Translate this page into:
Facile solvothermal synthesis of highly active monoclinic scheelite BiVO4 for photocatalytic degradation of methylene blue under white LED light irradiation
⁎Corresponding author at: Center of Excellence for Green Energy and Environmental Nanomaterials (CE@GrEEN), 300A Nguyen Tat Thanh, District 4, Ho Chi Minh City 755414, Viet Nam. ndtrinh@ntt.edu.vn (Trinh Duy Nguyen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, BiVO4 was successfully synthesized via the solvothermal process using a solvent mixture of ethylene glycol and water under different synthesis conditions of temperature and pH. Physicochemical properties such as crystal phase, morphology, and optical absorption of the as-synthesized BiVO4 samples were characterized by X-ray diffraction (XRD), Raman spectra, field emission scanning electron microscopy (FE-SEM), and ultraviolet–visible diffraction reflection spectroscopy (UV–vis DRS). The XRD analysis showed that different synthesis conditions of temperature and pH significantly affected the growth of monoclinic BiVO4 crystals oriented along (0 4 0) facets. Form SEM results, the synthesis conditions, including pH and temperature, have a great effect on the morphology of monoclinic structured BiVO4. As the pH value increases in the range of 0–9 and temperature increases from 80 °C to 180 °C, the morphology of BiVO4 changed from peanut-, rod-, and leaf-like shapes. The photocatalytic activities of as-synthesized BiVO4 photocatalysts were evaluated by the photodegradation of methylene blue (MB) dye under irradiation of white LED light. We have found that by using appropriate synthesis conditions (the synthesis temperature of 140 °C and the synthesis pH of 7) the BiVO4 exhibited high photocatalytic efficiency for MB degradation (about 82.30% after 180 min of irradiation). This result is due to the development of the BiVO4 crystals oriented along (0 4 0) facets with an increase in the intensity ratio of I(0 4 0)/I(1 2 1). The growth of BiVO4 crystals oriented along (0 4 0) facets may be beneficial to enhance the photocatalytic activity of monoclinic scheelite BiVO4.
Keywords
Solvothermal synthesis
monoclinic scheelite BiVO4
Photodegradation
LED light irradiation
1 Introduction
Concurrent rapid industrialization and population growth result in a substantial discharge of organic pollutants, causing severe environmental pollution and posing major health risks to human. Therefore, the remediation of toxic organic waste has been the focal research direction to ensure the process of sustainable development and limit the emission of environmental pollutants. In this research direction, photocatalytic techniques using semiconductor materials provide an ideal solution in terms of solar conversion and contaminant removal (Singh et al., 2020; Rajamanickam and Shanthi, 2016; Abdennouri et al., 2016). The main advantage of this technique is that organic pollutants can be converted into CO2, water, or other non-hazardous inorganic compounds and do not cause secondary pollution (Abbasi et al., 2018; Ameta and Ameta, 2016). Recently, monoclinic bismuth vanadate-based semiconductor photocatalyst (m-BiVO4) has attracted much attention from researchers due to its non-toxicity, low cost and high photocatalytic activity. m-BiVO4 has narrow band-gap energy, at about 2.4 eV, allowing photocatalysis to be directly exposed to visible light (the light area occupies 45% of the solar spectrum). Besides, it has been shown that the material could achieve high photocatalytic efficiency in water separation and decomposition of pollutants (Ressnig et al., 2012; Kohtani et al., 2003). However, due to some intrinsic properties within the structure of m-BiVO4, this material has some limitations, such as weak adsorption capacity, a difficulty for the electrical charge to move to the catalytic surface, and the recombination process of electron and hole pairs, thus reducing the photocatalytic activity. To improve photocatalytic activity, recent studies have shown that the surface structure of materials plays an essential role in their photocatalytic activities since photocatalytic reactions occur only when electrons and holes are created on the surface (Lim et al., 1995; Sun et al., 2009). For BiVO4 photocatalyst materials, Hui-Ling Tan and colleagues showed that BiVO4 synthesized with the appropriate crystal facet ratio of (0 1 0)/(1 1 0) would give superior photocatalytic activity (Tan et al., 2016).
According to previous studies, it has been shown that the method of synthesizing materials has a significant effect on morphology, particle size, specific surface and crystal structure of heterogeneous photocatalysts, which determines adsorption and photochemical activity of as-synthesized materials (Lim et al., 1995; Sun et al., 2009; Xi and Ye, 2010). Currently, m-BiVO4 can be synthesized by many methods such as solvothermal method (Zhao et al., 2013), co-precipitation method (Martínez-de la Cruz and Pérez, 2010), method of using microwave (Liu et al., 2010) and solution combustion synthesis method (Chen et al., 2015). In which, the synthesis of materials by solvothermal method allows the creation of materials with many different crystal morphologies through changing synthesis parameters. In the case of synthesis of catalytic materials by the solvothermal process, kind of solvents, synthesis temperature and time are essential factors related to the formation and development of crystals.
In this study, with the aim of obtaining BiVO4 with improved photochemical properties through control of crystal morphology and crystal faces, we adopted a BiVO4 synthesis routine that is based on the solvothermal method, allowing for close control of the synthesis and the structure and size of BiVO4 crystals. Solvents with high boiling temperature, high viscosity, polarity, and saturated vapor pressure, such as ethylene glycol (EG), were selected to synthesize BiVO4. The photocatalytic activity of the resulting materials was evaluated through the photodecomposition of methylene blue (MB) under the irradiation of LED light.
2 Experimental
2.1 Materials
Bismuth(III) nitrate pentahydrate (Bi(NO3)3·5H2O, ≥98.0%), ammonium metavanadate (NH4VO3, ≥98%) were purchased from Sigma-Aldrich. Ethylene glycol (C2H6O2, 99.0%), ethanol (CH3CH2OH, 99.7%) were obtained from Xilong Chemical Co., Ltd. (China). Methylene blue (MB, C16H18ClN3S, 99%) was purchased from HiMedia Laboratories Pvt. Ltd. (India).
2.2 Solvothermal synthesis of BiVO4 catalysts
The BiVO4 photocatalysts with highly photocatalytic performance were synthesized by a solvothermal method using the mixture of ethylene glycol and water as the mixed solvent under different synthesis conditions of temperature and pH values. In a typical synthesis, 4 mmol Bi(NO3)3·5H2O and 4 mmol NH4VO3 were dissolved in 40 mL of EG and 40 mL of H2O, respectively. These two solutions were mixed and stirred for 1 h to obtain a yellow homogeneous solution. Then, the pH of the mixed solution was adjusted to the desired value using NH3 solution. The mixture was transferred into an autoclave and heated at a specific temperature for 3 h. After that, the resultant suspension was centrifuged at 7000 rpm for 10 min, and the obtained BiVO4 powder was dried at 60 °C overnight. Finally, they were calcined at 400 °C for 3 h and denoted as BV-x-y (x is the synthesis temperature, and was chosen as 80, 120, 140, 160, and 180 °C; y is the pH values and was chosen as 0.5, 3, 7, and 9).
2.3 BiVO4 catalysts characterization techniques
Physicochemical properties such as crystal phase, morphology and optical absorption of the as-synthesized BiVO4 samples were characterized by X-ray Diffractometer (XRD, Bruker D8 Advance, with a Cu Kα excitation source at a scan rate of 0.030°/s in the 2-theta range of 10–50°), Raman spectra (Horiba Jobin-Yvon, with a laser beam of 633 nm in the wavenumber of 100–1000 cm−1), Scanning Electron Microscope (SEM, JEOL JSM 7401F), and ultraviolet–visible (UV–vis) diffuse reflectance spectroscopy (UV–vis DRS, Shimazu UV-2450, in the range 200–800 cm−1), respectively.
2.4 Photodegradation test
The photocatalytic activity of the resulting materials was evaluated based on the photocatalytic degradation of MB dye under the irradiation source as LED (six daylight Cree® Xlamp® XM-L2 LEDs; max power of 10 W and max light output of 1052 lm). The experimental process was as follows: 50 mg of catalyst was dispersed in 100 mL of MB solution (15 ppm) and stirred in the dark for 60 min. After that, the lamp was turned on to start the photocatalytic reaction. During irradiation, 3 mL of the reaction solution was withdrawn at equal intervals to investigate the change in MB concentration. This reaction solution was centrifuged at 7000 rpm for 15 min to remove the catalysts entirely. The concentration of MB was tested on an UV–Vis (Evolution 60S UV–Visible Spectrophotometer) instrument at the wavelength of maximum absorbance of 664 nm.
3 Results and discussion
3.1 BiVO4 catalysts characterization
3.1.1 XRD analysis
The effect of different synthesis conditions of temperature and pH values on the phase structure and morphology of BiVO4 were characterized by XRD. At a synthesis pH of 7, it can be observed from Fig. 1A that the crystalline phase of all BiVO4 samples can be well indexed according to monoclinic scheelite structure with the presence of weak peaks at 2θ = 15.5°, strong peaks at 2θ = 28.9° and the splitting of peaks at 2θ = 18.5°, 35°, and 47° (JCPDS no. 01-075-1867), corresponding with the XRD patterns of the study reported earlier (Zhao et al., 2016). Furthermore, the cell parameters of the BiVO4 samples are presented in Table 1. As shown in Table 1, the crystallographic parameter of the BiVO4 sample corresponds to the cell parameters of the monoclinic scheelite. Moreover, we observed that intensities of the major diffracted peaks changed remarkably under different synthesis conditions of temperature (Fig. 1A). A remarkable increase in the intensity ratio of I(0 4 0)/I(1 2 1) from 0.2212 to 1.1846 was observed when the synthesis temperature increased from 80 to 140 °C, whereas the change of the intensity ratio of I(0 1 1)/I(1 2 1) tended to be opposite (Table 1). However, with excessively high synthesis temperatures, the trend seemed to overturn. Especially, when the synthesis temperature was fixed at 140 °C, the intensity of (0 4 0) facets reaches the highest level while the intensity of (0 4 0) facets reaches the lowest level.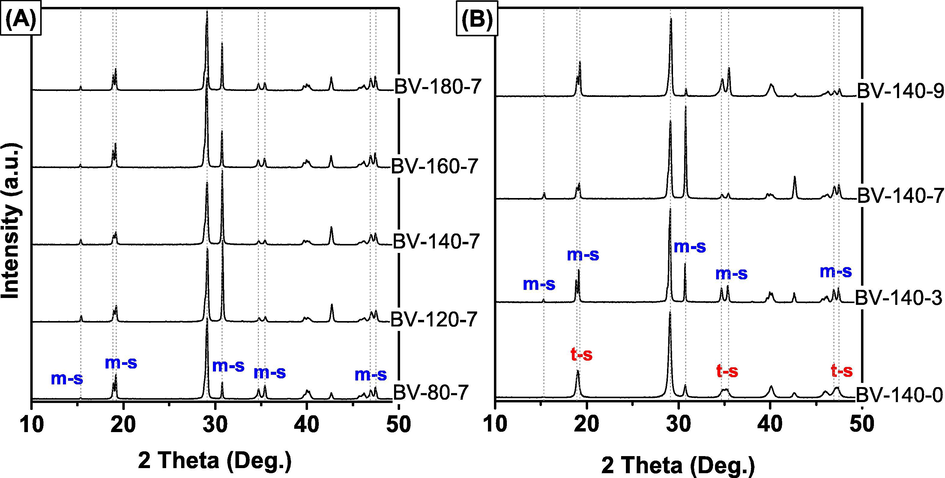
XRD patterns of BiVO4 synthesized at different temperatures (A) and pH (B).
Sample codes
Synthesis conditions
Unit cell parameters
LXRD (nm)
I040/I121
I011/I121
Temperature (°C)
pH
a (Å)
b (Å)
c (Å)
β (Å)
Vcell (Å3)
BV-80-7
80
7
5.1653
11.6327
5.0672
90.4065
304.460
31.64
0.2212
0.3105
BV-120-7
120
7
5.1567
11.6111
5.0629
90.3403
303.1358
52.90
1.1102
0.2348
BV-140-7
140
7
5.1646
11.6283
5.0677
90.3876
304.3328
49.11
1.1846
0.2096
BV-160-7
160
7
5.1689
11.6402
5.0746
90.4005
305.3124
32.70
0.4188
0.2563
BV-180-7
180
7
5.1645
11.6337
5.0805
90.5537
305.2350
32.41
0.5959
0.2823
BV-140-0
140
0
5.1949
11.7342
5.0967
91.1195
310.6215
25.20
0.1594
0.3237
BV-140-3
140
3
5.1700
11.6497
5.0753
90.4569
305.6720
35.24
1.0561
0.6582
BV-140-9
140
9
5.1597
11.6078
5.0562
90.5965
302.8113
29.28
1.0551
0.6596
Regarding the effects of pH (Fig. 1B), the as-synthesized BiVO4 samples exhibited a monoclinic scheelite structure when the pH of precursor solution was fixed as 3, 7, and 9, whereas the BiVO4 with tetragonal scheelite phase was observed when the pH value was 0. The XRD analysis showed that different synthesis conditions of temperature and pH significantly affected the growth of BiVO4 crystals oriented along (0 4 0) facets. These results are supported by results of previous studies, demonstrating that the growth of BiVO4 crystals oriented along (0 4 0) facets will be beneficial to enhance the photocatalytic activity of monoclinic scheelite BiVO4 (Tan et al., 2016).
3.1.2 Ramman analysis
Raman spectra of the BiVO4 samples were presented in Fig. 2, which demonstrated that BiVO4 was synthesized with a monoclinic crystalline structure. The monoclinic BiVO4 sample has six vibration modes including the external vibration mode of BiVO4 at 127 and 203 cm−1, the asymmetric and symmetric deformation vibration mode of VO4−3 groups at 324 cm−1 and 366 cm−1, the asymmetric stretching vibration mode of V—O bonds at 644 and 709 cm−1, and the symmetric stretching vibration mode of V—O bonds at 824 cm−1 (Phu et al., 2016). In addition, Raman signals corresponding to vanadium oxide or bismuth oxide was not observed in all samples. These results are in good agreement with the XRD results.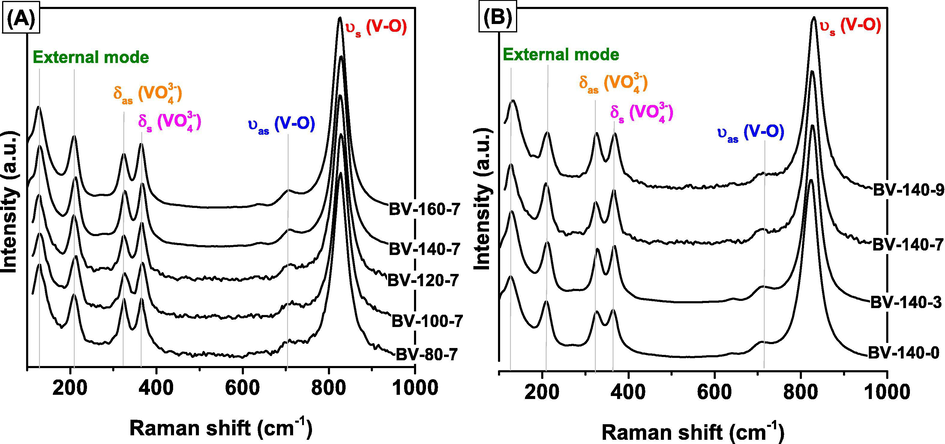
Ramman spectra of BiVO4 samples synthesized at different temperature (A) and pH (B).
3.1.3 FESEM analysis
The crystal shape, particle size and particle distribution of the material were observed through SEM images. The crystal shape of the resulting material varies widely when the solvothermal process is conducted at different temperatures and pH values (Fig. 3). This result proved that the morphology of BiVO4 is dependent on the solvothermal route. Especially, SEM images show that the synthesis pH has a significant influence on the crystal development process of materials. For samples synthesized at 140 °C, the crystal shape changed from a peanut-like to a rod when the synthesis pH increased from 0 to 9. At solvothermal pH of 7, it can be seen from Fig. 3 that the sample BV-140-7 shows a leaf-like structure. Furthermore, in this study, the SEM results indicate that different crystalline morphologies also relate to the temperature used for synthesis. With low temperatures corresponding to the low diffusion rate of crystal nuclei, crystal formation has a 3D structure and is relatively homogeneous. Meanwhile, the high synthetic temperature accelerates the diffusion rate of crystal nuclei, thus forming a leaf-like crystal with uneven size and heterogeneous morphology.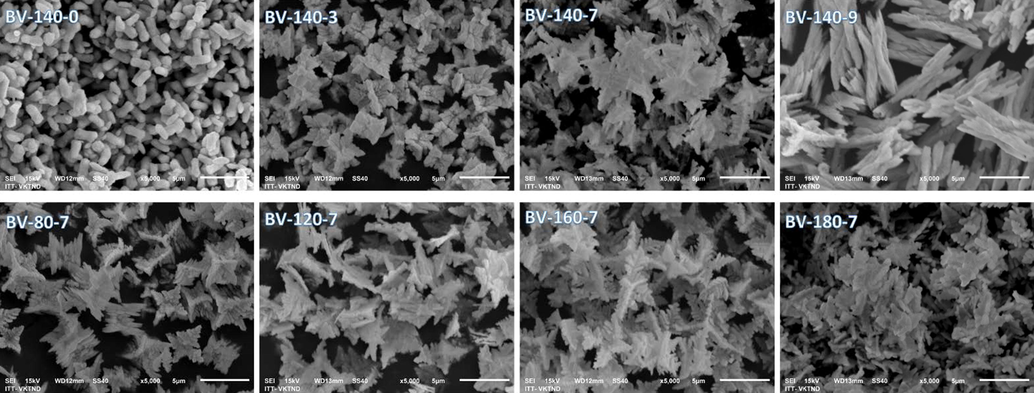
SEM images of BiVO4 samples synthesized at different temperature (A) and pH (B).
3.1.4 UV–vis DRS analysis
The light absorption properties of BiVO4 samples were examined by UV–vis DRS. Fig. 4 shows the UV–vis DRS of as-prepared BiVO4 samples, which were prepared using different synthesis conditions of temperature and pH. As shown in Fig. 4, all the BiVO4 exhibited absorption-edge in the visible light range at about λ = 535 nm. According to the absorbance of visible light of BiVO4 materials, the photocatalytic property was enhanced in the visible light region. The band-gap energy (Eg) of all the BiVO4 samples was calculated from the Tauc plot (Jo et al., 2015). The Eg value is presented in Table 2. As can be seen in Table 2, the Eg values are in the range from 2.29 to 2.41 eV, this result is consistent with Eg value of BiVO4 with the monoclinic crystal structure (Kohtani et al., 2003).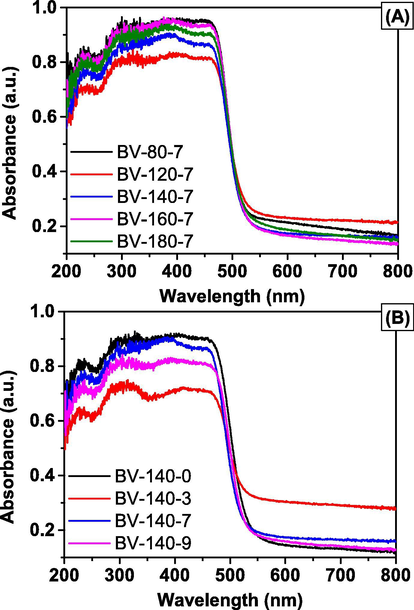
UV–Vis DRS spectra of the BiVO4 samples synthesized at different temperature (A) and pH (B).
Sample codes
Eg (eV)
kapp (10–3 min−1)
R2
BV-80-7
2.34
4.62
0.9979
BV-120-7
2.40
7.30
0.9431
BV-140-7
2.33
7.94
0.9526
BV-160-7
2.29
5.91
0.9182
BV-180-7
2.41
6.58
0.9207
BV-140-0
2.30
5.69
0.9467
BV-140-3
2.37
7.70
0.9765
BV-140-9
2.38
5.00
0.9751
3.2 Photocatalytic degradation of MB
The photocatalytic activities of as-synthesized BiVO4 photocatalysts were evaluated by the photodegradation of methylene blue (MB) dye in water solution in the presence of LED light. We have found that by using appropriate synthesis conditions (including temperature and pH) the BiVO4 exhibited high photocatalytic efficiency for MB degradation, as shown in Fig. 5. With different synthesis conditions of temperature, after visible light irradiation for 180 min, removal of MB was about 57.26%, 77.40%, 82.30%, 72.40%, and 73.80% in BV-80-7/visible light catalytic system, BV-120-7/visible light catalytic system, BV-140-7/visible light catalytic system, BV-160-7/visible light catalytic system, and BV-180-7/visible light catalytic system, respectively. From the obtained result, we observed that the BV-140–7 exhibited much higher activity than those of other samples and higher than that of previous studies (Chen et al., 2013; Zhao et al., 2018). In addition, the photocatalytic degradation of MB according to the first kinetics and the reaction rate constants of the samples are listed in Table 2. As shown in Table 2, the photodegradation rate constant (k) of BV-140–7 was higher than those of the other samples. Because of this synthetic condition the growth of BiVO4 crystals oriented along (0 4 0) facets. It will be beneficial to enhance the photocatalytic activity of monoclinic scheelite BiVO4.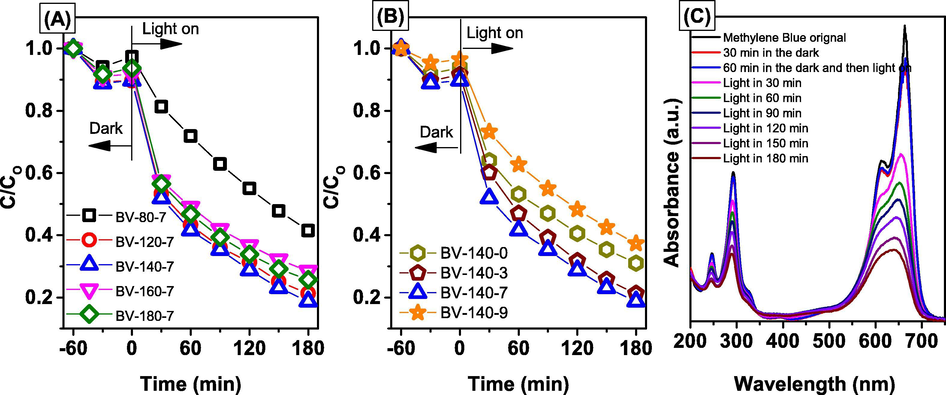
Photocatalytic degradation of MB over BiVO4 samples synthesized at difference temperature (A) and pH (B), and UV–vis absorption spectra (C) of MB solutions during illumination using BV-140-7 sample.
The change in the UV–vis absorption spectrum of CV over time in the presence of BV-140–7 is shown in Fig. 5C. As the irradiation duration was prolonged, the maximum absorption peak of MB at a wavelength of 664 nm decreased. Moreover, no increase in the absorption peak of MB in the UV region was found during irradiation, suggesting that most MB has completely decomposed without producing intermediate compounds.
The photocatalytic activities of as-synthesized BiVO4 photocatalysts were evaluated by the photodegradation of methylene blue (MB) dye in water solution in the presence of LED light. We have found that by using appropriate synthesis conditions (including temperature and pH) the BiVO4 exhibited high photocatalytic efficiency for MB degradation, as shown in Fig. 5. With different synthesis conditions of temperature, after visible light irradiation for 180 min, removal of MB was about 57.26%, 77.40%, 82.30%, 72.40%, and 73.80% in BV-80-7/light catalytic system, BV-120-7/light catalytic system, BV-140-7/light catalytic system, BV-160-7/light catalytic system, and BV-180-7/light catalytic system, respectively. From the obtained result, we observed that the BV-140-7 exhibited much higher activity than those of other samples. In addition, the photocatalytic degradation of MB according to the first kinetics and the reaction rate constants (k) of the samples are listed in Table 2. As shown in Table 2, the k value of BV-140-7 was higher than those of the other samples. This result could be explained as a consequence of the appropriate morphology under this synthetic condition.
Mechanism of photodegradation organic compounds using monoclinic scheelite BiVO4 crystals oriented along (0 4 0) facets was suggested in Fig. 6. Firstly, monoclinic scheelite BiVO4 crystals oriented along (0 4 0) facets are excited to generate the photoexcited electrons (e−) and the positive holes (h+) under the white LED light irradiation. Then, e− and h+ are transferred to the catalyst surface and reacted with O2 and H2O to yield −•O2 and OH•, respectively. e− and h+ tend to move towards (0 1 0) and (1 1 0) faces, leading to oxidation and reduction reactions that occur on {0 1 0} and (1 1 0) faces, respectively.(Tan et al., 2016) Finally, the MB molecules were oxidized by radicals such as OH• and/or h+.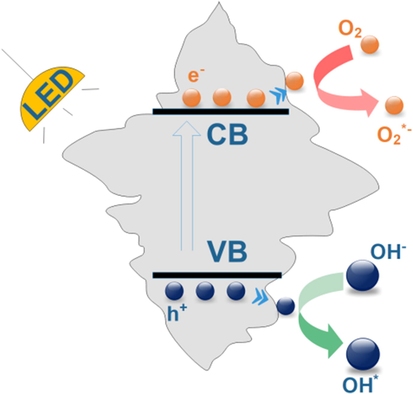
Mechanism of photodegradation organic compounds using monoclinic scheelite BiVO4 crystals oriented along (0 4 0) facets.
4 Conclusion
The monoclinic scheelite BiVO4 photocatalytic material with the high photocatalytic degradation efficiency of MB was successfully synthesized by a solvothermal process using a solvent mixture of ethylene glycol and water. The results indicate that the synthesis temperatures and pH exerted a strong influence on the morphology and crystal facet of BiVO4. The crystal facets were significantly affected by the separation of electron and hole pairs during the reaction process. In this study, the as-prepared BiVO4 with crystal facets ratio of (0 4 0)/(1 2 1) of 1.1846 (BV-140-7 sample) exhibited the high degradation of MB, in which 82.30% removal of MB was achieved within 180 min.
Acknowledgments
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.05-2017.315.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, characterisation and photocatalytic performance of ZnS coupled Ag2S nanoparticles: A remediation model for environmental pollutants. Arab. J. Chem.. 2018;11:827-837.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of pesticides by titanium dioxide and titanium pillared purified clays. Arab. J. Chem.. 2016;9:S313-S318.
- [CrossRef] [Google Scholar]
- Photocatalysis: Principles and Applications. Boca Raton: CRC Press; 2016. https://doi.org/10.1201/9781315372396
- Controllable synthesis of hollow and porous Ag/BiVO4 composites with enhanced visible-light photocatalytic performance. RSC Adv.. 2013;3:24354-24361.
- [CrossRef] [Google Scholar]
- Effects of citric acid and urea on the structural and morphological characteristics of BiVO4 synthesized by the sol–gel combustion method. J. Sol-Gel Sci. Technol.. 2015;76:562-571.
- [CrossRef] [Google Scholar]
- Phase transition-induced band edge engineering of BiVO4 to split pure water under visible light. Proc. Natl. Acad. Sci. U. S. A.. 2015;112:13774-13778.
- [CrossRef] [Google Scholar]
- Photodegradation of 4-alkylphenols using BiVO4 photocatalyst under irradiation with visible light from a solar simulator. Appl. Catal. B Environ.. 2003;46:573-586.
- [CrossRef] [Google Scholar]
- Prominent ferroelastic domain walls in BiVO4crystal. J. Phys. Condens. Matter.. 1995;7:7309-7323.
- [CrossRef] [Google Scholar]
- Ultrasound assisted synthesis of monoclinic structured spindle BiVO4 particles with hollow structure and its photocatalytic property. Ultrason. Sonochem.. 2010;17:669-674.
- [CrossRef] [Google Scholar]
- Photocatalytic properties of BiVO4 prepared by the co-precipitation method: Degradation of rhodamine B and possible reaction mechanisms under visible irradiation. Mater. Res. Bull.. 2010;45:135-141.
- [CrossRef] [Google Scholar]
- Control of crystal phase of BiVO4 nanoparticles synthesized by microwave assisted method. J. Mater. Sci. Mater. Electron.. 2016;27:6452-6456.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of an organic pollutant by zinc oxide – solar process. Arab. J. Chem.. 2016;9:S1858-S1868.
- [CrossRef] [Google Scholar]
- Morphology control of BiVO4 photocatalysts: pH optimization vs. self-organization. Mater. Chem. Phys. 135 2012:457-466.
- [CrossRef] [Google Scholar]
- Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arab. J. Chem.. 2020;13:3498-3520.
- [CrossRef] [Google Scholar]
- Synthetic loosely packed monoclinic BiVO4 nanoellipsoids with novel multiresponses to visible light, trace gas and temperature. Chem. Commun. 2009:4542-4544.
- [CrossRef] [Google Scholar]
- BiVO4 010 and 110 relative exposure extent: governing factor of surface charge population and photocatalytic activity. J. Phys. Chem. Lett.. 2016;7:1400-1405.
- [CrossRef] [Google Scholar]
- Synthesis of bismuth vanadate nanoplates with exposed 001 facets and enhanced visible-light photocatalytic properties. Chem. Commun.. 2010;46:1893-1895.
- [CrossRef] [Google Scholar]
- Effect of sulfur doping on the photocatalytic performance of BiVO4 under visible light illumination. Chin. J. Catal.. 2013;34:1617-1626.
- [CrossRef] [Google Scholar]
- Facile synthesis of hierarchically structured BiVO4 oriented along (010) facets with different morphologies and their photocatalytic properties. Appl. Surf. Sci.. 2016;390:531-539.
- [CrossRef] [Google Scholar]
- Nanostructured shuriken-like BiVO4 with preferentially exposed 010 facets: preparation, formation mechanism, and enhanced photocatalytic performance. Dalt. Trans.. 2018;47:1325-1336.
- [CrossRef] [Google Scholar]







