Translate this page into:
Experimental analysis and thermodynamic modelling of lenalidomide solubility in supercritical carbon dioxide
⁎Corresponding author at: Department of Chemical Engineering, Tarbiat Modares University, P.O. Box: 14115-111, Tehran, Iran. n.saadati@modares.ac.ir (Nedasadat Saadati Ardestani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Lenalidomide (LND) solubility in supercritical CO2 (sc-CO2) was measured for the first time. Solubility data were correlated by different density-based models. Experimental data was also correlated by Modeified Wilson and SRK models. The accuracy of the models was evaluated by statistical criteria (AARD%), (Radj). Thermodynamic enthalpies of Lenalidomide were estimated.
Abstract
Reduction of the size of pharmaceutical particles to micro/nano scale is an approved strategy to enhance their dissolution rate at decremented side effects. Production of drug micro/nanoparticles via a supercritical carbon dioxide (sc-CO2)- based process requires accurate data on the drug solubility in sc-CO2. In this study, the solubility of Lenalidomide (LND), an anti-cancer, was experimentally determined in sc-CO2 at various temperatures (308–338 K) and pressures (120–300 bar). Furthermore, the obtained solubility data were correlated by different theoretical methods. LND solubility, in terms of mole fraction, was obtained in the range of 0.02 × 10−4–1.08 × 10−4 depending on the conditions. The empirical models with different adjustable parameters, Soave–Redlich–Kwong equation of state (SRK-EoS) with two parameters van der Waals (vdW2) mixing rule, and expanded liquid theory (modified Wilson’s model) were applied for experimental data correlation. According to the results, modified Wilson’s model can correlate LND solubility in sc-CO2 with high accuracy. However, the SRK-EoS did not show acceptable accuracy in the correlation of LND solubility in sc-CO2. Among the empirical models, Alwi and Garlapati, Sung and Shim, Ch and Madras, Hozhabr et al., Garlapati and Madras, Keshmiri et al., Sparks et al., Reddy and Garlapati, Bian et al. (2011) and Belghait et al. showed Radj values higher than 0.99 and produced the best correlation with 3,4, 5, 6 and 8 adjustable parameters, respectively. Moreover, the approximate values of total mixing heat ( ) as well as vaporization ( ), and solvation ( ) enthalpies were computed.
Keywords
Lenalidomide
Solubility
Supercritical carbon dioxide
Empirical model
Modified Wilson’s model
Soave-Redlich-Kwong (SRK) equation of state
Nomenclature
-
Adjustable parameters of empirical model
- AARD%
-
Average absolute relative deviation
-
Energy parameter of the cubic EoS (Nm4 mol−2)
- b
-
Volume parameter for equations of state (m3 mol−1)
- f2L
-
The fugacity of the solid solute in the supercritical phase
- f2s
-
The fugacity of the solute in the solid phase
- Hf
-
Molar heat of fusion(kJ.mol−1)
- gE
-
Excess Gibbs free energy
- kij
-
Binary interaction parameters in the mixing rules
- lij
-
Binary interaction parameters in the mixing rules
- MSR
-
Mean square regression
- MSE
-
Mean square residual
- N
-
Number of data points, dimensionless
- Psub
-
Sublimation pressure (Pa)
- Q
-
Number of independent variables
- R2
-
Correlation coefficient
- Radj
-
Adjusted correlation coefficient
- S
-
Equilibrium solubility
- SSE
-
Error sum of squares
- SST
-
Total sum of squares
- SSR
-
Regression sum of squares
-
Critical temperature
- vs
-
Solid molar volume
- vdW2
-
Van der Waals mixing rule with two adjustable parameters
- y
-
Mole fraction solubility
- Z
-
Number of adjustable parameters
- α(Tr, ω)
-
Temperature-dependent function in the attractive parameter of the EoS
- φ
-
Fugacity coefficient
- ω
-
Acentric factor
- α
-
Regressed parameters of Wilson’s model
- β
-
Regressed parameters of Wilson’s model
- Λ′
-
Regressed parameters of Wilson’s model
- Λ
-
Adjustable parameters
-
The activity coefficient of the solid solute at infinite dilution
Greek symbols
- cal
-
Calculated
- exp
-
Experimental
- i, j
-
Component
Superscripts
1 Introduction
With the commercial name of Revlimid, Lenalidomide (LND) (C13H13N3O3) acts as an angiogenesis inhibitor, an antineoplastic agent as well as an immunomodulatory drug. It is the first FDA-approved oral medication for the treatment of multiple myeloma, since 2004 (Li et al., 2019). In recent years, it was also approved for the treatment of Mantle Cell Lymphoma, Myelodysplastic syndromes (MDS) and deletion 5q Myelodysplastic syndromes (Attal et al., 2012). This drug also exhibited promising therapeutic effects in other hematologic disorders. However, hydrophobicity and poor solubility of LND in water hinder its penetration into tumors; i.e. low bioavailability (less than 33%) (Yang et al., 2019). Therefore, long term usage or high doses of this drug may be accompanied by severe side effects including immune system depletion, metastasis to neighboring organs, cutaneous adverse neutropenia, deep vein thrombosis, infection, and hematological cancer (Patrizi et al., 2014).
Various approaches such as salt formation, co-crystallization, amorphous solid dispersion (ASD), and particle size reduction (micronization/nanonization) have been developed in medicinal chemistry, to enhance aqueous solubility and bioavailability (Liu et al., 2016). Diverse measures have been proposed to improve the aqueous solubility of LND and therapeutic efficacy; including, co-crystallization with various substances such as urea and 3,5-dihydroxybenzoic acid (Song et al., 2014) and gallic acid (GA), formation of different LND salts including methanesulfonate (Rangineni, 2010), sulfate, hydrochloride, and hydrosulphate (Stiegel, 2011), hydrates of benzene sulfonate and p-toluene sulfonate (Eupen, 2011) and acesulfame (Chen et al., 2019), as well as LND conjugation onto polymeric nanoparticles (such as chitosan (Gomathi et al., 2014) and LND complexing with gold ions (Arib and Spadavecchia, 2020).
Among the mentioned methods, micronization/nanonization has attracted a huge deal of attention due to enhancing the surface area of drug particles and incrementing drug solubility, hence lowering drug dosage and side effects. Supercritical fluid (SCF)-based processes are green and clean technologies for micro/nanoparticles formation. Low operational temperature, high quality products with uniform morphology and narrow size distribution, and elimination or significant reduction of used organic solvents are the main advantages of these processes (Ardestani and Amani, 2021). Supercritical carbon dioxide (sc-CO2) is the most commonly used SFC due to its low price, mild critical pressure and temperature, environmental compatibility, non-exclusivity, non-toxicity and chemical stability (Sodeifian et al., 2016; Sodeifian et al., 2018; Sodeifian et al., 2019). The solubility of a drug in sc-CO2 is the essential parameter in producing fine particles. This parameter determines the feasibility of using the sc-CO2 process for the considered drug and also the role of sc-CO2 in the supercritical process (as solvent, anti-solvent or reaction media) (Amani et al., 2021). Generally, RESS-based processes can be utilized for the preparation of nanoparticles and submicron drugs with high solubility in SC–CO2. In contrast, anti-solvent processes are recommended for the preparation of materials with low solubility in SC–CO2. To this end, the solubility of solid pharmaceuticals should be estimated in SCFs to select the proper method (Ardestani and Amani, 2021; Saadati Ardestani et al., 2020). Furthermore, solubility should be determined at a wide range of pressures and temperatures for the industrial development of supercritical processes. Given this necessity, the determination of the solubility of different drugs in sc-CO2 has become one of the most interesting pharmaceutical research topics during the past two decades. In this regard, solubility of various drugs has been assessed since the beginning of 2020, among which; Chloroquine (Pishnamazi, 2021), Capecitabine (Ardestani et al., 2020), Montelukast (Sajadian et al., 2022); Azathioprine (Sodeifian, 2020), Temozolomide (Zabihi, 2021), Lornoxicam (Pelalak, 2021), Aprepitant (Sodeifian et al., 2017); Gliclazide and Captopril (Wang et al., 2021), Glibenclamide (Esfandiari and Sajadian, 2022), Decitabine (Pishnamazi et al., 2021); Tamoxifen (Pishnamazi, 2020), Busulfan (Pishnamazi, 2020), Fenoprofen (Zabihi et al., 2020), Salsalate (Zabihi, 2021), Lornoxicam (Pelalak, 2021), Tolmetin (Pishnamazi et al., 2020), Tenoxicam (Zabihi et al., 2021); Carbamazepine (Kalikin, 2020); Sodium valproate (Sodeifian et al., 2020); Minoxidil (Sodeifian et al., 2020); Loxoprofen (Zabihi et al., 2020), Favipiravir (Sajadian et al., 2022); ipriflavone (Wang and Su, 2020), and Methylsalicylic Acid Isomers (Wang et al., 2021) can be mentioned.
However, experimental measurement of the sc-CO2 solubility of all the drugs in a wide range of pressure and temperature is costly, time-consuming, and even impossible in some cases. Therefore, several theoretical predictive models such as equation of states (EoSs), expanded liquid models and empirical models have been developed for correlating the sc-CO2 solubility of various substances at different operational conditions. EoS models (cubic and non-cubic) are one of the most popular theoretical models in which sc-CO2 is regarded as a condensed gas and the modeling is performed according to solute fugacity coefficient. Cubic EoSs can be rewritten as a cubic function of molar volume (e.g. Peng-Robinson (PR) and Soave- Redlich-Kowang (SRK)), while non-cubic models are based on statistical associating fluid theory (SAFT) (e.g. Perturbed-Chain Polar Statistical Associating Fluid Theory (PCP-SAFT)). In expanded liquid models (e.g. universal quasi-chemical (UNIQUAC) and modified Wilson's models), sc-CO2 is considered as an expanded liquid and modelling was based on the solute activity coefficient. The necessity of knowledge on the solute physicochemical properties such as acentric factor, critical pressure and temperature, sublimation pressure and its molar volume is the main challenge of EoS and expanded liquid models. These properties are usually unknown, especially for complex pharmaceutical components, and should be computed by various group contribution (GC) methods. In return, the empirical models have been developed according to linear relationship between the logarithm of solute solubility and the sc-CO2 density. These models only need to know pressure, temperature, and sc-CO2 density while their correlation accuracy is comparable to the EoSs method (Zabihi, 2021). Noteworthy, the accuracy of the proposed models could be different for each pharmaceutical component, making it impossible to specify the most accurate model for the proper correlation of the sc-CO2 solubility of all drugs (Zabihi et al., 2020). So, the correct predictive model indicating the best fitting with the experimental results was determined by comparison between the correlation and experimental results.
The sc-CO2 solubility of many drugs has been measured and correlated as a function of pressure and temperature while no report can be found on LND solubility in sc-CO2. Thus, this research is aimed to experimentally measure of LND solubility in sc-CO2 at various temperatures (308–338 K) and pressures (120–300 bar). Afterward, experimental solubility results were correlated by thirty well-known empirical density-based models, EoS model (SRK) and expanded liquid models (modified Wilson's model). The results of these models were validated by computing some statistical criteria including the average absolute relative deviation (AARD%), the adjusted correlation coefficient (Radj), and F-value.
2 Materials and methods
2.1 Materials
Lenalidomide (LND) (CAS No. 191732–72-6) was purchased from Abidi pharmaceutical company, with a minimum purity of 99%. Carbon dioxide (CO2), with a purity of 99.98% was provided from Oxygen Novin Co. (Shiraz, Iran). Analytical-grade methanol was provided by Merck (Darmstadt, Germany). All of these compounds were used with no additional purification.
2.2 Experimental procedure for solubility determination
The experimental setup for determining the solubility of LND in sc-CO2 is schematically depicted in Fig. 1. All the equipment, piping and connections were made from stainless steel 316 at 1/8″ in size. As can be seen in Fig. 1, after passing the CO2 gas from the molecular sieve filter (1 μm in pore size), it enters the refrigerator with an approximate temperature of −15 °C to be liquefied. The liquid CO2 was guided to the high-pressure reciprocating pump (an air-driven liquid pump, type-M64, Shineeast Co., Shandong, China), at the pressure of 60 bar (the CO2 tank pressure). Using the pressure gauge (Indumart pressure gauges, Canada), and transmitter, measurements were performed at a precision of ±1 bar. Afterward, liquid CO2 and LND (3000 mg) were homogenized with a magnetic stirrer (100 rpm) ((E-8, Alfa, D-500 180), in a cell with a capacity of 300 mL to reach an equilibrium phase. To keep the temperature at the desired level, the cell was placed in an oven equipped with a digital display whose temperature was measured with a precision of ±0.1 K. A porous filter (1 μm) was used on both sides of the cell to keep the LND in the cell and prevent its escape. CO2 was pressurized and transferred to the cell at the appropriate pressure. Based on preliminary experiments, the time required to reach the equilibrium, the static time, was considered as 120 min; after which, saturated sc-CO2 (600 μL) was introduced into the injection loop using a three-port two-position valve. By redirecting the injection valve, the loop was depressurized into the collection vial containing a certain volume of methanol (solvent). In this part, the micrometer valve was used for controlling the flow. In the final step, 1 mL of solvent was injected by an external needle-valve to wash the loop and the solution was collected in the vial. The final volume of the solution was 5 mL.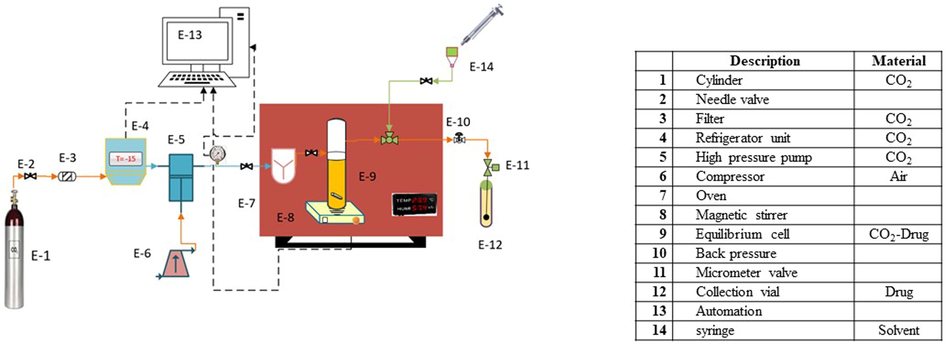
Experimental apparatus for supercritical solubility measurement.
Each experiment was carried out in triplicates and the solubility values were determined by the absorbance assays at λmax (250 nm) on a Perkin-Elmer UV–Vis spectrophotometer (LAMBDA 365, PerkinElmer-USA) with 1 cm long quartz cell. Finally, LND solubility was calculated from its concentration using the calibration curve (with a regression coefficient of 0.996) and the UV-absorbance. The values of LND solubility in sc-CO2 in terms of equilibrium mole fraction (y) and solubility (S (g L−1)) were computed at different temperatures and pressures via the equations in the literature (Saadati Ardestani et al., 2020).
The solubility measurement device was validated by evaluating the solubility of naphthalene at 308 K and different pressures and comparing them with the reports by Iwai et al. (Iwai et al., 1991), Yamini et al. (Yamini et al., 1998) and Sodeifian et al. (Sodeifian et al., 2017), as listed in Table 1.
Pressure (MPa)a
Iwai et al.36 (y × 103)
Yamini et al.37 (y × 103)
Sodeifian et al.38 (y × 103)
This work (y × 103) a
10.7
–
11.6
11.4
11.7 ± 0.2
13.8
14.1
15.2
14.3
14.8 ± 0.3
16.8
16.5
16.2
16.6
16.1 ± 0.3
20.4
17.6
17.4
17.7
17.9 ± 0.2
24.0
–
–
–
19.9 ± 0.4
2.3 Theoretical studies
2.3.1 Empirical models
Numerous empirical models have been proposed for correlating the solubility of a solid solute in sc-CO2. No need to solute properties (unlike the EoSs), simple application, and acceptable accuracy are the most important benefits, and the requirement of experimental solubility data is the only drawback of these models. Following the model proposed by Stahl et al. (Stahl et al., 1978) in 1978, several models have been developed with various adjustable parameters (in the range of 3 to 10) to enhance the correlation of experimental data.
In this research, thirty traditional empirical models are used to correlate the solubility of LND in sc-CO2, whose mathematical formulas are presented in Table 2. According to the solubility functions (Table 2), the proposed models can be classified into five groups; (i) solubility as a function of sc-CO2 density (Stahl et al., 1978), models with solubility dependency to sc-CO2 density and temperature (Chrastil, 1982; Kumar and Johnston, 1988; Andonova and Chandrasekhar, 2016; Alwi and Garlapati, 2021; Del Valle and Aguilera, 1988; Sung et al., 1999; Adachi and Lu, 1983; Garlapati and Madras, 2010; Bian et al., 2016; Sparks et al., 2008; Si-Moussa et al., 2017; Bian et al., 2011; Belghait et al., 2018; Amooey, 2014); (ii) solubility as a function of sc-CO2 density and pressure (Haghbakhsh et al., 2013); (iii) solubility as a function of pressure and temperature (Mitra and Wilson, 1991; Reddy et al., 2018; Gordillo et al., 1999; Yu et al., 1994; Reddy and Garlapati, 2019), and (iv) solubility as a function of sc-CO2 density, pressure and temperature (Bartle et al., 1991; Méndez-Santiago and Teja, 1999; Jafari Nejad et al., 2010; Ch and Madras, 2010; Hozhabr et al., 2014; Keshmiri et al., 2014; Asgarpour Khansary et al., 2015; Sodeifian et al., 2019; Jouyban et al., 2002).
In these models, y and S represent the equilibrium mole fraction and solubility of the solute (kg m−3), ρ, ρref” and ρr (=ρ/ρc) also denote the sc-CO2 density, the reference density (700 kg m−3) and reduced density in which ρc is the sc-CO2 critical density (467.6 kg m−3), respectively. T and Tr (=T/Tc) are temperature (K) and reduced temperature in which Tc is the sc-CO2 critical temperature (304 K), P, Pref, and Pr (=P/Pc) are pressure (bar), reference pressure (1 bar), and reduced pressure in which Pc is the sc-CO2 critical pressure (73.8 bar).
Empirical density-based models rely on simple error minimization and their adjustable parameters can be optimized through the simulated annealing (SA) algorithm in MATLAB software.
2.3.2 Equation of state-based (EOS) model (Soave- Redlich-Kowang (SRK))
Equality of the solute fugacity coefficient in the two phases (solvent (1) - solute (2)) is the vital condition to achieve equilibrium solubility. Equilibrium solute solubility in sc-CO2 (y2) can be expressed by Eq. (1), through considering some assumptions such as the insolubility of sc-CO2 in the solute phase, the purity and incompressibility of the solute, no dependency of the solute molar volume to pressure and very little solute vapor pressure.
Component
MWa (kg kmol−1)
Tbb (K)
Tmb (K)
Tcb (K)
Pc b (bar)
ωc
d (cm3 mol−1)
T (K)
308
318
328
338
Psub × 106(Pa)e
LND
259.25
663.20
560.65
1006.12
36.54
0.64
166.40
1.13
4.31
14.98
43.39
The relations of SRK-EoS is as follows:
Here, a(T) and b, can be defined by the following relations for a single component:
In this research, critical pressure (Pc), critical temperature (Tc), boiling point (Tb) and melting point (Tm) of LND were computed using Marrero and Gani contribution method (Marrero and Gani, 2001). Also, LND acentric factor (ω) was estimated by Constantinou - Gani method (Constantinou and Gani, 1994). All the mentioned physical and critical properties of LND are reported in Table 3.
For the binary system (LND/sc-CO2), a(T) and b can be defined by the van der Waals (vdW) mixing rule considering two parameters (Van der Waals, 1873):
2.3.3 Expanded liquid theory (Wilson’s model)
Due to the relatively high density of supercritical fluids and their proximity to liquids density, they can be considered as an expanded liquid (Higashi et al., 2001). In this case, equality of the fugacity of the solute in the solid phase
with the fugacity of the solute in the SCF (liquid) phase
was used for the thermo-dynamical description of the equilibrium state between the solute and sc-CO2. Regarding the negligible solubility of sc-CO2 in the pure solid phase, the term of
can be considered equal to the fugacity of the pure solid solute
. The fugacity of the solute in the liquid phase can be expressed based on the solute activity coefficient:
Here,
is the activity coefficient of the solid solute at the infinite dilution condition, which in this study was determined using the modified Wilson’s model (Nasri, 2018). This model is somewhat based on Flory’s theory (combinatorial contribution) with the following its usual form according to excess Gibbs energy (GE):
Here, Λ12 and Λ21 are the adjustable parameters which depend on the molar volume of the pure sc-CO2
and the solid solute
and also to characteristic energy differences as:
In the above relations, λ is the interaction energy between the specified species in the subscripts (sc-CO2 (1) and solid solute (2)) (Prausnitz et al., 1998). After differentiation of the excess Gibbs energy function (Eq. (11)) and rearrangement of the equation, the solute activity coefficient (
) can be expressed by:
According to Assael et al. (Assael et al., 1996), for infinite dilution, the above relation can be summarized:
Regardless of the terms of λ11 and λ22 at the infinite dilution condition, (Eq. (12)) and (Eq. (13)) can be written in reduced forms as:
Accordingly, the Eqs.16 and 17 become:
The accuracy and precision of the applied models in correlate the sc-CO2 solubility of solids were assessed by the statistical criteria including the AARD%, Radj and F-value, as follows (Jouyban et al., 2002):
Here, Q is the number of independent variables of each model and R2 indicates the correlation coefficient which can be calculated by the following equation:
In this relation, SSE and SST are the error and total sum of squares, respectively.
The parameter of F-value indicates the capability of the model in fitting the experimental data:
3 Results and discussions
3.1 Experimental solubility determination
The experimental solubility of LND in sc-CO2 was determined in terms of equilibrium mole fraction (y) at different temperatures (308, 318, 328, and 338 K) over a pressure range of 120–300 bar. Each experiment was repeated three times and the mean values with a relative standard deviation of less than 3% were reported in Table 4. Sc-CO2 density was obtained from the NIST chemistry web-book (http://webbook.nist.gov/chemistry). Furthermore, equilibrium solubility, S, of LND in sc-CO2 was also computed as presented in Table 4.
T (K) a
P (bar) a
ρ (kg m—3) b
y × 104c
Standard deviation of the mean, SD (
) × (1 0 4)
Expanded uncertainty × 104
S × 10 (g/l) d
308
120
768.42
0.17
0.0002
0.011
0.7704
150
816.06
0.22
0.0042
0.016
1.0596
180
848.87
0.31
0.0051
0.060
1.5526
210
874.40
0.36
0.0075
0.052
1.8567
240
895.54
0.45
0.0067
0.082
2.3765
270
913.69
0.56
0.0105
0.033
3.0168
300
929.68
0.70
0.0085
0.079
3.8354
318
120
659.73
0.09
0.0009
0.037
0.3506
150
743.17
0.17
0.0021
0.064
0.7458
180
790.18
0.30
0.0048
0.124
1.3987
210
823.71
0.39
0.0052
0.098
1.8949
240
850.10
0.51
0.0091
0.148
2.5567
270
872.04
0.67
0.0086
0.057
3.4449
300
890.92
0.83
0.0107
0.113
4.3582
328
120
506.85
0.04
0.0008
0.029
0.1201
150
654.94
0.14
0.0023
0.017
0.5415
180
724.13
0.31
0.0056
0.123
1.3246
210
768.74
0.44
0.0090
0.129
1.995
240
801.92
0.58
0.0103
0.094
2.7426
270
828.51
0.80
0.0099
0.119
3.9077
300
850.83
0.94
0.0189
0.079
4.7137
338
120
384.17
0.02
0.0032
0.327
0.0456
150
555.23
0.10
0.0019
0.109
0.3285
180
651.18
0.32
0.0063
0.058
1.2302
210
709.69
0.52
0.0096
0.109
2.1768
240
751.17
0.72
0.0136
0.049
3.1888
270
783.29
0.93
0.0153
0.100
4.2939
300
809.58
1.08
0.0129
0.122
5.1532
Based on Table 4, the maximum solubility of LND in sc-CO2 is 1.08 × 10−4 in terms of equilibrium mole fraction, which was achieved at the highest temperature and pressure (338 K and 300 bar). The influence of temperature and pressure on LND solubility in sc-CO2 can be analyzed in Fig. 2. Increasing the sc-CO2 pressure/density at a constant temperature enhanced the solubility in sc-CO2, which was intensified at higher temperatures. Reducing the intermolecular distance of CO2 molecules and increasing their density at higher pressures led to stronger LND (solute)/sc-CO2 (solvent) interactions, improving the solvation power of sc-CO2 (Dong et al., 2010). However, the effect of temperature on solute solubility in sc-CO2 is more complex, which can be explained according to its inverse effect on two competing parameters of solute vapor pressure (volatility) and sc-CO2 density (solvency power). Temperature elevation had a positive effect on solute solubility while reducing the second one and limiting the solubility. This opposite trend was usually demonstrated by the presence of a crossover point which is around 18 MPa for the LND/sc-CO2 binary system (Fig. 2b). At pressures lower than this point, the effect of sc-CO2 density is dominant and temperature increment declined the solubility. However, at higher pressures, the sensitivity of sc-CO2 density to temperature got lower, and solute volatility was the determinant parameter. Therefore, temperature increment above the crossover pressure enhanced the solubility (
). The dual influence of temperature on the solubility of different solutes in sc-CO2 was previously reported as well (Sodeifian et al., 2018; Jin et al., 2014).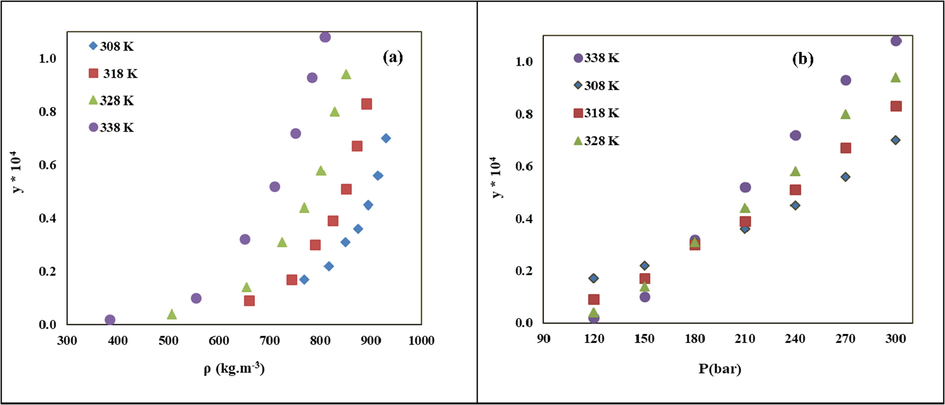
LND solubility at various temperatures vs. (a) sc-CO2 density and (b) pressure.
3.2 Correlation of solubility data
Due to the high time and cost required for solubility correlation, many theoretical models have been proposed. As previously mentioned, LND solubility in sc-CO2 was correlated using common empirical models, equation of state (SRK-EoS), and modified Wilson’s model.
3.2.1 Empirical (Density-based) models
In this study, thirty empirical models were applied for correlating the experimental solubility data of LND in sc-CO2 (Table 2). Obtained solubility values of these models were compared with the experimental data and the results were presented in terms of AARD%, Radj and F-value. The adjustable parameters along with AARD%, Radj and F-value of each empirical model are reported in Table 5. These models were compared in terms of Radj value in the range of 0.90 to 1.0, as shown in Fig. 3. Models proposed by Alwi and Garlapati, Sung and Shim, Ch and Madras, Hozhabr et al., Garlapati and Madras, Keshmiri et al., Sparks et al., Reddy and Garlapati, Bian et al. (2011) and Belghait et al. offered Radj value above 0.99. Among these, Keshmiri et al. model with the highest Radj of 0.995 and low AARD% value of 8.535 and the highest F-value of 553.991 showed the smallest deviation from the experimental data. After that, Bian et al. (2011) model (AARD% = 7.781) and Reddy and Garlapati model (AARD% = 10.589) with Radj values of 0.994 represent high correlation accuracy. Unexpectedly, the Haghbakhsh et al. model with the highest number of adjustable parameters did not represent an acceptable performance (AARD% = 17.47, Radj = 0.857). Moreover, the solubility values correlated via the best fitting models with different numbers of adjustable parameters were compared with the experimental findings in Fig. 4.
Model
Adjustable parameters
AARD(%)
Radj
F-value
Stahl et al.
−9.402
4.297
–
–
–
–
–
–
–
–
47.730
0.128
1.224
Chrastil
9.283
6.463
−5.747e + 3
–
–
–
–
–
–
–
15.093
0.983
263.918
Kumar and Johnston
0.594
9.177
−5.801e + 3
–
–
–
–
–
–
–
9.076
0.990
443.568
Bartle et al.
9.631e + 3
13.744
−8.357e + 3
–
–
–
–
–
–
–
16.409
0.982
249.423
Mendez-Santiago and Teja
Andonova and Garlapati−1.158e + 4
1.346e + 130.417e + 4
6.48020.901
17.890–
-–
-–
-–
-–
-–
-–
-11.867
14.6200.986
0.98336.071
263.97
Alwi and Garlapati
−5.555
−20.589
5.025e3
–
–
–
–
–
–
–
8.210
0.991
498.26
del Valle and Aguilera
73.310
6.531
−47149.226
6.683e + 6
–
–
–
–
–
–
12.738
0.979
159.949
Sung and Shim
3.089
−3734.650
–23.748
9921.15
–
–
–
–
–
–
11.268
0.993
515.400
Jafari Nedjad et al.
−16.307
6.968e-7
6.817e-4
5.247
–
–
–
–
–
–
9.886
0.987
269.413
Ch and Madras
1.314
−5.339
8.255
−1.789
–
–
–
–
–
–
8.525
0.992
408.803
Hozhabr et al.
6.460
−7881.083
2822.879
−0.170
–
–
–
–
–
–
7.998
0.993
513.477
Mitra and Wilson
8.660
2.65e-2
7.19e-5
−51.758
–
–
–
–
–
–
36.237
0.968
102.577
Adachi and Lu
9.804
1.934
11.798
−4.692
−5800.866
–
–
–
–
–
7.789
0.988
238.143
Garlapati and Madras
Keshmiri et al.
−231.566
1.366222.734
−3370.6157.938
5.486e-6−5228.331
−18.5322.953
7787.419–
-–
-–
-–
-–
-8.037
8.5350.991
0.995306.508
553.991
Khansary et al.
−3089.229
−7.56e-4
2.585e + 3
6.343
−0.0156
–
–
–
–
–
9.711
0.990
271.296
Bian et al. (2016)
10.248
−5861.46
−36.812
2.975
7.793
–
–
–
–
–
8.108
0.988
239.967
Reddy et al. (2018)
0.00145
0.000202
−0.00364
−0.000186
0.00218
–
–
–
–
–
12.412
0.985
174.016
Sodeifian et al.
−27.75
−0.012
2.414
−3.286
4.57e-3
242.495
–
–
–
–
11.450
0.990
231.4
Yu et al.
1.393e-5
−3.881e-8
2.474e-10
1.14e-9
4.031e-9
−5.11e-10
–
–
–
–
19.413
0.926
28.115
Gordillo et al.
3.484
−0.0748
−6.318e-5
3.546e-4
−0.0397
−6.054e-5
–
–
–
–
19.615
0.961
62.274
Jouyban et al.
0.151
−13.317
−2.37e-5
6.098e-5
−0.0835
11.273
–
–
–
–
11.836
0.990
238.13
Sparks et al.
2.309
10.820
−1.92e5
23.57
–22.35
1.127
–
–
–
–
8.330
0.993
330.33
Si-Moussa et al.
Reddy and Garlapati−15.335
8.766e-5−8.059
1.16–4−4.377
6.07e-50.0435
6.918e-50.0165
1.30e-414.814
6.11e-5–
-–
-–
-–
-10.115
10.5890.984
0.994146.014
353.960
Bian et al. (2011)
−20.634
−2.146
116.745
−9.455e3
4.196e3
16.837
–
–
–
–
7.781
0.994
430.118
Belghait et al.
−25.513
0.264
4.248
1.829ee4
0.0196
6.224e5
1.98
26.506
–
–
7.516
0.991
195.48
Amooey
−8.75e15
−1.824e15
8.547e14
1.657e14
−3.96e34
−1.920e14
−1.60e14
4.33e13
7.247e12
–
12.180
0.979
70.317
Haghbakhsh et al.
5.854
−0.238
25.612
7.32e4
−103.85
0.471
6.22e6
15.85
0.694
−5e-3
17.470
0.857
8.48
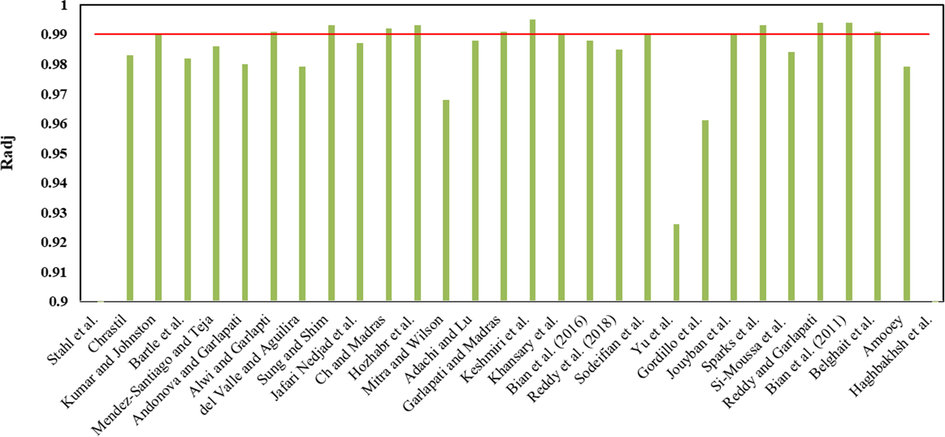
Comparison the empirical models for correlating the solubility of LND in sc-CO2 in terms of Radj.
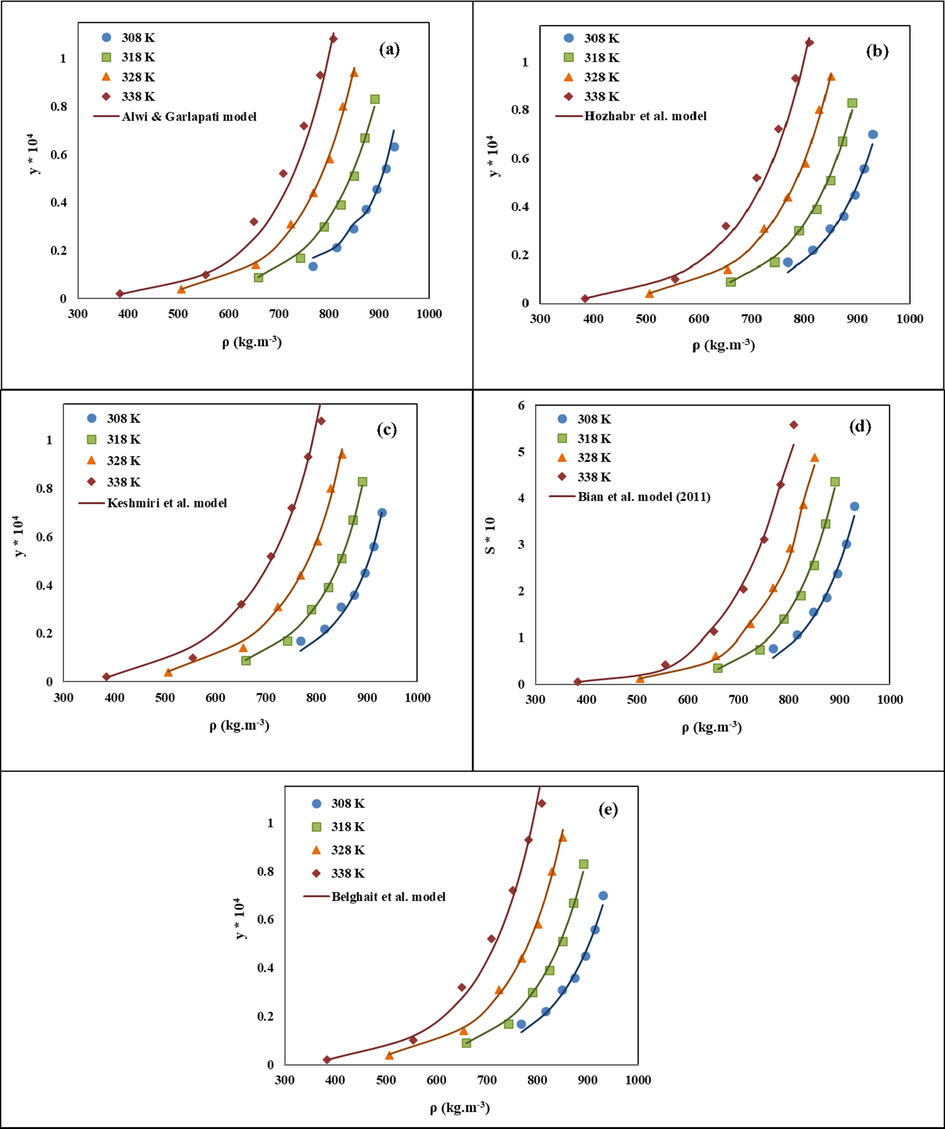
Comparison of experimental (points) and calculated (line) solubility of LND in the sc-CO2: a) Alwi and Garlapati, b) Hozhabr et al., c) Keshmiri et al., d) Bian et al., e) Belghait et al., models at various conditions.
The total mixing heat (ΔHt) of the LND/sc-CO2 binary system can be calculated using a2 adjustable parameter of Chrastil and also Kumar- Johnston models ( ). The vaporization enthalpy (ΔHvap) of this system can be estimated by utilization of the adjustable parameter of the Bartle model ( ). Furthermore, solvation enthalpy (ΔHsol) can be obtained by subtraction of vaporization enthalpy from the total reaction heat, based on Hess's rule. The total mixing heat of this binary system is obtained as 47.8 kJ mol−1 and 48.2 kJ mol−1 according to Chrastil and also Kumar- Johnston models, which exhibited good consistency with each other. Similarly, vaporization and solvation enthalpies were computed as 69.5 kJ mol−1 and −21.5 kJ mol−1 (based on the mean value of the obtained ΔHtotal (48 kJ mol−1)), respectively. LND evaporation and solvation were endothermic and exothermic processes, respectively. Also, the resulting solvation energy indicates the presence of remarkable intermolecular interactions between the LND and sc-CO2 molecules in the supercritical fluid phase (Hojjati et al., 2007).
3.2.2 Equation of state (SRK-EoS) based model
Among the available cubic equation of states, SRK-EoS combined with vdW2 mixing rule was selected for correlating of LND solubility in sc-CO2. The SRK-EoS-correlated solubility data are presented in Fig. 5 at different temperatures (308, 318, 328, and 338 K). As can be seen, correlated solubility values by SRK-EoS did not match the experimental data.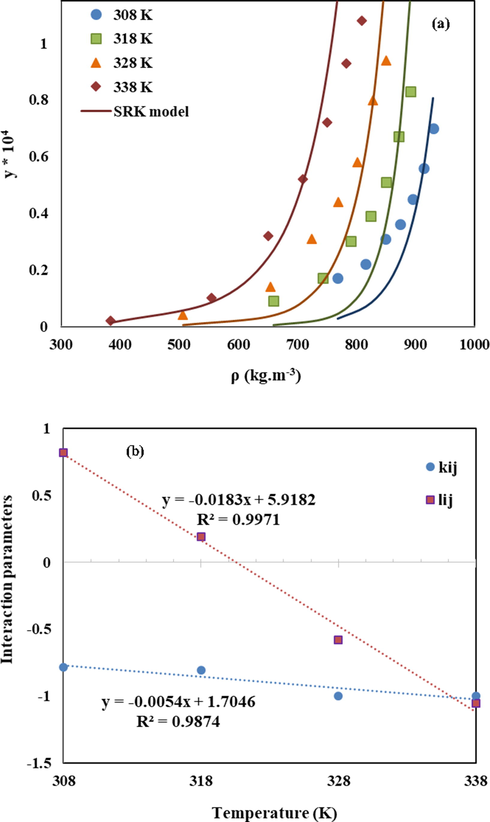
(a) Comparison of experimental (points) and calculated (line) solubility of LND in sc-CO2 based on SRK-EoS model. (b) Linear function of lij and kij versus temperature.
The optimum interaction parameters (
and
) of this model were obtained by minimizing the difference between the experimental LND solubility values and the calculated ones. The optimized values of these binary parameters as well as the statistical parameters (AARD%, Radj and F-value) of the SRK-EoS are reported in Table 6. Generally, the
and
interaction parameters are linear functions of temperature, in which the slope and intercept values of these linear functions were estimated by linear regression analysis. Obtained functions for LND/sc-CO2 binary system were as follows, as also shown in Fig. 5b:
Model
Parameter
T = 308 K
T = 318 K
T = 328 K
T = 338 K
SRK- vdW2
−0.7859
−0.8057
−0.9995
−1
0.8194
0.1946
−0.5784
−1.0564
AARD/%
39.17
49.89
45.50
29.27
F value
798.22
630.14
599.74
688.01
Radj
0.983
0.974
0.978
0.990
Accordingly, these equations can be applied for estimating the solubility of LND in sc-CO2 in the temperature range of 308–338 K. It is clear that both interaction parameters were descending functions of temperature.
3.2.3 Modified Wilson’s model
Optimized adjustable parameters (α, β, Λ'12 and Λ'21) and the statistical parameters (AARD%, Radj, and F-value) of the modified Wilson’s model are reported in Table 7 for the LND/sc-CO2 binary system. The correlation results are also represented in Fig. 6. According to the statistical parameters of AARD% (5.926), Radj (0.995) and F-value (1275.581), it can be concluded that Wilson’s model possesses proper accuracy and high precision for correlating the solubility of LND in sc-CO2. Furthermore, the interaction parameters (Λ12 and Λ21
were computed (Eqs. (19) and (20)) for each data point of the LND/sc-CO2 system and the obtained ranges for the Λ12 and Λ21 are 3.906 to 10.985 and 0.025 × 10−4 to 0.180 × 10−4, respectively. The parameter of Λ21is significantly smaller than Λ12 which is in accordance with the previously reported data, confirming the higher value of Λ12
as the interaction parameter of the solvent (1) around the solid solute (2), for complex solute molecules (Sodeifian et al., 2020; Higashi et al., 2001; Nasri, 2018).
Model
α
β
Λ'12
Λ'21
AARD/%
F value
Radj
Modified Wilson
−7.194
1.788
−1.166
11.803
5.926
1275.581
0.995
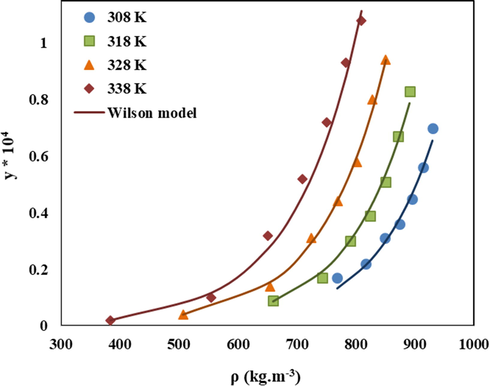
Comparison of experimental (points) and calculated (line) solubility of LND in sc-CO2 based on modified Wilson’s model.
Comparing the statistical criteria of all applied models, it can be concluded that the modified Wilson’s model with the lowest AARD% (5.926) along with the highest Radj (0.995) and F-value, followed by Keshmiri et al. model with the same Radj value and low AARD% (8.535) exhibited the lowest deviation from the experimental data and can be reliably used to correlate the solubility of LND in sc-CO2.
4 Conclusion
In the present research, the solubility of Lenalidomide (LND), as an anti-cancer drug, in supercritical CO2 (sc-CO2) was measured at different pressures (120–300 bar) and temperatures (308–338 K) using a statistical method. LND solubility in sc-CO2 was obtained in the range of 0.02 × 10-4 to 1.08 × 10-4 in terms of mole fraction. The maximum solubility was achieved at 338 K and 300 bar.
Furthermore, the experimental data were correlated with well-known empirical density-based models, SRK equation of state (SRK-EoS) with two parameters of van der Waals (vdW2) mixing rule, as well as, expanded liquid theory (modified Wilson’s model). According to the results, modified Wilson’s model exhibited the highest consistency with the experimental data for correlating the solubility of LND in sc-CO2. However, the SRK-EoS did not show acceptable accuracy in the correlation of LND solubility in sc-CO2. Among the empirical models, Alwi and Garlapati (AARD% = 8.210), Sung and Shim (AARD% = 11.268), Ch and Madras (AARD% = 8.525), Hozhabr et al. (AARD% = 7.998), Garlapati and Madras (AARD% = 8.037), Keshmiri et al. (AARD% = 8.535), Sparks et al. (AARD% = 8.330), Reddy and Garlapati (AARD% = 10.589), Bian et al. (2011) (AARD% = 7.781), and Belghait et al. (AARD% = 7.516) possessed Radj value above 0.99 and can be reliably employed to correlate the solubility of LND in sc-CO2. Various critical properties of LND were calculated by different group contribution methods. Also, the approximate values of total mixing heat ( 48 kJ mol−1) as well as vaporization ( = 69.5 kJ mol−1), and solvation ( = -21.5 kJ mol−1) enthalpies were computed via the obtained adjustable parameters of empirical models.
As mentioned previously, the appropriate supercritical method for producing LND micro/nanoparticles can be selected based on its solubility in sc-CO2. Due to the poor solubility of LND in sc-CO2, the supercritical gas anti-solvent (GAS) process can be considered a suitable choice. Therefore, producing LND micro/nanoparticles with desired morphology and narrow size distribution via the GAS process can be considered in future investigations.
Acknowledgements
The authors would like to thank the generous financial support provided by the Supercritical fluids development group, Shiraz, Iran.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Supercritical fluid extraction with carbon dioxide and ethylene. Fluid Phase Equilib.. 1983;14:147-156.
- [Google Scholar]
- A new semi empirical model for the solubility of dyestuffs in supercritical carbon dioxide. Chem. Pap.. 2021;75(6):2585-2595.
- [Google Scholar]
- Utilization of supercritical CO2 gas antisolvent (GAS) for production of Capecitabine nanoparticles as anti-cancer drug: Analysis and optimization of the process conditions. J. CO2 Util.. 2021;46:101465
- [Google Scholar]
- Investigation the phase equilibrium behavior in ternary system (CO2, DMSO, Capecitabine as anticancer drug) for precipitation of CPT Nanoparticle via the gas antisolvent supercritical process (GAS) Braz. J. Chem. Eng. 2021:1-12.
- [Google Scholar]
- A simple correlation to predict drug solubility in supercritical carbon dioxide. Fluid Phase Equilib.. 2014;375:332-339.
- [Google Scholar]
- A New Empirical Model to Correlate the Solubility of Penicillin G and Penicillin V in Supercritical Carbon Dioxide. J. Appl. Sci. Eng. Methodol. 2016:220-223.
- [Google Scholar]
- Production of Anthraquinone Violet 3RN nanoparticles via the GAS process: Optimization of the process parameters using Box-Behnken design. Dyes Pigm.. 2021;193:109471
- [Google Scholar]
- Experimental Measurement and Thermodynamic Modeling of Capecitabine (an Anticancer Drug) Solubility in Supercritical Carbon Dioxide in a Ternary System: Effect of Different Cosolvents. J. Chem. Eng. Data. 2020;65(10):4762-4779.
- [Google Scholar]
- Lenalidomide (LENA) Hybrid Gold Complex Nanoparticles: Synthesis, Physicochemical Evaluation, and Perspectives in Nanomedicine. ACS Omega. 2020;5(44):28483-28492.
- [Google Scholar]
- Representing solute solubility in supercritical carbon dioxide: A novel empirical model. Chem. Eng. Res. Des.. 2015;93:355-365.
- [Google Scholar]
- Thermophysical properties of fluids: An introduction to their prediction. Vol vol. 1. World Scientific; 1996.
- Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N. Engl. J. Med.. 2012;366(19):1782-1791.
- [Google Scholar]
- Solubilities of Solids and Liquids of Low Volatility in Supercritical Carbon Dioxide. J. Phys. Chem. Ref. Data. 1991;20(4):713-756.
- [Google Scholar]
- Semi-empirical correlation of solid solute solubility in supercritical carbon dioxide: Comparative study and proposition of a novel density-based model. C. R. Chim.. 2018;21(5):494-513.
- [Google Scholar]
- An improved density-based model for the solubility of some compounds in supercritical carbon dioxide. Thermochim. Acta. 2011;519(1-2):16-21.
- [Google Scholar]
- A five-parameter empirical model for correlating the solubility of solid compounds in supercritical carbon dioxide. Fluid Phase Equilib.. 2016;411:74-80.
- [Google Scholar]
- An association model for the solubilities of pharmaceuticals in supercritical carbon dioxide. Thermochim Acta. 2010;507-508:99-105.
- [Google Scholar]
- Lenalidomide acesulfamate: Crystal structure, solid state characterization and dissolution performance. J. Mol. Struct.. 2019;1175:852-857.
- [Google Scholar]
- Solubility of solids and liquids in supercritical gases. J. Phys. Chem.. 1982;86(15):3016-3021.
- [Google Scholar]
- New group contribution method for estimating properties of pure compounds. AIChE J.. 1994;40(10):1697-1710.
- [Google Scholar]
- An improved equation for predicting the solubility of vegetable oils in supercritical carbon dioxide. Ind. Eng. Chem. Res.. 1988;27(8):1551-1553.
- [Google Scholar]
- Measurement and correlation of solubilities of C.I. Disperse Red 73, C.I. Disperse Yellow 119 and their mixture in supercritical carbon dioxide. Fluid Phase Equilib.. 2010;297(1):46-51.
- [Google Scholar]
- Experimental and Modeling Investigation of Glibenclamide Solubility in Supercritical Carbon dioxide. Fluid Phase Equilib.. 2022;556:113408.
- [CrossRef] [Google Scholar]
- Eupen, J.T.H.V., 2011. Lenalidomide salts.
- New empirical expressions to correlate solubilities of solids in supercritical carbon dioxide. Thermochim Acta. 2010;500(1-2):123-127.
- [Google Scholar]
- Studies on drug-polymer interaction, in vitro release and cytotoxicity from chitosan particles excipient. Int. J. Pharm.. 2014;468(1-2):214-222.
- [Google Scholar]
- Solubility of the antibiotic Penicillin G in supercritical carbon dioxide. J. Supercrit. Fluids. 1999;15(3):183-190.
- [Google Scholar]
- Density estimation of pure carbon dioxide at supercritical region and estimation solubility of solid compounds in supercritical carbon dioxide: Correlation approach based on sensitivity analysis. Fluid Phase Equilib.. 2013;342:31-41.
- [Google Scholar]
- Solubilities and diffusion coefficients of high boiling compounds in supercritical carbon dioxide. Chem. Eng. Sci.. 2001;56(10):3027-3044.
- [Google Scholar]
- Solubility of some statin drugs in supercritical carbon dioxide and representing the solute solubility data with several density-based correlations. J. Supercrit. Fluids. 2007;41(2):187-194.
- [Google Scholar]
- Correlation of solute solubility in supercritical carbon dioxide using a new empirical equation. Chem. Eng. Res. Des.. 2014;92(11):2734-2739.
- [Google Scholar]
- Prediction of density in organic crystals. Acta Crystall. Sect. A. 1977;33(1):216-218.
- [Google Scholar]
- Solubilities of myristic acid, palmitic acid, and cetyl alcohol in supercritical carbon dioxide at 35.degree.C. J. Chem. Eng. Data. 1991;36(4):430-432.
- [Google Scholar]
- Prediction of solute solubility in supercritical carbon dioxide: A novel semi-empirical model. Chem. Eng. Res. Des.. 2010;88(7):893-898.
- [Google Scholar]
- Solubility of 4-Hydroxybenzaldehyde in Supercritical Carbon Dioxide with and without Cosolvents. J. Chem. Eng. Data. 2014;59(5):1521-1527.
- [Google Scholar]
- Mathematical representation of solute solubility in supercritical carbon dioxide using empirical expressions. J. Supercrit. Fluids. 2002;24(1):19-35.
- [Google Scholar]
- Carbamazepine solubility in supercritical CO2: A comprehensive study. J. Mol. Liq.. 2020;311:113104
- [Google Scholar]
- Development and evaluation of a new semi-empirical model for correlation of drug solubility in supercritical CO2. Fluid Phase Equilib.. 2014;363:18-26.
- [Google Scholar]
- Modelling the solubility of solids in supercritical fluids with density as the independent variable. J. Supercrit. Fluids. 1988;1(1):15-22.
- [Google Scholar]
- Effect of Cosolvents on the Solubility of Lenalidomide and Thermodynamic Model Correlation of Data. J. Chem. Eng. Data. 2019;64(10):4272-4279.
- [Google Scholar]
- Improving Oral Bioavailability of Sorafenib by Optimizing the “Spring” and “Parachute” Based on Molecular Interaction Mechanisms. Mol. Pharm.. 2016;13(2):599-608.
- [Google Scholar]
- Group-contribution based estimation of pure component properties. Fluid Phase Equilib.. 2001;183-184:183-208.
- [Google Scholar]
- The solubility of solids in supercritical fluids. Fluid Phase Equilib.. 1999;158-160:501-510.
- [Google Scholar]
- An Empirical Method to Predict Solubility in Supercritical Fluids. J. Chromatogr. Sci.. 1991;29(7):305-309.
- [Google Scholar]
- Design and Analysis of Experiments (eighth ed.). John Wiley; 2012.
- Modified Wilson's Model for Correlating Solubilities in Supercritical Fluids of Some Polycyclic Aromatic Solutes. Polycyclic Aromat. Compd.. 2018;38(3):244-256.
- [Google Scholar]
- Correlation and Prediction of the Solubility of Solid Solutes in Chemically Diverse Supercritical Fluids Based on the Expanded Liquid Theory. Adv. Chem. Eng. Sci.. 2013;03(04):255-273.
- [Google Scholar]
- Cutaneous adverse reactions linked to targeted anticancer therapies bortezomib and lenalidomide for multiple myeloma: new drugs, old side effects. Cutan. Ocul. Toxicol.. 2014;33(1):1-6.
- [Google Scholar]
- Predictive thermodynamic modeling and experimental measurements on solubility of active pharmaceutical ingredient: Lornoxicam case study. J. Mol. Liq.. 2021;326:115285
- [Google Scholar]
- Thermodynamic modelling and experimental validation of pharmaceutical solubility in supercritical solvent. J. Mol. Liq.. 2020;319:114120
- [Google Scholar]
- Measuring solubility of a chemotherapy-anti cancer drug (busulfan) in supercritical carbon dioxide. J. Mol. Liq.. 2020;317:113954
- [Google Scholar]
- Chloroquine (antimalaria medication with anti SARS-CoV activity) solubility in supercritical carbon dioxide. J. Mol. Liq.. 2021;322:114539
- [Google Scholar]
- Using static method to measure tolmetin solubility at different pressures and temperatures in supercritical carbon dioxide. Sci. Rep.. 2020;10(1)
- [CrossRef] [Google Scholar]
- Experimental and thermodynamic modeling decitabine anti cancer drug solubility in supercritical carbon dioxide. Sci. Rep.. 2021;11(1)
- [CrossRef] [Google Scholar]
- Properties of Gases and Liquids (fifth ed.). McGraw-Hill Education; 2001.
- Molecular thermodynamics of fluid-phase equilibria. Pearson Education; 1998.
- Rangineni, S., N.D., Mudapaka, V.K., Kadaboina, R., Murki, V., Manda, A., Badisa, V.R., Vemula, N., Pulla, R., Seshagiri, R., 2010. Lenalidomide solvates and processes.
- Dimensionless Empirical Model to Correlate Pharmaceutical Compound Solubility in Supercritical Carbon Dioxide. Chem. Eng. Technol.. 2019;42(12):2621-2630.
- [Google Scholar]
- Preparation of phthalocyanine green nano pigment using supercritical CO2 gas antisolvent (GAS): experimental and modeling. Heliyon. 2020;6(9):e04947.
- [CrossRef] [Google Scholar]
- Determination of Anthraquinone Violet 3RN solubility in supercritical carbon dioxide with/without co-solvent: Experimental data and modeling (empirical and thermodynamic models) Chem. Eng. Res. Des.. 2020;159:529-542.
- [Google Scholar]
- Solubility of favipiravir (as an anti-COVID-19) in supercritical carbon dioxide: An experimental analysis and thermodynamic modeling. J. Supercrit. Fluids. 2022;183:105539.
- [CrossRef] [Google Scholar]
- Solubility of montelukast (as a potential treatment of COVID -19) in supercritical carbon dioxide: Experimental data and modelling. J. Mol. Liq.. 2022;349:118219.
- [CrossRef] [Google Scholar]
- Novel density-based model for the correlation of solid drugs solubility in supercritical carbon dioxide. C. R. Chim.. 2017;20(5):559-572.
- [Google Scholar]
- Equilibrium constants from a modified Redlich-Kwong equation of state. Chem. Eng. Sci.. 1972;27(6):1197-1203.
- [Google Scholar]
- Experimental data and thermodynamic modeling of solubility of Azathioprine, as an immunosuppressive and anti-cancer drug, in supercritical carbon dioxide. J. Mol. Liq.. 2020;299:112179
- [Google Scholar]
- Application of supercritical carbon dioxide to extract essential oil from Cleome coluteoides Boiss: experimental, response surface and grey wolf optimization methodology. J. Supercrit. Fluids. 2016;114:55-63.
- [Google Scholar]
- Determination of solubility of Aprepitant (an antiemetic drug for chemotherapy) in supercritical carbon dioxide: empirical and thermodynamic models. J. Supercrit. Fluids. 2017;128:102-111.
- [Google Scholar]
- Experimental optimization and mathematical modeling of the supercritical fluid extraction of essential oil from Eryngium billardieri: Application of simulated annealing (SA) algorithm. J. Supercrit. Fluids. 2017;127:146-157.
- [Google Scholar]
- Properties of Portulaca oleracea seed oil via supercritical fluid extraction: Experimental and optimization. J. Supercrit. Fluids. 2018;135:34-44.
- [Google Scholar]
- Solubility measurement of a chemotherapeutic agent (Imatinib mesylate) in supercritical carbon dioxide: Assessment of new empirical model. J. Supercrit. Fluids. 2019;146:89-99.
- [Google Scholar]
- Production of Loratadine drug nanoparticles using ultrasonic-assisted Rapid expansion of supercritical solution into aqueous solution (US-RESSAS) J. Supercrit. Fluids. 2019;147:241-253.
- [Google Scholar]
- Prediction of Solubility of Sodium Valproate in Supercritical Carbon Dioxide: Experimental Study and Thermodynamic Modeling. J. Chem. Eng. Data. 2020;65(4):1747-1760.
- [Google Scholar]
- Experimental and thermodynamic analyses of supercritical CO2-Solubility of minoxidil as an antihypertensive drug. Fluid Phase Equilib.. 2020;522:112745.
- [CrossRef] [Google Scholar]
- Improving the Solubility of Lenalidomide via Cocrystals. Cryst. Growth Des.. 2014;14(6):3069-3077.
- [Google Scholar]
- Evaluation of density-based models for the solubility of solids in supercritical carbon dioxide and formulation of a new model. Chem. Eng. Sci.. 2008;63(17):4292-4301.
- [Google Scholar]
- A Quick Method for the Microanalytical Evaluation of the Dissolving Power of Supercritical Gases. Angew. Chem.. 1978;17(10):731-738.
- [Google Scholar]
- Stiegel, H.H., W.A., Brueck, S., Paetz, J., Meergans, D., 2011. Acid addition salts of lenalidomide.
- Solubility of C. I. Disperse Red 60 and C. I. Disperse Blue 60 in Supercritical Carbon Dioxide. J. Chem. Eng. Data. 1999;44(5):985-989.
- [Google Scholar]
- A new empirical model to correlate solubility of pharmaceutical compounds in supercritical carbon dioxide. J. Appl. Sci. Eng. Methodol.. 2018;4(2):575-590.
- [Google Scholar]
- Over de Continuiteit van den Gas-en Vloeistoftoestand (On the Continuity of the Gas and Liquid State). University of Leiden; 1873.
- Measurement and Correlation of Solubility of Methylsalicylic Acid Isomers in Supercritical Carbon Dioxide. J. Chem. Eng. Data. 2021;66(1):280-289.
- [Google Scholar]
- Measurement and modeling of solubility of gliclazide (hypoglycemic drug) and captopril (antihypertension drug) in supercritical carbon dioxide. J. Supercrit. Fluids. 2021;174:105244
- [Google Scholar]
- Solid solubility measurement of ipriflavone in supercritical carbon dioxide and microparticle production through the rapid expansion of supercritical solutions process. J. CO2 Util.. 2020;37:285-294.
- [Google Scholar]
- Solubility of dihydroxybenzene isomers in supercritical carbon dioxide. Fluid Phase Equilib.. 1998;152(2):299-305.
- [Google Scholar]
- Solubility parameter of lenalidomide for predicting the type of solubility profile and application of thermodynamic model. J. Chem. Thermodyn.. 2019;132:268-275.
- [Google Scholar]
- Solubilities of fatty acids, fatty acid esters, triglycerides, and fats and oils in supercritical carbon dioxide. J. Supercrit. Fluids. 1994;7(1):51-59.
- [Google Scholar]
- Thermodynamic study on solubility of brain tumor drug in supercritical solvent: Temozolomide case study. J. Mol. Liq.. 2021;321:114926
- [Google Scholar]
- Measuring salsalate solubility in supercritical carbon dioxide: Experimental and thermodynamic modelling. J. Chem. Thermodyn.. 2021;152:106271
- [Google Scholar]
- Loxoprofen Solubility in Supercritical Carbon Dioxide: Experimental and Modeling Approaches. J. Chem. Eng. Data. 2020;65(9):4613-4620.
- [Google Scholar]
- Experimental Solubility Measurements of Fenoprofen in Supercritical Carbon Dioxide. J. Chem. Eng. Data. 2020;65(4):1425-1434.
- [Google Scholar]
- Tenoxicam (Mobiflex) Solubility in Carbon Dioxide under Supercritical Conditions. J. Chem. Eng. Data. 2021;66(2):990-998.
- [Google Scholar]







