Translate this page into:
Exploring recent advances in silver halides and graphitic carbon nitride-based photocatalyst for energy and environmental applications
⁎Corresponding author at: School of Chemistry, Faculty of Basic Sciences, Shoolini University, Solan, HP 173229, India. pankajchem1@gmail.com (Pankaj Raizada)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Engineering visible light active photocatalytic systems for renewable energy production and environmental remediation has always been a promising technology to counter overall energy demands and pollution challenges. As a fascinating conjugated polymer graphitic carbon nitride (g-C3N4) has been developed as a hotspot in the research field as a metal-free semiconducting material with the appealing band gap of 2.7 eV. Recently, g-C3N4 has gained tremendous interest in photocatalytic wastewater abatement as well as for hydrogen (H2) generation, carbon dioxide (CO2) reduction, and pollutant degradation, under exposure to visible light. Plasmonic silver halides (AgX) such as AgCl, AgBr, and AgI as plasmonic photocatalyst have received immense research interest owing to their escalating photocatalytic efficacy and strong surface plasmon resonance effect (SPR). AgX is the photosensitive, broad bandgap semiconducting materials with effectual antimicrobial properties. This review summarizes the heterostructure of carbonaceous g-C3N4 with plasmonic AgX, to reduce the recombination of photo-generated charge carriers, thus enhancing the natural light absorption. g-C3N4 grafted AgX nanoarchitectures can be utilized for several potential applications, for instance, overall water splitting (OWS), CO2 conversion to hydrocarbon fuels, pollutant exclusion, and antibacterial disinfection. This review focuses on the evolution of g-C3N4 as well as AgX, facile, and synthetic routes for fabrication of g-C3N4 tailored AgX, construction of nano-junctions (AgX/g-C3N4) with various photocatalytic applications. Finally, we provided a viewpoint of current hassles and some perceptions of novel trends in this exciting as well as developing research arena.
Keywords
Semiconductor-based photocatalysis
Metal free graphitic carbon nitride (g-C3N4)
Localized surface plasmon resonance (LSPR)
Pollutant mineralization
Energy applications
Wastewater treatment
- AOT
-
Advanced oxidation technologies
- Eg
-
Band gap
- BET
-
Brunauer-Emmett-Teller
- CO2
-
Carbon dioxide
- CDs
-
Carbon Dots
- e−CB
-
Conduction band electrons
- DFT
-
Density functional theory
- DCF
-
Diclofenac
- EIS
-
Electrochemical impedance spectroscopy
- e−
-
Electron
- EHP
-
Electron-hole pair
- EDX
-
Energy dispersive X-ray analysis FTIR, Fourier-transform infrared spectroscopy
- g-C3N4
-
Graphitic carbon nitride
- h+
-
Hole
- H2
-
Hydrogen
- radOH
-
Hydroxyl radicals
- IBF
-
ibuprofen
- LSPR
-
Localized surface plasmon resonance
- MO
-
Methyl orange
- MB
-
Methylene blue
- NPs
-
Nanoparticles
- NS
-
Nanosheets
- NTP
-
Nitenpyram
- NOF
-
Norfloxacin
- OWS
-
Overall water splitting
- OA
-
Oxalic acid
- O2
-
Oxygen
- PL
-
Photoluminescence
- ROS
-
Reactive oxygen species
- RSs
-
Reactive species
- RhB
-
Rhodamine B
- SEM
-
Scanning electron microscopy
- SC
-
Semiconductor
- Ag
-
Silver AgBr, Silver Bromide
- AgCl
-
Silver chloride
- AgX
-
Silver halides
- AgI
-
Silver Iodide
- AgNO3
-
Silver nitrate
- radO2−
-
Superoxide radical
- SPR
-
Surface plasmon resonance
- TC
-
Tetracycline
- TGA
-
Thermal gravimetric analysis
- TiO2
-
Titanium dioxide
- TEM
-
Transmission electron microscopy
- UV
-
Ultraviolet
- UV–Vis DRS
-
UV–Visible diffuse reflectance spectroscopy
- e−VB
-
Valence band electrons
- VLT
-
Visible-light-triggered
- H2O
-
Water
- XRD
-
X-ray diffraction
- XPS
-
X-ray photoelectron spectroscopy
Abbreviations
1 Introduction
Global environmental pollution poses a severe hazard to the sustainable growth of the human community. Industrial development, economic expansion, and urbanization has turned into a big challenge (Bora and Mewada, 2017; Pawar et al. 2017). Lack of access to innocuous, reliable resources of water remains a critical hurdle worldwide, causing vector-borne syndromes alike cholera, jaundice, diarrhoea, etc. The release of agricultural run-off and organic pollutants such as antibiotics, chlorinated products, etc. decreases the oxygen level for aquatic systems (Banerjee et al., 2014). According to a report of The Times of India, ministry of urban development (2013), census (2011), as well as Central Pollution Control Board, determined that 75–80% of water effluence is due to household trash, although untreated effluent flowing into water bodies have increased in later years. Every day, 2 million tons of sewage, industrial as well as agricultural scrap is dispensed into the world's water (UNWWAP 2003). As reported, about 80% of India's surface water is contaminated, as evaluated by Water Aid, an international organization working for water sanitation and hygiene. Also, Environmental Protection Agency (EPA) sets legal limits on over 90 contaminants in drinking water. The legal limit for a contaminant reflects the level that protects human health and superior water systems can be achieved using the best available technology. Indeed, conservative approaches such as coagulation, flocculation, sedimentation, reverse osmosis, filtration, adsorption, boiling, etc. were adopted for wastewater refining (Singh et al., 2017, 2019a). These conventional methods were inherited with certain downsides, i.e., these are time-consuming, induces sludge formation, give rise to secondary pollutants, applicable at small scale and consume an intensive amount of energy. This calls for the development of innovative and greener water decontamination approaches, which are economical, sturdy, and energy efficient (Mamba and Mishra, 2016; Raizada et al., 2017a). Lately, advanced oxidation technologies (AOT’s) have been developed as effective filtration techniques for simulated water comprising of organic as well as inorganic toxins (Miklos et al., 2018; Pare et al., 2009). Reactive oxygen or free radicals are potent oxidants that establish AOTs to degenerate organic contaminants to reliable molecules, for instance, carbon dioxide (CO2) and water (H2O). Hydroxyl radicals (•OH) are highly reactive, owns non-selective nature, and possess compelling oxidizing efficacy (E0 = +2.80 V), and these species attack organic molecules with a rate of 106–109 M−1 s−1 (Miao et al., 2017b). •OH radicals rapidly disperse from the reaction solution, as a result of their short life span (ns) in water. AOTs can be categorized as homogenous and heterogeneous processes based on the phase of utilized catalyst. However, significant hindrances limiting their practical applications were a necessity of advanced paraphernalia, UV light absorption, high energy demands, partial decomposition of noxious waste as well as expensiveness of these techniques (Raizada et al., 2017b; Akhundi and Habibi-Yangjeh, 2016b; Sharma et al., 2019). To resolve these hitches, photocatalytic systems have fascinated enormous research interest as it offers greener and productive routes for various ecological issues (Raizada et al., 2020a; Shandilya et al., 2020). Among most of the alternatives, semiconductor mediated photocatalysts have appeared as the most enticing and propitious technologies employing natural energy for environmental restoration. In the year 1972, Fujishima and Honda introduced semiconductor photocatalysis for overall water splitting (OWS) to hydrogen (H2) production using a metal oxide, especially titanium dioxide (TiO2) (Fujishima and Honda, 1972). TiO2 has been widely employed for oxidative decay of pollutants due to its high abundance, inexpensiveness, non-toxic nature, insolubility in water, and high chemical inertness. However, lower absorption of visible light and less quantum yield may be accredited to a wide bandgap, i.e., 3.2 eV, which confines its applications in the visible range (Lai et al., 2016). The progressions in high-performance visible light-triggered (VLT) photocatalysts are more compelling due to pronounced visible light in the solar spectrum (43%) as compared with typically used photocatalytic materials, which absorb merely ultraviolet (UV) light (4% of solar spectrum). After that, heterogeneous photocatalysis has been enormously employed in water splitting to H2 and O2 (Mousavi and Habibi-Yangjeh, 2017; Appavu et al., 2016; Yamasita et al., 2004), water/air cleansing (Raizada et al., 2016; Singh et al., 2014; Fujishima et al., 2000), CO2 reduction (Wang et al., 2015; Liu et al., 2018), self-cleaning (Zhang et al., 2005; Malato et al., 2009), selective organic alterations (Lang et al., 2014) and high-efficiency solar cells (O'regan and Grätzel, 1991). Owing to properties such as; (i) complete mineralization of pollutants without the formation of any secondary product, (ii) economical, and (iii) viable operational temperature as well as pressure has prolonged the implementations of heterogeneous photocatalysis in wastewater treatment (Chong et al., 2010).
Fig. 1 (Singh et al., 2020) explicated the basic photocatalytic process, where semiconducting material upon illumination to UV light with equivalent or more than the bandgap energy (Eg) gets excited resulting in the generation of electrons (e−) and holes (h+) in their respective conduction band (CB) and valence band (VB). These valence band holes (h+VB) and conduction band electrons (e−CB) result in the formation of electron-hole pairs (EHP). The oxidative holes and reductive electrons contribute to the redox process over the surface of semiconducting material, respectively, (Xue et al., 2018). For mineralization of target pollutants, h+ could straight away oxidize contaminants or react with H2O/OH− to form •OH radicals. Also, e− capture oxygen (O2) to form superoxide radical (•O2−), which not only hinders the spatial charge separation of photo-induced charge carriers however prolongs the lifetime of h+, while this generated h+ as well as •O2− were responsible for pollutant deprivation. The generation of •O2− radical by a reaction of photo-induced e−CB with O2 plus formation of •OH radicals by the reaction among h+VB is specified by equation Eqs. (1) and (2);
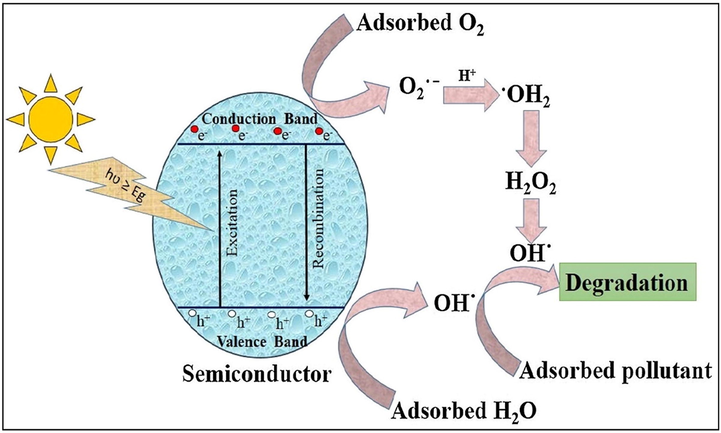
General photocatalytic mechanism of a photocatalyst explicating mineralization of organic pollutant; reprinted under licence from CC BY-NC-ND 4.0 (Singh et al., 2020).
Alternatively, for energy conversion, CO2 could be photo reduced to useful fuels, whereas H2O/OH− could be oxidized to O2. The reactive oxygen species (ROS) released during the photocatalytic process were h+, •O2−, H2O2, and •OH by reacting with O2 or H2O/OH−, facilitating the removal of organic/inorganic toxins present in water (Hasija et al., 2019b; Singh et al., 2020; Raizada et al., 2019c). ROS exhibits strong oxidizing ability, therefore increases reaction kinetics and effectively degrade the evolving pollutant (Zheng et al., 2017; Raizada et al., 2014; Gautam et al., 2017). Notably, not all photo-illuminated e− take part in surface reactions, but some of the photocarriers recombine with h+VB ensuing depressed photocatalytic ability. Hence, to boost the photocatalytic efficiency recombination rate of photo-charge carriers must be suppressed, promoting their transference to semiconductor surface where e− and h+ reacted with O2 and H2O to generate •O2− along with •OH, correspondingly. The reported literature revealed the exclusion of metal ions as well as inorganic ions by photocatalytic methods (Mousavi and Habibi-Yangjeh, 2018a). Several metal oxide semiconducting materials such as CaO, BiTiO3, ZnWO4, Fe2O3, WO3, Nb2O5, BiOCl, EuVO4, SrTiO3, ZrO2, Ag2CO3, Bi2WO6, BiOI, Ag3PO4, CaFe2O4, MnFe2O4, ZnFe2O4, CdS, AgI, SmVO4, MnFe2O4, BiVO4, g-C3N4, BiOBr, etc. have been utilized as photocatalysts for deterioration and is endlessly growing day by day (Sudhaik et al., 2018a; Yi et al., 2019; Raizada et al., 2019c; Zhang et al., 2017b; Zhang et al., 2009a). However, their wide bandgap, rapid recombination of photo-induced EHP, lower visible light absorption, and photo deterioration remains the obstacles of semiconductor photocatalysis for pollutant degradation and several other practical implementations (Raizada et al., 2017c; Shandilya et al., 2019; Hua et al., 2019; Singh et al., 2019b). Since past years, photocatalysis has been established as an efficient approach for OWS, CO2 conversion to energy fuels and wastewater treatment by photodegrading microbes, pharmacological leftover, antibiotics, dyes as well as phenols (Shandilya et al., 2018b; Priya et al., 2016; Patial et al., 2020). Ameliorated photocatalytic efficiency can be obtained by:
-
surface functionalization via integration of co-catalyst,
-
surface modulation by molecular as well as elemental doping, and
-
construction of typical and Z-scheme based nano-heterojunctions.
Out of all the above-mentioned strategies coupling of more than two semiconductors with appropriate band, gaps have achieved massive interest owing to (i) natural light absorption of a wide-ranging bandgap semiconductor can be enhanced by hybridizing with narrow bandgap semiconducting material, (ii) persuaded electric field excites the transference and segregation of photo-generated charge carriers, and (iii) in different semiconducting photocatalyst e− and h+ possessing greater redox as well as oxidative potentials simultaneously, enhances the redox capability (Chandel et al., 2020; Gautam et al., 2016; Shandilya et al., 2018a; Singh et al., 2019c).
In previous years, carbon-based materials showed tremendous ability to increase the photocatalytic activity of semiconducting materials. Carbon-based photocatalyst, for example, carbon nanotubes (CNTs), graphene, graphene oxide (GO), fullerene (C60), carbon quantum dots (CQDs), carbon fibers (CF), activated carbon (AC) and carbon black behaves as photosensitizers, act as co-catalyst and possesses large specific surface area for adsorption due to their active sites. Carbon materials have a band gap narrowing effect and act as supporting material. These carbon-based materials enhance the structural stability of semiconducting materials and minimize the aggregation of particles (Cao and Yu, 2016).
1.1 Polymeric graphitic carbon nitride (g-C3N4) as photocatalyst
Graphitic carbon nitride (g-C3N4), a 'golden photocatalyst,' has provoked ripples of excitement in research societies due to its maximum visible light absorption, cost-effectiveness, greater profusion, non-toxicity, л-conjugated assembly and thermal as well as chemical stability. g-C3N4 spurred our enormous attention in 1834 due to its tunable bandgap of 2.7 eV with VB and CB potentials positioned at −1.09 and +1.56 eV, respectively. Owing to these properties, g-C3N4 acts as a favourable photocatalytic material and has wider applications in electrochemical water splitting, act as a photocatalyst for H2 production as well as for photodegradation of noxious waste (Ong et al., 2016). Although, photocatalytic efficiency of bulk g-C3N4 is bounded due to (i) its inefficiency to absorb visible light (ii) higher photo-induced EHP recombination (iii) smaller surface area and (iv) lower electrical conductivity. Consequently, several modification approaches have been developed to accomplish spatial charge separation of photo-carriers such as; (1) deposition of metals/non-metals (doping), (2) fabricating porous structures and (3) coupling with alternative semiconductor (heterojunction) in order to boost the photoactivity of g-C3N4 (Sudhaik et al., 2018b). Hybridizing g-C3N4 with another semiconducting material is a notable technique because of its ability to achieve efficient transference/separation of photo-generated EHP and appropriate redox potential for photocatalytic implementations (Raizada et al., 2019d; Raizada et al., 2020).
1.2 Electronic structure of g-C3N4
In recent years’ carbon nitrides (C3N4) have gained remarkable attention as a polymeric semiconducting material. The structure of carbon nitride (C3N4) materials consist of two-dimensional (2D) sheets of triazine (1,3,5-triazine, C3N3) and heptazine (1,3,4,6,7,9,9b-heptaazaphenalene, C6N7) core (Kroke et al., 2002). These are aromatic molecules where carbon (C) and nitrogen (N) atoms are arranged in alternative positions in the ring. C3N4 exists in seven stages, and these are categorized as α-C3N4, β-C3N4, cubic C3N4, pseudocubic C3N4, g-h-triazine, go-triazine and g-h-heptatriazine having bandgap potentials of 5.49, 4.85, 4.30, 4.13, 2.97, 0.93 and 2.88 eV, correspondingly (Teter and Hemley, 1996). Several allotropes of C3N4 can be fabricated via temperature assisted process such as heating melamine to 317 °C leads to the formation of melam [(H2N)2(C3N3)2]NH which on further heating forms melem (tri amino-heptazine) C6N7(NH2)3 (Jürgens et al., 2003). Above 520 °C, melem condenses, and forms amine linked heptazine units, which is referred to as Liebig’s melon (Liebig, 1844). Pyrolysis of cyanamide, dicyanamide, urea, thiourea, and other molecules comprising of C and N above 520 °C are considered as Polymeric carbon nitride (PCN) called melon (Kessler et al., 2017). The condensation of melem under 520 °C forms triazine based graphitic carbon nitride (g-C3N4). g-C3N4 exists as the most substantial allotrope of carbon nitride, while tri-s-triazine based g-C3N4 was the utmost steady stage at ambient environments and was dynamically favourable. Furthermore, density functional theory (DFT) measurements were accomplished by Kroke and his research group to explore its electronic structure. Pz orbitals of N and C were mainly liable for the construction of VB and CB, as investigated by DFT calculations (Ong et al., 2016). As per reports, lone pair of e− on N atom was the reason for the formation of electronic structure and VB. Since 2009, g-C3N4, an n-type semiconducting material, has evolved as a prominent visible light active photocatalyst for degradation of organic toxins from aqueous phase (Kumar et al., 2019; Raizada et al., 2019a).
Fig. 2 illustrated various precursors for facile fabrication of g-C3N4 photocatalyst. Several physical as well as chemical approaches have been adopted for preparation of g-C3N4 photocatalyst. Mainly, top down and bottom up approaches have been embraced. Top down approach implicates breaking up of bigger g-C3N4 blocks into smaller units, namely g-C3N4 nanosheets, while bottom up approach involves incorporation of smaller molecules to form larger complex molecules. Moreover, thermal polymerization, solvothermal technique, sol-gel process, template assisted method and ionothermal approaches were commonly used for synthesis of g-C3N4 photocatalyst (Sudhaik et al., 2018b; Bojdys et al., 2008; Zhang et al., 2018).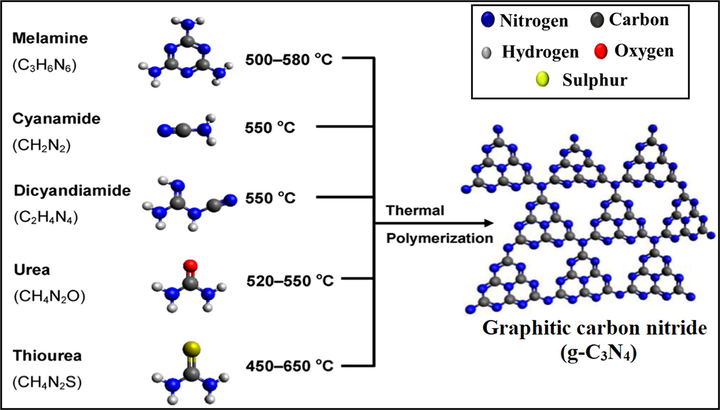
Schematic representation of synthesis process of g-C3N4 by thermal polymerization of various precursors (Reproduced with permission).
1.3 Silver halides (AgX) as potential visible light assisted photocatalyst
Fabrication of photocatalyst with extended visible light absorption, plasmonic photocatalytic materials have gained enormous research interests. The plasmonic photocatalyst, mainly silver (Ag) and gold (Au), unveil greater absorption coefficients in wide UV–visible-near infrared range (NIR). This can be accredited to their strong surface plasmon resonance (SPR) (Zhang et al., 2009b; Huang et al., 2006). Particularly, Ag NPs were chosen due to their inexpensiveness as compared to Au NPs, and they exhibited efficient plasmon resonance in solar light (Awazu et al., 2008; Georgekutty et al., 2008).
A collective oscillation of e−CB exhibited by noble metal NPs leads to an oscillating electric field. While these oscillations caused a rearrangement of charge density as well as electric field, therefore generating professed localized surface plasmon (LSP) through a coulombic refurbishing potency to repel the effect of positive nuclei. Plasmon resonant frequency of noble metal nano-junctions overlays the solar spectrum thereby, extending the natural light absorption. When oscillations befall at localized surface plasmon resonance (LSPR) wavelengths, i.e., 530–600 nm, the dissipated energy is assumed to be least, and consumption of natural light is maximum. Under exposure to solar light, SPR of noble metal NPs usually occurs when the frequency of stimulating light is equivalent to the rate of oscillating surface e− (Fig. 3a) (Ye et al., 2012; Wang et al., 2012). Noble metal NPs exhibiting LSPR effect, ameliorated the efficacy of semiconductor-based photocatalysis by interacting between free surface e− and photons, moreover tailoring novel plasmonic photocatalysts (Sarina et al., 2013; Ingram et al., 2011; Primo et al., 2011).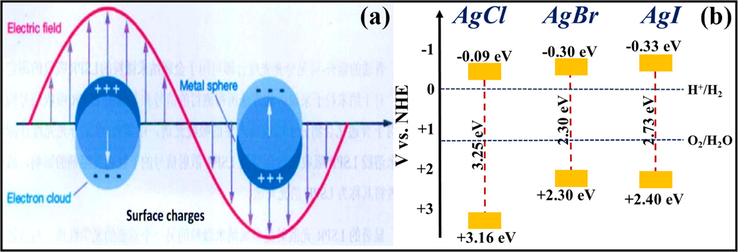
Schematic illustration of (a) Surface Plasmon Resonance (SPR) in a noble metal particle; reprinted with permission from Elsevier (Licence no. 4742480664982) (Ye et al., 2012), and (b) Band gap positions of AgCl, AgBr and AgI with their respective valence band and conduction band potentials (Ye et al., 2014; Yao et al., 2016; Islam et al., 2016).
Till date, much attention has been gained by Ag-based photocatalysts (such as Ag3PO4, AgVO3, Ag2CO3, AgX (X = Cl, Br, I), Ag@AgX (X = Cl, Br, I), AgIO3, and Ag2WO4) for photocatalytic degradation of organic toxins. Silver halides (AgX, X = Cl, Br, I) being plasmonic photocatalyst with antibacterial properties are the photosensitive semiconducting materials with ameliorated visible light absorption, and their photosensitivity proposes potential utilization in photocatalysis. They have been broadly exerted as source materials in photographic films and employed as photosensitizers. Also, they can be used as metallic silver precursors (Archana et al., 2015). Under exposure to visible light, AgX undergoes a reduction of Ag+ into Ag0, as depicted in equation Eqs. (3) and (4) (Tate, 2012);
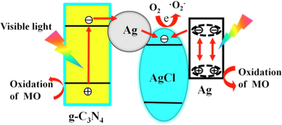
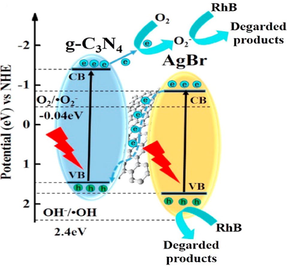
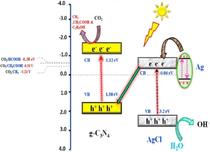
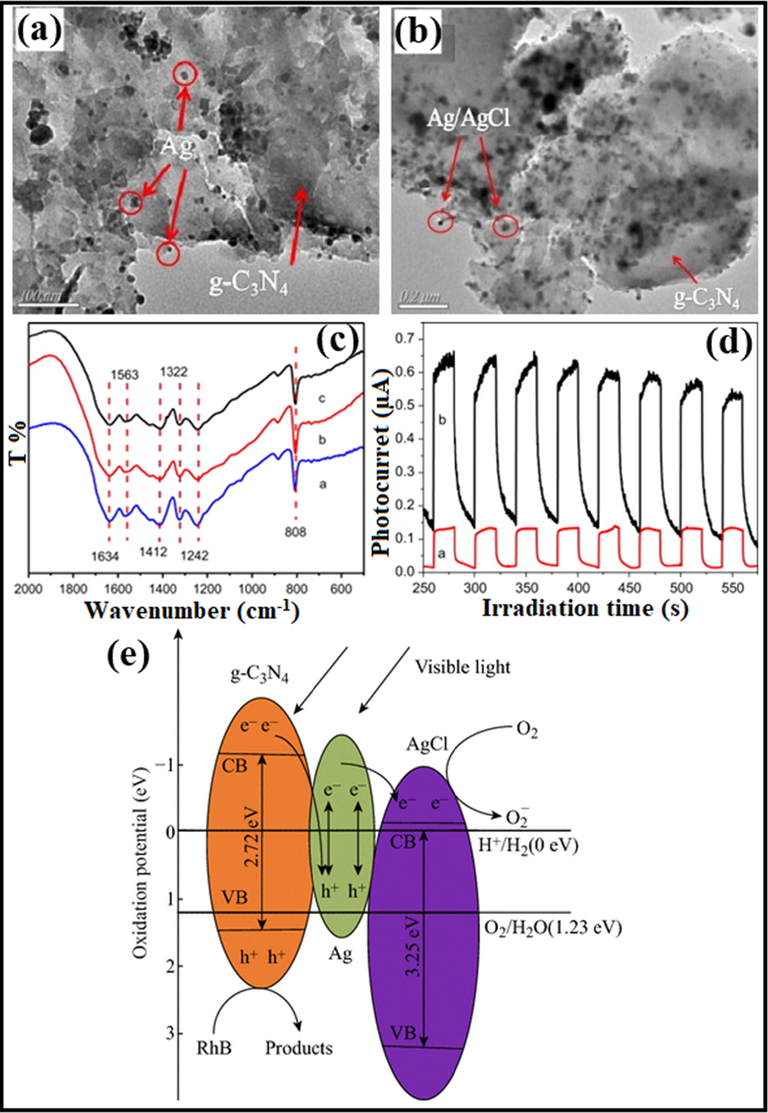
Ag/AgX photocatalyst can be attained from AgX by exploiting their photosensitivity. These photocatalysts are highly efficacious as well as stable under visible light radiation. Despite their broad bandgap, greater than the edge of natural light, AgX has been developed as visible light assisted photocatalytic material (Grzelczak and Liz-Marzán, 2014). However, the photo-corrosive nature of Ag-based compounds limits the practical implementations of AgX as photocatalysts. Therefore, silver chloride (AgCl) having bandgap of 3.25 eV with VB and CB potentials positioned at +3.16 eV and −0.09 eV, respectively (Ye et al., 2014), while silver bromide (AgBr) has a bandgap of 2.60 eV with VB and CB potential located at +2.30 eV and −0.30 eV, respectively (Yao et al., 2016). Also, silver iodide (AgI) possesses a band gap of 2.73 eV with VB potential at +2.40 eV and CB potential at −0.33 eV (Islam et al., 2016). Fig. 3b depicted the band gap positioning of AgCl, AgBr and AgI, respectively. Therefore, coupling of a semiconductor with another semiconducting material has emerged as a new technology for boosted photocatalytic performance (Hasija et al., 2019a). Precisely, coupling of Ag-based photocatalysts with another semiconductor material having appropriate band edge positioning can effectively migrate and separate charge carriers which further participate in photocatalytic reactions. As a consequence, the availability of electrons which participate in the photo-reduction of Ag+ into Ag0 reduced significantly providing superior stability to the system (Chen et al., 2015b; Liu et al., 2011). Chen et al. tailored plasmonic Ag/AgBr/g-C3N4 photocatalyst via a superficial means for mineralization of methylene blue (MB) under solar light irradiation. Ag/AgBr with 40% content exhibited superior photocatalytic activity, which was about 2.4 and 1.9 times greater than bare g-C3N4 and Ag/AgBr hybrid. Scanning electron microscopy (SEM) analysis confirmed the deposition of sphere-like Ag/AgBr particles on aggregated g-C3N4 nanosheets (NS) (Fig. 4a). Moreover, particle size of Ag/AgBr particles ranging between 50 nm and 200 nm was observed in transmission electron microscopy (TEM) images. The proposed mechanism (Fig. 4b) for Ag/AgBr/g-C3N4 nanocomposite, suggesting the boosted photocatalytic efficacy was attributable to the synergetic effect of Ag, AgBr, and g-C3N4. Under solar light illumination, both g-C3N4 (2.7 eV) and AgBr (2.6 eV) get excited, succeeding in the transference of photo-generated e− from g-C3N4 to CB of AgBr, thereby reacting with adsorbed O2 to produce CO2. Furthermore, metallic Ag produced photo-induced e− on the AgBr surface because of a strong SPR effect. AgBr possesses inferior fermi level than that of Ag ensuing transfer of e− from Ag to AgBr and lastly transference to adsorbed O2 to generate •O2− (Chen et al., 2015a, 2015b). Fig. 5(a–c) depicted facile routes for preparation of AgCl, AgBr and AgI, respectively, employing various precursors.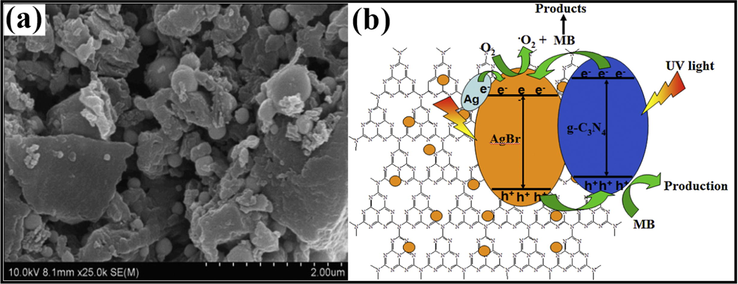
(a) SEM imageries of Ag/AgBr/g-C3N4 nanohybrid depicting deposition of Ag/AgBr particles on aggregated g-C3N4 NS, and (b) Mechanistic view of Ag/AgBr/g-C3N4 composite explicating boosted photocatalytic efficiency; reproduced with permission from Elsevier (Licence no. 4746461343390) (Chen et al., 2015).
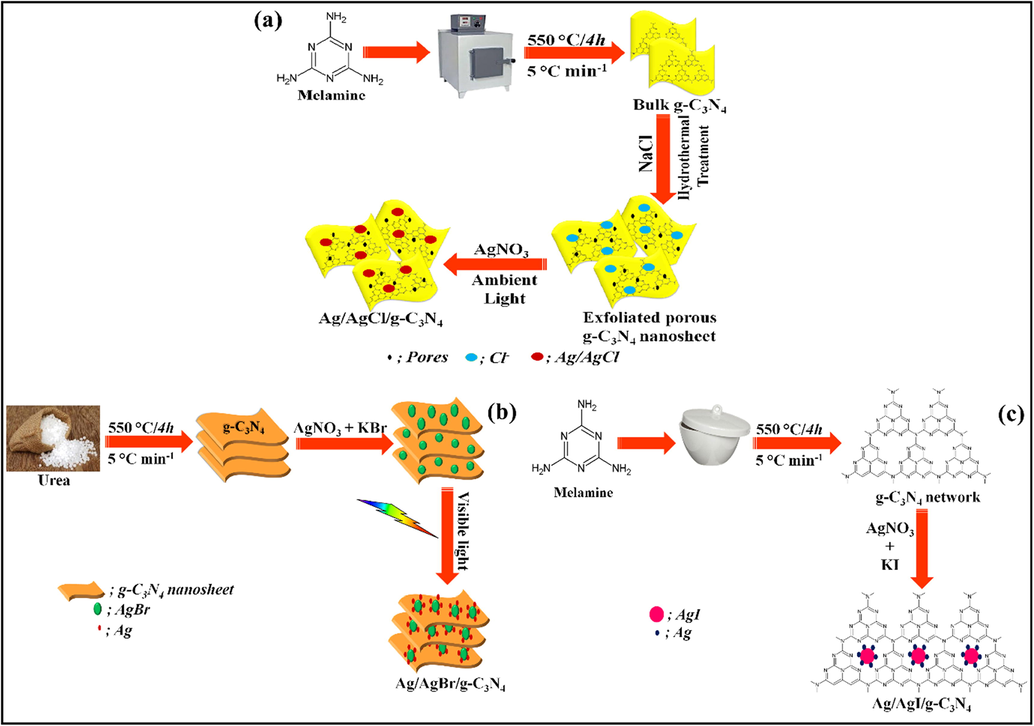
Synthesis scheme for facile fabrication of (a) AgCl, (b) AgBr and (c) AgI.
Recently, g-C3N4 grafted AgX heterojunctions have witnessed surge interest of the research community owing to its potential to minimalize the recombination of photo-excited charge carriers, thereby augmenting the solar light absorption. For example, Lan et al. delineated an integral report on highly efficient solar light assisted AgX@g-C3N4 (X = Cl, Br, I) photocatalyst for photodegradation of pollutants (Lan et al., 2013). In this research article, they discussed AgX and g-C3N4, preparation of AgX@g-C3N4 nanocomposite, briefly discussed the results along with the degradation mechanism for mineralization of methyl orange (MO) dye. In another report, Xu et al. explored AgX/g-C3N4 (X = Br, I) nanohybrid with synergistic photocatalytic efficiency (Xu et al., 2013a). Keeping in mind the studies mentioned above, we have tried to present an extensive report on cutting-edge trends in AgX/g-C3N4 hybrids emphasizing pollutant mineralization accompanied by energy applications. To best of our knowledge, no review article has been published to date, as per Scopus data. Therefore, in focus of this review, we have discussed comprehensively g-C3N4 and AgX nanostructures, synthetic routes for facile fabrication of AgX/g-C3N4 nanocomposite, engineering AgX/g-C3N4 nano-junctions involving type-ΙΙ and Z-scheme based heterostructures along with their practical implementations in photocatalytic degradation processes and energy conversion followed by bacterial disinfection. To get an overall idea of literature being reported on AgX/g-C3N4 composite “Scopus” database has been carried out to acquire effective outcomes. Consistent with Scopus data, we got 970, 165, 85 and 89 results employing keywords such as “g-C3N4 + AgCl photocatalyst”, “g-C3N4 + AgBr photocatalyst”, “g-C3N4 + AgI photocatalyst” and “g-C3N4 + AgX photocatalyst”, respectively, from 2009 to December 2019 (Fig. 6a–c). With such an escalating amount of reports on g-C3N4 tailored AgX, it is the apt and high time to impart an extensive review of AgX/g-C3N4 nanomaterials. Herein we presented a substantial and efficient review of this evolving hot research area.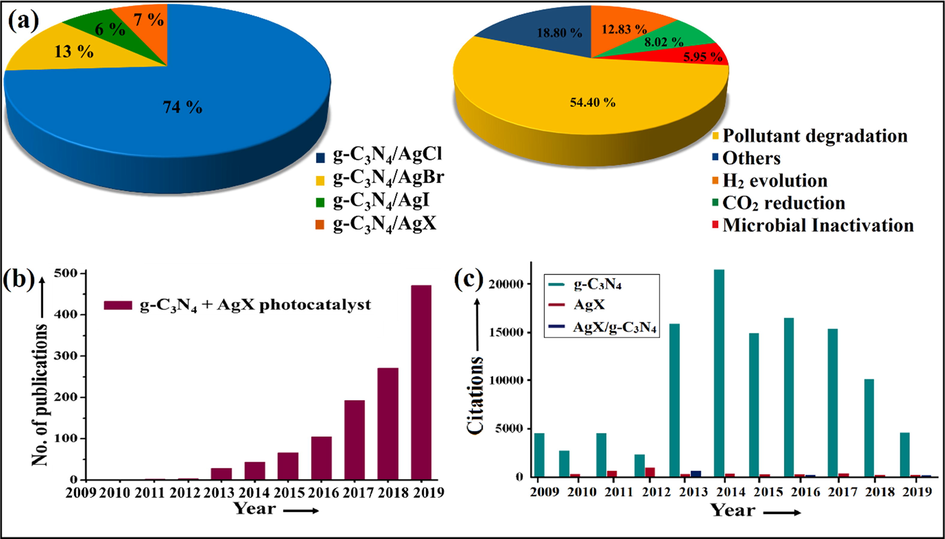
Scopus data analysis (a) Pie diagram depicting multifunctional applications of AgX/g-C3N4 photocatalyst, (b) Bar graph portraying annual publications, and (c) Citations of g-C3N4, AgX as well as AgX/g-C3N4 photocatalyst from year 2009 to December 2019.
2 Synthetic strategies for fabrication of AgX/g-C3N4 nanohybrids
2.1 Precipitation method
Precipitation method is often employed for AgX/g-C3N4 nanocomposite fabrication. Mohamed et al. explored AgCl@pg-C3N4 nano photocatalyst, where mesoporous graphitic carbon nitride (pg-C3N4) was fabricated via pyrolysis of dicyandiamide and urea at an atmospheric environment. Silver nitrate (AgNO3) and potassium chloride (KCl) were employed as starting materials, and the AgCl@pg-C3N4 composite was prepared in-situ. The photocatalytic ability of prepared samples was estimated for photodegradation of atrazine. No peak for AgCl was observed during X-ray diffraction (XRD) analysis due to lower AgCl content or successful dispersal of AgCl onto a g-C3N4 surface (Fig. 7a). UV–Visible diffuse reflectance spectroscopy (UV-DRS) spectra revealed that increased weight percentage of AgCl above 3% shifted the absorption edges of pg-C3N4 towards extended wavelengths because higher weight percentage increases the agglomeration and size of AgCl, which reduces the absorption edge (Mohamed, 2015). Yao et al. investigated novel Ag/AgCl/g-C3N4 nanocomposite by precipitation in geothermal water at ambient temperature. Geothermal water is a sort of natural heat energy and renewable energy resource, which provides green energy. Here, geothermal water was employed as a chlorine source for the preparation of AgCl NPs. 50% Ag/AgCl/g-C3N4 revealed more excellent photocatalytic capability for the elimination of organic dyes under illumination of natural light. SEM studies explained that the deposition of Ag/AgCl NPs onto the surface of g-C3N4 lead to an irregular surface and a close interface was formed among g-C3N4 and Ag/AgCl. Also, TEM analysis illustrated the decoration of several shady Ag/AgCl NPs on lamellar g-C3N4 nanosheets, with greater dispersion (Yao et al., 2014).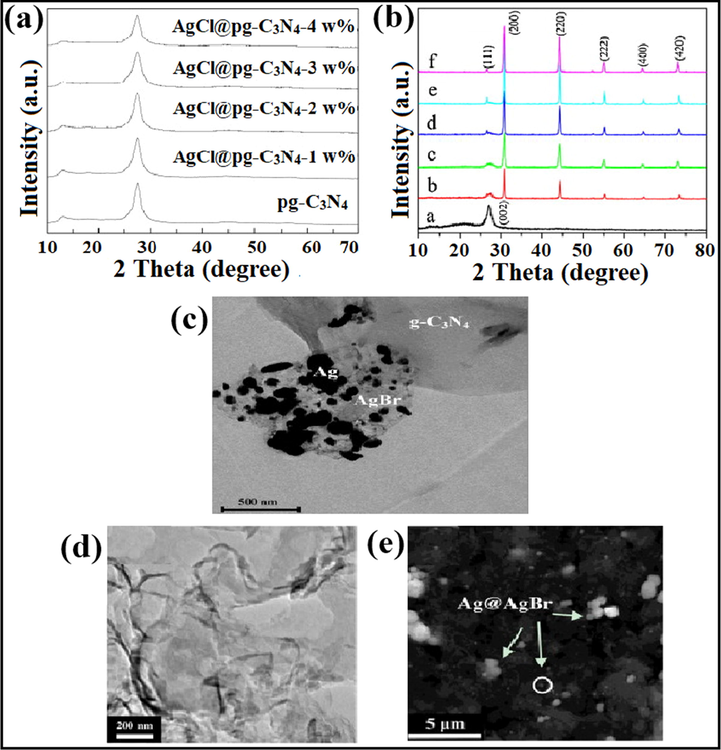
(a) XRD analysis of as-prepared AgCl@pg-C3N4; reprinted with permission from Elsevier (Licence no. 4746510793950) (Mohamed, 2015), (b) XRD spectra of Ag/AgBr/g-C3N4 photocatalyst showing face-centered cubic assembly of AgBr, (c) TEM studies confirming the formation of as-synthesized nanocomposite; reprinted with permission from Elsevier (Licence no. 4746550937986) (Cao et al., 2013), TEM images demonstrating (d) platelet-like morphology of bulk g-C3N4, and (e) particle size of Ag@AgBr/g-C3N4 composite; reprinted with permission from Elsevier (Licence no. 4746561201233) (Yang et al., 2014).
The advantages increasing the utilization of precipitation method for facile preparation of NPs are the relatively low reaction temperatures, fine and uniform particle size with weakly agglomerated particles, low cost and homogeneity of component distribution. Moreover, the precipitation method is not considered as a controlled process in terms of reaction kinetics. Also, the as-prepared solids have a wide particle size distribution, uncontrolled agglomerated morphology and incomplete precipitation of metal ions (Deraz, 2018).
2.2 Deposition precipitation method
A plasmonic Z-scheme based AgCl/Ag/g-C3N4 photocatalytic material was fabricated by Bao and his research group employing melamine (C3H6N6), hexadecyl trimethyl ammonium chloride (CTAC), AgNO3 as a precursor molecule. The photocatalytic efficiency of prepared nano-sample was explored for photodegradation of MO dye. The results revealed, Ag/AgCl NPs with a particle size of 5–15 nm were evenly distributed onto a g-C3N4 network. TEM studies demonstrated a lamellar sheet-like assembly and amorphous nature of pure g-C3N4. However, Ag/AgCl exhibits spherical morphology. It was observed that Ag/AgCl particles (dark spots) were evenly dispersed on the g-C3N4 surface. A slight redshift was found in the absorption edge by UV-DRS spectra of AgCl/Ag/g-C3N4 composite, which may be accredited to the SPR effect of Ag crystal, i.e., formed in-situ on AgCl surface. Also, superior photocatalytic efficacy of AgCl/Ag/g-C3N4 nanocomposite was indexed to the SPR of Ag nanocrystal (Bao and Chen, 2016).
Cao and co-workers fabricated a proficient VLT Ag/AgBr/g-C3N4 photocatalyst using C3H6N6, sodium bromide (NaBr), and AgNO3 as predecessors for the exclusion of MO dye. The superior photocatalytic ability was observed for 50% Ag/AgBr/g-C3N4 nanocomposite, which may be accredited to metallic Ag and synergistic effects of AgBr/g-C3N4 alliance. Fig. 7b explained XRD pattern indicating face-centered cubic assembly of AgBr, where small amounts of Ag+ were reduced to metallic Ag, and no prominent peaks for metallic Ag were obtained. TEM imaginings (Fig. 7c) confirmed the formation of as-synthesized nanocomposite possessing random microstructure (Cao et al., 2013). Ag/AgBr tailored graphite-alike carbon nitride was synthesized by Xu and his research group via deposition-precipitation method (Xu et al., 2013b). Yang et al. explored novel Z-scheme Ag@AgBr/g-C3N4 plasmonic photocatalyst by photoreduction of AgBr/g-C3N4 nanohybrid. Bulk g-C3N4 was synthesized by polymerization of urea (NH2CONH2), while cetyl methyl ammonium bromide (CTAB) and AgNO3 were the starting materials employed for the fabrication of Ag@AgBr hybrid. Ag@AgBr/g-C3N4 nanocomposite revealed stronger photocatalytic ability towards photo-degradation of MO and RhB. A diffraction peak at 38.1° attributed to metallic Ag was observed during XRD analysis. SEM and TEM analysis evaluated the morphological features of the Ag@AgBr/g-C3N4 composite. Fig. 7d displayed a platelet-like morphology of bulk g-C3N4, which provides more reactive sites for the growth of Ag@AgBr nanohybrids. Sphere-like Ag@AgBr hybrids with a particle size of 200 nm were formed, and the platelet-like structure remains inviolate after Ag@AgBr particle growth on the g-C3N4 surface (Fig. 7e) (Yang et al., 2014).
Zhang al. constructed a novel VLT AgI/g-C3N4 photocatalyst using C3H6N6, AgNO3, potassium iodide (KI) and polyvinylpyrrolidone (PVP) as precursors with enhanced photocatalytic efficiency for diclofenac (DCF) removal (Zhang et al., 2017a). Murugesan et al. synthesized a VLD AgI/g-C3N4 nanocomposite via deposition precipitation method (Murugesan et al., 2018a).
2.3 Hydrothermal method
The hydrothermal approach is utilizing the chemical reaction among reagents at a particular temperature and pressure (Ghosh and Pal, 2019). Using a hydrothermal approach for facile preparation of AgX/g-C3N4 photocatalyst is an unusual approach. For example,
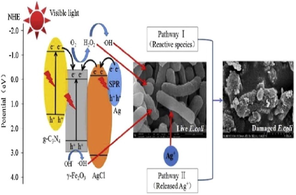
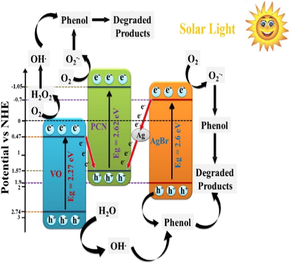
Akbarzadeh et al. employed bismuth (ΙΙΙ) chloride (BiCl3), ammonium metavanadate (NH4VO3), ethanolamine (C2H7NO), C3H6N6 and AgNO3 as precursors for the fabrication of g-C3N4/Ag/AgCl/BiVO4 micro-flowers viz. one-pot hydrothermal treatment. Photocatalytic performance of as-prepared nanocomposite was examined for photo-removal of ibuprofen (IBF). To BiCl3 solution, NH4VO3 was added to yield orange-coloured precipitates after that ethanolamine was added, and colour of the solution altered. Subsequently, the AgNO3 solution was added to the above mixture via the precipitation-photodeposition method, and the whole solution was exposed under visible light in a photoreactor. The g-C3N4 solution was prepared and was consequently added to the above-prepared mixture by undergoing ultrasonication. The mixture was autoclaved and heated at 180 °C for 12 h, and then the obtained precipitates were dried to obtain g-C3N4/Ag/AgCl/BiVO4 micro-flowers. XRD pattern (Fig. 8a) demonstrated a diffraction peak at 15.11°, which helped distinguish monoclinic scheelite BiVO4 (m-BiVO4) from tetragonal BiVO4 (t-BiVO4). While, peaks at 38.2° and 44.6° were attributed to metallic Ag, which indicated the photo-reduction of AgNO3 from Ag+ to Ag0 (Akbarzadeh et al., 2018). Raizada and research group explored BiOBr/PSCN/Ag/AgCl Z-scheme photocatalytic mechanism for degradation of phenol under visible light, via a facile hydrothermal technique. SEM images (Fig. 8b) depicted the successful loading of BiOBr/Ag/AgCl NPs on phosphorus (P) and sulfur (S) co-doped g-C3N4 (PSCN) nanosheet (Raizada et al., 2020b).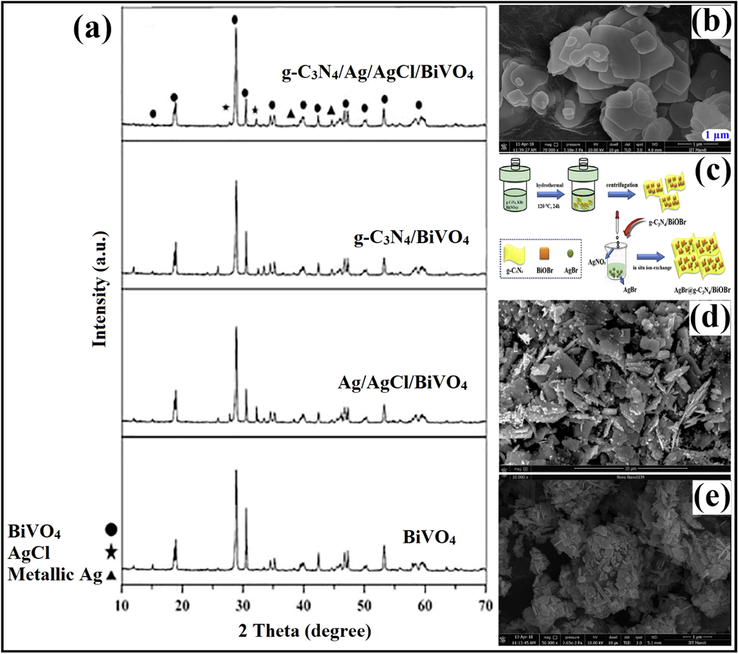
(a) XRD pattern distinguishing monoclinic scheelite BiVO4 from tetragonal BiVO4 in g-C3N4/Ag/AgCl/BiVO4 composite; reproduced with permission from Elsevier (Licence no. 4746601497828) (Akbarzadeh et al., 2018), (b) SEM images depicting successful loading of BiOBr/Ag/AgCl NPs on PSCN nanosheet; reproduced with permission from Elsevier (Licence no. 4754600966798) (Raizada et al., 2019b), (c) Detailed fabrication process of AgBr@g-C3N4/BiOBr nano-junction, (d) SEM imaginings revealing dispersal of AgBr particles on g-C3N4/BiOBr composite; reproduced with permission from Elsevier (Licence no. 4746611095568) (Tang et al., 2019), and (e) SEM studies confirming formation of PGCN/AgI/ZnO/CQDs heterostructure; reproduced with permission from Elsevier (Licence no. 4746620007509) (Hasija et al., 2019).
Tang et al. investigated AgBr@g-C3N4/BiOBr via hydrothermal route followed by the in-situ ion-exchange method, utilizing NH2CONH2, KBr, ethylene glycol (EG) and AgNO3 predecessors. The photocatalytic experimentations were carried out for RhB and tetracycline (TC) as target contaminants, under irradiation of visible light. Fig. 8c illustrated the detailed fabrication process of AgBr@g-C3N4/BiOBr nanoheterojunction. Fig. 8d explained SEM micrographs, which indicated the dispersal of AgBr particles on g-C3N4/BiOBr composite (Tang et al., 2019a). Chen et al. studied 3D Ag/AgBr/g-C3N4@NGA Z-scheme based photocatalyst for efficient MO removal and antimicrobial activities were also investigated (Chen et al., 2019).
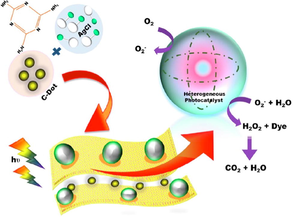
PGCN/AgI/ZnO/CQDs nanocomposite was developed by Hasija and co-workers employing NH2CONH2, diammonium hydrogen phosphate (NH4)2HPO4, AgI, zinc nitrate tetrahydrate Zn(NO3)2, sodium hydroxide (NaOH), sodium iodide (NaI) and zinc oxide (ZnO) as starting material. While CQDs were prepared using bamboo leaves through facile hydrothermal treatment. SEM images confirmed the formation of PGCN/AgI/ZnO/CQDs nano-junction, as shown in Fig. 8e (Hasija et al., 2019c). Xue et al. studied g-C3N4/Bi2WO6/AgI nanocomposite following a Z-scheme photocatalytic system for efficient photodegradation of TC through hydrothermal reaction followed by in-situ precipitation method (Xue et al., 2019). Zhang et al. developed a VLD Z-scheme AgI/LaFeO3/g-C3N4 photocatalyst for removal of norfloxacin (NOF) (Zhang et al., 2019).
During hydrothermal process, conditions of the reaction are very imperative. Solvent type, temperature as well as duration affect the synthesis of the products. Hydrothermal approach offers many advantages such as relatively mild operating conditions (reaction temperatures < 300 °C), one-step synthetic process, environmental friendly, and good dispersion in solution (Sōmiya and Roy, 2000). Besides, use of expensive autoclaves hinders its widespread applicability.
2.4 Ion-exchange method
An ion-exchange method is frequently employed for the synthesis of AgX/g-C3N4 composite. The operation circumstance is facile as well as lower in cost while choosing raw material is the crucial step in this scheme. For instance,
Zhang et al. employed C3H6N6, AgNO3, EG, and hydrochloric acid (HCl) as precursor molecules for the synthesis of plasmonic Ag@AgCl/g-C3N4 photocatalyst viz. in-situ ion exchange process. The photocatalytic performance of as-fabricated nanocomposite was estimated for degradation of RhB, MO, MB, and phenols under natural light illumination. The detailed synthesis of Ag@AgCl/g-C3N4 nanocomposite was illustrated in Fig. 9a. TEM imaginings explained the black Ag@AgCl hybrid with a particle size of ∼8–12 nm was evenly dispersed on the surface of exfoliated g-C3N4 (Fig. 9b). XRD analysis demonstrated the diffraction peaks for Ag@AgCl/g-C3N4, revealing face-centered cubic AgCl and peak intensities increased with increasing content of AgNO3 (Zhang et al., 2014). A novel AgCl/Ag3PO4/g-C3N4 ternary composite was explored by Zhou and research group, with enhanced photocatalytic efficiency towards sulfamethoxazole (SMX) degradation (Zhou et al., 2017).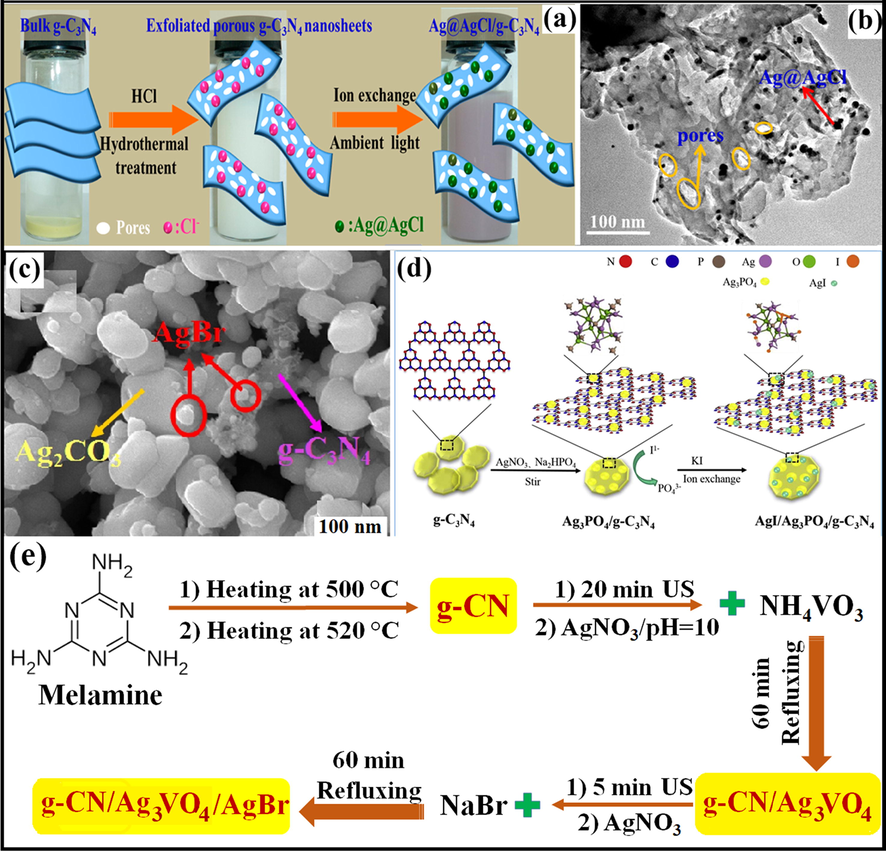
(a) Fabrication of Ag@AgCl/g-C3N4 nanocomposite, (b) TEM imageries showing dispersal of black Ag@AgCl particles onto g-C3N4 surface; reprinted with permission from Elsevier (Zhang et al., 2014) (c) SEM analysis representing loading of cubic AgBr particles on rod-like particles of Ag2CO3; reprinted with permission from Elsevier (Licence no. 4746900302562) (Tang et al., 2017), (d) Synthesis scheme of g-C3N4/Ag3PO4/AgI composite; reprinted with permission from Elsevier (Licence no. 4746910427726) (Tang et al., 2019), and (e) Preparation of g-CN/Ag3VO4/AgBr nanocomposite (Barzegar et al., 2018).
Tang et al. fabricated g-C3N4/Ag2CO3/AgBr heterostructure by surface alteration of g-C3N4/Ag2CO3 thru AgBr NPs via the ion-exchange process. The synthesis process involved starting materials such as NH2CONH2, sodium carbonate (Na2CO3), AgNO3, and NaBr. XRD pattern revealed the coexistence of Ag2CO3 and AgBr particles in the g-C3N4/Ag2CO3/AgBr composite. The Fourier-transform infrared spectroscopy (FTIR) results confirmed the presence of NPs and the formation of heterojunction. Irregular and cubic AgBr particles were observed on polyhedral rod-like particles of Ag2CO3, as explicated by SEM analysis (Fig. 9c) (Tang et al., 2017).
Tang and research group reported a Z-scheme based g-C3N4/Ag3PO4/AgI nano-photocatalyst for evaluating the photocatalytic ability for nitenpyram (NTP) degradation. Fig. 9d explained the preparation method of g-C3N4/Ag3PO4/AgI nanocomposite through the in-situ ion-exchange route (Tang et al., 2019b).
Ion exchange method is widely employed in industrial scale for waste water treatment. The process is environmental friendly, has low maintenance cost and can provide high flow rate to treated water. Along with these benefits, certain limitations associated with this process are bacterial contamination, chlorine adulteration and adsorption of organic matter.
2.5 Refluxing method
Refluxing is a process involving condensation of vapours and the return of this condensate to the system from which it is originated. g-C3N4/Ag3VO4/AgBr nanocomposites were studied by Barzegar et al. for the efficient elimination of toxins under visible light. The photocatalysts were synthesized by loading Ag3VO4 and AgBr particles onto a g-C3N4 surface. Moreover, the detailed fabrication process can be observed in Fig. 9e and the solution was refluxed at 96 °C for 1hr (Barzegar et al., 2018b).
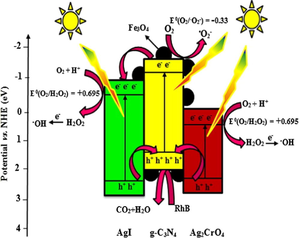
Akhundi et al. reviewed g-C3N4/Fe3O4/AgI nanocomposite by reflux method at 96 °C (Akhundi and Habibi-Yangjeh, 2016c). A visible-light-driven g-C3N4/Ag2CrO4/AgI photocatalyst was explored by Barzegar and co-workers via the refluxing process, for efficient photodegradation of RhB (Barzegar et al., 2018a). The typical process involved mixing of AgNO3 (0.109 g) with a suspension containing 0.35 g of g-C3N4/AgCrO (20%) sample. After that the sample was treated with 0.096 g of NaI and refluxed for 60 min at 96 °C.
The method requires the use of relatively cheaper equipment’s that are easy to operate and their setup is facile. Also, these methods are time consuming, involves the use of flammable solvents and sometimes these techniques may be destructive limiting their practical implementations.
Tables 1 and 2 illustrates various other methods for fabrication of AgX coupled g-C3N4 nano-hybrids with different photocatalytic applications.
S.No.
Photocatalyst
Fabrication routes
Targeted Pollutant
Light source
Degradation Mechanisms
References
1.
Ag/AgCl/g-C3N4
Facile preparation
MO
300 W Xe lamp with a 400 nm cut-off filter
__
Yang et al. (2014)
2.
g-C3N4/Fe3O4/AgCl
Facile preparation
RhB
50 W LED source
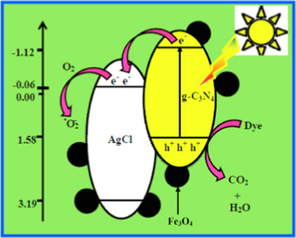
Akhundi and Habibi-Yangjeh (2015a)
3.
PoPD/AgCl/g-C3N4
Photoinitiated polymerization method
TC
250 W Xe arc lamp
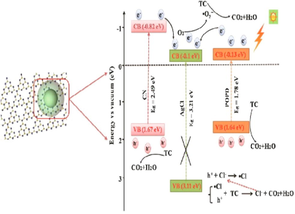
Sun et al. (2019)
4.
Ag/AgCl@ZIF-8/g-C3N4
Facile stirring method
LVF
150 W Xe lamp equipped with 420 nm filter
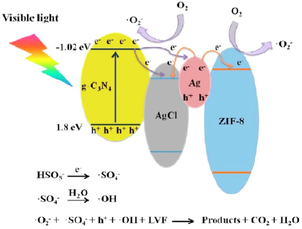
Zhou et al. (2019)
5.
AgCl-Ag/g-C3N4
Three step synthetic method
MO
350 W Xe lamp with UV cut-off filter (λ > 420 nm)
Yu et al. (2019)
6.
g-C3N4/AgCl/CDs
Impregnation method
MB, RhB
40 W LED bulb
Matheswaran et al. (2019)
7.
γ-Fe2O3/Ag/AgCl/g-C3N4
Solvothermal and photodeposition method
Escherichia Coli (E. coli)
500 W tungsten halogen lamp
Ai et al. (2019)
8.
Ag/AgBr/g-C3N4
Solvothermal method
RhB
300 W Xe arc lamp equipped with ultraviolet cut filter
Xu and Zhang (2013)
9.
g-C3N4/GO/AgBr
–
RhB
250 W Xe lamp wiyh a 420 nm cut-off filter
Miao et al. (2017b)
10.
Ag/AgBr/V2O5/PCN
Hydrothermal method
Phenol
35 W LED lamp
Raizada et al. (2019b)
11.
g-C3N4/AgBr/rGO
–
2,4-dichlorophe-nol
250 W Xe lamp
Zhou et al. (2018)
12.
g-C3N4/Fe3O4/AgI/Ag2CrO4
Refluxing Method
RhB
50 W LED lamp
Akhundi and Habibi-Yangjeh (2016a)
Photocatalyst
Methodology
Process Conditions
Major products
Charge transfer mechanism
Quantum efficiency
Reference
AgCl@g-C3N4
Deposition-precipitation method
11 W compact fluorescent lamp
Methane
Acetic acid
Formic acid
53.03 μmol g−1
6.01 μmol g−1
2.51 μmol g−1
Murugesan et al. (2018b)
g-C3N4/Ag/AgCl/BiVO4 (CAB)
Facile one-pot hydrothermal method
8 fluorescent lamps, 8 W each
Methane
205 μmol h−1 g−1
Rather et al. (2018)
AgBr/g-C3N4/NG (ACNNG)
Green and facile method
Xe arc lamp
Methanol
Ethanol
–
105.89 µmol g−1
256.45 µmol g−1
Li et al. (2015)
Ag/AgX(X = Cl, Br, I)/g-C3N4
In-situ solid phase method
300 W Xe lamp
H2
Ag/AgCl/CN-4; 26.39 μmol g−1h−1
Ag/AgBr/CN-4; 18.05 μmol g−1h−1
Ag/AgI/CN-4; 59.22 μmol g−1h−1
Bai et al. (2019)
PCN/GO/Ag/AgBr
Hard template method and selective chemical etching
300 W
Xe lamp with a light filter of 420 nmH2
3.69 mmol g−1h−1
Li et al. (2019)
g-C3N4-Ag/AgBr (CNAA)
Solvothermal method
Xe lamp
H2
47.84 μmol g−1h−1
Li et al. (2020)
3 AgX/g-C3N4 nano-junctions
Amongst several semiconductor mediated nanostructures, construction of type-II and Z-scheme based heterojunctions have turned up as a facile way for elevating the photocatalytic efficacy.
3.1 Type-ΙΙ heterojunctions
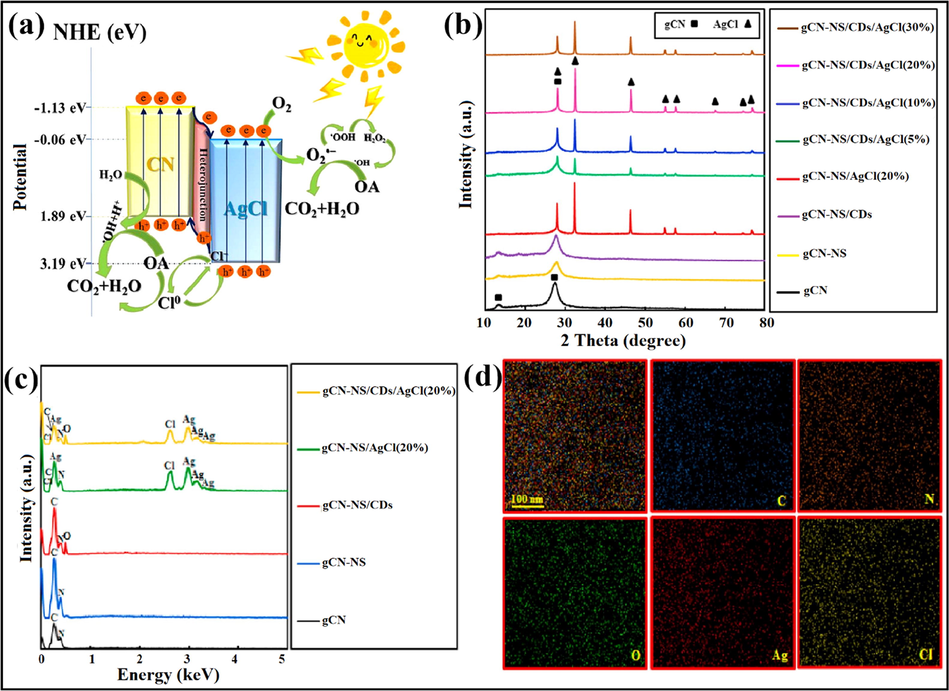
Combining two semiconducting materials where CB of semiconductor Ι (SC-Ι) is lower than that of semiconductor ΙΙ (SC-ΙΙ) and VB of SC-Ι has a higher value than that of SC-ΙΙ, shown in Fig. 10. In type-II heterostructures, the band potentials are positioned at optimum levels (staggered gap) that offer spatial charge carrier separation with enhanced photocatalytic efficacy. For instance, a novel work reporting deposition of AgCl NPs on the g-C3N4 surface was explored by Zhao et al. (2012). Shi and research group investigated AgCl decorated g-C3N4 (AgCl-CN), explicating the photodegradation process of oxalic acid (OA) under visible light irradiation. g-C3N4 and AgCl possess band gaps of 2.7 eV and 3.25 eV, respectively. Fig. 11a illustrated degradation mechanism explicating the transference of h+VB of AgCl to VB of CN while CN e−CB shift to CB of AgCl. Therefore, photo-generated e−CB of AgCl (ECB = −0.06 eV) reduces O2 to produce •O2− due to its more negative reduction potential, while h+VB of CN react with absorbed water to produce •OH radicals. These •O2− and •OH radicals were responsible for the photocatalytic degradation of OA (Shi et al., 2019).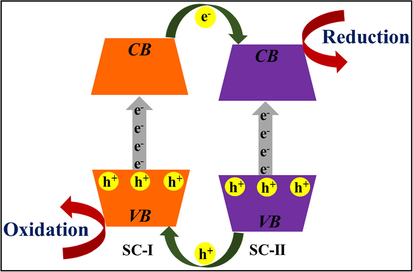
Diagrammatic representation of type- II heterojunction.
(a) Type- II degradation mechanism in AgCl-CN nanocomposite; reproduced with permission from Elsevier (Licence no. 4747851498621) (Shi et al., 2019), (b) XRD pattern for g-C3N4-NS/CDs/AgCl photocatalyst with no prominent peaks for CDs, (c) EDX spectra of as-prepared nanocomposite, and (d) EDX mapping confirming elemental distribution; reprinted with permission from Elsevier (Licence no. 4747860423948) (Asadzadeh-Khaneghah et al., 2018).
Asadzadeh-Khaneghah et al. explicated the loading of carbon dots (CDs) and AgCl over the g-C3N4 network by a simple approach for the photocatalytic degradation of RhB, MB, MO, and phenol. g-C3N4-NS/CDs/AgCl photocatalyst with 20% of AgCl exhibited maximum photocatalytic activity. CDs acted as electron mediator amongst g-C3N4-NS and AgCl semiconductors. XRD analysis illustrated (Fig. 11b) that the introduction of CDs does not affect the assembly of g-C3N4 and no diffraction peaks were detected for CDs. Due to the existence of several functional groups such as hydroxyl, carbonyl, carboxyl, and epoxy in CDs, excessive amounts of oxygen elements were observed in Energy Dispersive X-ray (EDX) spectra (Fig. 11c). Fig. 11d explicated elemental distribution in g-C3N4-NS/CDs/AgCl (20%) nanocomposite by EDX mapping. Further FTIR analysis demonstrated that exfoliation of g-C3N4/CDs and integration of CDs and AgCl particles does not have any significant influence on the g-C3N4 structure. As well, no peak for the Ag-Cl bond was noticed, as observed in Fig. 12a. The exfoliated nature of g-C3N4-NS was confirmed by the UV-DRS spectra, exhibiting a slight blue shift (Fig. 12b) (Asadzadeh-Khaneghah et al., 2018).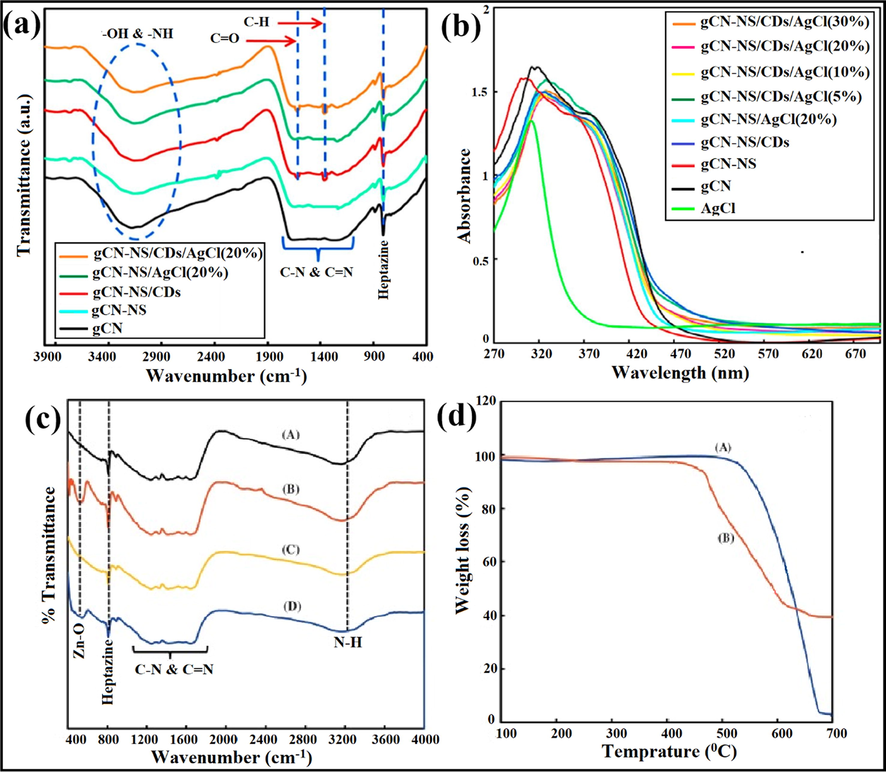
g-C3N4-NS/CDs/AgCl composite showing (a) FTIR analysis, (b) UV–visible spectra; reproduced with permission from Elsevier (Licence no. 4747860423948) (Asadzadeh-Khaneghah et al., 2018), (c) FTIR examinations confirming presence of Zn-O bond in g-C3N4/ZnO/AgCl hybrid, and (d) TGA curves explicating the reduced weight of g-C3N4 (Akhundi et al., 2015); reproduced with permission from Elsevier (Licence no. 4747860921119).
Akhundi and co-workers designed g-C3N4/ZnO/AgCl ternary nano photocatalyst employing a facile methodology for the photocatalytic degradation process. Typically, g-C3N4/ZnO/AgCl (40%) composite unveiled enhanced photocatalytic performance as compared to g-C3N4/ZnO and g-C3N4/AgCl nanocomposites. Further, FTIR studies (Fig. 12c) confirmed the presence of the ZnO sample with a characteristic peak at 540 cm−1 indexed to the Zn—O bond. Thermal gravimetric analysis (TGA) examinations (Fig. 12d) revealed that bulk g-C3N4 lost its 97% of weight when heated up-to 700 °C and decomposed to produce unsteady compounds. g-C3N4/ZnO/AgCl nanocomposite loses its weight from about 420 °C and lasts up-to 660 °C, while loading of ZnO/AgCl onto g-C3N4 sheet lead to decreased thermal stability. The photodegradation process of RhB was carried out at 25 °C revealing 9% of RhB was photolyzed in 420 min under natural light illumination. 40% g-C3N4/ZnO/AgCl composite exhibited maximum photocatalytic efficiency than pure g-C3N4, g-C3N4/ZnO, and g-C3N4/AgCl composites. It was observed that about 39%, 46%, 58%, and 99% RhB was photodegraded in 270 min over g-C3N4, g-C3N4/ZnO, and g-C3N4/AgCl and g-C3N4/ZnO/AgCl (40%) samples, respectively. Approximately 6.7% (in dark) RhB molecules were adsorbed over as-synthesized composite after 420 min (Akhundi and Habibi-Yangjeh, 2015c). Lv and research group prepared Ag@AgCl/g-C3N4/TiO2 ceramic films with superior photocatalytic abilities and a self-cleaning effect (Lv et al., 2018).
In another work, Miao and co-workers reported g-C3N4/AgBr photocatalyst loaded with CDs for the photocatalytic removal of organic contaminants. g-C3N4/CDs/AgBr photocatalyst revealed exceptional photocatalytic ability as comparable to pristine g-C3N4, AgBr, and g-C3N4/AgBr. Integration of CDs into g-C3N4/AgBr amended the photocatalytic efficacy due to light-absorbing and electron sinking nature of CDs amongst g-C3N4 and AgBr. TEM analysis clarified the size of CDs which was found to be 5 nm (Fig. 13a) and loading of AgBr NPs on the surface of CDs modified g-C3N4 NS was shown in Fig. 13b and c. XRD pattern explained the incorporation of CDs into g-C3N4 causing a shift of peaks with a slight distortion of the g-C3N4 lattice, as shown in Fig. 13d. Moreover, the FTIR examination described that no prominent peak for CDs was noticed in g-C3N4/CDs/AgBr nanocomposite (Fig. 13e). As reported earlier, Brunauer-Emmett-Teller (BET) specific surface area of g-C3N4/CDs (68.20 m2/g) was higher than that of pristine g-C3N4 (57.09 m2/g), demonstrating that addition of g-C3N4 lead to the boosted surface area. Consequently, it offers more reactive sites for adsorption and catalysis processes. Photoluminescence (PL) examinations (Fig. 13f) revealed that integration of CDs or AgBr onto g-C3N4 sheet inhibits the spatial charge separation of photo-induced charge carriers. Moreover, the photocatalytic ability of nanocomposite was enhanced, and insertion of CDs lessened the recombination of photo-induced EHP (Miao et al., 2017a).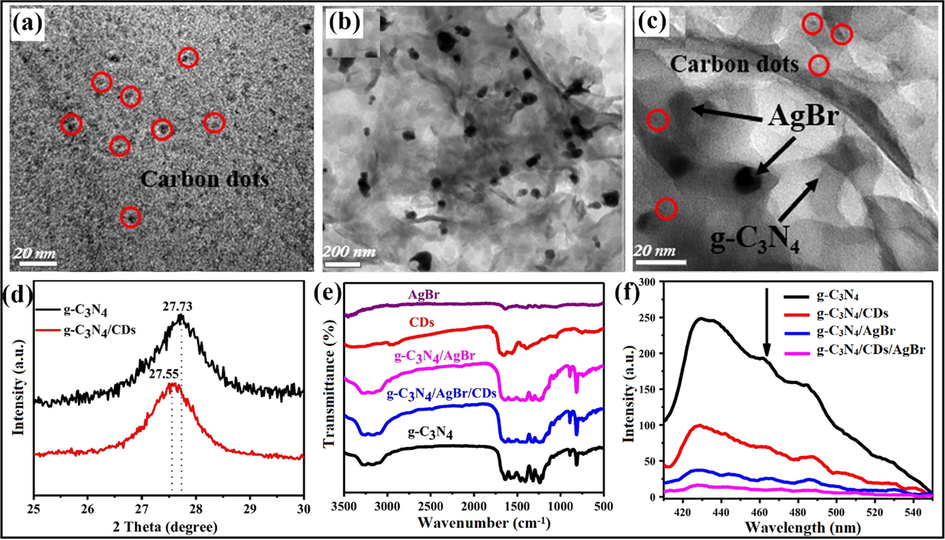
g-C3N4/CDs/AgBr nanocomposite presenting (a) TEM analysis of CDs, (b and c) TEM studies exhibiting loading of AgBr NPs on the surface of CDs modified g-C3N4 NS, (d) XRD pattern revealing shift of peaks by anchoring CDs into g-C3N4 sheet, (e) FTIR spectra describing no prominent peak for CDs, and (f) PL explorations of as-synthesized nanocomposite; reproduced with permission from Elsevier (Licence no. 4750670232021) (Miao et al., 2017).
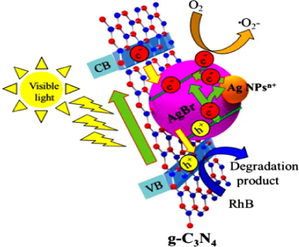
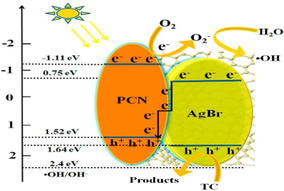
Chen et al. explored Ag/AgBr/g-C3N4 photocatalyst by a facile technique with greater photocatalytic ability for elimination of MB. The optimum Ag/AgBr content with maximum photocatalytic efficiency was determined to be 40%. The efficacious photocatalytic ability could be allocated to extended visible light absorption and effective spatial charge separation due to the SPR effect of Ag0. Ag/AgBr particles own sphere-like morphology with a particle size of 500 nm (Fig. 14a), and the intercalation of AgBr particles into g-C3N4 layers was explained in SEM images. With increased AgBr content, bigger AgBr particles were obtained and assembled instantaneously. Ag/AgBr NPs ranging from 50 nm to 200 nm were well distributed onto g-C3N4 sheet, deprived of noticeable accumulation. Fig. 14b explained that several Ag crystals with a particle size of 10 nm appeared along with AgBr particle. X-ray photoelectron spectroscopy (XPS) studies determined the elemental composition of as-synthesized composite. XPS peaks confirm that Ag/AgBr/g-C3N4 powders comprised of Ag, Br, O, N, and C elements and with the element content of 2.81%, 1.8%, 3.77%, 42.68%, and 48.93%, respectively. The diffraction peaks at 366.8 eV and 372.8 eV were allocated to Ag+ in AgBr, and the peaks at 374.5 eV and 368.5 eV were indexed to Ag0, as illustrated in Fig. 14c. Ag/AgBr/g-C3N4 nanocomposite displayed significant improvement of light absorption at wavelengths 500–900 nm (Fig. 14d), and upon aggregating the Ag/AgBr content absorption intensities improved, which could be indexed to the SPR effect of Ag nanocrystals. The improved photocatalytic ability of Ag/AgBr/g-C3N4 composite may be due to the synergetic effect of Ag, AgBr, and g-C3N4, as shown in. At the same time, metallic Ag on AgBr surface plays a crucial role in e− transformation (Cao et al., 2013). The band potentials of g-C3N4 and AgBr were 2.7 eV and 2.6 eV, respectively0, with more negative CB energies of g-C3N4 (−1.12 eV), than that of AgBr (−0.3 eV) and more positive VB energies of AgBr (+2.3 eV), than that of g-C3N4 (+1.58 eV). Therefore, photo-induced e−CB easily gets transferred from g-C3N4 to CB of AgBr, thereby producing CO2 by reacting with adsorbed O2. Meanwhile, photo-induced h+VB of AgBr relocates to the surface of g-C3N4. Furthermore, metallic Ag can provide photo-induced e− accredited to SPR effect, and Fermi level of AgBr lies inferior to that of Ag, consequently e− transferal from Ag to AgBr and lastly transferred to adsorbed O2 to generate •O2− (Chen et al., 2015a). Another work was explored by Akhundi and co-workers reporting the construction of g-C3N4/AgBr/Fe3O4 nano-composite with enhanced photocatalytic performance (Akhundi and Habibi-Yangjeh, 2015b). Mousavi et al. investigated a VLT magnetically recoverable g-C3N4/Fe3O4/Ag2WO4/AgBr quaternary photocatalyst for photodegradation of contaminants (Mousavi and Habibi-Yangjeh, 2018b).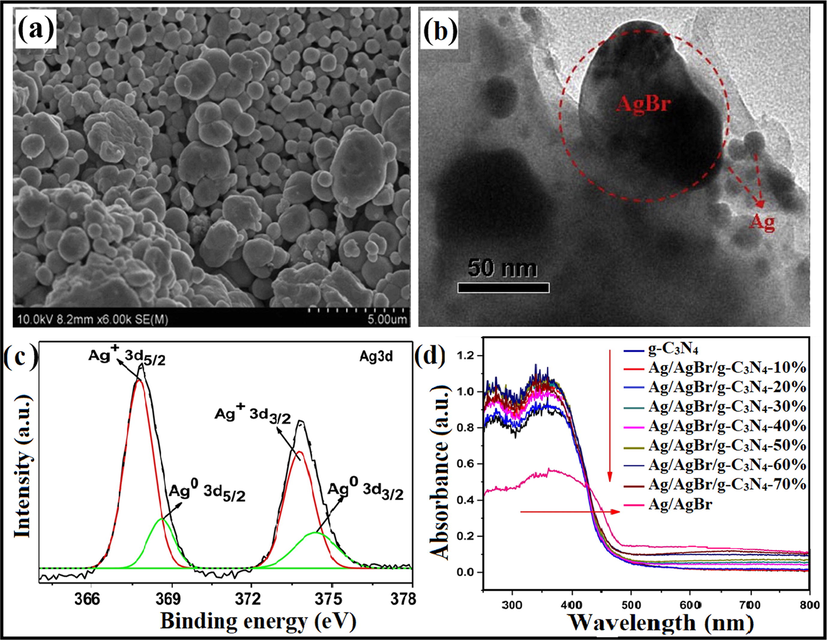
(a, b) SEM images of Ag/AgBr/g-C3N4 photocatalyst, (c) XPS examination revealing existence of Ag+ in AgBr, and (d) UV–Visible analysis of as-fabricated photocatalyst; reprinted with permission from Elsevier (Licence no. 4746461343390) (Chen et al., 2015).
Liu et al. reported AgI@g-C3N4 core-shell structures for efficient degradation of MB dye under the illumination of visible light (Liu et al., 2015). AgI/g-C3N4 nanocomposite was prepared by Lei and group, while TEM images (Fig. 15a) explicated that AgI NPs were stacked on g-C3N4 surface with increasing AgI content and particle size of AgI was observed to be about 30 nm (Lei et al., 2016). Akhundi and his research group explored recyclable g-C3N4/Fe3O4/AgI photocatalyst with superior photocatalytic activity for removal of RhB via the reflux method at 96°. The g-C3N4/Fe3O4/AgI (20%) composite illustrated more exceptional photocatalytic ability than bulk g-C3N4 and g-C3N4/Fe3O4 samples. XRD spectra for g-C3N4/Fe3O4/AgI nanocomposites revealed that diffraction peaks for AgI were associated to hexagonal crystal phase (Fig. 15b). SEM experimentations explained the loading of Fe3O4 and AgI particles on g-C3N4 sheet. Also, particle size was observed to be about 30 nm, as shown in Fig. 15c. Moreover, no peak for the Ag-I bond was observed, as illustrated by FTIR studies. Fig. 15d depicted two typical peaks at 430 and 620 cm−1 attributed to the Fe-O bond. The mechanistic view for g-C3N4/Fe3O4/AgI nanocomposite explicated the excitation of e− from VB to CB under visible light illumination. Attributable to confined band gaps of g-C3N4 and AgI, the e−CB reacts with adsorbed O2 to generate H2O2, and these H2O2 molecules produced •OH radicals by e− capturing. Though VB of g-C3N4 does not produce •OH radicals owing to lower VB energies of g-C3N4 (+1.58 eV) than adsorbed —OH (E°-OH/•OH = +2.38 eV) and H2O (E°H2O/•OH) = +2.72 eV). The O2, •O2− and h+ produced were the reactive species (RSs) accountable for photodegradation of RhB, as shown in Fig. 15e (Akhundi and Habibi-Yangjeh, 2016c).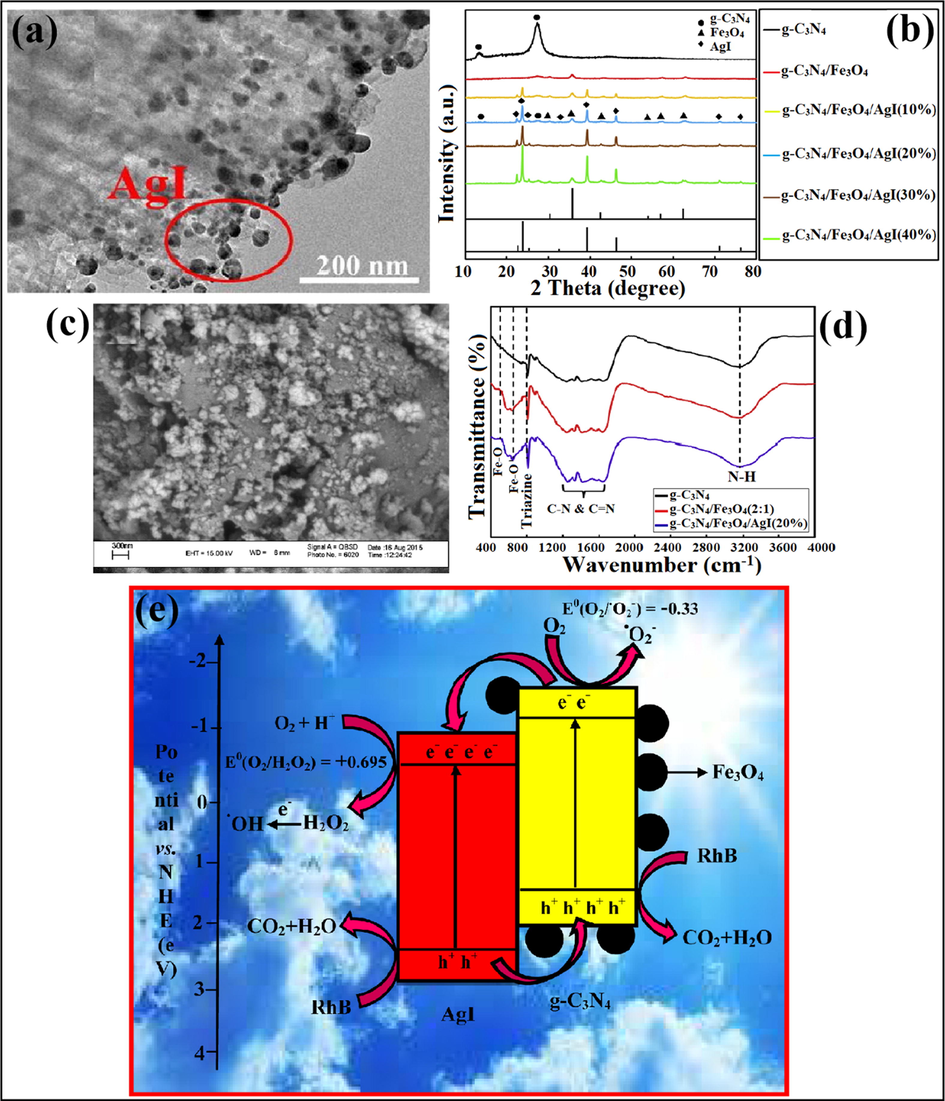
(a) TEM imaginings of AgI/g-C3N4 nanocomposite; reproduced with permission from Elsevier (Licence no. 4750100779496) (Lei et al., 2016), (b) XRD pattern of g-C3N4/Fe3O4/AgI nano-hybrid, (c) SEM analysis explained loading of Fe3O4 and AgI particles on g-C3N4 sheet, (d) FTIR studies confirming Fe-O bond, and (e) Photocatalytic degradation mechanism of as-prepared photocatalyst; reproduced with permission from Elsevier (Licence no. 4750110390251) (Akhundi and Habibi-Yangjeh, 2016).
3.2 Z-scheme based heterojunctions
Z-scheme heterojunction possesses more negative CB potentials and more positive VB potentials, thereby lowering the bandgap of the semiconductor. In Z-scheme arrangements, under natural light illumination, e−CB of SC-II merge with photo-generated h+VB of SC-I, as illustrated in Fig. 16. This caused the formation of sufficiently strong oxidative h+VB in SC-II and reductive e−CB of SC-I. This induced interface electric field accelerates the separation of EHP. For example, Zhou and co-workers investigated a plasmonic Ag/AgCl/g-C3N4 Z-scheme nano photocatalyst by an in-situ oxidation process. Ag/AgCl/g-C3N4 with an Ag/AgCl amount of 2.7 at % attained a more exceptional photocatalytic ability for mineralization of MO. TEM study demonstrated the dispersal of Ag NPs on the flake-like structure of g-C3N4, with a particle size of 10–50 nm, as shown in Fig. 17a. Consistent with Fig. 17b g-C3N4 acted as template for dispersion of Ag/AgCl crystals due to which agglomeration reduced and interface among Ag/AgCl augmented. No covalent bond was noticed amongst Ag/AgCl and g-C3N4, and the deposition of Ag/AgCl on the g-C3N4 surface was demonstrated by FTIR analysis, Fig. 17c. As per photocurrent examinations, the transient photocurrent response of Ag/AgCl/g-C3N4 composite is greater than that of bare g-C3N4, which means higher separation efficacy of EHP could be achieved, as illustrated in Fig. 17d (Zhou et al., 2014). Li et al. Ag@AgCl QDs decorated g-C3N4 nano-plates viz. oil-in-water self-assembly process (Li et al., 2017). Bao et al. explored a plasmonic AgCl/Ag/g-C3N4 composite ascribed to Z-scheme based mechanism, presented in Fig. 17e. Therefore, under exposure to solar light, both Ag and g-C3N4 get excited, thus producing photo-induced EHP. The photo-illuminated e− of Ag NPs travel to CB of AgCl to reduce O2. In the meantime, photo-induced e− of g-C3N4 go to Ag NPs to recombine with photo-generated h+ that were created by plasmonic absorption of Ag NPs. While the plasmon-induced h+VB of g-C3N4 reduces the organic pollutant (Bao and Chen, 2016).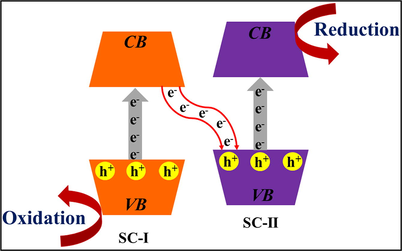
Diagrammatic representation of Z-scheme based heterojunction.
(a and b) TEM imageries of Ag/AgCl/g-C3N4 photocatalyst, (c) FTIR investigations of as-synthesized composite, (d) Photocurrent analysis depicting higher separation efficacy of EHP; reprinted with permission from Elsevier (Licence no. 4750660226117) (Zhou et al., 2014), and (e) Z-scheme photocatalytic mechanism of AgCl/Ag/g-C3N4 composite; reprinted under licence from CC BY 4.0 (Bao and Chen, 2016).
A Z-scheme Ag@AgBr grafted graphitic carbon nitride (Ag@AgBr/g-C3N4) by photo reducing AgBr/g-C3N4 nanohybrids was fabricated by deposition–precipitation process. The photocatalytic proficiency of the prepared sample was explored for the photo-degradation of MO and RhB dye. Under solar light irradiation, e−VB of both g-C3N4 and AgBr were excited to vacant CB producing EHP, respectively. As a result of more negative CB edges of AgBr (−0.3 V) comparable to fermi level of Ag (0.4 V), e− in CB of AgBr can simply migrate into Ag metal through Schottky barrier. Since the VB edge of g-C3N4 (1.4 V) is more favourable than the fermi level of Ag, therefore, e− from Ag travel to VB of g-C3N4. Subsequently, a Z-scheme mechanism was proposed, as shown in Fig. 18a. Accordingly, e−CB of g-C3N4 reacts with O2 forming O2−• radicals while h+VB of AgBr travel onto the surface forming Br0 atoms. •O2− and Br0 were the RSs accountable for the photodegradation of MO (Yang et al., 2014). In another work, Feng et al. constructed a g-C3N4/AgBr photocatalyst and concluded that integration of AgBr hybrid on g-C3N4 NS results in the formation of heterostructures, as elucidated by electrochemical impedance spectroscopy (EIS) Nyquist plots. A reduced arc radius of the EIS Nyquist plot reflects greater charge transference befalling at the interface. Owing to a smaller arc radius of as-prepared samples, these revealed more efficient spatial separation of plasmon-induced charge carriers (Feng et al., 2015). Azimi et al. proposed a ternary g-C3N4/AgBr/ZnO nanocomposite for active degradation of MB dye (Azimi et al., 2018).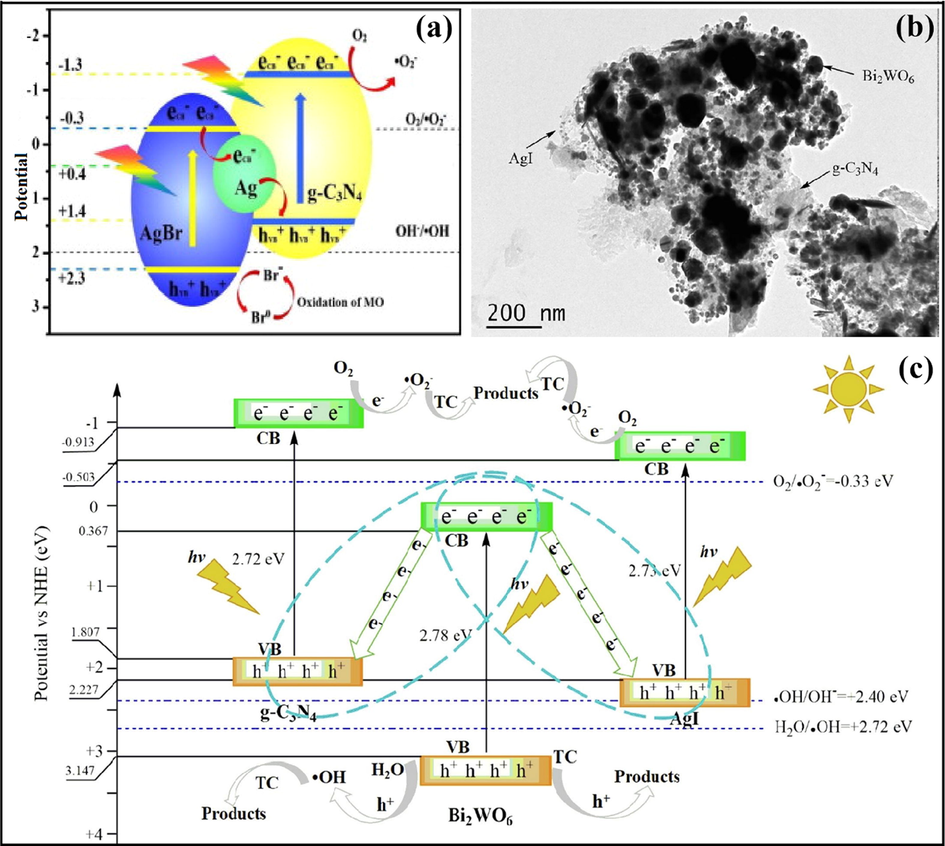
(a) Mechanistic view of Ag@AgBr/g-C3N4 nanocomposite; reproduced with permission from Elsevier (Licence no. 4751150026281) (Yang et al., 2014), (b) TEM studies explicating uneven dispersal of AgI NPs onto g-C3N4 surface, and (c) Z-scheme charge transfer mechanism in g-C3N4/Bi2WO6/AgI nanohybrid; reproduced with permission from Elsevier (Licence no. 4751171004447) (Xue et al., 2019).
Xue et al. explored a ternary g-C3N4/Bi2WO6/AgI Z-scheme photocatalytic system with improved photocatalytic activity towards the exclusion of TC. TEM analysis (Fig. 18b) depicted the microspheres of Bi2WO6, and it was observed that AgI NPs were unevenly deposited on g-C3N4 surface, with the existence of an active link among g-C3N4, AgI as well as Bi2WO6. Fig. 18c illustrated the proposed mechanism for photodegradation of organic pollutants. Under visible light excitation, the photo-illuminated e−CB of Bi2WO6 recombines with h+VB of g-C3N4 and AgI, resulting in spatial charge separation of photo-induced EHP. The transference of photo-charge carriers leads to retaining of e− at CB of g-C3N4 and AgI, while h+ remains accumulated at the VB of Bi2WO6, respectively. Owing to more negative CB energies of g-C3N4 (−0.913 eV) and AgI (−0.503 eV) than O2/•O2− (−0.33 eV vs. NHE), the photo-induced e−CB of g-C3N4 and AgI could reduce O2 to •O2−. Moreover, photo-induced h+ of Bi2WO6 can activate H2O molecules to produce •OH due to less negative VB potential of Bi2WO6 (3.147 eV) than that of H2O/•OH (+2.72 eV) (Xue et al., 2019). A dual Z-scheme g-C3N4/Ag3PO4/AgI photocatalyst with improved photocatalytic ability for removal of neonicotinoid pesticide, was explored (Tang et al., 2019b). In other work, Zhang et al. fabricated a ternary photocatalyst with greater photocatalytic activities for the elimination of NOF (Zhang et al., 2019).
4 Multifunctional applications
4.1 Reduction of carbon dioxide (CO2) to useful hydrocarbon fuels
A novel Z-scheme AgCl@g-C3N4 nanocomposite was fabricated via deposition-precipitation routes, thereby, loading different ratios of AgCl on g-C3N4 surface. The photocatalytic ability of as-prepared sample was investigated under visible light eradication for CO2 reduction in an aqueous medium (Fig. 19a). The VB and CB potentials for g-C3N4 were located at +1.58 eV and −1.12 eV, correspondingly, while for AgCl, these are located at 3.2 eV and −0.06 eV, respectively. As a result of a broad bandgap, AgCl (∼3.26 eV) could not absorb visible light. Attributable to the LSPR effect, Ag0 NPs absorb visible light, thereby producing EHP. Consequently, photo-generated e− can migrate from Ag0 NPs to AgCl hybrid. Thus the accumulated particles in AgCl CB cannot photo-reduce CO2 to methane (CH4), acetic acid (CH3COOH), and formic acid (HCOOH). Since, CB potentials of AgCl (−0.06 eV vs. NHE) is more favourable than the typical redox potential of CO2 to CH4 (−0.24 V Vs. NHE), CH3COOH (−0.31 V) and HCOOH (−0.58 V vs. NHE). The accumulated e− on the surface of AgCl can directly associate with the h+VB of g-C3N4. The spatial charge separation of photo-illuminated EHP does not follow a conservative double-transferal approach. Therefore, a possible Z-scheme mechanism for the reduction process has been suggested. The surface e− of AgCl directly associates with h+VB of g-C3N4. Meanwhile, the photo-generated e−CB of g-C3N4 (−1.12 eV) could react with adsorbed CO2 to reduce CH4, CH3COOH, and HCOOH. It can be inferred that the Z-scheme charge transference path in AgCl@g-C3N4 nanocomposite can effectually lower the recombination of photo-charge carriers (Murugesan et al., 2018b).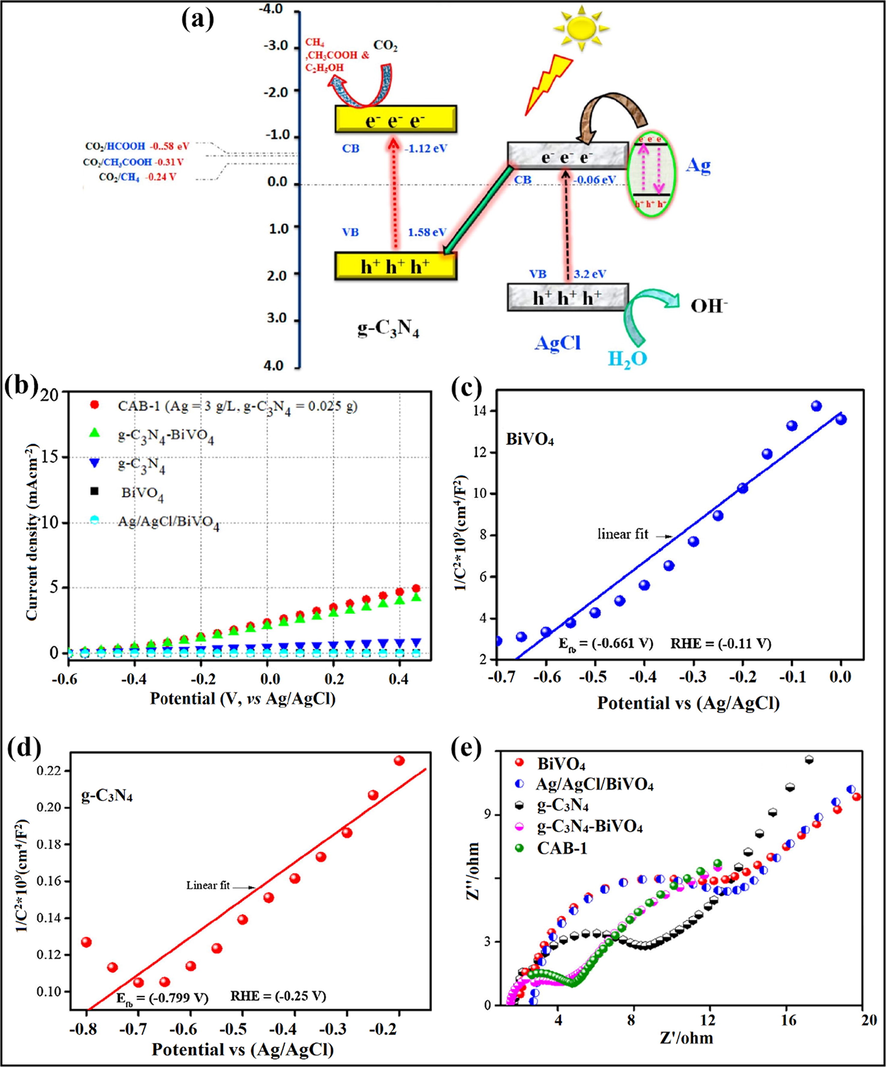
(a) Schematic representation of Z-scheme charge transferal mechanism in AgCl@g-C3N4 nanocomposite; reproduced with permission from Elsevier (Licence no. 4751201294275) (Murugesan et al., 2018a, 2018b), photocurrent density of photocatalyst in (b) Absence of light, Mott-Schottky slopes of (c) BiVO4, (d) g-C3N4, and (e) Nyquist plot of as-fabricated photocatalyst; reprinted with permission from Elsevier (Licence no. 4752980086692) (Rather et al., 2018).
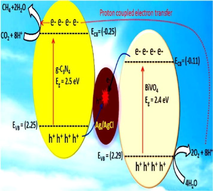
Rather et al. explored g-C3N4/Ag/AgCl/BiVO4 (CAB) composite for photo-reduction of CO2 to CH4 under alkali activation. The morphology of the CAB microstructure was reliant on anchoring of AgNO3 and g-C3N4, as explained by SEM studies. Pristine BiVO4 and g-C3N4 own sheet-like structure, while Ag/AgCl nanocrystals were formed by deposition of Ag0 and precipitation of AgCl onto BiVO4. The XPS spectra revealed the existence of all probable elements in the g-C3N4/Ag/AgCl/BiVO4 microstructure. The chemical shift near reduced binding energies was perceived after achieving the photocatalytic CO2 reduction, and downshift was observed because of the integration of sodium (Na) after alkali treatment. The interfacial possessions of the nanocomposite were scrutinized by observing their photoelectrochemical cell (PEC) response employing linear sweep voltammetry (LSV) technique mutually in the existence and nonexistence of light. CAB-1 (5.03 mA cm−1) and g-C3N4-BiVO4 (4.1 mA cm−2) witnessed small currents in the absence of light, succeeded by g-C3N4 (0.97 mA cm−2), BiVO4 (0.03 mA cm−2), and Ag/AgCl/BiVO4 (0.03 mA cm−2) at a potential ranging between −0.6 V and 0.5 V, as depicted in Fig. 19b. After exposure to light (150 W) the photocurrent density improved instantaneously in CAB-1 (17.5 mA cm−2) proceeded by g-C3N4-BiVO4 (12.67 mA cm−2), Ag/AgCl-BiVO4 (5.24 mA cm−2), g-C3N4 (4.25 mA cm−2) and BiVO4 (0.91 mA cm−2). Also, the positive Mott-Schottky (MS) slopes (Fig. 19c and d) specified that both BiVO4 and g-C3N4 were n-type semiconducting materials, as revealed by LSV measurements (Tachikawa et al., 2016) and retained the similar properties after the construction of flower-shaped microstructure. The PEC outcomes are in arrangement with EIS calculations. Nyquist studies (Fig. 19e) signified reduced charge transferal resistance (Rct) and segregation of photo-induced charge haulers in the as-prepared microstructure. g-C3N4/Ag/AgCl/BiVO4 composite follows the Z-scheme mechanism for the photocatalytic alteration of CO2 to CH4, where Ag/AgCl act as an e− sink. Both BiVO4 and g-C3N4 were excited under visible light resulting in the generation of h+ and e− in their particular EVB and ECB. Ag and AgCl were present in vicinity; the polarization effect of AgCl inclines to alter the charge density of Ag, followed by generation of various partial positive as well as negatively charged regions on its surface. Moreover, the Schottky barrier formed among BiVO4 and Ag/AgCl leads to the smooth flow of e−CB of BiVO4 onto Ag/AgCl surface. The h+VB of g-C3N4 transferred towards Ag/AgCl to eliminate the inward e−CB of BiVO4 and to counteract the polarization effect of AgCl. This mechanistic insight helped in the dissociation of h+ in EVB of BiVO4 and e− in ECB of g-C3N4 to accomplish the photocatalytic reaction. The water oxidation takes place at VB of BiVO4 to generate active protons and these protons couples through e− from ECB of g-C3N4 to reduce CO2 into CH4 (Rather et al., 2018).
4.2 Hydrogen (H2) evolution by OWS
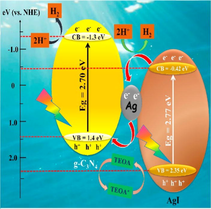
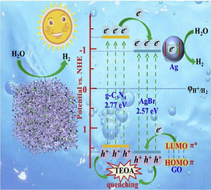
Li et al. tailored g-C3N4-Ag/AgBr (CNAA) heterostructure via solvothermal technique. N2 adsorption-desorption isotherms confirmed that g-C3N4 and CNAA own H3 kind of hysteretic hoop, which specified that porous arrangements were initiated from an accumulation of lamellar assemblies. Also, BET surface areas of g-C3N4 and CNAA were found to be 69.71 m2/g and 17.16 m2/g, correspondingly. The reduced BET surface area was because of the presence of AgBr NPs, which may obstruct the pore structure of g-C3N4 NS for apt particle size, shown in Fig. 20a. EDX mappings were employed to explore the elemental configuration of CNAA surface and confirmed the existence of C, N, Ag, and Br elements. UV–Vis DRS spectra (Fig. 20b) demonstrated that AgBr was in-situ deposited on the surface of g-C3N4 to enhance the light response. The detailed mechanism (Fig. 20c) explicated the transference of e−CB of g-C3N4 to more negative e−CB of AgBr, followed by the migration of photo-excited e− to Ag plasma. The hydrogen evolution rate (HER) (H2O + e− → H2) for high-density e−CB of AgBr, as well as Ag plasmas, can easily induce greater negative potential than hydrogen reduction potentials (φH2/H+). Instantaneously, h+VB of g-C3N4 and AgBr were straightaway apprehended and quenched by additional TEOA, thus obstructing the recombination of photo-carriers, thereby producing oxidative active radicals (Li et al., 2019). Li et al. explored construction g-C3N4/graphene oxide-Ag/AgBr Z-scheme composite for effective H2 evolution (Li et al., 2019). Another work by Bai and the research group reported Ag/AgX (X = Cl, Br, I)/g-C3N4 nanocomposite for photocatalytic H2 evolution (Bai et al., 2019).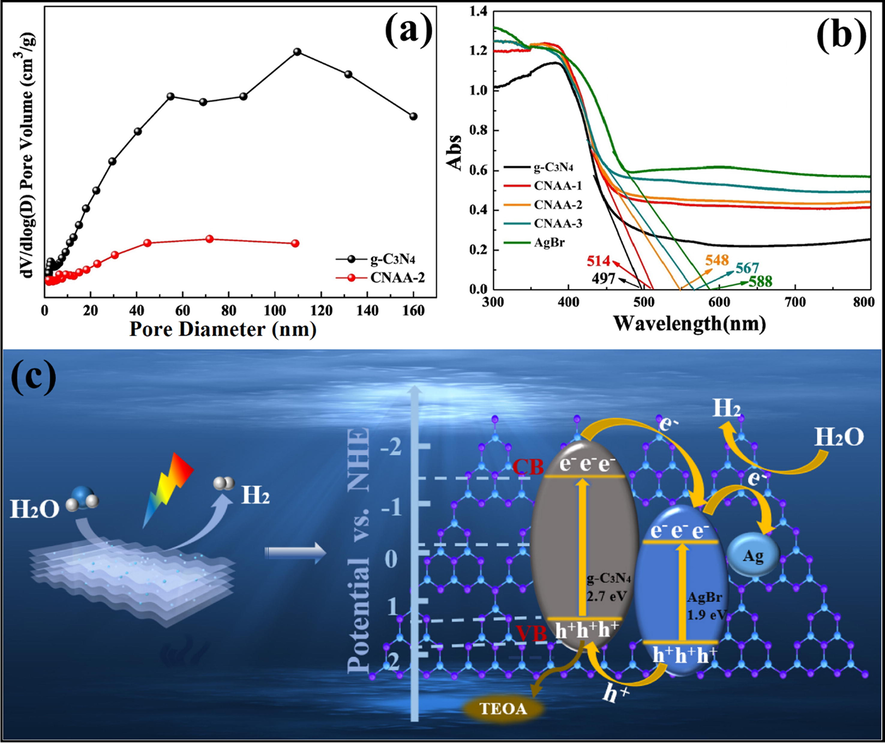
(a) BET surface area of g-C3N4-Ag/AgBr nano-heterostructure, (b) UV–Visible spectra revealing deposition of AgBr onto g-C3N4 NS, and (c) Mechanistic insight of charge transfer and generation of reactive radical species in g-C3N4-Ag/AgBr nanohybrid; reproduced with permission from Elsevier (Licence no. 4752990867947) (Li et al., 2019).
4.3 Microbial inactivation
Deng et al. synthesized g-C3N4-AgBr composite by anchoring AgBr NPs on the g-C3N4 surface for photocatalytic bacterial disinfection. The antibacterial activities for Gramnegative strain Escherichia coli (E. coli) and Gram-positive strain Staphylococcus aureus (S. aureus) were analyzed under illumination of solar light. Comprehensive deactivation of 3 × 106 CFU/mL feasible cell density was fulfilled in 60 min for E. coli and 150 min for S. aureus, correspondingly. During photocatalytic process, Ag+ released does not take part in disinfection activities. The photocatalytic microbial inactivation of g-C3N4-xAgBr (x = 0.5, 1, 2, 4) headed for E. coli was explored. g-C3N4-AgBr wholly deactivated 6.5 log E. coli in 60 min, whereas 1.2 log, 2.0 log, and 1.67 log reduction of feasible cell density were detected for g-C3N4-0.5 AgBr, g-C3N4-2 AgBr, and g-C3N4-4 AgBr within the elongated reaction time of 70 min, respectively. No considerable modification of viable cell density of Gram-positive S. aureus was perceived for the shady as well as blank control experimentations. The results suggested that the feasibility of S. aureus would be affected by either visible light emission or the direct contact with g-C3N4-AgBr NPs under dark surroundings. Minimal inactivation activities toward S. aureus were perceived for bulk g-C3N4 owing to its elevated rate of recombination. Comparable to E. coli, g-C3N4-AgBr photocatalyst unveiled exceptional germicidal effects to S. aureus. Deactivation kinetics of g-C3N4-AgBr for S. aureus was somewhat slower than that of E. coli (Deng et al., 2017).
Murugesan and research group investigated AgI@g-C3N4 photocatalyst exhibiting enhanced antimicrobial properties prepared by the deposition-precipitation approach. The photocatalytic performance of AgI@g-C3N4 nanocomposite for bacterial disinfection of E. Coli was studied employing the disc diffusion process (Murugesan et al., 2018a, 2018b). In another work, Raizada et al. explored highly efficient photocatalyst with superior visible light activity for pollutant exclusion and bacterial disinfection (Raizada et al., 2019d).
5 Conclusion and perspectives
Over past years, photocatalysis has been established as most alluring technologies that have spurred massive attention of investigators for various environmental remediations. A photocatalyst must possess physio-chemical stability, optimum redox potential, and more excellent solar light absorption for several applications. A multifunctional metal-free g-C3N4 has been developed as a cost-effective photocatalytic material since 2009, owing to its chemical stability, non-toxic nature, л-conjugated, and highly porous structure, facile preparation, and improved charge transference and separation. The electronic structure endows its potential implementations as a catalyst. The e−CB of g-C3N4 possesses the superior reducing ability, which can be employed for photocatalytic water splitting, H2 evolution, reduction of CO2 to hydrocarbon fuels, bacterial decontamination, and pollutant mineralization. The cornucopia possessions linked with g-C3N4 nanomaterial have unusually improved its photocatalytic efficacy. Moreover, for bulk g-C3N4, it would be problematic to own high stability, effective spatial charge separation, superior absorption range, and redox ability, which bounds its practical use. To overcome these deficits, specific criteria have to be satisfied these are; (i) natural light-harvesting (complete utilization of solar energy), (ii) facilitated the separation of photo-generated charge carriers, and (iii) enhancing surface area for surface adsorption and reaction. Moreover, g-C3N4 can be simply prepared via thermal polymerization of a nitrogen-rich precursor. Apart, g-C3N4 sheets act as deposition sites for the loading of NPs to form a hybrid system. Plasmonic photocatalyst composed of noble metal NPs (Ag, Au) revealed more excellent absorption in the visible range, which may be attributable to their strong SPR effect. Also, Ag NPs were preferred over Au NPs owing to their cost effectiveness comparable to Au NPs. We have encapsulated recent progressions on Ag-based compounds due to their potential applications in catalysis, biosensing, and biomedicines. To date, ample investigations based on AgX (X = Cl, Br, I) have been explored due to its various photocatalytic applications utilizing solar light for environmental restoration and energy output. However, due to the reactive nature of fluorine, AgF has demised its applicability as photocatalyst. Plasmonic AgX possessing antibacterial properties have emerged as photosensitive material with prolonged visible light absorption. Nevertheless, AgX being wide-ranging semiconductors, they have evolved as visible-light-driven photocatalyst. Due to strong photosensitivity, AgX undergoes a reduction of Ag+ to Ag° under exposure to visible light, thereby decreasing the stability and lifetime. AgX has wide-spread implementations in photographic films as well as used as photosensitizers and a metallic precursor for silver. However, Ag-based semiconductors were unstable and photochemically corrosive, restricting the practical applications of AgX as photocatalytic material. Also, bare AgX NPs can easily agglomerate any may cause decreased efficiency of a photocatalyst.
The evolution of nano-junctions by hybridizing a semiconductor with another semiconducting material of almost comparable energy, as co-catalyst to form various heterostructures, has been regarded as an advanced paradigm towards boosted photocatalytic efficiency. A tremendous work emphasizing AgX/g-C3N4 (X = Cl, Br, I) nanoarchitectures has been reported to date. g-C3N4 grafted AgX heterojunctions could depress spatial charge separation, thereby boosting visible light absorption. Moreover, g-C3N4 tailored AgX nano-junctions can be employed for various practical implementations, for instance, overall water splitting to H2 and O2, CO2 conversion to energy fuels, toxin removal, and microbial decontamination. In the focus of this review, we have divided heterostructures into two main classes, accurately type-ΙΙ heterojunctions and Z-scheme heterojunction process. Several reports based on type-ΙΙ and Z-scheme found AgX/g-C3N4 nanostructures exploring photodegradation of organic pollutants have been discussed in this review. At the same time, multifunctional applications for g-C3N4 anchored AgX nanohybrids have also been analyzed.
6 Challenges and outlooks
Despite admirable results stated, the investigations in this arena are still in initial phases, and more advances are prominently necessitated as discussed underneath;
The work suffers from lower efficacy as well as inferior stability of nanohybrid, which are distant from the necessities of industrial needs.
Advanced characterization of interfacial imageries remains a challenge for investigators. Subsequently, the decay of Ag-based compounds is often unpreventable when illuminated to a high energy e− beam.
For extensive fabrication of a nano photocatalyst, an expedient and facile approach is the Holy Grail.
Theoretically, the charge transfer and separation pathway could be well understood, but more experimental pieces of evidence are necessary to support these photochemical mechanisms and provided evidences to construct an effectual photocatalytic system.
Systematic estimation of photocatalytic material in a comprehensive way should be examined, for example overall waste water splitting, organic synthesis, and conversions, which usually occur by UV excitation.
Visible light assisted photocatalyst employed for various practical applications for environmental clean-up, utilizing green energy and light-reaping devices, there is still a long way to go.
So far, several issues need to be fixed for energy to fuel cells alteration before employing at commercial scale, to increase the quantum yield and reaction efficiency
Lesser absorption of solar light is the bottleneck of a photocatalytic material inhibiting the lifetime of the photocatalyst in everyday implementations.
The photo corrosive nature of Ag-based compounds owing to the reduction of Ag+ to Ag° is the obstruction limiting its potential applications.
Therefore, achievements and contributions in this field of exploration have aimed at a scientific community for commercial applications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel magnetically separable γ-Fe2O3/Ag/AgCl/g-C3N4 composite for enhanced disinfection under visible light. Colloids Surf., A. 2019;583:123981
- [Google Scholar]
- One-pot hydrothermal synthesis of g-C3N4/Ag/AgCl/BiVO4 micro-flower composite for the visible light degradation of ibuprofen. Chem. Eng. J.. 2018;341:248-261.
- [Google Scholar]
- Novel magnetic g-C3N4/Fe3O4/AgCl nanocomposites: Facile and large-scale preparation and highly efficient photocatalytic activities under visible-light irradiation. Mater. Sci. Semicond. Process.. 2015;39:162-171.
- [Google Scholar]
- Novel magnetically separable g-C3N4/AgBr/Fe3O4 nanocomposites as visible-light-driven photocatalysts with highly enhanced activities. Ceram. Int.. 2015;41(4):5634-5643.
- [Google Scholar]
- Ternary g-C3N4/ZnO/AgCl nanocomposites: synergistic collaboration on visible-light-driven activity in photodegradation of an organic pollutant. Appl. Surf. Sci.. 2015;358:261-269.
- [Google Scholar]
- Codeposition of AgI and Ag2CrO4 on g-C3N4/Fe3O4 nanocomposite: Novel magnetically separable visible-light-driven photocatalysts with enhanced activity. Adv. Powder Technol.. 2016;27(6):2496-2506.
- [Google Scholar]
- Novel g-C3N4/Ag2SO4 nanocomposites: fast microwave-assisted preparation and enhanced photocatalytic performance towards degradation of organic pollutants under visible light. J. Colloid Interface Sci.. 2016;482:165-174.
- [Google Scholar]
- Ternary magnetic g-C3N4/Fe3O4/AgI nanocomposites: novel recyclable photocatalysts with enhanced activity in degradation of different pollutants under visible light. Mater. Chem. Phys.. 2016;174:59-69.
- [Google Scholar]
- Enhanced visible light photocatalytic activities of template free mesoporous nitrogen doped reduced graphene oxide/titania composite catalysts. J. Ind. Eng. Chem.. 2016;36:184-193.
- [Google Scholar]
- Plasmonic enhancement of photoactivity by gold nanoparticles embedded in hematite films. J. Phys. Che. C. 2015;119(27):15506-15516.
- [Google Scholar]
- Decoration of carbon dots and AgCl over g-C3N4 nanosheets: novel photocatalysts with substantially improved activity under visible light. Sep. Purif. Technol.. 2018;199:64-77.
- [Google Scholar]
- A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc.. 2008;130(5):1676-1680.
- [Google Scholar]
- Dramatic visible photocatalytic performance of g-C3N4-based nanocomposite due to the synergistic effect of AgBr and ZnO semiconductors. J. Phys. Chem. Solids. 2018;122:174-183.
- [Google Scholar]
- In-situ solid-phase fabrication of Ag/AgX (X= Cl, Br, I)/g-C3N4 composites for enhanced visible-light hydrogen evolution. Int. J. Hydrogen Energy. 2019;44(39):21397-21405.
- [Google Scholar]
- New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett.. 2014;5(15):2543-2554.
- [Google Scholar]
- AgCl/Ag/g-C3N4 hybrid composites: preparation, visible light-driven photocatalytic activity and mechanism. Nano-micro Lett.. 2016;8(2):182-192.
- [Google Scholar]
- Novel ternary g-C3N4/Ag3VO4/AgBr nanocomposites with excellent visible-light-driven photocatalytic performance for environmental applications. Solid State Sci.. 2018;78:133-143.
- [Google Scholar]
- Combination of Ag2CrO4 and AgI semiconductors with g-C3N4: Novel nanocomposites with substantially improved photocatalytic performance under visible light. Solid State Sci.. 2018;77:62-73.
- [Google Scholar]
- Ionothermal synthesis of crystalline, condensed, graphitic carbon nitride. Chem.–A Eur. J.. 2008;14(27):8177-8182.
- [Google Scholar]
- Visible/solar light active photocatalysts for organic effluent treatment: Fundamentals, mechanisms and parametric review. Renew. Sustain. Energy Rev.. 2017;76:1393-1421.
- [Google Scholar]
- Ag/AgBr/g-C3N4: A highly efficient and stable composite photocatalyst for degradation of organic contaminants under visible light. Mater. Res. Bull.. 2013;48(10):3873-3880.
- [Google Scholar]
- Carbon-based H2-production photocatalytic materials. J. Photochem. Photobiol., C. 2016;27:72-99.
- [Google Scholar]
- Magnetically separable ZnO/ZnFe2O4 and ZnO/CoFe2O4 photocatalysts supported onto nitrogen doped graphene for photocatalytic degradation of toxic dyes. Arabian J. Chem.. 2020;13:4324-4340.
- [Google Scholar]
- In situ ionic-liquid-assisted synthesis of plasmonic photocatalyst Ag/AgBr/g-C3N4 with enhanced visible-light photocatalytic activity. Catal. Today. 2015;258:41-48.
- [Google Scholar]
- Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: a review. J. Alloy. Compd.. 2015;649:910-932.
- [Google Scholar]
- Fabrication of a three-dimensional porous Z-scheme silver/silver bromide/graphitic carbon nitride@ nitrogen-doped graphene aerogel with enhanced visible-light photocatalytic and antibacterial activities. J. Colloid Interface Sci.. 2019;536:389-398.
- [Google Scholar]
- Recent developments in photocatalytic water treatment technology: a review. Water Res.. 2010;44(10):2997-3027.
- [Google Scholar]
- Enhanced visible-light-driven photocatalytic bacteria disinfection by g-C3N4-AgBr. Colloids Surf., B. 2017;152:49-57.
- [Google Scholar]
- The comparative jurisprudence of catalysts preparation methods: I. Precipitation and impregnation methods. J Ind Environ Chem.. 2018;2(1):19-21.
- [Google Scholar]
- The preparation and properties of a g-C3N4/AgBr nanocomposite photocatalyst based on protonation pretreatment. New J. Chem.. 2015;39(2):1132-1138.
- [Google Scholar]
- Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238(5358):37-38.
- [Google Scholar]
- Superparamagnetic MnFe2O4 dispersed over graphitic carbon sand composite and bentonite as magnetically recoverable photocatalyst for antibiotic mineralization. Sep. Purif. Technol.. 2017;172:498-511.
- [Google Scholar]
- Solar photocatalytic mineralization of antibiotics using magnetically separable NiFe2O4 supported onto graphene sand composite and bentonite. J. Water Process Eng.. 2016;14:86-100.
- [Google Scholar]
- A highly efficient Ag-ZnO photocatalyst: synthesis, properties, and mechanism. J. Phys. Chem. C. 2008;112(35):13563-13570.
- [Google Scholar]
- Graphitic carbon nitride based Z scheme photocatalysts: Design considerations, synthesis, characterization and applications. J. Ind. Eng. Chem.. 2019;79:383-408.
- [Google Scholar]
- The relevance of light in the formation of colloidal metal nanoparticles. Chem. Soc. Rev.. 2014;43(7):2089-2097.
- [Google Scholar]
- Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: A review. Appl. Mater. Today. 2019;15:494-524.
- [Google Scholar]
- Fabrication of Ag/AgI/WO3 heterojunction anchored P and S co-doped graphitic carbon nitride as a dual Z scheme photocatalyst for efficient dye degradation. Solid State Sci. 2019106095
- [Google Scholar]
- Carbon quantum dots supported AgI/ZnO/phosphorus doped graphitic carbon nitride as Z-scheme photocatalyst for efficient photodegradation of 2, 4-dinitrophenol. J. Environ. Chem. Eng.. 2019;7(4):103272
- [Google Scholar]
- Highly efficient p-type Cu3P/n-type g-C3N4 photocatalyst through Z-scheme charge transfer route. Appl. Catal. B. 2019;240:253-261.
- [Google Scholar]
- Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc.. 2006;128(6):2115-2120.
- [Google Scholar]
- Predictive model for the design of plasmonic metal/semiconductor composite photocatalysts. ACS Catal.. 2011;1(10):1441-1447.
- [Google Scholar]
- An oxygen-vacancy rich 3D novel hierarchical MoS2/BiOI/AgI ternary nanocomposite: enhanced photocatalytic activity through photogenerated electron shuttling in a Z-scheme manner. PCCP. 2016;18(36):24984-24993.
- [Google Scholar]
- Melem (2, 5, 8-triamino-tri-s-triazine), an important intermediate during condensation of melamine rings to graphitic carbon nitride: Synthesis, structure determination by X-ray powder diffractometry, solid-state NMR, and theoretical studies. J. Am. Chem. Soc.. 2003;125(34):10288-10300.
- [Google Scholar]
- Functional carbon nitride materials—design strategies for electrochemical devices. Nat. Rev. Mater.. 2017;2(6):17030.
- [Google Scholar]
- Tri-s-triazine derivatives. Part I. From trichloro-tri-s-triazine to graphitic C3N4 structures. New J. Chem.. 2002;26(5):508-512.
- [Google Scholar]
- Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J.. 2019;123496
- [Google Scholar]
- Synthesis of surface molecular imprinted TiO2/graphene photocatalyst and its highly efficient photocatalytic degradation of target pollutant under visible light irradiation. Appl. Surf. Sci.. 2016;390:368-376.
- [Google Scholar]
- High performance visible light driven photocatalysts silver halides and graphitic carbon nitride (X= Cl, Br, I) nanocomposites. J. Colloid Interface Sci.. 2013;395:75-80.
- [Google Scholar]
- Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev.. 2014;43(1):473-486.
- [Google Scholar]
- Highly efficient visible-light photocatalytic performance based on novel AgI/g-C3N4 composite photocatalysts. Chem. Phys. Lett.. 2016;664:167-172.
- [Google Scholar]
- Intercorrelated superhybrid of AgBr supported on graphitic-C3N4-decorated nitrogen-doped graphene: high engineering photocatalytic activities for water purification and CO2 reduction. Adv. Mater.. 2015;27(43):6906-6913.
- [Google Scholar]
- Ag@ AgCl QDs decorated g-C3N4 nanoplates: the photoinduced charge transfer behavior under visible light and full arc irradiation. Appl. Surf. Sci.. 2017;422:626-637.
- [Google Scholar]
- Hydrogen evolution by catalyzing water splitting on two-dimensional g-C3N4-Ag/AgBr heterostructure. Appl. Surf. Sci.. 2019;494:275-284.
- [Google Scholar]
- Construction of Z-scheme and pn heterostructure: Three-dimensional porous g-C3N4/graphene oxide-Ag/AgBr composite for high-efficient hydrogen evolution. Appl. Catal. B. 2020;268:118384
- [Google Scholar]
- Ueber mellon und mellonverbindungen. Justus Liebigs Annalen der Chemie. 1844;50(3):337-363.
- [Google Scholar]
- Phosphorus-doped graphitic carbon nitride nanotubes with amino-rich surface for efficient CO2 capture, enhanced photocatalytic activity, and product selectivity. ACS Appl. Mater. Interfaces. 2018;10(4):4001-4009.
- [Google Scholar]
- Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method. Appl. Phys. Lett.. 2011;99(19):191903
- [Google Scholar]
- An AgI@ g-C3N4 hybrid core@ shell structure: stable and enhanced photocatalytic degradation. Appl. Surf. Sci.. 2015;358:319-327.
- [Google Scholar]
- Preparation of Ag@ AgCl/g-C3N4/TiO2 porous ceramic films with enhanced photocatalysis performance and self-cleaning effect. Ceram. Int.. 2018;44(8):9326-9337.
- [Google Scholar]
- Decontamination and disinfection of water by solar photocatalysis: recent overview and trends. Catal. Today. 2009;147(1):1-59.
- [Google Scholar]
- Graphitic carbon nitride (g-C3N4) nanocomposites: a new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B. 2016;198:347-377.
- [Google Scholar]
- Carbon dot sensitized integrative g-C3N4/AgCl Hybrids: An synergetic interaction for enhanced visible light driven photocatalytic process. Adv. Powder Technol.. 2019;30(8):1715-1723.
- [Google Scholar]
- g-C3N4/AgBr nanocomposite decorated with carbon dots as a highly efficient visible-light-driven photocatalyst. J. Colloid Interface Sci.. 2017;502:24-32.
- [Google Scholar]
- Fabrication of an all solid Z-scheme photocatalyst g-C3N4/GO/AgBr with enhanced visible light photocatalytic activity. Appl. Catal. A. 2017;539:104-113.
- [Google Scholar]
- Evaluation of advanced oxidation processes for water and wastewater treatment–a critical review. Water Res.. 2018;139:118-131.
- [Google Scholar]
- Synthesis and characterization of AgCl@ graphitic carbon nitride hybrid materials for the photocatalytic degradation of atrazine. Ceram. Int.. 2015;41(1):1197-1204.
- [Google Scholar]
- Novel magnetically separable g-C3N4/Fe3O4/Ag3PO4/Co3O4 nanocomposites: visible-light-driven photocatalysts with highly enhanced activity. Adv. Powder Technol.. 2017;28(6):1540-1553.
- [Google Scholar]
- Decoration of Fe3O4 and CoWO4 nanoparticles over graphitic carbon nitride: novel visible-light-responsive photocatalysts with exceptional photocatalytic performances. Mater. Res. Bull.. 2018;105:159-171.
- [Google Scholar]
- Magnetically recoverable highly efficient visible-light-active g-C3N4/Fe3O4/Ag2WO4/AgBr nanocomposites for photocatalytic degradations of environmental pollutants. Adv. Powder Technol.. 2018;29(1):94-105.
- [Google Scholar]
- A direct Z-scheme plasmonic AgCl@ g-C3N4 heterojunction photocatalyst with superior visible light CO2 reduction in aqueous medium. Appl. Surf. Sci.. 2018;450:516-526.
- [Google Scholar]
- Photocatalytic performance and antibacterial activity of visible light driven silver iodide anchored on Graphitic-C3N4 binary composite. Environ. Nanotechnol. Monit. Manage.. 2018;10:253-263.
- [Google Scholar]
- Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem. Rev.. 2016;116(12):7159-7329.
- [Google Scholar]
- A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991;353(6346):737.
- [Google Scholar]
- Pare, B., Singh, P., Jonnalgadda, S.B., 2009. Degradation and mineralization of victoria blue B dye in a slurry photoreactor using advanced oxidation process.
- Tunable photocatalytic activity of SrTiO3 for water splitting: Strategies and Future scenario. J. Environ. Chem. Eng.. 2020;103791
- [Google Scholar]
- Evaluation of a multi-dimensional hybrid photocatalyst for enrichment of H2 evolution and elimination of dye/non-dye pollutants. Catal. Sci. Technol.. 2017;7(12):2579-2590.
- [Google Scholar]
- Efficient visible-light photocatalytic water splitting by minute amounts of gold supported on nanoparticulate CeO2 obtained by a biopolymer templating method. J. Am. Chem. Soc.. 2011;133(18):6930-6933.
- [Google Scholar]
- Photocatalytic mineralization and degradation kinetics of ampicillin and oxytetracycline antibiotics using graphene sand composite and chitosan supported BiOCl. J. Mol. Catal. A: Chem.. 2016;423:400-413.
- [Google Scholar]
- Preparation and photocatalytic activity of hydroxyapatite supported BiOCl nanocomposite for oxytetracyline removal. Adv. Mater. Lett. 2016;7(4):312-318.
- [Google Scholar]
- Graphitic carbon nitride-based new advanced materials for photocatalytic applications. Curr. Anal. Chem.. 2020;16:1.
- [Google Scholar]
- Magnetically retrievable Bi2WO6/Fe3O4 immobilized on graphene sand composite for investigation of photocatalytic mineralization of oxytetracycline and ampicillin. Process Saf. Environ. Prot.. 2017;106:104-116.
- [Google Scholar]
- Kinetics of photocatalytic mineralization of oxytetracycline and ampicillin using activated carbon supported ZnO/ZnWO4. Desalin. Water Treat.. 2017;79:204-213.
- [Google Scholar]
- Solar light-facilitated oxytetracycline removal from the aqueous phase utilizing a H2O2/ZnWO4/CaO catalytic system. J. Taibah Univ. Sci.. 2017;11(5):689-699.
- [Google Scholar]
- Solar photocatalytic activity of nano-ZnO supported on activated carbon or brick grain particles: role of adsorption in dye degradation. Appl. Catal. A. 2014;486:159-169.
- [Google Scholar]
- Silver-mediated Bi2O3 and graphitic carbon nitride nanocomposite as all solid state Z scheme photocatalyst for imidacloprid pesticide abatement from water. Desalin. Water Treat.. 2019;171:344-355.
- [Google Scholar]
- Converting type II AgBr/VO into ternary Z scheme photocatalyst via coupling with phosphorus doped g-C3N4 for enhanced photocatalytic activity. Sep. Purif. Technol. 2019:115692.
- [Google Scholar]
- Ag3PO4 modified phosphorus and sulphur co-doped graphitic carbon nitride as a direct Z-scheme photocatalyst for 2, 4-dimethyl phenol degradation. J. Photochem. Photobiol., A. 2019;374:22-35.
- [Google Scholar]
- Fabrication of Ag3VO4 decorated phosphorus and sulphur co-doped graphitic carbon nitride as a high-dispersed photocatalyst for phenol mineralization and E. coli disinfection. Sep. Purif. Technol.. 2019;212:887-900.
- [Google Scholar]
- Visible light assisted photodegradation of 2, 4-dinitrophenol using Ag2CO3 loaded phosphorus and sulphur co-doped graphitic carbon nitride nanosheets in simulated wastewater. Arabian J. Chem.. 2020;13:3196-3209.
- [Google Scholar]
- Fabrication of dual Z-scheme photocatalyst via coupling of BiOBr/Ag/AgCl heterojunction with P and S co-doped g-C3N4 for efficient phenol degradation. Arabian J. Chem.. 2020;13:4538-4552.
- [Google Scholar]
- High charge transfer response of g-C3N4/Ag/AgCl/BiVO4 microstructure for the selective photocatalytic reduction of CO2 to CH4 under alkali activation. J. Catal.. 2018;366:28-36.
- [Google Scholar]
- Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem.. 2013;15(7):1814-1833.
- [Google Scholar]
- Fabrication of fluorine doped graphene and SmVO4 based dispersed and adsorptive photocatalyst for abatement of phenolic compounds from water and bacterial disinfection. J. Cleaner Prod.. 2018;203:386-399.
- [Google Scholar]
- Islanding of EuVO4 on high-dispersed fluorine doped few layered graphene sheets for efficient photocatalytic mineralization of phenolic compounds and bacterial disinfection. J. Taiwan Inst. Chem. Eng.. 2018;93:528-542.
- [Google Scholar]
- GdVO4 modified fluorine doped graphene nanosheets as dispersed photocatalyst for mitigation of phenolic compounds in aqueous environment and bacterial disinfection. Sep. Purif. Technol.. 2019;210:804-816.
- [Google Scholar]
- Synthesis of Eu3+-doped ZnO/Bi2O3 heterojunction photocatalyst on graphene oxide sheets for visible light-assisted degradation of 2, 4-dimethyl phenol and bacteria killing. Solid State Sci.. 2020;106164
- [Google Scholar]
- Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Cleaner Prod. 2019
- [Google Scholar]
- Band gap tuning of g-C3N4 via decoration with AgCl to expedite the photocatalytic degradation and mineralization of oxalic acid. J. Environ. Sci.. 2019;84:1-12.
- [Google Scholar]
- Graphene bentonite supported ZnFe2O4 as superparamagnetic photocatalyst for antibiotic degradation. Adv. Mater. Lett.. 2017;8(3):229-238.
- [Google Scholar]
- Photocatalytic mineralization of antibiotics using 60% WO3/BiOCl stacked to graphene sand composite and chitosan. Arabian J. Chem.. 2019;12(8):4627-4645.
- [Google Scholar]
- Solar-Fenton removal of malachite green with novel Fe0-activated carbon nanocomposite. Appl. Catal. A. 2014;476:9-18.
- [Google Scholar]
- Enhanced photocatalytic activity and stability of AgBr/BiOBr/graphene heterojunction for phenol degradation under visible light. J. Saudi Chem. Soc.. 2019;23(5):586-599.
- [Google Scholar]
- Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arabian J. Chem.. 2020;13(1):3498-3520.
- [Google Scholar]
- Systematic review on applicability of magnetic iron oxides–integrated photocatalysts for degradation of organic pollutants in water. Mater. Today Chem.. 2019;14:100186
- [Google Scholar]
- Hydrothermal synthesis of fine oxide powders. Bull. Mater. Sci.. 2000;23(6):453-460.
- [Google Scholar]
- Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem. 2018
- [Google Scholar]
- Magnetically recoverable graphitic carbon nitride and NiFe2O4 based magnetic photocatalyst for degradation of oxytetracycline antibiotic in simulated wastewater under solar light. J. Environ. Chem. Eng.. 2018;6(4):3874-3883.
- [Google Scholar]
- Fast electron transfer and enhanced visible light photocatalytic activity by using poly-o-phenylenediamine modified AgCl/g-C3N4 nanosheets. Chin. J. Catal.. 2019;40(1):80-94.
- [Google Scholar]
- Crystal-face-dependent charge dynamics on a BiVO4 photocatalyst revealed by single-particle spectroelectrochemistry. ACS Catal.. 2016;6(4):2250-2256.
- [Google Scholar]
- Constructing novel visible-light-driven ternary photocatalyst of AgBr nanoparticles decorated 2D/2D heterojunction of g-C3N4/BiOBr nanosheets with remarkably enhanced photocatalytic activity for water-treatment. Ceram. Int. 2019
- [Google Scholar]
- AgBr and g-C3N4 co-modified Ag2CO3 photocatalyst: a novel multi-heterostructured photocatalyst with enhanced photocatalytic activity. Appl. Surf. Sci.. 2017;391:440-448.
- [Google Scholar]
- Facile synthesis of dual Z-scheme g-C3N4/Ag3PO4/AgI composite photocatalysts with enhanced performance for the degradation of a typical neonicotinoid pesticide. Environ. Appl. Catal. B 2019:118395.
- [Google Scholar]
- Tate, E.W., 2012. Synthesis and Characterisation of Photocatalyst Silver/Silver Halide Nanocomposites
- Photochemical conversion of AgCl nanocubes to hybrid AgCl–Ag nanoparticles with high activity and long-term stability towards photocatalytic degradation of organic dyes. Can. J. Chem.. 2012;90(10):858-864.
- [Google Scholar]
- Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B. 2015;176:44-52.
- [Google Scholar]
- Novel visible-light-driven AgX/graphite-like C3N4 (X= Br, I) hybrid materials with synergistic photocatalytic activity. Appl. Catal. B. 2013;129:182-193.
- [Google Scholar]
- Ag/AgBr-grafted graphite-like carbon nitride with enhanced plasmonic photocatalytic activity under visible light. ChemCatChem. 2013;5(8):2343-2351.
- [Google Scholar]
- A plasmonic photocatalyst of Ag/AgBr nanoparticles coupled with g-C3N4 with enhanced visible-light photocatalytic ability. Colloids Surf., A. 2013;436:474-483.
- [Google Scholar]
- Assembly of AgI nanoparticles and ultrathin g-C3N4 nanosheets co-decorated Bi2WO6 direct dual Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced photocatalytic performance. Chem. Eng. J.. 2019;373:1144-1157.
- [Google Scholar]
- Performance and toxicity assessment of nanoscale zero valent iron particles in the remediation of contaminated soil: a review. Chemosphere 2018
- [Google Scholar]
- Recent progress of visible-light-driven heterogeneous photocatalysts for overall water splitting. Solid State Ionics. 2004;172(1–4):591-595.
- [Google Scholar]
- Fabrication of Z-scheme plasmonic photocatalyst Ag@ AgBr/g-C3N4 with enhanced visible-light photocatalytic activity. J. Hazard. Mater.. 2014;271:150-159.
- [Google Scholar]
- Characterization and mechanism analysis of AgBr mixed cuboid WO3 rods with enhanced photocatalytic activity. RSC Adv.. 2016;6(96):93436-93444.
- [Google Scholar]
- Synthesis of the Ag/AgCl/g-C3N4 composite with high photocatalytic activity under visible light irradiation. ChemCatChem. 2014;6(12):3409-3418.
- [Google Scholar]
- ACS Catal.. 2012;2:1677-1683.
- Recent advances in BiOX (X= Cl, Br and I) photocatalysts: synthesis, modification, facet effects and mechanisms. Environ. Sci. Nano. 2014;1(2):90-112.
- [Google Scholar]
- Nano-structured bismuth tungstate with controlled morphology: fabrication, modification, environmental application and mechanism insight. Chem. Eng. J.. 2019;358:480-496.
- [Google Scholar]
- Silver-melamine nanowire-assisted synthesis of net-like AgCl-Ag/g-C3N4 for highly efficient photocatalytic degradation ability. J. Alloy. Compd.. 2019;806:263-271.
- [Google Scholar]
- Ionothermal synthesis of triazine–heptazine-based copolymers with apparent quantum yields of 60% at 420 nm for solar hydrogen production from “Sea Water”. Angew. Chem. Int. Ed.. 2018;57(30):9372-9376.
- [Google Scholar]
- Fabrication of a novel AgI/LaFeO3/g-C3N4 dual Z-scheme photocatalyst with enhanced photocatalytic performance. Mater. Lett.. 2019;127029
- [Google Scholar]
- Synthesis of porous Bi2WO6 thin films as efficient visible-light-active photocatalysts. Adv. Mater.. 2009;21(12):1286-1290.
- [Google Scholar]
- Reconstruction of silver nanoplates by UV irradiation: tailored optical properties and enhanced stability. Angew. Chem. Int. Ed.. 2009;48(19):3516-3519.
- [Google Scholar]
- In situ ion exchange synthesis of strongly coupled Ag@ AgCl/g-C3N4 porous nanosheets as plasmonic photocatalyst for highly efficient visible-light photocatalysis. ACS Appl. Mater. Interfaces. 2014;6(24):22116-22125.
- [Google Scholar]
- Fabrication of novel visible-light-driven AgI/g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Colloid Interface Sci.. 2017;496:167-176.
- [Google Scholar]
- Self-cleaning particle coating with antireflection properties. Chem. Mater.. 2005;17(3):696-700.
- [Google Scholar]
- Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photocatalysts. Appl. Catal. B. 2017;202:620-641.
- [Google Scholar]
- Zhao, C., Yang, S. Y., Liu, Z. T., Fang, Y. P., 2012. AgCl Loaded Mesoporous Graphitic Carbon Nitride as Visible Light Photocatalyst. In: Advanced Materials Research, vol. 518. Trans Tech Publications, pp. 54–58.
- Emerging investigators series: advances and challenges of graphitic carbon nitride as a visible-light-responsive photocatalyst for sustainable water purification. Environ. Sci. Water Res. Technol.. 2017;3(6):982-1001.
- [Google Scholar]
- The synergistic effect of Ag/AgCl@ ZIF-8 modified g-C3N4 composite and peroxymonosulfate for the enhanced visible-light photocatalytic degradation of levofloxacin. Sci. Total Environ.. 2019;696:133962
- [Google Scholar]
- A novel ternary visible-light-driven photocatalyst AgCl/Ag3PO4/g-C3N4: synthesis, characterization, photocatalytic activity for antibiotic degradation and mechanism analysis. Catal. Commun.. 2017;100:191-195.
- [Google Scholar]
- In situ oxidation synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl/g-C3N4 and its activity. Ceram. Int.. 2014;40(7):9293-9301.
- [Google Scholar]
- Construction of 3D porous g-C3N4/AgBr/rGO composite for excellent visible light photocatalytic activity. Appl. Surf. Sci.. 2018;458:586-596.
- [Google Scholar]







