Translate this page into:
Extracts from Phyllanthus emblica L stem barks ameliorate blood glucose level and pancreatic and hepatic injuries in streptozotocin-induced diabetic rats
⁎Corresponding author. rosnani@usk.ac.id (Rosnani Nasution)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Phyllanthus emblica L is a promising medicinal plant for antidiabetic therapy but current research has not explored its stem bark potential as an antidiabetic agent despite the fact that the stem bark contains rich antioxidants. Herein, we aimed to investigate the antidiabetic potential of P. emblica stem barks in vivo. The dried simplicial powder of the stem barks was macerated sequentially using n-hexane, ethyl acetate, and methanol. The extracts were administered orally to streptozotocin (STZ)-induced diabetic rats, where the blood glucose levels were monitored every week. Histopathological analyses were performed for the pancreas and liver. The results revealed that ethyl acetate extract from P. emblica stem barks could lower the fasting blood glucose levels three weeks post-STZ injection (140.3 ± 38.99 versus 270.0 ± 50.51; p = 0.0375). Significant improvement on pancreas and liver histopathology at p < 0.05 was found in the group receiving P. emblica extracts. The IC50 of the ethyl acetate extract against DPPH free radical was 4.67 ± 1.2 mg/g, with total flavonoids, phenolics, and tannins content of 65.54 ± 0.11 QE mg/g, 35.72 ± 0.5 mg GAE/g, and 134.69 ± 0.7 mg TAE/g, respectively. Taken altogether, the ethyl acetate extract from P. emblica stem barks is potential as an antidiabetic drug candidate.
Keywords
Metabolic syndrome
Antioxidant
Diabetic hepatopathy
Ethyl acetate
Tannins
1 Introduction
The global trend of morbidity and mortality is predominated by type 2 diabetes mellitus (T2DM), which is expected to keep increasing in the next decades (Animaw and Seyoum, 2017). A report revealed that 451 million adults suffered from diabetes in 2017 (Cho et al., 2018). At the current available curative treatments, the number of diabetes-related morbidities are expected to keep increasing and even could reach 693 million cases in 2045 (Cho et al., 2018). Therefore, extensive studies to find more efficacious antidiabetic therapy is crucial to slow down the trend. Drug candidates from natural products are the promising solution (Andalia et al., 2022; Harahap et al., 2022). Metformin, the first line oral therapy for T2DM, is inspired by the discovery of blood glucose lowering extract of Galega officinalis L containing guanidine, galegine, as well as biguanide (Chaudhury et al., 2017). However, there is an urge to discover more efficacious blood glucose lowering drugs other than metformin (Grammatiki et al., 2021; Baker et al., 2021). In this light, Phyllanthus emblica has been reported to possess multiple pharmacological benefits such as antioxidant, anti-inflammatory, and antidiabetic activities (Ahmad et al., 2021).
Previously, researchers have reported that extracts from P. emblica fruits could lower blood glucose level and glycated hemoglobin (HbA1C) (Mohanty et al., 2021; Qureshi et al., 2009). These antidiabetic properties of P. emblica fruits might derived from the presence of antioxidant phytocompounds namely gallic acid, quercetin, kaempferol, and catechin (Saini et al., 2022). An in vitro study suggested that P. emblica fruit extract could decrease advance glycation end products (AGEs), where the extract contained high total phenolic and flavonoid contents (Alsahli et al., 2021). More interestingly, P. emblica fruit extract could inhibit dipeptidyl peptidase-IV (DPP-IV) and followed by the depletion of blood glucose and HbA1c (Mohanty et al., 2021; Borde et al., 2019).
However, the foregoing studies used the fruits, and no studies have reported the antidiabetic potential of P. emblica stem barks. Current published literature focused on the antioxidant and antimicrobial activities of P. emblica stem barks (Adak et al., 2018; Fitriansyah et al., 2018; Chaphalkar et al., 2017, 2017.). In addition to having rich phytocompounds, the stem barks are available throughout the seasons unlike the flowers making them as nearly unlimited resources. This is the first study to unveil the antidiabetic potential of the stem barks of P. emblica through an in vivo study design employing streptozotocin (STZ)-induced diabetic rat model.
2 Materials and methods
2.1 Materials
Plant specimen was collected from Aceh Besar Region, Aceh Province, Indonesia (Fig. 1) and the taxonomy was confirmed at Biology Laboratory, Universitas Syiah Kuala, Indonesia in October 2021. The cortex or stem bark was selected by examining its apparent maturity and removal easiness.
Geographical map of the plant specimen collection site (N: 503′1.2″- 5045′9.007″; E: 95055′43.6″ − 94059′50.13″).
Other materials included streptozotocin (STZ), carboxymethyl cellulose (CMC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), n-hexane, ethyl acetate, methanol, ethanol, NaHCO3, Na2CO3, Folin–Ciocâlteu reagent, potassium acetate, and AlCl3. All chemicals used were analytical grade and supplied from Merck (Selangor, Malaysia).
2.2 Extraction
The separated stem barks were air-dried for 24 h and subsequently oven-dried at 40–50 °C for 24 h. Thereafter, the stem barks were crushed into small pieces using an electronic crusher and filtered through a 60-mesh sieve to obtain the simplicial powder. As much as 400 g simplicial powder was initially macerated using n-hexane in a sealed glass container, stirred every 6 h and the filtrate was collected every 72 h. This procedure was repeated three times to obtain the n-hexane extract of P. emblica. The residue was sequentially re-macerated using ethyl acetate and methanol using the same protocols to produce ethyl acetate and methanol extracts, respectively. The filtrate of each extract was concentrated using a rotary evaporator and a water bath until the extract paste was produced.
2.3 Animal model and intervention
This study used 18 male Rattus norvegicus (Berkenhout) aged 2–3 months with body weights ranging from 200 to 300 g. The animals were kept in a cage with rice husk and size of 30x40x30 cm3 for every 4 rats. The animals were acclimated to the environment for 1 week and food and water were given ad libitum. During the adaptation phase, the animal was given feed that was comprised of 5% cheese, 10% egg yolk, 15% cow’s fat, 5% vegetable oil, 45% rice, and 20% commercial feed. The feed composition was chosen so that the feed would be rich in fat. Prior to the study, all animals were weighed to ensure the body weigh remained in the inclusion criteria.
All rats were randomized and evenly allocated into six groups. The randomization was performed based on the list generated on a web-based randomizer tool (https://www.randomizer.org/). The group allocations were blinded to the investigators performing the intervention and data collection. The six groups were labeled as normal (not receiving both STZ and treatment), STZ (receiving STZ injection only – negative control), metformin (receiving metformin therapy – positive control), n-hexane (receiving n-hexane extract from P. emblica stem barks), ethyl acetate (receiving ethyl acetate extract from P. emblica stem barks), and methanol (receiving methanol extract from P. emblica stem barks). Significant reduction of body weight and aggressive behavior were set as the humane endpoint.
Intraperitoneal injection of STZ was administered to all rats, except those in group normal. The STZ dosage used was 30 mg/kg body weight in a suspension of CMC 0.5%, as suggested by a previous study (El Maleky et al., 2021). The administration of STZ was performed after the rats were fastened for 10 h. The level of fasting blood glucose (FBG) was determined on day-4 post- STZ induction. Rats with FBG > 200 mg/dL were considered eligible for further intervention using metformin or P. emblica extracts. The therapies were administered once a day with a dosage of 60 mg/kg body weight for metformin and 200 mg/kg body weight (suspended in CMC 1%) for the extracts. The decision on selecting the extract dose was based on our preliminary study (unpublish), where we found that 200 mg/kg body weight was the optimum concentration and higher concentration resulted in toxic effects. The administration of the therapies was performed orally and assisted by nasogastric tube. During the intervention period, the animal was fed with standardized commercial feed. The venous blood from each rat was collected in alcohol-sterilized settings to measure the level of FBG. All rats were euthanized via cervical dislocation for organ histopathology analysis.
2.4 Determination of blood glucose level
Drawn fresh blood from the rat’s tail vein was used to determine the blood glucose level using an enzymatic assay-based commercial kit – GlucoDr.auto© following the instruction from the manufacturer (MDSS GmbH, Hannover, Germany).
2.5 Histopathological analysis
The histology slides of the liver and pancreas were stained using Mayer’s hematoxylin and Eosin. The observation was carried out under an Olympus CX21 light microscope (Waltham, MA, USA) with a magnification of 40x10 times. The occurrence of fat degeneration, hemorrhage, necrosis, and infiltration of inflammation cells were counted.
2.6 DPPH assay
2,2-diphenyl-1-picrylhydrazyl (DPPH) solution with a concentration of 40 μg/mL was initially prepared by dissolving DPPH powder (molecular weight of 394.32 g/mol) into n-hexane, ethyl acetate, and methanol solvents, respectively. Each extract from P. emblica was made varied in concentrations after the dissolution using their respective solvent (for example, the n-hexane extract was diluted in n-hexane). DPPH (1 mL) that had been dissolved in n-hexane, ethyl acetate, and methanol was mixed with the diluted n-hexane, ethyl acetate, and methanol extracts (5 mL), respectively. The mixture was homogenized on a vortex mixer and subsequently incubated at 37 °C for 31 min. The absorbance of the incubated mixture was measured on UV–vis spectrophotometer at 517 nm. The median inhibitory concentration (IC50) was calculated based on the DPPH scavenging activities by each extract at a concentration ranging from 2 to 10 mg/L. The positive control for this assay was ascorbic acid, in which its IC50 was calculated from the same concentration range. The protocol for the DPPH assay was in line with that used in a previous study (Yusnaini et al., 2023).
2.7 Determination of total phenolic content
Each extract from P. emblica stem barks, weighed 10 mg, was diluted in 10 mL pro-analytical ethanol. Subsequently, the diluted extract was pipetted as much as 100 μL and added into a reaction tube containing distilled water (1 mL), Folin–Ciocâlteu reagent (10%), and NaHCO3 2% (1.5 mL). The mixture was measured for its absorbance using UV–vis spectrophotometer at 763 nm. The protocol for the total phenolic content determination followed the suggestion from a previous study (Yusnaini et al., 2023).
2.8 Determination of total flavonoid content
TFC was determined following the recommendation from a previous study (Yusnaini et al., 2023). Each extract from P. emblica stem barks was weighed for 10 mg and subsequently added into a reaction tube containing 1 mL ethanol. Thereafter, 1 mL AlCl3 2% and 1 mL potassium acetate 120 nM were added to the reaction tube as the reagents before being incubated for 1 h. The absorbance of the mixture was measured on a UV–Vis spectrophotometer at 435 nm. The total flavonoid content (TFC) was expressed as quercetin equivalent (QE).
2.9 Determination of total tannin content
Each extract (0.5 g) was dissolved in double-distilled water until the volume reached 10 mL, where a sonicator was used to assist the homogenization if necessary. In a reaction tube consisting of 10 mL dissolved extract, Folin–Ciocâlteu reagent was added as much as 0.5 mL and left for 3 min before being added with 1 mL saturated Na2CO3. The mixture was incubated for 15 min, and the absorbance was subsequently measured on a UV–Vis spectrometer at 740 nm. The total tannin content (TTC) was expressed as tannic acid equivalent (TCE). The protocol used in the measurement of TTC was based on a previous study (Qarani et al., 2023).
2.10 Statistical analysis
Quantitative data obtained from the aforementioned protocols were presented as mean ± standard deviation (SD). The ANOVA and post-hoc Tukey analysis were performed to reveal the statistical significance between groups. All statistical data were computed on GraphPad Prism 9.2.0 software (GraphPad Software, San Diego, CA, USA). Statistical significance was considered reached if p < 0.05.
3 Results
3.1 The effect of P. emblica extract on blood glucose levels
The effects of P. emblica extract obtained by using various solvents through a sequential maceration on the fasting blood glucose (FBG) of STZ-induced diabetic rats are presented in Table 1. The FBG levels were elevated rapidly following the injection of STZ, resulting in a significant difference in average FBG levels between normal and STZ groups (p < 0.05). Even though it remained statistically significant at p < 0.05, these numbers were becoming less different with respect to observation time, suggesting the acute diabetic condition induced by the STZ. Administration of metformin as a positive control did not significantly reduce the FBG, though the numbers appear lower in metformin group than in STZ group. On Week 0, metformin group appeared to have higher FBD level than that in STZ group, but without statistical significance. Among the three extracts, the one obtained from ethyl acetate solvent was found to be able to significantly lower the FBG level (p < 0.0231) within 4 weeks of treatment and observation.
Group
Fasting blood glucose (mg/dL)
Week 0
Week 1
Week 2
Week 3
Week 4
Mean
SD
p-valuea
Mean
SD
p-valuea
Mean
SD
p-valuea
Mean
SD
p-valuea
Mean
SD
p-valuea
Normal
108.7
16.86
Ref
110.3
12.58
Ref
112.7
11.93
Ref
106.7
9.866
Ref
99.67
3.512
Ref
STZ
509.7
125.0
0.0035b*
336.0
93.72
0.0078b*
277.0
72.51
0.0185b*
270.0
50.51
0.0080b*
195.0
19.97
0.0077b*
Metformin
537.7
125.2
0.9992
328.0
52.46
>0.9999
254.3
50.56
0.9929
206.7
32.87
0.5423
158.3
33.17
0.5445
n-Hexane
415.3
57.93
0.8447
268.0
66.78
0.7560
292.7
84.39
0.9987
226.0
81.50
0.8297
182.0
44.31
0.9882
Ethyl acetate
493.0
75.72
>0.9999
207.3
79.78
0.1851
194.0
8.815
0.3985
140.3
38.99
0.0375*
113.7
15.04
0.0231*
Methanol
447.3
135.4
0.9677
288.3
21.50
0.9271
289.7
21.22
0.9995
242.0
15.72
0.9690
187.7
19.66
0.9992
3.2 The effect of P. emblica extract on histopathological profile of the pancreas
The results of histopathological analysis on the effect of P. emblica stem bark extracts on the pancreas are presented in Table 2. Number of necrosis and hemorrhage were significantly higher in STZ-induced diabetic rats (p < 0.001). The numbers reduced significantly when treated with metformin (p = 0.0001). Similarly, rats in groups n-hexane, ethyl acetate, and methanol experienced a significant decrease in cells with necrosis and hemorrhage (p < 0.05). The histopathological profiles of the pancreas between group metformin and n-hexane or ethyl acetate were identical (p > 0.05). Nevertheless, a significant difference at p < 0.05 was found between group metformin and methanol, where less amelioration observed in group methanol. The histopathological images of pancreas of STZ-induce diabetic rats treated with P. emblica stem bark extracts are presented in Fig. 2.
Group
Necrosis
Hemorrhage
Mean ± SD
p-valuea
Mean ± SD
p-valuea
Normal
5.778
0.5092
Ref
0.7778
0.3849
Ref
STZ
22.78
1.644
< 0.0001*
11
3.333
< 0.0001*
Metformin
11
0.6667
< 0.0001*
2.556
0.5092
0.0001*
n-Hexane
13.78
1.540
< 0.0001*
5.556
0.1925
0.0066*
Ethyl acetate
12.44
0.6939
< 0.0001*
2.778
0.6939
0.0002*
Methanol
17.44
1.836
0.0025*
6.667
0.8819
0.0316*
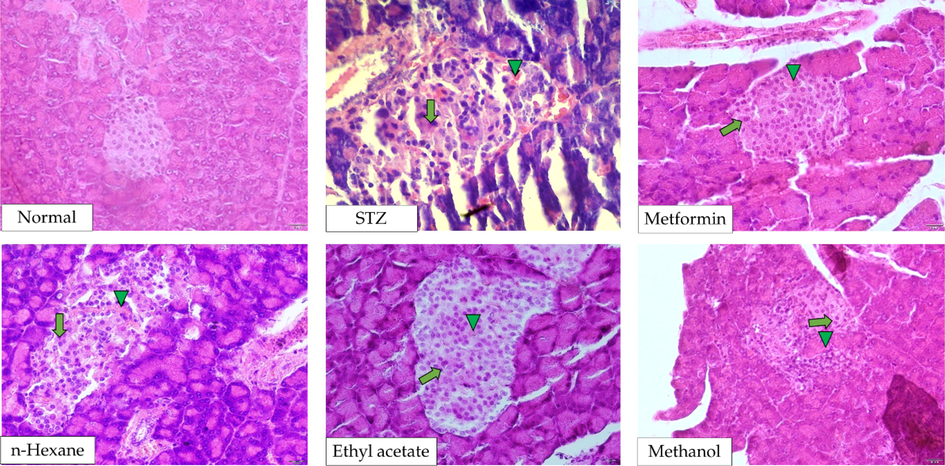
Histopathological images of pancreas after the administration of P. emblica extracts into STZ-induced diabetic rats. Necrosis and hemorrhage are indicated by arrow (↑) and triangle (▲) symbols, respectively.
3.3 The effect of P. emblica extract on histopathological profile of the liver
Drastic elevation of pathological findings (p < 0.0001) in the liver histology occurred once the STZ was injected into the rats (Table 3). Interestingly, the numbers of necrosis, inflammatory cells, and congestion reduced significantly (p < 0.0001) after the oral administration of metformin or P. emblica stem bark extracts. Among which, the lowest abnormality occurrence was found in group ethyl acetate. In fact, the p-value of group metformin versus ethyl acetate, in terms of cell necrosis, reached a statistical significance of p = 0.0439 (Table 3). The photographed representations of this analysis are presented in Fig. 3.
Group
Necrosis
Inflammation cell
Congestion
Mean ± SD
p-valuea
Mean ± SD
p-valuea
Mean ± SD
p-valuea
Normal
6.44 ± 1.02
Ref
9.89 ± 1.35
Ref
7.889 ± 1.07
Ref
STZ
47.89 ± 1.54
< 0.0001b
*37 ± 1.73
< 0.0001b
*35.67 ± 0.67
< 0.0001b*
Metformin
16 ± 2.85
< 0.0001*
17.33 ± 0.67
< 0.0001*
10.78 ± 1.50
< 0.0001*
n-Hexane
18.22 ± 1.58
< 0.0001*
17.89 ± 1.17
< 0.0001*
15.11 ± 1.26
< 0.0001*
Ethyl acetate
11.44 ± 1.17
< 0.0001*
18.22 ± 1.35
< 0.0001*
9.22 ± 0.84
< 0.0001*
Methanol
20.33 ± 0.67
< 0.0001*
22.22 ± 1.26
< 0.0001*
17.56 ± 1.58
< 0.0001*
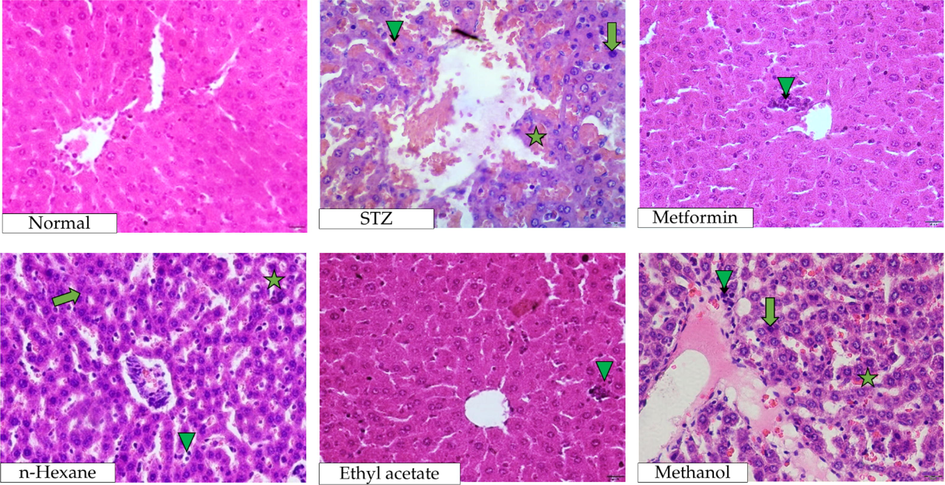
Histopathological images of liver after the administration of P. emblica extracts into STZ-induced diabetic rats. Necrosis, congestion, and inflammation are indicated by arrow (↑), star (★), and triangle (▲) symbols, respectively.
3.4 Antioxidant profile of P. emblica extracts
Antioxidant activity of the extracts were evaluated based on their ability in scavenging free radical DPPH, where the IC50s are presented in Fig. 4a. From the most active to the least active, the order is ethyl acetate > methanol > n-hexane. While the methanol extract has an IC50 of 5.99 ± 1.5 mg/g, the IC50 of the ethyl acetate extract is 4.67 ± 1.2 mg/g. The TFC, TPC, and TTC of each P. emblica extract have been presented in Fig. 4b—c. Despite being the most potent antioxidant in this present study, the ethyl acetate extract has the lowest TFC with only 65.54 ± 0.11 mg QE/g, whilst n-hexane and methanol extracts have TFC of 82.62 ± 0.08 and 99.52 ± 0.16 mg QE/g, respectively. Uniquely, the extract with the highest TPC is the one obtained using n-hexane (42.64 ± 0.17 mg GAE/g), followed by those using ethyl acetate (35.72 ± 0.5 mg GAE/g) and methanol (35.51 ± 0.5 mg GAE/g). The TTC was revealed to be the highest in ethyl acetate extract with a value of 134.69 ± 0.7 mg TAE/g, where methanol and n-hexane extracts of P. emblica stem barks have TCC of 83.06 ± 0.5 and 48.71 ± 1.77 mg TAE/g, respectively.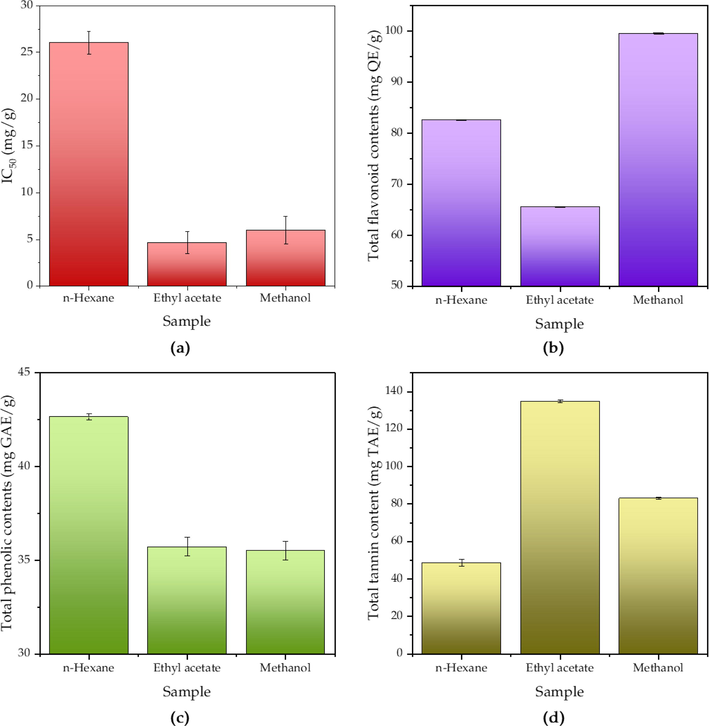
Antioxidant activity (a), total flavonoid content (b), total phenolic content (c), and total tannin content (d) of P. emblica extracts with respect to their solvents. The antioxidant activity is based on DPPH scavenging assay and expressed as median inhibitory concentration (IC50), with ascorbic acid as the positive control yielding IC50 = 9.59 mg/g.
4 Discussion
Results from this present study suggest that the ethyl acetate extract from P. emblica stem barks has a therapeutical efficacy against hyperglycemia in STZ-induced diabetic rats. STZ induces acute hyperglycemia and hypoinsulinemia attributed to its toxicity against pancreatic β cells (Lenzen, 2008). Herein, in addition to reducing the level of blood glucose, the ethyl acetate extract also significantly lowered the cell damage in the pancreas. The toxicity of STZ has been associated with its ability to alleviate oxidative molecules by lowering the synthesis of adenosine triphosphate (ATP) concomitant to DNA fragmentation (Szkudelski, 2001). Moreover, the release of nitric oxide could occur in N-methyl-N-nitrosourea side chain of the STZ (Friederich et al., 2009). Nitric oxide species (NOS), reactive oxygen species (ROS), and inflammatory reactions are thought to be responsible for the STZ-induced cellular damage (Friederich et al., 2009; Van Dyke et al., 2008). Improving the oxidative stress and inflammatory responses have been suggested as the therapeutical targets for both type 1 and type 2 diabetes (Tsalamandris et al., 2019).
In addition to the improvement of glycemia and pancreas, herein, we found the extracts of P. emblica stem barks are capable of ameliorating the diabetic hepatopathy. Our findings are in line with the previous study suggesting the ability P. emblica stem barks in ameliorating hepatic injury induced by ethanol in rat model (Chaphalkar et al., 2017). Patients with diabetes are at risk in developing advance liver diseases (Tolman et al., 2007). A review article has even called the relationship between diabetes and liver disease as ‘ominous’ (Moscatiello et al., 2007). Liver has been long suggested to play a key role in the pathogenesis of type 2 diabetes (DeFronzo, 2004). Steatosis, fat accumulation in the liver, underlies the reduction of hepatic insulin sensitivity which subsequently cause the hyperglycemic condition (Sattar and Gill, 2014).
To further investigate the underlying benefits of P. emblica stem bark extracts as antidiabetic agents, we evaluate the antioxidant capacity of the extracts. Herein, we found the extracts, especially ethyl acetate and methanol extracts, are potent antioxidant based on free radical DPPH scavenging activity. Antioxidant activity of an extract is usually dependent on the content quantities of flavonoids, phenols, and tannins (Halim et al., 2022; Sriwatcharakul, 2020). In this present study, ethyl acetate extract from P. emblica stem barks, the one with the highest antidiabetic potential, has a significantly higher quantity of tannin content as compared to others. This finding suggests that its antioxidant activity is dependent on the tannin content. Tannins as antioxidants have two underlying mechanisms in reducing the oxidative reaction; 1) acting as a primary antioxidant by donating hydrogen atom or electrons and 2) interfering the Fenton reaction by forming a chelation with metal ions including Fe(II) (Karamac et al., 2006; Amarowicz, 2007). The latter is more likely to be associated with the improvement of liver function as Fenton reaction produces ROS in the liver (Chen, 2022). It is worth noting that some in vitro and in vivo studies have found the positive correlation between non-alcoholic fatty liver disease and excessive free iron in the liver which contributes to the generation of oxidative stress and consequently increases the insulin resistance (Jahng et al., 2019; Dongiovanni et al., 2011). In a systematic review and studies cited therein, P. emblica fruit have been suggested to possess rich content of flavonoids, phenols, and tannins that are associated with its ability in improving diabetes (Huang et al., 2021).
Our study is the first reporting the antidiabetic properties of P. emblica stem barks in vivo, which is the strength of this study. We also found that the ethyl acetate extract is somewhat more efficacious in comparison with metformin. Nonetheless, there are some limitations in our study including the use of crude extract that limits us to conclude the role of specific phytometabolites (such as tannins) in attenuating hyperglycemia and diabetes-associated pancreas and liver damages. Moreover, we did not investigate the efficacy with a concentration range - only using a single dose. Additionally, we did not measure other blood markers such as ROS, inducible NOS, C-reactive protein, and advance glycation end products which could be essential to exhibit the anti-inflammatory activity of the extract.
5 Conclusion
P. emblica stem bark extracts, especially the one obtained using ethyl acetate, were potential as antidiabetic therapy. The ethyl acetate extract was the most potent candidate for antidiabetic agent since it could lower the blood glucose level more efficaciously than metformin, based on four-weeks treatment. The ethyl acetate extract was also found capable of ameliorating STZ-induced pancreas and liver damages, even somewhat more efficacious than metformin, which is important to reduce the complications in diabetes. Antioxidant property of the ethyl acetate extract was found high, which was predominantly contributed by the presence of tannins. Further research is required to investigate the antidiabetic potential of ethyl acetate extract from P. emblica stem barks at higher purity level.
6 Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Veterinary Ethics Committee of Universitas Syiah Kuala (149/KEPH/VI/2022 16 June 2022).
CRediT authorship contribution statement
Quranayati Quranayati: Conceptualization, Methodology, Investigation, Resources, Writing – original draft. Muhammad Iqhrammullah: Data curation, Writing – original draft, Visualization. Nurdin Saidi: Conceptualization, Writing – review & editing. Nurliana Nurliana: Conceptualization, Writing – review & editing. Rinaldi Idroes: Validation, Formal analysis, Writing – review & editing. Rosnani Nasution: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparison of the antimicrobial activity of the phyto-constituents obtained from the stem bark and leaf extracts of Phyllanthus emblica L. against different strains of Staphylococcus aureus and Salmonella typhi. Res. J. Pharmacol. Pharmacodyn.. 2018;10:53-60.
- [Google Scholar]
- Phyllanthus emblica: A comprehensive review of its therapeutic benefits. S. Afr. J. Bot.. 2021;138:278-310.
- [CrossRef] [Google Scholar]
- Health promoting effect of Phyllanthus emblica and Azadiractha indica against advanced glycation end products formation. Appl. Sci.. 2021;11:8819.
- [CrossRef] [Google Scholar]
- Amarowicz, R. Tannins: the new natural antioxidants? 2007, 109, 549-551.
- Molecular docking reveals phytoconstituents of the methanol extract from Muntingia calabura as promising α-glucosidase jnhibitors. Karbala Int. J. Mod. Sci. 2022:8.
- [Google Scholar]
- Increasing prevalence of diabetes mellitus in a developing country and its related factors. PLoS One. 2017;12:e0187670.
- [Google Scholar]
- Baker, C.; Retzik-Stahr, C.; Singh, V.; Plomondon, R.; Anderson, V.; Rasouli, N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Therapeutic Advances in Endocrinology and Metabolism 2021, 12, 2042018820980225.
- DPP-4 inhibitory activity and myocardial salvaging effects of Commiphora mukul in experimental diabetes. Int. J. Basic Clin. Pharmacol.. 2019;8
- [CrossRef] [Google Scholar]
- Antioxidants of Phyllanthus emblica L. bark extract provide hepatoprotection against ethanol-induced hepatic damage: A comparison with silymarin. Oxid. Med. Cell. Longev.. 2017;2017
- [Google Scholar]
- Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front. Endocrinol.. 2017;8:6.
- [Google Scholar]
- Iron metabolism in non-alcoholic fatty liver disease: A promising therapeutic target. Liver Res.. 2022;6:203-213.
- [CrossRef] [Google Scholar]
- IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract.. 2018;138:271-281.
- [CrossRef] [Google Scholar]
- Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J. Hepatol.. 2011;55:920-932.
- [Google Scholar]
- Investigation of the impacts of zamzam water on streptozotocin-induced diabetic nephropathy in rats. In-vivo and in-vitro study. Biomed. Pharmacother.. 2021;138:111474
- [CrossRef] [Google Scholar]
- Correlation of total phenolic, flavonoid and carotenoid content of Phyllanthus emblica extract from Bandung with DPPH scavenging activities. Pharmacogn. J. 2018:10.
- [Google Scholar]
- Diabetes, oxidative stress, nitric oxide and mitochondria function. Curr. Diabetes Rev.. 2009;5:120-144.
- [Google Scholar]
- Metformin: is it still the first line in type 2 diabetes management algorithm? Curr. Pharm. Des.. 2021;27:1061-1067.
- [Google Scholar]
- Determination of phytochemical constituent, antioxidant activity, total phenol and total flavonoid of extract ethanol Phyllanthus emblica Fruit. Pharmacogn. J. 2022:14.
- [Google Scholar]
- Antibacterial activities of seven ethnomedicinal plants from family Annonaceae. J. Adv. Pharm. Technol. Res.. 2022;13:148.
- [Google Scholar]
- Potential effect of tropical fruits Phyllanthus emblica L. for the prevention and management of type 2 diabetic complications: a systematic review of recent advances. Eur. J. Nutr. 2021:1-18.
- [Google Scholar]
- Iron overload inhibits late stage autophagic flux leading to insulin resistance. EMBO Rep.. 2019;20:e47911.
- [Google Scholar]
- Chelating of Fe (II), Zn (II) and Cu (II) by tannin fractions separated from hazelnuts, walnuts and almonds. Bromat. Chem. Toksykol.. 2006;39:257-260.
- [Google Scholar]
- The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia. 2008;51:216-226.
- [Google Scholar]
- Antidiabetic activity of Commiphora mukul and Phyllanthus emblica and Computational analysis for the identification of active principles with dipeptidyl peptidase IV inhibitory activity. Indian J. Pharmacol.. 2021;53:384-387.
- [CrossRef] [Google Scholar]
- Diabetes and liver disease: An ominous association. Nutr. Metab. Cardiovasc. Dis.. 2007;17:63-70.
- [CrossRef] [Google Scholar]
- Antioxidant and antiaging activity of Cinnamomum burmannii and Michelia champaca extract and combinations. Narra. J.. 2023;3:e111.
- [Google Scholar]
- The effect of Phyllantus emblica Linn on type - II diabetes, triglycerides and liver - specific enzyme. Pak. J. Nutr.. 2009;8:125-128.
- [CrossRef] [Google Scholar]
- Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol.. 2022;282:114570
- [CrossRef] [Google Scholar]
- Evaluation of bioactivities of Phyllanthus emblica seed. Energy Rep.. 2020;6:442-447.
- [Google Scholar]
- The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res.. 2001;50:537-546.
- [Google Scholar]
- Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30:734-743.
- [Google Scholar]
- The role of inflammation in diabetes: current concepts and future perspectives. Eur. Cardiol. Rev.. 2019;14:50.
- [Google Scholar]
- Luminescence experiments involved in the mechanism of streptozotocin diabetes and cataract formation. Luminescence. 2008;23:386-391.
- [Google Scholar]
- Yusnaini, R.; Nasution, R.; Saidi, N.; Arabia, T.; Idroes, R.; Ikhsan, I.; Bahtiar, R.; Iqhrammullah, M. Ethanolic Extract from Limonia acidissima L. Fruit Attenuates Serum Uric Acid Level via URAT1 in Potassium Oxonate-Induced Hyperuricemic Rats. Pharmaceuticals 2023, 16, 419.







