Translate this page into:
Evaluation of functional properties and freeze-thaw stability of Chinese quince (Chaenomeles sinensis) seed gum
⁎Corresponding authors at: College of Food Science and Engineering, Henan University of Technology, Zhengzhou 450001, China. liuhuamin5108@163.com (Hua-Min Liu), hou1269@126.com (Li-Xia Hou)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Experiments address the cryoprotective performance of Chinese quince seed gum. Flow behavior of the frozen-thawed solution followed the Ostwald-de Waele model. Freeze-thawing affects the rheological properties of Chinese quince seed gum. Cryoprotective properties of hydrophilic gum facilitates excellent food texture.
Abstract
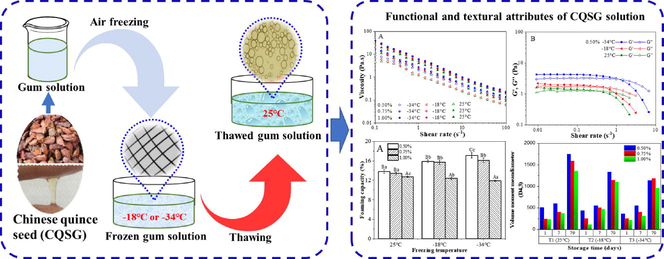
Hydrophilic natural gums have gained increasing attention as food cryoprotectants due to their special functional properties. In this study, the functional properties of Chinese quince seed gum (CQSG), i.e., rheological, textural, emulsifying and foaming properties, were characterized. Effects of gum concentration (0.50–3.00% in w/v) and freezing temperature (−18 °C and −34 °C) were investigated. During freeze–thaw process, there was insignificant change in the flow behavior of the CQSG solution, presumably due either to the random distribution or to the flexibility of the gum molecular chains. After freeze–thaw treatment, the textural properties were stable at low gum concentrations, and the cohesiveness and springiness were relatively stable at high gum concentrations. The hardness and gumminess were enhanced after being frozen at −34 °C and then thawed. The CQSG solutions at all concentrations exhibited enhanced emulsifying and foaming capacities after freeze–thaw treatment, and their stabilities were improved significantly. These results suggest that Chinese quince seed gum can be used as a cryoprotectant in frozen foods.
Keywords
Hydrocolloid
Rheology
Texture
Emulsifying
Foaming
Frozen food
Data availability
Data will be made available on request.
1 Introduction
Recently, seed gums have received much attention as novel biopolymers because of their functional properties (i.e., emulsifying, stabilizing, binding and thickening) and their potential utilization in the non-food and food industries (Soukoulis et al., 2018; Crispin-Isidro et al., 2019). Native seed gums are also easily available and sustainably sourced, biocompatible, biodegradable, and non-toxic (Beikzadeh et al., 2020).
Chinese quince (Chaenomeles sinensis) seed is a byproduct of quince fruit processing. On the seed surface, there is a large concentration of hydrophilic gum, which causes a gel-like layer to appear around the seeds when they are soaked in water at room temperature (25 °C). This gum, which will hitherto be referred to as CQSG (Chinese quince seed gum), are mainly consisted of water-soluble homogeneous polysaccharides (Wang et al., 2018). These kinds of polysaccharides are environmentally friendly and cost-effective (Yousuf and Maktedar, 2022; Farahmandfar et al., 2017), and have been widely used in food industry as edible biomaterial (i.e., coatings, emulsifiers, stablizers) for their functional, physicochemical, and rheological properties (Wang et al., 2018; Wang et al., 2019; Beikzadeh et al., 2020). CQSG is an anionic, high-molecular weight, branched polysaccharide mainly composed of xylose (37.1%), glucose (14.0%), arabinose (13.0%), and galacturonic acid (29.5%); the remaining 6.4% is dominated by galactose, mannose and glucuronic acid (Wang et al., 2019). In recent years, many scientists have been exploring new plant sources of gums and other plant components with potential applications in the food industry (Liu et al., 2018; Rashid et al., 2018; Rashid et al., 2019). CQSG is one such gum which has potential, but which has not been investigated.
Freezing is commonly used to preserve foods, such as cream-based items, meat, and cooked starchy foods, for later consumption. It effectively prevents food spoilage by inhibiting the growth of microorganisms in food, thus extending the shelf life of food products (Hu et al., 2022). However, freezing of foods with high water content, particularly foods with a cellular structure, causes irreversible changes in texture of the food. During freezing, ice crystals rupture cells in tissues such that when they are thawed, changes in the texture of food or tissue reduce the overall acceptability of the food (Williams et al., 2009; Upadhyay et al., 2012; Tan et al., 2021). There is wide evidence that biopolymers can change ice crystal shape and reduce ice crystal growth rate in supercooled solutions, such as milk or sucrose solutions, when stored at 2.5 °C (Sun et al., 2022; Buniowska-Olejnik et al., 2023). Hydrophilic gums are used to improve the texture of frozen foods because they can enhance viscosity by changing rheological properties (Maity et al., 2018; Hamdani et al., 2019). Many hydrophilic gums (e.g., carrageenan, xanthan, guar gum), and carboxymethyl and microcrystalline cellulose have been used for this purpose, that is, as cryopectants in frozen foods (Maity et al., 2018). Generally, −18 °C and −34 °C are the two temperatures commonly used for the preparation of frozen food (Zeynali et al., 2019). As far as we know, there has been no investigation evaluating CQSG as a cryoprotectant for frozen foods.
The ideal hydrophilic gum for use as a cryoprotectant has good viscoelastic and elastic properties, high emulsification and foaming capacities, and freeze–thaw stability (Saha and Bhattacharya, 2010; Zeynali et al., 2019). Based on the functional properties (e.g., rheological properties, water holding capacity, solubility) and their synergistic effects with other ingredients, hydrophilic gum (hydrocollid) can present different cryoprotective effects during freezing process (Maity et al., 2018). Therefore, in the present investigation, CQSG solutions with different concentrations were firstly frozen at −18 °C and −34 °C, and then thawed. The cryoprotectant capacities of various freeze-thawed CQSG solutions were evaluated by assessing their rheological, textural, emulsifying and foaming properties.
2 Materials and methods
2.1 Materials
Seeds were collected from mature Chinese quince (Chaenomeles sinensis) fruit grown at Zhengzhou, Henan province in China. The gum (CQSG) was obtained by soaking the seeds in de-ionized water at a ratio of 1:10 at 50 °C for 1 h. Separation and preliminary purification were performed as described in our previous report (Wang et al., 2018). De-ionized water was used, and all chemicals were of analytical grade.
The monosaccharide composition of CQSG was characterized by high-performance anion-exchange chromatography with pulsed amperometric detection (ICS-3000, Dionex, Sunnyvale, USA), and the molar weight was determined by high-performance size-exclusion chromatography (Malvern Instruments Ltd., Malvern, United Kingdom). As reported in our previous work, the CQSG was composed of rhamnose, arabinose, galactose, glucose, xylose, mannose, galacturonic acid, glucuronic acid and mannuronic acid in a molar ratio of 0.97: 13.00: 3.66: 31.67: 40.86: 2.56: 1.82: 4.85: 0.60 with a molecular weight (Mw) of 237 kDa (Ning et al., 2021). The main chain of CQSG are composed of → 4-b-D-Xylp-1 → and → 2,4-b-D-Xylp-1→, at a ratio of 5.9: 4.5, and the branched chain are composed of Arap-1→, a-D-GalpA-1→, →2-a-D-GlcpA-1→, →4-b-D-Glcp-1→, and → 2,3,5-L-Araf-1→ (Wang et al., 2018).
The thermogram of the CQSG was characterized by a Netzsch DSC 204 F1 Phoenix calorimeter (Netzsch-Gerätebau GmbH, Selb, Germany) equipped with an external liquid nitrogen cryostat. The CQSG (1.35 mg) were placed and sealed in an aluminium pan, and an empty pan was used as a reference sample. The thermogram data was obtained by heating the sample from −90 °C to 100 °C at a heating rate of 10 °C.min−1. Nitrogen was used as the purge gas at a flow rate of 20 mL.min−1. Netzsch Proteus software was used for data analyses. Fig. 1 shows the differential scanning calorimetry (DSC) data for CQSG, showing the thermograms corresponding to the change in heat flow (unit of mW.mg−1) versus temperature. Upon warming the CQSG from −90 °C to 100 °C, an exthothermic peak appears at the temperature range of 14.1–82.0 °C, which might be corresponding to the molecular structure rearrangement or recrystalization of CQSG.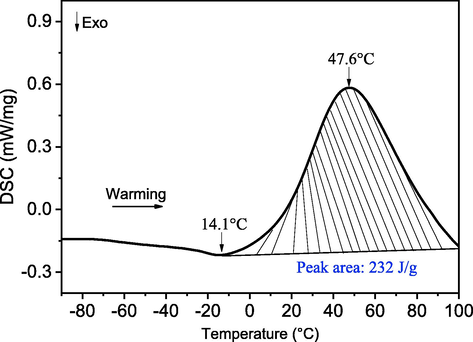
Low temperature differential scanning calorimetry (LT-DSC) scan of the CQSG within the temperature range of −90 °C to 100 °C heating stage.
2.2 Freeze-thaw treatment
CQSG solutions were prepared at various concentrations (0.50, 0.75 and 1.00% in w/v). The solutions were dealed with the traditional air freezing method (freezing rate: 0.194 cm/h) into −18 °C and −34 °C, thereafter thawed at room temperature (25 °C) for 10 h. The rheological, emulsifying and foaming properties of the freeze-thawed CQSG solutions were assessed and compared with the control samples. Freeze-thawed CQSG solutions at concentrations of 2.50, 2.75 and 3.00 wt% were prepared for the texture assessment in semi-solid state using the texture analyzer (TA.XTplusC, Stable Micro Systems, UK). Control samples were prepared without freeze–thaw treatment and stored at 25 °C.
2.3 Rheological characterization
The flow behavior of freeze-thawed CQSG solution is described by rheological properties, namely steady-shear properties and dynamic-shear properties. These properties were measured with a Haake-MARS60 rheometer (Thermo Fisher Scientific Inc., Waltham, USA), coupled with a 60/1° double cone and a serrated plate-plate geometry system (60 mm diameter). The stepwise flow curve was set from 0.01 to 10 Pa. Prior to the test, each sample was magnetically mixed for 2 min and equilibrated for 2 min. Biocompatible silicone oil was used to prevent evaporation. In the tests, stress sweeps ranged from 0.01 to 10 Pa at a fixed frequency of 1 Hz. All the tests were conducted at 25 °C in triplicate.
The Ostwald-de Waele model (Bouchendouka et al., 2022) was employed for fitting the experimental data to analyze and characterize the freeze-thawed CQSG solutions, as expressed in Eq. (1).
2.4 Texture measurements
Texture profile analysis (TPA) of CQSG solutions before and after freeze–thaw treatment was performed at 25 °C with a TA.XTplusC texture analyzer (Stable Micro Systems, UK) in four replicates, and the mean values were reported for adhesiveness, fracturability and hardness. A cylindrical probe (35 mm diameter) was applied at a rate of 33 mm/min for all TPA tests. Textural parameters were obtained by one-cycle compressing a CQSG sample of 20 mm in height by 50%.
2.5 Characterization of emulsifying properties
2.5.1 Emulsion preparation
For preparing oil-in water (O/W) emulsions, 6.67 mL of soy oil was mixed thoroughly in 20 mL of freeze-thawed CQSG solutions at different concentrations (0.50, 0.75 and 1.00% in w/v). To avoid microbial growth, 0.004 g of sodium azide was added to each solution. The mixed solutions were homogenized at a rate of 10000 rpm for 120 s at 25 °C by a Fluko high-shear homogenizer (Fluko Equipment Co., Ltd., Shanghai, China).
2.5.2 Emulsion stability
Emulsion stability indexes (ESI) of the prepared emulsions were determined based on our previous work (Liu et al., 2019). 26.67 mL of the prepared emulsions were stored in cylindrical glass tubes for 79 days at 25 °C, and the ESI was calculated by Eq. (2):
2.5.3 Emulsion droplet measurement
The analysis of particle size and size distribution of various emulsions were performed with a Zetasizer Nano ZS90 (Malvern Instruments Ltd., Worcestershire, UK). The detailed test procedure and methods for calculating average particle size and size distribution have been described in our previous report (Liu et al., 2019).
2.5.4 Microscopic observation
The microstructural observations of the prepared emulsions were carried out by using a BT-1600 optical microscope (Bettersize Instruments Ltd., Dandong, China). A drop of emulsion was placed on a microscope slide under a coverslip. Images were recorded (magnification of 40×) immediately at room temperature (25 °C).
2.6 Foaming properties
Foaming capacity (FC) and stability of the freeze-thawed CQSG solutions were determined according to the process reported previously (Rashid et al., 2019) with slight modification. The CQSG solutions with various gum concentrations (0.50, 0.75 and 1.00% in w/v) were whipped into foam at 15000 rpm for 2 min. The volumes of solutions were recorded before and after whipping. Foam capacity was calculated by Eq. (3):
To measure the foaming stability of the CQSG samples, the foams were transferred to and stored in 1000 mL graduated cylinders at room temperature (25 °C) for 2 h. Change in volume was recorded. Foaming stability was expressed as a percentage of the volume of retained foam to the initial volume of foam.
2.7 Statistical analysis
Experiments were performed in triplicate, except were indicated otherwise, and results are presented as mean ± SD (standard deviation). SPSS 19.0 (SPSS Inc., Chicage, USA) was used to analyse data. The Duncan test was used to determine significant differences (p < 0.05) by a post-hoc comparison of mean values.
3 Results and discussion
3.1 Rheological properties
Gums are commonly added to liquid foods to improve mouth-feel (Yaseen et al., 2005). Gums are also added to frozen foods, both those that are intended to be eaten frozen (e.g., ice cream) and those that are intended to be consumed after being thawed; in both cases the purpose of the gum is to improve mouth-feel, which is a function of viscosity. Rheological behaviors represent viscoelastic properties. Rheological properties of CQSG solutions with different gum concentrations (0.50% and 1.00% in w/v) were characterized to get an overall perspective on the rheology of CQSG.
3.1.1 Steady-shear properties
Fig. 2 presents the static rheological properties of CQSG solutions. As shown in Fig. 1A, the viscosity of CQSG solutions decreased as shear rate increased at all concentrations, which indicates that CQSG solution is a non-Newtonian fluid. This may be related to changes in the molecular structure of CQSG and interactions of molecular chains as shear rate increases (Hosseini-Parvar et al., 2010; Naji-Tabasi and Razavi, 2017). The increase in shear rate causes the breakdown of a gel’s molecular bonds, releasing molecules into solution and decreasing internal friction (Kutlu et al., 2021).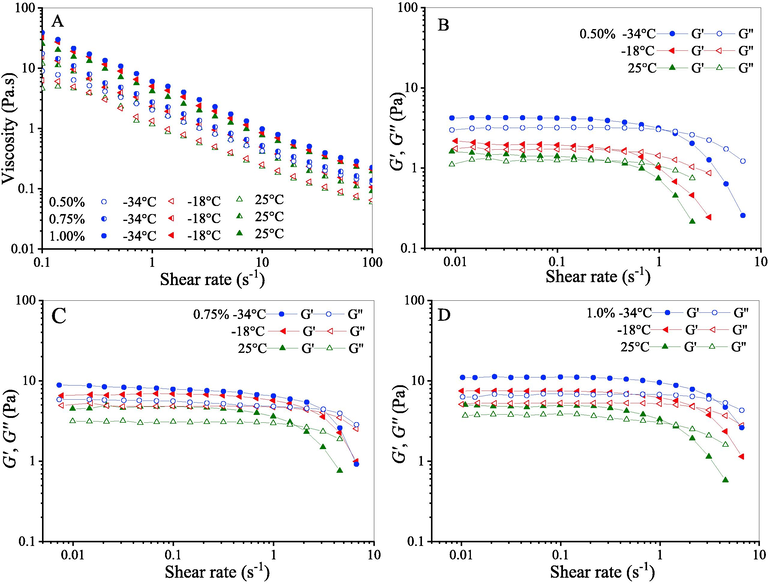
Effects of freeze-thawing on apparent viscosity (A), shear modulus (G′) and loss modulus (G″) at different CQSG concentrations (B, 0.50%; C, 0.75%; D, 1.00% in w/v).
It was found that CQSG solutions with the same gum concentrations exhibited higher viscosity when thawed, after freezing at −34 °C than those frozen at −18 °C, and higher than those prepared at 25 °C. At the same freezing temperature, the viscosity of thawed CQSG solutions increased with increasing gum concentration. We interpret this to mean that both freeze–thaw treatment and greater gum content enhanced film agglomeration at the molecular interface (Vardhanabhuti and Ikeda, 2006). After freeze–thaw process, the viscosity of CQSG solutions at lower gum concentration (e.g., 0.50% in w/v) varied considerably. This could be explained by increased fluidity due to weak molecular entanglements at low gum concentrations.
Shear modulus (G′) and loss shear modulus (G″) of the CQSG solutions, as shown in Fig. 2B-D, are two important parameters that affected the viscosity of the CQSG solution. This effect is because shear modulus relates to the adhesion force of the hydrocolloids in the CQSG solutions, and it is this adhesiveness that determines viscosity (Joseph and Phillip, 2006). In the case of CQSG solutions treated at the same temperature, significant increases in both moduli (G′ and G″) occurred with increasing concentration of CQSG, and G′ was higher than G′′ at the shear rate of 1 s−1 or less. When shear rate was higher than 1 s−1, all shear moduli (G′) decreased remarkably and were lower than G′′. These observations could be explained by the breakdown of gel molecular bonds as shear rate increased. The shear-thinning characteristic of CQSG decreases the viscosity of very viscous food, such that chewing them is more pleasant.
The flow behavior of freeze-thawed CQSG solution is described by the Ostwald-de Waele model (Bouchendouka et al., 2022), the so-called power law model, which is commonly employed for fitting non-Newtonian data with shear rates to analyze and characterize non-Newtonian fluids. As listed in Table 1, the coefficients of determination (R2) are higher than 0.99 indicating that the experimental data are well fitted. The n values of the CQSG solutions were less than 1 at all concentrations, indicating they are non-Newtonian or pseudoplastic fluids. The increase of CQSG concentration from 0.50% to 1.00% caused the decrease of n values and increase of K values for the solutions. A similar trend has been reported for asafoetida gum, cress seed gum and xanthan gum solutions (Saeidy et al., 2019; Naji-Tabasi et al., 2013). The colloidal thickening effect can be attributed to the increased apparent viscosity of CQSG solution at higher gum concentrations (Wang et al., 2009), leading to a weakening of the shear-thinning flow of the solutions.
Ostwald de Waele model τ = Kγn
Concentration (% w/v)
Temperature
(°C)n
K
R2
0.50
25
0.53
0.44
0.99
−18
0.50
0.56
0.99
−34
0.53
0.98
0.99
0.75
25
0.44
1.01
1.00
−18
0.49
1.26
1.00
−34
0.41
1.42
0.99
1.00
25
0.47
1.96
1.00
−18
0.36
2.66
1.00
−34
0.35
2.92
0.99
As one can see, the n values of freeze-thawed CQSG solutions with the same gum concentration differed only slightly at the two freeze–thaw temperatures (-18 °C and −34 °C), indicating that freeze–thaw treatment did not have a significant effect on the flow behavior of CQSG solutions, especially for the ones with low gum concentrations (e.g., 0.50% and 0.75% in w/v). The random distribution and/or the high flexibility of the CQSG molecular chains is/are probably the main reason(s) for this result. That is, the intermolecular forces maintain the structural stability of the gum even after freeze–thaw treatment, thereby preserving the stability of the CQSG solutions. This means that CQSG could be used as a cryoprotectant to preserve the texture of frozen foods.
The consistency coefficient (K value) was significantly higher for the CQSG solutions frozen at the lower temperature (−34 °C) and then thawed. This indicates those solutions had higher apparent viscosity, which can be attributed to greater pseudo-plasticity at lower temperatures. High K value indicates high apparent viscosity of a fluid, and thus protection against crystallization in frozen foods (e.g., ice-creams) (Zeynali et al., 2019).
3.1.2 Dynamic-shear properties
Fig. 3 shows the dynamic-shear rheological properties of freeze-thawed CQSG solutions. It can be seen that the storage modulus (G′) and loss modulus (G″) increase with increases of both angular frequency and gum concentration. The CQSG solutions freeze-thawed with gum concentration less than 1.00% (w/v) initially exhibit G′ higher than G′′. At higher angular frequency, there is a transition point, where G′′ exceeds G′. For example, the transition frequency for the freeze-thawed CQSG solution with gum concentration of 0.50% (w/v) was 0.75–1.00 HZ at 25 °C, and changed to 1–1.33 HZ after freezing at −18 °C and to 2.37–3.16 HZ after freezing at −34 °C, and then thawed. This indicates that viscoelastic properties dominate at the lower angular frequency while elastic properties dominate at higher angular frequency (Li et al., 2011). Freeze-thaw treatment enhances this pattern as the angular frequency required for achieving the transition point increases. Both G′ and G′′ of all freeze-thawed CQSG solutions increased slightly when angular frequency increased. This increase can be explained by the enhanced interactions between colloidal molecules due to freeze–thaw treatment, resulting in increased viscoelasticity and improved elasticity of the freeze-thawed CQSG solution (Naji-Tabasi et al., 2013; Dinesh Kumar et al., 2022; Khodaei et al., 2014).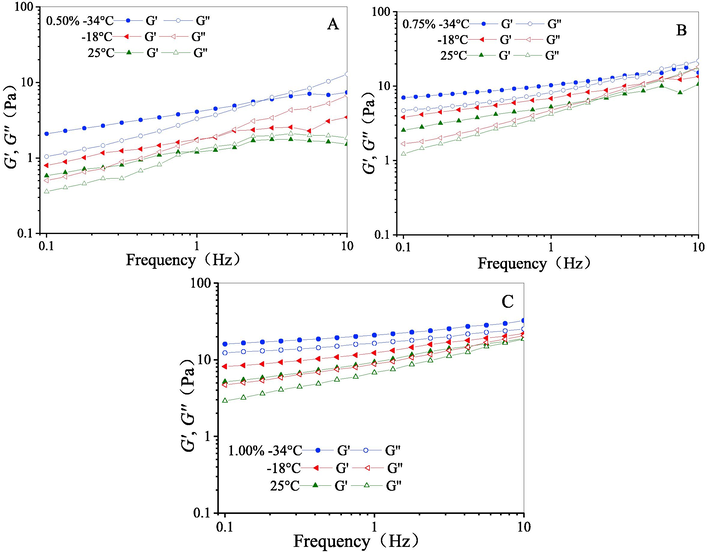
Effects of freeze-thawing on storage modulus (G′) and loss modulus (G″) changes of CQSG solutions at different CQSG concentrations. A, 0.50%; B, 0.75%; C, 1.00% in w/v.
The freeze-thawed CQSG solutions with gum concentration of 0.50% (w/v) exhibited viscous characteristics of a weak gel with smaller increase in G′′ than those with gum concentrations of 0.75% (w/v) and 1.00% (w/v). This result is consistent with the previous analysis of steady-shear properties. At the gum concentration of 1.00% (w/v), no transition is observed in the studied angular frequency range before and after freeze-thawing, indicating good elasticity and freezing stability of CQSG. As mentioned previously, the rheological properties including viscoelastic behavior and elastic behavior of CQSG are associated with cross-linking networks in the solutions; when these networks remain stable, the solution displays increased viscoelasticity and improved elasticity after freeze–thaw treatment. Therefore, the thickening property of CQSG imparts food matrices with increased viscosity, which impedes the mass transfer and diffusion of water molecules onto ice crystals, thereby preventing the formation, growth and the growth of ice crystals (recrystallisation), indicating its potential application as a cryoprotectant (Kadowaki et al., 2022).
3.2 Texture properties
In order to obtain more information about the texture properties of CQSG, and how these properties change after freeze–thaw, the textural attributes of hardness, cohesiveness, gumminess and springiness of the freeze-thawed CQSG solutions were analyzed. It can be seen from Fig. 4A that the hardness of the solutions slightly increased as the gum concentration increased from 2.50% to 2.75% (w/v), indicating stable gel strength. This result is comparable with the reports of locust bean gum, tamarind gum and xanthan gum (Lozinsky et al., 2000; Sae-Kang and Suphantharika, 2006; Sharma et al., 2014). However, the 3.00% (w/v) CQSG solution showed a significant increase in hardness value, with an even greater increase at the lower freezing temperature of − 34 °C. These results reveal a significant improvement of gel strength after freeze-thawing, and this may be related to stronger molecular cohesion at higher gum concentrations and the breakdown of internal cross-linked molecular structure caused by freeze-thawing (Naji-Tabasi and Razavi, 2017).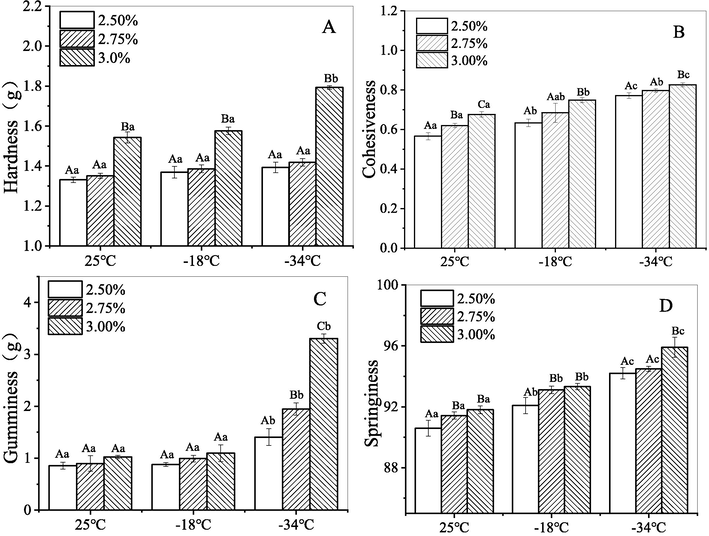
Textural properties (A: Hardness; B: Cohesiveness; C: Gumminess; D: Springiness) of CQSG solutions.
Cohesiveness is commonly used to describe the tendency of a substance to function as a united whole, and it is associated with the strength of molecular bonds within the substance’s structure. As can be seen in Fig. 4B, gum concentration had no significant effect on the cohesiveness of CQSG freeze-thawed at different temperatures. However, after freeze-thawing, the cohesiveness of the CQSG solutions freeze-thawed at the lower temperature was noticeably greater than those treated at the higher temperature. These findings indicate that freeze-thawing effectively improved the structure and texture of the CQSG solutions. It is probably because freezing strengthened the internal molecular bonds, thereby strengthening the cross-linked network. Similar enhancing effects of freezing on cohesiveness have been reported for cress seed gel (Naji-Tabasi and Razavi, 2017). Furthermore, this report suggests that freezing has an important impact on foods, and that cryopectants with high cohesion can maintain or improve food integrity and quality in the freeze–thaw process.
Gumminess describes the state or condition of being sticky or gluey; technically, it is defined as a function of hardness and cohesiveness. As shown in Fig. 4C, there was only a minor gumminess difference between the CQSG solutions at 25 °C and those frozen at −18 °C and then thawed. In contrast, the CQSG solutions frozen at −34 °C were noticeably gummier after thawing than those left at 25 °C. A similar trend has been observed for Balangu gum (Khodaei et al., 2014), cress seed gum and xanthan gum (Naji-Tabasi et al., 2013), where freezing/thawing cycles enhanced the gel gumminess, and the lower the freezing temperature down to −30 °C, the greater the effect on the textural attributes of gumminess.
Springiness is one of the textural attributes that describes the elasticity of a gum when compressed by mechanical force or by heat. As shown in Fig. 4D, it was found that freeze-thawing increased the springiness of CQSG, but there were no significant differences in springiness for gum concentrations in the range of 2.50% to 3.00% (w/v). The highest value was observed for the 3.00% in w/v CQSG solution frozen at the lower temperature, −34 °C.
Therefore, at the gum concentrations of 2.50% (w/v) and 2.75% (w/v), the textural properties (e.g., hardness and gumminess) of CQSG solution were stable when thawed, after freezing at −18 °C and −34 °C. This means CQSG easily maintained its original appearance and texture after being frozen and thawed. At the concentration of 3.00% (w/v), the cohesiveness and springiness of CQSG solution were stable when thawed, after freezing at −18 °C and −34 °C, and freeze-thawed solutions exhibited enhanced hardness and gumminess, indicating the texture of food would be retained or possibly improved with this concentration of CQSG solutions. These results suggest that CQSG can reduce the negative impact on food texture from the formation of ice crystals caused by freeze–thaw process.
3.3 Emulsifying properties
Emulsifying properties of hydrocolloid-based emulsifiers can be attributed to the chemical structure and components of hydrocolloids. This kind of emulsifier works by forming a three-dimensional network or film at the oil–water interface, thereby preventing the oil droplets from coalescing (Fu et al., 2022). The emulsifying properties of hydrocolloids are affected by the gum species used as emulsifier and processing conditions (e.g., temperature, gum concentration, mechanical forces) as these conditions determine how easily a three-dimensional cross-linked network or viscoelastic film can form at the oil/water interface. Emulsifying capacity and emulsifying stability are two indices generally used to evaluate the emulsifying properties of an emulsion system (Fu et al., 2022; Wang et al., 2022). As illustrated in Fig. 5, the freeze-thawed CQSG solutions presented excellent emulsifying capacity (>85%), with a dose-dependent trend (Fig. 5A). Khodaei et al. (2014) (Khodaei et al., 2014) reported that the emulsifying capacity is associated with an active molecule that forms a thick viscoelastic film at the oil/water interface to reduce the surface tension of the emulsion. Incremental increases of gum concentration led to similar incremental increases of CQSG solution viscosity. Formation of three-dimensional networks at high gum concentrations can prevent the coalescence of oil droplets and thus improve emulsification (Pereira et al., 2019; Yao et al., 2021). Some other hydrocolloids like gum Arabic (Golkar et al., 2018; Kadowaki et al., 2022), afzelia gum (Olorunsola and Majekodunmi, 2016), flaxseed gum (Zhao et al., 2015) and Balangu seed gum (Khodaei et al., 2014) have exhibited high emulsifying capacity that can be enhanced by increasing gum content. Freeze-thawing helped improve the emulsifying capacity of CQSG solutions at the gum concentrations of 0.75% (w/v) and 1.00% (w/v), which is consistent with a previous report for basil seed gum (Zeynali et al., 2019), in which emulsifying capacity was also improved by freeze-thawing and/or elevating gum concentration. However, there was no significant difference between the emulsifying capacity of CQSG at lower concentration (0.50% in w/v) after freezing at −18 °C (88.7%) and −34 °C (88.9%), and then thawing. This may be associated with lower CQSG content. Similar findings have been reported for curdlan gum by Williams et al. (Williams et al., 2010).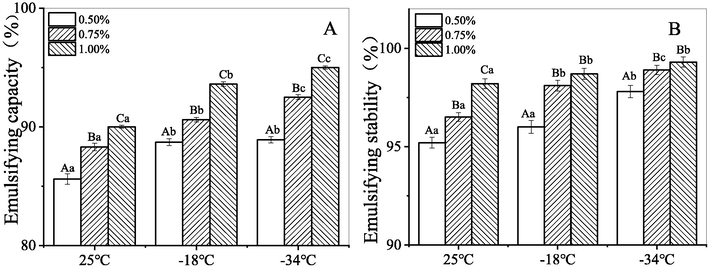
Emulsifying properties of the O/W emulsions. A: Emulsifying capacity; B: Emulsifying stability.
As mentioned in the literatures (Ravera et al., 2021), there are three main mechanisms governing the destabilisation of emulsions: flocculation, coalescence, and film-coarsening (Ostward ripening). The phenomena of coalescence and film-coarsening are affected by interfacial properties, like the rheological properties of the liquid systems, which are related to the presence of non-ionic emulsifiers like hydrocolloids, while flocculation is promoted by electrostatic droplet repulsion, induced by the adsorbed molecules like proteins on the surface of oil droplets. As can be seen from Fig. 5B, the emulsifying stability of O/W emulsions was enhanced by the addition of CQSG solutions at different concentrations, while at the same gum concentrations, O/W emulsions with the CQSG solutions freeze–thaw treated at −34 °C exhibited higher emulsifying stability than with those frozen at −18 °C, and than those that were not freeze-thawed. These results indicated an increased ability to hold oil droplets, which could be explained by the increasing emulsion viscosity due to either the gum concentration or the freeze–thaw process, since the viscosity of the fluid phases plays a major part in the emulsifying strength of emulsifiers (Khodaei et al., 2014). In other words, the CQSG solutions increased the apparent viscosity of the O/W emulsions, and thus stabilized emulsions against coalescence and coarsening.
3.4 Droplet size distribution
The literature mentions that the emulsion properties of hydrocolloids are reflected in their ability to hold oil droplets in the emulsion. This ability is affected by the viscosity of the emulsion, such that an increase in the viscosity will prevent droplet coalescence, and an increase in the interfacial viscosity will reduce the film-drainage rate, thus reflecting higher emulsifying capacity and long-term stability (Makri and Doxastakis, 2006). In Fig. 6, one can see the changes in the microstructures of the oil droplets of CQSG emulsions. CQSG emulsions with a gum concentration of 0.50% (w/v) had more oil droplets of irregular shapes and thinner walls than the emulsions at higher gum concentrations (e.g., 0.75% and 1.00% in w/v). Golkar et al. (Golkar et al., 2018) also reported for Persian gum that the emulsion droplet size decreased with gum concentration, which may be associated with insufficient emulsifier molecules to cover the newly created surfaces of O/W droplets. After storage for 79 days, the droplet sizes of CQSG emulsions increased significantly, while the total number of droplets decreased, especially at the lowest gum concentration of 0.50% (w/v). A similar observation was well reported in basil seed gum emulsions by Hosseini-Parvar et al. (Hosseini-Parvar et al., 2016).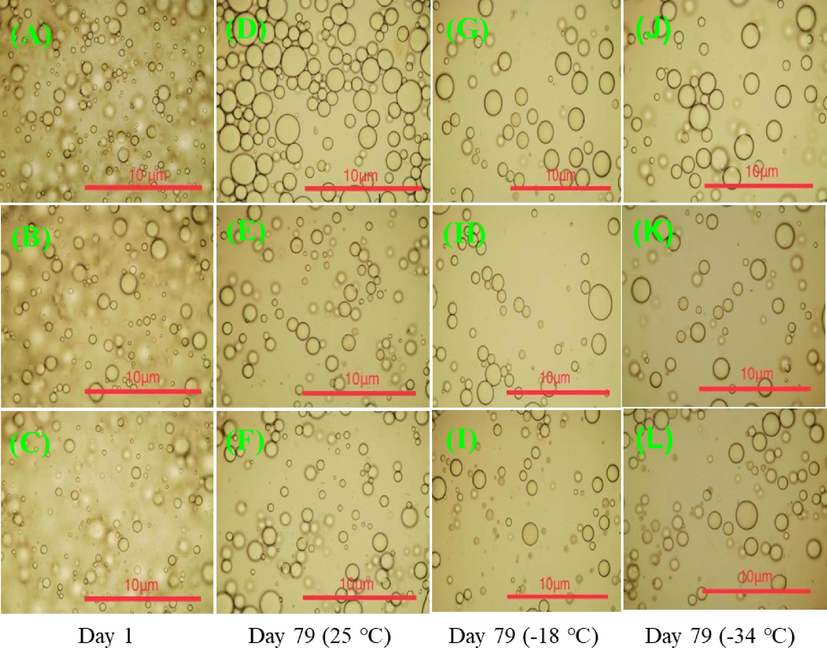
Microstructures of CQSG solutions of different concentration (A: 0.50%, B: 0.75%, and C: 1.00% in w/v) before (Day 1) and after 79 days of storage at different temperatures (D-F, 25 °C; G-I, −18 °C; J-L, −34 °C).
These findings are consistent with the droplet size analysis, in terms of the volume moment mean diameters (D4,3) of the solutions (Fig. 7). The faster defoaming rate in the emulsion with low CQSG content (e.g., 0.50% in w/v) could be attributed to its lower viscosity. The increase in the droplet size (D4,3) during the storage period could be explained by droplet aggregation induced by hydrophobic interactions. Freeze-thawed CQSG emulsions had smaller droplets of regular shapes with thinner walls and more even distribution. This could be explained by the increased interfacial tension, which is related to the alignment of interchains induced by freeze-thawing (Naji-Tabasi et al., 2013).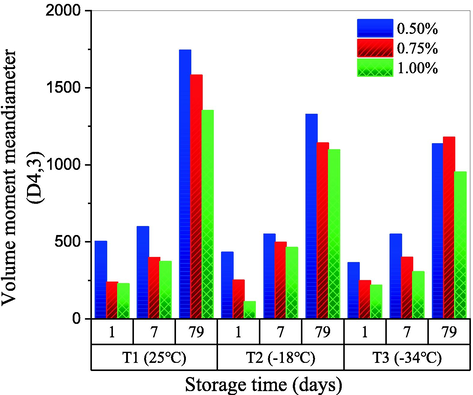
Volume moment mean diameters (D4,3) of CQSG solutions with different gum concentration. A: 0.50%, B: 0.75%, and C: 1.00% in w/v, before (Day 1), and after 7 and 79 days of storage.
3.5 Foaming properties
Foaming capacity and foaming stability are two important properties of hydrocolloids (Lastra-Ripoll et al., 2022). Foaming capacity is defined as the ability of a solution to form foam, and the amount of foam produced; foaming stability refers to the durability (how long it lasts) of the foam generated. Fig. 8 illustrates the impact of CQSG concentration and freeze–thaw temperature on the foaming properties of CQSG solutions. As shown in Fig. 8A, the foaming capacity of CQSG solutions decreased as gum concentration increased. After freezing at −34 °C and then thawing, the 0.50% (w/v) CQSG solution had the highest foaming capacity (17.1%), while the 1.00% (w/v) CQSG solution had the lowest value (11.9%). Surface active groups of CQSG help to decrease the air–water surface tension and trap gas bubbles, which enhance foaming capacity, but high concentration of gum tends to increase viscosity, which facilitates gas coalescence and thus the formation of thick layers, thereby reducing foaming capacity (Indrawati et al., 2008). The reduction of foaming capacity of CQSG solution was especially significant for the 1.00% (w/v) solution after freeze-thawing at −34 °C. This freeze-thawed CQSG solution was highly viscous, making the production of air bubbles through mechanical agitation more difficult (Naji-Tabasi and Razavi, 2017).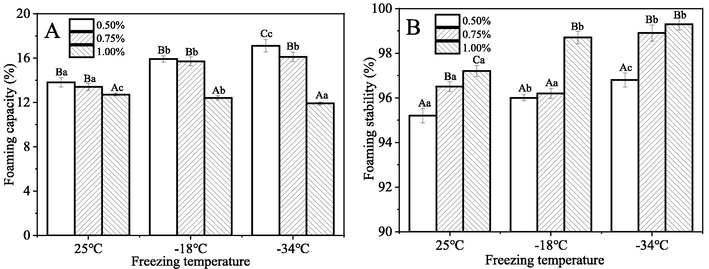
Foaming properties of CQSG solutions. A: Foaming capacity; B: Foaming stability.
As can be observed from Fig. 8B, the foaming stability of CQSG solution at 0.50% (w/v) was low (95.2%) before freezing, and increased with CQSG concentration. Increasing the concentration of CQSG increased foaming ability due to the increased viscosity of the gum solution. Previous investigations also mentioned the positive influences of viscosity on foaming stability, including the prevention of air-bubble coalescence and reduction of foam film permeability (Koocheki et al., 2013). After freeze-thawing, foaming stability of CQSG solutions increased significantly. The highest foaming stability (99.3%) was shown by the CQSG solution at 1.00% (w/v) after freeze-thawing at −34 °C. This could be explained by the fact that freeze-thawing restored foam stability due to the increased strength of internal bonds (Zeynali et al., 2019).
4 Conclusions
The present study investigated the properties of CQSG solutions in the context of the potential use of CQSG as a cryoprotectant in the food industry. CQSG solutions with different gum concentrations were frozen (−18 °C, −34 °C) and then thawed. The solutions before freezing and after thawing were characterized in terms of their functional properties including rheological, textural, emulsifying and foaming properties. CQSG exhibited good functional properties after freeze-thawing. Low freeze temperature resulted in higher apparent viscosity of CQSG solutions, which resulted in increased viscoelasticity and improved elasticity after thawing. Freeze-thawing had little effect on the flow behavior of CQSG solution; this is presumably related to the random distribution or the high flexibility of the CQSG molecular chains. After freeze-thawing, the structure and textural properties of CQSG were effectively improved. At low concentrations (2.50%–2.75% in w/v), the textural properties were stable. This means that, if CQSG were added to foods, the original appearance and textural stability of semi-solid foods would be maintained even after freezing and thawing. At high concentration (3.00% in w/v), the cohesiveness and springiness of the CQSG solutions were relatively stable, and the hardness and gumminess were enhanced after freeze-thawing at −34 °C. Thus, at high concentrations, CQSG would help to retain or improve the food’s texture. CQSG presented high emulsifying capacity (>85%) with a dose-dependent trend. The emulsifying and foaming capacity and stability of CQSG solutions were enhanced by freeze-thawing. These results suggest that the CQSG has the potential to be used as a food cryoprotectant. Future investigations could be done to investigate on the exthothermic behavior of CQSG during its freezing and thawing process, and to compare the functional properties of CQSG with different hydrophilic gums (e.g., carrageenan, xanthan, guar gum) as cryoprotectants in food. This would provide a comprehensive description of CQSG, as a potential cryoprotectant to be applied in the food industry.
CRediT authorship contribution statement
Xiao-Shuang Cai: Writing – review & editing, Funding acquisition. Yan-Yan Ning: Investigation, Formal analysis. Zhao Qin: Data curation, Methodology. Hua-Min Liu: Supervision, Validation. Xue-De Wang: Resources, Project administration. Li-Xia Hou: Supervision, Validation.
Acknowledgements
The authors thank the Henan University of Technology for supporting this work. Authors thank Dr. Yu-Xiang Ma for excellent technical assistance. Authors thank Dr. Run-Yang Zhang for providing help with writing assistance and proof reading the article.
Funding: This work was supported by the Doctor Scientific Research Start-up Foundation from Henan University of Technology [grant number 2019BS063].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Seed mucilages as the functional ingredients for biodegradable films and edible coatings in the food industry. Adv. Colloid Interface Sci.. 2020;280:102164
- [CrossRef] [Google Scholar]
- Flow of a self-Similar non-Newtonian fluid using fractal dimensions. Fractal Fract.. 2022;6:582.
- [CrossRef] [Google Scholar]
- Study of water freezing in low-fat milky ice cream with oat β-Glucan and its influence on quality indicators. Molecules. 2023;28:2924.
- [CrossRef] [Google Scholar]
- Influence of purification on physicochemical and emulsifying properties of tamarind (Tamarindus indica L.) seed gum. Food Hydrocoll.. 2019;93:402-412.
- [CrossRef] [Google Scholar]
- Studies on the consistency of jaggery-based hard-boiled candy by incorporating thickening and gelling agents. Sugar Tech.. 2022;24:1617-1623.
- [CrossRef] [Google Scholar]
- Effects of basil seed gum, Cress seed gum and Quince seed gum on the physical, textural and rheological properties of whipped cream. Int. J. Biol. Macromol.. 2017;98:820-828.
- [CrossRef] [Google Scholar]
- Effects of psyllium husk powder on the emulsifying stability, rheological properties, microstructure, and oxidative stability of oil-in-water emulsions. Food Control. 2022;134:108716
- [CrossRef] [Google Scholar]
- The emulsifying properties of persian gum (amygdalus scoparia spach) as compared with gum Arabic. Int. J. Food Prop.. 2018;21:416-436.
- [CrossRef] [Google Scholar]
- Sources, structure, properties and health benefits of plant gums: A review. Int. J. Biol. Macromol.. 2019;135:46-61.
- [CrossRef] [Google Scholar]
- Steady shear flow behavior of gum extracted from Ocimum basilicum L. seed: effect of concentration and temperature. J. Food Eng.. 2010;101:236-243.
- [CrossRef] [Google Scholar]
- Emulsifying properties of basil seed gum: effect of pH and ionic strength. Food Hydrocoll.. 2016;52:838-847.
- [CrossRef] [Google Scholar]
- Novel synergistic freezing methods and technologies for enhanced food product quality: A critical review. Compr. Rev. Food Sci. Food Saf.. 2022;21:1979-2001.
- [CrossRef] [Google Scholar]
- Effect of processing parameters on foam formation using a continuous system with a mechanical whipper. J. Food Eng.. 2008;88:65-74.
- [CrossRef] [Google Scholar]
- Adhesion properties of lightly crosslinked solvent-swollen polymer gels. J. Adhes.. 2006;82:945-971.
- [CrossRef] [Google Scholar]
- Extraordinary high preservation of the dispersion state of Au nanoparticles during freeze-thawing and freeze-drying with gum Arabic. Colloids Surf. A Physicochem. Eng. Asp.. 2022;639:128392
- [CrossRef] [Google Scholar]
- Functional properties of balangu seed gum over multiple freeze-thaw cycles. Food Res. Int.. 2014;66:58-68.
- [CrossRef] [Google Scholar]
- Studies on the steady shear flow behavior and functional properties of Lepidium perfoliatum seed gum. Food Res. Int.. 2013;50:446-456.
- [CrossRef] [Google Scholar]
- Rocket seed (Eruca Sativa Mill) gum: physicochemical and comprehensive rheological characterization. Food Sci. Technol.. 2021;42:e69620.
- [Google Scholar]
- Chemical, technological, and rheological properties of hydrocolloids from sesame (Sesamum indicum) with potential food applications. Arabian J. Chem.. 2022;15:104146
- [CrossRef] [Google Scholar]
- Rheological properties of aqueous solution of new exopolysaccharide secreted by a deep-sea mesophilic bacterium. Carbohydr. Polym.. 2011;84:1117-1125.
- [CrossRef] [Google Scholar]
- Flaxseed gum a versatile natural hydrocolloid for food and non-food applications. Trends Food Sci. Technol.. 2018;75:146-157.
- [CrossRef] [Google Scholar]
- Emulsifying and structural properties of polysaccharides extracted from Chinese yam by an enzyme-assisted method. LWT - Food Sci. Technol.. 2019;111:242-251.
- [CrossRef] [Google Scholar]
- Lozinsky, V.I., Damshkaln, L.G., Brown, R., Norton, I. T., 2000. Study of cryostructuring of polymer systems. xix. on the nature of intermolecular links in the cryogels of locust bean gum. John Wiley & Sons, Ltd., 49, 1434–1443. http://doi.org/10.1002/1097-0126(200011)49:113.0.CO;2-F.
- Use of hydrocolloids as cryoprotectant for frozen foods. Crit. Rev. Food Sci. Nutri.. 2018;58:420-435.
- [CrossRef] [Google Scholar]
- Study of emulsions stabilized with Phaseolus vulgaris or Phaseolus coccineus with the addition of Arabic gum, locust bean gum and xanthan gum. Food Hydrocoll.. 2006;20:1141-1152.
- [CrossRef] [Google Scholar]
- New studies on basil (Ocimum bacilicum L.) seed gum: part III - steady and dynamic shear rheology. Food Hydrocoll.. 2017;67:243-250.
- [CrossRef] [Google Scholar]
- Effect of freezing on functional and textural attributes of cress seed gum and xanthan gum. Food Bioproc. Tech.. 2013;6:1302-1311.
- [CrossRef] [Google Scholar]
- Effects of isolation conditions on structural and functional properties of the seed gum from Chinese quince (Chaenomeles sinensis) Carbonhydr. Polym.. 2021;273
- [CrossRef] [Google Scholar]
- Emusifying properties of afzelia gum in liquid paraffin emulsion. Int. J. Pharm. Pharm. Sci.. 2016;8:195-198.
- [CrossRef] [Google Scholar]
- Mutamba seed mucilage as a novel emulsifier: Stabilization mechanisms, kinetic stability and volatile compounds retention. Food Hydrocoll.. 2019;97:105190
- [CrossRef] [Google Scholar]
- Extraction purification and characterization of galactomannan from fenugreek for industrial utilization. Carbohydr. Polym.. 2018;180:88-95.
- [CrossRef] [Google Scholar]
- Linum usitatissimum L seeds: flax gum extraction, physicochemical and functional characterization. Carbohydr. Polym.. 2019;2015:29-38.
- [CrossRef] [Google Scholar]
- Emulsification and emulsion stability: the role of the interfacial properties. Adv. Colloid Interface Sci.. 2021;288:102344
- [CrossRef] [Google Scholar]
- Emulsion properties of Asafoetida gum: Effect of oil concentration on stability and rheological properties. Colloids Surf. A Physicochem. Eng. Asp.. 2019;560:114-121.
- [CrossRef] [Google Scholar]
- Influence of pH and xanthan gum addition on freeze-thaw stability of tapioca starch pastes. Carbohydr. Polym.. 2006;65:371-380.
- [CrossRef] [Google Scholar]
- Characteristics of gellan gum-based food gels. J. Texture Stud.. 2010;41:459-471.
- [CrossRef] [Google Scholar]
- Preparation of tamarind gum based soft ion gels having thixotropic properties. Carbohydr. Polym.. 2014;102:467-471.
- [CrossRef] [Google Scholar]
- Plant seed mucilage as emerging biopolymer in food industry applications. Curr. Opin. Food Sci.. 2018;22:28-42.
- [CrossRef] [Google Scholar]
- A review of natural polysaccharides for food cryoprotection: Ice crystals inhibition and cryo-stabilization. Bioact. Carbohydr. Diet. Fibre. 2022;27:100291
- [CrossRef] [Google Scholar]
- The formation and control of ice crystal and its impact on the quality of frozen aquatic products: A review. Crystals. 2021;11:68.
- [CrossRef] [Google Scholar]
- Characterization of bread dough: Rheological properties and microstructure. J. Food Eng.. 2012;109:104-113.
- [CrossRef] [Google Scholar]
- Isolation and characterization of hydrocolloids from monoi (cissampelos pareira) leaves. Food Hydrocoll.. 2006;20:885-891.
- [CrossRef] [Google Scholar]
- Chinese quince (Chaenomeles sinensis) seed gum: structural characterization. Food Hydrocoll.. 2018;75:237-245.
- [CrossRef] [Google Scholar]
- Chinese quince seed gum: flow behaviour, thixotropy and viscoelasticity. Carbohydr. Polym.. 2019;209:230-238.
- [CrossRef] [Google Scholar]
- Surface characteristics and emulsifying properties of whey protein/ nanoliposome complexes. Food Chem.. 2022;384:132510
- [CrossRef] [Google Scholar]
- Rheological properties of waxy maize starch and xanthan gum mixtures in the presence of sucrose. Carbohydr. Polym.. 2009;77:472-481.
- [CrossRef] [Google Scholar]
- Hydrocolloid gelling agents and their applications. In: Mcclements D.J., ed. Gums and Stabilisers for the Food Industry. Royal Society of Chemistry:; 2009. p. :23-31. Special Publications (Chapter 12)
- [CrossRef] [Google Scholar]
- Texture stability of hydrogel complex containing curdlan gum over multiple freeze-thaw cycles. J. Food Process. Preserv.. 2010;33:126-139.
- [CrossRef] [Google Scholar]
- Emulsifying properties of chinese quince seed gum in oil-in-water emulsions. LWT - Food Sci. Technol.. 2021;147:111560
- [CrossRef] [Google Scholar]
- Rheological properties of selected gum solutions. Food Res. Int.. 2005;38:111-119.
- [CrossRef] [Google Scholar]
- Influence of quince seed mucilage-alginate composite hydrogel coatings on quality of fresh walnut kernels during refrigerated storage. J. Food Sci. Technol.. 2022;59:4801-4811.
- [CrossRef] [Google Scholar]
- Investigation of basil (Ocimum bacilicum L.) seed gum properties as cryoprotectant for frozen foods. Food Hydrocoll.. 2019;90:305-312.
- [CrossRef] [Google Scholar]
- Sodium caseinate/flaxseed gum interactions at oil-water interface: effect on protein adsorption and functions in oil-in-water emulsion. Food Hydrocoll.. 2015;43:137-145.
- [CrossRef] [Google Scholar]







