Translate this page into:
Fabrication and characterizations of nanocomposite flexible films of ZnO and polyvinyl chloride/poly(N-vinyl carbazole) polymers for dielectric capacitors
⁎Corresponding authors at: Department of Physics, Faculty of Education and Applied Sciences at Arhab, Sana’a University, Sana’a, Yemen (A. Al-Muntaser); Nuclear Engineering Department, Faculty of Engineering, King Abdulaziz University, Jeddah 21589, Saudi Arabia (E. Banoqitah); Department of Physics, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia (A. Saeed). almuntser2015@gmail.com (A.A. Al-Muntaser), ali.almuntasser@su.edu.ye (A.A. Al-Muntaser), ebanoqitah@kau.edu.sa (Essam Banoqitah), Abdusaeed@tu.ed.ye (Abdu Saeed) Abdusaeed79@hotmail.com (Abdu Saeed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polymer-based energy storage has recently attracted the attention of researchers. Herein, via the sol–gel method, we prepared zinc oxide nanoparticles (ZnO NPs), as nanofiller up to 5 wt% filling levels, and a host polymeric blend matrix of polyvinyl chloride (PVC) and poly (N-vinyl carbazole) (PVK) were taken to synthesize nanocomposite films via the solution casting. The ratio of PVC: PVK was 90:10 wt%. The X-ray diffraction (XRD) results indicated that the prepared nanocomposite films’ crystallinity decreased as ZnO NPs content increased; it decreased from 54.23 to 22.14 %. The scanning electron microscope (SEM) micrographs revealed good miscibility of the prepared PVC/PVK blend and the homogeneous distribution of ZnO NPs within the host blend matrix. The thermal stability of the prepared nanocomposites was studied through the TGA technique, and the thermal activation energy was calculated using the Coats-Redfern method. In the optical results, the optical absorption spectra revealed that the energy gap for the allowed direct transition reduced under adding ZnO NPs; it reduced from 3.61 eV for the pure PVC/PVK blend to 2.96 eV for the nanocomposite film with ZnO nanofiller content of 5 wt%. AC conductivity experiments revealed that the electrical/dielectric properties of the nanocomposite films get enhanced by increasing the ZnO NPs content. Following the incorporation of the ZnO NPs, the AC electrical conductivity (σac), dc electrical conductivity (σdc), dielectric constant (ε′), and dielectric loss (ε″) values of PVC/PVK/ZnO nanocomposite films get improved; the σdc values increased from 6.46 × 10−16 S/cm for the blend PVC/PVK matrix to 3.63 × 10−13 S/cm for nanocomposites with ZnO NPs content of 5 wt%. Additionally, both ε′ and ε″ values increased while increasing the ZnO NPs content. These findings could suggest that the prepared PVC/PVK/ZnO nanocomposite films could be a promise for the applications of dielectric energy storage polymer-based capacitors.
Keywords
PVC
PVK
ZnO nanoparticles
Nanocomposites
Energy storage
1 Introduction
Polymer-based energy storage has become a popular topic among researchers lately (Michael and Prabaharan, 2004; Menazea et al., 2020; Tarabiah et al., 2022; Morsi et al., 2022; Al-Muntaser et al., 2023; Al-Muntaser et al., 2023; El Gohary et al., 2023; Saeed et al., 2023), as they offer a better alternative choice for electrical power systems and modern electronic applications. They have unique advantages, such as working at high voltage, low cost/simple processing, and fast charge/discharge (Liu et al., 2016). Therefore, the fabricating and processing of new polymers for polymer-based energy storage will contribute to the solution energy problem.
Polyvinyl chloride (PVC) is a valuable industrial polymer with numerous applications (Nikam and Deshpande, 2018). As a result of its low cost, high recovery rate, and excellent electrical and corrosion resistance (Miliute-Plepiene et al., 2021), it is commonly used in the industry. PVC is commonly mixed with several polymers (Zhang et al., 2020; Ranjan et al., 2021; Yang et al., 2009). The organic semiconducting polymer poly(N-vinyl carbazole) (PVK) contains molecules consisting of repeating groups of atoms (Das et al., 2017). It is the most vastly recognized polymeric photoconductor (Ahlatcıoğlu Özerol, 2019); here, its carbazole group allows it to be used in optical and electric devices (Lai et al., 2005; Choudhury et al., 2004). PVK has high thermal conductivity, low optical absorption, and a high refractive index in visible and infrared ranges (Alghunaim, 2018). Its photophysical characteristics make it an ideal polymer for electro-optically active material in xerography (Moisan et al., 1991) and light-emitting diodes (Das et al., 2016; Wang et al., 2000). Recently, it has been utilized as a matrix for assessing low molecular weight compounds. In the field of energy storage, it was used to design lithium-ion batteries (Menazea et al., 2020) and rechargeable lithium batteries based on hybrid polymer electrolytes (Michael and Prabaharan, 2004).
PVK is a carbazole derivative of PVC, where the chlorine atom in PVC [(C2H3Cl)n] is replaced by the carbazole group (C12H9N) to form PVK [(C14H11N)n] (Michael and Prabaharan, 2004). Therefore, they can be blended to produce composites with excellent compatibility. In most cases, mixing two or more polymers is cheaper and faster than discovering novel polymer chemistry to advance novel polymer composite with unique properties (El Miri et al., 2016). Many studies (Alghunaim, 2018; Krylov et al., 2016; Berestennikov and Aleshin, 2017) were concerned with analyzing the physical and chemical properties of the doped PVC/PVK mix with nanoparticles and carbon materials.
Nanoparticles (NPs) in the polymer can create nanocomposites exhibiting enhanced physical properties (Al-Muntaser et al., 2022b, 2022c; Bet-Moushoul et al., 2016). As a result of their blend of parts, nanocomposites can exhibit properties with new behaviors (Pleşa et al., 2016). A new substitute for conventionally filled polymers is introduced via nanocomposite of polymer and NPs (El-Kader et al., 2013). The development of novel and cutting-edge functions within the polymer matrix is made possible by including both organic polymers and NPs through these NPs. Alghunaim studied (Alghunaim, 2018) the impact of silicon carbide (SiC) NPs on the structural and optical properties, besides the electrical characteristics of the PVC/PVK blend, was studied. When the filler percentages boosted from 0.00 to 0.08 wt%, they discovered that SiC decreased the values of the energy gap from 3.82 to 0.73 eV. Besides, the PVC/PVK composite exhibited improved AC electrical conductivity as a result of the increased SiC content (Alghunaim, 2018). The impact of lead(II) oxide (PbO) NPs on the mechanical and thermal characteristics of a composite PVC/PMMA material has been studied by Gamal and Elsayed (El-Gamal and Elsayed, 2020), where the PVC/PMMA composite’s thermal stability and thermal expansion were improved.
Zinc oxide (ZnO) nanoparticles (NPs) possess remarkable electrochemical and physicochemical properties, particularly their optical characteristics and chemical stability (Tarabiah et al., 2022). It is a semiconductor with a wide bandgap and unique properties such as low toxicity and high optical/thermal stability (Wang et al., 2021). As a result, it has been used in many applications, such as a gas sensor (Onkar et al., 2020), cleaner for drinking water/wastewater (Dimapilis et al., 2018), solar cells, luminescence (Willander et al., 2010), UV-blocking (Sasani Ghamsari et al., 2017), and energy storage devices (Fahimi and Moradlou, 2020). ZnO NPs have been incorporated with different polymers for different purposes, such as producing high luminescence emitters (Abdullah et al., 2003), polymer thermal stabilization (Cho et al., 2004), optoelectronics devices (Willander et al., 2011), enhancing tribological properties (Sudeepan et al., 2014), and antibacterial applications (Kubacka et al., 2009).
After looking through the articles and extensive studies on the impact of ZnO in polymers, to the best of our knowledge, there is no research on the effects of ZnO NPs on the structural, optical, and electrical/dielectric properties of this blend (PVC/PVK). This study focuses on creating PVC/PVK/ZnO nanocomposite films using the solution casting process and analyzing their structural, optical, and electrical characteristics. We employed X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA) techniques to investigate the structural, morphological, and thermal characteristics of prepared nanocomposites. Transmission electron microscopy (TEM) was utilized to explore the ZnO NPs’ morphology prepared via the sol–gel method. UV/visible spectroscopy (UV/Vis) was used to determine the optical characteristics. Besides, we investigated the electrical/dielectric properties of nanocomposites made of PVK, PVC, and ZnO NPs to assess their promising applications in energy storage devices.
2 Experimental
2.1 Chemicals
In this study, the PVC and PVK were from Sigma Aldrich (Sigma–Aldrich, Germany). Ethanol (CH2COOH) and Zinc acetate dihydrate [Zn(O2CCH3)2(H2O)2] with 99% of purity (Hm bG Chemical, Germany), sodium hydroxide (NaOH) pellets reagent grade with purity ≥98% (Sigma Aldrich, Germany), were used to prepare the ZnO NPs. The PVK and PVC were dissolved in tetrahydrofuran (THF) from Al-Gamhoreih company (Al-Gamhoreih company, Mansoura, Egypt). The chemicals were utilized as received without any further purifications.
2.2 Nanocompostes’ preparation
First, we prepared ZnO NPs via the sol–gel method reported in the published work (Al-Muntaser et al., 2022a, 2022c). Then, in 40 mL of THF, 1.26 g of PVC and 0.14 g of PVK (90:10) were dissolved with stirring at 40 °C for about 6 h (h) to form a solution. The prepared ZnO NPs were suspended in THF and then exposed to a high-power probe sonicator, specifically the model Q500 Sonicator (20 kHz, 500 W) from (QSONICA, USA), for a duration of 9 min. Afterward, ZnO NPs were added to the PVC/PVK blend solution at 1, 3, and 5 wt%. The resulting solution was incorporated into a Petri dish. Nanocomposites, comprised of both pure blend and PVC/PVK/ZnO nanocomposite at ZnO NPs contents of 1, 3, and 5 wt%, were generated after three days of progressive solvent evaporation in the oven at a temperature of 50 °C. Peeled off the Petri plate, the resulting films ranged from 0.13 to 0.21 mm. Finally, the PVC/PVK/ZnO NPs nanocomposite films were used for characterizations and investigations. Scheme 1 illustrates the preparation steps of the PVC/PVK/ZnO NPs films.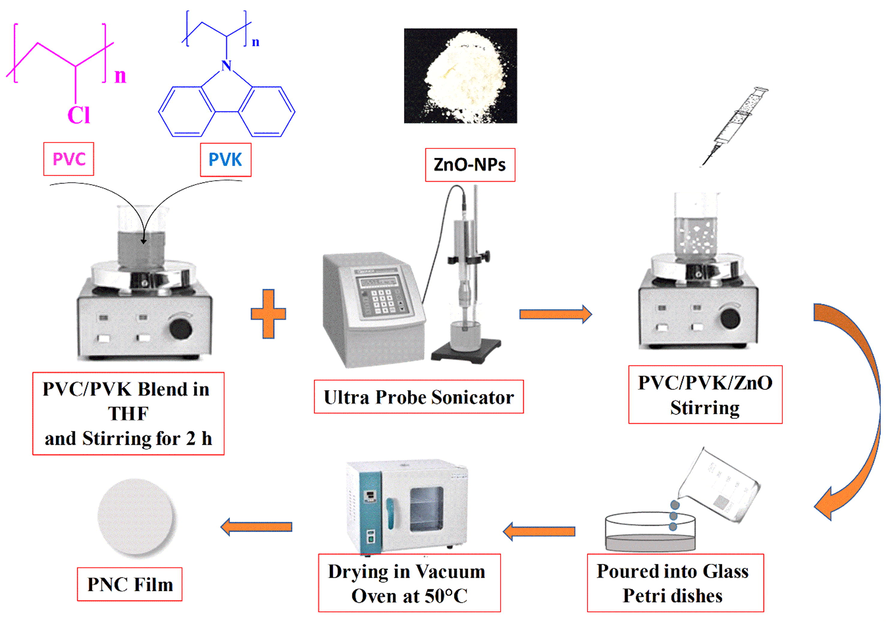
The preparation steps of the PVC/PVK/ZnO nanocomposite films.
2.3 Characterizations
We recorded the XRD patterns of prepared films (PVC/PVK/ZnO NPs) using x-ray diffractometer model X’Pert PRO (Malvern PANalytical, USA) with a Ni filter and Cu Kα radiation of λ = 15.406 Å. FTIR analysis using model Nicolet iS10 (Thermo Scientific, USA) was utilized to identify the functional groups in PVC/PVK/ZnO NPs. The nanocomposite films’ surfaces were investigated through SEM, model JEOL-JSM-7600f (JEOL, USA); their thermal features were analyzed using thermogravimetric analysis (TGA), model STA 409CD (NETZSCH, Germany) with 10 °C/min heating rate in a nitrogen environment. TEM model JEOL 1200 EX (JEOL, Japan) was employed to explore the size and form of ZnO NPs. By means of the UV/Vis spectrophotometer V-630 model (JASCO, Japan), the UV/Vis spectra of the prepared nanocomposite samples were investigated at room temperature. Concept Turnkey dielectric, conductivity, and impedance spectrometers with temperature control model Concept 40 (Novocontrol Technologies GmbH & Co. KG, Germany) was utilized to analyze the prepared nanocomposite films’ electric/dialectical properties over the frequency range of 0.1 Hz to 20 MHz.
3 Results and discussion
3.1 XRD and TEM
Fig. 1 shows the XRD patterns of prepared PVC/PVK blend, PVC/PVK/ZnO nanocomposite films (1, 3, and 5 wt%), and pure ZnO nanopowder. The XRD pattern of pure ZnO NPs shows reflection peaks at 2θ ≈ 31.61° (1 0 0), 34.20° (0 0 2), 36.11° (1 0 1), 47.45° (0 1 2), 56.54° (1 1 0), 62.68° (0 1 3), 66.14° (2 0 0), 67.80° (1 1 2), and 68.84° (2 0 1). Based on JCPDS Card No. 01–089-0510 and the published works (Soliman et al., 2020; Hezma et al., 2019; Jiménez-Rosado et al., 2022), these diffraction peaks were indexed to the atomic planes of (1 0 0), (0 0 2), (1 0 1), (0 1 2), (1 1 0), (0 1 3), (2 0 0), (1 1 2), and (2 0 1), depicting the hexagonal Wurtzite crystal structure with cell lattice parameters of a = b = 3.2493 Å and c = 5.2057 Å. Using Scherrer’s equation (Abdelrazek et al., 2018), the mean crystallite size was 18.07 nm. The XRD pattern of pure PVC/PVK blend exhibits two broad peaks at 2θ ≈17.97 and 25.63° explaining the amorphous nature of the polymer blend. Al-Muntaser et al. (Al-Muntaser et al., 2020) and Taha (Taha, 2017) reported that the amorphous PVC polymer shows two humps at 2θ = 17 and 25.50°. Abd El-kader et al. (Abd El-kader et al., 2015) and Menazea et al. (Menazea et al., 2018) indicated that the PVK polymer presented amorphous characteristics by showing two halos at 2θ = 7.90° and 23.10°. Therefore, the two broad peaks in the XRD scan of the amorphous PVC/PVK blend were attributed to the PVC polymer with content (90 wt%) within the blend structure and the disappearance of characterized halos of PVK results from the complexation between PVC/PVK chains. The reduction in the intensity and the increase of the two main peaks of PVC/PVK with the ZnO NPs addition imply the rise of the degree of amorphously of the filled samples because of the intermolecular interactions between these NPs and the host blend matrix that raise the amorphous regions’ content and soften the segmental motion of blend chains. From the XRD pattern of the nanocomposite sample (1 wt%), the diffraction peaks attributed to ZnO phases are not observed However, at ZnO NPs contents ≥3 wt%, diffraction peaks are observed at 2θ = 31.68, 34.54, 36.55, 47.71, 56.63, 63.12, 68.14, and 69.1° that can be attributed to ZnO NPs, and that their intensities increase largely at 5 wt% content. This result demonstrates the presence of ZnO NPs within the prepared nanocomposites.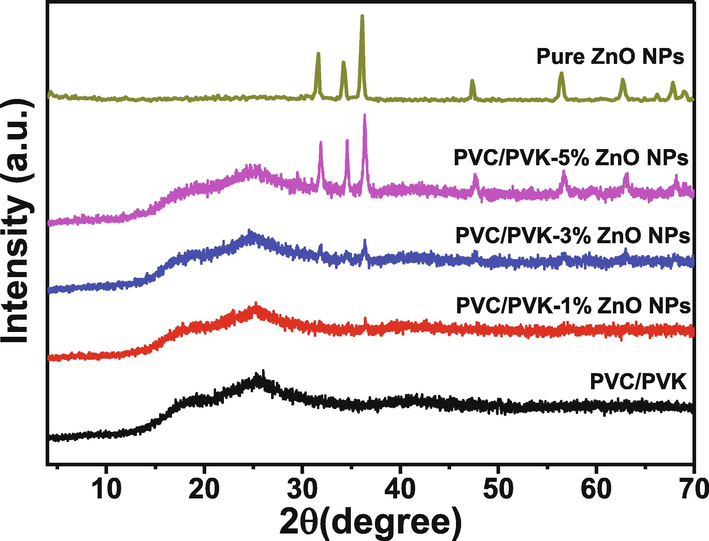
XRD patterns of the prepared PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt%, besides the pure PVC/PVK and pure ZnO NPs.
The broadening in the XRD patterns could be understood via Hodge et al. (Hodge et al., 1996), who found a relationship between peak intensity and crystallinity level, where the peak intensity of the XRD pattern might reduce as the amorphousness of the material increase as a result of the dopant addition. Therefore, the crystallinity (Xc) was determined by equation(1), reported by Hermans and Weidinger (Hermans and Weidinger, 1949):
Sample
Pure PVC/PVK
PVC/PVK-1% ZnO NPs
PVC/PVK-3% ZnO NPs
PVC/PVK-5% ZnO NPs
Crystallinity (%)
54.23
41.34
36.73
22.14
Fig. 2 shows the TEM results; here, Fig. 2a displays the TEM photomicrograph for the ZnO NPs prepared via the sol–gel method. The ZnO NPs appear to have an almost near-hexagonal shape; this result agrees with those reported shapes of ZnO NPs prepared using the sol–gel method (Chelouche et al., 2014; Zhang et al., 2010). The prepared NPs show aggregation behavior that could result from their suspension in deionized water before dropping in the carbon-coated copper grids (200 mesh) for TEM imaging. The particle sizes for ZnO NPs were in the range of 8–28 nm; Fig. 2b displays a histogram distribution for the particle sizes. The distribution was fitted using the lognormal distribution function; lognormal distribution is derived from a normal distribution using logarithmic mathematics, suggesting the prepared particles’ sizes distribution is nearly homogeneous. The ZnO NPs derived by sol–gel showed particle size with a mean value of 15 nm.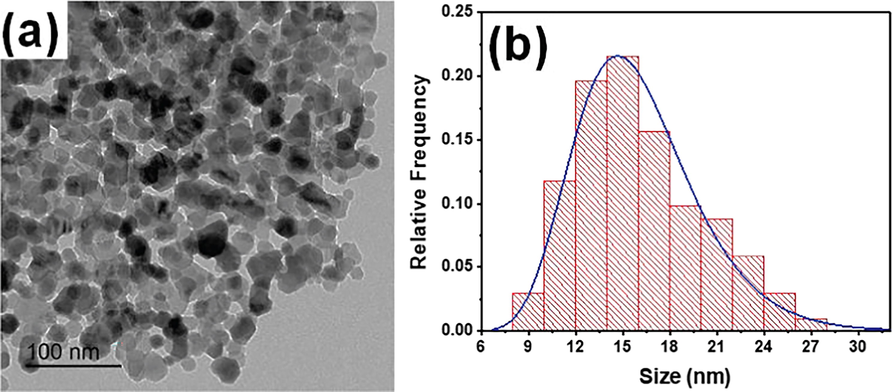
(a) TEM photomicrograph of the ZnO NPs prepared via sol–gel (b) histogram distribution of the ZnO NPs’ particle size.
3.2 SEM analysis
SEM technique is utilized to verify the distribution and dispersion of ZnO NPs within the PVC/PVK matrix and investigate the surface of the prepared films. Fig. 3 illustrates the SEM images for the two virgin polymers (PVC and PVK), pure PVC/PVK blend, and blend/ZnO nanocomposite samples. From Fig. 3a–b, the images of PVC and PVK polymers have homogeneous and smooth surfaces without cracks and pores, reflecting their amorphous structure as indicated in the XRD data. The same images were reported by Taha et al. (2019) and Menazea et al. (2018). From Fig. 3c, the surface of pure PVC/PVK also seems smooth; this could indicate a good miscibility/coherence between the blend’s components. With the addition of ZnO NPs, the surface of filled samples becomes rougher due to the existence of small white granules. The near disappearance of the ZnO from the surface of the nanocomposite film (1 wt%) is because of the good dispersion of the nanofiller and its presence in small filling levels. These morphological variations could imply a good dispersion of added NPs within the PVC/PVK structure and their uniform distribution on the surface, where the interbreeding between the PVC/PVK matrix and ZnO NPs is apparent.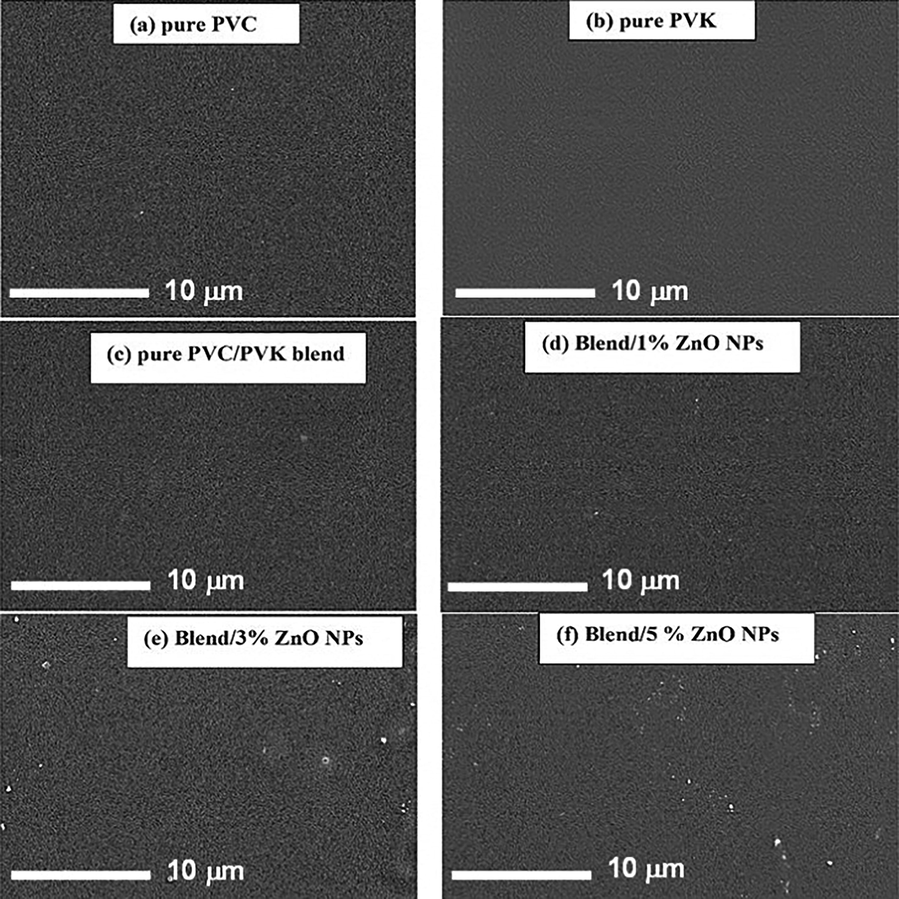
SEM micrographs of pure PVC, pure PVK, pure Pure PVC/PVK blend, and PVC/PVK/ZnO NPs nanocomposite samples.
3.3 FTIR study
FTIR investigation is an essential tool for establishing the interactions between PVC/PVK blend and ZnO NPs. FTIR spectra of both the PVC/PVK blend and the PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt% show the most characteristic infrared vibrational modes (Fig. 4). The major vibrational bands for PVC/PVK have been assigned. The bands, which appear in the spectral range of 3250–2750 cm−1, are due to the C—H bonds’ symmetric and asymmetric stretching vibration modes in the methyl and methylene groups of both PVC and PVK polymers (Malimabe et al., 2020; Kaur et al., 2022; Thinh et al., 2012). The bands between 1485 and 1398 cm−1 could be referred to as the symmetrical bending vibration modes of C—H bonds, whereas the bands in the range 1136–1049 cm−1 could result from the stretching of C—C bonds (Malimabe et al., 2020). The bands between 990 and 888 cm−1 are ascribed to C—H bond bending modes (Kaur et al., 2022). The bands in the range 790–700 cm−1 belong to out-of-plane bending vibration modes of the aromatic —C—H group in PVK, besides the less intensity band located at 1602 cm−1, which is attributed to the C⚌C stretching vibration of the vinylidene group (PVK) (Thinh et al., 2012). Before comparing the intensities of the peak of the samples' spectra, normalization, which is a spectroscopic correction to avoid if there is any difference in the concentrations of the samples (O’Rourke et al., 2019), was applied for all the samples' spectra. The spectra showed that the intensities of all the characteristic bands in prepared PVC/PVK/ZnO nanocomposite films weaken as ZnO NPs content increases. The decrease in the intensities of FTIR spectra peaks with the increase in the content of ZnO NPs could be explained through the decrease in the polymer blend ratio.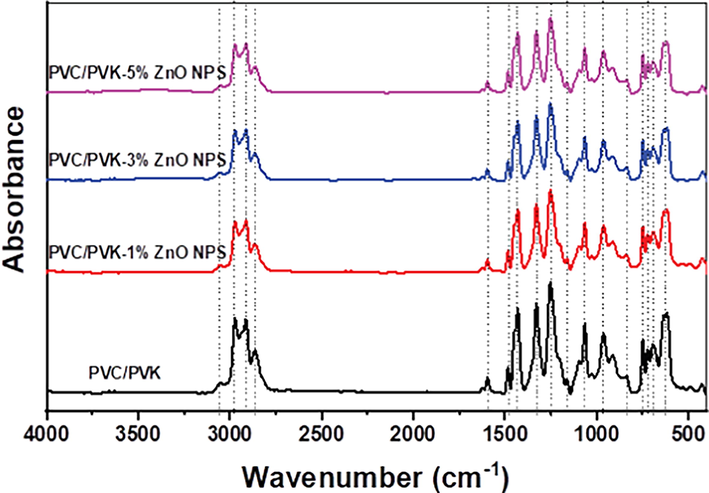
FT-IR spectra of the prepared PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt%, besides the pure PVC/PVK.
3.4 TGA
The TGA analysis of pure PVC, pure PVK, pure PVC/PVK blend, and PVC/PVK/ZnO nanocomposite films are indicated in Fig. 5. A three-stage decomposition steps can be observed in the TGA of pure PVC polymer (Fig. 5a) (Taha, 2017; Jakić et al., 2013; Nair et al., 2007), where the first stage (180–250 °C) of a weight loss (3%) attributes to the moisture evaporation and THF dispersal. The second (265–360 °C) and final (410–525 °C) stages correspond to weight loss values of 45% and 73%. The second stage was referred to as the HCl-elimination “dehydrochlorination,” leaving behind longer polyene chains. The thermal decomposition of the carbon chain and polyene sequences exists in the third thermal stage producing volatile aliphatic/aromatic compounds through the intramolecular cyclization of the conjugated sequences. The residual weight (9%) at temperature˃ 525 °C corresponds to the remaining char.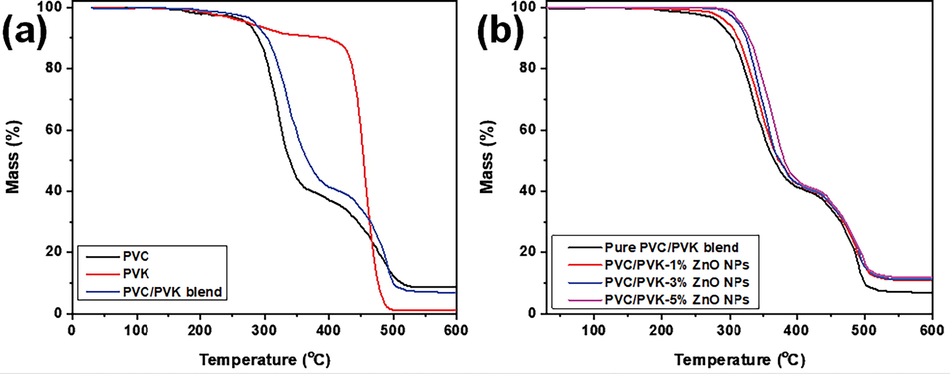
TGA of (a) pure PVC, pure PVK, and pure PVC/PVK blend, and (b) PVC/PVK/ZnO nanocomposite films.
Pure PVK exhibits a two-step decomposition pattern (Fig. 5a) that begins with a small, steady loss at ∼330 °C because of the decomposition of the PVK chains (Menazea et al., 2018; Cui et al., 2011; Santos et al., 2012). The fast second decomposition step reaches a 90% weight loss at 330 °C, corresponding to the cleavage backbone of the polymer as the PVK structure suffers from the random chain scission of C—H, N—H, and C⚌C groups during the thermal degradation.
The degradation of pure PVC/PVK blend shows a similar pattern as pure PVC polymer due to the high content of PVC within the prepared blend matrix and exhibits three-step decomposition. It is clear that the TGA curve of the blend possesses higher stability than that of pure PVC, which could be a sign of the PVK thermal stabilizing effect on PVC due to the interactions between the functional groups of the blend’s components delay the dehydrochlorination of PVC within the blend.
For the various compositions of PVC/PVK/ ZnO nanocomposite samples, the trend of TGA curves is similar to pure blend by showing three thermal degradation stages. These curves shift toward high-temperature values and show a lower weight loss (%) at ∼510 °C compared to the pure blend, which is assigned to the existence of thermally stable ZnO NPs that boost heat dissipation within the nanocomposite films. Table 2 reports the extracted mass loss values at temperatures (300, 400, and 500 °C) values and residual weight at 600 °C. From this table and Fig. 5b, the increased thermal stability of the filled samples can be attributed to the good dispersion of ZnO NPs (Hezma et al., 2019; Taha, 2017; Menazea et al., 2018; Abutalib and Rajeh, 2020), as it prevents the flux of decomposition product and, therefore, hinder the degradation process. The PVC/PVK chains around ZnO NPs may decompose more slowly, which assists in moving the thermal degradation temperatures to higher values. Thus, the PVC/PVK/ ZnO nanocomposite samples have good thermal stability, which is needed for their processing applications, such as polymer batteries.
Samples
Weight loss (%)
Residual weight (%)
(kJ/mol)
300 °C
400 °C
500 °C
Pure PVC/PVK
9.18
59.13
90.21
7.03
1.18
PVC/PVK-1% ZnO NPs
5.93
58.23
85.52
10.58
1.06
PVC/PVK-3% ZnO NPs
2.68
57.14
84.79
11.31
0.93
PVC/PVK-5% ZnO NPs
1.24
56.06
83.35
12.17
0.867
The thermal kinetics of the PVC/PVK/ZnO nanocomposite films can be obtained from the TGA curves at the main degradation step by using the Coats-Redfern method (Coats and Redfern, 1964) as follow:
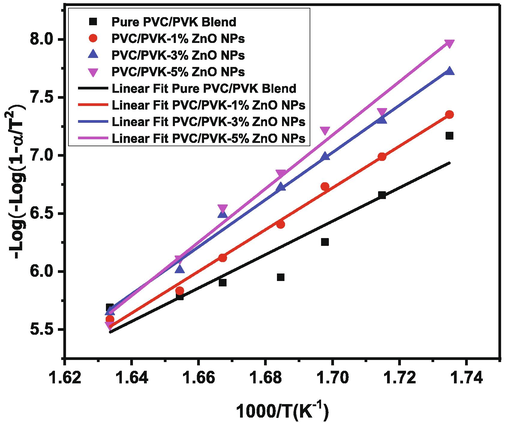
Plots of Coats-Redfern relation for pure PVC/PVK blend and PVC/PVK/ZnO nanocomposite films.
3.5 Optical measurement
Fig. 7 displays the UV/Vis spectra of the PVC/PVK blend, besides PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt% in the spectra range between 200 and 1000 nm. The tested samples’ UV/Vis spectra showed four absorption peaks; the broad peak centered at around 242 nm corresponded to π → π* electronic transition (Menazea et al., 2020). The second peak is a small but sharp absorption centered at 298 nm, and the third peak, which is also sharp, centered at around 362 nm; these two peaks were attributed to n → π* electronic transition; these two peaks, i.e., the second and third belong to the phenyl ring exists in each repeat unit in the structure of the polymers (Sonone et al., 2014). The third peak showed a weak absorbance peak at 337 nm; this peak was not observed in the pure PVC/PVK blend. This peak could be attributed to the surface plasmon resonance (SPR) of the ZnO NPs (Hezma et al., 2019; Abutalib and Rajeh, 2020; Choudhary, 2018), depicting NPs within the PVC/PVK structure. It is evident that the intensity of the second and fourth absorption peaks in the prepared nanocomposites faint with rising ZnO NPs concentrations; this result could be referred to as the interaction impacts of the ZnO NPs as fillers in the PVC/PVK polymers matrix. This result corresponds to and confirms the results obtained by FTIR.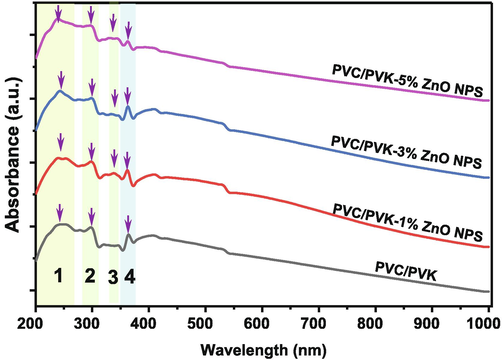
The UV/vis spectra of the pure PVC/PVK blend and PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt%.
It is essential to investigate and determine the average optical energy gap (Eg) values to comprehend the optical characteristics and parameters of the nanocomposite. The nature of the film and the distribution and arrangement of molecules within the polymeric chain all affect the Eg values. The energy gap of the PVC/PVK blend and PVC/PVK/ZnO nanocomposite films was studied using the absorption band at the UV area. Where the Eg was estimated via the Tauc equation:
Fig. 8 exhibits the Tauc plot of the PVC/PVK blend and PVC/PVK/ZnO nanocomposite films at ZnO NPs contents of 1, 3, and 5 wt% in both direct (Fig. 8a) and indirect (Fig. 8b) transitions. The value of Eg for each sample was assessed through the extrapolation of the plot of (αhυ)2 linear regions against hυ (direct transition) and (αhυ)1/2 against hυ (indirect transition). Both extracted optical band gap energy, direct (Egd) and indirect (Egi), are listed in Table 3. The values of Egd decreased from 3.61 eV for pure PVC/PVK polymers to 2.96 eV for the PVC/PVK/ZnO nanocomposite film at the highest ZnO NPs content, i.e., 5 wt%; also, the Egi values decreased from 0.56 to 0.13 eV.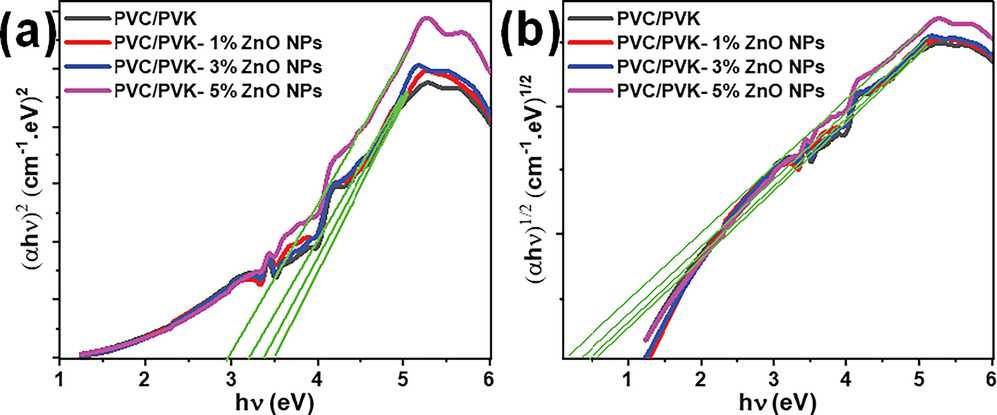
The optical band gap energy of the PVC/PVK blend and PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt% for both (a) direct and (b) indirect transitions.
Sample
Pure PVC/PVK
PVC/PVK-1% ZnO NPs
PVC/PVK-3% ZnO NPs
PVC/PVK-5% ZnO NPs
Egd (eV)
3.61
3.48
3.38
2.96
Egi (eV)
0.56
0.41
0.39
0.13
3.6 Electrical study
3.6.1 AC conductivity
Measuring the electrical conductivity of the materials is a significant step in determining the transport mechanisms in polymeric nanocomposites. Fig. 9 shows the AC conductivity at room temperature for pure PVC/PVK and PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt% versus logarithms of frequency (f). It can be seen that the pure PVC/PVK showed AC conductivity, which increased linearly as the frequency increased. This behavior can be understood by that the electrical field can induce charge carriers that contribute to and enhance the electrical conductivity (Saeed et al., 2021; Alharbi et al., 2018). The PVC/PVK/ZnO nanocomposite film at ZnO NPs content of 1 wt% showed nearly behavior and values similar to the pure PVC/PVK, suggesting no effect for ZnO NPs at the lowest content, i.e., 1%. However, The PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 3 and 5 wt% exhibited two behaviors, the first at lower frequencies, where the AC electrical conductivity appeared constant while increasing the frequency. The AC electrical conductivity increased linearly at higher frequencies in the second behavior.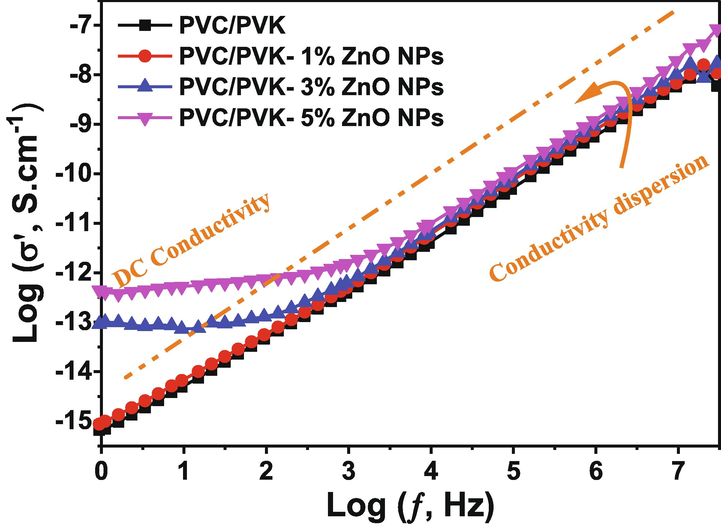
AC electrical conductivity of the prepared PVC/PVK blend and PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt%.
The AC electrical conductivity behaviors of the PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 3 and 5 wt% could occur due to the non-equilibrium trap charges in the polymeric materials, the AC conductivity in the lower frequencies region is nearly not frequency independent, where the conductivity decreases as a result of increased charge gathering at the electrode contact. Conductivity results in the high-frequency range rising exponentially as a function of frequency, which is compatible with charge carrier hopping. Hoping and Koop’s low can be used to explain how conductivity increases with frequency (Koops, 1951). According to Koop’s theory, conductivity is regulated and caused by grain boundaries with poor conductivity at lower frequencies. The hopping theory, which states that the electrical conductivity increases by improving the charge carrier’s hopping between the polymer materials of PVC/PVK and the ZnO NPs, also could explain why conductivity increases with frequency (Elashmawi and Al-Muntaser, 2021). Conducting grains are the reason for the conductivity dispersion seen at higher frequencies. Furthermore, the ZnO NPs integration into the PVC/PVK composite improves the charge conduction mechanism significantly more quickly, as evidenced by the higher σ values of the PVC/PVK doped films compared with the blend polymers. This improvement could be referred to as the decrease in the crystallinity (see the XRD results), which improves disorders, and subsequently enhances the mobility of charge carriers, particularly ions transport (Croce et al., 1998). Since the nanocomposite polymer electrolytes are a type of flexible solid-state or quasi-solid-state ion-dipolar complexes, and these nanocomposites allow ion transportation to happen alongside the movement of polymer chain segments, particularly within the amorphous polymer phase (Dhatarwal et al., 2018).
Also, the dc electrical conductivity of pure PVC/PVK and PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt% was measured; their obtained values are listed in Table 4. Pure PVC/PVK was 6.46 × 10−16 Ω−1.cm−1 as the lowest dc electrical conductivity in all tested samples; dc electrical conductivity increased while increasing the ZnO nanofillers to reach 3.63 × 10−13 Ω−1.cm−1 as the highest value for the PVC/PVK/ZnO nanocomposite film at ZnO NPs content of 3 wt%.
Sample
Pure PVC/PVK
PVC/PVK-1% ZnO NPs
PVC/PVK-3% ZnO NPs
PVC/PVK-5% ZnO NPs
σdc (Ω−1 cm−1)
6.46 × 10−16
8.71 × 10−16
4.37 × 10−14
3.63 × 10−13
3.6.2 Dielectric study
We investigated the dielectric properties to estimate whether the prepared PVC/PVK/ZnO nanocomposite films can be used in the polymer-based capacitors’ design. For the investigation of dielectric properties, both dielectric constant (ε′) and dielectric loss (ε″) are involved in to estimate. The dielectric constant measures the ability of the material to store energy, while the dielectric loss measures the energy dissipation in the material (Saeed et al., 2021, 2022). Fig. 10 displays the measured ε′ and ε″ of the prepared PVC/PVK blend and PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt%; both of them were measured in the frequency range starting from 0.1 Hz up to 20 MHz. The prepared PVC/PVK/ZnO nanocomposite films showed improvements in values of ε′ and ε″ compared with the prepared PVC/PVK blend polymer. This result could be due to the ZnO NPs, which were reported to have high orientation polarizability (Kurniawan et al., 2017), causing an enhancement in the dielectric properties in the nanocomposites. Also, it could result from the accumulation of the charges in the grain boundaries of the ZnO NPs, creating space charge polarization in the nanocomposites. The relation between the ε′ and the logarithm of the frequency for the prepared nanocomposite is shown in Fig. 10a. At the same time, Fig. 10b exhibits the relation between ε″ and the logarithm of the frequency. The values of ε′ and ε″ rise rapidly toward lower frequency because dipoles in the nanocomposite film could follow and track the orientation of the changeable applied electric field at the lower frequency. However, at high frequencies, the applied electric field changes very fast; as a result, the charge carriers couldn’t follow the change in the applied field, causing lowers values of both dielectric constants and dielectric losses (Ramesh and Liew, 2013). In the conclusion of dielectric properties, the ε′ and ε″ values increased while increasing the ZnO NPs content. This impact could be due to the free charge carriers, which build up at the electrode-sample interfaces (Alghunaim, 2018), suggesting the prepared PVC/PVK/ZnO nanocomposite films could be a promise for the applications of dielectric energy storage polymer-based capacitors.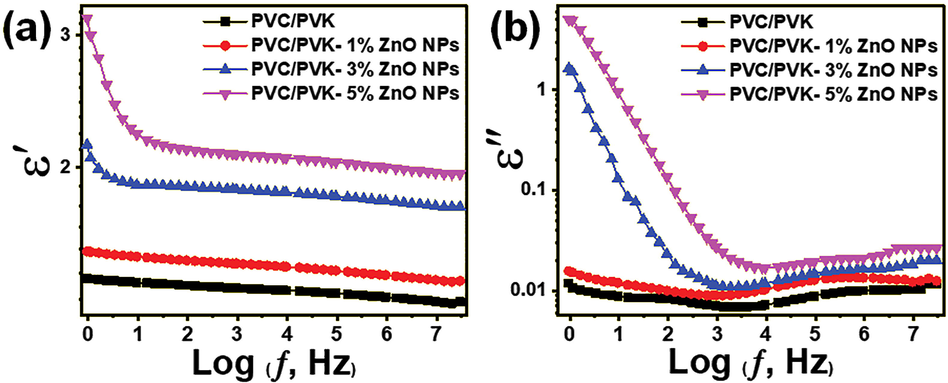
Dielectric results of the prepared PVC/PVK blend and PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt% (a) dielectric constant and (b) dielectric constant.
3.6.3 Electric modulus
The electric modulus (M*), a complex quantity, was utilized to study the phenomenon of conductivity relaxation and electrode effect (Yu et al., 2000). It is also useful to recognize and distinguish between electrode polarization and bulk effects. The formulas (3)–(5) are applied to determine the M′, M″, and M*:
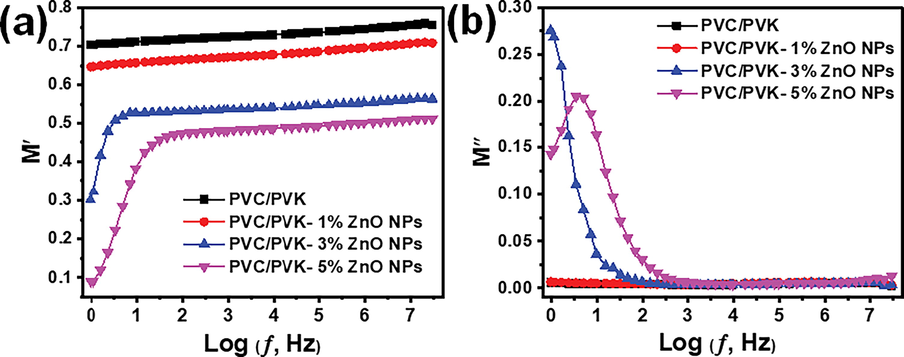
Electric modulus of the prepared PVC/PVK blend and PVC/PVK/ZnO nanocomposite films at ZnO NPs content of 1, 3, and 5 wt% (a) real part (b) imaginary part.
It can be observed that the values of M″ demonstrated, as expected, the nature of the relaxation processes with its broad, well-defined peaks at ZnO NPs content of 5 wt%. The obtained sample is most probably ionic conductors, according to the M″ relaxation peak. In contrast, the pure PVC/PVK blend and nanocomposite at ZnO NPs content of 1 wt% (lowest dropped) showed no peak maximum, suggesting that the ZnO-NPs contribute to the relaxation process.
4 Conclusion
The solution casting process was utilized to prepare PVC/PVK/ZnO nanocomposite films at ZnO NP contents of 1.0, 3.0, and 5.0 wt%; the ZnO NPs were prepared via the sol–gel method. XRD results show that adding ZnO NPs decreases the crystallinity degree for the polymer matrix in the nanocomposite. The prepared ZnO NPs, as a filler in the nanocomposites, have a spherical shape with a mean particle size of 15 nm. The SEM micrographs illustrated that ZnO NPs were well dispersed within the PVC/PVK blend. From the TGA curves, the prepared nanocomposites showed better thermal stability, depicting that the existence of ZnO NPs inhibited/delayed the breakdown of the PVC/PVK backbone. The optical results revealed that the direct and indirect optical band gaps for the prepared PVC/PVK/ZnO nanocomposite films were decreased with the addition of ZnO as a result of the creation of a charge transfer complex between the PVC/PVK polymer as a matrix and the nanohybrid atoms. AC conductivity experiments revealed that the ε′ and ε″ values of the PVC/PVK/ZnO nanocomposite films increased with increasing the ZnO NPs contents; besides, the σdc values of the PVC/PVK/ZnO nanocomposite films get improved nearly 2000 folds compared with the pure PVC/PVK blend polymers where the σdc values of PVC/PVK/ZnO NPs films improved from 6.46 × 10−16 S/cm for the blend of PVC/PVK to 3.63 × 10−13 S/cm for PVC/PVK/ZnO nanocomposite film at ZnO NPs content 5.0 wt% ZnO NPs. The findings of this study indicate that the prepared PVC/PVK/ZnO nanocomposite films could be a promise for the applications of flexible capacitors for dielectric energy storage polymer-based.
Acknowledgment
This work was funded by Institutional Fund Projects under no. (IFPIP: 1616-135-1443). The authors, therefore, gratefully acknowledge the technical and financial support provided by the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis and characterization of PVK/AgNPs nanocomposites prepared by laser ablation. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2015;138:331-339.
- [Google Scholar]
- Structural, optical, morphological and thermal properties of PEO/PVP blend containing different concentrations of biosynthesized Au nanoparticles. J. Mater. Res. Technol.. 2018;7(4):419-431.
- [Google Scholar]
- In situ synthesis of polymer nanocomposite electrolytes emitting a high luminescence with a tunable wavelength. J. Phys. Chem. B. 2003;107(9):1957-1961.
- [Google Scholar]
- Influence of ZnO/Ag nanoparticles doping on the structural, thermal, optical and electrical properties of PAM/PEO composite. Phys. B Condens. Matter. 2020;578:411796
- [Google Scholar]
- Electro-optical properties of poly(N-vinyl carbazole) nanoclay composites. Polym. Bull.. 2019;76(10):5301-5311.
- [Google Scholar]
- Structural, thermal, dielectric spectroscopic and AC impedance properties of SiC nanoparticles doped PVK/PVC blend. Results Phys.. 2018;9:1136-1140.
- [Google Scholar]
- Structural characterizations and electrical conduction mechanism of CaBi2Nb2O9 single-phase nanocrystallites synthesized via sucrose-assisted sol–gel combustion method. J. Mater. Sci.. 2018;53(16):11584-11594.
- [Google Scholar]
- Antibacterial and photocatalytic activities of controllable (anatase/rutile) mixed phase TiO2 nanophotocatalysts synthesized via a microwave-assisted sol–gel method. New J. Chem.. 2020;44(2):562-570.
- [Google Scholar]
- Enhancement of optical and electrical properties of PVC/PMMA blend films doped with Li4Ti5O12 nanoparticles. J. Mater. Res. Technol.. 2020;9(1):789-797.
- [Google Scholar]
- Reinforcement of structural, optical, electrical, and dielectric characteristics of CMC/PVA based on GNP/ZnO hybrid nanofiller: Nanocomposites materials for energy-storage applications. Int. J. Energy Res.. 2022;n/a(n/a)
- [Google Scholar]
- α-MoO3 nanobelts/CMC-PVA nanocomposites: hybrid materials for optoelectronic and dielectric applications. J. Polym. Res.. 2022;29(7):1-11.
- [Google Scholar]
- Reinforcement of structural, optical, electrical, and dielectric characteristics of CMC/PVA based on GNP/ZnO hybrid nanofiller: Nanocomposites materials for energy-storage applications. Int. J. Energy Res. 2022
- [Google Scholar]
- Boosting the optical, structural, electrical, and dielectric properties of polystyrene using a hybrid GNP/Cu nanofiller: novel nanocomposites for energy storage applications. J. Mater. Sci.-Mater. Electron.. 2023;34(7):678.
- [Google Scholar]
- Structural, morphological, optical, electrical and dielectric features based on nanoceramic Li4Ti5O12 filler reinforced PEO/PVP blend for optoelectronic and energy storage devices. Ceram. Int. 2023
- [Google Scholar]
- Mechanisms of charge transport and resistive switching in composite films of semiconducting polymers with nanoparticles of graphene and graphene oxide. J. Phys. Conf. Ser.. 2017;917(9):092019
- [Google Scholar]
- TiO2 nanocomposite based polymeric membranes: a review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J.. 2016;283:29-46.
- [Google Scholar]
- Synthesis and characterizations of new morphological ZnO and Ce-doped ZnO powders by sol–gel process. Optik. 2014;125(19):5626-5629.
- [Google Scholar]
- Effects of ZnO nano particles on thermal stabilization of polymers. Polym. Eng. Sci.. 2004;44(9):1702-1706.
- [Google Scholar]
- Structural, optical, dielectric and electrical properties of (PEO–PVP)–ZnO nanocomposites. J. Phys. Chem. Solid. 2018;121:196-209.
- [Google Scholar]
- Charge carrier transport in poly (N-vinylcarbazole): CdS quantum dot hybrid nanocomposite. J. Phys. Chem. B. 2004;108(5):1556-1562.
- [Google Scholar]
- Nanocomposite polymer electrolytes for lithium batteries. Nature. 1998;394(6692):456-458.
- [Google Scholar]
- PVK/MWNT electrodeposited conjugated polymer network nanocomposite films. ACS Appl. Mater. Interfaces. 2011;3(7):2300-2308.
- [Google Scholar]
- White polymer light emitting diodes based on PVK: the effect of the electron injection barrier on transport properties, electroluminescence and controlling the electroplex formation. Phys. Chem. Chem. Phys.. 2016;18(48):33077-33084.
- [Google Scholar]
- Strategy toward designing semiconducting polymer nanoparticle–multichomophoric dye assembly. J. Phys. Chem. C. 2017;121(7):4050-4059.
- [Google Scholar]
- Effectively improved ionic conductivity of montmorillonite clay nanoplatelets incorporated nanocomposite solid polymer electrolytes for lithium ion-conducting devices. SN Appl. Sci.. 2018;1(1):112.
- [Google Scholar]
- Zinc oxide nanoparticles for water disinfection. Sustainable Environ. Res.. 2018;28(2):47-56.
- [Google Scholar]
- Reinforcement of structural, thermal and electrical properties and antibacterial activity of PVA/SA blend filled with hybrid nanoparticles (Ag and TiO2 NPs): Nanodielectric for energy storage and food packaging industries. Ceram. Int.. 2023;49(12):20174-20184.
- [Google Scholar]
- Synergistic effect of cellulose nanocrystals/graphene oxide nanosheets as functional hybrid nanofiller for enhancing properties of PVA nanocomposites. J. Carbohydrate Polym.. 2016;137:239-248.
- [Google Scholar]
- Influence of Co3O4 nanoparticles on the optical, and electrical properties of CMC/PAM polymer: combined FTIR/DFT study. J. Inorg. Organometall. Polym. Mater.. 2021;31(6):2682-2690.
- [Google Scholar]
- Synthesis, structural, thermal, mechanical, and nano-scale free volume properties of novel PbO/PVC/PMMA nanocomposites. Polymer. 2020;206:122911
- [Google Scholar]
- Structural, optical and thermal characterization of ZnO nanoparticles doped in PEO/PVA blend films. Aust. J. Basic Appl. Sci. 2013;7(10):608-619.
- [Google Scholar]
- Fabrication of ZnO@C foam: A flexible free-standing electrode for energy storage devices. Mater. Des.. 2020;189:108525
- [Google Scholar]
- X-ray studies on the crystallinity of cellulose. J. Polym. Sci.. 1949;4(2):135-144.
- [Google Scholar]
- An insight into the effect of zinc oxide nanoparticles on the structural, thermal, mechanical properties and antimicrobial activity of Cs/PVA composite. Colloids Surf. A Physicochem. Eng. Asp.. 2019;581:123821
- [Google Scholar]
- Water absorption and states of water in semicrystalline poly (vinyl alcohol) films. Polymer. 1996;37(8):1371-1376.
- [Google Scholar]
- Thermal degradation of poly (vinyl chloride)/poly (ethylene oxide) blends: Thermogravimetric analysis. Polym. Degrad. Stab.. 2013;98(9):1738-1743.
- [Google Scholar]
- Green synthesis of ZnO nanoparticles using polyphenol extracts from pepper waste (Capsicum annuum) J. Clean. Prod.. 2022;350:131541
- [Google Scholar]
- Improvement in optical absorption and emission characteristics of polymethyl methacrylate in solution cast polymethyl methacrylate/polyvinyl carbazole polyblends. J. Thermoplast. Compos. Mater. 202208927057221115714
- [Google Scholar]
- On the dispersion of resistivity and dielectric constant of some semiconductors at audiofrequencies. J. Phys. Rev.. 1951;83(1):121.
- [Google Scholar]
- S-shaped current–voltage characteristics of polymer composite films containing graphene and graphene oxide particles. Phys. Solid State. 2016;58(12):2567-2573.
- [Google Scholar]
- Plasmonic nanoparticle/polymer nanocomposites with enhanced photocatalytic antimicrobial properties. J. Phys. Chem. C. 2009;113(21):9182-9190.
- [Google Scholar]
- Polarization behavior of zinc oxide thin films studied by temperature dependent spectroscopic ellipsometry. Opt. Mater. Express. 2017;7(11):3902-3908.
- [Google Scholar]
- Bistable resistance switching of poly (N-vinylcarbazole) films for nonvolatile memory applications. J. Appl. Phys. Lett.. 2005;87(12):122101
- [Google Scholar]
- Structure and electrical characterization of ZnO-Ag phosphate glasses. Results Phys.. 2017;7:1022-1029.
- [Google Scholar]
- Dielectric properties and energy storage densities of poly(vinylidenefluoride) nanocomposite with surface hydroxylated cube shaped Ba(0.6)Sr(0.4)TiO3 nanoparticles. Polymers (Basel). 2016;8(2):45.
- [Google Scholar]
- Characterization of the incorporated ZnO doped and co-doped with Ce3+ and Eu3+ nanophosphor powders into PVC polymer matrix. J. Mol. Struct.. 2020;1202:127339
- [Google Scholar]
- Nanosecond pulsed laser ablation in liquids as new route for preparing polyvinyl carbazole/silver nanoparticles composite: spectroscopic and thermal studies. J. Inorg. Organomet. Polym Mater.. 2018;28:2564-2571.
- [Google Scholar]
- The role of Li4Ti5O12 nanoparticles on enhancement the performance of PVDF/PVK blend for lithium-ion batteries. J. Mater. Res. Technol.-JMRT. 2020;9(3):5689-5698.
- [Google Scholar]
- Rechargeable lithium battery employing a new ambient temperature hybrid polymer electrolyte based on PVK+PVdF–HFP (copolymer) J. Power Sources. 2004;136(2):408-415.
- [Google Scholar]
- Overview of polyvinyl chloride (PVC) waste management practices in the Nordic countries. Clean. Eng. Technol.. 2021;4:100246
- [Google Scholar]
- Chapter 13 - Characterization of subsurface hydrocarbon/water saturation using Markov-chain Monte Carlo stochastic inversion of broadband electromagnetic logs. In: Misra S., Li H., He J., eds. Machine Learning for Subsurface Characterization. Gulf Professional Publishing; 2020. p. :369-402.
- [Google Scholar]
- Xerographic dark discharge of PVK:TNF photoconductive material. Chem. Phys.. 1991;153(1):305-312.
- [Google Scholar]
- Structural, optical, mechanical, and dielectric properties studies of carboxymethyl cellulose/polyacrylamide/lithium titanate nanocomposites films as an application in energy storage devices. Polym. Test.. 2022;114:107705
- [Google Scholar]
- Hybrid MWCNTs/Ag nanofiller reinforced PVP/CMC blend-based polymer nanocomposites for multifunctional optoelectronic and nanodielectric applications. J. Polym. Environ. 2022:1-13.
- [Google Scholar]
- Thermogravimetric analysis of PVC/ELNR blends. Polym. Degrad. Stab.. 2007;92(2):189-196.
- [Google Scholar]
- Dielectric behavior of plasticized PVC/alumina nanocomposites influenced with DC biasing field. Mater. Today: Proc.. 2018;5(1):2254-2262.
- [Google Scholar]
- What is Normalization? The strategies employed in top-down and bottom-up proteome analysis workflows. Proteomes 2019:29.
- [Google Scholar]
- Gas sensing behavior of ZnO thick film sensor towards H2S, NH3, LPG and CO2. J. Phys. Conf. Ser.. 2020;1644(1):012060
- [Google Scholar]
- Properties of polymer composites used in high-voltage applications. Polymers. 2016;8(5):173.
- [Google Scholar]
- Dielectric and FTIR studies on blending of [xPMMA–(1–x) PVC] with LiTFSI. J Measurement. 2013;46(5):1650-1656.
- [Google Scholar]
- On polyvinyl chloride-polypropylene composite matrix for 4D applications: Flowability, mechanical, thermal and morphological characterizations. J. Thermoplast. Compos. Mater.. 2021;08927057211059754
- [Google Scholar]
- Co3O4/NiO nanocomposite as a thermocatalytic and photocatalytic material for the degradation of malachite green dye. J. Mater. Sci.-Mater. Electron.. 2023;34(1):15.
- [Google Scholar]
- Electrical and dielectric properties of meridional and facial Alq3 nanorods powders. J. Mater. Sci.-Mater. Electron.. 2021;32(2):2075-2087.
- [Google Scholar]
- Electrical and dielectric properties of the natural calcite and quartz. SILICON. 2022;14(10):5265-5276.
- [Google Scholar]
- Improving the polyethylene oxide/carboxymethyl cellulose blend's optical and electrical/dielectric performance by incorporating gold quantum dots and copper nanoparticles: nanocomposites for energy storage applications. J. Mater. Res. Technol.-JMRT. 2023;24:8241-8251.
- [Google Scholar]
- Graphene nanocomposite for biomedical applications: fabrication, antimicrobial and cytotoxic investigations. Nanotechnology. 2012;23(39):395101
- [Google Scholar]
- Impact of nanostructured thin ZnO film in ultraviolet protection. Int. J. Nanomed.. 2017;12:207-216.
- [Google Scholar]
- Investigation of linear optical parameters and dielectric properties of polyvinyl alcohol/ZnO nanocomposite films. Phys. Status Solidi (a). 2020;217(19):2000321.
- [Google Scholar]
- Structural and electroluminescence properties of pure PVK and doped TiO2 polymer thin films. J. Adv. Res. Chem. Sci.. 2014;1(1):87-94.
- [Google Scholar]
- Study of friction and wear of ABS/Zno polymer composite using Taguchi technique. Procedia Mater. Sci.. 2014;6:391-400.
- [Google Scholar]
- Optical and thermogravimetric analysis of Pb3O4/PVC nanocomposites. J. Mater. Sci. Mater. Electron.. 2017;28:12108-12114.
- [Google Scholar]
- Effect of NiO NPs doping on the structure and optical properties of PVC polymer films. Polym. Bull.. 2019;76:4769-4784.
- [Google Scholar]
- Enhanced structural, optical, electrical properties and antibacterial activity of PEO/CMC doped ZnO nanorods for energy storage and food packaging applications. J. Polym. Res.. 2022;29(5):1-16.
- [Google Scholar]
- Characterization and electrochemical behaviors of honeycomb-patterned poly (N-vinylcarbazole)/polystyrene composite films. Polym. Bull.. 2012;69(1):81-94.
- [Google Scholar]
- Synthesis and applications of ZnO/polymer nanohybrids. ACS Mater. Lett.. 2021;3(5):599-621.
- [Google Scholar]
- Poly (N-vinylcarbazole)(PVK) photoconductivity enhancement induced by doping with CdS nanocrystals through chemical hybridization. J. Phys. Chem. B. 2000;104(50):11853-11858.
- [Google Scholar]
- Luminescence from zinc oxide nanostructures and polymers and their hybrid devices. Materials 2010:2643-2667.
- [Google Scholar]
- Zinc oxide nanorods/polymer hybrid heterojunctions for white light emitting diodes. J. Phys. D: Appl. Phys.. 2011;44(22):224017
- [Google Scholar]
- Poly(vinyl alcohol)/poly(vinyl chloride) composite polymer membranes for secondary zinc electrodes. J. Power Sources. 2009;191(2):669-677.
- [Google Scholar]
- Dielectric properties of polystyrene–aluminum-nitride composites. J. Appl. Phys.. 2000;88(1):398-404.
- [Google Scholar]
- Polymer composites based on polyvinyl chloride nanofibers and polypropylene films for terahertz photonics. Opt. Mater. Express. 2020;10(10):2456-2469.
- [Google Scholar]
- Sol−gel growth of hexagonal faceted ZnO prism quantum dots with polar surfaces for enhanced photocatalytic activity. ACS Appl. Mater. Interfaces. 2010;2(6):1769-1773.
- [Google Scholar]







