% by weight of Ti-NP did not show Ti-NP peaks due to the low ratio of Ti-NP to the components of the titanium peak. However, the Ti-NP peaks were observed in samples containing Ti-NP at 25.17°, 37.73°, 48.03°, 54.64°, 62.95°, 69.26°, and 69.83°, corresponding to the pages of crystalline (1 0 1), (0 0 4), (2 0 0), (2 1 1), (2 2 0), (2 0 4), (3 0 1), and (1 1 2), representing the anatase and rutile phases of Ti-NP. The addition of higher amounts of Ti-NP increased the crystalline phase of the samples, and the amorphous peak of hyaluronic acid was not observed. The main peaks of HA are within the range of 20-60°. A comparison of the obtained pattern with the existing standard pattern revealed a slight displacement of the peaks towards higher angles, indicating the replacement of calcium ions with a smaller ion radius instead of ions.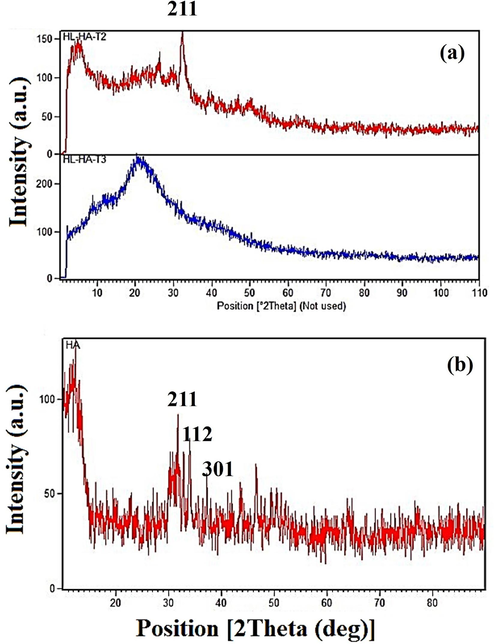
XRD pattern a) sample containing different amounts of TiNPs, and b) of HA nanoparticles.
3.1.2 X-ray diffraction spectrum analysis
The lattice of a material becomes less constant as the size of its constituent ions decreases, leading to a shift in the Bragg angle of its peaks. The Williamson-Hall method was employed to determine the crystallite size of HA powder, yielding an average size of 40–80 nm, confirming the powder's nanostructure. Analysis of functional groups and chemical interactions was performed using the FTIR spectrum in Fig. 3. Absorption bands at around 900 cm−1 indicate phenolic C—O tensile vibrations of hyaluronic acid. However, the presence of hyaluronic acid's 650 cm−1 bandwidth precludes the identification of phosphate peaks in this region due to overlap. A broad absorption band centered at 3425 cm−1 corresponds to the tensile vibrations of hydroxyl groups in all three materials. The peak at 3064 cm−1 is attributed to HA, displaying a wide absorption band in this region. Peaks at 2930 and 2861 cm−1 correspond to the tensile vibrations of CH and CH3 in hyaluronic acid, respectively. At 1641 cm−1, the carbonyl acid and amide groups of hyaluronic acid exhibit tensile vibrations, overlapping with Ti-OH-related tensile vibrations. Additionally, the peak at 1532 cm−1 is associated with the tensile vibrations of carbonate in apatite, while the 1406 cm−1 band reflects the C—O tensile vibration in the COO– group. Peaks at 1237 cm−1 and bands below 600 cm−1 correspond to the tensile vibrations of P⚌O. An absorption band at 1068 cm−1 indicates the C—O tensile vibration in alcohols, with the shoulder in this band arising from the C—O—C tensile vibration. As previously mentioned, phosphate peaks cannot be identified in this region due to overlap with hyaluronic acid's 650 cm−1 bandwidth.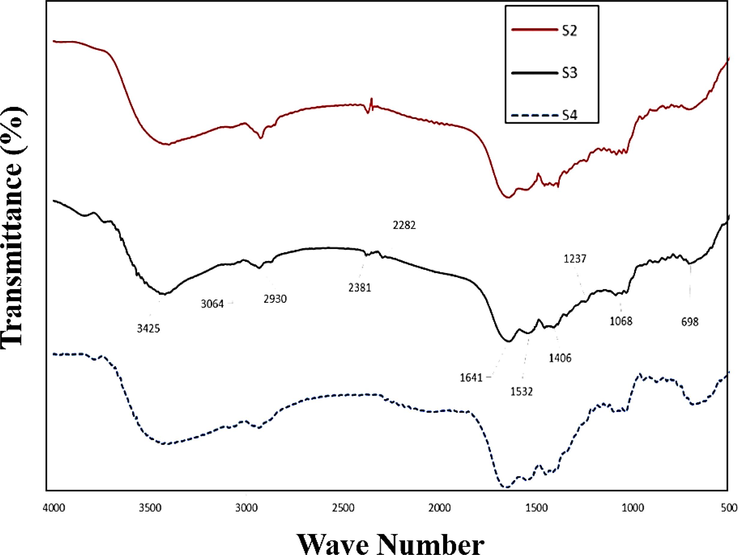
FTIR spectrum of samples containing various amount of TiNPs as S2, S3 and S4.
3.2 Morphology study and porosity analysis
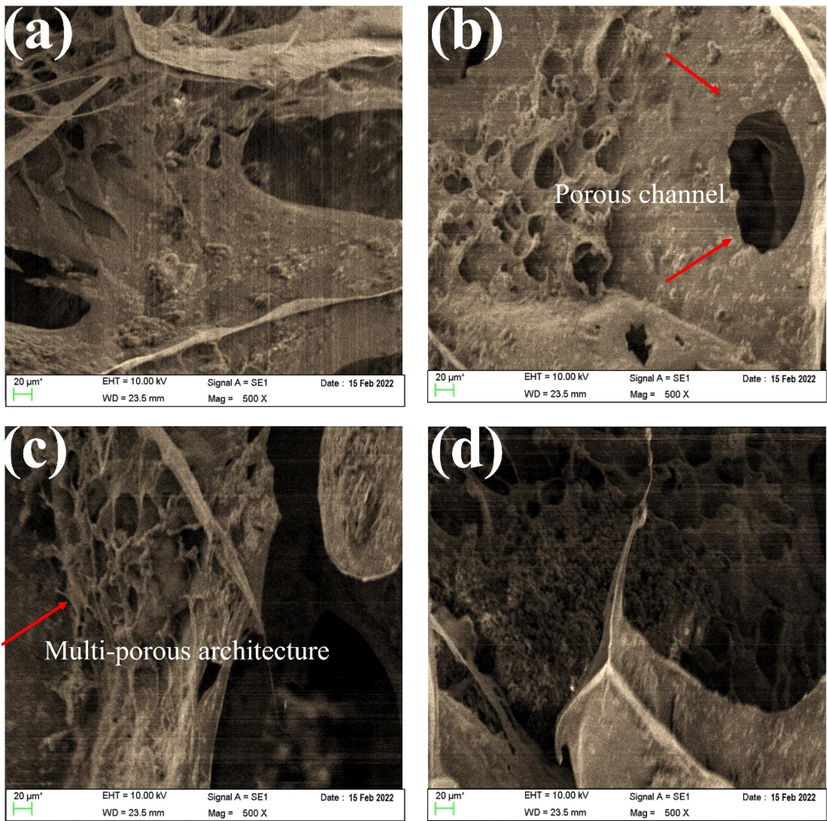
The microstructure of scaffolds with different amounts of Ti-NP was analyzed using SEM images, as shown in Fig. 4(a–c). The SEM images indicate the presence of a porous structure that meets the acceptable range for bone tissue engineering, with elliptical and interconnected cavities. These features play a crucial role in bone tissue growth and penetration into the scaffold. Interestingly, the porous structure emerged spontaneously, without any added porosity, and was the result of material dissolution in water. The SEM images provide a clearer view of the pore structure, which becomes more defined as the percentage of Ti-NP less than 50 nm increases, forming spherical particles and indicating better mechanical strength. Furthermore, the SEM images reveal interconnected pores that trap Ti-NP and enable internal communication. The pore walls consist of HLA-Alginate nanocomposites with CaP nanoparticles reinforced with Ti-NP. The quantity of nano Ti-NP directly affects the scaffold morphology, with porosity size and internal cohesion of pores being vital for cell growth, migration, nutrient flow, angiogenesis, and spatial organization for cell growth and ECM production. It is worth noting that porosities less than 100 µm do not facilitate sufficient transport of oxygen and nutrients, leading to limited osteoblast survival and bone formation, as shown in Fig. 4(a–d). The microstructure of scaffolds with varying Ti-NP amounts was examined through SEM images depicted in Fig. 4(a–d). The images confirm the presence of a porous structure with elliptical cavities suitable for bone tissue engineering. The porosity, created spontaneously without the addition of porosity, resulted from the material dissolution in water. As the Ti-NP concentration increased, the porosity percentage rose to approximately 76.5 %, marginally greater than that of hyaluronic acid/HA scaffolds. The interconnected porosity facilitates the exchange of nutrients, waste, water, and oxygen, essential for cell growth, angiogenesis, spatial organization, and ECM production. Scaffolds with a mean porosity diameter of 400 μm exhibit more bone growth, proliferation, angiogenesis, and are more conducive to nutrient transfer. CaP particles and Ti-NP are homogeneously dispersed throughout the scaffold, denoting their availability to create a bioactive surface. Porosity and pore interconnectivity are crucial factors in designing bone scaffolds for tissue engineering applications. Adequate porosity and interconnected pores can enhance nutrient and oxygen distribution within the scaffold, facilitating the growth and proliferation of stem cells. In studies that have used fillers in bone scaffolds, porosity and pore interconnectivity have played important roles. Several studies showed that porosity and pore interconnectivity in bone scaffolds with filler led to increased mechanical strength and biocompatibility of the material. Therefore, when designing bone scaffolds with fillers, porosity and pore interconnectivity should be considered as two critical factors (Rahman et al., 2009; Lison et al., 2008; Yang et al., 2023; Sanapalli et al., 2022; Bian and Xing, 2022). Fig. 5 shows as the amount of Ti-NP increases, the porosity percentage increases and the strength of the first two samples decreases while the sample with the highest percentage of Ti-NP may exhibit a significant increase in strength. Nevertheless, the porosity difference between the last two samples is relatively small and can be verified by the SEM outcomes. Furthermore, the augmentation of Ti-NP volume possessing catalytic features results in the enhancement of tensile strength in the scaffold network structure, and hence, the likelihood of calcium ion presence and its bonding with Ti-NP on the surface of the sample increases.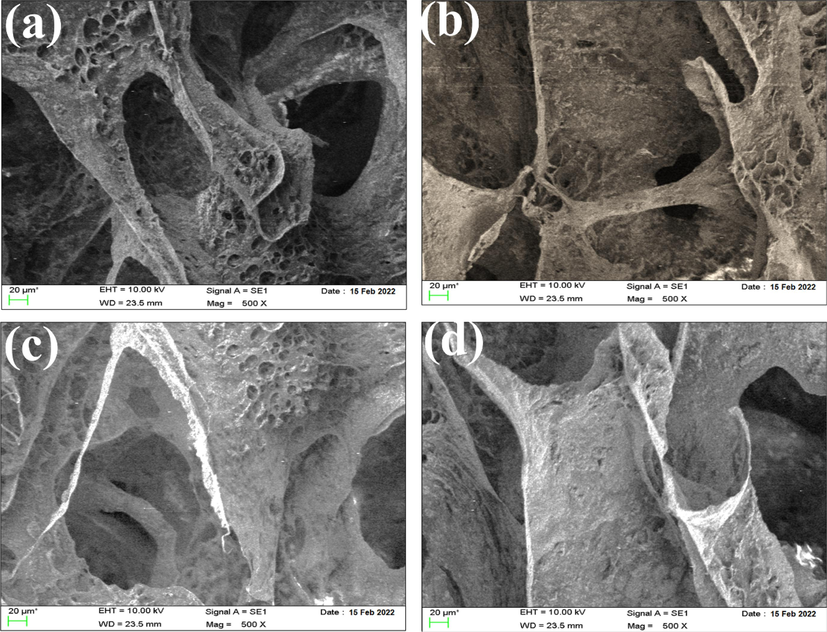
SEM images of hyaluronic acid-Alginate/HA scaffold specimens containing (a) 0 wt% (b) 5 wt%, (c) 10 wt% Ti-NP, and (d) 15 wt% Ti-NP.
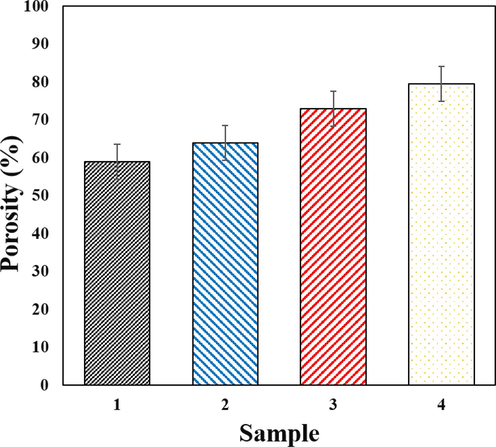
Results of porosity measurement in samples with different amounts of Ti-NP.
3.3 Mechanical properties results
The natural extracellular matrix (ECM) plays a crucial role in bone repair as it possesses conductive and inductive signals that can expedite bone repair and remodeling. In scaffold design for tissue engineering, mechanical properties are of paramount importance. The scaffold must be capable of bearing loads until new bone tissue forms, and the mechanical properties should be biomechanically compatible with the environment and capable of load transfer. Incompatibility can lead to tension shield or scaffold collapse. HA is a bioceramic that is chemically similar to the primary bone minerals and is frequently employed in bone and tooth compounds to improve bioactivity. The utilization of HA can improve the mechanical properties and biocompatibility of scaffolds (Al-Taweel and Saud, 2016; Smith et al., 2012; Mishra et al., 2019; Zhang et al., 2020; Wiedmer et al., 2018). Although porous scaffolds are favorable for osteogenic environment conducive to bone tissue growth and formation, excessively large pore size and porosity percentage can significantly impact mechanical properties, leading to the loss of mechanical integrity (Zhang et al., 2020; Wiedmer et al., 2018; Hasan and Fatalla, 2023; Alnajm and Shukri, 2023; Kim et al., 2017). Ti-NP ceramics have higher compressive strength than other bone conductor scaffolds, with pure Ti-NP scaffolds exhibiting a compressive strength of approximately 2.5 MPa. After the implantation of the scaffold in the body, the high strength is maintained. Scaffolds composed of CaP ceramics and polymer/CaP scaffolds such as HA, with a similar porosity percentage, have a strength value of approximately 2 MPa (Kopec et al., 2007; Li et al., 2020; Gao et al., 2017; Liu et al., 2004). This indicates that the presence of Ti-NP is more effective than bioceramics in enhancing the mechanical properties. Additionally, the compressive strength of normal spongy and dense bone has been reported to be about 2–12 MPa and 100–230 MPa, respectively (Rahman et al., 2009). While the addition of HA nanoparticles can improve the mechanical properties, it may also increase the brittleness of the scaffold. A single HA may not provide the desired mechanical properties for bone (Lison et al., 2008). Since Ti-NP alone lacks good toughness, it is usually combined with other materials to form bone scaffolds that provide a structure for bone conduction with adequate mechanical strength and load-bearing capacity necessary for bone repair. The addition of Ti-NP to polymers and hydrogels improves strength, mechanical properties, and bioactivity (Hasan et al., 2023; Shuai et al., 2017; Cui et al., 2020; Kopec et al., 2007; Li et al., 2020; Gao et al., 2017; Liu et al., 2004; AL-Anezi et al., 2023; Rahman et al., 2009). Therefore, the application of Ti-NP, HLA-Alginate, and n-HA together seems to enhance the mechanical properties and compensate for their respective weaknesses. In this study, the mechanical properties of scaffolds were investigated, and the results are depicted in Fig. 6(a–c). As shown in Fig. 5(a), the addition of 15 % Ti-NP increased the tensile strength of the specimens. The low tensile strength of ceramics such as HA is one of their challenges, and the addition of 5 % Ti-NP and 10 % Ti-NP does not have a significant effect on tensile strength. However, 15 % Ti-NP can compensate for this weakness and increase the tensile strength to about 100 kPa. A similar trend was observed in the elastic modulus, and the addition of different amounts of Ti-NP may increase the elastic modulus. The elastic modulus of HA-Alginate/HAp/15Ti was less than 1 MPa compared to other samples, making it more suitable for bone applications as show in Fig. 6(b). The results of fracture toughness evaluation are also presented in Fig. 6(c). The findings indicate that the inclusion of Ti-NP enhances fracture toughness, with the best results observed in the 15 % Ti-NP sample. HA exhibits bioactivity, non-inflammatory and non-toxic properties, and promotes ossification and differentiation of osteoblastic cells.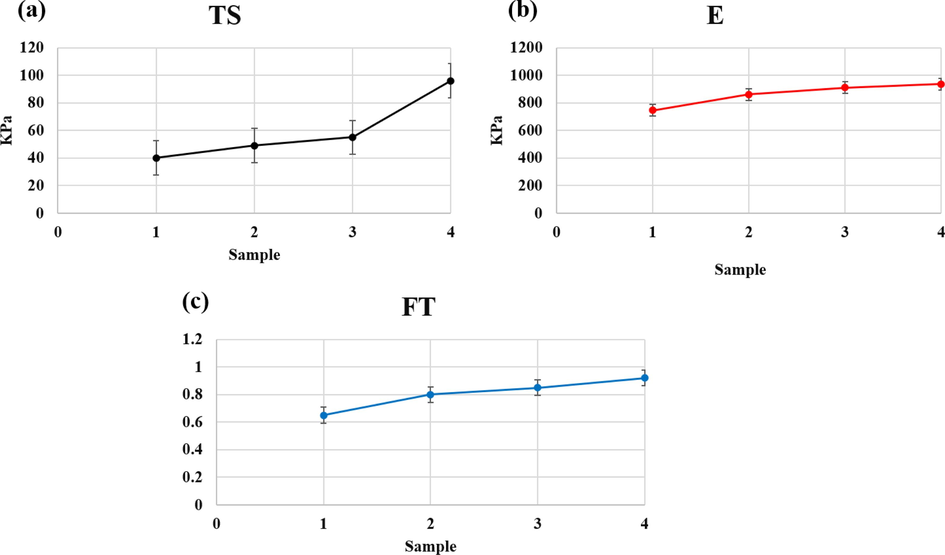
Results of a) Tensile strength, b) elastic modulus and c) fracture toughness of samples containing hyaluronic acid-Alginate/HA of different amounts of Ti-NP.
Various strategies have been considered, including adding different reinforcing agents to the structure of calcium phosphate ceramics and improving manufacturing and processing methods, to enhance their mechanical properties while maintaining biocompatibility. Reinforcing agents like metal particles, ceramic whiskers, biocompatible glass, and neutral ceramic phases such as alumina, zirconia, and Ti-NP have been added to improve the mechanical properties of HA. Ti-NP is naturally found in minerals like safety (FeTiO3), roulette (TiO2), and aspher (CaSiTiO5), and its density varies depending on the polymorph. Different techniques have been developed to create biodegradable three-dimensional porous scaffolds, each with unique porosity characteristics. In this study, the freeze-drying method was used, where the polymer was dissolved in a solvent, frozen at a low temperature, and then the solvent was sublimated under vacuum pressure to create a highly porous sponge. Traditional drying methods, such as heating, are not appropriate for drying heat-sensitive materials, such as polymers, biological and inorganic compounds, due to the risk of damage or degradation. Solvent extraction and water removal are critical processes in various industries, including pharmaceuticals, food, medical, petrochemicals, biochemicals, and nanotechnology. Freeze-drying is a widely used method for drying sensitive and valuable substances, such as drugs, vaccines, antibiotics, blood components, biological samples, microorganisms, sensitive inorganic substances, trace metal powders, and ceramics, particularly in the nanotechnology industry. The freeze-drying process offers several advantages over other drying methods, including no oxidation, no formation of new compounds, reduced adhesion and agglomeration of materials, uniform particle size distribution, extended shelf life, and low cost. This method also causes minimal damage to heat-sensitive products and preserves their sensory and nutritional attributes.
3.4 Contact angle of three-component scaffold
The wettability of the samples was evaluated by analyzing the contact angle as shown in Fig. 7. The findings from a single test on the samples in Fig. 7 indicate that the scaffold lacking pure nanoparticles is hydrophobic, resulting in poor absorption of the culture medium. After introducing Ti-NP, the contact angle decreased, except in sample four where there was a slight increase. However, compared to the scaffold without nanoparticles, there was a significant decrease in contact angle, indicating an enhancement in the hydrophilicity of the samples. Improved hydrophilicity leads to better penetration of fluids, nutrients, and removal of cellular wastes. Moreover, cells tend to adhere to hydrophilic surfaces and spread more readily.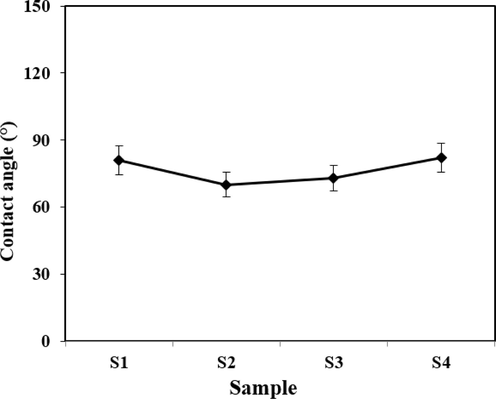
Contact angle of samples A: 1, B: 2, C: 3 and D: 4 with different amounts of Ti-NP.
3.5 Weight loss analysis
The porous scaffold used to encourage tissue growth and replacement within the body gradually degrades, allowing the inserted cells to multiply and eventually replace the damaged tissue. To facilitate bone formation, the scaffold or bone-replacement composite material should be able to ossify, either through containing growth factors and extracellular proteins or being made of bio-nanocomposite n-HA. Biodegradable polymers such as HLA-Alginate and gelatin are commonly used in bone replacement surgeries to avoid the need for additional surgical procedures. Hyaluronic acid, a molecule found in connective tissue, plays diverse roles in the body, including regulating tissue regeneration and cell movement, and it promotes composite material adhesion when in a solid state. Porous Ti-NP scaffolds are also biocompatible and have demonstrated promising results in promoting osteoblast cell growth and providing a suitable substrate for human seminal vesicles to thrive and multiply. Conversely, the HLA-Alginate/HAp/5Ti sample showed an increased slope of degradation owing to its improved hydrophilic properties, low crystallinity, and Ti-NP content. Furthermore, it should be noted that environmental changes and material release could greatly affect implant performance in vivo, with pH being one of the critical factors. The pH levels of different samples were assessed, and the results are presented in Fig. 8(a). The present study investigate the degradability of porous Ti-NP scaffolds in different compositions. To this end, the scaffolds were immersed in PBS and the weight loss was measured after a specific time, as illustrated in Fig. 8(b). The results demonstrated that adding small amounts of Ti-NP augmented the degradation process, whereas increasing the Ti-NP content decreased the process and reached a minimum in the HLA-Alginate/HAp/15Ti sample. This decrease in degradation could be ascribed to a decrease in pH acidity, an increase in the crystalline phase, and the low degradation rate of Ti-NP. During the calcium release test, it was observed that samples containing higher amounts of Ti-NP exhibit more intense calcium ion release, leading to the formation of a layer of HA on the samples, and reducing the degradation in the HLA-Alginate/HAP/15 TiNP, as depicted in Fig. 8(b). In this study, we evaluated the amount of ion release from different samples, as shown in Fig. 8(c). The findings indicate that the HLA-alginate/HAp/5Ti sample does not significantly differ in calcium ion release from the titanium-free sample. However, the release rate increases with increasing titanium content in the HLA-alginate /HAp/10 TiNP sample, and the HLA-alginate/HAp/15TiNP sample exhibits the highest release rate. Despite the positive effects of calcium ions, it should be noted that the HLA-alginate/HAp/15Ti sample shows better results than the other samples, while the amount of release does not exceed the allowable range to avoid forming a calcium carbonate layer. The maximum adsorption percentage is for the second sample, and the lowest attractive rate occurs in the sample containing the highest amount of Ti-NP. We observe an increase in the adsorption percentage of PBS solution by increasing the Ti-NP. Our results demonstrate that the HLA/HA samples create a somewhat acidic environment, which could cause cell damage after degradation (Rahman et al., 2009; Lison et al., 2008). On the other hand, the addition of Ti-NP increases the pH, and the HA/HAp/15Ti sample, in particular, exhibits the highest pH due to Ti-NP's somewhat alkaline property. This has been able to neutralize the relatively acidic environment, resulting in a pH close to 7, which is a more suitable neutral pH for the treatment of bone lesions. In this study, the bioactivity of the samples was evaluated by immersing them in SBF and analyzing the deposited layer using SEM imaging, as shown in Fig. 9(a–d). Results indicate that an increased percentage of Ti-NP led to an increase in the deposited HA layer, which can be attributed to the increase in sample bioactivity. Furthermore, the calcium release test demonstrated that an increased percentage of Ti-NP resulted in an increased amount of calcium released into the environment. The release of calcium ions can stimulate the deposition of calcium phosphates (CaPs) and accelerate the growth of HA, ultimately leading to the formation of a Ca-OH layer on the sample.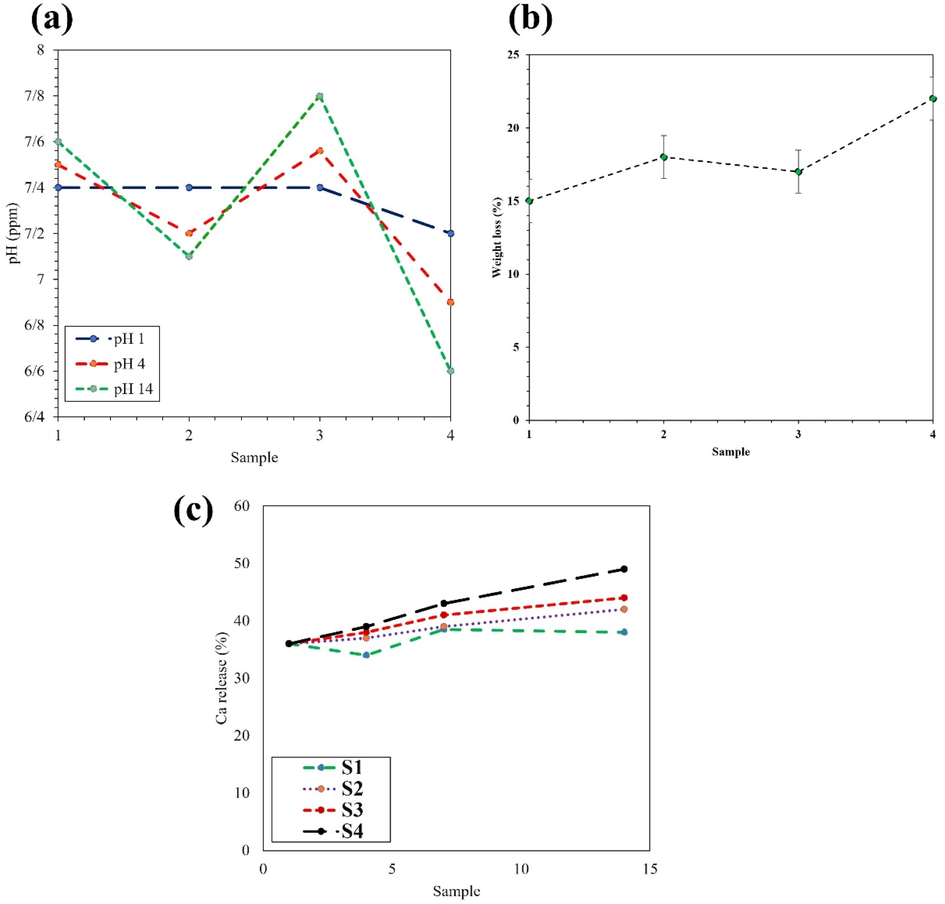
a) pH concentration b) weight loss, and c) Ca release of sample containing various amount of Ti-NP.
SEM images of the HA layer deposited on a hyaluronic acid-Alginate/HA sample containing (a) 0 wt%, (b) 5 wt%, and (c) 10 wt%, and d) 15 wt% Ti-NP.
Maji et al. (Maji et al., 2018) introduced a more straightforward method to create a three-dimensional microporous scaffold for bone tissue engineering. They used a foaming combination approach that involved intense agitation of gelatin/carboxymethyl chitosan (CMC)/nanoHA followed by freezing to create a scaffold with interconnected networks of macropores. The resulting built-in macro porous (SGC) scaffold had higher porosity, water holding capacity, slower enzyme degradation, and greater compressive strength than the non-porous (NGC) scaffold. Histopathology and radiographic examinations were performed on bone biopsy samples in the eighth week to assess fusion rate, ossification activity, and remodeling. Radiographic examination showed a significant difference between the groups, with the n-HA group exhibiting higher ossification activity in the sixth week.
Dennis et al. (Dennis et al., 2017) investigated the use of colloidal HLA-Alginate/HAp derivative gels in bone tissue engineering and found them to be non-cytotoxic and supportive of cell viability. Subramaniam et al. (Subramaniam et al., 2016) proposed an alternative approach to bone tissue engineering by combining HA/calcium sulfate/HLA with collagenase to enhance bone regeneration. The mechanical tests and WST-1 cell assays revealed high biocompatibility and mechanical strength of 6.69 MPa. The release of collagenase from the samples was found to accelerate the process of bone regeneration.
Fig. 10(a–d) displays the cell survival against different samples over 24 h, 48 h and 72 h. As the mineral phase of bone consists primarily of calcium and phosphorus, the measurement of calcium ions is a critical parameter in designing bone and knee fillers. The apatite layer is a calcium phosphate bioceramic that resembles the ECM of bone and functions as a bone conductor. The physicochemical properties of a synthesized scaffold are essential in determining its behavior and suitability for various applications, including molecular weight, solubility, chemical composition, morphology, and surface charge. The critical-sized bone defects are a significant challenge in bone damage, and bone scaffolds can provide mechanical support and act as a template for bone tissue formation. Additionally, stress shielding is an important phenomenon to consider when designing bone scaffolds, as it can cause bone resorption and eventual failure of the scaffold (Dennis et al., 2017). The intricate nature of bone tissue and its unique characteristics necessitate the use of an ex vivo model to incorporate all its properties. This is only achievable due to the significant resemblance between the external environment and the body's biological environment (Liu et al., 2004; AL-Anezi et al., 2023; Rahman et al., 2009; Lison et al., 2008). Current research is actively exploring this area, highlighting the importance of closely mimicking the biological environment when designing tissue engineering scaffolds to achieve favorable outcomes. Studies have shown that composite scaffolds hold the potential to stimulate and guide bone cell growth and proliferation.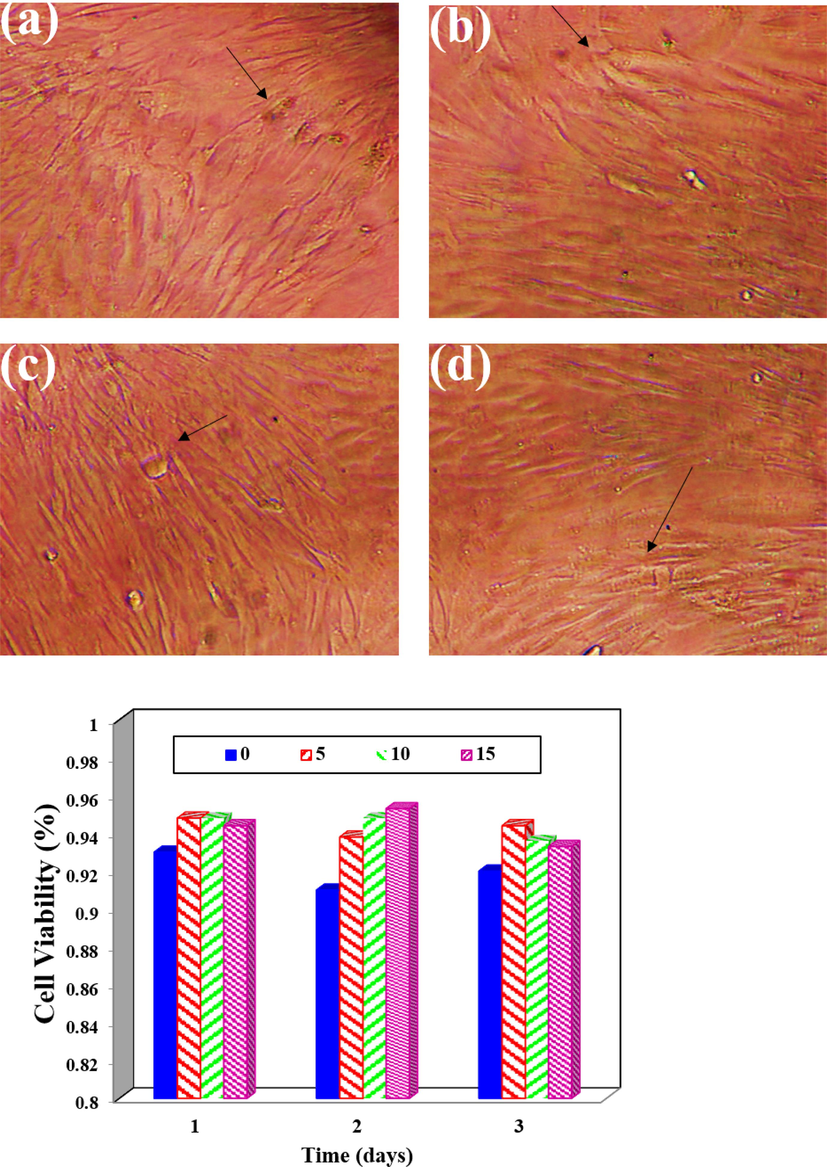
Cell Viability Analysis (a-d) The viability of cells is assessed at 24 h, 48 h, and 72 h for various samples containing Ti-NP.
In addition, the similarity in structure between calcium phosphate in scaffold structures and natural bone, along with the release of similar substances and ions in the body, promotes bone cell growth. To assess the capacity of samples to support cell growth and viability, a cell viability test was conducted on scaffolds with and without 15 % Ti-NP. The results demonstrated that both scaffolds exhibited high percentages of survival at all timepoints, with an increase in cell growth after 48 h. This outcome is attributed to the scaffolds' porous and biocompatible fabrication, critical for cell growth and nutrient exchange. The inclusion of 15 % Ti-NP further improved cell viability, confirming its positive effect on cell growth and survival. Fig. 11 highlights the specialized use of bio-nanocomposite hydrogels in repairing tissue damage resulting from sports injuries, particularly fractures and cartilage damage. These types of injuries are common in sports and can have a significant impact on an athlete's performance and overall quality of life. Bio-nanocomposite hydrogels, made up of a hydrogel matrix infused with nanoparticles, are increasingly being studied for their potential in tissue engineering and regenerative medicine. These hydrogels can provide additional structural support and enhance the material's bioactivity. The use of these hydrogels in repairing tissue damage caused by sports injuries is a promising area of research with the potential to revolutionize the treatment of these injuries. Additionally, there are several other treatment options available for sports-related joint injuries. Nonsteroidal anti-inflammatory drugs (NSAIDs) can effectively alleviate joint pain and inflammation. However, due to potential negative side effects, prolonged use of NSAIDs should only occur under the supervision of a healthcare professional. Corticosteroid injections can be highly effective in reducing joint inflammation and pain, but their use should be limited and only under the guidance of a healthcare professional, as overuse may result in joint damage. Platelet-rich plasma (PRP) therapy involves the injection of a concentrated solution of the patient's own platelets into the joint, containing growth factors that can promote joint healing and reduce inflammation. Metal and metal oxide nanoparticles (NPs) have advantages such as modulating ROS and RNS, disrupting cell membranes, and targeted delivery, but their excessive production of ROS and RNS can be cytotoxic, and the potential toxicity of released metal ions requires evaluation for long-term effects on the environment and human health (Martínez-Vázquez et al., 2010; Alavi and Yarani, 2023). Titanium dioxide nanoparticles have shown promising potential in promoting healing mechanisms related to bone injuries. These nanoparticles possess unique properties that make them suitable for bone tissue engineering applications, interacting with surrounding cells and tissues to trigger a cascade of biological responses that contribute to the healing process. One of their key healing mechanisms is the enhancement of osteogenesis, stimulating the differentiation and proliferation of osteoblasts responsible for bone formation. TiO2 NPs also upregulate the expression of genes and proteins involved in osteogenic differentiation, leading to increased bone matrix deposition and mineralization. Additionally, TiO2 NPs exhibit antibacterial properties, inhibiting the growth and activity of bacteria, thereby reducing the risk of infection and creating a more favorable environment for bone healing. Furthermore, these nanoparticles modulate the inflammatory response in bone injuries by suppressing the release of pro-inflammatory cytokines and promoting the secretion of anti-inflammatory factors. This balanced and controlled inflammatory reaction helps to reduce tissue damage and expedite the healing process. Through these mechanisms, TiO2 NPs hold promise for improving bone injury outcomes and advancing the field of bone tissue engineering.
Specialized use of bio-nanocomposite hydrogels in the repair of tissue damage resulting from sports injuries, including fracture or cartilage damage.
4 Conclusion
Sports-related injuries often lead to tissue damage that necessitates effective treatment strategies, and tissue engineering has emerged as a promising approach for repairing such injuries. One area of research focuses on the development of three-dimensional microporous scaffolds using a combination of alginate, hyaluronic acid, and nano hydroxyapatite (HA). This unique scaffold structure exhibits superior properties, including enhanced porosity, increased water holding capacity, reduced enzyme degradation, and improved compressive strength compared to non-porous scaffolds. Furthermore, studies have explored the potential of colloidal hyaluronic acid-alginate/HA derivative gels, HA/calcium sulfate/hyaluronic acid with collagenase, and platelet-rich plasma (PRP) therapy in promoting bone tissue regeneration and mitigating inflammation. With the growing population and the prevalence of bone-related issues, particularly in the knee joint, the exploration of alternative approaches has become imperative. In this research, we aimed to fabricate and evaluate hyaluronic acid and HA scaffolds with varying concentrations of titanium nanoparticles (Ti-NPs) as bone fillers. The scaffolds were successfully fabricated using freeze-drying and underwent various laboratory tests. Structural analysis revealed that the scaffolds had a combination of crystalline and amorphous phases, with higher concentrations of Ti-NP leading to increased crystallinity. The morphological analysis demonstrated that the scaffolds had a porous structure, which was further enhanced by the addition of Ti-NP. The Ti-NP was found to be distributed uniformly within the scaffold, which led to improved mechanical properties and biocompatibility. A 15 wt% Ti-NP additive was found to be the most effective, as it helped to neutralize pH, slow down degradation, increase calcium ion release, encourage osteoblast activity, and support cell viability. These results suggest that a hyaluronic acid/HA scaffold with a 15 % Ti-NP additive could be a promising bone filler, although additional studies are required to confirm its efficacy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fabrication of highly porous biodegradable biomimetic nanocomposite as advanced bone tissue scaffold. Arab. J. Chem.. 2017;10(2):240-252.
- [Google Scholar]
- Early and delayed effect of cavity disinfection with chlorhexidine and ozone on the shear bond strength of dentin to glass hybrid restoration: an in vitro study. Dental Hypotheses. 2023;14(1):16-18.
- [Google Scholar]
- ROS and RNS modulation: the main antimicrobial, anticancer, antidiabetic, and antineurodegenerative mechanisms of metal or metal oxide nanoparticles. Nano Micro Biosyst.. 2023;2(1):22-30.
- [Google Scholar]
- Serum and salivary vitamin B12 levels among iron deficiency anemia patient with recurrent aphthous stomatitis: an analytical cross-sectional study. Dental Hypotheses. 2023;14(2):55-58.
- [Google Scholar]
- HPMC crosslinked chitosan/hydroxyapatite scaffolds containing Lemongrass oil for potential bone tissue engineering applications. Arab. J. Chem.. 2022;15(7):103850
- [Google Scholar]
- Assessment of salivary interleukin-1β levels in patients with gingivitis and periodontitis: an analytical cross-sectional study. Dental Hypotheses. 2023;14(1):3-6.
- [Google Scholar]
- Assessment of the amount of apically extruded debris using three different reciprocating single endodontic file systems: an: ex vivo: study. Dental Hypotheses. 2023;14(1):25-28.
- [Google Scholar]
- New route for synthesis of pure anatase TiO2 nanoparticles via utrasound-assisted sol-gel method. J. Chem. Pharm. Res.. 2016;8(2):620-626.
- [Google Scholar]
- Nanofibrous nonmulberry silk/PVA scaffold for osteoinduction and osseointegration. Biopolymers. 2015;103(5):271-284.
- [Google Scholar]
- A collagen (Col)/nano-hydroxyapatite (nHA) biological composite bone scaffold with double multi-level interface reinforcement. Arab. J. Chem.. 2022;15(5):103733
- [Google Scholar]
- Titanium dioxide nanotubes enhance bone bonding in vivo. J. Biomed. Mater. Res. Part a: Official J. Soc. Biomater. Japanese Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater.. 2010;92(3):1218-1224.
- [Google Scholar]
- Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29:100587
- [Google Scholar]
- Syntheses and anti-microbial evaluation of new quinoline scaffold derived pyrimidine derivatives. Arabian J. Chem.. 2016;9:S451-S456.
- [Google Scholar]
- Hyaluronic-acid–hydroxyapatite colloidal gels combined with micronized native ECM as potential bone defect fillers. Langmuir. 2017;33(1):206-218.
- [Google Scholar]
- Electrophoretic deposition and characterization of thin hydroxyapatite coatings formed on the surface of NiTi shape memory alloy. Ceram. Int.. 2016;42(16):19124-19132.
- [Google Scholar]
- Effect of amoxicillin and azithromycin suspensions on microhardness of sliver reinforced and nano resin-modified glass ionomers: an in vitro study. Dental Hypotheses. 2023;14(1):32-35.
- [Google Scholar]
- Assessment of elongation percentage, tensile, and tear strength of filler particles: an in vitro study. Dental Hypotheses. 2023;14(1)
- [Google Scholar]
- Facile formation of anatase/rutile TiO2 nanocomposites with enhanced photocatalytic activity. Molecules. 2019;24(16):2996.
- [Google Scholar]
- Effect of gaseous ozone on transverse and impact strengths of heat cure acrylic resin: an in vitro study. Dental Hypotheses. 2023;14(1):13-15.
- [Google Scholar]
- Development of porous, antibacterial and biocompatible GO/n-HAp/bacterial cellulose/β-glucan biocomposite scaffold for bone tissue engineering. Arab. J. Chem.. 2021;14(2):102924
- [Google Scholar]
- Novel microstructure mechanical activated nano composites for tissue engineering applications. J. Bioeng. Biomed. Sci.. 2015;5(1):1.
- [Google Scholar]
- An innovative cell-laden α-TCP/collagen scaffold fabricated using a two-step printing process for potential application in regenerating hard tissues. Sci. Rep.. 2017;7(1):3181.
- [Google Scholar]
- Descriptive epidemiology of osteoarthritis in British Columbia, Canada. J. Rheumatol.. 2007;34(2):386-393.
- [Google Scholar]
- The prevalence of symptomatic knee osteoarthritis in relation to age, sex, area, region, and body mass index in China: a systematic review and meta-analysis. Front. Med.. 2020;7:304.
- [Google Scholar]
- Pectin-chitosan-PVA nanofibrous scaffold made by electrospinning and its potential use as a skin tissue scaffold. J. Biomater. Sci. Polym. Ed.. 2013;24(4):470-484.
- [Google Scholar]
- Nominal and effective dosimetry of silica nanoparticles in cytotoxicity assays. Toxicol. Sci.. 2008;104(1):155-162.
- [Google Scholar]
- Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R. Rep.. 2004;47(3–4):49-121.
- [Google Scholar]
- Soft Tissue Feature Tracking Based on Deep Matching Network. Comp. Model. Eng. Sci.. 2023;136(1):363-379.
- [CrossRef] [Google Scholar]
- Development of gelatin/carboxymethyl chitosan/nano-hydroxyapatite composite 3D macroporous scaffold for bone tissue engineering applications. Carbohydr. Polym.. 2018;189:115-125.
- [Google Scholar]
- Bilinear numerical analysis of the structural behavior of a dental implant applied as a biomaterial Carbon Fiber Reinforced Polyether-Ether-Ketone (CFR-PEEK): a finite element analysis. Dental Hypotheses. 2023;14(2):45-48.
- [Google Scholar]
- Improving the compressive strength of bioceramic robocast scaffolds by polymer infiltration. Acta Biomater.. 2010;6(11):4361-4368.
- [Google Scholar]
- Synthesis and characterization of gelatin-PVP polymer composite scaffold for potential application in bone tissue engineering. Eur. Polym. J.. 2019;119:155-168.
- [Google Scholar]
- Bioactive porous beads as an injectable urethral bulking agent: their in vitro evaluation on smooth muscle cell differentiation. Tissue Eng. A. 2011;17(5–6):655-664.
- [Google Scholar]
- Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in Syrian hamster embryo fibroblasts. Environ. Health Perspect.. 2009;117(5):709-715.
- [Google Scholar]
- Electrospun polycaprolactone fibrous membranes containing Ag, TiO2 and Na2Ti6O13 particles for potential use in bone regeneration. Membranes. 2019;9(1):12.
- [Google Scholar]
- Effect of addition of polymerized polymethyl methacrylate (PMMA) and zirconia particles on impact strength, surface hardness, and roughness of heat cure PMMA: an: in vitro: study. Dental Hypotheses. 2023;14(1):36-38.
- [Google Scholar]
- Addition of MgO nanoparticles and plasma surface treatment of three-dimensional printed polycaprolactone/hydroxyapatite scaffolds for improving bone regeneration. Mater. Sci. Eng. C. 2017;74:525-535.
- [Google Scholar]
- L-Glutamic acid loaded collagen chitosan composite scaffold as regenerative medicine for the accelerated healing of diabetic wounds. Arab. J. Chem.. 2022;15(6):103841
- [Google Scholar]
- Fabrication and characterization of novel nano-biocomposite scaffold of chitosan–gelatin–alginate–hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C. 2016;64:416-427.
- [Google Scholar]
- Calcium silicate improved bioactivity and mechanical properties of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds. Polymers. 2017;9(5):175.
- [Google Scholar]
- Developing scientific literacy in a primary school. Int. J. Sci. Educ.. 2012;34(1):127-152.
- [Google Scholar]
- Hydroxyapatite-calcium sulfate-hyaluronic acid composite encapsulated with collagenase as bone substitute for alveolar bone regeneration. Biomaterials. 2016;74:99-108.
- [Google Scholar]
- An improved method for soft tissue modeling. Biomed. Signal Process. Control. 2021;65
- [CrossRef] [Google Scholar]
- Conducting polymers, hydrogels and their composites: preparation, properties and bioapplications. Polymers. 2019;11(2):350.
- [Google Scholar]
- Antibacterial surface coating for bone scaffolds based on the dark catalytic effect of titanium dioxide. ACS Appl. Mater. Interfaces. 2018;10(42):35784-35793.
- [Google Scholar]
- Unraveling of Advances in 3D-Printed Polymer-Based Bone Scaffolds. Polymers. 2022;14(3)
- [CrossRef] [Google Scholar]
- In vitro and in vivo evaluation of hydrogel-based scaffold for bone tissue engineering application. Arab. J. Chem.. 2023;16(7):104799
- [Google Scholar]
- Traditional Chinese Sports under China’s Health Strategy. J. Environ. Public Health. 2022;2022:1381464
- [CrossRef] [Google Scholar]
- Local cellular responses to metallic and ceramic nanoparticles from orthopedic joint arthroplasty implants. Int. J. Nanomed. 2020:6705-6720.
- [Google Scholar]







