Translate this page into:
Ethanol leaf extract of Ruspolia hypocrateriformis abrogated hepatic redox imbalance and oxidative damage induced by heavy metal toxicity in rats
⁎Corresponding author. joshua.nonso@ebsu.edu.ng (J.N. Awoke)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Heavy metal exposure causes hepatic redox imbalance and oxidative damage in rats. Leaf extract of Ruspolia hypocrateriformis is highly rich in phenolic compounds. Leaf extract of Ruspolia hypocrateriformis has antioxidant potentials. The antioxidant potentials restored hepatic redox imbalance and oxidative damage in rats.
Abstract
Humans and animals are frequently exposed to heavy metals in the environment, which are highly toxic to the physiological milieu and organs of the body. We investigated the ameliorative potentials of ethanol leaf extract of Ruspolia hypocrateriformis against redox imbalance due to exposure of rats to heavy metals. The in vitro study explored the antioxidant potentials of the ethanol leaf extract using 1,1-diphenyl-2-picryl hydrazyl, nitric oxide and ferric reducing antioxidant potential assays respectively. HPLC was used to quantify the amount of flavonoids and phenolic acids in the extract. For in vivo study, 30 rats were randomly divided into 5 groups. Group A received normal saline. Group B received combined solution of Lead Nitrate and Mercury Chloride (11.25 mg/kg and 0.4 mg/kg) per Bwt/day. Group C, D and E were administered with the leaf extract at doses of 200, 400 and 600 mg/kg body weight respectively for 28 consecutive days. Biomarkers of hepatic dysfunctions and oxidative stress were investigated in the study rats. The HPLC study revealed high amount of gallic and ferulic acids (17.86 ± 2.68), which are the major phenolic compounds found in the extract. The extract further exhibited high antioxidant potentials in inhibiting the scavenging activity of free radicals produced in vitro. Interestingly, 600 mg/kg dosage of the leaf extract successfully ameliorated the distorted redox imbalance and oxidative damage in the liver of the rats caused by exposure to the heavy metals. Leaf extract of Ruspolia hypocrateriformis demonstrated strong antioxidant potentials, which could be exploited in pharmaceutical preparations.
Keywords
Ruspolia hypocrateriformis
Phenolic
Oxidative damage
Heavy metals
HPLC
- DPPH
-
2,2-diphenyl-1-picrylhydrazyl
- FRAP
-
Ferric Reducing Antioxidant Potential
- SOD
-
superoxide Dismutase
- MDA
-
Malondialdehyde
- NO
-
Nitric Oxide
- CAT
-
Catalase
- GSH
-
Reduced glutathione
- HPLC
-
High Performance Liquid Chromatography
- ROS
-
Reacting Oxygen Species
Abbreviations
1 Introduction
The presence of various heavy metals in the environment remains one of the major sources of environmental pollution worldwide (Jaishankar et al., 2014; Nagajyoti et al., 2010). Humans, animals and plants are frequently exposed to these heavy metals in the environment through various means at doses beyond the minimum permissively level, which lead to severe damage to relevant organs of the body (Assi et al., 2016; Tchounwou et al., 2012). Hence, heavy metal poisoning and toxicity have become major global health concern especially for miners and many others who are frequently exposed to them either due to their occupation or due to contact with heavy metal polluted environment (Morais et al., 2012). The deleterious effects of heavy metals in the physiological system are mostly mediated through abnormal increase in the production of reactive oxygen species (ROS) leading to depletion in endogenous cellular antioxidants, which further enhance redox imbalance and oxidative damage in various organs of the body (Sharma et al., 2014; Abdelhamid et al., 2020; Nishio et al., 2019). They also trigger some cascade signalling systems and transcription factors that elicit dysregulation of inflammatory mediators in the physiological system leading to DNA or protein damage, mitochondrial disruption, apoptosis and distortion in normal body metabolism resulting in disease condition (Fu and Xi, 2020; Aja et al., 2020; Sabath and Robles-Osorio, 2012).
Lead is one of these heavy metals whose widespread use has caused extensive environmental contamination and health problems in almost all parts of the world (Wani et al., 2015; Assi et al., 2016). It is a poisonous metal commonly found in the environment in different forms such as organic (tetraethyl Lead) and inorganic (Lead acetate, Lead chloride and Lead nitrate) (Ahamed and Siddiqui, 2007a, 2007b). Exposure to lead nitrate through major sources such as air, water and foods mainly occurs through the respiratory and gastrointestinal systems (Patra et al., 2011). Absorbed lead is conjugated in the liver and passed to the kidney; where little quantities are excreted in urine leaving the remnants to accumulate in various organs of the body (Jadhav et al., 2007). This further affects many biological activities at the molecular, cellular and intercellular levels, resulting in morphological alterations that could remain even after the level of lead have receded (Ibrahim et al., 2012; Garz et al., 2006).
Mercury is another important toxic heavy metal, which widely occurs in nature in three major forms i.e. elemental (or metallic) mercury, inorganic mercury compounds, and organic mercury compounds (Bernhoft, 2012; Park and Zheng, 2012). Human exposure to any of these forms of mercury would all cause major cellular, cardiovascular, hematological, pulmonary, renal, immunological, neurological, embryonic and reproductive toxic effects in addition to severe damage to the vital organs of the body (Rice et al., 2014; Carocci et al., 2014). Amongst the many heavy metals that frequently contaminate environments in developing countries, lead and mercury are the most important of all of them particularly due to sustained increase in illegal mining of these heavy metals in developing countries (Spiegel, 2009; Kortei et al., 2020; Duncan, 2020). This prompted our use of lead and mercury in this present study.
The use of natural products from plants for the amelioration of various physiological damages in the body due to heavy metals exposure is currently an emerging area of research particularly in developing countries where various human activities have led to severe contamination of the environment with heavy metals. There is also a growing body of scientific evidence in recent time that plant products with antioxidant potentials could ameliorate physiological damage caused by heavy metal exposure (Mirkov et al., 2020; Orji et al., 2016a; Yang et al., 2013, 2014). Interestingly, natural products from plants are easily available in various localities and are accessed easily without much cost. The limitation however, remains in the inadequate scientific evidence to support the efficacy of these local herbs and formulations.
Ruspolia hypocrateriformis (Vahl) Milne-Redh sometimes known as Justicia hypocrateriformis Vahl belongs to the family Acanthaceae. It is a straggling shrub of about one meters high found in the savanna, secondary and deciduous forest areas of Africa and India (Castleman, 2001). The plant is distinctly ornamental and are used for this biological role in most West African countries (Agbor et al., 2001). However, its medicinal values are been exploited by locals in folk medicine for the treatment of various diseases such as anaemia and diarrhoea (Adjanohoun et al., 1996; Orji et al., 2016b). It was previously demonstrated in our laboratory and that of others that leaf extract of Ruspolia hypocrateriformis has enormous endowment of phytochemical and nutritional compositions, which could further be exploited in pharmaceutical preparations for the treatment of various diseases (Orji et al., 2017; Guetchueng et al., 2019; Agbor et al., 2014). We have also reported the anti-anaemic potentials of the leaf extract against lead induced anaemia (Orji et al., 2016a,b). The leaf extract showed no toxicity through oral route of administration at LD50 of 14.35 g/kg and possesses strong antioxidant and antidiarrheal potentials (Agbor et al., 2014). As part of our research plan to fully explore the medicinal potentials of this plant and further provide scientific evidence for their use in folk medicine, we presently investigated the antioxidant potentials of the ethanol leaf extract against lead nitrate and mercury chloride induced oxidative damage in rats.
2 Materials and methods
2.1 Sample collection, identification and preparation
Fresh leaves of Ruspolia hypocrateriformis were collected from Afikpo, Ebonyi State, Nigeria in a location relatively free from heavy metal contamination. Taxonomical identification of the plant was done by Prof Onyekwelu of the Department of Applied Biology Ebonyi State University, Nigeria. A sample was deposited in the herbarium of the Department with voucher number of EBSU/APB/HB/0221. The plant extract was prepared according to the method used by Orji et al. (2017). The fresh leaves were thoroughly washed with clean distilled water and sun-dried for about 24 h. It was further pulverized with blender into powdery form and 300 g of the ground leaves were soaked in 1 litre of ethanol for two days with intermittent shaking. After thorough extractions, the mixture was further sieved using a clean muslin cloth and the ethanol was recovered under mild temperature of 30 °C to get the extract. The doses (200, 400 and 600 mg/kg) used for this present study were chosen based on the doses used in our previous studies (Orji et al., 2017, 2016b) and on the LD50 of 14.35 g/kg earlier reported by Agbor et al., (2014).
2.2 Chemicals and reagents
Flavonoids (quercetin and rutin hydrate) and phenolic acids standards (gallic, ferulic and caffeic acids) used as standards were purchased from sigma-Aldrich (UK). Methanol, acetonitrile, hydrochloric acid, phosphoric acid, lead nitrate, mercury chloride and water (HPLC grade) were also purchased from Thermo Fisher Scientific (UK). Other reagents used were of standard analytical grades.
2.3 HPLC apparatus and quantification of flavonoids and phenolic compounds in the leaf extract.
A quantitative analysis on the extract to determine bioactive compounds present in the sample was carried out using Agilent 1100 Series LC HPLC equipped with column thermostat, UV detector and a 20 μl injection loop (Agilent Technologies, CA, USA). Additionally, the chromatographic separation process was achieved using a ZORBAX SB-C18 analytical column (250 mm × 4.6 mm, 5 µm) at wavelength of 257 nm. The mobile phase consisted 0.01 mol.L–1 phosphoric acid–methanol-acetonitrile (72:14:14, v/v/v). The procedure followed was as described by Wang et al. (2010) and Agilent catalogue 2017. Briefly, 3 g of the extract was weighed into a 50 ml centrifuge tube and 12 ml of extraction solutions were added and thoroughly shaken for 30 sec in a vortex mixer at high speed. The pH of the mixture was adjusted to 2.0–2.2 before allowing for extraction for about 20 min with an oscillator on the maximum speed. After centrifugation at 13,000 rmp for 10 min (at 4 °C), the supernatants were filtered through 0.22 µm membrane filters (Thermo Fisher Scientific, UK), and 20 µl of the filtrate was injected into HPLC for further analysis.
2.4 Antioxidant in vitro studies
2.4.1 DPPH assay for free radical scavenging
The antioxidant potential of the leaf extract of Ruspolia hypocrateriformis was determined using DPPH (2,2-diphenyl-1-picrylhydrazyl) (Sigma Aldrich USA) as described by Mazimba et al (2011). Briefly, 2 ml of the extract samples at different concentrations of 25, 75 and 100 µg/ml respectively in test tubes were mixed with 2 ml DPPH (2%). Ascorbic acid and Gallic acids were used as standards. After 30 min, absorbance was determined at 517 nm using UV spectrophotometer. Radical scavenging activity was determined using the formula: % Scavenging Activity = [(Blank Absorbance − Sample Absorbance)/(Blank Absorbance)] × 100.
2.4.2 Nitric oxide radical scavenging activity assay
The nitric oxide free radical scavenging activity of the leaf extract was determined using the method described by Igbinosa et al (2011). Briefly, 50 µl of different concentrations of the extract (25, 75 and 100 µg/ml), ascorbic acid and Gallic acid (standards) in separate test tubes were dissolved in DMSO and uniformly made up to 150 µl with methanol. Furthermore, 2.0 ml of sodium nitroprusside (10 mM) in phosphate buffer saline was added to each of the solutions in the test tubes. The solutions were further incubated at room temperature for 150 min and 5 ml of griess reagent was finally added into each of the solution. The absorbance was measured at 546 nm on UV–visible spectrometer. The percentage nitric oxide inhibition by the extract was calculated using the formula: % scavenging/Reduction = [Absorbance of control − Absorbance of test sample/Absorbance of control] × 100
2.4.3 FRAP assay for total reducing power
The reducing power of the leaf extract of Ruspolia hypocrateriformis and standards (ascorbic acid and gallic acid) were determined by the method of Vijayalakshmi and Ruckmani, (2016). The extract at various concentrations (25, 75 and 100 μg/ml) were added to 2.5 ml of 0.2 M sodium phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide solution. The solution was thoroughly vortexed and incubated at 50 °C for 20 min on a vortex shaker. After the incubation, 2.5 ml of 10% trichloroacetic acid was added to the solution and centrifuged at 3000 rpm for 10 min. Then 2.5 ml of the supernatant mixed with 2.5 ml of deionised water and 0.5 ml of 0.1% ferric chloride. Absorbance of the solution was taken at 700 nm using UV Spectrophotometer.
2.5 Experimental animals for the antioxidant in vivo studies
The animal model, (30 male albino rats) with average body weight of 180–200 g was purchased from University of Nigeria Nsukka Enugu, Nigeria. The rats were kept in stainless steel rat cages in a well-ventilated animal house of Biochemistry Department, Ebonyi State University Abakaliki. They were acclimatized for 7 days under good standard laboratory conditions (12 h light/dark cycle; room temperature: 25–30 °C). They had access to standard rodent chow (Vital feed®, Grand Cereals Ltd, Jos, Nigeria) and water ad libitum. The International Standard Procedure for Experimental Animal Handling of National Institute of Health (NIH), USA (NIH Publications No. 8023, revised in 1978), adopted by Biochemistry Department, Ebonyi State University, Nigeria was followed after due approval by the Departmental Ethical Committee. The Ethical approval number for this present study is EBSU/BCH/ET/19/001.
2.5.1 Experimental design
After acclimatization, the rats were divided randomly into 5 groups (n = 6) with different treatment conditions. The combined solution of lead nitrate and mercury chloride dissolved in normal saline was administered through oral inturbation. The extract dissolved in normal saline was also administered through oral inturbation. Group A (normal control) received normal saline (2 mg/kg body weight) for 28 days. Group B, C, D and E were given combined solution of lead nitrate and mercury chloride (11.25 mg/kg and 0.4 mg/kg) per Bwt/day according to Goudarzi et al. (2017) for 28 days. However, Group C, D and E were administered with ethanol leaf extract of Ruspolia hypocrateriformis at daily doses of 200, 400 and 600 mg/kg body weight respectively for 28 consecutive days beginning from day 8 of combined lead nitrate and mercury chloride exposure until the end of the experiment. At the end of the experiment, the animals were allowed a day rest and then sacrificed under light ether anaesthesia. The organs of interest were excised, rinsed with normal saline and homogenized in ice-cold buffer and utilized for further studies.
2.6 Determination of markers of redox imbalance
2.6.1 Determination of malondialdehyde (MDA) level
The MDA level in the liver homogenates was measured using the method of Buege and Aust, (1978). Briefly, 1.0 ml each of the liver supernatant in test tubes was separately mixed with 2.0 ml of TCA reagent (10%, w/v). Each mixture was centrifuged at 3000g for about 10 min; and 2.5 ml of each sample supernatant was further transferred to a test tube containing 2.5 ml of TBA solution (0.67%, w/v). The mixture was heated for 30 min, cooled and the absorbance read at 532 nm by spectrophotometer. MDA level was calculated based on the absorbance coefficient of the TBA–MDA complex (ε = 1.56 × 105 cm−1 M−1) and expressed in nmol/mg protein.
2.6.2 Determination of superoxide dismutase (SOD) activity
SOD activity in the liver homogenates was assayed using the method of Sun and Zigma (1978). The sample volume of 0.2 ml was transferred into 2.5 ml of 0.05 phosphate buffer, pH 7.8 and 0.3 ml of newly prepared adrenaline solution was then added to the reaction mixture followed by quick mixing by inversion in the cuvette. The increase in absorbance was taken every 30 s for 3 min at 480 nm against blank. Blank contained 0.3 ml of adrenaline and 2.5 ml buffer.
2.6.3 Determination of catalase (CAT) activity
CAT activity was assayed according to the method used by Oyedemi et al (2010). The sample volume of 4.0 ml of hydrogen peroxide (H2O2) solution was added to 5.0 ml of phosphate buffer and 1.0 ml of the sample was mixed at room temperature. Afterwards, 1.0 ml of the reaction mixture was withheld and added to 2.0 ml dichromate/acetic acid reagent at one minute interval and the steady absorbance reading taken at 570 nm.
2.6.4 Determination of reduced glutathione determination (GSH) level
The GSH level in the liver homogenate was determined using Ellman's reagent (DTNB) according to the method used by Aguirre et al (2011). The sample volume of 1.0 ml of was added 4.0% sulfo-salicyclic acid and the mixture centrifuged at 3,000 rpm for 15 min at 2 °C. The sample was introduced to 4.5 ml of Ellman reagent and absorbance was measured at 412 nm. The blank was prepared by addition of 0.5 ml of 4% sulfo-salicyclic acid to 4.5 ml of Ellman reagent while absorbance was measured at 412 nm.
2.7 Determination of markers of hepatic dysfunctions
2.7.1 Determination of alkaline phosphatase (ALP) activity
Alkaline phosphatase activity was determined using commercial kit manual (Randox, UK) and according to the method of Wright et al. (1972). A sample of 0.05 ml of was transferred into a test tube, and then 3.0 ml of reagent (a mixture of diethanolamine buffer, MgCl2 and a substrate, p-nitrophenylphosphate) was added and mixed. The initial absorbance was taken and a timer was started simultaneously. The absorbance was read after 1, 2 and 3 min at the wavelength of 450 nm. Calculation: ALP (U/L) = 2790 × change in absorbance
2.7.2 Determination of bilirubin level
Serum bilirubin level was determined using the method of Jendrassik and Grof (1938), briefly the sample volume of 200 µl, R1 (1000 µl), R2 (50 µl) and R3 (1000 µl) were mixed and left for 10 min at 25 °C. Then R4 (1000 µl) was added, mixed and left for 30 min at 25 °C. Absorbance of the sample was read at 578 nm against the sample blank (ATB). R1-sulphanilic acid; R2-Nitrite; R3-Caffeine; R4-Tartrate. Calculation: Total bilirubin (mg/dL) = 10.8 × ATB (578 nm).
2.7.3 Determination of aspartate aminotransferase (AST) activity
The method of Reitman and Frankel (1957) was used to assay for the activity of aspartate aminotransferase using commercially available kit manual (Randox, UK). Briefly, sample volume of 0.5 ml was mixed with 0.5 ml of reagent 1 (Buffer: phosphate buffer, L-aspartate and α-oxoglutarate) and incubated for 30 mins at room temperature. Then 0.1 ml of reagent (2,4-dinitrophenylhydrazine) was added, mixed and allowed to stand for 20 min at a temperature of 20–25 °C. Furthermore, 5 ml of NaOH was then added, mixed and read after 5 min at a wavelength of 546 nm. The AST activity was obtained from the table in the manual.
2.7.4 Determination of alanine aminotransferase (ALT) activity
For alanine aminotransferase, 0.1 ml of sample was pipetted into a test tube and 0.5 ml of solution R1 (buffer containing phosphate buffer, L-alanine and α-oxoglutarate) was added, mixed and incubated for 30 min at 37 °C. Then, 0.5 ml of solution R2 (2,4-dinitrophenylhydrazine) was added and mixed and then allowed to stand for 20 min at 20 to 25 °C. Finally, 5.0 ml of NaOH was added, mixed and after 5 min the absorbance was read at wavelength of 546 nm (Reitman and Frankel, 1957).
2.7.5 Determination of serum albumin level
For albumin determination, three test tubes were prepared and designed as reagent blank, standard and sample test tubes. Then 0.01 ml of distilled water, standard and serum were added to the reagent blank, standard and sample test tubes respectively and 3.0 ml of BCG reagent was further added to the three test tubes. The tubes were mixed properly and incubated for 5 min at 20–25 °C. The absorbance of the sample and that of the standard were read against the reagent blank at the wavelength 630 nm. Calculations = .
2.7.6 Determination of total protein level
The total protein content was determined using the method of Tietz (1995). Briefly, 20 µl of serum, 10 µl standard and 10 µl distilled water were added to three test tubes respectively. Thereafter, 0.5 ml of reagent R1 was added to all the test tubes and incubated for 3 min at 25 0C. The absorbance was measured at 500 nm.
2.8 Statistical analysis
The statistical analysis was performed using Graph Pad Prism 5.04 (GraphPad, La Jolla, CA, USA). Data was expressed as Mean ± SEM. One-way ANOVA with Dunnett’s test was used for the statistical tests. In general, p < 0.05 was considered as the statistical level of significance. The IC50 for DPPH and NO was calculated using Linear Regression. Degree of significance was denoted using asterisk, while groups that were not significant were denoted with hash tag (#).
3 Results
See Table 1 and Fig. 1.
Compound
Amount (mg/100 g)
Gallic acid and Ferulic acid (Phenolic)
17.86 ± 2.68
Quercetin (Flavonoids)
2.07 ± 0.07
Rutin (Flavonoids)
*ND
Caffeic acid (Phenolic)
ND
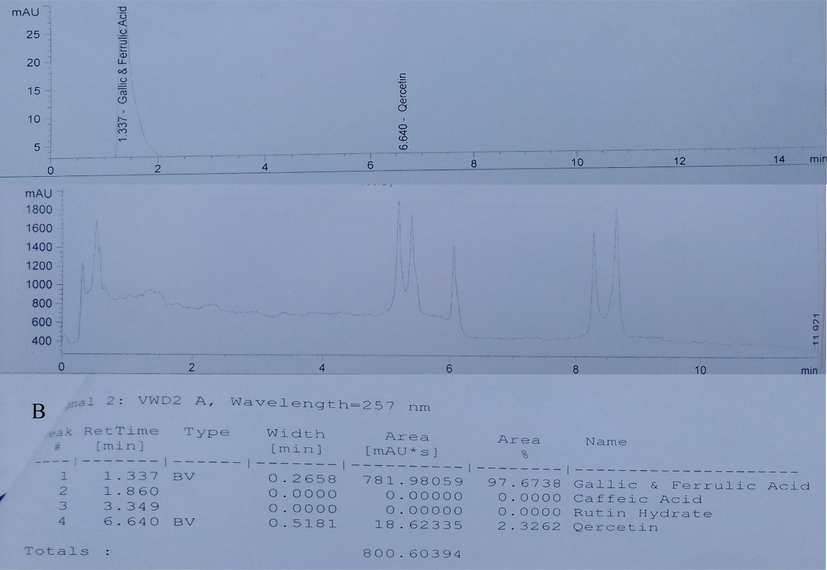
HPLC Chromatogram of Ethanol Leaf Extract of Ruspholia hypocrateriformis. (A) Chromatogram graph showing the peaks of the bioactive compounds identified in the leaf extract (B) Chromatogram data values. The result revealed the presence phenolic compounds (gallic acid and ferulic acid) and a flavonoid (quercetin). Caffeic acid and rutin were not detected in the extract. The area under the peak for gallic acid and ferulic acid was high, which indicates high concentration of these compounds in the leaf extract. Quercetin was present but in small concentration as seen in the area under the peak. The concentrations in mg/100 g are shown in Table 1.
3.1 Antioxidant in vitro studies
3.1.1 Free radical scavenging activity of ethanol leaf extract of Ruspholia hypocrateriformis
The antiradical scavenging activity of ethanol leaf extract of Ruspholia hypocrateriformis showed in Fig. 2 increased in a concentration dependent manner and slightly parallel compared to the standards (ascorbic acid and gallic acids). The difference in activities were however statistically significant amongst the three compounds. The IC50 for ascorbic acid is 27.5 ± 0.03 μg/ml, gallic acid is 37.65 ± 0.81 μg/ml and extract is 77.5 ± 0.10 μg/ml.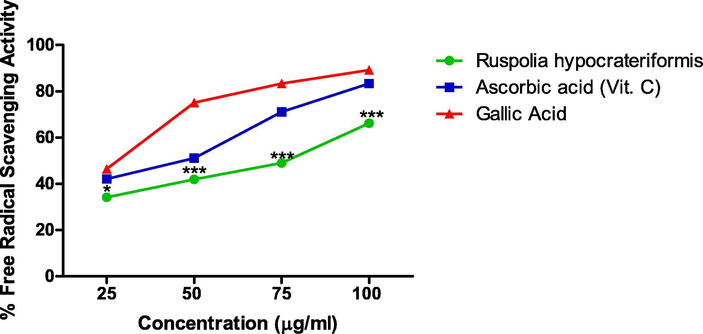
Free Radical scavenging Activity of Ethanol Leaf Extract of Ruspholia hypocrateriformis as compared with other standards (Ascorbic acid and Gallic acid).
3.1.2 Nitric oxide radical scavenging activity of ethanol leaf extract of Ruspholia hypocrateriformis
The result shown in Fig. 3 revealed a concentration dependent increase in the percentage inhibition of the NO scavenging activity by the extract in a near linear form. At the concentration of 100 μg/ml there was no statistical significant difference in the activity of the extract and the standards (ascorbic acid and gallic acid) albeit differences exist at the lower concentrations. The IC50 of the extract is 77.90 ± 0.04 μg/ml, ascorbic acid is 45.50 ± 0.05 μg/ml and gallic acid is 32.5 ± 0.01 μg/ml.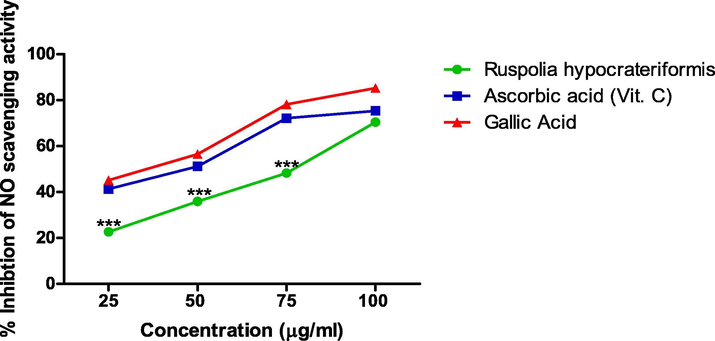
Nitric Oxide Scavenging Activity of Ethanol Leaf Extract of Ruspholia hypocrateriformis as compared with other standards (Ascorbic acid and Gallic acid).
3.1.3 Total reducing power of ethanol leaf extract of Ruspholia hypocrateriformis
The extract exhibited a rapid reducing impact as shown in Fig. 4, which was significantly higher compared to ascorbic acid albeit both began at the same absorbance level. The reducing impact of the extract was also concentration dependent. At 100 μg/ml which is the maximum concentration used, the reducing power of the extract was 0.524 ± 0.002, while gallic acid was 0.572 ± 0.013 and ascorbic acid was 0.196 ± 0.001.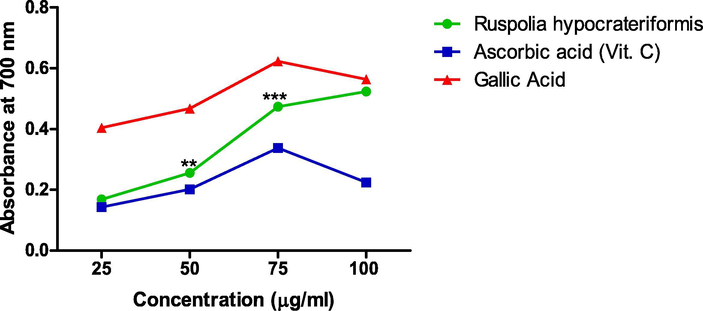
Total reducing power of ethanol leaf extract of Ruspholia hypocrateriformis as compared with other standards (Ascorbic acid and Gallic acid).
3.2 Antioxidant in vivo study
3.2.1 Effect of ethanol leaf extract of Ruspholia hypocrateriformis on malondialdehyde and glutathione levels in rats exposed to heavy metals
The result shown in Fig. 5(a and b) revealed that exposure of the rats to combined solution of lead nitrate and mercury chloride led to a significant elevation of MDA level in the hepatocytes of the rats while glutathione level was significantly reduced. Interestingly, treatment with various doses of ethanol leaf extract of Ruspholia hypocrateriformis caused a dose dependent decrease in the level of MDA and increase in the GSH level in the rats’ liver. Interestingly, 600 mg/kg dose of the leaf extract significantly reduced the abnormally elevated level of MDA in the liver of the rats to a level comparable to that of the normal control group. Extract dose of 600 mg/kg further elevated the GSH level in the hepatocyte to a level comparable to that in the normal control group.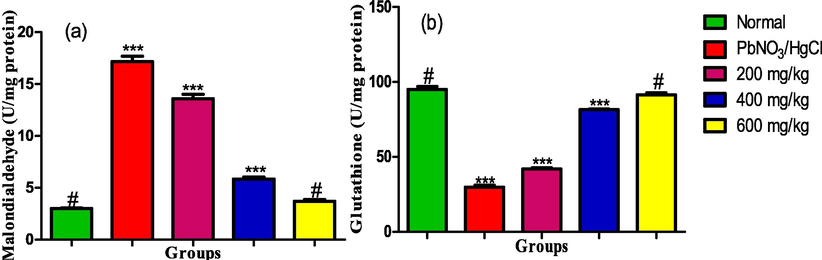
(a) MDA and (b) GSH levels in the liver homogenate of male albino rats exposed to heavy metals and treated with ethanol leaf extract of Ruspholia hypocrateriformis. Each group was compared with the normal control group and statistical significance levels indicated with the number of asterisk. Single asterisk indicates high level of significance while double asterisks indicate higher level of significance and triple asterisks indicate highest level of significance.
3.2.2 Effect of ethanol leaf extract Ruspholia hypocrateriformis on catalase and superoxide dismutase activities in rats exposed to heavy metals
The activities of catalase and superoxide dismutase shown in Fig. 6(a and b) decreased with the exposure of the rats to a solution of lead nitrate and mercury chloride. Treatment with the ethanol leaf extract of the Ruspholia hypocrateriformis led to dose dependent elevation in the activities of the enzymes. The elevation peaked at the maximum dose of 600 mg/kg, whose effect was also comparable with the catalase activity in the normal control rats.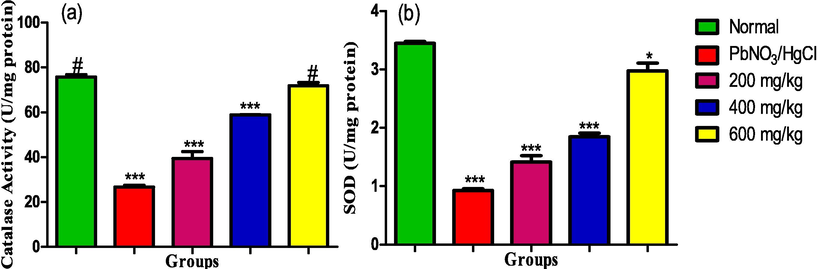
(a) Catalase (b) Superoxide dismutase activities in the liver homogenate of male albino rats exposed to heavy metals and treated with ethanol leaf extract of Ruspholia hypocrateriformis. Each group was compared with the normal control group and statistical significance levels indicated with the number of asterisk. Single asterisk indicates high level of significance while double asterisks indicate higher level of significance and triple asterisks indicate highest level of significance.
3.3 Liver function study
3.3.1 Effect of ethanol leaf extract Ruspholia hypocrateriformis on aspartate aminotransferase and superoxide Alkaline phosphatase activities in rats exposed to heavy metals
The serum activities of aspartate aminotransferase and alkaline aminotransferase showed in Fig. 7(a and b) was significantly elevated in the serum of rats exposed to the heavy metals. Conversely, all the administered doses of the leaf extract significantly decreased the serum activities of the enzymes and was comparable to the activities in normal rats.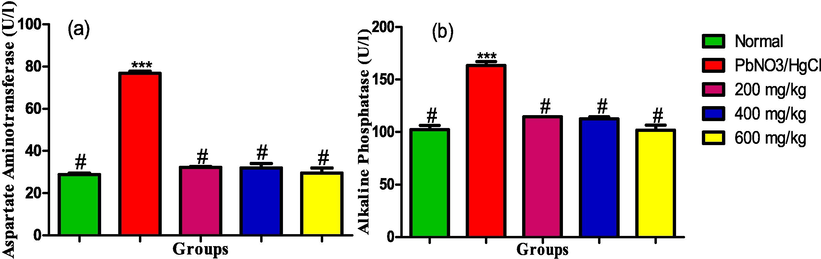
(a) Aspartate aminotransferase (b) Alkaline aminotransferase activities in the liver homogenate of male albino rats exposed to heavy metals and treated with ethanol leaf extract of Ruspholia hypocrateriformis. Each group was compared with the normal control group and statistical significance levels indicated with the number of asterisk. Single asterisk indicates high level of significance while double asterisks indicate higher level of significance and triple asterisks indicate highest level of significance.
3.3.2 Effect of ethanol leaf extract Ruspholia hypocrateriformis on alanine aminotransferase activity and level of total bilirubin in rats exposed to heavy metals
The serum activity of alanine aminotransferase and total bilirubin level showed in Fig. 8(a and b) significantly increased in the rats exposed to the heavy metals. However, administration of the leaf extract led to dose dependent reductions in the activity and level of the parameters respectively with 600 mg/kg effectively reducing the activity and level such that it was comparable with the rats in the normal control group.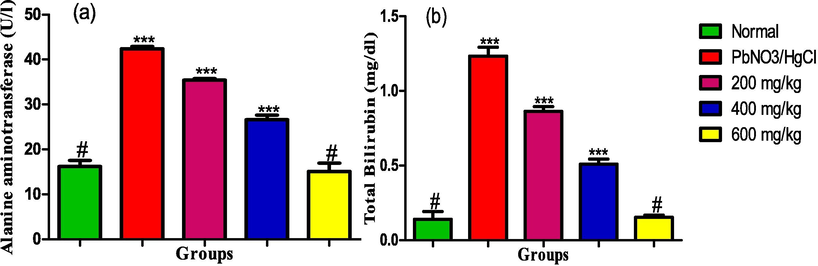
(a) Alanine aminotransferase activity (b) Total Bilirubin level in the liver homogenate of male albino rats exposed to heavy metals and treated with ethanol leaf extract of Ruspholia hypocrateriformis. Each group was compared with the normal control group and statistical significance levels indicated with the number of asterisk. Single asterisk indicates high level of significance while double asterisks indicate higher level of significance and triple asterisks indicate highest level of significance.
3.3.3 Effect of ethanol leaf extract Ruspholia hypocrateriformis on the levels of total protein and albumin in rats exposed to heavy metals
The serum levels of total protein and albumin were significantly decreased in the rats exposed to the heavy metals. However, administration of the extract significantly increased the levels of the biomarkers in dose dependent pattern. The maximum dose of 600 mg/kg elevated the serum levels of the biomarkers to the same levels in the serum of the rats in the normal control group (see Fig. 9).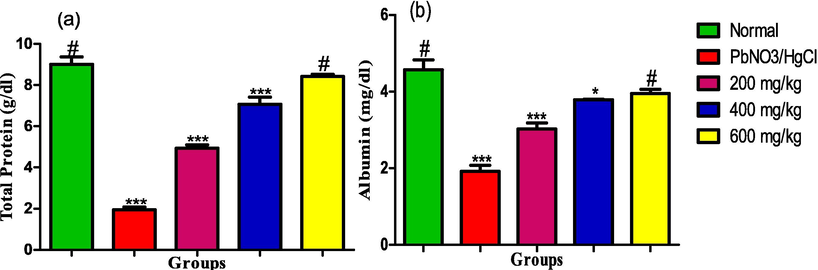
(a) Total Protein (b) Albumin levels in the liver homogenate of male albino rats exposed to heavy metals and treated with ethanol leaf extract of Ruspholia hypocrateriformis. Each group was compared with the normal control group and statistical significance levels indicated with the number of asterisk. Single asterisk indicates high level of significance while double asterisks indicate higher level of significance and triple asterisks indicate highest level of significance.
4 Discussion
The present study investigated the antioxidant potentials of ethanol leaf extract of Ruspholia hypocrateriformis against hepatic oxidative damage due to exposure to heavy metals. We first carried out HPLC study on the leaf extract to identify and quantify the various bioactive components in the leaf extract. Bioactive components in plant products are responsible for various medicinal and therapeutic potentials elicited by these plant products against disease conditions (Denaro et al., 2020; Mickymaray, 2019; Dahibhate et al., 2019). The presence of gallic acid, ferulic acid and quercetin in the leaf extract of Ruspholia hypocrateriformis (Fig. 1) depict that the leaf extract may have wider medicinal and therapeutic potentials when fully exploited. Studies have shown that gallic acid has high antioxidant, anti-inflammatory and anti-microbial properties respectively (BenSaad et al., 2017; Aytac et al., 2016). High concentration of gallic acid and ferulic acid in the extract would enhance the biological activities of the extract against several disease conditions. Quercetin is a flavonoid compound, which also has enormous anti-oxidant potential even at a little amount as has been shown by other studies (Kanimozhi et al., 2017; Lin and Zhou, 2018). Leaves of Ruspholia hypocrateriformis may serve as good source of gallic acid and quercetin for industrial uses and pharmaceutical preparations.
To verify whether the presence of these compounds (gallic acid and quercetin) in the ethanol leaf extract of Ruspholia hypocrateriformis could elicit antioxidant effect, we conducted an in vitro study using well known antioxidant assays (DPPH, FRAP and NO). DPPH (1,1-diphenyl-2-picryl hydrazyl) is an antioxidant assay method that works through the reduction of DPPH, a stable free radical leading to decolouration of the solution. We observed an increase in decolouration even at low concentration of the ethanol leaf extract of Ruspholia hypocrateriformis, indicating effectiveness of the antioxidant activity of the leaf extract against the free radicals generated in the assay. This further yielded an IC50 of 77.5 ± 0.10 μg/ml (Fig. 2), which is quite remarkable and showed that the extract at 77.5 ± 0.10 μg/ml was able to mop up or scavenge 50% of the free radicals generated during the reaction of the DPPH antioxidant assay system. This is lower than the IC50 of several plant extracts such as Alstonia Angustifolia (Rahim et al., 2018), Piper retrofractum (Jadid et al., 2017) and coumarin compounds (Patel and Patel, 2011) reported to have antioxidant potentials through DPPH assay method.
Additionally, the results obtained for Ferric Reducing Antioxidant Potential (FRAP) and Nitric Oxide assay were consistent with the result of DPPH assay for the extract. In FRAP assay, we reported a progressive dose dependent increase in absorbance due to the antioxidant activity of the extract, which is comparable to the absorbance of pure gallic acid and higher than ascorbic acid used as standards in the study. The result shown in Fig. 3 strongly suggests that ethanol leaf extract of Ruspholia hypocrateriformis possesses antioxidant properties, which reacted with the free radicals in the assay, stabilized and further terminated the free radical’s chain reactions. Albeit, nitric oxide has numerous beneficial effects in the physiological system, however, its significant contribution to oxidative damage has also been reported (Förstermann et al., 2017). We reported a concentration dependent inhibition of the scavenging activity of nitric oxide in the NO assay system by ethanol leaf extract of Ruspholia hypocrateriformis. Interestingly, the IC50 of ethanol leaf extract of Ruspholia hypocrateriformis was 77.90 ± 0.04 μg/ml, suggesting that at the concentration of 77.90 ± 0.04 μg/ml, the extract inhibited 50% of NO generated in the NO assay system. This was comparable to values obtained for the pure compounds used as standards vis-à-vis ascorbic acid (45.50 ± 0.05 μg/ml) and gallic acid (32.5 ± 0.01 μg/ml). Markedly, this low IC50 for NO and DPPH and increase in absorbance for the FRAP assay are strong indications that extract of Ruspholia hypocrateriformis possesses strong antioxidant potentials. We therefore went further to verify this antioxidant potential of Ruspholia hypocrateriformis through in vivo studies in animal model.
To create an in vivo oxidative stress in the rats, we exposed the rats to a combined solution of lead nitrate and mercury chlorides, which are heavy metal compounds. Humans are also frequently exposed to several lethal doses of heavy metals in the environment through various sources (Assi et al., 2016; Tchounwou et al., 2012). As one of their major mechanisms of toxicity to humans, animals and even plants, heavy metals are known to cause oxidative damage in the physiological system and in various organ systems of the body especially the liver (Elblehi et al., 2019; Cariccio et al., 2019). Upon entry into the physiological system, heavy metals accumulate preferentially in the liver and kidney and interfere with the physiological balance between pro-oxidant elements and antioxidant elements in the body. This critical interference causes an abnormal increase in the levels of pro-oxidant elements such as MDA and NO, and subsequent reduction in the levels of endogenous antioxidants such as SOD and GSH. This creates a redox imbalance in the physiological system leading to oxidative damage of various relevant organs in the body particularly the liver (hepatotoxicity) and kidney (nephrotoxicity) (Sharma et al., 2014; Aja et al., 2020; Valko et al., 2005). More so, accumulation of lead and mercury in the body trigger some cascade signalling systems leading to increase in the generation of free radicals and reactive oxygen species (ROS), which are mostly unpaired electron species that attack relevant biomolecules in their search for electrons. Heavy metals detoxification processes in the kupffer cells of the liver also lead to production of free radicals, which further enhances cellular redox imbalance and destroys the structural framework of the hepatocyte membrane (Juranek et al., 2013; Kim et al., 2019). Albeit, there are beneficial effects of these ROS particularly as signalling molecules, their hazard effects mostly occur when they are produced in large amount as with the case of exposure to heavy metals (Elblehi et al., 2019; Cariccio et al., 2019). Interestingly, our results revealed a state of redox imbalance in the animals exposed to heavy metals in this study, thus confirming this established scientific knowledge of mechanism of heavy metal toxicity. More interestingly, administration of various doses of extract of Ruspholia hypocrateriformis, which we previously established to be rich in some bioactive compounds, normalized this state of redox imbalance due to excess production of ROS in heavy metal exposed rats and protected the structural integrity of the hepatocytes. Albeit, there are many factors such as inflammatory mediators, and transcription factors that influence redox imbalance in the cellular system, however, in this present study, we established this using various in vivo antioxidant biomarkers (MDA, GSH, CAT and SOD) and hepatic dysfunction markers (ALT, AST, ALP, bilirubin, albumin and total protein). Our next study plan in this area of research would focus on other factors of cellular redox homeostasis with particular interest in inflammatory mediators and transcription factors.
Lipid peroxidation is one of the major consequences of excessive generation of ROS in the physiological system leading to the formation of malondialdehyde (MDA) (Cui et al., 2018; Misra et al., 2017). MDA is therefore a strong indicator of increase in lipid oxidation due to increased levels of free radicals in the form of ROS and subsequent oxidative stress in the body. It is a significant oxidative stress marker in the body and has been implicated in the aetiology of several diseases (Ali et al., 2018; Verma et al., 2019; Cui et al., 2018). Perhaps exposure of the rats to lead and mercury led to a significant increase in ROS in the physiological system of the rats, which subsequently increased the level of MDA in the exposed rats when compared with the normal rats group. This further caused the redox imbalance evidenced in the exposed animals and subsequent oxidative stress and damage to the liver as seen in the elevated serum activities of ALT, AST, ALP and total bilirubin level in addition to decrease in serum levels of albumin and total protein. Conversely, the bioactive antioxidant compounds present in the extract may have scavenged these ROS, restored the redox imbalance leading to a significant reduction in MDA level and normalization of markers of hepatic dysfunctions seen in the treated rats particularly at 600 mg/kg dose. Additionally, the extract may have protective and healing effects on the structural integrity of the hepatocytes perhaps through opposing the effect of oxidative damage caused by the heavy metals on the lipid components of the hepatocyte membrane through the actions of ROS. Our results also demonstrated a similar dose dependent effects for reduced glutathione (GSH), which is one of the most abundant important non-enzymatic antioxidant that plays major roles in the detoxification of free radicals in the physiological redox system (Winterbourn, 2019; Berndt and Lillig, 2017). In our study, GSH level in the hepatocyte was significantly reduced due to exposure to lead and mercury compared to the normal rats, which is consistent with the finding of others (Fan et al., 2020; Gogoi et al., 2019; Bozdağ and Eraslan, 2020; Zhao et al., 2020). Lead and mercury may have bound to the sulfhydryl subunit of glutathione, formed complexes with the biomolecule and subsequently inhibited its antioxidant actions (Bottari et al., 2020). However, extract administration significantly increased GSH level particularly at the dose of 600 mg/kg. Bioactive compounds present in the leaf extract may have increased the synthesis of GSH, disrupted the deleterious complexes formed between the heavy metals and GSH subunits and subsequently displaced the heavy metals from the sulfhydryl unit of GSH, hence enhancing its actions.
More so, catalase and superoxide dismutase, which are the two endogenous enzymatic antioxidants known to scavenge free radicals in the physiological milieu showed similar trend with GSH. Catalase and superoxide dismutase are the first-line antioxidant defense system that help in detoxifying the deleterious effects of hydrogen peroxide and superoxide produced endogenously in the body (Ighodaro and Akinloye, 2018; Fındıklı et al., 2018). Our study revealed that exposure of the rats to lead and mercury significantly reduced the activities of these antioxidant enzymes, which perhaps aggravated the distortion of redox imbalance in the hepatocytes of the exposed rats. Moreover, extract administration ameliorated these negative effects in a concentration dependent pattern. The markedly ameliorative effect of the extract demonstrated in this present study against lead and mercury toxicity may be through its scavenging action against various free radicals generated in vivo due to lead and mercury detoxification process in the liver. Perhaps, the bioactive compounds present in the extract through their antioxidant potentials already established by others, quenched free radicals generated in the kupffer cells and further improve the efficiency of the detoxification system of the liver. Furthermore, we reported that 600 mg/kg, which is the highest dose of the extract used in the in vivo study, consistently demonstrated maximum ameliorative effects against all the dysregulated oxidative stress biomarkers investigated in this study. Albeit, this is a high dose, which may be problematic in direct translation to humans, however, in line with US Food and Drug Administration regulation, it has been suggested that animal dose should not be directly extrapolated to a human equivalent dose (HED) by simple conversion based on body weights (Reagan-Shaw et al., 2008; Nair and Jacob, 2016; FDA, 2002). This is to avoid high attrition rate in drug development and translation. Perhaps using the body surface area (BSA) normalization method and allometric approach, which are the most appropriate methods, a dose formulation that would be lower and acceptable to humans upon translation may be developed.
5 Conclusion
In this study, we reported for the first time that ethanol leaf extract of Ruspholia hypocrateriformis contains relevant bioactive compounds, which perhaps were responsible for the high antioxidant potentials exhibited by the leaf extract both in the in vitro and in vivo studies respectively. We also demonstrated that various doses of the extract particularly 600 mg/kg ameliorated hepatic redox imbalance and protected the hepatocytes from oxidative damage due to heavy metal exposure in the rats. Perhaps, our extract may be endowed with unique combination of natural bioactive compounds, which possesses potent medicinal values especially in its native complex composition. Further molecular and mechanistic based studies on the extract would enhance the possibility of its translation to treatment of human diseases caused by heavy metal exposure.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ameliorative effect of curcumin against lead acetate–induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environ. Sci. Pollut. Res.. 2020;27:10950-10965.
- [CrossRef] [Google Scholar]
- Traditional medicine and pharmacopoeia: Contribution to ethnobotanical and floristic studies in Cameroon. Porto-Novo: Centre National de Production de Manuels Scolaires; 1996. p. :77. Org of Afric Unity Scient Technical and Research Commission
- Production of plant secondary metabolites: a historical perspective. J. Plant. Meta. 2001;161:839-885.
- [Google Scholar]
- Evaluation of the antidiarrheal and antioxidant properties of Justicia hypocrateriformis. Pharm. Biol.. 2014;52:1128-1133.
- [CrossRef] [Google Scholar]
- Beneficial effects of quercetin on obesity and diabetes. Open Nutraceut. J.. 2011;4:189-198.
- [Google Scholar]
- Low level lead exposure and oxidative stress: Current opinions. Clin. Chim. Acta. 2007;383:57-64.
- [Google Scholar]
- Hesperidin protects against cadmium-induced pancreatitis by modulating insulin secretion, redox imbalance and iNOS/NF-ĸB signaling in rats. Life Sci.. 2020;259:118268.
- [CrossRef] [Google Scholar]
- Determination of MDA as oxidative stress marker and most common type of blood group in asthmatic patients before and after treatment with montelukast drug. Tikrit J. Pure Sci.. 2018;20:84-87.
- [Google Scholar]
- The detrimental effects of lead on human and animal health. Veterin. World. 2016;9:660-671.
- [Google Scholar]
- Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng., C. 2016;63:231-239.
- [Google Scholar]
- Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med.. 2017;17:47.
- [CrossRef] [Google Scholar]
- Mercury toxicity and treatment: a review of the literature. J. Environ. Public Health. 2012;2012:1-10.
- [CrossRef] [Google Scholar]
- Behavior of glutathione as ligand of lead (II) Chemosphere. 2020;246:125718.
- [CrossRef] [Google Scholar]
- The effect of diosmin against lead exposure in rats. Naunyn-Schmiedeberg's Arch. Pharmacol.. 2020;393:639-649.
- [CrossRef] [Google Scholar]
- Mercury involvement in neuronal damage and in neurodegenerative diseases. Biol. Trace Elem. Res.. 2019;187:341-356.
- [CrossRef] [Google Scholar]
- Mercury toxicity and neurodegenerative effects. In: Whitacre D., ed. Reviews of Environmental Contamination and Toxicology. Reviews of Environmental Contamination and Toxicology (Continuation of Residue Reviews). Vol 229. Cham: Springer; 2014.
- [Google Scholar]
- The new healing herbs: The classic guide to nature's best medicines featuring the top 100 time-tested herbs. J. Med. Herbs.. 2001;15:59-68.
- [Google Scholar]
- Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers. Rockville, Maryland, USA: U.S Food and Drug Administration; 2002.
- Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J. Thoracic Dis.. 2018;10:3088-3097.
- [CrossRef] [Google Scholar]
- Mangrove plants as a source of bioactive compounds: A review. Natural Prod. J.. 2019;9:86-97.
- [CrossRef] [Google Scholar]
- Antiviral activity of plants and their isolated bioactive compounds: An update. Phytother. Res.. 2020;34:742-768.
- [CrossRef] [Google Scholar]
- Duncan, A.E. 2020. The dangerous couple: Illegal mining and water pollution—A case study in Fena river in the Ashanti Region of Ghana. J. Chem. 2020, Article ID 2378560, 9 pages. https://doi.org/10.1155/2020/2378560.
- L-α-Phosphatidylcholine attenuates mercury-induced hepato-renal damage through suppressing oxidative stress and inflammation. Environ. Sci. Pollut. Res.. 2019;26:9333-9342.
- [CrossRef] [Google Scholar]
- Lead-induced oxidative damage in rats/mice: A meta-analysis. J. Trace Elem. Med Biol.. 2020;58:1264.
- [CrossRef] [Google Scholar]
- The diagnostic value of malondialdehyde, superoxide dismutase and catalase activity in drug naïve, first episode, non-smoker generalized anxiety disorder patients. Clin. Psychopharmacol. Neurosci.: Off. Sci. J. Korean College Neuropsychopharmacol.. 2018;16:88-94.
- [CrossRef] [Google Scholar]
- Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res.. 2017;120:713-735.
- [Google Scholar]
- The effects of heavy metals on human metabolism. Toxicol. Mech. Methods. 2020;30:167-176.
- [CrossRef] [Google Scholar]
- Circulatory heavy metals (cadmium, lead, mercury, and chromium) inversely correlate with plasma GST activity and GSH level in COPD patients and impair NOX4/Nrf2/GCLC/GST signaling pathway in cultured monocytes. Toxicol. In Vitro. 2019;54:269-279.
- [CrossRef] [Google Scholar]
- The hepatoprotective effect of gallic acid on mercuric chloride-induced liver damage in rats. Jundishapur J. Nat. Pharm. Prod.. 2017;12:e12345.
- [Google Scholar]
- Justicialosides A and B, two new flavone glycosides from the leaves of Ruspolia hypocrateriformis (Vahl) Milne-Redh. (Acanthaceae) Phytochem. Lett.. 2019;31:101-103.
- [CrossRef] [Google Scholar]
- Impact of chronic lead exposure on selected biological markers. Indian J. Clin. Biochem.. 2012;27:83-89.
- [Google Scholar]
- Polyphenolic contents and antioxidant potential of stem bark extracts from Jatropha curcas (Linn) Int. J. Mol. Sci.. 2011;12:2958-2971.
- [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med.. 2018;54:287-293.
- [CrossRef] [Google Scholar]
- Effects of subchronic exposure via drinking water to a mixture of eight water contaminating metals: A biochemical and histopathological study in male rats. Arch. Environ. Con. Toxicol.. 2007;53:667-677.
- [Google Scholar]
- Antioxidant activities of different solvent extracts of Piper retrofractum Vahl. using DPPH assay. AIP Conf. Proc.. 2017;1854:1.
- [Google Scholar]
- Toxicity, mechanism and health effects of some heavy metals. Interdisciplin. Toxicol.. 2014;7:60-72.
- [Google Scholar]
- Colorimetric method of determination of bilirubin. Search results. BiochemischeZeitschrift.. 1938;297:81-82.
- [Google Scholar]
- Biological importance of reactive oxygen species in relation to difficulties of treating pathologies involving oxidative stress by exogenous antioxidants. Food Chem. Toxicol.. 2013;61:240-247.
- [Google Scholar]
- Influence of the flavonoid, quercetin on antioxidant status, lipid peroxidation and histopathological changes in hyperammonemic rats. Ind. J. Clin. Biochem.. 2017;32:275-284.
- [CrossRef] [Google Scholar]
- Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med Biol.. 2019;54:226-231.
- [CrossRef] [Google Scholar]
- Health risk assessment and levels of toxic metals in fishes (Oreochromis noliticus and Clarias anguillaris) from Ankobrah and Pra basins: Impact of illegal mining activities on food safety. Toxicol. Rep.. 2020;7:360-369.
- [CrossRef] [Google Scholar]
- Role of quercetin in the physicochemical properties, antioxidant and antiglycation activities of bread. J. Funct. Foods. 2018;40:299-306.
- [Google Scholar]
- Antioxidant and antibacterial constituents from Morusnigra. Afr. J. Pharm. Pharmacol.. 2011;5:751-754.
- [Google Scholar]
- Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics. 2019;8:257.
- [Google Scholar]
- Plant extracts and isolated compounds reduce parameters of oxidative stress induced by heavy metals: an up-to-date review on animal studies. Curr. Pharm. Des.. 2020;26
- [CrossRef] [Google Scholar]
- Oxidative stress markers in vitamin B12 deficiency. Mol. Neurobiol.. 2017;54:1278-1284.
- [Google Scholar]
- Morais, S., Costa, F.G., Pereira, M.L. 2012. Heavy metals and human health. In: Oosthuizen, J. (Ed.), Environmental Health – Emerging Issues and Practice. pp. 227–246.
- Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett.. 2010;8:199-216.
- [Google Scholar]
- A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm.. 2016;7:27-31.
- [CrossRef] [Google Scholar]
- Intake of heated leaf extract of Coriandrum sativum contributes to resistance to oxidative stress via decreases in heavy metal concentrations in the kidney. Plant Foods Hum. Nutr.. 2019;74:204-209.
- [CrossRef] [Google Scholar]
- Effect of ethanol extract of Ruspolia hypocrateriformis leaf on haematological parameters in lead poisoned albino rats. World J. Med. Sci.. 2016;13:225-235.
- [CrossRef] [Google Scholar]
- Biochemical assessment of ethanol leaf extract of Cnidoscolus aconitifolius on liver intergrity of albino rat treated with lead. Global J. Pharmacol.. 2016;10:108-113.
- [CrossRef] [Google Scholar]
- Investigations of phytochemical and nutritional composition of Ruspolia Hypocrateriformis leaf. J. Appl. Sci.. 2017;2:70-81.
- [Google Scholar]
- Effect of aqueous extract of Leonotis (L) leaves in male Wistar rats. Hum. Exp. Toxicol.. 2010;29:377-384.
- [Google Scholar]
- Human exposure and health effects of inorganic and elemental mercury. J. Prevent. Med. Public Health. 2012;45:344-352.
- [Google Scholar]
- In vitro antioxidant activity of coumarin compounds by DPPH, Super oxide and nitric oxide free radical scavenging methods. J. Adv. Pharm. Educ. Res.. 2011;1:52-68.
- [Google Scholar]
- Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int.. 2011;2011:457327.
- [Google Scholar]
- Antioxidant assay of alstonia angustifolia ethanolic leaf extract. J. Adv. Res. Fluid Mech. Therm. Sci.. 2018;42:80-86.
- [Google Scholar]
- Dose translation from animal to human studies revisited. FASEB J.. 2008;22:659-661.
- [CrossRef] [Google Scholar]
- Colorimetric methods for the determination of serum transaminases. Am. J. Clin. Pathol.. 1957;28:56-61.
- [Google Scholar]
- Environmental mercury and its toxic effects. J. Prevent. Med. Public Health. 2014;47:74-83.
- [Google Scholar]
- Renal health and the environment: heavy metal nephrotoxicity. Nefrologia. 2012;32:279-286.
- [CrossRef] [Google Scholar]
- Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res. Int.. 2014;2014:26.
- [CrossRef] [Google Scholar]
- Socioeconomic dimensions of mercury pollution abatement: Engaging artisanal mining communities in Sub-Saharan Africa. Ecol. Econ.. 2009;68:3072-3083.
- [CrossRef] [Google Scholar]
- An improved spectrophotometric assay of superoxide dismutase based on epinephrine autoxidation. Anal. Biochem.. 1978;90:81-89.
- [Google Scholar]
- Tchounwou, P.B., Yedjou, C.G., Patlolla, A.K., Sutton, D.J., 2012. Heavy metal toxicity and the environment. In: Luch A. (Eds.), Molecular, Clinical and Environmental Toxicology. Experientia Supplementum. 101. Springer, Basel
- Clinical Guide to Laboratory Tests (ELISA) (third ed.). Philadelphia: W.B. Saunders Co; 1995. p. :22-23.
- Oxidative stress and biomarker of TNF-α, MDA and FRAP in hypertension. J. Med. Life. 2019;12:253-259.
- [CrossRef] [Google Scholar]
- Ferric reducing anti-oxidant power assay in plant extract. Bangl. J. Pharmacol.. 2016;11:570-572.
- [Google Scholar]
- A new, simple and rapid HPLC method for determination of chlortetracycline in pig solid manure. Ital. J. Anim. Sci.. 2010;9:e37.
- [Google Scholar]
- Vitamin C from standardized water Spinach extract on inhibition of cytotoxicity and oxidative stress induced by heavy metals in HepG2 cells. J. Korean Soc. Appl. Biol. Chem.. 2014;57:161-166.
- [CrossRef] [Google Scholar]
- Protective effect of chlorophyllin and lycopene from water spinach extract on cytotoxicity and oxidative stress induced by heavy metals in human hepatoma cells. J. Toxicol. Environ. Health Part A. 2013;76:1307-1315.
- [CrossRef] [Google Scholar]
- Exposed to mercury-induced oxidative stress, changes of intestinal microflora, and association between them in mice. Biol. Trace Elem. Res.. 2020;2020
- [CrossRef] [Google Scholar]







