Translate this page into:
Facile Amberlyst A-21 catalyzed access of β-hydroxynitriles via epoxide opening in water
⁎Corresponding authors at: Department of Chemistry, Faculty of Applied Sciences, Umm Al-Qura University, 21955 Makkah, Saudi Arabia. Tel.: + 966 530435760 (S.A. Ahmed). msmalik@uqu.edu.sa (M. Shaheer Malik), bhasghar@uqu.edu.sa (Basim H. Asghar), saahmed@uqu.edu.sa (Saleh A. Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
β-Hydroxynitriles are essential intermediates in the synthesis of diverse bioactive compounds and clinical drugs. One of the precursor reactions to these intermediates is the opening of an epoxide ring with a cyanide nucleophile. In the present study, we report a milder and safer route to β-hydroxynitriles employing recyclable, Amberlyst A-21 resin in the ring-opening of epoxides with acetone cyanohydrin in water. A diverse range of substrates (fifteen), including aromatic epoxides, phenoxy epoxides, non-terminal, and terminal aliphatic epoxides, are investigated under the optimized conditions to afford the desired β-hydroxynitriles in good to excellent yield. In addition to this, the recyclability of the Amberlyst A-21 resin is also successfully demonstrated. This relatively safer methodology has the potential to be explored in other organic transformations.
Keywords
β-Hydroxynitriles
Epoxide opening
Amberlyst A-21
Acetone cyanohydrin
Water
1 Introduction
In recent decades, the development of environmentally less hazardous methods in organic synthesis has become critical because of the increased environmental regulations and awareness. The use of water as reaction media and recyclable reagents/catalysts in organic synthesis are two of the most promising approaches. The epoxide ring-opening reaction is one of the most versatile reactions in organic synthesis. It generates a diverse range of functionalities with controlled regiospecificity and stereospecificity. A broad range of nucleophiles such as alkoxides, Grignard’s reagent, hydroxides, hydrides, cyanides, azides, etc., are employed in epoxide ring-opening reaction. This results in the generation of various key intermediates that are further exploited in the synthesis of bioactive compounds, chiral auxiliaries, active pharmaceutical ingredients, and other useful compounds (Pastor and Yus, 2005; Schneider, 2006). One of such nucleophiles is cyanide ion that provides access to β-hydroxynitriles, a useful intermediate in the synthesis of other chiral intermediates, bioactive compounds and clinical drugs like fluoxetine, duloxetine, GABOB, orlistat, levamisole, β-adrenergic blocking agents etc, (Fig. 1) (Gregory, 1999; Kamal and Ramu, 2002; Kamal et al., 2003, 2006; Kamal et al., 2005a, 2005b; Kim et al., 2012). The cyanide ion, which acts as a nucleophile, is an inhibitor of the enzyme cytochrome c oxidase and is highly toxic (Hamel, 2011). In general, to generate a cyanide nucleophile for the epoxide opening reaction, three approaches are employed, as shown in Fig. 1. Primarily, the epoxide ring opening to access β-hydroxynitriles is carried out by using highly toxic inorganic salts such as NaCN, KCN, LiCN, and volatile HCN (Fülöp et al., 1991; Ciaccio et al., 1992; Jin and Weinreb, 1997; Kamal and Ramu, 2002, 2014). These cyanide-based salts are also studied in combination with additives such as metal complexes such as triflates, perchlorate salts, Yb(CN)3, cyanide ion exchange resin etc, to increase the efficiency of the reaction (Chini et al., 1991; Yamasaki et al., 2001; Iranpoor et al., 2003; Tamami et al., 2003; Naeimi and Karshenas, 2013).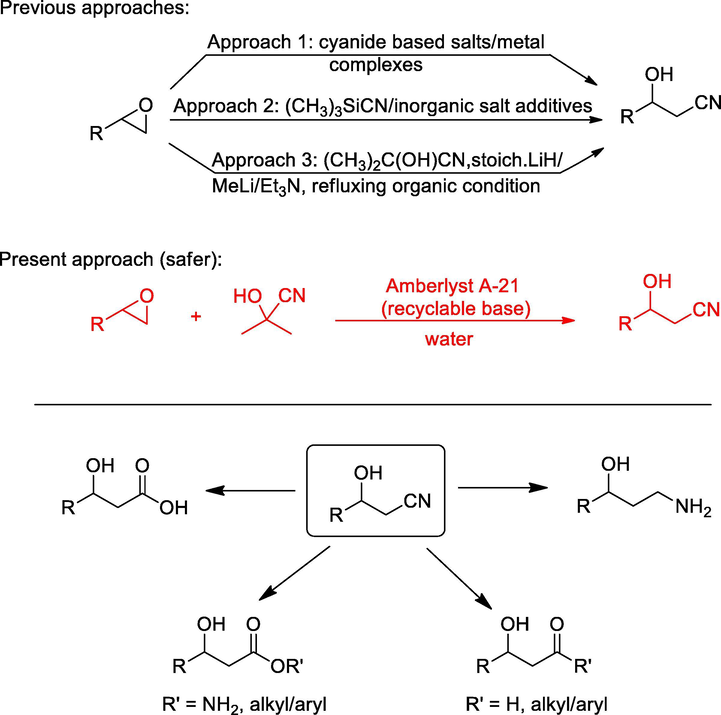
Epoxide opening approaches to β-hydroxynitriles and their versatility.
In the second approach, trimethylsilyl cyanide was developed as a cyanide source in the ring-opening of epoxide to avoid the use of more toxic cyanide-based salts; however, it resulted in the formation of undesired isonitriles as the main product. To overcome this problem, additives are required to obtain the desired hydroxynitriles as the main product. (Matsubara et al., 1990; Konno et al., 2003, 2014). Moreover, trimethylsilyl cyanide and its hydrolyzed products are also toxic. In addition to inorganic salts and trimethylsilyl cyanide, acetone cyanohydrin is an interesting cyanating agent that provides a steady source of cyanide ion. In these reactions, the cyanide ions are produced from acetone cyanohydrin in the presence of base along with the formation of acetone (Van Leeuwen (2014). However, the reported reactions with this reagent were carried out under reflux conditions and in the presence of lanthanide alkoxide, lithium hydride, methyl lithium, and triethylamine to generate the in-situ cyanide ion (Mitchell and Koenig, 1992; Hiroshi et al., 1993; Tsuruoka et al., 1997; Ciaccio et al., 2004). Therefore, there is a need for milder and environmentally safer methodologies that provides access to β-hydroxynitriles. In the present study, we report a milder and safer alternative methodology that allows access to β-hydroxynitriles via ring opening of the epoxide employing acetone cyanohydrin by introducing two crucial greener aspects. The first is the use of a recyclable Amberlyst A-21 resin, which acts as a base by abstracting the proton of acetone cyanohydrin thus facilitating the release of cyanide ion required for epoxide ring-opening. The second important aspect is avoiding the use of organic solvents as reaction medium, and all the reactions are successfully carried out in the water with excellent results.
2 Experimental
2.1 General
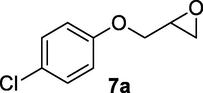
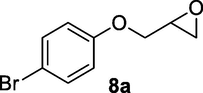
Reactions were monitored by thin layer chromatography (silica gel glass plates containing 60 F-254) and visualization was achieved by UV light or iodine indicator. Column chromatography was performed with Merck (Navi Mumbai, India) 60–120 mesh silica gel. Infrared (IR) spectra are recorded on Perkin-Elmer model 683 or 1310 spectrometers with sodium chloride optics. 1H NMR spectra were recorded on Gemini (200 MHz) (Varian Inc, Palo Alto, CA, USA) and chemical shifts (d) are reported in ppm, downfield from internal TMS standard. ESI spectra were recorded on Micro mass, Quattro LC using ESIþ software with capillary voltage of 3.98 kV and ESI mode positive ion trap detector. Elemental analyses were performed on an elemental analyzer (Model: VARIO EL, Elementar, Hanau, Germany). Starting materials and reagents were either commercially available or purchased from, Sigma Aldrich (St Louis, MO, USA), Lancaster (Alfa Aesar, Johnson Matthey Co, Ward Hill, MA, USA) and Spectrochem Pvt Ltd (Mumbai, India). The glycidyl phenyl ether substrates 3a-10a and 15a were prepared by following standard literature procedure (Zhu et al., 2019).
2.2 General procedure for the synthesis of β-hydroxynitriles (1b −12b)
To a mixture of substituted epoxides (1a-12a) (3 mmol) and acetone cyanohydrin (5 mmol) in 15 mL of deionized water in a 25 mL pressure flask, 1.5 gm of Amberlyst A-21 was added. The reaction was incubated at 45–48 °C in a shaker, and the progress of the reaction is monitored on thin layer chromatography with a solvent system of 25–30% ethyl acetate in hexane. After the completion of the reaction in 6–8 h, the reaction mixture is filtered under vacuum to separate Amberlyst A-21. The catalyst was washed thoroughly with ethyl acetate and also stirred in a small quantity of organic solvent. The water-based reaction mixture was extracted with ethylacetate (twice), and the combined organic layer was washed with brine solution and dried over with and. Na2SO4. The solvent was evaporated under pressure, and the obtained crude residues was purified by 60–120 silica-gel column chromatography with 20–25% ethylacetate in hexane to provide pure β-hydroxynitriles (1b-12b).
2.2.1 3-Hydroxy-3-phenylpropanenitrile (1b)
Following the general procedure, the reaction was carried out by employing epoxide 1a (0.36 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 1b (0.37 g, 85% yield) as yellow liquid. IR (Neat) 3438, 3046, 2954, 2923, 2238, 1092 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.67 (d, 2H, J = 5.8 Hz), 3.15 (br s, 1H), 4.94 (t, 1H, J = 5.8), 7.35 (s, 5H) [lit. (Yang et al., 2018): 1H NMR (400 MHz, CDCl3) δ (ppm): 2.58 (d, 2H, J = 6.0 Hz), 3.28 (br s, 1H), 4.85 (t, 1H, J = 6.2), 7.20–7.30 (m, 5H); 13C NMR (100 MHz, CDCl3) δ (ppm): 27.8, 69.7, 117.6, 125.6, 128.6, 128.8, 144.1]; EIMS (m/z): 147 (M+); Anal. Calcd for C9H9NO: C, 73.45; H, 6.16; N, 9.52. Found: C, 73.28; H, 6.03; N, 9.45%.
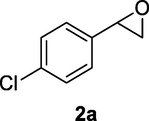
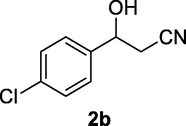
2.2.2 3-(4-Chlorophenyl)-3-hydroxypropanenitrile (2b)
Following the general procedure, the reaction was carried out by employing epoxide 2a (0.46 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 2b (0.45 g, 83% yield) as yellow liquid. IR (KBr) 3444, 3074, 2936, 2904, 2234, 1069 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.73 (d, 2H, J = 5.8 Hz), 3.19 (br s, 1H) 5.00 (t, 1H, J = 5.8 Hz), 7.32–7.39 (m, 4H); [lit. (Coady et al., 2015): 1H NMR (400 MHz, CDCl3) δ (ppm): 2.74 (d, 2H, J = 6.4 Hz), 5.04 (t, 1H, J = 6.4), 7.35 (m, 4H); 13C NMR (100 MHz, CDCl3) δ (ppm): 28.01, 69.9, 114.4, 126.9 (2Cs), 133.6 (2Cs), 160.2]; EIMS (m/z): 181 (M+); Anal. Calcd for for C9H8ClNO: C, 59.52; H, 4.44; N, 7.71. Found C, 59.72; H, 4.42; N, 7.68%.
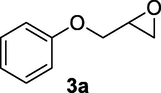
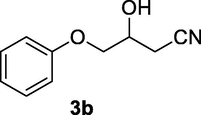
2.2.3 3-Hydroxy-4-phenoxybutanenitrile (3b)
Following the general procedure, the reaction was carried out by employing epoxide 3a (0.45 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 3b (0.48 g, 90% yield) as pale solid, mp 69–72 °C. IR (KBr) 3397, 3024, 2932, 2267, 1244, 1099 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.62–2.79 (m, 2H), 3.01–3.06 (m, 1H), 3.99–4.09 (m, 2H), 4.27–4.36 (m, 1H), 6.84–6.99 (m, 3H); 7.25–7.31 (m, 2H); [lit. (Azizi et al., 2015): 1H NMR (500 MHz, CDCl3) δ (ppm): 2.69–2.81 (m, 2H), 2.92 (m, 1H), 4.12–4.17 (m, 2H), 4.39 (br.s, 1H), 7.02–7.13 (m, 3H), 7.31–7.39 (m, 2H); 13C NMR (125 MHz, CDCl3) δ (ppm): 26.8, 28.7, 29.6, 31.2, 60.8, 116.1, 117.4, 119.2, 159.1]; EIMS (m/z): 177 (M+); Anal. Calcd for for C10H11NO2: C, 67.79; H, 6.21; N, 7.90. Found C, 67.75; H, 6.17; N, 7.82%.
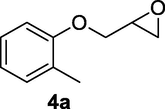
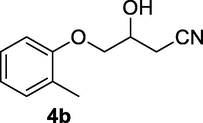
2.2.4 3-Hydroxy-4-(o-tolyloxy)butanenitrile (4b)
Following the general procedure, the reaction was carried out by employing epoxide 4a (0.49 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 4b (0.49 g, 86% yield) as gummy solid. IR (KBr) 3401, 3022, 2930, 2269, 1242, 1095 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.22 (s, 3H) 2.62–2.88 (m, 3H), 3.97–4.08 (m, 2H), 4.28–4.39 (m, 1H), 6.75–6.90 (m, 2H); 7.08–7.15 (m, 2H); [lit. (Kamal and Khanna, 2001): 1H NMR (200 MHz, CDCl3) δ (ppm): 2.22 (s, 3H), 2.60–2.84 (m, 2H), 3.16 (d, J = 4.76 Hz, 2H), 3.93–4.10 (m, 2H), 4.23–4.40 (m, 1H), 6.73–6.94 (m, 2H), 7.06–7.19 (m, 2H)]; EIMS (m/z): 191 (M+); Anal. Calcd for for C11H13NO2: C, 69.11; H, 6.80; N, 7.33. Found C, 68.93; H, 6.76; N, 7.19%.
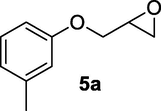
2.2.5 3-Hydroxy-4-(m-tolyloxy)butanenitrile (5b)
Following the general procedure, the reaction was carried out by employing epoxide 5a (0.49 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 5b (0.52 g, 92% yield) as gummy solid. IR (KBr) 3398, 3024, 2926, 2258, 1238, 1088 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.25 (s, 3H) 2.64–2.82 (m, 3H), 3.97–4.08 (m, 2H), 4.26–4.36 (m, 1H), 6.79–7.19 (m, 4H); EIMS (m/z): 191 (M+); Anal. Calcd for for C11H13NO2: C, 69.11; H, 6.80; N, 7.33. Found C, 68.94; H, 6.75; N, 7.19%.
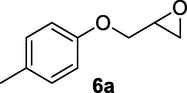
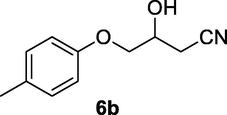
2.2.6 3-Hydroxy-4-(p-tolyloxy)butanenitrile (6b)
Following the general procedure, the reaction was carried out by employing epoxide 6a (0.49 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 6b (0.48 g, 85% yield) as white solid, mp 59–62 °C. IR (KBr) 3401, 3028, 2928, 2260, 1239, 1089 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.29 (s, 3H) 2.61–2.80 (m, 3H), 3.98–4.09 (m, 2H), 4.25–4.35 (m, 1H), 6.79 (d, 2H); 7.06 (d, 2H); [lit. (Azizi et al., 2015): 1H NMR (500 MHz, CDCl3) δ (ppm): 2.31 (s, 3H), 2.78–2.86 (m, 2H), 3.71–3.85 (m, 2H), 4.13 (m, 1H), 4.41 (br.s, 1H), 6.87 (m, 2H), 7.24 (m, 2H); 13C NMR (125 MHz, CDCl3) δ (ppm): 21.2, 23.1, 65.8, 71.2, 114.9, 117.8, 131.2, 132.4, 156.5]; EIMS (m/z): 191 (M+); Anal. Calcd for for C11H13NO2: C, 69.11; H, 6.80; N, 7.33. Found C, 68.92; H, 6.76; N, 7.11%.
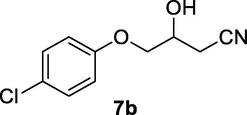
2.2.7 4-(4-Chlorophenoxy)-3-hydroxybutanenitrile (7b)
Following the general procedure, the reaction was carried out by employing epoxide 7a (0.55 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 7b (0.58 g, 92% yield) as yellowish solid. IR (KBr) 3404, 3025, 2933, 2258, 1245, 1089 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.63–2.81 (m, 3H), 3.96–4.05 (m, 2H), 4.21–4.32 (m, 1H), 6.82 (d, 2H); 7.15 (d, 2H); [lit. (Kamal and Khanna, 2001): 1H NMR (200 MHz, CDCl3): 2.63 (dd, 1H), 2.79 (dd, 1H), 3.19 (br.s, 1H), 3.99 (d, 2H), 4.24–4.34 (m, 1H), 6.84 (d, 2H), 7.24 (d, 2H)]; EIMS (m/z): 211 (M+); Anal. Calcd for for C10H10NO2Cl: C, 56.87; H, 4.74; N, 6.64. Found C, 56.78; H, 4.70; N, 6.61%.
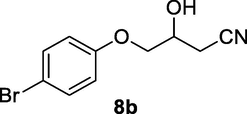
2.2.8 4-(4-Bromophenoxy)-3-hydroxybutanenitrile (8b)
Following the general procedure, the reaction was carried out by employing epoxide 8a (0.68 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 8b (0.68 g, 90% yield) as pale solid, mp 80–82 °C. IR (KBr) 3395, 3028, 2938, 2259, 1242, 1092 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.60–2. 77 (m, 3H), 3.98–4.11 (m, 2H), 4.20–4.31 (m, 1H), 6.81 (d, 2H); 7.18 (d, 2H); [lit. (Kamal and Khanna, 2001): 1H NMR (200 MHz, CDCl3) δ (ppm): 22.61 (dd, 1H), 2.74 (dd, 1H), 3.99 (d, 2H), 4.20–4.33 (m, 1H), 6.76 (d, 2H), 7.35 (d, 2H)]; EIMS (m/z): 255 (M+); Anal. Calcd for for C10H10NO2Br: C, 47.05; H, 3.92; N, 5.49. Found C, 47.01; H, 3.88; N, 5.53%.
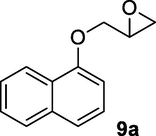
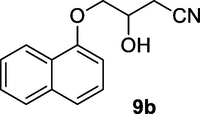
2.2.9 3-Hydroxy-4-(naphthalen-1-yloxy)butanenitrile (9b)
Following the general procedure, the reaction was carried out by employing epoxide 9a (0.6 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 9b (0.63 g, 93% yield) as white solid, mp 88-92 °C. IR (KBr) 3401, 3025, 2933, 2261, 1242, 1096 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.68–2.89 (m, 3H), 3.88–4.03 (m, 2H), 4.05–4.29 (m, 1H), 6.68–6.81 (m, 1H); 7.52–8.2 (m, 6H); [lit. (Kamal and Khanna, 2001): 1H NMR (200 MHz, CDCl3) δ (ppm): 2.70–2.91 (m, 2H), 4.21 (d, 2H), 4.39–4.50 (m, 1H), 6.8 (d, 1H), 7.25–7.51 (m, 4H), 7.76–7.81 (m,1H)]; EIMS (m/z): 227 (M+); Anal. Calcd for for C14H13NO2: C, 74.00; H, 5.72; N, 6.16. Found C, 73.85; H, 5.68; N, 6.19%.
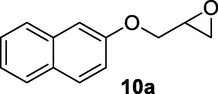
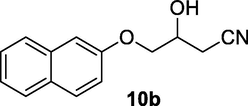
2.2.10 3-Hydroxy-4-(naphthalen-2-yloxy)butanenitrile (10b)
Following the general procedure, the reaction was carried out by employing epoxide 10a (0.6 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 10b (0.61 g, 90% yield) as white solid, mp 130–133 °C. IR (KBr) 3395, 3031, 2928, 2264, 1248, 1098 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.26–2.85 (m, 3H), 3.81–4.02 (m, 2H), 4.03–4.25 (m, 1H), 7.19–8.03 (m, 7H); [lit. (Kamal and Khanna, 2001): 1H NMR (200 MHz, CDCl3) δ (ppm): 2.69 (dd, 1H), 2.84 (dd, 1H), 3.99–4.33 (m, 3H), 7.14–7.20 (m, 2H), 7.26–7.55 (m, 2H), 7.66–7.78 (m, 3H)]; EIMS (m/z): 227 (M+); Anal. Calcd for for C14H13NO2: C, 74.00; H, 5.72; N, 6.16. Found C, 73.78; H, 5.64; N, 6.10%.
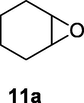
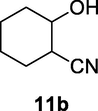
2.2.11 2-Hydroxycyclohexanecarbonitrile (11b)
Following the general procedure, the reaction was carried out by employing epoxide 11a (0.29 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 11b (0.29 g, 78% yield) as oily liquid. IR (KBr) 3420, 2970, 2256, 1060 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 1.15–1.40 (m, 3H), 1.52–1.83 (m, 3H), 1.96–2.16 (m, 2H), 2.36–2.47 (m, 1H); 3.48 (s, 1H); 3.63–3.75 (m, 1H); [lit. (Rocha et al., 2017): 1H NMR (400 MHz, CDCl3) δ (ppm): 1.25 (m, 3H), 1.56 (m, 1H), 1.71 (m, 1H), 2.06 (m, 2H), 2.41 (m, 1H), 2.85 (bs, 1H), 3.70 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 23.5, 24.0, 28.2, 33.8, 37.6, 70.6, 121.6]; EIMS (m/z): 125 (M+); Anal. Calcd for C7H11NO: C, 67.17; H, 8.86; N, 11.19. Found C, 67.20; H, 8.89; N, 10.99%.
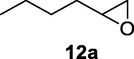
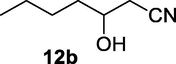
2.2.12 3-Hydroxyheptanenitrile (12b)
Following the general procedure, the reaction was carried out by employing epoxide 12a (0.3 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 12b (0.3 g, 80% yield) as oily liquid. IR (KBr) 3598, 3019, 2259, 1065 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 0.91 (t, 3H), 1.30 (m, 4H), 1.60 (m, 2H) 2.53 (m, 2H), 3.14 (bs, 1H); 3.82 (q, 1H); [lit. (Pollock et al., 2007): 1H NMR (400 MHz, CDCl3) δ (ppm): 0.93 (t, 1H), 1.25–1.5 (m, 4H), 1.55–1.70 (m, 2H), 2.1 (bs, 1H), 2.46 (dd, 1H), 2.52 (dd, 1H), 3.96 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 13.9, 22.4, 26.1, 27.5, 36.2, 67.7, 117.7]; EIMS (m/z): 127 (M+); Anal. Calcd for C7H13NO: C, 66.10; H, 10.30; N, 11.01. Found C, 65.81; H, 10.05; N, 10.95%0.
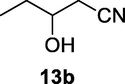
2.2.13 3-Hydroxypentanenitrile (13b)
Following the general procedure, the reaction was carried out by employing epoxide 13a (0.21 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 13b (0.22 g, 76% yield) as oily liquid. IR (KBr) 3485, 2989, 2285, 1071 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 0.96 (t, 3H), 1.51 (m, 2H), 2.58 (m, 2H), 3.29 (bs, 1H); 3.69 (q, 1H); EIMS (m/z): 99 (M+); Anal. Calcd for C5H9NO: C, 60.58; H, 9.15; N, 14.13. Found C, 60.53; H, 9.18; N, 14.10%.
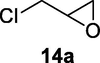
2.2.14 4-Chloro-3-hydroxybutanenitrile (14b)
Following the general procedure, the reaction was carried out by employing epoxide 14a (0.27 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 14b (0.29 g, 84% yield) as oily liquid. IR (KBr) 3458, 2985, 2289, 1058 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 2.68–2.71 (m, 2H), 3.60–3.71 (m, 3H); [lit. (Wan et al., 2015): 1H NMR (500 MHz, CDCl3) δ (ppm): 2.74–2.71 (t, 2H), 3.67–3.69 (dd, 2H), 4.22 (s, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 25.9, 50.1, 70.0, 119.1]. EIMS (m/z): 119 (M+); Anal. Calcd for C4H6ClNO: C, 40.19; H, 5.06; N, 11.72. Found C, 39.2; H, 5.02; N, 11.59%.
2.2.15 4-(tert-butoxy)-3-hydroxybutanenitrile (15b)
Following the general procedure, the reaction was carried out by employing epoxide 15a (0.39 g, 3 mmol), acetone cyanohydrin (0.45 mL, 5 mmol), 1.5 g Amberlyst A-21 in 25 mL deionized water to provide compound 15b (0.39 g, 83% yield) as oily liquid. IR (KBr) 3419, 2985, 2252, 1062 cm−1; 1H NMR (200 MHz, CDCl3) δ (ppm): 1.20 (s, 9H), 2.39–2.68 (m, 2H), 3.25–3.42 (m, 2H), 3.72–3.91 (m, 1H); [lit. (Naeimi and Moradian, 2006) 1H NMR (400 MHz, CDCl3) δ (ppm): 1.14 (s, 9H), 2.36–2.4 8 (m, 2H), 3.14 (m, 2H), 3.88 (m, 1H); 13C NMR (100 MHz, CDCl3) δ (ppm): 22.1, 27.4, 36.2, 66.5, 68.5, 73.2, 117.8]; EIMS (m/z): 157 (M+); Anal. Calcd for C8H15NO2: C, 61.12; H, 9.62; N, 8.91. Found C, 61.08; H, 9.58; N, 8.88%.
3 Results and discussion
Heterogeneous reactions employing ion exchange resins offer promising advantages over their homogenous counterparts. Environmental compatibility, simpler operating conditions, recyclability, non-corrosiveness, etc., are some of the advantages involving the use of ion exchange resins (Barbaro and Liguori, 2009). Amberlyst A-21 is a weakly basic ion exchange resin, which was primarily developed to remove acidic materials from a range of product streams. It is used as a recyclable base in the synthesis of diverse heterocyclic compounds such as substituted pyrans, chromenes, furans, indazolones etc, that avoids the use of hazardous solvents in chromatographic separation (Yadav et al., 2007; Palmieri et al., 2010; Bihani et al., 2013; Rao et al., 2014). Additionally, Amberlyst A-21 is utilized in different organic transformations such as the conversion of the carbonyl group to oximes, deprotection of N-Boc amines and Henry reaction (Ballini et al., 1996, 1997; Srinivasan et al., 2005). Amberlyst A-21 supported with copper iodide is also exploited in catalyzing Huisgen’s reaction, KA2 coupling reaction, and reaction involving the synthesis of propargyl amines (Girard et al., 2006; Bosica and Gabarretta, 2015; Bosica and Abdilla, 2017). Keeping in view the versatility of Amberlyst A-21, we investigated its efficiency as a recyclable base in the epoxide ring-opening reaction to obtain the useful key intermediates, β-hydroxynitriles.
In the present study, macro-reticular, commercially available Amberlyst A-21 resin in bead form was used without any further conditioning or purification for epoxide ring-opening by a cyanide nucleophile provided by acetone cyanohydrin. Amberlyst A-21 has a macroporous matrix, comprised of styrene divinylbenzene, with a moisture content of 56–62%. It is weakly basic with tertiary alkylamine functionality as a free base form with an added advantage of recyclability. The use of organic solvent as the reaction medium was completely circumvented, and the reaction was carried out in water, the most environmentally compatible solvent. To optimize the reaction, initially, the epoxide ring-opening reaction was carried out with styrene oxide as a model substrate. Acetone cyanohydrin was employed as a cyanide source in the presence of Amberlyst A-21 with water as the reaction medium (Table 1). The preliminary results showed that the progress of the reaction was promising, and the desired hydroxynitrile was formed with over 40% yield after 12 h at room temperature.
Entry
Solvent
Temperature
Time (h)
Yield (%)
1.
Water
rt
12
41
2.
Water
45 °C
8
85 (70)b
3.
Dichloromethane
45 °C
8
55
4.
Acetonitrile
45 °C
8
45
5.
Ethanol
45 °C
8
80
In subsequent optimization, the temperature was increased to 45–48 °C, which resulted in higher yield (over 85% in 8 h). However, there was no remarkable increase in the yield with a further rise in temperature. In addition to this, three different organic solvents were studied and compared with the water. The yields of the reactions in organic media were comparatively lower than water, and in this case, the use of an environmentally compatible water-based reaction medium was desired. The Amberlyst A-21 resin amount was also investigated, and the use of 1 g and 1.5 g of resin provided 70% and 85% of yields, respectively. However, a further increase in the amount of resin did not lead to any substantial increase in yield. In addition to this, the reaction progress was also studied under the optimized conditions with styrene oxide 1a as a model substrate (Fig. 2). The results showed that the reaction proceeded with 38% and 70% after 2 h and 6 h, respectively. After 8 h of reaction time, 85% yield of the product was obtained, and a further increase in reaction time resulted in no improvement in reaction yield.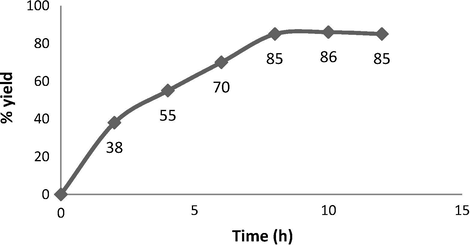
Reaction progress curve of epoxide opening of 1aa.
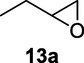
Finally, the Amberlyst A-21 mediated nucleophilic opening of different epoxides was carried out in the presence of acetone cyanohydrin at 45–48 °C with water as solvent (table 2). In this investigation, the aromatic epoxides acted as better substrates for nucleophilic opening with acetone cyanohydrin than the aliphatic epoxides. The styrene-based epoxides provided the corresponding β-hydroxynitriles in around 85% yields under the optimized conditions. The aromatic glycidyl phenyl ethers were comparatively more reactive than the styrene epoxides, and the glycidyl phenyl ethers afforded the desired products in 86–92% yields. The parent glycidyl phenyl ether 3a resulted in 90% of corresponding product 3b, and subsequently, the electronic effect of some selected electron-donating and withdrawing groups and their positions on 3a was studied. The substrate 5a with electron-donating methyl substitution on meta-position provided the best result with 92% yield compared to other isomeric substrates (4a and 6a). The glycidyl phenyl ether with electron-withdrawing groups like bromo and chloro substituents on para-position (7a and 8a) also provided excellent results. The glycidyl ethers with bulkier napthyl group were employed as substrates, and 1-substituted ether 9a produced slightly better yield than 2-substituted ether 10a. In addition to the aromatic substrate, some selected aliphatic epoxides were also investigated to establish the versatility of the methodology. Among the aliphatic substrates, the non-terminal cyclohexene oxide 11a afforded the desired product 11b in 78% yield. On the other hand, the terminal aliphatic epoxide, 1,2-epoxyhexane 12a and 1,2-epoxybutane 13a proceeded with 80% and 76% yield, respectively. The glycidyl based aliphatic substrates with chloride and tert-butoxy groups (14a and 15a) were also investigated affording the desire products in good yields. In these ring-opening reactions, the nucleophilic cyanide attacked the epoxide from the least hindered side, resulting in a single product. The formation of the products was confirmed with the characteristic IR stretching vibration bands around 3400 and 2200 cm−1 corresponding to hydroxyl and cyanide groups, respectively, in addition to other spectroscopic characterization.
Entry
Substrate
Product
Yield (%)b
Yield (%)c
1.
85
79d
80e,f
2.
83
78f
3.
90
88d
80g
72f
4.
86
86g
5.

90
–
6.
85
87g
78f
7.
92
90g
8.
90
82g
9.
93
70g
10.
90
55g
11.
78
89d
85h
12.
80
78d
70h
13.
76
–
14.
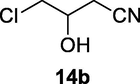
84
85d,h
15.
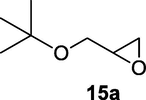
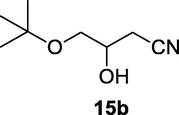
83
85d
The recyclability of the Amberlyst A-21 resin was demonstrated with glycidyl phenyl ether 3a as a model substrate. After the completion of the first run under the studied conditions, Amberlyst A-21 resin was recovered by simple filtration. It was washed with ethanol, followed by drying at 80 °C under reduced pressure (1 h) for subsequent use. The results showed that the efficiency of the resin was retained in the second run, and from the third run, the yields started to decrease marginally (table 3). After the fifth cycle, the resin was required to be reactivated to obtain the desired high yields. Amberlyst A-21 resin catalyzed the ring-opening of epoxide by the mechanism outlined in Fig. 3. The tertiary alkylamine functionality in the free base form of Amberlyst A-21 17a abstracts the active proton from acetone cyanohydrin 16, thereby releasing the cyanide ion besides the formation of acetone 18. The released cyanide ion than attacks the epoxide 1a from sterically unhindered side to provide the desired β-hydroxynitriles 1b. This recyclable Amberlyst A-21 offers a steady source of cyanide nucleophile in a minimal amount required for ring-opening of the epoxide.
Entry
Cycle
Yield (%)
1.
First
90
2.
Second
90
3.
Third
88
4.
Fourth
86
5.
Fifth
78
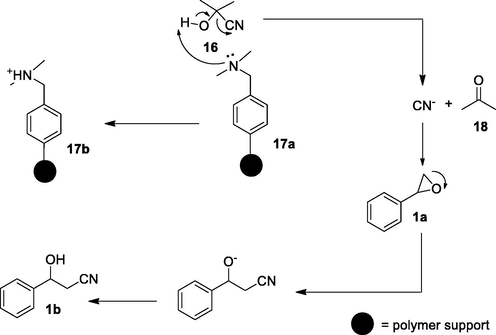
Plausible mechanism of β-hydroxynitrile formation.
4 Conclusion
The synthetic utility of β-hydroxynitriles necessitates the development of safer methodologies. Toxic cyanide salts or trimethylsilyl cyanide are primarily used as a source of cyanide ion in the ring-opening of epoxide to access β-hydroxynitriles. An alternate route to produce a cyanide nucleophile is the use of acetone cyanohydrin in the presence of a base. In the present investigation, a weakly basic, recyclable, ion exchange resin, Amberlyst A-21 is utilized for the opening of different aromatic and aliphatic oxiranes to access β-hydroxynitriles. To further enhance the environmental compatibility of the method, the reactions are performed in water with excellent results. The results showed that aromatic phenoxy epoxides were more susceptible to ring-opening than the aliphatic epoxides, and the aromatic epoxides with electron-withdrawing groups provided relatively better yields under the studied conditions. The present methodology offers a relatively greener alternative to the reported methods, which employs toxic cyanide sources, mostly in organic solvents. It has the potential to be explored in other organic transformations like ring-opening of azirines.
Acknowledgments
The authors are highly indebted to the Deanship of the Scientific Research (DSR), Umm Al-Qura University, for the full financial support through the project number 19-SCI-1-01-0020.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Van Leeuwen P.W.N.M., 2014. Cyanation of Epoxides. C-1 Building Blocks in Organic Synthesis 2. P. W. N. M. van Leeuwen. Stuttgart, Georg Thieme Verlag.
- A magnetic nanoparticle catalyzed eco-friendly synthesis of cyanohydrins in a deep eutectic solvent. RSC Adv.. 2015;5:61191-61198\&&&.
- [Google Scholar]
- Amberlyst A-21 an excellent heterogeneous catalyst for the conversion of carbonyl compounds to oximes. Chem. Lett.. 1997;26:475-476.
- [Google Scholar]
- Nitroaldol (Henry) reaction catalyzed by Amberlyst A-21 as a far superior heterogeneous catalyst. Tetrahedron. 1996;52:1677-1684.
- [Google Scholar]
- Amberlyst A21: a reusable solid catalyst for green synthesis of pyran annulated heterocycles at room temperature. C. R. Chim.. 2013;16:419-426.
- [Google Scholar]
- The KA2 coupling reaction under green, solventless, heterogeneous catalysis. J. Mol. Catal. A: Chem.. 2017;426:542-549.
- [Google Scholar]
- Unprecedented one-pot multicomponent synthesis of propargylamines using Amberlyst A-21 supported CuI under solvent-free conditions. RSC Adv.. 2015;5:46074-46087.
- [Google Scholar]
- Easy direct stereo- and regioselective formation of β-hydroxy nitriles by reaction of 1,2-epoxides with potassium cyanide in the presence of metal salts. Tetrahedron Lett.. 1991;32:4775-4778.
- [Google Scholar]
- Synthesis of β-hydroxy nitriles and 1,3-amino alcohols from epoxides using acetone cyanohydrin as a LiCN precursor. Tetrahedron Lett.. 2004;45:7201-7204.
- [Google Scholar]
- Facile conversion of epoxides to β-hydroxy nitriles under anhydrous conditions with lithium cyanide. Tetrahedron Lett.. 1992;33:1431-1434.
- [Google Scholar]
- Substrate evaluation of Rhodococcus erythropolis SET1, a nitrile hydrolysing bacterium, demonstrating dual activity strongly dependent on nitrile sub-structure. Eur. J. Org. Chem.. 2015;5:1108-1116.
- [Google Scholar]
- Trans-2-Cyanocycloalkanols: Versatile synthons for alicyclic cis- and trans-1,3-amino alcohols. Synthesis. 1991;01:43-46.
- [Google Scholar]
- Reusable polymer-supported catalyst for the [3+2] Huisgen cycloaddition in automation protocols. Org. Lett.. 2006;8:1689-1692.
- [Google Scholar]
- Cyanohydrins in nature and the laboratory: biology, preparations, and synthetic applications. Chem. Rev.. 1999;99:3649-3682.
- [Google Scholar]
- A review of acute cyanide poisoning with a treatment update. Crit. Care Nurse.. 2011;3:172-181. quiz 82
- [Google Scholar]
- Ring opening of epoxides with acetone cyanohydrin catalyzed by lanthanoid(III) alkoxides. Chem. Lett.. 1993;22:975-978.
- [Google Scholar]
- Micellar media for the efficient ring opening of epoxides with CN−, N3−, NO3−, NO2−, SCN−, Cl− and Br− catalyzed with Ce(OTf)4. Org. Biomol. Chem.. 2003;1:724-727.
- [Google Scholar]
- Ring opening of epoxides with sodium cyanide catalyzed with Ce(OTf)4. Synth. Commun.. 1999;29:2249-2254.
- [Google Scholar]
- Enantioselective total syntheses of the 5,11-methanomorphanthridine amaryllidaceae alkaloids (−)-pancracine and (−)-coccinine. J. Am. Chem. Soc.. 1997;119:2050-2051.
- [Google Scholar]
- A new facile chemoenzymatic synthesis of levamisole. Bioorg. Med. Chem. Lett.. 2005;15:613-615.
- [Google Scholar]
- Lipase-mediated resolution of 3-hydroxy-4-trityloxybutanenitrile: synthesis of 2-amino alcohols, oxazolidinones and GABOB. Tetrahedron Asymmetry. 2006;17:1281-1289.
- [Google Scholar]
- New chemoenzymatic pathway for β-adrenergic blocking agents. Tetrahedron Asymmetry. 2005;16:1485-1494.
- [Google Scholar]
- Chemoenzymatic synthesis2 of both enantiomers of fluoxetine, tomoxetine and nisoxetine: lipase-catalyzed resolution of 3-aryl-3-hydroxypropanenitriles. Tetrahedron Asymmetry. 2002;13:2039-2051.
- [Google Scholar]
- Chemoenzymatic synthesis of duloxetine and its enantiomer: lipase-catalyzed resolution of 3-hydroxy-3-(2-thienyl) propanenitrile. Tetrahedron Lett.. 2003;44:4783-4787.
- [Google Scholar]
- A facile preparation of (±)-β-hydroxy nitriles and their enzymatic resolution with lipases. Tetrahedron Asymmetry. 2001;12:405-410.
- [Google Scholar]
- Asymmetric total synthesis of ent-tetrahydrolipstatin from an epoxy alkenol. Synlett. 2012;23:1832-1834.
- [Google Scholar]
- An epoxide ring-opening reaction via hypervalent silicate intermediate: synthesis of statine. Synthesis. 2003;14:2161-2164.
- [Google Scholar]
- Reaction of cyanotrimethylsilane with oxiranes under Yb(CN)3 catalysis. Tetrahedron Lett.. 1990;31:6209-6212.
- [Google Scholar]
- Regiospecific opening of 1,2-expoxides with acetone cyanohydrin under mildly basic conditions. Tetrahedron Lett.. 1992;33:3281-3284.
- [Google Scholar]
- Highly regioselective conversion of epoxides to β-hydroxy nitriles using metal(II) Schiff base complexes as new catalysts under mild conditions. Polyhedron. 2013;49:234-238.
- [Google Scholar]
- Metal(II) Schiff base complexes as catalysts for the high-regioselective conversion of epoxides to β-hydroxy nitriles in glycol solvents. Can. J. Chem.. 2006;84:1575-1579.
- [Google Scholar]
- Efficient two-step sequence for the synthesis of 2,5-disubstituted furan derivatives from functionalized nitroalkanes: successive Amberlyst A21- and Amberlyst 15-catalyzed processes. Chem. Commun.. 2010;46:6165-6167.
- [Google Scholar]
- A mild biosynthesis of lactones via enantioselective hydrolysis of hydroxynitriles. Tetrahedron Asymmetry. 2007;18:1888-1892.
- [Google Scholar]
- Catalysis by Amberlyst A-21: a greener approach to 4,5,6,7-tetrahydro-1H-indazol-3(2h)-ones via construction of cyclohexanones. Synth. Commun.. 2014;44:1076-1083.
- [Google Scholar]
- Enzymatic kinetic resolution of secondary alcohols using an ionic anhydride generated in situ. ChemSusChem. 2017;10:296-302.
- [Google Scholar]
- Synthesis of 1,2-difunctionalized fine chemicals through catalytic, enantioselective ring-opening reactions of epoxides. Synthesis. 2006;23:3919-3944.
- [Google Scholar]
- Rapid deprotection of N-Boc amines by TFA combined with freebase generation using basic ion-exchange resins. Mol. Divers.. 2005;9:291-293.
- [Google Scholar]
- Highly regioselective conversion of epoxides to β-hydroxy nitriles with cyanide exchange resin. Synth. Commun.. 2003;33:3153-3157.
- [Google Scholar]
- Practical oxirane ring opening with in situ prepared LICN; synthesis of (2S,3R)-3-(2,4-difluorophenyl)-3-hydroxy-2-methyl-4-(1H–1,2,4-triazol-1-yl)-1-butanenitrile. Synth. Commun.. 1997;27:3547-3557.
- [Google Scholar]
- A one-step biocatalytic process for (S)-4-chloro-3-hydroxybutyronitrile using halohydrin dehalogenase: a chiral building block for atorvastatin. ChemCatChem. 2015;7:2446-2450.
- [Google Scholar]
- Amberlyst A-21®: An efficient, cost-effective and recyclable catalyst for the synthesis of substituted 4H-chromenes. Catal. Commun.. 2007;8:2208-2211.
- [Google Scholar]
- Novel multiaction of Zr catalyst: one-pot synthesis of β-cyanohydrins from olefins. J. Am. Chem. Soc.. 2001;123:1256-1257.
- [Google Scholar]
- Iridium catalysed highly efficient transfer hydrogenation reduction of aldehydes and ketones in water. Catal. Commun.. 2018;117:38-42.
- [Google Scholar]
- A facile and efficient method for synthesis of β-iodocarboxylates from terminal epoxides. Tetrahedron Lett.. 2019;60:151353
- [Google Scholar]







