Translate this page into:
Exploring the anti-inflammatory effect of Fructus Gleditsia sinensis Lam., Fructus Gleditsiae abnormalis, and Gymnocladus chinensis Baill. using SPME-GC-MS, network pharmacology, and molecular docking
⁎Corresponding author at: Department of Pharmacy, Guizhou University of Traditional Chinese Medicine, Dongqing South Road, Huaxi District, Guiyang City, Guizhou Province, People’s Republic of China. whm0425@126.com (Hong-Mei Wu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Fructus Gleditsia sinensis Lam. (FGSL), Fructus Gleditsiae abnormalis (FGA), and Gymnocladus chinensis Baill. (GCB) are fruits of leguminous plants that are used in traditional medicine. Among them, FGSL and FGA are developed to different degrees, and GCB is related to them. The literature records indicate their use in the external treatment of carbuncle. Modern pharmacological studies have shown that the formation of a carbuncle is closely related to the occurrence and development of inflammation, and the volatile components contained in the FGSL/FGA drugs have significant anti-inflammatory effects. The solid phase micro extraction-gas chromatography-mass spectrometry (SPME–GC–MS) method was used to analyze the volatile components contained in FGSL, FGA, and GCB. Moreover, the molecular mechanism underlying the anti-inflammatory effects was explored based on network pharmacology and molecular docking. The SPME-GC-MS demonstrated significant differences in the chemical constituents and percentage contents among FGSL, FGA, and GCB. 13 common volatile components were identified in FGSL, FGA, and GCB. Through network pharmacology and molecular docking, the differences in the anti-inflammatory mechanism of FGSL, FGA, and GCB were initially revealed. This study laid the foundation for further study of FGSL, FGA, and GCB. Simultaneously, it also provided a reference for the correct use of FGSL, FGA, and GCB in the clinic.
Keywords
Fructus Gleditsia sinensis Lam.
Fructus Gleditsiae abnormalis
Gymnocladus chinensis Baill.
Solid phase micro extraction-gas chromatography-mass spectrometry
Anti-inflammatory
Network pharmacology
Molecular mechanism
1 Introduction
Fructus Gleditsia sinensis Lam. (FGSL) and Fructus Gleditsiae abnormalis (FGA) are derived from the leguminous plant Gleditsia sinensis Lam. Where in the FGSL comprises mature fruits, and the FGA comprises sterile fruits. GCB is the fruit of the leguminous plant Gymnocladus chinensis Baill. The FGSL, FGA, and GCB have been used in traditional Chinese medicine and reported to possess the same effect, application, and dosage; the decoction can be used internally to expel phlegm and resuscitate, and externally to treat the swollen toxin in carbuncles (National Pharmacopoeia Committee, 2020a, 2020b; An Editorial Committee of the Administration Bureau of Traditional Chinese Medicine, 1999). In addition, modern research has shown that the saponins in FGSL, FGA, and GCB have anti-inflammatory and antiviral pharmacological effects. Furthermore, FGSL can regulate immune function and prevent cardiovascular disease (Sheng, 1997; Liu and Yuan,1996). FGA can resist allergies (Dai, et al., 2002; Hou et al., 2006a, 2006b; Xia et al., 2005). GCB has the effects of inhibiting enzyme activity, anti-AIDS, etc. (Zhu et al., 2011; Chen et al., 2000). Modern research revolves around the saponins of FGSL, FGA, and GCB. However, there are few studies on their volatile components.
Carbuncle is a common clinical disease in traditional Chinese medicine. It refers to the evil poison trapped in Qi and blood and results in the blockage of Qi and blood. It is mostly characterized by local redness, swelling, heat, pain, and dysfunction caused by inflammation of hair follicles, skin tissue abscesses, infections, etc. In addition, the main manifestations of heat toxicity in traditional Chinese medicine also include inflammatory reactions (Liu et al., 2017). In the clinic, inflammation is treated orally or by injecting anti-inflammatory drugs. In recent years, with in-depth research, traditional Chinese medicine has the characteristics of treating small side effects and offering effective symptomatic relief. According to the efficacy and clinical application, FGSL, FGA, and GCB display obvious anti-inflammatory effects. However, their mechanism of action is not clear.
The basic research on active substances of traditional Chinese medicine has received extensive attention in recent years. Network pharmacology is a new method that combines bioinformatics, pharmacology, computer science, and other disciplines to study the mechanism of drug action. Through multiple computer software and databases, it interprets the potential targets and mechanism of action of drugs in the treatment of diseases in a multi-directional manner. It is consistent with the characteristics of traditional Chinese medicine in reflecting the integrity and systematic role of drugs (Zhang, 2020; Zhang et al., 2020; Hou et al., 2019). Studies have shown that the gas chromatography-mass spectrometry (GC–MS) method is often combined with headspace solid-phase microextraction. It combines sample extraction, enrichment, and injection and thus, improves the analysis speed. It displays high detection sensitivity and good separation effect and is widely used in the analysis of volatile components and effective components of drugs. Therefore, this study employed the SPME–GC–MS method to analyze the active volatile components in FGSL, FGA, and GCB. Moreover, network pharmacology and molecular docking research ideas were incorporated to conduct information mining on the identified chemical components. The key active ingredients, key targets, and potential signal pathways involved in the anti-inflammatory action of FGSL, FGA, and GCB were screened. The study aims to provide a reference for the in-depth analysis and clinical use of the anti-inflammatory effects of FGSL, FGA, and GCB.
2 Materials and methods
2.1 Materials
The FGSL, FGA, and GCB were collected from Kaili city, Guizhou Province (China). They were identified by Prof. Xiangpei Wang (Guizhou Minzu University). The voucher specimens were deposited at the Herbarium of Guizhou University of traditional Chinese Medicine. Helium gas was purchased from Guizhou Yagang Gas Co., Ltd. (Guizhou, China).
2.2 SPME–GC–MS analysis
Analyses were carried out with the HP6890/5975C GC/MS combination instrument (Agilent Company, USA). 1 g of DZJ and ZYZ, 3 g of FZJ were crushed and placed in a 25-mL sample bottle of solid-phase microextraction. Next, a manual sampler equipped with a 2 cm Stable Flex fiber of 50/30 µm DVB/CAR/PDMS (divinylbenzene/Carboxen/polydimethylsiloxane) was inserted into the injection port of the gas chromatograph (250 °C) instrument after 60 min of headspace extraction under the heating condition of a flat plate at 60 °C; the sample was introduced to the GC/MS by thermal desorption. The volatile compounds were separated on an Agilent HP–5MS capillary column (60 m × 0.25 mm × 0.25 µm). High-purity helium (99.999%) served as the carrier gas. The temperature of the vaporization chamber was set at 250 °C, the column front pressure was15.85 psi, and the carrier gas flow rate was 1.0 mL/min. The column temperature was held at 40 °C for 2 min and then increased to 180 °C at the rate of 3.5 °C/min; this was followed by an increase to 260 °C at 10 °C/min. The running time was 50 min. The injection method was splitless. The solvent delay time was 3 min.
A full scan mode was applied to identify all target compounds. The parameters were as follows: ion source: EI, ion source temperature: 230 °C, quadrupole temperature: 150 °C, electron energy: 70 eV, emission current: 34.6 µA, multiplier voltage: 1847 V, interface temperature: 280 °C, and the mass scan range: 29–500 amu. The samples were analyzed under the above-mentioned GC–MS conditions, and the chromatograms and mass spectral data were recorded. The chromatographic peaks were identified based on MS computer data system retrieval, collating Nist2005 and Wiley 275 standard mass spectra. The peak area normalization method was used to determine the relative mass fraction of each chemical component.
2.3 Network pharmacology and molecular docking
2.3.1 Screening active ingredient
The compounds were imported into the Traditional Chinese Medicine System Pharmacology Analysis Platform (TCMSP, https://tcmspw.com/tcmsp.php) to screen the active ingredients with oral availability (OB > 30% (Bryan et al., 2014)) from FGSL, FGB, and GCB.
2.3.2 Retrieval of target proteins
The target proteins of these active ingredients in FGSL, FGB, and GCB were predicted using the TCMSP and PharmMapper (https://www.lilab-ecust.cn/pharmmapper/) database. The target was searched using “Homo sapiens” as the limited condition. Using “Inflammation” as the keyword, inflammation-related targets in Gene Cards (https://www.genecards.org/), OMIM (https://mirror.omim.org/), and other databases were retrieved after deduplication. The above targets were converted and queried into the UniProtID format with “Homo sapiens” as the qualifying condition in the UniProt database (https://www.uniprot.org/).
2.3.3 Target analysis and network diagram construction
The above targets were imported into the STRING database (https://string-db.org/) for protein interaction network analysis and with the species to “Homo sapiens.” The Degree, Betweenness centrality, and Closeness centrality for each node were evaluated by the Cytoscape 3.6.1 software, and the anti-inflammatory target points of FGSL, FGB, and GCB were obtained. The active ingredients, targets, and diseases of FGSL, FGB, and GCB were imported into the Cytoscape 3.6.1 software to construct a “drug–component–disease–target” network.
2.3.4 GO and KEGG enrichment analysis
The target points selected above were subjected to the Kyoto Encyclopedia of Genes and Genome (KEGG) pathway enrichment analysis and the Gene Ontology (GO) biological process analysis in the DAVID database (https://david.ncifcrf.gov/). The signal pathways closely related to the targets were imported into the Cytoscape 3.6.1 software to construct a “target-signal pathway” network diagram.
2.3.5 Molecular docking
The crystal structures of the targets and the chemical structures of the composition were obtained from the PDB (https://www.rcsb.org) and the ZINC database (https://zinc.docking.org/). Molecular docking was performed using the AutoDock software. The water molecules and atoms were removed from the target receptors by the PyMOL software. Docking was enabled using the AutoGrid and AutoDock modules to obtain the affinities.
3 Results
3.1 SPME–GC–MS analysis
The SPME–GC–MS total ion chromatograms of FGSL, FGA, and GCB are shown in Figs. 1–3. A total of 60 volatile components were isolated from FGSL, and 38 components were identified, 61 volatile components were isolated from FGA, and 34 components were identified, 38 volatile components were isolated from GCB, and 23 components were identified. There were eight terpenes and 26 other compounds such as aldehydes, alkanes, and alcohols in FGA. There were five aldehyde compounds, five esters, and 12 other compounds such as alcohols, alkanes, ethers, and terpenes in GCB. There were 13 common compounds in FGSL, FGA, and GCB, such as hexanal, linalool, limonene, etc. And there were eight unique ingredients in FGSL, six unique ingredients in FGA, and six unique ingredients in GCB. The Venn diagram of the ingredients of FGSL, FGA, and GCB is shown in Fig. 4. A normalization method was used to determine the relative mass fraction of each chemical component, and the information of the volatile components contained in the FGSL, FGA, and GCB was obtained. The results are shown in Table 1.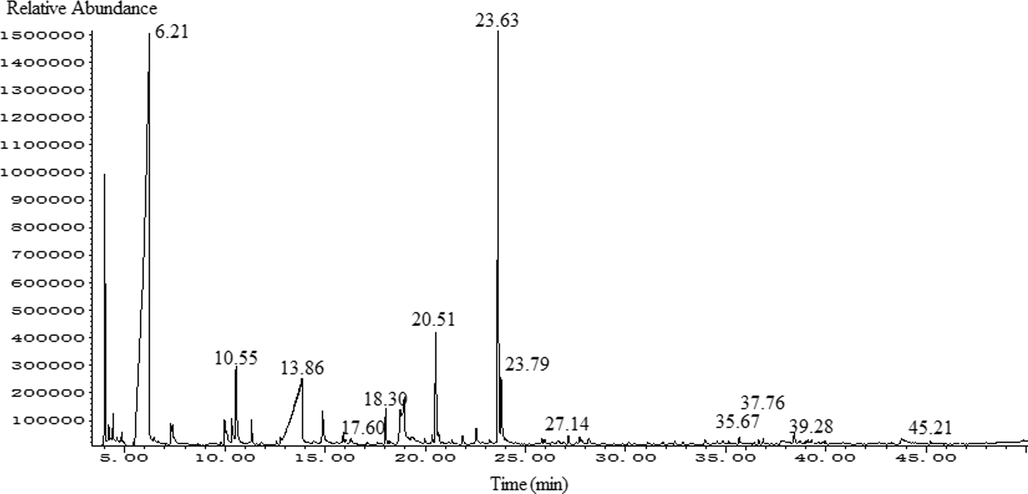
The total ion chromatogram of GC–MS of FGSL.
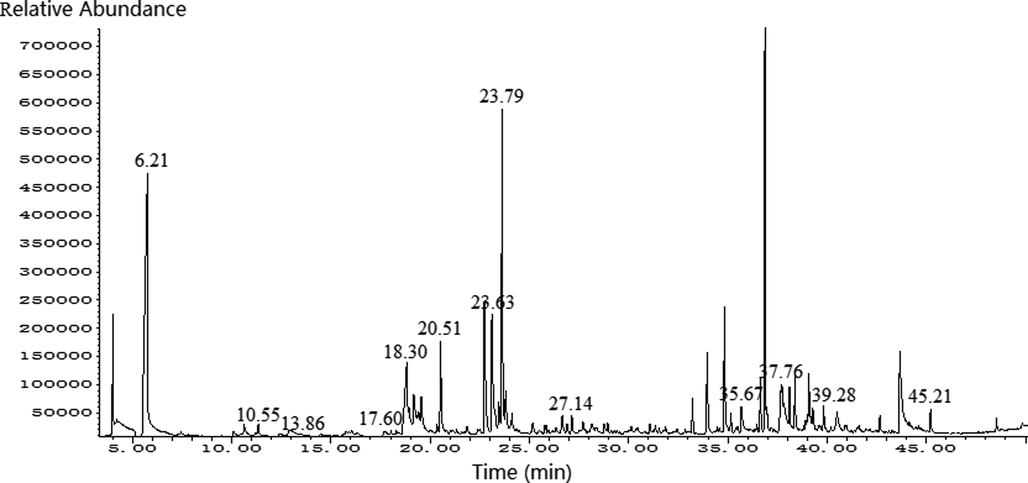
The total ion chromatogram of GC–MS of FGA.
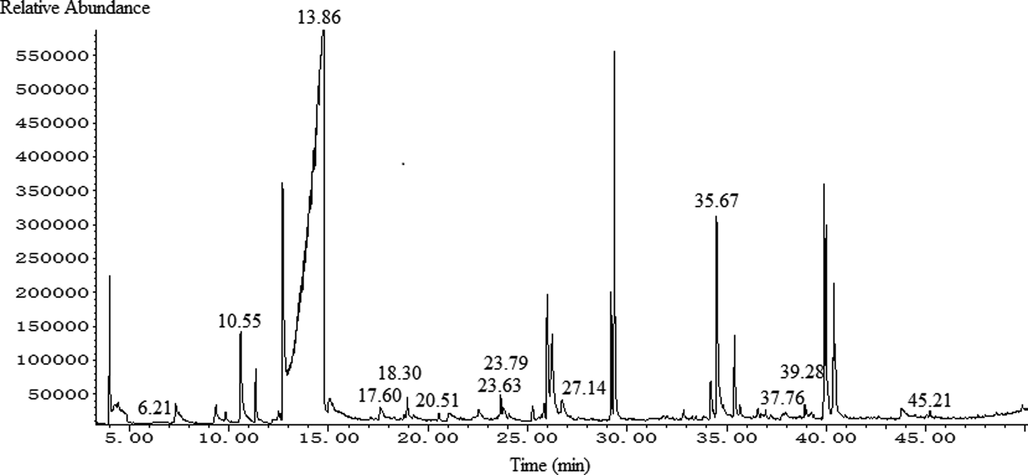
The total ion chromatogram of GC–MS of GCB.
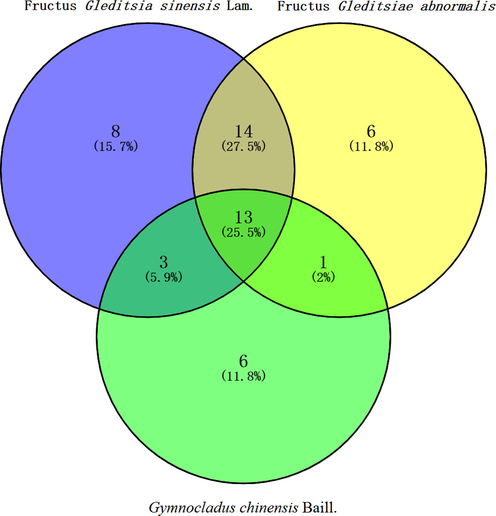
The venn diagram of the target points of FGSL, FGA, and GCB.
NO.
Retention time (min)
Name of compound
Molecular formula
Molecular weight
Retention index
Percentage content (%)
Fructus Gleditsia sinensis Lam.
Fructus Gleditsiae Abnormalis
Gymnocladus chinensis Baill.
1
4.78
Dimethyl sulfide
C2H6S
62
520
0.09
–
–
2
6.21
Acetic acid
C2H4O2
60
610
49.89
18.61
0.24
3
6.46
3-Methylbutanal
C5H10O
86
652
0.12
–
–
4
7.38
Pentanal
C5H10O
86
699
0.51
–
–
5
7.47
Furan, 2-ethyl-
C6H8O
96
703
0.23
–
0.60
6
9.81
methyl 2-methylbutanoate
C6H12O2
116
775
0.08
–
0.23
7
9.98
2,3-Butanediol
C4H10O2
90
788
0.80
0.39
–
8
10.55
Hexanal
C6H12O
100
800
2.49
0.89
2.07
9
12.44
Furfural
C5H4O2
96
833
–
0.34
0.15
10
12.57
Butanoic acid, 2-methyl-, ethyl ester
C7H14O2
130
849
0.11
–
0.10
11
13.86
Butanoic acid, 2-methyl-
C5H10O2
102
861
13.67
1.00
62.16
12
14.47
Styrene
C8H8
104
893
0.09
0.24
–
13
14.78
Heptanal
C7H14O
114
901
0.05
–
–
14
15.78
Pyrazine, 2,3-dimethyl-
C6H8N2
108
926
–
0.26
–
15
15.79
Hexanoic acid, methyl ester
C7H14O2
130
925
0.04
–
–
16
17.60
Benzaldehyde
C7H6O
106
962
0.17
0.49
0.46
17
18.02
sabinene
C10H16
136
974
0.79
0.19
–
18
18.30
1-Octen-3-ol
C8H16O
128
980
0.09
0.24
0.07
19
18.80
Furan, 2-pentyl-
C9H14O
138
993
–
–
0.10
20
18.95
Hexanoic acid
C6H12O2
116
990
3.35
4.85
–
21
19.33
Octanal
C8H16O
128
1003
0.23
–
–
22
19.39
Pyrazine, trimethyl-
C7H10N2
122
1004
–
1.18
–
23
20.34
p-Cymene
C10H14
134
1025
0.25
0.23
–
24
20.51
Limonene
C10H16
136
1030
3.09
2.52
0.14
25
20.66
Eucalyptol
C10H18O
154
1032
0.28
–
–
26
21.05
Benzyl alcohol
C7H8O
108
1036
–
–
0.51
27
21.87
γ-Terpinene
C10H16
136
1060
0.30
0.28
–
28
23.11
2,3-Dimethyl-5-ethylpyrazine
C8H12N2
136
1090
–
4.77
–
29
23.63
Linalool
C10H18O
154
1099
9.16
7.24
0.32
30
23.77
Butanoic acid, 2-methyl-, 2-methylbutyl ester
C10H20O2
172
1105
–
–
0.11
31
23.79
Nonanal
C9H18O
142
1104
1.97
1.31
0.12
32
25.76
(+)-2-Bornanone
C10H16O
152
1144
–
0.16
–
33
25.95
(+)-Citronellal
C10H18O
154
1152
0.19
–
–
34
26.66
endo-Borneol
C10H18O
154
1167
0.22
0.76
–
35
26.91
dl-Menthol
C10H20O
156
1174
0.08
0.34
–
36
27.14
Terpinen-4-ol
C10H18O
154
1177
0.28
0.55
0.07
37
27.82
Dodecane
C12H26
170
1200
0.13
0.16
–
38
28.15
Decanal
C10H20O
156
1206
0.27
0.51
–
39
28.94
β-Cyclocitral
C10H16O
152
1220
0.06
0.30
–
40
29.19
cis-3-Hexenyl-.alpha.-methylbutyrate
C11H20O2
184
1234
–
–
1.31
41
29.35
Butanoic acid, 2-methyl-, hexyl ester
C11H22O2
186
1236
–
–
3.93
42
30.14
Linalyl acetate
C12H20O2
196
1257
0.08
0.20
–
43
31.86
Tridecane
C13H28
184
1300
0.12
0.17
–
44
34.49
2(3H)-Furanone, dihydro-5-pentyl-
C9H16O2
156
1363
–
–
3.58
45
35.15
Copaene
C15H24
204
1376
0.11
0.55
–
46
35.67
Tetradecane
C14H30
198
1400
0.23
1.29
0.23
47
36.86
Caryophyllene
C15H24
204
1419
0.13
8.36
–
48
37.76
Ethanone, 1-(2-hydroxy-4-methoxyphenyl)-
C9H10O3
166
1438
0.42
1.14
0.13
49
38.38
Alloaromadendrene
C15H24
204
1461
–
1.34
–
50
39.28
Pentadecane
C15H32
212
1500
0.16
0.50
0.07
51
42.66
Caryophyllene oxide
C15H24O
220
1581
–
0.38
–
52
45.21
Heptadecane
C17H36
240
1700
0.08
0.58
0.11
Total
90.42
62.34
76.85
It can be seen from Table 1 that 40, 34, and 23 volatile components have been identified for FGSL, FGA, and GCB, respectively. Among them, there were 13 common components, mainly acetic acid, hexanal, butanoic acid, 2-methyl-, limonene, linalool. The content of acetic acid in FGSL, FGA, and GCB is 49.892%, 18.606%, 0.244% respectively. The content of hexanal in FGSL, FGA, and GCB is 2.489%, 0.894%, 2.073% respectively. The content of butanoic acid, 2-methyl- in FGSL, FGA, and GCB is 13.675%, 0.998%, 62.164% respectively. The content of limonene in FGSL, FGA, and GCB is 3.088%, 2.520%, 0.145% respectively. The content of linalool in FGSL, FGA, and GCB is 9.159%, 7.236%, 0.316% respectively.
FGSL mainly contain acetic acid (49.892%), butanoic acid, 2-methyl- (13.675%), linalool (9.159%), Among them, acetic acid is mainly present in the form of esters in the fruit, indicating that the FGSL are mainly esters and alcohol compounds. FGA mainly contain acetic acid (18.606%), caryophyllene (8.361%), linalool (7.236%), illustrating that FGA mainly contains esters, terpenes and alcohol compounds. GCB mainly contain butanoic acid,2-methyl- (62.164%), butanoic acid,2-methyl-, hexyl ester (3.930%), 2(3H)-Furanone, dihydro-5-pentyl- (3.580%), illustrating that GCB mainly contains esters, terpenes and alcohol compounds esters and aldehydes.
3.2 Network pharmacology and molecular docking
3.2.1 Construction of active components
There were 15, 11, and 7 active ingredients in FGSL, FGA, and GCB, respectively. They contained five common ingredients such as hexanal, linalool, nonanal, limonene, and benzaldehyde. The results are shown in Table 2.
NO.
MolID
Name of compound
OB (%)
Number of targets
Fructus Gleditsia sinensis Lam.
Fructus Gleditsiae Abnormalis
Gymnocladus chinensis Baill.
1
MOL002046
Hexanoic acid
73.08
3
3
–
2
MOL001335
Benzyl alcohol
58.68
–
–
10
3
MOL005970
Eucalyptol
60.62
26
–
–
4
MOL001604
Linalool
58.18
8
8
8
5
MOL002379
Pentanal
59.53
1
–
–
6
MOL000666
Hexanal
55.71
6
6
6
7
MOL000066
Alloaromadendrene
50.62
–
5
–
8
MOL000116
Nonanal
40.28
4
4
4
9
MOL004627
Hexanoic acid, methyl ester
52.44
2
–
–
10
MOL001193
Caryophyllene oxide
45.75
–
7
–
11
MOL000023
Limonene
39.84
13
13
13
12
MOL005315
(+)-Citronellal
50.78
1
–
–
13
MOL000722
β-Cyclocitral
40.36
0
0
–
14
MOL000172
Furfural
34.35
–
4
4
15
MOL000708
Benzaldehyde
32.63
5
5
5
16
MOL002484
3-Methylbutanal
44.71
87
–
–
17
MOL000202
γ-Terpinene
33.02
7
7
–
18
MOL002025
Acetic acid, methyl ester
40.17
37
–
–
19
MOL002850
Butylated Hydroxytoluene
40.02
19
–
–
3.2.2 Retrieval of target proteins
A total of 166 targets related to the volatile active ingredients of FGSL, 27 for FGA, and 29 for GCB, were obtained. From GeneCards and OMIM databases, 10,272 inflammation-related targets were obtained by deduplication. There were 128, 21, and 20 anti-inflammatory targets for the volatile active ingredients of FGSL, FGA, and GCB, respectively.
3.2.3 Target analysis and network diagram construction
The protein interaction analysis of the above targets was analyzed by the STRING database. The analysis results were imported into the Cytoscape 3.6.1 software to obtain the degree, betweenness centrality, and closeness centrality values of each target. The targets of FGSL (Degree ≥ 10), FGA, and GCB (Degree ≥ 5) are shown in Table 3. The network diagram of the “drug-component-disease-target” constructed in Cytoscape 3.6.1 is described in Fig. 5. The analysis results demonstrated the presence of 18 identical targets for FGSL, FGA, and GCB. Among these, TNF, JUN, and PTGS2 had the highest Degree value. There were 21 identical targets (TNF, ACHE, CHRM1, ADRA1B, and PTGS2) by paired comparison between FGSL and FGA, 20 identical targets (TNF, CHRM1, ADRB2, PTGS2, JUN) between FGSL and GCB, and 18 identical targets (TNF, JUN, PTGS2, CHRM1, CHRM2) between FGA and GCB. Thus, differences existed in the anti-inflammatory ingredients and targets of FGSL, FGA, and GCB.
NO.
Name
Protein names
Betweenness Centrality
Closeness Centrality
Degree
UniProtID
FGSL
1
SRC
Proto-oncogene tyrosine-protein kinase Src
0.1759636
0.52892562
21
P12931
2
TNF
Tumor necrosis factor
0.21411127
0.53781513
20
P01375
3
F2
0.13924273
0.52892562
20
P00734
4
ACHE
0.10423389
0.50793651
19
P22303
5
GPT
0.16118246
0.49612403
14
P24298
6
SLC6A4
0.03239261
0.41290323
14
P31645
7
CHRM1
0.04586799
0.46376812
14
P11229
8
ADRA1B
0.00617478
0.42384106
13
P35368
9
ADRB2
0.05708121
0.48120301
13
P07550
10
ADRA1D
0.00548488
0.42105263
12
P25100
11
ADRA1A
0.00548488
0.42105263
12
P35348
12
HTR2A
0.00622669
0.42105263
12
P28223
13
PTGS2
0.0198051
0.47058824
12
P35354
14
JUN
0.04380317
0.46376812
12
P05412
15
SLC6A3
0.00480293
0.38554217
10
Q01959
16
OPRM1
0.06479608
0.46715328
10
P35372
17
CHRM2
0.04180519
0.4
10
P08172
18
BCHE
0.01939574
0.42105263
10
P06276
FGA
1
TNF
0.41140351
0.45454545
8
P01375
2
JUN
0.42982456
0.5
6
P05412
3
PTGS2
0.04824561
0.41666667
5
P35354
4
CHRM1
0.47807018
0.46511628
5
P11229
GCB
1
TNF
0.46296296
0.51351351
8
P01375
2
JUN
0.37231969
0.5
6
P05412
3
CHRM2
0.35282651
0.42222222
5
P08172
4
PTGS2
0.03996101
0.41304348
5
P35354
20
PRSS3
0
0.26760563
1
P35030
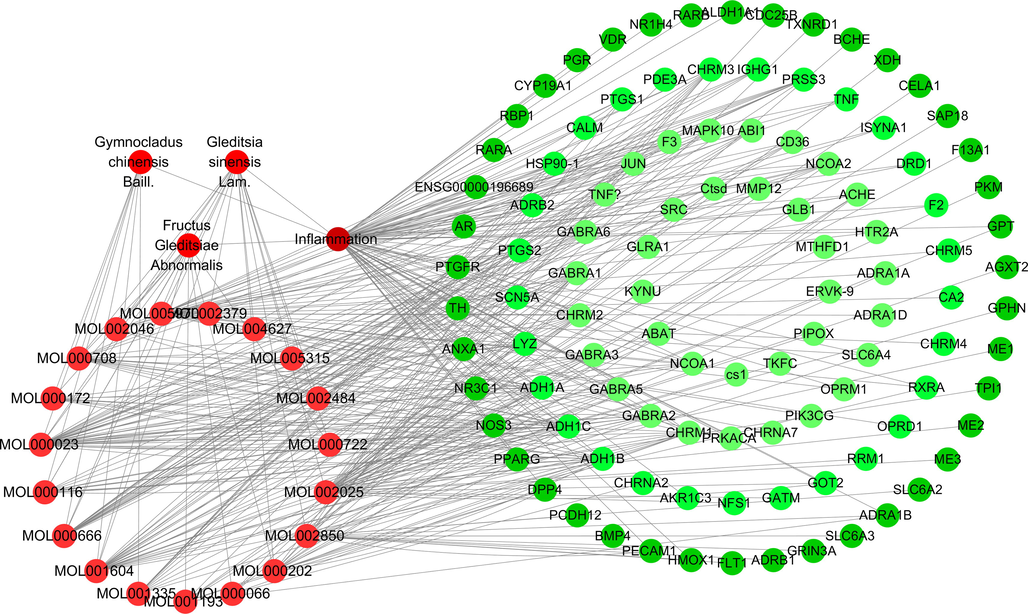
The “drug-component-disease-target” network diagram of the anti-inflammatory effects of FGSL, FGA and GCB.
3.2.4 GO and KEGG analysis
The target proteins were subjected to enrichment analysis of the signaling pathway and Gene Ontology biological process by the DAVID database. There were 33, 21, and 23 anti-inflammatory signal pathways for the volatile components of FGSL, FGA, and GCB (P < 0.05), the “drug-signal pathway-target” network diagram constructed by the Cytoscape 3.6.1 software is shown in Fig. 6. There were 37, 13, and 12 bioprocesses in FGSL, FGA, and GCB, respectively (P < 0.005) (Fig. 7). There were 27, 11, and 10 cell composition functions and 42, 19, and 15 molecular functions for FGSL, FGA, and GCB, respectively (Figs. 8, 9). A total of ten identical signal pathways, six biological processes, nine-cell composition, and ten molecular functions were identified in FGSL, FGA, and GCB. The targets involved in the most closely regulated signaling pathways and biological processes were “neuroactive ligand-receptor interaction” and “adenylate cyclase-activating adrenergic receptor” signaling pathways. The results indicated that the signal pathway was the same, but the number of targets involved in the signal pathway was different. There were 19, 6, and 6 targets involved in regulating the signal pathways of FGSL, FGA, and GCB.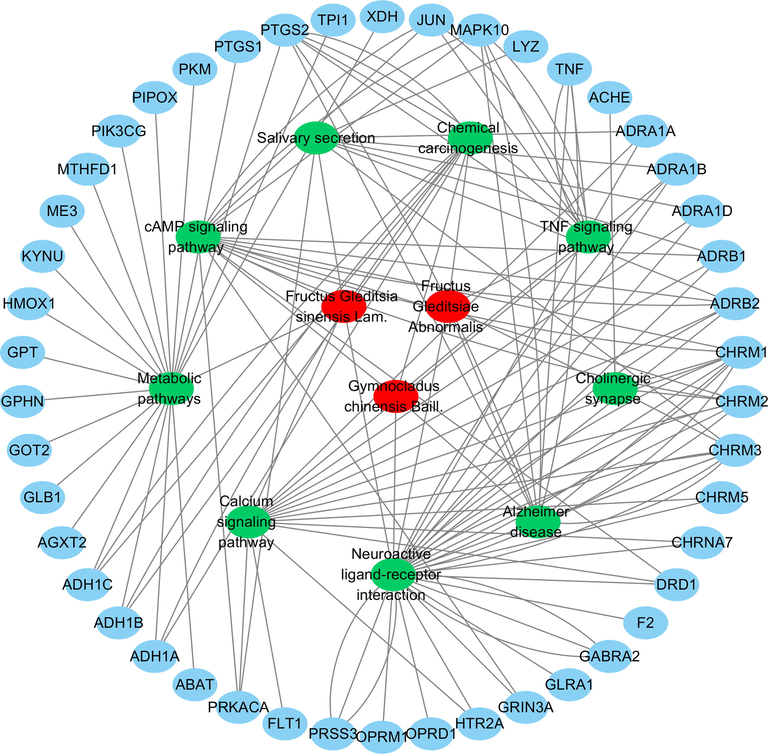
The “drug-signal pathway-target” network diagram of FGSL, FGA and GCB.
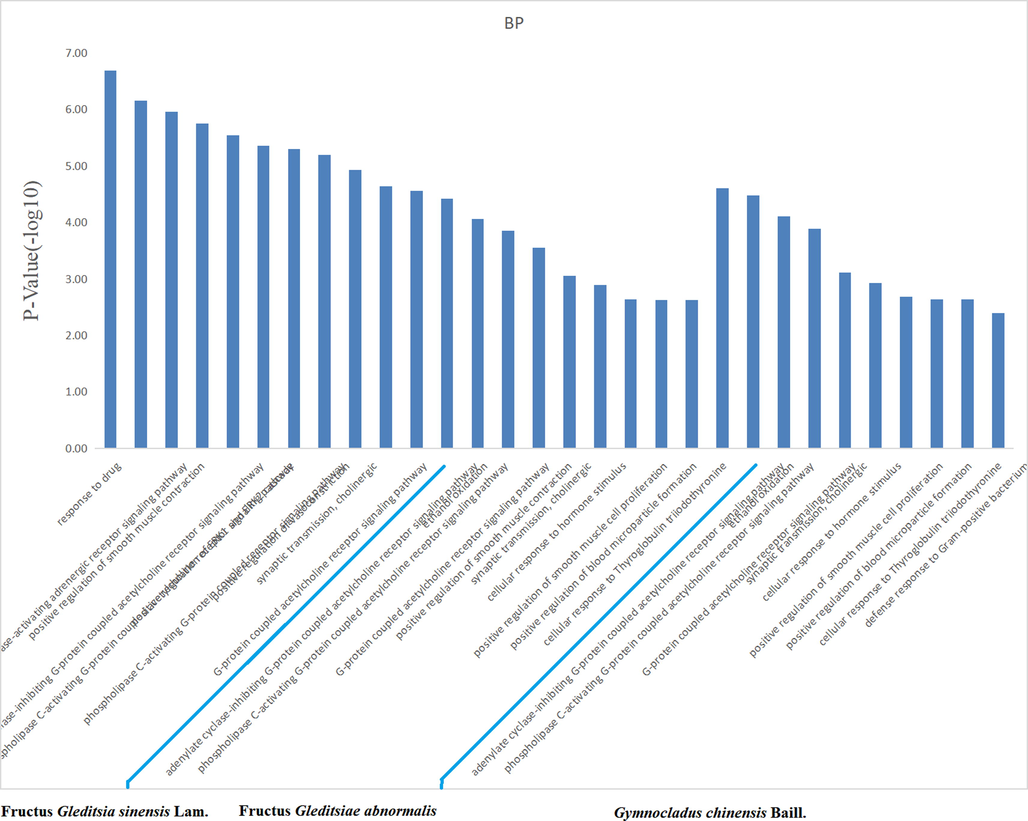
Enrichment of biological processes of FGSL, FGA and GCB.
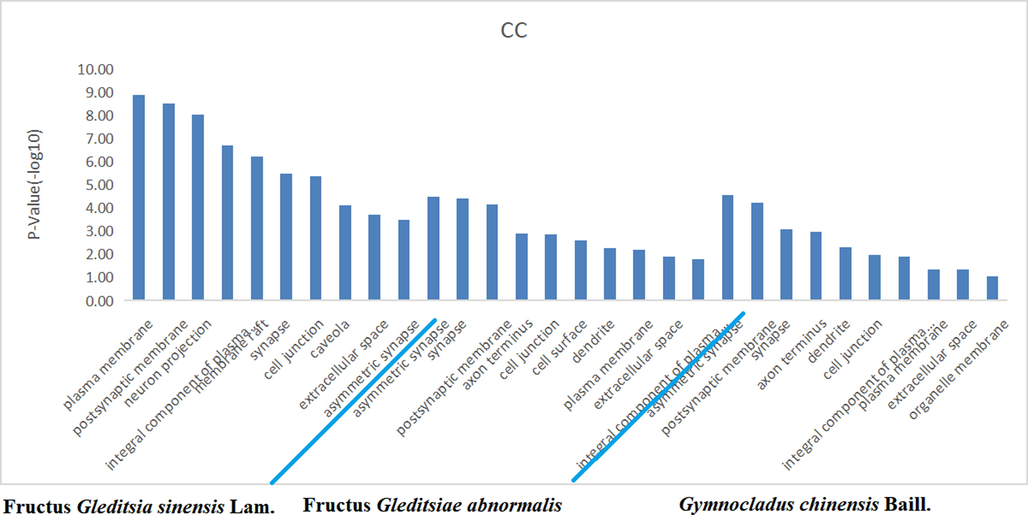
Enrichment of cell composition of FGSL, FGA and GCB.
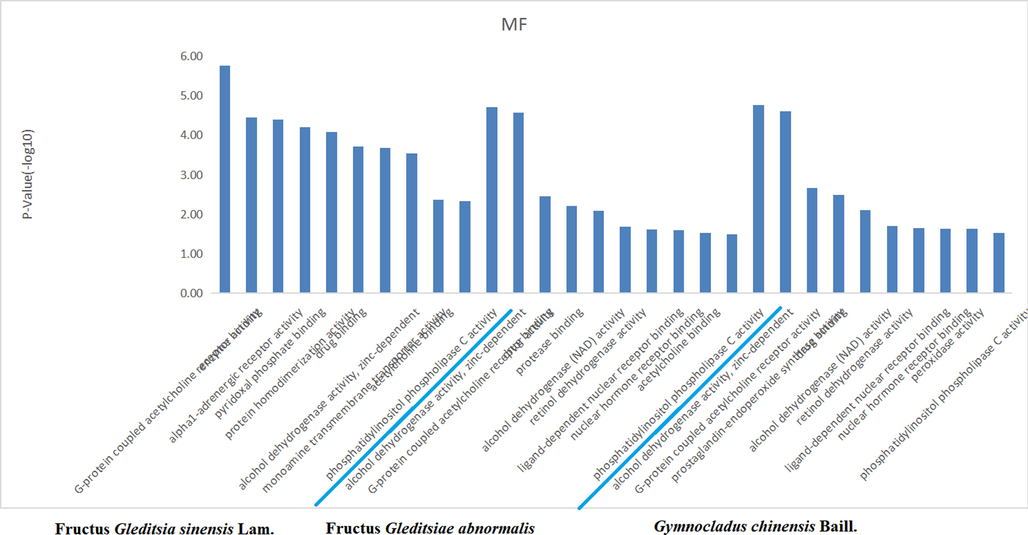
Enrichment of molecular functions of FGSL, FGA and GCB.
3.2.5 Molecular docking
The six targets at the top three levels in the degree value that docked with the active ingredients of FGSL, FGA, and GCB were named SRC, TNF, ACHE, JUN, PTGS2, and F2 in the protein network analysis results (Table 4). Binding energy less than 0 indicated that the ligand molecule could spontaneously bind to the receptor target. Binding energy less than –5 indicated a good binding (Li et al., 2021). All the active components in the table were combined with the targets, and the results are shown in Table 4. The best molecular docking diagrams are shown in Fig. 10.
Term
Name of compound
Affinity (kcal/mol)
SRC
TNF
ACHE
JUN
PTGS2
F2
FGSL&FGA&GCB
Hexanal
−2.25
−2.78
−2.69
−2.72
−2.49
−3.42
Linalool
−2.16
−2.39
−2.76
−3.45
−1.96
−2.75
Nonanal
−1.8
−1.95
−2.25
−2.3
−2.45
−2.23
Limonene
−3.83
−3.75
−3.96
−4.07
−6.13
−4.09
Benzaldehyde
−2.67
−2.92
−3.05
−2.93
−3.59
−3.25
FGSL&FGA
Hexanoic acid
−0.7
−0.84
−0.37
−1.65
−1.15
−1.74
.beta.-Cyclocitral
−3.48
−3.32
−4.7
−4.48
−3.64
−4.6
.gamma.-Terpinene
−3.59
−3.72
−4.32
−4.31
−3.64
−4.28
FGA&GCB
Furfural
—
−2.94
−3.51
−3.24
−2.98
–
FGSL
Eucalyptol
−3.69
−3.86
−4.7
–
–
−5.13
Pentanal
−2.01
−2.36
−2.39
–
–
−2.38
Hexanoic acid, methyl ester
−2.12
−2.76
−2.05
–
–
−2.58
(+)-Citronellal
−2.55
−2.76
−2.52
–
–
−3
3-Methylbutanal
−2.32
−2.73
−2.52
–
–
−2.5
Butylated Hydroxytoluene
−3.09
−3.09
−3.46
–
–
−3.51
Acetic acid, methyl ester
−2.25
−2.5
−2.36
–
–
−2.08
FGA
Caryophyllene oxide
–
−4.24
−5.19
−5.87
−4.12
–
Alloaromadendrene
–
−4.32
−5.39
−4.86
−4.22
–
GCB
Benzyl alcohol
–
−2.22
–
−2.93
−3.38
–
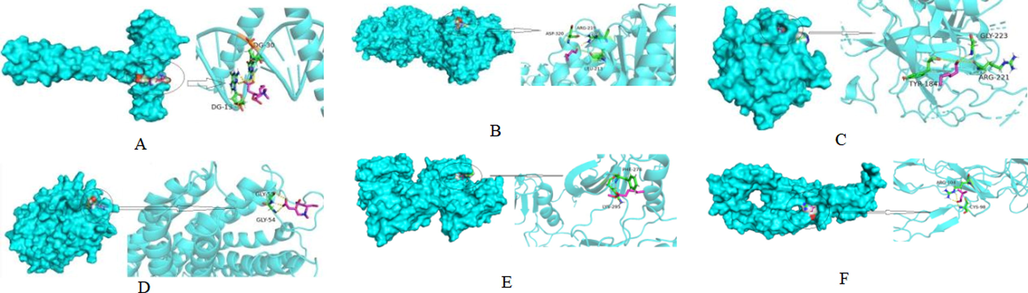
Molecular docking results of FGSL, FGA and GCB. (A: Hexanoic acid-JUN; B: Pentanal-ACHE; C: Hexanal-F2; D: Hexanoic acid-PTGS2; E: Hexanoic acid, methyl ester-SRC; F: 3-Methylbutanal-TNF).
4 Discussion
In this study, GC–MS was combined with network pharmacology and molecular docking technology to explore the potentially active anti-inflammatory ingredients and their molecular mechanisms in FGSL, FGA, and GCB preliminarily. Through GC–MS analysis, 15 active ingredients from FGSL, 11 from FGA, and 7 from GCB were identified to possess anti-inflammatory action. After mining and analyzing databases such as TCMSP, BATMAN–TCM, STRING, DAVID, etc., 65, 21, and 20 anti-inflammatory targets of FGSL, FGA, and GCB were predicted.
There was no significant difference between FGSL and FGA in terms of efficacy and dosage, but they are still used as two drugs in clinical practice. Additionally, modern studies have shown that the total saponins and the content of different solvent extracts in the two compounds are not significantly different (Yang, 2004). GCB is related to FGA and GCB and has similar functions and applications. However, the GC–MS analysis results of this study illustrated differences in the volatile components of FGSL, FGA, and GCB. Moreover, their anti-inflammatory active ingredients and target points were also different. There are five identical active ingredients of FGSL, FGA, and GCB (Hexanal, Linalool, Limonene, Nonanal, and Benzaldehyde). Among them, the specific active components in FGSL are eucalyptol, (+)-citronellal, pentanal, etc.; those in FGA are alloaromadendrene, caryophyllene oxide; and that in GCBs is benzyl alcohol. Studies have shown that linalool has broad-spectrum antibacterial and antifungal activity (Pattnaik et al., 1997). Limonene has bactericidal and antioxidant activities. Chen (Chen et al., 2019) and others found that the main component of rosemary herb essential oil is eucalyptol, which can be used to treat arthritis. Caryophyllene oxide has a good anti-gastric ulcer effect (Duan et al., 2015).
According to the analysis results of the target points, the FGSL, FGA, and GCB have the same target points (TNF, JUN, PTGS2, etc.). In the results of protein interaction analysis, the node with the highest Degree value is regarded as the “central node” of all nodes and occupies an important position in the target interaction. Here, SRC, a unique action target of FGSL, has the largest degree among the action targets, while TNF is the action target with the largest degree in FGA and GCB. SRC–1 from the steroid receptor co-activator (SRC) family acts as a transcriptional co-activator, which can simultaneously bind to the nuclear receptor GR and nuclear transcription factors NF–κB and AP–1 to play a dual role in anti-inflammation and inflammation (Leo and Chen, 2000; Tian, 2019). In addition, studies have shown that SRC–1 can also inhibit the expression of inflammatory factors such as IL–6, IL–1β, and TNF–α . TNF–α is an important pro-inflammatory cytokine. Many drugs exert anti-inflammatory effects by inhibiting the expression of TNF–α. For example, polysaccharides of the Ginkgo biloba leaf exert anti-inflammatory effects by inhibiting the expression of TNF–α (Martin et al., 2007).
The results of GO and KEGG analysis demonstrated that the FGSL, FGA, and GCB have the same signal pathway, the neuroactive ligand-receptor interaction signal pathway, which is more closely related; the same genes Chrm1, Chrm2, and Chrm3are enriched in this pathway. Studies have shown that the neuroactive ligand-receptor interaction is a collection of all receptors and ligands related to the intracellular and extracellular signaling pathways on the plasma membrane34. The expression of Chrm3, DRD5, and HTR1B affects the pathway. For example, the up-regulation of Chrm3 expression enhances cholinergic function and promotes the improvement of learning and memory abilities (Pan, 2011; Wang et al., 2007). The molecular docking results demonstrated that among the targets with the higher degree value, the SRC, PTGS2, F2, TNF, ACHE have better binding properties with the active components of FGSL, FGA, and GCB.
5 Conclusion
In this study, GC–MS was used to analyze the volatile components of different varieties (FGSL, FGA, and GCB). The results indicated differences in the species and contents of the volatile components in FGSL, FGA, and GCB. Network pharmacology and molecular docking analyzed the anti-inflammatory mechanism of the volatile components of FGSL, FGA, and GCB. This explained the characteristics of the multi–component, multi-target, and multi–pathway functions of FGSL, FGA, and GCB at the molecular level. The results also illustrated differences in the anti-inflammatory targets and signal pathways of the volatile active ingredients of FGSL, FGA, and GCB. Thus, these results have provided a reference for the correct clinical use and in–depth development and utilization of FGSL, FGA, and GCB.
Funding
This research was funded by the Guizhou Characteristic Forestry Industry Research Project [Te Lin Yan (2020) 13]. The funding bodies play no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chinese Materia Medica (Zhonghua Bencao). Shanghai: Shanghai Scientific & Technical Publishers; 1999. p. :24-25.
- Mechanoresponsive networks controlling vascular inflammation. Arterioscler. Thromb. Vasc. Biol.. 2014;34:2199-2205.
- [Google Scholar]
- A survey of studies on the pharmacological action of peppermint oil and its active components. Chin. J. Inf. Tradit. Chin. Med.. 2000;7:33.
- [Google Scholar]
- GC-MS analysis of essential oil components of Rosmarinus of ficinal is L. leaves before and after drying. Jiangsu Agric. Sci.. 2019;47:171-176.
- [Google Scholar]
- Antiallergic and anti-inflammatory properties of the ethanolic extract from Cleditsia sinensis. Biol. Phann. Bull.. 2002;25:1179-1182.
- [Google Scholar]
- GC-MS analysis on the content of Caryophyllin and trans-Caryophyllene in Caodoukou formula granule. China J. Chin. Med.. 2015;30:407-409.
- [Google Scholar]
- Amelioration of collagen-in-duced arthritis in mice by a saponin fraction from Cleditsia sinensis. Pharm. Biol.. 2006;44:651-656.
- [Google Scholar]
- Alleviation of picryl chlo-ride-induced delayed type hypersensitivity reaction by saponin fraction of Cleditsia sinensis. Biol. Pluum. Bull.. 2006;29:1056-1059.
- [Google Scholar]
- Analysis of anti-inflammation mechanism of Zukamu granules based on network pharmacology and HS-SPME-GC-MS. Int. J. Pharm. Sci. Res.. 2019;46:441-455.
- [Google Scholar]
- Properties and development of soybean saponin. Grain Sci. Technol. Econ.. 1996;4:35-36.
- [Google Scholar]
- Discussion on the significance of treating heart failure with clearing heat and removing toxicity from the inflammation. China J. Traditional Chin. Med. Pharm.. 2017;32(4901)
- [Google Scholar]
- Study on the mechanism of different parts of Morus alba L. in preventing and treating diabetes based on network pharmacology-molecular docking. Nat. Prod. Res.. 2021;33:291-303.
- [Google Scholar]
- The Pharmacopoeia of the People’s Republic of China (Part Ⅰ). Beijing: China Medical Science Press; 2020. p. :22.
- The Pharmacopoeia of the People’s Republic of China (Part Ⅰ). Beijing: China Medical Science Press; 2020. p. :331.
- Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios.. 1997;89:39-46.
- [Google Scholar]
- Influence of long-term usage of diazepam on neuroactive ligand-receptor interaction signaling pathway. J. China Pharm. Univ.. 2011;42:443-446.
- [Google Scholar]
- The role and mechanism of steroid receptor coactivator 1(SRC-1) in angiotensin II-induced hypertensive inflammation in mice. Xiamen University; 2019.
- Study of the effect of Daicong solution on gene expression of M1, M3 receptor in aged rat dementia model. Acta Acad. Med. Weifang.. 2007;29:392-394.
- [Google Scholar]
- Inhibition of allergic rhinitis by n-butanol fraction from anomalous fruits of Gleditsia sinensis. Chin. J. Clin. Pharmacol. Therapeut.. 2005;10:925-992.
- [Google Scholar]
- Analysis and comparison of the chemical composition of Gleditsia sinensis Lam. and Fructus gleditsiae abnormalis. J. Huaiyin Teachers College (Nat. Sci. Ed.). 2004;2:143-146.
- [Google Scholar]
- Isolation and characterization of a kunitz-typetrypsin inhibitor with antiproliferative activity from Gymnocladus chinensis (Yunnan Bean) seeds. Protein J.. 2011;30:240-246.
- [Google Scholar]
- Network pharmacology based virtual screening of active constituents of Prunella vulgaris L. and the molecular mechanism against breast cancer. Sci. Rep.. 2020;10(15730)
- [Google Scholar]
- A bioinformatics investigation into molecular mechanism of Yinzhihuang granules for treating hepatitis B by network pharmacology and molecular docking verification. Sci. Rep.. 2020;10:11448.
- [Google Scholar]







