Translate this page into:
Exploring natural essential oil components and antibacterial activity of solvent extracts from twelve Perilla frutescens L. Genotypes
⁎Corresponding author at: Sulaimani Polytechnic University, Slemani 46001, Kurdistan Region, Iraq. hiwa2009@yahoo.com (Hiwa M. Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Pharmaceutical active ingredients from medicinal plants can be seen as a gateway to drug discovery with the importance of medical and health care. Perilla frutescens has been traditionally used for medicinal and flavoring purposes, thus aromatic compounds and antimicrobial activity of twelve accessions of three solvent extracts (distilled water, methanol and ethyl acetate) of P. frutescens L. Britt grown under open field was studied. RauTiaTo showed relatively high essential oil (EO) content and EO yield, followed by 203P and J1 genotypes. Twenty four monoterpene hydrocarbons, eighteen sesquiterpenes, eight oxygenated compounds—alcohols, five aldehydes, three phenols, other relatively low contents such as four esters and one phenylpropanoid were found. The chief natural constituents of the EO extracted from perilla genotypes were perillaldehyde in (RauTiaTo, J1, 203P, PS1, JTD3, NP606 and 588P), ß-dehydro-elsholtzia ketone in GB, MP3, and PS3 respectively, followed by perilla ketone in PS2 and 465P. Other natural compounds were limonene, ß caryophyllene, (Z,E)-alpha-Farnesene, shisofuran and trans-shisool. Aqueous extract of PS2, 588P and JTD3, as well as antibiotic gentamicin, inhibited more strongly the growth of Klebsiella spp., than other solvent extracts using disc diffusion method. Gentamicin showed a very strong antibacterial activity against Staphylococcus aureus., Streptococcus pneumonia., Klebsiella spp., Shigella spp., Salmonella spp., Escherichia coli with varying degree of inhibition, while another amoxicillin was only active against bacterial infections Streptococcus pneumonia and Escherichia coli. The combination 2 and 3 of herbal preparation showed the antibacterial activity against Streptococcus pneumonia respectively. These results can be helpful for qualitative and quantitative analyses of this herbal drug and food additives in quality control for the standardisation of natural products.
Keywords
Active ingredient
Biological activity
Essential oil
Natural products
Antibacterial activity
1 Introduction
Perilla (Perilla frutescens L. Britt.) is an annual short‐day medicinal plant that belongs to the Lamiaceae plant family and has been used traditionally for medical and flavoring purposes. The leaves of the plant are applied against bronchitis or other problems of the respiratory system as well as in the treatment of skin allergic reactions (Ahmed, 2019). Recent publications have also pointed out its antiallergic, anticancer and immunostimulant properties (Khanaree et al., 2018). Because of the morphological variation of perilla, it can also be used for ornamental purposes cultivated as an annual plant. Perilla has been recorded in Chinese traditional medicinal system nearly 500 CE, particularly in records called “Ming Yi Bie Lu” [Renown Physicians' Extra Records), and other drugs such as “su” which means comforting our body and boosts the blood circulatory system. In Chinese traditional medicinal system, dried parts such as perilla leaf, perilla stalk and perilla seed are considered as a drug (Liu et al., 2013), and recorded in Chinese Pharmacopoeia (1990), and in the Japanese Pharmacopoeia (1991) only leaves and twigs of perilla are recorded as a drug (Chen, 1997). The perilla species are widely cultivated throughout China, Taiwan, Japan, Korea, Vietnam, Thailand and India as a medicinal plant and edible herb (Igarashi and Miyazaki, 2013; Ahmed, 2019). In China cultivated varieties can be classified to P. var. frutescens, var. arguta, var. crispa, var. auriculato-dentata and var. acuta (Brenner, 1993). In Japan, four perilla species are known: P. frutescens (cultivated) with the chromosome (2n = 40), P. citriodora, P. hirtella, and P. setoyensis (wild) species with the chromosomes (2n = 20) (Ito et al., 1998). It has been said that high-quality of perilla leaves are purple on both sides with a good aroma. These purple varieties have been used to relieve stomach disorders and induce sweating. It has been mixed with some Japanese folk drugs like Shimpito, Kososan and Hangekobokuto (Fujiwara et al., 2018). It has been also used in the traditional medicinal system to cure a few ailments such as allergy, cough, tumor and intoxication. In traditional medicine, perilla is often mixed with other oriental medicines named Kampo, which are used to treat depression-related diseases and asthma. These health benefits of perilla are due to natural compounds (secondary metabolites) synthesised by different organs in particular polyphenols, flavonoids, essential oils, triterpenes, carotenoids, phytosterols, fatty acids, tocopherols and policosanols (Ahmed, 2019). These bioactive compounds have been studied extensively, such as phenolic acids (Peng et al., 2005; Ahmed and Sarosi, 2019), flavonoids (Feng et al., 2011), anthocyanins (Fujiwara et al., 2018), essential oils (Ahmed and Sarosi, 2019), carotenoids (Müller-Waldeck et al., 2010), fatty acid (Ding et al., 2012). The perilla plant extracts and their active substances have been studied widely for pharmacological property and showed antioxidant activity (Gu et al., 2009), antibacterial and antifungal activity (Yamamoto and Ogawa, 2002; Tian et al., 2014), anti-allergic effect (Cota et al., 2013), anti-depressant activity (Nakazawa et al., 2003; Wei-Wei et al., 2014), anti-inflammatory activity (Kim et al., 2006), antitumor effect (Banno et al., 2004), anti-HIV-1 activity (Yamasaki et al., 1998), anti-aging (Bae et al., 2017). Moreover, the leaves, seeds, and stems of perilla species have been shown to exert antipyretic and antibiotic effects to cure intestinal disorders (Nakazawa and Ohsawa, 2000). The purple perilla varieties are known to be rich in anthocyanins, which are associated with high antioxidant activities (Yoshida et al., 1990). While green perilla varieties are rich in phenolic compounds (Lee et al., 2017).
The chemotypes variations of P. Frutescens, leading to a different concentration of phytochemicals and associated with various physiological effects for humans, as well as its utilization in food and pharmaceutical products. To increase the intake of health-related bioactive substances, the best strategy is to optimize bioactivity in medicinal, aromatic, and spice plants by increasing the phytochemical concentrations via proper cultivation approaches. To the best of our knowledge, no studies have been conducted on the qualitative and quantitative analysis and comparison of these different genotypes of P. frutescens. Thus, we carried out, quantitative and qualitative analysis of the aromatic compounds, the antibacterial activity of different solvent extractions, and mixed combinations raised from open field.
2 Materials and methods
2.1 Plant materials and reagents
Methanol for gas chromatography-MS SupraSolv®, Sodium sulphate (analytical grade), acetic acid (100%), n-hexane for analysis EMSURE® ACS, phenylmethyl siloxane (viscosity 450–550 cSt), helium Messer® CANGas, (99.999%) and aliphatic hydrocarbons (analytical standard), methanol (99.5%), ethyl acetate (99.5%) were bought from Merck (Darmstadt, Germany). Mueller-Hinton Broth (MHB) and Mueller-Hinton Agar (MHA) were purchased from (Difco, Becton Dickinson, Sparks, MD, USA). This investigation was carried out during 2017/2018, to evaluate essential oil content, yield, composition and antibacterial activity of solvent extracts from twelve perilla genotypes (Perilla frutescens L.) grown under open field condition.
2.2 Qualitative and quantitative analysis of volatile components
The fresh leaves of different perilla plants were randomly collected from the open field and separated from stems of Perilla L. Prior to this, leaf material was dried in shade at room temperature and used for laboratory measurements. The essential oil of each accession (PS1, PS2, PS3, 203P, 465P, 588P, J1, JTD3, NP-606, RauTiaTo, MP3 and GB) was extracted by hydrodistillation in a Clevenger-type apparatus for 3 h. The individual samples of the same accessions from an open field were mixed to get representative plant material, and (15 g) was accurately weighed and placed in a (1 L) round-bottomed flask filled with (500 mL distilled water) according to the described method (Ahmed and Sarosi, 2019). The essential oils were collected, and traces of water removed with anhydrous sodium sulphate then essential oils were stored in dark vials sealed with Teflon-faced septa (Supelco, Bellefonte, CA, USA) and kept at 4 °C until analysis. The essential oil content of each perilla plant accession was measured, and reported as a relative percentage (%, v/w) and expressed in mL/100 g dry matter (DM). The essential oil yield was calculated by multiplying the perilla drug yield with the corresponding oil content.
2.3 Gas chromatographic-mass spectrometric analysis
GC–MS analysis was performed for each accession (PS1, PS2, PS3, 203P, 465P, 588P, J1, JTD3, NP-606, RauTiaTo, MP3 and GB) separately, using an Agilent Technology 6890 N instrument equipped with an HP-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm) and an Agilent Technologies MS 5975 inert mass selective detector. The initial temperature was 60 °C and maintained for 5 min, then increased by a rate of 3 °C/min up to 240 °C. The carrier gas was helium (1 mL min−1), injector and detector temperatures were 250 °C. Split ratio: 30:1. Prior to injection volume 0.2 μl, the ten μl EO was diluted in1000 μl hexane. The percentage composition of the EO was calculated from the GC peak areas. Acquisition mass range 40–400 m/z; Ionization energy was 70 eV. The mass spectra and linear retention indices (LRI) of essential components were compared with (NIST; Wiley) and by matching their recorded mass spectra with literature data (Adams, 2017).
2.4 Preparation of extracts
The dried leaves were separated from the stems and ground using an electric blender into a fine powder and sifted by using a stainless-steel sieve (500 μm hole size). 50 mL of boiling distilled water was added into 0.5 g of the powder from samples of each accession. The plant test solution was shaken and stored at room temperature for 24 h. The extracts were filtered using filter paper and stored in the refrigerator (4 °C) until the measurements were performed. The preparations were carried out in 6 replications.
2.5 Antibacterial activity
2.5.1 Preparation of extracts
The fresh leaves of different perilla genotypes were randomly collected from the open field and separated from the stems of Perilla L. The plant material was dried in shade at room temperature and ground using an electric grinder into a fine powder and used for laboratory measurements. 5 g powder of each accession named (PS1, PS2, PS3, 203P, 465P, 588P, J1, JTD3, NP-606, RauTiaTo, MP3 and GB) was added to 100 mL of organic solvents from different polarity distilled water, methanol (99.5%) and ethyl acetate (99.5%) in a conical flask. The plant extracts were shaken for 24–48 h on an orbital shaker (150 rpm) at room temperature. After extractions, the solutions were filtered using Whatman No.1 filter paper (150 mm) and centrifuged at 1000 rpm for 10 min and then the filtrates were filtered again. The distilled water, methanol and ethyl acetate extracts were evaporated to dryness at 40 °C using a vacuum rotary evaporator and weighted with an analytical balance (W/W, dry base) to determine the yield of soluble constituents. For stock solutions, each crude extract was then dissolved in one ml distilled water and ethyl acetate separately to prepare the final concentration 98 mg/mL and stored at 4 °C. Determination of extraction yield was done using this formula: % Extract recovery = (X/Y) * 100 where X = weight of crude dried extract and y = weight of powdered plant material used for each extraction.
2.5.2 Microorganisms and culture media
In vitro antibacterial activity of the perilla solvent extracts evaluated against two groups of microorganisms were gram-positive bacteria [Staphylococcus aureus, Streptococcus pneumonia ] and gram-negative bacteria [Escherichia coli, Klebsiella spp, Shigella spp, Salmonella spp] by using the Kirby-Bauer disc diffusion method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2012). These bacterial strains were procured from the microbial research center, biology department, Raparin University.
2.5.3 Preparation of culture media
The medium was prepared by adding 500 mL distilled water into 38 g of Muller Hinton agar in a conical flask. Nutrient broth (No.2) was prepared by adding 100 mL. Nutrient broth (No.2) was prepared by suspending 2.5 g of Nutrient powder in100 mL of sterile distilled water in a conical flask. The solution was fully mixed and warmed slightly to dissolve thoroughly the powder before dispensing into universal bottles. Both solutions were autoclaved at 121 °C for 15 min, and allowed to cool at room temperature and unless the plates were used the same day, and stored at 4 °C for further experiment. The agar media was poured into sterile Petri dishes and allowed to solidify and dried for about 30 min before covering the plates to prevent the formation of water on the agar surface and stored for further use.
2.5.4 Maintenance and storage of stock cultures
The streak plate technique was used to obtain pure bacterial cultures. Bacterial strains were subcultured by transferring a single colony into the surface of the agar plates using a sterile inoculating loop. Streaked agar plates were incubated at 35 °C for 24 h using an incubator. After subculturing of the bacteria, the bacterial cell suspension was prepared by transferring a single isolated colony with a sterile inoculating loop from previous streaked plates into test tubes containing sterile nutrient broth. The prepared cell suspension was well-mixed using Vortex Mixer (Medline VX-3000 Digital Vortex mixer) and incubated at 35 °C for 24 h.
2.5.5 Preparation of impregnated filter paper discs
Filter paper discs were prepared using qualitative Whatman No.1 filter paper (Whatman International Ltd., England) with approximately 5 mm in diameter, and sterilised by autoclaving at 121 °C for 15 min prior to use. Sterile filter paper discs were impregnated with100 μL of each crude extract separately and with three combinations of extracts combination 1 (aqueous extracts of mixed twelve accessions) combination 2 (methanol extracts of mixed twelve accessions) and combination 3 (ethyl acetate extracts of mixed twelve accessions) and were left under laminar flow cabinet overnight to dry. Standard antibiotics such as Gentamicin (10 µg/disc) and Amoxicillin 25 µg (cmg) were served as a positive control.
2.5.6 Preparation of inoculum and application of discs
At least three to five well-isolated colonies of the same morphological type from a culture agar plate were selected and touched the top of colony with a loop and transferred to a test tube containing 4 mL of a suitable sterile broth. The suspension (broth) was incubated at 37 °C for 12 h. The fresh inoculums of tested microorganisms were standardised to the 0.5 McFarland standard turbidity by adjusting the optical density (OD) of the bacterial suspension to a turbidity corresponding to spectrophotometric absorbance OD = 0.08–0.1 at 630 nm. This bacterial suspension was equal to 1.5 × 106 bacterial cells per ml (CFU/ml). Approximately, (106 colonyforming units (CFU)/ml) were spread with a sterile cotton swab on the surface of Mueller-Hinton agar (MHA) plates at 37 °C and allowed to dry for 10 min. Six extract disks with one of the standard positive control disks were placed individually using sterile forceps on the inoculated Mueller-Hinton agar plate. Plates were incubated overnight at 37 °C. The formation of a clear zone surrounding the sample impregnated disks indicated antibacterial activity by subtracting the disc diameter (i.e. 5.0 mm) from the total zone of inhibition.
2.6 Statistical analysis
The data were analysed using the IBM SPSS Statistics 22 program. According to the Kolmogorov-Smirnov and Shapiro-Wilk, normality can be accepted because the p-value > 0.05. The results are reported as mean ± standard deviation (SD) of six replications and one-way analysis of variance (ANOVA) was employed for data comparison, following Tukey’s honestly significant difference (HSD) test. Significant differences (p < 0.05) within rows were represented by different superscript letters. The correlation between the studied variables (essential oil content and yield with biomass and drug) in the samples was also determined by a two-tailed Pearson correlation analysis. Principal component analysis (PCA) for sixteen major essential oil components was performed using Minitab® 19.2020.1 (64-bit) version 20. Hierarchical cluster analyses of the heatmap were done using (https://biit.cs.ut.ee/clustvis/).
3 Results and discussion
3.1 Essential oils content, yield and composition
Essential oils and other natural volatile compounds present in P. frutescens genotypes were collected via a steam distillation method and analyzed by GC–MS. The variations in essential oil content (EO), essential oil yield and essential oil composition of Perilla frutescens are reported in Tables 1 and 2 and Fig. 1, respectively. Perilla accession RauTiaTo (Purple colour) showed relatively high content of (1.35 ± 0.06 mL/100 g DM) and EO yield (2.61 ± 1.05) %, followed by 203P (Green colour) (1.21 ± 0.06 mL/100 g DM) and (3.55 ± 1.19) %, and J1 (Green colour) (1.03 ± 0.03 mL/100 g DM) and (2.37 ± 1.01) % for both EO content and yield respectively and statistically significant than all of them (P < 0.05). On the other hand, these phenotypes showed a very tiny amount of aromatic compounds compared to others such as GB (Green/purple) (0.07 ± 0.00 mL/100 g DM) and (0.09 ± 0.02) %, PS3 (Purple), (0.14 ± 0.00 mL/100 g DM) and (0.26 ± 0.12) %, JTD3 (Purple) (0.11 ± 0.03 mL/100 g DM) and (0.29 ± 0.13) % for both studied parameters EO content and yield correspondingly. –; absent.
No.
Accessions name, voucher number
Phenotypes colour
Statistics
Essential oil content (mL/100 g DM)
Essential oil yield %
Drug (g/plant)
1
PS1 N20-01
Green
Mean
0.92f
2.22c
69.80b
Std. Deviation
0.00
0.97
31.68
2
PS2 N20-02
Green
Mean
0.35e
1.11b
77.60b
Std. Deviation
0.01
0.48
38.34
3
PS3 N20-03
Purple
Mean
0.14c
0.26a
43.80b
Std. Deviation
0.00
0.12
18.12
4
203P N20-04
Green
Mean
1.21i
2.51c
83.00b
Std. Deviation
0.06
1.19
29.11
5
465P N20-05
Green
Mean
0.96g
2.28c
54.20b
Std. Deviation
0.09
1.49
35.54
6
588P N20-06
Purple/Green
Mean
1.00g
2.07c
56.00b
Std. Deviation
0.07
0.56
14.41
7
J1 N20-07
Green
Mean
1.03h
2.37c
62.40b
Std. Deviation
0.03
1.01
23.52
8
JTD3 N20-08
Purple
Mean
0.11b
0.29a
67.20b
Std. Deviation
0.03
0.13
17.96
9
NP606 N20-09
Green/Purple
Mean
0.91f
1.59a
50.00b
Std. Deviation
0.00
0.50
16.59
10
RauTiaTo N20-10
Purple
Mean
1.35j
2.61c
54.60b
Std. Deviation
0.06
1.05
24.77
11
MP3 N20-11
Green/Purple
Mean
0.25d
0.47a
50.60b
Std. Deviation
0.03
0.12
12.35
12
GB N20-12
Green/Purple
Mean
0.07a
0.09a
32.00a
Std. Deviation
0.00
0.02
7.77
No
Component
RTa
LRIb
PS1
PS2
PS3
203P
465P
588P
J1
JTD3
NP606
RauTiaTo
MP3
GB
1
α-pinene
5.56
938
0.02
–
–
0.04
–
–
0.12
–
–
0.01
–
–
2
Camphene
5.95
952
–
–
–
–
–
–
–
–
–
0.01
–
–
3
Sabinene
6.52
976
–
–
–
–
–
–
0.01
–
–
0.05
–
–
4
ß-pinene
6.64
981
0.09
–
–
0.11
–
–
0.21
–
0.03
0.07
–
–
5
Hexyl benzoate <n->
30,69
1586
–
–
–
–
–
–
–
0.06
–
–
–
–
6
Hexenyl benzoate <(2E)->
30.37
1595
–
–
0.25
–
–
–
0.06
–
–
0.25
–
7
ß-myrcene
6.99
995
–
0.06
–
–
–
–
–
–
–
0.06
–
–
8
α-phellandrene
7.43
1008
0.03
–
–
0.06
–
–
0.03
–
–
0.06
–
–
9
ß-ionon
26.55
1491
–
–
–
–
–
–
–
–
–
0.04
–
–
10
Limonene
8.19
1029
5.61
10.46
–
11
–
5.12
14.89
3.22
8.19
11.1
–
–
11
1,8-cineole
8.38
1034
–
–
–
–
–
–
–
–
0.09
–
–
12
α-terpinolene
10.29
1085
0.03
–
–
0.04
–
–
–
–
–
0.05
–
–
13
Perilla ketone
16.83
1243
–
75.19
0.19
0.68
97.9
–
–
0.17
–
0.19
0.20
0.18
14
Dihydro-carveole-acetate (neo)
19.44
1306
–
–
–
–
–
–
–
–
–
–
2.11
15
Shisofuran
14.77
1194
–
–
7.72
–
–
–
–
–
–
–
6.61
6.72
16
Elsholtzia keton
14.87
1197
–
–
3.43
–
–
–
–
–
–
–
3.34
–
17
Perilla alcohole
18.98
1294
0.21
–
–
0.19
–
–
0.18
0.35
0.19
0.40
–
–
18
1-Octen-3-yl acetate
15.25
1206
–
–
0.25
–
–
–
–
–
–
0.31
0.21
–
19
Furfural < 5-methyl->
5.445
933
–
–
0.08
–
–
–
–
–
–
–
0.03
0.05
20
Geranyl acetone
25.19
1457
–
–
–
–
–
–
–
–
–
0.05
–
–
21
ß-dehydro-elsholtzia ketone
19.11
1297
–
–
67.75
–
–
–
–
–
–
–
65.5
58.03
22
Trans-shisool
17.77
1266
3.55
0.03
–
1.42
–
1.76
1.25
4.65
2.5
1.01
–
–
23
Safranal
14.86
1196
0.06
–
–
–
–
–
–
0.2
–
0.17
–
–
24
Camphenilone
10.04
1078
–
–
0.06
–
–
–
–
–
–
–
0.05
0.3
25
ß-elemene
22.55
1391
–
–
–
–
–
–
–
–
–
0.04
–
–
26
ß caryophyllene
23.68
1420
4.89
2.69
6.85
5.45
1.8
3.19
5.91
5.09
2.61
4.98
6.81
9.75
27
Tau-cadinole
32.26
1644
–
0.07
0.13
0.43
–
–
0.31
0.15
–
0.09
0.11
–
28
Spatulenol
29.98
1584
–
–
0.46
–
–
–
–
–
–
0.06
0.52
0.43
29
α-humulene
25.07
1454
0.18
0.19
0.36
0.29
–
0.35
0.29
0.29
0.38
0.35
0.22
0.35
30
Bisabolol < epi-α->
33.73
1683
–
0.2
–
–
–
–
–
–
–
0.01
–
5.88
31
ß-cadiene
29.87
1581
–
–
–
–
–
–
–
–
–
0.05
–
–
32
Germakcrene-D
26.18
1482
0.41
0.14
0.15
0.27
–
0.2
0.28
0.13
1.47
1.29
0.15
0.55
33
Caryophyllene oxide
30.2
1590
0.15
–
2.23
0.22
–
–
0.1
0.22
–
0.29
2.22
5.35
34
Eremophila ketone
29.30
1558
0.02
–
–
0.03
–
–
–
–
–
–
–
–
35
Germacrene A
27.06
1504
–
0.02
–
0.02
–
–
–
–
–
0.2
–
–
36
(Z,E)-alpha-Farnesene
26.79
1498
5.05
1.56
3.87
2.89
1.02
6.85
3.19
2.37
1.55
4.48
3.82
–
37
(E,E) Farnesene
27.2
1507
–
–
–
–
–
–
–
–
–
0.07
–
5.01
38
Germacrene B
29.01
1557
–
–
–
–
–
–
–
–
–
0.02
–
–
39
δ-cadiene
27.8
1524
–
–
–
–
–
–
–
–
0.05
0.09
–
–
40
Eugenol < dihydro->
21.63
1366
0.05
–
–
–
–
–
–
–
–
–
–
–
41
α-copaene
22.03
1377
–
–
–
–
–
–
–
–
0.09
0.07
–
–
42
Thujanol acetate < neoiso-3->
18.44
1281
0.2
–
–
0.09
–
–
–
0.22
–
0.6
–
–
43
1-octen-3-ol
6.81
987
–
0.06
–
0.05
–
–
–
–
0.09
0.20
–
–
44
Linalool
10.76
1097
1.55
1.43
0.56
–
–
0.35
0.79
0.49
1.21
3.12
0.50
0.51
45
2-Nonyne-1-ol
12.91
1149
–
–
–
–
–
–
–
–
–
0.06
–
–
46
Cumenol < m->
16.01
1224
–
–
–
–
–
–
–
0.08
–
0.49
–
–
47
α-terpineole
14.55
1189
0.07
–
–
0.09
–
–
0.08
0.07
0.09
0.26
–
–
48
Linalool formate
15.69
1216
–
–
–
0.05
–
–
–
0.03
0.05
–
–
–
49
Nerolidol
29.35
1566
–
–
–
–
–
–
–
–
–
0.35
–
–
50
Phytol
47.67
2064
–
–
–
–
–
–
–
0.05
–
0.09
–
–
51
Benzaldehyde
6.28
967
0.18
0.65
0.86
0.39
–
–
0.24
0.51
0.06
0.11
0.80
0.75
52
Octanal
7.04
997
–
–
–
–
–
–
–
–
–
0.09
–
–
53
Perillaldehyde
17.89
1268
77.49
7.02
0.58
73.95
–
82.15
72.25
79.34
81.44
67.22
0.51
0.45
54
Limonene aldehyde
20.2
1327
0.08
–
–
0.04
–
–
–
–
–
–
–
–
55
Neral (citral-b)
16.58
1238
–
–
–
–
–
–
–
–
0.29
–
–
56
Thymole
18.81
1290
–
–
–
–
–
–
–
–
–
–
–
57
Carvacrol
19.2
1300
–
–
3.51
–
–
–
–
–
–
–
3.51
3.45
58
Eugenol
21.44
1361
0.08
0.06
–
–
–
–
–
–
–
0.51
–
–
59
Methyl geranate
19.99
1321
–
–
0.1
–
–
–
–
–
–
–
0.2
0.1
60
Benzyl benzoate
36.79
1772
–
–
0.21
–
–
–
–
–
–
–
0.22
–
61
Isomenthyl acetate
19.04
1296
–
–
–
–
–
0.03
–
–
–
–
–
–
62
Cis-3-Hexenyl benzoate
29.69
1576
–
–
0.39
0.04
–
–
–
0.2
–
0.01
0.31
–
63
Estragole
14.85
1196
–
–
–
–
–
–
–
–
–
0.15
–
–
Monoterpene hydrocarbons
9.6%
85.74%
79.73%
13.54%
97.9%
6.88%
16.96%
8.71%
10.91%
13.67%
76.19%
67.39%
Oxygenated Sesquiterpene Sesquiterpenes hydrocarbons
10.95%
4.87%
14.05%
9.69%
2.10%
10.59%
10.08%
8.47%
6.15%
12.69%
13.85%
27.32%
Oxygenated Compounds—Alcohols
1.62%
1.49%
0.56%
0.19%
0%
0.35%
0.83%
0.72%
1.44%
4.57%
0.50%
0.51%
Oxygenated Compounds—Aldehydes
77.75%
7.67%
1.44%
73.99%
0%
82.15%
72.49%
79.85%
82.96%
67.71%
1.31%
1.2%
Oxygenated Compounds—Phenols
0.08%
0.06%
3.51%
0%
0%
0%
0%
0%
0%
0.51%
3.51%
3.45%
Oxygenated Compounds—Esters
0%
0%
0.7%
0.04%
0%
0.03%
0%
0.2%
0%
0.01%
0.73%
0.1%
Phenylpropanoids
0%
0%
0%
0%
0%
0%
0%
0%
0%
0.15%
0%
0%
Total
100
99.83
99.99
97.45
100
100
100
97.95
100
99.31
96.09
99.97
NO of compounds
22
16
22
24
3
9
17
22
16
46
22
18
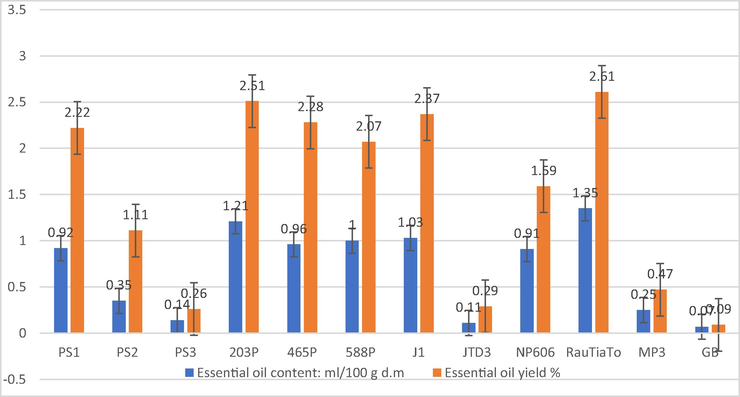
Essential oil content and yield of perilla genotypes cultivated in open field extracted by steam distillation method.
There was a broad range of variation appeared in the chemical composition of the natural volatile extracts (essential oils) among twelve P. frutescens phenotypes and genotypes. A total of 63 active ingredients Table 2 have been found and quantified in perilla accession, including twenty-four monoterpene hydrocarbons ranged from (9.6% to 97.6%), eighteen sesquiterpenes ranged from (2.10% to 27.32%), eight oxygenated compounds—alcohols ranged from (0.19% to 4.57%), five aldehydes ranged from (1.2% to 82.96%), three phenols ranged from (0.08% to 3.51%), other relatively low contents were four esters and one phenylpropanoid. The structures of the chief volatile compounds were shown in Fig. 2. The largest number of volatile compounds discovered in RauTiaTo taxes was forty-six components that represent nearly (99.31%) of the obtained oil, followed by twenty-four in 203P accession (97.45%), twenty-two for each different phenotype PS1, PS3, JTD3, and MP3 respectively. The main natural constituents of the EO extracted from the seven perilla genotypes (RauTiaTo, J1, 203P, PS1, JTD3, NP606 and 588P) was perillaldehyde with the percentage ranged from (67.22%), (72.25%), (73.95%), (77.49%), (79.34%), (82.12%) in the mentioned accessions.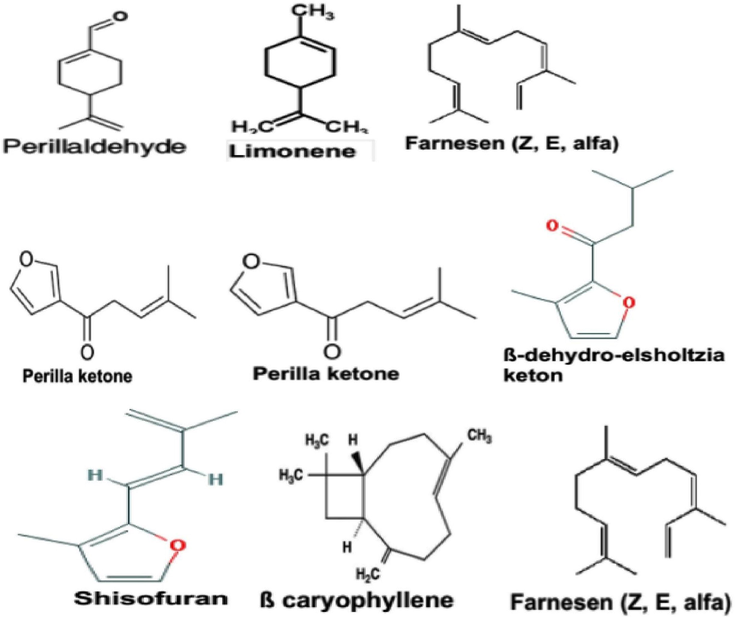
Structures of the chief volatile compounds identified in perilla genotypes.
ß-dehydro-elsholtzia ketone seems to be the second major component in the three perilla phenotypes GB, MP3, and PS3 with the percentage of (58.3%), (65.5%), (67.75%) respectively, followed by perilla ketone which was stood the third major chemical composition in PS2 and 465P (75.19–97.9%). Other natural compounds detected in various concentrations, including limonene, ß caryophyllene, (Z, E) -alpha-Farnesene, shisofuran and trans-shisool. Several chemically distinct chemovars (varieties) have been found in the studies P. frutescens (L.) Britton genotypes that can be classified based on the principal chemical components into three different chemotypes: PA – perillaaldehyde chemotype in PS1, 203P, 588P, J1, JTD3 and RauTiaTo populations where perilla aldehyde (67.22–82.12%), limonene (5.12–11%) and ß- caryophyllene (4.98–8.19%) were the major compounds. EK – elsholziaketone chemotype in PS3, MP3 and GB populations where ß dehydro- elsholtzia ketone (58.3–67.75%) was the major essential oil component. PK – perillaketone chemotype (or iso egomaketone) in PS2 and 465P population where perilla ketone (75.19–97.9%) was the major constituent.
In Asian countries, the dried leaves of perilla have been employed as raw material for crude drugs due to the higher potent content of bioactive substances, the author was also found the dried leaves of perilla accumulated more anticancer flavonoids apigenin (28-fold) and luteolin (86-fold) than other substances such as perillaldehyde and rosmarinic acid compared to fresh leaves (Kagawa et al., 2019). Lee et al., (2019) studied the chemical compounds of P. frutescens Britton var. acuta Kudo during roasting at 150 °C for various time points and found a total of one hundred and forty-two aromatic constituents with the majority of hydrocarbons followed by fewer alcohols, miscellaneous compounds, aldehydes, heterocyclic compounds, ethers, and ketones. The predominant compounds were methyl benzoate and limonene while in the current study perillaldehyde, ß-dehydro-elsholtzia ketone and perilla ketone were identified as major natural molecules present in the leaves of perilla genotypes. Perillaldehyde (PA) is a major volatile oil monoterpene compound found in the essential oil of P. frutescens (L.) Britton (Ahmed and Sarosi 2019), and has a unique and faintly sweet flavor and possesses antimicrobial and antidepressant properties (Ahmed, 2019). Limonene is an aliphatic terpene and major constituent usually derived from citrus oils (orange, lemon, mandarin, lime, and grapefruit). Limonene is widely utilized as a flavor and fragrance additive in consumer products, such as beverages, perfumes, detergents, and soaps (Erasto and Viljoen, 2008). One of the forms of limonene is d-limonene that has been used clinically to dissolve cholesterol-containing gallstones. Moreover, it has been used for relief of heartburn and gastroesophageal reflux (GERD) and has a broad range of chemopreventive and therapeutic activities against many types of cancer (Sun, 2007).
In our study, three different chemotypes were detected (PA – perillaaldehyde, EK – elsholziaketone, PK – perillaketone), while in the previous study in addition to the mentioned ones other chemotypes were described such as citral (C), phenylpropanoids (PP), perillene (PL), piperitenone (PT), shisofuran (SF), beta-caryophyllene, myristicine (MT), limonene, furylketone (FK) (Koezuka et al., 1986; Ito et al., 2002; Zhang et al., 2009; Ghimire et al., 2017; Ahmed, 2019).
Perilla genotypes possess a characteristic odor owing to various essential oil ingredients that affect nutritional and medicinal properties (Zhang et al., 2009). Rouphael et al., (2019) showed that green perilla genotypes resulted in a higher content of perilla ketone and cis-jasmone. Whereas red perilla accumulated higher content of perilla aldehyde and benzaldehyde in response to salinity applied as chemical eustressor. In our study, there was a very large intraspecific variation concerning active ingredients among studied species. For instance, two green perilla accessions PS2 and 465P produced the higher content of perilla ketone ranged from (75.19–97.9%) similar to the previous study, while in the current study perilla aldehyde can be detected in both phenotypes and benzaldehyde accumulated in very low content compared to previous research. Seo and Baek, (2009) found thirty-three aromatic compounds in the leaf of P. frutescens Britton using four different extraction methods and found chief compounds, including perilla ketone, (Z)-3-hexenol, and 1-octen-3-ol. These variations in the results of the study were slightly due to different cultivation methods, environmental conditions, genetic factors, physiology of the plant, post-harvest technology, storage conditions, extraction and process of the samples. All together can affect the yields, morphology, plant-derived bioactive agents and biological properties of plant quality (Ahmed and Sarosi, 2019; Getahun et al., 2019).
3.2 Multivariate data analysis of essential oil components of perilla
Differences between samples in their essential oil components are obvious as seen in Table 2. To visualize the underlying structure in experimental data and relationships between the data and samples, a multivariate statistical analysis of the essential oil components was adopted. The principal components analysis (PCA) is a significant and broadly utilized device for getting a diagram of an unpredictable informational index. It has likewise been utilized for lessening the measurements and for uncovering the connections among the information items. Therefore, PCA was applied to assess the variations in 16 volatile compounds traits in the 12 genotypes of perilla. The principal component (PC) analysis of the essential oil components data provided three principal components with eigenvalue ≥1, accounting 66.5% of the total variance across the whole dataset. The first principal component and the second principal component showed 48.1% and 18.4% of the total variance, respectively. N = 12 data points. In this loading plot (Fig. 3a), seven perilla genotypes PS1, 203P, 588P, J1, JTD3, NP606 and RauTiaTo have large positive loadings on principal component 1 (PC1), so these variables have the largest effect on each component primarily. GB, PS3 and MP3 have large negative loadings on component 2. The score plot (Fig. 3b), generated based on essential oil components, of the studied perilla genotypes, is seen significantly distributed with four prominent groupings which are limonene, perilla ketone, ß-dehydro-elsholtzia ketone and perillaldehyde. Limonene and perillaldehyde are situated to the far positive axis of PC1. According to the hierarchical clustering of the heatmap (Fig. 3c), the perilla genotypes were clearly separated and clustered into four main groups. Group one (PS1, 203P, 588P, J1, JTD3, NP606 and RauTiaTo) were contained a high amount of perillaldehyde compound and located on the positive region of PC1. Group two (PS3, GB and MP3) were constituted by high ß-dehydro-elsholtzia ketone, shisofuran and ß caryophyllene essential oil compounds and located in the negative region of PC2. Group three (PS2 and 465P) were rich in perilla ketone essential oil compound. Group four (PS1, PS2, 203P, 588P, J1, JTD3, NP606 and RauTiaTo) were including other volatile compounds.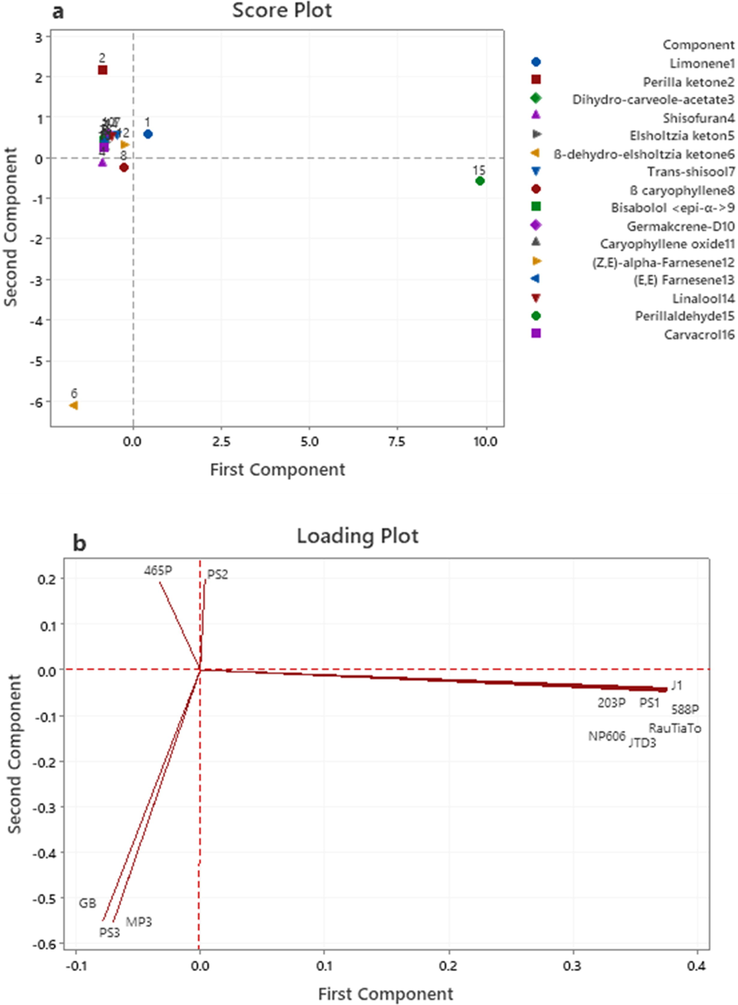
a) PCA Score plot for the main variation of essential oil compositions among twelve Perilla populations. b) Loading plot for volatile constituents explaining the variation on PC1 and PC2 axes. C) A dendrogram obtained by hierarchical cluster analyses of the heatmap on essential oil components of 12 Perilla samples.
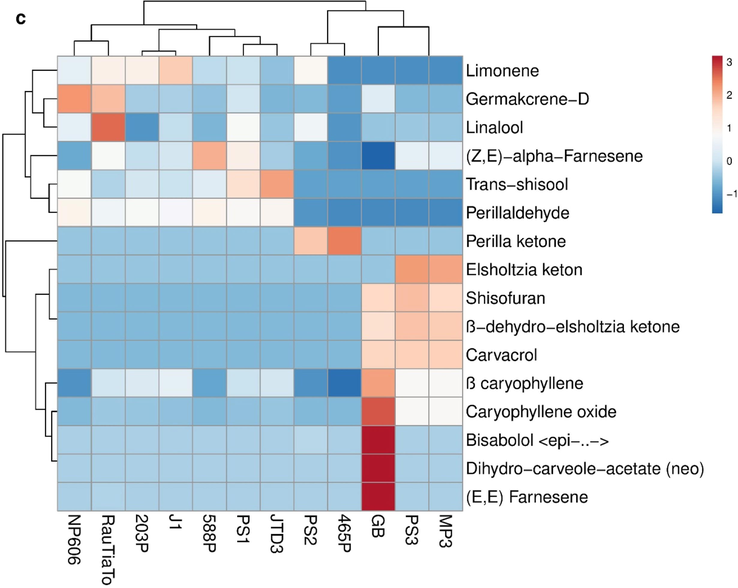
a) PCA Score plot for the main variation of essential oil compositions among twelve Perilla populations. b) Loading plot for volatile constituents explaining the variation on PC1 and PC2 axes. C) A dendrogram obtained by hierarchical cluster analyses of the heatmap on essential oil components of 12 Perilla samples.
3.3 Antibacterial activity
The antimicrobial activity of various accessions of P. frutescens (L.) Britton using three different solvent polarity was evaluated against two gram-positive bacteria [Staphylococcus aureus, Streptococcus pneumonia] and four gram-negative bacteria [Escherichia coli, Klebsiella spp, Shigella spp, Salmonella spp] by using the Kirby-Bauer disc diffusion method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI). As it can be seen from the results of the current study (Table 3), extracts of perilla genotypes were more active against Klebsiella spp., than any other organisms with varying degree of inhibition. The aqueous extract of PS2 (Green colour), 588P (Purple/Green colour) and JTD3 (Purple colour) inhibited more strongly the growth of Klebsiella spp, with the inhibition zone measured (9 ± 0.96, 9 ± 0.6 mm) for both extracts respectively than methanol and ethyl acetate extracts. In contrast, the combination (1, 2 and 3) of twelve accessions of three different solvents (water, methanol and ethyl acetate) extracts showed the lowest zone of inhibition (6 mm) against Klebsiella spp. and had no inhibition against other studied infectious organisms such as Staphylococcus aureus, Shigella spp., Salmonella spp., and Escherichia spp.
Samples
Dry matter %
Staphylococcus aureus +
Streptococcus pneumonia +
Klebsiella spp–
Shigella spp-
Salmonella spp-
Escherichia coli -
Water extracts
PS1
0.6253
–
–
–
–
–
–
PS2
0.7332
–
–
9 ± 0.96
–
–
–
PS3
0.7248
–
–
8 ± 0.98
–
–
–
203P
0.7214
–
–
8 ± 07
–
–
–
465P
0.1437
–
–
8 ± 0.9
–
–
–
588P
0.9325
–
–
9 ± 1.7
–
–
–
J1
0.986
–
–
–
–
–
–
JTD3
0.7894
–
–
9 ± 0.6
–
–
–
NP606
0.6989
–
–
7 ± 0.78
–
–
–
RauTiato
0.7331
–
–
6 ± 0.8
–
–
6 ± 0.6
MP3
0.4807
–
–
7 ± 0.96
–
6 ± 0.89
6 ± 0.8
GB
0.8799
–
–
8 ± 1.01
–
–
–
Methanol extracts
PS1
0.684
–
–
–
–
–
–
PS2
1.1004
–
–
6 ± 0.72
–
–
PS3
0.7837
–
–
6 ± 0.78
–
6 ± 0.25
–
203P
0.8233
–
–
6 ± 0.14
–
6 ± 0.33
–
465P
0.7304
–
–
6 ± 0.70
–
–
588P
0.7075
–
–
6 ± 0.79
–
6 ± 0.78
–
J1
–
–
–
–
–
–
JTD3
0.7917
–
–
6 ± 0.11
–
–
–
NP606
0.5298
–
–
6 ± 0.4
–
6 ± 0.31
–
RaTiato
0.7947
–
–
6 ± 0.32
–
–
–
MP3
1.0749
–
–
6 ± 0.45
6 ± 0.7
–
GB
1.0298
–
–
6 ± 0.78
–
–
–
Ethyl acetate
PS1
0.258
–
–
–
–
–
–
PS2
0.2951
–
–
6 ± 0.6
–
–
–
PS3
0.3179
–
–
6 ± 0.14
–
–
6 ± 0.6
203P
0.4193
–
–
6 ± 0.11
–
–
–
465P
0.3258
–
–
6 ± 0.3
6 ± 0.36
–
588P
0.3073
–
–
–
–
–
–
J1
–
–
–
–
–
–
JTD3
0.3265
–
–
–
–
–
–
NP606
0.3852
–
–
6 ± 0.7
–
–
–
RaTiato
0.4281
–
–
6 ± 0.9
–
–
–
MP3
0.2637
–
–
6 ± 0.7
–
–
–
GB
0.1586
–
–
6 ± 0.94
–
–
–
Combination
Combination1
–
–
6 ± 0.6
–
–
–
Combination2
–
12 ± 0.77
6 ± 0.8
–
–
–
Combination3
–
12 ± 0.66
6 ± 0.23
–
–
–
Positive control
Gentamicin (10 µg/disc)
20 ± 1.2
22 ± 0.81
20 ± 1.01
20 ± 0.98
22 ± 0.8
20 ± 0.75
Amoxicillin (25 µg/disc)
–
19 ± 0.74
–
–
–
17 ± 0.65
Among standard antibiotics, gentamicin could suppress strongly Klebsiella spp., even than any other studied herbal extracts which were (20 ± 1.01 mm), opposite to amoxicillin which was showed resistance to the studied bacteria Klebsiella spp. The combination 2 (methanol extracts of mixed twelve accessions) and 3 (ethyl acetate extracts of mixed twelve accessions) showed the antibacterial activity against gram-positive Streptococcus pneumonia, with the zone of inhibition (12 ± 0.77, 12 ± 0.66) mm, while the combination 1 (aqueous extracts of mixed twelve accessions) showed resistance against the same gram-positive bacteria. Moreover, both gentamicin and amoxicillin exhibited very strong activity against Streptococcus pneumonia, with higher inhibition zone (22 ± 0.81, 19 ± 0.74 mm) respectively.
The individual perilla extracts were resistant for Staphylococcus aureus, Streptococcus pneumonia, Shigella spp., Salmonella spp., E. coli except for the Klebsiella spp. Generally, Gentamicin showed a very strong antibacterial activity against all the studied microorganisms with varying degree of inhibition for Staphylococcus aureus., (20 ± 1.2), Streptococcus pneumonia, (22 ± 0.81), Klebsiella spp., (20 ± 1.01), Shigella spp., (20 ± 0.98), Salmonella spp., (22 ± 0.8), Escherichia coli (20 ± 0.75) in mm respectively, while another antibiotic amoxicillin was appeared to be active only against two bacterial strains including Streptococcus pneumonia., (19 ± 0.74 mm) Escherichia coli (17 ± 0.65 mm), while the same antibiotic was resistant for the rest of the infectious bacteria such as Staphylococcus aureus., Klebsiella spp., Shigella spp., Salmonella spp.
Nowadays, the research for alternative antibacterial agents derived from herbal extractions is growing against a broad range of human infection and for food preservation in the control of food spoilage and food-borne diseases. Antibacterial activity of Perilla frutescens var. acuta leaf ethyl acetate extractions was studied against Staphylococcus aureus using evolutionary operation (EVOP) factorial design technique. It was found that the population of S. aureus was reduced from 7.535 log CFU/mL in the initial set to 4.865 log CFU/mL in the third set by using this technique, as well as to 2.600 log CFU/mL by extraction with the used solvent and the morphology of the bacteria was also affected (Kim et al., 2011). On the other hand, in the current study, only gentamicin could inhibit the same bacteria than any other herbal preparations. The ethyl acetate seed extracts of Perilla frutescens Britton var. japonica Hara was showed strong antibacterial activity against oral cariogenic Streptococci and periodontopathic Porphyromonas gingivalis especially, luteolin one of the polyphenols have been shown remarkable antibacterial activity against the oral bacteria tested while ethanol extracts of defatted perilla seed showed low activity against oral pathogenic bacterial strains (Yamamoto and Ogawa, 2002).
Essential oils extracted from perilla leaves exhibited antibacterial activity against Gram-positive and Gram-negative bacteria. The minimum inhibitory concentration (MIC) on Staphylococcus aureus and Escherichia coli were 500 µg/mL and 1250 µg/mL respectively (Qunqun, 2003). Perillaldehyde is the most abundant terpene-type compound, moderately inhibits a broad range of both bacteria in the range of 125–1000 pg/mL (Kang et al., 1992). This antimicrobial activity of plant extracts is probably due to the presence of secondary metabolites that act to suppress or inhibit the growth of bacterial strains either individually, synergistically, or antagonistically.
4 Conclusion
Aromatic compounds and antimicrobial activity of twelve genotypes of Perilla frutescens L. Britt organically grown under open field were studied. There was great variability in the essential oil content, yield and composition according to studied genotypes. RauTiaTo (Purple colour) showed relatively high EO content and EO yield, followed by 203P (Green colour), and J1 (Green colour). The obtained essential oils were belonging to monoterpene hydrocarbons, sesquiterpenes, oxygenated compounds—alcohols, aldehydes, phenols ranged, other relatively low contents were four esters and one phenylpropanoid. The largest number of volatile compounds discovered in RauTiaTo taxes was forty-six components that represent nearly (99.31%) of the obtained oil. The chief volatiles oils were perillaldehyde, ß-dehydro-elsholtzia ketone, perilla ketone, limonene, ß caryophyllene, (Z,E)-alpha-Farnesene, shisofuran and trans-shisool among all the genotypes.
Regarding antibacterial activity, extracts of perilla genotypes were more active against Klebsiella spp., than any other organisms with varying degree of inhibition. The aqueous extract of PS2 (Green colour), 588P (Purple/Green colour) and JTD3 (Purple colour) inhibited more strongly the growth of Klebsiella spp, for both extracts respectively than methanol and ethyl acetate extracts. Among standard antibiotics, gentamicin showed a very strong antibacterial activity against all the studied microorganisms with varying degree of inhibition for Staphylococcus aureus., Streptococcus pneumonia., Klebsiella spp., Shigella spp., Salmonella spp., Escherichia coli respectively, while another antibiotic amoxicillin was appeared to be active only against two bacterial strains including Streptococcus pneumonia, Escherichia coli. The combination 2 (methanol extracts of mixed twelve accessions) and 3 (ethyl acetate extracts of mixed twelve accessions) showed the antibacterial activity against gram-positive Streptococcus pneumonia. These results could be useful for further evaluation of pharmacologically active components in perilla materials, especially for qualitative and quantitative analyses of this herbal drug and food additives, in quality control for standardisation of natural products.
Acknowledgements
The author thanks Stipendium Scholarship and Newcasle Center for Natural Therapy for fellowships and financial support. The author also thanks Dr. Szilvia Sarosi and Dr Peter Radacsi for helping during fieldwork and analysing the samples.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adams, R.P., 2017. Identification of essential oil components by gas chromatography/mass spectrometry. 5 online ed.
- Ethnomedicinal, phytochemical and pharmacological investigations of perilla frutescens (L.) Britt. Molecules. 2019;24(1):102.
- [Google Scholar]
- Identification and quantification of essential oil content and composition, total polyphenols and antioxidant capacity of Perilla frutescens (L.) Britt. Food Chem.. 2019;275:730-738.
- [Google Scholar]
- Anti-allergic potential of herbs and herbal natural products-activities and patents. Recent patents on endocrine. Metab. Immune Drug Discov.. 2013;7(1):26-56.
- [Google Scholar]
- Perilla frutescens leaves extract ameliorates ultraviolet radiation-induced extracellular matrix damage in human dermal fibroblasts and hairless mice skin. J. Ethnopharmacol.. 2017;195:334-342.
- [Google Scholar]
- Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem.. 2004;68(1):85-90.
- [Google Scholar]
- Perilla, Botany, Uses and Genetic Resources. J. Janick and JE; 1993.
- Application and prescriptions of Perilla in traditional Chinese medicine. In: Kosuna K., Haga M., Yu H.C., eds. Perilla: the genus Perilla. Amsterdam: Harwood Academic Publishers; 1997. p. :37-45.
- [Google Scholar]
- Clinical and Laboratory Standards Institute CLSI, 2012. Performance standards for antimicrobial disk susceptibility tests; approved standard. CLSI document M02-A11. 11th Ed. Volume 32. Clinical and Laboratory Standards Institute, pp. 11–13, 41, 43.
- Characterization of fatty acid composition from five perilla seed oils in China and its relationship to annual growth temperature. J. Med. Plants Res.. 2012;6(9):1645-1651.
- [Google Scholar]
- Limonene-A review: biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun.. 2008;3(7) 1934578X0800300728
- [Google Scholar]
- Hypolipidemic and antioxidant effects of total flavonoids of Perilla Frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res. Int.. 2011;44(1):404-409.
- [Google Scholar]
- The genus Laggera (Asteraceae)–ethnobotanical and ethnopharmacological information, chemical composition as well as biological activities of its essential oils and extracts: a review. Chem. Biodivers.. 2019;16(8):e1900131
- [Google Scholar]
- GC–MS analysis of volatile compounds of Perilla frutescens Britton var. Japonica accessions: morphological and seasonal variability. Asian Pacific J. Trop. Med.. 2017;10(7):643-651.
- [Google Scholar]
- TLC bioautography-guided isolation of antioxidants from fruit of Perilla frutescens var. acuta. LWT-Food. Sci. Technol.. 2009;42(1):131-136.
- [Google Scholar]
- Igarashi, M., Miyazaki, Y., 2013. A review on bioactivities of perilla: progress in research on the functions of perilla as medicine and food. Evidence-based complementary and alternative medicine, 2013.
- Phylogenetic analysis of Japanese Perilla species by using DNA polymorphisms. Nat. Med.= 生薬學雜誌. 1998;52(3):248-252.
- [Google Scholar]
- A new type of essential oil from Perilla frutescens from Thailand. J. Essent. Oil Res.. 2002;14(6):416-419.
- [Google Scholar]
- Drying the leaves of Perilla frutescens increases their content of anticancer nutraceuticals. Food Sci. Nutrit. 2019
- [Google Scholar]
- Antimicrobial activity of the volatile constituents of Perilla frutescens and its synergistic effects with polygodial. J. Agric. Food. Chem.. 1992;40(11):2328-2330.
- [Google Scholar]
- The effect of Perilla frutescens leaf on 1, 2-dimethylhydrazine-induced initiation of colon carcinogenesis in rats. J. Food Biochem.. 2018;42(2):e12493
- [Google Scholar]
- Optimization of antibacterial activity of Perilla frutescens var. acuta leaf against Staphylococcus aureus using evolutionary operation factorial design technique. Int. J. Mol. Sci.. 2011;12(4):2395-2407.
- [Google Scholar]
- Luteolin inhibits LPS-stimulated inducible nitric oxide synthase expression in BV-2 microglial cells. Planta Med.. 2006;72(01):65-68.
- [Google Scholar]
- Genetic control of the chemical composition of volatile oils in Perilla frutescens. Phytochemistry. 1986;25(4):859-863.
- [Google Scholar]
- Perilla frutescens britton: a comprehensive study on flavor/taste and chemical properties during the roasting process. Molecules. 2019;24(7):1374.
- [Google Scholar]
- Characterization of metabolite profiles from the leaves of green perilla (Perilla frutescens) by ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry and screening for their antioxidant properties. J. Food Drug Anal.. 2017;25(4):776-788.
- [Google Scholar]
- Determination of the content of rosmarinic acid by HPLC and analytical comparison of volatile constituents by GC-MS in different parts of Perilla frutescens (L.) Britt. Chem. Cent. J.. 2013;7(1):61.
- [Google Scholar]
- Determination of toxic perilla ketone, secondary plant metabolites and antioxidative capacity in five Perilla frutescens L. varieties. Food Chem. Toxicol.. 2010;48(1):264-270.
- [Google Scholar]
- Metabolites of orally administered Perilla frutescens extract in rats and humans. Biol. Pharm. Bull.. 2000;23(1):122-127.
- [Google Scholar]
- Antidepressant-like effects of apigenin and 2, 4, 5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull.. 2003;26(4):474-480.
- [Google Scholar]
- Determination of phenolic compounds in Perilla frutescens L. by capillary electrophoresis with electrochemical detection. J. Agric. Food. Chem.. 2005;53(21):8141-8147.
- [Google Scholar]
- Antibacterial activity of Perilla Frutescens leaf essential oil. Sci. Technol. Food Industry 2003:9.
- [Google Scholar]
- Chemical eustress elicits tailored responses and enhances the functional quality of novel food Perilla frutescens. Molecules. 2019;24(1):185.
- [Google Scholar]
- Characteristic aroma-active compounds of Korean perilla (Perilla frutescens Britton) leaf. J. Agric. Food. Chem.. 2009;57(24):11537-11542.
- [Google Scholar]
- Regional variation in components and antioxidant and antifungal activities of Perilla frutescens essential oils in China. Ind. Crops Prod.. 2014;59:69-79.
- [Google Scholar]
- Antidepressant-like effect of essential oil of Perilla frutescens in a chronic, unpredictable, mild stress-induced depression model mice. Chinese J. Nat. Med.. 2014;12(10):753-759.
- [Google Scholar]
- Antimicrobial activity of perilla seed polyphenols against oral pathogenic bacteria. Biosci. Biotechnol. Biochem.. 2002;66(4):921-924.
- [Google Scholar]
- Structure of anthocyanins isolated from purple leaves of Perilla ocimoides L. var. crispa Benth and their isomerization by irradiation of light. Agric. Biol. Chem.. 1990;54(7):1745-1751.
- [Google Scholar]
- Essential oil variations in different Perilla L. accessions: chemotaxonomic implications. Plant Syst. Evol.. 2009;281(1–4):1-10.
- [Google Scholar]







