Translate this page into:
Facile synthesis and characterization of copper oxalate/cobalt oxalate/manganese oxalate and copper oxide/cobalt manganese oxide/manganese oxide as new nanocomposites for efficient photocatalytic degradation of malachite green dye
⁎Corresponding author. chem241@ksu.edu.sa (Salwa AlReshaidan) chem241@ksu.edu.sa (Salwa AlReshaidan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Scientists seek to synthesize new catalysts with simple methods to treat water pollution from organic dyes using photocatalytic degradation technology. In this technology, when light falls on the catalyst, the produced hydroxyl free radicals convert the dye into non-toxic gases such as CO2 and H2O. So, in this work, copper oxalate/cobalt oxalate/manganese oxalate (Abbreviated as P1) and copper oxide/cobalt manganese oxide/manganese oxide (Abbreviated as P2) new nanocomposites were fabricated via precipitation of Cu2+/Co2+/Mn2+ solution using oxalic acid and ignition of precipitate at 550 °C for 4 hrs, respectively. Some tools, involving X-ray diffraction (XRD), UV–vis spectrophotometer, energy dispersive X-ray spectroscopy (EDX), nitrogen gas sorption analyzer, transmission electron microscope (TEM), and field emission scanning electron microscope (FE-SEM), were used for characterizing the fabricated nanocomposites. The EDX spectra confirmed that the P1 composite consist of C (26.28 %), oxygen (46.66 %), manganese (7.27 %), cobalt (7.59 %), and copper (12.20 %). Also, the P2 composite consist of oxygen (8.23 %), manganese (31.34 %), cobalt (27.19 %), and copper (33.24 %). A transmission electron microscope shows that the P1 and P2 composites consist of polyhedral and spherical shapes with an average diameter of 28.13 and 14.37 nm, respectively. The BET surface area, average pore size, and total pore volume of the P1 composite are 29.0725 m2/g, 2.0749 nm, and 0.0302 cc/g, respectively. Besides, the BET surface area, average pore size, and total pore volume of the P2 composite are 58.1088 m2/g, 1.6087 nm, 0.0467 cc/g, respectively. 60 mg of the synthesized nanocomposites completely decompose 60 mL of 15 mg/L of malachite green dye solution within 20 min in the presence of hydrogen peroxide and UV light. The synthesized catalysts outperformed many other catalysts published in previous studies.
Keywords
Nanoparticles
Photocatalytic degradation
Analytical parameters
Malachite green dye
1 Introduction
Water contamination by harmful industrial wastes is the most serious environmental issue. Numerous approaches and substances are being researched to purify the water (Adel et al., 2022; Bi et al., 2022; Rayaroth et al., 2022). Thousands of synthetic dyes are the primary cause of water pollution. Since the majority of these dyes are nonbiodegradable, they stay in the water for an extended period of time. In addition to their toxicity, even low quantities of these substances harm aquatic life by blocking sunlight. Malachite green is among the industrial organic dyes that are harmful to mammalian cells. Malachite green is a dye used in the paper, cotton, jute, wool, silk, leather, and acrylic industries, as well as an antibacterial and fungicide (Raval et al., 2017). Since the pollutants are transformed from one phase to another in the adsorption method, a secondary pollutant issue occurs despite the fact that adsorption methods are the most extensively utilized for dye removal as they are efficient and economical (Abdelrahman, 2018; Abdelrahman et al., 2019a). Consequently, intensive research is being conducted on the removal of contaminants via photodegradation (Abdelrahman et al., 2019b; Alharbi and Abdelrahman, 2020; Hegazey et al., 2020). In recent years, advanced oxidation technologies have been utilized efficiently for the degradation of hazardous chemicals in wastewater (Abdelrahman and Hegazey, 2019). Using semiconductor catalyst, heterogeneous photocatalysis completely converts organic contaminants to CO2 and H2O with the help of the generated free radicals (Abdelrahman and Hegazey, 2019). There are many catalysts for degrading malachite green dyes such as Fe(III)-cross-linked alginate-carboxymethyl cellulose composite (Karadeniz et al., 2022), cobalt oxide/citric acid nanocomposite (Verma et al., 2021), sn-doped TiO2 (Sayilkan et al., 2007), Fe3O4/ SiO2/TiO2 composite (Farhadian and Kazemzad, 2016), and chitosan supported Ce–ZnO (Saad et al., 2020). However, most of these catalysts require a high cost to prepare them, as well as their ability to degrade a small concentration of malachite green dye in a large time. Therefore, scientists strive to prepare effective catalysts in simple ways using low-cost chemicals. Copper, cobalt, and manganese salts are cheap, available, and easy to purchase. Also, oxalic acid is among the cheapest organic precipitants for the preparation of low crystalline nanomaterials. Precipitation and/or ignition method is an effective method for producing many nanomaterials such as iron oxide (AL-Harbi and Darwish, 2022), ferromagnetic nanoparticles (Kumar and Gangawane, 2022), zinc oxide (Mahmood et al., 2022), Mn3O4 (Altiner et al., 2022), cerium oxide (Aseena et al., 2021), borohydride (Wang and Aguey-Zinsou, 2021), CuCo2O4 (Sun et al., 2021), and cadmium oxide (Sujatha et al., 2021). So, in this work, copper oxalate/cobalt oxalate/manganese oxalate and copper oxide/cobalt manganese oxide/manganese oxide new nanocomposites were synthesized by precipitation of Cu2+/Co2+/Mn2+ solution using oxalic acid and ignition of precipitate at 550 °C for 4 hrs, respectively. Some tools, involving X-ray diffraction (XRD), UV–vis spectrophotometer, energy dispersive X-ray spectroscopy (EDX), nitrogen gas sorption analyzer, transmission electron microscope (TEM), and field emission scanning electron microscope (FE-SEM), were used for characterizing the fabricated nanocomposites. The produced nanocomposites were employed as new photocatalysts for the degradation of malachite green dye. Furthermore, analytical parameters impacting the degradation efficiency of malachite green dye, such as pH, irradiation time, dye concentration, and catalyst quantity, have been investigated.
2 Experimental
2.1 Utilized chemicals
Copper(II) acetate monohydrate (Cu(CH3COO)2·H2O), cobalt(II) acetate tetrahydrate (Co(CH3COO)2·4H2O), hydrochloric acid (HCl), oxalic acid (C2H2O4), sodium hydroxide (NaOH), manganese(II)acetate tetrahydrate (Mn(CH3COO)2·4H2O), hydrogen peroxide (H2O2), and malachite green dye (C23H25ClN2) were acquired from Sigma Aldrich Company (Purity = 99.99 %) and utilized without further purification.
2.2 Synthesis of composites
5.00 g of copper(II) acetate monohydrate, 5.00 g of manganese(II) acetate tetrahydrate, and 5.00 g of cobalt(II) acetate tetrahydrate were dissolved in 250 mL of distilled water for getting the Cu/Co/Mn solution. Additionally, 8.50 g of oxalic acid was dissolved in 120 mL of distilled water. After that, the oxalic acid solution was added to the Cu/Co/Mn solution drop by drop with constant stirring for 30 min. Moreover, the produced precipitate was separated, washed several times with distilled water, and dried at 60 °C for 24 hrs. Additionally, the dried precipitate was ignited at 550 °C for 4 hrs.
2.3 Instrumentation
Using a PANalytical XRD diffractometer with Kα Cu line equal to 1.5 Å, the average crystallite size and crystal structure of the P1 and P2 composites were determined. Using an Ultra 55 Gemini-Zeiss scanning electron microscope, the surface shape and constituents of the P1 and P2 composites were determined. Using a Talos F200iS transmission electron microscope, the morphologies of the P1 and P2 composites were obtained. The surface textures of the P1 and P2 composites were determined using a Quantachrome TouchWin nitrogen gas sorption analyzer after 12 hrs of degassing at 60 °C. The energy gap of the P1 and P2 composites and concentration of the malachite green dye was obtained using a Shimadzu-M160 PC UV–vis spectrophotometer. Maximum malachite green dye wavelength is 620 nm.
2.4 Photocatalytic degradation of malachite green dye
The P1 or P2 catalyst is combined with the malachite green dye solution in a 250 mL beaker and stirred for 4 hrs in a dark location. During stirring, the beaker is then exposed to ultraviolet light (Wavelength = 254 nm, Length = 40 cm, Power = 10 Watt). According to Table 1, several parameters impacting the photocatalytic degradation of malachite green dye using the P1 and P2 composites have been examined.
Parameter
Experimental conditions
Concentration of dye (mg/L)
Volume of dye (mL)
Amount of catalyst (mg)
Irradiation time (min)
pH
pH (2.5–8.5)
15
60
60
180
----
Time (4–40 min)
15
60
60
----
8.5
Amount of catalyst (0.02–0.1 g)
15
60
----
32 min and 20 min in the absence and presence of H2O2, respectively.
8.5
Concentration of dye (5–25 mg/L)
---
60
60
32 min and 20 min in the absence and presence of H2O2, respectively.
8.5
Regeneration and reusability
15
60
60
32 min and 20 min in the absence and presence of H2O2, respectively.
8.5
The preceding experimental procedures were repeated with the addition of 0.5 mL of a 1 M hydrogen peroxide solution. In the instance of regeneration and reusability, the P1 or P2 catalysts were regenerated by washing them with hot distilled water after every cycle and then drying them at 60 °C. Four cycles were then utilized for the degradation of the malachite green dye.
The percentage of photocatalytic degradation (% Degradation Efficiency) of malachite green dye was calculated using Eq. (1).
Bo (mg/L) is the concentration of the malachite green dye after the end of its stirring period in a dark place. Be (mg/L) is the concentration of the malachite green dye after the expiration of the period of stirring in the presence of ultraviolet rays.
3 Results and discussion
3.1 Characterization of the synthesized composites
The appearance of XRD peaks at 2Θ = 18.84°, 22.79°, 24.66°, 30.05°, 33.58°, and 34.62° confirms that the P1 composite consists of copper oxalate hydrate (CuC2O4·H2O, JCPDS No. 00–022-1089), cobalt oxalate hydrate (CoC2O4·2H2O, JCPDS No. 00–025-0250), and manganese oxalate hydrate (MnC2O4·2H2O, JCPDS No. 00–025-0544) as clarified in Fig. 1A. Also, the P2 composite consists of copper oxide (CuO, JCPDS No. 00–005-0661), cobalt manganese oxide ((Co,Mn)(Co,Mn)2O4, JCPDS No. 00–018-0408), and manganese oxide (Mn5O8, JCPDS No. 00–018-0801) as clarified in Fig. 1B. Characteristic peaks of copper oxide appear at 2Θ = 35.69°, 38.91°, 45.77°, 48.93°, 61.70°, and 66.17°. Characteristic peaks of manganese oxide appear at 2Θ = 15.98°, 31.14°, 44.32°, and 64.54°. Characteristic peaks of cobalt manganese oxide appear at 2Θ = 36.63° and 58.76°. The average crystallite size of the P1 and P2 composites is 30.12 and 18.54 nm, respectively.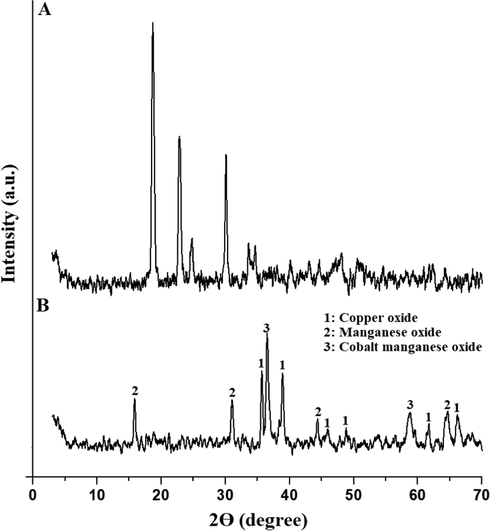
XRD patterns of the P1 (A) and P2 (B) composites.
As seen from EDX spectra in Fig. 2A, the P1 composite consist of C (26.28 %), oxygen (46.66 %), manganese (7.27 %), cobalt (7.59 %), and copper (12.20 %). Also, the P2 composite consist of oxygen (8.23 %), manganese (31.34 %), cobalt (27.19 %), and copper (33.24 %) as seen from EDX spectra in Fig. 2B.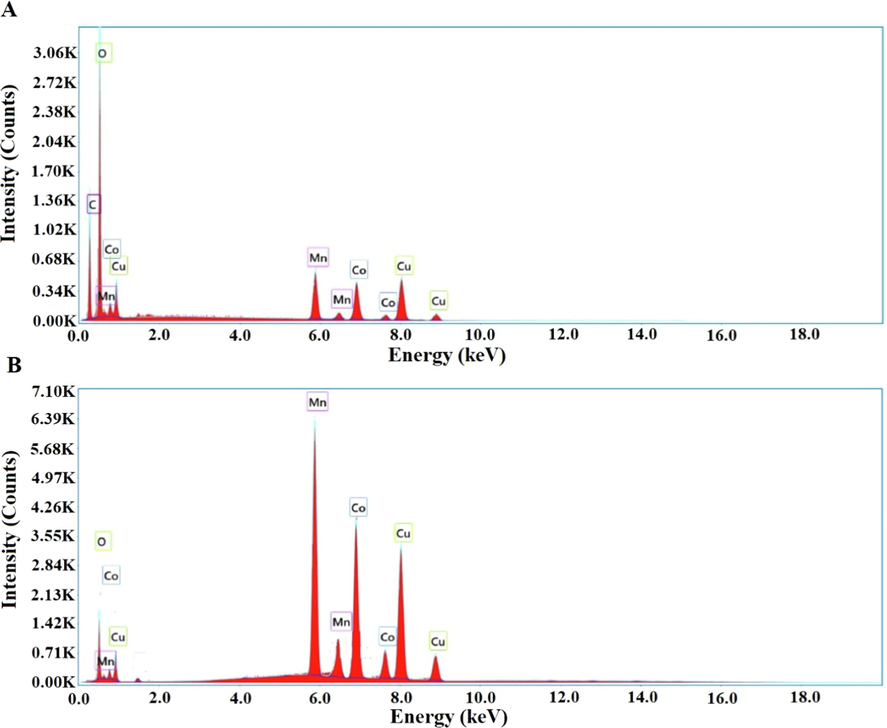
EDX patterns of the P1 (A) and P2 (B) composites.
A scanning electron microscope shows that the P1 composite consists of irregular shapes with an average diameter of 3.57 µm, as seen in Fig. 3A. Also, the P2 composite consists of irregular and spherical shapes with an average diameter of 1.26 µm, as seen in Fig. 3B. A transmission electron microscope shows that the P1 and P2 composites consist of polyhedral and spherical shapes with an average diameter of 28.13 and 14.37 nm, as seen in Fig. 4A-B, respectively.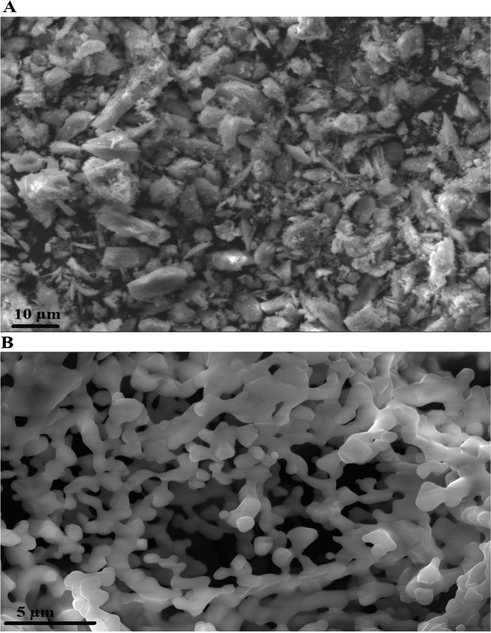
Scanning electron microscope images of the P1 (A) and P2 composites.
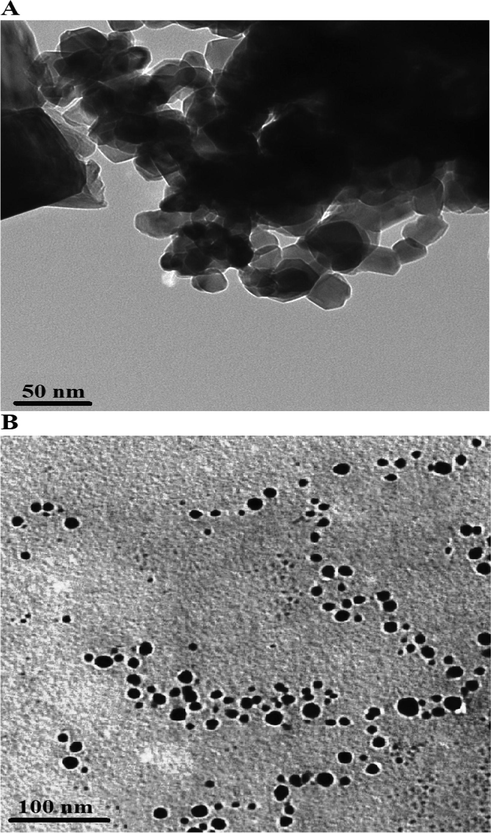
Transmission electron microscope images of the P1 (A) and P2 composites.
The N2 adsorption/desorption isotherms of the P1 and P2 composites belong to type IV as seen in Fig. 5A-B, respectively (Abdelrahman et al., 2019b). Also, the BET surface area, average pore size, and total pore volume of the P1 composite are 29.0725 m2/g, 2.0749 nm, and 0.0302 cc/g, respectively. Besides, the BET surface area, average pore size, and total pore volume of the P2 composite are 58.1088 m2/g, 1.6087 nm, 0.0467 cc/g, respectively.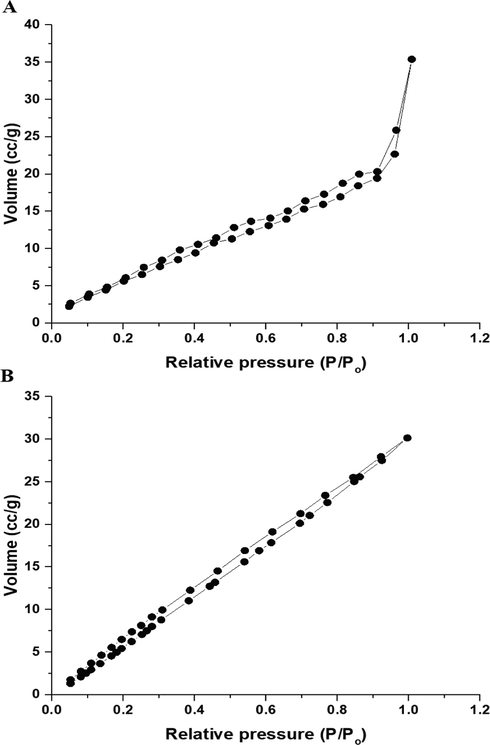
N2 adsorption/desorption isotherms of the P1 (A) and P2 (B) composites.
By exploiting the spectrum of the P1 and P2 composites in paraffin oil, the optical energy gap (Egap) was calculated using Eq. (2) (Abdelrahman et al., 2019b; Hegazey et al., 2020).
Ko, α, P are a constant, the absorption coefficient, and an integer based on the nature of the transition. P = 0.5 for indirect permitted transitions, whereas P = 2 for direct permitted transitions. As seen in Fig. 6A-B, direct permitted transitions predominated in the P1 and P2 composites. The energy gap of the P1 composite is 1.96 eV. Also, the energy gaps of the P2 composite are 2.29 and 3.53 eV.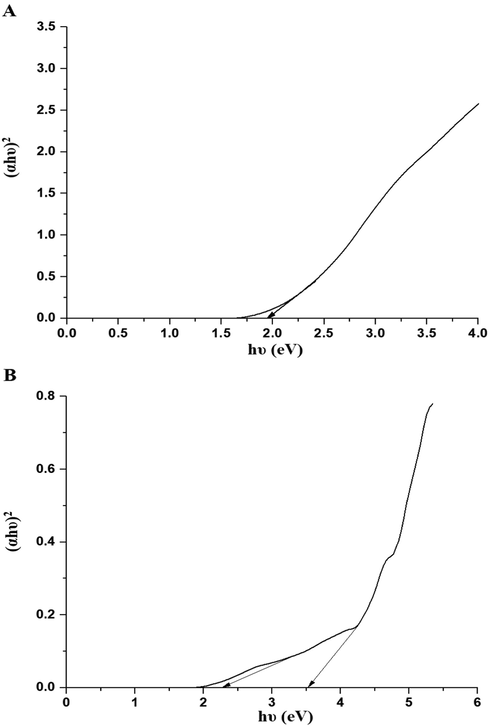
The plot of (αhυ)2 versus hυ for the P1 (A) and P2 (B) composites.
3.2 Photocatalytic degradation of malachite green dye
3.2.1 Effect of pH
The effect of pH on the degradation efficiency of malachite green dye using the P1 and P2 composites was studied with pH varying from 2.5 to 8.5 as seen in Fig. 7A-B, respectively. The optimal pH for the degradation of malachite green dye in the absence and presence of hydrogen peroxide is 8.5. The degradation efficiency of malachite green dye, at pH = 8.5 using the P1 composite in the absence and presence of hydrogen peroxide, is 29.58 and 100 %, respectively. The degradation efficiency of malachite green dye, at pH = 8.5 using the P2 composite in the absence and presence of hydrogen peroxide, is 36.50 and 100 %, respectively. The ability of hydrogen peroxide under the effect of UV rays to form more hydroxyl free radicals, which accelerate the degradation of the malachite green dye, boosted the degradation efficiency as compared to the absence of hydrogen peroxide. The point of zero charge of the P1 and P2 composites was determined as described by Khalifa et al (Khalifa et al., 2020). The point of zero charge (pHPZC) of the P1 and P2 composites is 3.96 and 4.28, respectively. Malachite green dye is a cationic dye. Due to the attraction forces, it is therefore adsorbable on the surface of a catalyst at pH values greater than pHPZC. Thus, the efficiency of malachite green dye degradation improves at pH values greater than pHPZC. Malachite green dye is a cationic dye. Consequently, electrostatic repulsion prevents it from being favorably adsorbed on the surface of a catalyst at pH values below pHPZC. Thus, at pH levels below pHPZC, the degradation efficiency of malachite green dye decreases (Abdelwahab et al., 2022).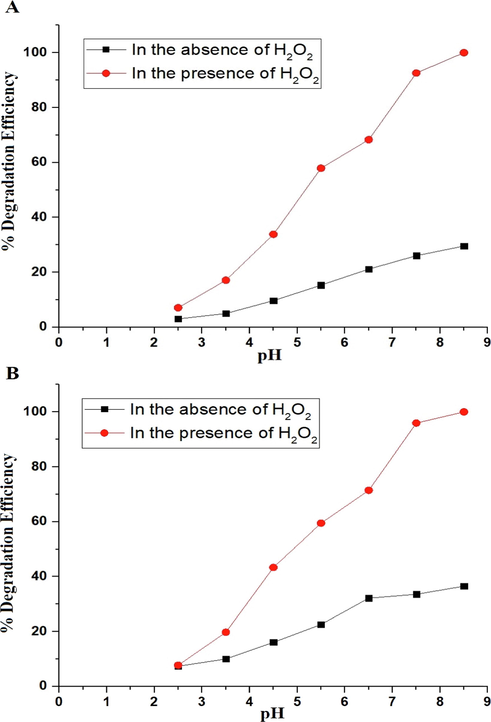
The effect of pH on the degradation efficiency of malachite green dye using the P1 (A) and P2 (B) composites.
3.2.2 Effect of time
The effect of time on the degradation efficiency of malachite green dye using the P1 and P2 composites was studied with time varying from 4 min to 40 min as seen in Fig. 8A-B, respectively. The optimal period for the degradation of malachite green dye utilizing the P1 and P2 composites in the absence of hydrogen peroxide was determined to be 32 min. Due to the saturation of the active sites, it was observed that the degradation efficiency of malachite green dye remains rather stable when the irradiation time increases from 32 min to 40 min. The optimal period for the degradation of malachite green dye utilizing the P1 and P2 composites in the presence of hydrogen peroxide was determined to be 20 min. In the absence of hydrogen peroxide, the degradation efficiency of malachite green dye using the P1 and P2 composites is 29.58 and 37.39 %, respectively. In the presence of hydrogen peroxide, the degradation efficiency of malachite green dye using the P1 and P2 composites is 100 %.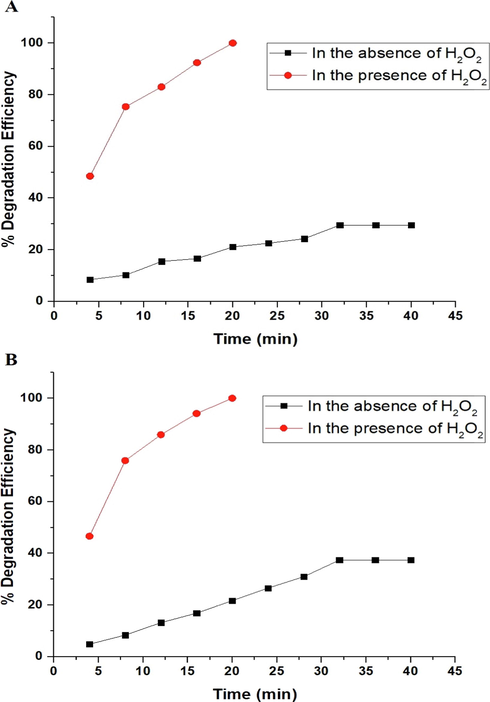
The effect of time on the degradation efficiency of malachite green dye using the P1 (A) and P2 (B) composites.
Using Eq. (3), the photocatalytic degradation processes of malachite green dye using the P1 and P2 composites were found to obey the first-order kinetic model as seen in Fig. 9A-B, respectively (Abdelwahab et al., 2022).
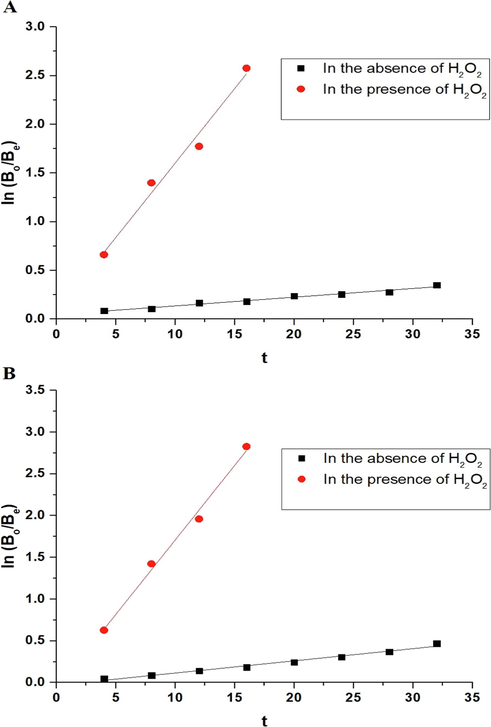
The plots of ln (Bo/Be) versus irradiation time using the P1 (A) and P2 (B) composites.
Composite
K1 (1/min)
R2
Without H2O2
With H2O2
Without H2O2
With H2O2
P1
0.0089
0.1529
0.9735
0.9743
P2
0.01461
0.1784
0.9814
0.9891
3.2.3 Effect of amount of catalyst
The effect of amount of catalyst on the degradation efficiency of malachite green dye using the P1 and P2 composites was studied with amount varying from 0.02 g to 0.10 g as seen in Fig. 10A-B, respectively. The optimal amount of catalyst for the degradation of malachite green dye utilizing the P1 and P2 composites was determined to be 0.06 g due to the increase in the number of catalyst particles, which improves photon absorption. In addition, it was noticed that the degradation efficiency of malachite green dye decreased when the amount of catalyst was increased from 0.06 g to 0.10 g due to the screening of incident light by the excess catalyst particles and the catalyst aggregation (Abdelwahab et al., 2022).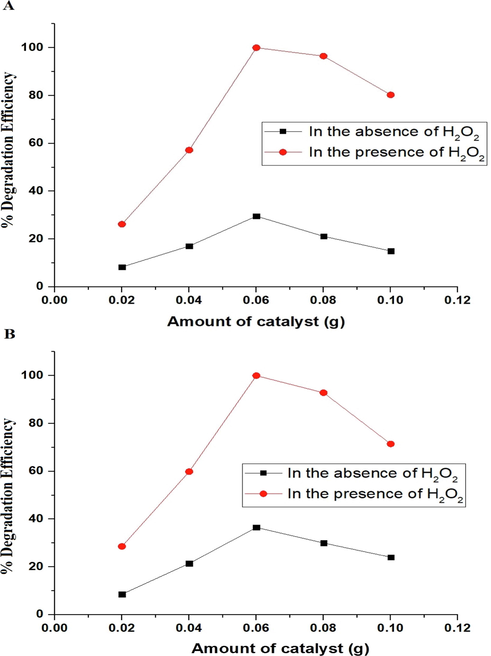
The effect of amount of catalyst on the degradation efficiency of malachite green dye using the P1 (A) and P2 (B) composites.
3.2.4 Effect of initial malachite green dye concentration
The effect of initial concentration of malachite green dye on the degradation efficiency using the P1 and P2 composites was studied with concentration varying from 5 mg/L to 25 mg/L as seen in Fig. 11A-B, respectively. In addition, it was observed that the degradation efficiency of malachite green dye decreases as the dye concentration increases from 5 mg/L to 25 mg/L. This is as a result of the shorter path length of photons entering the malachite green dye solution, which limits photon absorption by the catalyst and, consequently, photocatalytic degradation (Abdelwahab et al., 2022).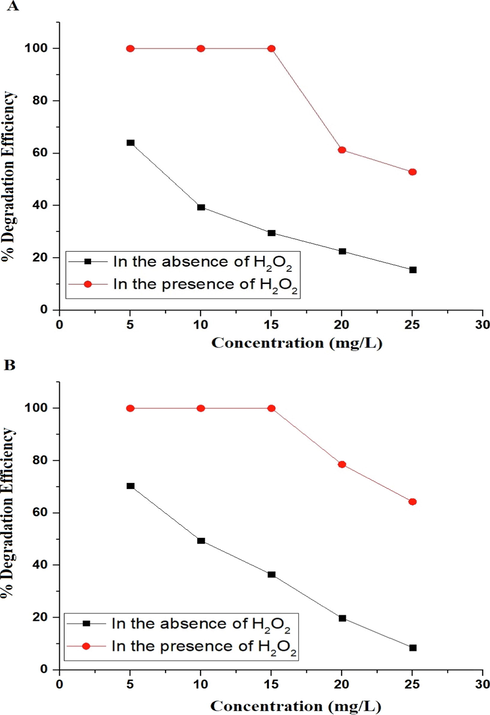
The effect of malachite green dye concentration on the degradation efficiency using the P1 (A) and P2 (B) composites.
3.2.5 Effect of regeneration and reusability
The effect of regeneration and reusability on the degradation efficiency of malachite green dye using the P1 and P2 composites was studied for four cycles as seen in Fig. 12A-B, respectively. The results indicated that the degradation efficiency of malachite green dye has been slightly modified. This shows that these catalysts can be utilized repeatedly without diminishing in efficacy.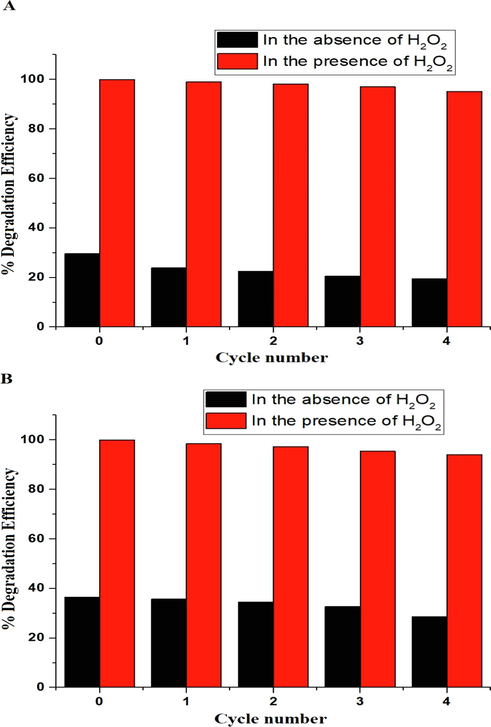
The effect of regeneration and reusability on the degradation efficiency of malachite green dye using the P1 (A) and P2 (B) composites.
3.2.6 Mechanism of photocatalytic degradation of malachite green dye
As illustrated in Fig. 13, when UV rays excite electrons in the valence band, they transfer to the conduction band, resulting in the production of holes at the valence band and electrons at the conduction band. The conduction and valence bands then produced oxygen anion free radicals (O2.-) and hydroxyl free radicals (.OH), respectively. Free radicals eventually convert malachite green dye to volatile gases (CO2 and H2O). Hydrogen peroxide inhibits the rapid recombination of electrons and holes and increases the quantity of hydroxyl free radicals. Therefore, this addition improves the catalytic efficiency of malachite green dye degradation (Abdelwahab et al., 2022).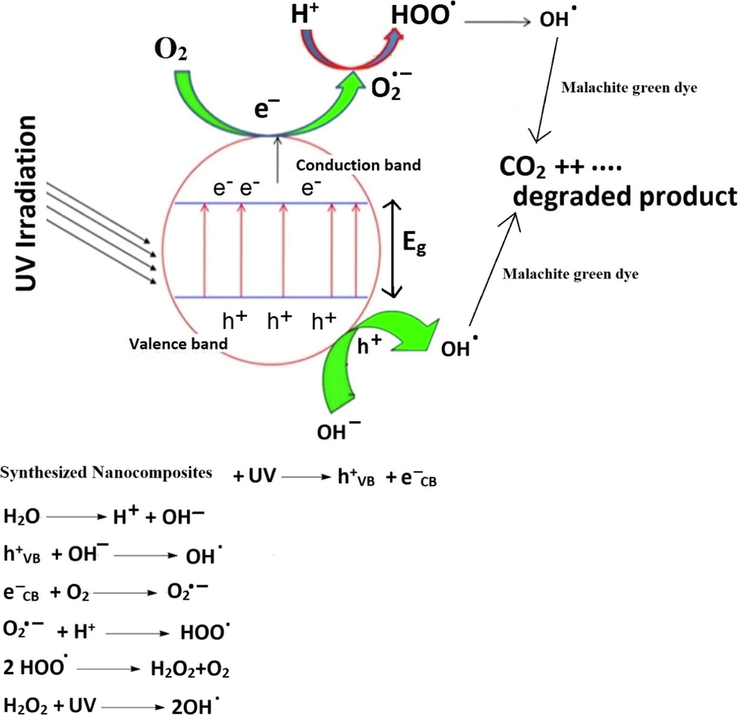
The suggested mechanism for the degradation of malachite green dye.
3.2.7 Comparison of photocatalytic degradation of malachite green dye using the produced composites with other photocatalysts
As clarified in Table 3, the photocatalytic degradation of malachite green dye using the P1 and P2 composites has been compared with that of other photocatalysts reported in the literature such as Fe(III)-cross-linked alginate-carboxymethyl cellulose composite (Karadeniz et al., 2022), cobalt oxide/citric acid nanocomposite (Verma et al., 2021), sn-doped TiO2 (Sayilkan et al., 2007), Fe3O4/ SiO2/TiO2 composite (Farhadian and Kazemzad, 2016), and chitosan supported Ce–ZnO (Saad et al., 2020). The results demonstrated that the P1 and P2 composites are superior to other catalysts in their capacity to rapidly degrade a significant volume and concentration of malachite green dye.
Catalyst
Amount of catalyst (g)
Concentration of dye (mg/L)
Volume of dye (mL)
% Degradation
Time (min)
Ref
Fe(III)-cross-linked alginate-carboxymethyl cellulose
0.10
10
50
98.8
30
(Karadeniz et al., 2022)
Cobalt oxide/citric acid
0.05
10
100
91.20
100
(Verma et al., 2021)
Sn-doped TiO2
0.10
20
25
100
340
(Sayilkan et al., 2007)
Fe3O4/ SiO2/TiO2
0.10
10
100
100
150
(Farhadian and Kazemzad, 2016)
Chitosan supported Ce–ZnO
0.02
5
100
83
180
(Saad et al., 2020)
P1
0.06
15
60
100
20
This work
P2
0.06
15
60
100
20
This work
4 Conclusions
Copper oxalate/cobalt oxalate/manganese oxalate (Abbreviated as P1) and copper oxide/cobalt manganese oxide/manganese oxide (Abbreviated as P2) new nanocomposites were fabricated via precipitation of Cu2+/Co2+/Mn2+ solution using oxalic acid and ignition of precipitate at 550 °C for 4 hrs, respectively. The average crystallite size of the P1 and P2 composites is 30.12 and 18.54 nm, respectively. The P1 composite consist of C (26.28 %), oxygen (46.66 %), manganese (7.27 %), cobalt (7.59 %), and copper (12.20 %). Also, the P2 composite consist of oxygen (8.23 %), manganese (31.34 %), cobalt (27.19 %), and copper (33.24 %). The P1 composite consists of irregular shapes with an average diameter of 3.57 µm. The P2 composite consists of irregular and spherical shapes with an average diameter of 1.26 µm. 60 mg of the synthesized nanocomposites completely decompose 60 mL of 15 mg/L of malachite green dye solution within 20 min in the presence of hydrogen peroxide and UV light.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of zeolite nanostructures from waste aluminum cans for efficient removal of malachite green dye from aqueous media. J. Mol. Liq.. 2018;253:72-82.
- [CrossRef] [Google Scholar]
- Facile synthesis of HgO nanoparticles using hydrothermal method for efficient photocatalytic degradation of crystal violet dye under UV and sunlight irradiation. J. Inorg. Organomet. Polym. Mater.. 2019;29
- [CrossRef] [Google Scholar]
- Efficient removal of methylene blue dye from aqueous media using Fe/Si, Cr/Si, Ni/Si, and Zn/Si amorphous novel adsorbents. J. Mater. Res. Technol.. 2019;8:5301-5313.
- [CrossRef] [Google Scholar]
- Facile synthesis of Fe2O3 nanoparticles from Egyptian insecticide cans for efficient photocatalytic degradation of methylene blue and crystal violet dyes. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2019;222:117195
- [CrossRef] [Google Scholar]
- Encapsulation of NiS and ZnS in analcime nanoparticles as novel nanocomposites for the effective photocatalytic degradation of orange G and methylene blue dyes Encapsulation of NiS and ZnS in analcime nanoparticles as novel nanocomposites for the effectiv. Int. J. Environ. Anal. Chem.. 2022;00:1-18.
- [CrossRef] [Google Scholar]
- Removal of heavy metals and dyes from wastewater using graphene oxide-based nanomaterials: a critical review. Environ. Nanotechnology, Monit. Manag.. 2022;18:100719
- [CrossRef] [Google Scholar]
- Efficient photocatalytic degradation of malachite green dye using facilely synthesized hematite nanoparticles from Egyptian insecticide cans. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2020;226:117612
- [CrossRef] [Google Scholar]
- Functionalized iron oxide nanoparticles: synthesis through ultrasonic-assisted co-precipitation and performance as hyperthermic agents for biomedical applications. Heliyon. 2022;8:e09654.
- [CrossRef] [Google Scholar]
- Production of Mn3O4 nanoparticles from a manganiferous iron ore via reductive leaching, precipitation, and calcination. Hydrometallurgy. 2022;208:105810
- [CrossRef] [Google Scholar]
- Aseena, S., Abraham, N., Suresh Babu, V., 2021. Morphological and optical studies of zinc doped cerium oxide nanoparticles prepared by single step co-precipitation method. Mater. Today Proc. https://doi.org/10.1016/j.matpr.2021.05.636.
- Mercaptocarboxylic acid intercalated MgAl layered double hydroxide adsorbents for removal of heavy metal ions and recycling of spent adsorbents for photocatalytic degradation of organic dyes. Sep. Purif. Technol.. 2022;289:120741
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of malachite green by magnetic photocatalyst. Synth. React. Inorg. Met. Nano-Metal Chem.. 2016;46:458-463.
- [CrossRef] [Google Scholar]
- Facile fabrication of hematite nanoparticles from Egyptian insecticide cans for efficient photocatalytic degradation of rhodamine B dye. J. Mater. Res. Technol.. 2020;9:1652-1661.
- [CrossRef] [Google Scholar]
- Effective photocatalytic degradation of malachite green dye by Fe(III)-Cross-linked Alginate-Carboxymethyl cellulose composites. J. Photochem. Photobiol. A Chem.. 2022;428:113867
- [CrossRef] [Google Scholar]
- Application of mesoporous silica nanoparticles modified with Dibenzoylmethane as a novel composite for efficient removal of Cd(II), Hg(II), and Cu(II) ions from aqueous media. J. Inorg. Organomet. Polym. Mater.. 2020;30:2182-2196.
- [CrossRef] [Google Scholar]
- Effect of precipitating agents on the magnetic and structural properties of the synthesized ferrimagnetic nanoparticles by co-precipitation method. Powder Technol.. 2022;401:117298
- [CrossRef] [Google Scholar]
- Synthesis and characterization of zinc oxide nanoparticles via oxalate co-precipitation method. Mater. Lett. X. 2022;13:100126
- [CrossRef] [Google Scholar]
- Malachite green “a cationic dye” and its removal from aqueous solution by adsorption. Appl. Water Sci.. 2017;7:3407-3445.
- [CrossRef] [Google Scholar]
- Simultaneous removal of heavy metals and dyes in water using a MgO-coated Fe3O4 nanocomposite: role of micro-mixing effect induced by bubble generation. Chemosphere. 2022;294:133788
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce–ZnO nano-flowers under visible light. J. Environ. Manage.. 2020;258:110043
- [CrossRef] [Google Scholar]
- Photocatalytic performance of Sn-doped TiO2 nanostructured mono and double layer thin films for Malachite Green dye degradation under UV and vis-lights. J. Hazard. Mater.. 2007;144:140-146.
- [CrossRef] [Google Scholar]
- Surfactant assisted spectroscopic application of cadmium oxide nanoparticles prepared via co-precipitation method. Mater. Today Proc.. 2021;50:48-52.
- [CrossRef] [Google Scholar]
- Co-precipitation synthesis of CuCo2O4 nanoparticles for supercapacitor electrodes with large specific capacity and high rate capability. Electrochim. Acta. 2021;397:139306
- [CrossRef] [Google Scholar]
- Efficient photocatalytic degradation of Malachite green dye using facilely synthesized cobalt oxide nanomaterials using citric acid and oleic acid. J. Phys. Chem. Solids. 2021;155:110125
- [CrossRef] [Google Scholar]
- Synthesis of borohydride nanoparticles at room temperature by precipitation. Int. J. Hydrogen Energy. 2021;46:24286-24292.
- [CrossRef] [Google Scholar]







