Translate this page into:
Facile synthesis and characterization of β-cobalt hydroxide/hydrohausmannite/ramsdellitee/spertiniite and tenorite/cobalt manganese oxide/manganese oxide as novel nanocomposites for efficient photocatalytic degradation of methylene blue dye
⁎Corresponding author at: Department of Chemistry, College of Science, Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh 11623, Saudi Arabia. EAAAhmed@imamu.edu.sa (Ehab A. Abdelrahman) ehab.abdelrahman@fsc.bu.edu.eg (Ehab A. Abdelrahman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this paper, our research team has synthesized new nanocomposites by simple precipitation/ignition method and using low-cost chemicals. Hence, β-cobalt hydroxide/hydrohausmannite/ramsdellitee/spertiniite and tenorite/cobalt manganese oxide/manganese oxide new nanocomposites were synthesized by precipitation of Mn(II)/Co(II)/Cu(II) solution using sodium hydroxide and ignition of precipitate at 700 °C for 3 hrs, respectively. The synthesized nanocomposites were characterized using different instruments such as energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), field emission scanning electron microscope (FE-SEM), transmission electron microscope (TEM), nitrogen gas sorption analyzer, and UV–vis spectrophotometer. Energy dispersive X-ray analysis revealed that the nanocomposite formed as a result of precipitation consists of copper, cobalt, manganese, and oxygen where the weight percentages are equal to 31.73, 27.01, 17.26, and 24 %, respectively. Also, the nanocomposite formed as a result of ignition consists of copper, cobalt, manganese, and oxygen where the weight percentages are equal to 31.26, 23.87, 14.56, and 30.31 %, respectively. Transmission electron microscope revealed that the nanocomposites formed as a result of precipitation and ignition consist of polyhedral and spherical shapes with an average diameter of 34.50 and 28.56 nm, respectively. The synthesized nanocomposites were used as new photocatalysts for the efficient degradation of methylene blue dye. 0.05 g of the synthesized nanocomposites degrade 100 % of 50 mL of 15 mg/L of methylene blue dye solution within 25 min in the presence of H2O2 under UV light.
Keywords
New photocatalysts
Methylene blue dye
Precipitation
Ignition
1 Introduction
Energy and environmental pollution challenges are already becoming among the world's most difficult problems. Plastics, printing, textiles, and other industries use untreated synthetic organic dyes that are mixed with water sources. The environment and humans may be adversely affected by synthetic organic dyes (Tang et al., 2022; Xie et al., 2022). Methylene blue is among the industrial organic dyes. It is amongst the most often used dyes for wood, cotton, and silk. Excessive concentrations of methylene blue dye in the water supplies can cause a range of diseases, such as troubles with the respiratory and digestive tracts, as well as profuse sweating, vomiting, and nausea (Kenawy et al., 2022). Various treatment procedures, such as biological processes, chemical oxidation, membrane filtration, coagulation, and advanced oxidation processes, are frequently employed to get rid of various organic dyes (Bustos-Terrones et al., 2021; Ihaddaden et al., 2022; Ismail and Sakai, 2022; Javanbakht and Mohammadian, 2021; Kim et al., 2004; Subrahmanya et al., 2022). Advanced oxidation processes, for instance, have an enormous ability to control many kinds of pollution and can decrease the generation of secondary contaminants since they produce carbon dioxide and water. In the presence of proper wavelengths of light, superoxide anion and hydroxyl radical production can attack organic molecules in wastewater during the photocatalytic process. These organic molecules can be decomposed into non-hazardous substances (Alharbi and Abdelrahman, 2020; Hegazey et al., 2020). Several composites were used as photocatalysts to degrade the methylene blue dye such as Bi/Carbon nanotubes/α-Fe2O3 nanocomposite (Manda et al., 2022), MgSO4/g-C3N4 composite (Zeng et al., 2022), ZnWO4/SnS2 nanocomposite (Kumar et al., 2022), α-Fe2O3/ZnO nanocomposite (Harijan et al., 2022), multi-walled carbon nanotube/WO3 nanocomposite (Stan et al., 2022), multi-walled carbon nanotube/TiO2 nanocomposite (Jiang et al., 2011), multi-walled carbon nanotube/ZnO nanocomposite (Chaudhary et al., 2018), multi-walled carbon nanotube/SnO2/TiO2 nanocomposite (Réti et al., 2013), and WO3/Cu2O nanocomposite (Li et al., 2022). These nanocomposites reduce electron/hole recombination and thus increase the efficiency of photocatalytic degradation of the methylene blue dye (Harijan et al., 2022). However, most of these nanocomposites require expensive chemicals to prepare. The precipitation and/or ignition method has proven its efficiency in preparing many nanomaterials such as ferrimagnetic nanoparticles (Kumar and Gangawane, 2022), CeO2 nanoparticles (Shibeshi et al., 2022), copper ferrite nanoparticles (Subha et al., 2022), zinc oxide nanoparticles (Mahmood et al., 2022), bismuth oxychloride nanoparticles (Puttaraju et al., 2022), Co3O4 nanoparticles (Nassar et al., 2017), NiO nanoparticles (Nassar et al., 2017), and CuO nanoparticles (Aly et al., 2015). So, in this paper, our research team has synthesized new nanocomposites by simple precipitation/ignition method and using low-cost chemicals. In this concern, β-cobalt hydroxide/hydrohausmannite/ramsdellitee/spertiniite and tenorite/cobalt manganese oxide/manganese oxide new nanocomposites were synthesized by precipitation of Mn(II)/Co(II)/Cu(II) solution using sodium hydroxide and ignition of precipitate at 700 °C for 3 hrs, respectively. The synthesized nanocomposites were characterized using different instruments such as energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), field emission scanning electron microscope (FE-SEM), transmission electron microscope (TEM), nitrogen gas sorption analyzer, and UV–vis spectrophotometer. The synthesized nanocomposites were used as new photocatalysts for the degradation of methylene blue dye. Moreover, analytical factors affecting the degradation efficiency of methylene blue dye have been studied, such as pH, time, concentration of dye, and amount of catalyst.
2 Experimental
2.1 Chemicals
Copper(II) acetate monohydrate (Cu(CH3COO)2·H2O), cobalt(II) acetate tetrahydrate (Co(CH3COO)2·4H2O), manganese(II) acetate tetrahydrate (Mn(CH3COO)2·4H2O), sodium hydroxide (NaOH), methylene blue dye (C16H18ClN3S), hydrogen peroxide (H2O2), and hydrochloric acid (HCl) were purchased from Sigma Aldrich Company (Purity = 99.99 %) and utilized as received without further purification.
2.2 Synthesis of nanocomposites
10 g of copper(II) acetate monohydrate, 10 g of cobalt(II) acetate tetrahydrate, and 10 g of manganese(II) acetate tetrahydrate were dissolved in 300 mL of distilled water for obtaining the mixed metal solution. 11 g of sodium hydroxide was dissolved in 100 mL of distilled water. Then, the sodium hydroxide solution was added to the mixed metal solution drop by drop with constant stirring for 2 hrs. After that, the formed precipitate was filtered, washed with hot distilled water, and dried at 70 °C for 12 hrs. Furthermore, the dried precipitate was ignited at 700 °C for 3 hrs.
2.3 Characterization
X-ray diffraction patterns of the synthesized nanocomposites were obtained utilizing a Bruker D8 Advance X-ray diffractometer adopting copper target with Kα line have a wavelength equal to 0.15 nm. The surface morphology and EDX spectra of the synthesized nanocomposites were obtained utilizing a JSM-IT800 Schottky field emission scanning electron microscope (FE-SEM). The morphologies of the synthesized nanocomposites were obtained utilizing a Talos F200iS transmission electron microscope (TEM). The synthesized nanocomposites were degassed at 60 °C for 24 hrs then a nitrogen gas sorption analyzer (Quantachrome TouchWin, Austria, Graz) was utilized for the determination of the average pore radius, BET surface area, and total pore volume. The concentration of the methylene blue dye and energy gap of the synthesized nanocomposites were determined utilizing a Jasco V-670 UV–vis spectrophotometer. The maximum wavelength of methylene blue dye is 663 nm.
2.4 Photocatalytic degradation of methylene blue dye using the synthesized nanocomposites
Photocatalytic degradation of methylene blue dye using the synthesized nanocomposites has been studied at different pH values (3–8) as follows: 0.05 g of the synthetic catalyst was added to 50 mL of a methylene blue dye solution with a concentration of 15 mg/L. Then, the catalyst/methylene blue dye mixture was stirred in a dark place for 2 hrs. After that, the catalyst/methylene blue dye mixture was exposed to ultraviolet rays, under stirring for 2 hrs, using a UV lamp (Wavelength = 254 nm, Power = 8 Watt, Length = 30 cm, Model G8W, Sylvania lighting, Japan) located 5 cm away from the beaker containing the catalyst/methylene blue dye mixture. The previous experimental steps were repeated but in the presence of 1 mL of 2 M of hydrogen peroxide solution.
Photocatalytic degradation of methylene blue dye using the synthesized nanocomposites has been studied at different UV irradiation time values (5–60 min) as follows: 0.05 g of the synthetic catalyst was added to 50 mL of a methylene blue dye solution with a concentration of 15 mg/L. It is worth noting that the pH of the methylene blue dye solution was adjusted to 8 before the addition of the catalyst. Then, the catalyst/methylene blue dye mixture was stirred in a dark place for 2 hrs. After that, the catalyst/methylene blue dye mixture was exposed to ultraviolet rays during stirring for different times. The previous experimental steps were repeated but in the presence of 1 mL of 2 M of hydrogen peroxide solution.
Photocatalytic degradation of methylene blue dye using the synthesized nanocomposites has been studied using different amounts of catalysts (0.0125–0.2 g) as follows: A definite amount of the synthetic catalyst was added to 50 mL of a methylene blue dye solution with a concentration of 15 mg/L. It is worth noting that the pH of the methylene blue dye solution was adjusted to 8 before the addition of the catalyst. Then, the catalyst/methylene blue dye mixture was stirred in a dark place for 2 hrs. After that, the catalyst/methylene blue dye mixture was exposed to ultraviolet rays during stirring for 40 min and 25 min in the absence and presence of H2O2, respectively.
Photocatalytic degradation of different concentrations of methylene blue dye (5–30 mg/L) using the synthesized nanocomposites has been studied as follows: 0.05 g of the synthetic catalyst was added to 50 mL of a methylene blue dye solution. It is worth noting that the pH of the methylene blue dye solution was adjusted to 8 before the addition of the catalyst. Then, the catalyst/methylene blue dye mixture was stirred in a dark place for 2 hrs. After that, the catalyst/methylene blue dye mixture was exposed to ultraviolet rays during stirring for 40 min and 25 min in the absence and presence of H2O2, respectively.
The photodegradation efficiency (% P) of methylene blue dye was determined using Eq. (1).
3 Results and discussion
3.1 Characterization of the synthesized nanocomposites
X-ray diffraction analysis revealed that the nanocomposite formed as a result of precipitation using sodium hydroxide has an average crystallite size of 33.40 nm and consists of β-cobalt hydroxide (β-Co(OH)2 as clarified from JCPDS No. 00-051-1731 and JCPDS No. 00-030-0443), hydrohausmannite ((Mn4-2xMnx) Mn8O16-x(OH)x as clarified from JCPDS No. 00–012-025), ramsdellitee (MnO2 as clarified from JCPDS No. 00–004-0378), and spertiniite (Cu(OH)2 as clarified from JCPDS No. 00–035-0505), as presented in Fig. 1. The miller indices of β-cobalt hydroxide at 2θ = 9.5°, 18.9°, and 58° are (0 0 1), (0 0 2), and (1 1 0), respectively. The miller indices of β-cobalt hydroxide at 2θ = 32.5° and 59° are (1 0 0) and (0 0 3), respectively. The miller indices of hydrohausmannite at 2θ = 28.7°, 30.9°, 49.2°, and 53.9° are (1 1 2), (2 0 0), (2 0 4), and (3 1 2), respectively. The miller indices of spertiniite at 2θ = 39.7°, 43.2°, and 47° are (1 3 0), (1 3 1), and (1 1 2), respectively.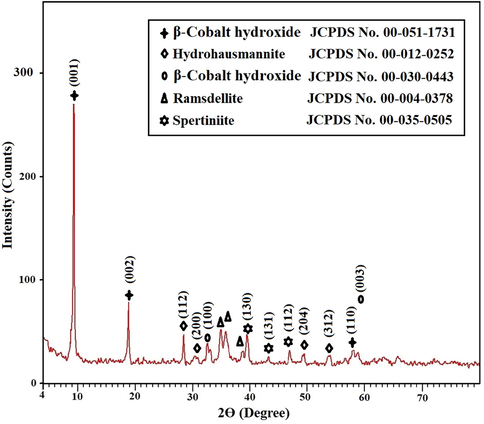
X-ray diffraction analysis of the nanocomposite formed as a result of precipitation using sodium hydroxide.
X-ray diffraction analysis revealed that the nanocomposite formed as a result of ignition at 700 °C for 3 hrs has an average crystallite size of 24.28 nm and consists of tenorite (CuO as clarified from JCPDS No. 00-005-0661), cobalt manganese oxide ((Co,Mn) (Co,Mn)2O4 as clarified from JCPDS No. 00-018-0408), and manganese oxide (Mn5O8 as clarified from JCPDS No. 00-018-0801), as presented in Fig. 2. The miller indices of tenorite at 2θ = 35.5°, 38.7°, 46.3°, 48.8°, 61.5°, 66.2°, and 68.1° are (−1 1 1), (1 1 1), (−1 1 2), ( −2 0 2), (−1 1 3), (−3 1 1), and (2 2 0), respectively. The miller indices of cobalt manganese oxide at 2θ = 18.5°, 32.9°, 36.4°, and 59° are (1 1 1), (1 1 3), (3 1 1), and (5 1 1), respectively. The miller indices of manganese oxide at 2θ = 18.1°, 31.2°, 31.9°, 44.3°, 55.1°, and 64.4° are (2 0 0), (0 2 0), (−3 1 1), (−4 0 2), (−4 2 2), and (4 0 2), respectively.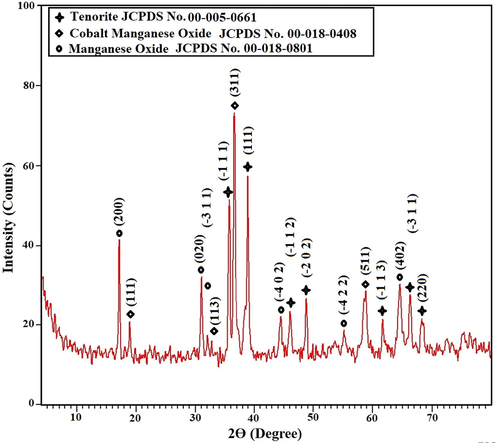
X-ray diffraction analysis of the nanocomposite formed as a result of ignition at 700 °C for 3 hrs.
Energy dispersive X-ray analysis revealed that the nanocomposite formed as a result of precipitation using sodium hydroxide consists of copper, cobalt, manganese, and oxygen where the weight percentages are equal to 31.73, 27.01, 17.26, and 24 %, respectively, as shown in Fig. 3A. Also, energy dispersive X-ray analysis revealed that the nanocomposite formed as a result of ignition at 700 °C for 3 hrs consists of copper, cobalt, manganese, and oxygen where the weight percentages are equal to 31.26, 23.87, 14.56, and 30.31 %, respectively, as shown in Fig. 3B.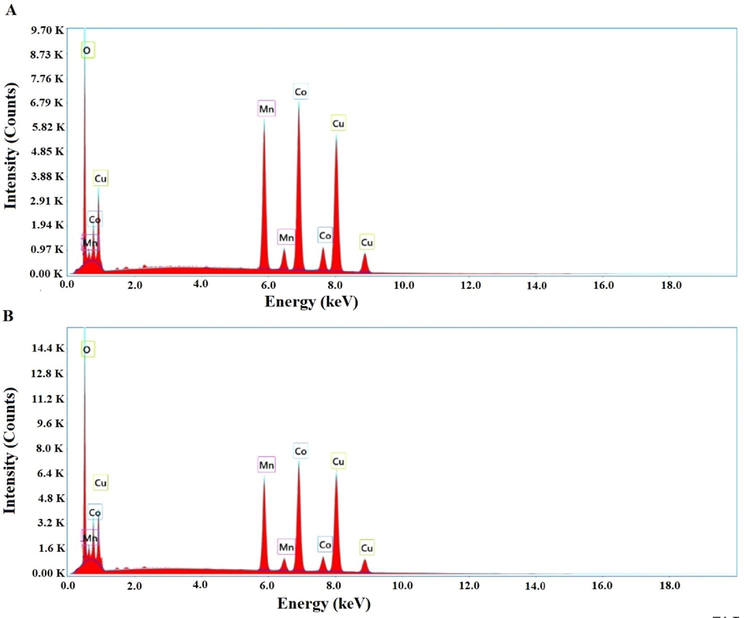
Energy dispersive X-ray analysis of the nanocomposites formed as a result of precipitation (A) and ignition (B).
Scanning electron microscope analysis revealed that the nanocomposites formed as a result of precipitation using sodium hydroxide and ignition at 700 °C for 3 hrs consist of irregular and spherical shapes with an average diameter of 3.63 and 0.83 µm, as shown in Fig. 4A-B, respectively.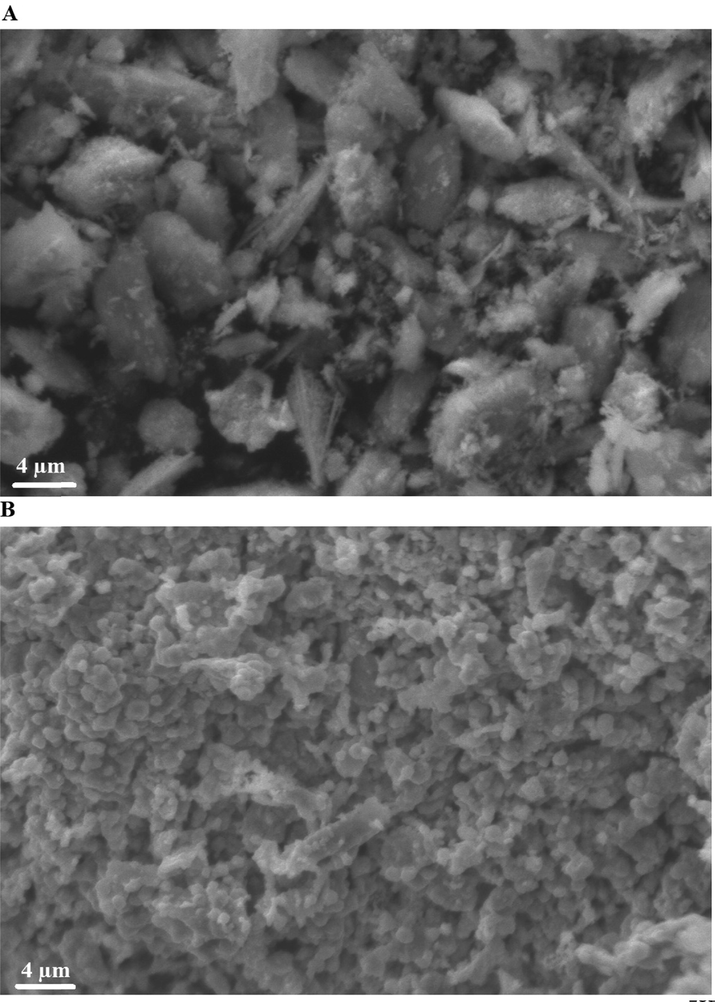
Scanning electron microscope analysis of the nanocomposites formed as a result of precipitation (A) and ignition (B).
Transmission electron microscope analysis revealed that the nanocomposites formed as a result of precipitation using sodium hydroxide and ignition at 700 °C for 3 hrs consist of polyhedral and spherical shapes with an average diameter of 34.50 and 28.56 nm, as shown in Fig. 5A-B, respectively.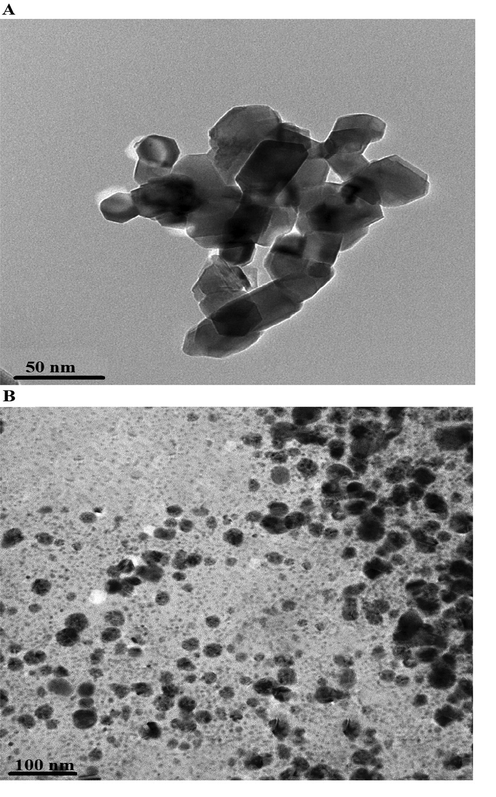
Transmission electron microscope analysis of the nanocomposites formed as a result of precipitation (A) and ignition (B).
N2 adsorption/desorption analyzer revealed that the isotherms of nanocomposites formed as a result of precipitation using sodium hydroxide and ignition at 700 °C for 3 hrs belong to type IV as shown in Fig. 6A-B, respectively (Abdelwahab et al., 2022; Khalifa et al., 2020). The BET surface area, total pore volume, and average pore size of the nanocomposite formed as a result of ignition at 700 °C for 3 hrs are 37.4716 m2/g, 0.0415 cc/g, and 2.2148 nm, respectively. Also, the BET surface area, total pore volume, and average pore size of the nanocomposite formed as a result of precipitation using sodium hydroxide are 30.7931 m2/g, 0.0307 cc/g, and 1.9938 nm, respectively.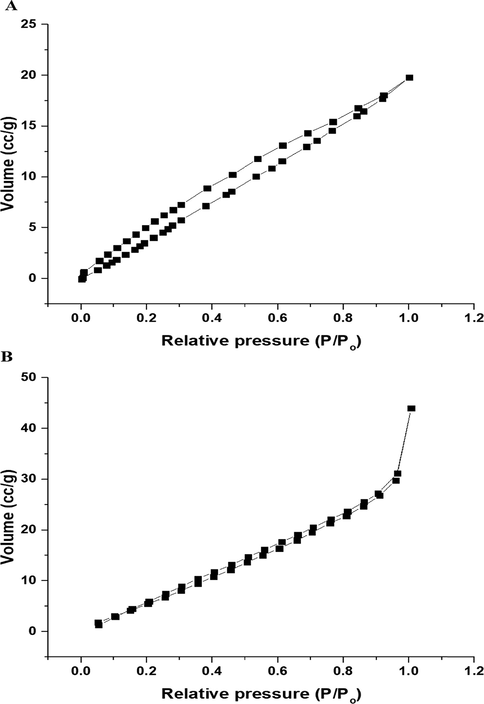
N2 adsorption/desorption isotherms of the nanocomposites formed as a result of precipitation (A) and ignition (B).
By exploiting the spectrum of the synthesized nanocomposites in paraffin oil, the optical energy gap (Eg) of the synthesized nanocomposites was obtained using Eq. (2) (Alharbi and Abdelrahman, 2020).
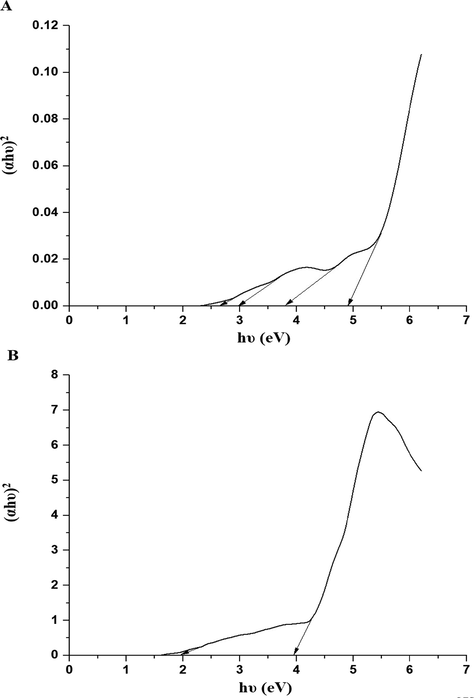
The plot of (αhυ)2 versus hυ for the nanocomposites formed as a result of precipitation (A) and ignition (B).
3.2 Photocatalytic degradation of methylene blue dye
3.2.1 Effect of pH
The effect of pH on the photodegradation efficiency (% P) of methylene blue dye, via the nanocomposites formed as a result of precipitation using sodium hydroxide and ignition at 700 °C for 3 hrs, can be seen in Fig. 8A-B, respectively. The figure indicates that pH = 8 helps to reach maximum degradation efficiency for methylene blue dye. The maximum photodegradation efficiency (% P) of methylene blue dye, using the nanocomposite formed as a result of precipitation using sodium hydroxide in the absence and presence of H2O2, is 24.70 and 100 %, respectively. Also, the maximum photodegradation efficiency (% P) of methylene blue dye, using the nanocomposite formed as a result of ignition at 700 °C for 3 hrs in the absence and presence of H2O2, is 28.57 and 100 %, respectively. It is noticeable that the photodegradation efficiency increases in the case of using hydrogen peroxide because it produces more hydroxyl free radicals when exposed to ultraviolet rays. The point of zero charge of the nanocomposites formed as a result of precipitation using sodium hydroxide and ignition at 700 °C for 3 hrs was determined as reported by Khalifa et al (Khalifa et al., 2020) and found to be 4.20 and 4.43, respectively. The electrostatic interactions between the negative catalyst surface and the cationic methylene blue dye at pH > pHPZC lead to an increase in the photodegradation efficiency of methylene blue dye. The electrostatic repulsions between the positive catalyst surface and the cationic methylene blue dye at pH < pHPZC lead to a decrease in the photodegradation efficiency of methylene blue dye (Abdelwahab et al., 2022).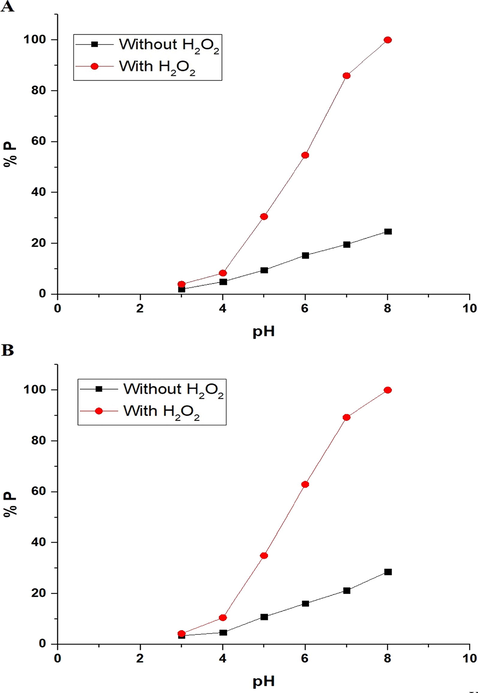
The effect of pH on the photodegradation efficiency (% P) of methylene blue dye using the nanocomposites formed as a result of precipitation (A) and ignition (B).
3.2.2 Effect of time
The effect of UV irradiation time on the photodegradation efficiency (% P) of methylene blue dye, via the nanocomposites formed as a result of precipitation using sodium hydroxide and ignition at 700 °C for 3 hrs, can be seen in Fig. 9A-B, respectively. The figure indicates that time = 40 min and 25 min helps to reach maximum degradation efficiency for methylene blue dye using the synthesized nanocomposites in the absence and presence of H2O2, respectively. It is noticeable that the photodegradation efficiency, using the synthesized nanocomposites in the absence of hydrogen peroxide, did not change when increasing the time from 40 min to 60 min due to the saturation of active sites (Abdelwahab et al., 2022). Utilizing Eq. (3), the photocatalytic degradation process of methylene blue dye, via the nanocomposites formed as a result of precipitation by sodium hydroxide and ignition at 700 °C for 3 hrs, was found to follow the first-order kinetic model as seen in Fig. 10A-B, respectively (Alharbi and Abdelrahman, 2020).
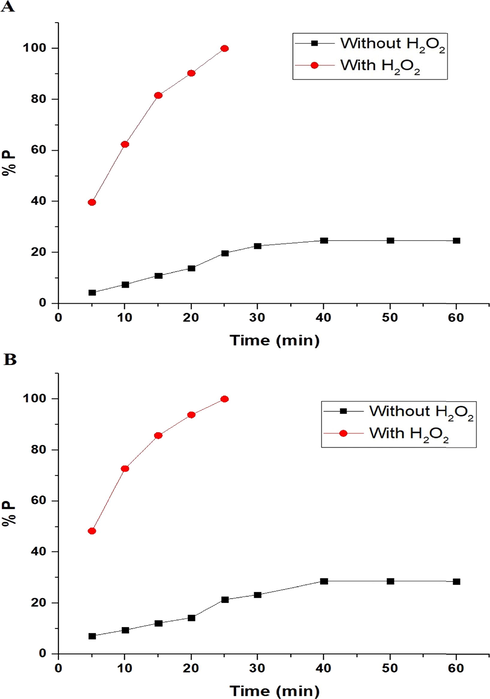
The effect of time on the photodegradation efficiency (% P) of methylene blue dye using the nanocomposites formed as a result of precipitation (A) and ignition (B).
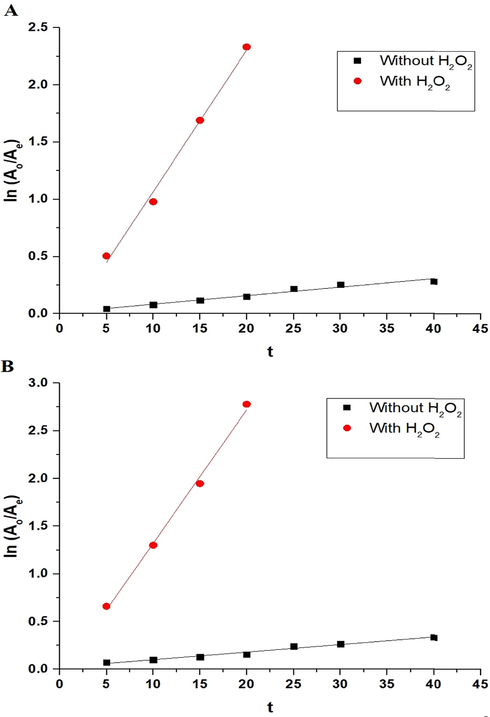
The plots of ln (Ao/Ae) versus irradiation time using the nanocomposites formed as a result of precipitation (A) and ignition (B).
Catalyst produced due to
KF (1/min)
R2
Without H2O2
With H2O2
Without H2O2
With H2O2
Precipitation
0.0075
0.1238
0.9553
0.9908
Ignition
0.0079
0.1401
0.9688
0.9934
3.2.3 Effect of amount of catalyst
The effect of the amount of photocatalyst on the photodegradation efficiency (% P) of methylene blue dye, via the nanocomposites formed as a result of precipitation using sodium hydroxide and ignition at 700 °C for 3 hrs, can be seen in Fig. 11A-B, respectively. The figure indicates that the amount of photocatalyst = 0.05 g helps to reach maximum degradation efficiency for methylene blue dye using the synthesized nanocomposites. This is a result of the rise in the number of photocatalyst particles, which boosts photon absorption. It is noticeable that the photodegradation efficiency decreases when increasing the amount of photocatalyst from 0.05 g to 0.10 g as a result of the scattering of incident UV light via the excess photocatalyst particles and the aggregation of the photocatalyst (Abdelwahab et al., 2022).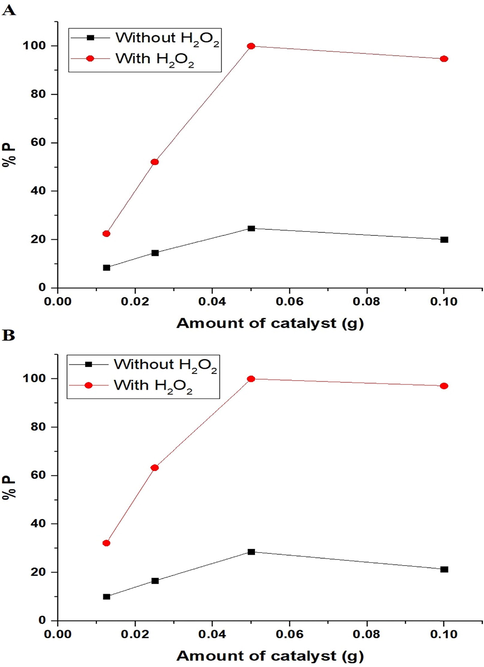
The effect of the amount of photocatalyst on the photodegradation efficiency (% P) of methylene blue dye using the nanocomposites formed as a result of precipitation (A) and ignition (B).
3.2.4 Effect of initial concentration of methylene blue dye
The effect of the initial concentration of methylene blue dye on the photodegradation efficiency (% P), via the nanocomposites formed as a result of precipitation using sodium hydroxide and ignition at 700 °C for 3 hrs, can be seen in Fig. 12A-B, respectively. It is noticeable that the photodegradation efficiency decreases when increasing the initial concentration of methylene blue dye as a result of the reduced path length of the UV photons arriving at the methylene blue dye solution, which decreases the photon absorption by the photocatalyst and consequently decreases the photocatalytic degradation (Abdelwahab et al., 2022).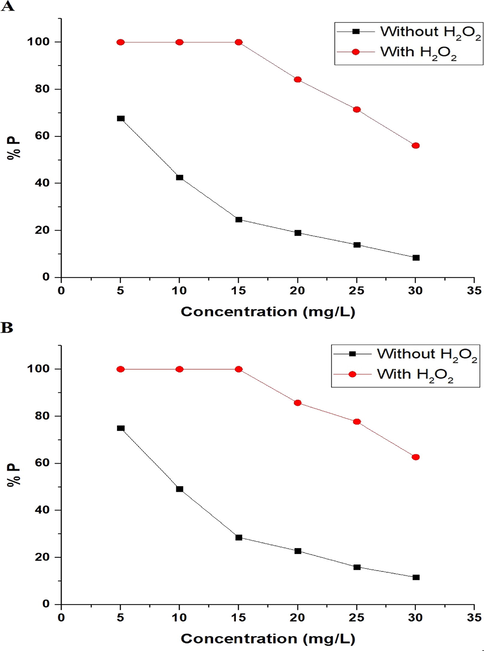
The effect of the initial concentration of methylene blue dye on the photodegradation efficiency (% P) using the nanocomposites formed as a result of precipitation (A) and ignition (B).
3.2.5 Mechanism of photocatalytic degradation of methylene blue dye
Under the effect of UV irradiation, the photogenerated electrons stimulate to conduction band from the valence band, resulting in the creation of an electron/hole pair on the surface of the photocatalyst. The hydroxyl free radicals can be produced with the help of the holes, which react with the surface-bound water, whereas electrons of the conduction band can react with oxygen to form the superoxide radical anion of oxygen. The superoxide radical anion of oxygen can react with water to form hydroxyl free radicals. Under the effect of hydroxyl free radicals, the methylene blue dye can be decomposed into volatile gases such as CO2 and H2O as clarified in Fig. 13 (Abdelwahab et al., 2022; Alharbi and Abdelrahman, 2020).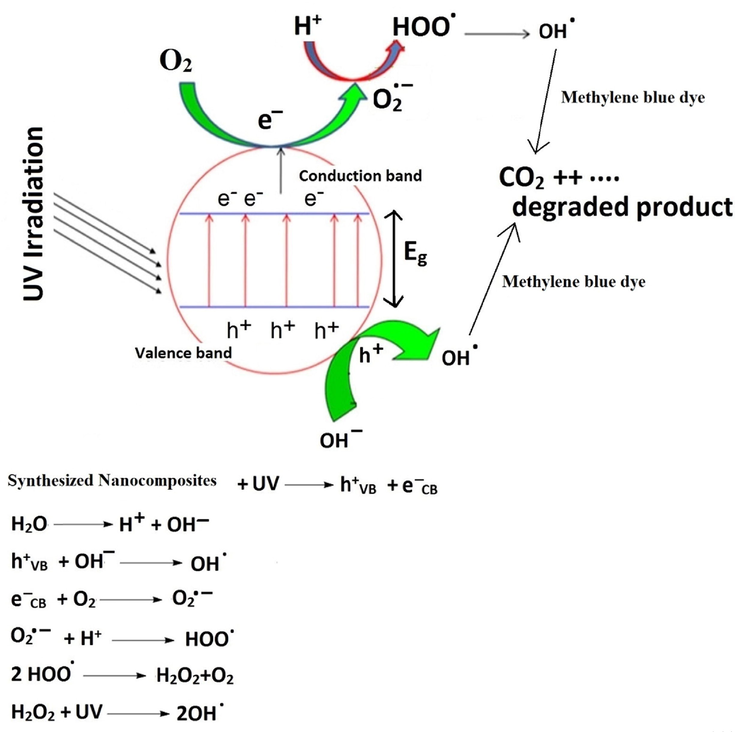
Proposed mechanism for the degradation of methylene blue dye using the synthesized nanocomposites.
3.2.6 Effect of regeneration and reusability
The effect of the regeneration and reusability on the photodegradation efficiency (% P) of methylene blue dye, via the nanocomposites formed as a result of precipitation using sodium hydroxide and ignition at 700 °C for 3 hrs, can be seen in Fig. 14A-B, respectively. It is noticeable that the photodegradation efficiency is weakly affected when increasing the cycle number (Abdelwahab et al., 2022). Thus, the synthesized photocatalysts can be used many times without losing their efficiency.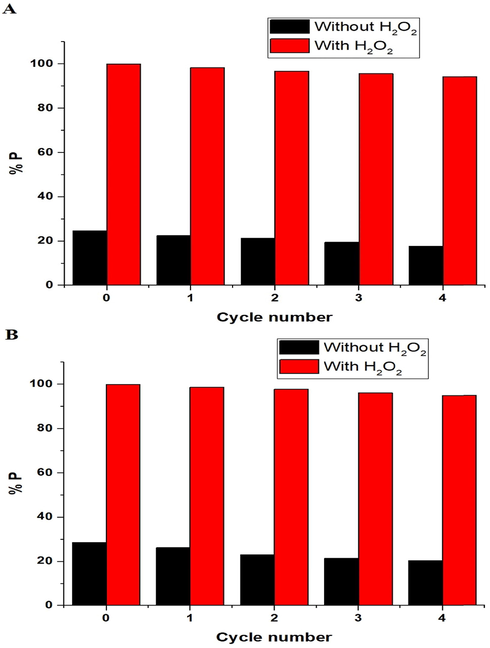
The effect of the regeneration and reusability on the photodegradation efficiency (% P) of methylene blue dye using the nanocomposites formed as a result of precipitation (A) and ignition (B).
3.2.7 Comparison of photocatalytic degradation of methylene blue dye using the synthesized nanocomposites with other photocatalysts in the literature
As clearly seen in Table 2, the photocatalytic efficiency of methylene blue dye using the synthesized nanocomposites has been compared with that of other photocatalysts reported in the literature such as Bi/Carbon nanotubes/α-Fe2O3 nanocomposite (Manda et al., 2022), MgSO4/g-C3N4 composite (Zeng et al., 2022), ZnWO4/SnS2 nanocomposite (Kumar et al., 2022), multi-walled carbon nanotube/WO3 nanocomposite (Stan et al., 2022), and multi-walled carbon nanotube/TiO2 nanocomposite (Jiang et al., 2011). The results demonstrated that the synthesized nanocomposites were superior to other photocatalysts in their capacity to rapidly degrade a significant volume and concentration of methylene blue dye.
Catalyst
Amount of catalyst (g)
Concentration of dye (mg/L)
Volume of dye (mL)
% Degradation
Time (min)
Ref
Bi/Carbon nanotubes/α-Fe2O3
0.06
20
100
100
20
(Manda et al., 2022)
MgSO4/g-C3N4
0.03
20
50
98.45
90
(Zeng et al., 2022)
ZnWO4/SnS2
0.03
20
50
99.60
75
(Kumar et al., 2022)
Multi-walled carbon nanotube/WO3
0.02
3.19
10
85.20
300
(Stan et al., 2022)
Multi-walled carbon nanotube/TiO2
0.1
30
100
92.5
60
(Jiang et al., 2011)
Composite formed due to precipitation
0.05
15
50
100
25
This work
Composite formed due to ignition
0.05
15
50
100
25
This work
4 Conclusions
Our research team has manufactured novel nanocomposites consist of copper, cobalt, manganese, and oxygen utilizing a simple precipitation/ignition technique and inexpensive ingredients. The synthesized nanocomposites were characterized using different instruments such as energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD), field emission scanning electron microscope (FE-SEM), transmission electron microscope (TEM), nitrogen gas sorption analyzer, and UV–vis spectrophotometer. 0.05 g of the produced nanocomposites degrade 100 % of 50 mL of 15 mg/L of methylene blue dye solution within 25 min in the presence of H2O2 under UV light.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Encapsulation of NiS and ZnS in analcime nanoparticles as novel nanocomposites for the effective photocatalytic degradation of orange G and methylene blue dyes Encapsulation of NiS and ZnS in analcime nanoparticles as novel nanocomposites for the effectiv. Int. J. Environ. Anal. Chem.. 2022;00:1-18.
- [CrossRef] [Google Scholar]
- Efficient photocatalytic degradation of malachite green dye using facilely synthesized hematite nanoparticles from Egyptian insecticide cans. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2020;226:117612
- [CrossRef] [Google Scholar]
- Synthesis and characterization of novel Cu (II) complexes with 3-substituted-4-amino-5-mercapto-1,2,4-triazole Schiff bases: A new route to CuO nanoparticles. J. Mol. Struct.. 2015;1086:223-231.
- [CrossRef] [Google Scholar]
- Removal of BB9 textile dye by biological, physical, chemical, and electrochemical treatments. J. Taiwan Inst. Chem. Eng.. 2021;121:29-37.
- [CrossRef] [Google Scholar]
- ZnO nanoparticles decorated multi-walled carbon nanotubes for enhanced photocatalytic and photoelectrochemical water splitting. J. Photochem. Photobiol. A Chem.. 2018;351:154-161.
- [CrossRef] [Google Scholar]
- High photocatalytic efficiency of α-Fe2O3 - ZnO composite using solar energy for methylene blue degradation. Phys. B Condens. Matter. 2022;627:413567
- [CrossRef] [Google Scholar]
- Facile fabrication of hematite nanoparticles from Egyptian insecticide cans for efficient photocatalytic degradation of rhodamine B dye. J. Mater. Res. Technol.. 2020;9:1652-1661.
- [CrossRef] [Google Scholar]
- Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica) J. Water Process Eng.. 2022;49:102952
- [CrossRef] [Google Scholar]
- Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere. 2022;291:132906
- [CrossRef] [Google Scholar]
- Photo-assisted advanced oxidation processes for efficient removal of anionic and cationic dyes using Bentonite/TiO2 nano-photocatalyst immobilized with silver nanoparticles. J. Mol. Struct.. 2021;1239:130496
- [CrossRef] [Google Scholar]
- Photo-degradation of methylene blue by multi-walled carbon nanotubes/TiO2 composites. Powder Technol.. 2011;207:465-469.
- [CrossRef] [Google Scholar]
- Highly efficient adsorbent material for removal of methylene blue dye based on functionalized polyacrylonitrile. Eur. Polym. J.. 2022;169:111138
- [CrossRef] [Google Scholar]
- Application of Mesoporous Silica Nanoparticles Modified with Dibenzoylmethane as a Novel Composite for Efficient Removal of Cd(II), Hg(II), and Cu(II) Ions from Aqueous Media. J. Inorg. Organomet. Polym. Mater.. 2020;30:2182-2196.
- [CrossRef] [Google Scholar]
- Comparison of disperse and reactive dye removals by chemical coagulation and Fenton oxidation. J. Hazard. Mater.. 2004;112:95-103.
- [CrossRef] [Google Scholar]
- Effect of precipitating agents on the magnetic and structural properties of the synthesized ferrimagnetic nanoparticles by co-precipitation method. Powder Technol.. 2022;401:117298
- [CrossRef] [Google Scholar]
- Solar light induced photocatalytic process for reduction of hexavalent chromium and degradation of tetracycline and methylene blue by heterostructures made of SnS2 nanoplates surface modified by ZnWO4 nanorods. Sep. Purif. Technol.. 2022;292:121040
- [CrossRef] [Google Scholar]
- Construction of Z-scheme WO3-Cu2O nanorods array heterojunction for efficient photocatalytic degradation of methylene blue. Inorg. Chem. Commun.. 2022;138:109248
- [CrossRef] [Google Scholar]
- Synthesis and characterization of zinc oxide nanoparticles via oxalate co-precipitation method. Mater. Lett. X. 2022;13:100126
- [CrossRef] [Google Scholar]
- Manda, A.A., Elsayed, K.A., Ibrahim, U., Haladu, S.A., Alshammery, S., Altamimi, N.A., Al-otaibi, A.L., 2022. Enhanced photocatalytic degradation of methylene blue by nanocomposites prepared by laser ablation of Bi on CNT- α -Fe2O3 nanoparticles 155, 1–13. https://doi.org/10.1016/j.optlastec.2022.108430.
- Synthesis, characterization, and biological activity of some novel Schiff bases and their Co(II) and Ni(II) complexes: A new route for Co3O4 and NiO nanoparticles for photocatalytic degradation of methylene blue dye. J. Mol. Struct.. 2017;1143:462-471.
- [CrossRef] [Google Scholar]
- Synthesis of bismuth oxychloride nanoparticles via co-precipitation method: Evaluation of photocatalytic activity. Mater. Today Proc.. 2022;62:5533-5539.
- [CrossRef] [Google Scholar]
- Preparation of SnO2-TiO2/MWCNT nanocomposite photocatalysts with different synthesis parameters. Phys. Status Solidi Basic Res.. 2013;250:2549-2553.
- [CrossRef] [Google Scholar]
- Study of Fe-doped and glucose-capped CeO2 nanoparticles synthesized by co-precipitation method. Chem. Phys.. 2022;561:111617
- [CrossRef] [Google Scholar]
- Facile synthesis of MWCNT-WO3 composites with enhanced photocatalytic degradation of methylene blue dye. Synth. Met.. 2022;288:117117
- [CrossRef] [Google Scholar]
- Role of surface defects and anisotropy variation on magnetic properties of copper ferrite nanoparticles prepared by co-precipitation method. Mater. Chem. Phys.. 2022;286:126212
- [CrossRef] [Google Scholar]
- Subrahmanya, T.M., Widakdo, J., Mani, S., Austria, H.F.M., Hung, W.S., H K, M., Nagar, J.K., Hu, C.C., Lai, J.Y., 2022. An eco-friendly and reusable syringe filter membrane for the efficient removal of dyes from water via low pressure filtration assisted self-assembling of graphene oxide and SBA-15/PDA. J. Clean. Prod. 349, 131425. https://doi.org/10.1016/j.jclepro.2022.131425.
- Application of natural minerals in photocatalytic degradation of organic pollutants: A review. Sci. Total Environ.. 2022;812:152434
- [CrossRef] [Google Scholar]
- Progress of graphite carbon nitride with different dimensions in the photocatalytic degradation of dyes: A review. J. Alloys Compd.. 2022;901
- [CrossRef] [Google Scholar]
- Sulfate doped graphitic carbon nitride with enhanced photocatalytic activity towards degradation of methylene blue. Mater. Lett.. 2022;309:131310
- [CrossRef] [Google Scholar]







