Translate this page into:
Exploration of spray pyrolysis technique in preparation of absorber material CFATS: Unprecedented hydrophilic surface and antibacterial properties
⁎Corresponding authors at: Université Tunis El Manar, Faculté des Sciences de Tunis, Département de Physique, LR99ES13 Laboratoire de Physique de la Matière Condensée (LPMC), 2092 Tunis Tunisie, Tunisia (C. Nefzi); Physics Department, Common First Year Deanship, Umm Al-Qura University, Makkah 21955, Saudi Arabia (B. Yahmadi); Department of Chemistry, Faculty of Applied Science, Umm Al-Qura University, 21955 Makkah, Saudi Arabia (S.A. Ahmed). nefzichaima@fst.utm.tn (Chayma Nefzi), bmyahmadi@uqu.edu.sa (Bechir Yahmadi), saahmed@uqu.edu.sa (Saleh A. Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, we achieve the production of nontoxic Cu2Fe1-xAlxSnS4 films (x = 0, 0.25, 0.50, 0.75 and 1) by substituting Fe with Al atoms. Physical properties of the investigated films were studied using: Energy dispersive X-ray spectrometry (EDX), scanning electron microscopy (SEM), X-ray diffraction (XRD), Raman spectroscopy, spectrophotometer and drop shape analysis system (DSA). The formation of new quaternary Cu3Al0.6Sn1S6 (CATS) chalcogenide for x = 1 was proven from EDX study. Notably, the major diffraction peaks were located at 2θ = 28.34°, 47.43° and 55.93° which are respectively tagged as (1 1 2), (2 0 4), and (3 1 2) plans, confirming the stannite crystal structure of Cu3Al0.6Sn1S6 film. The morphological states show a nanofiber structure accompanied with voids and cavities for CATS films. Tauc-relation plot reveals direct energy bandgap, close to 1.52 eV, which proves the absorber film type of Cu3Al0.6Sn1S6. The effluent toxicity of the obtained thin films has been assessed using the inhibition of Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria and indicated good antibacterial activity of the CATS/SnO2:F heterojunction. The viability rates against S. aureus achieved 40 %, 31 % and 15% for SnO2:F, Cu3Al0.6Sn1S6 films and CATS/SnO2:F heterojunction. These results highlight the great antibacterial activity of coupled CATS/SnO2:F. Therefore this research underscores the effectiveness of CATS/SnO2:F surface which demonstrates self-disinfecting and self-cleaning with hydrophilicity and high antibacterial activity.
Keywords
Cu2Fe1-xAlxSnS4 films
Spray pyrolysis
Antibacterial activity
Hydrophilicity
1 Introduction
The I-III-VI semiconductors with chalcogenide structure have sparked great interest owning to their good semiconducting and optical properties useful for optoelectronic and biomedicine applications (Koç et al., 2021; Shin et al., 2013; Mokurala et al., 2016; Ozel, 2016; Ali et al., 2020). Much research has been dedicated to the exploration of abundant and low-cost semiconductors to produce renewable and clean energy for solar cells (Betul et al., 2021; Benyahia et al., 2021; Sadanand et al., 2021; Khemiri et al., 2020; Hussein et al., 2021; Victoria et al., 2021; Sun et al., 2021). In recent years, CdTe and Cu (In,Ga)Se2 (CIGSe) have been used as effective absorbers which may be engineered and tuned for solar cells with high power conversion efficiency (Shin et al., 2022; Amoupour et al., 2021). Nevertheless, the expensive cost and scarcity of In, Ga and Te elements and the toxicity of Cd present major issues that limit the widespread fabrication of CdTe and CIGS semiconductors. Quaternary I2–II–IV–VI4 (I = Cu, Ag, II = Fe, Zn, Mn, Cd, IV = Si, Ge, Sn, VI = S, Se, Te) chalcogenides create a large group of semiconductors with different structural, optical, and electrical properties. Among them, Cu2MgSnS4, Cu2FeSnS4, Cu2InSnS4 and Cu2CoSnS4 semiconductors have recently emerged as promising light-absorber semiconductors for solar cells to replace the remarkable properties of CIGS (Shin et al., 2022; Amoupour et al., 2021; Nefzi et al., 2021a; Nefzi et al., 2021b; Nefzi et al., 2020a; Nefzi et al., 2020b).
In addition, wetting behavior is one of the most influential surface properties of a solid and therefore is of great relevance in many applications from both practical and fundamental point of view. The water contact angle θ (WCA) determines the surface wettability by the angle between solid/air and liquid/air interfaces. Taking advantages of their properties, hydrophilicity and hydrophobicity impact applications including self-cleaning (Wan et al., 2011), antimicrobial property (Kim et al., 2021; Sopata et al., 2020), medical supplies (Sui et al., 2020), and friction reduction (Wang et al., 2011).
A relevant field of applications involves using nanoparticles semiconductors as bactericides and insecticides (Shin et al., 2022; De Falco et al., 2018; Qi et al., 2019; Karam et al., 2013). Nanomaterials exhibiting antimicrobial property can be used for the purpose of combatting the proliferation of pathogenic microorganisms such as bacteria and fungi. Based on literature, Gram-negative bacteria present less resistance against nanoparticles material than Gram-positive ones (Naderi et al., 2020). The Gram-negative bacteria Escherichia coli (E.coli) is a cause of concern for humanity and the Gram-positive bacteria Staphylococcus aureus (S. aureus) is responsible for food poisoning, hospital infections, ulcers, skin infections and abdominal abscesses (Davarpanah et al., 2022).
In this work, we report, for the first time, on new promising candidate Cu2Fe1-xAlxSnS4 (CFATS) thin films using spray pyrolysis technique (for x = 0, 0.25, 0.50, 0.75 and 1) where × is the ratio of between Al and Fe concentration in the spray solution (x = [Al3+]/ [Fe3+]). We have synthesized absorber CATS film with direct band gap and studied the surface wettability of CFATS. Then, the antimicrobial activities of SnO2:F, CATS and CATS/SnO2:F against Staphylococcus aureus and Escherichia coli were investigated. The overarching aim of our research is to explore the physical properties of novel material for different applications like biomaterials.
2 Experimental
2.1 Chemicals
Copper (II) chloride dihydrate (CuCl2·2H2O, 99.99%), aluminum (III) chloride hexahydrate (AlCl3·6H2O, 95%), iron (III) chloride hexahydrate (FeCl3, 6H2O, 0.004 M), tin (II) chloride dehydrate (SnCl2, 2H2O, 98%) and thiourea (CH2N2S, 98%) were purchased from Sigma Aldrich and Fluka. All chemicals products were used without any further purification for the elaboration of Cu2Fe1-x AlxSnS4 (CFATS) thin layers. The methanol (CH4O, 99.99%) was purchased from Fisher Chemical society and used as a solvent.
2.2 Deposition of Cu2Fe1-x AlxSnS4 (CFATS) thin films
Cu2Fe1-xAlxSnS4 (CFATS) thin films deposited on glass substrates using chemical spray pyrolysis technique in ambient air. The glass substrates were cleaned in ultrasonic bath using double-distilled water (Bi-distiller water GFL), followed by isopropyl alcohol for 10 min. All cleaned substrates were dried in a furnace at 80 °C for 15 min. Typically, the sprayed solution was prepared by mixing CuCl2 (0.01 M), AlCl3 (0.004 M), FeCl3 (0.004 M), SnCl2 (0.004 M) and CH2N2S (0.04 M) in 300 ml of methanol. This process was repeated three times to obtain approximately good physical properties. Then, the solutions were varied with and without aluminum concentrations by maintaining × = 0, 0.25, 0.50, 0.75, and 1 for the growth of Cu2Fe1-x AlxSnS4 layers. The obtained solutions were stirred for 15 min until they became clear and transparent without impurities which can potentially block the nozzle of the sprayer. Therefore, this solution is sprayed with a flow rate of 25 ml/min onto preheated substrates (Ts = 240 °C) as fine droplets by means of compressed air as carrier gas. The substrates and nozzle distance is equal to 28 cm. The possible proposed growth mechanism may follow the reactions as follows (Orletskii et al., 2018): 2Cu2+ + Sn2+→2Cu+ + Sn4+ Cu+ + Cl-→CuCl CuCl + 2SC(NH2)2 → [Cu(SC(NH2)2)2]Cl Sn4+ + 2SC(NH2)2 → [Sn(SC(NH2)2)2]4+ Al3+ + 2SC(NH2)2 → [Al(SC(NH2)2)2]3+
The formation of CATS is the result of the following reaction: Cu(SC(NH2)2)2]+ + [Sn(SC(NH2)2)2]4++[Al(SC(NH2)2)2]2+→ Cu2AlSnS4 precursor solution.
2.3 Bacterial adhesion analysis
Basically, the Gram-negative bacterium Escherichia coli (E.coli) and the Gram-positive bacterium Staphylococcus aureus (S. aureus) have been utilized as the experimental strains by counting forming unity (CFU). Overnight the growth of E. coli and S. aureus were attained at 37 °C in 5 ml of Tryptic Soya Broth (TSB) medium under agitation. Spread 10 µl of each culture on the chemically coated thin films and other uncoated glass slides that will serve as a control (previously sterilized in glass petri dishes) make a series of dilutions of the two cultures to spread it and count it on Tryptic Soya Agar TSA (up to 10-6 is sufficient). Incubate the slides at 37 °C for 48 h, taking care not to tip them over when moving to the oven. After 48 h, immerse each film in Eppendorf tube filled with 1 ml of physiological water (using sterile forceps without touching the surface on which you have deposited the culture) and vortex it well to detach the slide bacteria. Therefore, this is a stock solution. Carry out successive dilutions and spread on (TSA) for enumeration. Also, spread 100 µl of the stock solution (normally a dilution up to 10-4 is sufficient). The decrease in CFU/mL is considered as the effect of thin films against the bacterial growth. Calculate the viability (%) with the following formula;
2.4 Characterization methods
The crystalline phases were studied using an XPERT-PRO diffractometer system with λ = 1.5406 Å and 2θ varied from 20° to 60° at room temperature. The purity of films was investigated by Raman spectrometer type Jobin Yvon technology T 64,000 with 488 nm argon in laser like excitation source. Elemental compositions were explored using Energy dispersive X-ray spectroscopy (EDX). Film morphology and thickness was examined using scanning electron microscopy (SEM) type Zeiss EVO MA10 microscope. Optical behavior was recorded using spectrophotometer type Perkin Elmer Lambda 950 in the wavelength range 250–2500 nm at room temperature. The surface wettability of Cu2Fe1-xAlxSnS4 (x = 0, 0.25, 0.50, 0.75 and 1) thin films were investigated by Water Contact angle θ (WCA) measurements using Drop Shape Analysis System type DSA100 (Kruss GmbH, Hamburg).
3 Results and discussion
3.1 Chemical composition
The composition of synthesized Cu2Fe1-xAlxSnS4 thin layers (x = 0–0.25 – 0.50–0.75 and 1) has been determined using energy-dispersive X-ray (EDX) spectroscopy and the results are provided in Table 1. This table indicates the existence of copper, iron, aluminum, tin and sulfur comprising the Cu2Fe1-xAlxSnS4 molecular structure. As expected, the rise in aluminum concentration seems to change the elemental composition of Cu, Sn and S contents in the films. It is shown that copper and tin amounts increased with Al inclusion. Fig. 1 shows the elements composition of Cu2AlxSnS4 (where x = 1) thin layer. Moreover, copper, aluminum, tin and sulfur ratios in Cu2AlSnS4 (where x = 1) are about 28.7: 5.92: 10.38: 54.99, which are nearly in accordance with the stoichiometric ratio of Cu3Al0.6Sn1S6 (CATS) composition.
x
0
0.96
0.82
---
0.90
0.25
1.32
0.17
0.60
0.80
0.50
1.74
0.41
0.25
0.84
0.75
1.58
0.50
0.41
0.78
1
1.76
---
0.57
1.20
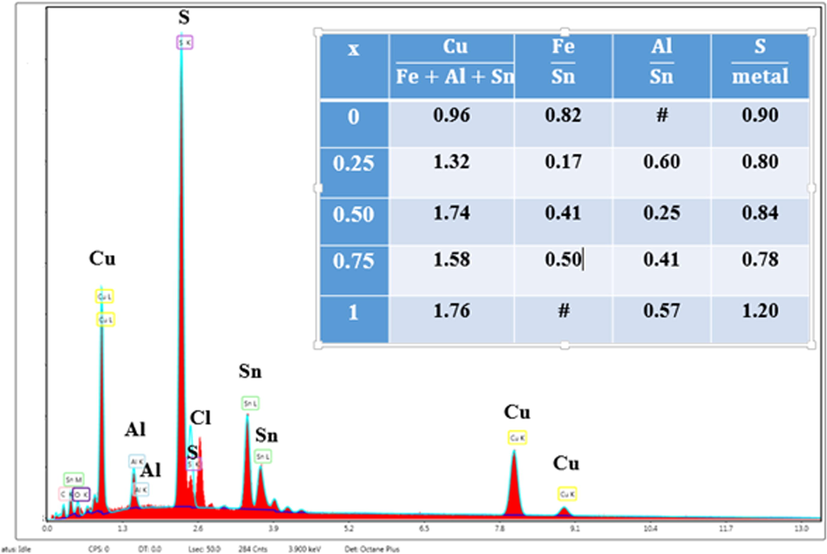
EDAX spectrum of CATS (where x = 1) film.
3.2 Structural characterization
The crystallographic structure of Cu2Fe1-xAlxSnS4 thin layers (x = 0–0.25 – 0.50–0.75 and 1) has been studied by XRD with Cu (kα) radiation source in the range of 2θ [10°-70°] as shown in Fig. 2. The major diffraction peaks 2θ = 18.20°, 28.77°, 47.86° and 56.87° were indexed respectively to (0 0 2), (1 1 2), (2 0 4), and (3 1 2) plans of stannite CFTS structure, coordinated with the standard (JCPDF card No. 74-1025). In stannite structure the space group is I
2 m, each atom has four nearest neighbors and each anion S is surrounded by two Cu one Fe and one Sn atoms. On the other hand, each cation is tetrahedrally coordinated by four anions. After aluminum inclusion in Cu2Fe1-xAlxSnS4 (CFATS) matrix, the peak intensities are slightly decreased accompanied with broad full width at half-maximum (FWHM), designed a decline of the crystallinity. The poor crystalline quality at x = 0.25 indicates that some quantities of Al atoms prefer to situate in or near the grain boundary regions (Venkatachalam et al., 2008). Meanwhile, the large FWHM may be originated to a combination of grains and possible assembling of the micro-strains at several planes as crytallographic defects form (Das Bakshi et al., 2018). It is also evidenced that all peaks are shifted toward lower angles, which is due to an increment of lattice parameters for CFATS materials. This trend may be ascribed to the ionic radii of Al3+ (0.53 Å) that is lower to that of Fe3+ (0.64 Å). However, for x = 1, the patterns of CATS thin film revealed a polycrystalline nature with diffraction peaks 2θ = 28.34°, 47.43° and 55.93° that are identified respectively to (1 1 2), (2 0 4), and (3 1 2) plans. No sign of contamination CATS thin film.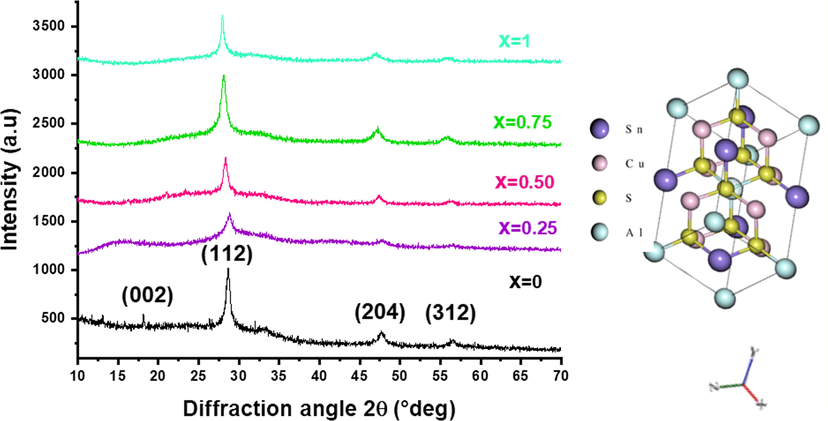
XRD patterns of Cu2Fe1-xAlxSnS4 films (x = 0–0.25 – 0.50–0.75 and 1) films. Inset presents the Stannite Type Structure of CATS material.
Crystallite size (D), dislocation density (δdis), crystallites number (Nc) and strain (ε) were calculated for (1 1 2) plane using the following relations (Monisha et al., 2021; Agawane et al., 2014) and tabulated in Table 2:
x
0
0.25
0.50
0.75
1
D (nm)
60
23
19
19
45
δdis (1011 cm−2)
3
19
28
28
5
Nc (1012 cm−2)
4
25
44
44
15
ε (10−3)
6
15
18
18
8
FWHM
0.4338
0.3524
0.4132
0.4132
0.1771
d (Å)
3.11
3.08
3.17
3.15
3.19
Where: k is the shape factor, λ is the wavelength of incident X-ray, β is the full-width at half maximum, e is the film thickness and θ is the Bragg angle. The average crystallite size decreases from 60 nm for CFTS film to 45 nm for CATS film. This trend suggests the high crystallinity at x = 1 than the other concentration. Consequently, this enhancement leads to lesser crystal defects and for small recombination centers at the grain boundaries. Additionally, micro-strain and dislocation densities calculated to investigate the film quality such as crystal defects, dislocations and the presence of lattice imperfections. Therefore, we can say that CATS deposited with high quality and lesser defects, where δdis and Nc were equal to 5*1011 cm−2 and 15*1012 cm−2 respectively.
XRD data used to evaluate Lattice parameters ‘a’ and ‘c’, Unit cell volume (V), Molecular weight (M), X- ray density (dx) and Porosity (P) from the following expressions (Somvanshi et al., 2020):
where, d, (h, k, l), M, Z, NA and dB are the inter-planar spacing, miller indices, molecular weight, atoms number per unit cell, Avogadro’s number and bulk density, respectively. The structural parameters are tabulated in Table 3. Generally, it is found that Al concentration promotes a remarkable increment of lattice parameters ‘a’ and ‘c’ that correlated with the shift of diffraction peaks toward lower angles. Consequently, ‘a’ and ‘c’ lead to increase in the unit cell volume that decreases the X- ray density. Notably, the highest film porosity found for CATS film may be ascribed to the presence of major void space.
x
d (1 1 2)(Å)
d (2 0 4) (Å)
V (Å3)
M (g mol−1)
dx × 10-23 (g/Å3)
P(%)
0
3.1161
1.9056
313.1
430
3.66
34
0.25
3.0899
1.9881
310.2
423
3.62
35
0.50
3.1625
1.9301
329.3
415
3.35
37
0.75
3.1724
1.9241
329.2
408
3.29
38
1
3.1898
1.9364
337.3
401
3.16
43
3.3 Raman spectroscopy
XRD patterns of all chalcogenides Cu2XSnS4 (where X is a metal) are similar and close to that of FeS and Cu3SnS4 (Mokurala et al., 2016). Raman spectroscopy utilized 488 nm as excitation wavelength and shown in Fig. 3. As mentioned from our previous work and literature (Nefzi et al., 2018), quaternary chalcogenides show principally two Raman peaks. For example, CFTS is kknown to show two Raman peaks at 285 cm−1 and 319 cm−1, which are observed here. Fig. 3 demonstrates that CATS displays two principal peaks at 335 cm−1 and 295 cm−1 which may be identified as the A1 and the B/E modes respectively. In our knowledge, there is no work, which identified the Raman peaks of CATS material before. As a consequence, Raman peak matched at 295 cm−1 may correspond to S2- around Cu2+ (Lee et al., 2012). Then, the principal peak at 335 cm−1 may be attributed to the strongest asymmetric vibrational mode of S2- around Sn (Lee et al., 2012).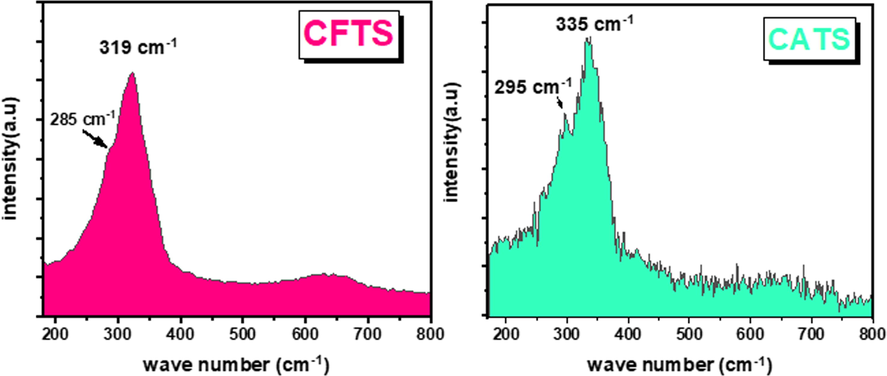
Raman scattering results for CFTS and CATS films.
3.4 Morphological analysis
Fig. 4 shows the morphological states of CFATS thin layers (x = 0 – 0.25 – 0.50–0.75 and 1) extracted from SEM, revealing a transformation in film morphology. It is evident that Cu2FeSnS4 film appears with smooth and uniform surface. At x = 0.25, it is clear that the surface morphology is uniform and dense with some cracks. A granulated structure with small grain size distribution was observed at x = 0.50. The micrographs at x = 0.75 reveal that the film surface is covered with large numbers of rod-shaped grains that are closely attached to each other. By contrast, the surface morphology of CATS appears as well as a nanofibers structure accompanied with voids and cavities. This trend is beneficial for photovoltaic applications as motioned by Hao Guan et al. (Guan et al., 2014). These obtained results are similar to those reported in other researches (Guan et al., 2014; Ozel et al., 2015). Fig. 5 indicates that film thickness of CATS slightly decreased to 723 nm compared to CFTS which mainly associates with the decrease of crystallite size.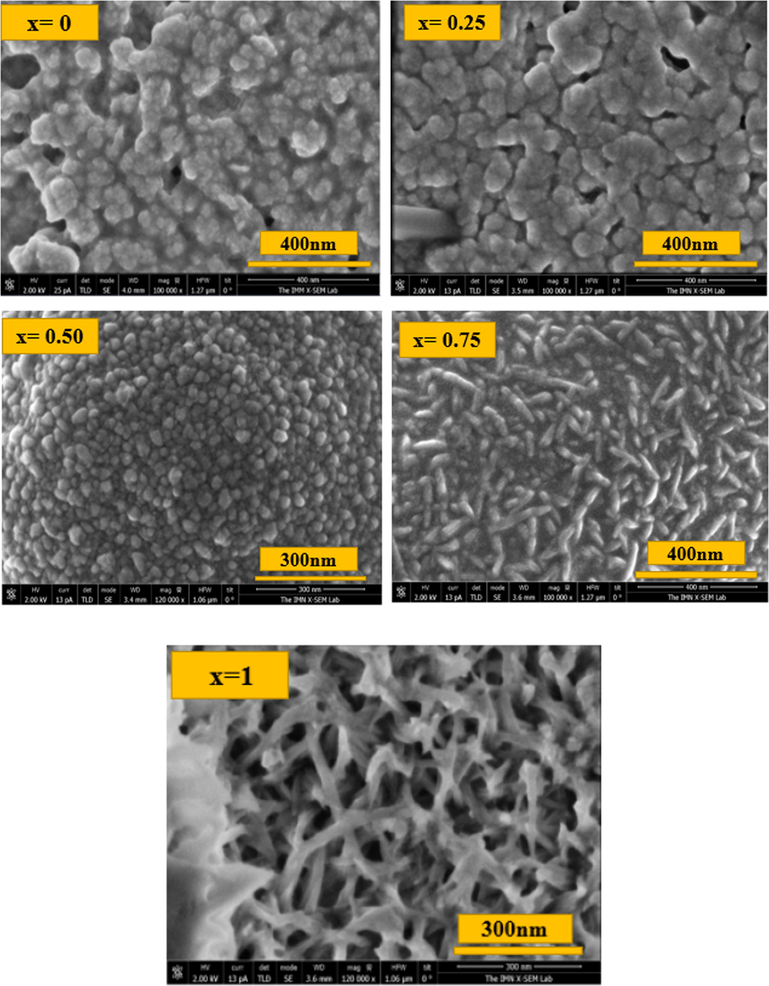
Morphology of the as-synthesized Cu2Fe1-xAlxSnS4 (CFATS) films (x = 0 – 0.25 – 0.50–0.75 and 1).
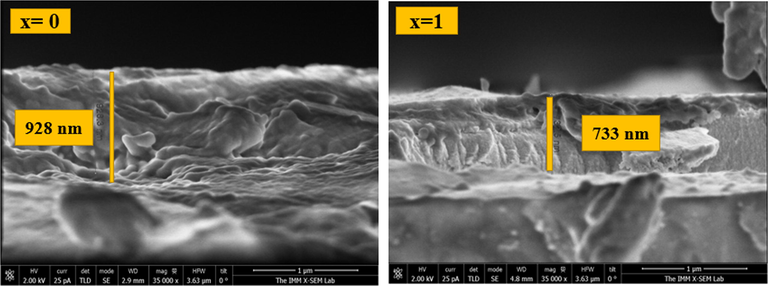
Cross-section views of CFTS and CATS films for scale of 1 µm.
3.5 Optical properties
The optical band gap (Eg) of sprayed CFATS thin films was deduced by utilizing Tauc’s relationship (Jrad et al., 2016):
Where A is a constant and n presents the nature of transition. After fitting, all Cu2Fe1-xAlxSnS4 layers were found to obey equation (3) with n = 2, which presents the direct transition type as shown in Fig. 6. Generally, the molarity of Al in the solution precursors affects the optical band gap values of elaborated CFATS thin films. The optical band gap of CFATS was varied with incorporating more Al atoms in the Fe lattices. This increment is due to the modification in composition and surface morphology (Fig. 4; Table 1). Eg is found to be increased from 1.47 eV to a maximum value about 2.1 eV with decrement of crystallite size, which may be attributed to the quantum confinement effect (Beraich et al., 2020). The films grown at x = 0.50 and 1 with optimum band gaps are well suited to be used as absorber layers in solar cell applications. In addition, the increase in Eg can also be originated to the less structural defects in samples that accompanied by the decrease in the density of defect states due to the decrease in film thickness of CFATS, as well as during the growth process. Hence, the optical band gap Eg = 1.52 eV of CATS is tuned to make it optically active as absorber layer for solar cell application.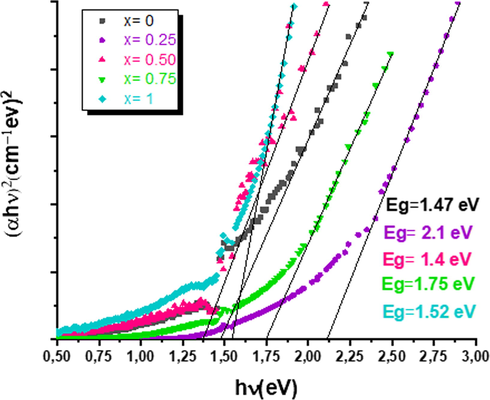
Tauc-plot of the as-deposited Cu2Fe1-xAlxSnS4 (CFATS) films (x = 0–0.25 – 0.50–0.75 and 1).
3.6 Wettability measurement
The surface wettability of materials is considered as a significant parameter to exploring their performance. It let to investigate the difference of energy between the molecules, which exist on the surface and those of material. Thomas Young described the contact angle θY in 1805 using the following equation (Ben Ameur et al., 2020):
Where
correspond to the interfacial tensions with solid/vapor, liquid/vapor and solid/liquid respectively. Fig. 7 presents water contact form on CFATS thin layers. It is clear that CFATS thin films exhibit a hydrophilic character with
. According to the curve, after inclusion of aluminum atoms, CATS (x = 1) material exhibits lowest contact angle near 44.6°, because CATS appears as nanofibers accompanied with voids and cavities. As stated by the figure, the droplets on materials surface exhibited elliptical form. The surface wetting behavior is controlled by various parameters such as micro/nanotexture and chemical composition. The variation of water contact angle θY is related to the change of surface free energy and surface morphology. It can also be explained by the weak adhesive force accompanied with Van der Waals forces between the water and material surfaces (Geim et al., 2003). However, it has been demonstrated that films with hydrophilic character produce large surface area between solid and liquid (Mrabet et al., 2016). This surface type favors the physisorption processes, which are necessary for antibacterial and photocatalytic activities (Dridi et al., 2017).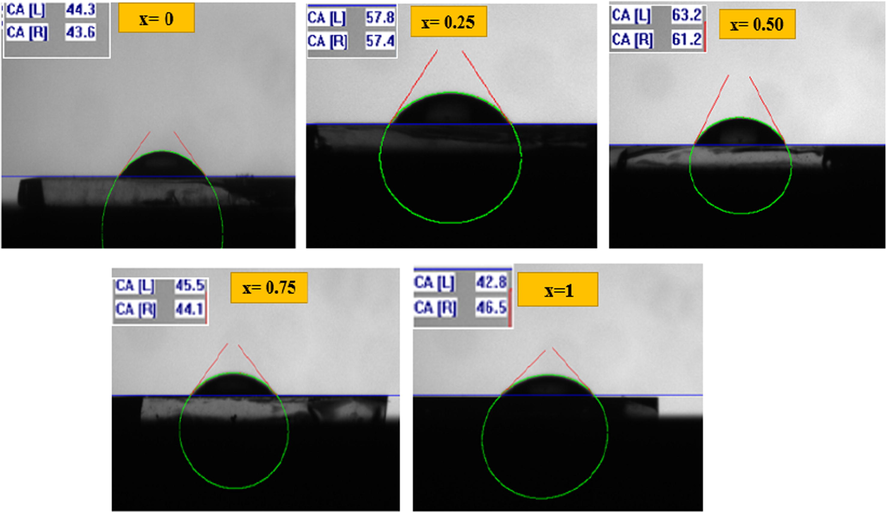
Water contact angle of Cu2Fe1-xAlxSnS4 (CFATS) films deposited by spray pyrolysis (x = 0–0.25 – 0.50–0.75 and 1).
3.7 Cyclic voltammetry (CV) analysis
See Fig. 8a represents the cyclic voltammogram plot for CATS thin layer.The electrochemical energy level of CATS thin film was extracted from the first oxidation onset potentials peak. It is shown that the valance-band edge (VB) equals to 0.29 eV. Conduction-band edge was given from the following expression: ECB = EVB – Eg, whereas EVB = 0.29 eV and Eg = 1.52 eV so ECB = -1.23 eV. From our previous work (Nefzi et al., 2020c), the valence and conduction band edges of SnO2:F were located respectively at −3.96 eV and 0.1 eV.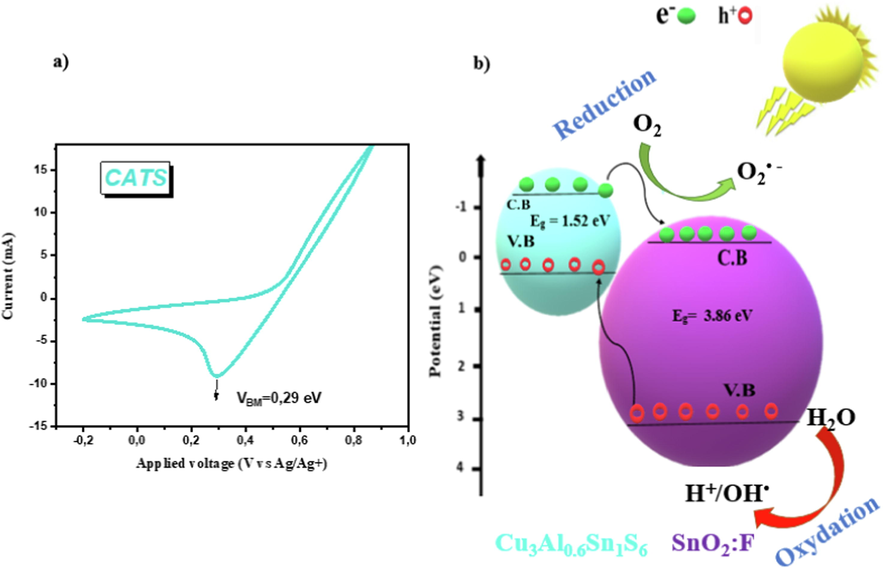
a) Cyclic voltammogram of CATS thin film, b) Mechanism of visible light antibacterial activity of CATS/SnO2:F heterojunction.
3.8 Antibactetrial and hydrophilicity properties
Antibacterial tests were carried on Escherichia coli (E.coli) and Staphylococcus aureus (S. aureus) because they are culprits in various infections. The antibacterial activities of SnO2:F, CATS films and CATS/SnO2:F heterojunction against the degradation of E. coli and S. aureus were examined, and the results are presented in Fig. 9a. The evaluation of antibacterial activity has been performed by counting forming unity (CFU). SnO2:F and CATS films exhibit stronger antibacterial activities for Gram positive S. aureus than that of Gram negative E. coli, while the opposite trend was found for CATS/SnO2:F heterojunction. These behaviors are due to the antibacterial mechanisms of films and the different bacteria structures (Qi et al., 2019). Generally, a greater inhibition of both S. aureus and E. coli was attained by CATS/SnO2:F heterojunction which may be attributed to its fast carriers generation (Ali et al., 2020). Deposition of p-type CATS on the surface of n-type SnO2:F caused generation of the space charge layer (SCL) that affected the charge carriers diffusion. Therefore, it will increase the negative and positive carrier’s separation. The viability rates achieved against S. aureus was 40 %, 31 %, and 15% for SnO2:F, CATS films and CATS/SnO2:F heterojunction, indicating the excellent results of antibacterial activity of coupled CATS/SnO2:F. A poor inhibition of E. coli bacterium implying just 90 % and 71% reductions to the surface of SnO2:F and CATS films. However, CATS/SnO2:F heterojunction has good effect in the antibacterial activity reaching 5 % as viability rate of E. coli. It has been proposed that the cation released from materials (in our case Cu2+, Al3+ and Sn2+) could bind to thiol groups (SH) found in proteins and enzymes on the surface of cellule. Then, they can intervene in with cell division and conductive to bacterial cell death (Shao et al., 2015). Antibacterial activity assay of the membranes by CATS/SnO2:F heterojunction against the degradation of E. coli and S. aureus are shown in Fig. 9b.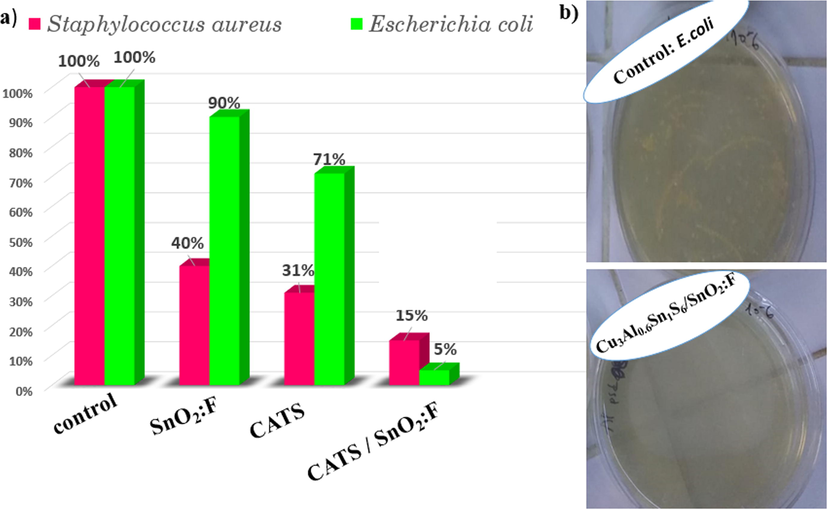
Percentage of bacterial inhibition of E. coli and S. aureus bacteria against SnO2:F and CATS films and CATS/SnO2:F heterojunction by monitoring the optical density (OD) at 600 nm. Antibacterial activity assay of CATS/SnO2:F heterojunction against E. coli.
See Fig. 8b reports the antibacterial activity mechanism of CATS/SnO2:F heterojunction. Beside, CATS presents narrow band gap, the electron can jumped from its valance-band (VB) to its conduction band (CB) by the generation of same contents of holes in its VB. The photogenerated electrons located in CB of CATS can migrate to the CB of SnO2:F, because of the more positive CB of SnO2:F than that of CATS. Therefore, the photogenerated holes located at the VB of SnO2:F achieve the VB of CATS. Thus, the efficient charge separation enhances the lifetime of charge carriers, increasing the reduction and oxidation process that produce the reactive oxygen species (ROS). Meanwhile, the death of bacteria is mostly ascribed to the destruction of their outer membrane. The crust damage instigated by physical or chemical modification of the surface membrane because it consists of lipid along a polyunsaturated fatty acid (Ali et al., 2020). Then, a reaction of free radicals with polyunsaturated acids and initiation of lipid peroxide formation modifies the fluidity of membrane and interrupts protein bonds. This rapid augmentation of radicals induces oxidative stress that results in the degradation of polyunsaturated fatty acids in harmful products (Ali et al., 2020). CATS/SnO2:F heterojunction is easy to penetrate the cell wall of E. coli than S. aureus, SnO2:F and CATS films present lower antibacterial activity to E. coli.
3.9 Correlation between antibactetrial and hydrophilicity
It has been demonstrated here that CATS was characterized with hydrophilic surface. This surface type may be caused by the nanofiber structure accompanied with voids and cavities, which have been clearly observed in SEM images. It has been already established in the literature (De Falco et al., 2018; Qi et al., 2019; Karam et al., 2013), that materials with hydrophilic surfaces display higher antibacterial activity than those with hydrophobic surfaces. Contrary to the mechanism that conduct wettability, holes which exist in the valence band react with the adsorbed H2O to generate hydroxyl radicals, whereas electrons contribute to reduction processes with oxygen molecules generating superoxide anions O2− (Chen et al., 2012; Cho et al., 2004). Such responsible species for the inactivation of bacteria through oxidative damages (Cai et al., 2014) are also capable in damaging the cytoplasmic membrane and the outer membrane of microorganisms, consequently, deteriorate essential cellular components leading to cell death.
4 Conclusion
In summary, a simple and facile method was developed to synthesize Cu2Fe1-xAlxSnS4 (CFATS). The aluminum content was varied to obtain new quaternary chalcogenide named CATS. XRD spectra and EDX revealed the formation of this new quaternary Cu3Al0.6Sn1S6 chalogenide which crystalized with stannite structure. Two Raman peaks located at 335 cm−1 (A1mode) and 295 cm−1 (B/E mode) have been assigned to CATS material. A nanofiber structure coupled with voids and cavities was noted in SEM image of CATS, prompting us to investigate the antibacterial properties of CATS film and improve its antibacterial activity by coupling it with SnO2:F. This novel chalcogenide features optimum band gap near 1.52 eV, which renders it as absorber layer for photovoltaic application. CATS grows with the high porosity, explicating its hydrophilic surface and provides a way for the possibilities of using CATS in several applications such as self-disinfecting and self-cleaning. Overall, this work reveals also that the surface of CATS/SnO2:F heterojunction displayed higher inhibition against S. aureus and E. coli bacteria.
Acknowledgments
The authors would like to acknowledge the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant code: 22UQU4350568DSR01. Also, the author would thank Dr. Ziad Moussa, United Arab Emirates University, as native English specker (Canadian) for his valuable editing and revision the manuscript
References
- Next generation promising Cu2(ZnxFe1-x)SnS4 photovoltaic absorber material prepared by pulsed laser deposition technique. Mater. Lett.. 2014;137:147-149.
- [Google Scholar]
- Mutual contaminants relational realization and photocatalytic treatment using Cu2MgSnS4 decorated BaTiO3. Appl. Mater. Today. 2020;18:100534
- [Google Scholar]
- Numerical simulations of ultrathin CdTe solar cells with a ZnxCd1−xS window layer and a Cu2O hole transport layer. J. Comput. Electron.. 2021;20:2501-2510.
- [Google Scholar]
- Impact of substrate nature and film thickness on physical properties of antimony trisulphide (Sb2S3) thin films for multifunctional device applications. Superlattices Microstruct.. 2020;142:106473
- [Google Scholar]
- Microstructured ZnO-ZnS composite for earth-abundant photovoltaics: elaboration, surface analysis and enhanced optical performances. Sol. Energy. 2021;218:312-319.
- [Google Scholar]
- Synthesis of tetragonal Cu2NiSnS4 thin film via low-cost electrodeposition method: effect of Ni2+ molarity. J. Electron. Mater.. 2020;49:728-735.
- [Google Scholar]
- Optoelectronic and material properties of solution-processed Earth-abundant Cu2BaSn(S, Se)4 films for solar cell applications. Nano Energy. 2021;80:105556
- [Google Scholar]
- Disinfection kinetics and contribution of reactive oxygen species when eliminating bacteria with TiO2 induced photocatalysis. J. Biomater. Nanobiotechnol.. 2014;5:200.
- [Google Scholar]
- Titanium dioxide photocatalysis in atmospheric chemistry. Chem. Rev.. 2012;112:5919-5948.
- [Google Scholar]
- Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res.. 2004;38:1069-1077.
- [Google Scholar]
- Anisotropic broadening of XRD peaks of α′-Fe: Williamson-Hall and Warren-Averbach analysis using full width at half maximum (FWHM) and integral breadth (IB) Mater. Charact.. 2018;142:144-153.
- [Google Scholar]
- Iranian native medicinal plants affecting staphylococcus aureus as septic pathogens. Egypt. J. Vet. Sci.. 2022;53:1-8.
- [Google Scholar]
- TiO2 nanoparticle coatings with advanced antibacterial and hydrophilic properties prepared by flame aerosol synthesis and thermophoretic deposition. Surf. Coat. Technol.. 2018;349:830-837.
- [Google Scholar]
- Electrical conductivity of Zn2SnO4 thin films along with wettability and EtOH-sensing. J. Alloy. Compd.. 2017;708:769-779.
- [Google Scholar]
- Structural and optical properties of Cu2FeSnS4 thin film synthesized via a simple chemical method. Mater. Sci. Semicond. Process.. 2014;25:159-162.
- [Google Scholar]
- Optoelectronic properties of solar cell materials based on copper-zinc-tin-sulfide Cu2ZnSn(SxTe1-x)4 alloys for photovoltaic device applications. Sol. Energy. 2021;225:851-862.
- [Google Scholar]
- Effect of copper concentration on the physical properties of ZnS: Cu alloys prepared by chemical bath deposition. J. Mater. Sci.: Mater. Electron.. 2016;27:10684-10695.
- [Google Scholar]
- Activated hydrophobic and hydrophilic surfaces: assessment of peptide adsorption and antibacterial activity against some food pathogens. Appl. Microbiol. Biotechnol.. 2013;97:10321-10328.
- [Google Scholar]
- Properties of thermally evaporated CZTS thin films and numerical simulation of earth abundant and non toxic CZTS/Zn(S, O) based solar cells. Sol. Energy. 2020;207:496-502.
- [Google Scholar]
- Supersonically sprayed washable, wearable, stretchable, hydrophobic, and antibacterial rGO/AgNW fabric for multifunctional sensors and supercapacitors. ACS Appl. Mater. Interfaces. 2021;13:10013-10025.
- [Google Scholar]
- Optoelectronic investigation of Cu2FeSnS4 quaternary functional photodiodes with IR detection capabilities. J. Mol. Struct.. 2021;1246:131265
- [Google Scholar]
- Biomineralized N-doped CNT/TiO2 core/shell nanowires for visible light photocatalysis. ACS Nano. 2012;6:935-943.
- [Google Scholar]
- Alternative quaternary chalcopyrite sulfides (Cu2FeSnS4 and Cu2CoSnS4) as electrocatalyst materials for counter electrodes in dyesensitized solar cells. J. Power Sources. 2016;305:134-143.
- [Google Scholar]
- Influence of Mn dopant on the crystallite size, optical and magnetic behaviour of CoFe2O4 magnetic nanoparticles. J. Phys. Chem. Solids. 2021;148:109654
- [Google Scholar]
- Effects of surface oxygen vacancies content on wettability of zinc oxide nanorods doped with lanthanum. J. Alloy. Compd.. 2016;688:122-132.
- [Google Scholar]
- Preparation and identification of nanoparticle of lanthanum oxide and its application as antibacterial agent. Eurasian Chem. Commu. 2020:265-271.
- [Google Scholar]
- Synthesis of new promising quaternary Cu2InSnS4 absorber layer: physical behaviors, wettability and photocatalysis applications. J. Alloy. Compd.. 2021;162771
- [Google Scholar]
- Nefzi, C., Souli, M., Dotor Castilla M.L., Garcia, J.M., Kamoun-Turki, N., 2020c. CFTS-3/In2S3/SnO2:F heterojunction structure as eco-friendly photocatalytic candidate for removing organic pollutants. Arab. J. Chem. 13, 6366–6378.
- Effect of sulfur concentration on structural, optical and electrical properties of Cu2FeSnS4 thin films for solar cells and photocatalysis applications. Superlattices Microstruct.. 2018;124:17-29.
- [Google Scholar]
- Effect of substrate temperature on physical properties of Cu2FeSnS4 thin films for photocatalysis applications. Mater. Sci. Eng., B. 2020;254:114509
- [Google Scholar]
- Growth of the next generation promising Cu2 Fe1-xCoxSnS4 thin films and efficient p-CCTS/n-In2S3/n-SnO2F heterojunction for optoelectronic applications. Mater. Res. Bull.. 2020;133:111028
- [Google Scholar]
- Exploitation of irradiated Cu2InSnS4 semiconductor: a successful attempts with high efficacy towards water treatment under photocatalysis approach. J. Mol. Struct.. 2021;131943
- [Google Scholar]
- Electrical and optical properties of Cu2Zn(Fe, Mn)SnS4 films prepared by spray pyrolysis. Optics. 2018;63:251-257.
- [Google Scholar]
- Earth-abundant quaternary semiconductor Cu2MSnS4 (M = Fe Co, Ni and Mn) nanofibers: fabrication, characterization and band gap arrangement. J. Alloy. Compd.. 2016;657:157-162.
- [Google Scholar]
- Fabrication of quaternary Cu2FeSnS4 (CFTS) nanocrystalline fibers through electrospinning technique. J. Mater. Sci.. 2015;50:777-783.
- [Google Scholar]
- Hydrophilic and antibacterial modification of poly(lactic acid) films by γ–ray irradiation. ACS Omega. 2019;4:21439-21445.
- [Google Scholar]
- Sadanand, Singh, P.K., Rai, S., Lohia, P., Dwivedi, D.K., 2021. Comparative study of the CZTS, CuSbS2 and CuSbSe2 solar photovoltaic cell with an earth-abundant non-toxic buffer layer. Solar Energy 222, 175–185.
- Preparation, characterization, and antibacterial activity of silver nanoparticle-decorated graphene oxide nanocomposite. ACS Appl. Mater. Interfaces. 2015;7:6966-6973.
- [Google Scholar]
- Thin film solar cell with 8.4% power conversion efficiency using an earth-abundant Cu2ZnSnS4 absorber. Prog. Photovoltaics Res. Appl.. 2013;21:72-76.
- [Google Scholar]
- Ultrathin Cu(In, Ga)Se2 transparent photovoltaics: an alternative to conventional solar energy-harvesting windows. Nano Energy. 2022;92:106711
- [Google Scholar]
- Hyperthermic evaluation of oleic acid coated nano-spinel magnesium ferrite: enhancement via hydrophobic-to-hydrophilic surface transformation. J. Alloy. Compd.. 2020;835:155422
- [Google Scholar]
- Development of tantalum with highly hydrophilic surface and antimicrobial properties obtained by micro-arc oxidation process. J. Biomed. Mater. Res. 2020:1-12.
- [Google Scholar]
- Plasma treatment of polymethyl methacrylate to improve surface hydrophilicity and antifouling performance’ wileyonlinelibrary. Soc. Plastics Eng. 2020:1-8.
- [Google Scholar]
- Enhancing the performance of Cu2ZnSnS4 solar cell fabricated via successive ionic layer adsorption and reaction method by optimizing the annealing process. Sol. Energy. 2021;220:204-210.
- [Google Scholar]
- Preparation and characterization of Al doped ZnO thin films by PLD. Superlattices Microstruct.. 2008;44:127-135.
- [Google Scholar]
- Victoria, M., Haegel, N., Peters, I.M., Sinton, R., Jäger-Waldau, A., del Cañizo, C., Breyer, C., Stocks, M., Blakers, A., Kaizuka, I., Komoto, K., Smets, S., 2021. Solar photovoltaics is ready to power a sustainable future, Joule 5, 1041–1056, May 19, 1043.
- Fabrication and wear protection performance of superhydrophobic surface on zinc. Appl. Surf. Sci.. 2011;257:7486-7489.
- [Google Scholar]
- Super-hydrophobic film prepared on zinc as corrosion barrier. Corros. Sci.. 2011;53:2080-2086.
- [Google Scholar]







