Translate this page into:
Essential oil from Artemisia herba-alba Asso grown wild in Algeria: Variability assessment and comparison with an updated literature survey
*Corresponding author. Tel.: +213 36 62 01 04; fax: +213 36 62 01 09 rbelhat@yahoo.fr (Rachid Belhattab)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 7 May 2012
Abstract
The chemical variability of the essential oils of Artemisia herba-alba Asso aerial parts, collected at Algeria was evaluated. A. herba-alba populations were collected in four regions, Benifouda; Bougaa; Boussaada and Boutaleb, at two different periods, July (flowering phase), and October and November (vegetative phase). The essential oils were isolated by hydrodistillation and analyzed by Gas Chromatography (GC) and Gas Chromatography-Mass Spectrometry (GC-MS). The essential oils yield ranged between 0.2% and 0.9% (v/d.w.). Fifty components were identified in A. herba-alba oils, oxygen-containing monoterpenes being dominant in all cases (72–80%). Camphor (17–33%), α-thujone (7–28%) and chrysanthenone (4–19%) were the major oil components. Despite the similarity in main components, three types of oils could be defined, (a) α-thujone : camphor (23–28:17–28%), (b) camphor : chrysanthenone (33:12%) and (c) α-thujone : camphor : chrysanthenone (24:19:19%). The comparison between the present data and an updated survey of the existing literature reinforces the major variability of A. herba-alba essential oils and stresses the importance of obtaining a defined chemical type crop production avoiding the wild harvest.
Keywords
Asteraceae
Artemisia herba-alba Asso
Essential oil
Chemical variability
Algeria
1 Introduction
Asteraceae Martinov (= Compositae Giseke) is a family of herbs, shrubs or trees, commonly known as Aster or Compositae family, comprising about 1535 genera and 23,000 species. Artemisia (wormwood, tarragon), one of the most economically important and widespread of this family genus, includes 400 species (Judd et al., 2002).
Dobignard, (1977) has shown the taxonomic complexity of A. herba-alba lato sensu described in North Africa, and the need for a taxonomic study of the whole group. In the present study we followed the traditional criterion of this species delimitation. Eleven spontaneous Artemisia species are present in the Algerian flora (Quezel and Santa 1963). Artemisia herba-alba Asso = [Artemisia aragonensis Lam., Seriphidium herba-alba (Asso) Soják] (Greuter, 2006–2009), commonly known as white wormwood or desert wormwood (Arabic name chih), is a greyish-strongly aromatic dwarf shrub native to the South western Europe, Northern Africa, Arabian Peninsula and Western Asia.
The economic value, the local medicinal uses, the disappearance in some areas due to pasture and over-collection, as well as the potential use to restore degraded ecosystems support the large number of studies on A. herba-alba. A recent review detailed the distribution, taxonomy, morphology, phytochemistry and biological activities of A. herba-alba and its different extracts (Mohamed et al., 2010).
Among A. herba-alba phytochemical constituents, essential oils have been extensively studied, with several chemotypes being recognized. The variability from the essential oils isolated from A. herba-alba collected at Algeria, Israel, Morocco and Spain was revised by Dob and Benabdelkader in 2006, but, since then, many other studies reinforced its high chemical polymorphism (Table 1, and references therein). Plants were collected from wild in different countries, grown under controlled experimental field conditions, collected at different harvesting times, subject to different drying periods and processes, extracted fresh or dry, by hydrodistillation or steam-distillation under different experimental conditions, and different aerial plant parts have been used (Table 1). ∗ (a) data in Dob and Benabdelkader (2006). CS: Collection site. UG: University garden. S: Sub-cultured plants with different origins. EF: Experimental field. PMS: Plant material status. IP: Isolation Procedure. H: hydrodistillation. SD: steam-distillation. F/D: Fresh and different drying processes.
Country / Plant part
CS
PMS
IP
Oil yield
Main components (⩾5%)
Reference
Algeria
Aerial part
Wild
0.1–0.7
Chrysanthenone 5–55, α-thujone t-26, β-thujone 6–16, camphor 6–16, bornyl acetate t-8, 1,8-cineole t-6
Boutekedjiret et al. 1992 in (a)
n.r.
Wild
0.7
Camphor 2–48, α-thujone 2–27, chrysanthenone 5–23, β-thujone 2–22, 1,8-cineole 8–18
Vernin et al. 1995 in (a)
Leaves and stems
Wild
Dry
H
1.0 (w/w)
Camphor 19, trans-pinocarveol 17, chrysanthenone 16, β-thujone 15
Dob and Benabdelkader (2006)
n.r.
Wild
n.r.
H
n.r.
β-Thujone 32–41, camphor 16–25, cineol 0.1–10
Benabdellah et al. (2006)
Aerial parts
Wild
n.r.
SD*
1.5–3.3 (w/w)
Chrysanthenone 31–54, camphor 11–27, filifolone 5–9, 1,8-cineole 2–9
Boutemak et al. (2009)
Aerial parts flowering phase
Wild
Dry
H
0.6 (w/w)
Camphor 49, 1,8-cineole 13, borneol 7, pinocarvone 6, camphene 5
Dahmani-Hamzaoui and Baaliouamer (2010)
Flowering tops
Wild
Dry
H
1.0 (v/w)
cis-Chrysanthenyl acetate 25, α-thujone 8, 2E,3Z-2-ethyliden-6-methyl-3,5-heptadienal 8, verbenone 7, myrtenyl acetate 7, chrysanthenone 5
Bezza et al. (2010)
Egypt
Leaves
Wild
Fresh
SD
1.6 (v/w)
Carvone, piperitone (no% given)
Saleh et al. (2006)
Israel and Sinai
Leaves, stems and flowers
Wild
Dry
SD
0.1–1.7 (v/w)
1,8-cineole 5–50, thujone n.d. 27, camphor 0.1–25, cis-chrysanthenol n.d. 25, cis-chrysanthenyl acetate n.d. 25, iso-thujone n.d. 12, borneol n.d. 11, artemisia alcohol n.d. 10, santolina alcohol n.d. 6, yomogi alcohol n.d. 9, xanthoxylin n.d. 9, lyratol n.d. 6, terpinen-4-ol 1–5
Feuerstein et al. (1986)
n.r.
Wild
Fresh
H
0.1–1.9
cis-Chrysanthenyl acetate n.d. 69, β-thujone t-44, camphor n.d. 42, α-thujone n.d. 41, cis-chrysanthenol n.d. 30, 1,8-cineole 0.2–27, cis-chrysanthenyl propionate n.d. 7, camphene n.d. 5
Fleisher et al. (2002)
Jordan
Leaves, stems and flowers
UG
Dry
H
1.3 (v/w)
α-Thujone 16, santolina alcohol 13, artemisia ketone 12, β-thujone 9, trans-sabinyl acetate 5, germacrene-D 5, caryophyllene acetate 5
Hudaib and Aburjai (2006)
Morocco
Aerial parts
Wild
Dry
H
n.r.
α-Thujone 2–74, β-thujone 1–84, camphor 5–70
Benjilali and Richard (1980)
Aerial parts
Wild
Dry
H
2.0
Chrysanthenone 31, camphor 24, camphene 5
Ouachikh et al. (2009)
Aerial parts flowering phase
Wild
Fresh
H
1.3–3.3 (v/w)
α-Thujone n.d. 74, chrysanthenone n.d. 53, camphor 9–46, β-thujone n.d. 16, borneol t-10, 1,8-cineole 0.3–8, camphene 0.2–8
Paolini et al. (2010)
Leaves
Wild
Dry
H
022
Verbenol 22, bisabolene oxide 18, farnesene epoxide 17, β-thujone 6, camphor 5
Tilaoui et al. (2011)
Spain
Leaves
Wild
Dry
SD
0.6
Camphor 15, 1,8-cineole 13, α-terpineol 6, borneol 5, chrysanthenone 5, terpinen-4-ol 5
Feuerstein et al. (1988)
Aerial parts flowering phase**
Wild
Dry
H
0.8(w/w)
Davanone 18, p-cymene 14, 1,8-cineole 10, chrysanthenone 7, cis-chrysanthenyl acetate 6, γ-terpinene 6, myrcene 5
Salido et al. (2001)
Flowering tops
Wild
Dry
SD
0.4–2.3 (w/w)
Davanone n.d. 51, chrysanthenone n.d. 36, cis-chrysanthenol n.d. 28, 1,8-cineole 2–26, p-cymene 5–21, cis-chrysanthenyl acetate n.d. 18, α-pinene t-17, camphor n.d. 17, myrcene 1–11, bornyl acetate n.d. 9, γ-terpinene 1–6, γ-muurolene 1–5, spathulenol 1–5, davana ether n.d. n.d. 5
Salido et al. (2004)
Tunisia
Leaves
Wild
n.r.
H
1.9 (v/w)
Pinocarvone 38, isoamyl 2-methylbutyrate 20, α-copaene 12, limonene 11
Neffati et al. (2008)
Leaves and flowers
S
Dry
H
0.7–1.9 (v/w)
α-Thujone n.d. 42, β-thujone n.d. 24, 1,8-cineole 1–28, sabinyl acetate n.d. 23, davanone n.d. 20, camphor n.d. 18, davana ether isomers n.d. 16, chrysanthenone n.d. 17, borneol n.d. 11, cis-chrysanthenyl acetate n.d. 10, yomogi alcohol n.d. 10, terpinen-4-ol 1–9, germacrene-D n.d. 7, bicyclogermacrene 1–6, cis-sabinol n.d. 6, davana ether n.d. 6, 3-hydroxyisodavanone n.d. 5, trans-pinocarveol n.d. 5
Mohsen and Ali (2009)
Leaves and flowers
EF
F/D
H
1.7–2.5 (v/w)
β-Thujone 18–25, α-thujone 13–23, camphor 9–13, chrysanthenone 7–11, 1,8-cineole 7–9, trans-sabinyl acetate 4–7, terpinen-4-ol 3–5
Mighri et al. (2009a)
Aerial parts (flowering phase and vegetative phase)
EF
Dry***
H
1.6–2.2 (v/w)
β-Thujone 16–34, α-thujone 14–24, 1,8-cineole 6–12, camphor 5–10, trans-sabinyl acetate 3–6, terpinen-4-ol 2–5
Mighri et al. (2009c)
Leaves and flowers
EF
Dry
H
0.9–2.4 (w/w)
β-Thujone 20–34, α-thujone 12–26, 1,8-cineole 6–23, camphor 5–12, chrysanthenone 1–9, trans-sabinyl acetate 1–7, terpinen-4-ol 2–5
Mighri et al. (2009b)
Leaves and flowers
Wild
Dry
H
1.1–2.3
β-Thujone 17–58, α-thujone 7–44, 1,8-cineole 6–17, camphor 4–11, trans-sabinyl acetate 5–7, chrysanthenone 3–7
Mighri et al. (2010a)
Leaves and flowers
EF
Dry
H
n.r.
β-Thujone 14–58, α-thujone 6–49, 1,8-cineole 5–18, camphor 4–11, trans-sabinyl acetate 3–8
Mighri et al. (2010b)
Leaves and flowers
Wild
Dry
H
1.2–4.9 (v/w)
α-Thujone n.d. 80, chrysanthenone n.d. 65, camphor n.d. 48, trans-sabinyl acetate n.d. 44, 1,8-cineole 1–24, davanone n.d. 21, β-thujone n.d. 18, trans-pinocarveol n.d. 15, borneol n.d. 11, cis-chrysanthenyl acetate n.d. 11, camphene n.d. 10, p-cymene n.d. 9, germacrene-D 1–5, terpinen-4-ol n.d. 6, pinocarvone n.d. 5
Boukrich et al. (2010)
Leaves and flowers
Wild
Dry
H
1.5 (v/w)
cis-Chrysanthenyl acetate 11, α-thujone 9, sabinyl acetate 9, davana ether 6, chrysanthenone 5
Zouari et al. (2010)
Aerial parts
Wild
Dry
H
n.r.
α-Thujone 25, germacrene-D 15, camphor 11, 1,8-cineole 9, β-thujone 8, lepidozene 6, chrysanthenone 5, sabinyl acetate 5
Kadri et al. (2011)
The aim of the present study was both to evaluate the chemical composition of the essential oils isolated from A. herba-alba collected at different locations in Algeria, and to compare these data with an updated survey on the chemical variability of this species essential oils.
2 Experimental
2.1 Plant material
The aerial parts of A. herba-alba were collected during the flowering (July, 2008) and vegetative phase of the plant (October and November, 2008) at different localities in Algeria (Benifouda; Bougaa; Boussaada and Boutaleb), characterized by diverse geographic and climate conditions (Fig. 1, Table 2). Plant material was dried in the dark, at room temperature. Certified voucher specimens have been deposited in the Herbarium of the Faculty of Nature and Life Sciences at F. A. University, Setif, Algeria. Data gathered from official Headquarter Maps[Carte d’État Major (CEM); CEM SoukNadjaa paper 169, CEM SaintArnaud, paper 98, CEM AinRoua, paper 69] and from the National Meteorology Office (Office National de la Météorologie).
Algeria geographical location (a) and collection sites (b) of Artemisia herba-alba. Benifouda (Be); Bougaa (Boug); Boussaada (Bous) and Boutaleb (Bout).
Average/Year
Artemisia herba-alba
Benifouda (Be)
Bougaa (Boug)
Boussaada (Bous)
Boutaleb (Bout)
Altitude (m)
821
914
459
1321
Precipitation (mm)
500
500
17
300
Temperature (°C)
2–38
2–38
3–42
−2– 40
2.2 Essential oil isolation
The essential oils were isolated from the dried plant material by hydrodistillation for 3 h, at a distillation rate of 3 ml.min−1, using a Clevenger-type apparatus according to the European Pharmacopoeia (Council of Europe, 2007). The essential oils were stored at −20 °C in the dark until analysis.
2.3 Essential oil analysis
2.3.1 Gas chromatography (GC)
Gas chromatographic analyses were performed using a Perkin Elmer Autosystem XL gas chromatograph equipped with two flame ionization detectors (FIDs), a data handling system and a vaporizing injector port into which two columns of different polarities were installed: a DB-1 fused-silica column (polydimethylsiloxane, 30 m × 0.25 mm i.d., film thickness 0.25 μm; J & W Scientific Inc., Rancho Cordova, CA, USA) and a DB-17HT fused-silica column[(50% phenyl)-methylpolysiloxane, 30 m × 0.25 mm i.d., film thickness 0.15 μm; J & W Scientific Inc.]. Oven temperature was programed, 45–175 °C, at 3 °C min−1, subsequently at 15 °C.min−1 up to 300 °C, and then held isothermal for 10 min; injector and detector temperatures, 280 °C and 300 °C, respectively; carrier gas, hydrogen, adjusted to a linear velocity of 30 cm.s−1. The samples were injected using split sampling technique, ratio 1:50. The volume of injection was 0.1 μL of a pentane-volatiles solution (1:1). The percentage composition of the essential oils was computed by the normalization method from the GC peak areas, calculated as mean values of two injections from each sample, without using correction factors.
2.3.2 Gas chromatography–Mass spectrometry (GC-MS)
The GC–MS unit consisted of a Perkin Elmer Autosystem XL gas chromatograph, equipped with DB-1 fused-silica column (30 m × 0.25 mm i.d., film thickness 0.25 μm; J & W Scientific, Inc.), and interfaced with a Perkin–Elmer Turbomass mass spectrometer (software version 4.1, Perkin Elmer, Shelton, CT, USA). Injector and oven temperatures were as above; transfer line temperature, 280 °C; ion source temperature, 220 °C; carrier gas, helium, adjusted to a linear velocity of 30 cm.s−1; split ratio, 1:40; ionization energy, 70 eV; scan range, 40-300 u; scan time, 1 s. The identity of the components was assigned by comparison of their retention indices, relative to C9–C16 n-alkane indices and GC-MS spectra from a home-made library, constructed based on the analyses of reference oils, laboratory-synthesized components and commercial available standards.
2.4 Statistical analysis
The percentage composition of the isolated essential oils was used to determine the relationship between the different samples by cluster analysis using Numerical Taxonomy Multivariate Analysis System (NTSYS-pc software, version 2.2, Exeter Software, Setauket, New York) (Rohlf, 2000). For cluster analysis, correlation coefficient was selected as a measure of similarity among all accessions, and the Unweighted Pair Group Method with Arithmetical Averages (UPGMA) was used for cluster definition. The degree of correlation was evaluated according to Pestana and Gageiro (2000) and classified as very high (0.9-1), high (0.7-0.89), moderate (0.4-0.69), low (0.2-0.39) and very low (<0.2).
3 Results and discussion
The aerial parts of A. herba-alba were collected at different localities in Algeria (Benifouda; Bougaa; Boussaada and Boutaleb), characterized by diverse geographic and climate conditions (Fig. 1, Table 2).
A. herba-alba populations studied afforded oils in a yield ranging from 0.2% (Boutaleb) to 0.9% (Bougaa) (v/d.w.), respectively, (Table 3). The oil yields recorded in the present study were within the ranges reported in the literature[0.1–4.9% (v/w), (Table 1)], and, as referred by Mighri et al. (2009b), where higher at the flowering phase. RI = Retention index relative to C9–C16 n-alkanes on the DB-1 column, t = trace (<0.05%).
Components
RI
Artemisia herba-alba
Benifouda
Bougaa
Boussaada
Boutaleb
Nov-08
Jul-08
Oct-08
Nov-08
Santolina triene
911
0.7
0.2
1.3
t
Tricyclene
921
0.2
0.3
0.2
t
α-Thujene
924
t
t
t
t
α-Pinene
930
0.4
0.8
0.8
t
Camphene
938
7.1
4.2
4.1
0.7
Sabinene
958
0.2
0.2
0.7
0.3
1-Octen-3-ol
961
t
t
t
t
β-Pinene
963
0.1
0.3
0.3
t
1,2,4-Trimethyl benzene
978
0.4
0.4
0.2
0.2
1-Decene
995
t
t
t
t
α-Phellandrene
995
0.2
0.8
0.3
t
1,2,3-Trimethyl benzene
1001
0.2
0.7
t
1.0
α-Terpinene
1002
0.3
0.1
0.1
0.6
p-Cymene
1003
0.8
0.9
0.4
0.6
1,8-Cineole
1005
8.6
8.2
9.8
3.0
Limonene
1009
t
t
t
t
Santolina alcohol
1011
t
t
0.7
t
γ-Terpinene
1035
0.3
0.8
0.6
0.3
trans-Sabinene hydrate
1037
t
0.8
0.4
0.2
Filifolene*
1074
3.9
1.0
1.1
2.8
α-Thujone
1074
6.9
28.1
27.7
23.5
β-Thujone
1081
1.9
7.8
3.4
3.0
Chrysanthenone*
1081
12.2
3.9
7.6
19.0
α-Campholenal
1088
t
0.1
0.4
0.3
trans-p-2-Menthen-1-ol
1095
0.7
0.5
1.0
0.5
Camphor
1095
33.1
22.8
17.3
18.7
trans-Pinocarveol
1106
1.1
0.7
0.5
0.3
cis-Verbenol
1110
0.9
0.3
t
0.4
trans-Verbenol
1114
1.1
0.1
0.7
t
Pinocarvone
1121
1.7
1.5
1.1
0.6
Borneol
1134
2.5
2.0
1.9
1.5
Terpinen-4-ol
1148
0.6
0.6
1.0
0.7
Myrtenal
1153
0.2
0.2
t
0.2
Myrtenol
1168
t
0.1
0.6
t
trans-Carveol
1189
t
0.1
0.3
t
cis-Carveol
1202
0.1
0.2
t
0.1
Carvone
1206
t
0.1
0.1
t
cis-Ocimenone
1206
t
0.1
0.2
t
Piperitone
1211
0.4
0.3
t
0.2
cis-Chrysanthenyl acetate
1241
0.5
0.2
1.2
t
Bornyl acetate
1265
0.3
0.3
0.2
0.2
Carvacrol
1286
0.3
0.7
t
t
β-Copaene
1426
t
t
0.1
0.1
β-Ylangene
1435
t
t
0.2
t
allo-Aromadendrene
1456
t
t
t
t
γ-Muurolene
1469
2.4
0.7
3.5
7.1
Bicyclogermacrene
1487
0.7
0.4
1.1
2.5
δ-Cadinene
1505
0.1
t
0.2
0.5
Spathulenol
1551
0.1
0.4
0.2
1.0
Ledol
1580
t
t
0.1
0.2
% of identification
91.2
91.9
91.6
90.3
Grouped components
Monoterpene hydrocarbons
14.2
9.6
9.9
5.3
Oxygen-containing monoterpenes
73.1
79.7
76.1
72.4
Sesquiterpene hydrocarbons
3.2
1.1
5.1
10.2
Oxygen-containing sesquiterpenes
0.1
0.4
0.3
1.2
Others
0.6
1.1
0.2
1.2
Oil Yield (%, v/dry weight)
0.79
0.94
0.72
0.16
A. herba-alba identified oil components are listed in Table 3 in order of their elution on the DB-1 column. Monoterpenes (78–89%) and particularly oxygen-containing monoterpenes (72–80%) dominated all oils. Sesquiterpenes ranged from 2–11% (Table 3).
A. herba-alba essential oils percentage composition was used to determine the relationship between the different samples, and allowed the definition of two clusters, Fig. 2. Cluster I was a one sample group as included only the Benifouda (Be) oil, which was dominated by camphor (33%) and chrysanthenone (12%), Table 3. Cluster II included the more correlated oil samples (Scorr > 0.88) from Bougaa (Boug), Boussaada (Bous) and Boutaleb (Bout). α-Thujone, which was < 7% in Be oil, was dominant in Cluster II sample oils (24–28%). Boug, Bous and Bout samples from Cluster II showed also large percentages of camphor (17–23%), and a wide range in chrysanthenone (4–19%) relative amount, Table 3.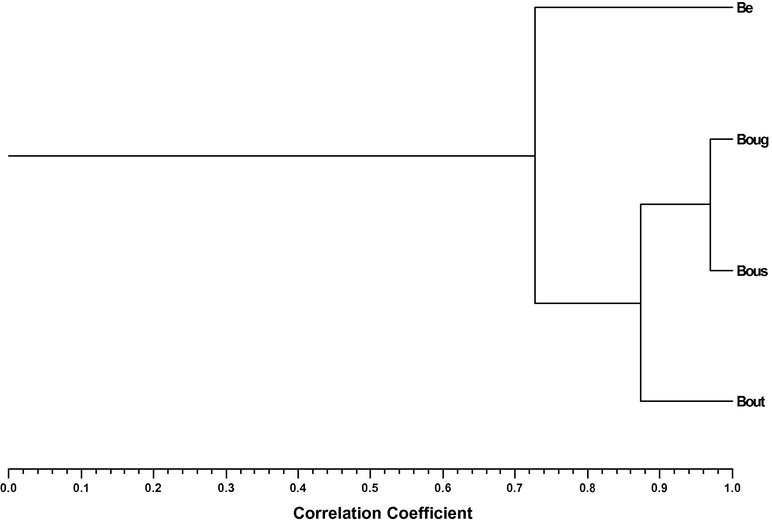
Dendrogram obtained by cluster analysis of the percentage composition of the essential oils isolated from Artemisia herba-alba samples based on correlation and using unweighted pair-group method with arithmetic average (UPGMA). Benifouda (Be); Bougaa (Boug); Boussaada (Bous) and Boutaleb (Bout).
Overall, the essential oil profiles of all samples were similar (Scorr > 0.7), although with some variation in the relative amount of the three main components, camphor (17–33%), α-thujone (7–28%) and chrysanthenone (4–19%). Despite the global resemblance, three types of oils could be defined, (a) α-thujone : camphor (Bougaa and Boussaada plants oils), (b) camphor : chrysanthenone (Benifouda plant oil) and (c) α-thujone : camphor : chrysanthenone (Boutaleb plant oil), Fig. 2.
No relationship could be drawn between the chemical composition of A. herba-alba essential oils and the four regions of Algeria (Benifouda; Bougaa; Boussaada and Boutaleb) where the samples were collected, nor with the altitude of the collection sites, temperature or humidity ranges (Tables 2 and 3, Fig. 2). This was confirmed by the fact that the most similar essential oil profiles were those isolated from plants collected during the flowering phase in Bougaa and during the vegetative phase in Boussaada (Tables 2 and 3, Fig. 2).
A review of the existing literature on A. herba-alba essential oils afforded a large number of studies, particularly in the last two years (Table 1). Although the isolation procedure was similar in most cases, the plant parts, the physiological stage, the plant status (fresh or dry), and the geographical origin were quite diverse, in addition to the use of collective samples and to the possibility of existing different subspecies. Only seldom studies have used individual plants, and even in those cases no clear correlation between plant oil types and environmental conditions was established (Fleisher et al., 2002).
With high percentage variability, α-thujone (n.d. 80%) and β-thujone (n.d. 58%) were reported in studies from all countries (Table 1). Although not mentioned in Table 1, because this table includes only components with a relative amount ⩾5%, the occurrence of α-thujone and β-thujone was also reported in one study from Spain (Villar et al., 1983; in Salido et al., 2001). With exception of the work published on Jordanian A. herba-alba (Hudaib and Aburjai, 2006) (Table 1), chrysantenone (n.d. 65%), camphor (n.d. 49%) and 1,8-cineole (n.d. 28%) occurrence was always mentioned in, at least, some studies from all other countries (Table 1). In addition, with a more restricted occurrence, davanone (Spain and Tunisia) and cis-chrysanthenyl acetate (Israel, Sinai, Spain and Tunisia) attained also relatively high percentages (n.d. 51% and n.d. 69%, respectively) (Table 1). Usually one of these seven compounds or some of them, in different proportions, dominate A. herba-alba essential oils, which is in agreement with the results here reported for samples collected at different Algerian sites.
The chemical variability of essential oils from Algerian A. herba-alba emphasizes the importance of evaluating individual plant samples, as well as the worth of avoiding wild plant material collection, not only due to the innate variation, which has a negative market impact, but also to impair biodiversity depletion. In view of this, it seems most adequate to recognize which chemovariety best fits the market demands and develop sustainable culture methodologies for local A. herba-alba crop production.
Acknowledgements
The invaluable help of Dr Carles Benedí (Universidade de Barcelona) on providing information on updated international Floras, and Dr Maria de Lurdes Saramago (Biblioteca de Biologia da Faculdade de Ciências da Universidade de Lisboa) on making available several references is greatly acknowledged. Rachid Belhattab acknowledges MESRS (Algeria) the financial support that allowed the training period at the FCUL/CBV/IBB, Portugal.
References
- Inhibition of steel corrosion in 2 M H3PO4 by artemisia oil. Appl. Surf. Sci.. 2006;252:6212-6217.
- [Google Scholar]
- Etude de quelque peuplements d’Armoise blanche du Maroc, Artemisia herba-alba. Riv. Ital. E.P.P.O.S.. 1980;62:69-74.
- [Google Scholar]
- Chemical composition of the essential oils of Artemisia herba-alba issued from the district of Biskra (Algeria) Phytothérapie. 2010;8:277-281.
- [Google Scholar]
- Chemical variability of Artemisia herba-alba Asso growing wild in Semi-arid and Arid Land (Tunisia) J. Essent. Oil Res.. 2010;22:331-335.
- [Google Scholar]
- Extraction by steam distillation of Artemisia herba-alba essential oil from Algeria: kinetic study and optimization of the operating conditions. J. Essent. Oil Bearing Plants. 2009;12:640-650.
- [Google Scholar]
- Council of Europe (COE) European Directorate for the Quality of Medicines (2007). European Pharmacopoeia, 6th ed. COE, Strasbourg.
- Chemical composition of Algerian Artemisia herba-alba essential oils isolated by microwave and hydrodistillation. J. Essent. Oil Res.. 2010;22:514-517.
- [Google Scholar]
- Chemical composition of the essential oil of Artemisia herba-alba Asso grown in Algeria. J. Essent. Oil Res.. 2006;18:685-690.
- [Google Scholar]
- Constitution of essential oils from Artemisia herba-alba population of Spain. Phytochemistry. 1988;27:433-434.
- [Google Scholar]
- The constitution of essential oils from Artemisia herba-alba populations of Israel and Sinai. Phytochemistry. 1986;25:2343-2347.
- [Google Scholar]
- Chemovariation of Artemisia herba alba Asso aromatic plants of the Holy Land and Sinais. J. Essent. Oil Res.. 2002;14:156-160.
- [Google Scholar]
- Greuter, W. (2006–2009): Compositae (pro parte majore). In: Greuter, W. & Raab-Straube, E. von (ed.): Compositae. Euro+Med Plantbase - the information resource for Euro-Mediterranean plant diversity. http://ww2.bgbm.org/EuroPlusMed/[accessed 07.11].
- Composition of the essential oil from Artemisia herba-alba grown in Jordan. J. Essent. Oil Res.. 2006;18:301-304.
- [Google Scholar]
- Plant systematics: a phylogenetic approach (2nd ed.). USA: Sinauer Associates Inc.; 2002.
- Chemical constituents and antioxidant activity of the essential oil from aerial parts of Artemisia herba-alba grown in Tunisian semi-arid region. Afr. J. Biotechnol.. 2011;10:2923-2929.
- [Google Scholar]
- Influence of drying time and process on Artemisia herba-alba Asso essential oil yield and composition. Journal of Essential Oil Bearing Plants. 2009;12:358-364.
- [Google Scholar]
- Impact of season and harvest frequency on biomass and essential oil yields of Artemisia herba-alba cultivated in southern Tunisian. Exp. Agric.. 2009;4–5:499-508.
- [Google Scholar]
- Composition and intraspecific chemical variability of the essential oil from Artemisia herba alba growing wild in a Tunisian arid zone. Chem. Biodivers.. 2010;7:2709-2717.
- [Google Scholar]
- The essential oil from Artemisia herba-alba Asso cultivated in Arid Land (South Tunisia) J. Essent. Oil Res.. 2009;21:453-456.
- [Google Scholar]
- Antimicrobial and antioxidant activities of Artemisia herba-alba essential oil cultivated in Tunisian arid zone. C. R. Chim.. 2010;13:380-386.
- [Google Scholar]
- Chemical constituents and biological activities of Artemisia herba-alba. Records of Natural Products. 2010;4:1-25.
- [Google Scholar]
- Essential oil composition of Artemisia herba-alba from Southern Tunisia. Molecules. 2009;14:1585-1594.
- [Google Scholar]
- Chemical composition, mutagenic and antimutagenic activities of essential oils from (Tunisian) Artemisia campestris and Artemisia herba-alba. J. Essent. Oil Res.. 2008;20:471-477.
- [Google Scholar]
- Application of essential oil of Artemisia herba alba as green corrosion inhibitor for steel in 0.5M H2SO4. Surf. Rev. Lett.. 2009;16:49-54.
- [Google Scholar]
- Chemical variability of Artemisia herba-alba Asso essential oils from East Morocco. Chem. Pap.. 2010;64:550-556.
- [Google Scholar]
- Pestana, M. H., Gageiro, J.N. (2000). Análise de dados para ciências sociais. A complementaridade do SPSS. Edições Sílabo, Lisboa, Portugal.
- Nouvelle flore de l’Algérie et des régions désertiques méridionales. Paris: CNRS; 1963.
- NTSYS-pc, Numerical Taxonomy and Multivariate Analysis System. New York: Applied Biostatistics; 2000.
- Fungicidal activity of Artemisia herba alba Asso (Asteraceae) J. Environ. Sci. Health. 2006;41:237-244.
- [Google Scholar]
- Chemical composition of the essential oil of Artemisia herba-alba Asso ssp. valentine (Lam.) Marcl. J. Essent. Oil Res.. 2001;13:221-224.
- [Google Scholar]
- Composition and infraspecific variability of Artemisia herba-alba from southern Spain. Biochem. Syst. Ecol.. 2004;32:265-277.
- [Google Scholar]
- A. Chemical composition and antiproliferative activity of essential oil from aerial parts of a medicinal herb Artemisia herba-alba. Rev. Bras. de Farmacognosia. 2011;21:781-785.
- [Google Scholar]
- Chemical composition and biological activities of a new essential oil chemotype of Tunisian Artemisia herba alba Asso. J. Med. Plants Res.. 2010;4:871-880.
- [Google Scholar]







