Translate this page into:
Evaluation of ORAC methodologies in determination of antioxidant capacity of binary combinations of quercetin and 3-(3,4,5-trihydroxybenzoyl) coumarin derivatives
⁎Corresponding author. mmoncadab@utem.cl (Mauricio Moncada-Basualto)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Antioxidant capacity is used to refer to ability of compounds to react with free radicals, it is also described as ability to inhibit oxidation processes. There are different methods that evaluate the antioxidant capacity of compounds of natural origin or extracts. ORAC methodologies measure the ability to transfer hydrogen atoms to RO·/ROO· radicals generated by the AAPH thermolysis, in presence of a probe that accounts for oxidation of antioxidant. Despite extensive use of these methods to assess antioxidant capacity, they have been questioned by type of radical generated and information they can deliver, especially if it is a mixture of compounds. In this work, antioxidant capacity of binary combinations of quercetin with synthetic 3-phenylcoumarins was evaluated through ORAC-FL, PGR and quantification techniques after oxidation kinetics via free radicals, through HPLC. It was found through ORAC-FL that derived 3-(3,4,5-trihydroxybenzoyl) coumarin have greater antioxidant capacity than Trolox: 1 < 2 < 3 < quercetin. Determination of ORAC-FL indices of combination showed an antagonistic effect among the antioxidants. Also, ORAC-PGR method did not allow determination of antioxidant capacity of compounds, separately, indicated high reactivity of compounds. However, unusual behaviors were observed in combinations, unable to explain antagonistic effect observed in ORAC-FL. HPLC oxidation kinetics analysis showed that in the combination the consumption of the most reactive antioxidant dominated the antioxidant capacity and followed a similar trend as observed by ORAC-FL. Therefore, ORAC methodologies would not be useful in characterizing antioxidant capacity of mixture in relation to reactivity of metabolites present therein, but in relation to amount of hydroxyl groups available.
Keywords
Antioxidant capacity
ORAC assay
Oxidation kinetics
Combination
Free radicals
1 Introduction
Antioxidant capacity is used to refer to capacity of compounds to react with free radicals, but in other cases it is described as capacity to inhibit oxidation processes. This is determinate by two factors: (i) the rate of scavenging that refers to the quenching rate of free radicals formed and (ii) amount of radicals trapped by the antioxidant, generating more stable and therefore less reactive compounds (Jensen, 2003; Cheeseman and Slater, 1993; Halliwell and Gutteridge, 2015). The efficacy of an anti-oxidant is characterized by the period of inhibition, where it stops the formation of free radicals (Dai et al., 2008); being polyphenolic compounds of natural origin those that stand out for a high activity. Most of these compounds are present in the diet, so it is essential to evaluate their antioxidant properties to understand their behavior in more complex matrices such as food. As well as the application of these in biomedicine (Sánchez-Moreno, 2002).
Several methods have been used to determine antioxidant capacity in different compounds; some common experiments based on hydrogen atom transfer (HAT) are Oxygen Radical Absorbance Capacity (ORAC) and Total Radical-Trapping Antioxidant Parameter (TRAP), the methods based in Electron Transfer (ET) are Trolox Equivalent Antioxidant Capacity (TEAC), 1,1-diphenyl-2-picrylhydracil (DPPH) and Ferric Reducing Antioxidant Power (FRAP) (Prior et al., 2005). The ORAC method is one of the most used to establish antioxidant capacity of polyphenolic compounds (Speisky et al., 2012). Currently, it has been known that ORAC index is strongly influenced by type of probe used (López-Alarcón et al., 2012). Dorta et al. (2015) indicated that ORAC index is not directly related to ability to eliminate free radicals present in sample, because their determination does not it only involves the concentration of antioxidant, but also the chemical nature of compound and its possible interaction between antioxidants presents in sample. The ORAC assay measures the ability to transfer hydrogen atoms to peroxyl radicals (ROO·) generated by AAPH thermolysis. When using fluorescein as a probe the test is called ORAC-fluorescein (ORAC-FL) deliver results regarding the stoichiometry of reaction while if pyrogallol red is used the assay is called ORAC-pyrogallol red (ORAC-PGR) indicates reactivity of antioxidant (López-Alarcón et al., 2012).
The rate of scavenging is evaluated by oxidation of probe with high reactivity, as is case of PGR and amount of radical trap is evaluated by oxidation of a probe molecule with low reactivity such as fluorescein (Takashima et al., 2012; Matos et al., 2013). Prior (2015) indicated that ORAC assay provides valuables results in analysis of sample of natural products, provided that advantages and disadvantages of in vitro assay are understood. Based on the above, Journal of Food Composition and Analysis will no longer accept papers for review that use only assays for total phenolic compounds and antioxidants (Harnly, 2017). The existence of thousands of phenolic compounds makes the analysis an expensive and slow process, hence the popularity of direct colorimetric methods for all antioxidants, although these methods are not specific and very prone to interference, since any reducing compound gives a positive response (‘AOAC SMPR 2011.011 Standard Method Performance Requirements for in Vitro Determination of Total Antioxidant Activity in Foods, Beverages, Food Ingredients, and Dietary Supplements’, 2012; Ruiz, 2001). Likewise, Association of Official Agricultural Chemists (AOAC International) concluded that the term “antioxidant” is primarily a marketing tool and that ORAC-FL and ORAC-PGR produce values that cannot be compared (‘AOAC SMPR 2011.011 Standard Method Performance Requirements for in Vitro Determination of Total Antioxidant Activity in Foods, Beverages, Food Ingredients, and Dietary Supplements’, 2012). In based on the above, the US Department of Agriculture (USDA) deleted of internet ORAC database due to the growing evidence that the values indicating antioxidant capacity are not relevant for the effects of specific bioactive compounds, including polyphenols in human health ((U.S.), 2010).
However, it has not been completely elucidated that evaluate ORAC techniques in extracts or mixture of compounds, since although the antioxidant capacity is not directly related to biological activity. The antioxidant capacity and total polyphenolic content have found use as a simple means to quantify a wide range of secondary metabolites, especially polyphenolic compounds, in a single measurement. These methodologies have been used to study the inhibition of peroxyl radical mediated oxidation of tryptophan residues in myofibrillar proteins through the use of mange by products. Where, inhibition of tryptophan oxidation was correlated with the antioxidant capacity of mango by products determined by ORAC-PGR (Jongberg et al., 2014; Rodríguez-Carpena et al., 2011; Dorta et al., 2017). However, the effect of the components of the extracts on the observed antioxidant capacity is unknown.
Currently, the study of combinations of antioxidant compounds is of great interest, to determine if the relationship between the components is antagonistic (negative effect) or synergistic (positive effect), (Kancheva et al., 2018) they indicated that the combination of 4-methylcoumarins with l-ascorbic acid and α-tocopherol showed a strong synergism as a result of the continuous regeneration of α-tocopherol, from l-ascorbic acid and coumarins. The latter have stood out for their multiple biological properties and antioxidant capacity.
It should be noted that electron paramagnetic resonance (EPR), high performance liquid chromatography (HPLC), nuclear magnetic resonance (RMN), among others, have been useful tools in the direct and indirect detection of radicals of biological interest in different complex matrices (biological and others) (Zweier et al., 1987; Blasig et al., 1990; Arpad et al., 1993; Komarov et al., 2021).
Coumarins are compounds that are characterized by having a high antioxidant capacity, possibly due to their structural similarity with polyphenolic compounds such as chalcones or resveratrol. Based on this, the antioxidant capacity of some coumarin chalcones has been studied, finding high antioxidant capacity values even higher than recognized antioxidants such as catechin and quercetin (Pérez-Cruz et al., 2013). In addition, this type of compound confers cytoprotection against Reactive Nitrogen Species (RNS) and Reactive Oxygen Species (ROS) in cells, MAO inhibitory activity, trypanocidal activity, anti-inflammatory activity, among others (Matos et al., 2013; Matos et al., 2011; Vazquez-Rodriguez et al., 2013; Vazquez-Rodriguez et al., 2015). Likewise, coumarins are widely distributed in the plant kingdom and can contribute greatly to the antioxidant capacity and/or biological properties of many extracts and products of natural origin.
Based on the above, it is interesting to study the antioxidant behavior of binary mixtures of coumarins with compounds of recognized antioxidant capacity, evaluated through ORAC methodologies. Therefore, in this work, the antioxidant capacity of three new 3-(3,4,5-trihydroxybenzoyl) coumarin with small structural variations in position 6 to influence their antioxidant capacity and their equimolar combination with quercetin was synthesized and evaluated, by means of ORAC-FL and ORAC-PGR. In addition, the persistence of these compounds after accelerated oxidation via free radicals was quantified by HPLC.
2 Experimental section
2.1 Chemistry
2.1.1 Materials and methods
Reagent grade chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and solvents were purchased from Merck (Darmstadt, Germany). Melting points (Mp) are uncorrected and were determined using a Reichert Kofler Thermopan or in capillary tubes in a Büchi 510 apparatus. 1H NMR (250 MHz) and 13C NMR (75.5 MHz) spectra were recorded using a Bruker AMX spectrometer using CDCl3 or DMSO‑d6 as solvent. Chemical shifts (δ) are expressed in parts per million (ppm) using TMS as an internal standard. Coupling constants J are expressed in Hertz (Hz). Spin multiplicities are given as s (singlet), d (doublet), dd (doublet of doublets) and m (multiplet). Mass spectrometry was carried out using a Hewlett-Packard 5988 A spectrometer, this system is an automated service utilizing electron impact (EI) ionization. Elemental analyses were performed by using a Perkin-Elmer 240B microanalyzer and the results are within ± 0.4 % of calculated values in all cases. The analytical results document ≥ 98 % purity for all compounds. Flash chromatography (FC) was performed on silica gel (Merck 60, 230–400 mesh); analytical TLC was performed on precoated silica gel plates (Merck 60 F254). Organic solutions were dried over anhydrous sodium sulfate. Concentration and evaporation of the solvent after reaction or extraction were carried out on a rotary evaporator (Büchi Rotavapor) operating under reduced pressure.
The fluorescence and absorbance were measured with a Synergy™ HT multidetection microplate reader from Bio-Tek Instruments, Inc. (Winooski, VT, USA) and a EnSpire multimode plate reader from PerkinElmer, Inc. (Waltham, Massachusetts, USA), using white polystyrene 96-well plates by fluorescent and transparent polystyrene 96-well plates by absorbance, purchased from Nunc (Roskilde, Denmark). For the ORAC-FL and ORAC-PGR techniques, the fluorescence was read from the top, with an excitation wavelength of 485/20 nm and an emission filter of 528/20 nm, and the absorbance was read from the bottom at 525 nm, respectively. The plate reader was controlled by Gen 5 software.
For the quantification of antioxidants was used 1260 Infinity II Series chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA) with DAD diode array detector (Santa Clara, CA, USA). For the identification, a Chromolith® C18 endcapped column was used (150 × 4.60 mm, 5.0 µm) (Merck, KGaA-Germany), quaternary pump system and au-tomatic injection, the injection volume was 20 µL.
2.2 General procedure for the synthesis of 3-(3,4,5-trihydroxybenzoyl)coumarins (1–3)
To a solution of the appropriate β-ketoester (1 mmol) and the corresponding salicylaldehyde (1 mmol) in ethanol (3 mL) was added piperidine in a catalytic amount. The mixture was refluxed for 2–5 h. After completion (indicated by TLC), the reaction was cooled, and the precipitated was filtered and washed with cold ethanol and ether to afford the methoxylated coumarins 1a-3a. The compounds were further recrystallized from MeOH/CH2Cl2. Then, to the corresponding methoxy-3-benzoylcoumarin (1 mmol), BBr3 in DCM (20 mmol, 1 M) was added in a Schlenk tube. The tube was sealed, and the reaction mixture was heated at 80 °C for 48 h. The resulting crude product was treated with MeOH and rotated to dryness. The obtained precipitate was recrystallized from MeOH or purified by flash chromatography using hexane/ethyl acetate mixtures as the eluent to afford the desired hydroxy derivatives 1–3.
6-Bromo-3-(3,4,5-trihydroxybenzoyl)coumarin (1): Yield 70 %. 1H NMR (250 MHz DMSO‑d6) δ ppm 9.33 (bs, 3H, 3x OH), 8.19 (s, 1H, H-4), 8.06 (s, 1H, H-5), 7.84 (d, J = 8.3 Hz, 1H, H-7), 7.45 (d, J = 8.3 Hz, 1H, H-8), 6.90 (s, 2H, H-2′, H-6′). m/z (%): 377 ([M + 2]+, 27), 375 ([M]+, 32), 297 (41), 251 (59), 249 (63), 153 (100), 125 (25), 79 (18).
6-Chloro-3-(3,4,5-trihydroxybenzoyl)coumarin (2): Yield 58 %. 1H NMR (250 MHz, DMSO‑d6) δ ppm 9.32 (bs, 3H, 3x OH), 8.20 (s, 1H, H-4), 7.93 (d, J = 3.1 Hz, 1H, H-5), 7.73 (d, J = 9.2, 2.9 Hz, 1H, H-7), 7.52 (d, J = 9.0 Hz, 1H, H-8), 6.90 (s, 2H, H-2′, H-6′). m/z (%): 334 ([M + 2]+, 24), 332 ([M]+, 70), 266 (18), 207 (48), 153 (100), 125 (21), 79 (15).
6-Hydroxy-3-(3,4,5-trihydroxybenzoyl)coumarin (3): Yield 81 %. 1H NMR (250 MHz, DMSO‑d6) δ ppm 9.85 (s, 1H, –OH), 9.29 (d, J = 19.0 Hz, 3H, 3X –OH), 8.17 (s, 1H, H-4), 7.33 (d, J = 9.6 Hz, 1H, H-8), 7.18–7.06 (m, 2H, H-5, H-7), 6.87 (s, 2H, H-2′, H-6′). m/z (%): 315 ([M + 1]+, 31), 314 ([M]+, 92), 189 (73), 161 (39), 153 (100), 125 (21), 79 (16).
2.3 Oxygen radical antioxidant capacity (ORAC)
In general, the reaction was carried out in 75 mM sodium phosphate buffer (pH 7.4), in a 200 μL final volume. The 3-(3,4,5-trihydroxybenzoyl) coumarins and combinations with quercetin were prepared in mixture buffer phosphate pH 7.4/MeOH (70/30).
2.3.1 ORAC-FL
Fluorescein (FL, 40 nM, final concentration) and 3-(3,4,5-trihydroxybenzoyl) coumarins and combinations with quercetin (0.1 – 2.0 µM) solutions were placed in each well of plate. The mixture was pre-incubated at 40 °C for 15 min, and 2,2′-azo-bis(2-amidinopropane) dihydrochloride (AAPH) solution (18 mM, final concentration) was added (Alarcón et al., 2008). The microplate was immediately placed in the reader and automatically shaken prior to each reading. The fluorescence was recorded every 1 min for 50 min. A blank with FL and AAPH using buffer phosphate instead of the compound solution was used in each assay. Five calibration solutions of commercial Trolox (2.0 – 20.0 µM) as the antioxidant were also used in each assay. The inhibition capacity was ex-pressed as ORAC-FL values, and it was quantified by integration of the area under the fluorescence decay curve (AUC). All reaction mixtures were prepared in triplicate and at least three independent assays were performed for each sample. The ORAC-FL indexes were calculated as described by (Pérez-Cruz et al., 2018; Moncada-Basualto et al., 2018).
2.3.2 ORAC-PGR
Pyrogallol red (PGR, 70 µM, final concentration) and 3-(3,4,5-trihydroxybenzoyl) coumarins and combinations with quercetin (0.10 – 0.75 µM) solutions were placed in each well of plate. The mixture was pre-incubated at 40 °C for 15 min, and AAPH solution (800 mM, final concentration) was added. The microplate was immediately placed in the reader and automatically shaken prior to each reading. The absorbance was recorded every 35 s for 50 min. A blank with FL and AAPH using buffer phosphate instead of compound solution was used in each assay (López-Alarcón and Lissi, 2006). Five calibration solutions of Trolox (15 – 200 µM) as the antioxidant were also used in each assay. The inhibition capacity was expressed as ORAC-PGR values (Dorta et al., 2015; Romero et al., 2010). All reaction mixtures were prepared in triplicate and at least three independent assays were performed for each sample.
2.4 Oxidation kinetics
The oxidation kinetics of the 3-(3,4,5-trihydroxybenzoyl) coumarins and combinations with quercetin were performed on the Perkin-Elmer EnSpire plate spectrophotometer (Shelton, CT, USA). The absorbance was read in a wavelength range of 280 nm to 550 nm. The reaction was carried out in 75 mM phosphate buffer (pH = 7.4). In the plate 150 µL of buffer solution and 25 µL of the samples and their respective mixtures in phosphate buffer were added at a concentration of 30 µM in each well of the plate. The plate was incubated for 5 min, then 25 µL of 1.2 mM AAPH was added. Registration of 100 spectra as blank was used phosphate buffer with AAPH (Zúñiga-Núñez et al., 2018). Data processing was performed with the Origin Pro 8.5 SR2 program (Origin Lab Corporation, Washington, USA).
2.5 Quantification of antioxidants by HPLC-DAD after oxidation by free radicals
The concentration of 3-(3,4,5-trihydroxybenzoyl)coumarins and quercetin was determined after oxidation at different times. At 900 µL of 3-trihydroxybenzoylcumarins and equimolar mixtures with quercetin at 5 ppm. Then, 200 µL of AAPH was added at stoichiometrically equivalent concentration.
Subsequently, oxidation was carried out at 0, 30, 60 and 120 min at 40 °C and the persistence of antioxidant compounds after treatment was quantified by HPLC. For the quantification was used 1260 Infinity II Series chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA) using a Chromolith® C18 endcapped column (150 × 4.60 mm, 5.0 µm) (Merck, KGaA-Germany) with DAD diode array detector (Santa Clara, CA, USA). The data was acquired at a wavelength of 272 nm and separation was performed in a gradient system with an optimal mobile phase consisting of: channel (A) acetonitrile and channel (B) 2 % acetic acid in water. The gradient solvent system used was a follows: 0 min, 10 % A; subsequently mobile phase A was increased to 50 % at 30 min. The flow rate was adjusted to 1.0 mL/min using an injection volume of 20 µL.
3 Results and discussion
3.1 Synthesis of 3-(3,4,5-trihydroxybenzoyl)coumarins
Polyhydroxylated coumarin derivatives 1–3 were efficiently synthesized in two steps (Matos et al., 2011), which are briefly described as follows: (i) the synthesis of methoxy-3-benzoylcoumarins 1a-3a and (ii) the synthesis of hydroxy-3-benzoylcoumarins 1–3. These steps are outlined in Scheme 1. Based on the widely used Knoevenagel condensation reaction for the preparation of coumarin derivatives (Brunet et al., 2001; Borges et al., 2005), we used an efficient one-step synthesis to obtain the main core of the methoxylated coumarin precursors 1a − 3a, which were synthesized in good yields (74–98 %). The methoxy derivatives were obtained using the appropriately substituted salicylaldehyde and the corresponding β-ketoester in the presence of piperidine in ethanol under reflux for 2–5 h, obtaining the desired compound as a precipitate that was separated by filtration and further purified by recrystallization in MeOH/DCM. Final compounds 1–3 were then synthesized via hydrolysis of the corresponding methoxy precursors 1a − 3a by treating them with an excess of BBr3 in DCM at 80 °C in a Schlenk tube for 48 h. Final quenched with MeOH and the crude was purified by flash chromatography (58–81 %).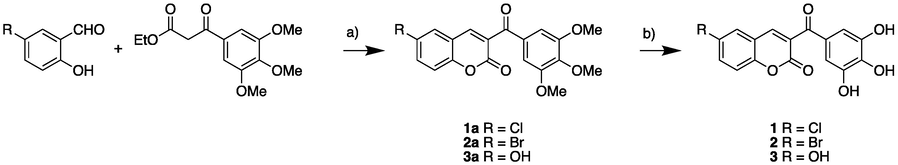
Synthesis of polyhydroxycoumarin derivatives. Reagents and conditions: (a) piperidine, EtOH, reflux, 2–5 h; (b) BBr3, DCM, 80 °C, 48 h.
3.2 Determination of oxygen radical absorbance capacity (ORAC)
3.2.1 ORAC-FL
Quercetin and coumarin derivatives were selected as antioxidants under study. Due to the description that these compounds have had in different applications in biomedicine: antihypertensive, antiparasitic, neuroprotective, anti-inflammatory activity, among others (Kim et al., 2001; Duarte et al., 2001; Bak et al., 2010; Babaei et al., 2018; Chang et al., 2021; Moncada-Basualto et al., 2018). Although they cannot be directly related to antioxidant capacity, they are of great importance in the chemistry of natural products and in the search for functional foods.
The antioxidant capacity of three 3-(3,4,5-trihydroxybenzoyl) derived coumarins, quercetin and their equimolar combinations, was determined through the ORAC-FL methodology.
Fig. 1 shows the kinetics of fluorescence decay of the FL probe via free radicals in the presence of increasing amounts of coumarin 2, which follows the same profile as the rest of the compounds. Table 1 shows that quercetin has the highest peroxyl radical quenching capacity and the longest induction time (time in which the FL probe is protected by the antioxidant from oxidation mediated by the peroxyl radical); followed by the 3 > 2 > 1 compounds.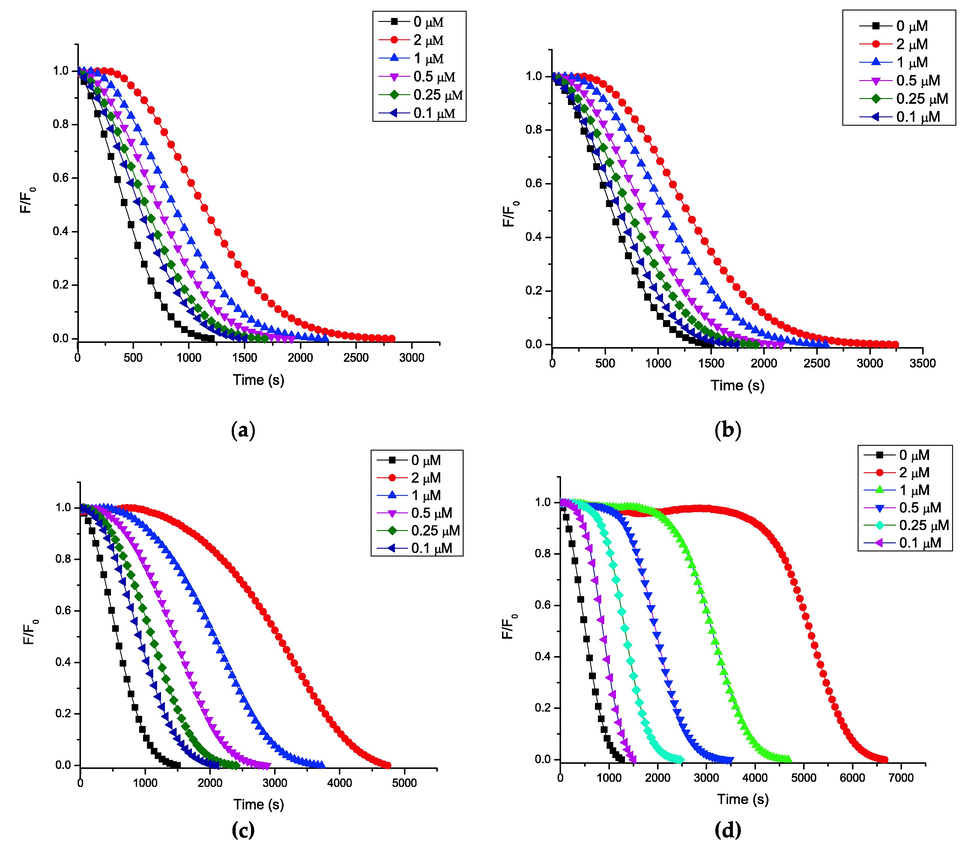
ORAC-FL profile of the compounds: (a) 1; (b) 2; (c) 3; and (d) quercetin.
Compound
Induction time (s)
ORAC-FL
indexstoichiometry
ORAC-FL
[2 µM]
ORAC-PGR
[0.75 µM]
1
300
420
1.7 ± 0.1
4
2
532
350
1.9 ± 0.1
7
3
1006
420
5.5 ± 0.2
12
quercetin
4200
525
11.1 ± 0.1
50
Trolox
4080
210
1.0 ± 0.1
2
21
1200
–
5.47 ± 0.31
15
Additionally, Table 1 shows the ORAC-FL indices determined according to the antioxidant TROLOX. These values indicate that the compounds under study have a higher antioxidant capacity than TROLOX. Structurally, the 3-(3,4,5-trihydroxybenzoyl) coumarin derivatives studied present a trihydroxy-benzaldehyde group at position 3 of the basic coumarin structure. The small structural variation of each of the derivatives is found in position 6 of the base skeleton, compound 3 has a hydroxyl group, while derivatives 1 and 2 have bromine (Br) and chlorine (Cl), respectively. Compound 3 has better anti-oxidant capacity, due to the presence of the hydroxyl group in position 6, which facilitates the HAT-type reaction, since it is in the para position with respect to the carbonyl group, favoring the stabilization of the generated radical (Robledo-O’Ryan et al., 2017). In the case of compounds 1 and 2, the antioxidant capacity is lower due to the halogens (Br and Cl), since being groups with high electronegativity, they behave as electron accepting groups and leave the hydrogens of the hydroxyl groups unprotected. in the trihydroxy-benzaldehyde substituent in position 3 with greater lability. In agreement with was postulated by (Pérez-Cruz et al., 2013), who determined that the presence of a catechol group in the benzoyl substituent and a hydroxyl group in position 6 of the base skeleton of coumarin increases the antioxidant capacity, since catechol allows the stabilization of generated radical, while the hydroxyl group in the position 6 has greater lability to react. The presence of a trihydroxy-benzaldehyde group in position 3 of the coumarin skeleton provides antioxidant capacity, since the oxidation of the catechol group leaves an ortho-semiquinone radical stabilized by delocalization of the unpaired electron in the aromatic ring. In turn, the presence of hydroxyl groups in the meta position is unfavorable in oxidation process, in agreement whit Mura (Mura et al., 2014), who indicated that the presence of 3 hydroxyl groups in a benzene ring decreases the antioxidant capacity, since that can generate hydrogen bond interactions between them. The antioxidant capacity of compound 3 was similar to that determined by Robledo-O'Ryan for coumarin 5-hydroxy-3-(3-hydroxyphenyl) coumarin, which contains fewer hydroxyl groups (Robledo-O’Ryan et al., 2017). Studies conducted by China (Raju et al., 2010) on substituted chromone derivates determined that the presence of halogens or electro-withdrawing groups in position para to the carbonyl group of the skeleton base of coumarin decreases antioxidant activity, while the absence of substituent increases activity.
On the other hand, Niki postulated that shape of curve decay and the values of area under the curve (AUC) depend on the probe molecule, for which longer induction times are related to a low reactivity of the probe in its reaction with free radicals. The AUC considers the induction time, initial speed and the extension of total inhibition in a single value (Niki, 2010; Ou et al., 2001). Therefore, the induction time is determined by amount of radicals that react with the antioxidant molecule and therefore with the stoichiometry of the reaction and the rate of generation of peroxyl radicals (Dai et al., 2008), which is given by equation (1).
Table 1 shows the stoichiometries determined for each of the compounds from equation (1). The values obtained do not agree with a 1:1 stoichiometry according to the HAT mechanism, which may account for the existence of other associated mechanisms (vide infra).
3.2.2 ORAC-PGR
The antioxidant capacity of all the compounds under study was determined using the ORAC-PGR methodology, this probe has a high reactivity with the peroxyl radical (López-Alarcón and Lissi, 2005). Fig. 2 shows the curves of absorbance decrease as a function of time at different concentrations (higher concentrations were also tested obtaining similar results, data not shown), these curves were similar for all the compounds under study; with the exception of quercetin whose ORAC-PGR index (11.5 ± 0.4) has been described by (López-Alarcón and Lissi, 2005). The non-dependence of the area under the curve in relation to the concentration of the 3-(3,4,5-trihydroxybenzoyl) coumarin derivatives can be explained by a very high reactivity of the system since the radicals generated by thermolysis of AAPH completely consume to the antioxidant and after this they degrade the probe. This postulate is since despite the increase in the concentration of antioxidants, there was no increase in the area under the curve. Additionally, this explains the high initial rate of antioxidant consumption, evidenced by the low induction times (Table 1).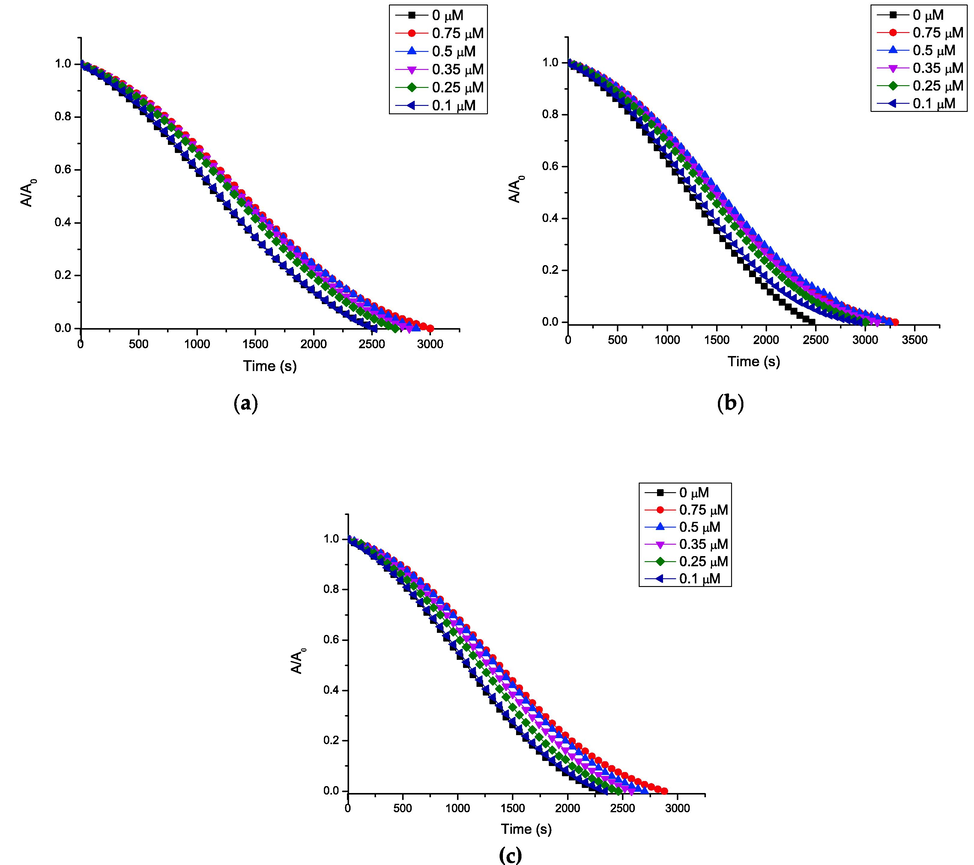
ORAC-PGR profile of the compounds: (a) 1; (b) 2 and (c) 3.
Therefore, the concentrations used were different from those used in the ORAC-FL assay, since at a concentration>0.75 µM the linear relationship between absorbance and concentration was lost, preventing the determination of the ORAC-PGR indices of coumarin derivatives.
The studies carried out by (Vazquez-Rodriguez et al., 2013) on coumarin-chalcone hybrids determined that all the compounds under study were inactive in the ORAC-PGR analyses, except compounds 4 (0.52 ± 0.05) and 5 (1.71 ± 0.10). Based on the results, they indicated that the compounds have a low reactivity towards peroxyl radicals. However, these results can be strongly influenced by the reactivity of the system, preventing an ORAC-PGR value from being obtained.
Based on the results obtained in ORAC-FL and PGR and considering the characteristics of each of the tests, it has been proposed that both indices may be complementary in determining antioxidant capacity. However, like all antioxidant capacity assays, it is influenced by the antioxidant mechanism, which, for example, is different from the DPPH assay (SET mechanism). Therefore, the results obtained are not comparable with each other.
3.3 Determination of antioxidant capacity of combinations
The antioxidant capacity of combinations of 3-(3,4,5-trihydroxybenzoyl) coumarins derived with quercetin in a 1:1 ratio was studied, through the previously used methodologies. Fig. 3 shows the ORAC-FL curves corresponding to the combinations ranging from 0.1 to 2.0 µM. Where, it is observed that the best quenching kinetics corresponds to the combination of compound 3 with quercetin, since it has an approximate induction time of 4260 s at 2 µM.
ORAC-FL profile of the combinations: (a) 1-quercetin; (b) 2-quercetin; and (c) 3-quercetin.
Likewise, it was determined through the ORAC-FL index values that all the combinations have a higher antioxidant capacity than the Trolox reference (Table 2); following the same trend as the 3-(3,4,5-trihydroxybenzoyl) coumarin derivatives alone. However, the ORAC values determined were higher relative to coumarins alone. Where, the combination of compound 1 increased the antioxidant capacity by 206 %, while the combination with compound 3 had an antioxidant capacity similar to that of quercetin alone. This indicates that there is an increase in antioxidant capacity relative to 3-(3,4,5-trihydroxybenzoyl) coumarins alone, but a decrease relative to quercetin alone; indicating that there is no antioxidant synergy between the compounds. This result indicates that there is a certain influence of the reactivities of the antioxidants through this methodology, which differs in part from that described by (López-Alarcón and Lissi, 2005).
sample
ORAC Induction time (s)
ORAC index
stoichiometry
FL1
PGR2
FL
PGR
1
1680
315
5.1 ± 0.3
120.3 ± 14.1
21
2
2160
490
6.5 ± 0.1
130.4 ± 5.4
26
3
4260
805
12.9 ± 0.2
220.9 ± 8.8
52
Similarly, the combinations of 3-(3,4,5-trihydroxybenzoyl) coumarin derivatives with quercetin increased the reaction stoichiometry values with respect to the coumarins alone, being 21, 26 and 52 for the combinations of compounds 1, 2 and 3, respectively. This would indicate the existence of other associated mechanisms, vide infra, since the observed values do not correspond to the stoichiometric relationship with respect to the hydroxyl groups. Therefore, the calculation of the reaction stoichiometry from the induction times both for the single compounds and for the combinations is not a quantitative parameter that describes the HAT reaction for this type of compound. This is possibly since the choice of the probe molecule for the ORAC assays promotes the presence or absence of induction times and modifies the values of ORAC indices (López-Alarcón et al., 2012; Martin et al., 2009; POBLETE et al., 2009), which makes it impossible to compare between ORAC indices determined using different probes.
Subsequently, we studied the effect of the combinations on the reactivity of the system, through ORAC-PGR. Fig. 4 shows the ORAC-PGR curves for the combinations under study at the same concentrations as the ORAC-PGR analysis of the compounds alone. Where, it is determined that the highest antioxidant capacity against peroxyl radicals has the combination of compound 3 with quercetin, with an induction time of 770 s at 0.75 µM, which indicates that at that time the probe is protected by the molecules antioxidants of oxidation mediated by the peroxyl radical. Moncada-Basualto and Olea-Azar (2020), indicate that the inhibition of oxidation mediated by peroxyl radicals in myofibrilar proteins from mango extracts showed differences between extracts in the ORAC-PGR assays, while in ORAC-FL there were not differences. Likewise, (Quezada and Cherian, 2012), studied the antioxidant capacity of camelia sativa and flax flours extracts by ORAC-PGR, determining that the high-fat methanolic extract of camelia flour presented the hidghest ORAC index, indicating that the ORAC-PGR methodology allows the determination of the antioxidant capacity of complex systems (natural extracts). However, it cannot be used as an assay for the total determination of antioxidant capacity, since it uses a HAT reaction mechanism, measuring only the activity on peroxyl radicals and not on other relevant oxygen species such as O2·-, HO·, ONOO– and singlet oxygen.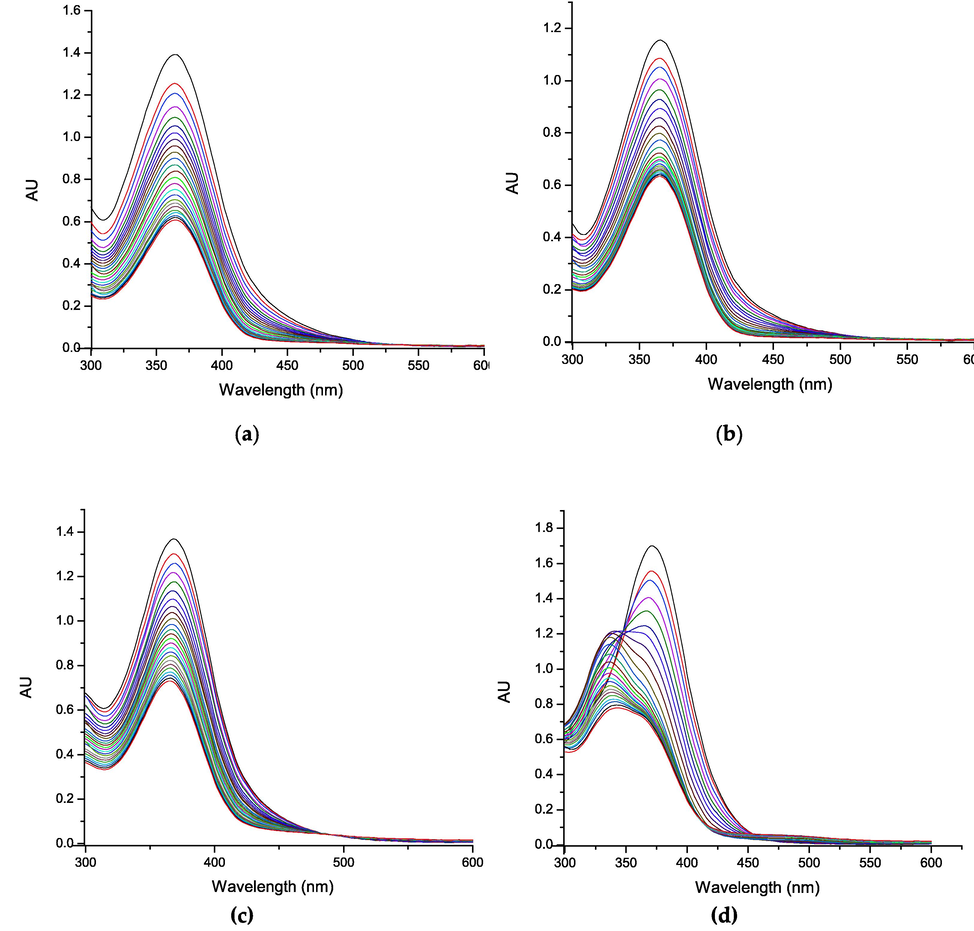
Oxidation kinetic of the compounds: (a) 1; (b) 2; (c) 3; and (d) quercetin by UV–vis.
Table 2 shows the ORAC-PGR index values relative for compounds in combination. Where, all the combinations present greater antioxidant capacity than Trolox, following order: 3 > 2 > 1. However, these values are not representative of the system since the high values indicate a decrease in the reactivity of the system in relation to the compounds alone. Therefore, it is postulated that this methodology is not suitable for describing the antioxidant capacity of binary mixtures of compounds with high reactivity towards peroxyl radicals. This could be one of the reasons why such high ORAC-PGR values are found in some complex blends such as tea. Given that the values of the antioxidants described for this drink present lower values in many cases; this could erroneously lead to assume a synergism of the components present in extracts of natural origin.
As a complementary analysis to the two ORAC methodologies, HPLC was used as well as UV–vis spectrophotometry, where the reaction kinetics of the pure samples and combinations were evaluated. In the latter, it was evaluated whether the antioxidant capacity observed corresponded to a synergistic or antagonistic effect.
The antioxidant capacity of this type of compound can also be affected by the pro-oxidant activity itself, since it has been described that hydroxycoumarins can be potentially pro-oxidant, since some of them can act as photosensitizers and generate singlet oxygen in the presence of light UV–vis (Lagunes and Trigos, 2015; Guerrero et al., 2021). Therefore, the antioxidant capacity could be affected under these conditions, and it would be interesting to be able to evaluate this type of process and its influence on the antioxidant capacity determined by ORAC techniques or another appropriate methodology.
3.4 Quantification of antioxidants alone and in combination during radical oxidation
3.4.1 UV–vis spectrophotometry
In order to obtain more information about the effect of the combinations on the antioxidant capacity of the compounds under study, the absorption spectra were recorded at different times while the reaction with the peroxyl radicals generated by thermolysis of AAPH was taking place.
First, the molar absorptivity coefficients of the 3-(3,4,5-trihydroxybenzoyl) coumarins derived and quercetin were determined. Next, the oxidation of all the antioxidants under study was carried out by reaction with peroxyl radicals. In Fig. 4, a decrease in the intensity of the absorption spectra is observed as a function of time. Additionally, for quercetin, the formation of an absorption band at a lower wavelength is observed as oxidation proceeds, forming an isosbestic point at 340 nm; indicating that there are two or more species in equilibrium whose stoichiometry remains constant over time, evidencing the formation of an oxidation product that could be quantifiable, unlike the oxidation of 3-(3,4,5-trihydroxybenzoyl) coumarins derived, where the formation of this point was not observed.
Table 3 shows the percentage of consumption of antioxidants as a function of time. Where, quercetin has the highest percentage of consumption, indicating greater reactivity. In contrast, for the 3-(3,4,5-trihydroxybenzoyl) coumarins derived, the order of consumption and reactivity is: 3 > 2 > 1. Therefore, the persistence over time of these compounds is less.
Compound
Time (s)
Absorbance (UA)
Concentration (µM)
% antioxidant consumption
1
(366 nm)0
1.39
0.15
0 ± 1 %
6600
0.96
0.12
20 ± 1 %
15,000
0.61
0.09
40 ± 3 %
2
(366 nm)0
1.16
0.21
0 ± 1 %
6600
0.80
0.17
19 ± 1 %
15,000
0.63
0.14
33 ± 2 %
3
(368 nm)0
1.37
0.16
0 ± 1 %
6600
0.96
0.12
25 ± 1 %
15,000
0.73
0.09
44 ± 3 %
Quercetin
(370 nm)0
1.70
0.70
0 ± 1 %
6600
1.15
0.47
33 ± 1 %
15,000
0.63
0.25
64 ± 2 %
If the percentage of consumption is compared with the determined ORAC-FL indices; a direct relationship is obtained in terms of the antioxidant capacity and the reactivity of the compounds. Where, the compound with higher reactivity has a longer induction time in ORAC-FL. Therefore, this methodology not only considers the number of labile hydrogens in the antioxidant molecule but also the reactivity in terms of persistence over time against a free radical.
When analyzing the combinations (Fig. 5), it is observed that there is no formation of absorption maxima that allows each compound to be differentiated. In addition, it is evident that while the reaction time increases, there is a decrease in the absorption band of the combination, forming an isosbestic point in all the combinations under study, which could correspond to quercetin.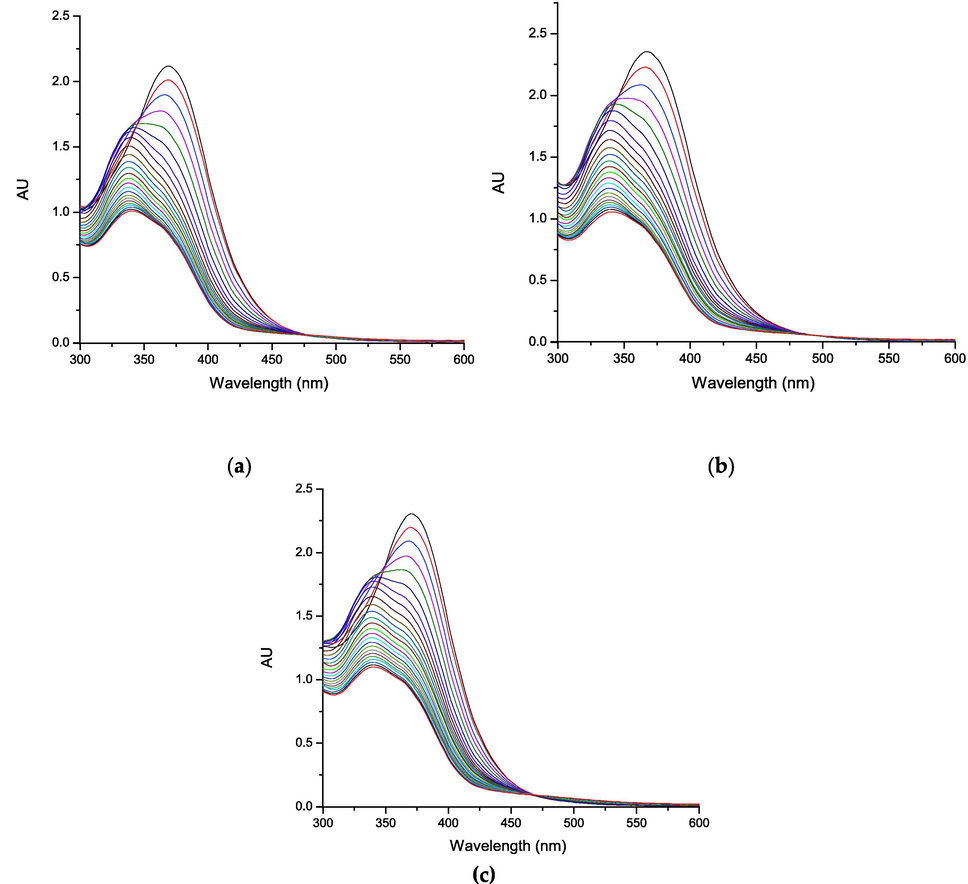
Oxidation kinetic of the combinations: (a) 1-quercetin; (b) 2-quercetin; and (c) 3-quercetin by UV–vis.
The Table 4 shown, that consumption percentages of the antioxidant compounds in combination correspond to the average of the consumption percentages of the pure compounds separately, this would indicate a possible antagonism. Since the spectra obtained from the combinations did not present a deconvolution of the absorption bands corresponding to the pure compounds, an analysis was carried out by means of liquid chromatography.
Time (s)
Component
Abs. (UA)
Concentration (µM)
% antioxidant consumption
1
0
1
(370 nm)2.12
0.21
0 ± 1 %
6600
1.60
0.17
19 ± 1 %
15,000
0.82
0.11
48 ± 1 %
0
Quercetin
(370 nm)2.12
0.88
0 ± 1 %
6600
1.60
0.66
25 ± 1 %
15,000
0.82
0.33
63 ± 1 %
2
0
2
(370 nm)2.34
0.36
0 ± 1 %
6600
1.63
0.27
25 ± 1 %
15,000
0.89
0.18
50 ± 1 %
0
Quercetin
(370 nm)2.34
0.97
0 ± 1 %
6600
1.63
0.67
31 ± 1 %
15,000
0.89
0.36
63 ± 1 %
3
0
3
(370 nm)2.31
0.27
0 ± 1 %
6600
1.60
0.19
29 ± 1 %
15,000
0.92
0.11
59 ± 1 %
0
Quercetin
(370 nm)2.31
0.96
0 ± 1 %
6600
1.60
0.66
32 ± 1 %
15,000
0.92
0.37
61 ± 1 %
3.4.2 HPLC-DAD
The oxidation process of the pure compounds and combination was carried out using the same methodology described for point 3.4.1. The consumption kinetics of the pure 3-(3,4,5-trihydroxybenzoyl) coumarins and the combinations with quercetin were monitored in 4 steps: 0, 30, 60 and 120 min. Prior to the analysis of the oxidation kinetics, the chromatograms of each of the 3-(3,4,5-trihydroxybenzoyl) coumarins derived and quercetin were obtained, identifying the retention times (Table 5).
Compound
Retention time (min)
1
19.46
2
17.83
3
5.80
Quercetina
6.63
16.43
Then, the chromatographic separation of a multicomponent mixture of all the antioxidants under study at a concentration of 5 mg/L was performed. As can be seen in Fig. 6, there is no peak overlap that affects the identification of the compounds.
Chromatogram of the compounds 1, 2, 3 and quercetin by HPLC-DAD.
In Fig. 7, the chromatograms of the pure compounds at different times of the reaction kinetics are observed. It is possible to notice that as the reaction time increases, there is a decrease in the absorbance intensity of each of the compounds; in the case of quercetin, the formation of a peak was observed (RT. 5.1 min) that increased the intensity of absorbance as the reaction time progressed.
Oxidation kinetic of the compounds: (a) 1; (b) 2; (c) 3; and (d) quercetin by HPLC-DAD.
In the chromatogram obtained after oxidation for 60 min, the formation of a broad peak was observed at 25 min, which could correspond to an oxidation product of the compounds; Similarly, in the reaction time of 120 min, all the compounds present a characteristic peak at 35 min, which could correspond to the oxidation products. For the structural determination of these products, it would be necessary in future studies to carry out a fragmentation analysis of each of the compounds by means of liquid chromatography coupled to mass spectrometry.
Table 6 shows the variation of the areas of the characteristic peaks of each of the pure compounds; it is postulated that all the compounds except for compound 3 have been completely consumed after 120 min of reaction. For compounds 1 and 2, the percentage of consumption at 30 min was 90 %, which indicates a low antioxidant capacity against the peroxyl radical, while compound 3 had a percentage of consumption of 31 % at 120 min. reaction; indicating the high antioxidant capacity. For quercetin, it is observed that the peak with a retention time of 6.63 min at 60 min of reaction increased its area with respect to 30 and 0 min, which demonstrates the formation of a product with a different molecular structure. When comparing the results obtained for the oxidation kinetics with those of the UV–vis spectrophotometry, differences are observed, since the consumption of compound 3 was greater than for the rest of the coumarins under study. However, the impossibility of discriminating the formation of oxidation products makes the HPLC methodology more valid in the evaluation of oxidation products.
Compound
Retention time (rt) (min)
Area
% antioxidant consumption
1
0
94,46
0 ± 1 %
30
5,45
94 ± 2 %
60
0
100 ± 2 %
120
0
100 ± 32 %
2
0
106,03
0 ± 1 %
30
90,94
14 ± 3 %
60
0
100 ± 1 %
120
0
100 ± 2 %
3
0
101,51
0 ± 1 %
30
91,93
10 ± 1 %
60
78,13
25 ± 3 %
120
72,28
31 ± 3 %
Quercetin
0
13,90 (rt:6.63 min)
105,26 (rt:16.43 min)0 ± 1 %
0 ± 1 %
30
2,86 (rt:6.63 min)
41,35 (rt:16.43 min)79 ± 2 %
61 ± 2 %
60
78,17 (rt:6.63 min)
0 (rt:16.43 min)462 ± 3 %
100 ± 3 %
120
0 (rt:6.63 min)
0 (rt:16.43 min)100 ± 2 %
100 ± 1 %
In the analysis of the combinations (Fig. 8), it was observed that there is a high consumption of quercetin, since at 60 min of reaction the quercetin peak (RT: 15 min) is not quantifiable, while the peak at 3-(3,4,5-trihydroxybenzoyl) coumarins persist. Likewise, it was observed that at 120 min of oxidation there is presence of the coumarin compound, which would indicate a greater reactivity of quercetin. This is related to what was obtained by ORAC-FL for the combinations, where the indices obtained were similar to quercetin alone. Therefore, there was a protection of coumarins by quercetin to oxidation by peroxyl radicals; being the ORAC-FL methodology not useful in the determination of antioxidant combinations, since it depends on the reactivity of the antioxidants, unlike what was previously described (Dorta et al., 2015).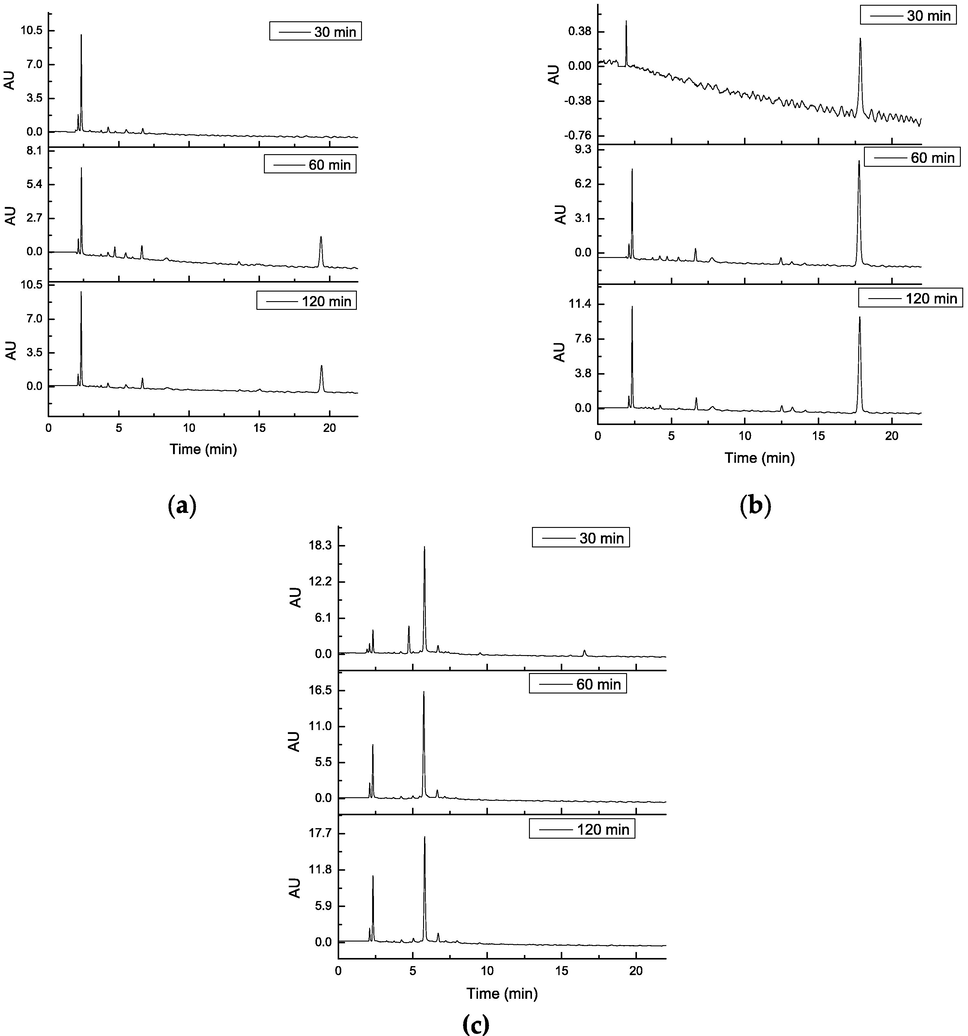
Oxidation kinetic of the compounds and quercetin combination: (a) 1-quercetin; (b) 2-quercetin; (c) 3-quercetin by HPLC-DAD.
As in the chromatograms of the pure compounds, the presence of a broad peak is observed at an approximate time of 25 min, which could be due to a convolution of peaks of compounds generated in the oxidation, in addition to the peak observed in the pure compounds with a retention time of 35 min, it only appears in the combination of compound 3 with quercetin. Table 7 shows the percentages of consumption of each of the compounds in combination.
Time
(min)
Component
Area
% antioxidant consumption
1
0
1
94.46
0 ± 1 %
30
50.02
47 ± 1 %
60
27.86
71 ± 3 %
120
32.83
65 ± 2 %
0
Quercetin
13.90 (rt:6.63 min)
105.26 (rt:16.43 min)0 ± 1 %
0 ± 1 %
30
7.40 (rt:6.63 min)
50.02 (rt:16.43 min)47 ± 3 %
53 ± 2 %
60
7.04 (rt:6.63 min)
N/A1 (rt:16.43 min)47 ± 1 %
100 ± 1 %
120
6.81 (rt:6.63 min)
N/A1 (rt:16.43 min)51 ± 1 %
100 ± 1 %
2
0
2
106.03
0 ± 1 %
30
106.03
0 ± 1 %
60
109.16
+ 3 ± 1 %
120
117.64
+11 ± 2 %
0
Quercetin
13.90 (rt:6.63 min)
105.26 (rt:16.43 min)0 ± 1 %
0 ± 1 %
30
8.89 (rt:6.63 min)
N/A1 (rt:16.43 min)36 ± 2 %
100 ± 3 %
60
7.29 (rt:6.63 min)
N/A1 (rt:16.43 min)48 ± 1 %
100 ± 2 %
120
8.6 (rt:6.63 min)
N/A1 (rt:16.43 min)38 ± 1 %
100 ± 1 %
3
0
3
101.51
0 ± 1 %
30
110.95
+9 ± 1 %
60
102.98
+1 ± 1 %
120
104.52
+3 ± 1 %
0
Quercetin
13.90 (rt:6.63 min)
105.26 (rt:16.43 min)0 ± 1 %
0 ± 1 %
30
7.10 (rt:6.63 min)
9.73 (rt:16.43 min)49 ± 1 %
91 ± 1 %
60
7.08 (rt:6.63 min)
N/A1 (rt:16.43 min)49 ± 1 %
100 ± 1 %
120
9.13 (rt:6.63 min)
N/A1 (rt:16.43 min)34 ± 1 %
100 ± 1 %
With the data obtained, it is determined that in combinations, quercetin is the one that reacts first with the radical generated by AAPH. Additionally, it is observed that in the combinations with compounds 2 and 3 there are percentages that increase, due to the formation of oxidation products that absorb at the same wavelength, for which the peak area increases.
Based on the above, it was determined that the ORAC methodologies would not be useful in the characterization of antioxidant capacity of combinations in relation to the reactivity of the metabolites present in it.
However, the ORAC-FL methodology is useful in explaining the antioxidant capacity of pure compounds in terms of the number of hydroxyl groups they contain. This is not the case with the ORAC-PGR methodology, where the analysis of the results is difficult due to the dependence on the reactivity of the compounds; the analysis of the results can be confusing. An interesting methodology to analyze would be the ORAC-EPR, where it would be possible to differentiate between the type of radical formed by thermolysis through spin trapping and deliver relevant information regarding the reactivity of the system and quantification of the antioxidant capacity. Likewise, the HPLC technique would allow an indirect analysis of the antioxidant capacity of the compounds, being complementary methodologies to the ORAC studies.
It is interesting to note that previously it was described that the ORAC-FL methodology only provided information regarding the stoichiometry of the antioxidant reaction. However, we have shown that in combinations there is a strong dependence on the reactivity of the system, and it could be used to determine this parameter in combinations, since the ORAC-PGR methodology presents greater dependence on this parameter and in combination it presents greater interferences that lead to ambiguous values.
4 Conclusions
The ORAC-FL methodology is useful for determining the antioxidant capacity of pure compounds, while it is difficult in mixtures, since the antioxidant capacity cannot be characterized in relation to the metabolites present in it. Likewise, the ORAC-PGR methodology is not optimal for the determination of the antioxidant capacity in pure compounds of high reactivity and binary combinations, since it presents an unusual behavior, which does not account for the antioxidant capacity or reactivity of the studied combination.
The study of the reaction kinetics allowed to evaluate the oxidation process of pure coumarins and combinations; evidencing the formation of oxidation products through variations in the absorption spectra, without being able to attribute these bands to compounds of known structure.
The HPLC methodology determined the formation of oxidation products as the reaction time increased in pure coumarins and combinations; observing in the latter that quercetin reacted first with the radical generated by the thermolysis of AAPH, protecting coumarin, indicating protection by quercetin over coumarin. Therefore, ORAC methodologies would not be useful in characterizing the antioxidant capacity of combinations in relation to the reactivity of the metabolites present in them. Therefore, the description of the antioxidant capacity of extracts of natural origin through these methodologies is not recommended, since it could only account for the antioxidant capacity of the metabolite with greater reactivity against the oxygen-centered radicals formed. Instead, the use of more specific methods such as ORAC-EPR is suggested.
Acknowledgments
This research was funded by Agencia Nacional de Investigación y Desarrollo, ANID, Chileunder the project Fondecyt Postdoctoral N° 3190449 and project Fondecyt Regular N° 1190340.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- (U.S.), N. D. L. (U. S. . A.-N. D. L. (2010). USDA database for the oxygen radical absorbance capacity (ORAC) of selected foods. USDA. https://handle.nal.usda.gov/10113/43336.
- Antioxidant capacity of herbal infusions and tea extracts: A comparison of ORAC-fluorescein and ORAC-pyrogallol red methodologies. Food Chem.. 2008;107(3):1114-1119.
- [CrossRef] [Google Scholar]
- AOAC SMPR 2011.011 Standard Method Performance Requirements for in vitro Determination of Total Antioxidant Activity in Foods, Beverages, Food Ingredients, and Dietary Supplements. (2012). J. AOAC Int., 95(6), 1557. https://doi.org/10.5740/jaoac.int.SMPR_2011_011.
- Comparisons of ESR and HPLC methods for the detection of OH. radicals in ischemic/reperfused hearts: A relationship between the genesis of free radicals and reperfusion arrhythmias. Biochem. Pharmacol.. 1993;45(4):961-969.
- [CrossRef] [Google Scholar]
- A critical appraisal of the use of the antioxidant capacity (TEAC) assay in defining optimal antioxidant structures. Food Chem.. 2003;80(3):409-414.
- [CrossRef] [Google Scholar]
- Quercetin in Food: Possible Mechanisms of Its Effect on Memory. J. Food Sci.. 2018;83(9):2280-2287.
- [CrossRef] [Google Scholar]
- Isolation and Analysis of Bioactive Constituents of Sour Cherry (Prunus cerasus) Seed Kernel: An Emerging Functional Food. J. Med. Food. 2010;13(4):905-910.
- [CrossRef] [Google Scholar]
- Inverse relationship between ESR spin trapping of oxyradicals and degree of functional recovery during myocardial reperfusion in isolated working rat heart. Cardiovasc. Res.. 1990;24(4):263-270.
- [CrossRef] [Google Scholar]
- Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Current Med. Chem.. 2005;12(8):887-916.
- [CrossRef] [Google Scholar]
- Novel polyaminocarboxylate chelates derived from 3-aroylcoumarins. Tetrahedron. 2001;57(15):3105-3116.
- [CrossRef] [Google Scholar]
- SIRT5-Related Desuccinylation Modification Contributes to Quercetin-Induced Protection against Heart Failure and High-Glucose-Prompted Cardiomyocytes Injured through Regulation of Mitochondrial Quality Surveillance. Oxid. Med. Cell. Longevity. 2021;2021:5876841.
- [CrossRef] [Google Scholar]
- An introduction to free radical biochemistry. Br. Med. Bull.. 1993;49(3):481-493.
- [CrossRef] [Google Scholar]
- Antioxidant synergism of green tea polyphenols with α-tocopherol and l-ascorbic acid in SDS micelles. Biochimie. 2008;90(10):1499-1505.
- [CrossRef] [Google Scholar]
- The ORAC (oxygen radical absorbance capacity) index does not reflect the capacity of antioxidants to trap peroxyl radicals. RSC Adv.. 2015;5(50):39899-39902.
- [CrossRef] [Google Scholar]
- Use of the Oxygen Radical Absorbance Capacity (ORAC) Assay to Predict the Capacity of Mango (Mangifera indica L.) By-Products to Inhibit Meat Protein Oxidation. Food Anal. Methods. 2017;10(2):330-338.
- [CrossRef] [Google Scholar]
- Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol.. 2001;133(1):117-124.
- [CrossRef] [Google Scholar]
- Antagonistic activity of hydroxycoumarin-based antioxidants as possible singlet oxygen precursor photosensitizers. Dyes Pigm.. 2021;192:109447
- [CrossRef] [Google Scholar]
- Halliwell, B., Gutteridge, J.M.C., 2015. Ageing, nutrition, disease, and therapy: a role for antioxidants? In: Free Radicals in Biology and Medicine, 5th ed.. Oxford University Press. https://doi.org/10.1093/acprof:oso/9780198717478.003.0011.
- Oxidative stress and free radicals. J. Mol. Struct. (Thoechem). 2003;666–667:387-392.
- [CrossRef] [Google Scholar]
- Competitive Reduction of Perferrylmyoglobin Radicals by Protein Thiols and Plant Phenols. J. Agric. Food. Chem.. 2014;62(46):11279-11288.
- [CrossRef] [Google Scholar]
- Protective effects of new antioxidant compositions of 4-methylcoumarins and related compounds with dl-α-tocopherol and l-ascorbic acid. J. Sci. Food Agric.. 2018;98(10):3784-3794.
- [CrossRef] [Google Scholar]
- Neuroprotective Constituents from Hedyotis diffusa. J. Nat. Prod.. 2001;64(1):75-78.
- [CrossRef] [Google Scholar]
- High fidelity triangular sweep of the magnetic field for millisecond scan EPR imaging. J. Magn. Reson.. 2021;329:107024
- [CrossRef] [Google Scholar]
- Photo-oxidation of ergosterol: Indirect detection of antioxidants photosensitizers or quenchers of singlet oxygen. J. Photochem. Photobiol., B. 2015;145:30-34.
- [CrossRef] [Google Scholar]
- Interaction of pyrogallol red with peroxyl radicals. A basis for a simple methodology for the evaluation of antioxidant capabilities. Free Radical Res.. 2005;39(7):729-736.
- [CrossRef] [Google Scholar]
- Influence of the Target Molecule <break></break>on the ORAC Index. Emerging Trends in Dietary Components for Preventing and Combating Disease. 2012;vol. 1093:24-417.
- [Google Scholar]
- A novel and simple ORAC methodology based on the interaction of Pyrogallol Red with peroxyl radicals. Free Radical Res.. 2006;40(9):979-985.
- [CrossRef] [Google Scholar]
- Influence of the Target Molecule on the Oxygen Radical Absorbance Capacity Index: A Comparison Between Alizarin Red- and Fluorescein-Based Methodologies. J. Med. Food. 2009;12(6):1386-1392.
- [CrossRef] [Google Scholar]
- MAO inhibitory activity modulation: 3-Phenylcoumarins versus 3-benzoylcoumarins. Bioorg. Med. Chem. Lett.. 2011;21(14):4224-4227.
- [CrossRef] [Google Scholar]
- Remarkable antioxidant properties of a series of hydroxy-3-arylcoumarins. Bioorg. Med. Chem.. 2013;21(13):3900-3906.
- [CrossRef] [Google Scholar]
- Spectrophotometric Methods and Electronic Spin Resonance for Evaluation of Antioxidant Capacity of Food BT - Spectroscopic Techniques & Artificial Intelligence for Food and Beverage Analysis. Singapore: Springer; 2020. p. :53-75.
- [CrossRef]
- Evaluation of Trypanocidal and Antioxidant Activities of a Selected Series of 3-amidocoumarins. Medicinal Chemistry. 2018;14(6):573-584.
- [CrossRef] [Google Scholar]
- New insights into the antioxidant activity of hydroxycinnamic and hydroxybenzoic systems: Spectroscopic, electrochemistry, and cellular studies. Free Radical Res.. 2014;48(12):1473-1484.
- [CrossRef] [Google Scholar]
- Assessment of Antioxidant Capacity in vitro and in vivo. Free Radical Biol. Med.. 2010;49(4):503-515.
- [CrossRef] [Google Scholar]
- Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food. Chem.. 2001;49(10):4619-4626.
- [CrossRef] [Google Scholar]
- Synthesis and antioxidant study of new polyphenolic hybrid-coumarins. Arabian J. Chem.. 2018;11(4):525-537.
- [CrossRef] [Google Scholar]
- Synthesis and Electrochemical and Biological Studies of Novel Coumarin-Chalcone Hybrid Compounds. J. Med. Chem.. 2013;56(15):6136-6145.
- [CrossRef] [Google Scholar]
- Oxygen radical antioxidant capacity (ORAC) values of herbal teas obtained employing different Methodologies can provide complementary data. J. Chil. Chem. Soc.. 2009;54:154-157. scielocl
- [Google Scholar]
- Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods. 2015;18:797-810.
- [CrossRef] [Google Scholar]
- Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food. Chem.. 2005;53(10):4290-4302.
- [CrossRef] [Google Scholar]
- Lipid characterization and antioxidant status of the seeds and meals of Camelina sativa and flax. Eur. J. Lipid Sci. Technol.. 2012;114(8):974-982.
- [CrossRef] [Google Scholar]
- α-Glucosidase inhibitory antihyperglycemic activity of substituted chromenone derivatives. Bioorg. Med. Chem.. 2010;18(1):358-365.
- [CrossRef] [Google Scholar]
- Synthesis, antioxidant and antichagasic properties of a selected series of hydroxy-3-arylcoumarins. Bioorg. Med. Chem.. 2017;25(2):621-632.
- [CrossRef] [Google Scholar]
- Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Sci.. 2011;89(2):166-173.
- [CrossRef] [Google Scholar]
- Antioxidant Capacity of Pure Compounds and Complex Mixtures Evaluated by the ORAC-Pyrogallol Red Assay in the Presence of Triton X-100 Micelles. Molecules. 2010;15(9)
- [CrossRef] [Google Scholar]
- Ruiz, R.P., 2001. Gravimetric Determination of Water by Drying and Weighing. Current Protocols Food Anal. Chem., 00(1), A1.1.1-A1.1.1. https://doi.org/10.1002/0471142913.faa0101s00.
- Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Sci. Technol. Int.. 2002;8(3):121-137.
- [CrossRef] [Google Scholar]
- First Web-Based Database on Total Phenolics and Oxygen Radical Absorbance Capacity (ORAC) of Fruits Produced and Consumed within the South Andes Region of South America. J. Agric. Food. Chem.. 2012;60(36):8851-8859.
- [CrossRef] [Google Scholar]
- Assessment of antioxidant capacity for scavenging free radicals in vitro: A rational basis and practical application. Free Radical Biol. Med.. 2012;52(7):1242-1252.
- [CrossRef] [Google Scholar]
- Synthesis of coumarin–chalcone hybrids and evaluation of their antioxidant and trypanocidal properties. MedChemComm. 2013;4(6):993-1000.
- [CrossRef] [Google Scholar]
- Design, synthesis and antibacterial study of new potent and selective coumarin–chalcone derivatives for the treatment of tenacibaculosis. Bioorg. Med. Chem.. 2015;23(21):7045-7052.
- [CrossRef] [Google Scholar]
- Atypical antioxidant activity of non-phenolic amino-coumarins. RSC Adv.. 2018;8(4):1927-1933.
- [CrossRef] [Google Scholar]
- Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci.. 1987;84(5):1404-1407.
- [CrossRef] [Google Scholar]







