Translate this page into:
Evaluation of CD93hi macrophage on atherosclerosis through dynamic cells adoptive transfer
⁎Corresponding authors. yeminghan100@163.com (Yeming Han), ghhou@sdu.edu.cn (Guihua Hou), rggaofeng@sdu.edu.cn (Feng Gao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Macrophage (MΦ) with different surface marker exhibits diversity effect on the development of atherosclerosis (AS). Recently CD93high MΦ was reported tightly related with inflammatory diseases, however, its effect on the development of AS remains unclear. Here, we adoptive transferred with prepared CD93 high and low expressed (CD93hi, CD93lo) MΦ in establishing ApoE-/- mice AS model at the week of 1, 4, 8, 12, 16, respectively. At w12 and 20, pathology change and the expression of CD93, F4/80, CD31, ApoE in related vessels were detected. Pro-inflammatory cytokines and related signal pathway were also analyzed. Finally, CD93 targeting radionuclide imaging in AS model was performed with prepared 125I-anti-CD93 mAb injection. The results showed that all pathological changes in diseased vessels showed much severe in w20 (the time that AS model was established successfully) than w12. In contrast to group with CD93lo MΦ adoptive transferred, much serious inflammatory infiltration, increased pro-inflammatory factors and elevated CD93, F4/80, CD31 expression in clamped carotid artery were detected, and also higher Tumor necrosis factor-α (TNF-α) and Interleukin-1β (IL-1β) in serum were measured in ApoE-/- model with CD93hi MΦ adoptive transferred in w20. Furthermore, obviously high expressed p-STAT3 and p-NF-κB were detected in CD93hi MΦ, compared with CD93lo MΦ. Ex vivo phosphor-autoradiography showed higher radioactivity accumulation in diseased vessels in ApoE-/- model with CD93hi MΦ adoptive transferred after 125I-anti-CD93 mAb injection, compared with CD93lo MΦ adopted group in w20. In conclusion, CD93hi MΦ subset promoted the development of AS and aggravated the disease severity mainly through p-STAT3/p-NF-κB pathways. CD93 on MΦ may be as a novel target for AS modulation.
Keywords
CD93
Macrophage
Adoptive transfer
Atherosclerosis
Radionuclide
Molecular imaging
1 Introduction
Atherosclerosis (AS) is the leading cause of cardiovascular disease, resulting in ischemic heart disease, strokes, aneurysms and life-threatening plaque rupture (Libby et al., 2019). Inflammation initiated lipid entering into the arterial wall, causes thrombosis (Kyaw et al., 2013), which has been recognized as a major risk factor in progression and rupture of atherosclerotic plaque (Grebe et al., 2018; Wolf and Ley, 2019). The early lesion of AS is mainly dependent upon continuously monocyte influx into the vessel wall, and macrophage (MΦ) is essential in both lipid handing and inflammation (Patel et al., 2017), which exists in all stages of AS progression and responsible for many kinds of pro-inflammatory cytokines secretion (Apostolakis et al., 2008). It is well known that inflammation could be induced by a variety of pro-inflammatory factors, such as Interleukin-1 (IL-1), Tumor necrosis factor-α (TNF-α), Matrix metalloproteinases (MMPs) and others (Qiao et al., 2021; Zhu et al., 2018). Inflammatory signaling pathways reported include Toll-Like Receptor 4 (TLR4) signaling, Janus Kinase-Signal Transducer and Activator of Transcription Signaling (JAK-STAT) (Zhu et al., 2018) and NF-κB pathway. Anti-inflammation therapy has been proved favor to prognosis of AS (Tabas and Glass, 2013).
CD93, a transmembrane protein, is mainly expressed on the surface of MΦ and epithelial cells. Recently, CD93 was reported to promote phagocytosis of apoptotic cells within atherosclerotic plaques, aggregate the progression of plaque (Mälarstig et al., 2011). And more importantly, the epidermal growth factor domains of CD93 are positive related with angiogenesis (Kao et al., 2012), the new capillary vessels formation has been proved to play a pivotal role in atherosclerotic plaques formation (Jaipersad et al., 2014). So, whether and how this MΦ with CD93 high expression (CD93hi MΦ) aggregates the progression of AS remain unclear. Here, we explored the impact of CD93hi MΦ on the development of AS and underlying mechanisms through adoptive transferred CD93hi and CD93lo MΦ during the modeling process of AS.
2 Materials and methods
2.1 Establishment of AS model with ApoE-/- mice
This study was approved by the institutional Animal Care and Use Committee of Shandong University. 8-week-old ApoE-/- mice (apolipoprotein E knockout, derived from C57BL/6 mice, (Beijing Vital River Laboratory Animal Technology Co., Ltd.)) were used to establish AS models through high-fat diet (10% fat, 5% sucrose, 3% cholesterol, 0.5% bile salt and 0.2% propylthiouracil); inflammatory stimulation (ovalbumin, 2.5 mg/kg, Solarbio; bovine serum albumin, 40 mg/kg, Solarbio); calcium overload (Vitamin D3, 10w IU/kg, Solarbio) (Zhao et al., 2020) and mechanical damage (left carotid artery was clamped). C57BL/6 mice were used as control. Atherosclerotic lesions were assessed by H&E and Oil Red O staining along with serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL) and low-density lipoprotein cholesterol (LDL) measurements, respectively. The AI, which is the ratio of TC level to HDL level (AI = TC/HDL), has been suggested to be an important marker in the identification of AS risk, was calculated according to reference (Peters et al., 2010).
2.2 CD93hi/lo MΦ adoptive transfer
MΦ from naive C57BL/6 mice were collected from peritoneal cavity after injection with 6% potato starch for 72 h. CD93hi MΦ was prepared with 0.1 μg/ml of lipopolysaccharide (LPS, Sigma) stimulation for 12 h in complete RPMI 1640 medium at 37 °C and 5% CO2, while CD93lo MΦ without LPS stimulation. CD93hi and CD93lo MΦ were evaluated through Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Western blot as the same way to our previous study (Su et al., 2022). At week of 1, 4, 8, 12 and 16 during model establishment, fresh prepared CD93hi and CD93lo MΦ (5 × 106/each mouse) were adoptive transferred to ApoE-/- model mice through intraperitoneal injection, respectively. Aortic arch and carotid arteries were isolated 72 h after MΦ adoptive transferred at checking time points.
2.3 Immunohistochemical staining
CD93, F4/80, ApoE and CD31 immunohistochemical (IHC) staining were performed ex vivo for left carotid artery and aorta, IHC staining of STAT3 was performed for aorta (SP-9002 Histostain™ Plus kits (ZSGB-BIO)), stained by rabbit-anti-mouse mAb (1:500 dilution, Servicebio, China), and Horseradish Peroxidase (HRP) conjugated goat anti-mouse secondary antibody (1:200 dilution, Servicebio, China). Finally, diaminobenzidine (DAB) was added to produce brown precipitate. Images were subsequently taken under microscope at 400 × magnifications and the IOD/Area (Optic Density/Area) of corresponding positive areas was analyzed by Image-Pro Plus software version 4.5.0.29 (Media Cybernetics).
2.4 ELISA
Culture supernatants of CD93hi and CD93lo MΦ were collected, and centrifugation at 300 × g for 10 min after 12 h stimulation with LPS (0.1 μg/ml),.Serum samples from AS model and control mice were obtained at 1000 × g. TNF-α and IL-1β were detected by Enzyme-linked immunosorbent assay (ELISA, MULTI SCIENCES), briefly, 100 μl standard and samples were added into each well, incubated at 37 ℃ for 2 h. Then washed, followed by the addition of HRP-labeled detected antibody and incubated for an additional 45 min. After 100 μl 3, 3′, 5, 5′-tetramethylbenzidine (TMB) was added into each well in a dark room, incubated for 20 min then stopped with adding 100 μl of stop solution. The optical density (OD) of each well was read at 450 nm in the Tecan Microplate Reader (Bio-Rad).
2.5 Protein microarray assay
Left carotid artery proteins from model mice were extracted with RIPA lysate (15 μl RIPA lysate/mg tissues) and protein concentration was measured using BCA kit. Protein microarrays (R&D) were closed with 2 ml array buffer on a shaker for 1 h. 250 μg protein samples and 15 μl detected antibody were added on the microarray overnight, then the corresponding washing liquid was used to wash the membrane three times (5 min/time) and added HRP labeled streptavidin for 30 min. Finally, the microarrays were placed on chemiluminescence instrument (Chemi Scope 6300) for color development.
2.6 Western blot
The RIPA reagent (Servicebio, China) was used to extract protein from cells. Briefly, 20 μg total proteins were run on SDS-PAGE gel (10%). The samples were transferred from the gel onto the PVDF membrane and then 5% defatted milk powder was used to seal the PVDF membrane for 2 h. The blots were incubated with primary antibodies (i) mouse anti-mouse CD93 antibody (1:200 dilution, Santa, America), (ii) rabbit anti-mouse β-Actin antibody (1:3000 dilution, Bioworld, America), (iii) rabbit anti-mouse p-STAT3 antibody (1:2000 dilution, CST, America) and (iv) mouse anti-mouse STAT3 antibody (1:1000 dilution, CST, America), (v) rabbit anti-mouse p-NF-κB antibody (1:2000 dilution, CST, America), (vi) rabbit anti-mouse NF-κB antibody (1:1000 dilution, CST, America) at 4 ℃ overnight. Later, the blots were incubated with appropriate HRP conjugated secondary antibodies (1:10000 dilution, Abways) at room temperature for an hour. ECL reagent (HONBIOTECH) was used for imaging the blots with a Tanon 4200 imaging system (Tanon Science and Technology Co., Ltd., Shanghai, China).
2.7 Preparation of 125I-anti-CD93 mAb and 125I-IgG
Iodogen method was used to prepare 125I-anti-CD93 mAb with 3 μg anti-CD93 mAb and 3.7 MBq Na125I (China Isotope & Radiation Corporation, Specific Activity 13.6 GBq/ml), and control 125I-IgG with 3 μg IgG and 3.7 MBq Na125I in the same way. The labeled tracer was purified by PD-10 (GE Healthcare), the labeling rate (the ratio of 125I-anti-CD93 mAb or 125I-IgG to total input Na125I) was calculated. The radiochemical purity (the ratio of 125I-anti-CD93 mAb or 125I-IgG to total radioactivity) was determined by paper chromatography with methanol/saline (v/v, 2:1) as the mobile phase. The stability of 125I-anti-CD93 mAb and control 125I-IgG in 0.9% saline and mouse serum was detected.
2.8 Phosphor-autoradiography
To block the thyroid gland uptake of radioiodine, 4 % sodium iodide (Meilunbio) was given in drinking water 24 h before radiotracers injection. Dynamic phosphor-autoradiography were performed at 24, 48 and 72 h after 125I-anti-CD93 mAb or 125I-IgG (0.37 MBq each mouse) injection and anesthetized with 0.6% pentobarbital sodium (0.01 ml/g), scanning with phosphorus screen imaging system (PerkinElmer) after 20 min lying on the super sensitive screen (TR, PerkinElmer), respectively. Ex vivo bilateral carotid artery and aorta phosphor-autoradiography were performed in the same way at 72 h after injection. DLU/mm2 (digital light units/mm2) was obtained through OptiQuant™ image analysis software 5.0 (PerkinElmer Life Sciences) for semi-quantity analysis.
2.9 Statistical analysis
All data were presented as mean ± standard deviation (SD). Statistically significant differences were conducted on Student′s t-test from at least three independent groups with statistical significance p value ≤ 0.05. The Pearson correlation analysis between protein expression in IHC and the radioactivity in aorta in CD93hi MΦ adoptive transferred group at w20 was calculated. Graph Pad Prism 9 software (GraphPad Software, Inc) was used.
3 Results
3.1 CD93hi MΦ adoptive transfer promoted the development of AS
To investigate whether CD93hi MΦ could promote the development of AS, we performed MΦ adoptive transfer in ApoE-/- mice from first week during model induction.
At w12, 72 h after the 4th time of CD93hi/lo MΦ adoptive transfer, artery was isolated to do pathology analysis. H&E staining showed that no AS plaque in related arteries, even in the clamped left carotid artery, neither CD93hi nor CD93lo MΦ adoptive transfer group. No intimal thickening, no apparently inflammatory cells infiltrating and no foam cells/cholesterol cracks beneath the intima (Fig. 1-A), which indicated 12 weeks modelling were too short to form AS plaque, even with repeated 4 times MΦ adoptive transfer. Lipid measurement showed no apparently difference between groups of CD93hi MΦ and CD93lo MΦ adoptive transferred, (TC, 8.89 ± 1.13 to 7.96 ± 0.79 mmol/L, p > 0.05; LDL, 6.25 ± 1.18 to 6.64 ± 1.26 mmol/L, p > 0.05), even no obviously difference in AS index (AI, TC/HDL, 4.76 to 4.64) (Supplementary Table 1).
H&E staining of related arteries of modeling mice CD93hi macrophages (MΦ) means CD93hi MΦ adoptive transferred in ApoE-/- mice. CD93lo MΦ means CD93lo MΦ adoptive transferred in ApoE-/- mice. Non-MΦ means no MΦ was adoptive transferred in ApoE-/- mice. Representative H&E staining images were under 400 ×. Black arrow points to foam cells, blue arrow points to cholesterol cracks. The data come from three independent experiments.
However, at w20, after the 5th time of CD93hi/lo MΦ adoptive transfer, the pathology analysis for isolated artery showed in Fig. 1-B. The left carotid artery was almost completely blocked by plaques (over 99%) and the aorta was to 91.4%. The intima was thickened with a large number of inflammatory cells infiltrating, foam cells and cholesterol cracks were detected in group of CD93hi MΦ adoptive transferred ApoE-/- mice. While, in contrast to group of CD93lo MΦ adoptive transfer, H&E staining only found disordered arrangement and a loss of endothelial cells in clamped left carotid artery and aorta, inflammatory cells and foam cells were also existed in subendothelium. In CD93hi MΦ adoptive transfer group, TC were 12.91 ± 1.90 and LDL were 10.54 ± 1.41 (mmol/L), AI was 8.12, respectively, obviously higher than those in CD93lo MΦ group (TC, 10.32 ± 1.49; LDL, 6.58 ± 1.97; AI, 7.37), the higher AI scores, the much severer the AS.
Compared with w12, a considerable increasing of TC and LDL was observed in CD93hi MΦ adoptive transferred group at w20 (p < 0.05; p < 0.05). At w12, only slight lipid droplets were visible both in CD93hi and CD93lo MΦ adoptive transfer ApoE-/- mice in Oil Red O staining, Per Area (Ratio of red area of object to total area of artery) was 2.03 % in CD93hi MΦ adoptive transfer group and 1.40% in CD93lo MΦ group. At w20, abundant lipid droplets were more clearly formed with Per Area of 8.4% in CD93hi MΦ adoptive transfer group and 4.8% in CD93lo MΦ group. The Per Area of lipid droplets was 5.09% in non-MΦ adoptive transfer ApoE-/- mice AS model at w20.
For control Non-MΦ adoptive ApoE-/- mice AS model, small AS lesions were also detected in clamped left carotid artery and aorta with a few inflammatory cells’ infiltration and foam cells, but much weaker than that in groups with MΦ adoptive transfer (Fig. 1-B).
3.2 Expression of CD93, F4/80, ApoE, and CD31 in related artery
Compared with w12, CD93 and F4/80 (MΦ marker) positive cells in related artery were significantly increased at w20 in CD93hi MΦ adoptive transferred ApoE-/- mice, which suggested a serious inflammation and MΦ infiltration, together with advancing of AS. Also, at w20, IHC staining confirmed higher expression of F4/80 and CD93-positive cells in related artery in CD93hi MΦ adoptive transferred ApoE-/- mice than CD93lo MΦ group and non-MΦ adoptive group, which indicated CD93hi MΦ adoptive transferred promoted AS development (Fig. 2).
Immunohistochemistry (IHC) staining of CD93 and F4/80 in related arteries of model mice CD93hi MΦ/CD93hi means CD93hi MΦ adoptive transferred ApoE-/- mice. CD93lo MΦ/CD93lo means CD93lo MΦ adoptive transferred ApoE-/- mice. Non-MΦ/Non means no MΦ adoptive transferred ApoE-/- mice. L means left carotid artery; A means aorta. Positive staining showed in brown color. Representative pictures photographed under 400×, Data derived from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
To confirm these infiltrated MΦ was mainly come from adoptive transferred, artery tissue sections from AS ApoE-/- model were stained with anti-ApoE antibody. The result showed that the expression of ApoE in the artery was increased in CD93hi MΦ adoptive transferred ApoE-/- mice compared with group of CD93lo MΦ, while no ApoE expression was detected in non-MΦ adoptive transferred ApoE-/- AS mice. Which further indicated that adoptive transferred CD93hi MΦ could arrive at plaque-artery and infiltrated into the diseased-vascular intima, and moreover, CD93hi MΦ exhibited a stronger chemotasix to plaque (Fig. 3).
Immunohistochemistry staining of ApoE in related arteries of model mice at w20 Positive staining showed in brown color, CD93hi MΦ means CD93hi MΦ adoptive transferred ApoE-/- mice. CD93lo MΦ means CD93lo MΦ adoptive transferred ApoE-/- mice. Non-MΦ means no MΦ adoptive transfer ApoE-/- mice. Representative pictures photographed under 400×, Data derived from three independent experiments.
Further, we detected the expression of CD31, which represented new blood vessels. The result showed that the highest CD31 expression was detected in CD93hi MΦ adoptive transferred ApoE-/- mice at w20, the number of neocapillaries was also higher in CD93hi MΦ group than the groups of CD93lo MΦ and Non-MΦ adoptive transferred (p < 0.05, p < 0.05), which suggested that CD93hi MΦ promoted plaque angiogenesis (Fig. 4).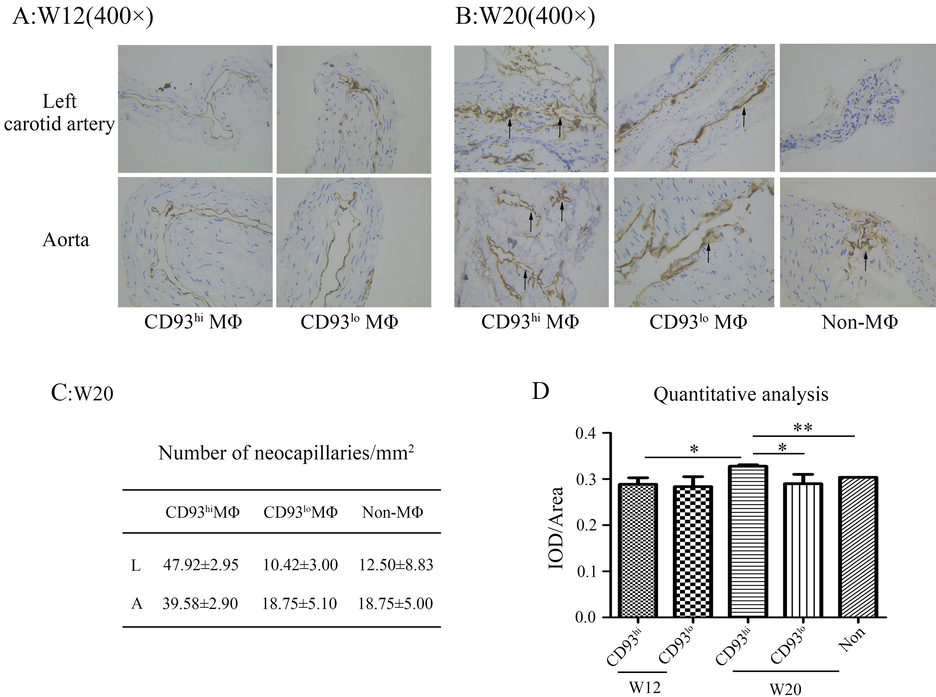
Immunohistochemistry staining of CD31 in related arteries of model mice CD93hi MΦ/CD93hi means CD93hi MΦ adoptive transferred ApoE-/- mice. CD93lo MΦ/CD93lo means CD93lo MΦ adoptive transferred ApoE-/- mice. Non-MΦ:Non means no MΦ adoptive transferred ApoE-/- mice. Positive staining showed in brown color. Black arrows point to neocapillaries. Representative pictures photographed under 400 ×. (C) Number of neocapillaries at w20, L means left carotid artery, A means aorta. (D) Quantitative analysis of CD31 in IHC staining. Data derived from three independent experiments. *p < 0.05, **p < 0.01.
3.3 CD93hi MΦ adoptive transfer increased inflammatory factors
To further understand how CD93hi MΦ promotes AS development, we detected the secretion of pro-inflammatory cytokine both in vitro and in vivo. The results showed higher TNF-α and IL-1β secretion in CD93hi MΦ than CD93lo MΦ in vitro. And more importantly, the level of TNF-α and IL-1β obviously increased in serum in the group of CD93hi MΦ adoptive transfer than CD93lo MΦ group at w20, and higher than control non-MΦ adoptive control group (p < 0.01, Fig. 5-A, B).
Cytokines in culture supernatant of MΦ and serum of AS model and proinflammatory factor protein expression ELISA for (A) TNF-α and (B) IL-1β. (C) Relative protein expression of left carotid artery in CD93hi and CD93lo MΦ adoptive transferred group at w20. NT means non-treated which from control group. n = 3, *p < 0.05, **p < 0.01, ***p < 0.001.
And also, the result of Protein microarray showed that IL-1β, IL-6, IL-12, IL-17, IL-23, chemotactic factors, monocyte chemoattractant protein-5 (MCP-5), macrophage inflammatory protein-1β (MIP-1β) and TNF-α, interferon-γ (IFN-γ), triggering receptor expressed on myeloid cells 1 (TREM-1), tissue inhibitor of metallo-proteinase 1 (TIMP-1) were apparently increased, which suggested pro-inflammation factors were higher expressed in plaque formed vessels in CD93hi MΦ adoptive transferred group than CD93lo MΦ group at w20. Pro-angiogenic factors, keratinocyte-derived chemokine (KC) and soluble intercellular adhesion molecule-1 (sICAM-1), regulated on activation normal T-cells expressed and secreted (RANTES), which may further promote IFN-γ, MIP-1β secretion, were also higher in CD93hi MΦ adoptive transferred group. While, anti-inflammatory factor IL-10, and anti-angiogenic factor, Interferon-γ-inducible protein (IP-10), were apparently decreased (Fig. 5-C).
3.4 MΦ with CD93 high expression promoted AS development through p-NF-κB and p-STAT3 pathway
It was reported that the inflammation signal is mainly through STAT3 (Yeung et al., 2018) and NF-κB (Lawrence, 2009) pathways. To further investigate which signal pathway of CD93hi MΦ is through, we detected the expression of CD93, STAT3, p-STAT3, NF-κB and p-NF-κB. The result showed that LPS stimulation significantly increased the expression of CD93, p-STAT3 and p-NF-κB in MΦ, without obviously influence on total STAT3. Compared with group of LPS stimulation alone, the expression of p-STAT3 was obviously decreased with SH-4–54 treatment (STAT inhibitor, 1.5 µmol/L, 24 h), (p < 0.05, Fig. 6-A, B), which indicated that CD93 in MΦ promoted AS development may through p-STAT3 and p-NF-κB pathway. IHC staining confirmed STAT3 was expressed on aorta both in CD93hi/and CD93lo MΦ adoptive transferred model mice (Fig. 6-C).
Pathway-related protein expression (A) Western blot: SH (SH-4–54, STAT inhibitor), LPS group was treated with 0.1 μg/ml LPS for 15 min, LPS + SH group was treated with 1.5 μg/ml SH-4–54 for 24 h and then 0.1 μg/ml LPS for 15 min, control group was not treated. (B) Western blot: LPS group was treated with 0.1 μg/ml LPS for 15 min, control group was not treated. (C) Immunohistochemistry staining of STAT3 in aorta of CD93hi/lo MΦ adoptive transferred model mice. n = 3, *p < 0.05, **p < 0.01.
3.5 Successfully preparation of 125I-anti-CD93 mAb and 125I-IgG
To further investigate the high chemotaxis of adoptive transferred MΦ to plaque, and confirm CD93 in plaque as a novel biomarker for AS diagnosis and treatment, we successfully prepared CD93-specific targeting probe, 125I-anti-CD93 mAb, and control isotype 125I-IgG. Labeled rates were 96.33 ± 1.50% and 90.47 ± 0.61%, and the radiochemical purities were 95.81 ± 1.48% and 95.61 ± 0.73%, respectively. The stability was both higher than 95% in serum and PBS until 72 h. Our previous published data confirmed that CD93hi MΦ has a higher 125I-anti-CD93 mAb-binding ability than CD93lo MΦ (Su et al., 2022).
3.6 Phosphor-autoradiography in groups of CD93hi and CD93lo MΦ adoptive transfer ApoE-/- mice
To validate the specific targeting and monitoring effect on CD93 positive cells in related arteries for prepared tracer, we carried out the whole-body phosphor-autoradiography noninvasively after two tracers injected both in groups of CD93hi and CD93lo MΦ adoptive transfer. The results showed that no significant radioactivity accumulation in related arteries with whole-body phosphor-autoradiography scanning neither at w12 nor w20 (Fig. 7-A, B). However, ex vivo artery phosphor-autoradiography showed higher radioactivity accumulation in CD93hi MΦ adoptive transferred group, compared with CD93lo MΦ and Non-MΦ at w20. Radioactivity (DLU/mm2) was 24824, 7837, 5338, respectively. The results indicated that CD93hi MΦ adoptive transferred were more plaque-chemotaxis. In addition, the radioactivity accumulation of 125I-anti-CD93 mAb was higher than isotype control 125I-IgG in CD93hi MΦ adoptive transferred group, which confirmed the specific targeting of 125I-anti-CD93 mAb (Fig. 7-C).
Phosphor-autoradiography of 125I-anti-CD93 mAb/125I-IgG in ApoE-/- mice CD93hi MΦ/CD93hi means CD93hi MΦ adoptive transferred ApoE-/- mice. CD93lo MΦ/CD93hi means CD93lo MΦ adoptive transferred ApoE-/- mice. Non-MΦ means no MΦ was adoptive transferred in ApoE-/- mice. (A) w12 whole-body autoradiography was performed at 24, 48, 72 h. (B) w20 whole-body autoradiography was performed at 24, 48, 72 h. (C) Ex vivo aorta and bilateral carotid arteries autoradiography was performed at 72 h. (D) Correlation analysis between CD93 expression in IHC and the radioactivity in aorta in CD93hi MΦ adoptive transferred group at w20. (E) Correlation analysis between CD31 expression and the radioactivity in aorta in CD93hi MΦ adoptive transferred group at w20. Data derived from three independent experiments.
And also we found that the expression of CD93 and CD31detected with IHC and the radioactivity of phosphor-autoradiography ex vivo in aorta were highly positive related (p < 0.05, r = 0.999, Fig. 7-D, p < 0.05, r = 0.998 Fig. 7-E) in CD93hi MΦ adoptive transferred group at w20, which indicated that CD93hi MΦ adoptive transfer apparently increased the CD93 expression in plaque (high chemotax to plaque), and moreover, the high expressed CD93 expression in plaque tissue promoted plaque neovessels growth in CD93hi MΦ adoptive transferred group.
4 Discussion
This study successfully established an AS mice model with adoptive transferred CD93hi and CD93lo MΦ. The results indicated that CD93hi MΦ adoptive transfer was chemotactic accumulated at inflammatory vessels, promoted the development of atherosclerotic plaque, aggregated its severity. Underlying mechanisms are mainly through enhancing inflammatory factors expression in artery, increasing pro-inflammatory TNF-α and IL-1β secretion, promoting STAT3 and NF-κB phurspholysis, and also promoting plaque angiogenesis. And more importantly, through targeting radionuclide imaging with prepared probe 125I-anti-CD93 mAb, we found that CD93 within plaque may be as a novel biomarker for AS diagnosis and treatment.
Nowadays, the major effect pathway for treatment of AS is to weaken the arteries vessels atherogenesis through reducing LDL cholesterol, but with uncontrollable complications. Therefore,it is urgent to develop new therapy (Geovanini and Atherosclerosis, 1979). Anti-inflammatory therapy provided possible way to prevent atherothrombotic events, without accompanied bleeding complications. Canakinumab as drug to treat inflammatory diseases targeting IL-1β, has been proved effective in lowing C-reaction protein (CRP), fibrinogen, and IL-6 (Geovanini and Atherosclerosis, 1979). A large number of MΦ infiltrated the arterial wall and secreted MMPs, lead to extracellular matrix degradation, promoted atherosclerotic lesions unstable (Libby and Theroux, 2005). CRP could bind to LDL in atherosclerotic plaques, positively correlated with the risk of AS (Blaha et al., 2011). CRP induced by IL-6, IL-1β, and TNF-α stimulating, was found synthesized in the liver. And moreover, during the inflammatory process, MΦ accumulated in the plaque could release IL-6 and TNF-α (Calabro et al., 2005), MΦ over expressed Toll-like receptor 4 (TLR4), could be activated by its ligand LPS or heat shock protein (HSP), resulted in metabolic rewiring, increased glycolysis and block certain steps in the tricarboxylic acid (TCA) cycle (Tabas and Bornfeldt, 2020). Dysregulation of uncontrol over diverse MΦ activation plays a major role in the pathogenesis of AS (Kuznetsova and Prange, 2020). Exosomal miR-21-3p from nicotine-treated MΦ may facilitate atherogenesis by increasing vascular smooth muscle cell (VSMC) migration and proliferation via the targeting of phosphatase and tension homologue (PTEN) (Zhu et al., 2019). Consequently, MΦ plays a pivotal role in accelerating the development of AS.
Based on expression of CD14/CD16, human monocyte subsets were distinguished into three subsets: “classical” CD14++CD16– cells, which are the majority of total monocytes, have pro-inflammatory features, and differentiate into macrophages (M1-like property) and dendritic cells (DCs) (Ghattas et al., 2013); “Nonclassical” CD14+CD16++ cells, present more M2-like properties (Geissmann et al., 2003) and “Intermediate monocytes” CD14++CD16+ cells which account for approximately 5% of the total monocyte population and considered to be positive relationships with cardiovascular disease (CVD) events and plaque thinning (Biessen and Wouters, 2017). According to the difference of cytokine secretion, macrophage could be classified into 2 groups: M1 macrophages, typically polarized by Th1 cytokines, produce high levels of pro-inflammatory cytokines, such as IL-6, IL-12, IL-23, TNF-α, and IL-1β (Verreck et al., 2004). M2 macrophages, mainly induced by Th2-related cytokines, including IL-4, IL-33 and IL-13, have the ability to counter-balance the pro-inflammatory response and function to modulate inflammation, scavenge apoptotic cells, accelerate angiogenesis and fibrosis, and promote tissue repair (Gordon and Martinez, 2010). There are many complicated MΦ subsets in AS except for typical, non-typical subsets, M1-M2 subsets. For example, Mhem macrophages and M (Hb) macrophages could resistant to lipid uptake, suppress oxidative stress. M4 macrophages are pro-atherosclerosis and pro-inflammatory subsets. Mox macrophages have the ability to reduce phagocytosis and pro-inflammatory (Stöger et al., 2012; Chinetti-Gbaguidi et al., 2015). MΦ which accumulated through different mechanisms can perform wide different functions in intra-plaque angiogenesis and tissue repair, and it has long been assumed that plaques contain several phenotypically heterogeneous MΦ subsets.
CD93 is a type 1 transmembrane glycoprotein, consists of a C-type lectin-like domain (CTLD), five epidermal growth factor-like domains, a mucin domain, a single transmembrane domain and an intracellular domain (Lugano et al., 2018). The ligand of CD93 is traditionally considered as C1q, however, it is reported recently that IL-17D bound CD93 expressed on mature ILC3 (group 3 innate lymphoid cells), IL-17D-CD93 axis regulated ILC3 function to preserve intestinal homeostasis (Huang et al., 2021). CD93 regulates various processes involved in innate immunity, inflammation reaction and induced differentiation of monocytes to macrophage-like cells, as shown by activated cell adhesion and increased phagocytic activities. MΦ could be divided into two types: CD93hi MΦ and CD93lo MΦ. Nevertheless, the functional role of CD93hi MΦ in the pathogenesis of AS has not been reported yet. CD93 promoted β1-integrin activation and fibronectin fibrillogenesis, contributing to tumor angiogenesis metastasis, CD93 was able to interact with CpG motifs then delivered bacterial DNA to endosomal TLR9 as a novel receptor (Nativel et al., 2019).Our study showed that CD93hi subtype excibited the similar inflammatory factor expression as the M1 type macrophage,so its effective pathway may be also similar as M1 subtype in the development of.AS. However, the role and the molecular mechanism of CD93hi MΦ in development of AS is not clearly defined until now.
To confirm the effect of CD93hi MΦ on AS, we adoptive transferred with CD93hi or CD93lo MΦ for 5 times during the atherosclerotic modeling in ApoE-/- mice. Artery samples were collected, H&E staining, Oil Red O staining, and pathological analysis were conducted, and also, serum lipid and cytokine were measured. Through analysis at w12 and w20, we found that artery pathological changes were increasing from w12 to w20, and CD93hi MΦ adoptive transfer aggregated plaque formation with much more CD93hi macrophage infiltration. And more importantly, we found that, CD31 also was high expressed on plaque in group of CD93hi MΦ adoptive model mice and positively related with the new vessels number within plaque at w20 in group with CD93hi MΦ adoptive transfer. The results suggested that CD93hi MΦ acted as a promoter in AS development.
And furthermore, we try to investigate how this CD93hi MΦ aggregate plaque formation. The results indicated that pro-inflammatory cytokine TNF-α and IL-1β apparently increased in serum and plaque in CD93hi MΦ and model mice with CD93hi MΦ adoptive transferred. Interleukins such as IL-1β, IL-6, IL-12, IL-17, IL-23, chemotactic factors, MCP-5, MIP-1β and pro-angiogenic factors, KC, sICAM-1 were apparently increased in plaque formed artery in CD93hi MΦ adoptive transferred ApoE-/- models. While, anti-inflammatory factor, IL-10, and anti-angiogenic factor, IP-10, were decreased. p-STAT3 and p-NF-κB were apparently up-regulated, STAT3 was expressed on aorta. Our results suggested that CD93hi MΦ subset could promote the development of AS through enhancing the inflammatory reaction in diseased vessels, which may be through STAT3/p-STAT3 and NF-κB/p-NF-κB pathways. Recently, it was reported that the Src-dependent phosphorylation of CD93 and the adaptor protein Cbl lead to the recruitment of Crk, which worked as a downstream integrator in the CD93-mediated signaling and promoted the establishment of cell polarity and adhesion (Barbera et al., 2021 Nov 17). So, we may further investigate whether CD93hi macrophage also affects AS development through Src-dependent phospharylation.
Noninvasive radionuclide molecular imaging AS plaque is of great importance for prognosis, while no suitable target was detected until now. Gezim Bala used 99mTc labeled MΦ mannose receptor-specific nanobodies as molecular tracers for in vivo nuclear imaging in AS model, but with a limitation of non-negligible background (Bala et al., 2018). Some scholars confirmed the specificity of 99mTc- hydrazinonicotinic acid (HYNIC)-IL-2 for imaging activated T lymphocytes infiltration in carotid plaques in vivo and ex vivo studies (Glaudemans et al., 2014), while the metabolism of IL-2 in vivo is too fast to be used for imaging. 99mTc labeled Annexin V showed an effective apoptotic Single-Photon Emission Computed Tomography (SPECT)/CT imaging by monitoring vulnerable atherosclerotic plaques in early time points (Hu et al., 2018), while the apoptosis signal from plaque is too weak to be detected with high efficiency. Nynke A. Jager used 99mTc-Folate to explore distribution of polarized MΦ populations in human atherosclerotic plaque and showing a positive correlation between accumulation of a 99mTc-folate compound in atherosclerotic plaques and FR-b expression on M2-like MΦ, however, the study divided macrophages solely into M1 and M2 subtypes, as we all know, the local macrophages microenvironment in atherosclerosis plaque was complex, rather than simply M1 and M2 subsets (Jager et al., 2014). The molecular probes 99mTc-scFv vascular cell adhesion protein 1 (VCAM1) showed the favorable quality of detecting vulnerable plaques and provided the prospect for early diagnosis and treatment of AS, but suboptimal T/B (Target to Blood) ratios and low resolution in SPECT imaging could not be avoided (Liu et al., 2016). Thereby, a suitable radionuclide imaging of using a radionuclide-labeled molecule or cell is unavailable until now. So in this study, we successfully prepared 125I-anti-CD93 mAb tracer with high specificity and affinity, phosphor-autoradiography ex vivo exhibited a better targeting to AS plaque, and positively correlated with CD93hi MΦ in local plaque tissue in animal models, which suggested that CD93 within plaque may be as a novel biomarker for noninvasively molecular imaging of AS.
In conclusion, our results suggested that CD93hi MΦ subsets contributed to AS lesion development, aggravated local inflammation, and promoted plaque instability. Furthermore, the effect of CD93hi MΦ on AS may be mediated by activation of the STAT3/p-STAT3 and NF-κB/p-NF-κB pathway. Our finding indicated that targeting plaque-CD93 expression may be a new route for diagnosis and treatment of AS.
Funding
This research was funded by Natural Science Foundation of Shandong Province, grant number ZR2019MH019, to G.H.
CRediT authorship contribution statement
Chen Su: Methodology, Software, Data curation, Writing – original draft. Ting Liang: Methodology, Data curation, Writing – original draft. Bin Qu: Software, Writing – original draft. Chao Zhang: Data curation, Writing – original draft. Yeming Han: Data curation, Writing – review & editing. Guihua Hou: Conceptualization, Writing – review & editing, Funding acquisition. Feng Gao: Data curation, Writing – original draft, Writing – review & editing.
Acknowledgments
We thank Translational Medicine Core Facility of Shandong University for consultation and instrument availability that supported this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- IL-1 cytokines in cardiovascular disease: diagnostic, prognostic and therapeutic implications. Cardiovasc. Hematol. Agents Med. Chem.. 2008;6(2):150-158.
- [Google Scholar]
- Evaluation of [(99m)Tc]radiolabeled macrophage mannose receptor-specific nanobodies for targeting of Atherosclerotic lesions in mice. Mol. Imag. Biol.. 2018;20(2):260-267.
- [Google Scholar]
- CD93 signaling via rho proteins drives cytoskeletal remodeling in spreading endothelial cells. Int J Mol Sci.. 2021 Nov 17;22(22):12417.
- [Google Scholar]
- Macrophage complexity in human atherosclerosis: opportunities for treatment? Curr. Opin. Lipidol.. 2017;28(5):419-426.
- [Google Scholar]
- Association between obesity, high-sensitivity C-reactive protein ≥2 mg/L, and subclinical atherosclerosis: implications of JUPITER from the Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol.. 2011;31(6):1430-1438.
- [Google Scholar]
- Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J. Am. Coll. Cardiol.. 2005;46(6):1112-1113.
- [Google Scholar]
- Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71-82.
- [Google Scholar]
- Geovanini GR, Libby P. Atherosclerosis and inflammation: overview and updates. Clinical science (London, England: 1979). 2018;132(12):1243-52.
- Monocytes in coronary artery disease and atherosclerosis: where are we now? J. Am. Coll. Cardiol.. 2013;62(17):1541-1551.
- [Google Scholar]
- In vivo and in vitro evidence that 99mTc-HYNIC-interleukin-2 is able to detect T lymphocytes in vulnerable atherosclerotic plaques of the carotid artery. Eur. J. Nucl. Med. Mol. Imaging.. 2014;41(9):1710-1719.
- [Google Scholar]
- Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593-604.
- [Google Scholar]
- NLRP3 inflammasome and the IL-1 pathway in Atherosclerosis. Circ. Res.. 2018;122(12):1722-1740.
- [Google Scholar]
- A Comparison of [(99m)Tc]Duramycin and [(99m)Tc]Annexin V in SPECT/CT Imaging Atherosclerotic Plaques. Mol. Imag. Biol.. 2018;20(2):249-259.
- [Google Scholar]
- Interleukin-17D regulates group 3 innate lymphoid cell function through its receptor CD93. Immunity. 2021;54(4):673-686.
- [Google Scholar]
- Folate receptor-β imaging using 99mTc-folate to explore distribution of polarized macrophage populations in human atherosclerotic plaque. J. Nucl. Med.. 2014;55(12):1945-1951.
- [Google Scholar]
- The role of monocytes in angiogenesis and atherosclerosis. J. Am. Coll. Cardiol.. 2014;63(1):1-11.
- [Google Scholar]
- The epidermal growth factor-like domain of CD93 is a potent angiogenic factor. PLoS One. 2012;7(12):e51647.
- [Google Scholar]
- Kuznetsova T, Prange KHM. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. 2020;17(4):216-28
- BAFF receptor mAb treatment ameliorates development and progression of atherosclerosis in hyperlipidemic ApoE(-/-) mice. PLoS One. 2013;8(4):e60430.
- [Google Scholar]
- The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol.. 2009;1(6):a001651
- [Google Scholar]
- SPECT and fluorescence imaging of vulnerable atherosclerotic plaque with a vascular cell adhesion molecule 1 single-chain antibody fragment. Atherosclerosis. 2016;254:263-270.
- [Google Scholar]
- CD93 promotes β1 integrin activation and fibronectin fibrillogenesis during tumor angiogenesis. J. Clin. Invest.. 2018;128(8):3280-3297.
- [Google Scholar]
- Mälarstig A, Silveira A, Wågsäter D, .et al. Plasma CD93 concentration is a potential novel biomarker for coronary artery disease. Journal of internal medicine. 2011;270(3):229-36.
- CD93 is a cell surface lectin receptor involved in the control of the inflammatory response stimulated by exogenous. DNA. 2019;158(2):85-93.
- [Google Scholar]
- Monocyte inflammatory profile is specific for individuals and associated with altered blood lipid levels. Atherosclerosis. 2017;263:15-23.
- [Google Scholar]
- EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann. Rheum. Dis.. 2010;69(2):325-331.
- [Google Scholar]
- Qiao L, Ma J, Zhang Z, Sui W, Zhai C, Xu D, et al. Deficient Chaperone-Mediated Autophagy Promotes Inflammation and Atherosclerosis. 2021;129(12):1141-57
- Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225(2):461-468.
- [Google Scholar]
- Su C, Han Y, Qu B, Zhang C, Liang T, Gao F. CD93 in macrophages: A novel target for atherosclerotic plaque imaging? 2022.
- Intracellular and intercellular aspects of macrophage immunometabolism in Atherosclerosis. Circ. Res.. 2020;126(9):1209-1227.
- [Google Scholar]
- Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science (New York, N.Y.). 2013;339(6116):166-172.
- [Google Scholar]
- Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. PNAS. 2004;101(13):4560-4565.
- [Google Scholar]
- Signaling pathways in inflammation and anti-inflammatory therapies. Curr. Pharm. Des.. 2018;24(14):1449-1484.
- [Google Scholar]
- Small rodent models of atherosclerosis. Biomed. Pharmacother. = Biomed. Pharmacother.. 2020;129:110426.
- [Google Scholar]
- Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9(23):6901-6919.
- [Google Scholar]
- Research progress on the relationship between Atherosclerosis and inflammation. Biomolecules. 2018;8(3)
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104796.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







