Translate this page into:
Extraction and carrier mediated transport of urea using noncyclic receptors through liquid membrane systems
⁎Corresponding author. komalsharma1412@gmail.com (Komal Sharma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Extraction and carrier mediated transport through bulk liquid membrane and supported liquid membrane systems have wide applications in separation technology. This paper highlights the use of six noncyclic receptors (podands) having variations in chain length and end group for the removal of urea using liquid membrane system. These receptors R1, R2, R3, R4, R5, R6 are diethylene glycol dimethyl ether, diethylene glycol dibutyl ether, diethylene glycol dibenzoate, diethylene glycol, triethylene glycol and tetraethylene glycol respectively. The sequence of extraction and transport of urea by BLM system using various receptors is R2 > R3 > R1 > R4 > R5 > R6 and R6 ≈ R3 > R5 > R4 > R1 > R2 respectively. Receptor R2 containing butyl end group is best extractant while receptor R6 with flexible backbone is best carrier and this carrier efficiency is used to remove urea using BLM system from the feed phase by recyclization process up to 88.16%. The experimental results influenced by concentration of receptors and urea. Effect of time was also studied.
Keywords
Liquid-liquid extraction
Bulk liquid membrane (BLM) and supported liquid membrane (SLM) transport system
Hydrophobicity
Podands
Recyclization
1 Introduction
Carrier facilitated transport of biomolecules and metal ions through liquid membrane system using different receptors as an extractant as well as carrier plays a significant role in simulating biological membrane functions and separation technologies. Liquid membranes are selective because of high transport efficiency, minimum sample consumption, and economic superiority of liquid membrane over other separation techniques. (Clark et al., 2005; Chakraborty et al., 2009).
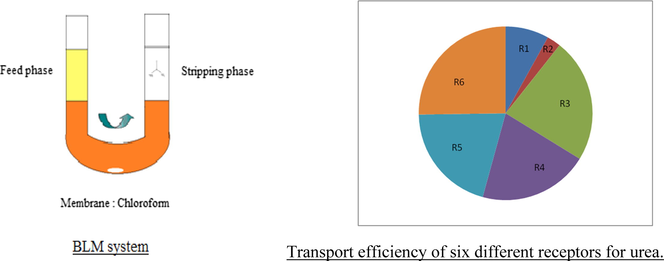
Urea is the nitrogenous product of protein metabolism. It is used for preparing formaldehyde-Urea resin -plastics (Chen and Wang, 2017), barbiturates (Dixit et al., 2010), and fertilizers (Rahman et al., 1994; George et al., 1997). Urea is also used at large scale in the paper industry to soften cellulose and is being used to promote healing in infected wounds and other vast applications in the field of medicine (Gnewuch and Sosnovsky, 1997). Hence industrial waste water contains a large amount of urea which could be removed by membrane separation technique. This is also important for the treatment of brackish and seawater. Azizian and Nabati, 2012; Zhang et al., 2013 Several other methods reported for the removal of urea are adsorption, biological decomposition, chemical oxidation, and enzymatic decomposition etc. (Magne et al., 2002; Sugiyama et al., 2013; Rahimpour, 2004; Nicolau et al., 2014; Wu et al., 2017; Yang et al., 2018). Other then urea, removal of some heavy metals from wastewater using extraction methods has also been reported. (Citak and Tuzen, 2010; Tuzen et al., 2013). Fig. 1 shows the structure of various receptors used and Fig. 2 shows the supported liquid membrane transport system used in our study.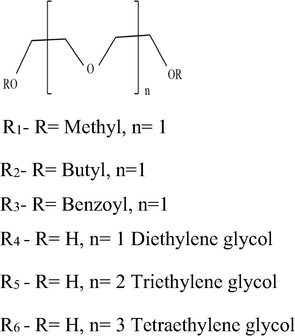
Structure of six noncyclic receptors.
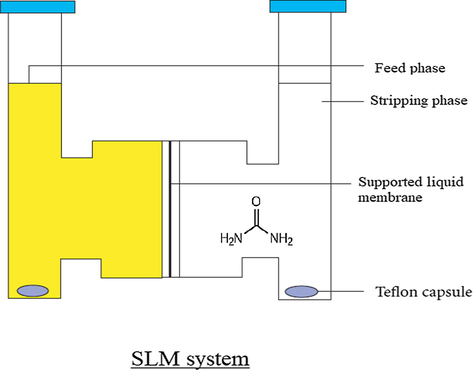
Supported liquid membrane (SLM) system for the transport of urea.
This reactive liquid membrane system has emerged as a novel and effective tool in separation technique and display high selectivity due to specific interaction with guest molecules by the carrier which facilitates the separation through membranes. Supramolecular receptors like crown ethers (Pedersen, 1967) podands (Vögtle and Weber, 1979), lariat ethers and calixarenes (Gutsche, 1989) have found applications for the selective transport of ions and biomolecules through bulk and supported liquid membrane systems. (Anchaliya and Sharma, 2014, 2017; Bhatnagar et al., 2008; Raizada and Sharma, 2013; Robak et al., 2009).
Earlier reports indicate that 86% of urea was removed using urea bioreactor (Nicolau et al., 2014) and 80% urea was removed using urease-immobilized fibers. (Sugiyama et al., 2013). In the present work, the removal of urea through extraction and carrier facilitated transport using chloroform bulk liquid membrane and supported liquid membrane system containing a series of noncyclic receptors is performed and urea was removed from feed phase up to 88.16% by recyclization process. The stripping phase containing urea can be used directly as fertilizer.
2 Experimental
2.1 Reagents and instruments
Diethylene glycol dimethyl ether, Diethylene glycol dibutyl ether, Diethylene glycol dibenzoate, Diethylene glycol, Triethylene glycol, Tetraethylene glycol and P-(N, N- dimethyl amino) benzaldehye were purchased from Fluka (USA). Urea was obtained from Merck (Germany) and used without further purification. Chloroform and ethanol were obtained from Qualigens (Worli, Mumbai, India). Systronic – spectrophotometer: 106 (Ahmdabad, India) used for the estimation of urea.
2.2 Estimation of urea
A stock solution of p-(N, N- dimethyl amino) benzaldehye was prepared by dissolving 8 g in 400 mL of ethanol and 40 mL of concentrated hydrochloric acid. 1 mL of aqueous urea solution of various concentrations (0.5 M −10 M) was added to 10 mL of this stock solution and the total volume make up to 25 mL in volumetric flask, results in orange colored solution λmax = 440 nm. The calibration curve was obtained with various concentration of urea and used for the estimation of urea in feed phase and stripping phase.
2.3 Extraction studies
10 mL of aqueous solution of urea (0.5 M−10 M) and 10 mL of receptor (0.1 M) solution in chloroform were taken in a 50 mL beaker and stirred on a magnetic stirrer for 24 h at room temperature. After stirring, the mixture was allowed to stand for 5 min for the separation of two phases, and the aqueous phase was analyzed for extracted urea by determining the difference in the concentration of urea in aqueous phase before and after extraction. The distribution coefficient was calculated shown in Table 2.
Conc of urea
Receptors
Amount of urea extracted
(×10−3M)by R1
Amount of urea extracted
(×10−3M)by R2
Amount of urea extracted
(×10−3M) by R3
Amount of urea extracted
(×10−3M) by
R4
Amount of urea extracted
(×10−3M) by
R5
Amount of urea extracted
(×10−3M) by R6
0.5 M
3.50
1.10
2.05
1.95
1.80
1.55
1 M
25.00
17.50
20.00
25.00
30.00
40.00
2 M
50.00
37.50
70.00
50.00
30.00
60.00
3 M
75.00
5.00
120.00
75.00
27.50
20.00
4 M
170.00
40.00
107.00
95.00
67.50
75.00
5 M
267.00
42.50
92.00
120.00
107.50
127.00
6 M
275.00
12.50
70.00
100.00
87.50
112.00
7 M
2200.00
2400.00
2250.00
1875.00
1850.00
1825.00
8 M
2250.00
1875.00
1650.00
1555.00
1725.00
1625.00
9 M
2275.00
1925.00
1700.00
1625.00
1775.00
1650.00
10 M
2350.00
1975.00
1750.00
1675.00
1800.00
1700.00
Conc of urea
Receptors
Du by R1
Du by R2
Du by R3
Du by R4
Du by R5
Du by R6
0.5 M
0.001
0.0008
0.001
0.001
0.001
0.0009
1 M
0.02
0.02
0.02
0.02
0.30
0.04
2 M
0.03
0.02
0.04
0.01
0.01
0.38
3 M
0.03
0.002
0.05
0.03
0.01
0.02
4 M
0.06
0.03
0.03
0.03
0.02
0.02
5 M
0.08
0.01
0.02
0.03
0.03
0.38
6 M
0.07
0.003
0.01
0.25
0.02
0.02
7 M
0.06
0.81
0.72
0.45
0.55
0.51
8 M
0.52
0.39
0.335
0.3
0.35
0.32
9 M
0.51
0.40
0.29
0.3
0.36
0.33
10 M
0.51
0.40
0.3
0.31
0.36
0.33
2.4 Transport studies
Transport studies for BLM system were performed in a “U” tube glass cell. (Anchaliya and Sharma, 2014)15 mL of chloroform containing (0.1 M) concentration of receptors (R1- R6) was used as membrane phase, feed phase was composed of 10 mL of various concentrations of urea in one limb of the “U” tube and 10 mL of deionised water served as the striping phase in another limb. The membrane phase was constantly stirred for 24 h and the feed phase was analyzed for the concentration of urea. Now striping phase was replaced by double distilled water keeping the same feed phase and membrane system was stirred for another 24 h. This recyclization process was continued up to 168 h shown in Table 6 and Fig. 7.
In SLM system egg shell membrane was impregnated by dipped overnight in chloroform containing receptors (R1- R6) and used as membrane support which positioned between two cylindrical half-cells. One cell compartment (feed phase) is filled with water containing urea (50 mL) and the other cell compartment (stripping phase) filled with double distilled water (50 mL), separated by membrane. Both phases were stirred on magnetic stirrer (120 rpm) at 25 °C and the striping phase was analyzed after 24 h for the concentration of urea and flux was calculated.
3 Results and discussion
The blank experiments were performed with the concentration of urea from 1 M to 10 M separately in which membrane was devoid of carrier. No detectable amount of urea across chloroform membrane was observed in striping phase which proved that there was no leakage. All measurements were performed in triplicate and average values are shown in the tables.
3.1 Effect of urea concentration
In order to find out the optimum concentration of urea for extraction, we have varied the concentration of urea from 0.5 M to 10 M and the receptor concentration was kept constant at 0.1 M. The amount of urea extracted given in Table 1 and Fig. 3. From the results it is observed that the amount of urea extracted increase with increase in the concentration of urea. A sudden increase in the amount of urea extracted was observed at 7 M.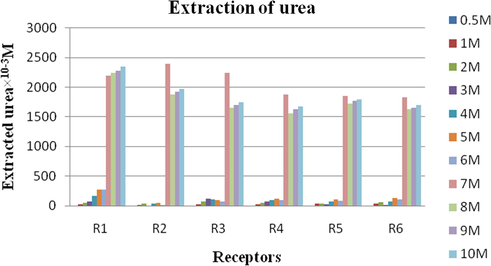
Amount of urea extracted after 24 h with noncyclic receptors. Conditions: Urea concentration: 0.5 to 10 M, Receptors concentration in chloroform: 0.1 M, Stirring speed = 120 rpm (revolutions per minute) at room temperature.
3.2 Effect of receptor concentration
For optimization of receptor concentration, its concentration was varied from 1 × 10−3M to 1 × 10−1M. At the lower concentration, there was no considerable amount of urea extracted, therefore the optimal concentration of the receptor is 0.1 M and urea interacts with the receptors and results in the formation of urea- receptor complex in the membrane phase. Receptor R2 possesses butyl end group is best extractant.
3.3 Effect of time
Fig. 5 shows the time dependence of urea extraction through liquid membrane containing receptor R2 under the optimized experimental conditions. It is clear from the fig. that the amount of urea extracted was increased with time. Maximum amount of urea extracted was observed within 4–5 h, after this a gradual increase in the amount of extraction up to 20 h and then it becomes constant and observed up to 24 h.
3.4 Back extraction
For back extraction studies, after 24 h of extraction the aqueous phase and organic phase were separated. The organic phase was mixed with 10 mL of double distilled water and the system was stirred for another 24 h and then aqueous phase was analyzed for the amount of urea back extracted. From the results shown in Table 3 and Fig. 4, it is clear that for the urea concentration 1 M–6 M the amount of urea back extracted is higher in comparison to 7 M–10 M. This indicates that the back extraction is also concentration dependent.
Amount of urea back extracted ×10−3M
Conc of urea
Receptors
R1
R2
R3
R4
R5
R6
0.5 M
1.02
0.85
0.95
0.80
0.80
0.60
1 M
10.95
16.60
17.50
18.85
18.45
19.35
2 M
12.17
17.20
18.92
17.47
17.80
18.30
3 M
23.50
44.50
45.00
22.50
24.65
17.15
4 M
109.65
37.15
102.15
57.15
59.65
64.65
5 M
179.65
37.15
87.15
114.65
102.15
122.15
6 M
197.15
9.65
67.15
89.65
77.15
94.65
7 M
222.15
412.15
377.15
282.15
302.15
284.65
8 M
132.15
184.65
242.15
204.65
177.15
189.65
9 M
145.35
200.00
195.65
195.15
275.15
205.50
10 M
300.15
255.75
190.15
185.15
215.50
210.50
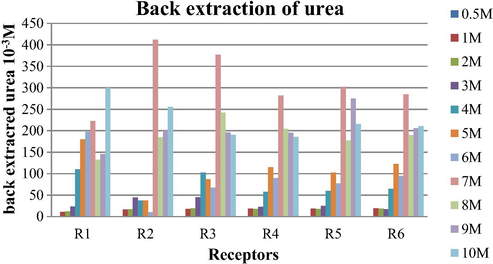
Amount of urea back extracted after 24 h with noncyclic receptors. Conditions: Urea concentration: 0.5–10 M, Receptors concentration in chloroform: 0.1 M, Stirring speed = 120 rpm at room temperature.
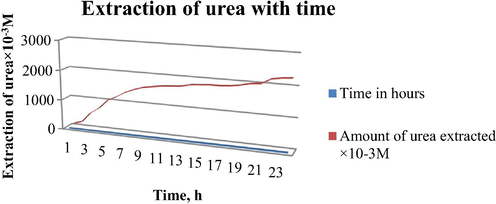
Extraction of urea with time using receptor R2. Conditions: Urea concentration: 7 M, Receptor concentration in chloroform: 0.1 M, Stirring speed = 120 rpm at room temperature.
On the basis of results of back extraction, it is clear that receptors R1- R6 have a tendency to complexation- decomplexation under optimum conditions.
3.5 Transport of urea through liquid membrane system
The results of transport of urea by receptors R1-R6 through chloroform bulk liquid membrane after 24 h are shown in Table 4 and Fig. 6. The sequence of transport of urea by six receptors was observed R6≈R3 > R5 > R4 > R1 > R2. The sudden increase in the amount of urea transported was observed at 7 M concentration for receptor R1, at 9 M for receptor R2 and for the rest it was observed at 6 M. The results of removal of urea indicate that this technique can be used for the removal of urea from feed phase using these receptors.
Conc of urea
Receptors
Amount of urea transported
(×10−3 M) by R1
Amount of urea transported
(×10−3 M) by R2
Amount of urea transported
(×10−3 M) by R3
Amount of urea transported
(×10−3 M) by
R4
Amount of urea transported
(×10−3 M) by
R5
Amount of urea transported
(×10−3 M) by R6
1 M
0.27
2.75
0.95
0.03
0.00
0.12
2 M
2.85
3.70
3.80
0.77
1.05
0.67
3 M
2.17
2.62
4.60
1.80
2.37
1.32
4 M
20.75
7.15
17.55
11.67
23.82
22.00
5 M
33.20
4.90
17.40
14.65
59.65
25.90
6 M
39.40
12.60
122.90
114.05
110.15
123.15
7 M
118.65
21.15
124.90
121.40
124.15
124.90
8 M
121.4
20.9
135.4
131.9
127.4
135.9
9 M
124.9
22.4
139.15
135.4
131.1
138.9
10 M
129.9
25.8
144.9
138.15
135.65
145.15
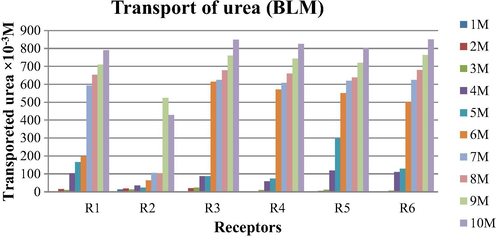
Amount of urea transported by BLM system after 24 h with noncyclic receptors. Conditions: Urea concentration (feed phase): 0.5 to 10 M, Receptors concentration in chloroform (membrane phase): 0.1 M, Stirring speed = 120 rpm at room temperature.
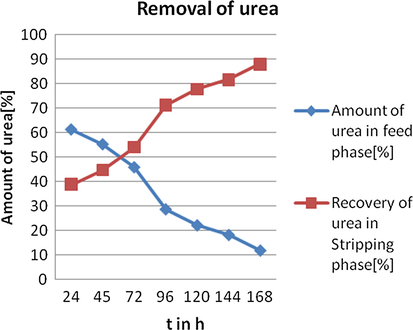
Recyclization of urea through bulk liquid membrane system. Conditions: Urea concentration (feed phase): 7 M, Receptors concentration in chloroform (membrane phase): 0.1 M, Stirring speed = 120 rpm at room temperature.
The transport efficiency of reactive liquid membranes was improved by structural variations in the receptors i.e. change in end group and by varying chain length of the backbone of receptors. Receptor R6 possesses a tetraethylene glycol backbone hence flexible with more number of donor sites which enhances the transport efficiency. Receptor R3 possesses same number of donor sites as R6 and shows almost same transport efficiency.
The results of transport of urea through supported liquid membrane (SLM) studies with different receptors are shown in Table 7 and Fig. 8. No effective transport was observed in concentration range from 1 M to 5 M of urea. As we increase the concentration of urea from 6 M to 9 M the transport efficiency of six different receptors for urea has also increased. Flux values are shown in Tables 5 and 8 for BLM and SLM system respectively.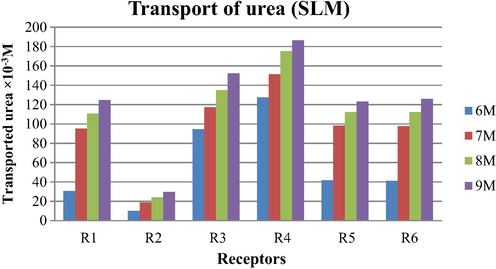
Amount of urea transported by SLM system after 24 h with noncyclic receptors. Conditions: Urea concentration (feed phase): 0.5–10 M, Receptors concentration: 0.1 M, Stirring speed = 120 rpm at room temperature.
Conc of urea
Receptors
Jm[×10−7] (mol/m2/s) by R1
Jm[×10−7] (mol/m2/s) by R2
Jm[×10−7] (mol/m2/s) by R3
Jm[×10−7] (mol/m2/s) by R4
Jm[×10−7] (mol/m2/s) by R5
Jm[×10−7] (mol/m2/s) by R6
1 M
1.00
13.00
4.00
0.00
0.00
0.00
2 M
14.00
18.00
19.00
3.00
5.00
3.00
3 M
10.00
13.00
23.00
9.00
11.00
6.00
4 M
103.00
35.00
87.00
58.00
119.00
110.00
5 M
166.00
24.00
87.00
73.00
298.00
129.00
6 M
197.00
63.00
614.00
570.00
550.00
500.00
7 M
593.00
105.00
624.00
607.00
620.00
624.00
8 M
652.00
104.00
677.00
659.00
63.00
679.00
9 M
710.00
523.00
760.00
742.00
720.00
764.00
10 M
789.00
428.00
849.00
825.00
803.00
850.00
Time in hour
Amount of urea in feed phase [%]
Recovery of urea in Stripping phase [%]
24
61.33
38.67
45
55.3
44.7
72
45.92
54.08
96
28.72
71.28
120
22.18
77.82
144
18.25
81.75
168
11.87
88.16
Conc of urea
Receptors
Amount of urea transported
Amount of urea transported
Amount of urea transported
Amount of urea transported
Amount of urea transported
Amount of urea transported
(×10−3M)by R1
(×10−3M)by R2
(×10−3M) by R3
(×10−3M) by R4
(×10−3M) by R5
(×10−3M) by R6
6 M
118.9
40.15
369.4
498.15
162.65
160.4
7 M
371.65
73.65
457.65
590.65
383.65
381.65
8 M
432.4
94.15
526.4
684.15
437.65
438.15
9 M
487.4
115.65
594.9
728.15
481.4
491.65
Conc of urea
Receptors
Jm[×10−5] (mol/m2/s)
by R1
Jm[×10−5] (mol/m2/s)
by R2
Jm[×10−5] (mol/m2/s)
by R3
Jm[×10−5] (mol/m2/s)
by R4
Jm[×10−5] (mol/m2/s)
by R5
Jm[×10−5] (mol/m2/s)
byR6
6 M
30.43
10.27
94.56
127.52
41.63
41.06
7 M
95.14
18.85
117.15
151.2
98.21
97.7
8 M
110.69
24.1
134.75
175.14
112.03
112.16
9 M
124.77
29.6
152.29
186.4
123.23
125.86
4 Conclusion
The efficiency of six different receptors (R1 to R6) for extraction and transport of urea were studied. Non- cyclic receptor R2 containing butyl end group is best extractant while receptor R6 with flexible backbone is best carrier. Receptor R3 and R6 having same number of donor sites and show equal transport efficiency. The results here led to the conclusion that the structure and design of receptors/carrier (end group and flexible backbone) play an important role in separation and results may help in designing of more specific carrier for the substrate. The BLM system is more effective for transport of urea than SLM system as 88.16% urea removed from urea contaminated water through BLM by recyclization process.
Acknowledgements
We are thankful to Prof. and Head Dr. Shubha Jain, School of Studies in Chemistry and Biochemistry, Vikram University, Ujjain for providing research facilities.
References
- Napthaquinone derived ionophores: interaction with biologically important metal ions (Li+, Na+, K+, Mg2+ and Ca2+) J. Incl. Phenom. Macrocycl. Chem.. 2014;79:465-471.
- [Google Scholar]
- Selective bulk liquid membrane carrier facilitated transport of Mg2+ over Li+, Na+, K+ and Ca2+ metal ions using naphthaquinone derived redox-switchable ionophores. Main Group Met. Chem.. 2017;40:27-33.
- [Google Scholar]
- Large-scale virtual screening for the identification of new helicobacter pylori urease inhibitor scaffolds. J Mol Model.. 2012;18:2917-2927.
- [Google Scholar]
- Redox chemical switching of cation transport in liquid membrane system. Main Group Met. Chem. 2008:203-210.
- [Google Scholar]
- Extraction and recovery of lignosulfonate from its aqueous solution using bulk liquid membrane. J. Membrane Sci.. 2009;330:135-144.
- [Google Scholar]
- Urea-formaldehyde resins : production, application, and testing. Mater. Sci. Eng.. 2017;223:012053
- [Google Scholar]
- A novel preconcentration procedure using cloud point extraction for determination of lead, cobalt and copper in water and food samples using flame atomic absorption spectrometery. Food Chem Toxicol.. 2010;48:1399-1404.
- [Google Scholar]
- Amino acid resolution using supported liquid membranes. Sep. Purif. Technol.. 2005;42:201-211.
- [Google Scholar]
- Binding of urea and thiourea with a barbiturate derivative : experimental and theoretical approach. J. Phys Chem.. 2010;114(1):97-104.
- [Google Scholar]
- A new process for the treatment of fertilizer effluent using immobilized urease. Bioprocess. Eng.. 1997;16:83-85.
- [Google Scholar]
- A Critical Appraisal of the evolution of N-nitrosoureas as anticancer drugs. Chem. Rev.. 1997;97(3):829-1013.
- [Google Scholar]
- Calixarenes RSC. Cambridge University; 1989.
- Enzyme textile for removal of urea with coupling process : enzymatic reaction and electrodialysis. Desalination. 2002;144:164-167.
- [Google Scholar]
- Evaluation of a urea bioelectrochemical system for wastewater treatment process. ACS Sustain. Chem. Eng.. 2014;2:749-754.
- [Google Scholar]
- Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc.. 1967;89:7017-7036.
- [Google Scholar]
- A non-ideal rate-based model for industrial urea thermal hydrolyser. Chem. Eng. Proc.. 2004;43:1299-1307.
- [Google Scholar]
- Studies on the effects of some additives on the physicomechanical properties of urea-ammonium sulphate (UAS) pellets. Fertilizer Res.. 1994;38:89-93.
- [Google Scholar]
- Extraction and transport of amino acids using kryptofix 5 as carrier through liquid membrane. J. Chem.. 2013;2013:1-7.
- [CrossRef] [Google Scholar]
- Electrochemical treatment of aqueous solutions containing urea. J. Appl. Electrochem.. 2009;39:1137-1143.
- [Google Scholar]
- Removal of urea from water using urease- immobilized fibers. J. Chem. Eng. Jpn.. 2013;46:509-513.
- [Google Scholar]
- Pressure assisted ionic liquid dispersive microextraction of vanadium coupled with electrothermal atomic absorption spectrometery. J. Anal. At. Spectrom.. 2013;28:1441-1445.
- [Google Scholar]
- Multidentate acyclic neutral ligands and their complexation. Angew. Chem. Int. Ed.. 1979;18:753-776.
- [Google Scholar]
- Electrocatalytic oxidation of urea in alkaline solution using nickel/nickel oxide nanoparticles derived from nickel-organic framework. Electrochim. Acta.. 2017;17:32235-32311.
- [Google Scholar]
- Urea electro-oxidation efficiently catalyzed by nickel-molybdenum oxide nanorods. Electrochim. Acta.. 2019;295:524-531.
- [Google Scholar]
- Silver – PEGylated dendrimer nanocomposite coating for anti-fouling thin film composite membranes for water treatment. Colloid Surf. A Physicochem. Eng. Asp.. 2013;436:207-214.
- [Google Scholar]







